Multiscale interfacial engineering strategies for inorganic all-solid-state lithium batteries
Min
Xu
a,
Hongmin
Liu
a,
Xinran
Gao
a,
Yitao
Lou
a,
Huakun
Liu
a,
Shixue
Dou
a,
Nana
Wang
 b and
Zhongchao
Bai
b and
Zhongchao
Bai
 *a
*a
aInstitute of Energy Materials Science (IEMS), University of Shanghai for Science and Technology, 516 Jungong Road, Shanghai, 200093, China. E-mail: baizhongchao@tyut.edu.cn
bCentre for Clean Energy Technology, School of Mathematical and Physical Sciences, Faculty of Science, University of Technology Sydney, Sydney, NSW 2007, Australia. E-mail: nanaw@uow.edu.au
First published on 25th November 2025
Abstract
All-solid-state lithium batteries (ASSLBs) offer exceptional energy density and safety, yet interfacial instability at both cathode and anode remains a major challenge. This review pioneers a unified, multiscale framework that systematically dissects interfacial failure mechanisms across mainstream inorganic solid-state electrolytes (SSEs)—including oxides, sulfides, and halides—under multiphysics coupling. Unlike previous studies focusing on isolated materials or single strategies, we provide a critical, side-by-side comparison of leading interface engineering routes—electrode engineering, interlayer construction, electrolyte regulation, and integrated dual-interface design—emphasizing their respective merits, limitations, and application boundaries. Furthermore, advanced characterization techniques are summarized to reveal dynamic interfacial evolution and validate the effectiveness of various engineering approaches, thereby bridging fundamental understanding with practical design. Finally, future perspectives highlight multiphysics characterization, machine-learning-assisted interface material screening, and scalable process integration. This review offers a comprehensive, comparative, and practical guide to interface innovation in high-performance ASSLBs.
Broader contextWith the global push toward carbon neutrality and the rapid integration of renewable energy, advanced storage systems are required to deliver higher energy density, enhanced safety, and scalable manufacturing. While commercially mature, conventional lithium-ion batteries are intrinsically limited by the flammability of liquid electrolytes and their poor compatibility with high-capacity electrodes, restricting their suitability for next-generation applications such as electric vehicles and grid storage. ASSLBs enabled by thermally stable SSEs offer improved intrinsic safety, structural robustness, and access to the full capacity of lithium metal. However, the solid–solid nature of the electrode–electrolyte interface gives rise to coupled electrochemical, chemical, and mechanical challenges. Poor physical contact, interfacial reactions, and uncontrolled lithium dendrite growth critically limit the rate capability and long-term cycling stability of ASSLBs. Overcoming these issues requires holistic interface design strategies that integrate structural optimization, interfacial chemistry regulation, and advanced multiscale characterization. This review examines three major classes of SSEs—oxides, sulfides, and halides—systematically analyzing their interfacial contact behavior, electrochemical compatibility, and dendrite-related failure modes. It further summarizes mainstream interface engineering approaches and clarifies their applicability across different SSE systems, providing mechanistic understanding and practical guidance for the development of high-performance ASSLBs. |
1. Introduction
Against the backdrop of carbon neutrality and the rapid expansion of renewable energy, energy storage technologies are facing unprecedented demands for both enhanced performance and intrinsic safety.1–3 Conventional lithium-ion batteries, limited by their energy density and the safety risks associated with liquid electrolytes, can no longer meet the growing requirements for high energy and high reliability in electric transportation, grid regulation, and large-scale energy storage systems.4,5 In this context, ASSLBs have emerged as a promising candidate for next-generation high-energy-density storage systems, owing to the ultra-high theoretical specific capacity (3860 mAh g−1) and the lowest electrochemical potential (−3.04 V vs. SHE) of lithium metal, as well as the enhanced thermal stability and safety enabled by replacing flammable liquid electrolytes with SSEs.6–9 Within ASSLBs, SSEs not only serve as ionic conductors but also fulfill critical roles in interfacial contact, mechanical support, and dendrite suppression, with their properties directly governing the overall energy output and cycling stability of the battery.10,11 SSEs can be broadly classified into organic polymer-based and inorganic crystalline systems, depending on their chemical composition and phase structure.12–15 Polymer electrolytes based on Poly(ethylene oxide) (PEO) and Poly(methyl methacrylate) (PMMA) offer good flexibility and film-forming ability, facilitating intimate contact with electrodes. However, their inherently limited ionic conductivity and electrochemical stability hinder their compatibility with high-voltage cathodes or lithium metal anodes.16–19 In contrast, inorganic SSEs exhibit highly ordered crystalline frameworks and diverse topological Li+ conduction pathways, providing fast and well-defined ionic transport through interconnected tetrahedral and octahedral sites. Their rigid inorganic lattices and strong ionic bonding confer high thermal and electrochemical stability, enabling operation over wide voltage windows (>4 V) and elevated temperatures without degradation. Meanwhile, the high mechanical modulus of inorganic SSEs ensures structural stability and stable electrode–electrolyte contact, effectively suppressing lithium dendrite penetration.20–22 Depending on their anionic frameworks and crystal symmetries, inorganic SSEs can be divided into three major families: oxide, sulfide, and halide systems, each possessing unique conduction mechanisms and structural advantages (Table 1). Oxide SSEs (e.g., Li7La3Zr2O12, Li1.3Al0.3Ti1.7(PO4)3) feature strong structural rigidity, broad electrochemical stability, and excellent compatibility with high-voltage cathodes; sulfide SSEs (e.g., Li10GeP2S12, Li6PS5Cl) offer superionic conductivity up to 10−2 S cm−1, high deformability, and facile processability; while halide SSEs (e.g., Li3InCl6, Li3YCl6) combine high ionic conductivity (∼10−3 S cm−1) with exceptional oxidative stability and favorable mechanical compliance.23–33 These collective merits endow inorganic SSEs with the ability to simultaneously achieve fast Li+ transport, wide electrochemical operation windows, and robust mechanical reliability, establishing them as one of the most promising material systems for next-generation solid-state batteries.| Electrolyte system | Type | Crystal structure | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|
| Oxide SSEs | Garnet | Cubic/Tetragonal (e.g. Li7La3Zr2O12) | High ionic conductivity (10−2–10−3 S cm−1) | High sintering temperature | 23 |
| Wide electrochemical window | Li2CO3 surface formation | ||||
| Stable vs. Li metal | Poor Li wettability | ||||
| Good mechanical strength | |||||
| Perovskite | Layered ABO3 structure (e.g. Li0.33La0.56TiO3) | High bulk conductivity (∼10−4 S cm−1) | Ti4+ reduction by Li | 24 | |
| Dense microstructure | Large grain-boundary resistance | ||||
| Stable framework | |||||
| LISICON | Orthorhombic/Tetragonal (e.g. Li4SiO4) | Stable | Moderate conductivity (∼10−6–10−4 S cm−1) | 25 | |
| Low-cost | Brittle and rigid | ||||
| Easy synthesis | |||||
| NASICON | Rhombohedral/Orthorhombic (e.g. Li1.3Al0.3Ti1.7(PO4)3) | Good chemical stability | Poor compatibility with Li (Ti4+/Ge4+ reduction) | 26 | |
| Air-stable | High interfacial impedance | ||||
| Strong mechanical strength | |||||
| Sulfide SSEs | Glasses state | Amorphous Li2S–P2S5 glass network | High formability | Low ionic conductivity (∼10−4 S cm−1) | 27 |
| Good interparticle contact | Poor structural stability | ||||
| Simple synthesis | |||||
| Glasses-ceramics state | Mixed amorphous-crystalline (e.g. Li7P3S11) | High ionic conductivity (∼10−3 S cm−1) | Moisture sensitive | 28 | |
| Low interfacial resistance | Narrow electrochemical window | ||||
| Easy densification | |||||
| Thio-LISICON | Monoclinic (e.g. Li10GeP2S12) | Superionic conductivity (∼10−2 S cm−1) | Air-sensitive (H2S release) | 29 | |
| 3D Li+ pathways | Unstable vs. Li metal | ||||
| Soft and ductile | |||||
| Li-argyrodite | Cubic (e.g. Li6PS5Cl) | 3D Li+ migration channels | Limited chemical stability | 30 | |
| Tunable anion disorder | Interfacial decomposition with electrodes | ||||
| High conductivity (∼10−3 S cm−1) | |||||
| Halide SSEs | LISICON-like | Trigonal (e.g. Li3YCl6) | High oxidative stability (>4 V) | Anisotropic Li+ diffusion (1D channels) | 31 |
| Low migration barrier | Moderate ionic conductivity (∼10−3 S cm−1) | ||||
| Tunable by cation substitution | |||||
| Good deformability | |||||
| Orthorhombic (e.g. Li3LuCl6) | Air-tolerant | Low ionic conductivity (∼10−4 S cm−1) | 32 | ||
| Stable framework | Hygroscopic under humid conditions | ||||
| Compatible with high-voltage cathodes | |||||
| Monoclinic (e.g. Li3InCl6) | High ionic conductivity (2–3 × 10−3 S cm−1) | Unstable vs. Li metal | 33 | ||
| 3D Li+ migration network | Moisture-sensitive | ||||
| Facile wet-chemical synthesis | High In cost |
However, the inherent structural rigidity and electronic bandwidth narrowing resulting from the high lattice order and chemical stability of inorganic SSEs also give rise to a series of interfacial challenges. On one hand, these materials often exhibit significant mismatches with electrode components in terms of mechanical modulus, electrochemical potential, and lattice structure. Such disparities lead to stress concentration, abrupt ionic flux transitions, and distorted electric field gradients at solid–solid interfaces, ultimately causing poor interfacial contact, ion transport bottlenecks, and localized electrochemical instability.34–38 On the other hand, their intrinsically low electronic conductivity, while beneficial for suppressing the propagation of parasitic reactions, also promotes the formation of space charge layers (SCL) and electron–ion decoupling at the interface, further exacerbating interfacial polarization and the heterogeneity of interphase evolution.39–41 Moreover, under extreme chemical potential conditions such as at the interfaces with lithium metal or high-voltage cathodes, the active metal centers in inorganic SSEs (e.g., Ti4+, Ge4+, In3+) are often subject to valence state changes, leading to the formation of mixed ionic-electronic conducting (MIEC) phases. These transformations result in increased interfacial impedance and continuous performance degradation.42–44 Therefore, establishing stable interfaces between inorganic SSEs and electrodes has become a key scientific challenge in advancing ASSLBs from laboratory research toward practical engineering applications.
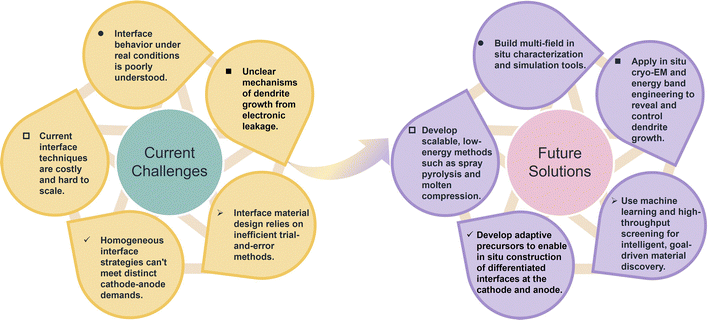
Under this background, numerous strategies have been proposed in recent years to engineer stable interfaces between inorganic SSEs and electrodes, which can be roughly summarized into the following aspects: (1) introducing interfacial buffer layers to create graded transitions in electrochemical potential and mechanical modulus, using materials such as alloys, oxides and polymers.45–49 (2) modifying the electrode materials via surface coating, elemental doping, or crystal structure reconstruction to suppress interfacial reactivity and enhance compatibility with ionic flux.50–54 (3) applying surface in situ chemical pretreatment, elemental tuning, or grain boundary engineering to the SSEs in order to enhance its tolerance to extreme chemical environments.55–60 (4) engineering composite lithium metal anodes or incorporating three-dimensional host structures to relieve deposition stress, enhance interfacial wettability, and enable uniform lithium plating.61–65 Despite substantial advances at the material level, most existing approaches remain confined to a single class of SSEs or isolated physicochemical factors. Current reviews typically focus on specific SSE families or individual interfacial engineering strategies, lacking a comprehensive framework for cross-system comparison and critical evaluation. More importantly, the advantages, limitations, and practical boundaries of various interfacial strategies have rarely been systematically summarized, hindering the transfer and generalization of interfacial mechanism understanding. In addition, conventional methods often fall short in addressing increasingly complex application scenarios—such as high current densities, extreme temperatures, or the integration of high-voltage cathodes—thus exposing core challenges in stability, universality, and scalability.
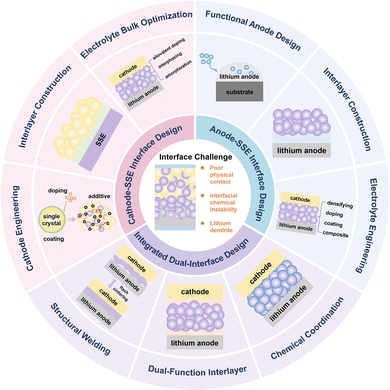
To address these gaps, this review proposes, for the first time, a multiscale, multiphysics-coupled analytical framework that systematically dissects the key interfacial failure mechanisms in inorganic ASSLBs, comprehensively covering the three major classes of SSEs: oxides, sulfides, and halides. By integrating interfacial challenges at both the cathode and anode—such as structural mismatch, interfacial reactions, electronic leakage, and lithium dendrite evolution—with the intrinsic properties of each SSE system and their coupling mechanisms under multiphysical fields, this review offers a holistic and in-depth analysis. Furthermore, this work categorizes and critically compares state-of-the-art interfacial optimization strategies from four key dimensions: electrode engineering, interlayer construction, electrolyte regulation, and integrated dual-interface design (Fig. 1). Of particular note, the review provides a thorough comparative analysis of the strengths, limitations, and applicability of each strategy—not only elucidating their fundamental mechanisms but also clarifying their material compatibility and potential for scalable engineering. These systematic comparisons and critical insights contribute to the establishment of a unified and logically coherent framework for interfacial design, alongside actionable guidance for engineering practice. Together, they lay a solid theoretical foundation for achieving high energy density and long cycle life in ASSLBs, while clearly highlighting the distinctive innovation and integrated perspective of this review in research scope, methodological approach, and strategic synthesis.
2. Cathode–electrolyte interface
2.1. Challenges
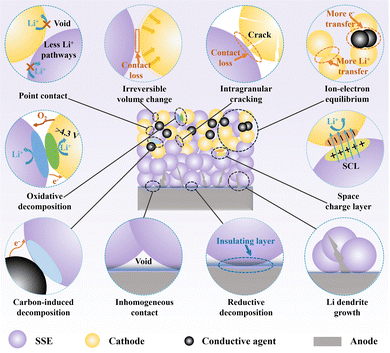
In ASSLBs, the interfaces between the cathode and SSEs represent a critical bottleneck that limits both energy density utilization and long-term cycling stability. Despite the distinct properties and application merits of the three major SSE families—oxides, sulfides, and halides—they face several common interfacial challenges when integrated with high-voltage cathodes, including mechanical mismatch, chemical instability, and ion transport barriers induced by SCL (Fig. 2).Oxide SSEs generally exhibit a wide electrochemical stability window and excellent thermal robustness, demonstrating strong oxidation resistance under high-voltage cathode conditions.76,77 Nevertheless, achieving sufficient densification and high ionic conductivity often necessitates high-temperature sintering to promote grain growth and eliminate porosity. During this process, interdiffusion and interfacial reactions may occur between the oxide SSEs and cathode active materials (e.g., LiCoO2), causing the emergence of undesirable secondary phases such as LaCoO3 and LiTi2(PO4)3—where LaCoO3 acts as an ionic insulating phase impeding Li+ transport, while LiTi2(PO4)3, though a NASICON-type Li+ conductor, can undergo reduction of Ti4+ to Ti3+, leading to irreversible lithium consumption and interfacial instability—which disrupt the structural integrity of the interface.78,79
Sulfide SSEs offer high room-temperature ionic conductivity and good moldability, which facilitate the formation of dense structures and continuous lithium-ion transport pathways, thereby enabling low interfacial resistance. Although their thermodynamic electrochemical stability window is relatively narrow (approximately 1.7–2.2 V vs. Li+/Li), sulfide SSEs can still stably operate with high-voltage cathodes because, during the initial charging process, limited interfacial redox reactions occur between the oxidized cathode surface and the sulfide framework. These reactions generate a thin layer of partially oxidized species (e.g., Li2S, Li3P, and Li–S–O/P–S–O compounds) that electronically isolate the bulk electrolyte while maintaining Li+ conduction.80,81 Once this layer forms, it kinetically suppresses further electron transfer and electrolyte decomposition, thus acting as a self-passivating, metastable interphase that effectively extends the apparent electrochemical stability window beyond the thermodynamic limit. However, this kinetic stabilization is not permanent. During prolonged cycling, mechanical stress and repeated lithiation/delithiation induce microcracks at the electrode–electrolyte interface, exposing fresh reactive surfaces that locally rupture the passivation layer. Meanwhile, high-voltage operation continuously generates reactive oxygen species, which further attack P–S and Ge–S bonds in the sulfide framework, triggering progressive oxidative degradation and producing electronically insulating by-products such as Li2SO4 and Li3PO4. These insulating phases accumulate at the interface, thickening the cathode electrolyte interphase (CEI) and increasing interfacial impedance.82,83 Ultimately, localized electron penetration and spatially inhomogeneous reactions lead to excessive polarization, loss of ionic percolation pathways, and gradual deterioration of interfacial stability.
Halide SSEs have recently attracted significant attention as emerging electrolyte systems, owing to their excellent ionic conductivity and theoretical stability against high-voltage cathodes. However, recent studies have revealed that halide SSEs still face risks of interfacial chemical evolution under practical operating conditions. Under high electrochemical potentials, the release of high-energy lattice oxygen from the cathode generates a strongly oxidative environment that can trigger the oxidation of halide ions (Cl− or Br−) within the electrolyte, converting them into neutral Cl0 or Br0 species. These neutral intermediates are often volatile or chemically unstable, compromising the electronic insulation of the electrolyte, inducing electron leakage, and driving continuous evolution of the interfacial electronic structure.84 In parallel, high-valence transition metal ions (e.g., Ni3+/Ni4+) present in high-voltage cathodes possess strong electrostatic modulation capabilities. These species can trigger valence fluctuations and charge redistribution of halide anions at the interface, promoting the gradual formation of non-uniform reaction layers. Such interfacial inhomogeneity distorts the potential landscape and hinders efficient lithium-ion transport.85 Therefore, although the interfacial reaction kinetics of halide SSEs are generally slower than those of sulfide counterparts, the progressive accumulation of degradation products during cycling can still result in impedance growth and capacity fading over time.
In summary, the multifaceted interfacial evolution at the cathode side originates from the synergistic effects of mechanical mismatch, electrochemical imbalance, and charge redistribution. Although the specific manifestations vary depending on the types of SSEs, they fundamentally converge on two critical issues: the disruption of structural continuity and the obstruction of lithium-ion transport pathways. A deeper mechanistic insight into interface degradation across different SSE systems, coupled with the deliberate construction of interfacial microenvironments that offer mechanical compliance, chemical robustness, and electrostatic compatibility, will be pivotal for enabling the long-term stability of cathode interfaces in high-performance ASSLBs.
2.2. Optimization strategies
In ASSLBs, the interfacial stability between high-voltage cathodes and SSEs is a critical factor governing overall cell performance. To address common challenges at the cathode–SSE interface—such as mechanical stress mismatch, interfacial chemical reactivity, and impeded ion transport—various interfacial engineering strategies have been proposed. These approaches can be broadly categorized into three main types: cathode engineering, interlayer construction and electrolyte bulk optimization. The following sections provide a detailed discussion of each approach, accompanied by comparative analyses of representative pathways and case studies.Single-crystal design. Given that grain boundaries in polycrystalline materials often act as initiation sites for stress concentration and structural degradation during cycling, the use of single-crystal particles has been proposed as an effective approach to improve the structural robustness of cathode materials. For instance, in contrast to polycrystalline counterparts where anisotropic lattice expansion often induces microcrack formation and interfacial delamination, micron-sized single-crystal LiNi0.83Co0.11Mn0.06O2 (SC-N83) particles effectively suppress these degradation mechanisms, thereby exhibiting excellent cycling stability even at high voltages (up to 4.4 V) (Fig. 3a).90 However, due to the limitations of large single-crystal particles in terms of ion transport kinetics and interfacial contact, the development of smaller single-crystal cathodes has gained increasing attention. Tian et al. reported that ∼1 µm single-crystal LiNi0.8Co0.1Mn0.1O2 (NCM811) particles not only display superior structural integrity compared to conventional large-particle designs, but also offer enhanced interfacial contact and electrochemical stability under high-loading and high-rate conditions, attributed to their larger specific surface area and shorter diffusion pathways.91
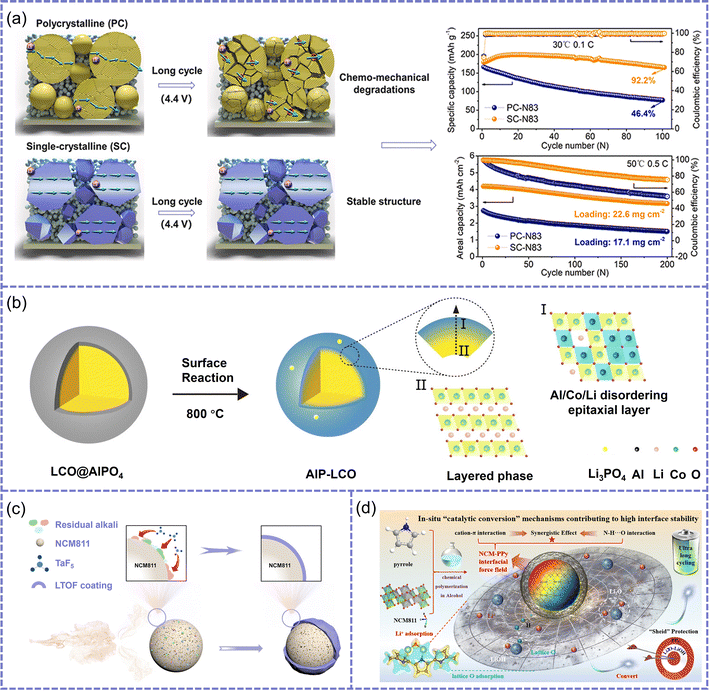 | ||
| Fig. 3 (a) The structural evolution and cycling performance of the PC-N83 and SC-N83 electrodes. Reproduced with permission.90 Copyright 2021, Elsevier. (b) Schematic illustration of the surface reconstruction of LCO. Reproduced with permission.92 Copyright 2024, John Wiley and Sons. (c) Schematic diagram of the synthesis process for Li–Ta–O–F (LTOF) oxyhalide coating through an in situ gas–solid reaction. Reproduced with permission.97 Copyright 2024, John Wiley and Sons. (d) Schematic of the preparation process and principle of LCO@PPy. Reproduced with permission. Reproduced with permission.103 Copyright 2024, Royal Society of Chemistry. | ||
Structural and surface regulation. Although single-crystal engineering helps preserve the internal structural coherence of cathode particles, the surface remains highly susceptible to severe interfacial side reactions under high-voltage conditions. To address this, Zhang et al. proposed a surface lattice doping strategy for LiCoO2 (LCO) cathode.92 By introducing AlPO4 precursor and inducing Al3+ ion doping into the surface lattice of the cathode through high-temperature annealing, cation-disordered layer with a rock-salt-like structure is formed (Fig. 3b). This Al/Co/Li mixed atomic layer exhibits low electrochemical activity and exceptional interface stability, effectively inhibiting the oxidative decomposition of Li3InCl6 under high voltage and maintaining the structural integrity and reversible lithium-ion transport capability of the cathode–SSE interface at 4.5 V. However, doping strategies are primarily oriented toward lattice-level modifications and remain insufficient to mitigate direct interfacial degradation between the cathode and SSE. To tackle this issue, the construction of physical and chemical buffering layers on particle surfaces is still imperative.
In this context, inorganic coating layers have emerged as a preferred approach.93–98 Zhang et al. employed a gas–solid interfacial reaction to construct a CoO/Li2CO3 dual-phase layer on the surface of LCO (SR-LCO), which effectively passivates reactive oxygen species and blocks the diffusion of active anions such as S2− and PS43− from the sulfide electrolyte.96 As a result, the SR-LCO cathode retains 84.63% of its capacity after 400 cycles at 0.2C. Unfortunately, despite the advantages of facile fabrication and in situ reconstruction, this strategy still faces challenges under long-term high-voltage cycling, such as interfacial stress concentration and compositional inhomogeneity arising from the dual-phase crystalline structure. To further improve interfacial structural continuity and regulation precision, Zhang et al. employed volatile TaF5 to react with surface residues on the cathode, thereby in situ forming an amorphous Li–Ta–O–F coating (Fig. 3c).97 This layer is ion-conductive but electronically insulating, enabling the battery to retain 80.4% of its capacity after 200 cycles. It is worth noting that although the amorphous inorganic interfacial layer significantly mitigates chemical instability, it still exhibits limitations in terms of strain accommodation and interfacial compliance. To this end, flexible organic coatings have been proposed as a complementary strategy.99–103 Zhou et al. constructed an organic interfacial layer using polypyrrole (PPy), where −NH groups form hydrogen bonds with lattice oxygen, suppressing oxygen release and enabling the in situ formation of a Li2O–LiOH buffer layer to mitigate the oxidative decomposition of the SSE (Fig. 3d).103 More importantly, the flexible structure of PPy significantly enhances physical contact between the cathode and the SSE, effectively accommodating volume changes during cycling. As a result, the NCM@PPy cathode achieves a capacity retention of 81.2% after 300 cycles. Since neither inorganic nor organic coatings alone can simultaneously ensure chemical stability and mechanical flexibility, Su et al. employed molecular layer deposition (MLD) to construct an aluminum-glycerol (Al-GL) inorganic–organic hybrid coating on the surface of NCM811 (Fig. 4a).104 This coating shows an elastic modulus of 0.17 GPa and demonstrates excellent adhesion and strain adaptability, effectively preventing structural cracking and electrolyte delamination. Thus, the battery retains 88% of its capacity after 100 cycles at 0.2C (Fig. 4b).
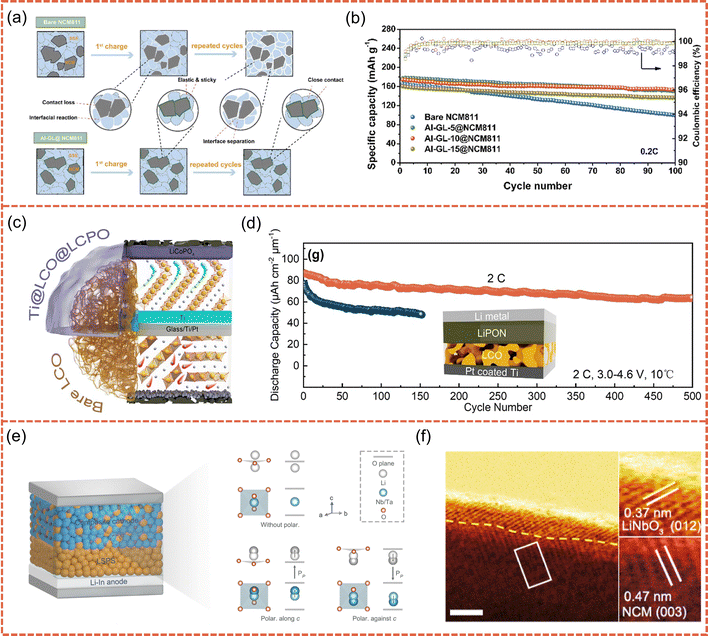 | ||
| Fig. 4 (a) Schematic diagram of the functioning mechanism for the Al-GL coating. (b) The long-term cycling performance tests of the bare NCM811, Al-GL-5@NCM811, Al-GL-10@NCM811, and Al-GL-15@NCM811 at 0.2C. Reproduced with permission.104 Copyright 2023, Elsevier. (c) The scheme of dual-modified layer strategy for LCO thin film. (d) The cycling stability of bare LCO and Ti@LCO@LCPO under the rate of 2C at 10 °C. Reproduced with permission.106 Copyright 2024, Elsevier. (e) Schematic illustration of LMO3 (M = Ta and Nb) polarization in ASSBs. (f) Corresponding HAADF-STEM image for modified NCM. Reproduced with permission.107 Copyright 2024, American Chemical Society. | ||
In fact, isolated coating or doping strategies still face constraints in addressing structural degradation and interfacial instability under high-voltage conditions. As a result, synergistic designs that combine bulk doping with surface modification have gradually emerged as a mainstream approach.105–107 Qiu et al. stabilized the lattice structure through Ti doping and introduced a LiCoPO4 coating layer epitaxially matched with the LCO lattice, thereby constructing a Ti@LCO@LiCoPO4 cathode with dual stabilization effects (Fig. 4c).106 This design markedly boosts cycling stability at high voltage and achieves a capacity retention of 75% after 500 cycles under 2C at 10 °C (Fig. 4d). In contrast to conventional static regulation strategies that rely on lattice doping and surface coating to achieve interfacial stability, Dai et al. proposed a dynamic “chemical competitive diffusion” mechanism, where heteroatoms with distinct migration kinetics and bonding strengths spontaneously distribute along a bulk-to-surface gradient during annealing.107 Ta5+ ions, possessing stronger Ta–O bonding and higher lattice-oxygen affinity, remain anchored within the bulk to suppress oxygen evolution and enhance structural integrity. In contrast, Nb5+ ions, with higher diffusivity, migrate outward and react with residual Li to form a self-assembled LiNbO3 piezoelectric layer (Fig. 4e and f). This diffusion-driven reconstruction produces a graded interface endowed with spontaneous polarization that regulates the SCL, aligns interfacial electric fields, and promotes Li+ transport across the cathode–electrolyte interface. With this regulation, the NCM cathode paired with Li10SnP2S12 electrolyte demonstrates significantly enhanced capacity retention and cycling stability at 4.5 V.
Functional network and structural integration. In composite cathode systems, even with effective surface regulation of active particles, the continuity of electronic and ionic pathways, along with the mechanical cohesion of the entire electrode, remains critical to interfacial performance and long-term stability. Although conventional carbon black exhibits high electronic conductivity, its large specific surface area and low crystallinity introduce numerous structural defects and oxygen-containing functional groups, which tend to generate localized electron accumulation regions at the carbon–electrolyte interface, thereby triggering the electrochemical decomposition of sulfide SSEs. To address this issue, Kim et al. systematically investigated the interfacial chemical stability of carbon materials with different degrees of crystallinity.108 They compared amorphous carbon black (CB), graphitized carbon black (GCB) obtained via high-temperature treatment, and carbon nanofibers (CNF) possessing long-range ordered sp2 carbon frameworks in Li6PS5Cl (LPSC) systems (Fig. 5a). The results reveal that high-crystallinity carbons (such as GCB and CNF), owing to their more ordered graphitic layers, lower surface defect densities, and reduced chemical reactivity, effectively suppress charge-transfer-induced electrolyte decomposition, thereby reducing interfacial resistance and improving cycling stability. On this basis, to further balance the construction of conductive networks with electrolyte stability, Saqib et al. proposed a hybrid conductive architecture comprising a minimal amount (0.3 wt%) of carbon nanotubes (CNT) and a higher proportion (4.7 wt%) of CNF, establishing a synergistic long-short electron transport network (Fig. 5b).109 This approach avoids the electrolyte instability typically induced by conventional carbon additives such as Super P, while delivering superior rate capability and capacity retention, thereby validating the importance of optimizing both the geometry and loading ratio of carbon components in composite cathode systems. To further eliminate interfacial instability associated with carbon-based conductors, Fang et al. proposed the use of Ti2O3 as a substitute for conventional conductive carbon (Fig. 5c).110 Ti2O3 not only exhibits metallic-level electronic conductivity (10–102 S cm−1) but also effectively scavenges lattice oxygen released from high-voltage cathodes during cycling, thereby suppressing interfacial oxidative reactions. A composite cathode constructed with Ti2O3, NCM811, and LPSC demonstrates excellent interfacial stability, delivering an initial capacity of 192 mAh g−1 and retaining 86.5% of its capacity after 140 cycles. These results confirm the dual functionality of non-carbon conductors in enhancing both interfacial stability and electronic conductivity in ASSLB systems.
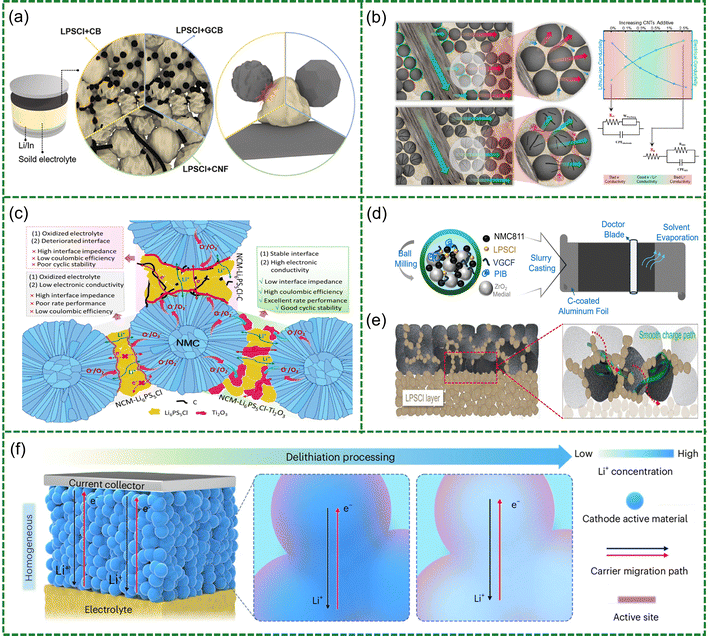 | ||
| Fig. 5 (a) Schematic showing cell structure containing sulfide electrolyte and different types of conductive materials. Reproduced with permission.108 Copyright 2025, Elsevier. (b) Schematic illustration highlighting the mechanisms of ionic and electrical conductivities in a cathode composite. Reproduced with permission.109 Copyright 2024, American Chemical Society. (c) Schematic illustration, showing the interfaces in NCM-00, NCM-C, and NCM-Ti2O3 cathodes. Reproduced with permission.110 Copyright 2023, Elsevier. (d) Schematic diagram of fabricating slurry-cast NMC811 cathode. (e) Schematic diagram of 1270PIB improves the integrity of cathode that facilitates the Li transport over cycling. Reproduced with permission.112 Copyright 2024, John Wiley and Sons. (f) Schematic illustration of homogeneous cathode microstructure evolution during charging. Reproduced with permission.114 Copyright 2024, Springer Nature. | ||
Beyond conductive additives, binders also play an essential role in determining the film-forming quality, ionic transport pathways, and interfacial stability of composite cathodes.111–113 Li et al. found that high-molecular-weight polyisobutylene (PIB) facilitates particle adhesion and strain-buffering capability (Fig. 5d and e), resulting in 44.77% increase in capacity under high mass loading conditions (8.51 mg cm−2).112 Unlike other high-molecular-weight binders, PIB possesses a non-polar hydrocarbon backbone that is chemically compatible with sulfide SSEs and the non-polar solvent (toluene) used in slurry processing. This unique combination enables uniform particle wetting, continuous coating formation, and robust chain entanglement, thereby strengthening interparticle adhesion and suppressing interfacial voids. Nevertheless, the absence of ionic conductivity in PIB limits its overall performance enhancement. Toward this end, Charlesworth et al. developed a single-ion-conducting polycarbonate binder—poly(5-methyl-5-allyloxycarbonyl-trimethylene carbonate) (PMAC)—which combines excellent mechanical flexibility with interfacial adaptability, significantly improving lithium-ion transport and cycling stability.113
Still, the aforementioned strategies still rely on multiphase composite architectures, where the separation of electronic and ionic pathways and the complexity of interfacial configurations hinder structural integration and long-term stability. To address these limitations more fundamentally, intrinsic structural design of cathode materials offers a more holistic solution. Cui et al. proposed a “homogeneous cathode” concept and developed a Li1.75Ti2(Ge0.25P0.75S3.8Se0.2)3 material featuring zero-strain characteristics and intrinsic mixed conductivity (Fig. 5f).114 Unlike conventional layered oxide cathodes that suffer from anisotropic lattice expansion and interfacial delamination, this sulfide-based Ti–P–S–Se framework originates from LiTi2(PS4)3, a three-dimensional open network composed of edge-sharing TiS6 octahedra and PS4 tetrahedra. The partial substitution of P by Ge optimizes Li+ diffusion channels (DLi+ = 6.5 × 10−8 cm2 s−1), while S-to-Se anion modulation narrows the bandgap from 1.29 eV to 0.52 eV, achieving high mixed ionic-electronic conductivities (σLi+ = 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mS cm−1, σe = 225 mS cm−1). Owing to its low Young's modulus (17 GPa) and reversible Ti4+/Ti3+/Ti2+ and P5+/Px+ redox couples, Li1.75Ti2(Ge0.25P0.75S3.8Se0.2)3 undergoes only 1.2% volume change during cycling, maintaining structural integrity and interfacial conformity. Batteries constructed solely from this material, without any conductive or ionic additives, exhibit over 20
mS cm−1, σe = 225 mS cm−1). Owing to its low Young's modulus (17 GPa) and reversible Ti4+/Ti3+/Ti2+ and P5+/Px+ redox couples, Li1.75Ti2(Ge0.25P0.75S3.8Se0.2)3 undergoes only 1.2% volume change during cycling, maintaining structural integrity and interfacial conformity. Batteries constructed solely from this material, without any conductive or ionic additives, exhibit over 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 stable cycles and an energy density of 390 Wh kg−1, pushing the boundaries of conventional cathode structure and functionality.
000 stable cycles and an energy density of 390 Wh kg−1, pushing the boundaries of conventional cathode structure and functionality.
Polymer interlayers. As one of the earliest approaches explored, polymer interlayers have been widely employed to construct initial interfaces due to their inherent flexibility and film-forming capability. Wang et al. developed a PLC60 interlayer, composed of PEO, lithium salt, and C60, which forms an interfacial film rich in LixPOyFz, LiPxFy, and C60Fn species on the surface of NCM811 cathodes, effectively suppressing cathode structural degradation (Fig. 6a and b).115 However, such interlayers generally suffer from low ionic conductivity, narrow electrochemical windows, and poor tolerance to extreme temperatures, limiting their applicability under harsh conditions. To improve transport properties and adaptability, Wu et al. designed an ether-based gel polymer interlayer featuring a flexible ion-conducting network, which boosts interfacial wettability and ion transport (Fig. 6c).116 The interlayer displays a room-temperature ionic conductivity of 2 mS cm−1, enabling the cell to stably cycle for over 100 cycles at 0.3C (Fig. 6d).
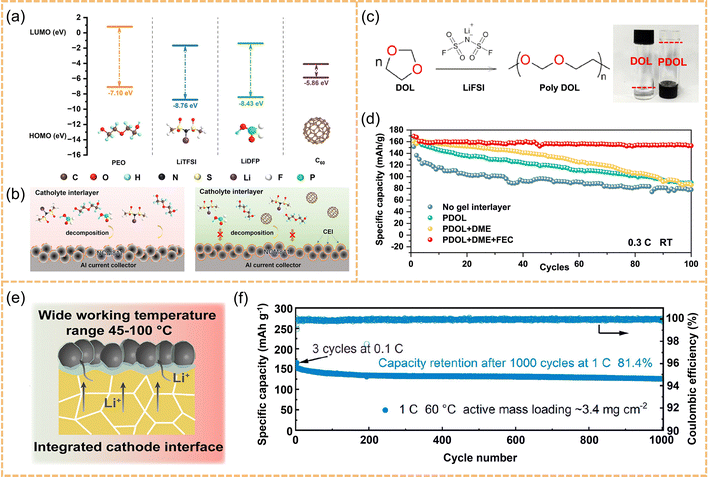 | ||
| Fig. 6 (a) Structural formulas and calculated HOMO/LUMO energies (eV) of the components of the catholyte buffer layer (PEO, LiTFSI, LiDFP, and C60). (b) Schematic diagram showing the positive effects of C60 additives on the catholyte interlayer and the NCM811 cathode. Reproduced with permission.115 Copyright 2024, American Chemical Society. (c) The diagram of the in situ polymerization process and optical photos. (d) Battery cycling of LFP@GPE/LSPCl/3D LSLL coin cells using different type of GPE interlayer at a rate of 0.3C. Reproduced with permission.116 Copyright 2024, Elsevier. (e) Structure for the solid-state battery with (Li,K,Cs)FSI molten salt. (f) Long cycling performance of the Li//LFP batteries modified by (Li,K,Cs)FSI molten salt at the rate of 1C at 60 °C. Reproduced with permission.117 Copyright 2023, American Chemical Society. | ||
Molten salt interlayers. Although gel polymer interlayers enhance interfacial ion transport and adhesion, their organic matrices still suffer from limited thermal stability and poor oxidative resistance, hindering long-term operation under high-temperature or high-voltage conditions. To address these challenges, Yu et al. constructed a partially molten interlayer based on a low-melting (Li,K,Cs)FSI system, which features a wide electrochemical stability window of up to 4.5 V and can adaptively fill interfacial voids while forming continuous ion-conduction pathways at operating temperatures (Fig. 6e).117 This strategy achieves the cell to retain over 80% of its capacity after 1000 cycles at 60 °C and 1C, demonstrating outstanding thermal stability and electrochemical compatibility (Fig. 6f).
Aliovalent doping. Regulating lithium-vacancy concentration and local electronic environments through aliovalent substitution induces controlled lattice distortion that generates low-barrier Li+ migration pathways and optimizes the electronic potential landscape of the anion framework, thereby achieving concurrent enhancement of ionic conductivity and oxidative stability.118–120 For instance, in the Li3−xLu1−xZrxCl6 system, substituting Lu3+ with Zr4+ precisely tunes the Li/vacancy ratio, yielding a high ionic conductivity of 1.5 mS cm−1 and a low activation energy of 0.285 eV.119 The balanced distribution of Li+ and vacancies within the hexagonal close-packed (hcp) anion framework minimizes structural bottlenecks and ensures continuous Li+ transport through face-sharing octahedra, while Zr4+-induced lattice contraction and electrostatic perturbation strengthen the Cl–M bonds (M = Lu3+ or Zr4+), thereby suppressing anion oxidation and enhancing high-voltage stability. This electrolyte shows nearly decay-free cycling over 1000 cycles with high-voltage cathodes. Furthermore, incorporating multiple metal cations (e.g., Y, Er, Yb, In, and Zr) produces a high-entropy electrolyte (Li2.75Y0.16Er0.16Yb0.16In0.25Zr0.25Cl6), whose configurational-entropy-driven cation disorder endows the lattice with distortion-stabilized characteristics (Fig. 7a and b).120 This entropy-mediated structural distortion confines Cl− vibrations and elongates Li–Cl bonds, promoting Li+ activation and inhibiting Cl− oxidation. As a result, this high-entropy halide achieves both high ionic conductivity (1.171 mS cm−1) and an expanded electrochemical stability window (4.6 V), markedly improving interfacial compatibility and structural robustness.
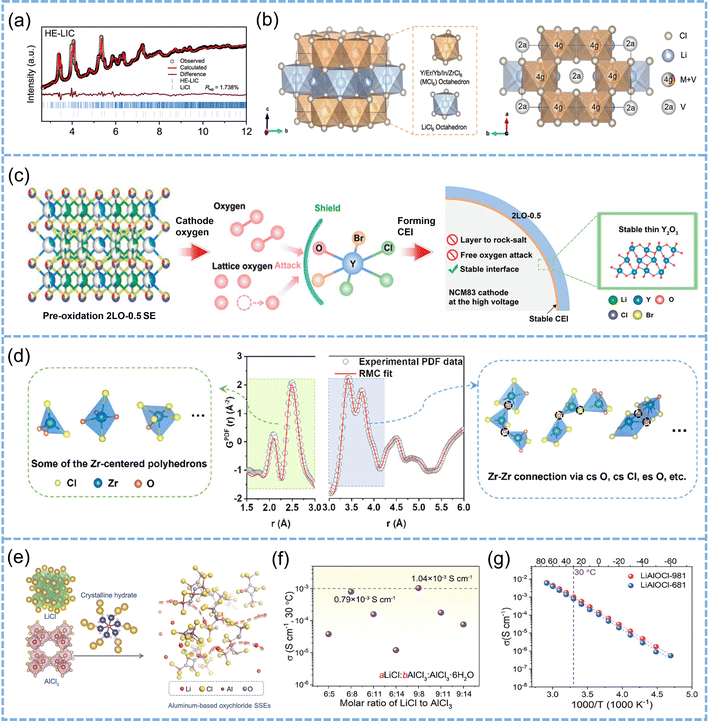 | ||
| Fig. 7 (a) Neutron diffraction patterns and the corresponding refinement of HE-LIC. (b) Graphical representations of a HE-LIC unit cell from the refinement, showing MCl6 (M = Y, Er, Yb, In, Zr) octahedral and LiCl6 octahedral framework. Reproduced with permission.120 Copyright 2024, Springer Nature. (c) Schematic illustration of the CEI evolution in Li2YCl2.5Br1.5O0.5 (2LO-0.5) ASSLBs. Reproduced with permission.123 Copyright 2025, John Wiley and Sons. (d) RMC fit (red line) to the experimental G(r) (gray circle) of Li3ZrCl4O1.5. Several possible basic building blocks in Li3ZrCl4O1.5 are shown on the left side. On the right side, the schemes show the connectivity that leads to the edge-sharing and corner-sharing Zr-centered polyhedra in Li3ZrCl4O1.5, respectively. Reproduced with permission.124 Copyright 2024, American Chemical Society. (e) Illustration of a hydrate-assisted synthesis route for aluminum-based oxychloride SSEs. (f) The ionic conductivity of the LiAlOCl-ab1 SSEs at 30 °C. (g) Arrhenius plots of LiAlOCl-981 (a = 9, b = 8) and LiAlOCl-681 (a = 6, b = 8) SSEs. Reproduced with permission.125 Copyright 2024, John Wiley and Sons. | ||
Interface-guided bulk modification. Unlike conventional doping strategies that originate from crystal lattice design, interface-induced bulk modification emphasizes the design of spontaneous reaction pathways at the operating interface.121–123 For example, Gao et al. introduced F− into a Ta-based oxychloride electrolyte, leading to the in situ formation of a LiF-rich passivation layer at the interface, which effectively suppresses the oxidative decomposition of Cl−.122 Liu et al. incorporated O2− into Li3YCl3Br3 to form a Y–O covalent network, which induces the formation of a Y2O3-dominated passivation layer at the interface, successfully preventing detrimental reactions between cathode-derived active oxygen species and Y3+ (Fig. 7c).123 As a result, the battery retains 80.6% of its capacity after 1000 cycles. This class of strategies enables the in situ construction of stable interphases through “pre-programmed interfacial reactions”, providing an effective buffer against high-voltage electrochemical environments.
Amorphization engineering. Compared to the localized rigidity and ion channel constraints of crystalline electrolyte structures, amorphous structures exhibit stronger interfacial adaptability and diffusion flexibility due to their short-range order and long-range disorder characteristics. In Li3ZrCl4O1.5, oxygen incorporation increases the amorphous content to 89.5%, producing predominantly corner-sharing Zr–O/Cl polyhedra that form a three-dimensional (3D) interconnected network (Fig. 7d).124 This configuration weakens lattice constraints and lowers the Li+ migration barrier (Ea = 0.10 eV vs. 0.12 eV in crystalline Li2ZrCl6), thereby enhancing ionic conductivity from the 10−4 S cm−1 level to (1.3 ± 0.1) × 10−3 S cm−1 at 25 °C. The flexible 3D network accommodates local stress, ensuring intimate contact and chemical stability with high-voltage cathodes. Building on this concept, Wang et al. synthesized amorphous-rich LiAlOCl via a hydrate-assisted route that introduces [AlaObClc](2b+c−3a)− polyanionic clusters into a disordered LiCl-like matrix (Fig. 7e).125 The resulting material contains 59 wt% amorphous component, exhibits Li+ conductivity >1 mS cm−1 (Ea = 0.48 eV) (Fig. 7f and g), and maintains a wide electrochemical stability window up to 4.8 V. The combination of reduced structural rigidity and mixed O/Cl coordination diversifies Li+ environments, weakens Li+–X− interactions, and stabilizes the amorphous network under high-voltage operation. These quantitative comparisons confirm that amorphization—by flattening the energy landscape for Li+ diffusion and providing elastic structural buffering—establishes a direct link between structural flexibility and high-voltage interfacial stability.
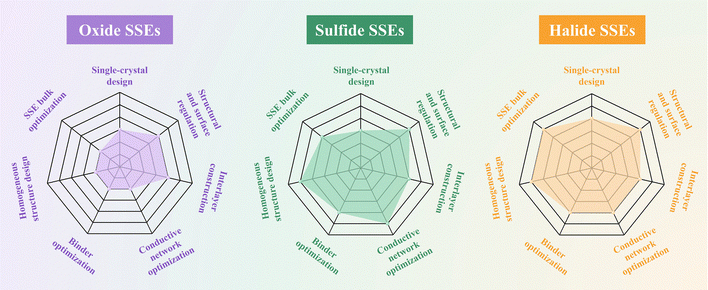
In summary, optimization strategies for the cathode–SSE interface can be broadly categorized into three pathways: cathode engineering, interlayer construction, and electrolyte bulk optimization (Table 2). In terms of general applicability, single-crystal cathode design, structural and surface regulation, as well as interlayer construction, are the main strategies commonly adopted for all three types of SSEs (oxides, sulfides, and halides). Specifically, mainstream cathode materials such as LCO and NCM typically encounter interfacial challenges including structural mismatch, oxygen release, and related side reactions when paired with inorganic SSEs. Additionally, NCM—particularly with high nickel content and polycrystalline structures—is prone to microcrack formation during cycling, further exacerbating interfacial discontinuity and chemical instability. To address these issues, single-crystal strategies, by reducing grain boundaries and microcracks, significantly enhance the mechanical robustness of cathode structures, with especially pronounced benefits for NCM. However, in the case of rigid oxide SSEs, achieving intimate contact between single-crystal particles and the electrolyte remains difficult, and interfacial resistance continues to be a limiting factor. Moreover, direct contact between single-crystal cathodes and SSEs tends to induce more severe high-voltage interfacial side reactions in sulfide systems; for oxide SSEs, interdiffusion and secondary phase formation during high-temperature processing are more prevalent, compromising interfacial electrochemical stability. On this basis, structural and surface regulation of the cathode—such as doping or introducing physical/chemical buffer layers—not only helps maintain the intrinsic cathode structure but also effectively suppresses interfacial side reactions, thereby improving overall interfacial stability. Nevertheless, the ability of these methods to accommodate strain is limited and may introduce compositional inhomogeneity. Building upon this, interlayer construction employing flexible, dense, and chemically passivating designs stands out for its ability to buffer stress, enhance wettability, and inhibit parasitic reactions. However, its long-term chemical stability and ionic conductivity under extreme conditions such as high temperature or voltage still require systematic optimization.
| Interface optimization strategies | Cathode material | SSE type | Modification approaches | Initial capacity (mAh g−1) | Cycling performance | Ref. |
|---|---|---|---|---|---|---|
| Single-crystal design | LiNi0.83Co0.11Mn0.06O2 | Li9.54Si1.74P1.44S11.7Cl0.3 | Grain-boundary-free particles | ∼150 | 85.1% after 500 cycles at 0.5C | 90 |
| LiNi0.8Co0.1Mn0.1O2 | Li9.54Si1.74P1.44S11.7Cl0.3 | Small particle (∼1 µm) | ∼138 | 100% after 500 cycles at 1C | 91 | |
| Surface lattice doping | LiCoO2 | Li3InCl6 | Al3+ doping | 72.4 | 88.5% after 2000 cycles at 3C | 92 |
| Inorganic coating | LiNi0.88Co0.11Al0.01O2 | Li6PS5Cl | Li3YCl6 coating | ∼185 | 84.7% after 200 cycles at 0.5C | 93 |
| LiNiO2 | Li6PS5Cl | LixAlyZnzOδ coating | ∼203.08 | 83.1% after 200 cycles at 0.2C | 94 | |
| LiCoO2 | Li10GeP2S12 | Li7.5La3Zr1.5Co0.5O12 coating | 121 | 96% after 100 cycles at 0.2C | 95 | |
| LiNi0.8Co0.1Mn0.1O2 | Li1.3Al0.3Ti1.7(PO4)3 | La4NiLiO8 coating | 171.49 | 89.47% after 100 cycles at 0.1C | 98 | |
| LiCoO2 | Li6PS5Cl | CoO/Li2CO3 coating | 149.7 | 84.63% after 400 cycles at 0.2C | 96 | |
| LiNi0.8Co0.1Mn0.1O2 | Li6PS5Cl | Li–Ta–O–F oxyhalide coating | 151.5 | 94% after 500 cycles at 1 mA cm−2 | 97 | |
| Organic coating | LiCoO2 | Li5.5PS4.5Cl1.5 | Polyaniline (PANI) coating | ∼131 | 85.5% after 200 cycles at 0.5C | 99 |
| LiNi0.83Co0.11Mn0.06O2 | Li6PS5Cl | Poly((4-vinyl benzyl)trimethylammonium bis(trifluoromethanesulfonylimide)) (PVBTA-TFSI) coating | ∼185 | 86% after 100 cycles at 0.1C | 100 | |
| LiNi0.83Co0.12Mn0.05O2 | Li6PS5Cl | Succinonitrile (SN)-based coating | 168 | 80% after 100 cycles at 0.1C | 101 | |
| LiNi0.95Co0.04Al0.01O2 | Li6PS5Cl | Poly(propylene carbonate)-based ion-conductive polymer (PPC-ICP) coating | 125 | 84% after 100 cycles at 0.5C | 102 | |
| LiNi0.8Co0.1Mn0.1O2 | Li6PS5Cl | Polypyrrole (PPy) coating | 133.2 | 81.2% after 300 cycles at 2C | 103 | |
| Inorganic–organic hybrid coating | LiNi0.8Mn0.1Co0.1O2 | Li9.54Si1.74P1.44S11.7Cl0.3 | Al-GL coating | 174.3 | 88% after 100 cycles at 0.2C | 104 |
| Lattice stabilization and surface coating | LiNi0.9Co0.05Mn0.05O2 | Li6PS5Cl | B doping and coating | ∼185 | 90.7% after 300 cycles at 0.5C | 105 |
| LiCoO2 | LiPON | Ti doping and LiCoPO4 coating | ∼170 | 75% after 500 cycles at 2C | 106 | |
| LiNi0.8Co0.1Mn0.1O2 | Li10SnP2S12 | Ta5+ doping and Nb5+ migration to form LiNbO3 coating | ∼198 | 100% after 100 cycles at 0.2C | 107 | |
| Conductive network design | LiNi0.8Co0.1Mn0.1O2 | Li6PS5Cl | Hybrid conductive network with CNTs (0.3 wt%) and CNFs (4.7 wt%) | ∼150 | 90.1% after 50 cycles at 0.5C | 109 |
| Non-carbon conductor integration | LiNi0.8Co0.1Mn0.1O2 | Li6PS5Cl | Ti2O3 as a substitute for carbon conductors | 192 | 86.5% after 140 cycles at 0.1C | 110 |
| Binder optimization | LiNi0.8Co0.1Mn0.1O2 | Li6PS5Cl | high-molecular-weight PIB | 206.25 | 90.42% after 90 cycles at 0.1C | 112 |
| LiNi0.8Co0.1Mn0.1O2 | Li6PS5Cl | Lithium borate polycarbonate binder | 203 | 94% after 300 cycles at 1.75 mA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm−2 cm−2 |
113 | |
| Homogeneous structure design | Li1.75Ti2(Ge0.25P0.75S3.8Se0.2)3 | Li6PS5Cl | Key elements introduced (Ge, Se) | ∼175 | 70% after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 2.5C 000 cycles at 2.5C |
114 |
| Polymer interlayer | LiNi0.8Co0.1Mn0.1O2 | Li1.4Al0.4Ti1.6(PO4)3 | PLC60 interlayer | 150.3 | 85% after 200 cycles at 0.5C | 115 |
| LiFePO4 | Li6PS5Cl | Ether-based gel polymer interlayer | 159.5 | 96% after 100 cycles at 0.3C | 116 | |
| Molten salt interlayer | LiFePO4 | Li6.4La3Zr1.4Ta0.6O12 | Inorganic (Li,K,Cs)FSI ternary system | ∼151 | 81.4% after 1000 cycles at 1C | 117 |
| Aliovalent doping | LiNi0.7Co0.1Mn0.2O2 | Li3.25InCl5.75O0.25 | O doping to replace partial Cl in Li3InCl6 | 224.97 | 82.9% after 100 cycles at 0.2C | 118 |
| LiMn2O4 | Li2.5Lu0.5Zr0.5Cl6 | Zr substitution in Li3−xLu1−xZrxCl6 | ∼70 | 100% after 1000 cycles at 0.3C | 119 | |
| LiCoO2 | Li2.75Y0.16Er0.16Yb0.16In0.25Zr0.25Cl6 | High-entropy doping with multiple metal cations (Y, Er, Yb, In, Zr) | ∼131 | 88.9% after 500 cycles at 0.5C | 120 | |
| Interface-guided bulk modification | LiNi0.9Co0.05Mn0.05O2 | Li2.5ZrCl5F0.5O0.5 | F−/O2− anion engineering to form F-rich CEI | ∼113 | 70.4% after 2000 cycles at 2C | 121 |
| LiCoO2 | nLi2O–TaCl5 (LTOC)-10%F | Introduction of F− to form a LiF-rich passivation layer | ∼115 | 81% after 300 cycles at 1 mA cm−2 | 122 | |
| LiNi0.83Co0.11Mn0.06O2 | Li2YCl2.5Br1.5O0.5 | Pre-oxidation to form Y2O3-based CEI | 208 | 80.6% after 1000 cycles at 0.5C | 123 | |
| Amorphization engineering | LiNi0.83Co0.11Mn0.06O2 | Li3ZrCl4O1.5 | Amorphization of Li2ZrCl6via O incorporation | ∼138 | 90.1% after 300 cycles at 1C | 124 |
| LiNi0.88Co0.09Mn0.03O2 | LiAlOCl | Hydrate-assisted synthesis of aluminum-based oxychloride SSEs | ∼163 | 84.86% after 1500 cycles at 0.5C | 125 |
For sulfide and halide SSEs, their lower modulus, greater interfacial compliance, and wider compositional tunability not only allow the adoption of general interface engineering methods but also enable a range of differentiated optimization strategies. Incorporating composite conductive networks and non-carbon conductors facilitates continuous electronic pathways, suppresses electron leakage and side reactions typically induced by high-surface-area carbon materials, and achieves synergistic transport of electrons and ions—thereby greatly enhancing interfacial stability and rate performance. However, it is essential to ensure chemical compatibility between the components and structure of the conductive network and the SSE, as incompatibility may trigger the formation of new interfacial byproducts. In addition, binder optimization improves particle adhesion and strain buffering, while single-ion-conducting designs enable directional Li+ transport, further stabilizing the interface. Nevertheless, such binders still face challenges in terms of ionic conductivity, functional synthesis, and scalable application. Furthermore, homogeneous cathode architectures with zero strain and intrinsic mixed conductivity help eliminate three-phase boundaries and transport separation. They are highly compatible with flexible SSEs, though the related materials and processing technologies still need further advancement. At the same time, electrolyte bulk optimization, by tailoring the composition and crystalline/amorphous structure of the SSE, can significantly enhance its compatibility with high-voltage cathodes. In particular, halide SSEs, with their open frameworks and excellent tunability, exhibit unique engineering advantages in terms of aliovalent or high-entropy doping, interface-induced passivation, and amorphization strategies.
Overall, sulfide and halide SSEs, owing to their lower modulus, higher formability, and excellent compositional tunability, offer broader engineering latitude and innovation potential for cathode interfacial modification. By contrast, oxide SSEs, which are rigid, thermally robust, and possess wide electrochemical windows while typically requiring high-temperature densification, are better served by a combined strategy of single-crystal design, precise structural and surface regulation, and construction of thin, high-voltage-stable interlayers (Fig. 8). Single crystallization helps alleviate interfacial stress concentrations and crack initiation; surface doping, graded architectures, and inorganic-dominant hybrid coatings effectively suppress interdiffusion and parasitic reactions at elevated temperature and high voltage, while band alignment and polarization tuning mitigate SCL effects. Interlayers should be thin, dense, and stable under high voltage, and the fraction of flexible organic constituents should be limited to avoid undermining solid–solid interfacial continuity. Accordingly, strategies must be tailored to the intrinsic attributes of each SSE, with precise matching of materials and processing, to fully unlock the energy density and rate capability of high-voltage cathodes and advance the practical deployment of ASSLBs.
3. Anode–electrolyte interface
3.1. Challenges
Anodes in SSBs often experience substantial volumetric fluctuations and high reactivity during cycling, which readily trigger interfacial structural disruption and performance degradation upon contact with SSEs. The associated failure mechanisms at the interface primarily stem from two key factors: First, due to mechanical mismatch, surface contamination, or accumulation of reaction byproducts, a stable and conformal interface is difficult to achieve, interrupting continuous ionic pathways and increasing interfacial resistance. Second, localized defects can serve as nucleation sites for lithium dendrites, which propagate into the SSE bulk driven by electronic leakage and concentrated mechanical stress, ultimately leading to internal short circuits and structural breakdown (Fig. 2).Taking lithium metal as a representative example, its intrinsically low modulus leads to severe mechanical mismatch when interfaced with most inorganic SSEs. Due to insufficient compaction, surface roughness, or the accumulation of reaction-induced byproducts, the interface often fails to form a continuous and stable ionic transport pathway. For instance, garnet-type oxides such as Li7La3Zr2O12 (LLZO) possess excellent chemical stability but often exhibit interfacial voids due to their high hardness and poor wettability, which hinders intimate contact with lithium metal. In addition, the interfacial layer at the Li–LLZO interface typically forms through reactions between lithium and surface impurities such as carbonates and hydroxides, generating byproducts like Li2CO3, LiOH, and Li2O. These contamination phases differ markedly in ionic conductivity and structural order, with Li2CO3-rich regions in particular exhibiting poor Li-ion conductivity, which can lead to localized current density accumulation, electronic leakage, and anisotropic ion migration pathways—ultimately exacerbating interfacial instability.126 By comparison, sulfide SSEs, with their lower Young's modulus and superior compressibility, are more likely to form intimate contact with lithium metal under cold-pressing conditions, typically exhibiting lower initial interfacial resistance. However, their strong reductive reactivity with lithium often leads to the formation of porous and brittle interphases (e.g., Li2S, Li3P), whose ionic conductivity and transport behavior are governed by the distribution and crystallinity of these phases—crystalline Li3P provides preferential Li+ conduction pathways due to its ordered lattice, whereas amorphous Li2S acts as a bottleneck with lower mobility, resulting in a spatially heterogeneous percolation network that promotes nonuniform current distribution and compromises interfacial integrity during cycling.60,127,128 Similar to sulfide-based systems, halide SSEs demonstrate favorable initial densification. However, their interfacial interactions with lithium metal remain non-negligible. Upon contact, high-valence metal cations (e.g., In3+, Y3+) are prone to reduction, leading to elemental metal precipitation and microstructural reorganization at the interface, thereby weakening interfacial compactness. Concurrently, reduction byproducts such as LiX (e.g., LiCl, LiBr) persist as discontinuous and ionically resistive interphases. As a result, the interphase evolves into a heterogeneous metal-in-salt composite, where lithium-ion migration is restricted to narrow percolation channels within the LiX matrix. These tortuous paths, shaped by the spatial distribution of LiX and embedded metal particles, limit effective ionic transport and give rise to increased interfacial resistance and localized overpotentials.129
It is worth noting that even in chemically more stable systems, such as silicon-based anodes, interfacial degradation remains inevitable, but the dominant mechanism transitions from chemical reactivity to mechanical mismatch. Unlike lithium metal, silicon undergoes enormous volume changes of up to ∼300% during lithiation and delithiation.130 Its rigid structure and significant elastic disparity with inorganic SSEs induce severe interfacial stress accumulation and delamination upon cycling. In situ tomography and finite-element simulations have revealed that the Si–SSE interface is prone to microcrack initiation, contact loss, and fragmentation of ionic transport pathways.131 Although silicon shows excellent chemical compatibility with inorganic SSEs, stress-driven interfacial fracture caused by volume expansion remains the major bottleneck restricting the long-term stability of silicon anodes in solid-state systems.
Overall, whether arising from interfacial reactions that induce chemical phase evolution or from volume-change-driven mechanical delamination, the multiscale chemo-mechanical mismatch between anodes and SSEs constitutes the fundamental challenge governing interfacial stability and cycling durability in ASSLBs.
In contrast, sulfide SSEs possess higher interfacial compliance and lower interfacial impedance, yet their intrinsically low fracture toughness makes them prone to stress-induced cracking during lithium deposition. Consequently, they exhibit a coupled instability in which mechanical fracture and dendrite penetration co-evolve. In situ transmission electron microscopy (TEM) and focused ion beam-scanning electron microscopy (FIB-SEM) observations reveal that dendrites preferentially propagate along stress-induced microcracks, which in turn facilitate further dendrite growth—forming positive feedback between interfacial cracking and metallic penetration. Singh et al. systematically investigated the effect of microstructure on dendrite evolution by tuning the grain size of LPSC and found that both interfacial roughness and bulk defects jointly determine the stress distribution and dendrite propagation.134 Fine-grained LPSC exhibits higher fracture toughness and more homogeneous stress transmission, effectively enhancing the CCD, whereas coarse-grained samples suffer from stress localization and crack percolation, leading to pronounced instability. Moreover, during lithium deposition, the formation of MIEC phases such as Li3P and Li2S provides additional electronic pathways, accelerating dendrite extension and interconnection within the electrolyte bulk.135 Hence, dendrite formation in sulfide SSEs mainly originates from a mechanical-chemical synergistic instability arising from low fracture toughness and interfacial reaction by-products, characterized by a “crack-first, dendrite-following” evolution behavior.
Similar to sulfide SSEs, halide SSEs also feature a mechanically soft and chemically reactive nature, yet their dendrite instability stems from distinct interfacial dynamics. Existing evidence suggests that reduced metal particles and electrically insulating byproducts formed at the Li–halide interface are often heterogeneously distributed, promoting non-uniform lithium deposition.136 Once a continuous metallic network develops within the interphase, electrons can penetrate deep into the bulk electrolyte, initiating spontaneous nucleation and internal lithium growth. Moreover, the inherently low shear modulus of halide SSEs is insufficient to effectively relieve localized stress induced by lithium deposition, making the interfacial region prone to expansion and pore accumulation, which further facilitates secondary dendrite formation.137 Broadly speaking, though the three classes of SSEs exhibit distinct interfacial characteristics, their dendrite growth mechanisms consistently evolve from interface-initiated processes to bulk-phase penetration, revealing a universal feature of multiscale-coupled instabilities underlying lithium dendrite behavior.
In summary, failure phenomena at the anode–SSE interface highlight the need for synergistic regulation among structural integrity, chemical stability, and electronic insulation. Owing to the limited self-adaptability of solid–solid interfaces, it is often challenging for a single-phase material to simultaneously fulfill the mechanical conformity, interfacial stability, and dendrite suppression requirements. Consequently, the core of interfacial design lies in constructing composite architectures capable of accommodating mechanical deformation, mediating interfacial reactions, and enabling selective transport, thereby achieving integrated stability across structural, chemical, and electronic dimensions within the interfacial region.
3.2. Optimization strategies
To address the common challenges at the anode–SSE interface, including poor interfacial contact, parasitic reactions, and dendrite-induced failure, research has focused on three main directions: functional anode design, interlayer construction and electrolyte engineering. The following sections analyze the mechanistic features and application potential of these strategies across different classes of SSEs.Interfacial layer construction. Functional interfacial layers can be constructed on the Li metal surface via physical coating or in situ chemical reactions, featuring high ionic conductivity, electronic insulation, and chemical stability. These layers effectively improve interfacial wettability, suppress parasitic reactions, and regulate Li deposition behavior.138–140 For example, Liu et al. proposed an in situ conversion strategy using P2S5 as a precursor to construct a dense and uniform Li3PS4 passivation layer on the Li surface, which significantly reduces interfacial reactivity.139 The resulting P2S5@Li symmetric cell exhibits stable cycling for over 500 h at 0.1 mA cm−2, and the full cell retains 75.5% of its capacity after 400 cycles at 0.5C. However, this strategy primarily relies on the intrinsic chemical affinity of a single inorganic component and lacks multifunctional synergistic regulation, especially in controlling Li nucleation and deposition morphology. To this end, Wu et al. introduced a LiF–LiPO3–Ag composite interfacial layer, which integrates the electronic insulation of LiF, the amorphous compliance of LiPO3, and the strong Li affinity of Ag to enable synergistic regulation of electron blocking, interfacial adaptability, and nucleation behavior (Fig. 9a).140 By integrating multiple functionalities, this strategy overcomes the limitations of conventional single-component passivation layers and significantly expands the tunability of interfacial engineering.
 | ||
| Fig. 9 (a) Schematic illustration of the whole fabrication procedure for the L-A@Li interphase via solution coating method. Reproduced with permission.140 Copyright 2025, Elsevier. (b) Schematic of LiAl-p. (c) CCD testing of the LiAl-p//LiAl-p symmetric cell, conducting with a fixed Li plating/stripping time of 1 h. Reproduced with permission.141 Copyright 2024, John Wiley and Sons. (d) CCD comparison for three types of solid-state electrolytes with an Li(110) anode strategy and other reported modification methods. RT, room temperature. Reproduced with permission.142 Copyright 2025, Springer Nature. | ||
Composite anode design. Functional interfacial layers primarily act on the surface and are often insufficient to suppress long-term dendrite growth and structural degradation. Therefore, research has focused on composite anode design to enable synergistic regulation of bulk and interfacial stability. In this design, metallic, nonmetallic, or compound components with specific structural or chemical properties are incorporated into the anode to reconstruct Li-ion transport pathways, mitigate mechanical stress, and regulate Li nucleation and deposition behavior within the bulk phase, thereby significantly enhancing interfacial stability and cycling durability.
In practical implementation, composite anodes are generally developed following two main design strategies. The first involves alloy-type composite anodes, which achieve structural reinforcement and interfacial stability by forming alloys or multiphase interpenetrating structures between lithium and other elements.141,143–146 For example, Zhu et al. fabricated a porous Li–Al alloy anode (∼19% porosity) that effectively relieves volumetric stress (Fig. 9b).141 Moreover, surface oxides react in situ with sulfide electrolytes to form a robust inorganic solid electrolyte interphase (SEI), greatly boosting interfacial stability. This structure enables ultra-long cycling over 5000 h at 0.5 mA cm−2 and achieves a critical current density (CCD) of 6.0 mA cm−2 (Fig. 9c). Building upon this, Li–Sn alloys integrate relatively high lithium storage capacity with favorable interfacial compatibility.143 Prelithiated phases such as Li22Sn5 can effectively buffer volume changes during cycling and establish stable interfaces with sulfide-based SSEs (e.g., LPSC), thereby mitigating interfacial side reactions. This synergistic effect leads to excellent long-term cycling performance, with full cells retaining 91% of their capacity after 650 cycles. Extending this concept, Li–Ag alloys introduce a dual-phase structure (Li3Ag/Li0.98Ag0.02) that enables both efficient Li transport and interfacial stabilization.144 During cycling, Li3Ag serves as a lithiophilic channel to guide uniform Li plating, while Li0.98Ag0.02 acts as the primary Li deposition host. Meanwhile, Ag at the interface reacts with LPSC to form a stable, electrochemically inert Ag–P–S–Cl interphase, which effectively suppresses dendrite growth and preserves interfacial integrity.
Beyond conventional metal-based systems, silicon-based alloy architectures have attracted increasing attention for achieving higher energy density and improved chemical stability. In Li–Si alloys, the formation of Li-rich phases (e.g., Li15Si4, Li22Si5) provides both high theoretical capacity and chemical compatibility with sulfide electrolytes. The partial lithiation of Si produces a ductile Li–Si framework that maintains intimate contact at the interface.147 However, the enormous volumetric expansion and associated stress accumulation can still induce particle pulverization, interfacial cracking, and contact loss, leading to impedance growth and capacity fading. To address these limitations, Li et al. developed an In–Si composite anode featuring a low-melting-point In network that forms a 3D mixed ionic-electronic conducting framework during operation.148 The In–Li alloy network not only enhances ionic and electronic conductivity but also redistributes local stress and stabilizes interfacial contact. This design delivers outstanding cycling stability (98.5% capacity retention after 2000 cycles at 3.69 mA cm−2) and high-rate capability (80% capacity retention at 6C), demonstrating the effectiveness of ductile metal reinforcement in semiconductor-based anodes.
The second approach employs reactive components such as metal phosphides or nitrides (e.g., GaP, GaN, Mg3N2), which react with Li to form ion-conductive phases and nucleation-inducing domains.149–151 For instance, Li et al. introduced GaP into molten lithium to in situ generate Li3P and Li2Ga, which not only lower the surface tension but also build a lithiophilic network and favorable nucleation structure.149 The resulting composite anode delivers stable cycling for 5700 h at 0.3 mA cm−2, and the full cell retains 97.32% of its capacity after 490 cycles at 1C, demonstrating the long-term stability and practical potential of this interfacial engineering strategy.
In summary, alloy-type and reactive-component composite anodes share a common design philosophy: both rely on the formation and evolution of alloy phases to achieve synergistic optimization of structural integrity, ionic transport, and interfacial stability. However, alloy-based anodes are not without limitations. The large volumetric changes and stress accumulation during repeated alloying/dealloying can induce interfacial delamination, pore formation, or structural fatigue, particularly under low stack pressures.152 These effects may lead to impedance growth and gradual capacity decay. Therefore, future design efforts should aim to balance the structural reinforcement benefits of alloy phases with strategies that mitigate their intrinsic mechanical instability—such as elastic buffering frameworks, graded architectures, or low-yield-strength interlayers—to ensure long-term stability and practical operation in all-solid-state configurations.
Crystal structure modulation. Despite the significant progress achieved by the above composite structural strategies in improving interfacial wettability, ion diffusion capability, and local reaction stability, the intrinsic polycrystalline nature of lithium metal still imposes critical limitations. The presence of discontinuous grain boundaries and anisotropic diffusion behavior within the bulk remains difficult to eliminate, posing persistent risks of dendrite formation and interfacial instability. To further improve the uniformity of lithium deposition and the coherence of ion diffusion, Chen et al. synthesized single-crystal lithium foils with exposed Li(110) facets via a thermally induced recrystallization process.142 The Li(110) surface features an ultralow self-diffusion energy barrier and a relatively low Young's modulus, which significantly enhance in-plane Li-ion diffusion while mitigating interfacial stress concentration during deposition. In solid-state systems, the single-crystal lithium anode markedly increases the CCD compared to polycrystalline lithium, reaching 6.0, 5.0, and 5.0 mA cm−2 in LLZTO, LPSC, and Li3InCl6 systems, respectively—far exceeding the 0.5–1.0 mA cm−2 typical of polycrystalline lithium (Fig. 9d). Compared to structural composite strategies, single-crystal lithium fundamentally avoids grain boundary defects and electrochemical anisotropy, thereby enhancing interfacial electric field uniformity and promoting cooperative ion transport. This crystal engineering approach offers an ideal solution for achieving high current density and long cycle life in ASSLBs.
Flexible organic interlayers. Owing to their excellent film-forming ability and segmental mobility, flexible organic interlayers can effectively mitigate physical mismatches during the early stages of interface formation, making them particularly suitable for systems with porous interfaces or uneven Li deposition. For example, Li et al. employed poly(1,3-dioxolane) (PDOL) as an interlayer to prevent solvent-induced degradation of the Li5.4PS4.4Cl1.6, while its flexible polymer chains adaptively filled interfacial voids (Fig. 10a).153 During cycling, the interlayer transformed into a stable SEI rich in Li2S and LiF, permitting dendrite-free operation for over 1200 h (Fig. 10b). Building upon such structural adaptability, Yang et al. introduced poly(lithium 4-styrenesulfonate) (PLSS) between LLZTO and Li to further enhance interfacial compatibility at the atomic level.154 The sulfonate (–SO3Li) groups in PLSS strongly coordinate with metal atoms (La, Zr, Ta) on the LLZTO surface, forming an ion-conductive “bridge” that accelerates Li+ transport across the ceramic–polymer interface. This coordination not only ensures intimate contact and rapid ion migration but also endows the interlayer with strong electron-blocking capability, effectively preventing electron tunneling and dendrite initiation within LLZTO. As a result, PLSS-based symmetric cells achieve long-term stable cycling exceeding 4700 h at room temperature.
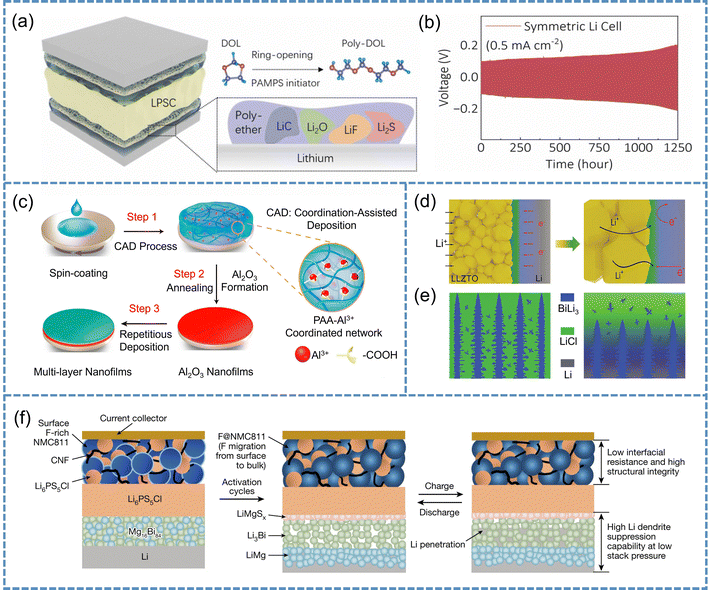 | ||
| Fig. 10 (a) The polydioxolane interlayer formed by in situ polymerization provides a robust solid electrolyte interphase between the lithium anode and Li5.4PS4.4Cl1.6. (b) Voltage profiles of the Li/PDOL/LPSC/PDOL/Li cells at a current density of 0.5 mA cm−2 with a cut-off capacity of 0.5 mAh cm−2. Reproduced with permission.153 Copyright 2024, Elsevier. (c) Schematic diagram for the CAD process to form metal oxide nanofilms. Reproduced with permission.156 Copyright 2022, American Chemical Society. (d) Multifunctional double-layer with interfacial design coupling lithiophilic and electron-insulating. (e) Interlayers formed at different cooling rates, fast cooling on the left and slow cooling on the right. Reproduced with permission.157 Copyright 2024, John Wiley and Sons. (f) Illustrations of the in situ formation of F@NMC811/Li6PS5Cl/LiMgSx/Li3Bi/LiMg. Reproduced with permission.158 Copyright 2023, Springer Nature. | ||
With structural and chemical compatibility effectively optimized, research attention has shifted toward electronic-level modulation to achieve deeper interfacial stabilization. To this end, Nie et al. designed a biphenyl (BP)–Li composite interlayer, where π–π stacking of BP molecules and Li coordination induce a built-in electric field at the interface, effectively homogenizing local charge density and suppressing dendrite tip effects during Li deposition.155 Upon electrochemical evolution, the interlayer converted into a gradient SEI with a “rigid core-soft shell” architecture, combining electronic passivation with mechanical compliance, thereby extending the symmetric cell lifespan to over 1800 h.
Inorganic oxide interlayers. Similar to the evolution pathway of cathode interfaces, flexible organic interlayers offer favorable interfacial conformity at early stages but are still constrained by limited thermal stability and inadequate electronic insulation. These challenges have progressively redirected research efforts toward oxide-based interlayers with superior interfacial passivation performance. Representative oxides such as Li2O, Al2O3 and ZnO demonstrate outstanding electronic insulating characteristics and chemical inertness, effectively suppressing electron leakage and enhancing interfacial robustness.156,159,160 For example, Jiang et al. constructed a bilayer structure on the surface of LLZTO, consisting of a spontaneously formed Li2O buffer layer and an electronically insulating Li2O layer deposited via atomic layer deposition (ALD).159 The buffer layer improves wettability and fills interfacial voids, while the ALD-grown layer effectively blocks electron penetration while maintaining ionic conductivity, enabling Li//Li symmetric cells to cycle stably for over 1000 h at 0.5 mA cm−2. However, the high cost and energy consumption of ALD hinder its feasibility for large-scale applications. To overcome this limitation, Guo et al. developed a solution-based wet-chemical strategy, in which polyacrylic acid (PAA) was used to coordinate Al3+ ions, enabling the formation of a uniform and flexible Al2O3 nanolayer on the LLZTO surface (Fig. 10c).156 This interfacial modification significantly reduces the interfacial resistance from 2079.5 Ω cm2 to 8.4 Ω cm2, and the resulting ASSLB exhibits a long cycling lifespan comparable to that of conventional lithium-ion batteries.
Unlike these conventional oxides, lithium phosphorus oxynitride (LiPON), combines strong electronic insulation with measurable Li+ conductivity (∼0.7–1.0 mS cm−1 at 25–30 °C) and an amorphous, grain-boundary-free structure that promotes conformal wetting and mitigates contact loss. This dual function—passivating electrons while conducting Li+—makes LiPON particularly suited to stabilize Li–SSE interfaces rather than acting as a purely inert electronic barrier like Li2O, Al2O3, or ZnO. Su et al. deposited an ultrathin (∼30 nm) LiPON interlayer by radio frequency (RF) sputtering on argyrodite LPSC and demonstrated markedly improved interfacial contact and wettability.161 As a result, the interfacial resistance drops to ∼1.3 Ω cm2, symmetric cells exhibit stable Li plating/stripping for >1000 h at 0.5 mA cm−2, and the CCD reaches 4.1 mA cm−2 at 30 °C.
Functional composite interlayers. While oxide-based interlayers offer chemical stability, their limited functionality makes them insufficient to accommodate interfacial evolution and regulate dendrite growth. This limitation has stimulated the development of functional composite interlayers. By integrating lithium-conductive phases, electronically insulating components, and buffering structures, these strategies realize spatially resolved multifunctionality and have emerged as a key approach for constructing stable lithium-metal interfaces. Depending on differences in structural design and adaptive response mechanisms, such interlayers can be broadly classified into two representative types: pre-designed configurations and dynamically evolving architectures.
In pre-designed configurations, the spatial arrangement and functional zoning are defined during the fabrication stage, offering clear structural delineation and controllable regulation pathways.157,162–166 A representative example is the BiLi3/LiCl3 bilayer interlayer (Fig. 10d and e), where BiLi3, positioned adjacent to the lithium metal, displays strong lithiophilicity and facilitates uniform lithium deposition. LiCl3, in contact with the SSE, provides electronic insulation and interfacial passivation.157 This functionally stratified design enables electronic-ionic decoupling, significantly enhancing both the CCD and interfacial stability. However, due to its inherently stable configuration during operation, it lacks dynamic adaptability to evolving interface conditions.
Dynamically evolving architectures harness interfacial reactivity, electrochemical driving forces, or fluidic mobility to achieve the in situ generation, adaptive regulation, and self-healing reconstruction of functionally stratified structures, thereby exhibiting superior interfacial responsiveness and structural stability under dynamic operating conditions.158,167–169 For example, Wan et al. proposed a Mg16Bi84 alloy interlayer that undergoes an interfacial reaction with the LPSC, forming an electronically insulating LiMgSx layer in situ, accompanied by the migration of Mg atoms toward the lithium metal. This process induces the construction of a tri-layer composite structure comprising LiMg–Li3Bi–LiMgSx (Fig. 10f).158 In this configuration, the Li3Bi layer serves as a lithium-conductive framework that guides lithium deposition into the interlayer and accommodates interfacial volume changes. Meanwhile, the LiMgSx and LiMg layers provide electronic passivation and mechanical support, respectively, working synergistically to form a gradient-functional interface. Driven by a solid-solution mechanism, this structure effectively suppresses interfacial side reactions and dendrite formation, enabling symmetric cells to cycle stably for over 2700 h at room temperature. While such solid-phase reaction-based designs offer promising adaptability, their inherent rigidity still limits real-time stress accommodation and defect healing during prolonged cycling. To address this limitation, recent research has extended dynamic interlayer design toward liquid-metal-driven adaptive architectures, in which fluidic metallic phases not only ensure conformal contact but also participate in in situ alloying and redox conversion to achieve self-regulating interfacial stabilization. A representative example is the Ga–In liquid metal (G–LM) interlayer, which couples metallic fluidity with electrochemical reactivity to build an intelligent, self-healing interface.169 The G–LM can conformally wet the Li–LLZTO interface at room temperature, filling nanoscale voids and establishing intimate contact without high-temperature processing. During lithiation, it undergoes in situ phase transformation to form a multiphase alloy-oxide hybrid composed of Li2Ga, Li13In3, LiGaO2, and LiInO2. The alloy phases provide fast Li+ transport channels, while the oxide components homogenize the interfacial electric field and block electronic leakage, collectively functioning as an ionic-electronic rectification layer. Moreover, the liquid metal's intrinsic fluidity allows continuous self-reconstruction under cycling, dissipating interfacial stress and maintaining uniform Li plating/stripping. Consequently, G–LM-modified Li-LLZTO interfaces achieve ultralow interfacial resistance (12 Ω cm2) and long-term stability exceeding 1400 h at 1.0 mA cm−2.
Overall, this liquid-metal-mediated dynamic interfacial strategy extends the concept of functional composite interlayers from solid-state reaction systems to fluidic, self-regulating architectures capable of in situ phase transformation and autonomous defect healing, providing a robust design paradigm for next-generation dendrite-free ASSLBs.
Microstructural regulation. To reduce the risk of dendrite penetration and improve interfacial uniformity between the SSE and lithium metal, research has first focused on densifying the microstructure of the electrolyte. In oxide-based systems, Accardo et al. developed a high-pressure low-temperature (HPLT) sintering strategy for LLZO garnet electrolytes.170 By applying a quasi-isostatic pressure of 5.5 GPa at 500 °C, followed by a brief post-annealing at 800 °C, they fabricated dense LLZO ceramics with a relative density above 97% and an impressive ionic conductivity of 3.1 × 10−4 S cm−1 at room temperature. This process effectively suppresses lithium volatilization and abnormal grain growth while maintaining a homogeneous submicron grain structure, which strengthens the mechanical resistance against dendrite penetration and ensures stable Li–LLZO interfacial cycling. Inspired by these advances, sulfide-based electrolytes have also been optimized through microstructural densification strategies. Liu et al. employed a “pressing-then-sintering” approach to fabricate a densified LPSC nanorod-based electrolyte.171 By enhancing particle uniformity and surface flatness, this strategy effectively minimized interparticle voids and improved the degree of cold-press compaction, significantly increasing the CCD to 1.05 mA cm−2. Nevertheless, the structure still suffers from broad particle size distribution and disordered crystallographic orientation, which limit its ability to further support higher current densities. To address this, Zhong et al. regulated the crystallization kinetics of Li5.3PS4.3ClBr0.7 to construct well-defined cubic crystallites with uniform particle size and oriented crystallographic alignment, forming a highly ordered three-dimensional stacking structure (Fig. 11a).172 This crystal morphology facilitates the formation of a denser and more isotropic ionic transport network during cold pressing, resulting in a remarkably high CCD of 3.8 mA cm−2 (Fig. 11b). Such microstructural homogenization strategies fundamentally eliminate dendrite initiation pathways by promoting structural continuity and provide a stable morphological foundation for subsequent chemical-level interface engineering.
 | ||
| Fig. 11 (a) Schematic of the Li–electrolyte interface with SS-LPSCB and BMAN-LPSCB. (b) Galvanostatic cycling of Li–Li symmetric cells with BMAN-LPSCB electrolyte at step-increased current densities with a step size of 0.2 mA cm−2 at room temperature. Reproduced with permission.172 Copyright 2024, John Wiley and Sons. (c) Crystal structures of Li10GeP2S12 and Li9.98Ge0.99Sn0.01P2S11.98F0.02. Reproduced with permission.173 Copyright 2024, John Wiley and Sons. (d) Diagram of interface reaction and Li dendrite growth at the Li–Li6.16P0.92In0.08S4.88O0.12Cl interface. Reproduced with permission.174 Copyright 2024, John Wiley and Sons. (e) A Scheme of the trade-off principle between Li vacancy and concentration toward an optimized ionic conduction for LixYI3+x (x = 2, 3, 4, or 9) SEs. Reproduced with permission.175 Copyright 2024, John Wiley and Sons. | ||
Doping engineering. Although microstructural optimization improves interfacial densification, chemical incompatibility between the SSE and lithium metal remains a primary cause of interfacial failure—particularly in sulfide and halide systems, where electronically conductive side phases are prone to formation. To address this issue, research has shifted toward doping strategies that enhance the intrinsic stability of the electrolyte and improve interfacial tolerance by modulating the crystal structure and local bonding environment.
Early studies primarily focused on point-defect-mediated doping strategies, in which aliovalent substitution introduces lithium vacancies or modifies local lattice disorder to enhance ionic conductivity and interfacial compatibility. For instance, in Li10SnP2S12, partial substitution of S2− with Cl− increases lithium vacancy concentration and shortens Li+ diffusion pathways along the c-axis, resulting in enhanced ionic conductivity and reduced electronic leakage.176 Similarly, in Li10GeP2S12, the substitution of Ge4+ with Sn2+ introduces lithium vacancies to enhance ionic transport, while the replacement of S2− with F− effectively suppresses moisture-induced reactions and H2S release (Fig. 11c).173 Such doping modulates the lattice structure and electronic distribution, effectively lowering the reduction tendency of the electrolyte upon contact with lithium metal and thereby mitigating interfacial side reactions. However, the regulatory effects of these strategies are primarily confined to the bulk phase, with limited capacity to directly control the interfacial reaction processes.
To further enhance interfacial regulation, doping strategies have gradually evolved toward structure-interface co-regulated approaches, wherein dopants not only stabilize the bulk framework but also actively participate in the formation of beneficial interfacial phases. A representative example is the In3+/O2− co-doping of LPSC, which introduces InS45− and PS3O4− structural units to reinforce the framework rigidity while simultaneously inducing the formation of a Li–In alloy layer at the interface (Fig. 11d).174 This dual function improves both the bulk stability of the electrolyte and the interfacial wettability with lithium metal. In another case, Al3+/F− co-doping in the halide electrolyte Li2ZrCl6 not only broadens the electrochemical window to 4.04 V, but also promotes the in situ formation of a Li–Al alloy and LiF-based electronically insulating SEI layer at the interface, effectively suppressing electron leakage and interfacial side reactions in synergistic fashion.177
Building upon previous efforts, recent research has begun to explore defect-tolerant, intrinsically stabilized doping strategies aimed at fundamentally resolving interfacial instability at the structural level. A notable case is the superionic conductor Li4YI7 proposed by Zhang et al., whose highly symmetric lattice structure confers excellent defect tolerance and enables three-dimensional lithium-ion transport pathways (Fig. 11e).175 Upon contact with lithium metal, this material avoids the formation of electrically conductive by-products (e.g., Li–Y alloys) and instead promotes the stable formation of inert YI6 octahedral units composed of Y3+ and I−, thereby effectively preventing undesirable interfacial reactions. Benefiting from its intrinsic high ionic conductivity (1.04 × 10−3 S cm−1) and interfacial chemical stability, Li/Li4YI7/Li symmetric cells achieve over 1000 h of stable, dendrite-free cycling at 0.1 mA cm−2, indicating outstanding interfacial compatibility and long-term operational reliability.
These advances collectively highlight a critical shift in doping strategies from isolated bulk-phase regulation toward integrated structure-interface engineering. A key takeaway is that effective doping no longer solely relies on enhancing bulk ionic conductivity or structural robustness, but increasingly aims to engineer the interfacial chemistry in situ. Co-doping schemes, in particular, demonstrate the ability to induce favorable interphases—such as Li–In or Li–Al alloys and LiF-rich SEI layers—that simultaneously suppress electron leakage and stabilize electrochemical cycling. Moreover, the development of intrinsically stable frameworks like Li4YI7 underscores the importance of lattice symmetry and defect tolerance in achieving durable lithium compatibility without relying on additional interfacial constructs. These insights suggest that future doping strategies should emphasize multifunctional dopant roles—those that synergistically tailor the bulk structure, interfacial reactivity, and electrochemical properties—to enable robust and scalable ASSLB systems.
Functional coating design. While doping strategies enhance interfacial stability primarily by modulating the bulk structure of the material, surface coating approaches act directly on the electrolyte surface and grain boundaries. This method is not only applicable to a wide range of material systems but also offers greater flexibility for practical engineering applications.
In halide-based electrolytes, Liu et al. in situ constructed a dense and uniform LiF–ZrF4 (LFZ) fluorinated coating on the surface of Li2ZrCl6 (LZC) (Fig. 12a).178 This layer exhibits ultralow electronic conductivity (7.1 × 10−10 S cm−1) and excellent thermal stability, effectively suppressing lithium reduction side reactions and enhancing moisture resistance. As a result, Li/LFZ-LZC/Li symmetric cells demonstrate stable cycling for over 800 h at 0.1 mA cm−2, with the CCD increased to 1.8 mA cm−2.
 | ||
| Fig. 12 (a) Schematic diagram of the LCO/LZC/LFZ@LZC/Li ASSLMBs. Reproduced with permission.178 Copyright 2025, Elsevier. (b) Ameliorated Li+-ion diffusion kinetics of a CoxB-coated LLZTO pellet through the grain boundaries. Reproduced with permission.179 Copyright 2023, Royal Society of Chemistry. (c) Schematic diagram of the interfacial and bulk chemistry of the PPA-LLZTO pellet. (d) Cycling test of Li/PPA-LLZTO/Li under a current density of 0.2 mA cm−2 at 65 °C. Reproduced with permission.180 Copyright 2023, American Chemical Society. (e) Schematic of the interfacial modification of LLZTO by ZrO2 and Li2CO3 co-sintering. Reproduced with permission.181 Copyright 2024, John Wiley and Sons. | ||
Compared with halide SSEs, which are prone to interfacial reactions and moisture-induced decomposition, oxide SSEs such as LLZTO offer superior chemical stability. However, high-temperature sintering often leads to microcracks at grain boundaries and surface defects, which can trigger electron leakage and interfacial degradation. As a result, coating strategies for oxide SSEs place greater emphasis on the synergistic regulation of electronic passivation and grain boundary buffering. Chen et al. introduced an amorphous CoxB nanocoating on the surface of LLZTO (Fig. 12b).179 Owing to its excellent electronic shielding capability and moderate interfacial flexibility, the coating effectively alleviates electron leakage and prevents microcrack propagation at the interface, thereby markedly improving the cycling stability of lithium symmetric cells. To further strengthen continuous coverage along grain boundaries and establish efficient ion transport pathways, Xiong et al. employed a polyphosphoric acid (PPA) infiltration method.180 The PPA penetrates the interparticle voids of LLZTO and in situ forms a Li3PO4 coating, which creates an interfacial network that is electronically insulating and ionically conductive (Fig. 12c). This design effectively inhibits dendrite growth, reduces interfacial resistance, and enables stable cycling for over 2000 h (Fig. 12d). However, such chemical infiltration treatments are inherently passive in nature, and their effectiveness is limited by the penetration depth and uniformity of the solution reaction, making it difficult to achieve comprehensive regulation of grain boundary structures and energy band alignment. To overcome these limitations, Zhou et al. employed a co-sintering approach using ZrO2 and Li2CO3 to in situ generate a Li2ZrO3 coating at the surface and grain boundaries of LLZTO (Fig. 12e).181 This process removes interfacial impurities and reconstructs the grain boundary structure, thereby significantly enhancing interfacial insulation and stability. The treated LLZTO shows over 2000 h of stable, short-circuit-free cycling in symmetric cells and retains 70.5% of its capacity after 5800 cycles in full cells, demonstrating excellent interfacial durability and strong potential for practical applications.
In air-sensitive sulfide systems, Luo et al. introduced a g-C3N4 coating with excellent air stability, which in situ transforms into Li3N during electrochemical cycling (Fig. 13a).182 This layer simultaneously provides electronic passivation and lithium-ion conductive, enabling lithium symmetric cells to operate stably for over 1000 h (Fig. 13b). Considering the rigidity of dry coatings, Su et al. further applied a flexible encapsulation using melt-processable polycaprolactone (PCL) (Fig. 13c).183 During hot pressing, PCL forms a dense anchoring network that fills interparticle voids. Meanwhile, its C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O–C groups promote Li+ transport via dipole-ion interactions. As a result, the symmetric cell achieves stable cycling for over 1300 h at 0.2 mA cm−2, offering combined benefits of structural buffering and ionic conductivity.
O and C–O–C groups promote Li+ transport via dipole-ion interactions. As a result, the symmetric cell achieves stable cycling for over 1300 h at 0.2 mA cm−2, offering combined benefits of structural buffering and ionic conductivity.
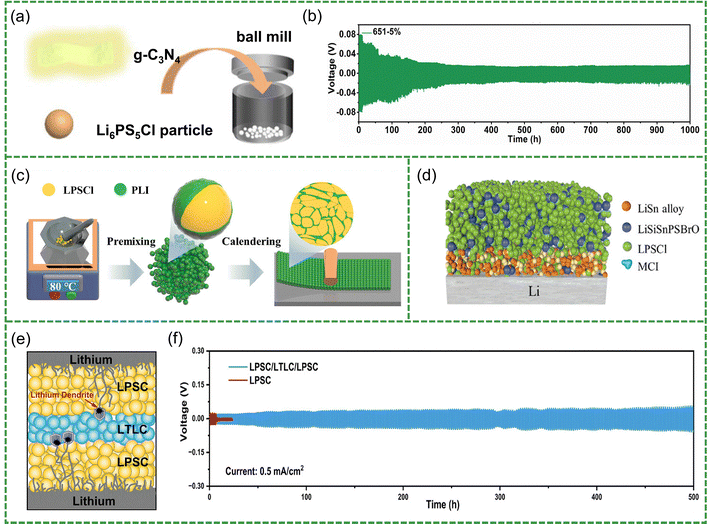 | ||
Fig. 13 (a) Schematic diagram of g-C3N4-coated Li6PS5Cl (651–x%) SSEs preparation. (b) Cycle performance of Li//Li symmetric cell with 651–5% SSE at 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mA cm−2. Reproduced with permission.182 Copyright 2024, John Wiley and Sons. (c) Illustration of the fabrication process of LPSCl-PLI electrolyte. Reproduced with permission.183 Copyright 2024, John Wiley and Sons. (d) Batteries containing multifunctional composite sulfide electrolyte. Reproduced with permission.184 Copyright 2025, John Wiley and Sons. (e) The schematic illustrating the dendrite-scavenging effect by LTLC. (f) Galvanostatic cycling performance of lithium symmetric cells at a 0.5 mA cm−2. Reproduced with permission.185 Copyright 2024, John Wiley and Sons. mA cm−2. Reproduced with permission.182 Copyright 2024, John Wiley and Sons. (c) Illustration of the fabrication process of LPSCl-PLI electrolyte. Reproduced with permission.183 Copyright 2024, John Wiley and Sons. (d) Batteries containing multifunctional composite sulfide electrolyte. Reproduced with permission.184 Copyright 2025, John Wiley and Sons. (e) The schematic illustrating the dendrite-scavenging effect by LTLC. (f) Galvanostatic cycling performance of lithium symmetric cells at a 0.5 mA cm−2. Reproduced with permission.185 Copyright 2024, John Wiley and Sons. | ||
Integrated composite architectures. When conventional structural modulation and localized interfacial engineering fail to simultaneously address the multiple demands of ionic conductivity, interfacial compatibility, and dendrite suppression, the design of integrated composite electrolyte architectures emerges as a promising solution. This strategy leverages spatial coupling and functional synergy between heterogeneous phases to construct unified electrolyte systems with multidimensional performance advantages, enabling comprehensive optimization of both intrinsic properties and interfacial behavior.
The multifunctional composite sulfide electrolyte (M-CSE) developed by Xu et al. exemplifies this integrated strategy.184 By co-blending highly conductive Li5.3PS4.3Cl1.7 with passivating Li9.54[Si0.5Sn0.5]PSBrO in a controlled ratio followed by cold pressing, a dense dual-scale microstructure is constructed (Fig. 13d). At the Li interface, the passivating component in situ forms a Li–Sn alloy and multicomponent buffer phases, which effectively absorb local electronic and mechanical disturbances to establish a stable dendrite-suppressing interphase. Hence, Li/M-CSE/Li symmetric cells achieve dendrite-free cycling for 650 h at a high current density of 3.76 mA cm−2, while full cells retain 95.04% of their capacity after 500 cycles at 1C. Another representative strategy involves the introduction of an interfacial reaction-regulating layer via a sandwiched architecture. Xu et al. embedded the halide superionic conductor Li0.388Ta0.238La0.475Cl3 (LTLC), which features high ionic conductivity and strong reductive stability, between layers of the LPSC to construct an LPSC/LTLC/LPSC trilayer structure (Fig. 13e).185 Upon contact with lithium dendrites, the LTLC layer in situ generates Ta and La metal nanoparticles along with LiCl, forming a dendrite-trapping interphase that enables self-passivating interfacial reactions. This structure mitigates the tip effect and delays dendrite penetration pathways. As a result, Li/LPSC/LTLC/LPSC/Li symmetric cells achieve a lifespan exceeding 500 h at 0.5 mA cm−2—over 70 times longer than that of conventional LPSC (Fig. 13f)—while full cells maintain 87.6% capacity after 200 cycles, featuring excellent interfacial protection and high-current adaptability.
In summary, the core of anode–SSE interface optimization lies in the coordinated achievement of mechanical adaptability, chemical stability, ionic conductivity, and electronic insulation, with representative strategies and electrochemical metrics summarized in Table 3. Among them, functional anode design and interlayer construction are the two most widely applied and system-compatible strategies for interface regulation. The former, through surface passivation, composite structures, and crystallographic orientation optimization, can effectively modulate lithium nucleation and deposition behaviors, fundamentally suppressing dendrite growth and interface failure; however, challenges remain in terms of fabrication complexity and chemical compatibility between the anode and SSE, and inappropriate material selection may trigger adverse interfacial reactions. The latter, benefiting from its tunable mechanical and chemical properties, demonstrates remarkable immediate effects in stress buffering, wettability enhancement, and electron blocking, with strong adaptability across various inorganic SSE systems. Nonetheless, during prolonged operation, interlayers may face issues such as structural evolution and increased resistance, thereby imposing stricter requirements on the process window for material composition and thickness. Achieving an optimal balance among ionic conductivity, mechanical compliance, and interfacial connectivity is therefore essential.
| Interface optimization strategies | Representative system | CCD (mA cm−2) | Symmetric cell lifespan | Full cell lifespan | Ref. |
|---|---|---|---|---|---|
| Interfacial layer construction | P2S5@Li/Li6PS5Cl | 0.4 | >500 h at 0.1 mA cm−2 | 75.5% over 400 cycles at 0.5C | 139 |
| LiF/LiPO3–Ag @Li/Li5.5PS4.5Cl1.5 | 2.4 | 500 h at 0.1 mA cm−2 | 82.6% after 400 cycles at 0.5C | 140 | |
| Composite anode design | LiAl-p/Li6PS5Cl | 6 | 5000 h at 0.5 mA cm−2 | 83% after 1800 cycles at 1C | 141 |
| LiSn/Li6PS5Cl | 2 | >1100 h at 1 mA cm−2 | 91% after 650 cycles at 1C | 143 | |
| Li–Ag/Li6PS5Cl | — | >1000 h at 0.1 mA cm−2 | 83.86% after 150 cycles at 0.1C | 144 | |
| In–Si/Li5.5PS4.5Cl1.5 | — | — | 98.5% after 2000 cycles at 3.69 mA cm−2 | 148 | |
| Li–GaP/Li6.4La3Zr1.4Ta0.6O12 | 2.5 | >5700 h at 0.3 mA cm−2 | 97.32% after 490 cycles at 1C | 149 | |
| Li–Mg/Li6.4La3Zr1.4Ta0.6O12 | 1 | >400 h at 0.3 mA cm−2 | 82% after 300 cycles at 0.5C | 151 | |
| Crystal structure modulation | Li(110)/Li6.75La3Zr1.75Ta0.25O12, Li6PS5Cl, Li3InCl6 | 6.0, 5.0, and 5.0 | — | 350 cycles at 1C | 142 |
| Flexible organic interlayers | Li/PDOL/Li5.4PS4.4Cl1.6 | 2.8 | >1200 h at 0.5 mA cm−2 | 95.4% after 200 cycles at 0.1C | 153 |
| Li/PLSS/Li6.5La3Zr1.5Ta0.5O12 | 1.1 | 4800 h at 0.1 mA cm−2 | 400 cycles at 0.5C | 154 | |
| Li/BP–Li/Li3GaF5.3Cl0.7 | 0.6 | 1800 h at 0.1 mA cm−2 | 100 cycles at 0.5C | 155 | |
| Inorganic oxide interlayers | Li/Li2O/Li6.4La3Zr1.4Ta0.6O12 | 2.4 | >1000 h at 0.5 mA cm−2 | 84% after 600 cycles at 1C | 159 |
| Li/Al2O3/Li6.5La3Zr1.5Ta0.5O12 | 1 | >1000 h at 0.3 mA cm−2 | 500 cycles at 1C | 156 | |
| Li/LiPON/Li6PS5Cl | 4.1 | >1000 h at 0.5 mA cm−2 | — | 161 | |
| Functional composite interlayers | Li/BiLi3–LiCl3/Li6.5La3Zr1.5Ta0.5O12 | 2.9 | >10000 h at 0.3 mA cm−2 | 88.5% after 400 cycles at 0.5C | 157 |
| Li/LiSn–LiCl/Li6.5La3Zr1.5Ta0.5O12 | 0.8 | >6000 h at 0.2 mA cm−2 | 94.7% after100 cycles at 0.2C | 162 | |
| Li/SbLi3–LiCl/Li6.25Ga0.25La3Zr2O12 | 6 | >1000 h at 2 mA cm−2 | 94.7% at 1C after 600 cycles | 163 | |
| Li/LiSn–Li2S/Li6.5La3Zr1.5Ta0.5O12 | 0.9 | >1500 h at 0.2 mA cm−2 | 99.8% after 100 cycles at 1C | 164 | |
| Li/LiIn–Li3N/Li6.4La3Zr1.4Ta0.6O12 | 1.8 | >2000 h at 0.5 mA cm−2 | 92.4% after 110 cycles at 0.2C | 165 | |
| Li/LiMg–Li3Bi–LiMgSx/Li6PS5Cl | 2.6 | >2700 h at 1.2 mA cm−2 | 681 cycles at 5C | 158 | |
| Li/LiF–C–Li3N–Bi/Li6PS5Cl | >3.5 | >500 h at a step increased current density | 854 cycles at 150 mA g−1 | 167 | |
| Li/G-LM/LLZTO | 2.7 | >1000 h at 0.5 mA cm−2 | 90% after 200 cycles at 2.32 mA cm−2 | 169 | |
| Microstructural regulation | HPLT Li7La3Zr2O12 | — | 150 h at 0.1 mA cm−2 | — | 170 |
| Nanorod-based Li6PS5Cl | 1.05 | 3000 h at 0.5 mA cm−2 | 80.3% after 100 cycles at 1C | 171 | |
| Li5.3PS4.3ClBr0.7 | 3.8 | >150 h at 3 mA cm−2 | 96% after 1000 cycles at 1C | 172 | |
| Doping engineering: | Li/Li9.9SnP2S11.9Cl0.1 | 0.15 | 350 h at 0.05 mA cm−2 | 60% after 25 cycles at 0.1C | 176 |
| Li/Li9.98Ge0.99Sn0.01P2S11.98F0.02 | 2.1 | 900 h at 0.1 mA cm−2 | 80.1% after 600 cycles at 1C | 173 | |
| Li/Li6.16P0.92In0.08S4.88O0.12Cl | 1.4 | 3000 h at 0.1 mA cm−2 | 82.9% after 100 cycles at 0.1C | 174 | |
| Li–In/LPSC–LZC-0.1AlF3 | 3 | >2000 h at 0.5 mA cm−2 | 87.5% after 50 cycles at 0.5 mA cm−2 | 177 | |
| Functional coating design | Li/LiF–ZrF4@Li2ZrCl6 | 1.8 | 1 mA cm−2 for over 400 h | 81.9% after 200 cycles at 1C | 178 |
| Li/Co2B@Li6.4La3Zr1.4Ta0.6O12 | — | 1450 h at 0.1 mA cm−2 | 98.51% after 300 cycles at 32 mA g−1 | 179 | |
| Li/PPA@Li6.4La3Zr1.4Ta0.6O12 | 1.8 | 2000 h at 0.2 mA cm−2 | 85.9% after 200 cycles at 0.2C | 180 | |
| Li/LLZTO-(ZrO2 and Li2CO3) | 2.1 | 2000 h at 0.8 mA cm−2 | 70.5% after 5800 cycles at 4C | 181 | |
| Li/g-C3N4-coated Li6PS5Cl | 1.5 | 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h at 0.2 h at 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mA mA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm−2 cm−2 |
99.1% after 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cycles at 0.1C cycles at 0.1C |
182 | |
| Li/LPSCl-polycaprolactone-based binder | 0.5 | 1300 h at 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mA mA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm−2 cm−2 |
89.9% after 300 cycles at 0.1C | 183 | |
| Integrated composite architectures | Li/Li6PS5Cl-M-CSE | 3.76 | 650 h at 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mA mA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm−2 cm−2 |
95.04% after 500 cycles at 0.5C | 184 |
| Li/LPSC–Li0.388Ta0.238La0.475Cl3 | 1.52 | >500 h at 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mA mA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm−2 cm−2 |
87.6% after 200 cycles at 0.5C | 185 |
By comparison, electrolyte engineering strategies exhibit stronger system dependence. For oxide-based SSEs, microstructural regulation remains the most effective approach, as these materials rely heavily on structural densification to achieve intimate interfacial contact and mechanical integrity. The intrinsic rigidity and high sintering temperature of oxide electrolytes make their interfacial properties particularly sensitive to porosity and grain connectivity; thus, optimizing particle packing, reducing voids, and balancing densification with mechanical compliance are crucial to enhance ionic conduction and suppress dendrite penetration. In contrast, functional coatings and integrated composite architectures are more suitable for sulfide and halide systems, which are typically softer, more deformable, and more chemically reactive. Functional coatings can effectively passivate reactive surfaces, improve chemical compatibility with lithium, and enhance long-term interfacial stability, while integrated composite architectures leverage multiphase spatial coupling to achieve simultaneous improvements in ionic conductivity, interfacial uniformity, and mechanical buffering. Furthermore, doping regulation is primarily applied to sulfide and halide SSEs, where aliovalent ion substitution can tune defect structures, regulate lattice polarizability, and significantly enhance intrinsic ionic conductivity as well as interfacial tolerance. These materials possess relatively open and flexible frameworks that can accommodate defect modulation without compromising structural integrity. In contrast, oxide SSEs exhibit densely packed and rigid lattices with limited defect mobility, restricting the extent to which doping can alter transport properties or interfacial chemistry.
Overall, the regulation of the anode–SSE interface is a multidimensional dynamic equilibrium involving material structure, interfacial chemistry, and processing conditions. The intrinsic properties of different inorganic SSEs dictate both the diversity and limitations of available strategies (Fig. 14), making it difficult for any single approach to address all interfacial failure bottlenecks. Looking ahead, advancing the synergy among materials innovation, interface engineering, and multiscale process integration will enable full-process optimization—from material selection and structural design to manufacturing. This holistic approach ensures unified control of mechanical, chemical, and electrical properties, paving the way for highly efficient and long-lasting ASSLBs.
4. Integrated dual-interface design
SSEs often face highly heterogeneous interfacial challenges at both the cathode and anode sides: oxidative decomposition driven by high voltage at the cathode interface, and severe reduction reactions and dendrite penetration at the anode interface. Conventional interfacial engineering typically focuses on stabilizing single interfaces, making it difficult to achieve balanced performance across the entire cell. The integrated dual-interface design strategy enables simultaneous regulation of both interfaces through structural configuration optimization or the introduction of functional materials. Without requiring additional fabrication steps, this approach constructs efficient ion transport pathways and a long-term stable chemical potential platform spanning the entire cell, representing a key avenue toward the practical realization of ASSLBs. According to differences in construction logic and functional mechanisms, current integrated dual-interface design strategies can be categorized into three main pathways: structural integration, functional interlayer regulation, and chemical synergistic modification.Structural integration
Conventional sintering-based densification and rapid bonding techniques, such as thermal-pulse welding (TPW) or ultrafast Joule-heating sintering (UHS), have been widely employed to construct continuous, low-resistance interfaces between the SSE and electrodes.186,187 These thermally induced integration methods effectively improve interfacial connectivity and ion-transport continuity without compromising the bulk structure, as exemplified by the LATP-based TPW process, which achieves 185.9 mAh g−1 and 90.9% capacity retention after 100 cycles (Fig. 15a).186 However, such physical densification approaches mainly focus on improving contact and neglect the electro-chemo-mechanical coupling effects that arise from mismatched volumetric strain between the cathode and anode during cycling. Recent studies by Gu et al. revealed that when electrodes exhibit opposite volume-change directions (e.g., sulfur or NCM811 vs. Li metal), strong inter-electrode stress crosstalk develops within the SSE, leading to grain-boundary cracking and dendrite penetration.188 In contrast, cathodes such as LiCoO2, which expand in the same direction as Li metal, induce milder mechanical damage and longer cycling life. Furthermore, incorporating active pressure-control systems can dynamically balance stack pressure and effectively suppress stress accumulation, extending the lifespan of sulfide-based ASSLBs. These findings suggest that structural integration should not be limited to densification alone but must also consider stress-adaptive design, ensuring mechanical compatibility across both interfaces for long-term durability of dual-interface solid-state systems. Even so, structural integration primarily enhances physical contact while offering limited capability to regulate interfacial chemistry and long-term reactivity. | ||
| Fig. 15 (a) Schematic of UHS and TPW technique for integrated anode/SSE/cathode. Reproduced with permission.186 Copyright 2025, Elsevier. (b) Solid-state structure of the Li–SN complex in stick and ball style shows 2D Li–SN molecules interlocked together, forming a long-range ordered 3D Li–SN complex. Reproduced with permission.189 Copyright 2024, American Chemical Society. (c) Schematic illustrating the comparison of the electrode–LLZTO interfaces. (d) The comparison of cycling performance between in situ polymerization and in situ polymerization-fluorination strategies at different cut-off voltages. Reproduced with permission.190 Copyright 2025, American Chemical Society. (e) Schematic of the electrochemical reduction of the electrophile, where the reductive electrophiles gain electron and Li+ from LPSC SSE or cathode powders upon contact with them, thus being electrochemically reduced on the surface of these materials. r.t., room temperature. Reproduced with permission.191 Copyright 2025, Springer Nature. | ||
Functional interlayer regulation
To overcome the intrinsic limitation of structural integration in governing interfacial chemistry, functional interlayer materials can be introduced to simultaneously enable interfacial passivation and ion transport pathway reconstruction at both interfaces. For instance, Luo et al. proposed the use of lithium-rich succinonitrile (Li–SN45) as a dual-interface modification material (Fig. 15b).189 This material exhibits high ionic conductivity (3.38 mS cm−1) and excellent resistance to lithium corrosion. With the introduction of the Li–SN45 interlayer, the battery achieves a discharge capacity of 170 mAh g−1 at 20 mA g−1, with 97% capacity retention after 100 cycles. Unfortunately, the functionality of Li–SN45 is primarily concentrated at the anode side and fails to effectively address structural instability and gas evolution at the cathode interface under high-voltage conditions.Chemical synergistic modification
Building on this, researchers further developed chemically synergistic strategies based on anion chemistry regulation and interfacial self-passivation mechanisms. Umeshbabu et al. introduced Cl− to partially substitute F−, constructing a dual-halide SSE, Li2ZrF6−xClx, which enables lattice parameter tuning and electronic structure optimization.192 The high electronegativity of F− facilitates the in situ formation of a dense and stable LiF interfacial layer at the anode side, effectively suppressing reduction-induced decomposition. Meanwhile, Cl− doping significantly enhances ionic mobility and structural stability of the crystal, thereby maintaining a wide electrochemical window and improving high-voltage compatibility at the cathode side. However, this strategy mainly relies on intrinsic structural regulation of the material, while offering limited control over the interfacial reactivity at the electrode surface. In this regard, Yin et al. proposed a polymerization-fluorination coupled strategy (Poly-FR), in which initiators induce the in situ formation of LiF-rich interfacial layers at both the cathode and anode sides (Fig. 15c).190 These layers provide multiple functions, including electronic passivation, structural buffering, and impurity removal, enabling the full cell to maintain 83.4% capacity retention over 400 cycles at 4.5 V (Fig. 15d). Yet, the process involves thermal initiation and monomer infiltration, making the fabrication relatively complex. Furthermore, Zhang et al. reported a class of reductive electrophilic reagents (e.g., diphosphoryl fluoride, DPF), which, when interacting with metal-nucleophilic materials (e.g., LPSC), can extract electrons and Li+ from the material's surface.191 This process triggers electrochemical reduction reaction, leading to the in situ formation of dense, ultrathin solid reductive-electrophilic interface (SREI) on the material's surface (Fig. 15e). The growth of the SREI is governed by electron migration and exhibits self-limiting characteristics. Once the interface layer reaches certain thickness, it automatically transforms into an amorphous inorganic interface layer rich in LiF–LixPyOzF. This interface demonstrates excellent lithium-ion repellency and electron-blocking properties, effectively mitigating lithium dendrite formation, preventing the reduction decomposition of the SSEs, and greatly enhancing compatibility with high-voltage cathodes. As a result, DPF-treated LPSC (DPF-LPSC) exhibits high lithium reversibility and a high CCD (>3.4 mA cm−2) when paired with the lithium metal anode. Under high-loading conditions, the Li//LiNi0.8Co0.15Al0.05O2 full cell achieves stable cycling over 600 cycles with a Coulombic efficiency exceeding 99.9%. Importantly, the construction of this SREI does not require the application of external electric field or complex equipment; it relies solely on the contact reactions between materials. This approach offers highly simplified process and broad material applicability, providing new pathway and theoretical foundation for the design of next-generation interface engineering.In the pursuit of high-performance ASSLBs, integrated dual-interface design strategies are evolving from structural construction toward chemical synergy and self-regulated interfacial design, reflecting a deeper trend of multi-mechanism cooperative integration. In particular, when addressing critical challenges such as interfacial reactivity asymmetry between electrodes, local stress mismatch, and discontinuous ion transport across interfaces, these strategies not only overcome the limitations of conventional single-interface approaches but also offer systematic solutions for achieving stable operation under high voltage, high current, and high loading conditions. Looking ahead, such strategies are expected to integrate closely with multiscale interfacial characterization, in situ evolution monitoring, and artificial intelligence (AI)-assisted materials design, paving the way for a more precise, intelligent, and scalable new phase in application-oriented interfacial engineering.
5. Advanced characterization techniques in SSBs interface
Understanding the intricate physicochemical processes occurring at solid–solid interfaces is pivotal to advancing ASSLBs. However, the buried nature and multiscale complexity of these interfaces pose significant challenges for direct observation and quantitative analysis. To overcome these barriers, a suite of advanced characterization techniques—spanning spectroscopic, microscopic, synchrotron-based, and neutron or ion beam-based methods—has been developed to probe interfacial structures, reactions, and dynamics with unprecedented spatial and temporal resolution. These approaches collectively enable the visualization of ion transport pathways, mapping of interphase formation, and elucidation of electro-chemo-mechanical coupling effects under realistic operating conditions. The following subsections summarize representative methodologies and their key contributions to understanding interfacial evolution and degradation mechanisms in ASSLBs, providing a comprehensive framework for correlating atomic-scale chemistry with macroscopic electrochemical behavior.5.1. In situ and operando spectroscopic techniques
In situ and operando spectroscopic techniques have become indispensable tools for elucidating the dynamic interfacial processes governing the performance and degradation of ASSLBs. By enabling real-time observation of chemical, structural, and electronic evolutions under electrochemical operation, these methods bridge the gap between static post-mortem analyses and true interfacial dynamics. Among them, X-ray photoelectron spectroscopy (XPS), Raman spectroscopy, solid-state nuclear magnetic resonance (NMR), and X-ray absorption spectroscopy (XAS) provide complementary insights spanning from surface chemistry and lattice vibrations to atomic-scale lithium distribution and local electronic structure. Together, these techniques offer a multidimensional understanding of interfacial reactions, transport phenomena, and degradation pathways, thereby guiding the rational design of stable and high-performance solid-state interfaces.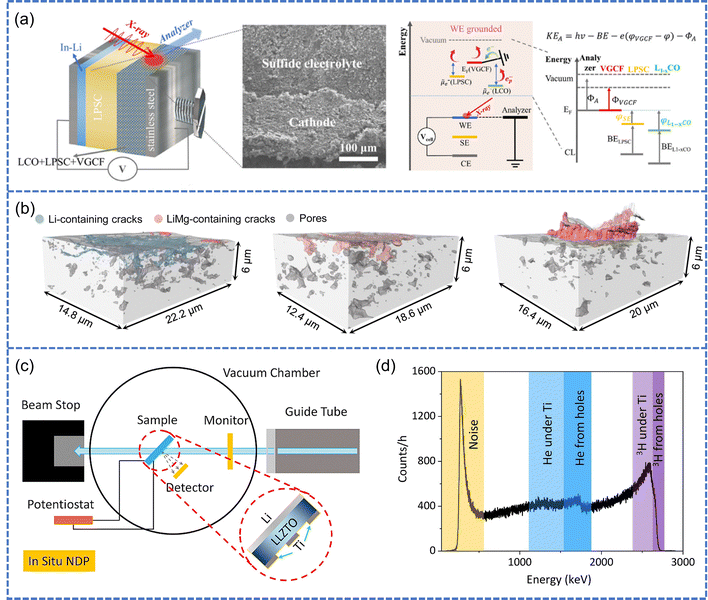 | ||
| Fig. 16 (a) Schematic diagram of the LCO/LPSC/Li–In batteries for the operando XPS measurements and grounded mode. Reproduced with permission.193 Copyright 2024, Elsevier. (b) Three-dimensional views of the surface cracks identifying those filled with Li or LiMg. Reproduced with permission.199 Copyright 2024, Royal Society of Chemistry. (c) Experimental setup for the in situ NDP measurements. (d) A typical NDP spectrum of ASSLMB w/3D Ti electrode. Reproduced with permission.209 Copyright 2019, Elsevier. | ||
Moreover, the chemical shift evolution of metallic lithium—from ∼230 ppm (flat deposits) to ∼270 ppm (dendritic structures)—indicates that NMR can sensitively track morphological transitions correlated with dendrite growth and interfacial degradation. The study also identifies two distinct dead Li formation modes: electronically disconnected Li trapped inside the SSE bulk and ionically isolated Li residing on the Cu current collector. Importantly, operando NMR further demonstrates that dendritic lithium exhibits a faster corrosion rate than planar lithium during calendar aging, reflecting its higher reactivity and instability. Collectively, these findings establish solid-state NMR as a unique, nondestructive probe for simultaneously quantifying lithium species, mapping transport pathways, and elucidating dynamic interfacial failure mechanisms in ASSLBs.
5.2. Advanced electron microscopy
Advanced electron microscopy enables direct, high-resolution observation of buried interfaces and microstructural evolution in ASSLBs. Techniques such as focused ion beam (FIB) cross-sectioning and cryogenic transmission electron microscopy (Cryo-TEM) provide precise visualization of interfacial structures and degradation processes, offering critical insights into the nanoscale mechanisms that govern battery stability and performance.5.3. Synchrotron and X-ray-based techniques
Advanced synchrotron and X-ray-based techniques have emerged as indispensable tools for probing the buried interfaces and structural dynamics in ASSLBs. Their high spatial resolution, tunable photon energy, and compatibility with in situ and operando measurements enable direct correlation between electrochemical behavior and crystallographic or morphological evolution. The following sections summarize representative synchrotron and X-ray characterization methods that have been applied to elucidate interfacial reactions, phase transformations, and mechanical degradation processes in ASSLBs.Recent developments have extended XCT to post-mortem and in situ analyses of interlayer-modified interfaces. For instance, laboratory-scale XCT combined with a high-pressure xenon contrast method successfully differentiated lithium metal from voids, overcoming the challenge of lithium's low X-ray absorption.207 This approach has been applied to visualize lithium deposition morphology in ASSLBs with carbon interlayers formed by different compaction processes. The technique demonstrates that simultaneous compression of the SSE and carbon layer produces intimate interfacial contact and uniform lithium deposition, while sequential pressing results in poor adhesion and dendritic intrusion. Overall, XCT serves as a crucial bridge between electrochemical performance and microstructural evolution in ASSLBs. Its ability to provide real-time, three-dimensional, and quantitative insight into interfacial morphology and internal stress evolution significantly advances the mechanistic understanding of lithium penetration, interlayer function, and failure processes, offering indispensable guidance for rational interfacial engineering in next-generation SSBs.
5.4. Neutron and ion beam analysis
Neutron and ion beam techniques offer powerful tools to visualize and quantify lithium distribution and interfacial evolution in ASSLBs. Their high sensitivity to light elements and excellent spatial or depth resolution enable nondestructive analysis of buried interfaces. Among them, neutron depth profiling (NDP), neutron imaging and tomography, and time-of-flight secondary ion mass spectrometry (TOF-SIMS) provide complementary information across multiple scales, revealing lithium transport pathways and interphase formation processes.In earlier studies, Li et al. employed in situ NDP to investigate lithium plating behavior in Li/LLZTO/Ti SSBs with a 3D Ti framework (Fig. 16c, d).209 They found that most lithium was deposited within the void spaces of the 3D electrode rather than directly at the solid electrolyte interface. The results show that such deposition effectively mitigates interfacial degradation and suppresses lithium dendrite growth. The NDP spectra record the evolution of 3H and 4He signals at various energies corresponding to lithium accumulation at different depths, confirming that lithium preferentially fills the 3D voids while its penetration into the electrolyte is significantly reduced. More recently, Westover et al. conducted comparative measurements combining NDP and neutron reflectometry (NR) on model Li-LiPON interfaces.208 They observed that NDP resolved lithium concentration profiles through Li and LiPON layers several hundred nanometers thick but could not distinguish ultrathin interphases below approximately 10 nm. The results indicate that NDP possesses excellent resolution within the 50 nm–1 mm range, while NR provides complementary sensitivity to nanometer-scale variations (0.1–200 nm). Together, these two techniques yield a comprehensive picture of the interfacial structure and lithium distribution across SSB stacks. Overall, NDP provides direct visualization of lithium migration, interphase formation, and dendrite evolution beneath buried interfaces. It demonstrates high sensitivity to light elements, excellent depth resolution, and a nondestructive nature, making it a powerful tool for probing ion transport and interfacial dynamics in ASSLBs, particularly when integrated with complementary neutron or X-ray-based techniques.
Technically, neutron imaging and tomography enable real-time monitoring of lithium migration, dendrite propagation, and interfacial reaction fronts within SSBs. The use of isotope labeling (6Li/7Li contrast enhancement) can further improve image contrast and provide insights into lithium diffusion behavior inside SSEs. Compared with NDP, neutron imaging and tomography offer a broader visualization range and 3D reconstruction capability, while NDP is more suitable for quantitative depth analysis. The integration of these techniques enables multiscale characterization of lithium distribution from the nanometer to micrometer level, providing a powerful approach for elucidating ion transport and interfacial dynamics in SSBs.
In the study by Otto et al., in situ TOF-SIMS was employed to investigate Li–SSE interfaces that were prepared by lithium vapor deposition or electrochemical plating.211 The technique reveals a continuous Li2S-rich interlayer (∼250 nm) at the Li–LPSC interface, which demonstrates the formation of a chemically stable yet ionically conductive SEI. Depth-profile analyses through micrometer-thick Li layers further enable classification of the interfacial stability of different electrolytes—LLZO (thermodynamically stable), LPSC (SEI-forming), and LATP (mixed-conducting interphase). Similarly, Sastre et al. utilized FIB-TOF-SIMS to visualize the element distribution and lithium gradient in doped LLZO thin films, and the analysis shows a homogeneous elemental distribution and a slight lithium gradient, demonstrating the capability of TOF-SIMS for nanoscale depth profiling of oxide SSEs.212 Overall, TOF-SIMS provides 3D chemical mapping of interfacial reactions, elemental diffusion, and degradation layers with nanometer precision, playing a crucial role in elucidating ion transport mechanisms and interfacial evolution processes.
Taken together, these advanced characterization techniques establish a comprehensive toolbox for deciphering the intricate interfacial phenomena in ASSLBs. From atomic-scale spectroscopy and electron microscopy to mesoscale X-ray tomography and macroscopic neutron analysis, each method contributes complementary information toward building a holistic understanding of interfacial chemistry, structure, and dynamics. The integration of in situ, operando, and multimodal approaches enables direct correlation between electrochemical behavior and structural evolution, thus revealing the origins of interfacial instability and guiding the rational design of robust interfaces. Moving forward, the convergence of these high-resolution tools with computational modeling and machine learning is expected to accelerate the development of predictive frameworks for interfacial engineering, paving the way for safer, more durable, and high-energy SSBs.
6. Summary and outlook
Inorganic ASSLBs are considered promising direction for next-generation energy storage systems due to their potential for high energy density and excellent safety. As the critical pathway for energy and material transfer within ASSLBs, the stability of the interface directly determines the cycling life and rate performance of the entire battery system. However, the inherent mismatch between the SSEs and the electrodes at the physical, chemical, and electrochemical levels presents significant challenges. Achieving stable control of the electrode–SSE interfaces under complex and dynamic operating conditions remains central issue in this field of research. In this review, we systematically integrate the coupled interfacial failure mechanisms encountered by inorganic SSEs at both electrode interfaces—including stress mismatch, interfacial reactions, electron leakage, and dendrite growth—and, crucially, conduct a side-by-side and critical comparison of leading interface engineering strategies. Four key approaches—electrode engineering, interlayer construction, electrolyte regulation, and integrated dual-interface design—are comparatively assessed, with special emphasis on their respective advantages, limitations, and application boundaries across different material systems and operational scenarios. This establishes a novel interfacial regulation framework that encompasses structural modulation, chemical stabilization and multifunctional synergy. Multiscale evolution of interfacial degradation and the functional complementarity among various strategies are highlighted, offering a forward-looking and integrated solution for realizing ASSLBs with high energy density and long-term stability. However, despite the significant progress made in interface design, several key issues remain that require further in-depth research and optimization (Fig. 17):(1) Current interface control primarily focuses on static structural design and enhancing chemical stability. However, in actual operating conditions, the interface environment is influenced by the synergistic effects of multiple physical fields, including electric fields, stress fields, concentration gradients, and temperature gradients. This is particularly pronounced under high-rate or wide-temperature range operating conditions. Therefore, there is urgent need to establish an interface characterization and simulation platform that couples multiple physical fields. This should incorporate in situ nano-mechanical-electrochemical coupling characterization methods (such as in situ electrochemical stretching and synchronized nano-indentation-impedance spectroscopy measurements), alongside multi-scale models combining finite element analysis, density functional theory, and phase field methods. Such approach would reveal the coupling evolution mechanisms of interfaces under real working conditions, providing essential physical insights for the design of interface materials and structures.
(2) Although many current interlayers and composite anode designs have shown some success in mitigating lithium dendrite growth, the initiation mechanisms, evolution pathways, and failure thresholds of dendrites remain inadequately understood. This is especially true regarding the issue of bulk-phase dendrite formation caused by electronic leakage, for which direct evidence and quantifiable standards are lacking. Therefore, it is recommended that future studies employ in situ cryo-electron microscopy and cryo-electron tomography techniques to visualize lithium deposition pathways and grain boundary penetration behaviors. In parallel, operando X-ray spectroscopy—such as X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS), complemented by grazing-incidence X-ray diffraction (GI-XRD) and small-angle X-ray scattering (SAXS) when appropriate—should be used to track real-time changes in interfacial valence states, coordination environments, and decomposition products, enabling correlation between the onset of electronic leakage and the emergence of mixed-conducting interphases. Based on this multimodal evidence, adopting an energy-band-engineering strategy offers a promising pathway forward. By leveraging interface dipole induction, block-copolymer interlayers, and electronegativity-gradient materials, it is possible to simultaneously optimize electronic barriers and ion flux regulation. Operando spectral parameters—such as edge shifts and pre-edge features—can then quantitatively verify band alignment and stability under cycling.
(3) Current designs for interface interlayers, electrode coatings, and doping modifications primarily rely on empirical screening and limited first-principles calculations, which are inefficient and lack transferability. As material databases (such as Materials Project, Open Catalyst Project, etc.) continue to expand, it is imperative to establish multi-parameter high-throughput screening system focused on indicators such as interface compatibility, conductivity, interface reaction enthalpy, and mechanical modulus. By leveraging machine learning and graph neural network (GNN) models, intelligent and collaborative screening of interfacial materials—such as those at cathode-electrolyte and interlayer-lithium metal anode interfaces—can be effectively realized. Furthermore, reverse design algorithms can be developed, combining battery performance objectives (such as high rate capability, long cycle life, low impedance, etc.) to deduce the material compositions and structural parameters that meet specific performance windows, thereby accelerating the transition of interface-building materials from experimental validation to engineering application.
(4) The present dual-interface synergistic construction strategies primarily rely on symmetric interlayers or electrolyte structural modifications to simplify the process and maintain stability at both the anode and cathode interfaces. However, such “homogeneous synergy” approaches are insufficient under extreme operating conditions—e.g., high current densities and low temperatures—where electro-chemo-mechanical coupling intensifies and the inherent differences in chemical potential, reactivity, and mechanical behavior between the two electrodes become more pronounced, limiting gains in rate capability and low-temperature robustness. To align with practical application needs, future designs should pursue “asymmetric yet coordinated” interfaces that are explicitly engineered for extremes: (i) low-temperature, fast-ion pathways and reliable wetting to minimize cold-start interfacial impedance; (ii) strong electronic blocking and Li-flux homogenization to suppress electronic-leakage-induced bulk dendrites at high rates; and (iii) modulus/thermal-expansion-graded architectures with stress-relief or self-healing chemistries to prevent contact loss across wide temperature ranges. A promising strategy involves developing adaptive interface precursors that spontaneously convert during processing or cycling into targeted interphases—such as lithiophilic, electron-insulating layers at the anode and ion-conductive, stable layers at the cathode. This self-evolving design streamlines fabrication while ensuring coordinated interfacial evolution and durable performance, even under high-current and low-temperature operation.
(5) At this stage, laboratory techniques such as atomic layer deposition (ALD) and molecular layer deposition (MLD) are commonly used; however, they are expensive and difficult to scale up for industrial production. To promote the commercialization of ASSLBs, it is essential to accelerate the development of process-friendly interface construction technologies. These should include low-energy, high-throughput engineering strategies, such as spray-pyrolysis-based interlayer construction, fusion of interfaces via molten compression, and the introduction of pressure-sensitive-adhesive-based polymer interfaces. Alongside these efforts, pilot-scale fabrication trials (e.g., roll-to-roll lamination, slot-die/spray coating, tape casting, and hot-press/melt-fusion under dry-room conditions) should be conducted to translate laboratory protocols to manufacturing-relevant throughputs, quantify yield and uniformity over tens-to-hundreds of meters, and establish robust process windows (temperature, pressure, line speed) that preserve interfacial integrity. Additionally, it is important to explore the stability and controllability of the interface layers under real processing conditions, such as rolling and laminating, using statistically designed experiments during pilot runs to assess reproducibility, aging, and thermal excursions. Furthermore, building a comprehensive framework that integrates a cross-scale interface-failure database, process maps, and lifetime prediction models will bridge the gap between laboratory research and engineering practice. Continuous data feedback from pilot production lines can then close the design-manufacturing loop, paving the way for the large-scale deployment of ASSLBs.
In summary, the stable construction of electrode-inorganic SSE interfaces still faces challenges regarding the multi-physical field coupling evolution, unclear dendrite formation mechanisms, insufficient intelligent design of interface materials, and difficulty in controlling the differences between dual interfaces. Moving forward, the focus should be on the synergistic advancement of mechanism analysis, material screening, and process integration, aiming to establish high-performance, scalable interface engineering systems.
Conflicts of interest
There are no conflicts to declare.Data availability
This article is a review and does not report new original data. All data supporting the findings of this study are available within the cited references.Acknowledgements
Z. B. thanks the financial support of National Natural Science Foundation of China (No. 22272093), Research Fund for International Scientists National Natural Science Foundation of China (52350710795), and Natural Science Foundation of Shandong Province (ZR2021MB127). N. W. acknowledge support from the Australian Research Council (DP240102926 and FT240100596).References
- Y. Qin, M. Zhou, Y. Hao, X. Huang, D. Tong, L. Huang, C. Zhang, J. Cheng, W. Gu, L. Wang, X. He, D. Zhou, Q. Chen, A. Ding and T. Zhu, Nat. Geosci., 2024, 17, 411–418 CrossRef CAS.
- H. J. Xu, X. C. Han, W. S. Hua, D. Friedrich, G. Santori, E. Bevan, K. Vafai, F. Q. Wang, X. L. Zhang, G. J. Yu and H. F. Xu, Renewable Sustainable Energy Rev., 2025, 215, 115587 CrossRef CAS.
- J. Aslam, M. A. Waseem, X.-M. Lu, W. Sun and Y. Wang, Chem. Eng. J., 2025, 505, 159556 CrossRef CAS.
- X. Xu, X. Han, L. Lu, F. Wang, M. Yang, X. Liu, Y. Wu, S. Tang, Y. Hou, J. Hou, C. Yu and M. Ouyang, J. Power Sources, 2024, 603, 234445 CrossRef CAS.
- N. Nasajpour-Esfahani, H. Garmestani, M. Bagheritabar, D. J. Jasim, D. Toghraie, S. Dadkhah and H. Firoozeh, Renewable Sustainable Energy Rev., 2024, 203, 114783 CrossRef CAS.
- J. Huang, C. Li, D. Jiang, J. Gao, L. Cheng, G. Li, H. Luo, Z.-L. Xu, D.-M. Shin, Y. Wang, Y. Lu and Y. Kim, Adv. Funct. Mater., 2025, 35, 2411171 CrossRef CAS.
- J. Li, Y. Li, Y. Wang, X. Wang, P. Wang, L. Ci and Z. Liu, Energy Storage Mater., 2025, 74, 103962 CrossRef.
- Y. Xu, Z. Fang, J. Yue, L. Wang, Z. Wang, S. Zhang, M. Liu, Z. Ye, R. Liu, X. Yan, X. Han, Y. Wang, C. Zhao, B. Xiao, X. Wang, W. Xiao, X. Li, T. Mei and J. Liang, Small, 2025, 21, 2408824 CrossRef CAS.
- N.-L. Shen, F. Jiang, J.-X. Guo, Y.-F. Du, Z.-Y. Qu, X. Shen, Y. Zhou, Z. Lyu, G. Xiao, X.-B. Cheng and Y. Wu, Adv. Funct. Mater., 2025, 35, 2505437 CrossRef CAS.
- D. Kim, X. Hu, B. Yu and Y. I. Chen, Adv. Mater., 2024, 36, 2401625 CrossRef CAS PubMed.
- M. Muzakir, K. Manickavasakam, E. J. Cheng, F. Yang, Z. Wang, H. Li, X. Zhang and J. Qin, J. Mater. Chem. A, 2025, 13, 73–135 RSC.
- X. Dai, K. Zhou, L. Zhang, T. Wu, H.-M. Ye, X. Cao, Y. Han, G. Huang and S. Xu, Nano Energy, 2025, 133, 110475 CrossRef CAS.
- H. Jamal, F. Khan, J. H. Kim, E. Kim, S. U. Lee and J. H. Kim, Small, 2024, 20, 2402001 CrossRef CAS.
- N. Sarfraz, N. Kanwal, M. Ali, K. Ali, A. Hasnain, M. Ashraf, M. Ayaz, J. Ifthikar, S. Ali, A. Hendi, N. Baig, M. F. Ehsan, S. S. Shah, R. Khan and I. Khan, Energy Storage Mater., 2024, 71, 103619 CrossRef.
- Z. Xiao, L. Jiang, L. Song, T. Zhao, M. Xiao, Q. Yan and L. Li, J. Energy Storage, 2024, 96, 112696 CrossRef.
- Y. Guo, M. Zhang, Z. Ge, Z. Fang, Z. Xu, J. Wu and M. Wu, Adv. Funct. Mater., 2025, 35, 2419998 CrossRef CAS.
- M.-S. Tu, Z.-H. Wang, Q.-H. Chen, Z.-P. Guo, F.-F. Cao and H. Ye, Energy Environ. Sci., 2025, 18, 2873–2882 RSC.
- S. Qin, M. Wu, H. Zhao, J. Li, M. Yan, Y. Ren and Y. Qi, J. Mater. Sci. Technol., 2024, 199, 197–205 CrossRef CAS.
- S. Venkatesan, R.-Y. Hong, H. Teng and Y.-L. Lee, Mater. Today Energy, 2025, 48, 101761 CrossRef CAS.
- J. Wang, L. Chen, H. Li and F. Wu, Energy Environ. Mater., 2023, 6, e12613 CrossRef CAS.
- J. H. Kim, D. Kim, S. Nanda and H. Khani, Adv. Funct. Mater., 2024, 34, 2410845 CrossRef CAS.
- S. Wang, Y. Liao, S. Li, C. Cui, J. Liang, G. Du, Z. Tong, L. Yuan, T. Zhai and H. Li, Adv. Energy Mater., 2024, 14, 2303641 CrossRef CAS.
- S. Kim, G. Yoon, S.-K. Jung, S. Park, J.-S. Kim, K. Yoon, S. Lee and K. Kang, ACS Energy Lett., 2023, 8, 9–20 CrossRef CAS.
- Z. Hu, J. Sheng, J. Chen, G. Sheng, Y. Li, X.-Z. Fu, L. Wang, R. Sun and C.-P. Wong, New J. Chem., 2018, 42, 9074–9079 RSC.
- Y. Deng, C. Eames, J.-N. Chotard, F. Lalère, V. Seznec, S. Emge, O. Pecher, C. P. Grey, C. Masquelier and M. S. Islam, J. Am. Chem. Soc., 2015, 137, 9136–9145 CrossRef CAS PubMed.
- X. Wang, X. Xu, W. Hou, Y. Chen, Y. Yang, Y. Wang, Z. Guo, Z. Song and Y. Liu, Adv. Energy Mater., 2025, 15, 2402731 CrossRef CAS.
- M. Murayama, N. Sonoyama, A. Yamada and R. Kanno, Solid State Ionics, 2004, 170, 173–180 CrossRef CAS.
- Y. Seino, T. Ota, K. Takada, A. Hayashi and M. Tatsumisago, Energy Environ. Sci., 2014, 7, 627–631 RSC.
- S. Jian, H. Li, X. Jia, D. Zhong, B. Tao, X. He, G. Wang and H. Chang, FlatChem, 2024, 46, 100693 CrossRef CAS.
- Y. Chen, X. Gao, Z. Zhen, X. Chen, L. Huang, D. Zhou, T. Hu, B. Ren, R. Xu, J. Chen, X. Chen, L. Cui and G. Wang, Energy Environ. Sci., 2024, 17, 9288–9302 RSC.
- C. Wang, J. Liang, J. Luo, J. Liu, X. Li, F. Zhao, R. Li, H. Huang, S. Zhao, L. Zhang, J. Wang and X. Sun, Sci. Adv., 2021, 7, eabh1896 CrossRef CAS.
- L. Zhou, T. Zuo, C. Li, Q. Zhang, J. Janek and L. F. Nazar, ACS Energy Lett., 2023, 8, 3102–3111 CrossRef CAS.
- W. Kim, J. Noh, S. Lee, K. Yoon, S. Han, S. Yu, K.-H. Ko and K. Kang, Adv. Mater., 2023, 35, 2301631 CrossRef CAS.
- P. Huang, L. T. Gao and Z.-S. Guo, Electrochim. Acta, 2023, 463, 142873 CrossRef CAS.
- T. Yang, H. Pei, H. Lv, S. Lu, Q. Liu and D. Mu, J. Energy Chem., 2025, 101, 233–262 CrossRef CAS.
- T.-T. Wu, S. Guo, B. Li, C.-Y. Shen, X.-H. Liu and A.-M. Cao, Rare Met., 2023, 42, 3177–3200 CrossRef CAS.
- L. Ma, Y. Dong, N. Li, W. Yan, S. Ma, Y. Fang, Y. Li, L. Xu, C. Liu, S. Chen, R. Feng, L. Chen, D. Cao, Y. Lu, Q. Huang, Y. Su and F. Wu, eTransportation, 2024, 20, 100312 CrossRef.
- J. Zhang, J. Fu, P. Lu, G. Hu, S. Xia, S. Zhang, Z. Wang, Z. Zhou, W. Yan, W. Xia, C. Wang and X. Sun, Adv. Mater., 2025, 37, 2413499 CrossRef CAS PubMed.
- A. Banerjee, X. Wang, C. Fang, E. A. Wu and Y. S. Meng, Chem. Rev., 2020, 120, 6878–6933 CrossRef CAS PubMed.
- H. Liu, Y. Wang, L. Chen, H. Li and F. Wu, Adv. Funct. Mater., 2024, 34, 2315701 CrossRef CAS.
- K. Ma, B. Chen, C.-X. Li and V. Thangadurai, Adv. Sustainable Syst., 2024, 8, 2300656 CrossRef CAS.
- Y. Liang, M. Burton, B. Jagger, H. Guo, J. Ihli and M. Pasta, Chem. Commun., 2024, 60, 12597–12600 RSC.
- Q. Xia, S. Yuan, Q. Zhang, C. Huang, J. Liu and H. Jin, Adv. Sci., 2024, 11, 2401453 CrossRef CAS PubMed.
- G. H. Chun, J. H. Shim and S. Yu, ACS Appl. Mater. Interfaces, 2022, 14, 1241–1248 CrossRef CAS.
- Q. Fan, W. Zhang, Y. Jin, D. Zhang, X. Meng, W. Peng, J. Wang, J. Mo, K. Liu, L. Liu and M. Li, Chem. Eng. J., 2023, 477, 147179 CrossRef CAS.
- M. Gao, P. Li, S. Fu, S. Yu, Y. Hu, D. Chen, Y. Wei, D. Li, N. Wang, L. Yang and Y. Chen, ACS Appl. Mater. Interfaces, 2024, 16, 69253–69261 CrossRef CAS.
- L. Zhao, Y. Zhong, C. Cao, T. Tang and Z. Shao, Nano-Micro Lett., 2024, 16, 124 CrossRef CAS PubMed.
- J. Cui, J. H. Kim, S. Yao, A. Guerfi, A. Paolella, J. B. Goodenough and H. Khani, Adv. Funct. Mater., 2023, 33, 2210192 CrossRef CAS.
- L. Nie, S. Chen, M. Zhang, T. Gao, Y. Zhang, R. Wei, Y. Zhang and W. Liu, Nano Res., 2024, 17, 2687–2692 CrossRef CAS.
- J. Shi, Z. Ma, D. Wu, Y. Yu, Z. Wang, Y. Fang, D. Chen, S. Shang, X. Qu and P. Li, Small, 2024, 20, 2307030 CrossRef CAS.
- S. Kim, M. Kim, M. Ku, J. Park, J. Lee and Y.-B. Kim, ACS Nano, 2024, 18, 25096–25106 CrossRef CAS.
- G. Liu, Z. Li, L. Zeng, J. Lin, B. Zheng, H. Liu, L. Chen and F. Wu, Nano Energy, 2025, 137, 110798 CrossRef CAS.
- M. K. Jangid, T. H. Cho, T. Ma, D. W. Liao, H. Kim, Y. Kim, M. Chi and N. P. Dasgupta, Nat. Commun., 2024, 15, 10233 CrossRef CAS.
- K. Chen, Y. Tang, S. Zhang, X. Hao, X. Zhao, L.-Q. Cheng, Y. Xiao and Z. Wen, ACS Appl. Mater. Interfaces, 2024, 16, 45459–45472 CrossRef CAS PubMed.
- L. Hu, Q. Duan, Y. Li, S. Huang, W. Li, X. Liu, Y. Xu, B. Zhao, J. Zhang and Y. Jiang, J. Power Sources, 2024, 619, 235220 CrossRef CAS.
- B. Li, L. Xian, J. Peng and L.-B. Kong, ACS Appl. Energy Mater., 2024, 7, 3550–3557 CrossRef CAS.
- R. Chen, C. Yao, Q. Yang, H. Pan, X. Yu, K. Zhang and H. Li, ACS Appl. Mater. Interfaces, 2021, 13, 18743–18749 CrossRef CAS.
- X. Wang, W. Jiang, X. Zhu, S. Li, S. Zhang, Q. Wu, J. Zhang, W. Zhong, S. Zhao, H. Cheng, Y. Tan, M. Ling and Y. Lu, Small, 2024, 20, 2306763 CrossRef CAS.
- Z. Jiang, J. Yang, C. Liu, C. Wei, Z. Wu, Q. Luo, L. Zhang, X. Chen, L. Li, G. Li, S. Cheng and C. Yu, Nano Energy, 2024, 128, 109926 CrossRef CAS.
- J. Yang, Z. Jiang, C. Liu, L. Li, S. Li, Z. Wu, C. Wei, Q. Luo, L. Zhang, S. Cheng and C. Yu, Chem. Eng. J., 2024, 500, 157027 CrossRef CAS.
- P. Ren, S. Zhang, B. Li, R. Liu, N. Wu and Y. Li, Nano Energy, 2024, 130, 110127 CrossRef CAS.
- Y. Liang, C. Shen, H. Liu, C. Wang, D. Li, X. Zhao and L.-Z. Fan, Adv. Sci., 2023, 10, 2300985 CrossRef CAS.
- Y.-E. Park, M.-K. Oh, H.-T. Sim, H.-J. Kim, Y.-S. Cho, S.-J. Park and D.-W. Kim, ACS Appl. Mater. Interfaces, 2024, 16, 39460–39469 CrossRef CAS PubMed.
- L. Zhang, D. Xu, H. Chen, R. Li, Z. Hu, B. Wen, A. Chen, X. Liu, J. Luo and A. Wang, Adv. Funct. Mater., 2025, 35, 2416013 CrossRef CAS.
- X. Zhang, H. Yu, L. Ben, G. Cen, Y. Sun, L. Wang, J. Hao, J. Zhu, Q. Sun, R. Qiao, X. Yao, H. Zhang and X. Huang, Adv. Mater., 2025, 37, 2506298 CrossRef CAS.
- J.-H. Kim, J. Sun, J.-S. Jang, D.-H. Park, S.-Y. Ahn, W.-C. Kim, K. Min and K.-W. Park, Energy Storage Mater., 2024, 64, 103080 CrossRef.
- Y. He, C. Wang, R. Zhang, P. Zou, Z. Chen, S.-M. Bak, S. E. Trask, Y. Du, R. Lin, E. Hu and H. L. Xin, Nat. Commun., 2024, 15, 10015 CrossRef CAS PubMed.
- G. Pustorino, H. Jagad, W. Li, M. Feng, M. Poma, J. Ko, P. Johari and Y. Qi, Chem. Mater., 2025, 37, 313–321 CrossRef CAS.
- C. Wang, J. Liang, J. T. Kim and X. Sun, Sci. Adv., 2022, 8, eadc9516 CrossRef CAS PubMed.
- H. Su, J. Li, Y. Zhong, Y. Liu, X. Gao, J. Kuang, M. Wang, C. Lin, X. Wang and J. Tu, Nat. Commun., 2024, 15, 4202 CrossRef CAS PubMed.
- C. Liu, F. Roters and D. Raabe, Nat. Commun., 2024, 15, 7970 CrossRef CAS PubMed.
- J. Fu, S. Wang, D. Wu, J. Luo, C. Wang, J. Liang, X. Lin, Y. Hu, S. Zhang, F. Zhao, W. Li, M. Li, H. Duan, Y. Zhao, M. Gu, T.-K. Sham, Y. Mo and X. Sun, Adv. Mater., 2024, 36, 2308012 CrossRef CAS.
- Q. Lin, X. Kang, L. Li, J. Yao, Y. Chen, X. Yan, C. Yu, L. Zhang and Z. Huang, Energy Storage Mater., 2025, 75, 104062 CrossRef.
- S. Huo, L. Sheng, W. Xue, L. Wang, H. Xu, H. Zhang, B. Su, M. Lyu and X. He, Adv. Energy Mater., 2023, 13, 2204343 CrossRef CAS.
- R. Xu, J. Yao, Z. Zhang, L. Li, Z. Wang, D. Song, X. Yan, C. Yu and L. Zhang, Adv. Sci., 2022, 9, 2204633 CrossRef CAS PubMed.
- C. Zhang, J. Yu, Y. Cui, Y. Lv, Y. Zhang, T. Gao, Y. He, X. Chen, T. Li, T. Lin, Q. Mi, Y. Yu and W. Liu, Nat. Commun., 2024, 15, 5325 CrossRef CAS PubMed.
- J. Wu, L. Shi, J. Li, J. Xu, T. Feng and W. Guo, J. Energy Storage, 2025, 120, 116487 CrossRef.
- A. Müller, F. Okur, A. Aribia, N. Osenciat, C. A. F. Vaz, V. Siller, M. El Kazzi, E. Gilshtein, M. H. Futscher, K. V. Kravchyk, M. V. Kovalenko and Y. E. Romanyuk, Mater. Adv., 2023, 4, 2138–2146 RSC.
- Y. Jiang, A. Lai, J. Ma, K. Yu, H. Zeng, G. Zhang, W. Huang, C. Wang, S.-S. Chi, J. Wang and Y. Deng, ChemSusChem, 2023, 16, e202202156 CrossRef CAS PubMed.
- Y. Li, D. Zhang, X. Xu, Z. Wang, Z. Liu, J. Shen, J. Liu and M. Zhu, J. Energy Chem., 2021, 60, 32–60 CrossRef CAS.
- T.-T. Zuo, R. Rueß, R. Pan, F. Walther, M. Rohnke, S. Hori, R. Kanno, D. Schröder and J. Janek, Nat. Commun., 2021, 12, 6669 CrossRef CAS PubMed.
- X. Liu, Y. Cheng, Y. Su, F. Ren, J. Zhao, Z. Liang, B. Zheng, J. Shi, K. Zhou, Y. Xiang, J. Zheng, M.-S. Wang, J. Huang, M. Shao and Y. Yang, Energy Storage Mater., 2023, 54, 713–723 CrossRef.
- M. J. Joo, M. Kim, S. Chae, M. Ko and Y. J. Park, ACS Appl. Mater. Interfaces, 2023, 15, 59389–59402 CrossRef CAS.
- S. Lee, Y. Kim, C. Park, J. Kim, J.-S. Kim, H. Jo, C. J. Lee, S. Choi, D.-H. Seo and S.-K. Jung, ACS Energy Lett., 2024, 9, 1369–1380 CrossRef CAS.
- F. Zhao, S. Zhang, S. Wang, C. M. Andrei, H. Yuan, J. Zhou, J. Wang, Z. Zhuo, Y. Zhong, H. Su, J. T. Kim, R. Yu, Y. Gao, J. Guo, T.-K. Sham, Y. Mo and X. Sun, Energy Environ. Sci., 2024, 17, 4055–4063 RSC.
- J. Li, J. Chen, X. Xu, Z. Wang, J. Shen, J. Sun, B. Huang and T. Zhao, Adv. Energy Mater., 2025, 15, 2402746 CrossRef CAS.
- N. J. J. de Klerk and M. Wagemaker, ACS Appl. Energy Mater., 2018, 1, 5609–5618 CAS.
- J. Yi, P. He, H. Liu, H. Ni, Z. Bai and L.-Z. Fan, J. Energy Chem., 2021, 52, 202–209 CrossRef CAS.
- Z. Wang, T. Wang, N. Zhang, W. Zhang, Y. Liu and C. Wang, Adv. Mater., 2025, 37, 2501838 CrossRef CAS.
- W. Jiang, X. Fan, X. Zhu, Z. Wu, Z. Li, R. Huang, S. Zhao, X. Zeng, G. Hu, B. Zhang, S. Zhang, L. Zhu, L. Yan, M. Ling, L. Wang and C. Liang, J. Power Sources, 2021, 508, 230335 CrossRef CAS.
- R. Tian, Z. Wang, J. Liao, H. Zhang, D. Song, L. Zhu and L. Zhang, Adv. Energy Mater., 2023, 13, 2300850 CrossRef CAS.
- H.-S. Zhang, X.-C. Lei, D. Su, S.-J. Guo, J.-C. Zhu, X.-F. Wang, X. Zhang, T.-T. Wu, S.-Q. Lu, Y.-T. Li and A.-M. Cao, Angew. Chem., Int. Ed., 2024, 63, e202400562 CrossRef CAS PubMed.
- J. S. Kim, S. Jung, H. Kwak, Y. Han, S. Kim, J. Lim, Y. M. Lee and Y. S. Jung, Energy Storage Mater., 2023, 55, 193–204 CrossRef.
- L. Wang, A. Mukherjee, C.-Y. Kuo, S. Chakrabarty, R. Yemini, A. A. Dameron, J. W. DuMont, S. H. Akella, A. Saha, S. Taragin, H. Aviv, D. Naveh, D. Sharon, T.-S. Chan, H.-J. Lin, J.-F. Lee, C.-T. Chen, B. Liu, X. Gao, S. Basu, Z. Hu, D. Aurbach, P. G. Bruce and M. Noked, Nat. Nanotechnol., 2024, 19, 208–218 CrossRef CAS PubMed.
- B. Shen, L. Li, X. Yao and B. Huang, Ceram. Int., 2024, 50, 7150–7155 CrossRef CAS.
- B. Zhang, Z. He, T. Liu, Z. Li, S. Zhang, W. Zhao, Z.-W. Yin, Z. Zhuo, M. Zhang, F. Pan, S. Zhang, Z. Lin and J. Lu, Adv. Mater., 2024, 36, 2305748 CrossRef CAS.
- Y. Liu, T. Yu, S. Xu, Y. Sun, J. Li, X. Xu, H. Li, M. Zhang, J. Tian, R. Hou, Y. Rao, H. Zhou and S. Guo, Angew. Chem., Int. Ed., 2024, 63, e202403617 CrossRef CAS.
- L. Wang, D. Gong, S. Niu, L. Wang, Q. Shi, X. Wang, J. Qiao, G. Liu and C. Zhan, Appl. Surf. Sci., 2023, 619, 156741 CrossRef CAS.
- Z. Li, J. Miao, W. Hu, Y. Liu, M. Li, M. Zhao, J. Liu and L. Xiao, Chem. Commun., 2023, 59, 5627–5630 RSC.
- B.-X. Shi, Y. Yusim, S. Sen, T. Demuth, R. Ruess, K. Volz, A. Henss and F. H. Richter, Adv. Energy Mater., 2023, 13, 2300310 CrossRef CAS.
- R. Ma, Y. Liu, R. Fang, J. Zhang, Y.-H. Wang, H. Huang, Y. Gan, X. He, X. Xia, W. Zhang, Y. Xia and S. Xin, ChemSusChem, 2024, 17, e202400840 CrossRef CAS PubMed.
- J. Kim, W. Choi, S.-J. Hwang and D. W. Kim, Energy Environ. Mater., 2024, 7, e12776 CrossRef CAS.
- X. Zhou, B. Zhang, P. Lyu, L. Xi, F. Li, Z. Ma, M. Zhu and J. Liu, Energy Environ. Sci., 2024, 17, 8174–8188 RSC.
- Y. Su, X. Liu, H. Yan, J. Zhao, Y. Cheng, Y. Luo, J. Gu, H. Zhong, A. Fu, K. Wang, M.-S. Wang, J. Huang, J. Yan and Y. Yang, Nano Energy, 2023, 113, 108572 CrossRef CAS.
- U.-H. Kim, T.-Y. Yu, J. W. Lee, H. U. Lee, I. Belharouak, C. S. Yoon and Y.-K. Sun, ACS Energy Lett., 2023, 8, 809–817 CrossRef CAS.
- J. Qiu, H. Li, Y. Zhao, R. Xu, K. Wei, Y. Cui, J. Shu and Y. Cui, Mater. Today, 2024, 80, 342–352 CrossRef CAS.
- Z. Dai, X. Sun, R. Chen, F. Wu and L. Li, J. Am. Chem. Soc., 2024, 146, 34517–34527 CrossRef CAS.
- J. Kim, W. Choi, Y.-P. Jeon, S.-J. Hwang and D. W. Kim, J. Power Sources, 2025, 658, 238306 CrossRef CAS.
- K. S. Saqib, T. J. Embleton, J. H. Choi, S.-J. Won, J. Ali, K. Ko, S. Choi, M. Jo, S. Park, J. Park, W. Kaveevivitchai, Y. Son, W.-J. Lee and P. Oh, ACS Appl. Mater. Interfaces, 2024, 16, 47551–47562 CrossRef CAS PubMed.
- R. Fang, Y. Liu, Y. Li, A. Manthiram and J. B. Goodenough, Mater. Today, 2023, 64, 52–60 CrossRef CAS.
- J. Oh, S. H. Choi, H. Kim, W. J. Chung, M. Kim, I. Kim, T. Lee, J. Lee, D. O. Kim, S. Moon, D. Kim and J. W. Choi, ACS Energy Lett., 2025, 10, 2831–2838 CrossRef CAS.
- Y. Li, C. Kim, E. Williams, Y. Su, J. Nanda and G. Yang, Energy Environ. Mater., 2025, 8, e12858 CrossRef CAS.
- T. Charlesworth, K. Yiamsawat, H. Gao, G. J. Rees, C. K. Williams, P. G. Bruce, M. Pasta and G. L. Gregory, Angew. Chem., Int. Ed., 2024, 63, e202408246 CrossRef CAS PubMed.
- L. Cui, S. Zhang, J. Ju, T. Liu, Y. Zheng, J. Xu, Y. Wang, J. Li, J. Zhao, J. Ma, J. Wang, G. Xu, T.-S. Chan, Y.-C. Huang, S.-C. Haw, J.-M. Chen, Z. Hu and G. Cui, Nat. Energy, 2024, 9, 1084–1094 CAS.
- X. Wang, S. Huang, B. Wei, M. Liu, B. Yang, R. Liu and H. Jin, ACS Appl. Mater. Interfaces, 2024, 16, 44912–44920 CrossRef CAS PubMed.
- D. Wu, J. Peng, Z. Jiang, L. Zhu, Y. Wu, C. Xu, Z. Wang, M. Yang, H. Li, L. Chen and F. Wu, Energy Storage Mater., 2024, 72, 103749 CrossRef.
- J. Yu, W. Zhai, C. Zhang, C. Wu, R. Wei, S. Chen, Y. He, Q. Hu, Y. Yu and W. Liu, ACS Energy Lett., 2023, 8, 1468–1476 CrossRef CAS.
- Q. Luo, C. Liu, L. Li, Z. Jiang, J. Yang, S. Chen, X. Chen, L. Zhang, S. Cheng and C. Yu, J. Energy Chem., 2024, 99, 484–494 CrossRef CAS.
- C. Wang, S. Wang, X. Liu, Y. Wu, R. Yu, H. Duan, J. T. Kim, H. Huang, J. Wang, Y. Mo and X. Sun, Energy Environ. Sci., 2023, 16, 5136–5143 RSC.
- Z. Song, T. Wang, H. Yang, W. H. Kan, Y. Chen, Q. Yu, L. Wang, Y. Zhang, Y. Dai, H. Chen, W. Yin, T. Honda, M. Avdeev, H. Xu, J. Ma, Y. Huang and W. Luo, Nat. Commun., 2024, 15, 1481 CrossRef CAS PubMed.
- L. Shen, J.-L. Li, W.-J. Kong, C.-X. Bi, P. Xu, X.-Y. Huang, W.-Z. Huang, F. Fu, Y.-C. Le, C.-Z. Zhao, H. Yuan, J.-Q. Huang and Q. Zhang, Adv. Funct. Mater., 2024, 34, 2408571 CrossRef CAS.
- Y. Gao, S. Zhang, F. Zhao, J. Wang, J. Zhou, W. Li, S. Deng, J. Fu, X. Hao, R. Li and X. Sun, ACS Energy Lett., 2024, 9, 1735–1742 CrossRef CAS.
- H. Liu, Y. Lu, Y. Liu, S. Jing, Z. Zhang, S. Liu, Y. Liu, Y. Chen, K. Zhang, S. Yin, F. Li and F. Liu, Small, 2025, 21, 2412647 CrossRef CAS PubMed.
- S. Zhang, F. Zhao, L.-Y. Chang, Y.-C. Chuang, Z. Zhang, Y. Zhu, X. Hao, J. Fu, J. Chen, J. Luo, M. Li, Y. Gao, Y. Huang, T.-K. Sham, M. D. Gu, Y. Zhang, G. King and X. Sun, J. Am. Chem. Soc., 2024, 146, 2977–2985 CrossRef CAS PubMed.
- G. Wang, S. Zhang, H. Wu, M. Zheng, C. Zhao, J. Liang, L. Zhou, J. Yue, X. Zhu, Y. Xu, N. Zhang, T. Pang, J. Fu, W. Li, Y. Xia, W. Yin, X. Sun and X. Li, Adv. Mater., 2025, 37, 2410402 CrossRef CAS.
- T. Wang, J. Duan, B. Zhang, W. Luo, X. Ji, H. Xu, Y. Huang, L. Huang, Z. Song, J. Wen, C. Wang, Y. Huang and J. B. Goodenough, Energy Environ. Sci., 2022, 15, 1325–1333 RSC.
- C. Fan, N. Ahmad, T. Song, C. Zeng, X. Liang, Q. Dong and W. Yang, Nano Res., 2024, 17, 9640–9650 CrossRef CAS.
- S. Narayanan, U. Ulissi, J. S. Gibson, Y. A. Chart, R. S. Weatherup and M. Pasta, Nat. Commun., 2022, 13, 7237 CrossRef CAS PubMed.
- A. Golov, J. X. Lian and J. Carrasco, ACS Appl. Mater. Interfaces, 2024, 16, 57870–57877 CrossRef CAS.
- H. Huo, M. Jiang, Y. Bai, S. Ahmed, K. Volz, H. Hartmann, A. Henss, C. V. Singh, D. Raabe and J. Janek, Nat. Mater., 2024, 23, 543–551 CrossRef CAS PubMed.
- D. L. Nelson, S. E. Sandoval, J. Pyo, D. Bistri, T. A. Thomas, K. A. Cavallaro, J. A. Lewis, A. S. Iyer, P. Shevchenko, C. V. Di Leo and M. T. McDowell, ACS Energy Lett., 2024, 9, 6085–6095 CrossRef CAS.
- Y. You, D. Zhang, Z. Wu, T.-Y. Lü, X. Cao, Y. Sun, Z.-Z. Zhu and S. Wu, Nat. Commun., 2025, 16, 4630 CrossRef CAS PubMed.
- H. Liu, Y. Chen, P.-H. Chien, G. Amouzandeh, D. Hou, E. Truong, I. P. Oyekunle, J. Bhagu, S. W. Holder, H. Xiong, P. L. Gor’kov, J. T. Rosenberg, S. C. Grant and Y.-Y. Hu, Nat. Mater., 2025, 24, 581–588 CrossRef CAS.
- D. K. Singh, A. Henss, B. Mogwitz, A. Gautam, J. Horn, T. Krauskopf, S. Burkhardt, J. Sann, F. H. Richter and J. Janek, Cell Rep. Phys. Sci., 2022, 3, 101043 CrossRef CAS.
- G. G. Serbessa, Y. Nikodimos, B. W. Taklu, S. K. Merso, Z. B. Muche, B. D. Dandena, S. A. Vallal, T.-I. Yeh, F. Valencia, Y.-F. Hung, J.-H. Hsu, C.-M. Lee, S.-H. Wu, W.-N. Su, C.-C. Yang and B. J. Hwang, Energy Storage Mater., 2025, 76, 104103 CrossRef.
- X. Nie, J. Hu and C. Li, Interdiscip. Mater., 2023, 2, 365–389 Search PubMed.
- L. Jia, J. Zhu, X. Zhang, B. Guo, Y. Du and X. Zhuang, Electrochem. Energy Rev., 2024, 7, 12 CrossRef CAS.
- M. Wu, M. Li, Y. Jin, X. Chang, X. Zhao, Z. Gu, G. Liu and X. Yao, J. Energy Chem., 2023, 79, 272–278 CrossRef CAS.
- T. Liu, L. Zhang, J. Li, Y. Li, X. Zhang, K. Lai, S. Zhang, G. Zhao and L. Ci, J. Power Sources, 2023, 580, 233290 CrossRef CAS.
- Z. Wu, Z. Jiang, S. Li, Z. Lu, M. Deng, C. Liu, C. Wei, Z. Peng, Z. Wang, L. Zhang and C. Yu, Nano Energy, 2025, 138, 110840 CrossRef CAS.
- J. Zhu, J. Luo, J. Li, S. Huang, H. Geng, Z. Chen, L. Jia, Y. Fu, X. Zhang and X. Zhuang, Adv. Mater., 2024, 36, 2407128 CrossRef CAS PubMed.
- H. Chen, Y. Zhao, X. Zhang, R. Li, A. Wang, H. Zhang, J. Liu, B. Wen, L. Zhang, Q. Hua, T. Liu, K. Wu, K. Amine and J. Luo, Nat. Synth., 2025, 4, 552–561 CrossRef CAS.
- H. Geng, S. Huang, Z. Zhang, S. Han, R. Miao, J. Zhu and X. Zhuang, Chem. Commun., 2025, 61, 5027–5030 RSC.
- B. Li, Z. Sun, N. Lv, Y. Hu, L. Jiang, Z. Zhang and F. Liu, ACS Appl. Mater. Interfaces, 2022, 14, 37738–37746 CrossRef CAS.
- D.-S. Ko, S. Kim, S. Lee, G. Yoon, D. Kim, C. Shin, D. Kim, J. Lee, S. Sul, D.-J. Yun and C. Jung, Nat. Commun., 2025, 16, 1066 CrossRef CAS PubMed.
- Y.-G. Lee, S. Fujiki, C. Jung, N. Suzuki, N. Yashiro, R. Omoda, D.-S. Ko, T. Shiratsuchi, T. Sugimoto, S. Ryu, J. H. Ku, T. Watanabe, Y. Park, Y. Aihara, D. Im and I. T. Han, Nat. Energy, 2020, 5, 299–308 CrossRef CAS.
- D. H. S. Tan, Y.-T. Chen, H. Yang, W. Bao, B. Sreenarayanan, J.-M. Doux, W. Li, B. Lu, S.-Y. Ham, B. Sayahpour, J. Scharf, E. A. Wu, G. Deysher, H. E. Han, H. J. Hah, H. Jeong, J. B. Lee, Z. Chen and Y. S. Meng, Science, 2021, 373, 1494–1499 CrossRef CAS.
- R. Li, J. Zeng, P. Wang, T. He, L. Rao, L. Zhou, M. Hou, X. Zhang, T. Lin, Y. Zhang and N. Zhang, Adv. Energy Mater., 2025, 15, e02913 CrossRef CAS.
- Z. Li, W. Zheng, G. Lu, M. Li, D. Tang, Q. Zhao, Y. Wang, C. Xu and R. Wang, Adv. Funct. Mater., 2024, 34, 2309751 CrossRef CAS.
- B. Chen, J. Zhang, D. Wong, T. Wang, T. Li, C. Liu, L. Sun and X. Liu, Angew. Chem., Int. Ed., 2024, 63, e202315856 CrossRef CAS PubMed.
- L. Chen, R.-A. Tong, J. Zhang, H. Wang, G. Shao, Y. Dong and C.-A. Wang, Angew. Chem., Int. Ed., 2023, 62, e202305099 CrossRef CAS PubMed.
- C. Wang, Y. Liu, W. J. Jeong, T. Chen, M. Lu, D. L. Nelson, E. P. Alsaç, S. G. Yoon, K. A. Cavallaro, S. Das, D. Majumdar, R. Gopalaswamy, S. Xia and M. T. McDowell, Nat. Mater., 2025, 24, 907–916 CrossRef CAS PubMed.
- G. Li, S. Wu, C. Gao, Y. Shen, H. Zheng, M. Yang, H. Liu and H. Duan, Nano Energy, 2024, 127, 109786 CrossRef CAS.
- G. Yang, X. Bai, Y. Zhang, Z. Guo, C. Zhao, L. Fan and N. Zhang, Adv. Funct. Mater., 2023, 33, 2211387 CrossRef CAS.
- X. Nie, J. Hu, M. Lei, G. Li, Y. Zeng and C. Li, Adv. Energy Mater., 2025, 15, 2402997 CrossRef CAS.
- S. Guo, Y. Li, B. Li, N. S. Grundish, A.-M. Cao, Y.-G. Sun, Y.-S. Xu, Y. Ji, Y. Qiao, Q. Zhang, F.-Q. Meng, Z.-H. Zhao, D. Wang, X. Zhang, L. Gu, X. Yu and L.-J. Wan, J. Am. Chem. Soc., 2022, 144, 2179–2188 CrossRef CAS PubMed.
- X. Bai, G. Zhao, G. Yang, M. Wang, Q. Lin and N. Zhang, Adv. Energy Mater., 2024, 14, 2304112 CrossRef CAS.
- H. Wan, Z. Wang, W. Zhang, X. He and C. Wang, Nature, 2023, 623, 739–744 CrossRef CAS PubMed.
- H. Jiang, J. Liu, B. Tang, Z. Yang, X. Liang, X. Yu, Y. Gao, J. Wei and Z. Zhou, Adv. Funct. Mater., 2024, 34, 2306399 CrossRef CAS.
- C.-F. Li, R. Muruganantham, W.-C. Hsu, M. Ihrig, C.-T. Hsieh, C.-C. Wang and W.-R. Liu, J. Taiwan Inst. Chem. Eng., 2023, 144, 104681 CrossRef CAS.
- J. Su, M. Pasta, Z. Ning, X. Gao, P. G. Bruce and C. R. M. Grovenor, Energy Environ. Sci., 2022, 15, 3805–3814 RSC.
- Y. Yuan, Q. Liu, C. Ji, Z. Xiang, S. Feng and X. Xiong, J. Mater. Chem. A, 2025, 13, 6804–6812 RSC.
- G. Zhao, C. Luo, B. Wu, M. Zhang, H. Wang and Q. Hua, ACS Appl. Mater. Interfaces, 2023, 15, 50508–50521 CrossRef CAS PubMed.
- F. Zhu, W. Deng, B. Zhang, H. Wang, L. Xu, H. Liu, Z. Luo, G. Zou, H. Hou and X. Ji, Nano Energy, 2023, 111, 108416 CrossRef CAS.
- Y. Chen, J. Qian, X. Hu, Y. Ma, Y. Li, T. Xue, T. Yu, L. Li, F. Wu and R. Chen, Adv. Mater., 2023, 35, 2212096 CrossRef CAS.
- S. Lee, K.-s Lee, S. Kim, K. Yoon, S. Han, M. H. Lee, Y. Ko, J. H. Noh, W. Kim and K. Kang, Sci. Adv., 2022, 8, eabq0153 CrossRef CAS PubMed.
- H. Wan, B. Zhang, S. Liu, Z. Wang, J. Xu and C. Wang, Adv. Energy Mater., 2024, 14, 2303046 CrossRef CAS.
- R. J. Y. Park, C. M. Eschler, C. D. Fincher, A. F. Badel, P. Guan, M. Pharr, B. W. Sheldon, W. C. Carter, V. Viswanathan and Y.-M. Chiang, Nat. Energy, 2021, 6, 314–322 CrossRef CAS.
- W. Ji, B. Luo, Z. Zhang, Y. Tian, Q. Wang, G. Yu, X. He, Z. Liu, Z. Zhao, R. Zhao, S. Wang, J. Zheng, X. Wang, B. Zhang, J. Zhang, J. Liang and R. Jin, Energy Storage Mater., 2025, 81, 104488 CrossRef.
- G. Accardo, A. Orue, D. Chatzogiannakis, P. Gluchowski, M. Casas-Cabanas and P. López-Aranguren, J. Power Sources, 2023, 585, 233632 CrossRef CAS.
- G. Liu, W. Weng, Z. Zhang, L. Wu, J. Yang and X. Yao, Nano Lett., 2020, 20, 6660–6665 CrossRef CAS PubMed.
- Y. Liu, H. Su, Y. Zhong, M. Zheng, Y. Hu, F. Zhao, J. T. Kim, Y. Gao, J. Luo, X. Lin, J. Tu and X. Sun, Adv. Energy Mater., 2024, 14, 2400783 CrossRef CAS.
- N. Zhang, Q. He, L. Zhang, J. Zhang, L. Huang and X. Yao, Adv. Mater., 2024, 36, 2408903 CrossRef CAS PubMed.
- C. Wang, J. Hao, J. Wu, H. Shi, L. Fan, J. Wang, Z. Wang, Z. Wang, L. Yang, Y. Gao, X. Yan and Y. Gu, Adv. Funct. Mater., 2024, 34, 2313308 CrossRef CAS.
- S. Zhang, F. Zhao, H. Su, Y. Zhong, J. Liang, J. Chen, M. L. Zheng, J. Liu, L.-Y. Chang, J. Fu, S. H. Alahakoon, Y. Hu, Y. Liu, Y. Huang, J. Tu, T.-K. Sham and X. Sun, Angew. Chem., Int. Ed., 2024, 63, e202316360 CrossRef CAS.
- Q. Wang, D. Liu, X. Ma, X. Zhou and Z. Lei, ACS Appl. Mater. Interfaces, 2022, 14, 22225–22232 CrossRef CAS.
- H. Yan, J. Yao, Z. Ye, Q. Lin, Z. Zhang, S. Li, D. Song, Z. Wang, C. Yu and L. Zhang, Chin. Chem. Lett., 2025, 36, 109568 CrossRef CAS.
- H. Liu, Y. Li, C. Dong, H. Yuan, D. Yu, P. Ding, Y. Li, R. Cao, Q. Wu, Y. Liang, H. Luo, Z. Qin, L. Gao, Y. Ren, L.-Z. Fan and C.-W. Nan, Energy Storage Mater., 2025, 75, 104107 CrossRef.
- M. Chen, J. Zhang, J. Zhang, B. Yu, L. Zhou, Y. Xiao, X. Gao, J. Xiao, C. Li, Y. Sun, H. Liu, S. Dou and S. Chou, Nanoscale, 2023, 15, 13076–13085 RSC.
- B.-Q. Xiong, Q. Nian, X. Zhao, Y. Chen, Y. Li, J. Jiang, S. Jiao, X. Zhan and X. Ren, ACS Energy Lett., 2023, 8, 537–544 CrossRef CAS.
- X. Zhou, J. Liu, Z. Ouyang, F. Liu, Z. Zhang, Y. Lai, J. Li and L. Jiang, Small, 2024, 20, 2402086 CrossRef CAS PubMed.
- M. Luo, C. Wang, Y. Duan, X. Zhao, J. Wang and X. Sun, Energy Environ. Mater., 2024, 7, e12753 CrossRef CAS.
- Z. Su, Q. Zhou, J. Jin, S. Yang, G. Li and J. Zhang, Adv. Sci., 2024, 11, 2407798 CrossRef CAS PubMed.
- F. Xu, Y. Wu, L. Wang, Z. Zhang, G. Liu, C. Guo, D. Wu, C. Yi, J. Luo, W. He, C. Xu, M. Yang, H. Li, L. Chen and F. Wu, Adv. Energy Mater., 2025, 15, 2405369 CrossRef CAS.
- S. Xu, X. Cheng, S. Yang, Y. Yin, X. Wang, Y. Zhang, D. Ren, Y. Sun, X. Sun, H. Yao and Y. Yang, Adv. Mater., 2024, 36, 2310356 CrossRef CAS PubMed.
- X. Cui, X. Chen, C. Lin, J. Wang, Z. Guo, M. Zhou, Z. Yao, J. Zhang, S. Sun, J. Wang and W. Yan, J. Colloid Interface Sci., 2025, 686, 660–671 CrossRef CAS.
- X. Yao, S. Chen, C. Wang, T. Chen, J. Li, S. Xue, Z. Deng, W. Zhao, B. Nan, Y. Zhao, K. Yang, Y. Song, F. Pan, L. Yang and X. Sun, Adv. Energy Mater., 2024, 14, 2303422 CrossRef CAS.
- J. Gu, X. Chen, R. Ma, Z. He, Z. Liang, H. Zhong, Y. Su, J. Shi and Y. Yang, Energy Storage Mater., 2023, 63, 103052 CrossRef.
- J. Luo, Y. Chang, J.-W. Shi, X. Wang, H. Huang, Y. Zhang, X. Wang, J. Zhang, Y.-X. Huang and R. Zhao, Nano Lett., 2024, 24, 15035–15042 CrossRef CAS PubMed.
- X. Yin, Y. Guo, S. Chi, Y. Jia, F. Li, J. Qi, X. Yi, S. Wu and Q.-H. Yang, J. Am. Chem. Soc., 2025, 147, 4393–4402 CrossRef CAS PubMed.
- W. Zhang, Z. Wang, H. Wan, A.-M. Li, Y. Liu, S.-C. Liou, K. Zhang, Y. Ren, C. Jayawardana, B. L. Lucht and C. Wang, Nat. Mater., 2025, 24, 414–423 CrossRef CAS PubMed.
- E. Umeshbabu, S. Maddukuri, Y. Hu, M. Fichtner and A. R. Munnangi, ACS Appl. Mater. Interfaces, 2022, 14, 25448–25456 CrossRef CAS PubMed.
- W. Zhong, J. Tao, Y. Chen, R. G. White, L. Zhang, J. Li, Z. Huang and Y. Lin, Adv. Powder Mater., 2024, 3, 100184 CrossRef.
- J. Morey, J.-B. Ledeuil, H. Martinez and L. Madec, J. Mater. Chem. A, 2023, 11, 9512–9520 RSC.
- Y. Chen, L. Huang, D. Zhou, X. Gao, T. Hu, Z. Zhang, Z. Zhen, X. Chen, L. Cui and G. Wang, Adv. Energy Mater., 2024, 14, 2304443 CrossRef CAS.
- Z. Liang, Y. Xiang, K. Wang, J. Zhu, Y. Jin, H. Wang, B. Zheng, Z. Chen, M. Tao, X. Liu, Y. Wu, R. Fu, C. Wang, M. Winter and Y. Yang, Nat. Commun., 2023, 14, 259 CrossRef CAS.
- Y. Morino and S. Kanada, ACS Appl. Mater. Interfaces, 2023, 15, 2979–2984 CrossRef CAS.
- M. Kazemian, M. Kiskinova and B. Bozzini, J. Power Sources Adv., 2022, 17–18, 100106 CrossRef CAS.
- M. Siniscalchi, Y. Shi, G. Li, J. S. Gibson, R. S. Weatherup, R. S. Bonilla, S. C. Speller and C. R. M. Grovenor, Energy Environ. Sci., 2024, 17, 2431–2440 RSC.
- J. Z. Lee, T. A. Wynn, M. A. Schroeder, J. Alvarado, X. Wang, K. Xu and Y. S. Meng, ACS Energy Lett., 2019, 4, 489–493 CrossRef CAS.
- X. Peng, Q. Tu, Y. Zhang, K. Jun, F. Shen, T. Ogunfunmi, Y. Sun, M. C. Tucker, G. Ceder and M. C. Scott, Sci. Adv., 2023, 9, eabq3285 CrossRef CAS PubMed.
- O. Sheng, J. Zheng, Z. Ju, C. Jin, Y. Wang, M. Chen, J. Nai, T. Liu, W. Zhang, Y. Liu and X. Tao, Adv. Mater., 2020, 32, 2000223 CrossRef CAS PubMed.
- D. Cheng, T. A. Wynn, X. Wang, S. Wang, M. Zhang, R. Shimizu, S. Bai, H. Nguyen, C. Fang, M.-C. Kim, W. Li, B. Lu, S. J. Kim and Y. S. Meng, Joule, 2020, 4, 2484–2500 CrossRef CAS.
- J. Hu, Z. Sun, Y. Gao, P. Li, Y. Wu, S. Chen, R. Wang, N. Li, W. Yang, Y. Shen and S.-H. Bo, Cell Rep. Phys. Sci., 2022, 3, 100938 CrossRef CAS.
- Y. Liang, T. Zheng, K. Sun, Z. Xu, T. Guan, F. A. C. Apfelbeck, P. Ding, I. D. Sharp, Y. Cheng, M. Schwartzkopf, S. V. Roth and P. Müller-Buschbaum, ACS Appl. Mater. Interfaces, 2024, 16, 33307–33315 CrossRef CAS PubMed.
- S. Hao, S. R. Daemi, T. M. M. Heenan, W. Du, C. Tan, M. Storm, C. Rau, D. J. L. Brett and P. R. Shearing, Nano Energy, 2021, 82, 105744 CrossRef CAS.
- M. Kodama, N. Uno, Y. Takase, O. Aoki, R. Iwamura, T. Kotaka, K. Aotani and S. Hirai, J. Power Sources Adv., 2024, 26, 100142 CrossRef CAS.
- A. S. Westover, K. L. Browning, A. Cannavó, R. Gilles, J. Vacik, J. F. Browning, N. Paul, G. Ceccio and V. Lavrentiev, J. Mater. Chem. A, 2025, 13, 35435–35446 RSC.
- Q. Li, T. Yi, X. Wang, H. Pan, B. Quan, T. Liang, X. Guo, X. Yu, H. Wang, X. Huang, L. Chen and H. Li, Nano Energy, 2019, 63, 103895 CrossRef CAS.
- T. Ji, Y. Zhang, J. Torres, A. S. Mijailovic, Y. Tang, X. Zhao, J.-C. Bilheux, J. Wang, B. W. Sheldon, O. Oyedeji and H. Zhu, Nat. Commun., 2025, 16, 7667 CrossRef CAS PubMed.
- S.-K. Otto, L. M. Riegger, T. Fuchs, S. Kayser, P. Schweitzer, S. Burkhardt, A. Henss and J. Janek, Adv. Mater. Interfaces, 2022, 9, 2102387 CrossRef CAS.
- J. Sastre, A. Priebe, M. Döbeli, J. Michler, A. N. Tiwari and Y. E. Romanyuk, Adv. Mater. Interfaces, 2020, 7, 2000425 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |