Probing the proton exchange kinetics of BaZr0.1Ce0.7Y0.1Yb0.1O3−δ ceramic electrolyte by operando diffuse reflectance infrared Fourier transform spectroscopy
Yuqing
Meng
a,
Fan
Liu
*a,
Meng
Li
 a,
Zixian
Wang
ab,
Hao
Deng
ab,
Qian
Zhang
a,
Haixia
Li
a,
Wanhua
Wang
a,
Quanwen
Sun
a,
Joshua
Gomez
a,
Zeyu
Zhao
a,
Zixian
Wang
ab,
Hao
Deng
ab,
Qian
Zhang
a,
Haixia
Li
a,
Wanhua
Wang
a,
Quanwen
Sun
a,
Joshua
Gomez
a,
Zeyu
Zhao
 a,
Haiyan
Zhao
*c and
Dong
Ding
a,
Haiyan
Zhao
*c and
Dong
Ding
 *a
*a
aEnergy & Environmental Science and Technology, Idaho National Laboratory, Idaho Falls, Idaho 83415, USA. E-mail: fan.liu@inl.gov; dong.ding@inl.gov
bTim Taylor Department of Chemical Engineering, Kansas State University, Manhattan, KS 66506, USA
cChemical and Biological Engineering Department, The Critical Materials and Energy Systems Innovation Center (CMESIC), University of Idaho, Idaho Falls, ID 83401, USA. E-mail: haiyanz@uidaho.edu
First published on 12th December 2025
Abstract
Proton exchange kinetics plays an important role in governing the performance of intermediate-temperature protonic ceramic electrolysis cells (PCECs) for hydrogen production. Our understanding of the nature of the surface hydration reaction at the single-cell level, however, remains very limited, hampering further efficiency improvements. Here, we developed a custom operando diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) platform that operates under high temperature and steam conditions with applied bias. Quantitative investigations of surface H2O/D2O isotope exchange in a BaZr0.1Ce0.7Y0.1Yb0.1O3−δ (BZCYYb1711) protonic electrolyte-based single cell were conducted under different applied voltages using this DRIFTS platform, to gain molecular-level insight into hydration kinetics. The findings show that the application of an external voltage significantly enhances the surface proton exchange rate, decreasing the apparent activation energy from 29.1 kJ mol−1 at open-circuit voltage (OCV) to 6.8 kJ mol−1 at 1.3 V. In addition, distinct voltage-induced spectral shifts in O–D vibrations point to dynamic changes in surface hydration. These findings demonstrate a sensitive spectroscopic platform for probing interfacial proton processes and reveal strong electrochemical control over surface proton kinetics, offering new opportunities for probing electrolyte hydration behavior in PCECs.
Broader contextProtonic ceramic electrolysis cells (PCECs) are a promising technology for efficient hydrogen production at intermediate temperatures. A critical bottleneck lies in the hydration processes, which govern the concentration and mobility of protons and directly influence protonic conductivity and Faradaic efficiency. While recent studies have advanced materials and device performance, the fundamental understanding of surface hydration dynamics under realistic single-cell operation remains very limited, hampering further efficiency improvements. Protonic conduction mechanisms inferred from previous studies, mainly on electrolytes alone, may not accurately represent device conditions, leaving local proton dynamics largely unresolved. Thus, gaining direct insight into hydration and exchanging kinetics under practical cell operation is highly desirable. In this work, we developed a custom operando DRIFTS platform capable of operating under high temperature and steam conditions with applied bias. The platform also incorporated H2O/D2O isotope exchange for molecular-level insight into the hydration exchange kinetics. Our findings demonstrated that applied bias significantly accelerates surface hydration, providing direct evidence of how the electrical field impacts surface hydration reactions at the single-cell level. This discovery may have a profound impact on electrocatalysis and the use of PCECs in various energy-related applications. |
Intermediate-temperature protonic ceramic electrolysis cells (PCECs), employing a protonic conductor as the electrolyte, represent a promising technology for efficient hydrogen production through steam electrolysis.1–5 Currently, the acceptor-doped barium zirconates, typically formulated as BaZr1−xMxO3−δ (M = trivalent cation, 0 < x < 1), have been intensively investigated as the electrolyte because of their higher protonic conductivity, good sinterability and chemical stability.6,7 Proton carriers in these materials are introduced through a thermally activated hydration reaction, wherein water molecules react with oxygen vacancies to form hydroxide defects:
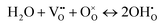 .8,9 At elevated temperatures, the electrolyte can facilitate proton diffusion through the hopping mechanism between neighboring oxygen ions in the presence of hydroxide defects
.8,9 At elevated temperatures, the electrolyte can facilitate proton diffusion through the hopping mechanism between neighboring oxygen ions in the presence of hydroxide defects 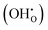 .10 In other words, the protons are not stuck to any particular oxygens but are rather free to move from one oxygen to another, resulting in high proton mobility. Hydration in PCECs plays a central role in determining the concentration of mobile protons directly influencing proton conductivity and Faradaic efficiency.11 While recent studies have demonstrated promising PCEC development, most have focused on materials development, fabrication, and device-level metrics with less attention to the fundamental hydration processes.12–19 The limited understanding of surface hydration dynamics in practical single-cell operation, however, has hampered improvement of the electrolysis performance.
.10 In other words, the protons are not stuck to any particular oxygens but are rather free to move from one oxygen to another, resulting in high proton mobility. Hydration in PCECs plays a central role in determining the concentration of mobile protons directly influencing proton conductivity and Faradaic efficiency.11 While recent studies have demonstrated promising PCEC development, most have focused on materials development, fabrication, and device-level metrics with less attention to the fundamental hydration processes.12–19 The limited understanding of surface hydration dynamics in practical single-cell operation, however, has hampered improvement of the electrolysis performance.
Proton conduction in ceramic oxides typically involves two coupled elementary steps: (i) proton transfer via hydrogen bonding between adjacent oxygens, and (ii) rotational reorientation of hydroxyl groups.20 The rates of these processes are highly sensitive to the local bonding environment.21 Stronger hydrogen bonds may facilitate proton transfer but hinder OH group reorientation, introducing a trade-off that modulates macroscopic transport properties. Meanwhile, diverse surface hydration conduction phenomena were discussed over ceramic oxides in previous studies. For instance, by leveraging thermal desorption spectroscopy, Miyoshi et al.22 proposed that hydroxyl-terminated nanoparticle surfaces formed hydrogen bonds with water molecules, creating water-retaining nanochannels that enabled surface protonic conduction. Norby et al.23 applied impedance spectra to simulate the equivalent circuit to separate the volume and surface protonic transportation. However, these protonic conduction mechanisms based on the electrolyte alone may not be accurately represented or adopted at the single-cell device level due to significantly different working conditions, leaving the local proton dynamics largely unresolved. Therefore, gaining direct insight into surface hydration and exchange kinetics under applied electrochemical bias in PCEC operation mode is highly desirable for the rational design of next-generation hydrogen production systems.24,25
In situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) is especially effective for detecting surface intermediate species under actual working conditions (i.e., operando).26–29 While it has been widely used in room or low-temperature electrochemical systems, its application under intermediate-temperature conditions, particularly in the presence of steam and electrochemical bias, remains limited.30–32 This gap is particularly significant for the investigation into the PCECs given that they are an emerging technology for hydrogen production.33–35 For example, how surface hydration responds to electrochemical potential and how surface dynamics evolve under operando conditions have not been observed experimentally thus far. Therefore, coupling DRIFTS with electrochemical control and isotope labeling (e.g., H2O/D2O exchange) can enable real-time, molecular-level insight into surface reaction pathways and hydration kinetics in the protonic electrolyte of PCECs, unveiling key pieces of the puzzle. However, designing a high-temperature chamber that supports such electrochemical testing under humidified conditions while maintaining gas sealing, and thermal/electrical insulation is nontrivial, and requires extensive design effort and customized modification of standard testing fixtures.
To address the aforementioned challenges, we developed a custom operando DRIFTS platform tailored for intermediate-temperature PCECs. As shown in Fig. 1 and Fig. S1, the ceramic half-cell consisted of a thin, dense, single-phase BaZr0.1Ce0.7Y0.1Yb0.1O3−δ (BZCYYb1711) electrolyte layer supported on a porous Ni–BZCYYb1711 hydrogen electrode that was prepared for operando DRIFTS experiments (Fig. S2 and S3). The cell was mounted inside the custom-designed high-temperature reaction chamber, with the electrolyte surface exposed to humidified air and aligned with the incident IR beam for direct spectral acquisition. The bottom hydrogen electrode was exposed to flowing dry hydrogen gas, thereby reproducing asymmetric gas conditions relevant to practical steam electrolysis operation. Silver paste was applied to both surfaces to serve as current collectors.
This platform enables time-resolved DRIFTS detection of hydration behavior under controlled temperature, distinct gas environments, and simultaneous electrochemical operation (Fig. 1b). By employing H2O/D2O isotope exchange, we monitored the interconversion between O–H and O–D vibrational modes and carried out quantitative kinetic analysis, providing direct insight into surface hydration exchange kinetics.36,37 This integrated approach not only elucidates how electrochemical potential modulates interfacial proton exchange but also establishes a quantitative framework linking local surface chemistry with macroscopic electrochemical performance in PCECs. To the best of our knowledge, this is the first operando DRIFTS study to probe the hydration dynamics of a proton-conducting ceramic electrolyte operating in the single-device mode.
To validate the origin of the surface hydroxyl signals, DRIFTS were first collected under dry air at 600 °C. As shown in Fig. S4, the BZCYYb1711 electrolyte exhibits a weak yet distinct –OH stretching band centered around ∼3379 cm−1,38 even in the absence of externally introduced steam. No corresponding signal is observed from the ceramic sealing material (Fig. S5), confirming that the hydroxyl features originate intrinsically from the BZCYYb1711 surface.30 While most previous studies have suggested that steam is required to initiate hydration, our results provide direct evidence that intrinsic hydration can occur without externally supplied H2O. This observation underscores the electrolyte's strong hydration affinity, even under nominally dry conditions.
Time-resolved isotope exchange experiments were subsequently conducted by switching the feed gas from 3% H2O to 3% D2O in humidified air under open-circuit conditions. As shown in Fig. 2a and Fig. S6, the –OH band (∼3379 cm−1) gradually decreases in intensity, while a corresponding –OD band (∼2500 cm−1) simultaneously emerges and intensifies.39 The absence of intermediate HDO features suggests a direct, one-to-one replacement of surface protons with deuterons.40,41 Minor spectral features attributed to molecular H2O (∼1650 cm−1 and ∼3700 cm−1) and CO2 (∼2370 cm−1) are also present but do not interfere with the analysis.42 It is notified that even after long-time equilibrium, a small residual –OH band remains, indicating that a fraction of strongly bonded hydroxyl species persist in the subsurface that are less accessible to D2O molecules.43 The normalized intensities of the –OH and –OD signals intersect at approximately 0.5, marking the crossover point. This crossover occurs more rapidly at elevated temperatures, indicating thermally activated surface exchange kinetics (Fig. 2b and Fig. S7). For example, such equilibrium is reached within 5 min at 600 °C but requires more than 25 min at 400 °C. To quantify the exchange kinetics, the natural logarithm of the normalized –OH intensity [ln(Rel. OH)] was plotted as a function of time (Fig. 2c).36 The resulting linear trends demonstrate a first-order kinetic process, in which the exchange rate is directly proportional to the surface –OH concentration. We also conducted time-resolved isotope exchange experiments by switching the feed gas from 3% H2O to 3% D2O while monitoring the decay of the –OH band, and vice versa by tracking the decay of the –OD band, to determine the surface isotope exchange rate constants under each condition. As illustrated in Fig. 2d, the extracted rate constants were k (OH) = 0.1294 min−1 and k (OD) = 0.0914 min−1, yielding a kinetic isotope ratio of 1.41, closely matching the theoretical value of 1.4 based on the mass difference between H and D, i.e., (mD/mH)1/2.36 This agreement supports a proton hopping mechanism consistent with Grotthuss-like transport under open-circuit conditions.22,44
While open-circuit measurements provide fundamental insight into proton exchange, practical PCECs operate under applied voltage or current. To investigate how electrochemical bias affects surface hydration kinetics, operando DRIFTS isotope exchange experiments were conducted under varying applied potentials (Fig. 3). As shown in Fig. 3a, isotope exchange at 600 °C proceeds significantly faster under 1.3 V compared to OCV conditions, indicating accelerated surface proton exchange. This voltage-enhanced behavior persists across the full temperature range studied (Fig. S8). At 400 °C, the time to reach 90% isotopic equilibration is reduced by ∼73%, and even at 600 °C, a ∼19% reduction is observed (Fig. 3b and Fig. S9). This substantial improvement highlights the critical role of electrochemical potential in promoting surface proton exchange beyond thermal activation alone. Additionally, increasing the potential led to a distinct redshift and broadening of the –OD stretching band, along with the appearance of a low-wavenumber shoulder near ∼2450 cm−1 (Fig. 3c), indicating a weakening of the –OD bond strength.22,36,45 It was reported that the changes of hydrogen-bond interactions could promote reorientation of surface hydroxyl groups, thereby facilitating proton (or deuteron) transfer along the surface.21 Correspondingly, the surface exchange rate constant (k) increased monotonically with the applied voltage, reaching a maximum at 1.3 V (Fig. 3d). Beyond this potential, no further enhancement was observed, indicating a transition from voltage-limited to diffusion- or site-limited proton exchange, where proton availability might become the dominant constraint.
The activation energy (Ea) for proton exchange in PCECs is a critical descriptor that governs reaction kinetics and overall electrochemical performance. As shown in Fig. 4a, the apparent Ea under OCV is 29.1 kJ mol−1. In stark contrast, application of a 1.3 V bias reduces Ea to just 6.8 kJ mol−1, which represents a direct experimental quantification enabled by operando DRIFTS of voltage-induced enhancement in proton exchange dynamics. As summarized in Table 1, most previously reported isotope-exchange measurements were conducted at lower temperatures, whereas the current work demonstrates the feasibility of performing kinetic measurements at elevated temperatures (up to 600 °C). The surface Ea obtained under OCV in this work is comparable to values from in situ Raman methods, while its difference from the bulk conductivity method indicates the surface sensitivity of the operando DRIFTS. It should be noted that the nearly fourfold decrease in the Ea under 1.3 V highlights the significant role of electrochemical potential in accelerating surface hydration kinetics, providing new insights into practical PCEC operation enabled by such operando characterization. A mechanistic illustration is proposed in Fig. 4b to explain this behavior. Under OCV, surface proton exchange proceeds via a thermally activated Grotthuss-type mechanism, in which proton transfer is limited by local hydrogen bonding strength and OH group orientation. Upon applying a bias, the electric field induces charge redistribution at the surface, which in turn lowers the energy barrier for proton reorientation, thereby promoting faster proton exchange.
| Materials | Atmosphere | Tools | k (min−1) | E a (kJ mol−1) | Ref. |
|---|---|---|---|---|---|
| BaZr0.1Ce0.7Y0.1Yb0.1O3−δ | 3% D2O | Operando DRIFTS | 0.1311 (600 °C); | 29.1 | This work |
| 0.1384 (600 °C and 1.3 V) | 6.8 (1.3 V) | ||||
| BaZr0.1Ce0.7Y0.1Yb0.1O3−δ | 3% D2O | In situ Raman | 0.0137 (300 °C) | 21.0 | 46 |
| SrCe0.95Yb0.05O3−δ | P H2O = 0.0004 Pa in vacuum | Elastic recoil detection | 0.0084 (25 °C) | N/A | 43 |
| P D2O = 0.0004 Pa in vacuum | 0.00084 (25 °C) | ||||
| SrZr0.9Sc0.1O3−δ | Saturated D2O | Conductivity measurement | N/A | 59.8 | 47 |
| Saturated H2O | 56.9 | ||||
| BaZr0.1Ce0.9Y0.1O3−δ | 1.5% H2O | Conductivity measurement | N/A | 56.9 | 48 |
| 1.5% D2O | N/A | 60.8 | |||
| SrCe0.95Yb0.05O2.975 | ∼7% H2O | Conductivity measurement | N/A | 74 | 49 |
This operando DRIFTS study provides direct evidence that applied bias accelerates surface proton exchange in PCECs, while more in-depth kinetics and the proton conduction mechanism need further investigation. Looking ahead, the effects of ceramic structure such as grain size, and steam concentration on hydration reactions are not yet well understood. Another key step will be to couple these surface hydration dynamics with bulk proton conductivity to understand the transport pathways across the electrolyte. In addition, combining operando DRIFTS with artificial intelligence (AI) offers a promising way to predict interfacial behavior under diverse conditions. Beyond the electrolyte, this operando DRIFTS platform can also be extended to probe intermediate species in triple-conducting steam electrodes and electrocatalysts, paving the way for more efficient and durable systems for hydrogen production and beyond.
Author contributions
Y. M.: conceptualization, data curation, formal analysis, investigation, methodology and writing – original draft; F. L.: conceptualization, formal analysis, investigation, methodology, supervision, writing – original draft and writing – review and editing; M. L.: methodology, writing – original draft and writing – review and editing; Z. W.: methodology, visualization, writing – original draft and writing – review and editing; H. D.: methodology; Q. Z.: methodology; H. L.: methodology; W. W.: methodology; Q. S.: methodology; J. G.: methodology; Z. Z.: methodology; H. Z.: conceptualization, investigation, methodology, supervision and writing – review and editing; D. D.: conceptualization, funding acquisition, investigation, methodology, project administration, resources, supervision, writing – original draft, and writing – review and editing.Conflicts of interest
The authors declare no competing financial interest.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5ee05957g.
Acknowledgements
This work is supported by the HydroGEN Advanced Water Splitting Materials Consortium, established as part of the Energy Materials Network under the U.S. Department of Energy (USDOE); the Office of Energy Efficiency and Renewable Energy (EERE); the Hydrogen and Fuel Cell Technologies Office (HFTO) under DOE Idaho Operations Office under contract no. DE-AC07-05ID14517.References
- F. Liu, H. Deng, D. Diercks, P. Kumar, M. H. A. Jabbar, C. Gumeci, Y. Furuya, N. Dale, T. Oku, M. Usuda, P. Kazempoor, L. Fang, D. Chen, B. Liu and C. Duan, Lowering the operating temperature of protonic ceramic electrochemical cells to <450 °C, Nat. Energy, 2023, 8(10), 1145–1157 CrossRef CAS.
- W. Bian, W. Wu, B. Wang, W. Tang, M. Zhou, C. Jin, H. Ding, W. Fan, Y. Dong, J. Li and D. Ding, Revitalizing interface in protonic ceramic cells by acid etch, Nature, 2022, 604(7906), 479–485 CrossRef CAS PubMed.
- C. Duan, J. Huang, N. Sullivan and R. O'Hayre, Proton-conducting oxides for energy conversion and storage, Appl. Phys. Rev., 2020, 7(1), 011314 CAS.
- Y. Wang, Y. Ling, B. Wang, G. Zhai, G. Yang, Z. Shao, R. Xiao and T. Li, A review of progress in proton ceramic electrochemical cells: material and structural design, coupled with value-added chemical production, Energy Environ. Sci., 2023, 16(12), 5721–5770 RSC.
- M. Li, F. Liu and D. Ding, Critical insights into the steam electrolysis electrode in protonic ceramic cells for hydrogen production, Nat. Catal., 2025, 8(4), 293–300 CrossRef CAS.
- I. Jang, J. S. A. Carneiro, J. O. Crawford, Y. J. Cho, S. Parvin, D. A. Gonzalez-Casamachin, J. Baltrusaitis, R. P. Lively and E. Nikolla, Electrocatalysis in Solid Oxide Fuel Cells and Electrolyzers, Chem. Rev., 2024, 124(13), 8233–8306 CrossRef CAS PubMed.
- W. Zhang, M. Liu, X. Gu, Y. Shi, Z. Deng and N. Cai, Water Electrolysis toward Elevated Temperature: Advances, Challenges and Frontiers, Chem. Rev., 2023, 123(11), 7119–7192 CrossRef CAS.
- K. D. Kreuer, Proton-Conducting Oxides, Annu. Rev. Mater. Res., 2003, 33, 333–359 CrossRef CAS.
- A. S. Nowick and Y. Du, High-temperature protonic conductors with perovskite-related structures, Solid State Ionics, 1995, 77, 137–146 CrossRef CAS.
- C. Zhou, X. Wang, D. Liu, M. Fei, J. Dai, D. Guan, Z. Hu, L. Zhang, Y. Wang, W. Wang, R. O'Hayre, S. P. Jiang, W. Zhou, M. Liu and Z. Shao, New Strategy for Boosting Cathodic Performance of Protonic Ceramic Fuel Cells Through Incorporating a Superior Hydronation Second Phase, Energy Environ. Mater., 2024, 7(4), e12660 CrossRef CAS.
- C. Duan, R. Kee, H. Zhu, N. Sullivan, L. Zhu, L. Bian, D. Jennings and R. O’Hayre, Highly efficient reversible protonic ceramic electrochemical cells for power generation and fuel production, Nat. Energy, 2019, 4(3), 230–240 CrossRef CAS.
- H. Tian, W. Li, Y.-L. Lee, H. Zheng, Q. Li, L. Ma, D. Bhattacharyya, X. Chen, D. Zhang, G. Li, Y. Wang, L. Li, Q. Wang, F. Xia, M. Kartal, Z. Shao, M. R. Rowles, W. Li, W. A. Saidi, C. Liu, X. Li, J. Luo, X. Li, K. He and X. Liu, Conformally coated scaffold design using water-tolerant Pr1.8Ba0.2NiO4.1 for protonic ceramic electrochemical cells with 5000-h electrolysis stability, Nat. Energy, 2025, 10(7), 890–903 CrossRef CAS.
- H. Ding, W. Wu, C. Jiang, Y. Ding, W. Bian, B. Hu, P. Singh, C. J. Orme, L. Wang, Y. Zhang and D. Ding, Self-sustainable protonic ceramic electrochemical cells using a triple conducting electrode for hydrogen and power production, Nat. Commun., 2020, 11(1), 1907 CrossRef CAS PubMed.
- F. Liu, D. Diercks, P. Kumar, A. Seong, M. H. A. Jabbar, C. Gumeci, Y. Furuya, N. Dale, T. Oku, M. Usuda, P. Kazempoor, I. Ghamarian, L. Liu, L. Fang, D. Chen, Z. Wang, S. Skinner and C. Duan, Redesigning protonic ceramic electrochemical cells to lower the operating temperature, Sci. Adv., 2025, 11(2), eadq2507 CrossRef CAS PubMed.
- H. Yu, I. Jeong, S. Jang, D. Kim, H.-N. Im, C.-W. Lee, E. D. Wachsman and K. T. Lee, Lowering the Temperature of Solid Oxide Electrochemical Cells Using Triple-Doped Bismuth Oxides, Adv. Mater., 2024, 36(5), 2306205 CrossRef CAS PubMed.
- M. Papac, V. Stevanović, A. Zakutayev and R. O’Hayre, Triple ionic–electronic conducting oxides for next-generation electrochemical devices, Nat. Mater., 2021, 20(3), 301–313 CrossRef CAS PubMed.
- W. Zhang, Q. Zhang, P. Zhu, Y. Meng, Z. Zhao, W. Wang, Y. Ding, Q. Sun, Y. Zhang, M. Li, H. Deng, B. Liu, W. Wu and D. Ding, An Active Oxygen Electrode for Proton-Conducting Solid Oxide Electrolysis Cells with High Faradaic Efficiency, Adv. Energy Mater., 2025, 15(27), 2500852 CrossRef CAS.
- W. Tang, W. Bian, H. Ding, Y. Ding, Z. Zhao, Q. Sun, S. Koomson, Y. Wang, B. Xu, P. Dong, D. Chen, J. Y. Gomez, W. Feng, W. Wu, M. Zhou, Y. Dong, H. Luo, J. Li and D. Ding, Sintering protonic zirconate cells with enhanced electrolysis stability and Faradaic efficiency, Nat. Synth., 2025, 4(5), 592–602 CrossRef CAS.
- D. Ding, X. Li, S. Y. Lai, K. Gerdes and M. Liu, Enhancing SOFC cathode performance by surface modification through infiltration, Energy Environ. Sci., 2014, 7(2), 552–575 RSC.
- A. S. Nowick and A. V. Vaysleyb, Isotope effect and proton hopping in high-temperature protonic conductors, Solid State Ionics, 1997, 97(1), 17–26 CrossRef CAS.
- W. Münch, G. Seifert, K. D. Kreuer and J. Maier, A quantum molecular dynamics study of proton conduction phenomena in BaCeO3, Solid State Ionics, 1996, 86–88, 647–652 CrossRef.
- S. Miyoshi, Y. Akao, N. Kuwata, J. Kawamura, Y. Oyama, T. Yagi and S. Yamaguchi, Low-Temperature Protonic Conduction Based on Surface Protonics: An Example of Nanostructured Yttria-Doped Zirconia, Chem. Mater., 2014, 26(18), 5194–5200 CrossRef CAS.
- S. Ø. Stub, E. Vøllestad and T. Norby, Protonic surface conduction controlled by space charge of intersecting grain boundaries in porous ceramics, J. Mater. Chem. A, 2018, 6(18), 8265–8270 RSC.
- T. Norby, Proton Conduction in Solids: Bulk and Interfaces, MRS Bull, 2009, 34(12), 923–928 CrossRef CAS.
- J. Gu, L. Jiang, S. A. Ismail, H. Guo and D. Han, Surface Protonic Conduction on Oxide Ceramics: Mechanism, Materials, and Method for Characterization, Adv. Mater. Interfaces, 2023, 10(1), 2201764 CrossRef CAS.
- Q. Fan, C. Pu and E. S. Smotkin, In Situ Fourier Transform Infrared-Diffuse Reflection Spectroscopy of Direct Methanol Fuel Cell Anodes and Cathodes, J. Electrochem. Soc., 1996, 143(10), 3053 CrossRef.
- F. Liu, H. Deng, H. Ding, P. Kazempoor, B. Liu and C. Duan, Process-intensified protonic ceramic fuel cells for power generation, chemical production, and greenhouse gas mitigation, Joule, 2023, 7(6), 1308–1332 CrossRef CAS.
- F. Liu, H. Deng, Z. Wang, A. M. Hussain, N. Dale, Y. Furuya, Y. Miura, Y. Fukuyama, H. Ding, B. Liu and C. Duan, Synergistic Effects of In-Situ Exsolved Ni–Ru Bimetallic Catalyst on High-Performance and Durable Direct-Methane Solid Oxide Fuel Cells, J. Am. Chem. Soc., 2024, 146(7), 4704–4715 CrossRef CAS PubMed.
- M. Li, B. Hua, W. Wu, L.-C. Wang, Y. Ding, M. M. Welander, R. A. Walker and D. Ding., Activating nano-bulk interplays for sustainable ammonia electrosynthesis, Mater. Today, 2022, 60, 31–40 CrossRef CAS.
- Z. Wang, F. Liu, Y. Meng, W. Bian, H. Zhao, C. Duan, M. T. Benson, M. Li, B. Liu and D. Ding, A comprehensive review of diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) techniques in protonic ceramic cells (PCCs): Current status and future perspective, eScience, 2025, 5(5), 100437 CrossRef.
- C. Mu, C. Lv, X. Meng, J. Sun, Z. Tong and K. Huang, In Situ Characterization Techniques Applied in Photocatalysis: A Review, Adv. Mater. Interfaces, 2023, 10(3), 2201842 CrossRef.
- D. Li, R. Qiu, B. M. Moskowitz, Z. Jiang, H. Gu, Q. Wen, I. E. Wachs and M. Zhu, Nature of the Active Sites and Reaction Mechanism during Methanol Steam Reforming over Cu/ZnO: An Isotopic Modulated Excitation Diffuse Reflectance Infrared Fourier Transform Spectroscopy Study, J. Am. Chem. Soc., 2025, 147(27), 24040–24049 CrossRef CAS PubMed.
- H. Liu, M. Yu, X. Tong, Q. Wang and M. Chen, High Temperature Solid Oxide Electrolysis for Green Hydrogen Production, Chem. Rev., 2024, 124(18), 10509–10576 CrossRef CAS PubMed.
- M. Li, B. Hua, L.-C. Wang, J. D. Sugar, W. Wu, Y. Ding, J. Li and D. Ding, Switching of metal–oxygen hybridization for selective CO2 electrohydrogenation under mild temperature and pressure, Nat. Catal., 2021, 4(4), 274–283 CrossRef CAS.
- W. Wu, H. Ding, Y. Zhang, Y. Ding, P. Katiyar, P. K. Majumdar, T. He and D. Ding, 3D Self-Architectured Steam Electrode Enabled Efficient and Durable Hydrogen Production in a Proton-Conducting Solid Oxide Electrolysis Cell at Temperatures Lower Than 600 °C, Adv. Sci., 2018, 5(11), 1800360 CrossRef PubMed.
- J. Gao, Y. Meng, A. Benton, J. He, L. G. Jacobsohn, J. Tong and K. S. Brinkman, Insights into the Proton Transport Mechanism in TiO2 Simple Oxides by In Situ Raman Spectroscopy, ACS Appl. Mater. Interfaces, 2020, 12(34), 38012–38018 CrossRef CAS PubMed.
- M. Chen, K. Zhao, J. Li, G. Lin, D. Banham, L. Du and M. Chen, Separation of hydrogen isotopes using a protonic ceramic fuel cell, J. Mater. Chem. A, 2025, 13(11), 7687–7691 RSC.
- K. Arai, M. Saito, K. Suganami, M. Inada, K. Hayashi and T. Motohashi, Proton conductive behaviors of Ba(ZnxNb1−x)O3−δ−y(OH)2y studied by infrared spectroscopy, J. Solid State Chem., 2022, 308, 122913 CrossRef CAS.
- N. Bonanos, A. Huijser and F. W. Poulsen, H/D isotope effects in high temperature proton conductors, Solid State Ionics, 2015, 275, 9–13 CrossRef CAS.
- H. H. Shin and S. McIntosh, On the H2/D2 isotopic exchange rate of proton conducting barium cerates and zirconates, J. Mater. Chem. A, 2013, 1(26), 7639–7647 RSC.
- I. Litvak, Y. Anker and H. Cohen, On-line in situ determination of deuterium content in water via FTIR spectroscopy, RSC Adv., 2018, 8(50), 28472–28479 RSC.
- F. Azzolina-Jury and F. Thibault-Starzyk., Mechanism of Low Pressure Plasma-Assisted CO2 Hydrogenation Over Ni-USY by Microsecond Time-resolved FTIR Spectroscopy, Top. Catal., 2017, 60(19), 1709–1721 CrossRef CAS.
- E. Iizuka, T. Horikawa, B. Tsuchiya, K. Soda, K. Morita and H. Iwahara, Anomalous Difference between Replacements of D–H and H–D in Oxide Ceramics by Exposure to Air Containing H2O and D2O Vapors, Jpn. J. Appl. Phys., 2001, 40(5R), 3343 CrossRef CAS.
- N. Akbar, Q. Pang, Y. Lu, Y. Jing, M. Singh, J. Wang, B. Zhu, F. Wang and S. Yun, Synergistic proton conduction via Ca-vacancy coupled with Li+-bridge in Ca5(PO4)3OH, Commun. Mater., 2025, 6(1), 7 CrossRef CAS.
- M. Glerup, F. W. Poulsen and R. W. Berg, Vibrational spectroscopy on protons and deuterons in proton conducting perovskites, Solid State Ionics, 2002, 148(1), 83–92 CrossRef CAS.
- J. Gao, Y. Meng, J. H. Duffy and K. S. Brinkman, Low-Temperature Protonic Ceramic Fuel Cells through Interfacial Engineering of Nanocrystalline BaCe0.7Zr0.1Y0.1Yb0.1O3−δ Electrolytes, Adv. Energy Sustainability Res., 2021, 2(11), 2100098 CrossRef CAS.
- K. C. Liang, I. Y. Lee and A. S. Nowick, Protonic Conduction in Y-, Yb- and Sc-Doped SrZrO3 Ceramics, MRS Online Proc. Libr., 1992, 293(1), 355–360 CrossRef.
- S. Ricote, N. Bonanos and G. Caboche, Water vapour solubility and conductivity study of the proton conductor BaCe(0.9−x)ZrxY0.1O(3−δ), Solid State Ionics, 2009, 180(14), 990–997 CrossRef CAS.
- R. A. De Souza, J. A. Kilner and C. Jeynes, The application of secondary ion mass spectrometry (SIMS) to the study of high temperature proton conductors (HTPC), Solid State Ionics, 1997, 97(1), 409–419 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |




