Superbasic CO2-concentrating layer-protected nickel catalyst for solar CH4 synthesis via direct air capture
Weimin
Ma
ab,
Shidi
Gui
a,
Jingyang
Zhu
a,
Huaisuo
Yao
a,
Jingxue
Sun
a,
Jian
Pei
 a and
Yingxuan
Li
a and
Yingxuan
Li
 *a
*a
aState Key Laboratory of Space Power-Sources, MIIT Key Laboratory of Critical Materials Technology for New Energy Conversion and Storage, School of Chemistry and Chemical Engineering, Harbin Institute of Technology, Harbin, 150001, China. E-mail: liyingxuan@hit.edu.cn
bShaanxi Key Laboratory of Catalysis, School of Chemical & Environment Sciences, Shaanxi University of Technology, Hanzhong, 723000, P.R. China
First published on 17th December 2025
Abstract
Although direct air capture technology shows promise for atmospheric CO2 reduction, it is hindered by the energy-intensive CO2 concentration processes and unresolved long-term storage risks. As an alternative approach, direct conversion of atmospheric CO2 into solar fuels could simultaneously achieve carbon neutrality and energy storage, yet existing conversion technologies predominantly require high-concentration CO2 streams. Herein, we demonstrate a nickel-encapsulated mesoporous nitrogen-doped carbon (NC) architecture that enables integrated CO2 capture from air and CH4 production via in situ catalysis of the captured CO2 with H2 under solar irradiation. The engineered mesoporous NC framework with superbasic sites achieves exceptional CO2 capture capacity (55 cm3 g−1) and ultrafast adsorption–desorption kinetics (equilibrium attained in ∼1 min) under ambient conditions. The Ni nanoparticles and NC layers function as tandem catalytic sites for CH4 production, where photogenerated electrons drive H2 dissociation on Ni sites, while the adsorbed CO2 on NC undergoes photothermal reduction to CH4 by the spilled hydrogen. This mechanism enables a record CH4 production rate of 339 mmol g−1 h−1 (nearly identical with that obtained using pure CO2), with perfect selectivity through atmospheric CO2 conversion. Furthermore, the hydrophobic NC overlayers effectively prevent Ni sintering via physical confinement effects and inhibit oxidative deactivation by dynamically scavenging the H2O byproduct, enabling the catalyst to maintain stability for over 100 cycles of atmospheric CO2 capture and conversion. Our temporal-decoupling strategy for converting atmospheric CO2 eliminates oxygen interference in ambient air and energy-intensive concentration steps, thereby establishing an innovative paradigm for producing carbon-neutral fuels.
Broader contextThe solar-powered conversion of atmospheric CO2 is a promising approach for the production of truly carbon-neutral fuels and mitigation of global warming. Over the past decades, tremendous CO2 utilization technologies predominantly required a concentrated CO2 feed or high temperature. However, the efficient conversion of CO2 in air has been rarely achieved due to the difficulty in adsorption and the activation of ultralow concentrations of CO2 (∼0.04%). Herein, we developed a catalyst that synergistically integrates atmospheric CO2 capture with in situ conversion and achieved CH4 formation with near-perfect selectivity under solar irradiation. The CO2-enriched Ni@NC-600 system therefore achieves a catalytic enhancement of approximately 100–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times higher than traditional approaches. Notably, the catalyst also achieves exceptional CO2 capture capacity (55 cm3 g−1) and ultrafast adsorption–desorption kinetics (equilibrium attained in ∼1 min) under ambient conditions. Furthermore, the hydrophobic NC overlayers effectively prevent Ni sintering via physical confinement effects and inhibit oxidative deactivation through dynamically scavenging the H2O byproduct, enabling the catalyst to maintain stability for over 100 cycles of atmospheric CO2 capture and conversion. This temporal-decoupling strategy establishes a new framework for solar-powered carbon-neutral fuel production without atmospheric CO2 separation constraints. Significantly, this work pioneers a solar-driven technology for atmospheric CO2-to-CH4 conversion, offering a transformative solution to the critical energy-environment nexus between climate change mitigation and renewable energy storage. 000 times higher than traditional approaches. Notably, the catalyst also achieves exceptional CO2 capture capacity (55 cm3 g−1) and ultrafast adsorption–desorption kinetics (equilibrium attained in ∼1 min) under ambient conditions. Furthermore, the hydrophobic NC overlayers effectively prevent Ni sintering via physical confinement effects and inhibit oxidative deactivation through dynamically scavenging the H2O byproduct, enabling the catalyst to maintain stability for over 100 cycles of atmospheric CO2 capture and conversion. This temporal-decoupling strategy establishes a new framework for solar-powered carbon-neutral fuel production without atmospheric CO2 separation constraints. Significantly, this work pioneers a solar-driven technology for atmospheric CO2-to-CH4 conversion, offering a transformative solution to the critical energy-environment nexus between climate change mitigation and renewable energy storage.
|
Introduction
The continuous high consumption of fossil fuels has caused annual atmospheric CO2 emissions of 4.4 Gt,1,2 intensifying the urgency for carbon removal technologies. Direct air capture (DAC) offers a pathway to mitigate climate change by extracting ambient CO2, yet its widespread adoption is hindered by energy-intensive CO2 concentration processes and expensive collection systems.3,4 Although geological sequestration of captured CO2 is technically feasible, unresolved concerns about its environmental risks and long-term storage integrity persist.4Direct catalytic conversion of atmospheric CO2 presents an alternative strategy for both removing excess CO2 from air and producing fuel. However, this process typically demonstrates reaction rates confined to the micromolar regime (µmol g−1 h−1) due to the ultralow atmospheric CO2 concentration (0.04%) and sluggish mass-transport kinetics.5–9 Furthermore, the presence of O2 in air is generally considered as a threat to CO2 reduction.4 Therefore, catalytic CO2 reduction is currently based almost exclusively on the use of pure or concentrated CO2 as the feed gas,1,10–12 necessitating energy-intensive pre-concentration steps for atmospheric CO2 (440–600 kJ mol−1, including CO2 adsorption, adsorbent regeneration, and the movement of large quantities of air).13 The massive energy input required for the CO2 concentration and conversion processes can lead to additional CO2 emissions and increased costs, thus considerably reducing the benefits of catalytic CO2 conversion.
To address these challenges, integrated CO2 capture and utilization has emerged as a transformative strategy that enables the in situ catalytic conversion of captured CO2 into value-added chemicals. This dual-functional approach eliminates energy-intensive CO2 concentration and transportation steps compared to conventional decoupled systems. Recently, Reisner and co-authors developed a dual-bed flow reactor for gas-phase direct air capture and utilization.4 This system first captures atmospheric CO2 using a solid silica-amine adsorbent, releasing CO2-free air. Concentrated light then simultaneously releases the captured CO2 and converts it directly into syngas (CO + H2) over a Co-based molecular-semiconductor photocatalyst. Although this dual-bed flow reactor can operate without requiring high temperature or pressure, this system is complex with poor infrared-light (comprises ∼50% of the solar spectrum) utilization and slow capture kinetics (12 h). An alternative approach is to develop dual-functional light-driven catalysts with high capacity for capturing atmospheric CO2. When powered by renewable energy such as sunlight, such catalysts can produce real carbon-neutral fuels.14 Despite their potential, dual-functional catalysts remain scarce due to the intrinsic challenge of integrating multifunctional components within a single material.15–18
For dual-functional catalysts, the strongly adsorbed CO2 covers the active sites of the catalysts, thus impeding H2 adsorption and activation and subsequent CO2 hydrogenation.19 Therefore, how to balance CO2 adsorption and H2 activation is a primary challenge for atmospheric CO2 hydrogenation on a dual-functional catalyst. Developing catalysts with isolated active sites can provide an effective approach for reducing the co-adsorption coverage effects of CO2 and H2 in the hydrogenation reaction. We recently developed a platinum-loaded nickel metal–organic framework (Pt/Ni-MOF) that enables simultaneous CO2 capture from the atmosphere at the Ni sites and photocatalytic H2 activated at the Pt sites.2 However, Pt/Ni-MOF operates only under near-infrared light (≤940 nm), leaving most of the infrared light (accounting for about 50% of the solar energy) unutilized. Moreover, it requires an external heat source (160 °C) for CO2 activation, which limits its practical application. Critically, developing dual-functional catalysts with isolated active sites that synergize high atmospheric CO2 capture capacity, rapid sorption kinetics, and high activity driven by full solar spectrum remains a challenge.
Beyond the conventional photocatalysis that is dependent on photoinduced charge carriers, solar energy can drive CO2 reduction not only through the isolated photothermal effect but also via synergistic photochemical–photothermal activation pathways, offering enhanced efficiency and tailored reaction control. The photothermal catalysis operates via synergistic coupling of photon-to-heat conversion and thermally activated CO2 reduction, providing an opportunity for converting underutilized infrared photons in traditional photocatalysis. For example, a series of Ni-,20 Co-,21 Cu-,22 Fe-,23 Ir-,24 and Ru25-based catalysts have recently been reported for CO2 reduction driven by light-induced heating. Under light irradiation, the metal nanoparticles (NPs) can generate the so-called “hot electrons” by localized surface plasmon resonance (LSPR), which can catalyse CO2 conversion reactions and/or the localized heating of nanoparticles from electron-lattice collisions. By coupling the photothermal and photochemical effects, the metal NPs effects can achieve an outstanding enhancement of the catalytic CO2 reduction activity. Recently, catalytic methanation of CO2 has been achieved over Ru@Ni2V2O726 and Au/Ce0.95Ru0.05O2,27 which effectively converts high-energy photons into electronic excitations while transforming low-energy infrared photons into localized heat, demonstrating a unique advantage in harnessing the full solar spectrum. Consequently, this synergistic photochemical–photothermal activation enables efficient CO2 reduction at lower temperatures28 and reduced activation barriers compared to thermocatalysis,26,27,29,30 while delivering much higher activity compared to single-mode photocatalytic systems.
Beyond metal-based photothermal catalysts, carbon-coated metal NPs have emerged as promising candidates owing to their broadband light absorption, high photothermal conversion efficiency, large surface area, and suitable porosity.21 The application of a carbon coating to metal sites is an effective strategy for mitigating metal oxidation by the H2O formed during CO2 hydrogenation, thereby ensuring long-term stability under reaction conditions. Such a core–shell structure can also provide two distinct active sites for CO2 adsorption (on the carbon layer) and H2 activation (on the metal site). Despite these advantages, the existing systems still fail to address the critical challenge of reducing ultralow-concentration atmospheric CO2 (0.04%) under full solar spectrum irradiation.
Herein, we develop a Ni-encapsulated mesoporous nitrogen-doped carbon (NC) architecture that synergistically combines atmospheric CO2 capture with solar-driven catalytic conversion. This design achieves exceptional CO2 capture kinetics (55 cm3 g−1 capacity, about 1 min equilibrium) and enables direct methane synthesis from captured CO2 under sunlight irradiation. The CH4 production occurs via a dual-site mechanism: the NC sites adsorb atmospheric CO2, while the Ni sites activate H2. Under ultraviolet light irradiation, H2 dissociates into H atoms. These atoms then spill over to the CO2 adsorption sites, where they facilitate CH4 generation via photothermal reduction. Under synergetic photochemical–photothermal activation, the system attains a record CH4 production rate of 339 mmol g−1 h−1 with perfect selectivity, matching pure CO2 feedstock performance while eliminating energy-intensive concentration requirements. Furthermore, the catalyst maintains 100-cycle operational stability, overcoming a critical durability bottleneck in direct atmospheric CO2 conversion systems.
Results and discussion
Synthesis and characterization of the Ni@NC catalysts
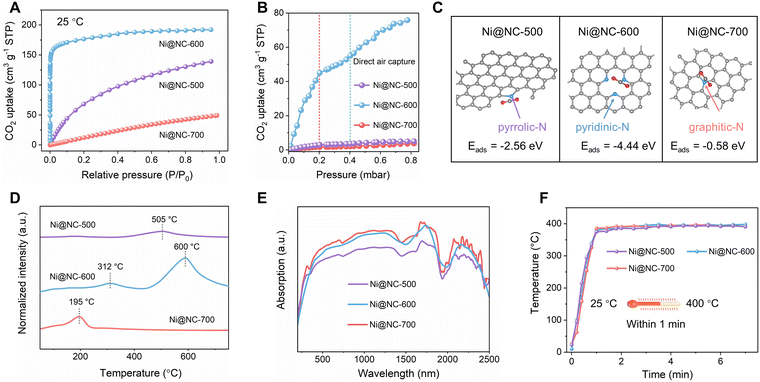
The two-step process for synthesizing the Ni@NC catalyst is schematically depicted in Fig. 1A. First, Ni-MOF was synthesized according to a previously reported method.2 Thermogravimetric analysis of Ni-MOF in nitrogen gas indicated that decomposition occurred above 500 °C (Fig. S1). Therefore, Ni-MOF was pyrolyzed in Ar gas at temperatures of 500, 600 and 700 °C for 30 minutes to produce Ni@NC-X (X = 500, 600 and 700). In this process, the reduction of the Ni2+ species in the MOF was achieved along with the decomposition and carbonization of the organic linker, as observed in previous reports.31–33 Simultaneously, N was doped in the carbon shells by using coordinated N,N-dimethylformamide (DMF) on the Ni-MOF as the N source under carbonization conditions.Scanning electron microscopy (SEM) images of Ni@NC-X (X = 500, 600 and 700) are shown in Fig. 1B and Fig. S2; the samples exhibit a distinct prism-shaped morphology, consistent with that of the Ni-MOF precursor (Fig. S3). However, compared to Ni-MOF with a smooth surface, the surfaces of the pyrolyzed samples were relatively rough. The transmission electron microscopy (TEM) images (Fig. S4), high-resolution TEM (HRTEM) images (Fig. 1C) and X-ray diffraction (XRD) patterns (Fig. 1D) indicate that carbon-coated Ni NPs with an average size of approximately 10 nm were produced over the N-doped carbon support during heat treatment at different temperatures (Fig. S4). The structure of the NC layers was also confirmed by Raman spectroscopy (Fig. 1E).
The HRTEM image (Fig. 1C) reveals that the thickness of the NC shell was 1–3 nm and that irregular nanopores were formed among the core–shell Ni@NC NPs. N2 adsorption–desorption isotherms reveal the mesoporous structure of the pyrolyzed samples (Fig. S5). The pore size distribution ranges from 3 to 12 nm, while the pore volumes of Ni@NC-X (X = 500, 600 and 700) are 0.55, 0.67 and 0.48 cm3 g−1, respectively (Fig. 1F). High-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) imaging coupled with energy-dispersive X-ray (EDX) mapping confirmed that C, N and Ni are distributed throughout the NC matrix (Fig. 1G). The Ni contents of Ni@NC-X (X = 500, 600 and 700) determined via inductively coupled plasma-mass spectrometry are almost the same, with values of ∼18.9%, 18.5%, and 18.4%, respectively.
As shown in Fig. 1H, the X-ray photoelectron spectroscopy (XPS) spectrum of Ni 2p reveals that the Ni species exist in the metallic state (at 852.6 and 870.2 eV),31–33 which is consistent with the results of the HRTEM and XRD analyses. The shoulder peaks in Fig. 1H are attributed to the satellite peaks (at 858.5 and 874.3 eV) and the formation of Ni–C bonding (at 854.6 eV).33 As shown in Fig. 1I, the N 1s spectrum of Ni@NC-500 exhibits two distinct peaks corresponding to pyridinic–N (398.2 eV) and pyrrolic–N (400.1 eV),34 among which pyrrolic–N (86%) represents the majority. In contrast, peaks attributed to pyridinic–N (398.2 eV) and graphitic–N (401.2 eV) are observed for Ni@NC-600 and Ni@NC-700, respectively.35,36 The N 1s spectra confirm that the N species doped in the C layers were effectively tuned by adjusting the pyrolysis temperature.
As shown in Fig. 2A, the CO2 adsorption capacity of Ni@NC-600 (191 cm3 g−1) at 25 °C is greater than those of Ni@NC-500 (135 cm3 g−1) and Ni@NC-700 (49 cm3 g−1). Impressively, Ni@NC-600 exhibits a sharp increase in the low-pressure region, indicating its remarkable capacity to adsorb CO2 at low concentrations. As shown in Fig. 2B and Fig. S6, Ni@NC-600 demonstrated a superior ability for direct CO2 capture from air, achieving an adsorption capacity of 55 cm3 g−1 at 25 °C and 52.8 cm3 g−1 at 400 °C under 0.4 mbar, respectively, which is approximately equivalent to the partial pressure of atmospheric CO2. This CO2 adsorption capacity is much larger than that of most solid sorbents (Table S1), suggesting the superior activity of Ni@NC-600 for direct capture of CO2 from air. In N-doped carbon materials, pyridinic–N, as a Lewis base site, has much stronger alkalinity than pyrrolic–N and graphitic–N and is therefore responsible for capturing low-concentration CO2 molecules and stabilizing *CO2 intermediates (where * represents the active site on the surface).37–39 These results are consistent with the calculated adsorption energies of CO2 on different types of N (Fig. 2C). Furthermore, as shown in Fig. S7, Ni-MOF undergoes solvent replacement for 12 h and 48 h with dichloromethane (CH2Cl2) to gradually remove DMF molecules.2 Then, the obtained Ni-MOF without DMF was pyrolyzed at 600 °C, which produced the carbon-coated Ni NPs (denoted as Ni@C-600). Negligible CO2 adsorption capacity and nearly no activity for photothermal or thermal conversion of CO2 were observed on Ni@C-600 (Fig. S8), indicating that the presence of N in Ni@NC-X (X = 500, 600 and 700) plays a crucial role in CO2 adsorption.
CO2 temperature-programmed desorption (CO2-TPD) of Ni@NC-500 catalyst shows a small desorption peak at 505 °C (Fig. 2D), indicating a low ability to adsorb CO2. For Ni@NC-600, the material displayed two evident CO2 desorption peaks. The peak at 312 °C is assigned to medium adsorption sites on pyridinic–N, which stems from the weak Lewis basicity of the N lone pair electrons interacting with CO2 to form the weakly bound surface species.40 The higher temperature peak, observed at approximately 600 °C, is attributed to chemisorbed CO2 molecules. Furthermore, as shown in Fig. S9, Ni@NC-600 demonstrates a significant capacity for CO2 adsorption, even at the elevated temperature of 400 °C. This phenomenon can be explained by the electron-withdrawing inductive effect of pyridinic–N, which substantially alters the electron density of its neighboring carbon atoms.37 This result confirms that the pyridinic–N in Ni@NC-600 results in the formation of superbasic sites on the NC layers.41 However, for Ni@NC-700, only a small CO2 desorption peak at 195 °C is observed, which may be induced by physical adsorption of CO2. Although the CO2 adsorbed on the sample prepared at 700 °C desorbs at 195 °C, the desorbed CO2 can still react with hydrogen on the catalyst surface due to the confined nature of the reactor. Therefore, this sample retains a certain level of methanation activity even when the photothermal temperature reaches 400 °C. However, the CH4 production rate remains low (0.06 mmol g−1 h−1), which is attributed to the low concentration of released CO2 in the gas phase. This observation further confirms the important role of strong CO2 adsorption in enhancing its conversion performance. Compared to the other two samples, Ni@NC-600 displays a much stronger CO2 desorption peak, suggesting that Ni@NC-600 has a much higher CO2 adsorption capacity. These results suggest that the pyridinic–N species promote the generation of solid superbasic sites,42 which characteristically adsorb and activate CO2, especially for low concentrations of CO2.
As shown in Fig. 2E, the Ni@NC-X (X = 500, 600 and 700) samples exhibit broad light absorption from 200 to 2500 nm, implying their potential for high solar energy utilization efficiency. This characteristic is essential for converting absorbed solar energy to simultaneously stimulate photochemical and thermochemical reactions. As depicted in Fig. 2F, the temperatures of the catalysts (measured in situ with a thermal imaging camera) increase rapidly and reach the equilibrium temperature (∼400 °C) within the first minute under 300 W Xe lamp irradiation. To gain further insight into the role of carbon coating, finite-difference time-domain (FDTD) simulations (Fig. S10) were performed. The results reveal that the NC-encapsulated structure of Ni@NC-600 triples the electric field intensity compared to that of the bare metallic Ni nanoparticles under resonant photon excitation. This substantial enhancement of the localized surface plasmon resonance (LSPR) intensity significantly promotes the plasmon–photon coupling effect of the metallic nanoparticles,43 thereby efficiently converting light into heat to locally elevate the catalyst's temperature for photothermal CO2 conversion (Fig. S11). Considering the ability of Ni@NC-600 to capture trace CO2 (Fig. 2B), we carried out solar-driven CO2 conversion by using atmospheric-level CO2 (0.04%) as the feed gas in the next experiment.
Direct capture and conversion of atmospheric CO2 on Ni@NC-600
Ni@NC-600 demonstrates exceptional CO2 capture performance, achieving an uptake capacity of 55 cm3 g−1 at 25 °C and 0.4 mbar (Fig. 2B) with pronounced selectivity for CO2 over O2/N2 in ambient air (CO2/N2 selectivity = 41; CO2/O2 selectivity = 36; Fig. S12). This dual functionality prompted its application as an integrated capture-conversion catalyst in a batch reactor equipped with a top quartz window for light irradiation (Fig. S13). Under Xe lamp irradiation, the catalyst surface temperature was stabilized at approximately 400 °C across the entire sample area. This uniform temperature profile confirms the spatial homogeneity of the incident light (Fig. S14).Initially, the CO2 conversion performance of Ni@NC-600 was evaluated under irradiation intensities ranging from 1 to 21 suns. The temperatures of the Ni@NC-600 sample increase from 25.6 °C (the ambient temperature) to maximum values between 93.3 and 419.8 °C within 1 minute, depending on the used Xe lamp with different intensities (Fig. S15). As shown in Fig. S16, the highest CH4 formation rate was achieved at 18 suns (399.6 °C), and similar activity is exhibited when the irradiation intensity is further increased to 21 suns (419.8 °C). This result proves that the photothermal effect of the Xe lamp play a vital role on the CO2 reduction on Ni@NC-600. Under a reaction temperature of approximately 400 °C, the adsorbed CO2 is effectively and nearly completely converted, and further increasing the temperature beyond this point does not improve the conversion but instead decreases the overall energy efficiency. Moreover, the selectivity toward CH4 remained nearly unchanged (close to 100%) across all irradiation intensities (Fig. S16). Therefore, from an energy-efficiency perspective, an intensity of 18 suns was selected for all subsequent CO2 conversion experiments.
Under light illumination, the maximum CH4 formation (∼100% selectivity) on Ni@NC-600 in a H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ar gas mixture (4
Ar gas mixture (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
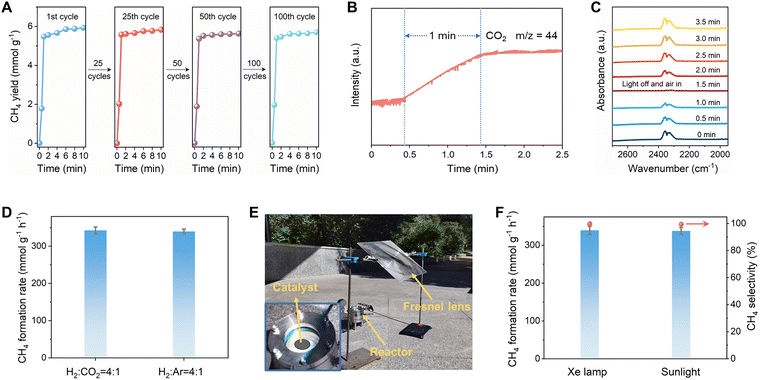
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) reaches 5.6 mmol g−1 in 1 min (Fig. 3A). Notably, this high activity was maintained even under humid conditions of 50% and 75% RH (Fig. S17). Moreover, O 1s XPS analysis revealed no detectable changes in the oxygen species following CO2 capture-conversion across varying humidity levels, further confirming the stability of the Ni@NC-600 surface (Fig. S18). The superior performance of this photothermal catalyst surpasses that of many previously reported photocatalytic systems for CO2 methanation around 400 °C (Table S2). As shown in Fig. 3A, no decrease in CH4 generation was observed after 100 CO2 capture-convention cycles, demonstrating the unprecedented stability for the atmospheric CO2 utilization systems based on Ni@NC-600. Moreover, XRD, SEM, TEM, and XPS analysis revealed negligible changes in the Ni@NC-600 sample after 100 cycles (Fig. S19). Online gas chromatography (GC) analysis (Fig. S20) and an isotope labelling experiment (Fig. S21) verified the high selectivity of CH4, further confirming the stability of Ni@NC-600. The excellent stability of Ni@NC-600 can be attributed to the existence of NC layers that can suppress the sintering of Ni NPs.
1 ratio) reaches 5.6 mmol g−1 in 1 min (Fig. 3A). Notably, this high activity was maintained even under humid conditions of 50% and 75% RH (Fig. S17). Moreover, O 1s XPS analysis revealed no detectable changes in the oxygen species following CO2 capture-conversion across varying humidity levels, further confirming the stability of the Ni@NC-600 surface (Fig. S18). The superior performance of this photothermal catalyst surpasses that of many previously reported photocatalytic systems for CO2 methanation around 400 °C (Table S2). As shown in Fig. 3A, no decrease in CH4 generation was observed after 100 CO2 capture-convention cycles, demonstrating the unprecedented stability for the atmospheric CO2 utilization systems based on Ni@NC-600. Moreover, XRD, SEM, TEM, and XPS analysis revealed negligible changes in the Ni@NC-600 sample after 100 cycles (Fig. S19). Online gas chromatography (GC) analysis (Fig. S20) and an isotope labelling experiment (Fig. S21) verified the high selectivity of CH4, further confirming the stability of Ni@NC-600. The excellent stability of Ni@NC-600 can be attributed to the existence of NC layers that can suppress the sintering of Ni NPs.
Remarkably, the Ni@NC-600 catalyst achieves CO2 adsorption–desorption equilibrium within 1 minute after the exhaustion of the captured CO2 (Fig. 3B), surpassing conventional benchmarks by 30–300 times in equilibration kinetics (Table S3). The kinetics for CO2 capture on Ni@NC-600 was also studied by in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) spectroscopy. As shown in Fig. 3C, the intensity of the CO2 absorption peak gradually decreases under light irradiation, and then disappears after 1 min reaction. Upon dark cycling, rapid *CO2 signal recovery occurs within 1 min of air exposure, which is in accordance with the result shown in Fig. 3B.
As shown in Fig. 3D, the Ni@NC-600 catalyst demonstrates an exceptional ability to convert atmospheric CO2. In batch reactor tests, it achieved a high CH4 production rate of 339 mmol g−1 h−1 using CO2 captured directly from air, a value nearly identical to the 342 mmol g−1 h−1 obtained with high-purity CO2 (Fig. 3D). This parity confirms that Ni@NC-600 has the remarkable ability to effectively capture and convert highly diluted CO2 from the atmosphere. Furthermore, Ni@NC-600 exhibits significantly higher CH4 production activity compared to Ni@NC-500 (0.13 mmol g−1 h−1) and Ni@NC-700 (0.06 mmol g−1 h−1) under identical experimental conditions, which may be attributed to the superior atmospheric CO2 adsorption capacity of Ni@NC-600 (Fig. 2B). Strikingly, conventional solar-driven catalysts for atmospheric-concentration CO2 reduction typically exhibit activity levels in the micromolar range (µmol g−1 h−1; Table S4). The CO2-enriched Ni@NC-600 system therefore achieves a catalytic enhancement of approximately 100–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times higher than that achieved using traditional approaches. These results establish that Ni@NC-600 enables efficient atmospheric CO2 conversion at ultralow concentrations (∼0.04%). The integrated CO2 capture-conversion approach circumvents the need for transportation and storage infrastructure, bypasses the energy-intensive regeneration of the capture media, and avoids molecular CO2 release steps, thereby significantly enhancing the energy efficiency of atmospheric CO2 conversion processes.
000 times higher than that achieved using traditional approaches. These results establish that Ni@NC-600 enables efficient atmospheric CO2 conversion at ultralow concentrations (∼0.04%). The integrated CO2 capture-conversion approach circumvents the need for transportation and storage infrastructure, bypasses the energy-intensive regeneration of the capture media, and avoids molecular CO2 release steps, thereby significantly enhancing the energy efficiency of atmospheric CO2 conversion processes.
The ultimate goal of artificial photosynthesis is to harness natural solar energy as the exclusive driving force. Given the remarkable performance of the Ni@NC-600 catalyst for light-driven CH4 production, the catalytic CO2 methanation reaction was evaluated under natural sunlight utilizing a Fresnel lens as concentrator. The experiment was carried out on May 6th, 2023 from 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 to 12
30 to 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 under natural sunlight in Harbin, China (Fig. 3E). Under these conditions, the temperature of the Ni@NC-600 catalyst rapidly increased from ambient condition (29 °C) to 402.2 °C upon continuous irradiation for 1 min (Fig. S22), achieving a remarkable CH4 production rate of 338.1 mmol g−1 h−1 under concentrated sunlight, comparable to Xe lamp-driven conditions (Fig. 3F). As depicted in Fig. S23, outdoor solar-driven photoreforming maintained 100% CH4 selectivity throughout the day. To evaluate the practical application of Ni@NC-600, the device based on this catalyst for the large-scale methanation of CO2 in air is designed and depicted in Fig. S24. Based on this device, we calculated the surface area required to process 1 kg of CO2. This calculation was based on a cyclic procedure comprising CO2 adsorption (1 min), N2 purging (3 min), and photocatalytic conversion under illumination (1 min). Under these conditions, the catalyst exhibited a CO2 capture capacity of 55 cm3 g−1 and achieved a high CO2-to-CH4 conversion efficiency of 96%. When normalized to 8 hours of daily operation, the required catalyst area for methanation of 1 kg atmospheric CO2 is calculated to be approximately 2.83 m2. This performance starkly contrasts with that obtained through natural photosynthesis. For instance, the most productive terrestrial ecosystems, such as tropical forests, exhibit a maximum carbon fixation rate of about 2200 g C m−2 year−1, equivalent to ∼8074 g CO2 m−2 year−1.44 On an annualized and per-unit-area basis, our catalyst demonstrates a CO2 fixation rate that is approximately 16 times greater. Furthermore, a critical advantage of our technology lies in the product outcome: it selectively transforms captured CO2 into readily usable CH4 fuel, whereas natural photosynthesis produces recalcitrant biomass that is challenging to utilize efficiently.
30 under natural sunlight in Harbin, China (Fig. 3E). Under these conditions, the temperature of the Ni@NC-600 catalyst rapidly increased from ambient condition (29 °C) to 402.2 °C upon continuous irradiation for 1 min (Fig. S22), achieving a remarkable CH4 production rate of 338.1 mmol g−1 h−1 under concentrated sunlight, comparable to Xe lamp-driven conditions (Fig. 3F). As depicted in Fig. S23, outdoor solar-driven photoreforming maintained 100% CH4 selectivity throughout the day. To evaluate the practical application of Ni@NC-600, the device based on this catalyst for the large-scale methanation of CO2 in air is designed and depicted in Fig. S24. Based on this device, we calculated the surface area required to process 1 kg of CO2. This calculation was based on a cyclic procedure comprising CO2 adsorption (1 min), N2 purging (3 min), and photocatalytic conversion under illumination (1 min). Under these conditions, the catalyst exhibited a CO2 capture capacity of 55 cm3 g−1 and achieved a high CO2-to-CH4 conversion efficiency of 96%. When normalized to 8 hours of daily operation, the required catalyst area for methanation of 1 kg atmospheric CO2 is calculated to be approximately 2.83 m2. This performance starkly contrasts with that obtained through natural photosynthesis. For instance, the most productive terrestrial ecosystems, such as tropical forests, exhibit a maximum carbon fixation rate of about 2200 g C m−2 year−1, equivalent to ∼8074 g CO2 m−2 year−1.44 On an annualized and per-unit-area basis, our catalyst demonstrates a CO2 fixation rate that is approximately 16 times greater. Furthermore, a critical advantage of our technology lies in the product outcome: it selectively transforms captured CO2 into readily usable CH4 fuel, whereas natural photosynthesis produces recalcitrant biomass that is challenging to utilize efficiently.
The photochemical–photothermal effect on Ni@NC-600 for atmospheric CO2 methanation
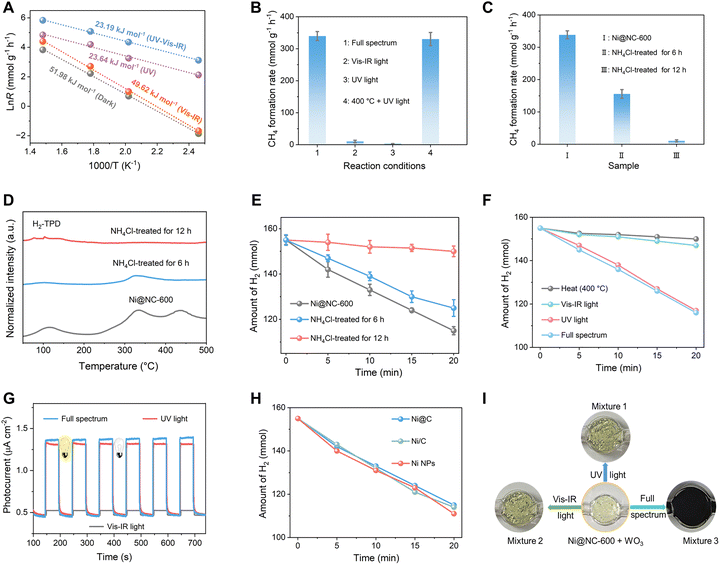
To investigate the electron transfer pathway on Ni@NC-600 under light irradiation, quasi in situ XPS measurements were conducted to examine the charge transfer between the Ni core and carbon shell in Ni@NC-600. Under light irradiation, the Ni 2p spectra show a positive binding energy shift of roughly 0.42 eV (Fig. S25a), and the pyridinic–N peak in the N 1s spectra shows a negative shift of about 0.41 eV (Fig. S25b). These shifts confirm that photogenerated electrons are transferred from the Ni core to the NC surface, which is beneficial for CO2 photoreduction. Furthermore, the prolonged fluorescence lifetime of Ni@NC-600 (28.2 ns), as shown by the time-resolved PL spectra in Fig. S26, is notably longer than the lifetime observed for Ni/C (14.6 ns), which endows photogenerated carriers with adequate time to migrate to the NC surface and participate in the photocatalytic CO2 reduction.To study the effect of light irradiation, we calculated the Ea of Ni@NC-600 for CO2 methanation using the Arrhenius equation (Fig. 4A) based on the CH4 formation rates and surface temperatures (Ts) obtained with light irradiation and/or electrical heating. As shown in Fig. 4A, the Ea calculated under full-spectrum irradiation with an intensity of 1.8 W cm−2 is 23.19 kJ mol−1, which is significantly lower than that in the dark (51.98 kJ mol−1, grey line in Fig. 4A). This finding reveals that CO2 methanation is kinetically favorable under irradiation, which is a key factor for the greater increase in the reaction rate compared to that of thermocatalysis on Ni@NC-600. This result also proves that the photoelectric (nonthermal) effect plays a critical role in CO2 methanation over Ni@NC-600, indicating the distinct advantage of light-driven catalysis over thermocatalysis.
To clarify the contribution of light to CH4 formation kinetics, the Ea values in the ultraviolet (UV) and visible-infrared (vis-IR) ranges at a fixed intensity of the full-spectrum (1.8 W cm−2) were measured.29 As shown in Fig. 4A, the Ea under vis-IR light was 49.62 kJ mol−1 (orange line), which is similar to that under thermocatalysis (51.98 kJ mol−1, gray line). In sharp contrast, UV light illumination causes a reduction in Ea to 23.64 kJ mol−1 (purple line), which is similar to that under full-spectrum irradiation (23.19 kJ mol−1, blue line). The wavelength dependence of Ea indicates that UV and vis-IR light illumination are responsible for electronic and thermal excitation, respectively, in the CO2 methanation reaction on Ni@NC-600.29 This result is also proved by the comparable CO2 conversion activities of Ni@NC-600 under full-spectrum illumination and UV light + 400 °C (electrical heating) conditions (Fig. 4B). Furthermore, the CH4 formation rate on Ni@NC-600 driven by UV or vis-IR light is much lower than that driven by full-spectrum irradiation (Fig. 4B), implying that the CO2 reduction kinetics over Ni@NC-600 is determined primarily by the synergistic effect of photochemical and photothermal processes.
Generally, CO2 activation is considered as the rate-determining step in the methanation reaction.45 Considering the Ea values in different conditions (Fig. 4A), we can speculate that photogenerated electrons under UV light play a critical role in activating CO2. In addition to CO2 adsorption/activation, H2 activation is a key factor determining the CO2 methanation performance. In the CO2 methanation reaction, Ni NPs are usually regarded as the active sites for dissociating H2 molecules (H2 → 2H*).46–48 To investigate the role of Ni NPs in CO2 methanation, the Ni@NC-600 catalyst was treated with ammonium chloride (NH4Cl) at 350 °C for 6 h and 12 h to gradually remove the embedded Ni NPs.49 ICP analysis confirmed that the Ni content in the Ni@NC-600 catalyst was 8.8 wt% and 0.27 wt% after treatment times of 6 h and 12 h, respectively (Fig. S27). Under the same conditions, the light-powered CO2 reduction performance of Ni@NC-600 was approximately 2.2 and 33.6 times higher than that of the catalysts treated with NH4Cl for 6 h and 12 h, respectively (Fig. 4C). This confirms the crucial role of the Ni content in CO2 methanation. Combined H2-TPD and decomposition analyses of Ni@NC-600 and the NH4Cl-treated catalysts (Fig. 4D and E) reveal that the H2 activation step on the catalyst is governed by its Ni content, a conclusion consistent with the observed methanation performance in Fig. 4C. XRD, SEM, TEM, and XPS analyses revealed that the NH4Cl treatment selectively removed the encapsulated Ni NPs while preserving the outer NC layers (Fig. S28), a finding consistent with prior reports that this etching process does not alter the porous structure of the carbon support.49 Under this constant pore-size condition, the H2 dissociation activity showed a direct correlation with the amount of remaining Ni NPs (Fig. 4E), thus playing a decisive role in the CO2 methanation performance of Ni@NC-600 (Fig. 4C).
The wavelength-dependent H2 decomposition abilities of Ni@NC-600 suggest that the H2 dissociation on Ni sites is driven mainly by UV light illumination, whereas vis-IR light has a negligible effect (Fig. 4F). This result is in good agreement with the wavelength-dependent photocurrent measurement of Ni@NC-600 (Fig. 4G). Furthermore, as shown in Fig. 4H, the hydrogen dissociation activities of Ni nanoparticles (Ni NPs, Fig. S29), Ni@C (Ni NPs encapsulated in carbon, Fig. S29), and Ni/C (Ni NPs loaded on carbon support, Fig. S30) with an average size of approximately 10 nm are independent of the carbon support under Xe lamp irradiation. This finding indicates that hydrogen dissociation is governed by the Ni NPs themselves, not by their loading configuration or the support material. Therefore, to isolate the size effect of Ni on CO2 methanation, we investigated the correlation between the size of bare Ni NPs and their H2 dissociation activity. Ni nanoparticles with mean sizes of 5.6, 10.2, and 20.8 nm were synthesized via the acid–base-mediated alcohol reduction method (Fig. S31).50 Ni NPs with a middle size of approximately 10.2 nm exhibit optimal activity for H2 dissociation (Fig. S32). Smaller Ni NPs are rich in low-coordinated edge/corner sites that facilitate H2 adsorption, while larger NPs offer more high-coordinated terrace sites that are active for H–H bond cleavage.51 Hence, Ni NPs of intermediate size (10.2 nm) achieve peak activity by providing a balanced ensemble of sites for both essential steps—adsorption and dissociation.
Dissociated H atoms transfer from the Ni core to the NC shell to induce the CO2 methanation reaction because CO2 molecules are adsorbed mainly on the NC shell of Ni@NC-600. The diffusion of H atoms can be improved by the photothermal heating effect of vis-IR light, which was proven by the adoption of a WO3 chromogenic agent. As shown in Fig. 4I, the colours of Mixtures 1 and 2 were nearly unchanged under only UV or vis-IR light irradiation, suggesting that H spill over did not occur under those conditions. Considering that the UV light of the Xe-lamp dominates the H2 dissociation on Ni NPs (Fig. 4F), the unchanged colour of Mixture 1 indicates that the H* spill over can be hardly induced with UV light input alone. The unchanged colour of Mixture 2 is understandable because H2 cannot be decomposed on Ni@NC-600 under vis-IR light irradiation (Fig. 4F). In sharp contrast, the colour of Mixture 3 under full spectrum irradiation turns dark, indicating that the vis-IR light-induced heat input can drive the diffusion of the dissociated H* species by UV light to react with WO3 to form HxWO3. Based on the observations in Fig. 4I, we can conclude that the photothermal effect of vis-IR light helps the dissociated H* species on Ni cores to spill toward the NC shell on Ni@NC-600 for reducing the adsorbed CO2. This result is consistent with the fact that the CO2 methanation on Ni@NC-600 was induced by the synergistic effect of photochemical and photothermal processes. Therefore, the enhanced kinetics was achieved on Ni@NC-600 under combined thermal and electronic excitation. Furthermore, the isolated active sites limit the coadsorption of the H2 and CO2 reactants, which also boosts the overall kinetics of solar-driven CH4 formation on Ni@NC-600.
The roles of hydrophobicity NC layers on the stability of CO2 methanation
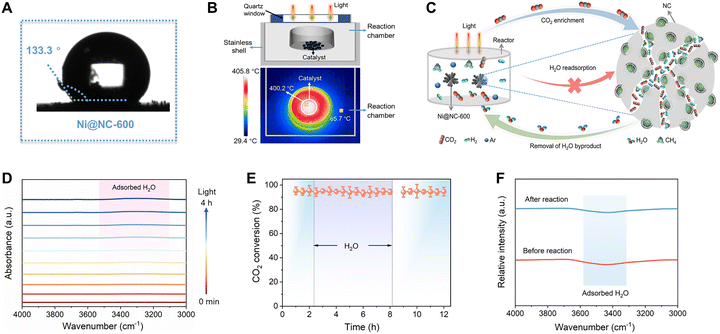
Ni@NC-600 has excellent hydrophobicity with a contact angle of 133.3° (Fig. 5A), which might be caused by its high surface roughness (Fig. 1B).52 Hydrophobicity is conducive to preventing direct contact with water and protecting catalysts from corrosion because H2O severely affects the deactivation of Ni and other metal catalysts for hydrogenation reactions by changing the surface structure of the catalyst or blocking active sites.53–57The infrared image clearly shows that under Xe lamp irradiation (1.8 W cm−2), the temperature on the surface of Ni@NC-600 reached ∼400 °C due to the localized photothermal effect, while the temperature of the reaction chamber was only 65 °C (Fig. 5B). This large spatial temperature difference (335 °C) provides a strong driving force for pumping water byproducts out of the nanopores and promoting its removal from the Ni@NC-600 surface (denoted as *H2O ⇌ * + H2O), which facilitates the concentration and localization of the produced H2O in the low-temperature zone of the reactor (Fig. 5B). Unfortunately, this type of temperature gradient cannot be induced in traditional thermocatalytic systems with a uniform heat distribution. Moreover, the hydrophobic surface helps prevent the readsorption of H2O on Ni@NC-600 and recover surface active sites (Fig. 5C).55 As a result of the combined effects of local photothermal heating and hydrophobicity, the local H2O concentration on the Ni@NC-600 surface should be significantly decreased. The Ni@NC-600 samples before and after 4 h of photoreaction (Fig. 5D) show similar FT-IR spectra at 4000–3000 cm−1 (belong to –OH groups) after the CO2 methanation reaction, indicating that the produced water can be readily removed once it is formed on the catalyst.
To further understand this mechanism, we performed a solar-powered CO2 methanation reaction on Ni@NC-600 in a flow reactor with a volume of 100 mL by adding water at a rate of 0.01 mL min−1, which is approximately 22 times greater than the rate of water formation in the CO2 methanation reaction if all the CO2 is converted into CH4. During the 12-hour test, no decline in CH4 formation was detected compared with that in the control experiment without the addition of H2O (Fig. 5E). After this reaction, no obvious changes in the shape of the FT-IR spectrum of Ni@NC-600 were observed in the wavenumber range of 4000–3000 cm−1 (Fig. 5F). These results indicate that the removed H2O byproducts can be readily confined to the low-temperature zone of the reactor and cannot reach the catalyst surface; therefore, the local H2O concentration on Ni@NC-600 can be greatly reduced during the solar-driven CO2 methanation reaction.
The mechanism for solar-driven catalytic CO2 methanation on Ni@NC-600
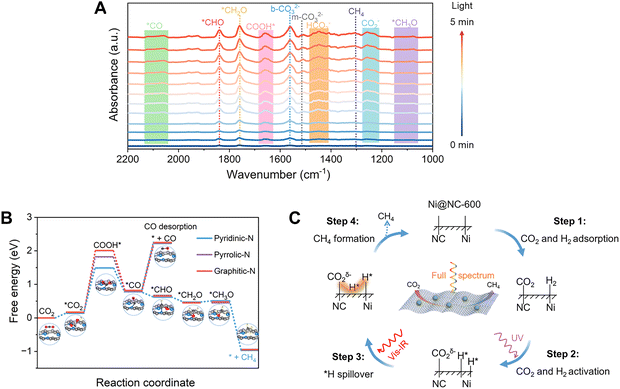
To study the catalytic mechanism, in situ DRIFTS measurement was employed to monitor the formed intermediates on Ni@NC-600 during atmospheric-level CO2 methanation powered by solar light. As shown in Fig. 6A, several new infrared peaks were detected under light irradiation. The peaks around 1253, 1446 and 1509 and 1562 cm−1 are attributed to the stretching of active *CO2−, *HCO3−, m-CO32− (monodentate carbonate), and b-CO32− (bidentate carbonate),54 respectively, indicating that CO2 was efficiently absorbed and activated on the surface of Ni@NC-600. The peaks at 1649 cm−1 and 2101 cm−1 can be ascribed to the formation of the COOH* and *CO species,58–61 respectively. Therefore, we speculate that CO* was formed by the dehydration of COOH* due to the fact that no peaks belonging to the formate species were observed in Fig. 5B. Furthermore, the peaks at 1838, 1760, and 1098 cm−1 correspond to the formation of the *CHO, *CH2O, and *CH3O species, respectively.59,61 Based on the in situ DRIFTS test, we propose that the solar-driven CO2 methanation on Ni@NC-600 was achieved by a stepwise hydrogenation process.Next, we carried out density functional theory (DFT) calculations of the CO2 methanation on Ni@NC-600 based on the in situ DRIFTS results. As shown in Fig. 6B, the conversion of CO2 to COOH* is the rate-determining step of the CO2 methanation on the catalysts. The formation of COOH* on pyridinic–N of Ni@NC-600 had a free-energy barrier of 1.35 eV, which is much lower than that on pyrrolic–N of Ni@NC-500 (1.66 eV) and graphitic–N of Ni@NC-700 (1.84 eV). This result indicates that the types of nitrogen in the NC layers primarily influence the rate-determining step of the CO2 methanation. The DFT study is in good agreement with the experimental observation on the CO2 adsorption and methanation properties of the three catalysts. Moreover, the energy barriers of *CO hydrogenation on the three catalysts are exothermic (Fig. 6B), which are much lower than that for CO desorption (*CO → * + CO). This phenomenon indicates that *CO is energetically favourable to hydrogenation, further supporting the catalyst's selectivity for CH4. Based on this mechanistic insight, further product selectivity modulation can be achieved by precisely tuning the chemisorption energy of crucial reaction intermediates. Specifically, a weak adsorption energy of *CO will favour CO product formation.62 Based on the above discussions, the proposed pathway for CO2 methanation on Ni@NC-600 is illustrated in Fig. 6C.
Conclusions
In conclusion, we developed a Ni@NC-600 core–shell catalyst via the pyrolysis of a Ni-MOF precursor for light-driven methanation of atmospheric CO2. The Ni core is mainly responsible for H2 dissociation when excited by UV light under a Xe lamp. The dissociated H2 spills to the NC shell, driven by light-induced heating, and enables methanation of the adsorbed CO2via the coupled photo/photothermal effect. In this process, the isolated active sites limit the coadsorption of the H2 and CO2 reactants, and the NC shell plays four critical roles. First, the mesoporous structure and super-basicity of NC allow easy adsorption of the diluted CO2 in the nanopores of Ni@NC-600. Second, the local photothermal heating effect and the hydrophobic properties of NC facilitate the removal of H2O byproducts in the CO2 methanation reaction. Third, the photothermal heating effect of NC energizes the active sites to boost CH4 production with the help of photogenerated electrons from Ni, which reduce the Ea of the CO2 methanation reaction. Fourth, the hydrophobic NC shell not only inhibit the sintering of the Ni catalyst but also prevent it from reacting with H2O byproducts during the methanation reaction. As a result, a 339 mmol g−1 h−1 CH4 production rate with ∼100% CH4 selectivity is achieved on Ni@NC-600 under light irradiation for over 100 capture-convention cycles, representing a promising approach for producing truly carbon-neutral fuels.Author contributions
Y. L. and W. M. conceived the project and designed the experiments. S. G., W. M., and H. Y. synthesized the catalysts and conducted the structure analysis and photocatalytic CO2 reduction tests. H. Y. performed the TPD measurements. J. Z. performed the theoretical calculations. J. P., J. S., and Y. L. co-wrote the paper. All authors commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article, titled “Superbase CO2-concentrating layers protected nickel catalyst for solar CH4 synthesis via direct air capture,” have been included as part of the supplementary information (SI). The supplementary information file includes detailed experimental procedures, catalysts characterization, catalytic performance, density functional theory (DFT) calculations and catalytic comparison tables. See DOI: https://doi.org/10.1039/d5ee06482a.Acknowledgements
This work is jointly supported by the National Natural Science Foundation of China (22372050), the Fundamental Research Funds for the Central Universities (HIT.OCEF.2023012) and the Doctor Research Start Foundation of Shaanxi University of Technology (SLGRCQD038).Notes and references
- I. Sullivan, A. Goryachev, I. A. Digdaya, X. Li, H. A. Atwater, D. A. Vermaas and C. Xiang, Nat. Catal., 2021, 4, 952–958 CrossRef.
- W. Ma, J. Sun, S. Yao, Y. Wang, G. Chen, G. Fan and Y. Li, Angew. Chem., Int. Ed., 2023, 62, e202313784 CrossRef.
- B. Parkinson, ACS Energy Lett., 2016, 1, 1057–1059 CrossRef.
- S. Kar, D. Kim, A. Bin Mohamad Annuar, B. B. Sarma, M. Stanton, E. Lam, S. Bhattacharjee, S. Karak, H. F. Greer and E. Reisner, Nat. Energy, 2025, 10, 448–459 CrossRef CAS PubMed.
- J. Ding, P. Du, P. Li, W. Liu, J. Xu, W. Yan, Y. Pan, J. Hu, J. Zhu, Q. Chen, X. Jiao and Y. Xie, Angew. Chem., Int. Ed., 2025, 64, e202414453 CrossRef CAS PubMed.
- P. Du, J. Ding, C. Liu, P. Li, W. Liu, W. Yan, Y. Pan, J. Hu, J. Zhu, X. Li, Q. Chen and X. Jiao, Angew. Chem., Int. Ed., 2025, 64, e202421353 CrossRef CAS PubMed.
- Y. Ma, X. Yi, S. Wang, T. Li, B. Tan, C. Chen, T. Majima, E. R. Waclawik, H. Zhu and J. Wang, Nat. Commun., 2022, 13, 1400 CrossRef CAS PubMed.
- Y. Wei, J. Li, D. Zhao, Y. Zhao, Q. Zhang, L. Gu, J. Wan and D. Wang, CCS Chem., 2024, 6, 3065–3076 CrossRef CAS.
- X. Wu, Y. Li, G. Zhang, H. Chen, J. Li, K. Wang, Y. Pan, Y. Zhao, Y. Sun and Y. Xie, J. Am. Chem. Soc., 2019, 141, 5267–5274 Search PubMed.
- F. Wang, S. He, H. Chen, B. Wang, L. Zheng, M. Wei, D. G. Evans and X. Duan, J. Am. Chem. Soc., 2016, 138, 6298–6305 CrossRef CAS PubMed.
- M. Ghoussoub, M. Xia, P. N. Duchesne, D. Segal and G. Ozin, Energy Environ. Sci., 2019, 12, 1122–1142 Search PubMed.
- W. Gao, Y. Li, D. Xiao and D. Ma, J. Energy Chem., 2023, 83, 62–78 Search PubMed.
- F. E. Osterloh, ACS Energy Lett., 2016, 1, 1060–1061 CrossRef CAS.
- R. Schäppi, D. Rutz, F. Dähler, A. Muroyama, P. Haueter, J. Lilliestam, A. Patt, P. Furler and A. Steinfeld, Nature, 2022, 601, 63–68 Search PubMed.
- D. Sengupta, S. Bose, X. Wang, N. M. Schweitzer, C. D. Malliakas, H. Xie, J. Duncan, K. O. Kirlikovali, T. Yildirim and O. K. Farha, J. Am. Chem. Soc., 2024, 146, 27006–27013 CrossRef CAS PubMed.
- Y. Wang, J.-X. Wei, H.-L. Tang, L.-H. Shao, L.-Z. Dong, X.-Y. Chu, Y.-X. Jiang, G.-L. Zhang, F.-M. Zhang and Y.-Q. Lan, Nat. Commun., 2024, 15, 8818 Search PubMed.
- Y. Tian, R. Wang, S. Deng, Y. Tao, W. Dai, Q. Zheng, C. Huang, C. Xie, Q. Zeng, J. Lin and H. Chen, Nano Lett., 2023, 23, 10914–10921 Search PubMed.
- Y. Yang, H.-Y. Zhang, Y. Wang, L.-H. Shao, L. Fang, H. Dong, M. Lu, L.-Z. Dong, Y.-Q. Lan and F.-M. Zhang, Adv. Mater., 2023, 35, 2304170 CrossRef CAS.
- H. Jia, X. Feng, X. Du, L. Lin, R. Mu and Q. Fu, Angew. Chem., Int. Ed., 2025, 64, e202503319 Search PubMed.
- X. Zhu, H. Zong, C. J. V. Pérez, H. Miao, W. Sun, Z. Yuan, S. Wang, G. Zeng, H. Xu, Z. Jiang and G. A. Ozin, Angew. Chem., Int. Ed., 2023, 62, e202218694 CrossRef CAS PubMed.
- J. Ma, J. Yu, G. Chen, Y. Bai, S. Liu, Y. Hu, M. Al-Mamun, Y. Wang, W. Gong, D. Liu, Y. Li, R. Long, H. Zhao and Y. Xiong, Adv. Mater., 2023, 35, 2302537 CrossRef CAS PubMed.
- Y. Li, X. Bai, D. Yuan, C. Yu, X. San, Y. Guo, L. Zhang and J. Ye, Nat. Commun., 2023, 14, 3171 CrossRef CAS PubMed.
- H. Zhang, T. Wang, J. Wang, H. Liu, T. D. Dao, M. Li, G. Liu, X. Meng, K. Chang, L. Shi, T. Nagao and J. Ye, Adv. Mater., 2016, 28, 3703–3710 CrossRef CAS.
- Y. Tang, T. Zhao, H. Han, Z. Yang, J. Liu, X. Wen and F. Wang, Adv. Sci., 2023, 10, 2300122 CrossRef CAS PubMed.
- Z. Chen, X. Dong, Z.-X. Sun, X. An, C. Li, S. Liu, J. Shen, C. Wu, J. Wang, Z. Wang, Z. Zhu, Y. Zhou, K. Yu, Y. Ma, J. He, K. Feng, L. He and Z. Hu, ACS Nano, 2024, 18, 19672–19681 CAS.
- Y. Chen, Y. Zhang, G. Fan, L. Song, G. Jia, H. Huang, S. Ouyang, J. Ye, Z. Li and Z. Zou, Joule, 2021, 5, 3235–3251 CrossRef CAS.
- H. Jiang, L. Wang, H. Kaneko, R. Gu, G. Su, L. Li, J. Zhang, H. Song, F. Zhu, A. Yamaguchi, J. Xu, F. Liu, M. Miyauchi, W. Ding and M. Zhong, Nat. Catal., 2023, 6, 519–530 CrossRef CAS.
- K. Feng, S. Wang, D. Zhang, L. Wang, Y. Yu, K. Feng, Z. Li, Z. Zhu, C. Li, M. Cai, Z. Wu, N. Kong, B. Yan, J. Zhong, X. Zhang, G. A. Ozin and L. He, Adv. Mater., 2020, 32, 2000014 CrossRef CAS PubMed.
- L. Zhou, D. F. Swearer, C. Zhang, H. Robatjazi, H. Zhao, L. Henderson, L. Dong, P. Christopher, E. A. Carter, P. Nordlander and N. J. Halas, Science, 2018, 362, 69–72 CrossRef CAS PubMed.
- L. Kang, X. Y. Liu, A. Wang, L. Li, Y. Ren, X. Li, X. Pan, Y. Li, X. Zong, H. Liu, A. I. Frenkel and T. Zhang, Angew. Chem., Int. Ed., 2020, 59, 12909–12916 CrossRef CAS PubMed.
- I. S. Khan, D. Mateo, G. Shterk, T. Shoinkhorova, D. Poloneeva, L. Garzón-Tovar and J. Gascon, Angew. Chem., Int. Ed., 2021, 60, 26476–26482 CrossRef CAS PubMed.
- M. Mukoyoshi, H. Kobayashi, K. Kusada, M. Hayashi, T. Yamada, M. Maesato, J. M. Taylor, Y. Kubota, K. Kato, M. Takata, T. Yamamoto, S. Matsumura and H. Kitagawa, Chem. Commun., 2015, 51, 12463–12466 RSC.
- X. Lin, S. Wang, W. Tu, H. Wang, Y. Hou, W. Dai and R. Xu, ACS Appl. Energy Mater., 2019, 2, 7670–7678 CrossRef CAS.
- M. Zhang, Y.-G. Wang, W. Chen, J. Dong, L. Zheng, J. Luo, J. Wan, S. Tian, W.-C. Cheong, D. Wang and Y. Li, J. Am. Chem. Soc., 2017, 139, 10976–10979 CrossRef CAS PubMed.
- A. Aijaz, N. Fujiwara and Q. Xu, J. Am. Chem. Soc., 2014, 136, 6790–6793 CrossRef CAS PubMed.
- Z. Zhang, L. Yu, Y. Tu, R. Chen, L. Wu, J. Zhu and D. Deng, Cell Rep. Phys. Sci., 2020, 1, 100145 CrossRef CAS.
- D. Guo, R. Shibuya, C. Akiba, S. Saji, T. Kondo and J. Nakamura, Science, 2016, 351, 361–365 CrossRef CAS PubMed.
- T. Muthusamy, S. Sethuram Markandaraj and S. Shanmugam, J. Mater. Chem. A, 2022, 10, 6470–6474 RSC.
- Y. Dong, Q. Zhang, Z. Tian, B. Li, W. Yan, S. Wang, K. Jiang, J. Su, C. W. Oloman, E. L. Gyenge, R. Ge, Z. Lu, X. Ji and L. Chen, Adv. Mater., 2020, 32, 2001300 CrossRef CAS PubMed.
- Q. Lu, C. Chen, Q. Di, W. Liu, X. Sun, Y. Tuo, Y. Zhou, Y. Pan, X. Feng, L. Li, D. Chen and J. Zhang, ACS Catal., 2022, 12, 1364–1374 Search PubMed.
- L. B. Sun, J. Yang, J. H. Kou, F. N. Gu, Y. Chun, Y. Wang, J. H. Zhu and Z. G. Zou, Angew. Chem., Int. Ed., 2008, 47, 3418–3421 CrossRef CAS PubMed.
- B. Li, X. Sun and D. Su, Phys. Chem. Chem. Phys., 2015, 17, 6691–6694 Search PubMed.
- J. Ma, X. Mao, C. Hu, X. Wang, W. Gong, D. Liu, R. Long, A. Du, H. Zhao and Y. Xiong, J. Am. Chem. Soc., 2024, 146, 970–978 CrossRef CAS PubMed.
- F. Stuart Chapin, P. A. Matson and P. M. Vitousek, Principles of Terrestrial Ecosystem Ecology, Springer Science & Business Media, 2011, pp. 157–181 Search PubMed.
- C. Vogt, M. Monai, G. J. Kramer and B. M. Weckhuysen, Nat. Catal., 2019, 2, 188–197 Search PubMed.
- L. Ma, R. Ye, Y. Huang, T. R. Reina, X. Wang, C. Li, X. L. Zhang, M. Fan, R. Zhang and J. Liu, Chem. Eng. J., 2022, 446, 137031 Search PubMed.
- P. A. U. Aldana, F. Ocampo, K. Kobl, B. Louis, F. Thibault-Starzyk, M. Daturi, P. Bazin, S. Thomas and A. C. Roger, Catal. Today, 2013, 215, 201–207 CrossRef CAS.
- V. Golovanova, M. C. Spadaro, J. Arbiol, V. Golovanov, T. T. Rantala, T. Andreu and J. R. Morante, Appl. Catal., B, 2021, 291, 120038 CrossRef CAS.
- M.-X. Chen, M. Zhu, M. Zuo, S.-Q. Chu, J. Zhang, Y. Wu, H.-W. Liang and X. Feng, Angew. Chem., Int. Ed., 2020, 59, 1627–1633 Search PubMed.
- C. Dong, C. Lian, S. Hu, Z. Deng, J. Gong, M. Li, H. Liu, M. Xing and J. Zhang, Nat. Commun., 2018, 9, 1252 CrossRef PubMed.
- Z. Zou, Y. Shen, C. Chen, W. Li, Y. Zhang, H. Zhang, Z. Yu, H. Zhao and G. Wang, Adv. Funct. Mater., 2025, 35, 2417584 Search PubMed.
- Z. Zhao, L. Duan, Y. Zhao, L. Wang, J. Zhang, F. Bu, Z. Sun, T. Zhang, M. Liu, H. Chen, Y. Yang, K. Lan, Z. Lv, L. Zu, P. Zhang, R. Che, Y. Tang, D. Chao, W. Li and D. Zhao, J. Am. Chem. Soc., 2022, 144, 11767–11777 Search PubMed.
- L. C. Buelens, V. V. Galvita, H. Poelman, C. Detavernier and G. B. Marin, Science, 2016, 354, 449–452 Search PubMed.
- D. Mateo, N. Morlanes, P. Maity, G. Shterk, O. F. Mohammed and J. Gascon, Adv. Funct. Mater., 2021, 31, 2008244 Search PubMed.
- W. Fang, C. Wang, Z. Liu, L. Wang, L. Liu, H. Li, S. Xu, A. Zheng, X. Qin, L. Liu and F.-S. Xiao, Science, 2022, 377, 406–410 Search PubMed.
- M. Wolf, N. Fischer and M. Claeys, Nat. Catal., 2020, 3, 962–965 CrossRef CAS.
- H. Li, C. Qiu, S. Ren, Q. Dong, S. Zhang, F. Zhou, X. Liang, J. Wang, S. Li and M. Yu, Science, 2020, 367, 667–671 Search PubMed.
- X. Zu, Y. Zhao, X. Li, R. Chen, W. Shao, Z. Wang, J. Hu, J. Zhu, Y. Pan, Y. Sun and Y. Xie, Angew. Chem., Int. Ed., 2021, 60, 13840–13846 CrossRef CAS.
- W. Zhang, C. Deng, W. Wang, H. Sheng and J. Zhao, Adv. Mater., 2024, 36, 2405825 CrossRef CAS.
- L. Ran, Z. Li, B. Ran, J. Cao, Y. Zhao, T. Shao, Y. Song, M. K. H. Leung, L. Sun and J. Hou, J. Am. Chem. Soc., 2022, 144, 17097–17109 CrossRef CAS PubMed.
- S. Karmakar, S. Barman, F. A. Rahimi, D. Rambabu, S. Nath and T. K. Maji, Nat. Commun., 2023, 14, 4508 CrossRef CAS PubMed.
- K. Lin, P. Qiao, Q. Liu, Y. He, X. Kang, D. Wang, C. Tian, A. Wu and H. Fu, Adv. Funct. Mater., 2025, e15276 CrossRef.
| This journal is © The Royal Society of Chemistry 2026 |