High-voltage lithium-ion battery with a wide operating temperature range and fast-charging ability
Wen
Zhou
a,
Gaohong
Liu
a,
Yanbing
Mo
a,
Xiao
Zhu
a,
Kaiyue
Zhu
b and
Xiaoli
Dong
 *a
*a
aDepartment of Chemistry and Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Institute of New Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Fudan University, Shanghai 200433, China. E-mail: xldong@fudan.edu.cn
bState Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China
First published on 25th November 2025
Abstract
The slow desolvation process and low ionic conductivity of lithium-ion batteries (LIBs) pose significant challenges, severely limiting their performance in extreme environments. Herein, we propose a weakly solvating electrolyte system based on methyl difluoroacetate (MDFA) and isobutyronitrile (IBN) solvents to exert a synergistic effect, where the weak solvation affinity of MDFA facilitates a rapid desolvation process and the high-permittivity of IBN ensures a high ionic conductivity, setting a solid foundation for smooth ion transport under fast-charging and operations in a wide temperature range. The as-formulated electrolyte endows graphite (Gr) with a capacity of 192 mAh g−1 at 10C and achieves a high reversible capacity retention of 91.7% at −40 °C. Benefitting from the high-voltage endurance of both solvents and the electrolyte, the LiNi0.8Co0.1Mn0.1O2||Gr full cells can be stably operated at 4.5 V and display a high capacity retention of 58.7% at 10C. Moreover, the full cell exhibits an excellent reversible capacity of 101 mAh g−1 at −60 °C. Moreover, the practical 1.2 Ah pouch cell exhibits a capacity retention of 75.3% at 3C and 45.2% at −50 °C. Such an electrolyte design presents a promising strategy to develop electrolytes for LIBs that operate under harsh scenarios.
Broader contextThe expanding application of lithium-ion batteries (LIBs) in electronic devices and electric vehicles raises the demand for reliable performance across all-climate scenarios. However, this remains challenging due to the narrow liquidus range and low ionic conductivity of commercial carbonate electrolytes. After screening solvents with wide liquidus ranges, we combined a weak-solvating fluorinated carboxylate ester and a high-permittivity nitrile to jointly address the issues. In electrolytes, the slow desolvation of Li+ is a key factor inducing sluggish electrochemical kinetics during fast charging and low temperatures. Although solvents with weak solvating capability can facilitate faster ion desolvation to mitigate this issue, they trigger strong ion aggregation and sluggish ion transport. Incorporating high-permittivity co-solvents offers a compelling strategy to overcome this trade-off, enhancing ion dissociation and mobility while simultaneously weakening Li+–solvent interactions. Meanwhile, this tailored solvation environment not only enables efficient ion transport across a broad temperature range but also reshapes interfacial chemistry. Specifically, it promotes the formation of robust, anion-derived solid electrolyte interphases enriched in LiF and Li3N, which lower interfacial energy barriers and suppress parasitic reactions at elevated temperatures. By addressing both bulk transport and interfacial stability, this study enables batteries with high performance and provides a new paradigm for electrolyte design, offering a versatile pathway toward next-generation LIBs capable of reliable performance in extreme thermal environments. |
Introduction
Lithium-ion batteries (LIBs) have become indispensable in modern life, powering a wide array of devices from portable electronics to electric vehicles.1–4 However, the commonly used graphite (Gr) anode-based LIBs suffer from poor performance under extreme scenarios, limiting their practical applications in military and outer space, which are mainly restrained by the low intercalation potential of the Gr anode.5 With decreasing temperature, the polarization in LIBs increases, reducing the Li+ intercalation potential at the Gr anode.6,7 This even potentially leads to lithium precipitation on the Gr surface, causing a sharp decline in both the capacity and stability of batteries.8 Noteworthily, the sluggish electrochemical kinetics at subzero temperatures are primarily attributed to the impaired mass transport of Li+,9 slow charge transfer at the electrode/electrolyte interfaces,10 and the emergence of lithium plating,11–13 all of which are closely related to the properties of liquid electrolytes.14–16 Specifically, the performance degradation of LIBs due to electrolyte failure at low temperatures is manifested in three key aspects: reduced bulk ionic conductivity, severe interphase degradation, and slow lithium ion desolvation.17–20 At elevated temperatures, capacity degradation of batteries primarily arises from the parasitic reactions of electrolytes and the instability of interfacial films. Therefore, electrolyte engineering is vital to improve the wide-temperature performance of Gr anodes and Gr-based LIBs.21,22To satisfy ion transport and desolvation processes over a wide temperature range, electrolytes should exhibit weak solvation energy and high ionic conductivity in both the liquid electrolyte and solid electrolyte interphase (SEI) layer.23–25 Currently, commercial electrolytes predominantly utilize cyclic and linear carbonates as solvents.26–28 Cyclic carbonates, like ethylene carbonate (EC) and propylene carbonate (PC), feature high dielectric constants but suffer from high viscosity. Additionally, EC has a relatively high melting point, severely limiting its performance in low-temperature applications.29–31 On the contrary, linear carbonates, such as dimethyl carbonate (DMC) and ethyl methyl carbonate (EMC), are favored for their reduced viscosity, which enhances electrolyte conductivity.32–34 Nevertheless, their lower dielectric constants significantly impede ion migration rates, restricting the operation of LIBs with DMC or EMC electrolytes to extreme temperatures.35–37 To address these limitations, alternative solvents with moderate dielectric constants to dissociate the lithium salt, low freezing points, and weak solvation affinity should be explored.38,39 Compared to the commonly used carbonate solvents, fluorinated carboxylates, such as methyl difluoroacetate (MDFA), offer a promising option due to their low melting points and high boiling points. MDFA exists in a liquid state at temperatures spanning from −124 °C to 85 °C. The fluorinated group in MDFA exhibits strong electron-withdrawing properties, reducing electron density at the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond and weakening the interaction between the C
O bond and weakening the interaction between the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond and Li+, resulting in weak solvation capability. However, this weak solvation affinity gives rise to ion aggregation, which may negatively impact ionic conductivity and the overall electrochemical performance. Considering the limitations of single-solvent systems, researchers can customize electrolyte characteristics to meet specific requirements by combining mixed solvents with complementary properties.40,41 Mixed-solvent electrolytes offer the advantage of optimizing the balance between ionic conductivity and viscosity, while also enabling the modulation of solvation structure and interfacial properties.42 This approach has demonstrated great potential in enhancing the overall performance of LIBs, especially in harsh environments. Inspired by this, another class of solvents, nitriles, can be introduced to make a compensation owing to their high dielectric constant and broad liquid-phase range. Isobutyronitrile (IBN) stands out for its liquid state over a wide temperature range (−72 °C to 108 °C) and higher permittivity than MDFA (21 for IBN vs. 10.1 for MDFA), which can facilitate better salt dissociation and ensure higher ionic conductivity. In addition, IBN exhibits a low viscosity of 0.456 mPa s,18 outperforming common solvents, such as EMC (0.65 mPa s) and DMC (0.59 mPa s), thereby facilitating ion movement in solvents. However, the synergistic combination of MDFA and IBN and their effect on wide-temperature performance have not been investigated.
O bond and Li+, resulting in weak solvation capability. However, this weak solvation affinity gives rise to ion aggregation, which may negatively impact ionic conductivity and the overall electrochemical performance. Considering the limitations of single-solvent systems, researchers can customize electrolyte characteristics to meet specific requirements by combining mixed solvents with complementary properties.40,41 Mixed-solvent electrolytes offer the advantage of optimizing the balance between ionic conductivity and viscosity, while also enabling the modulation of solvation structure and interfacial properties.42 This approach has demonstrated great potential in enhancing the overall performance of LIBs, especially in harsh environments. Inspired by this, another class of solvents, nitriles, can be introduced to make a compensation owing to their high dielectric constant and broad liquid-phase range. Isobutyronitrile (IBN) stands out for its liquid state over a wide temperature range (−72 °C to 108 °C) and higher permittivity than MDFA (21 for IBN vs. 10.1 for MDFA), which can facilitate better salt dissociation and ensure higher ionic conductivity. In addition, IBN exhibits a low viscosity of 0.456 mPa s,18 outperforming common solvents, such as EMC (0.65 mPa s) and DMC (0.59 mPa s), thereby facilitating ion movement in solvents. However, the synergistic combination of MDFA and IBN and their effect on wide-temperature performance have not been investigated.
By integrating the unique strengths of MDFA and IBN, an electrolyte was herein designed with weak solvation ability while synergistically achieving high ionic conductivity over a wide temperature range. The optimized electrolyte can form an anion-dominated solvation structure and an inorganic-enriched interfacial film, which will significantly enhance fast-charging and wide-temperature performance. This electrolyte imparts the Gr anode with a specific capacity of 192 mAh g−1 at a high rate of 10C and an impressive capacity retention of 70% after 1000 cycles at 25 °C, indicating robust long-cycling stability. Furthermore, it retains a specific capacity of 334 mAh g−1 at 100 °C. Benefitting from the high oxidative stability, LiNi0.8Co0.1Mn0.1O2 (NCM811)||Gr full cells can be operated at a high voltage of 4.5 V and exhibit a high specific capacity of 101 mAh g−1 at −60 °C. The 1.2 Ah pouch cells display decent capacity retention of 75.3% and 51% at a high rate of 3C and 4C, respectively. The well-designed electrolytes operating under extreme conditions pave the way for broader applications of LIBs in wide-temperature environments.
Results and discussion
Physicochemical and spectral characterization of electrolytes
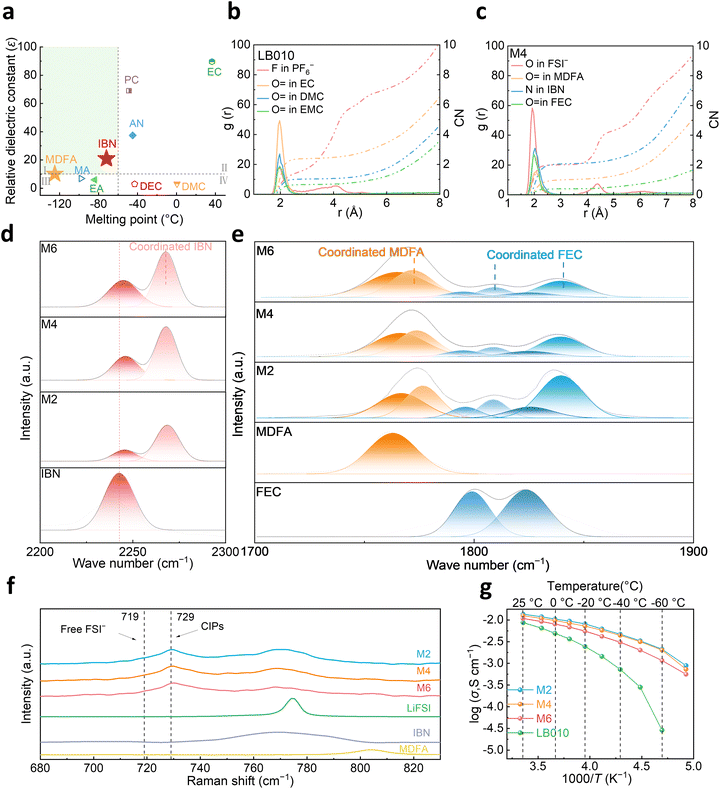
Electrolyte solvents play an important role in determining the liquidus temperature range and ionic conductivity of the electrolyte.43,44 To ensure the stable operation of batteries under cold scenarios, electrolytes should basically exhibit a high dielectric constant and a low melting point.45–48Fig. 1a shows a comparison among several typical carbonates, nitriles, and carboxylic esters. Cyclic carbonates, such as EC and PC, possess high dielectric constants, which facilitate the dissociation of lithium salts. However, the high melting points limit their suitability for subzero-temperature applications. Carboxylic esters, such as methyl acetate (MA) and ethyl acetate (EA), exhibit low melting points but low dielectric constants, which hinder the complete dissociation of lithium salts. In contrast, IBN and MDFA combine the advantages of a broad liquid phase range and a moderate dielectric constant (zone I). The slow desolvation of Li+ at low temperatures is a significant limiting factor for LIBs, emphasizing the need for designing molecules with weak solvation properties. As illustrated in (Fig. S1), electrostatic potential (ESP) diagrams reveal the molecular structures and charge distributions of MA and MDFA. In the MA molecule, the negative charges are concentrated on the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond. In the MDFA molecule, the negative charges are predominantly located on the fluorine (F) atom of the fluorinated group and the oxygen (O) atom. The electron-withdrawing effect of the fluorinated group effectively attenuates the charge density of the C
O bond. In the MDFA molecule, the negative charges are predominantly located on the fluorine (F) atom of the fluorinated group and the oxygen (O) atom. The electron-withdrawing effect of the fluorinated group effectively attenuates the charge density of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, thereby weakening the electrostatic attraction between the C
O bond, thereby weakening the electrostatic attraction between the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond and Li+. The binding energy further verified the weaker solvation affinity of MDFA and IBN compared to that of carbonate esters (Fig. S2), which would benefit the desolvation process during fast-charging and low-temperature scenarios. Considering the features of MDFA and IBN, MDFA-based electrolytes were formulated at different ratios (accordingly named as M2, M4 and M6 electrolytes), while the commonly used EC-based carbonate electrolyte (LB010) served as the reference electrolyte (see more details in the Methods part of Supplementary Information). Given the weak solvation properties of MDFA and IBN solvents, the coordination environment of Li+ would be different from the carbonate-based electrolytes, which can be compared via the radial distribution functions (RDFs) of Li+ through molecular dynamics calculations (Fig. 1b and c). It can be observed that the carbonate electrolyte exhibits a preferential coordination of the EC solvent with Li+ owing to its high permittivity (89.6). In the MDFA-based electrolyte, the Li+–O (MDFA) peak appears at 2.07 Å—longer than the Li+–O (FSI−) peak at 1.93 Å—implying the preferential coordination of FSI− molecules with Li+. The coordination number of Li+–FSI− is 0.5, reflecting the higher proportion of Li+–anions in the first solvation sheath and the enhanced Li+–anion interactions driven by the weak solvation of MDFA and IBN. Furthermore, the Li+ solvation sheath composition quantified using molecular dynamics simulations clearly indicated an anion-dominated structure in the M4 electrolyte (Fig. S3). Fourier transform infrared (FTIR) and Raman spectroscopy were further employed to investigate the solvation structures of electrolytes containing IBN and MDFA (Fig. 1d–f). Fig. 1d and e present the FTIR spectra of C
O bond and Li+. The binding energy further verified the weaker solvation affinity of MDFA and IBN compared to that of carbonate esters (Fig. S2), which would benefit the desolvation process during fast-charging and low-temperature scenarios. Considering the features of MDFA and IBN, MDFA-based electrolytes were formulated at different ratios (accordingly named as M2, M4 and M6 electrolytes), while the commonly used EC-based carbonate electrolyte (LB010) served as the reference electrolyte (see more details in the Methods part of Supplementary Information). Given the weak solvation properties of MDFA and IBN solvents, the coordination environment of Li+ would be different from the carbonate-based electrolytes, which can be compared via the radial distribution functions (RDFs) of Li+ through molecular dynamics calculations (Fig. 1b and c). It can be observed that the carbonate electrolyte exhibits a preferential coordination of the EC solvent with Li+ owing to its high permittivity (89.6). In the MDFA-based electrolyte, the Li+–O (MDFA) peak appears at 2.07 Å—longer than the Li+–O (FSI−) peak at 1.93 Å—implying the preferential coordination of FSI− molecules with Li+. The coordination number of Li+–FSI− is 0.5, reflecting the higher proportion of Li+–anions in the first solvation sheath and the enhanced Li+–anion interactions driven by the weak solvation of MDFA and IBN. Furthermore, the Li+ solvation sheath composition quantified using molecular dynamics simulations clearly indicated an anion-dominated structure in the M4 electrolyte (Fig. S3). Fourier transform infrared (FTIR) and Raman spectroscopy were further employed to investigate the solvation structures of electrolytes containing IBN and MDFA (Fig. 1d–f). Fig. 1d and e present the FTIR spectra of C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in the formulated electrolytes. As shown in Fig. 1d, the peak at 2242 cm−1 corresponds to the C
O bonds in the formulated electrolytes. As shown in Fig. 1d, the peak at 2242 cm−1 corresponds to the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond. Upon the addition of Li+ to the solvent, a new peak appeared at 2268 cm−1, indicating the coordination of Li+ with IBN, which also led to a slight redshift of the peak of 2242 cm−1. Moreover, as the proportion of IBN increases, the area under the C
N bond. Upon the addition of Li+ to the solvent, a new peak appeared at 2268 cm−1, indicating the coordination of Li+ with IBN, which also led to a slight redshift of the peak of 2242 cm−1. Moreover, as the proportion of IBN increases, the area under the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N peak gradually enlarges from M6 to M2 electrolytes, suggesting the increased coordination of IBN with Li+. The coordination of Li+ with MDFA is shown in Fig. 1e. The peak at 1763 cm−1 corresponds to the C
N peak gradually enlarges from M6 to M2 electrolytes, suggesting the increased coordination of IBN with Li+. The coordination of Li+ with MDFA is shown in Fig. 1e. The peak at 1763 cm−1 corresponds to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond. As lithium salts dissolved, a new peak emerged at 1777 cm−1, signifying the coordination of Li+ with MDFA. Additionally, with the increase in the MDFA content, the intensity of the coordination peak increases. Furthermore, the solvent peaks of FEC appear at 1798 cm−1 and 1822 cm−1, with new coordination peaks of FEC with Li+ appearing at 1809 cm−1 and 1841 cm−1, respectively. Raman spectroscopy further demonstrated the solvation structure shown in FTIR spectra (Fig. 1f). Compared to pure solvents, the three electrolytes exhibit the emergence of new peaks. It is evident that the anions primarily exist as the contact-ion pair (CIP, 729 cm−1, one anion coordinating with one Li+).3 This suggests that most FSI− anions are engaged in the solvated sheath to coordinate with Li+, demonstrating that Li+ exhibits a weaker interaction with solvents. This facilitates ion desolvation and lays a foundation for excellent wide-temperature performance.
O bond. As lithium salts dissolved, a new peak emerged at 1777 cm−1, signifying the coordination of Li+ with MDFA. Additionally, with the increase in the MDFA content, the intensity of the coordination peak increases. Furthermore, the solvent peaks of FEC appear at 1798 cm−1 and 1822 cm−1, with new coordination peaks of FEC with Li+ appearing at 1809 cm−1 and 1841 cm−1, respectively. Raman spectroscopy further demonstrated the solvation structure shown in FTIR spectra (Fig. 1f). Compared to pure solvents, the three electrolytes exhibit the emergence of new peaks. It is evident that the anions primarily exist as the contact-ion pair (CIP, 729 cm−1, one anion coordinating with one Li+).3 This suggests that most FSI− anions are engaged in the solvated sheath to coordinate with Li+, demonstrating that Li+ exhibits a weaker interaction with solvents. This facilitates ion desolvation and lays a foundation for excellent wide-temperature performance.
To ensure a wide-temperature operation, electrolytes should exhibit a wide liquid-phase range. Differential scanning calorimetry (DSC) results reveal that all three electrolytes maintain a wide liquid-phase range with freezing points below −140 °C and boiling points above 100 °C (Fig. S4), demonstrating excellent temperature adaptability. Moreover, a high ionic conductivity is the basis for fast ion transport under fast-charging and wide-temperature scenarios. Fig. 1g illustrates the temperature dependence of conductivity for the different electrolytes. At room temperature, the conductivity of the electrolyte increases with the rising proportion of high-permittivity IBN. Consequently, the M2 electrolyte exhibits the highest conductivity of 13.6 mS cm−1 and M4 with 12.6 mS cm−1, higher than that of the commercial carbonate electrolyte LB010 (8.7 mS cm−1). At −60 °C, the ionic conductivity of the M4 electrolyte still reaches 2 mS cm−1, ensuring rapid ion transport at low temperatures. Notably, the ionic conductivity of the formulated M4 electrolyte not only outperforms that of the commercial LB010 electrolyte but also excels among the recently reported electrolytes (Fig. S5). Together with the weak solvation affinity, the as-formulated MDFA-based electrolytes are anticipated to realize excellent electrochemical performance in Gr and Gr-based batteries.
Electrochemical performance of Gr anodes
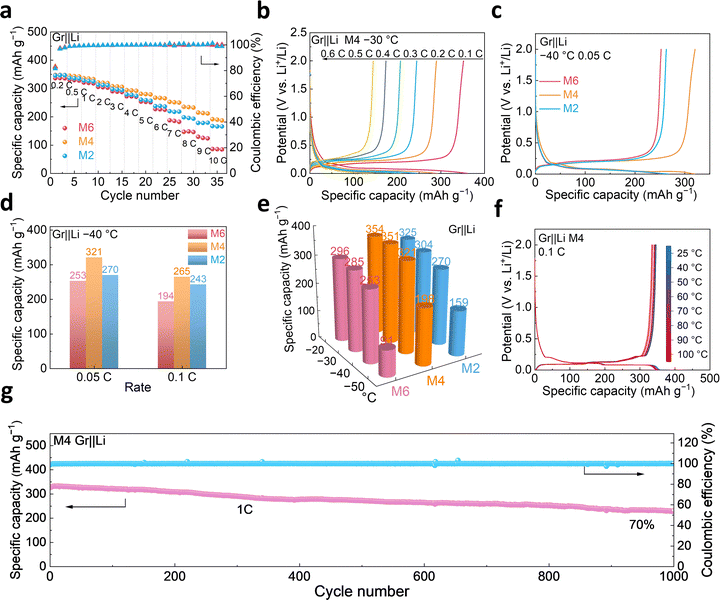
To investigate the practical applications of the formulated electrolytes, Gr||Li half-cells were assembled to assess the suitability. As shown in (Fig. 2a), Gr electrodes with the three electrolytes exhibit a specific capacity of approximately 350 mAh g−1 at 0.2C (1C = 372 mA g−1). As the charge/discharge rate increases, the Gr||Li half-cell with M4 electrolyte demonstrates an excellent fast-charging ability with specific capacities of 267 mAh g−1 (a capacity retention of 76.7% compared to that at 0.2C at room temperature) at 6C and 192 mAh g−1 (a capacity retention of 54.9%) at an even higher rate of 10C (Fig. S6). Meanwhile, with LB010 electrolyte, only a specific capacity of 78 mAh g−1 can be achieved at 6C (Fig. S7). These demonstrate the enhancement of the rate performance of Gr by the synergetic-effect electrolyte design.Regarding the physicochemical properties of the formulated electrolytes, we know that all three electrolytes exhibit a broad operational temperature range and high ionic conductivities across different temperatures. However, cell polarization gradually increases as the temperature decreases, lowering the Li+ intercalation potential at the Gr anode and leading to a potential precipitation of lithium. It is thus crucial to investigate the performance of Gr||Li half-cells at low temperatures. Low-temperature charge/discharge tests were then conducted with Gr||Li half-cells (Fig. 2b and Fig. S8). At −30 °C, the cell assembled with M4 electrolyte was evaluated. At 0.1C, it demonstrates nearly 100% capacity retention, with specific capacities of 245 mAh g−1 at 0.3C, 145 mAh g−1 at 0.6C, and 55 mAh g−1 at 1.0C. At −40 °C, the cell with M4 electrolyte exhibits the highest specific capacity of 321 mAh g−1 at 0.05C among the three electrolytes (91.7% retention) (Fig. 2c and Fig. S9b). While for LB010 electrolyte, the Gr||Li half-cell shows only 139 mAh g−1 at −30 °C (Fig. S9d). Meanwhile, the charge/discharge curves are similar to those at room temperature, exhibiting minimal overpotentials and no lithium dendrite precipitation. Additionally, the Gr||Li half-cell with M4 electrolyte at −40 °C also maintains the highest specific capacity of 265 mAh g−1 at 0.1C among the electrolytes (Fig. 2d and Fig. S10b), highlighting the fastest Li+ intercalation and deintercalation at Gr anodes using M4 electrolyte. As shown in Fig. 2e, even at −50 °C, the Gr anode with M4 electrolyte maintains a specific capacity of 198 mAh g−1 without the precipitation of lithium.
The performance of the Gr||Li half-cell across a wide temperature range was also evaluated with the commonly used protocol of discharging at room temperature and charging at various temperatures (Fig. S11). The Gr||Li half-cell with M4 electrolyte displays the prime charging performance at −20 °C, delivering the same capacity as at 25 °C. Although the capacity declines with decreasing temperature, a decent capacity retention of 97.7% is maintained at −40 °C. Even at −60 °C, Gr with M4 electrolyte can still deliver a charging capacity of 323 mAh g−1, with an exceptional capacity retention of 92.3%, indicating its feasibility at subzero temperatures. In addition to its outstanding performance at low temperatures, M4 electrolyte also equips Gr with excellent stability at elevated temperatures, benefiting from its high boiling point (Fig. 2f). The charge/discharge curves show minimal changes as the temperature increases, suggesting negligible parasitic reactions in the cell at high temperatures. Even at 0.1C and 100 °C, the cell retains a reversible specific capacity of 334 mAh g−1, with a high capacity retention of 95.4%.
In addition to its outstanding rate and wide-temperature performance, Gr with M4 electrolyte exhibits excellent long-cycling stability. The Gr||Li half-cell was subjected to a long-cycling test, retaining 70% of its initial capacity after 1000 cycles, highlighting the good stability of the Gr||Li half-cell with M4 electrolyte (Fig. 2g). As for fast-charging cycling tests, the cell with M4 electrolyte was cycled at 3C, retaining 90% of its initial capacity after 600 cycles. Whereas the cell with M6 electrolyte shows 36% retention after 600 cycles, indicating the superior cycling stability and fast-charging performance of the cell with M4 electrolyte (Fig. S12). Furthermore, the Gr||Li half-cell with M4 electrolyte maintains stable cycling performance even at subzero and elevated temperatures. The cell can realize a stable cycling performance at −30 °C with excellent capacity retention of 79.2% after 150 cycles (Fig. S13). Cycling stability was also assessed at an elevated temperature of 45 °C, exhibiting a capacity retention of 77% after 100 cycles at 45 °C and an average Coulombic efficiency of 99.5% (Fig. S14). This exceptional performance indicated the adaptability of M4 electrolyte over a wide temperature range. The resulting low-temperature and rate performance of the cell with M4 electrolyte is much superior to that of the recently reported Gr||Li half-cells (Tables S1 and S2). This well indicates that the rational electrolyte design enables excellent performance of Gr half-cells in terms of rate capability, cycling stability, and wide-temperature operation.
Interfacial properties of Gr anodes
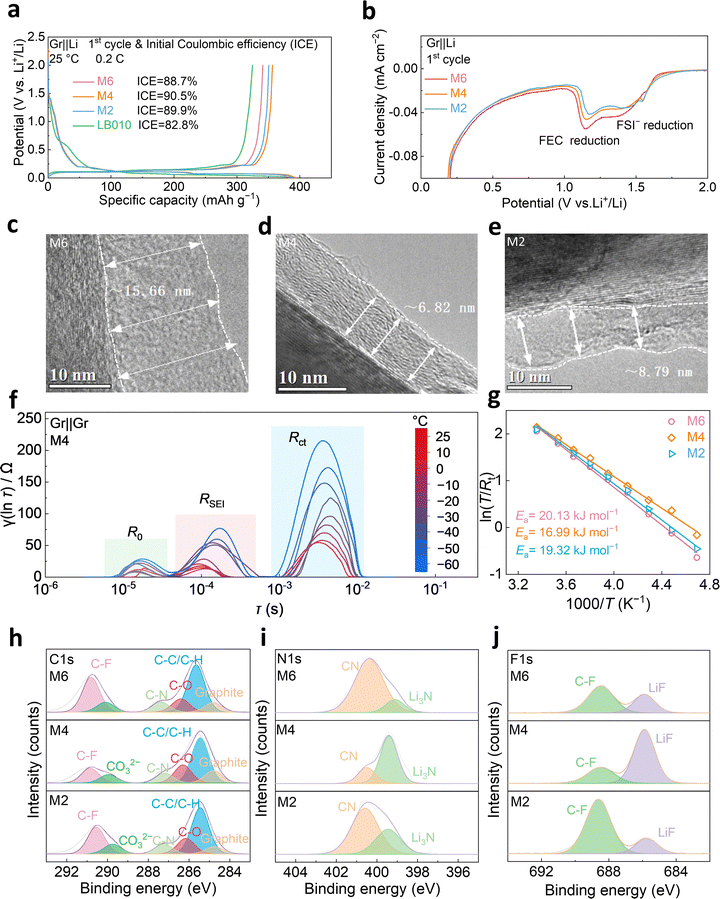
The solid electrolyte interphase (SEI) layer on the Gr anode serves to separate the electrode from the electrolyte, preventing continuous parasitic reactions at the Gr surface and governing the kinetics of ion transport. Therefore, a comprehensive investigation of the SEI layer is of critical importance. As shown in Fig. 3a, the initial charge–discharge curves and the corresponding initial Coulombic efficiencies (ICEs) of Gr||Li half-cells with the electrolytes are presented. At 0.2C, the Gr||Li half-cell with M4 electrolyte displays the highest ICE of 90.5%, indicating the least irreversible capacity loss during the initial charge–discharge process. To further investigate the impact of electrolyte on electrochemical performance, a series of characterizations were conducted on the SEI layer formed during the first cycle of the Gr||Li half-cell. Cyclic voltammetry (CV) curves reveal three pairs of redox peaks, clearly indicating the processes of Li+ intercalation and deintercalation at Gr anodes (Fig. 3b and Fig. S15). An enlarged plot of the first laps for the CV curves reveals that FSI− anions and FEC are reduced at 1.5 V and 1.2 V (vs. Li+/Li) on the Gr anode, respectively, where FEC has a significant influence on the electrochemical performance (Fig. S16). Among the three electrolytes, the reduction of M4 electrolyte is moderate, revealing that it is reduced to form the SEI layer with modest electrolyte decomposition.The morphology and thickness of SEI layers significantly influence Li+ transport and interfacial impedance. Transmission electron microscopy (TEM) was employed to examine the SEI layers (Fig. 3c–e and Fig. S17). For M4 electrolyte, the average thickness of the SEI layer is the thinnest (about 6.82 nm), enabling the shortest Li+ diffusion pathway and reducing interfacial impedance. To evaluate the interfacial impedance of SEI layers quantitatively, symmetric Gr||Gr cells were assembled, and the resulting Nyquist plots were analyzed (Fig. S18). Before assembly, Gr anodes were pre-cycled at 0.2C to form the SEI layers. The electrodes were then disassembled from the cells.38 The Li+ migration rates across different SEI layers can be investigated by analyzing the Nyquist plots. At room temperature, the SEI in M4 electrolyte exhibits minimal impedance. As the temperature decreases, the ionic mobility slows, increasing interfacial resistance. However, the SEI formed in M4 electrolyte still maintains the lowest impedance, corresponding to the best Gr||Li half-cell performance. Further insights into the effect of varying solvent volume ratios on the Gr interface are provided by the analysis of the distribution of relaxation times (DRT). DRT was derived from the Nyquist plots of Gr anodes with the three electrolytes at various temperatures, employing a continuous distribution function to identify distinct electrochemical processes (Fig. 3f and Fig. S19). Peaks in the relaxation time range of 10−5 to 10−4 s correspond to ohmic resistance (R0), while peaks in the range of 10−4 to 10−3 s represent Li+ transfer across the SEI (RSEI). Additionally, peaks in the range of 10−3 to 10−2 s are associated with the charge transfer process (Rct).39 A comparison of the three electrolytes reveals that both Rct and RSEI of the Gr anode with M4 electrolyte exhibit the lowest resistance under different temperatures. Notably, as the temperature decreases, the difference in Rct between cells with M4 electrolyte and other electrolytes becomes more obvious compared to that of RSEI, indicating that charge transfer kinetics dominates at low temperatures. To further assess the difficulties of Li+ diffusion through SEI layers, the activation energy for each electrolyte was calculated based on the Nyquist plots of the Gr anode at varying temperatures (Fig. 3g and Fig. S20). The results indicate that M4 electrolyte exhibits the lowest activation energy (16.99 kJ mol−1), facilitating the smoothest Li+ diffusion across the SEI layer. Furthermore, X-ray photoelectron spectroscopy (XPS) was performed to analyze the composition of SEI layers. As shown in (Fig. 3h–j and Fig. S21), the contents of LiF and Li3N with M4 electrolyte are the highest, forming a LiF-rich and Li3N-rich SEI. LiF is known for its high mechanical strength and electronic insulating properties, which effectively suppress parasitic reactions in cells. On the other hand, Li3N exhibits high ionic conductivity (5.767 × 10−4 S cm−1), which significantly reduces interfacial impedance across the SEI layer. Simultaneously, the increased incorporation of inorganic components into the SEI layer enhances the thermal tolerance of cells, as these species exhibit significantly higher decomposition temperatures (>700 °C) compared to organic compounds (∼60 °C).49 Therefore, these characteristics jointly underpin the stable performance of M4-based cells at a wide temperature range.
Electrochemical compatibility of high-voltage cathodes and assessment of full cells
The hybrid system comprising MDFA, IBN, and FEC is compatible with Gr||Li half-cells, facilitating the formation of thin and low-impedance SEI layers. Meanwhile, MDFA-based electrolytes exhibit excellent high-voltage tolerance, which is achieved via rational molecular design of fluorinated carboxylate and nitrile solvents. As shown in Fig. S22, the energies of the highest occupied molecular orbitals (HOMO) for different solvents are calculated and compared, including EC, DMC, EMC, FEC, MA, MDFA, and IBN. Besides the well-known oxidation stability of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group, the strong electron-withdrawing property of the fluorinated group reduces the electron density of carbonyl oxygen, which can further enhance the high-voltage tolerance of MDFA. Therefore, MDFA exhibits comparatively high oxidation stability in carbonate solvents. Meanwhile, the IBN solvent displays the lowest energy level among all these solvents, indicating its excellent high-voltage tolerance. Furthermore, the oxidation potentials of MDFA and IBN molecules are calculated, indicating the excellent anti-oxidation ability of the two solvent molecules (Fig. S23). On these foundations, the formulated MDFA-based electrolyte is applicable under high-voltage operating conditions. To further evaluate the oxidative stability of the three electrolytes, the electrochemical windows of the electrolytes were further assessed through linear scanning voltammetry (LSV) (Fig. S24), revealing that the oxidative decomposition voltages of all three electrolytes exceed 4.5 V (vs. Li+/Li). Notably, M4 electrolyte demonstrates the highest oxidative decomposition voltage (4.7 V vs. Li+/Li). The excellent anti-oxidative ability of M4 electrolyte is also confirmed by chronoamperometry (CA) tests with Li||Al half-cells shown in Fig. S25, where the residual current of the cell with M4 electrolyte remains stably low compared with the reference LB010 electrolyte. To further clarify the outstanding oxidative stability of the M4 electrolyte, the layered NCM811 electrode, a commonly employed cathode material in rechargeable batteries for its high energy density and rate performance, is used to assess the compatibility of the three electrolytes with the cathode. Notably, the NCM811||Li half-cell with M4 electrolyte exhibited the highest ICE of 91.5% (Fig. S26), confirming the favorable compatibility between M4 electrolyte and the NCM811 cathode. Moreover, the CV curve of the NCM811||Li half-cell with M4 electrolyte (Fig. S27) shows nearly identical profiles between the second and third cycles, confirming the high reversibility and stability of the cell. Furthermore, we conducted XPS analysis of the CEI layers of NCM811 cathodes with M4 and LB010 electrolytes (Fig. S28). The C1s spectrum of M4 electrolyte indicates a higher proportion of CO32− species, reflecting a larger inorganic content. The N1s and F1s spectra further confirm a higher percentage of Li3N and LiF in the M4 electrolyte, enhancing its Li+ interfacial transport rate and high-voltage tolerance.50–56 Therefore, the NCM811||Li half-cell with M4 electrolyte shows superior electrochemical performance under high potential (Fig. 4a). At a cut-off potential of 4.5 V, the NCM811 cathode with M4 electrolyte exhibits a high reversible specific capacity of 230 mAh g−1 at 0.2C (1C corresponds to 200 mA g−1). Furthermore, it retains a reversible specific capacity of 160 mAh g−1 at 6C, corresponding to a high capacity retention of 70% (compared to that at 0.2C under 25 °C), whereas the cell with LB010 electrolyte delivers only 58 mAh g−1 at 6C (Fig. S29). These results highlight the superior performance of the M4 electrolyte in NCM811||Li half-cells at high voltages, thus supporting the rationality behind the electrolyte design.
O group, the strong electron-withdrawing property of the fluorinated group reduces the electron density of carbonyl oxygen, which can further enhance the high-voltage tolerance of MDFA. Therefore, MDFA exhibits comparatively high oxidation stability in carbonate solvents. Meanwhile, the IBN solvent displays the lowest energy level among all these solvents, indicating its excellent high-voltage tolerance. Furthermore, the oxidation potentials of MDFA and IBN molecules are calculated, indicating the excellent anti-oxidation ability of the two solvent molecules (Fig. S23). On these foundations, the formulated MDFA-based electrolyte is applicable under high-voltage operating conditions. To further evaluate the oxidative stability of the three electrolytes, the electrochemical windows of the electrolytes were further assessed through linear scanning voltammetry (LSV) (Fig. S24), revealing that the oxidative decomposition voltages of all three electrolytes exceed 4.5 V (vs. Li+/Li). Notably, M4 electrolyte demonstrates the highest oxidative decomposition voltage (4.7 V vs. Li+/Li). The excellent anti-oxidative ability of M4 electrolyte is also confirmed by chronoamperometry (CA) tests with Li||Al half-cells shown in Fig. S25, where the residual current of the cell with M4 electrolyte remains stably low compared with the reference LB010 electrolyte. To further clarify the outstanding oxidative stability of the M4 electrolyte, the layered NCM811 electrode, a commonly employed cathode material in rechargeable batteries for its high energy density and rate performance, is used to assess the compatibility of the three electrolytes with the cathode. Notably, the NCM811||Li half-cell with M4 electrolyte exhibited the highest ICE of 91.5% (Fig. S26), confirming the favorable compatibility between M4 electrolyte and the NCM811 cathode. Moreover, the CV curve of the NCM811||Li half-cell with M4 electrolyte (Fig. S27) shows nearly identical profiles between the second and third cycles, confirming the high reversibility and stability of the cell. Furthermore, we conducted XPS analysis of the CEI layers of NCM811 cathodes with M4 and LB010 electrolytes (Fig. S28). The C1s spectrum of M4 electrolyte indicates a higher proportion of CO32− species, reflecting a larger inorganic content. The N1s and F1s spectra further confirm a higher percentage of Li3N and LiF in the M4 electrolyte, enhancing its Li+ interfacial transport rate and high-voltage tolerance.50–56 Therefore, the NCM811||Li half-cell with M4 electrolyte shows superior electrochemical performance under high potential (Fig. 4a). At a cut-off potential of 4.5 V, the NCM811 cathode with M4 electrolyte exhibits a high reversible specific capacity of 230 mAh g−1 at 0.2C (1C corresponds to 200 mA g−1). Furthermore, it retains a reversible specific capacity of 160 mAh g−1 at 6C, corresponding to a high capacity retention of 70% (compared to that at 0.2C under 25 °C), whereas the cell with LB010 electrolyte delivers only 58 mAh g−1 at 6C (Fig. S29). These results highlight the superior performance of the M4 electrolyte in NCM811||Li half-cells at high voltages, thus supporting the rationality behind the electrolyte design.
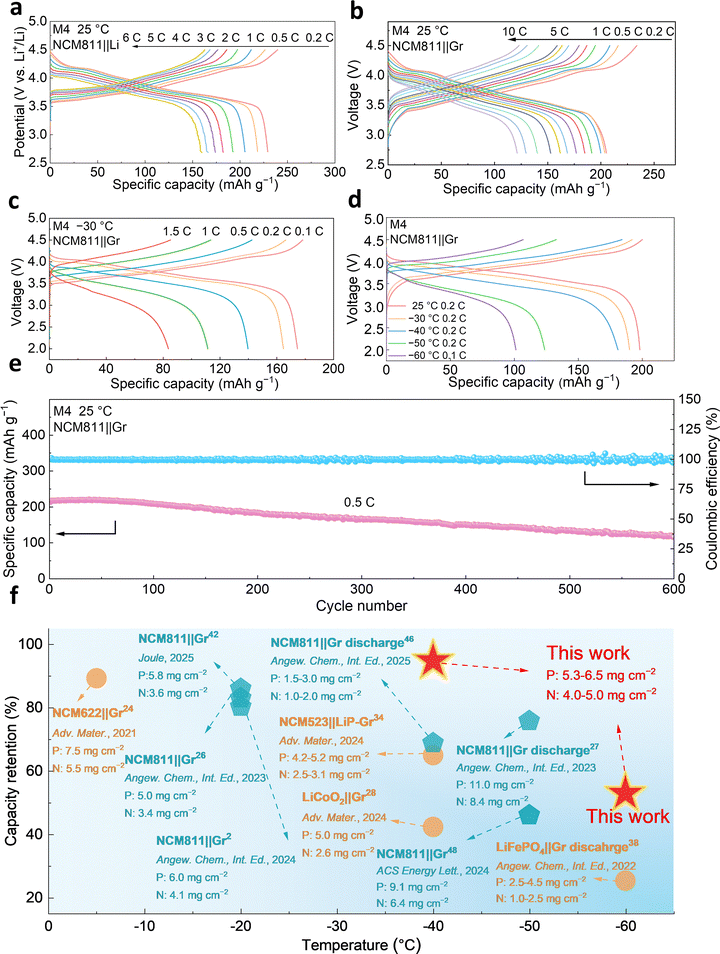
Given the favorable performance of both the anode and cathode, full cells were assembled with NCM811 and Gr. At 0.2C, the full cell with M4 electrolyte exhibits a high reversible specific capacity of 206 mAh g−1. At higher rates of 6C and 10C, it maintains reversible capacities of 162 mAh g−1 and 121 mAh g−1, corresponding to a high capacity retention of 78.6% and 58.7%, respectively (compared to that at 0.2C under room temperature) (Fig. 4b and Fig. S30). In contrast, the cell with LB010 electrolyte achieves only 60 mAh g−1 at 5C (Fig. S31). Additionally, specific capacities of the full cell can maintain 140 mAh g−1 and 111 mAh g−1 at 0.5C and 1C, respectively, even charged/discharged at the low temperature of −30 °C (Fig. 4c). The full cell still exhibits a high reversible specific capacity of 84 mAh g−1 at a higher rate of 1.5C, indicating excellent rate performance under extreme conditions. Further reversible charge/discharge tests were performed at a lower temperature, showing that the full cell with M4 electrolyte maintains a high specific capacity of 181 mAh g−1 at 0.2C at −40 °C (96% retention). Moreover, the cell retains 101 mAh g−1 at 0.1C at −60 °C (Fig. 4d). In sharp contrast, the full cell with LB010 electrolyte sustains a mere 101 mAh g−1 at 0.2C at −30 °C (Fig. S32). Moreover, the full cell with M4 electrolyte can maintain high capacity retention at 25 °C and be charged/discharged at 0.5C and −50 °C with a higher cut-off voltage of 4.6 V (Fig. S33), demonstrating its excellent temperature adaptability. Furthermore, the NCM811||Gr full cell was subjected to a cycling test, during which the M4 electrolyte demonstrated favorable performance (Fig. 4e). Over 600 cycles within a voltage range of 2.7–4.4 V, the full cell retained 54% of its initial capacity. In contrast, under the same conditions, the NCM811||Gr full cell with LB010 electrolyte can only maintain a capacity retention of 36% after 100 cycles (Fig. S34). Meanwhile, to evaluate the cycling performance of the M4 electrolyte under both high-voltage and high-rate conditions, the NCM811||Gr full cell with M4 electrolyte is cycled at a higher rate and higher voltage at 25 °C (Fig. S35). At 3C, the full cell with M4 electrolyte still retains 84.4% and 90% capacity of its initial capacity after 350 cycles and 100 cycles at 4.4 V and 4.5 V, respectively, demonstrating the excellent rate capability and voltage tolerance of the full cell based on M4 electrolyte. To investigate the morphology of NCM811 particles after long-term cycling in M4 and LB010 electrolytes, scanning electron microscopy (SEM) analysis was first conducted (Fig. S36a and b). The NCM811 particles cycled in M4 electrolyte retain the relatively intact morphology after long cycling. In contrast, LB010 electrolyte leads to a significant structural collapse and surface degradation of NCM811 particles. Furthermore, the TEM characterization of the CEI layers of the two electrolytes is also conducted. As shown in Fig. S36c and d, the CEI layer formed in M4 electrolyte is thinner (2.43 nm) and more uniform than that formed in LB010 electrolyte (6.72 nm), reducing interface impedance and increasing ion migration rate across the CEI layers. Moreover, to gain a more comprehensive understanding of the CEI composition formed by the two electrolytes after long cycling, we additionally performed XPS characterization (Fig. S36e–g). F1s and N1s spectra indicate a higher proportion of inorganic species (e.g., LiF, Li3N) in the CEI layer of M4 electrolyte, laying a foundation for its high-voltage stability. Therefore, compared with the LB010 electrolyte, the M4 electrolyte leads to better cathode structural integrity, a thinner CEI layer with a higher inorganic content, which collectively contribute to significantly enhanced long-term cycling performance at high voltage. To evaluate the cycling performance of the cell under cold conditions, a low-temperature cycling test was also performed, where the NCM811||Gr full cell exhibited a high capacity retention of 87% after 70 cycles at 0.2C at −30 °C within a voltage range from 2–4.5 V (Fig. S37a). Notably, no voltage plateaus indicative of lithium plating are observed in the curves (Fig. S37b). Meanwhile, the discharge mid-voltage decreases slowly, showing low polarization during low-temperature cycling of the full cell (Fig. S37c). Moreover, at an elevated temperature of 45 °C, the full cell with M4 electrolyte displayed excellent capacity retention of 56.1% after 400 cycles (Fig. S38). In addition, the low-temperature and rate performance of the full cell with high areal loadings using M4 electrolyte outperforms those in most recent reports in terms of both discharge and reversible capacity at subzero temperatures (Fig. 4f and Tables S3, S4).
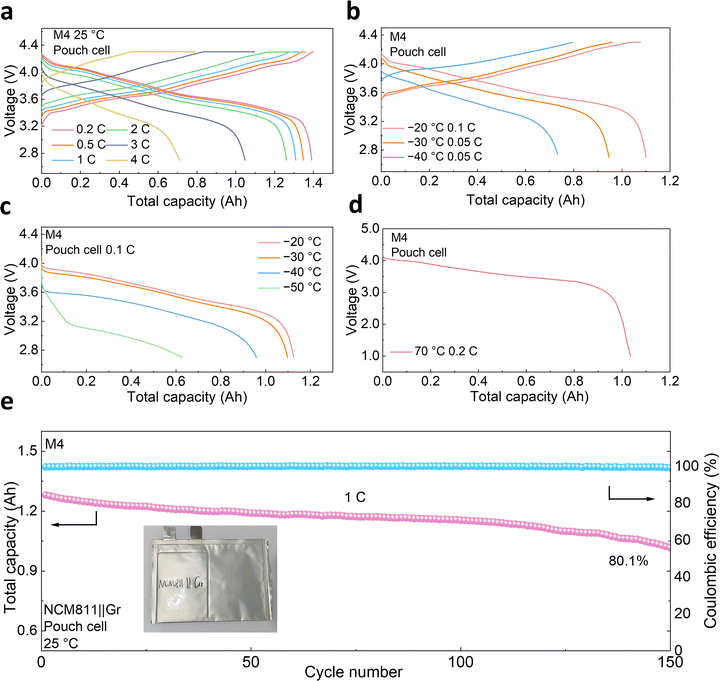
Stimulated by the prime performance of the full cells, Ah-level pouch cells were further assembled to assess the practicality of the electrolyte. The 1.2 Ah NCM811||Gr pouch cells were prepared with a negative/positive (N/P) capacity ratio of 1.05, a slight excess of the Gr anode. The pouch cell with M4 electrolyte delivers a decent capacity of 1391 mAh at 0.2C with an ICE of 93.1% (1C = 1200 mA, Fig. 5a). Besides, the pouch cell exhibits an exceptional rate capability similar to the coin cells. A reversible capacity of 1261 mAh can still be attained at a rate of 2C, correlating with an impressive capacity retention of 90.7% compared to that at 0.2C. The pouch cell with M4 electrolyte also maintained high capacity at subzero temperatures (Fig. 5b). A high capacity of 1100 mAh was achieved at 0.1C and −20 °C, with a capacity retention of 79.2%. Even at −40 °C, a specific capacity of 729 mAh is observed, corresponding to a capacity retention of 52.4%. These results exceed those reported for pouch cells in recent studies (Table S5). Meanwhile, with LB010 electrolyte, the pouch cell only retains 552 mAh at 0.1C at −20 °C, lower than the obtained capacity of 1100 mAh based on M4 electrolyte (Fig. S39), indicating the excellent low-temperature performance of M4 electrolyte. Furthermore, the pouch cells were tested under a general charge/discharge protocol, which includes room-temperature charging and temperature-varying discharge.16,57–60 As illustrated in Fig. 5c, the pouch cell comprising M4 electrolyte shows a high capacity of 1173 mAh at 0.1C at −20 °C. Despite a decrease in capacity with decreasing temperature, a decent capacity retention of 69% is retained at −40 °C. Even at 70 °C, the pouch cell exhibits a high discharge capacity of 1035 mAh (Fig. 5d). Unlike sluggish ion movement at low temperatures, elevated temperatures can improve the kinetics and accelerate the electrode kinetics to some extent. This is a double-edged sword for batteries because this can enhance the output capacity and power density, but it would also bring severe side effects, including electrolyte evaporation and an unstable interphase. Fortunately, the as-formulated M4 electrolyte not only possesses a high boiling point but also facilitates the formation of stable SEI and CEI layers rich in inorganic compounds (Fig. S4, Fig. 3h–j and Fig. S28). These advantages jointly underpin the stable performance of M4-based cells even at 70 °C, illustrating the robustness of the M4 electrolyte from low-temperature to high-temperature conditions. Therefore, the wide-temperature performance of the pouch cell with M4 electrolyte surpasses that reported in many recent studies (Table S6). In addition, we conducted cycling performance tests on pouch cells. The pouch cell using LB010 electrolyte exhibited only 8.2% capacity retention after 150 cycles at 1C (Fig. S40). However, the pouch cell assembled with M4 electrolyte displays 80.1% capacity retention after 150 cycles under the same conditions (Fig. 5e), highlighting its excellent reversibility and stability, confirming the rationality of a complementary synergy-driven electrolyte design.
After a comprehensive comparison with recent literature (Table S1–S7), it was observed that the performance of our cells surpasses that of the reported state-of-the-art cells. This exceptional performance can be attributed to the novel design strategies (Fig. 6). Firstly, the use of MDFA as the primary solvent imparts a weak solvation feature to the well-formulated electrolyte, while the cosolvent strategy involving IBN significantly enhances ionic conductivity. Notably, both components exhibit a wide liquid-phase window and excellent high-voltage tolerance. Thus, the synergistic effect arising from these characteristics collectively contributes to the overall performance improvement of the designed electrolyte. Meanwhile, M4 electrolyte forms an anion-derived solvation sheath, which promotes the formation of a robust SEI layer enriched in LiF and Li3N. This SEI layer reduces the interfacial ion-transport barrier and facilitates faster Li+ migration across the interface. As a consequence, the promising application of M4 electrolyte featuring synergistic interactions enable high-rate and long-cycling batteries to operate at high voltages over a wide temperature range.
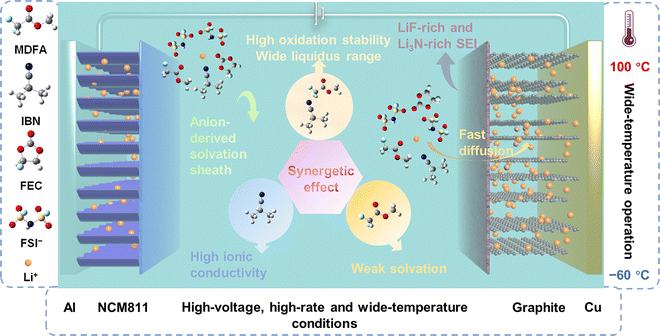 | ||
| Fig. 6 Schematic of the synergetic-effect design principle for high-rate, high-voltage, and wide-temperature LIBs. | ||
Conclusions
In summary, the synergistic combination of weakly solvating MDFA and high-permittivity IBN can fully exert the individual strengths of the solvents, leading to outstanding performance of LIBs in high-rate, high-voltage, and wide-temperature environments. The optimized M4 electrolyte promotes the formation of a low-impedance and inorganic-rich SEI, endowing Gr with fast-charging ability and achieving a reversible specific capacity of 192 mAh g−1 at 10C. Furthermore, Gr exhibits a capacity retention of 79.2% at 0.2C at −30 °C after 150 cycles and a high reversible capacity of 321 mAh g−1 at a lower temperature of −40 °C. The electrolyte exhibits a high boiling point and imparts Gr with excellent stability at a high temperature of 100 °C. The NCM811||Gr full cell can be stably operated at a high voltage of 4.5 V and exhibits outstanding fast-charging ability at a rate of 10C. Besides, the full cell shows impressive low-temperature performance, delivering 101 mAh g−1 at −60 °C and achieving stable cycling performance over 600 cycles. Additionally, the Ah-level pouch cell maintains impressive capacity retention of 75.3% at 3C and exhibits high capacities within a wide temperature range from −50 °C to 70 °C. This weak solvation synergetic effect offers novel insights into the design of electrolytes for extreme conditions.Author contributions
X. Dong conceived the idea and supervised the work. W. Zhou carried out the experiments, characterization and electrochemical measurements. G. Liu and W. Zhou conducted the theoretical calculations. X. Zhu and K. Zhu helped discuss and analyze the data. W. Zhou and X. Dong wrote the manuscript. All authors discussed the data and commented on the manuscript.Conflicts of interest
The authors declare no competing financial interests.Data availability
The authors will supply the relevant data in response to reasonable requests.The data supporting this article have been included as part of the supplementary information (SI). detailed experimental procedures, additional figures, and tables supporting the main text. See DOI: https://doi.org/10.1039/d5ee05191f.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2022YFB3803400), the National Natural Science Foundation of China (22422902, 22379028, and 22109028), the Natural Science Foundation of Shanghai Municipality (22ZR1404400), the Science & Technology Commission of Shanghai Municipality (24DZ3001300), and the State Key Laboratory of Catalysis (2024SKL-B-009).Notes and references
- M. J. Zachman, Z. Tu, S. Choudhury, L. A. Archer and L. F. Kourkoutis, Nature, 2018, 560, 345–349 CrossRef CAS PubMed.
- L. Dong, H.-J. Yan, Q.-X. Liu, J.-Y. Liang, J. Yue, M. Niu, X. Chen, E. Wang, S. Xin, X. Zhang, C. Yang and Y.-G. Guo, Angew. Chem., Int. Ed., 2024, 63, e202411029 CAS.
- Y. Mo, G. Liu, Y. Yin, M. Tao, J. Chen, Y. Peng, Y. Wang, Y. Yang, C. Wang, X. Dong and Y. Xia, Adv. Energy Mater., 2023, 13, 2301285 CrossRef CAS.
- D. Hubble, D. E. Brown, Y. Zhao, C. Fang, J. Lau, B. D. McCloskey and G. Liu, Energy Environ. Sci., 2022, 15, 550–578 RSC.
- J. B. Goodenough and K.-S. Park, J. Am. Chem. Soc., 2013, 135, 1167–1176 CrossRef CAS PubMed.
- Y. Feng, L. Zhou, H. Ma, Z. Wu, Q. Zhao, H. Li, K. Zhang and J. Chen, Energy Environ. Sci., 2022, 15, 1711–1759 RSC.
- Y. Du, S. Shironita, E. Hosono, D. Asakura, Y. Sone and M. Umeda, J. Power Sources, 2023, 556, 232513 CrossRef CAS.
- J. Chen, Y. Zhang, H. Lu, J. Ding, X. Wang, Y. Huang, H. Ma and J. Wang, eScience, 2023, 3, 100135 CrossRef.
- J. Wang, Q. Zheng, M. Fang, S. Ko, Y. Yamada and A. Yamada, Adv. Sci., 2021, 8, 2101646 CrossRef CAS.
- R. Han, Z. Wang, D. Huang, F. Zhang, A. Pan, H. Song, Y. Wei, Y. Liu, L. Wang, Y. Li, J. Xu, J. Hu and X. Wu, Small, 2023, 19, 2300571 CrossRef CAS PubMed.
- M. Li, J. Lu, Z. Chen and K. Amine, Adv. Mater., 2018, 30, 1800561 CrossRef PubMed.
- G.-L. Xu, Q. Liu, K. K. S. Lau, Y. Liu, X. Liu, H. Gao, X. Zhou, M. Zhuang, Y. Ren, J. Li, M. Shao, M. Ouyang, F. Pan, Z. Chen, K. Amine and G. Chen, Nat. Energy, 2019, 4, 484–494 CrossRef CAS.
- L. Suo, W. Xue, M. Gobet, S. G. Greenbaum, C. Wang, Y. Chen, W. Yang, Y. Li and J. Li, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 1156–1161 CrossRef CAS PubMed.
- B. Liao, H. Li, M. Xu, L. Xing, Y. Liao, X. Ren, W. Fan, L. Yu, K. Xu and W. Li, Adv. Energy Mater., 2018, 8, 1800802 CrossRef.
- Z. M. Konz, E. J. McShane and B. D. McCloskey, ACS Energy Lett., 2020, 5, 1750–1757 CrossRef CAS.
- C. S. Rustomji, Y. Yang, T. K. Kim, J. Mac, Y. J. Kim, E. Caldwell, H. Chung and Y. S. Meng, Science, 2017, 356, eaal4263 CrossRef PubMed.
- N. Piao, X. Ji, H. Xu, X. Fan, L. Chen, S. Liu, M. N. Garaga, S. G. Greenbaum, L. Wang, C. Wang and X. He, Adv. Energy Mater., 2020, 10, 1903568 CrossRef CAS.
- L. Luo, K. Chen, H. Chen, H. Li, R. Cao, X. Feng, W. Chen, Y. Fang and Y. Cao, Adv. Mater., 2024, 36, 2308881 CrossRef CAS.
- S. Yuan, S. Cao, X. Chen, J. Wei, Z. Lv, H. Xia, L. Chen, R. B. F. Ng, F. L. Tan, H. Li, X. J. Loh, S. Li, X. Feng and X. Chen, J. Am. Chem. Soc., 2025, 147, 4089–4099 CrossRef CAS PubMed.
- D. Lu, R. Li, M. M. Rahman, P. Yu, L. Lv, S. Yang, Y. Huang, C. Sun, S. Zhang, H. Zhang, J. Zhang, X. Xiao, T. Deng, L. Fan, L. Chen, J. Wang, E. Hu, C. Wang and X. Fan, Nature, 2024, 627, 101–107 CrossRef CAS PubMed.
- X. Zheng, Z. Cao, W. Luo, S. Weng, X. Zhang, D. Wang, Z. Zhu, H. Du, X. Wang, L. Qie, H. Zheng and Y. Huang, Adv. Mater., 2023, 35, 2210115 CrossRef CAS PubMed.
- J. Xu, J. Zhang, T. P. Pollard, Q. Li, S. Tan, S. Hou, H. Wan, F. Chen, H. He, E. Hu, K. Xu, X.-Q. Yang, O. Borodin and C. Wang, Nature, 2023, 614, 694–700 CrossRef CAS PubMed.
- M. Mao, X. Ji, Q. Wang, Z. Lin, M. Li, T. Liu, C. Wng, Y.-S. Hu, H. Li, X. Huang, L. Chen and L. Suo, Nat. Commun., 2023, 14, 1082 CrossRef CAS PubMed.
- Y. Zou, Z. Cao, J. Zhang, W. Wahyudi, Y. Wu, G. Liu, Q. Li, H. Cheng, D. Zhang, G. T. Park, L. Cavallo, T. D. Anthopoulos, L. Wang, Y.-K. Sun and J. Ming, Adv. Mater., 2021, 33, 2102964 CrossRef CAS PubMed.
- M.-T. F. Rodrigues, F. N. Sayed, H. Gullapalli and P. M. Ajayan, J. Power Sources, 2018, 381, 107–115 CrossRef CAS.
- J.-Y. Liang, Y. Zhang, S. Xin, S.-J. Tan, X.-H. Meng, W.-P. Wang, J.-L. Shi, Z.-B. Wang, F. Wang, L.-J. Wan and Y.-G. Guo, Angew. Chem., Int. Ed., 2023, 62, e202300384 CrossRef CAS PubMed.
- Y. Zou, Z. Ma, G. Liu, Q. Li, D. Yin, X. Shi, Z. Cao, Z. Tian, H. Kim, Y. Guo, C. Sun, L. Cavallo, L. Wang, H. N. Alshareef, Y.-K. Sun and J. Ming, Angew. Chem., Int. Ed., 2023, 62, e202216189 CrossRef CAS PubMed.
- S. Yuan, S. Cao, X. Chen, J. Wei, Z. Lv, H. Xia, J. Li, H. Zhang, L. Liu, C. Tian, L. Chen, W. Zhang, Z. Xing, H. Li, S. Li, Q. Zhu, X. Feng and X. Chen, Adv. Mater., 2024, 36, 2311327 CrossRef CAS PubMed.
- Y. Jie, S. Wang, S. Weng, Y. Liu, M. Yang, C. Tang, X. Li, Z. Zhang, Y. Zhang, Y. Chen, F. Huang, Y. Xu, W. Li, Y. Guo, Z. He, X. Ren, Y. Lu, K. Yang, S. Cao, H. Lin, R. Cao, P. Yan, T. Cheng, X. Wang, S. Jiao and D. Xu, Nat. Energy, 2024, 9, 987–998 CrossRef CAS.
- X. Liu, J. Zhang, X. Yun, J. Li, H. Yu, L. Peng, Z. Xi, R. Wang, L. Yang, W. Xie, J. Chen and Q. Zhao, Angew. Chem., Int. Ed., 2024, 63, e202406596 CrossRef CAS PubMed.
- D. Zhang, L. Li, W. Zhang, M. Cao, H. Qiu and X. Ji, Chin. Chem. Lett., 2023, 34, 107122 CrossRef CAS.
- Y.-G. Cho, M. Li, J. Holoubek, W. Li, Y. Yin, Y. S. Meng and Z. Chen, ACS Energy Lett., 2021, 6, 2016–2023 CrossRef CAS.
- X. Huang, R. Li, C. Sun, H. Zhang, S. Zhang, L. Lv, Y. Huang, L. Fan, L. Chen, M. Noked and X. Fan, ACS Energy Lett., 2022, 7, 3947–3957 CrossRef CAS.
- Y. Huang, C. Wang, H. Lv, Y. Xie, S. Zhou, Y. Ye, E. Zhou, T. Zhu, H. Xie, W. Jiang, X. Wu, X. Kong, H. Jin and H. Ji, Adv. Mater., 2024, 36, 2308675 CrossRef CAS PubMed.
- L.-L. Jiang, C. Yan, Y.-X. Yao, W. Cai, J.-Q. Huang and Q. Zhang, Angew. Chem., Int. Ed., 2021, 60, 3402–3406 CrossRef CAS PubMed.
- H. Su, H. Zhang, Z. Chen, M. Li, J. Zhao, H. Xun, J. Sun and Y. Xu, Chin. Chem. Lett., 2023, 34, 108640 CrossRef CAS.
- Y. Mo, G. Liu, J. Chen, X. Zhu, Y. Peng, Y. Wang, C. Wang, X. Dong and Y. Xia, Energy Environ. Sci., 2024, 17, 227–237 RSC.
- Y. Yang, Z. Fang, Y. Yin, Y. Cao, Y. Wang, X. Dong and Y. Xia, Angew. Chem., Int. Ed., 2022, 61, e202208345 CrossRef CAS PubMed.
- Y. Chen, Q. He, Y. Zhao, W. Zhou, P. Xiao, P. Gao, N. Tavajohi, J. Tu, B. Li, X. He, L. Xing, X. Fan and J. Liu, Nat. Commun., 2023, 14, 8326 CrossRef CAS PubMed.
- D. Lu, X. Lei, S. Weng, R. Li, J. Li, L. Lv, H. Zhang, Y. Huang, J. Zhang, S. Zhang, L. Fan, X. Wang, L. Chen, G. Cui, D. Su and X. Fan, Energy Environ. Sci., 2022, 15, 3331–3342 RSC.
- L. X. Dang, J. Chem. Phys., 1992, 97, 1614 CrossRef CAS.
- Q. Yang, X. Lv, Y. Fu, K. Xu and W. Guo, Joule, 2025, 9, 101821 CrossRef CAS.
- S. Li, H. Zhu, C. Gu, F. Ma, W. Zhong, M. Liu, H. Zhang, Z. Zeng, S. Cheng and J. Xie, ACS Energy Lett., 2023, 8, 3467–3475 CrossRef CAS.
- W. Yang, W. Chen, H. Zou, J. Lai, X. Zeng, Y. Zhang, X. Zeng, K. Ding, S. Zhang, L. Ma, Z. Li and Q. Zheng, Angew. Chem., Int. Ed., 2025, 64, e202424353 CrossRef CAS PubMed.
- J. B. Goodenough and Y. Kim, Chem. Mater., 2009, 22, 587–603 CrossRef.
- F. Su, Y. Lin, X. Dou, H. You, S. Gao, Z. Bai, J. Jiang, L. Chen and C. Li, Angew. Chem., Int. Ed., 2025, e202510647 CAS.
- K. Xu, Chem. Rev., 2014, 114, 11503–11618 CrossRef CAS PubMed.
- P. Ma, C. Xue, K.-H. Wang, P. Mirmira, M. C. Vu, O. Rivera and C. V. Amanchukwu, ACS Energy Lett., 2024, 9, 6144–6152 CrossRef CAS.
- C. Shi, Z. Li, M. Wang, S. Hong, B. Hong, Y. Fu, D. Liu, R. Tan, P. Wang and Y. Lai, Energy Environ. Sci., 2025, 18, 3248–3258 RSC.
- X. Y. Huang, C. Z. Zhao, W. J. Kong, N. Yao, Z. Y. Shuang, P. Xu, S. Sun, Y. Lu, W. Z. Huang, J. L. Li, L. Shen, X. Chen, J. Q. Huang, L. A. Archer and Q. Zhang, Nature, 2025, 646, 343–350 CrossRef CAS PubMed.
- H. Wan, J. Xu and C. Wang, Nat. Rev. Chem., 2024, 8, 30–44 CrossRef CAS PubMed.
- Q. Xiong, D. Li, S. Li, D. Zhang, R. Li, S. Zhang, S. Wang, H. Hong, D. Zhu, Q. Liu, X. Fan and C. Zhi, Sci. Adv., 2025, 11, eadx5020 CrossRef CAS PubMed.
- F. Zhang, P. Zhang, W. Zhang, P. R. Gonzalez, D. Q. Tan and Y. Ein-Eli, Adv. Mater., 2024, 36, 2410277 CrossRef CAS PubMed.
- Y. Zhang, Y. Zhang, X. Wang, H. Gong, Y. Cao, K. Ma, S. Zhang, S. Wang, W. Yang, L. Wang and J. Sun, Adv. Energy Mater., 2025, 13, 2403751 CrossRef.
- Y. He, P. Zou, S.-M. Bak, C. Wang, R. Zhang, L. Yao, Y. Du, E. Hu, R. Lin and H. L. Xin, ACS Energy Lett., 2022, 7, 2866–2875 CrossRef CAS.
- J. Liu, M. Wu, X. Li, D. Wu, H. Wang, J. Huang and J. Ma, Adv. Energy Mater., 2023, 13, 2300084 CrossRef CAS.
- Y. Lu, Q. Cao, W. Zhang, T. Zeng, Y. Ou, S. Yan, H. Liu, X. Song, H. Zhou, W. Hou, P. Zhou, N. Hu, Q. Feng, Y. Li and K. Liu, Nat. Energy, 2025, 10, 191–204 CAS.
- X. Fan, X. Ji, L. Chen, J. Chen, T. Deng, F. Han, J. Yue, N. Piao, R. Wang, X. Zhou, X. Xiao, L. Chen and C. Wang, Nat. Energy, 2019, 4, 882–890 CrossRef CAS.
- R. Gu, D. Zhang, S. Xu, X. Guo, Y. Xiao, Z. Sheng, Q. Xu, J. Xu, S. Zhu, K. Liao, S. Gong, P. Shi and Y. Min, Nat. Commun., 2025, 16, 5474 CrossRef CAS PubMed.
- J. Holoubek, M. Yu, S. Yu, M. Li, Z. Wu, D. Xia, P. Bhaladhare, M. S. Gonzalez, T. A. Pascal, P. Liu and Z. Chen, ACS Energy Lett., 2020, 5, 1438–1447 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |