Addressing the fundamental issues in Ni-rich cathodes: degradation mechanisms and mitigation strategies
Ziqi
Liu†
 a,
Yiming
Zhang†
a,
Shanshan
Pan
d,
Yong
Chen
a,
Yiming
Zhang†
a,
Shanshan
Pan
d,
Yong
Chen
 *c,
Keer
Yang
a,
Shanxi
Wu
a,
Musong
Liu
a,
Lei
Hu
e,
Shuaicheng
Jiang
*f,
Xiaopeng
Wang
a,
Guoxiu
Wang
*c,
Keer
Yang
a,
Shanxi
Wu
a,
Musong
Liu
a,
Lei
Hu
e,
Shuaicheng
Jiang
*f,
Xiaopeng
Wang
a,
Guoxiu
Wang
 *c and
Meng
Yao
*c and
Meng
Yao
 *ab
*ab
aCollege of Materials Science and Engineering, Sichuan University, 610064 Chengdu, P. R. China. E-mail: yaomeng@scu.edu.cn
bEngineering Research Center of Alternative Energy Materials & Devices, Ministry of Education, Sichuan University, 610064 Chengdu, P. R. China
cCentre for Clean Energy Technology, School of Mathematical and Physical Science, Faculty of Science, University of Technology Sydney, Sydney, 2007, Australia. E-mail: Yong.chen@student.uts.edu.au; Guoxiu.Wang@uts.edu.au
dSchool of Energy Science and Technology, Henan University, 450000 Zhengzhou, P. R. China
eSchool of Energy Materials and Chemical Engineering, Hefei University, 340100 Hefei, P. R. China
fState Key Laboratory of Efficient Production of Forest Resources & MOE Key Laboratory of Wood Material Science and Application, Beijing Forestry University, 100083, Beijing, P. R. China. E-mail: jiangsc@bjfu.edu.cn
First published on 8th December 2025
Abstract
The rapid growth of electric vehicles (EVs) is driving an urgent demand for lithium-ion batteries (LIBs) with higher specific energy, longer life, and uncompromised safety. Ni-rich layered oxides (LiNixCoyMn(1−x−y)O2, x ≥ 0.8) have emerged as leading cathode materials for next-generation LIBs, owing to their high capacity and energy density. Further increasing Ni content is essential for improved performance and cost reduction. However, it also introduces new obstacles, necessitating thoughtful design of cathode composition, morphology, and microstructure, as well as the development of electrolyte formulations. In this review, we discuss the multiple failure mechanisms of Ni-rich cathodes in terms of two major aspects: structural degradation and gas release. We elucidate the key factors contributing to chemical, crystallographic, and microstructural degradation in Ni-rich cathodes, and summarize the various origins of gas evolution associated with these materials. Another key theme of this review is the modification of Ni-rich cathodes to address the practical hurdles that limit their use in long-range and high-safety EVs. Accordingly, we present a comprehensive overview of the latest Ni-rich cathode modification strategies for next-generation EV platforms.
Broader contextLithium-ion batteries (LIBs), serving as the cornerstone of contemporary electrochemical energy storage systems, have gained widespread adoption across consumer electronics and electric vehicles (EVs) owing to their superior energy density, extended cycle life, and minimal self-discharge characteristics. Cathode materials play a decisive role in determining key battery performance metrics. Their technological evolution is connected to the realization of global energy transition objectives and carbon neutrality targets. Innovations in cathode materials will continue to push the boundaries of battery performance. From a sustainable development perspective, the development of cathode materials must balance resource availability, environmental friendliness, and ease of recycling. Among various cathode materials, Ni-rich materials have emerged as a promising LIB cathode material due to their high energy density, low cost, and strong industrial compatibility. However, the high nickel content leads to severe structural degradation and vigorous gas evolution, limiting their practical application. This paper aims to comprehensively elucidate the reaction mechanisms underlying structural degradation and gas release in Ni-rich cathode materials and summarize recent solutions addressing these two issues. We hope to provide valuable references for future research on Ni-rich cathode materials from a practical application perspective. |
1. Introduction
With the continuous transformation of the global energy system, conventional fossil fuels are progressively being displaced by cleaner, low-carbon alternatives to meet growing energy demands.1–4 The development of efficient energy conversion and storage devices is a critical approach to addressing the intermittency and uncertainty shortcomings of clean energy itself.5,6 Among numerous energy storage technologies, lithium-ion batteries (LIBs) have been widely adopted in various essential domains, due to their high energy density, long cycle life, and lack of memory effect.7 Especially, LIBs are also considered the battery of choice for powering the next generation of hybrid electric vehicles and plug-in hybrid electric vehicles and the global energy storage capacity of electric vehicles (EVs) has grown rapidly in recent years,8 with projections estimating approximately 240 million units by 2030 (Fig. 1a).9 This surge in demand presents unprecedented opportunities for the development of LIBs. Therefore, to meet the growing demands for longer driving range and enhanced safety in EVs, the development of high-performance LIBs has become a key focus for both academia and industry.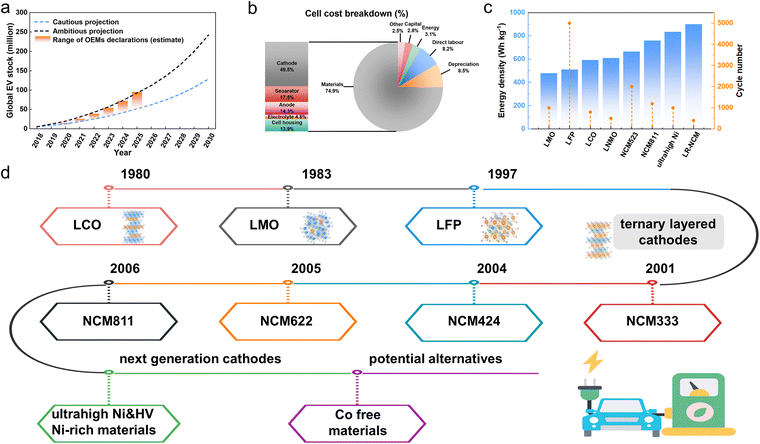 | ||
| Fig. 1 (a) Projected global electric car stock compared with original equipment manufacturer (OEM) targets (2020–2025).9 Copyright 2019, International Energy Agency. (b) A breakdown of production/material costs of a typical LIB cell.10 Copyright 2018, Springer Nature. (c) Cycle life and energy density of a range of fully or partially commercialized LIB cathodes. (d) History of LIB cathodes. | ||
As a key component of LIBs, the cathode materials are the main cost driver of the battery (Fig. 1b), and are the main factor determining the energy density and safety of the battery.10,11 The commercial LIB cathode materials mainly include lithium manganate (LiMn2O4, LMO),12,13 lithium iron phosphate (LiFePO4, LFP),14,15 lithium cobalt oxide (LiCoO2, LCO),16,17 and ternary cathode materials (LiNixCoyMn/AlzO2, x + y + z = 1, NCM/NCA) with different Ni contents.18–24 Considering the actual needs of power batteries and the current technical sophistication, the Ni-rich cathode (LiNixCoyMn/AlzO2, x + y + z = 1, x ≥ 0.8) is becoming one of the most promising cathode materials for the next generation of power batteries due to its high theoretical discharge capacity (275 mAh g−1), high operating voltage (3.8 V), and low cost.25–27 To further increase the capacity and energy density, ultrahigh Ni cathodes with a Ni content of more than 90% are also promising next-generation cathodes, especially under high voltage (HV Ni-rich cathodes).28–31 In addition, considering the high price of Co, Co-free materials are also potential options for future LIB cathodes (Fig. 1d).32–40 As shown in Fig. 1c, Ni-rich cathodes and ultrahigh Ni-rich cathodes exhibit satisfactory cycle stability and excellent energy density, and are expected to be applied on a large scale in EVs. On the other hand, Co-free cathodes, such as lithium-rich NCM (LR-NCM) and LiNi0.5Mn1.5O4 (LMNO), are only considered as emerging cathode materials due to their shorter cycle life or lower energy density.29,41–44
Although Ni-rich cathodes promise compelling energy gains, their large-scale deployment demands stringent mitigation of the diverse degradation phenomena that escalate with higher Ni content. Previous studies typically summarized the problems of Ni-rich cathodes as independent points, including cation mixing, excessive residual lithium contents on the surface, phase transition and microcrack generation, poor safety performance, etc.45–48 However, after comprehensive analysis in this review, it is found that the problems arising from Ni-rich cathodes can be divided into two major categories: structural degradation problems, including (1) generation of residual lithium compounds (RLCs) on the surface,49–52 (2) interfacial side reactions,53–57 (3) surface phase transition,58–62 (4) microcracks and particle cracking,63–66 and (5) cation mixing,60,67–70 and gas production issues, including gases such as CO, CO2, and O2.62,71,72 In order to alleviate the structural decay and gas release existing in Ni-rich cathodes, the researchers adopted various strategies. Surface coating could prevent electrolyte erosion and oxygen release by forming a protective layer on the surface of the cathodes.73–76 Element doping is also an important means to improve structural stability and inhibit gas production. Doping of low valence metal ions (e.g., Na+, Mg2+) could inhibit the collapse of layered structures in the form of “pillars”.77–81 Some high valence transition metal ions (e.g., Ta5+, Nb5+, W6+, Mo6+, etc.) could refine the primary grains, ease stress accumulation, and enhance the bonding with oxygen atoms, thus improving the lattice oxygen stability.28,46,78,81–86 Besides, optimization of the electrolyte by adding specific additives (e.g., fluorine-rich additives, phosphate additives, and nitrile-based additives)87–91 and rational structural design of the Ni-rich cathodes64,92–95 could also effectively inhibit the release of gases and stabilize the structure of the cathode materials.
Focusing on the structural stability and gas emission behavior of Ni-rich layered cathodes, this review analyses the detailed mechanisms of structural damage as well as the gas production mechanisms and modification progress of Ni-rich cathodes. Firstly, we systematically summarize the causes, processes and effects of various types of structural damage, including chemical composition changes and lattice structure transformation on the surface/interface of Ni-rich cathodes, as well as microcracks and cation mixing problems throughout the whole Ni-rich cathodes. Then, multiple mechanisms of gas emission from Ni-rich cathodes are summarized in detail, including the decomposition of RLCs and electrolytes as well as the release of lattice oxygen and the effects of gas emission. Finally, this paper also summarizes strategies to suppress the structural damage and gas emission behavior of Ni-rich cathodes based on the failure mechanism. Through surface modification, element doping, electrolyte design and structural engineering, the safety and cycle life of the battery can be effectively improved. By understanding the failure mechanism of Ni-rich cathodes coupled with each other, researchers can adopt richer and targeted modification strategies to provide scientific basis and technical support for the commercial application of Ni-rich cathode materials.
2. Structural damage mechanisms of Ni-rich cathodes
2.1. Degradation of the interface/surface structure
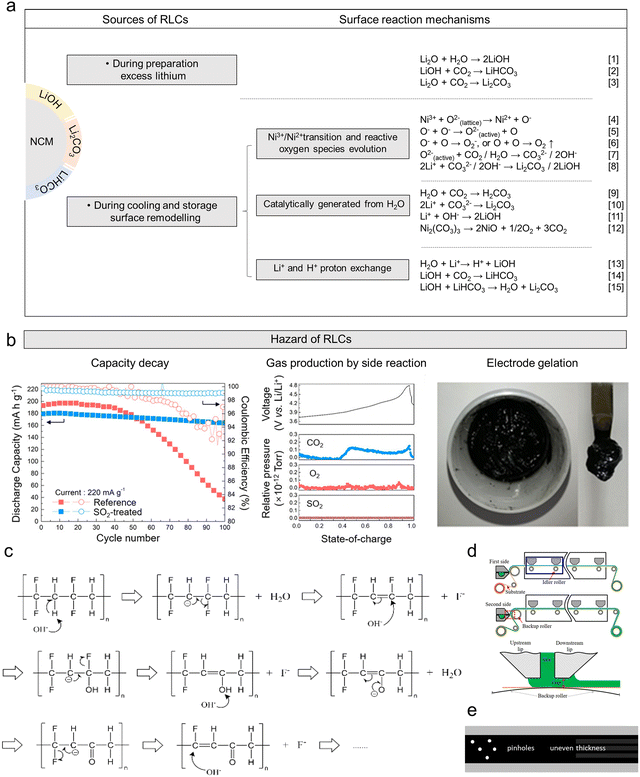 | ||
| Fig. 2 (a) Formation process of RLCs on the surface of Ni-rich cathodes. (b) Sources of surface RLCs and their multiple formation mechanisms.107 Copyright 2020, John Wiley and Sons. (c) Molecular mechanism of PVDF degradation.108 Copyright 2020, American Chemical Society. (d) Schematic illustration of slit extrusion coating operation.109 Copyright 2020, Elsevier. (e) Schematic diagram of electrode surface defects. | ||
The second viewpoint, on the other hand, suggests that H2O in the air could interact with CO2 to form Li2CO3 on the surface of the Ni-rich cathodes. This viewpoint assumes that Li2CO3 on the surface of the Ni-rich cathodes is generated in the presence of H2O at room temperature. H2O has a catalytic effect and could react with CO2 in the air to form H2CO3, which leads to material surface corrosion due to the acidic nature of the surface water. As a result, Li+ is easily leached from the material surface lattice and reacts with CO32− to form LiOH and Li2CO3. The reaction process is described by eqn (9)–(12) in Fig. 2a.103–105
The third viewpoint suggests that the formation of RLCs may be due to the exchange of Li+ and H+ protons near the surface of the Ni-rich cathodes. During storage, Li+ migrates to the surface of the material and reacts with H2O and CO2 in the air to form RLCs, and the released H+ occupies the Li+ position. The reaction process is described by eqn (13)–(15)106 in Fig. 2a.
With the formation of RLCs, the Li+ in the cathode surface structure would continue to be lost, which would cause the degradation of the electrochemical performance of the battery. Moreover, reactive RLC tends to undergo parasitic reactions with the electrolyte, resulting in gas generation and interfacial instability. RLCs (LiOH, Li2CO3) exhibit alkaline properties. While the widely used polyvinylidene fluoride (PVDF) binder demonstrates strong resistance to acids, oxidants, and organic solvents, it undergoes significant structural degradation in alkaline environments, leading to the phenomenon of slurry gelation (Fig. 2b).107,108,110–112 The PVDF molecular chain contains carbon atoms that are not fluorinated. Under the strong electron-withdrawing inductive effect of fluorine atoms, the hydrogen atoms on the carbon atoms become positively charged. Consequently, they readily undergo attack by strong nucleophiles (OH−), leading to a hydrogen fluoride elimination reaction that generates a conjugated polyene structure. This polyene structure, composed of conjugated double bonds, makes it susceptible to subsequent nucleophilic attack by OH− ions. The generated double bonds induce cross-linking reactions between PVDF molecular chains, ultimately forming a gel as shown in Fig. 2c.108,113 The high regularity of the PVDF chain leads to a chain reaction after dehydrofluorination, accelerating both the dehydrofluorination and gelation processes.114
In actual production, the gelation of cathode slurry caused by RLCs leads to a sharp increase in its viscosity, causing the slurry to lose its fluidity. When preparing electrode sheets using the currently mainstream slit extrusion coating apparatus as shown in Fig. 2d,109 this may result in the slurry becoming impossible to pump through pipelines and may cause uneven flow resistance or even complete blockage during the coating process. This unpredictable processability makes stable, continuous slurry delivery and coating impossible. Furthermore, partially gelled slurry leads to gel particles forming on the coated electrode surface, resulting in abnormal porosity and uneven thickness (Fig. 2e).115 These macro defects directly cause uneven current distribution within the battery, accelerate performance degradation, and lead to poor consistency between individual cells.116 Furthermore, slurry gelation may also force production lines to frequently clean slurry delivery and coating systems, significantly reducing production efficiency and substantially increasing manufacturing costs due to slurry waste and lost production capacity.
However, recent studies have shown that Li2CO3 is the most thermodynamically stable RLC, and its solubility in organic solvents is much lower than that of LiOH, which could prevent OH− from attacking the PVDF. In addition, Li2CO3 would react with HF to form a F-rich cathode electrolyte interface (CEI). Therefore, an appropriate amount of Li2CO3 can effectively enhance the air stability and cycling performance of Ni-rich cathodes.117
Interfacial side reactions between the cathode and the electrolyte are responsible for the formation and evolution of the CEI on the surface of Ni-rich cathodes. The formation of the CEI is closely related to the oxidation of the electrolyte. Primary CEI formation occurs when the cathode material is in contact with the electrolyte. It has been shown that LiNiO2 leads to the oxidation of the electrolyte and thus the formation of the CEI due to its strong nucleophilic oxygen atoms and Lewis base properties.118 In addition, according to previous studies, electrochemical oxidation of the electrolyte occurs when the lowest unoccupied molecular orbital (LUMO) energy level of the cathode active material is lower than the highest occupied molecular orbital (HOMO) energy level of the electrolyte. Since the energy of the antibonding hybridization orbitals of the TM 3d-O 2p of the Ni-rich cathode in the charging state corresponds to the LUMO of the holes and the concentration of holes inside the Ni-rich cathode increases, the LUMO energy level of the cathode decreases continuously, gradually approaching the HOMO level of the electrolyte, which may lead to the oxidation of the electrolyte as shown in Fig. 3a.119 In this case, the CEI of the Ni-rich cathode consists of Li2CO3 and alkyl carbonate oxidized from the electrolyte solvent and LixPOyFz oxidized from the electrolyte salt, and it continues to grow during the charge/discharge cycle. Besides, in the deep delithiation state, the presence of highly oxidized and unstable Ni4+ further promotes the oxidation of the electrolyte. As the Fermi energy level of the material decreases, the O2− ions lose electrons to become highly reactive O or O2, which also exacerbates electrolyte depletion and the formation of a thick and inhomogeneous CEI.
