Open Access Article
Open Access ArticleHyaluronic acid/chitosan/pectin based edible composite antioxidant coatings for the preservation of fresh apricots
Anfal A.
Al-Temimi
 a,
Sawsan A.
Al-Hilifi
a,
Sawsan A.
Al-Hilifi
 *a,
Rawdah M.
Al-Ali
*a,
Rawdah M.
Al-Ali
 a,
Le N. M.
Dinh
b,
Yin
Yao
c and
Vipul
Agarwal
a,
Le N. M.
Dinh
b,
Yin
Yao
c and
Vipul
Agarwal
 *b
*b
aDepartment of Food Science, College of Agriculture, University of Basrah, Iraq. E-mail: sawsan.hameed@uobasrah.edu.iq; agarwalvipul84@gmail.com
bCluster for Advanced Macromolecular Design (CAMD), School of Chemical Engineering, University of New South Wales, Sydney, NSW 2052, Australia
cMark Wainwright Analytical Centre, University of New South Wales, Sydney, NSW 2052, Australia
First published on 16th October 2025
Abstract
Apricots have a considerably short shelf-life with high sensitivity to degradation. In this study, hyaluronic acid is blended with pectin and chitosan to produce active edible coatings for the preservation of fresh postharvest apricots and extend their shelf-life. The objective of this study is to study the interplay between hyaluronic acid and pectin in edible coatings. The blended coatings are designed to take advantage of the antioxidant properties of both hyaluronic acid and pectin. The fruit preservation effectiveness of developed coatings is investigated over a 21 days storage period. The developed edible coatings exhibit significant fruit preservation characteristics in terms of weight loss, pH, titratable acidity and total solids content, and maintaining the antioxidant properties of coated fruits as measured through total phenolic content, change in ascorbic acid content and 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay compared to uncoated control fruits. We observed that a fine balance between the amount of hyaluronic acid and pectin is required to achieve optimal fruit preservation during the 21 days of storage with the best performance recorded for coatings containing marginally higher concentration of hyaluronic acid than pectin.
Sustainability spotlightThe significance of the present study lies in developing natural active biodegradable food packaging coatings. This study addresses the ever-growing problem of fresh food spoilage reducing their shelf-life and associated economic and environmental burden by developing active edible coatings based on polysaccharides hyaluronic acid and pectin and a natural polymer chitosan. The present study reports the intricate interplay between the different polysaccharides used in developed edible coatings. Based on the prepared active edible coatings, it was observed that to obtain the best apricot fruit preservation performance a fine balance in the amount of hyaluronic acid and pectin is required. However, a relatively higher amount of hyaluronic acid compared to pectin is required for the best fruit preservation performance during the 21 days storage period. Furthermore, any deviation in edible coatings from this fine balance led to a significantly lower fruit preservation. This study highlights the need to consider interplay between different constituents in edible coatings and is therefore expected to make a valuable contribution to the field of edible food packaging. |
1. Introduction
Food packaging has become a necessity to prolong the shelf life and for long-distance transportation of perishable products such as fruits and vegetables.1–4 Given the increasing urbanization of the global population, perishable food items have to be transported for long-distances from farms to markets. Globalization has further aided the shipping of fresh produce from source to market, pushing the demand for effective packaging materials.1 A commercial food packaging material is expected to preserve coated fruits by limiting their ripening during transport, should be easy to apply and remove, and must be cheap and environmentally friendly. While the commercial interest in plastic packaging material is persistent, it has a major limitation of inedibility and adverse environmental impact.2 This has inspired interest in packaging material made from natural sources leading to the development of a class of active coatings.5 Such active coatings are edible and enhance the properties of coated food by preventing moisture loss and avoiding oxidation mediated degradation while inducing additional benefits such as antioxidant properties.3,4,6 The active coatings are also expected to be a biodegradable, semi-permeable barrier to gases and water vapor and also decrease the growth of microbes, delay dehydration and enzymatic browning during processing, and retain the natural sensory characteristics of coated products while extending their shelf-life.7–9 A range of natural proteins and polysaccharides-based active edible coatings have been developed made predominantly from chitosan, gelatin, alginate, starch and cellulose.7–11 There are additional advantages to using natural proteins and polysaccharides in active edible coatings such as optical transparency, barrier properties (oxygen and carbon dioxide permeability), sensory score (flavorless, tasteless, odorless) and antimicrobial properties due to their natural antioxidant characteristics.9,12Pectin is a naturally occurring heteropolysaccharide present in the cell walls of apple, pear, and citrus peel.13 Due to its natural origin, it has been approved by the Food and Drug Administration (FDA). This has led to its (pectin) widespread use in the food manufacturing industry as a stabilizer, thickening, and gelling agent in products such as beverages, jams, yogurt, fruity milk drinks, and ice cream. The FDA approval also inspired its (pectin) exploration as an active edible coating material.14 The neat pectin forms brittle coatings which in combination with its high hydrophilicity makes it unusable in edible coatings. Neat pectin-based coatings are highly moisture sensitive, making coated fruits vulnerable to moisture loss leading to their (fruit) degradation.15 Despite these disadvantages, pectin has several advantages such as good oxygen barrier and surface adhesion properties. To circumvent limitations with neat pectin, it is either chemically modified to introduce hydrophobicity or blended with other polysaccharides and natural extracts to prepare composite edible coatings.13,15–17
Hyaluronic acid is a natural polymer of disaccharides found in a range of products such as green leafy vegetables, root vegetables including potatoes and carrots, citrus fruits and soy products. It is also naturally found in skin, connective, epithelial and neural tissues. Hyaluronic acid is widely used in pharmaceutical and biomedical research in drug development and cosmetic industries. In our previous work, we reported the first example of the use of hyaluronic acid in active edible coatings.18 In that study, we demonstrated that hyaluronic acid-based coatings exhibit a significant preservation of postharvest strawberries thus enhancing their (coated strawberries) shelf-life. Hyaluronic acid improved intrinsic coated fruit properties such as weight loss, pH, titratable acidity (TA) and total solids content (TSS) but also significantly enhanced antioxidant properties of developed edible coatings.18
Apricots are globally the third most economical stone fruit.19 Apricots are rich in phytochemicals such as carotenoids, flavonoids, phenolics, and antioxidants which determine their taste, color, and nutrition. Some of the examples of phenolics in apricots include chlorogenic, gallic, ferulic, caffeic, 4-aminobenzoic, procatechin, salicylic, and p-coumaric acid while the flavonoids include quercetin, glycoside rutin, resveratrol, and vanillin.19 The change in phytochemical concentrations indicates the ripening and degradation of the fruit. Apricots are also highly perishable with limited storage life, attributed to the high respiratory and metabolic rate of fruits. The high metabolic rate induces rapid ripening of the fruit to the overripen stage resulting in textural softening, loss of flavor and decay.7 Edible coatings have been proposed as the most effective strategy to extend the shelf-life of fresh apricots. For example, Morsy and Rayan explored alginate/chitosan/gellan gum coatings to preserve postharvest apricots and compared their performance against uncoated control fruits.7 They observed that coated fruits retained their biochemical characteristics (e.g. pH, titratable acidity (TA), total soluble solids (TSS) and vitamin C), physical properties such as textural color and firmness compared to uncoated control fruits. The alginate/chitosan/gellan gum coatings also significantly inhibited oxidative enzymes including peroxidase and polyphenol oxidase, known to be the major degradation pathway in apricots, thus extending the shelf-life of coated fruits.7 Other examples of edible coatings specific to apricots have been based on blended chitosan, methyl cellulose and alginate showing the effectiveness of edible coatings in extending the shelf-life of coated fruits.20–25 It has been reported that pectin modification, which is attributed to the loss of chelate-soluble pectin from fruit cell walls, during storage is also a reason behind the softening of fruit.17 The use of both pectin and hyaluronic acid in edible coatings is still in its infancy in general but has not been explored for apricot preservation.
In this study, we investigated the effects of pectin and hyaluronic acid in edible coatings. The active edible coatings were prepared by blending different ratios of pectin and hyaluronic acid while keeping the concentration of chitosan the same. Chitosan was included due to its well-established intrinsic antioxidant and antimicrobial activity.11,18,26 The ratio of pectin and hyaluronic acid were varied to investigate (i) the substitution effect of one polysaccharide over other, (ii) which polysaccharide is more important for the preservation of fresh postharvest apricots. The obtained coatings were assessed for physicochemical properties and fruit preservation effectiveness on fresh apricots. We observed that the best fruit preservation effectiveness in edible coatings with marginally higher concentration of hyaluronic acid than pectin. It can be hypothesized that potential interactions between the polysaccharides present in coatings with the surface of coated fruits could have influenced the observed positive preservation outcome.
2. Materials and methods
2.1 Materials
Hyaluronic acid (HA), pectin (P; galacturonic acid ≥74), 2,6-dichloroindophenol and gallic acid (GAE) were purchased from Sigma. Chitosan (CS; 91.3% degree of deacetylation), 1,1-diphenyl-2-picrylhydrazyl (DPPH), ascorbic acid, Folin–Ciocalteu reagent, sodium hydroxide, sodium carbonate, oxalic acid, methanol and phenolphthalein were purchased from Merck (Merck, Germany). All commercial chemicals used in this work were analytical grade. The packaging properties of the developed edible coatings were investigated on locally purchased apricots from Basrah city markets (Basrah, Iraq).2.2 Coating formation
The pectin:chitosan:hyaluronic acid (P:CS:HA) coatings were prepared by mixing varying amounts of pectin and hyaluronic acid while maintaining the concentration of chitosan consistent. We first prepared individual solutions of pectin (2 wt% in distilled water), chitosan (0.5 g in 1% acetic acid) and hyaluronic acid (0.2 wt% in distilled water). The different volumes of three components (P, C, HA) were added to prepare different coatings, i.e. 7P:1CS:2HA, 6P:1CS:3HA, 5P:1CS:4HA, 4P:1CS:5HA, 3P:1CS:6HA and 2P:1CS:7HA. The mixed solutions of each composition were divided into two halves with one aliquot poured into a 15 mL Petri dishes to prepare standalone coatings and the other half used to coat fresh fruits.2.3 Fourier transform infrared spectrometer (FTIR)
The FTIR analysis was conducted on standalone coatings by using a Bruker ATR-FTIR (Germany) operating in the wavelength range of 400 to 4000 cm−1 at a resolution of 4 cm−1. Data is presented as the average 32 scans on each coating sample.2.4 Thermogravimetric analysis (TGA)
The thermal properties of coatings were investigated on standalone coatings by using a TA Instruments TGA Q5000. Each coating sample (2–5 mg) was loaded a platinum sample holder and subjected to a temperature ramp from room temperature (20–30 °C) to 800 °C at a heating rate of 10 °C min−1 and a flow rate of 25 mL min−1 under an air atmosphere.2.5 Fruits
Fresh apricots were purchased from a local market in Basra, Iraq. The ripe, intact fruits of roughly similar size with an approximately weight of ∼35 g each were selected. They were carefully washed with distilled water to remove dirt and impurities, then dried with paper towels and arranged in metal trays prior to coating them. The fruits were divided into seven groups each comprising 40 fruits.2.6 Coating of fruits
The apricots were completely immersed for 3 min in each coating solution. After that, the fruits were placed on a metal clip and the excess coating solution was allowed to drip off. Next, the fruits were left to dry for 1 h at room temperature (∼25 °C). Uncoated fruits were taken as control. All untreated (control) and treated fruits were preserved in cork boxes, and stored in refrigeration at ∼5 °C. The fruit quality assessment of each treatment was evaluated regularly after 0, 3, 6, 9, 12,15, 18 and 21 days.2.7 Total soluble solids (TSS), pH, titratable acidity (TA)
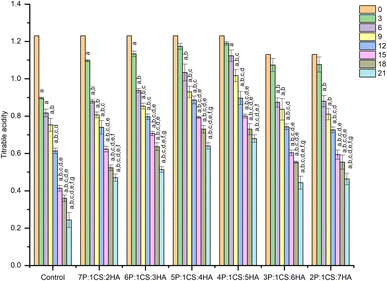
Apricot juice of fruits from each group was used to determine TSS, pH and TA. Fruit juice was obtained by mashing 25 g of fruit pulp in 100 mL of distilled water and the washed mixture was filtered using the Whatman paper. A digital refractometer (A87117, Bellingham, UK) was used to determine the TSS with the data presented as °Brix. In the case of TA, fruit juice was titrated against 0.1 N NaOH using phenolphthalein as an indicator until a permanent pink color appeared. The data is presented as a percentage of citric acid equivalent based on fresh fruit weight. To determine the pH, juice samples were subjected to a digital pH meter (pH-EMCO-256071, Japan) at an ambient temperature.2.8 Weight loss
The change in the weight of coated and uncoated fruits was studied to determine the loss in weight over the 21 days storage period. The fruits were weighed at different time points and the difference between the weight at day 0 and the specific day was recorded. Data is presented as a percentage (n = 5).2.9 Determination of total phenolic content (TPC)
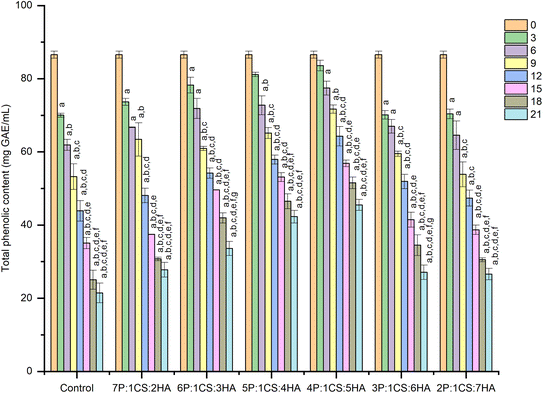
The Folin–Ciocalteu method was used to determine the TPC of coated and uncoated fruits using gallic acid as a standard. At every specified time point, freshly obtained fruit juice (0.5 mL) was mixed with Folin–Ciocalteu reagent (0.5 mL) and allowed to react in the dark for 10 min. Next, to this mixture, 1 mL of sodium carbonate solution (30%) was added and allowed to further react for 1 h in the dark. After which the obtained coloured solution was subjected to UV/Vis analysis with the absorbance measured at 760 nm. Gallic acid was used as a standard to determine the TPC content in untreated and treated fruits. Data is presented as mg of gallic acid equivalent to apricot fruit (mg GAE per mL) (n = 3).2.10 Determination of ascorbic acid
At specific time point ascorbic acid content was determined by first mashing 2 g of fruit pulp with 50 mL of oxalic acid (2% (v/v) in water), following which 10 mL of the solution was titrated against 2,6-dichloroindophenol (0.25 g L−1 in distilled water) to obtain a pink endpoint (color should persist for ≥15 s). Data is presented as mg of ascorbic acid per 100 mL sample (n = 3).2.11 Antioxidant activity
The antioxidant activity was determined using the standard 2,2-diphenyl-1-picryhydrazyl (DPPH) assay using the previously reported method.18,27 Briefly, a fresh fruit extract was prepared by smashing 25 g of fruit pulp in 100 mL of distilled water. Next, 1 mL of DPPH solution (0.01% in methanol) was mixed with 1 mL of fresh fruit extract and allowed to react for 30 min in dark at room temperature. After which the absorbance of the obtained colored solution was recorded at 517 nm.2.12 Statistics
The results for coating performance on treated fruits are expressed as mean ± standard deviation and analyzed using one-way analysis of variance (ANOVA). Significance was evaluated using a Bonferroni posthoc analysis and set at 95% confidence (p < 0.05).3. Results & discussion
3.1 Physicochemical characterisation of composite coatings
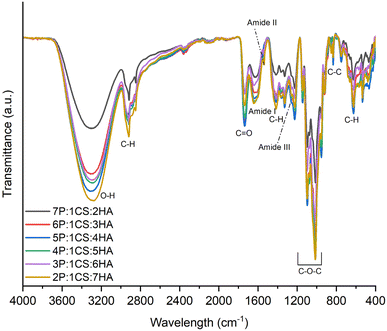
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional groups contributed from the carboxyl functional groups present on pectin and hyaluronic acid.28,29 We observed bands at ∼1645, 1538 and 1258 cm−1 which are typically associated with the three characteristic amide bands –C
O functional groups contributed from the carboxyl functional groups present on pectin and hyaluronic acid.28,29 We observed bands at ∼1645, 1538 and 1258 cm−1 which are typically associated with the three characteristic amide bands –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations (amide I), –N–H bending vibrations and –C–N stretching vibrations (amide II) and –C–N and –C–O stretching vibrations, –N–H and –O–C–N bending vibrations (amide III), respectively.18,30–33 The presence of amide groups (from the N-acetyl-D-glucosamine units) in hyaluronic acid could be attributed to the observed bands.28 The possibility of potential chemical interactions between the nucleophilic primary amine groups in chitosan with the carboxylic acid functional groups in pectin and hyaluronic acid forming some amide bonds is also of consideration. In all coatings, characteristic bands for polysaccharides are observed including at 1120, 1055, and 1014 cm−1 ascribed to –C–O–C– stretching vibrations, bands at ∼1415, 950, 830 and 625 cm−1 can be assigned to –C–H deformation, –CH3 rocking, –C–C rocking, –C–C stretching, respectively.32–35 The bands specific to the –CH3 functional group found in pectin and hyaluronic acid are observed at 980, 1325 and 2850 cm−1 attributed to –CH3 rocking, symmetrical bending and stretching, respectively.28,29 Based on the FTIR analysis, it can be deduced that all three major constituents (pectin, chitosan and hyaluronic acid) are present in prepared coatings particularly due to the presence of nitrogen containing functional groups associated with chitosan, amide groups of hyaluronic acid and methyl groups contributed by pectin and hyaluronic acid.
O stretching vibrations (amide I), –N–H bending vibrations and –C–N stretching vibrations (amide II) and –C–N and –C–O stretching vibrations, –N–H and –O–C–N bending vibrations (amide III), respectively.18,30–33 The presence of amide groups (from the N-acetyl-D-glucosamine units) in hyaluronic acid could be attributed to the observed bands.28 The possibility of potential chemical interactions between the nucleophilic primary amine groups in chitosan with the carboxylic acid functional groups in pectin and hyaluronic acid forming some amide bonds is also of consideration. In all coatings, characteristic bands for polysaccharides are observed including at 1120, 1055, and 1014 cm−1 ascribed to –C–O–C– stretching vibrations, bands at ∼1415, 950, 830 and 625 cm−1 can be assigned to –C–H deformation, –CH3 rocking, –C–C rocking, –C–C stretching, respectively.32–35 The bands specific to the –CH3 functional group found in pectin and hyaluronic acid are observed at 980, 1325 and 2850 cm−1 attributed to –CH3 rocking, symmetrical bending and stretching, respectively.28,29 Based on the FTIR analysis, it can be deduced that all three major constituents (pectin, chitosan and hyaluronic acid) are present in prepared coatings particularly due to the presence of nitrogen containing functional groups associated with chitosan, amide groups of hyaluronic acid and methyl groups contributed by pectin and hyaluronic acid.
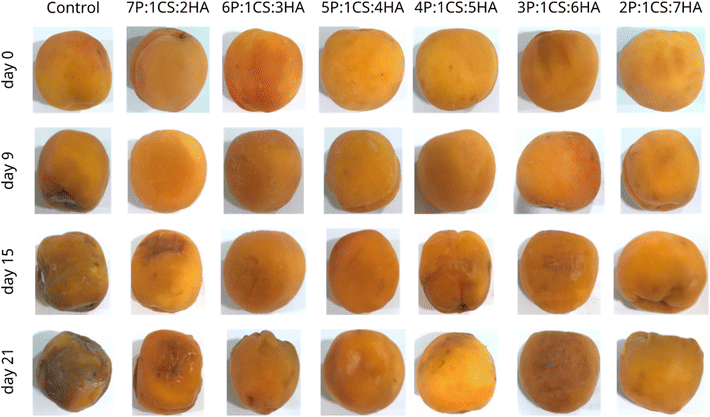
3.2 Effect of composite edible coatings on postharvest quality of apricots
Based on coating performance results on postharvest apricots, it is hypothesized that pectin and hyaluronic acid are not replaceable by each other as increasing pectin or hyaluronic acid while reducing the other polysaccharide (hyaluronic acid and pectin) in developed coatings significantly reduce their effectiveness in preserving the coated fruits.
4. Conclusion
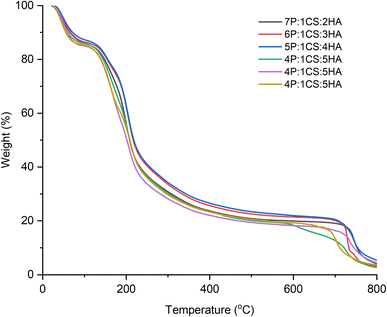
Food packaging has become pivotal for commercial sales of fresh produce, to extend the shelf-life of fruits and vegetables to account for transportation time from farms to markets. Edible coatings have become a necessity in preserving fresh fruits to preserve them from decay and potential bruising and damage from handling and transport. In this study, active edible coatings were developed from natural polymer polysaccharides for the preservation of apricots during a 21 days storage period. Getting inspiration from our previous work showing the protective effect of hyaluronic acid in edible coatings, we explored the interplay between it (hyaluronic acid) and pectin in this study. The aim of this work was to investigate if hyaluronic acid can be substituted with pectin without impacting the fruit preservation characteristics in edible coatings. The presence of the three polysaccharides was determined using FTIR. No significant impact of changing hyaluronic acid and pectin in coatings was observed on their thermal stability. Compared to uncoated fruits, a significant enhancement in the shelf-life was observed in coated fruits as studied from weight loss, changes in pH, titratable acidity and total soluble solids, and overall antioxidant characteristics including total phenolic content, ascorbic acid amount and DPPH activity. The best fruit preservation performance was observed for the 4P:1CS:5HA followed by 5P:1CS:4HA coatings indicating that a fine balance in the amount of hyaluronic acid and pectin is required to achieve optimal response. However, a relatively higher amount of hyaluronic acid compared to pectin is required for the best fruit preservation performance. Furthermore, any deviation in edible coatings from this fine balance led to significantly lower fruit preservation.Conflicts of interest
Authors declare no conflict of interest.Data availability
Data will be made available upon reasonable request from the authors.Acknowledgements
V.A. acknowledges the National Health and Medical Research Council (NHMRC), Australia, for an Early Career Fellowship (GNT1139060) and UNSW Safety Net Fellowship. The authors are grateful to the College of Agriculture, Basrah University for their infrastructural support.References
- J.-W. Han, L. Ruiz-Garcia, J.-P. Qian and X.-T. Yang, Compr. Rev. Food Sci. Food Saf., 2018, 17, 860–877 CrossRef PubMed.
- A. Trajkovska Petkoska, D. Daniloski, N. M. D'Cunha, N. Naumovski and A. T. Broach, Food Res. Int., 2021, 140, 109981 CrossRef CAS PubMed.
- S. F. Abdulla, R. Shams and K. K. Dash, Environ. Sci. Pollut. Res., 2024 DOI:10.1007/s11356-024-32806-z.
- P. R. Yaashikaa, R. Kamalesh, P. Senthil Kumar, A. Saravanan, K. Vijayasri and G. Rangasamy, Food Res. Int., 2023, 173, 113366 CrossRef CAS.
- S. Yildirim, B. Röcker, M. K. Pettersen, J. Nilsen-Nygaard, Z. Ayhan, R. Rutkaite, T. Radusin, P. Suminska, B. Marcos and V. Coma, Compr. Rev. Food Sci. Food Saf., 2018, 17, 165–199 CrossRef PubMed.
- F. J. Blancas-Benitez, B. Montaño-Leyva, L. Aguirre-Güitrón, C. L. Moreno-Hernández, Á. Fonseca-Cantabrana, L. D. C. Romero-Islas and R. R. González-Estrada, Food Control, 2022, 139, 109063 CrossRef CAS.
- N. E. Morsy and A. M. Rayan, J. Food Meas. Char., 2019, 13, 3173–3182 CrossRef.
- J. Peerzada Gh, B. J. Sinclair, G. K. Perinbarajan, R. Dutta, R. Shekhawat, N. Saikia, R. Chidambaram and A.-T. Mossa, Eur. Food Res. Technol., 2023, 249, 1935–1952 CrossRef CAS.
- L. Kumar, D. Ramakanth, K. Akhila and K. K. Gaikwad, Environ. Chem. Lett., 2022, 20, 875–900 CrossRef CAS.
- S. A. Al-Hilifi, R. M. Al-Ali, O. T. Al-Ibresam, N. Kumar, S. Paidari, A. Trajkovska Petkoska and V. Agarwal, Gels, 2022, 8, 645 Search PubMed.
- S. A. Al-Hilifi, O. T. Al-Ibresam, R. R. Al-Hatim, R. M. Al-Ali, N. Maslekar, Y. Yao and V. Agarwal, J. Comput. Sci., 2023, 7, 94 Search PubMed.
- H. Yong and J. Liu, Compr. Rev. Food Sci. Food Saf., 2021, 20, 2106–2145 CrossRef CAS PubMed.
- H. Rohasmizah and M. Azizah, Appl. Food Res., 2022, 2, 100221 CrossRef CAS.
- A. Roman-Benn, C. A. Contador, M.-W. Li, H.-M. Lam, K. Ah-Hen, P. E. Ulloa and M. C. Ravanal, Food Chem. Adv., 2023, 2, 100192 CrossRef.
- S. Roy, R. Priyadarshi, Ł. Łopusiewicz, D. Biswas, V. Chandel and J.-W. Rhim, Int. J. Biol. Macromol., 2023, 239, 124248 CrossRef CAS PubMed.
- J. R. Nastasi, V. Kontogiorgos, V. D. Daygon and M. A. Fitzgerald, Trends Food Sci. Technol., 2022, 120, 193–211 CrossRef CAS.
- Y. Chen, F. Chen, S. Lai, H. Yang, H. Liu, K. Liu, G. Bu and Y. Deng, Eur. Food Res. Technol., 2013, 237, 987–993 Search PubMed.
- S. A. Al-Hilifi, R. M. Al-Ali, L. N. M. Dinh, Y. Yao and V. Agarwal, Int. J. Biol. Macromol., 2024, 259, 128932 CrossRef CAS.
- O. Alajil, V. R. Sagar, C. Kaur, S. G. Rudra, R. R. Sharma, R. Kaushik, M. K. Verma, M. Tomar, M. Kumar and M. Mekhemar, Foods, 2021, 10, 1344 CrossRef CAS.
- L. Zhang, F. Chen, S. Lai, H. Wang and H. Yang, LWT, 2018, 96, 604–611 CrossRef CAS.
- A. Ayoubi, M. Balvardi and F. Mahmoudi-kordi, J. Food Meas. Char., 2022, 16, 4025–4035 CrossRef.
- E. H. A. Algarni, I. A. Elnaggar, A. E.-w. N. Abd El-wahed, I. M. Taha, H. A. AL-Jumayi, S. M. Elhamamsy, S. F. Mahmoud and A. Fahmy, Polymers, 2022, 14, 2227 CrossRef CAS.
- E. Ayranci and S. Tunc, Food Chem., 2004, 87, 339–342 CrossRef CAS.
- A. Gull, N. Bhat, S. M. Wani, F. A. Masoodi, T. Amin and S. A. Ganai, Food Chem., 2021, 349, 129149 CrossRef CAS PubMed.
- L. Monjazeb Marvdashti, S. Abdulmajid Ayatollahi, B. Salehi, J. Sharifi-Rad, A. Abdolshahi, R. Sharifi-Rad and F. Maggi, J. Food Saf., 2020, 40, e12805 CrossRef CAS.
- M. S. Nair, M. Tomar, S. Punia, W. Kukula-Koch and M. Kumar, Int. J. Biol. Macromol., 2020, 164, 304–320 CrossRef CAS PubMed.
- A. Nagarajan, N. Rizwana, M. Abraham, M. Bhat, A. Vetekar, G. Thakur, U. Chakraborty, V. Agarwal and M. Nune, J. Mater. Sci.: Mater. Med., 2023, 34, 49 CrossRef CAS PubMed.
- A.-M. Vasi, M. I. Popa, M. Butnaru, G. Dodi and L. Verestiuc, Mater. Sci. Eng., C, 2014, 38, 177–185 CrossRef CAS PubMed.
- A. Baum, M. Dominiak, S. Vidal-Melgosa, W. G. T. Willats, K. M. Søndergaard, P. W. Hansen, A. S. Meyer and J. D. Mikkelsen, Food Bioproc. Tech., 2017, 10, 143–154 CrossRef CAS.
- V. Agarwal, A. G. Panicker, S. Indrakumar and K. Chatterjee, Int. J. Biol. Macromol., 2019, 133, 382–390 CrossRef CAS.
- D. S. McLean, V. Agarwal, K. R. Stack, J. Horne and D. E. Richardson, Bioresources, 2011, 6, 4168–4180 CAS.
- T. Ghosh and V. Katiyar, Int. J. Biol. Macromol., 2022, 211, 116–127 CrossRef CAS PubMed.
- F. Li, Y. Yan, C. Gu, J. Sun, Y. Han, Z. Huangfu, F. Song and J. Chen, Foods, 2022, 11, 3548 CrossRef CAS PubMed.
- L. N. Ramana, L. N. M. Dinh and V. Agarwal, Nanoscale Adv., 2021, 3, 3513–3521 RSC.
- O. T. Al-Ibresam, R. M. Al-Ali, A. A. Al-Temimi, L. N. M. Dinh, Y. Yao, S. A. Al-Hilifi and V. Agarwal, Adv. Sustainable Syst., 2025, 9, 2400928 CrossRef CAS.
- I. N. Nasef, Postharvest Biol. Technol., 2018, 138, 1–10 CrossRef CAS.
- D. Valero, H. M. Díaz-Mula, P. J. Zapata, F. Guillén, D. Martínez-Romero, S. Castillo and M. Serrano, Postharvest Biol. Technol., 2013, 77, 1–6 CrossRef CAS.
- A. A. Al-Temimi, A.-E.-B. Al-Mossawi, S. A. Al-Hilifi, S. A. Korma, T. Esatbeyoglu, J. M. Rocha and V. Agarwal, Metabolites, 2023, 13, 465 CrossRef CAS.
- N. Rizwana, V. Agarwal and M. Nune, Antioxidants, 2022, 11, 72 CrossRef CAS.
- N. Rizwana, N. Maslekar, K. Chatterjee, Y. Yao, V. Agarwal and M. Nune, ACS Appl. Nano Mater., 2024, 7, 18177–18188 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |