Transformation and immobilization of sedimental galena (PbS) by phosphate from surface runoff in simulated storm suspensions
Yi-Pin
Lin
 *ab and
Ze-Xuan
Tan
a
*ab and
Ze-Xuan
Tan
a
aGraduate Institute of Environmental Engineering, National Taiwan University, No. 1, Sec. 4, Roosevelt Road, Taipei 10617, Taiwan. E-mail: yipinlin@ntu.edu.tw
bNTU Research Center for Future Earth, National Taiwan University, Taipei, Taiwan
First published on 10th November 2025
Abstract
Sedimental galena (PbS) is an important sink for lead in natural waters. Resuspension of PbS caused by storm disturbance can lead to its oxidative dissolution, resulting in higher ecological risks. Surface runoff resulting from intensive storms can also carry phosphate into receiving water bodies. It is hypothesized that phosphate from surface runoff can regulate Pb concentration and speciation during storm events. To test the hypothesis, the dissolution and transformation of PbS were investigated in the absence and presence of orthophosphate under different pH (5–8) and dissolved oxygen (0–8.4 mg L−1) conditions to simulate the behaviors of suspended PbS. The results indicated that the kinetics of PbS dissolution was mainly controlled by pH while dissolved oxygen played a minor role when orthophosphate was absent. In the presence of orthophosphate (0.5 mg-P L−1), the soluble lead concentration as high as 990 ppb resulting from PbS dissolution decreased immediately to ND (<5.1 μg L−1), except at pH 5, due to the formation of pyromorphite. A saturation index (SI) greater than 16.16 was required to initiate pyromorphite precipitation. The results suggested that phosphate, which is often associated with eutrophication, could sequester soluble lead and reduce associated ecological risks resulting from sedimental PbS dissolution in storm events.
Water impactSedimental galena (PbS) is an important sink for Pb in natural waters but its oxidative dissolution during storm events can release Pb. This study investigates the kinetics of PbS dissolution in simulated storm suspensions and explores whether phosphate in surface runoff can immobilize released Pb. It was found that the kinetics of PbS oxidative dissolution was mainly regulated by pH and soluble Pb decreased immediately after phosphate addition due to pyromorphite precipitation. Although phosphate is often associated with eutrophication, it could sequester soluble Pb and reduce ecological risks resulting from sedimental PbS dissolution in storm events. |
1. Introduction
Sediments resulting from weathering and erosion are the habitats of many aquatic organisms.1,2 However, heavy metal pollution caused by anthropogenic activities has negative impacts on the quality of sediments and has become a threat to ecosystems and human health.3–6Sediments are typically anoxic and reduced species such as sulfide are commonly present. The concentrations of heavy metals in the sediments could surpass those in the overlying water by several orders of magnitude because metal ions can quickly precipitate with sulfide to form low solubility metal sulfides, which are stable and considered as the major sink for heavy metals in anoxic sediments.7–9 It has been suggested that the bioavailability and toxicity of heavy metals in sediments can be predicted using the molar ratio of simultaneous extraction metals (SEM) to acid volatile sulfides (AVS).3,7,10 If [SEM]/[AVS] is less than 1, stable metal sulfides can form and reduce the acute toxicity for benthic organisms. On the other hand, if [SEM]/[AVS] is greater than 1, no sufficient sulfide is available and metal ions can become bioavailable, thereby increasing the toxicity to sensitive benthic species.4,11 It should be noted that the dissolution of metal sulfides is also affected by water chemistry such as pH, dissolved oxygen (DO) and other complexing agents.12–15 Therefore, the influences of water chemistry on this process should be considered in addition to the [SEM]/[AVS] ratio.
The dissolution of metal sulfides can occur due to resuspension of sediments resulting from turbulence or bioturbation, leading to the release of toxic metal ions into oxic water columns.14,16,17 For example, lead levels in water and suspended sediment can fluctuate by 10-fold during the dredging of contaminated sediment.17,18 A significant release of lead from contaminated creek and marine sediments due to resuspension has also been reported.19,20 Such events can elevate lead levels in water that cause adverse effects on the metabolism, survival, growth, development and reproduction of fish, wildlife and invertebrates.21
In areas affected by human activities, phosphate can be easily flushed into water bodies by surface runoff during storm events.22–25 The total phosphate level in the first flush runoff can be up to several mg L−1.26–28 In typical surface waters, phosphate is considered as the limiting nutrient regulating algae growth and the main contributor to the eutrophication of water bodies.29–31 The presence of phosphate, however, may lead to the formation of low solubility phosphate minerals that sequester toxic metal ions. For example, addition of phosphate has been considered as an effective remediation technology to reduce the mobility of lead in soils and drinking water by forming pyromorphites including Pb5(PO4)3Cl and Pb5(PO4)3OH according to the following reactions: 5 Pb2+ + 3 PO43− + Cl− → Pb5(PO4)3Cl(s); 5 Pb2+ + 3 PO43− + OH− → Pb5(PO4)3OH(s).32–36
Given the increasing frequency and intensity of storm events driven by climate change, disturbances of anoxic sediments and the flushing of phosphate through surface runoff are expected to occur more often. It is therefore hypothesized that phosphate transported by surface runoff may sequester lead ions released from the oxidative dissolution of sedimental PbS during such storm events. The coupled processes of PbS dissolution and pyromorphite precipitation could ultimately control the concentration of toxic lead ions in the water column. However, these interactions have not yet been thoroughly investigated.
The objectives of this study are to verify the above hypothesis and explore the fate of sedimental PbS during storm events. Specifically, the kinetics of PbS dissolution under different DO and pH conditions is examined. The influences of phosphate on the immobilization of lead and transformation of lead species during PbS dissolution are explored. Overall, the insights into the potential influences of phosphate-containing surface runoff on the lead level and the fate and transformation of sedimental PbS in storm suspensions are provided in this study.
2. Materials and methods
2.1 Chemicals and solution preparation
PbS (99.9%) was purchased from Sigma-Aldrich (USA). KCl (>99.0%), NaHCO3 (>99.7%), CaCl2·2H2O (>99.0%) and MgSO4·7H2O (>98.0%), all purchased from Merck (Germany), were used to prepare experimental solutions. Organic buffers including 2-(N-morpholino)ethanesulfonic acid (MES) (>99.5%, Merck, Germany) and 3-morpholinopropane-1-sulfonic acid (MOPS) (>99.5%, Merck, Germany) were used for pH control as they do not form complexes with Pb ions.37 10 mM MES was used to buffer the pH for experiments at pH 5 and 6. 5 mM MOPS was used for those at pH 7 and 8. 1 M HCl and 1 M NaOH (Honeywell Fluka, USA) were used for pH adjustments. 69% HNO3 (J.T. Baker, USA) was used to acidify samples. Combined reagents composed of ascorbic acid (>99.0%, Merck, Germany), H2SO4 (Honeywell Fluka, USA), K(SbO)C4H4O6·½H2O (99–103%, Acros Organics, USA) and (NH4)6Mo7O24·4H2O (81–83%, J.T. Baker, USA) were used in the ascorbic acid method for phosphate measurement. KH2PO4 (>99.5%, Nacalai Tesque, Japan) was used to prepare the stock orthophosphate solution (100 mg-P L−1). All solutions were prepared using deionized water generated from a PURELAB Classical system (ELGA, UK).2.2 Experimental apparatus and methods
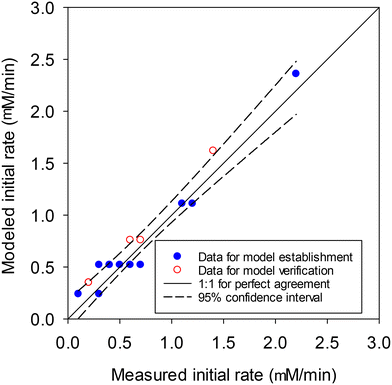
The schematic and photograph of the batch aeration setup used in this study are shown in Fig. S1. 1000 mL amber serum bottles were used as the reactors. Synthetic freshwater (Table S1) was prepared as the experimental solution.38 To simulate different DO concentrations of the water column, different partial pressures of pure N2 and O2 were mixed using a mass flow controller (MFC, Protec Instrument Corp., USA). The air mixture was passed through an O2 sensor (PrimaX® I Gas Transmitter, MSA, The Netherlands) before passing through ultra-pure water to minimize the decrease in the solution volume caused by contacting dry gas. The air mixture was purged into the experimental solution with a flow velocity of 3 L min−1 until the DO concentration reached the target level. The flow velocity was then adjusted to 1 L min−1 to keep the desired partial pressure of O2 in the headspace to retain a steady DO concentration during the experiments.After the addition of 100 mg L−1 of PbS, the solution was stirred with a magnetic bar at 200 rpm to suspend the particles. The experiments were conducted at different pH (5, 6, 7 and 8) and DO concentrations (0, 3, 5 and 8.4 mg L−1) for 72 h. The PbS concentration and experimental period employed were to simulate the sulfide level in the sediments17,18 and the suspension period in storm events.39 Different concentrations of orthophosphate (0.25, 0.50, 1 and 2 mg-P L−1) were added to simulate the surface runoff with different phosphate levels. A 9 mL sample was withdrawn and filtered through a 0.22 μm pore size nylon syringe filter at designated time intervals. 4.5 mL of the filtrate was acidified to a pH < 4 with 69% HNO3 and stored at 4 °C before soluble Pb(II) analysis. The other 4.5 mL filtrate was used for orthophosphate measurement. A polynomial regression was used to fit the results of soluble Pb(II) release versus time. The initial rate of PbS dissolution was determined from the initial slope of the polynomial regression curve at time zero. After the 72 h experiment, the solids in the reactors were collected by filtering the solution through a 0.22 μm pore size nylon membrane and dried at 80 °C in an oven for 24 h. The solids were stored in a dry cabinet prior to scanning electron microscopy (SEM) analysis. All experiments were conducted in duplicate and the error bar represents the range of duplicate data.
2.3 Analytical methods
The surface morphology of the solids was analyzed using a Field-Emission Scanning Electron Microscope (FE-SEM, ZEISS ΣIGMA Essential, Germany). A Brunauer–Emmett–Teller surface area analyzer (Micromeritics ASAP 2420, USA) and Particle Size Analyzer (CILAS 1090L, France) were used to determine the specific surface area and particle size distribution of PbS, respectively. The Pb concentration was measured using an inductively coupled plasma optical emission spectrometer (ICP-OES, Agilent 700 series, USA), with a method detection limit (MDL) of 5.1 μg L−1. The solution pH was measured using a pH meter (SUNTEX sp2100, Taiwan) equipped with an Ag/AgCl electrode (Sensorex SG200C, Taiwan) calibrated using pH 4, 7, and 10 standard buffer solutions. The DO concentration was measured using a DO meter (Thermo Scientific, USA) equipped with a membrane electrode calibrated using air saturation and zero DO solutions. The orthophosphate concentration was measured using the ascorbic acid method following the Standard Method 4500-P E.403. Results and discussion
3.1 Kinetics of PbS dissolution under different DO and pH conditions
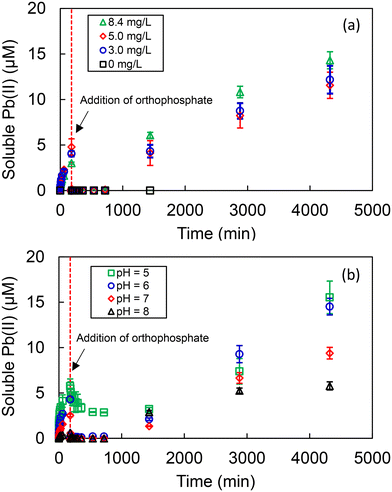
The PbS used in this study possessed a specific surface area of 0.9 m2 g−1 and a mean size of 5.9 μm (Fig. S2). The dissolution of PbS under different DO and pH conditions was first examined to establish the dissolution rate law of PbS and the results are shown Fig. 1. The initial dissolution rates calculated from the initial slopes of the soluble Pb(II) vs. time curves are summarized in Table S2. For the DO levels investigated (DO = 0–8.4 mg L−1), the released soluble Pb(II) levels were similar, particularly in the first 5 min (Fig. 1(a)). This result suggested that the dissolution of PbS was not significantly affected by the DO level, especially at the initial stage. For the pH investigated (pH 5–8), the rate of PbS dissolution increased with decreasing pH, implying that pH was the dominant factor regulating PbS dissolution (Fig. 1(b)). The strong influence of pH and the weak influence of DO on the dissolution of PbS were consistent with the results reported in the literature.41–43 | ||
| Fig. 1 Effects of (a) DO (pH fixed at 7) and (b) pH (DO fixed at 8.4 mg L−1) on the dissolution of PbS. | ||
It has been proposed that the dissolution of PbS proceeds via the following steps:41
1. Protonation of the PbS surface due to the adsorption of H+:
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) PbS + 2H+ → PbS + 2H+ → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) PbSH22+ PbSH22+ | (1) |
2. In the absence of DO, ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) PbSH22+ dissolves directly to release Pb2+:
PbSH22+ dissolves directly to release Pb2+:
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) PbSH22+ → Pb2+ + H2S PbSH22+ → Pb2+ + H2S | (2) |
3. In the presence of DO, O2 adsorbs onto ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) PbSH22+, followed by the oxidation of surface S2− to SO42− and the release of Pb2+:
PbSH22+, followed by the oxidation of surface S2− to SO42− and the release of Pb2+:
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) PbSH22+(s) + 2O2 → PbSH22+(s) + 2O2 → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) PbSH22+–2O2 PbSH22+–2O2 | (3) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) PbSH22+–2O2 → Pb2+ + SO42− + 2H+ PbSH22+–2O2 → Pb2+ + SO42− + 2H+ | (4) |
Based on the results in Fig. 1, it can be deducted that the rate of PbS dissolution is mainly controlled by proton attacks (eqn (1) and (2)), while that induced by DO attacks (eqn (3) and (4)) played a minor role. The initial dissolution rate can then be fitted to eqn (5) considering the overall influence of [H+] on the adsorption of H+ to form ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) PbSH22+ and its subsequent dissolution:
PbSH22+ and its subsequent dissolution:
| r = d[Pb(II)]/dt = k[H+]n | (5) |
Based on the least-square regression, k and n were determined to be 1.08 × 10−5 M0.67 min−1 and 0.33, respectively. Consequently, the initial dissolution rate law of PbS can be described as follows:
| r = 1.08 × 10−5 [H+]0.33 | (6) |
The dissolution rate of PbS decreased gradually with increasing time. De Giudici and Zuddas47 used atomic force microscopy to investigate the changes in surface microtopography during PbS dissolution. They found that PbS dissolution could result in the formation of protrusions, which were composed of secondary minerals such as lead oxydihydroxide (Pb2O(OH)2) and lead oxide (PbO) under their experimental conditions. The accumulation of protrusions could attenuate the PbS surface reactivity and hinder PbS dissolution. Visual MINTEQ 3.1 was used to determine the potential mineral supersaturation at the end of our experiments (Tables S3–S6). The results showed that no supersaturation was observed at pH 5 and 6 (Tables S3 and S4). At pH 7, hydrocerussite (Pb3(CO3)2(OH)2) and cerussite (PbCO3) were slightly supersaturated with a saturation index 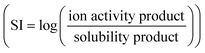 of 0.72 and 0.25, respectively (Table S5). At pH 8, Pb3(CO3)2(OH)2 is mostly supersaturated, followed by Pb(OH)2 and PbCO3, with an SI of 1.08, 0.51 and 0.03, respectively. These results indicated that it was thermodynamically possible to precipitate these secondary minerals at the end of the experiments. The potential changes in surface morphology due to the formation of secondary minerals as examined by SEM are discussed later.
of 0.72 and 0.25, respectively (Table S5). At pH 8, Pb3(CO3)2(OH)2 is mostly supersaturated, followed by Pb(OH)2 and PbCO3, with an SI of 1.08, 0.51 and 0.03, respectively. These results indicated that it was thermodynamically possible to precipitate these secondary minerals at the end of the experiments. The potential changes in surface morphology due to the formation of secondary minerals as examined by SEM are discussed later.
3.2 Effects of orthophosphate addition on PbS dissolution
It has been shown that the first flush in the agricultural area reaches the receiving water bodies in the first few hours of the rainfall events.48,49 Therefore, following the experiments conducted under different DO and pH conditions, 0.5 mg-P L−1 of orthophosphate was added at 180 min to simulate the influences of phosphate-containing surface runoff on the release of soluble Pb(II), and the results are shown in Fig. 3.For the experiments with different DO, soluble Pb(II) decreased immediately to ND (<5.1 μg L−1) for all DO levels after orthophosphate addition (Fig. 3(a)). The period of ND lapsed for about 720 min for DO = 3.0, 5.0 and 8.4 mg L−1. For DO = 0 mg L−1, soluble Pb(II) remained as ND till the end of the experiment. It has been proposed that PbS could be passivated by pyromorphite grown on its surfaces so that dissolution of PbS was hindered.50 Visual MINTEQ 3.1 was used to examine the degree of supersaturation with respect to different lead minerals after orthophosphate addition (Tables S8–S10). The solutions were highly supersaturated with respect to chloropyromorphite (Pb5(PO4)3Cl) (SI = 19.86–20.88) and hydroxypyromorphite (Pb5(PO4)3OH) (SI = 8.54–9.56). The solutions were also supersaturated with respect to Pb3(PO4)2 (SI = 4.65–5.26), Pb3(CO3)2(OH)2 (SI = 4.65–5.26), PbCO3 (SI = 0.53–0.73), PbHPO4 (SI = 0.29–0.49) and Pb(OH)2 (SI = 0.01–0.21). Given the much higher SI, the two pyromorphites were more thermodynamically favorable to precipitate.
For the experiments conducted at different pH levels, soluble Pb(II) decreased immediately to ND for all pH conditions except pH 5. The period of ND for pH = 6, 7 and 8 also lapsed for about 720 min, followed by a gradual increase of soluble Pb(II), in which the rate of increase was higher at a lower pH. Visual MINTEQ 3.1 was used to examine the degree of supersaturation with respect to different lead minerals after orthophosphate addition (Tables S11–S14). At pH < 6, only lead phosphate minerals, including Pb5(PO4)3Cl, Pb5(PO4)3OH and Pb3(PO4)2 were supersaturated. At pH > 6, in addition to the three lead phosphate minerals, the solutions were also supersaturated with the respective lead carbonate minerals, i.e., Pb3(CO3)2(OH)2 and PbCO3. However, the SI was much higher for the two pyromorphites, in which the SI values for Pb5(PO4)3Cl and Pb5(PO4)3OH were 10.99–19.46 and 3.84–8.14, respectively. It should be noted that the gradual increase of soluble Pb(II) after the period of ND under different DO and pH conditions could be due to two reasons: 1. the orthophosphate was completely consumed, therefore no orthophosphate was available to suppress the release of soluble Pb(II) and 2. the residual orthophosphate was not high enough to induce precipitation, in which a meta-saturated status with respect to pyromorphites may exist. This was further examined using different concentrations of orthophosphate shown in the following section.
3.3 Effects of orthophosphate concentrations on PbS dissolution
Different concentrations of orthophosphate (0.25–2.0 mg-P L−1) were added at 180 min to simulate the entry of surface runoff with different strengths. The calculations using Visual MINTEQ 3.1 indicated that the solutions were highly supersaturated with respect to the two pyromorphites, Pb5(PO4)3Cl and Pb5(PO4)3OH, after orthophosphate was introduced (Tables S15–S18).The profiles of soluble Pb(II) as a function of time are shown in Fig. 4. After orthophosphate addition, soluble Pb(II) decreased immediately to ND (<5.1 μg L−1) for all orthophosphate levels, and then a gradual increase was observed. The periods of ND lapsed for orthophosphate = 0.25, 0.5, 1, and 2 mg-P L−1 were 360, 720, 1440 and 2880 min, respectively. The variations of the orthophosphate concentration as a function of time for 0.25, 0.5 and 1.0 mg-P L−1 were also determined and the results are shown in Fig. S3. For the period when soluble Pb(II) was ND, there was always a sufficient level of orthophosphate in the solution. For example, when 0.25 mg-P L−1 was introduced at 180 min, during the period of ND soluble Pb(II) (180–360 min), the orthophosphate concentration was greater than 0.10 mg-P L−1. The orthophosphate concentration continued to decrease and a gradual increase of soluble Pb(II) was then observed. The same trend applied when 0.5 and 1.0 mg as P L−1 of orthophosphate were introduced (Fig. S3(b) and (c)). Therefore, it can be concluded that the gradual increase of soluble Pb(II) after ND could be attributed to the complete consumption of orthophosphate.
 | ||
| Fig. 4 Effects of orthophosphate concentrations on the dissolution of PbS. Orthophosphate was introduced at 180 min. pH 7, DO = 8.4 mg L−1. | ||
3.4 Changes in surface morphology
The SEM images of PbS before and after 60 min of the dissolution experiment without the addition of orthophosphate under different DO and pH conditions are shown in Fig. 5. Before the experiment, PbS displayed a cubic structure (Fig. 5(a)), which was consistent with the characteristic shape of PbS reported in the literature.51,52 The SEM images of PbS after dissolution under DO = 0, 5 and 8.4 mg L−1 at pH 7 are shown in Fig. 5(b)–(d) and those under DO = 8.4 mg L−1 at pH 5 and 8 are shown in Fig. 5(e) and (f), respectively. Based on the Visual MINTEQ 3.1 analysis, PbCO3 and Pb3(CO3)2(OH)2 were found to be supersaturated in these experiments except at pH < 6. The highest SI values with respect to PbCO3 and Pb3(CO3)2(OH)2 were 0.42 and 1.24, respectively. PbCO3 possesses a prismatic shape and Pb3(CO3)2(OH)2 possesses a hexagonal plate shape.53,54 No newly formed particles with these shapes were observed in the SEM images, suggesting that PbCO3 and Pb3(CO3)2(OH)2 did not precipitate and a meta-saturated status existed for these two lead carbonate minerals.55 These SEM results imply that when the sedimental PbS is disturbed, the dissolution of PbS could release substantial soluble Pb(II) which is more bioavailable than particulate Pb for a certain period of time and poses a higher ecological risk to the aquatic environment.The SEM images of PbS after the dissolution experiments in the presence of orthophosphate under different DO and pH conditions are shown in Fig. 6 and S4. The two pyromorphites (Pb5(PO4)3Cl or Pb5(PO4)3OH) are needle-shaped crystals with a hexagonal structure.35,56,57 Needle-shaped crystals were observed when DO > 0 and pH > 6 for all concentrations of orthophosphate employed (0.25–2 mg-P L−1). It was not possible to distinguish between Pb5(PO4)3Cl and Pb5(PO4)3OH due to their similar crystal shapes.54,58 However, the much higher SI for Pb5(PO4)3Cl (Tables S7–S18) leads us to believe that the needle-shaped crystals observed were more likely to be Pb5(PO4)3Cl. It should be noted that since needle-shaped pyromorphites were not observed at pH 6 with an SI = 16.16 (Table S12), the solution exhibited a meta-saturated status, indicating that a higher degree of supersaturation would be required to initiate the precipitation. Further confirmation of the mineralogy of the needle-shaped crystals using XRD, unfortunately, was not possible due to limited amounts of the minerals in the samples.
Based on the SEM observations and the soluble Pb(II) measurements, it can be deduced that orthophosphate in surface runoff during storm events could sequester soluble Pb(II) resulting from sedimental PbS dissolution and reduce the associated ecological risks due to pyromorphite precipitation.
4. Conclusions
The effects of orthophosphate on PbS dissolution and transformation under different DO and pH conditions during simulated storm suspensions were investigated in this study. In the absence of orthophosphate, the rate of PbS dissolution was mainly controlled by proton attacks and increased with decreasing pH. After orthophosphate addition, the soluble Pb(II) resulting from PbS dissolution decreased immediately to ND (<5.1 μg L−1) except at pH 5 due to the precipitation of pyromorphites. The soluble Pb(II) then gradually increased because of the consumption of orthophosphate. A higher concentration of orthophosphate could prolong the period of ND soluble Pb(II), in which an SI greater than 16.16 was required to initiate the precipitation of pyromorphites. The results of this study implied that sedimental PbS resuspended in storm suspensions could dissolve and elevate the soluble Pb(II) levels in the water column. However, phosphate-containing surface runoff resulting from the storm, especially in agricultural areas, could sequester soluble Pb(II) by forming low solubility pyromorphites to reduce Pb-associated ecological risks, although phosphate is generally considered as the main nutrient contributing to eutrophication.Several limitations still exist given the complexity of natural waters. For example, the dynamic roles of natural organic matter (NOM) in PbS dissolution and pyromorphite precipitation was not considered. NOM is known to adsorb on the mineral surfaces to protect them from proton or oxygen induced dissolution,59,60 as well as to initiate ligand-induced dissolution and metal complexation to enhance the solubility of the minerals.9,61 In addition, other metals such as Cu and Cd that were not investigated in this study may compete and consume orthophosphate.62 Therefore, the influences of NOM and other metals on the dissolution and transformation of PbS should be further investigated. Finally, field studies in water bodies experiencing lead contamination and surface runoff especially in agricultural areas could be conducted to verify the findings of this study.
Author contributions
Yi-Pin Lin: conceptualization, formal analysis, writing – original draft and review & editing, funding acquisition, and supervision. Ze-Xuan Tan: methodology, investigation, and formal analysis.Conflicts of interest
There are no conflicts to declare.Data availability
All data included in this study are available upon request by contact with the corresponding author.Supplementary information (SI): synthetic freshwater composition. The initial dissolution rates of PbS under different DO and pH. Lead mineral saturation index at PbS = 100 mg L−1, pH 5–8, DO = 8.4 mg L−1 and t = 60 min. Lead mineral saturation index right after phosphate addition (t = 180 min): PbS = 100 mg L−1, pH 7, DO = 0–8.4 mg L−1 and 0.5 mg-P L−1 phosphate. Lead mineral saturation index right after phosphate addition (t = 180 min): PbS = 100 mg L−1, pH 5–8, DO = 8.4 mg L−1 and 0.5 mg-P L−1 phosphate. Lead mineral saturation index right after phosphate addition (t = 180 min): PbS = 100 mg L−1, pH 7, DO = 8.4 mg L−1 and 0.25–2 mg-P L−1 phosphate. The schematic and photograph of the experimental setup. Particle size distribution of PbS. The variations of the orthophosphate concentration as a function of time for the addition of 0.25, 0.5 and 1.0 mg L−1 as P of orthophosphate. SEM images of PbS after the experiment for (a) DO = 3.0 mg L−1, pH 7, P = 0.5 mg L−1, (b) DO = 5.0 mg L−1, pH 7, P = 0.5 mg L−1, (c) DO = 8.4 mg L−1, pH 6, P = 0.5 mg L−1, (d) DO = 8.4 mg L−1, pH 7, P = 0.5 mg L−1, (e) DO = 8.4 mg L−1, pH 7, P = 0.5 mg L−1 and (f) DO = 8.4 mg L−1, pH 7, P = 1 mg L−1. Reaction time = 72 h. P indicates orthophosphate added at 180 min. See DOI: https://doi.org/10.1039/d5ew00329f.
Acknowledgements
This work was supported by the NTU Research Center for Future Earth from The Featured Areas Research Center Program (113 L901002) and the Excellence Research Program - Core Consortiums (NTU-CC-114 L892601), within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan.References
- K. M. Dernie, M. J. Kaiser, E. A. Richardson and R. M. Warwick, Recovery of soft sediment communities and habitats following physical disturbance, J. Exp. Mar. Biol. Ecol., 2003, 285, 415–434, DOI:10.1016/s0022-0981(02)00541-5.
- S. M. Yarnell, J. F. Mount and E. W. Larsen, The influence of relative sediment supply on riverine habitat heterogeneity, Geomorphology, 2006, 80(3–4), 310–324, DOI:10.1016/j.geomorph.2006.03.005.
- D. M. DiToro, J. D. Mahony, D. J. Hansen, K. J. Scott, A. R. Carlson and G. T. Ankley, Acid volatile sulfide predicts the acute toxicity of cadmium and nickel in sediments, Environ. Sci. Technol., 1992, 26(1), 96–101, DOI:10.1021/es00025a009.
- G. T. Ankley, D. M. DiToro, D. J. Hansen and W. J. Berry, Assessing the ecological risk of metals in sediments, Environ. Toxicol. Chem., 1996, 15(12), 2053–2055, DOI:10.1002/etc.5620151201.
- C. Diop, D. Dewaele, F. Cazier, A. Diouf and B. Ouddane, Assessment of trace metals contamination level, bioavailability and toxicity in sediments from Dakar coast and Saint Louis estuary in Senegal, West Africa, Chemosphere, 2015, 138, 980–987, DOI:10.1016/j.chemosphere.2014.12.041.
- Z. G. Jiang, N. Xu, B. X. Liu, L. Z. Zhou, J. Wang, C. Wang, B. G. Dai and W. Xiong, Metal concentrations and risk assessment in water, sediment and economic fish species with various habitat preferences and trophic guilds from Lake Caizi, Southeast China, Ecotoxicol. Environ. Saf., 2018, 157, 1–8, DOI:10.1016/j.ecoenv.2018.03.078.
- D. M. DiToro, J. D. Mahony, D. J. Hansen, K. J. Scott, M. B. Hicks, S. M. Mayr and M. S. Redmond, Toxicity of cadmium in sediments - the role of acid volatile sulfide, Environ. Toxicol. Chem., 1990, 9(12), 1487–1502, DOI:10.1002/etc.5620091208.
- J. W. Morse and G. W. Luther, Chemical influences on trace metal-sulfide interactions in anoxic sediments, Geochim. Cosmochim. Acta, 1999, 63(19–20), 3373–3378, DOI:10.1016/s0016-7037(99)00258-6.
- A. Charriau, L. Lesven, Y. Gao, M. Leermakers, W. Baeyens, B. Ouddane and G. Billon, Trace metal behaviour in riverine sediments: Role of organic matter and sulfides, Appl. Geochem., 2011, 26(1), 80–90, DOI:10.1016/j.apgeochem.2010.11.005.
- W. J. Berry, D. J. Hansen, J. D. Mahony, D. L. Robson, D. M. DiToro, B. P. Shipley, B. Rogers, J. M. Corbin and W. S. Boothman, Predicting the toxicity of metal-spiked laboratory sediments using acid-volatile sulfide and interstitial water normalizations, Environ. Toxicol. Chem., 1996, 15(12), 2067–2079, DOI:10.1002/etc.5620151203.
- D. J. Hansen, W. J. Berry, J. D. Mahony, W. S. Boothman, D. M. DiToro, D. L. Robson, G. T. Ankley, D. Ma, Q. Yan and C. E. Pesch, Predicting the toxicity of metal-contaminated field sediments using interstitial concentration of metals and acid-volatile sulfide normalizations, Environ. Toxicol. Chem., 1996, 15(12), 2080–2094, DOI:10.1002/etc.5620151204.
- Y. P. Lin, I. C. Li and P. I. Chou, Dissolution of PbS, CuS, and ZnS in oxic waters: effects of adsorbed natural organic matter, Environ. Sci. Pollut. Res., 2021, 28(14), 18102–18110, DOI:10.1007/s11356-020-11970-y.
- P. I. Chou, D. Q. Ng, I. C. Li and Y. P. Lin, Effects of dissolved oxygen, pH, salinity and humic acid on the release of metal ions from PbS, CuS and ZnS during a simulated storm event, Sci. Total Environ., 2018, 624, 1401–1410, DOI:10.1016/j.scitotenv.2017.12.221.
- J. M. C. Araujo, T. O. Ferreira, M. Suarez-Abelenda, G. N. Nobrega, A. Albuquerque, A. D. Bezerra and X. L. Otero, The role of bioturbation by Ucides cordatus crab in the fractionation and bioavailability of trace metals in tropical semiarid mangroves, Mar. Pollut. Bull., 2016, 111(1–2), 194–202, DOI:10.1016/j.marpolbul.2016.07.011.
- S. Wang, K. Zheng, Q. Y. Liu, L. Y. Wang, X. N. Feng and H. P. Li, Galena weathering in simulated alkaline soil: Lead transformation and environmental implications, Sci. Total Environ., 2021, 755, 142708, DOI:10.1016/j.scitotenv.2020.142708.
- S. L. Simpson, S. C. Apte and G. E. Batley, Effect of short term resuspension events on trace metal speciation in polluted anoxic sediments, Environ. Sci. Technol., 1998, 32(5), 620–625, DOI:10.1021/es970568g.
- M. Caetano, M. J. Madureira and C. Vale, Metal remobilisation during resuspension of anoxic contaminated sediment: short-term laboratory study, Water, Air, Soil Pollut., 2003, 143(1–4), 23–40, DOI:10.1023/a:1022877120813.
- Y. S. Hong, K. A. Kinney and D. D. Reible, Effects of cyclic changes in ph and salinity on metals release from sediments, Environ. Toxicol. Chem., 2011, 30(8), 1775–1784, DOI:10.1002/etc.584.
- P. Chanpiwat, M. Ponsin and A. Numprasanthai, Effects of sediment resuspension and changes in water nutrient concentrations on the remobilization of lead from contaminated sediments in Klity Creek, Thailand, J. Environ. Manage., 2023, 339, 117909, DOI:10.1016/j.jenvman.2023.117909.
- D. H. Dang, N. Layglon, N. Ferretto, D. Omanovic, J. U. Mullot, V. Lenoble, S. Mounier and C. Garnier, Kinetic processes of copper and lead remobilization during sediment resuspension of marine polluted sediments, Sci. Total Environ., 2020, 698, 134120, DOI:10.1016/j.scitotenv.2019.134120.
- R. Eisler, Lead Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review, in Contaminant Hazard Reviews, Laurel, MD, 1988 Search PubMed.
- V. S. Andrade, M. F. Gutierrez, L. Regaldo, A. R. Paira, M. R. Repetti and A. M. Gagneten, Influence of rainfall and seasonal crop practices on nutrient and pesticide runoff from soybean dominated agricultural areas in Pampean streams, Argentina, Sci. Total Environ., 2021, 788, 147676, DOI:10.1016/j.scitotenv.2021.147676.
- T. Kato, H. Kuroda and H. Nakasone, Runoff characteristics of nutrients from an agricultural watershed with intensive livestock production, J. Hydrol., 2009, 368(1–4), 79–87, DOI:10.1016/j.jhydrol.2009.01.028.
- I. L. Lacher, E. Ahmadisharaf, C. Fergus, T. Akre, W. J. McShea, B. L. Benham and K. S. Kline, Scale-dependent impacts of urban and agricultural land use on nutrients, sediment, and runoff, Sci. Total Environ., 2019, 652, 611–622, DOI:10.1016/j.scitotenv.2018.09.370.
- T. Kizuka, H. Mikami, S. Kameyama, S. Ono and H. Suzuki, Hydrological environment affects the nutrient retention and runoff function of naturally re-wetted agricultural peatland in lowland river floodplain, Sci. Total Environ., 2023, 857, 159483, DOI:10.1016/j.scitotenv.2022.159483.
- S. Chaudhary, L. H. Chua and A. Kansal, Event mean concentration and first flush from residential catchments in different climate zones, Water Res., 2022, 219, 118594, DOI:10.1016/j.watres.2022.118594.
- S. M. Li, X. L. Wang, B. Qiao, J. S. Li and J. M. Tu, First flush characteristics of rainfall runoff from a paddy field in the Taihu Lake watershed, China, Environ. Sci. Pollut. Res., 2017, 24(9), 8336–8351, DOI:10.1007/s11356-017-8470-2.
- W. T. Peterjohn and D. L. Correll, Nutrient dynamics in an agricultural watershed: observations on the role of a riparian forest, Ecology, 1984, 65(5), 1466–1475, DOI:10.2307/1939127.
- J. H. Ryther and W. M. Dunstan, Nitrogen,phosphorus, and eutrophication in coastal marine environment, Science, 1971, 171(3975), 1008–1013, DOI:10.1126/science.171.3975.1008.
- K. L. Seip, Phosphorus and nitrogen limitation of algal biomass across trophic gradients, Aquat. Sci., 1994, 56(1), 16–28, DOI:10.1007/bf00877432.
- P. M. Gilbert, Eutrophication, harmful algae and biodiversity - Challenging paradigms in a world of complex nutrient changes, Mar. Pollut. Bull., 2017, 124(2), 591–606, DOI:10.1016/j.marpolbul.2017.04.027.
- M. V. Ruby, A. Davis and A. Nicholson, In-situ formation of lead phosphates in soils as a method to immobilize lead, Environ. Sci. Technol., 1994, 28(4), 646–654, DOI:10.1021/es00053a018.
- J. A. Ryan, P. C. Zhang, D. Hesterberg, J. Chou and D. E. Sayers, Formation of chloropyromorphite in a lead-contaminated soil amended with hydroxyapatite, Environ. Sci. Technol., 2001, 35(18), 3798–3803, DOI:10.1021/es010634l.
- M. Edwards and L. S. McNeill, Effect of phosphate inhibitors on lead release from pipes, J. - Am. Water Works Assoc., 2002, 94(1), 79–90, DOI:10.1002/j.1551-8833.2002.tb09383.x.
- D. Q. Ng, T. J. Strathmann and Y. P. Lin, Role of orthophosphate as a corrosion inhibitor in chloraminated solutions containing tetravalent lead corrosion product PbO2, Environ. Sci. Technol., 2012, 46(20), 11062–11069, DOI:10.1021/es302220t.
- Y. Bae, J. D. Pasteris and D. E. Giammar, The ability of phosphate to prevent lead release from pipe scale when switching from free chlorine to monochloramine, Environ. Sci. Technol., 2020, 54(2), 879–888, DOI:10.1021/acs.est.9b06019.
- H. Soares, P. Conde, A. A. N. Almeida and M. Vasconcelos, Evaluation of n-substituted aminosulfonic acid pH buffers with a morpholinic ring for cadmium and lead speciation studies by electroanalytical techniques, Anal. Chim. Acta, 1999, 394(2–3), 325–335, DOI:10.1016/s0003-2670(99)00294-9.
- V. L. Snoeyink and D. Jenkins, Water Chemistry, Wiley, 1980 Search PubMed.
- A. S. Ogston, D. A. Cacchione, R. W. Sternberg and G. C. Kineke, Observations of storm and river flood-driven sediment transport on the northern California continental shelf, Cont. Shelf Res., 2000, 20(16), 2141–2162, DOI:10.1016/s0278-4343(00)00065-0.
- APHA, AWWA and WEF, Standard Methods for the Examination of Water and Wastewater, Washington DC: American Public Health Association, American Water Works Association, Water Environment Federation, 21st edn, 2017 Search PubMed.
- Y. H. Hsieh and C. P. Huang, The dissolution of PbS(S) in dilute aqueous-solutions, J. Colloid Interface Sci., 1989, 131(2), 537–549, DOI:10.1016/0021-9797(89)90196-3.
- D. Fornasiero, F. S. Li, J. Ralston and R. S. C. Smart, Oxidation of galena surfaces .1. X-Ray photoelectron spectroscopic and dissolution kinetics studies, J. Colloid Interface Sci., 1994, 164(2), 333–344, DOI:10.1006/jcis.1994.1175.
- Y. J. Peng and S. Grano, Dissolution of fine and intermediate sized galena particles and their interactions with iron hydroxide colloids, J. Colloid Interface Sci., 2010, 347(1), 127–131, DOI:10.1016/j.jcis.2010.03.027.
- J. Cama, P. Acero and C. Ayora, Galena dissolution in acidic pH at 25° C, in Water Rock Interaction, ed. R. B. Wanty and R. R. Seal, Balkema, 2004, pp. 731–734 Search PubMed.
- G. De Giudici, A. Rossi, L. Fanfani and P. Lattanzi, Mechanisms of galena dissolution in oxygen-saturated solutions: Evaluation of pH effect on apparent activation energies and mineral-water interface, Geochim. Cosmochim. Acta, 2005, 69(9), 2321–2331, DOI:10.1016/j.gca.2004.12.003.
- S. Zhang, J. P. Li, Y. R. Wang and G. Q. Hu, Dissolution kinetics of galena in acid NaCl solutions at 25-75 °C, Appl. Geochem., 2004, 19(6), 835–841, DOI:10.1016/j.apgeochem.2003.10.005.
- G. De Giudici and P. Zuddas, In situ investigation of galena dissolution in oxygen saturated solution: Evolution of surface features and kinetic rate, Geochim. Cosmochim. Acta, 2001, 65(9), 1381–1389, DOI:10.1016/s0016-7037(00)00605-0.
- J. H. Lee and K. W. Bang, Characterization of urban stormwater runoff, Water Res., 2000, 34(6), 1773–1780, DOI:10.1016/s0043-1354(99)00325-5.
- X. L. Yang, T. K. Li, K. K. Hua and Y. L. Zhang, Investigation of First Flushes in a Small Rural-Agricultural Catchment, Pol. J. Environ. Stud., 2015, 24(1), 381–389, DOI:10.15244/pjoes/28299.
- A. G. Stack, R. Erni, N. D. Browning and W. H. Casey, Pyromorphite growth on lead-sulfide surfaces, Environ. Sci. Technol., 2004, 38(21), 5529–5534, DOI:10.1021/es049487s.
- F. Davar, M. Mohammadikish, M. R. Loghman-Estarki and M. Masteri-Farahani, Synthesis of micro-and nanosized PbS with different morphologies by the hydrothermal process, Ceram. Int., 2014, 40(6), 8143–8148, DOI:10.1016/j.ceramint.2014.01.009.
- H. Karami, M. Ghasemi and S. Matini, Synthesis, characterization and application of lead sulfide nanostructures as ammonia gas sensing agent, Int. J. Electrochem. Sci., 2013, 8(10), 11661–11679, DOI:10.1016/S1452-3981(23)13213-5.
- Y. Zhang and Y. P. Lin, Determination of PbO2 formation kinetics from the chlorination of Pb(II) carbonate solids via direct PbO2 measurement, Environ. Sci. Technol., 2011, 45(6), 2338–2344, DOI:10.1021/es1039826.
- Y. C. Peng, Y. F. Lu and Y. P. Lin, Release of particulate lead from four lead corrosion products in drinking water: A laboratory study coupled with microscopic observations and computational fluid dynamics, Environ. Sci. Technol., 2022, 56(17), 12218–12227, DOI:10.1021/acs.est.2c02461.
- J. W. Mullin, Crystallization, Butterworth-Heinemann, 4th edn, 2001 Search PubMed.
- D. A. Lytle, M. R. Schock and K. Scheckel, The inhibition of Pb(IV) oxide formation in chlorinated water by orthophosphate, Environ. Sci. Technol., 2009, 43(17), 6624–6631, DOI:10.1021/es900399m.
- D. Q. Ng, C. Y. Chen and Y. P. Lin, A new scenario of lead contamination in potable water distribution systems: Galvanic corrosion between lead and stainless steel, Sci. Total Environ., 2018, 637, 1423–1431, DOI:10.1016/j.scitotenv.2018.05.114.
- J. D. Pasteris, Y. Bae, D. E. Giammar, S. N. Dybing, C. H. Yoder, J. T. Zhao and Y. D. Hu, Worth a closer look: Raman spectra of lead-pipe scale, Minerals, 2021, 11(10), 1047, DOI:10.3390/min11101047.
- Y. P. Lin, P. C. Singer and G. R. Aiken, Inhibition of calcite precipitation by natural organic material: Kinetics, mechanism, and thermodynamics, Environ. Sci. Technol., 2005, 39(17), 6420–6428, DOI:10.1021/es050470z.
- Q. Y. Liu, H. P. Li, G. H. Jin, K. Zheng and L. Y. Wang, Assessing the influence of humic acids on the weathering of galena and its environmental implications, Ecotoxicol. Environ. Saf., 2018, 158, 230–238, DOI:10.1016/j.ecoenv.2018.04.030.
- M. Ravichandran, G. R. Aiken, M. M. Reddy and J. N. Ryan, Enhanced dissolution of cinnabar (mercuric sulfide) by dissolved organic matter isolated from the Florida Everglades, Environ. Sci. Technol., 1998, 32(21), 3305–3311, DOI:10.1021/es9804058.
- R. L. Gao, H. Q. Hu, Q. L. Fu, Z. H. Li, Z. Q. Xing, U. Ali, J. Zhu and Y. H. Liu, Remediation of Pb, Cd, and Cu contaminated soil by co-pyrolysis biochar derived from rape straw and orthophosphate: Speciation transformation, risk evaluation and mechanism inquiry, Sci. Total Environ., 2020, 730, 139119, DOI:10.1016/j.scitotenv.2020.139119.
| This journal is © The Royal Society of Chemistry 2026 |