 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recent advances in chemical upcycling of plastic waste: microwave-assisted heating and heterogeneous catalysis
Rachel
Breen
ab,
Justin D.
Holmes
 ab and
Gillian
Collins
ab and
Gillian
Collins
 *ab
*ab
aSchool of Chemistry, University College Cork, Cork T12 YN60, Ireland. E-mail: g.collins@ucc.ie
bAMBER Centre, Sustainability Institute, University College Cork, Cork T23 XE10, Ireland
First published on 17th November 2025
Abstract
The chemical upcycling of plastic waste offers a sustainable pathway to transform discarded polymers into valuable chemical feedstocks and fuels. Among emerging approaches, microwave (MW)-assisted heating has gained significant attention for its ability to deliver rapid, uniform energy input, dramatically reducing reaction times compared with conventional thermal methods. When coupled with heterogeneous catalysis, MW irradiation can lower activation barriers for depolymerisation, enhance product selectivity and reduce overall energy demand. Heterogeneous catalysts also provide advantages in recovery, reuse and process scalability, making them attractive for industrial adoption. Recent research has focused on tailoring catalyst composition, structure, and surface properties to optimise MW–catalyst interactions across a wide range of polymer types, including polyesters, polyolefins, polystyrene, polyamides and polyvinyl chlorides. This review summarises the fundamental principles of MW-assisted catalysis and critically examines recent advances in catalyst design, reaction optimisation and mechanistic understanding.
Broader contextThe accumulation of plastic waste is a pressing environmental challenge that demands comprehensive, multifaceted solutions. Meaningful progress will combine design for circularity, improved collection, sorting and conversion strategies that upgrade mixed streams into higher-value products with reduced emissions. In this context, microwave-assisted heating paired with heterogeneous catalysis provides rapid, targeted energy input and controllable pathways using recoverable catalysts, enabling plastic depolymerisation and upgrading under energy-efficient conditions. These advances support circular economy objectives by converting low-value waste into useful chemicals and materials. More broadly, integrating catalyst design, reactor engineering, realistic feedstocks and robust techno-economic and life-cycle assessment will be key to scaling low-emission solutions to plastic pollution. |
1. Introduction
A material once described as the ‘greatest finding in the millennium’, now poses an undeniable threat to the future of our planet. Owing to its tensile strength, lightweight and versatility, plastic production has increase 200-fold since the 1940's.1 Plastic pollution has become widespread across the environment, accumulating in both terrestrial and marine ecosystems and even within human bodies. It poses significant risks due to the release of chemical additives through leaching and degradation, as well as the formation of microplastics. These issues stem largely from the global mismanagement of plastic waste and the limitations of current recycling methods.About 400 million tons of plastic is produced globally each year. Worldwide, it is estimated that only 9% of plastic waste is recycled by mechanical recycling.2,3 This involves sorting, washing, grinding, melting and reprocessing of plastic waste into pellets for new products which often results in a downcycled, lower quality product. Polyolefins such as low-density polyethylene (LDPE) and polypropylene (PP) are generally unsuitable for mechanical recycling due to degradation of mechanical properties during processing as well as the presence of additives and multi-layered plastic structures.4 While advancements in sorting technologies, decontamination protocols and pre-treatments have enhanced the viability of mechanical recycling as a valorisation pathway for certain plastic types, significant challenges remain including the limited capacity to process mixed and contaminated plastic wastes.5 Incineration allows for energy recovery and it is estimated that about 10% of household energy requirements in Europe are currently fulfilled using this method.6 However, incineration contributes to greenhouse gas emissions through the release of CO2 and may also emit persistent organic pollutants (POP) which pose significant environmental and health risks.7 According to reports by the Organisation for Economic Co-operation and Development (OECD),3 50% of global waste plastic is disposed of in regulated landfill sites.2 While landfilling is a low energy method that does not require sorting of plastic waste, it poses long term environmental risks such as the leaching of microplastics, additives and plasticisers into soil and water streams. The drawbacks associated with the current plastic waste management techniques have resulted in increasing attention towards chemical recycling of plastic waste. Chemical recycling enables upcycling of plastic waste by breaking them into original monomers (closed-loop recycling), which can be repolymerised into high-quality polymers. Alternatively, plastics can be converted into high quality fuels, feedstock chemicals or materials (open-loop recycling).8–10 While energy requirements for chemical recycling methods are generally high to overcome polymer stability, approaches such as MW-assisted heating and catalyst development are being investigated to reduce energy usage and maximise efficiency.11,12 The European Commission's Joint Research Centre found that based on life cycle assessments (LCA), mechanical and chemical recycling are far more beneficial from a climate perspective than landfill and incineration for energy recovery.13 To address the limitations inherent to mechanical recycling, chemical recycling has emerged as a complementary approach capable of processing plastic waste streams for the recovery of monomers or valuable chemical feedstock. A summary of current plastic waste management methods compared with chemical recycling is presented in Fig. 1(a).
 | ||
| Fig. 1 (a) Outline of current plastic waste management strategies compared with chemical recycling.3 (b) Summary of key chemical recycling pathways for polyesters, polyolefins and other plastics. | ||
A summary of chemical recycling pathways for polyesters, polyolefins and other plastics is illustrated in Fig. 1(b). Polyesters such as PET, PLA, PES and polycarbonates (PC) are well-suited to selective depolymerisation methods such as alcoholysis, glycolysis, hydrolysis and aminolysis. These processes yield defined monomers, green solvents and chemical feedstocks that can be directly repolymerised, enabling closed-loop recycling. In contrast, polyolefins such as LDPE, PP, and high-density polyethylene (HDPE), due to their non-polar, saturated hydrocarbon backbones, primarily degrade via energy-intensive processes such as pyrolysis, catalytic cracking or oxidative degradation. These methods yield a broader spectrum of products such as gases, liquid fuels, olefins, aromatics, waxes, organic acids and carbon nanomaterials, making them better suited for open-loop recycling. Other polymers include polystyrene (PS), polyvinyl chloride (PVC), and polyamide (PA) and high temperature pyrolysis and cracking are typically reported for PS and PVC, while PA can be depolymerised in a closed-loop system, similar to polyesters.
Among the various chemical recycling technologies, MW-assisted heterogeneous catalysis has emerged as an energy-efficient approach by reducing reaction times and allowing for catalyst recyclability.14 Heterogeneous catalysts are particularly advantageous for industrial scale-up due to their ease of recovery and regeneration.15 Additionally, MW-assisted heating has been shown to significantly reduce reaction times when compared with conventional thermal heating for plastic degradation. When coupled with MW-assisted heating, heterogeneous catalysts have demonstrated excellent stability and catalytic performance. Interactions between MW and solid catalysts can produce special thermal effects or ‘hot spots’ as well as MW plasmas, accelerating reactions at the catalyst surface.16 MW-assisted heating has been shown to enhance activity, selectivity and stability of heterogeneous catalysts.
The importance of chemical recycling is emphasised by various review articles that have emerged in recent years. Several comprehensive reviews on the chemical recycling of polyethylene terephthalate (PET) have been reported.17–21 Broader reviews on the catalytic depolymerisation of polyesters have also been conducted which include reports on polycarbonates towards closed-loop recycling and upcycling.22–24 Li et al.25 carried out a comprehensive review on closed and open-loop recycling methods for biodegradable polylactic acid (PLA) plastics. A number of focused reviews have also explored chemical recycling routes for polyolefins.15,26–28 Qin and co-workers reviewed advanced catalytic systems for the chemical recycling of different plastic types,29 while Chu et al. emphasised the significance of rational catalyst design in chemical recycling processes.30 Luo and co-workers provided a comparative review of mechanical recycling and recent advances in chemical recycling technologies.31 Additionally, there is a growing recognition of the importance of undertaking assessments of economic and environmental sustainability of proposed methodologies.32–34
This review provides an overview of recent developments in chemical recycling technologies, with a particular emphasis on heterogeneous catalysts coupled with MW-assisted heating. The focus is placed on the role of MW in enhancing energy efficiency of catalytic depolymerisation reactions and recent advances in the design and application of advanced heterogeneous catalysts to promote MW absorption and improve process yield and selectivity. We will discuss the fundamentals of MW-assisted heating for catalytic applications and explore MW-assisted chemical recycling strategies for a range of polymer types including polyesters (PET, PLA, PES and PC), polyolefins (LDPE, HDPE, PP) and other plastics such as PA, PS and PVC.
2. Fundamentals of MW-assisted catalysis
MWs are a type of electromagnetic radiation, and range in wavelengths from 1 mm to 1 m and frequencies from 300 MHz to 300 GHz, with most lab-based MW reactors operating at a frequency of 2.45 GHz. When materials are subject to MW irradiation, the polarisation of dipoles or charges within the material cannot instantly follow the rapidly oscillating electric field, leading to a lag known as dielectric relaxation. This results in heat generation due to energy loss from the friction between molecules. The quantity of energy loss from the system due to electromagnetic relaxation depends on the dielectric and magnetic properties of a material.Selective heating of materials under MW irradiation can be related to the complex permeability and permittivity of the specific material, which affects magnetic and dielectric heating, respectively.35 The complex permittivity of a material determines a material's dielectric heating by a MW-electric field, and is defined as:
| ε* = ε′ − jε″ | (1) |
As dielectric heating is commonly reported for catalytic processes carried out under MW irradiation, most literature reported use of the loss tangent (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) to assess a materials susceptibility to penetration by electric field and subsequently dissipate the energy as heat. The loss tangent of a material can be calculated using:
δ) to assess a materials susceptibility to penetration by electric field and subsequently dissipate the energy as heat. The loss tangent of a material can be calculated using:
 | (2) |
A material with a high loss tangent can therefore be easily heated in a MW-electric field, while materials with low loss tangents are less susceptible to heating in a MW-electric field.
The complex permeability of a material determines the material's magnetic heating in a MW-magnetic field, and is defined as:
| μ* = μ′ − jμ″ | (3) |
The quantity of energy absorbed by the material which is dissipated as heat can be expressed as:
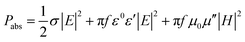 | (4) |
Solid materials have been classified as absorbers (dielectric), perfect conductors or insulators based on how they interact with MW-electric fields, illustrated in Fig. 2. Absorbers such as dielectric materials allow MWs to penetrate below their surface, perfect conductors such as metals reflect MWs with no penetration and insulators allow MWs to pass through them with little or no losses, for example, glass.
 | ||
| Fig. 2 Classification of solid materials and their interactions with MW conductors, dielectric and insulators, adapted from ref. 38 with permission from MDPI, Copyright 2020. | ||
In heterogeneous catalysis, MW heating has been reported to increase reaction efficiency and product selectivity due to rapid heating and hotspot formation at the catalyst surface, as well as the generation of micro-plasmas leading to accelerated activation of reactants.35Fig. 3(a) illustrates the distinct mechanisms of MW-induced hotspots compared with micro-plasmas highlighting their respective thermal and non-thermal catalytic activation pathways. Hot spots are localised regions of elevated temperature withing the catalyst or at its surface, arising from non-uniform MW absorption in areas with high dielectric losses.39 In contrast, micro-plasmas are localised electrical discharges consisting of ionised gas, typically composed of high energy electrons, ions and radicals. Micro-plasma formation occurs in regions of enhanced electromagnetic field often associated with sharp points or defects on a catalyst surface.40 Hotspots and micro-plasmas can coexist on a catalyst particle when a region contains both electric field enhancement points and strong dielectric zones. Thermal imaging has been used to capture hotspot formations at the contact point of silicon carbide (SiC) spheres with diameters ranging from 2.38 to 3.97 mm, as shown in Fig. 3(b).41 The purpose of this study was to directly observe local high temperature regions using in situ emission spectroscopy. The images and the corresponding temperature distribution profile along the contact region reveals localised high temperature zones resulting from increased electromagnetic fields at particle contact points under MW-irradiation. The temperature at the contact point was approximately 150 °C higher than surrounding regions. In the same study the temperature distributions were also modelled for the vicinal contact points of magnetite (Fe3O4) catalyst spheres under MW-irradiation shown in Fig. 3(c). The surface temperature at the contact point was reported to be 190 °C higher than surrounding regions due to the localised concentration of electromagnetic fields at the particle interfaces. The morphology of a solid catalyst material has also been shown to influence their dielectric properties, thereby affecting the spatial distribution of the electromagnetic field. A comparison between Cu2O cubes and spiked morphologies (Fig. 3d) showed that the complex permittivity of the spiked particles was 20% higher than cube-shaped particles. This enhancement was attributed to the increased electric field intensity at the sharp tips of the spiked nanostructures, which alters the polarisability and subsequently enhances dielectric properties beneficial for catalytic applications, as shown in Fig. 3(e).42
 | ||
| Fig. 3 (a) Schematic representation of hot spot and plasma formation on catalyst surface under MW irradiation, (b) thermal imaging to reveal hotspot formation on SiC spheres under MW-irradiation and temperature distribution profile, (c) temperature simulation data for hotspot formation on magnetite spheres under MW-irradiation reproduced from ref. 41 with permission from Nature Copyright 2019. (d) SEM images of Cu2O cubes and spikes and (e) illustration of calculated dipole density for spiked Cu2O at different volume fractions, reproduced from ref. 42 with permission from Elsevier Copyright 2018. | ||
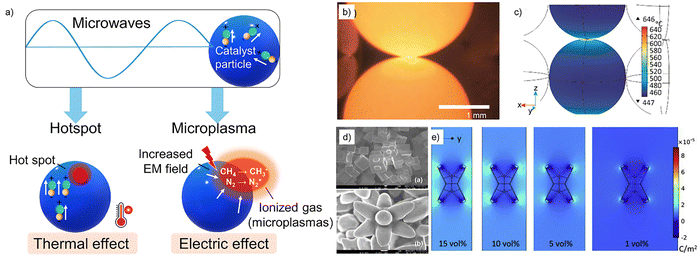
MW-assisted heating has gained significant attention for plastic depolymerisation reactions due to its rapid and volumetric heating capabilities, which can help to overcome the inherent thermal stability of polymers. MW-assisted heating has been widely reported to outperform conventional thermal heating for PET, PLA, LDPE and PP degradation reactions by reducing reaction time and lowering the overall energy consumption.43,44 Most plastics are generally poor MW absorbers because of long polymer chains which limit dipole rotation under an electromagnetic field resulting in low dielectric losses.45 However, the MW absorbing properties of polymers is highly dependent on their chemical composition. Polymers containing polar functionalities such as PET and PC exhibit an increased dielectric loss with increasing temperature, particularly above their glass transition temperature (Tg), under MW-irradiation.45 Polar solvents such as water or ethylene glycol are beneficial as they exhibit high dielectric losses under MW-irradiation leading to enhanced heating of the reaction solution. Additionally, MW-absorbing catalysts such as carbon nanomaterials46 or metal oxides can be used to further improve heating efficiency.47 In solvent-free plastic degradation reactions such as pyrolysis, solid MW absorbers are often added to facilitate efficient heating of the polymer. Solid MW absorbers convert MW irradiation to thermal energy by two mechanisms: dipole polarisation and dipole rotation, which are illustrated schematically in Fig. 4. In dipole polarisation, permanent or induced dipoles attempt to align with the rapidly oscillating electric field which generates internal friction and heat. In dipole rotation, the fluctuating electric field causes polar molecules to rotate back and forth as they attempt to align with the changing electric field.48
 | ||
| Fig. 4 Dipole polarisation and dipole rotation mechanisms of MW heating on AC as a MW-absorber for polyolefin pyrolysis, reproduced from ref. 48 with permission from Nature Copyright 2023. | ||
Effective MW heating in polymer degradation reactions typically requires the use of MW-absorbing catalysts, the addition of non-catalytic susceptors such as activated carbon, or the use of polar solvents. These strategies compensate for the inherently poor MW absorption of most polymers and enable efficient conversion of electromagnetic energy to heat. Catalytic MW-assisted degradation therefore represents a promising and potentially sustainable approach for converting waste polymers into valuable chemicals, offering rapid, energy-efficient processing with reduced environmental impact.
Microwave reactors are classified as multi-mode or single-mode systems based on their electromagnetic field distribution. Multi-mode reactors generate multiple field modes within a large cavity, enabling larger reaction volumes but resulting in non-uniform heating and limited reproducibility. In contrast, single-mode reactors maintain a single, well-defined electromagnetic field that provides uniform and reproducible heating, though they are restricted to small reaction volumes due to waveguide size limitations.49 Both multi-and single-mode reactors are used for lab-scale depolymerisation reactions. Despite limited reproducibility, multi-mode reactors would be most suited to industry scale up as they generally consist of large microwave cavities suitable for large scale or multiple samples, and they can operate at higher power maximums than single-mode reactors. For some microwave instruments, power is initially applied up to a set maximum and then reduced once the target temperature is reached, demonstrating a direct power–temperature relationship but limiting power input control in the system. The use of pressurised reaction vessels accelerates depolymerisation by enabling solvent superheating, which allows reactions to proceed at temperatures above the normal boiling point of the solvent. These vessels are typically equipped with built-in safety mechanisms such as spring-loaded pressure release systems to prevent over-pressurisation. For safety, especially when working with oxidisers such as peroxides or reactions that generate gas or volatiles, reaction vessels should generally be filled to no more than half of their total volume to accommodate thermal expansion and provide space for controlled pressure release if necessary. This may also limit scaling capacity of closed-system microwave depolymerisation reactions. In microwave-assisted pyrolysis, closed systems are less practical as condensation setups are typically required for collection of products. Microwave generators, typically multimode magnetrons, are generally coupled to the pyrolysis setup as a replacement for conventional thermal heating, rather than the reaction occurring within a dedicated microwave reactor as in depolymerisation studies. Recently, an increasing number of studies and emerging companies have focused on efficiently scaling up microwave-assisted depolymerisation processes. Ramopoulos et al.50 developed an industrial-scale microwave reactor for continuous alkaline hydrolysis of >12.5 kg h−1 PET, using a dual mode cavity and screw conveyor to ensure uniform heating and efficient microwave coupling. Microwave heating is being implemented commercially in the US in continuous pyrolysis systems converting polyolefins (HDPE, LDPE, PP, PS) into pyrolysis oil in volumes of 5 tons per day modules to serve local material recovery facilities.51
3. MW-assisted heterogeneous catalytic upcycling of polymers
In recent years, many studies have been conducted on MW-assisted catalytic degradation of different types of plastic. Here, we summarise some of the findings based on plastic types including polyesters, polyolefins and other plastics.3.1 MW-assisted heterogeneous catalytic upcycling of polyesters
Polyesters are a class of synthetic polymers characterised by the presence of ester functional groups in their main chain. Polyesters are formed through a condensation reaction between diols and dicarboxylic acids or their derivatives, resulting in long chains linked by ester bonds.52 They can be broadly categorised into thermoplastic and thermosetting types, each with distinct physical properties and applications.53 Polyesters are widely used in textiles, packaging, bottles, containers and engineering materials due to their durability, chemical resistance, and versatility.The chemical recycling of polyesters is well reported due to the presence of reactive ester bonds, allowing them to be efficiently broken down into their monomers and repolymerised, making them particularly suitable for closed loop recycling systems. Polyester linkages can be cleaved through trans-esterification reactions with a range of nucleophiles, enabling a variety of different depolymerisation routes such as hydrolysis,21 alcoholysis,54 glycolysis55 and amminolysis.56
The most common polyester is PET, known for its use in beverage bottles and synthetic fibres. PET is commonly synthesised via the condensation reaction of dimethyl terephthalate (DMT) or terephthalic acid (TPA) with EG.53 Glycolysis and aminolysis are among the most reported chemical recycling routes for PET. In glycolysis, PET undergoes a transesterification reaction with diols, typically EG, to yield the monomer bis(hydroxyethyl) terephthalate (BHET).55 In aminolysis, amines such as ethanolamine (EA) or methylamine (MA) act as nucleophiles which form amide linkages to yield terephthalamide derivates such as bis(2-hydroxyethyl) terephthalamide (BHETA) which are valuable chemicals for the synthesis of polymers, resin and surfactants.57
ZnO and ZnO-composites are well reported heterogeneous catalysts for PET glycolysis owing to their Lewis acidic nature, high thermal stability, selectivity, low toxicity and exhibit high performance after multiple cycles.58–60 The influence of catalyst morphology on MW-assisted PET glycolysis using EG was demonstrated in a study using ZnO platelets, revealing a strong correlation between ZnO morphology and catalytic activity.61 The favourable orientation of the Zn(001) surface facet on the nanoplatelets showed enhanced catalytic activity over other morphologies of ZnO. A size effect was also observed with yield increasing by more than 20% with particle size. The enhanced catalytic activity was attributed to smaller particle size for ZnO platelets over other morphologies such as plates, hexagonal disks and prisms resulting in a higher surface area. Additionally, the favouring exposure of the (001) facet for the platelets was attributed to the non-centrosymmetric crystal structure of ZnO resulting in a more polar hexagonal (001) facet increasing its ability to form hydrogen bonds. This work also demonstrated the effect of rapid MW heating, achieving a BHET yield of >95% in 45 min, compared with 60% yield under the same conditions using conventional thermal heating. Lastly, LCA found that BHET production via ZnO-catalysed glycolysis resulted in a significant decrease in greenhouse gas emissions when compared with current DMT-based synthesis methods and two Zn(OAc)2 homogeneous catalysts reported in the literature.62,63
Dispersing metal catalysts on a support material can enhance their stability, increase surface area and improve catalytic efficiency by maximising active site exposure. In this case, Kaolin supported-Nb2O5 is an effective catalyst for PET aminolysis using EA as a reagent reported by Revathi et al.,64 achieving high yields (95%) of bis(2-hydroxyethyl)terephthalamide (BHETA) in 30 min. For this work, PET was obtained from beverage bottles as well as coloured polyester fibres, and the catalyst also acted as an efficient absorbent of the colorant impurities. A Kaolin clay support was used to enhance the surface parameters of Nb2O5 and improve catalytic activity. The BET surface area increased from 13.54 m2 g−1 for Nb2O5 to 30.62 m2 g−1 for the Kaoiln–Nb2O5 and the BHETA yield obtained from both catalysts were 82% and 95%, respectively, which was attributed to the enhanced specific surface area.
The use of a multifunctional heterogeneous catalysts that can drive multiple reaction pathways is highly advantageous and significantly increases the practical value of the catalyst. A study by Anbarasu et al.65 reported the design and synthesis of In–ZnO/g-C3N4 and Sb–ZnO nanocomposites for both glycolysis and aminolysis of PET fibres. The addition of In and Sb were reported to improve the Lewis-acidity of the catalyst and lower the band gap energy, while immobilisation on a graphitic carbon nitride support was to enhance MW-absorbing properties of the catalyst. A 95% yield of BHETA was obtained after 15 min for the aminolysis reaction using EA while 92% BHET was obtained after 60 min for the glycolysis reaction. While these catalysts showed excellent activity and recyclability, shifting away from heavy and toxic metallic catalysts is essential for advancing green and sustainable chemistry in the chemical depolymerisation of plastics.
Similarly, Vinitha and coworkers66 reported the use of Ag/ZnO nanoparticles for the chemical recycling of coloured PET textiles. The catalysts showed bifunctionality as they were effective in both MW-assisted aminolysis and glycolysis of PET. Glycolysis was carried out using EG to yield BHET and diethylene glycol (DEG) to yield bis-[2-(2-hydroxy-ethoxy)-ethyl]terephthalate (BHEOET). Aminolysis was carried out using EA to yield BHETA and diethanolamine (DEA) to produce N1,N1,N4N4-tetrakis-(2-hydroxy-ethyl)-terephthalamide (BDHETA). The Ag/ZnO catalyst outperformed the ZnO catalyst for both glycolysis and aminolysis reactions which was attributed to a decrease in bandgap energy and decrease in crystallite size on incorporation of the Ag, enhancing the MW heating properties and Lewis acidity of the catalyst. The Ag/ZnO catalyst achieved a 90% yield of BHET using EG and 86% maximum yield of BHEOET using DEG after 30 min. The use of EA as an aminolysis reagent was more effective than DEA and achieved a maximum BHETA yield of 96% while the maximum yield of BDHETA achieved was 90% in 15 min of reaction time.
The development of metal-free catalysts is important in the transition towards more sustainable and cost-effective catalysis. Casey and coworkers43 reported mesoporous SiO2 functionalised with guanidine ligands as metal free organocatalysts. Guanidine compounds are strong bases that are effective homogeneous organocatalysts for PET glycolysis due to their strong Lewis base properties, enabling them to act as nucleophiles that increase the reactivity of EG towards the carbonyl group of PET.67 The guanidine-functionalised SiO2 catalysts were effective for the glycolysis of PET using EG as a solvent achieving an isolated BHET yield of 80% after 30 min, while 3 h was required to achieve similar yields under conventional thermal heating. Additionally, the catalysts displayed superior recyclability over successive MW cycles when compared with conventional thermal heating which resulted in faster catalyst deactivation. Another advantage of basic guanidine catalysts is their ability to promote the glycolysis of PET to bis(4-hydroxybutyl terephthalate) (BHBT), a useful monomer for synthesising polybutylene terephthalate (PBT). This reaction is achieved using 1,4-butanediol (BD) instead of EG as the glycolysis reagent. When PET glycolysis with 1,4-BD is carried out in the presence of a Lewis acid catalyst, such as a metal salt, the 1,4-BD tends to undergo an undesirable cyclisation reaction to form tetrahydrofuran (THF). This highlights the benefit of using a Lewis base catalyst like guanidine, which avoids this side reaction.
PLA is a bio-based aliphatic polyester synthesised primarily through the polymerisation of lactic acid derived from renewable resources.68 Production and use of PLA has increased as its bio-based and technically biodegradable nature, may make it a more sustainable option than traditional fossil derived polymers. While PLA can undergo industrial composting under controlled conditions its biodegradability is generally limited under ambient or marine environments, and concerns about its environmental persistence if improperly managed have been growing.69 Therefore, it is also critical to develop chemical recycling pathways for bio-based polymers such as PLA. Hydrolysis of PLA, typically carried out under acidic or basic conditions at elevated temperatures offers a pathway for lactic acid recovery, which can be repolymerised to produce PLA.70 Alcoholysis using ethanol or methanol in the presence of a catalyst such as Zn(OAc) can produce methyl lactate (MeLa) or ethyl lactate (EtLa), which are high value green solvents.71
The use of a phase-transfer catalyst such as hexadecyltrimethylammonium bromide (HTMAB) has been reported to enhance MW-assisted hydrolysis of PLA in aqueous NaOH to yield lactic acid.72 The catalyst facilitates the reaction by transporting hydroxide ions from the aqueous phase to the solid organic phase, enabling easier attack on the PLA chains. Optimal conditions for achieving over 90% PLA degradation involved heating at 100 °C for 10 min, with a maximum MW power of 10 W to reach this temperature. This represents a relatively mild and efficient depolymerisation pathway for PLA.
Lewis acid salts such as Zn(OAc) and Zn(II) triflate are excellent homogeneous catalysts for the methanolysis of PLA to yield MeLa.73 Under MW-assisted heating, the conversion of PLA to MeLa over these Zn salts was successful with >99% yield monomer yield achieved in 10 min at 160 °C. Under conventional heating, the methanolysis of PLA over a Zn(II) imino monophenolate complex catalyst was reported to yield 63% monomer in 30 min at 130 °C.74 While homogeneous catalysts such as the above mentioned metal salts are effective and result in short reaction times, heterogeneous catalysts provide the key advantage of easy separation and recovery after reaction. Guanidine functionalised porous SiO2 are effective heterogeneous catalysts for PLA methanolysis.43 A MeLa yield of 42% was achieved after 72 h of thermal heating at 130 °C, while a 30% MeLa yield was achieved after 30 min of MW-assisted heating at 130 °C, substantially reducing the reaction time. Density functional theory (DFT) modelled the interaction between the guanidine ligand (dicyanamide) with PLA and found that the N-rich environments in the ligand promoted the nucleophilic attack of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group on PLA.
O group on PLA.
Polyethylene furan-2,5-dicarboxylate (PEF) is also a bio-based polyester used in packaging, textiles and electronics. PEF can undergo glycolysis in the presence of EG to yield bis(2-hydroxyethyl) furan-2,5-dicarboxylate (BHEF) which can be repolymerised to PEF in a single condensation step. The MW-assisted glycolysis of PEF to BHEF over a ZnO catalyst was reported by Najmi and co-workers.75 Yields of BHEF of ∼90% were achieved in 40 min at 175 °C with 100 W of MW irradiation. In absence of the catalyst, large quantities of oligomers were present, however the addition of ZnO facilitated the rapid depolymerisation of oligomeric species to monomeric BEF owing to its Lewis acid properties.
Polycarbonates, PCs are polyesters typically made through a chemical reaction called condensation polymerisation, usually involving bisphenol A (BPA) and either carbonyl chloride or diphenyl carbonate. Owing to a combination of high impact resistance, optical clarity and thermal stability they are widely used in applications such as automotive components, optical lenses, electronic housings and construction materials.76 Recycling of PC presents significant challenges due to its susceptibility to hydrolytic and thermal degradation during mechanical processing recycling, leading to molecular weight reduction and deterioration of physical properties.77 Chemical recycling methods, such as hydrolysis,78 alcoholysis,79 and glycolysis,80 have been developed to depolymerise PC into monomers such as BPA, or oligomers. BPA is a valuable product due to its role as a precursor in the production of polymers and epoxy resins. The methanolysis of PC was reported by Chen et al.81 over ultrasmall (∼4 nm) ZnO nanoparticle catalysts, yielding 98% (BPA) after 1 h at 120 °C conventional thermal heating. The proposed mechanism involves the interaction of the carbonyl group of PC with the Zn2+ of the catalyst, resulting in an electropositive carbon atom which is subsequently attacked by the electronegative O atom in MeOH. This leads to the cleavage of the C–O bond, depolymerising the PC into BPA and dimethyl carbonate (DMC). The alkaline hydrolysis of PC has also been reported using a cationic surfactant, 1-hexadecyl trimethylammonium bromide (HTMAB) as a phase transfer catalyst.82 The cationic functionality of the catalyst transports the OH anion to the surface of the PC via interfacial mechanism, resulting in attack of the C–O groups and depolymerisation of PC. The PTC returns to the aqueous phase and forms BPA. Full PC depolymerisation was achieved in 10 min at 160 °C MW heating, which was significantly shorter than 1 h reported previously using thermal heating. A similar report found the uncatalysed methanolysis of PC under MW irradiation to achieve yields of 94% BPA at 190 °C in 3 h of reaction time.83Table 1 summarises reaction conditions and catalyst type reported in the literature for MW-assisted conversion of polyesters to value-added chemicals.
| Polymer | Catalyst | Conditions | Products | Ref. |
|---|---|---|---|---|
| Abbreviations: PET = polyethylene terephthalate; BHET = bis(2-hydroxyethyl) terephthalate; PES = polyester; BHETA = bis(2-hydroxyethyl) terephthalamide; PLA = polylactic acid; HTMAB = hexadecyl(trimethyl)ammonium bromide; MeLa = methyl lactate; PEF = polyethylene furonate; BPA = bisphenol A; PC = polycarbonate. | ||||
| PET | ZnO | 210 °C, 30 min, 850 W | BHET | 61 |
| PET, PES | Kaolin–Nb2O5 | 140 °C, 30 min, 180 W | BHETA | 64 |
| PES | In–ZnO–g-C3N4 | 180–200 °C, 60 min, 300 W | BHET | 65 |
| PES | In–ZnO–g-C3N4 | 125–150 °C, 15 min, 180 W | BHETA | 65 |
| PET, PES | Ag–ZnO | 150–180 °C, 15 min, 180 W | BHETA | 66 |
| PET, PES | Ag–ZnO | 180–200 °C, 30 min, 300 W | BHET | 66 |
| PET | SiO2–guanidine | 210 °C, 30 min, 800 W (upper limit) | BHET | 43 |
| PLA | HTMAB | 100 °C, 10 min, 2–10 W | Lactic acid | 72 |
| PLA | Zn(OAc), Zn(II) triflate | 160 °C, 10 min, n/a | MeLa | 73 |
| PLA | SiO2–guanidine | 130 °C, 30 min, 800 W (upper limit) | MeLa | 43 |
| PEF | ZnO | 175 °C, 40 min 100 W | BHEF | 75 |
| PC | HTMAB | 160 °C, 10 min, 17 W | BPA | 82 |
MW-assisted catalysis has emerged as a highly effective and sustainable approach for the chemical recycling of polyesters as described above. This chemical recycling method enables the rapid depolymerisation of these polymers into valuable monomers under relatively mild conditions which enables a closed-loop system. The use of MW heating significantly shortens reaction times compared to conventional methods, improving energy efficiency and process scalability. MW-assisted heterogeneous catalysis offers as a promising strategy for addressing plastic waste and supporting circular material use.
3.2 MW-assisted heterogeneous catalytic upcycling of polyolefins
Polyolefins are a major class of commodity polymers and include common plastics such as LDPE, HDPE and PP. Polyethylene accounts for roughly one third of overall plastic pollution workdwide.84 Polyolefins are synthesised through the chain growth polymerisation of simple olefins like ethylene and propylene using catalyst systems under controlled conditions. Known for their chemical resistance, durability and low-cost, polyolefins are used extensively in packaging, textiles and automotive parts. However, their saturated hydrocarbon backbone structures make them particularly resistant to chemical degradation, posing significant challenges for waste management and sustainability. As a result, developing efficient catalytic chemical recycling pathways for polyolefins is a growing area of interest.Catalytic pyrolysis is the most extensively reported chemical recycling technique for polyolefin recycling and involves heating the polymers to high temperatures (typically >300 °C) in the presence of a catalyst under inert atmosphere. The product distribution depends strongly on the catalyst used and the specific pyrolysis conditions. Catalytic pyrolysis over zeolite catalysts will typically yield a range of short chain hydrocarbon gases such as methane, ethylene and propylene, longer chain gasolene range hydrocarbons and aromatics such as benzene, toluene and xylene (BTX) derivatives which are useful fuels, and waxes.85,86 In contrast, Fe or other metal-containing catalysts typically favour the formation of solid carbon materials, such as carbon nanotubes (CNT), along with hydrogen gas (H2), rather than short chain hydrocarbons.87 This is because Fe (or other metals) can promote the dehydrogenation of the hydrocarbon chain in the polyolefin to form H2 and carbon, while the strong Lewis acid sites on zeolite catalysts can protonate the long hydrocarbon chains in polyolefins leading to carbocation formation and subsequently β-scission reactions that break the long chains into smaller hydrocarbon fractions.86
MW-assisted catalytic pyrolysis methods have been reported to achieve lower energy consumption, enhanced yield and improved quality of the liquid oil products obtained from polyolefin pyrolysis when compared with conventional pyrolysis due to rapid heating effects and hotspot formation associated with MW.88 Zeolites are widely used in catalytic pyrolysis due to the ease with which their structures can be tuned to optimise porosity and stability.89 Luong et al.90 reported catalytic pyrolysis of LDPE over zeolite H-ZSM-5 in a MW reactor. Optimal conditions were 300–400 °C, 30 min, to yield C1–C3 paraffinic hydrocarbons (37%), smaller amounts of BTX (12%) and coke (<10%). While MW heating achieved 100% conversion, under comparable conditions with conventional heating only 90% conversion was achieved, as well as lower BTX yields, highlighting the energy efficiency of MW-assisted pyrolysis. Infrared imaging revealed hotspot formation on the catalyst surface during the reaction.
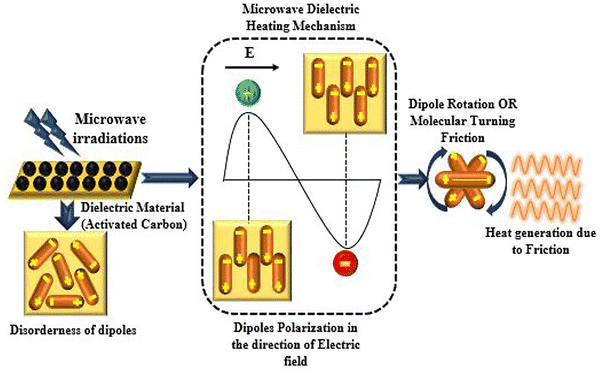
Vatankhah and coworkers91 reported the MW-assisted pyrolysis of LDPE over a metal-promoted zeolite catalyst, illustrated in Fig. 5(a). Zn was incorporated into the zeolite structure on a SiC support which was employed as an efficient MW absorber. The MW was reported to selectively heat the SiC, creating a temperature gradient between the catalyst and the plastic surfaces meaning only the LDPE that is in direct contact with the catalyst surface underwent cracking which increases process selectivity by minimising random scissions within the polymer. The simultaneous cracking and catalytic aromatisation of LDPE was achieved at 360 °C in 8 min. In the absence of Zn, the HZSM-5 catalyst achieved 75% selectivity towards C2–C4 alkenes. Incorporation of the Zn to HZSM-5 resulted in a liquid product consisting of 94% aromatics with 64% selectivity towards BTX. The enhanced aromatisation performance of the Zn-incorporated catalyst was attributed to the synergistic effect of Brønsted acid sites from the zeolite and Lewis acid sites from Zn2+ and [ZnOH]+ species.
 | ||
| Fig. 5 (a) Proposed mechanism for the MW-assisted cracking of LDPE over Zn–HZSM-5 catalyst, reproduced from ref. 91 with permission from American Chemical Society Copyright 2024. (b) IR camera images showing hotspot formation on Ru/ZSM-5 catalyst surface during catalytic pyrolysis of LDPE, reproduced from ref. 93 with permission from Elsevier Copyright 2024. (c) Proposed mechanism for MW-assisted pyrolysis of HDPE and PP using a Na/ZSM-5 catalyst, reproduced from ref. 48 with permission from Nature Copyright 2023. | ||
Similarly, Ding and coworkers92 reported a two-step MW-assisted catalytic pyrolysis of LDPE utilising the synergistic effect between a zeolite and NiO. The first step involved the conversion of LDPE to aliphatic compounds over the NiO catalyst under MW-assisted heating at 500 °C, followed by the conversion of these products to high octane number (ON) oil compounds over a HY zeolite at 450 °C. While it did not significantly increase the yield, the addition of NiO to the system increased the selectivity towards valuable high ON compounds via a H abstraction mechanism which proceeded due to accelerated reaction rates that arose from hot spot generation by the NiO under MW heating.
The MW-assisted catalytic pyrolysis of LDPE over a ruthenium-modified zeolite (Ru/ZSM-5) was reported by Tuli et al.93 The pyrolysis reactions were carried out at 300 °C with a 30 min hold time. A relatively high loading of 50 wt% catalyst was used. The Ru–ZSM-5 catalyst was easily regenerated at 550 °C after completing a reaction and showed excellent recyclability. A comparative study of thermal and MW reactions demonstrated that MW heating resulted in higher selectivity towards aromatic products while conventional heating favoured aliphatic compounds. Hot spot formation was observed on the catalyst particles under MW-irradiation using an IR camera (see Fig. 5b) and increased with successive cycles owing to Ru aggregation within the catalyst. Irfan et al.48 reported the catalytic MW pyrolysis of HDPE and PP over a Na-modified ZSM-5 catalyst. The reactions were carried out at 400–450 °C, 1000 W for 24 min and yielded mainly liquid oil consisting of aromatic hydrocarbons with some carbon nanotube (CNT) formation and H2 gas. The Na-modified zeolite acted as a nucleation site for CNT growth, which was driven by H2 formation. The proposed mechanism for CNT formation is shown in Fig. 5(c). SEM analysis showed the formation of crystalline CNTs with a maximum outer diameter measurement of 93 nm.
The use of transition metal catalysts such as Fe, Ni, and Co during the catalytic pyrolysis of polyolefins has been commonly reported to yield predominantly CNTs and H2via a catalytic chemical vapor deposition type mechanism. During pyrolysis, the catalyst breaks down the hydrocarbon molecules into H2 gas and reactive carbon species which dissolve into the metal catalyst. Once saturated, excess carbon precipitates out as the catalyst acts as a nucleation site for the growth of CNTs. Bimetallic catalysts are commonly reported for polyolefin degradation reactions due to synergistic and stabilisation effects. Effective solid state MW dehydrogenation of PE over Ni–Fe, Ni–Co and Co–Fe catalysts (average diameter 8.3 nm) has been demonstrated.94 The reaction produced H2, a solid fraction consisting of CNTs and an oil fraction consisting of C20+ linear hydrocarbons. The optimal conditions for the MW reactor were 500 °C, 800 W with 5 min reaction time under an argon flow of 100 mL min−1. Notably, MW-assisted catalytic dehydrogenation promoted the formation of carbon nanomaterials, while conventional thermal heating favoured a larger oil yield. A comparison of the solid carbon products obtained from MW versus thermal heating is showing in Fig. 6(a). During MW-assisted dehydrogenation, multiwalled CNTs are formed via selective growth on metal catalyst particle surfaces driven by rapid heating effects, while thermal heating results in poor CNT production efficiency as very few tubular structures were observed. A related study by Shoukat and co-workers95 reported the use of magnetic ferrite catalysts, namely NiZnFe2O4, NiMgFe2O4 and MgZnFe2O4 for the catalytic conversion of HDPE waste into H2 and CNT at 450 °C. The ferrite catalysts played a dual role, facilitating both catalytic growth of CNTs and MW absorption to enable rapid heating. The NiZnFe2O4 and MgZnFe2O4 catalysts gave higher yields of H2 and CNT than the NiMgFe2O4 catalyst. Jie et al.96 reported the conversion of HDPE and PP to H2 and multiwalled CNTs over a FeAlOx catalyst. The reactions were performed at a power of 1000 W for 3–5 min with temperatures reaching ∼350 °C. Hydrogen was rapidly extracted via an interfacial polarisation and a dehydrogenation mechanism with 97% of the H2 released from the plastic in just 20 s. The solid carbon residue was composed of 92 wt% multi-walled CNTs. Shen et al.39 investigated the catalytic performance of Fe immobilised on various supports – SiC, AC, and SiO2, for the MW-assisted pyrolysis of HDPE to produce H2 and CNTs. The study revealed a strong correlation between the catalyst's dielectric loss and the rate of the pyrolysis reaction, with higher dielectric loss leading to faster reaction rates and increased yields of H2 and solid CNTs, as opposed to liquid products. SEM images of the resulting CNTs are presented in Fig. 6(b). CNTs synthesised using the Fe@AC catalyst had an average diameter of 141 ± 20 nm, whereas a much smaller diameter was observed for those produced with the Fe@SiC, averaging 26 ± 6 nm. This size difference was attributed to the variation in dielectric loss values as Fe@AC exhibited a high value of 156.79, while Fe@SiC had a significantly lower value of 3.95. The Fe@SiO2 exhibited a dielectric loss value of 0.11, which did not support CNT formation. The high dielectric loss of Fe@AC facilitated rapid heating of the catalyst particles under MW heating, accelerating CNT growth. The mechanism is illustrated in Fig. 6(c) and can be described in three steps. The first step involves the rapid heating of the Fe catalyst particle under MW-irradiation which induces cracking on neighbouring HDPE particles producing volatile hydrocarbons (C9–C40). Secondly, the volatile hydrocarbons are further broken down into shorter chain hydrocarbons such as methane and ethane. In the last step, short chain hydrocarbons (C1–C5) decompose on the Fe-based catalyst surface, breaking C–H bonds to produce H2 and solid carbon. Unlike thermal pyrolysis, MW-induced local heating on the catalyst surface promotes the crystallisation of amorphous carbon into cylindrical networks, leading to CNT formation.
 | ||
| Fig. 6 (a) TEM images of solid carbon product obtained from MW and thermal heating, reproduced from ref. 94 with permission from Elsevier Copyright 2024 (b) SEM images of solid carbon products obtained on Fe@SiC, Fe@AC and Fe@SiO2 catalysts, respectively. (c) Schematic diagram for the 3-step mechanism of the MW-assisted pyrolysis of HDPE over Fe-based catalysts, reproduced from ref. 39 with permission from Elsevier Copyright 2022. | ||
Zhao et al.44 used ZnO nanorods for conversion of LDPE and PP to hydrocarbons at 280 °C, yielding 46–60 wt% C20+ products, within 30 min. Interestingly, TEM analysis revealed progressive etching of the ZnO rods with each pyrolysis cycle. This was attributed to reduction of ZnO to Zn clusters by H2 released during dehydrogenation. The Zn clusters were identified as effective active sites for C–C cleavage.
Innovative approaches for MW depolymerisation of polyolefins include the use of MW-absorbing heating elements (MWAHE) and liquid metal catalysts to maximise utilisation of the MW heating effect. Activated carbon and silicon carbide (SiC) were evaluated as MWAHE,97 where their role is not to directly catalyse the reaction, but to effectively heat the HDPE in MW-assisted pyrolysis. The process consisted of two steps: an initial crude cracking at 620 °C, followed by secondary cracking at 950 °C, resulting in gasification yields of 95% using a low power input of 80 W. The main products obtained were carbon monoxide, methane, carbon dioxide, acetylene, ethylene, ethane, propane, propylene, n-butane and i-butane. The AC MWAHE resulted in higher gas yields (84%) than the SiC MWAHE (66%), however, SiC resulted in higher selectivity towards ethylene (64 vol%) compared to AC (33 vol%). The superior performance of the AC was attributed to the emission of discharge plasmas from the AC when irradiated with MWs due to its high dielectric loss properties, which has been reported to increase microscopic surface temperatures to >750 °C, as shown in Fig. 7(a), while this effect was not observed for SiC.
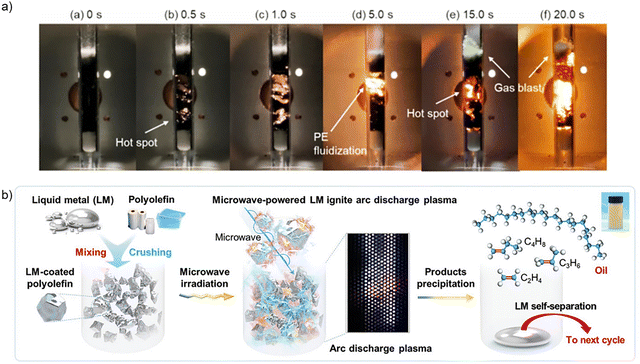 | ||
| Fig. 7 (a) Hot spot and discharge plasma observation for pyrolysis of PE with AC MWAHE, reproduced from ref. 97 with permission from Nature Copyright 2022 (b) reaction scheme of polyolefin pyrolysis using liquid metal catalyst, reproduced from ref. 98 with permission from John Wiley and Sons Copyright 2025. | ||
Fig. 7(b) illustrates a unique approach reported by Gao and coworkers98 utilising a liquid metal eutectic gallium indium alloy (EGaIn) as an effective catalyst for LDPE, HDPE and PP conversion to olefin monomers. The use of a liquid metal catalyst is reported to eliminate the deactivation that is commonly reported for solid heterogeneous catalysts. Additionally, their properties such as fluidity, high electrical and thermal conductivities make them excellent MW catalysts. The liquid metal catalyst was first coated onto the plastic particles, the thickness of which was carefully tailored to match the MW penetration depth to facilitate efficient MW absorption, a consideration that is rarely reported in MW studies. Due to the conductivity of the liquid metal, the coated plastic particles generated a high temperature arc discharge plasma when subject to 600 W of MW power, resulting in full depolymerisation of the polyolefins in 25 min, with high selectivity towards C2–4 olefins (50% for PE and 65% for PP). The liquid-metal catalyst was also self-separating and demonstrated excellent recyclability over 30 cycles.
Outside of catalytic pyrolysis, another chemical recycling route reported for polyolefins is oxidative degradation. This method involves the use of oxidising agents such as oxygen gas (O2), ozone (O3), acids, like nitric acid (HNO3) and peroxides, in the presence of catalysts. The oxidative degradation mechanism typically proceeds via radical oxidation followed by C–C chain scission, and yields small, oxygenated molecules such as carboxylic acids, ketones and alcohols which serve as valuable chemical intermediates.27,99 Oxidative degradation is emerging as a milder depolymerisation method of polyolefins. The MW-assisted oxidative degradation of LDPE in nitric acid has been reported to proceed at 180 °C in 1 h at 1200 W to yield dicarboxylic acids via a chain scission and radical oxidation mechanism.100 Fenton and Fenton-like processes, which use aqueous H2O2 and a redox catalyst to generate reactive oxygen species have also been reported for the conversion of polyolefins into oxygenated products such as carboxylic acids and esters101,102 and also for the destruction of microplastics to gaseous products.103,104 Fenton oxidation pathways facilitate the oxidative degradation of polymers while eliminating the use of hazardous or toxic reagents. Breen et al.105 reported the use of Fenton oxidation to convert LDPE to carboxylic acid products over a Fe–CeO2 solid solution catalyst. A synergistic effect was reported between Ce and Fe in the catalyst achieving acid yields of 71% under neutral pH. The main products obtained were formic acid, acetic acid, propionic acid and succinic acid. 90% LDPE degradation was achieved at 180 °C in 3 h with an average power usage of 300 W. The Fenton oxidation of LDPE was carried out under conventional thermal heating and was reported to not proceed to any extent. Table 2 summarises reaction conditions and catalysts reported in the literature for MW-assisted conversion of polyolefins to value-added product streams such as fuels and commodity chemicals.
| Polymer | Catalyst | Conditions | Main product | Ref. |
|---|---|---|---|---|
| Abbreviations: LDPE = low-density polyethylene; HZSM = hydrogen zeolite Socony Mobil-5; BTX = benzene, toluene, xylene; MAH = monocyclic aromatic hydrocarbons; HDPE = high-density polyethylene; PP = polypropylene; MWAHE = microwave-absorbing heating element. | ||||
| LDPE | HZSM-5 | 300–400 °C, N2, 30 min, 1000 W (max W) | Paraffinic (C1–C4), BTX, olefins | 90 |
| LDPE | Zn–HSM-5 | 360 °C, N2, 8 min, 1000 W (max W) | Gas (H2, C2–C4), oil (MAH, BTX) | 91 |
| LDPE | (1) Pyrolysis NiO | (1) 450–600 °C | Gasoline (C5–C12), aromatics, aliphatics | 92 |
| (2) Catalysis HY (2 stage) | (2) 350–500 °C | |||
| LDPE | Ru/ZSM-5 | 300 °C, N2, 30 min, 900 W (max W) | BTX, olefins | 93 |
| HDPE, PP | Na/ZSM-5 | 400–450 °C, N2, 24 min, 1000 W | H2, carbon nanotubes, liquid oil | 48 |
| LDPE | Ni3Co2Ox | 500 °C, Ar, 5 min, 800 W | H2, carbon nanotubes | 94 |
| HDPE | NiZnFe2O4, NiMgFe2O4, MgZnFe2O4 | 450 °C, N2, 8 min, 1000 W | H2, carbon nanotubes | 95 |
| HDPE | FeAlOx | 350 °C, Ar, 5 min, 1000 W | H2, carbon nanotubes | 96 |
| LDPE, PP | Zn/b-ZnO | 30 min, 280 °C, 240–400 W | Lubricant-base oil | 44 |
| HDPE | Fe@SiC, Fe@AC, Fe@SiO2 | 500 °C, N2, 1 h, 800 W | H2, carbon nanotubes | 39 |
| HDPE | SiC/AC MWAHE | 620 °C (1), 950 °C (2), N2, 3 min, 500 W | C2H4, CH4 | 97 |
| PP, LDPE, HDPE | EGaIn | 300 °C, Ar, 25 min, 600 W | C2–C4 | 98 |
| LDPE | Fe–CeO2 | 180 °C, 3 h, 300 W | RCOOH, R(COOH)2 (C1–C7) | 105 |
The use of MW heating is an effective method for upcycling of polyolefins to value-added fuels and carbon materials through open-loop pathways. Product selectivity can be influenced by tuning the reaction conditions including the choice of catalyst used. MW-assisted heterogeneous catalysis has potential for effectively converting waste polyolefin streams to valuable chemicals via open-loop pathways.
3.3 MW-assisted heterogeneous catalytic upcycling of other plastics
In addition to polyesters and polyolefins, the chemical recycling of other plastics such as PS, PAs PUR and PVC, is gaining increasing attention. These polymers pose unique challenges due to their diverse chemical structures and additives. MW-assisted processes offer a promising route by enabling rapid, energy-efficient depolymerisation tailored to these complex polymer types.Polystyrene (PS) is a widely used thermoplastic polymer composed of a saturated hydrocarbon backbone with conjugated benzene rings as side groups. Its chemical structure imparts rigidity, clarity, and good insulation properties, making it suitable for a broad range of applications, including packaging materials, disposable food containers and insulation.106 While PS can be mechanically recycled, its low density and contamination issues often make this route inefficient.107 As a result, chemical recycling methods such as thermal depolymerisation, pyrolysis, and MW-assisted depolymerisation have been explored to recover monomeric styrene and valuable aromatic compounds such as BTX,108 offering a more sustainable solution for PS waste.
PS can undergo catalytic pyrolysis reactions like polyolefins but typically yield higher amounts of aromatic products such as BTX. An investigation into the effect of Fe-based MW absorbers – Fe, Fe3O4 and FeS2 for the MW-assisted conversion of PS to aromatic hydrocarbons was explored by Fan et al.109 High oil yields of >92% were achieved for all 3 Fe-based materials. The dominant product obtained for Fe and Fe3O4 was aromatic hydrocarbons arising from the aromatic group of the PS chain. FeS2 showed a high selectivity towards aliphatic hydrocarbons compared to the other Fe-based catalysts. Luong et al.90 reported the catalytic pyrolysis of PS over zeolite H-ZSM-5 in a MW reactor. Due to the aromaticity of PS, the main product obtained was BTX (65%) with small amounts of coke (11%) and paraffin (5%), with selectivity towards BTX increasing with temperature from 300 °C to 400 °C. Optimal conditions were achieved at 400 °C in 30 min. The use of biochar, a sustainable carbon material obtained from the pyrolysis of biomass, was implemented as a MW-absorbing material to enhance the MW-assisted catalytic pyrolysis of PS to yield aromatic products.110 The product obtained was mainly pyrolysis oil which was composed of styrene, cyclopropyl methylbenzene, and alkylbenzene derivates. The oil was rich in carbon content (∼92 wt%) with C8–C24 aromatic hydrocarbons. At 300 and 450 W of MW power, the oil yield was higher than that of the gas yield, however, at 600 W of MW power, the gas yield exceeded the oil yield. The pyrolysis experiments were carried out at 600 °C in a time range of 14–38 min.
Polyamides (PA) are characterised by the presence of recurring amide linkages (–CONH–) in their backbone. They can be synthesised via condensation polymerisation of diamines with dicarboxylic acids or via ring-opening polymerisation of lactams. They exhibit excellent mechanical strength, abrasion resistance, thermal stability, and chemical resistance, making them widely used in automotive components, textiles, films, electrical connectors.111 PA can be chemically recycled by methods such as ammonolysis, alcoholysis and can be readily hydrolysed in the present of an acid or a base to yield the monomeric lactams.112
Klun et al.113 reported the phosphoric acid catalysed hydrolysis of polyamide-6 (PA-6) to yield ε-aminocaproic acid (ACA), the aliphatic form of the PA-6 monomer. PA-6 underwent full depolymerisation in 20 min with a MW input of 200 W and a temperature of 260 °C. Some cyclic species were also detected at lower phosphoric acid concentrations (10 wt%), however higher acid concentrations (>70 wt%) resulted in their conversion to linear species. The MW-assisted, HCl catalysed hydrolysis of a range of aliphatic PAs including PA-66, PA-1010, PA-11 and PA-12, to dicarboxylic acids and diamines has been reported.114 These PAs are comprised of different chain lengths obtained from polymerisation of diamines and carboxylic acids with different number of carbon atoms. PA-66 is composed of a hexamethylene diamine (C6) group and one adipic acid (C6) group, and PA-1010 is composed of one decamethylene diamine (C10) group and one sebacic group (C10). On the other hand, PA-11 and PA-12 refer to repeating units of 11-aminoundecanoic acid and 12-aminododecanoic acid, respectively. In the HCl-catalysed hydrolysis, PA-66 and PA-1010 can be depolymerised into their corresponding dicarboxylic acid and diamine chloride counterparts, while PA 11 and PA 12 can be depolymerised into their amino acid chloride counterparts. Owing to the presence of shorter chains between reactive functional groups, PA-66 was depolymerised 10 min at 200 °C (∼150 W), while PA 1010, PA 11 and PA 12 are composed of less polar long chains and required 15–20 min to achieve full depolymerisation. It was found that the rate of reaction was dependent on the molar ratio of HCl to amide bonds in the polymer. For PA66 as at equimolar ratios of HCl to amide groups, a 96% degree of PA degradation was observed from NMR. When a 0.5 molar equivalent of HCl to amide was used, this decreased to 50% degree of degradation.
Frisa-Rubio et al.115 developed a mathematical model for the scaled-up multi-frequency MW depolymerisation of PA-6 and PA-66 via HCl catalysed hydrolysis to yield 6 aminocaproic acid (6-ACA), and hexamethylenediamine (HDMA) and adipic acid (AA), respectively. The simulation demonstrated complete PA degradation and high monomer yields in under 10 min. The study also modelled the glycolysis of polyurethane (PU) to polyol diamine toluene (DAT), which the optimal conditions were simulated to be 230 °C for 40 min. A different approach was reported by Alberti and co-workers,116 who reported a ring closing transformation of PA-6 over a 4-dimethylaminopyridine organo-catalyst to form the monomer N-acetylated caprolactam. Additionally, the acetyl group can react with 2-aminoethanol to form N-acetyled 2-aminoethanol which is a starting material for poly(vinyl) amides. The depolymerisation of PA-6 to N-acetylcaprolactam proceeded after 15 min at 250 °C, while the acyl transfer step proceeded after 2 h at 180 °C. The successful use of a homogeneous organo-catalyst suggests potential for developing heterogeneous organo-catalysts for easier catalyst separation and recycling for this reaction pathway. Furthermore, implementation of MW heating could enhance reaction efficiency. Poly(p-phenylene terephthalamide) (PPTA), or commercially known as Kevlar or Twaron, is a highly crystalline aromatic polyamide that can be spun into fibres. Despite its lightweight characteristics, it exhibits significantly higher specific strength than steel, making it favourable for applications such as ropes, cables and advanced composites.117 Recycling of waste fibres poses significant challenges due to a large decrease in material quality after the mechanical recycling process and are usually not recycled after being used.118 Benninga et al.119 reported the use of MW-assisted heating for the alkaline hydrolysis of PPTA to monomers TPA and p-phenyldiamine (PPD) achieving full conversion in 15 min at 260 °C.
PVC is a widely used polymer consisting of repeating chloroalkane units produced via the free-radical polymerisation of vinyl chloride monomers. Due to its chemical resistance, flame retardancy and durability, combined with the ability to tailor its mechanical characteristics through plasticisation, have led to extensive use in applications such as construction (e.g. pipes, window frames, flooring), medical devices, packaging, and cables.120,121 Mechanical recycling of PVC is limited by the thermal instability of the polymer as PVC tends to undergo dehydrochlorination, releasing HCl gas.122 Chemical recycling of PVC often require a dechlorination step and can be carried out using techniques such as hydrogenation, pyrolysis, gasification and catalytic degradation.123 Pyrolysis and gasification of PVC is complicated by the release of HCl and the potential formation of hazardous byproducts including dioxins and furans.124
Ye et al.125 reported the pyrolysis of PVC over Fe3O4, where the catalyst reacts with Cl2 gas at high temperatures (400 °C) forming FeCl2, thereby mitigating harmful HCl emissions typically formed during PVC pyrolysis. The process proceeds in two stages described in Scheme 1.
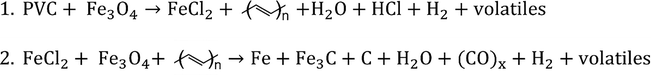 | ||
| Scheme 1 Reaction equations for stage 1 and 2 of co-pyrolysis of PVC with Fe3O4 to mitigate Cl2 emissions. | ||
The first stage occurs below 673 K involving dechlorination of PVC to form FeCl2 and HCl, leaving a solid residue of polyenes. The second stage occurs above 673 K where further reaction between FeCl2, Fe3O4 and polyenes yield solid carbon char, iron carbides and volatiles. Complete PVC decomposition was achieved within 5 min using 1800 W h−1 and an estimated temperature of 300 °C. PVC-coated power cables were also recycled via MW-assisted pyrolysis at 250 °C and 100–300 W for 120 s, to recover HCl, carbon black and the interior copper.126Table 3 summarises literature reports on MW-assisted chemical upcycling pathways including conditions and catalyst type for PS, PA and PVC.
| Polymer | Catalyst | Conditions | Products | Ref. |
|---|---|---|---|---|
| Abbreviations: PS = polystyrene; HZSM = hydrogen zeolite Socony Mobil-5; BTX = benzene, toluene, xylene, PA = polyamide; ACA = aminocaproic acid; PPTA = poly(p-phenylene terephthalamide); TPA = terephthalic acid; PPD = p-phenyldiamine PVC = polyvinyl chloride. | ||||
| PS | Fe/Fe3O4/FeS2 | 460 °C, 60 min, 650 W | Aromatic hydrocarbons (C8–C16) | 109 |
| PS | H-ZSM-5 | 400 °C, 30 min | BTX | 90 |
| PS | Biochar susceptor | 600 °C, 14–38 min, 300–600 W | Styrene, cyclopropyl methylbenzene, and alkylbenzene | 110 |
| PA-6 | H3PO4 | 260 °C, 20 min, 200 W | ACA | 113 |
| PA-66, PA-1010, | HCl | 200 °C, 10/17 min, ∼150 W | Adipic acid, hexamethylene diamine sebacic acid, 1,10 decanediamine | 114 |
| PA-11, PA-12 | HCl | 200 °C 15/20 min, ∼150 W | 11-Aminoundecanoic acidxHCl | 114 |
| 12-Aminododecanoic acidxHCl | ||||
| PA-6 | 4-Dimethylaminopyridine | 250 °C, 15 min (thermal) | Caprolactam, N-acetyled 2-aminoethanol | 116 |
| PPTA | NaOH | 240–260 °C, 15 min, 500 W | TPA, PPD | 119 |
| PVC | Fe3O4 | 400 °C (thermal) | Fe3C, solid carbon char, FeCl2 | 125 |
| PVC/Cu cable | — | 250 °C, 2 min, 100–300 W, | HCl, carbon black | 126 |
These findings highlight the applicability of MW-assisted heating for a wide range of polymers including PS, PA, and PVC. PAs can be depolymerised to monomers in a closed-loop chemical recycling system, while PS and PVC can be converted to fuel and solid carbon in open-loop systems. Further research is essential to explore more pathways for MW-assisted heterogeneous catalysis for the chemical recycling of these plastics.
4. Summary & outlook
This review has highlighted recent advances in MW-assisted heterogeneous catalysis as a transformative approach for the chemical upcycling of plastics. MW heating offers rapid, energy-efficient and selective activation of catalysts and reaction media, overcoming some of the limitations of conventional thermal processes. A wide range of catalyst types, including metal oxides, organocatalysts, modified zeolite and carbon-based materials have demonstrated effectiveness in catalytic pyrolysis and closed loop depolymerisation reactions. MW-assisted catalytic depolymerisation has been successfully applied to a broad spectrum of polymers, including polyolefins, polyesters, polystyrene, polyamides, polycarbonates, and PVC. In many studies, the catalysts show excellent recyclability over multiple cycles, a key advantage of heterogeneous catalysts and a valuable characteristic when considering scale-up. Mechanistic insights such as hotspot formation, microplasmas and the strategic use of MW-absorbing materials have enabled rapid depolymerisation of plastics with poor intrinsic MW absorption such as polyolefins. A discrepancy in MW power input for reactions can be observed in the reviewed articles when comparing polyesters (typically <300 W) and polyolefins (typically >800 W), likely owing to the harsher conditions required for polyolefin pyrolysis compared with polyester depolymerisation.Across the available literature, MW-assisted heating consistently demonstrates notable environmental and economic advantages over conventional thermal depolymerisation methods. Reported studies on LCA and technoeconomic assessment (TEA) show significant reductions in energy demand, greenhouse gas emissions, and processing time, alongside improved product selectivity and potential cost savings. MW-assisted aminolysis of PU foams achieved up to a 38% reduction in global warming potential (GWP) and 50% lower fossil resource use compared to virgin polyol production, while energy consumption decreased by 80% upon scale-up.127 Similarly, MW co-pyrolysis of food waste and LDPE exhibited a GWP of only 38.9 kg CO2 eq. per 100 kg feedstock, substantially below that of anaerobic digestion (840 kg CO2 eq.), and achieved economic feasibility with an 18% internal rate of return when coproducts were competitively priced.128 MW-assisted glycolysis of PET reached 95% BHET yield within 10 minutes, with lower environmental impacts and minimum selling prices than the conventional DMT route, attributed to modular reactor design and selective heating.129 Despite these results, LCA and TEA remain limited in scale and scope. Most studies rely on laboratory or pilot-scale data, with few analyses extending to industrial-scale scenarios that capture realistic processes. Comprehensive LCAs and TEAs at commercial scales are therefore needed to validate the environmental and economic potential of MW-assisted depolymerisation.
MW-assisted chemical recycling also faces several challenges associated with expensive catalysts, difficulty scaling or limited selectivity when dealing with mixed or contaminated plastic streams. To overcome these challenges, future research should prioritise the development of robust, selective and low-cost catalysts, reactor designs that can efficiently handle real-world waste plastics. Additionally, the use of metal organic frameworks (MOFs) as catalysts in MW-assisted depolymerisation reactions could provide a low-cost, robust and versatile catalyst as they are well reported heterogeneous catalysts for plastic depolymerisation reactions owing to their structural control, stability, reactivity and porosity,130 but not well reported in MW-assisted depolymerisation reactions. MW-assisted chemical plastic recycling aligns with the future direction of designing energy efficient plastic management strategies. Challenges will arise regarding suitable reactor scale-up design and sourcing cheap yet effective catalysts. To overcome these, it is crucial to promote interdisciplinary collaborations in this research area and advance research into sustainable catalyst materials such as biomass-derived catalysts. With continued advancements in catalyst design, reactor engineering and integrated sustainability assessments, MW-assisted catalytic upcycling has potential to be an important valorisation technology for transition toward a circular and more sustainable plastic economy.
Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included, and no new data were generated or analysed as part of this review.Acknowledgements
This research was funded by Research Ireland grant number (AMBER Grant No: 12/RC2278_P2).References
- A. Rafey and F. Z. Siddiqui, A review of plastic waste management in India – challenges and opportunities, Int. J. Environ. Anal. Chem., 2023, 103(16), 3971–3987, DOI:10.1080/03067319.2021.1917560.
- E. Naderi Kalali, S. Lotfian, M. Entezar Shabestari, S. Khayatzadeh, C. Zhao and H. Yazdani Nezhad, A critical review of the current progress of plastic waste recycling technology in structural materials, Curr. Opin. Green Sustainable Chem., 2023, 40, 100763, DOI:10.1016/j.cogsc.2023.100763.
- Development, O. f. E. C.-o. a. Global Plastics Outlook: Economic Drivers, Environmental Impacts and Policy Options, Paris, 2022. https://www.oecd.org/en/publications/global-plastics-outlook_de747aef-en/full-report.html (accessed 27/07/2025) Search PubMed.
- S. Bertin and J.-J. Robin, Study and characterization of virgin and recycled LDPE/PP blends, Eur. Polym. J., 2002, 38(11), 2255–2264, DOI:10.1016/S0014-3057(02)00111-8.
- K. Ragaert, L. Delva and K. Van Geem, Mechanical and chemical recycling of solid plastic waste, Waste Manage., 2017, 69, 24–58, DOI:10.1016/j.wasman.2017.07.044.
- P. G. C. Nayanathara Thathsarani Pilapitiya and A. S. Ratnayake, The world of plastic waste: A review, Cleaner Mater., 2024, 11, 100220, DOI:10.1016/j.clema.2024.100220.
- N. Li, H. Liu, Z. Cheng, B. Yan, G. Chen and S. Wang, Conversion of plastic waste into fuels: A critical review, J. Hazard. Mater., 2022, 424, 127460, DOI:10.1016/j.jhazmat.2021.127460.
- J. Jiang, K. Shi, X. Zhang, K. Yu, H. Zhang, J. He, Y. Ju and J. Liu, From plastic waste to wealth using chemical recycling: A review, J. Environ. Chem. Eng., 2022, 10(1), 106867, DOI:10.1016/j.jece.2021.106867.
- G. Martínez-Narro, S. Hassan and A. N. Phan, Chemical recycling of plastic waste for sustainable polymer manufacturing – A critical review, J. Environ. Chem. Eng., 2024, 12(2), 112323, DOI:10.1016/j.jece.2024.112323.
- A. Rahimi and J. M. García, Chemical recycling of waste plastics for new materials production, Nat. Rev. Chem., 2017, 1(6), 0046, DOI:10.1038/s41570-017-0046.
- Z. Jiang, Y. Liang, F. Guo, Y. Wang, R. Li, A. Tang, Y. Tu, X. Zhang, J. Wang and S. Li, et al., Microwave-Assisted Pyrolysis-A New Way for the Sustainable Recycling and Upgrading of Plastic and Biomass: A Review, ChemSusChem, 2024, 17(21), e202400129, DOI:10.1002/cssc.202400129 (accessed 2025/03/20).
- A. Schade, M. Melzer, S. Zimmermann, T. Schwarz, K. Stoewe and H. Kuhn, Plastic Waste Recycling—A Chemical Recycling Perspective, ACS Sustainable Chem. Eng., 2024, 12(33), 12270–12288, DOI:10.1021/acssuschemeng.4c02551.
- European Commission: Joint Research, C. P. Garcia-Gutierrez, A. Amadei, D. Klenert, S. Nessi, D. Tonini, D. Tosches, F. Ardente and H. Saveyn, Environmental and economic assessment of plastic waste recycling – A comparison of mechanical, physical, chemical recycling and energy recovery of plastic waste, Publications Office of the European Union, 2023, DOI:10.2760/0472.
- M. A. M. Rashid, K. H. Kim and K. Jeong, Microwave-Assisted Depolymerization of Natural and Synthetic Polymers, Depolymerization: Concepts, Progress, and Challenges Volume 3: Emerging Trends, ACS Symposium Series, American Chemical Society, 2025, vol. 1501, pp. 15–31 Search PubMed.
- M. Chu, W. Tu, S. Yang, C. Zhang, Q. Li, Q. Zhang and J. Chen, Sustainable chemical upcycling of waste polyolefins by heterogeneous catalysis, SusMat, 2022, 2(2), 161–185, DOI:10.1002/sus2.55 (accessed 2025/02/11).
- H. Li, C. Zhang, C. Pang, X. Li and X. Gao, The Advances in the Special Microwave Effects of the Heterogeneous Catalytic Reactions, Front. Chem., 2020, 8, 355 CrossRef CAS PubMed Review.
- A. Bohre, P. R. Jadhao, K. Tripathi, K. K. Pant, B. Likozar and B. Saha, Chemical Recycling Processes of Waste Polyethylene Terephthalate Using Solid Catalysts, ChemSusChem, 2023, 16(14), 1864–5631, DOI:10.1002/cssc.202300142.
- M. Babaei, M. Jalilian and K. Shahbaz, Chemical recycling of Polyethylene terephthalate: A mini-review, J. Environ. Chem. Eng., 2024, 12(3), 112507, DOI:10.1016/j.jece.2024.112507.
- T. Muringayil Joseph, S. Azat, Z. Ahmadi, O. Moini Jazani, A. Esmaeili, E. Kianfar, J. Haponiuk and S. Thomas, Polyethylene terephthalate (PET) recycling: A review, Case Stud. Chem. Environ. Eng., 2024, 9, 100673, DOI:10.1016/j.cscee.2024.100673.
- Z. Guo, J. Wu and J. Wang, Chemical degradation and recycling of polyethylene terephthalate (PET): a review, RSC Sustainability, 2025, 3(5), 2111–2133, 10.1039/D4SU00658E.
- H. Abedsoltan, A focused review on recycling and hydrolysis techniques of polyethylene terephthalate, Polym. Eng. Sci., 2023, 63(9), 2651–2674, DOI:10.1002/pen.26406 (accessed 2025/05/09).
- Y. Weng, C.-B. Hong, Y. Zhang and H. Liu, Catalytic depolymerization of polyester plastics toward closed-loop recycling and upcycling, Green Chem., 2024, 26(2), 571–592, 10.1039/D3GC04174C.
- S. Wang, J. Li, X. Li and Y. Tu, Recent advances in the chemical recycling of polyesters, Fundam. Res., 2025, 5, 395–950, DOI:10.1016/j.fmre.2024.05.014.
- J. Payne and M. D. Jones, The Chemical Recycling of Polyesters for a Circular Plastics Economy: Challenges and Emerging Opportunities, ChemSusChem, 2021, 14(19), 4041–4070, DOI:10.1002/cssc.202100400 (accessed 2025/05/09).
- Y. Li, S. Wang, S. Qian, Z. Liu, Y. Weng and Y. Zhang, Depolymerization and Re/Upcycling of Biodegradable PLA Plastics, ACS Omega, 2024, 9(12), 13509–13521, DOI:10.1021/acsomega.3c08674.
- K. Faust, P. Denifl and M. Hapke, Recent Advances in Catalytic Chemical Recycling of Polyolefins, ChemCatChem, 2023, 15(13), e202300310, DOI:10.1002/cctc.202300310 (accessed 2024/07/04).
- J. Sun, J. Dong, L. Gao, Y.-Q. Zhao, H. Moon and S. L. Scott, Catalytic Upcycling of Polyolefins, Chem. Rev., 2024, 124(16), 9457–9579, DOI:10.1021/acs.chemrev.3c00943.
- L. Zou, R. Xu, H. Wang, Z. Wang, Y. Sun and M. Li, Chemical recycling of polyolefins: a closed-loop cycle of waste to olefins, Natl. Sci. Rev., 2023, 10(9), nwad207, DOI:10.1093/nsr/nwad207 (accessed 5/9/2025).
- J. Qin, F. Wu, Y. Dou, D. Zhao, C. Hélix-Nielsen and W. Zhang, Advanced Catalysts for the Chemical Recycling of Plastic Waste, Adv. Mater., 2025, 2418138, DOI:10.1002/adma.202418138 (accessed 2025/05/09).
- M. Chu, Y. Liu, X. Lou, Q. Zhang and J. Chen, Rational Design of Chemical Catalysis for Plastic Recycling, ACS Catal., 2022, 12(8), 4659–4679, DOI:10.1021/acscatal.2c01286.
- H. Luo, H. Tyrrell, J. Bai, R. Ibrahim Muazu and X. Long, Fundamental, technical and environmental overviews of plastic chemical recycling, Green Chem., 2024, 26(23), 11444–11467, 10.1039/D4GC03127J.
- K. D. Nixon, Z. O. G. Schyns, Y. Luo, M. G. Ierapetritou, D. G. Vlachos, L. T. J. Korley and I. I. I. T. H. Epps, Analyses of circular solutions for advanced plastics waste recycling, Nat. Chem. Eng., 2024, 1(10), 615–626, DOI:10.1038/s44286-024-00121-6.
- G. Yadav, A. Singh, A. Dutta, T. Uekert, J. S. DesVeaux, S. R. Nicholson, E. C. D. Tan, C. Mukarakate, J. A. Schaidle and C. J. Wrasman, et al., Techno-economic analysis and life cycle assessment for catalytic fast pyrolysis of mixed plastic waste, Energy Environ. Sci., 2023, 16(9), 3638–3653, 10.1039/D3EE00749A.
- P. Huang, A. Ahamed, R. Sun, G. X. De Hoe, J. Pitcher, A. Mushing, F. Lourenço and M. P. Shaver, Circularizing PET-G Multimaterials: Life Cycle Assessment and Techno-Economic Analysis, ACS Sustainable Chem. Eng., 2023, 11(42), 15328–15337, DOI:10.1021/acssuschemeng.3c04047.
- P. D. Muley; Y. Wang; J. Hu and D. Shekhawat, Microwave-assisted heterogeneous catalysis, in Catalysis, ed. J. Spivey, Y.-F. Han and D. Shekhawat, The Royal Society of Chemistry, 2021, vol. 33 Search PubMed.
- E. Meloni; G. Iervolino and V. Palma, Basics of Microwave Heating and Recent Advances, in Advances in Microwave-assisted Heterogeneous Catalysis, ed. J. Hu and B. M. Reddy, Royal Society of Chemistry, 2023, vol. 45 Search PubMed.
- J. Sun, J. S. Hayward, M. Barter, D. R. Slocombe and J. K. Bartley, Designing Heterogeneous Catalysts for Microwave Assisted Selective Oxygenation, ChemCatChem, 2024, 16(19), e202301586, DOI:10.1002/cctc.202301586 (accessed 2025/02/19).
- V. Palma, D. Barba, M. Cortese, M. Martino, S. Renda and E. Meloni, Microwaves and Heterogeneous Catalysis: A Review on Selected Catalytic Processes, Catalysts, 2020, 10, 246, DOI:10.3390/catal10020246.
- X. Shen, Z. Zhao, H. Li, X. Gao and X. Fan, Microwave-assisted pyrolysis of plastics with iron-based catalysts for hydrogen and carbon nanotubes production, Mater. Today Chem., 2022, 26, 101166, DOI:10.1016/j.mtchem.2022.101166.
- F. Zhang, X. Zhang, Z. Song, X. Li, X. Zhao, J. Sun, Y. Mao, X. Wang and W. Wang, Promotion of microwave discharge over carbon catalysts for CO2 reforming of CH4 to syngas, Fuel, 2023, 331, 125914, DOI:10.1016/j.fuel.2022.125914.
- N. Haneishi, S. Tsubaki, E. Abe, M. M. Maitani, E.-I. Suzuki, S. Fujii, J. Fukushima, H. Takizawa and Y. Wada, Enhancement of Fixed-bed Flow Reactions under Microwave Irradiation by Local Heating at the Vicinal Contact Points of Catalyst Particles, Sci. Rep., 2019, 9(1), 222, DOI:10.1038/s41598-018-35988-y.
- T. D. Musho, C. Wildfire, N. M. Houlihan, E. M. Sabolsky and D. Shekhawat, Study of Cu2O particle morphology on microwave field enhancement, Mater. Chem. Phys., 2018, 216, 278–284, DOI:10.1016/j.matchemphys.2018.05.059.
- É. Casey, R. Breen, G. Pareras, A. Rimola, J. D. Holmes and G. Collins, Guanidine functionalized porous SiO2 as heterogeneous catalysts for microwave depolymerization of PET and PLA, RSC Sustainability, 2024, 2(4), 1040–1051, 10.1039/D3SU00425B.
- J. Zhao, B. Liu, L. Xiong, W. Liu, D. Wang, W. Ma, L. Jiang, J. Yang, P. Wang and T. Xiao, et al., Highly selective upcycling of plastic mixture waste by microwave-assisted catalysis over Zn/b-ZnO, Nat. Commun., 2025, 16(1), 1726, DOI:10.1038/s41467-024-55584-1.
- M. Adam, N. Hjalmarsson, C. S. Lee, D. J. Irvine, J. Robinson and E. Binner, Understanding microwave interactions with polymers to enable advanced plastic chemical recycling, Polym. Test., 2024, 137, 108483, DOI:10.1016/j.polymertesting.2024.108483.
- F. Zhang, N. Li, J.-F. Shi, L. Xu, L.-C. Jia, Y.-Y. Wang and D.-X. Yan, Recent progress on carbon-based microwave absorption materials for multifunctional applications: A review, Composites, Part B, 2024, 283, 111646, DOI:10.1016/j.compositesb.2024.111646.
- T. Hou, B. Wang, Z. Jia, H. Wu, D. Lan, Z. Huang, A. Feng, M. Ma and G. Wu, A review of metal oxide-related microwave absorbing materials from the dimension and morphology perspective, J. Mater. Sci.: Mater. Electron., 2019, 30(12), 10961–10984, DOI:10.1007/s10854-019-01537-0.
- M. Irfan, R. Saleem, B. Shoukat, H. Hussain, S. Shukrullah, M. Y. Naz, S. Rahman, A. A. J. Ghanim, G. Nawalany and T. Jakubowski, Production of combustible fuels and carbon nanotubes from plastic wastes using an in-situ catalytic microwave pyrolysis process, Sci. Rep., 2023, 13(1), 9057, DOI:10.1038/s41598-023-36254-6.
- W. Zhang, L. Wu, Y. Zhou, Y. Xu, J. Deng, Z. Yang and H. Sun, Design of a capacity-enhanced single-mode reactor for microwave chemistry researches, Chem. Eng. J., 2022, 427, 131898, DOI:10.1016/j.cej.2021.131898.
- V. Ramopoulos, G. Link, S. Soldatov and J. Jelonnek, Industrial scale microwave applicator for high temperature alkaline hydrolysis of PET, Int. J. Microwave Wireless Technol., 2018, 10(5–6), 709–716, DOI:10.1017/S1759078718000727 From Cambridge University Press Cambridge Core.
- Resynergi. Resynergi Homepage. 2025. https://www.resynergi.com/ (accessed 10/10/2025).
- T. Katoh, Y. Ogawa, Y. Ohta and T. Yokozawa, Synthesis of polyester by means of polycondensation of diol ester and dicarboxylic acid ester through ester–ester exchange reaction, J. Polym. Sci., 2021, 59(9), 787–797, DOI:10.1002/pol.20210032 (accessed 2025/07/01).
- B. L. Deopura and N. V. Padaki, Synthetic Textile Fibres: Polyamide, Polyester and Aramid Fibres, in Textiles and Fashion, ed. R. Sinclair, Woodhead Publishing, 2015, ch. 5, pp. 97–114 Search PubMed.
- J. Cao, H. Liang, J. Yang, Z. Zhu, J. Deng, X. Li, M. Elimelech and X. Lu, Depolymerization mechanisms and closed-loop assessment in polyester waste recycling, Nat. Commun., 2024, 15(1), 6266, DOI:10.1038/s41467-024-50702-5.
- A.-C. Enache, I. Grecu and P. Samoila, Polyethylene Terephthalate (PET) Recycled by Catalytic Glycolysis: A Bridge toward Circular Economy Principles, Materials, 2024, 17, 2991, DOI:10.3390/ma17122991.
- S. R. Shukla and A. M. Harad, Aminolysis of polyethylene terephthalate waste, Polym. Degrad. Stab., 2006, 91(8), 1850–1854, DOI:10.1016/j.polymdegradstab.2005.11.005.
- D. S. Achilias, G. P. Tsintzou, A. K. Nikolaidis, D. N. Bikiaris and G. P. Karayannidis, Aminolytic depolymerization of poly(ethylene terephthalate) waste in a microwave reactor, Polym. Int., 2011, 60(3), 500–506, DOI:10.1002/pi.2976 (accessed 2025/07/01).
- H. Yao, L. Liu, D. Yan, Q. Zhou, J. Xin, X. Lu and S. Zhang, Colorless BHET obtained from PET by modified mesoporous catalyst ZnO/SBA-15, Chem. Eng. Sci., 2022, 248, 117109, DOI:10.1016/j.ces.2021.117109.
- J. Cao, Y. Lin, W. Jiang, W. Wang, X. Li, T. Zhou, P. Sun, B. Pan, A. Li and Q. Zhang, Mechanism of the Significant Acceleration of Polyethylene Terephthalate Glycolysis by Defective Ultrathin ZnO Nanosheets with Heteroatom Doping, ACS Sustainable Chem. Eng., 2022, 10(17), 5476–5488, DOI:10.1021/acssuschemeng.1c08656.
- J.-T. Du, Q. Sun, X.-F. Zeng, D. Wang, J.-X. Wang and J.-F. Chen, ZnO nanodispersion as pseudohomogeneous catalyst for alcoholysis of polyethylene terephthalate, Chem. Eng. Sci., 2020, 220, 115642, DOI:10.1016/j.ces.2020.115642.
- E. Selvam, Y. Luo, M. Ierapetritou, R. F. Lobo and D. G. Vlachos, Microwave-assisted depolymerization of PET over heterogeneous catalysts, Catal. Today, 2023, 418, 114124, DOI:10.1016/j.cattod.2023.114124.
- G. Xi, M. Lu and C. Sun, Study on depolymerization of waste polyethylene terephthalate into monomer of bis(2-hydroxyethyl terephthalate), Polym. Degrad. Stab., 2005, 87(1), 117–120, DOI:10.1016/j.polymdegradstab.2004.07.017.
- F. Chen, G. Wang, C. Shi, Y. Zhang, L. Zhang, W. Li and F. Yang, Kinetics of glycolysis of poly(ethylene terephthalate) under microwave irradiation, J. Appl. Polym. Sci., 2013, 127(4), 2809–2815, DOI:10.1002/app.37608 (accessed 2025/06/17).
- M. Revathi, V. Sivamurugan, R. Dhanalakshmi, R. Biju Bennie and C. Joel, Kaolin supported niobia for microwave-assisted aminolytic depolymerization in the chemical recycling of polyester waste, Ceram. Int., 2024, 50(17, Part B), 30483–30492, DOI:10.1016/j.ceramint.2024.05.346.
- M. Anbarasu, V. Vinitha, M. Preeyanghaa, B. Neppolian and V. Sivamurugan, Valorization of PES Textile Waste Through Glycolysis and Aminolysis Using Bimetallic ZnO and Carbon Nitride Nanocomposites, J. Cluster Sci., 2024, 35(4), 1105–1125, DOI:10.1007/s10876-023-02534-4.
- V. Vinitha, M. Preeyanghaa, M. Anbarasu, B. Neppolian and V. Sivamurugan, Chemical recycling of polyester textile wastes using silver-doped zinc oxide nanoparticles: an economical solution for circular economy, Environ. Sci. Pollut. Res., 2023, 30(30), 75401–75416, DOI:10.1007/s11356-023-27567-0.
- K. Fukushima, O. Coulembier, J. M. Lecuyer, H. A. Almegren, A. M. Alabdulrahman, F. D. Alsewailem, M. A. McNeil, P. Dubois, R. M. Waymouth and H. W. Horn, et al., Organocatalytic depolymerization of poly(ethylene terephthalate), J. Polym. Sci., Part A:Polym. Chem., 2011, 49(5), 1273–1281, DOI:10.1002/pola.24551 (accessed 2025/07/28).
- H. Ramezani Dana and F. Ebrahimi, Synthesis, properties, and applications of polylactic acid-based polymers, Polym. Eng. Sci., 2023, 63(1), 22–43, DOI:10.1002/pen.26193 (accessed 2025/07/02).
- M. Hussain, S. M. Khan, M. Shafiq and N. Abbas, A review on PLA-based biodegradable materials for biomedical applications, Giant, 2024, 18, 100261, DOI:10.1016/j.giant.2024.100261.
- W. Limsukon, M. Rubino, M. Rabnawaz, L.-T. Lim and R. Auras, Hydrolytic degradation of poly(lactic acid): Unraveling correlations between temperature and the three phase structures, Polym. Degrad. Stab., 2023, 217, 110537, DOI:10.1016/j.polymdegradstab.2023.110537.
- P. McKeown and M. D. Jones, The Chemical Recycling of PLA: A Review, Sustainable Chemistry, 2020, vol. 1, pp. 1–22 Search PubMed.
- M. N. Siddiqui, L. Kolokotsiou, E. Vouvoudi, H. H. Redhwi, A. A. Al-Arfaj and D. S. Achilias, Depolymerization of PLA by Phase Transfer Catalysed Alkaline Hydrolysis in a Microwave Reactor, J. Polym. Environ., 2020, 28(6), 1664–1672, DOI:10.1007/s10924-020-01716-9.
- E. Cheung, C. Alberti and S. Enthaler, Chemical Recycling of End-of-Life Poly(lactide) via Zinc-Catalyzed Depolymerization and Polymerization, ChemistryOpen, 2020, 9(12), 1224–1228, DOI:10.1002/open.202000243 (accessed 2025/07/02).
- L. A. Román-Ramírez, P. McKeown, M. D. Jones and J. Wood, Poly(lactic acid) Degradation into Methyl Lactate Catalyzed by a Well-Defined Zn(II) Complex, ACS Catal., 2019, 9(1), 409–416, DOI:10.1021/acscatal.8b04863.
- S. Najmi, D. Huang, A. Duncan, D. Slanac, K. Hutchenson, J. Hughes, R. Poladi and D. G. Vlachos, Closed-loop chemical recycling of polyethylene furan-2,5-dicarboxylate (PEF) under microwave-assisted heating, Green Chem., 2025, 27, 5753–5763, 10.1039/D5GC01583A.
- K. Takeuchi, 5.16 – Polycarbonates, in Polymer Science: A Comprehensive Reference, ed. K. Matyjaszewski and M. Möller, Elsevier, 2012, pp. 363–376 Search PubMed.
- P. Sambyal, P. Najmi, D. Sharma, E. Khoshbakhti, H. Hosseini, A. S. Milani and M. Arjmand, Plastic recycling: Challenges and opportunities, Can. J. Chem. Eng., 2025, 103(6), 2462–2498, DOI:10.1002/cjce.25531 (accessed 2025/07/02).
- G. Grause, N. Tsukada, W. J. Hall, T. Kameda, P. T. Williams and T. Yoshioka, High-value products from the catalytic hydrolysis of polycarbonate waste, Polym. J., 2010, 42(6), 438–442, DOI:10.1038/pj.2010.21.
- J. Guo, M. Liu, Y. Gu, Y. Wang, J. Gao and F. Liu, Efficient Alcoholysis of Polycarbonate Catalyzed by Recyclable Lewis Acidic Ionic Liquids, Ind. Eng. Chem. Res., 2018, 57(32), 10915–10921, DOI:10.1021/acs.iecr.8b02201.
- D. Kim, B.-K. Kim, Y. Cho, M. Han and B.-S. Kim, Kinetics of Polycarbonate Glycolysis in Ethylene Glycol, Ind. Eng. Chem. Res., 2009, 48(2), 685–691, DOI:10.1021/ie8010947.
- T.-B. Chen, L.-X. Yun, B. Zhang, Y.-H. Gu, H.-T. Zhang, X.-F. Zeng and J.-X. Wang, Highly Efficient Depolymerization of Polycarbonate Enabled by Dispersions of Ultrasmall ZnO Nanoparticles, Ind. Eng. Chem. Res., 2024, 63(42), 17868–17877, DOI:10.1021/acs.iecr.4c03059.
- G. P. Tsintzou, E. V. Antonakou and D. S. Achilias, Environmentally friendly chemical recycling of poly(bisphenol-A carbonate) through phase transfer-catalysed alkaline hydrolysis under microwave irradiation, J. Hazard. Mater., 2012, 241–242, 137–145, DOI:10.1016/j.jhazmat.2012.09.027.
- K. Ikenaga, K. Higuchi, S. Kohri and K. Kusakabe, Depolymerization of polycarbonate by methanol under pressurized microwave irradiation, IOP Conf. Ser.:Mater. Sci. Eng., 2018, 458(1), 012037, DOI:10.1088/1757-899X/458/1/012037.
- S. T. Schwab, M. Baur, T. F. Nelson and S. Mecking, Synthesis and Deconstruction of Polyethylene-type Materials, Chem. Rev., 2024, 124(5), 2327–2351, DOI:10.1021/acs.chemrev.3c00587.
- G. Yang, P. Peng, H. Guo, H. Song and Z. Li, The catalytic pyrolysis of waste polyolefins by zeolite-based catalysts: A critical review on the structure-acidity synergies of catalysts, Polym. Degrad. Stab., 2024, 222, 110712, DOI:10.1016/j.polymdegradstab.2024.110712.
- B. Thangaraj and Y.-K. Lee, Review on recent development in catalytic cracking of waste polyolefins: Effect of zeolite-based catalysts and reaction parameters, Fuel, 2025, 380, 133220, DOI:10.1016/j.fuel.2024.133220.
- S. Tasleem, A. Soliman and E. H. Alsharaeh, Recent developments in catalytic materials and reactors for the catalytic pyrolysis of plastic waste into hydrogen: a critical review with a focus on the circular economy, RSC Adv., 2025, 15(26), 20881–20907, 10.1039/D5RA03170B.
- P. H. M. Putra, S. Rozali, M. F. A. Patah and A. Idris, A review of microwave pyrolysis as a sustainable plastic waste management technique, J. Environ. Manage., 2022, 303, 114240, DOI:10.1016/j.jenvman.2021.114240.
- Z. Qie, H. Xiang, H. Xiang, R. Zou, A. Alhelali, H. Alhassawi, S. Ding, Y. Jiao, S. M. Holmes and A. A. Garforth, et al., Catalytic pyrolysis of high-density polyethylene (HDPE) over hierarchical ZSM-5 zeolites produced by microwave-assisted chelation-alkaline treatment, Fuel, 2024, 368, 131532, DOI:10.1016/j.fuel.2024.131532.
- T. Luong, C. Luo, B. Robinson, J. Hu and Y. Wang, Microwave Catalytic Co-Upcycling of Polyethylene and Polystyrene, ACS Sustainable Resour. Manage., 2024, 1(8), 1750–1758, DOI:10.1021/acssusresmgt.4c00110.
- F. Vatankhah, A. Carrillo García and J. Chaouki, Role of Bifunctional Metal-Promoted Zeolitic Catalyst in Microwave-Assisted Pyrolysis of Polyethylene to Monocyclic Aromatics, Energy Fuels, 2024, 38(21), 20562–20576, DOI:10.1021/acs.energyfuels.4c03692.
- K. Ding, S. Liu, Y. Huang, S. Liu, N. Zhou, P. Peng, Y. Wang, P. Chen and R. Ruan, Catalytic microwave-assisted pyrolysis of plastic waste over NiO and HY for gasoline-range hydrocarbons production, Energy Convers. Manage., 2019, 196, 1316–1325, DOI:10.1016/j.enconman.2019.07.001.
- V. Tuli, C. Luo, B. Robinson, J. Hu and Y. Wang, Microwave-assisted catalytic technology for sustainable production of valuable chemicals from plastic waste with enhanced catalyst reusability, Chem. Eng. J., 2024, 489, 151551, DOI:10.1016/j.cej.2024.151551.
- J. Zhao, J. Gao, D. Wang, Y. Chen, L. Zhang, W. Ma and S. Zhao, Microwave-intensified catalytic upcycling of plastic waste into hydrogen and carbon nanotubes over self-dispersing bimetallic catalysts, Chem. Eng. J., 2024, 483, 149270, DOI:10.1016/j.cej.2024.149270.
- B. Shoukat, H. Hussain, M. Y. Naz, A. A. Ibrahim, S. Shukrullah, Y. Khan and Y. Zhang, Microwave-Assisted Catalytic Deconstruction of Plastics Waste into Nanostructured Carbon and Hydrogen Fuel Using Composite Magnetic Ferrite Catalysts, Scientifica, 2024, 2024(1), 3318047, DOI:10.1155/2024/3318047 (accessed 2025/03/25).
- X. Jie, W. Li, D. Slocombe, Y. Gao, I. Banerjee, S. Gonzalez-Cortes, B. Yao, H. AlMegren, S. Alshihri and J. Dilworth, et al., Microwave-initiated catalytic deconstruction of plastic waste into hydrogen and high-value carbons, Nat. Catal., 2020, 3(11), 902–912, DOI:10.1038/s41929-020-00518-5.
- H. Kondo, A. Sawai, K. Hirabayashi, A. Nakanishi, K. Fukumoto, Y. Tanaka and S. Horikoshi, Continuous process design of the microwave chemical recycling of waste plastics using microwave-absorbing heating elements, Sci. Rep., 2024, 14(1), 21952, DOI:10.1038/s41598-024-71958-3 From NLM.
- J. Gao, J. Zhao, Z. Xing, M. Guo, H. Xie, W. Ma and J. Liu, Microwave-Powered Liquid Metal Degradation of Polyolefins, Adv. Mater., 2025, 37(6), 2412539, DOI:10.1002/adma.202412539 (accessed 2025/03/19).
- S. Oh and E. E. Stache, Recent advances in oxidative degradation of plastics, Chem. Soc. Rev., 2024, 53(14), 7309–7327, 10.1039/D4CS00407H.
- E. Bäckström, K. Odelius and M. Hakkarainen, Trash to Treasure: Microwave-Assisted Conversion of Polyethylene to Functional Chemicals, Ind. Eng. Chem. Res., 2017, 56(50), 14814–14821, DOI:10.1021/acs.iecr.7b04091.
- C.-F. Chow, W.-L. Wong, K. Y.-F. Ho, C.-S. Chan and C.-B. Gong, Combined Chemical Activation and Fenton Degradation to Convert Waste Polyethylene into High-Value Fine Chemicals, Chem. – Eur. J., 2016, 22(28), 9513–9518, DOI:10.1002/chem.201600856 (accessed 2024/01/23).
- S. Ghatge, Y. Yang, Y. Ko, Y. Yoon, J.-H. Ahn, J. J. Kim and H.-G. Hur, Degradation of sulfonated polyethylene by a bio-photo-Fenton approach using glucose oxidase immobilized on titanium dioxide, J. Hazard. Mater., 2022, 423, 127067, DOI:10.1016/j.jhazmat.2021.127067.
- N. D. O. Dos Santos, R. Busquets and L. C. Campos, Insights into the removal of microplastics and microfibres by Advanced Oxidation Processes, Sci. Total Environ., 2023, 861, 160665, DOI:10.1016/j.scitotenv.2022.160665.
- K. Hu, P. Zhou, Y. Yang, T. Hall, G. Nie, Y. Yao, X. Duan and S. Wang, Degradation of Microplastics by a Thermal Fenton Reaction, ACS ES&T Eng., 2022, 2(1), 110–120, DOI:10.1021/acsestengg.1c00323.
- R. Breen, J. D. Holmes and G. Collins, Heterogeneous Fenton-type oxidative degradation of low-density polyethylene to valuable acid products using a nanostructured Fe–CeO2 solid solution catalyst, Catal. Sci. Technol., 2025, 15, 920–932, 10.1039/D4CY01384K.
- Z. Xu, D. Sun, J. Xu, R. Yang, J. D. Russell and G. Liu, Progress and Challenges in Polystyrene Recycling and Upcycling, ChemSusChem, 2024, 17(17), e202400474, DOI:10.1002/cssc.202400474 (accessed 2025/07/07).
- Commission, E. Proposal for a regulation of the European Parliament and of the Council on packaging and packaging waste, amending Regulation (EU) 2019/1020 and Directive (EU) 2019/904, and repealing Directive 94/62/EC. 2022.
- A. M. Gonzalez-Aguilar, V. Pérez-García and J. M. Riesco-Ávila, A Thermo-Catalytic Pyrolysis of Polystyrene Waste Review: A Systematic, Statistical, and Bibliometric Approach, Polymers, 2023, 15, 1582 CrossRef CAS.
- S. Fan, Y. Zhang, L. Cui, Q. Xiong and T. Maqsood, Conversion of Polystyrene Plastic into Aviation Fuel through Microwave-Assisted Pyrolysis as Affected by Iron-Based Microwave Absorbents, ACS Sustainable Chem. Eng., 2023, 11(3), 1054–1066, DOI:10.1021/acssuschemeng.2c05880.
- K. Kachhadiya, D. Patel, G. J. Vijaybhai, P. Raghuvanshi, D. V. Surya, S. Dharaskar, G. P. Kumar, B. R. Reddy, N. Remya and T. H. Kumar, et al., Conversion of waste polystyrene into valuable aromatic hydrocarbons via microwave-assisted pyrolysis, Environ. Sci. Pollut. Res., 2024, 31(46), 57509–57522, DOI:10.1007/s11356-023-28294-2.
- M. Kohutiar, L. Kakošová, M. Krbata, R. Janík, J. J. Fekiač, A. Breznická, M. Eckert, P. Mikuš and Ľ. Timárová, Comprehensive Review: Technological Approaches, Properties, and Applications of Pure and Reinforced Polyamide 6 (PA6) and Polyamide 12 (PA12) Composite Materials, Polymers, 2025, 17, 442, DOI:10.3390/polym17040442.
- V. Hirschberg and D. Rodrigue, Recycling of polyamides: Processes and conditions, J. Polym. Sci., 2023, 61(17), 1937–1958, DOI:10.1002/pol.20230154 (accessed 2025/07/07).
- U. Klun and A. Kržan, Rapid microwave induced depolymerization of polyamide-6, Polymer, 2000, 41(11), 4361–4365, DOI:10.1016/S0032-3861(99)00658-8.
- U. Češarek, D. Pahovnik and E. Žagar, Chemical Recycling of Aliphatic Polyamides by Microwave-Assisted Hydrolysis for Efficient Monomer Recovery, ACS Sustainable Chem. Eng., 2020, 8(43), 16274–16282, DOI:10.1021/acssuschemeng.0c05706.
- A. Frisa-Rubio, C. González-Niño, P. Royo, N. García-Polanco, D. Martínez-Hernández, L. Royo-Pascual, S. Fiesser, E. Žagar and T. García-Armingol, Chemical recycling of plastics assisted by microwave multi-frequency heating, Cleaner Eng. Technol., 2021, 5, 100297, DOI:10.1016/j.clet.2021.100297.
- C. Alberti, R. Figueira, M. Hofmann, S. Koschke and S. Enthaler, Chemical Recycling of End-of-Life Polyamide 6 via Ring Closing Depolymerization, ChemistrySelect, 2019, 4(43), 12638–12642, DOI:10.1002/slct.201903970 (accessed 2025/05/15).
- D. Tanner, J. A. Fitzgerald and B. R. Phillips, The Kevlar Story—an Advanced Materials Case Study, Angew. Chem., Int. Ed. Engl., 1989, 28(5), 649–654, DOI:10.1002/anie.198906491 (accessed 2025/03/21).
- P. M. Gore and B. Kandasubramanian, Functionalized Aramid Fibers and Composites for Protective Applications: A Review, Ind. Eng. Chem. Res., 2018, 57(49), 16537–16563, DOI:10.1021/acs.iecr.8b04903.
- J. Benninga, B. Gebben, R. Folkersma, V. S. D. Voet and K. Loos, Rapid Microwave-Assisted Chemical Recycling of Poly(p-Phenylene Terephthalamide), J. Am. Chem. Soc., 2025, 147(9), 7191–7195, DOI:10.1021/jacs.4c17791.
- L. Campisi, C. La Motta and D. Napierska, Polyvinyl chloride (PVC), its additives, microplastic and human health: Unresolved and emerging issues, Sci. Total Environ., 2025, 960, 178276, DOI:10.1016/j.scitotenv.2024.178276.
- X. Zhang, X. Feng, W. Guo, C. Zhang and X. Zhang, Chemically recyclable polyvinyl chloride-like plastics, Nat. Commun., 2024, 15(1), 8536, DOI:10.1038/s41467-024-52852-y.
- M. Havaei, O. Akin, A. Locaspi, R. John Varghese, F. Minette, E. Romers, S. De Meester and K. M. Van Geem, Beyond the Landfill: A critical review of techniques for End-of-Life Polyvinyl chloride (PVC) valorization, Waste Manage., 2025, 193, 105–134, DOI:10.1016/j.wasman.2024.11.023.
- L. Lu, W. Li, Y. Cheng and M. Liu, Chemical recycling technologies for PVC waste and PVC-containing plastic waste: A review, Waste Manage., 2023, 166, 245–258, DOI:10.1016/j.wasman.2023.05.012.
- W. Jerzak and A. Magdziarz, Dehydrochlorination of polyvinyl chloride and co-pyrolysis with maize cob: Insight into product composition and thermal behaviour, Thermochim. Acta, 2025, 749, 180023, DOI:10.1016/j.tca.2025.180023.
- L. Ye, T. Li and L. Hong, Co-pyrolysis of Fe3O4-poly(vinyl chloride) (PVC) mixtures: Mitigation of chlorine emissions during PVC recycling, Waste Manage., 2021, 126, 832–842, DOI:10.1016/j.wasman.2021.04.021.
- S. Horikoshi, N. Hachisuga and N. Serpone, Recycling of e-waste power cables using microwave-induced pyrolysis – process characteristics and facile recovery of copper metal, RSC Adv., 2024, 14(41), 29955–29964, 10.1039/D4RA05602G.
- M. Figueira-Magalhães, D. Martinez-Hernandez and I. Julian, Microwave-Assisted PUF Aminolysis: Experimental Validation, Scaling Process Assessment and LCA Evaluation, Sustainability, 2025, 17, 4091, DOI:10.3390/su17094091.
- S. Neha, K. Prasanna Kumar Ramesh and N. Remya, Techno-economic analysis and life cycle assessment of microwave co-pyrolysis of food waste and low-density polyethylene, Sustainable Energy Technol. Assess., 2022, 52, 102356, DOI:10.1016/j.seta.2022.102356.
- Y. Luo, E. Selvam, D. G. Vlachos and M. Ierapetritou, Economic and Environmental Benefits of Modular Microwave-Assisted Polyethylene Terephthalate Depolymerization, ACS Sustainable Chem. Eng., 2023, 11(10), 4209–4218, DOI:10.1021/acssuschemeng.2c07203.
- B.-N. T. Nguyen, T. T. Y. Tan, K.-I. Otake, S. Kitagawa and J. Y. C. Lim, MOF Catalysts for Plastic Depolymerization, Angew. Chem., Int. Ed., 2025, 64(25), e202504017, DOI:10.1002/anie.202504017 (accessed 2025/10/10).
| This journal is © The Royal Society of Chemistry 2026 |
