Chitosan-modified biochar enhanced PFAS degradation in UV/sulfite: impact of environmental conditions and applicability across different PFAS
Ziteng
Song
 *a,
Jianzhou
He
*a,
Jianzhou
He
 b,
Steven
Mai
a,
Thorsten
Knappenberger
a and
Yaniv
Olshansky
b,
Steven
Mai
a,
Thorsten
Knappenberger
a and
Yaniv
Olshansky
 *a
*a
aDepartment of Crop, Soil, and Environmental Sciences, Auburn, AL 36849, USA. E-mail: zzs0048@auburn.edu; yzo0015@auburn.edu
bDepartment of Biochemistry, Chemistry & Physics, Georgia Southern University, Savannah, GA 31419, USA
First published on 5th November 2025
Abstract
The persistence of per- and polyfluoroalkyl substances (PFAS) in aquatic environments poses significant environmental and health risks, necessitating the development of effective and sustainable remediation strategies. This study evaluated the combined use of chitosan-modified biochar (Chi-BC) and ultraviolet advanced reduction processes (UV-ARP) for PFAS degradation, focusing on environmental influences and varying PFAS chemistries. Chi-BC effectively adsorbed and concentrated PFAS onto its surface, enhancing localized radical activity and enabling efficient defluorination. The Chi-BC/UV-ARP system achieved high degradation and defluorination rates, notably with long-chain PFAS, where adsorption facilitated radical access to C–F bonds. Environmental factors, including ionic strength, nitrate, and natural organic matter (NOM), impacted system efficiency by altering radical availability and PFAS interactions. Interestingly, nitrate enhanced PFAS adsorption onto Chi-BC, indirectly promoting defluorination, while NOM showed mixed effects depending on concentration. Overall, this work presented Chi-BC/UV-ARP as an energy-efficient PFAS treatment strategy, where Chi-BC's adsorption characteristics enabled the use of compact reactors and lower energy inputs, advancing practical applications for diverse water chemistries.
Water impactThis study demonstrates that chitosan-modified biochar (Chi-BC) enhances the degradation and defluorination of diverse PFAS—including PFBS, PFHxS, and PFOS—under UV/sulfite-based advanced reduction processes (UV-ARP). Chi-BC facilitates PFAS pre-concentration and electron transfer, promoting efficient C–F bond cleavage. While humic acid and high ionic strength hinder degradation, nitrate improves defluorination by promoting hydroxylation. The system maintains high removal efficiency in simulated groundwater, highlighting its robustness under realistic conditions. These findings support the practical application of Chi-BC/UV-ARP as a low-cost, energy-efficient technology for PFAS remediation in complex water environments. |
Introduction
Per- and polyfluoroalkyl substances (PFAS) have been widely used in global industries and consumer products since the 1940s.1–3 The widespread use of PFAS is attributed to their exceptional surfactant properties, heat resistance, and chemical and biological degradation resistance, particularly due to their strong C–F bond.4–8 However, due to their extreme chemical stability, PFAS can persist in the environment for a long time, making them resistant to natural degradation.4,5,8 The half-life of PFAS in soil and water can range from decades to centuries.9,10 This degradation persistence has led to their widespread global distribution, with detectable levels even in remote regions like the Arctic.11,12 Their high mobility in aquatic environments and degradation resistance allow them to spread over long distances, contaminating drinking water sources and bioaccumulating in the food chain.11–13 Growing awareness of PFAS contamination resulted in regulation worldwide. In 2024, the U.S. Environmental Protection Agency (EPA) enacted stringent drinking water regulations for six high-priority PFAS compounds: perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA), perfluorononanoic acid (PFNA), perfluorohexane sulfonate (PFHxS), perfluorobutanesulfonate (PFBS), and hexafluoropropylene oxide dimer acid (HFPO-DA, commonly known as GenX) (USEPA, 2024). With the introduction of these regulations, there is an urgent need for the scientific community to develop effective treatment technologies to mitigate the long-term environmental and health impacts of PFAS.Traditional water treatment technologies, such as activated carbon adsorption, ion exchange, and membrane filtration (e.g., reverse osmosis), have been applied to remove PFAS from water.14–16 However, these methods primarily concentrate PFAS on the adsorbent surfaces rather than degrading them into harmless compounds, resulting in secondary waste streams that require further treatment.17,18 Consequently, research has gradually shifted toward destructive technologies that can break the persistent C–F bonds in PFAS. In recent years, advanced reduction processes (ARPs) have emerged as a promising approach.19,20 Specifically, UV/sulfite-based ARP utilizes ultraviolet light to activate sulfite ions, generating the strong reductant - hydrated electrons (eaq−, −2.9 V) and sulfite radicals (SO3˙−). eaq− can effectively break the highly stable C–F bonds in PFAS molecules.20–22
While UV/sulfite-based ARP systems have been studied as remediation technology for PFAS destruction, they have demonstrated limited efficiency due to the low concentration of PFAS in environmental waters, inefficient contact between PFAS and reactive species, and the risk of secondary pollution from residual sulfite or transformation.23 To enhance the performance of UV/sulfite-based ARP systems, integrating adsorbents can address key limitations by concentrating PFAS, facilitating interfacial electron transfer, and minimizing secondary pollution.24 Ideal adsorbents possess high specific surface areas, tunable surface charges, and the capacity to form reactive interfaces without scavenging reactive species.25,26 Among various adsorbents, biochar derived from agricultural waste offers a low-cost and sustainable alternative.21,27,28 Our previous work has shown that functionalized biochar, particularly chitosan-modified biochar (Chi-BC), significantly enhances the adsorption of several PFAS and the degradation efficiency of PFOS in ARP systems.27 This combined approach leverages the adsorption capacity of biochar to capture PFAS, followed by degradation through reduction processes, offering a sustainable and effective solution for PFAS removal from water.29–32 However, this approach still faces certain limitations and challenges.
The efficacy of biochar-enhanced ARP may be influenced by environmental factors such as ionic strength and the presence of organic matter, which can interfere with PFAS adsorption and degradation processes.33,34 Our previous research demonstrated the effectiveness of combining Chi-BC with ARP, particularly UV-sulfite systems, for the degradation of long-chain PFAS, such as PFOS.27 This approach achieved up to 85% defluorination at pH > 10 and nearly 100% PFAS removal across a pH range of 6 to 11. However, our study primarily focused on long-chain and anionic PFAS, leaving the behavior of short-chain and cationic PFAS and the system's performance under realistic environmental conditions largely unexplored.
Given the growing concern over short-chain PFAS and their diverse chemical behaviors, this study built upon our previous findings by investigating the degradation of PFBS, PFHxS, PFOS, and perfluorooctane sulfonamide (PFOSA) using Chi-BC in a UV-ARP system at pH 10.27 PFBS is a short-chain perfluoroalkyl sulfonate used as a substitute for phased-out long-chain PFOS. It remains highly mobile and persistent in groundwater.26 PFHxS and PFOS are long-chain sulfonates. While PFOS is one of the most frequently detected legacy PFAS and is exceptionally resistant to degradation, PFHxS has recently been designated a compound of emerging concern by several regulatory agencies.25,35 PFOSA is commercially manufactured as a distinct PFAS used for its grease- and water-repellent properties, particularly in food packaging and other consumer products.36 In the environment, it serves as a key precursor that can transform into PFOS and other perfluoroalkyl acids, making it critical for understanding PFAS transformation pathways.36 Collectively, these compounds represent both emerging short-chain and legacy long-chain PFAS, as well as precursor species. It is a representative and challenging test set for evaluating the robustness of Chi-BC/UV-sulfite systems under environmentally relevant conditions. Their structural diversity also enables mechanistic insights into the effects of chain length and headgroup chemistry on PFAS degradation.
In this study, we explored the effects of ionic strength, nitrate (NO3−), and humic acid (HA) on PFAS removal. Nitrate was included because it is prevalent in groundwater. In UV/sulfite systems, it can be photolyzed to generate ˙OH and ˙NO2 radicals, introducing an oxidative contribution. Additionally, as a background electrolyte, it can influence the adsorption of anionic PFAS on the positively charged Chi-BC surface. We further simulated groundwater conditions to evaluate the system's efficacy in environmental interferences, thereby advancing the practical application of Chi-BC/UV-ARP for PFAS remediation.
Materials and methods
PFAS and Chi-BC
Four anionic PFAS were selected with C4, C6, and C8 fluorocarbon chains and one uncharged compound (Table S1). PFBS and PFOS were sourced from Matrix Scientific (Elgin, SC) and Matrix Laboratories (Mount Prospect, IL), respectively, while PFHxS and PFOSA were obtained from SynQuest Laboratories (Alachua, FL). All PFAS standard solutions were purchased from Wellington Laboratories (>98%). Chitosan, sodium chloride (NaCl), and sodium sulfite (Na2SO3) were obtained from Thermo Fisher Scientific (Ward Hill, MA). Calcium chloride (CaCl2), magnesium sulfate (MgSO4), and HPLC-grade methanol were supplied by VWR Chemicals (Radnor, PA). Suwannee River humic acid was purchased from the International Humic Substances Society.The biochar produced from Douglas Fir feedstock at a pyrolysis temperature of 900 °C was tested because of its extraordinary performance in removing various PFAS from water in our previous studies.21,27,35,37 Details on the production process and physicochemical properties of this Douglas Fir 900 biochar can be found in previous work.35 The Chi-BC, prepared with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of biochar to chitosan, followed the same procedure.27 Comprehensive characterization data for Chi-BC, including its surface area, pore size distribution, and elemental composition, are also available in our previous publication.25 Briefly, the point of zero charge (PZC) of BC and Chi-BC are 1.05 and 7.95, respectively. Their specific surface areas (SSA) are 271 and 8.26 m2 g−1 (see Table S2 for details). Biochar was thoroughly rinsed with deionized (DI) water and centrifuged at 10
1 ratio of biochar to chitosan, followed the same procedure.27 Comprehensive characterization data for Chi-BC, including its surface area, pore size distribution, and elemental composition, are also available in our previous publication.25 Briefly, the point of zero charge (PZC) of BC and Chi-BC are 1.05 and 7.95, respectively. Their specific surface areas (SSA) are 271 and 8.26 m2 g−1 (see Table S2 for details). Biochar was thoroughly rinsed with deionized (DI) water and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm until the pH stabilized at 7.0 ± 0.1. The supernatant was carefully decanted, and the modified biochar was dried at 70 °C and ground into fine powder.
000 rpm until the pH stabilized at 7.0 ± 0.1. The supernatant was carefully decanted, and the modified biochar was dried at 70 °C and ground into fine powder.
PFAS destruction by UV-sulfite ARP system
The UV-sulfite ARP system was described in previous work, and a schematic of the experimental system is presented in Fig. S1. Briefly, PFAS, sodium sulfite, and biochar were mixed in 15 mL quartz tubes. Unless otherwise specified, the initial concentrations were 1 mg L−1 for PFAS, 10 mM for sodium sulfite, and 0.1 g L−1 for biochar. PFAS solutions included either single PFOS (used for ionic strength, nitrate, and humic acid effects) or a mixture of four PFAS (used in artificial groundwater experiments). Adsorbent conditions included unmodified biochar (BC), chitosan-modified biochar (Chi-BC), and a control with no adsorbent. The relatively high PFAS concentration (1 mg L−1), which exceeds typical environmental levels, was chosen to ensure sufficient fluoride ion (F−) generation for detection using an ion-selective electrode (ISE), as described in a previous study.21 Degradation and defluorination experiments for different PFAS compounds were conducted at pH 10, adjusted using 0.1 M NaOH, with and without adding BC or Chi-BC at a concentration of 0.1 g L−1. After fully deoxygenating the mixture by purging with high-purity N2 gas for 1 hour, the quartz tube was placed in a Rayonet photochemical reactor (Southern New England Ultraviolet Company, Branford, CT) and irradiated at 254 nm for 0 to 180 min. To investigate the effects of the solution chemistry on PFOS degradation and defluorination, additional experiments were conducted with varying concentrations of NaCl (1, 5, and 10 mM), NaNO3 (10 mM), and HA (0.1, 1, and 5 mg L−1). The HA solution contains 5 mM NaCl to maintain a constant ionic strength. Artificial groundwater was also prepared in the lab to simulate environmental conditions, containing the same concentration (∼1.43 mM) of CaCl2, MgSO4, and NaHCO3, with a total ionic strength of 10 mM and 1 mg L−1 of HA.35Following the ARP, the reaction suspension was divided into two parts. One part was centrifuged and filtered through a 0.22 μm polypropylene (PP) syringe filter (hereafter solution 1). The supernatant was carefully decanted to minimize the loss of the solid adsorbent at the bottom. The adsorbed PFAS were extracted by adding 3 mL of methanol to the second part solution, and the mixture was rotated for 24 hours to facilitate PFAS desorption. The methanol desorption method has been validated in prior PFAS studies with high reported recoveries.7,8 The mass of the second part solution was recorded before and after to determine the volume of the solution. The solution was then centrifuged and filtered using a PP syringe filter (hereafter, solution 2). The concentration of PFAS in solutions 1 and 2 was quantified using ultra-performance liquid chromatography-high-resolution tandem mass spectrometry (UPLC-HRMS/MS) (see SI for details). Fluoride concentration was measured in solution 1 with ISE.
The total removal efficiency was calculated by eqn (1):
 | (1) |
The adsorption efficiency was calculated by eqn (2):
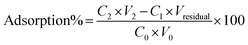 | (2) |
The defluorination ratio was calculated using eqn (3):
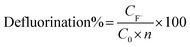 | (3) |
The degradation efficiency was calculated by eqn (4):
| Degradation% = Removal% − Adsorption% | (4) |
Statistical analyses
Raw data were processed using Microsoft Excel, and statistical analyses were conducted in Origin (version 9.0). Factorial treatment effects were evaluated using analysis of variance (ANOVA), with an confidence level of p < 0.05.Results
Impact of BC and Chi-BC on PFAS removal and defluorination efficiency
Fig. 1 shows the removal and defluorination efficiency of PFBS, PFHxS, PFOS, and PFOSA over a 180-minute reaction period using UV/sulfite, UV/sulfite/BC, and UV/sulfite/Chi-BC systems. The results highlight the differences in PFAS removal and defluorination efficiency among these systems and the varying degradation behaviors between short- and long-chain PFAS in biochar-enhanced ARP. The UV/sulfite system exhibited varying degradation efficiencies for different PFAS compounds. For the short-chain PFBS, the system achieved a removal rate of 41.6% within the first 120 minutes, with defluorination reaching 40.3%. In contrast, PFHxS undergoes rapid removal (66.8% within 30 minutes), followed by a plateau, with defluorination efficiency reaching 55.5% by 60 minutes. The long-chain PFOS is almost completely removed (98.5%) within 60 minutes, with defluorination reaching a stable phase earlier, attaining 40.3% at 20 minutes. PFOSA exhibits a degradation pattern similar to PFHxS under the UV/sulfite system alone, achieving removal and defluorination efficiencies of 81.3% and 58.6%, respectively, within 120 minutes.Introducing unmodified biochar (UV/sulfite/BC system) generally diminishes degradation performance. For PFBS, the removal rate decreases by 18.3%, while defluorination efficiency drops significantly to 9.6%. PFHxS removal declines to 60.2% (compared to 86.7% without BC), with defluorination reduced to 42.3%. Although PFOS removal remains nearly unchanged (98.9%), no enhancement in defluorination is observed. For PFOSA, removal efficiency shows minimal variation; however, defluorination decreases markedly to approximately 43%, considerably lower than that achieved in the BC-free system.
Conversely, chitosan-modified biochar (UV/sulfite/Chi-BC system) substantially enhances degradation for PFBS, PFHxS, and PFOS. The removal rate of PFBS increased to 67.6% by 180 minutes, with defluorination reaching 47.6%. PFHxS removal was similar to the UV/sulfite system (84.8% at 120 minutes), but defluorination efficiency surpasses all other systems, reaching 73.4% at 180 minutes. PFOS degradation is further optimized, achieving 98.9% removal with a significant improvement in defluorination (87.8% within 30 minutes). However, PFOSA remained an exception, showing no enhancement in either removal or defluorination efficiency under the Chi-BC system.
Fig. S2 illustrates the concentrations of various intermediate products during the degradation of PFHxS, PFOS, and PFOSA. Trace amounts of PFBS were detected as degradation products of PFHxS. Similarly, minor amounts of PFHxS and PFBS were observed as degradation products of PFOS. For PFOSA, degradation products included PFOS and PFHxS, while PFBS was not detected. These findings indicate that, in addition to the release of fluoride ions, cleavage of C–C bonds occurs during the reaction, forming other intermediate products.
Influence of various environmental conditions on PFOS removal and defluorination in Chi-BC/UV-ARP system
Fig. 2 illustrates the adsorption, degradation, and defluorination efficiencies of PFOS using Chi-BC under various concentrations of NaCl, NaNO3, and HA. At concentrations of 1, 5, and 10 mM NaCl, PFOS removal efficiencies remained consistently high. Both the degradation efficiency at 5 mM and 10 mM was around 95.0%, indicating that the presence of chloride ions or the ionic strength did not influence PFAS removal significantly. Our results show 89.9% and 100% recovery for the experiment conducted with 1 mM NaCl and 10 mM NaNO3. Nevertheless, an incomplete mass balance of PFOS is presented for the 5 and 10 mM NaCl and for all NOM treatments. This discrepancy may result from the formation of PFOS-DOM complexes or other PFOS transformation products that were not measured in our analysis.9–11 The residual adsorbed PFOS on Chi-BC showed minimal variation with increasing NaCl concentration, with 0.5% residual adsorption at 1 mM NaCl and 0.9% at 10 mM NaCl. However, the defluorination efficiency decreased as NaCl concentration increased. At 1 mM NaCl, the defluorination efficiency was approximately 87.3%, which declined to 63.2% at 5 mM and further to 53.1% at 10 mM. This trend suggests that although ionic strength does not affect overall degradation efficiency, it inhibits defluorination. This consistency implies that chloride ions significantly impact the generation or maintenance of reactive species (e.g., hydrated electrons) necessary for defluorination. In contrast, the introduction of NaNO3 had different effects on PFOS removal. Compared with 10 mM NaCl, the defluorination efficiency at 10 mM NaNO3 increased to 82.7%. The total removal efficiency was 86.3%, with degradation at 82.8% and adsorption at 3.5%.The influence of HA on PFOS removal efficiency was similar to NaNO3. At the lowest HA concentration (0.1 mg L−1), the removal efficiency showed no significant difference compared to the treatment without HA, achieving 96%, with adsorption and degradation accounting for 0.5% and 95.0%, respectively. However, as HA concentration increased to 1 mg L−1, the total removal efficiency decreased to 93.1%, with adsorption efficiency rising to 0.96% and degradation efficiency declining to approximately 92.2%. At 5 mg L−1, the total removal efficiency remained comparable to that at 1 mg L−1, at 92.0%, with a slight increase in adsorption efficiency to 1.0% and degradation efficiency to 91.0%. The presence of HA also significantly affected defluorination efficiency. At 0.1 mg L−1, defluorination efficiency was approximately 45.0%, the lowest among all experimental conditions. However, as HA concentration increased to 1 and 5 mg L−1, the defluorination efficiency significantly improved to 60.9% and 62.0%, respectively. However, the defluorination efficiency across all HA treatments remained lower than that of the no-HA condition (63.2%).
Degradation of PFAS in artificial groundwater
As illustrated in Fig. 3, under simple background conditions, the removal efficiency of all three PFAS compounds is relatively high, particularly for PFOS, which reaches approximately 81.0%, with the majority (over 80.1%) attributed to degradation. The removal efficiency of PFHxS is slightly lower than that of PFOS, with an overall rate of approximately 62.3%, and degradation remains the primary removal mechanism. PFBS shows the lowest removal efficiency at around 46.1%, which is entirely dependent on degradation, with no detectable adsorption of PFBS on Chi-BC.In contrast, the removal efficiencies for all three PFAS compounds decrease under artificial groundwater conditions. The total removal efficiency of PFOS declines to approximately 70.9%, with adsorption increasing to 5.3% and degradation efficiency significantly dropping to 66.2%. This trend is less pronounced for PFHxS, with its total removal efficiency showing a slight decrease to 62.0%, which is not statistically significant. Both adsorption and degradation proportions remain stable for PFHxS. For PFBS, the total removal rate drops to 42.3%, with degradation remaining the sole mechanism, as no adsorbed PFBS was detected on Chi-BC. These findings suggest that the ions or chemical components present in artificial groundwater may inhibit the degradation process while enhancing the interaction between PFAS and the Chi-BC surface. This pattern suggests that in more complex water chemistries, the removal mechanism for PFAS may shift from degradation to adsorption.
Finally, the removal efficiencies of the three PFAS compounds show significant variation under both conditions. PFOS consistently exhibits the highest removal efficiency, demonstrating strong degradability and reactivity in both environments. PFHxS did not show a significant decrease in removal efficiency under artificial groundwater conditions, indicating lower sensitivity to changes in water chemistry. PFBS has the lowest removal efficiency, and its performance varies significantly between conditions, suggesting it is more susceptible to environmental influences. Additionally, PFBS is the least likely to be adsorbed by Chi-BC among the three PFAS. Overall, the removal efficiency of PFAS is significantly influenced by its structure, chain length, and reactivity. Water chemistry further amplifies or inhibits these differences, especially under more complex conditions.
Discussion
Chi-BC mediated PFAS degradation efficiency in UV-ARP system: chain length effect
The reductive degradation of PFAS is significantly influenced by chain length and functional groups, which collectively determine the bond dissociation energy (BDE), electron affinity, and reactivity towards hydrated electrons (eaq−).4,21,27 This is primarily due to the abundance of –CF2– units along the fluorinated backbone, which possess relatively low BDE of C–F bonds (∼106–107 kcal mol−1).4 Hydrated electrons show limited reactivity toward the α-position of perfluoroalkyl sulfonates (PFSA) due to the strong electron-withdrawing sulfonate group and the high BDE of adjacent C–F bonds. As a result, PFSA degradation typically proceeds via alternative pathways such as stepwise H/F exchange and, in some cases, spontaneous C–S bond cleavage, rather than chain-shortening initiated at the α-position.38–40 Minor C–C scission is consistent with trace short-chain sulfonates (e.g., PFHxS) observed among PFOS degradation products, suggesting that minor C–C bond cleavage may occur under reductive conditions.39,40In contrast, short-chain PFAS (e.g., PFBS) present a considerable challenge due to their limited number of reactive –CF2– units and high BDE associated with terminal –CF3 groups (up to 490 kJ mol−1), rendering direct cleavage energetically unfavorable.4 Additionally, their high solubility and mobility limit their adsorption on the Chi-BC surfaces. Advanced strategies such as electron beam irradiation combined with sulfite activation have been employed to enhance their degradation.21,27,38 Transient radical control techniques (e.g., pulse radiolysis) further assist in capturing short-lived intermediates, which contribute to C–F bond cleavage in otherwise resistant compounds.41
Additionally, the hydrophobicity and extended chain structure of long-chain PFAS promote stronger binding to Chi-BC, increasing interfacial residence and contact with reactive radicals. However, the use of sole BC diminished PFAS removal efficiency, as suspended carbon particles can suppress reaction rates in solution by inducing light attenuation, scavenging eaq−, and consuming sulfite.42 The magnitude of these effects highly depends on the adsorbent's properties and surface chemistry. Notably, chitosan functionalization introduces amine groups that modify the surface chemistry and enhance the potential for hydrogen bonding with polar functional groups, thereby altering the reaction pathways and outcomes. Consequently, in UV-ARP systems enhanced with Chi-BC, long-chain PFAS generally demonstrate higher degradation efficiencies than short-chain PFAS. Notably, under conditions with BC or Chi-BC, the concentration of PFOS in the reaction solution following PFOSA degradation was significantly lower compared to treatments without adsorbents. This suggests that the adsorption of PFAS by the adsorbent and their degradation by free radicals occur simultaneously and are in a dynamic equilibrium. Future research could focus on leveraging specific adsorbents to enhance the adaptability and efficiency of ARP processes, providing new strategies for removing other water contaminants.
Chi-BC mediated PFAS degradation in UV-ARP system: functional group effect
The type of functional group in PFAS directly influences molecular reactivity and degradation efficiency. Different functional groups' electronic properties and polarity lead to distinct reactivity patterns with hydrated electrons.43 This study includes three sulfonates (PFBS, PFHxS, and PFOS) and a sulfonamide (PFOSA). Due to the presence of a nitrogen atom, the sulfonamide group confers distinct chemical properties compared to sulfonate groups. Although PFOSA is also a highly stable compound, its sulfonamide group can undergo hydrolysis under certain conditions, converting to PFOS. Thus, PFOSA is commonly regarded as a precursor to PFOS.44 In our system, PFOS was consistently detected as a transformation product of PFOSA in both BC and Chi-BC treatments, indicating that S–N bond cleavage is one degradation pathway. While PFHxS was also observed as a product of PFOSA degradation, its concentration was approximately 100 times lower than in the PFOS degradation process, suggesting that C–S bond cleavage and subsequent chain shortening occurred only to a limited extent.This disparity implies that the sulfonamide group in PFOSA may interact differently with the biochar surface compared to the sulfonate group in PFOS, possibly hindering effective C–S cleavage. The sulfonamide functional group has a larger molecular size and polarity compared with sulfonate, hence lowering the accessibility of the perfluoroalkyl chain to reactive sites.45 This structural characteristic may introduce steric hindrance during interaction with the surface functional groups of Chi-BC and, consequently, limit electron transfer in reductive processes.45,46 Moreover, the amide group may weaken electrostatic and hydrogen-bonding interactions with Chi-BC, thereby reducing adsorption affinity and diminishing the pre-concentration effect that facilitates degradation of other PFAS. In systems without biochar, the defluorination efficiency reached 56% at 180 minutes; however, adding BC and Chi-BC reduced the defluorination efficiency to approximately 43%. This indicates that while partial degradation of PFOSA occurs, the cleavage of C–F bonds is not significantly enhanced. These findings suggest that the specific functional groups and properties of perfluorooctane sulfonate may limit its interactions with biochar and reactive species in these systems.
As shown in Fig. 1, adding Chi-BC improved the degradation efficiencies of the three traditional PFAS. The chitosan coating on BC inhibits the negative surface potential and raises the point of zero charge (pHpzc = 7.95) compared with BC (pHpzc = 1.05),27 making the adsorption of PFBS, PFHxS, and PFOS anions more favorable. The introduced amino groups can also facilitate hydrogen bond formation with the oxygen in sulfonate groups. However, PFOSA displayed an opposite trend, with its degradation efficiency decreasing upon Chi-BC addition. Hydrophobic adsorbents generally capture PFAS through hydrophobic interactions with the PFAS hydrophobic chain (–CF3(CF2)6) and anion–π interactions.47 The synergistic adsorption mode anchors PFAS to the Chi-BC surface through head-group interactions, allowing PFAS molecules to bind closely to the surface.27 Li et al. investigated the electrostatic potential distribution of PFAS molecules and found that the head group region exhibited the highest electrostatic potential, which gradually decreased toward the tail.48–51 This close adsorption enhances direct electron transfer between PFAS and photo-generated hydrated electrons, significantly promoting PFAS decomposition and mineralization.27,51 Since the primary reactive species in photodegradation systems are water molecules and electrons are electrophilic, regions with higher negative electrostatic potential are more readily targeted for attack.52,53
Notably, while the –NH2 groups in Chi-BC may not directly initiate PFAS degradation, they play an important role in the stepwise defluorination process following the electron-mediated activation of PFAS. These amines can engage in hydrogen bonding with polar functional groups such as sulfonates and carboxylates, thereby enhancing PFAS adsorption at the biochar interface, by stabilizing PFAS at the solid–liquid boundary and increasing their residence time at the biochar surface.54,55 These effects lower the effective barrier for C–F bond cleavage once electron uptake has occurred. The activation energy of C–F bonds decreases significantly following electron transfer, a critical prerequisite for defluorination.48 It is hypothesized that the defluorination reaction follows a two-step process. First, the amino groups on the Chi-BC surface form hydrogen bonds or electrostatic interactions with polar functional groups, such as the sulfonate group in PFAS. These interactions may enhance PFAS adsorption onto Chi-BC, increasing the likelihood of electron-mediated activation and subsequent defluorination.56,57 When water and electrons interact with PFAS molecules, the C–F and C–S bonds are cleaved, releasing fluoride and sulfate ions.
Influence of various environmental conditions on PFOS removal and defluorination in chi-BC/UV-ARP system
Various factors influence the degradation and adsorption of PFAS in water. At higher ionic strengths (5 and 10 mM NaCl), the defluorination efficiency of PFOS slightly decreases, consistent with previous findings.37,58 Elevated ionic strength may alter solution conductivity and charge interactions, affecting the hydrated electrons' reaction rate.59 Increasing ionic strength generally reduces the availability of water molecules and electrons for PFAS degradation, as ionic interactions stabilize certain reaction intermediates or byproducts, decreasing the concentration of free water molecules and electrons. The ion competition and charge shielding effects of high ionic strength also inhibit PFAS adsorption.60 This reduced the enhancement effect of micro localization on degradation and defluorination.Under the same ionic strength conditions, the defluorination rate of PFOS in a 10 mM NaNO3 solution was significantly higher, comparable to that in NaCl. Previous studies have suggested that NO3− acts as a scavenger of reactive species in reductive processes, thereby inhibiting PFAS degradation by hydrated electrons.20,35 Conversely, other research has demonstrated that under 254 nm UV irradiation, nitrate (NO3−) undergoes photolysis, generating highly reactive oxygen and nitrogen species, including hydroxyl radicals (˙OH) and nitrogen dioxide radicals (˙NO2).61,62 A recent study aligns with this study, showing that in the presence of 100 μM NO3−, 50% defluorination was achieved with only 3 mM sulfite, compared to ≥10 mM required without NO3−. This indicates that NO3− can reduce sulfite demand by ∼70%, offering a strategy to minimize secondary pollution while meeting discharge standards.23
Hydroxyl radicals, as potent oxidants, can attack C–F and C–C bonds.63 Thus, in nitrate-containing water systems, UV irradiation can induce an advanced oxidation process, leveraging multiple reactive species to enhance PFAS degradation and mineralization efficiency. However, under pH 10 and anaerobic conditions, SO32− is the dominant reactant, shifting the radical system towards a reductive environment, where the ARP remains the prevailing pathway.59,64,65 Furthermore, studies have shown that nitrate can enhance the adsorption capacity of adsorbents for PFAS.35,37 Kim et al. (2024) revealed that in the presence of 10 mM NaNO3, hydrotalcite exhibited a greater adsorption capacity for PFOS compared to no-salt conditions. Consequently, at an optimal concentration, NO3− can enhance the adsorption of PFAS onto Chi-BC, indirectly facilitating the gradual defluorination of adsorbed PFAS within the microenvironment. Collectively, these findings suggest that the enhancement observed in this study may arise from a combination of radical-mediated oxidation and improved adsorption, although quantitative validation (e.g., radical probe experiments) is warranted to confirm the relative contributions.
HA is a significant scavenger of eaq−, which reduces the degradation rate of target contaminants in ARP.65 HA enhances the scavenging capacity of water and electrons and reduces their production rate by shielding UV irradiation of sulfite.59,64 In this experiment, a comparison of light absorption data (Fig. S3) confirmed that ionic strength, NO3−, and HA all affect light utilization. The results for HA showed an inhibitory effect on overall degradation efficiency but a slight enhancement in defluorination. Additionally, there was a slight increase in PFAS remaining on biochar. Studies have shown that HA often competes with PFAS for adsorption sites on biochar, thereby reducing PFAS adsorption efficiency.66 However, in some instances, HA may enhance hydrophobic interactions by increasing the hydrophobicity of the adsorbent surface, thereby improving PFAS adsorption under specific conditions.35,67 Under these conditions, Chi-BC creates a high-concentration microenvironment by adsorbing PFAS, thereby enhancing the attack efficiency of water and electrons.
Additionally, PFAS adsorbed onto Chi-BC may experience changes in intramolecular electron distribution due to electrostatic adsorption, altering C–F bond lengths and angles, which makes these bonds more susceptible to cleavage. Furthermore, chitosan functional groups may activate alternative, unidentified reaction pathways involving other reactive species.68 Thus, Chi-BC control offers potential energy savings. The adsorption process pre-concentrates PFAS from a large volume of water onto a smaller amount of Chi-BC, enabling subsequent ARP treatment in a compact photoreactor. Compared to the direct treatment of large volumes of raw water, this approach significantly reduces energy consumption.
Conclusion
Chitosan-modified biochar (Chi-BC) synergizes with UV/sulfite-ARP to accelerate PFAS degradation and defluorination. It does so by coupling electrostatic pre-concentration of anionic PFAS at positively charged surfaces with near-surface electron delivery, which lowers the barrier for C–F bond cleavage. Across the solution chemistry conditions tested, this interfacial mechanism was chain-length dependent for the perfluoroalkyl sulfonates (PFOS > PFHxS > PFBS), and negatively impacts the destruction of PFOSA, underscoring the joint control of site-specific bond energetics and adsorption affinity. Humic substances and elevated ionic strength (NaCl) dampen defluorination by scavenging/reactive-species attenuation and charge-screening, whereas nitrate produces modest enhancement under our conditions. Artificial groundwater tests further reveal decreased removals relative to simple backgrounds, highlighting the need to tune operating windows to realistic matrices. Practically, Chi-BC enables a capture-then-destroy strategy in compact photoreactors, pre-concentrating PFAS to reduce the treatment volume and potentially the energy footprint. While direct evidence for the electron transfer mechanism is yet to be elucidated, this study emphasizes the importance of surface adsorption coupled with ARP systems for PFAS remediation. Future work should focus on new adsorbents to accommodate a larger range of PFAS.Author contributions
Conceptualization: Z. S. and J. H.; methodology: Z. S., J. H. and S. M.; validation: Z. S., J. H. and Y. O.; formal analysis: Z. S., J. H. and Y. O.; investigation: Z. S., J. H. and Y. O.; resources: Z. S., J. H., Y. O. and T. K.; data curation: Z. S., J. H., Y. O. and T. K.; writing – original draft: Z. S.; writing – review and editing: Z. S., J. H., Y. O. and T. K.; visualization: Z. S., J. H., Y. O. and T. K.; supervision: Y. O.; funding acquisition: Y. O. and T. K. All authors read and approved the final manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The datasets generated during and/or analyzed during the current study are available in the AU Scholarly Repository, https://aurora.auburn.edu. Supplementary information (SI): provides additional methodological and analytical details supporting the main text. It includes: a description of PFAS analytical procedures (Text S1); a summary of acronyms, CAS numbers, and chemical formulas of the PFAS compounds investigated (Table S1); physicochemical properties of the biochars employed in this study (Table S2); instrumental parameters for HPLC-MS/MS analysis (Tables S3 and S4); and supplementary figures illustrating the experimental setup (Fig. S1), transformation product formation under optimized conditions (Fig. S2), and UV absorbance spectra under varying background chemistries (Fig. S3). See DOI: https://doi.org/10.1039/d5ew00661a.Acknowledgements
This research was supported by the National Institute of Food and Agriculture grant number 2022-09250 and the Hatch project ALA0 12-1-19155. We thank Melissa Boersma in the Mass Spectrometry Center at Auburn Univeristy for her assistance in analyzing PFAS.References
- Z. Wang, J. C. DeWitt, C. P. Higgins and I. T. Cousins, A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)?, Environ. Sci. Technol., 2017, 51, 2508–2518 CrossRef CAS.
- S. Kurwadkar, J. Dane, S. R. Kanel, M. N. Nadagouda, R. W. Cawdrey, B. Ambade, G. C. Struckhoff and R. Wilkin, Per- and polyfluoroalkyl substances in water and wastewater: A critical review of their global occurrence and distribution, Sci. Total Environ., 2022, 809, 151003 CrossRef CAS PubMed.
- P. Ramos, M. P. Schmidt, R. Xuan and D. J. Ashworth, Biochar selection for removal of perfluoroalkyl substances from reclaimed water for agricultural irrigation, Biochar, 2025, 7, 56 CrossRef CAS.
- M. J. Bentel, Y. Yu, L. Xu, Z. Li, B. M. Wong, Y. Men and J. Liu, Defluorination of Per- and Polyfluoroalkyl Substances (PFASs) with Hydrated Electrons: Structural Dependence and Implications to PFAS Remediation and Management, Environ. Sci. Technol., 2019, 53, 3718–3728 CrossRef CAS.
- M. G. Alalm and D. C. Boffito, Mechanisms and pathways of PFAS degradation by advanced oxidation and reduction processes: A critical review, Chem. Eng. J., 2022, 450, 138352 CrossRef.
- Z. Abbasian Chaleshtari and R. Foudazi, A review on per-and polyfluoroalkyl substances (PFAS) remediation: separation mechanisms and molecular interactions, ACS ES&T Water, 2022, 2, 2258–2272 Search PubMed.
- Y. Bao, S. Deng, X. Jiang, Y. Qu, Y. He, L. Liu, Q. Chai, M. Mumtaz, J. Huang, G. Cagnetta and G. Yu, Degradation of PFOA Substitute: GenX (HFPO-DA Ammonium Salt): Oxidation with UV/Persulfate or Reduction with UV/Sulfite?, Environ. Sci. Technol., 2018, 52, 11728–11734 CrossRef CAS PubMed.
- T. Zhou, X. Li, H. Liu, S. Dong, Z. Zhang, Z. Wang, J. Li, L. D. Nghiem, S. J. Khan and Q. Wang, Occurrence, fate, and remediation for per-and polyfluoroalkyl substances (PFAS) in sewage sludge: A comprehensive review, J. Hazard. Mater., 2024, 466, 133637 CrossRef CAS PubMed.
- J. W. Washington, K. Rankin, E. L. Libelo, D. G. Lynch and M. Cyterski, Determining global background soil PFAS loads and the fluorotelomer-based polymer degradation rates that can account for these loads, Sci. Total Environ., 2019, 651, 2444–2449 CrossRef CAS.
- B. J. Ruyle, C. P. Thackray, C. M. Butt, D. R. LeBlanc, A. K. Tokranov, C. D. Vecitis and E. M. Sunderland, Centurial Persistence of Forever Chemicals at Military Fire Training Sites, Environ. Sci. Technol., 2023, 57, 8096–8106 CrossRef CAS.
- K. Y. Kwok, E. Yamazaki, N. Yamashita, S. Taniyasu, M. B. Murphy, Y. Horii, G. Petrick, R. Kallerborn, K. Kannan, K. Murano and P. K. Lam, Transport of perfluoroalkyl substances (PFAS) from an arctic glacier to downstream locations: implications for sources, Sci. Total Environ., 2013, 447, 46–55 CrossRef CAS PubMed.
- L. Ahrens, J. Rakovic, S. Ekdahl and R. Kallenborn, Environmental distribution of per- and polyfluoroalkyl substances (PFAS) on Svalbard: Local sources and long-range transport to the Arctic, Chemosphere, 2023, 345, 140463 CrossRef CAS PubMed.
- X. Lyu, F. Xiao, C. Shen, J. Chen, C. M. Park, Y. Sun, M. Flury and D. Wang, Per- and Polyfluoroalkyl Substances (PFAS) in Subsurface Environments: Occurrence, Fate, Transport, and Research Prospect, Rev. Geophys., 2022, 60(3), e2021RG000765 CrossRef.
- V. Franke, M. Ullberg, P. McCleaf, M. Wålinder, S. J. Köhler and L. Ahrens, The Price of Really Clean Water: Combining Nanofiltration with Granular Activated Carbon and Anion Exchange Resins for the Removal of Per- And Polyfluoralkyl Substances (PFASs) in Drinking Water Production, ACS ES&T Water, 2021, 1, 782–795 Search PubMed.
- M. F. Rahman, S. Peldszus and W. B. Anderson, Behaviour and fate of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in drinking water treatment: a review, Water Res., 2014, 50, 318–340 CrossRef CAS PubMed.
- T. D. Appleman, C. P. Higgins, O. Quinones, B. J. Vanderford, C. Kolstad, J. C. Zeigler-Holady and E. R. Dickenson, Treatment of poly- and perfluoroalkyl substances in U.S. full-scale water treatment systems, Water Res., 2014, 51, 246–255 CrossRef CAS.
- G. Liu, C. Feng and P. Shao, Degradation of Perfluorooctanoic Acid with Hydrated Electron by a Heterogeneous Catalytic System, Environ. Sci. Technol., 2022, 56, 6223–6231 CrossRef CAS.
- M. Hušek, J. Semerád, S. Skoblia, J. Moško, J. Kukla, Z. Beňo, M. Jeremiáš, T. Cajthaml, M. Komárek and M. Pohořelý, Removal of per- and polyfluoroalkyl substances and organic fluorine from sewage sludge and sea sand by pyrolysis, Biochar, 2024, 6(1), 31 CrossRef.
- E. Houtz, D. Kempisty and Y. Lester, Poly- and perfluoroalkyl substances destruction via advanced reduction processes: assessing scientific and commercial progress and prospects, Curr. Opin. Chem. Eng., 2024, 44, 101022 CrossRef.
- J. Cui, P. Gao and Y. Deng, Destruction of Per- and Polyfluoroalkyl Substances (PFAS) with Advanced Reduction Processes (ARPs): A Critical Review, Environ. Sci. Technol., 2020, 54, 3752–3766 CrossRef CAS.
- J. He, M. Boersma, Z. Song, S. Krebsbach, D. Fan, E. C. Duin and D. Wang, Biochar and surfactant synergistically enhanced PFAS destruction in UV/sulfite system at neutral pH, Chemosphere, 2024, 141562, DOI:10.1016/j.chemosphere.2024.141562.
- B. P. Vellanki, B. Batchelor and A. Abdel-Wahab, Advanced Reduction Processes: A New Class of Treatment Processes, Environ. Eng. Sci., 2013, 30, 264–271 CrossRef CAS PubMed.
- Z. Feng, Y. Fu, J. Li, X. Lu, S. Wang, Y. Chen, W. Wang, Z. Sun and J. Ma, Deep Insight of the Mechanism for Nitrate-Promoted PFASs Defluorination in UV/Sulfite ARP: Activation of the Decarboxylation-Hydroxylation-Elimination-Hydrolysis Degradation Pathway, Environ. Sci. Technol., 2025, 59, 10087–10097 CrossRef CAS.
- M. He, P. Zhao, R. Duan, S. Xu, G. Cheng, M. Li and S. Ma, Insights on the electron transfer pathway of phenolic pollutant degradation by endogenous N-doped carbonaceous materials and peroxymonosulfate system, J. Hazard. Mater., 2022, 424, 127568 CrossRef CAS PubMed.
- A. Cerlanek, Y. Liu, N. Robey, A. S. Timshina, J. A. Bowden and T. G. Townsend, Assessing construction and demolition wood-derived biochar for in-situ per- and polyfluoroalkyl substance (PFAS) removal from landfill leachate, Waste Manage., 2024, 174, 382–389 CrossRef CAS.
- J. Deng, J. Han, C. Hou, Y. Zhang, Y. Fang, W. Du, M. Li, Y. Yuan, C. Tang and X. Hu, Efficient removal of per- and polyfluoroalkyl substances from biochar composites: Cyclic adsorption and spent regenerant degradation, Chemosphere, 2023, 341, 140051 CrossRef CAS PubMed.
- Z. Song, J. He, S. M. T. Kouzehkanan, T. S. Oh, Y. Olshansky, E. C. Duin, K. C. Carroll and D. Wang, Enhanced sorption and destruction of PFAS by biochar-enabled advanced reduction process, Chemosphere, 2024, 363, 142760 CrossRef CAS PubMed.
- Y. Lu, Y. Cai, S. Zhang, L. Zhuang, B. Hu, S. Wang, J. Chen and X. Wang, Application of biochar-based photocatalysts for adsorption-(photo)degradation/reduction of environmental contaminants: mechanism, challenges and perspective, Biochar, 2022, 4(1), 45 CrossRef CAS.
- X. Zhang, S. Wang, X. Zhu, D. Zhu, W. Wang, B. Wang, S. Deng and G. Yu, Efficient removal of per/polyfluoroalkyl substances from water using recyclable chitosan-coated covalent organic frameworks: Experimental and theoretical methods, Chemosphere, 2024, 356, 141942 CrossRef CAS PubMed.
- R. Shahrokhi and J. Park, Enhanced removal of short- and long-chain per- and poly-fluoroalkyl substances from aqueous phase using crushed grafted chitosan beads: Performance and mechanisms, Environ. Pollut., 2024, 340, 122836 CrossRef CAS PubMed.
- C. He, Y. Yang, Y.-J. Hou, T. Luan and J. Deng, Chitosan-coated fluoro-functionalized covalent organic framework as adsorbent for efficient removal of per- and polyfluoroalkyl substances from water, Sep. Purif. Technol., 2022, 294, 121195 CrossRef CAS.
- Y. Zhang, X. Tan, R. Lu, Y. Tang, H. Qie, Z. Huang, J. Zhao, J. Cui, W. Yang and A. Lin, Enhanced Removal of Polyfluoroalkyl Substances by Simple Modified Biochar: Adsorption Performance and Theoretical Calculation, ACS ES&T Water, 2023, 3, 817–826 Search PubMed.
- A. K. Ilango and Y. Liang, Surface modifications of biopolymers for removal of per- and polyfluoroalkyl substances from water: Current research and perspectives, Water Res., 2024, 249, 120927 CrossRef CAS.
- B. Chen, H. Zeng, F. Yang, Y. Yang, Z. Qiao, X. Zhao, L. Wang and F. Wu, Functional biochar as sustainable precursors to boost the anaerobic digestion of waste activated sludge from a circular economy perspective: a review, Biochar, 2024, 6(1), 60 CrossRef CAS.
- S. Krebsbach, J. He, T.-S. Oh and D. Wang, Effects of Environmental Factors on the Sorption of Per- and Polyfluoroalkyl Substances by Biochars, ACS ES&T Water, 2023, 3, 3437–3446 Search PubMed.
- W. Zhang and Y. Liang, Interactions between Lemna minor (common duckweed) and PFAS intermediates: Perfluorooctanesulfonamide (PFOSA) and 6:2 fluorotelomer sulfonate (6:2 FTSA), Chemosphere, 2021, 276, 130165 CrossRef CAS.
- S. Krebsbach, J. He, S. Adhikari, Y. Olshansky, F. Feyzbar, L. C. Davis, T. S. Oh and D. Wang, Mechanistic understanding of perfluorooctane sulfonate (PFOS) sorption by biochars, Chemosphere, 2023, 330, 138661 CrossRef CAS PubMed.
- H. Hori, E. Hayakawa, H. Einaga, S. Kutsuna, K. Koike, T. Ibusuki, H. Kiatagawa and R. Arakawa, Decomposition of Environmentally Persistent Perfluorooctanoic Acid in Water by Photochemical Approaches, Environ. Sci. Technol., 2014, 38, 9 Search PubMed.
- K. Nohara, M. Toma, S. Kutsuna, K. Takeuchi and T. Ibusuki, Cl atom-initiated oxidation of three homologous methyl perfluoroalkyl ethers, Environ. Sci. Technol., 2001, 35, 114–120 CrossRef CAS.
- Z. Song, H. Tang, N. Wang and L. Zhu, Reductive defluorination of perfluorooctanoic acid by hydrated electrons in a sulfite-mediated UV photochemical system, J. Hazard. Mater., 2013, 262, 332–338 CrossRef CAS PubMed.
- Z. Jiang, D. Adjei, S. A. Denisov, M. Mostafavi and J. Ma, Transient kinetics of short-chain perfluoroalkyl sulfonate with radiolytic reducing species, Environ. Sci. Technol. Lett., 2022, 10, 59–65 CrossRef.
- H. A. Santiago-Cruz, Z. Lou, J. Xu, R. C. Sullivan, B. B. Bowers, R. A. Mole, W. Zhang, J. Li, J. S. Yuan, S. Y. Dai and G. V. Lowry, Carbon Adsorbent Properties Impact Hydrated Electron Activity and Perfluorocarboxylic Acid (PFCA) Destruction, ACS ES&T Eng., 2024, 4, 2220–2233 Search PubMed.
- A. P. Folkerson, S. R. Schneider, J. P. D. Abbatt and S. A. Mabury, Avoiding Regrettable Replacements: Can the Introduction of Novel Functional Groups Move PFAS from Recalcitrant to Reactive?, Environ. Sci. Technol., 2023, 57, 17032–17041 CrossRef CAS PubMed.
- R. Xuan, X. Qiu, J. Wang, S. Liu, J. T. Magnuson, B. Xu, W. Qiu and C. Zheng, Hepatotoxic response of perfluorooctane sulfonamide (PFOSA) in early life stage zebrafish (Danio rerio) is greater than perfluorooctane sulfonate (PFOS), J. Hazard. Mater., 2024, 461, 132552 CrossRef CAS.
- F. Wang, K. M. Shih and X. Y. Li, The partition behavior of perfluorooctanesulfonate (PFOS) and perfluorooctanesulfonamide (FOSA) on microplastics, Chemosphere, 2015, 119, 841–847 CrossRef CAS PubMed.
- C. Gong, X. Huang, S. Lv, J. Li, J. Tang and F. Huang, Poly(silylene arylacetylene)s containing hexafluoroisopropylidene with attractive mechanical properties and dielectric performance for wave-transparent composites, Mater. Chem. Front., 2023, 7, 5015–5027 RSC.
- T. Xu, H. Ji, Y. Gu, T. Tong, Y. Xia, L. Zhang and D. Zhao, Enhanced adsorption and photocatalytic degradation of perfluorooctanoic acid in water using iron (hydr)oxides/carbon sphere composite, Chem. Eng. J., 2020, 388, 124230 CrossRef CAS.
- Y. Su, U. Rao, C. M. Khor, M. G. Jensen, L. M. Teesch, B. M. Wong, D. M. Cwiertny and D. Jassby, Potential-Driven Electron Transfer Lowers the Dissociation Energy of the C-F Bond and Facilitates Reductive Defluorination of Perfluorooctane Sulfonate (PFOS), ACS Appl. Mater. Interfaces, 2019, 11, 33913–33922 CrossRef CAS.
- Y. Lai, Y. Wang, S. Zhang and A. Duan, Kinetics and mechanism analysis of advanced oxidation degradation of PFOA/PFOS by UV/Fe(3+) and persulfate: A DFT study, Chemosphere, 2024, 357, 141951 CrossRef CAS PubMed.
- S. Yamijala, R. Shinde, K. Hanasaki, Z. A. Ali and B. M. Wong, Photo-induced degradation of PFASs: Excited-state mechanisms from real-time time-dependent density functional theory, J. Hazard. Mater., 2022, 423, 127026 CrossRef CAS PubMed.
- F. Li, Z. Wei, K. He, L. Blaney, X. Cheng, T. Xu, W. Liu and D. Zhao, A concentrate-and-destroy technique for degradation of perfluorooctanoic acid in water using a new adsorptive photocatalyst, Water Res., 2020, 185, 116219 CrossRef CAS.
- S. P. Mezyk, T. J. Neubauer, W. J. Cooper and J. R. Peller, Free-Radical-Induced Oxidative and Reductive Degradation of Sulfa Drugs in Water: Absolute Kinetics and Efficiencies of Hydroxyl Radical and Hydrated Electron Reactions, J. Phys. Chem. A, 2007, 111, 6 CrossRef PubMed.
- B. Galabov, D. Nalbantova, P. Schleyer and H. F. Schaefer, 3rd, Electrophilic Aromatic Substitution: New Insights into an Old Class of Reactions, Acc. Chem. Res., 2016, 49, 1191–1199 CrossRef CAS PubMed.
- S. T. McBeath, A. Serrano Mora, F. Asadi Zeidabadi, B. K. Mayer, P. McNamara, M. Mohseni, M. R. Hoffmann and N. J. D. Graham, Progress and prospect of anodic oxidation for the remediation of perfluoroalkyl and polyfluoroalkyl substances in water and wastewater using diamond electrodes, Curr. Opin. Electrochem., 2021, 30, 100865 CrossRef CAS.
- X. Liang, J. Cheng, C. Yang and S. Yang, Factors influencing aqueous perfluorooctanoic acid (PFOA) photodecomposition by VUV irradiation in the presence of ferric ions, Chem. Eng. J., 2016, 298, 291–299 CrossRef CAS.
- Y. Chen, H. Ma, J. Zhu, Y. Gu and T. Liu, New insights into ferric iron-facilitated UV(254) photolytic defluorination of perfluorooctanoic acid (PFOA): Combined experimental and theoretical study, J. Hazard. Mater., 2022, 434, 128865 CrossRef CAS PubMed.
- C. Yan, Y. Yang, J. Wei, J. Hou and Z. Shao, N self-doped multifunctional chitosan biochar-based microsphere with heterogeneous interfaces for self-powered supercapacitors to drive overall water splitting, Biochar, 2023, 5(1), 90 CrossRef CAS.
- J. Fabregat-Palau, M. Vidal and A. Rigol, Examining sorption of perfluoroalkyl substances (PFAS) in biochars and other carbon-rich materials, Chemosphere, 2022, 302, 134733 CrossRef CAS.
- B. D. Fennell, A. Odorisio and G. McKay, Quantifying Hydrated Electron Transformation Kinetics in UV-Advanced Reduction Processes Using the R(e-,UV) Method, Environ. Sci. Technol., 2022, 56, 10329–10338 CrossRef CAS PubMed.
- M. L. Brusseau and S. Van Glubt, The influence of molecular structure on PFAS adsorption at air-water interfaces in electrolyte solutions, Chemosphere, 2021, 281, 130829 CrossRef CAS PubMed.
- G. Sara and R. Joseph, Mechanism of Nitrite Formation by Nitrate Photolysis in Aqueous Solutions: The Role of Peroxynitrite, Nitrogen Dioxide, and Hydroxyl Radical, J. Am. Chem. Soc., 2007, 129, 10597–10601 CrossRef.
- Y. Huang, M. Kong, D. Westerman, E. G. Xu, S. Coffin, K. H. Cochran, Y. Liu, S. D. Richardson, D. Schlenk and D. D. Dionysiou, Effects of HCO(3)(−) on Degradation of Toxic Contaminants of Emerging Concern by UV/NO(3)(), Environ. Sci. Technol., 2018, 52, 12697–12707 CrossRef CAS PubMed.
- E. Banayan Esfahani, F. Asadi Zeidabadi and M. Mohseni, Vacuum-UV Radiation Capable of Catalyst-Free Decomposition of 6:2 FTSA: The Transformation Mechanism and Impacts of the Water Matrix, ACS ES&T Water, 2023, 3, 3614–3625 Search PubMed.
- B. D. Fennell, S. P. Mezyk and G. McKay, Critical Review of UV-Advanced Reduction Processes for the Treatment of Chemical Contaminants in Water, ACS Environ. Au, 2022, 2, 178–205 CrossRef CAS PubMed.
- B. D. Fennell, D. Fowler, S. P. Mezyk and G. McKay, Reactivity of Dissolved Organic Matter with the Hydrated Electron: Implications for Treatment of Chemical Contaminants in Water with Advanced Reduction Processes, Environ. Sci. Technol., 2023, 57, 7634–7643 CrossRef CAS.
- X. Lei, Q. Lian, X. Zhang, T. K. Karsili, W. Holmes, Y. Chen, M. E. Zappi and D. D. Gang, A review of PFAS adsorption from aqueous solutions: Current approaches, engineering applications, challenges, and opportunities, Environ. Pollut., 2023, 321, 121138 CrossRef CAS.
- Y. Li, Y. Zhu, J. Liu, W. Fan, Y. Cao, Y. Huo and J. Wei, Review of modified biochar for removing humic acid from water: analysis of structure-activity relationship, Biochar, 2025, 7(1), 1 CrossRef.
- C. K. Amador, H. Cavalli, R. Tenorio, H. Tetu, C. P. Higgins, S. Vyas and T. J. Strathmann, Influence of Carbonate Speciation on Hydrated Electron Treatment Processes, Environ. Sci. Technol., 2023, 57, 7849–7857 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |