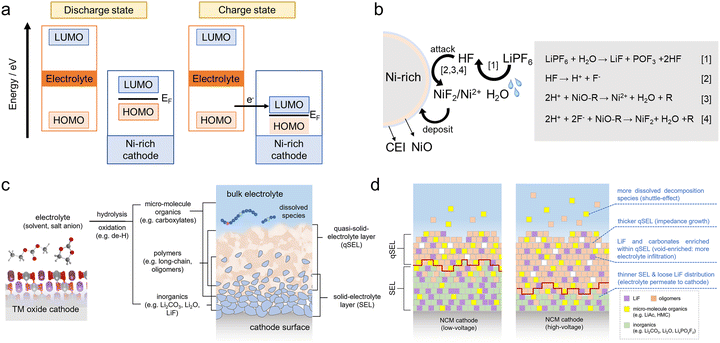 | ||
| Fig. 3 (a) Schematic of the oxidation decomposition of the electrolyte on the surface of cathodes. (b) Transition metal dissolution mechanisms in Ni-rich cathodes. (c) Schematic illustration of the origin and component of the CEI layer. (d) Architecture comparison of CEI layers formed at low/high voltage.123 Copyright 2023, American Chemical Society. | ||
In addition, the dissolution of TMs is a typical interfacial side reaction and promotes the formation of a CEI. After prolonged cycling, the TMs on the surface of the Ni-rich cathode will dissolve out of the lattice. This phenomenon is mainly affected by two factors: on the one hand, the cation mixing and oxygen evolution reactions generated during the cycling process lead to the formation of low-valent transition metal oxides, which have high solubility in the electrolyte.62 On the other hand, the electrolyte solvent contains traces of water, and these traces of water would react with LiPF6 to form HF, which dissociates into H+ and F− and frequently attacks the O–Li and O–TM (TM: Ni, Co, Mn) bonds to break them. The fractured O2− ions combine with H+ to form H2O, and the TM ions are detached from the crystals, which eventually dissolve in the electrolyte or combine with F− to form TMF2, which is deposited on the surface of the Ni-rich cathode to form a CEI. In the process, H2O is generated, which further promotes the solvation of LiPF6, forming a self-driven cyclic reaction as shown in Fig. 3b.120 However, in the case of NCM type cathodes, the direct loss of the active substance plays only a small role in the capacity decay. The secondary effects caused by the migration and deposition of dissolved TMs on the cathode surface are the main cause of the degradation of Ni-rich cathode performance. As a result, the active lithium on the anode side would be consumed and produce uneven deposition, resulting in lithium dendrites, which would lead to poor battery cycling performance as well as short circuits and thermal runaway, thus posing safety risks.121,122
The above interfacial side reactions yield abundant organic fragments and inorganic by-products, ultimately forming a composite CEI in which an inorganic-rich inner solid-electrolyte layer (SEL) and an organic-rich outer quasi-solid-electrolyte layer (qSEL) merge without a distinct boundary. The specific components of the CEI are shown in Fig. 3c.123 In particular, the SEL consists mainly of oxides, fluorides, fluorophosphates, carbonates, and other dense inorganic materials derived from the complete decomposition of salts and solvents, while the qSEL consists mainly of incompletely decomposed organic products such as polyolefins, semi carbonates and polymers.124,125 In fact, the specific structure and composition of the CEI are affected by the charging and discharging voltages. High voltage will lead to more electrolyte decomposition, causing most of the products to accumulate in the form of a qSEL, while the SEL is relatively thin. Meanwhile, the gas release at high voltage will cause the CEI to become more porous, making it easier for the electrolyte to penetrate. Excessive thickening of the qSEL elevates interfacial impedance, whereas a thin and porous SEL accelerates electrolyte decomposition and drives further qSEL build-up, ultimately precipitating pronounced capacity fade in Ni-rich cathodes under high-voltage operation (Fig. 3d).123
Although interfacial side reactions are usually viewed as detrimental, the literature offers two contrasting perspectives on the CEI. One academic thought posits that the CEI scavenges active Li+ and TM species, lowering coulombic efficiency and raising cell impedance.126–128 Another view is that due to the ionic conduction and electronic insulation properties of the CEI, it can ensure the rapid migration of Li+, prevent the continued decomposition of electrolyte components, suppress the co-embedding of solvent molecules and thereby aviods the irreversibly damaging of the electrode material. That is, the CEI can actually play a protective role against further electrolyte erosion and effectively inhibit the continuation of interfacial side reactions.129
This phase transition is related to the state of charge (SOC) and charge/discharge rate, and intensifies as the SOC and charge rate increase.131 At low SOC, this irreversible phase transition is achieved by Ni disproportionation and oxygen release (eqn (1) and (2)); at high SOC, especially in the H3 phase, this irreversible phase transition is mainly caused by oxygen release (eqn (3) and (4)).132 In addition, this irreversible phase transition occurs during thermal decomposition, intensifies with increasing Ni content, and leads to the release of O2 and CO2.133,134 In the practical application of Ni-rich cathodes, due to the slow solid phase diffusion kinetics of Li+, the surface structure of the material will be more likely to form a lithium-poor region and accelerate the structural decline. The phase transitions coupled with lattice-oxygen loss on the surface trigger electrolyte oxidation and vigorous exothermic reactions, severely undermining both cycling stability and safety.135,136
| 3LixNiO2 (layered) → LixNi2O4 (spinel) + NiO (rocksalt) + xLi2O | (1) |
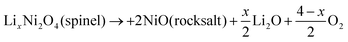 | (2) |
| 3NiO2 (layered) → Ni3O4 (spinel) + 2[O] | (3) |
| Ni3O4 (spinel) → 3NiO (rocksalt) + 2[O] | (4) |
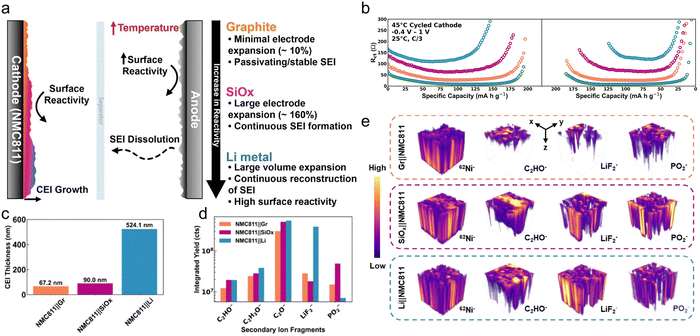 | ||
| Fig. 4 (a) Schematic of the temperature-accelerated degradation pathway. Heat amplifies reactivity at both electrodes, leading to increased SEI generation and soluble byproduct formation at the anode. These species then crossover to the cathode, driving excessive CEI formation, with the severity scaling with anode reactivity (graphite, SiOx, Li-metal). (b) Fitted Rct values plotted versus specific capacity (curve colors correspond to the paired anode: orange for Gr, purple for SiOx, blue for Li metal, and green for pristine). (c) Thickness of the CEI layer for cathodes cycled against graphite, SiOx, and Li metal anodes. (d) Integrated yield comparison of selected secondary fragments. (e) 3D reconstruction of spatial distribution for selected CEI components in cycled cathodes.138 Copyright 2025, John Wiley and Sons. | ||
2.2. Damage throughout the structure
 | ||
| Fig. 5 (a) Schematic diagram of the two cracks.142 Copyright 2023, Elsevier. (b) Comparison of the c-axis lattice parameters, a-axis lattice parameters, and unit cell volumes for LiNiO2. For comparison, the converted lattice parameters of the monoclinic phase are plotted together with the hexagonal unit cell parameters.156 Copyright 2019, Royal Society of Chemistry. (c) Secondary particles consist of (top) randomly oriented, (middle) radially oriented, and (bottom) size-refined primary particles. Depending on the microstructure, the build-up of local stress concentrations and stress distribution is different during charging.157 Copyright 2019, John Wiley and Sons. (d) 2D mapping of mesoscale SOC heterogeneity in cycled NCM secondary particles.160 Copyright 2019, John Wiley and Sons. | ||
2.2.1.1. Intergranular cracks. Intergranular cracking is more detrimental to the material than intragranular cracking; the latter originates mainly from the anisotropic shrinkage and expansion of primary particles during irreversible phase transitions.143–149 The intergranular cracks generated along the grain boundaries hinder the contact of the primary particles and therefore have an adverse effect on the internal conductivity of the secondary particles, which will result in an uneven charging state within the Ni-rich cathode particles.150 In addition, the generation of intergranular cracks allows the electrolyte to penetrate further into the interior of the particles, where more surface side reactions occur, accelerating the collapse of the Ni-rich cathode structure and the decay of the performance.151 Specifically, there are two factors that contribute to the formation of intergranular cracks: first, the irreversible H2–H3 phase transition.152–155 This leads to anisotropic lattice strains and sudden changes in lattice parameters within the Ni-rich cathode. As shown in Fig. 5b, the a-axis length and c-axis length of the Ni-rich cathode keep changing with continuous delithiation, and the change in the lattice volume follows the trend of the c-axis length. For the LiNiO2 cathode, unlike the overall decreasing trend of the a-axis, the c-axis will undergo a sudden change during the H2–H3 phase transition, and this sudden change will lead to localized strain concentration, which will initiate microcracks. In addition, due to the irregular shape of the primary particles of the Ni-rich cathode, their anisotropic deformation due to the phase transition is random.156 Meanwhile, the irregular arrangement of the primary particles leads to the interaction of forces generated by the expansion and contraction of two neighboring primary particles at their grain boundaries (Fig. 5c). This anisotropic stress and strain can be mitigated by refining the primary particle morphology.157 The second reason for the formation of intergranular cracks is the different SOC between different primary particles and even within individual active particles.158 According to previous studies, cracks expand from the core to the surface,159 which is due to the high degree of reduction on the surface of the Ni-rich cathode, while the interior is highly oxidized and inhomogeneous (Fig. 5d).160 Firstly, when the SOC is inhomogeneous, a charge compensation effect occurs in the Li-poor regions of the particles, causing Ni2+ near Li-vacancies to be oxidized to Ni4+.161,162 Similarly, O will also have a charge compensation effect,163 forming O vacancies and pores, which leads to microcracks. This also lowers the migration barrier of Ni, leading to easier migration of Ni2+ to the Li layer and the formation of disordered spinel and rock salt phases. During the charging and discharging process, neighboring primary particles with layered and disordered phases produce different volume changes, leading to forces at the grain boundaries of the two primary particles, which in turn produce intergranular cracks along the boundaries.164 In addition, under high SOC conditions, the de-embedding of Li+ effectively reduces the shielding effect between neighboring oxygen planes, leading to an increase in the electrostatic repulsion between the oxygen planes, which makes it easier for oxygen to lose electrons and produce O2 gas with the formation of oxygen vacancies.165 This is also the reason for the sudden contraction of the c-axis.70,144,166 Porosity in Ni-rich cathodes increases when O is continuously oxidized and oxygen is produced. Porosity is associated with the nucleation of microcracks and is negatively correlated with SOC, which accelerates the decay of the structure.159 In addition, there is a rock salt phase region formed around the pores,167 and the pores can disrupt the diffusion pathway of Li+ during cycling, leading to inhomogeneous electrochemical activity of the particles and increasing the likelihood of intergranular cracks.168 In addition, there is a significant effect of the difference in SOC on the formation of intergranular cracks. At high SOC, the NCM secondary particles tend to rupture and produce a high density of cracks during the initial cycling. The cracks nucleate at the end of the charging process and grow/expand during the discharging process. At low SOC, the NCM secondary particles, on the other hand, exhibit fatigue cracking behavior with chronic degradation.169
2.2.1.2. Intragranular cracks. Intragranular cracks are another type of crack in Ni-rich cathode materials. The causes of intragranular cracks are relatively complex. The root cause of these cracks is localized stress. As shown in Fig. 6a,170 specifically, they may be due to fatigue cracking caused by the expansion and contraction of primary particles during charging and discharging. They may also be induced by inhomogeneous expansion and contraction within the crystal as the SOC varies due to TM inhomogeneity. They may also occur due to tensile stresses in the fragile rock salt phase region formed by Li/Ni mixing. In addition, since the diffusion ability of Li+ in the rock salt phase generated by interface side reactions is different from that in the layered structure, Li+ delithiation is uneven in the extremely lithium deficient region, resulting in a stress field inside the particles. Intragranular cracks occur when the stress exceeds the fracture strength of the crystal plane.
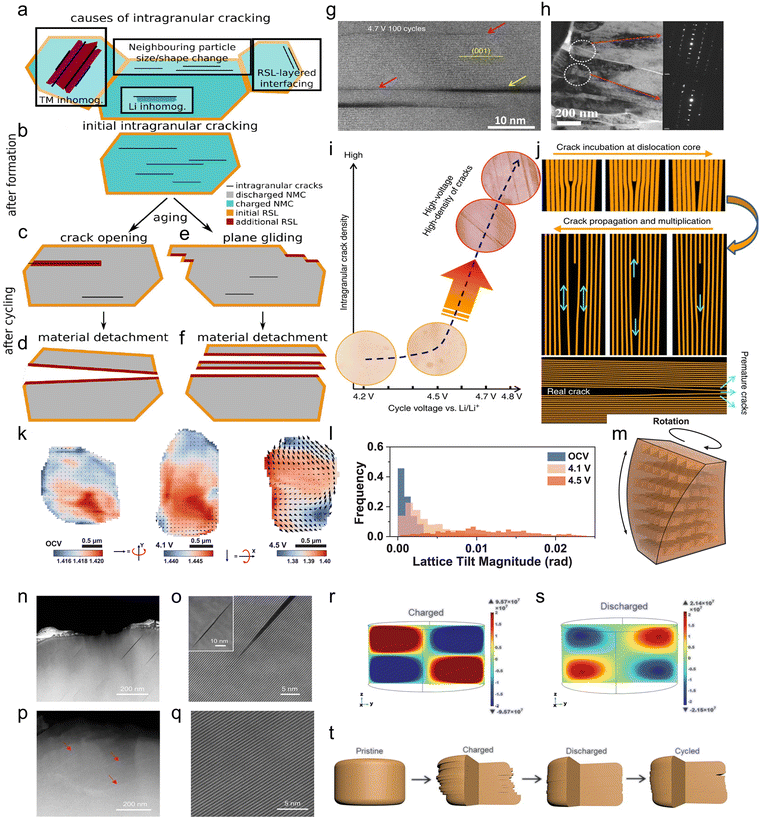 | ||
| Fig. 6 Schematic representation of the evolution mechanisms of intragranular cracks. (a) Charged NCM single crystal viewed along the (003) plane showing initial intragranular cracking and an initial rock salt-like layer (RSL) on the outer surface (orange). (b) Discharged NCM crystals showing the detrimental effects of intragranular cracking after aging. (c) and (d) Effects of crack reaching the outer surface and flooding with the electrolyte. This leads to the formation of an additional RSL (dark red) and eventually detachment of a segment of the original crystal. (e) and (f) Effects of plane gliding, which also leads to detachment of sections of the original crystal and formation of an additional RSL.170 Copyright 2024, American Chemical Society. (g) High angle angular dark field (HAADF) images from cycled LiNi1/3Mn1/3Co1/3O2 cathode particles, showing intragranular cracks along the (001) plane. The yellow arrows indicate real cracks and the pink arrows indicate incubation cracks. Scale bars: 10 nm. (h) Transmission electron microscope (TEM) image of a damaged primary particle in the cycled NCM cathode with diffraction patterns (inset) from the marked regions.164 Copyright 2016, John Wiley and Sons. (i) HAADF images overlaid diagram showing the apparent dependence of intragranular cracking on the cycle voltage; when cycled below 4.5 V, intragranular cracks can be hardly generated, while above 4.7 V, the density of intragranular cracks shows a drastic increase. (j) Schematic diagrams illustrating the dislocation-assisted crack incubation, propagation and multiplication process. Intragranular cracking as a critical barrier for high-voltage usage of layer-structured cathodes for LIBs.147 Copyright 2017, Springer Nature. (k) Scanning diffraction X-ray microscopy (SDXM) images of the (003) peak at OCV, charged to 4.1 V, and charged to 4.5 V. The d-spacing variation is expressed by blue and red colors. The length and orientation of the quiver represent the magnitude and direction of lattice rotation, respectively. A fixed scale is used for all three figures. (l) Lattice rotation magnitude. (m) Schematic illustration of lattice rotation.173 Copyright 2024, The American Association for the Advancement of Science. (n) and (o) Scanning transmission electron microscopy (STEM) images of single-crystalline NCM at the 4.4 V charge status (cycled in a full cell between 2.7 and 4.4 V for 120 cycles). (p) and (q) STEM images of single-crystalline NCM at the discharge status (cycled in a full cell between 2.7 and 4.4 V for 120 cycles). The red arrows indicate the gliding marks inside the single crystal. (r) COMSOL-simulated shear stress along the yz direction during charge (delithiation). (s) COMSOL-simulated shear stress along the yz direction during discharge (lithiation). (t) Schematic illustration of the structural evolution of single-crystalline NCM upon cycling.65 Copyright 2020, The American Association for the Advancement of Science. | ||
Furthermore, at the atomic scale, Lin et al.146 found that Li/Ni anti-site defect-rich regions with lattice distortions are the nucleation sites for intragranular cracks in primary particles. The strain difference between the layered phase and the rock salt phase and the coulomb repulsion in the cation-rich region are the two driving forces for the growth of intragranular cracks, which originate from the Li/Ni anti-site defects in the transformed structure. Moreover, in terms of specific elemental considerations, Guo et al.171 found that Co is responsible for the intensification of the intragranular cracking phenomenon. In the deeply charged state, Co exacerbates the transition from the layered phase to the rock salt phase and the accumulation of non-homogeneous strain, which ultimately drives intragranular cracking in the rock salt phase domain along the (003) plane at the Ni-rich cathode. In addition, oxygen vacancy formation and their subsequent migration along the (003) plane introduce pronounced lattice strain and expansion, which in turn promote the propagation of intragranular cracks. Unlike intergranular cracks, intragranular cracks begin inside the grain and the size of intragranular cracks is smaller than that of intergranular cracks, but the number of cracks is larger, so they are able to expose more of the fresh material surface in contact with the electrolyte. In addition, it is worth noting that intragranular cracking is not only a mechanical failure but also a structural degradation.147 According to the crack generation mechanism and morphology, intragranular cracks can be classified into three types: (1) classic cracks (indicated by yellow arrows in Fig. 6g);147 (2) dark contrast stripes (indicated by pink arrows in Fig. 6g); and (3) fine-serried low angle grain boundary (white circles in Fig. 6h).164
Currently, the first two types of intragranular cracks are believed to be formed due to heterogeneous delithiation. The internal structural properties of dark contrast stripes were analyzed by Yan et al. It was confirmed that the dark contrast stripes are premature classic cracks, and the authors constructed a model of the crack formation process.147 According to the model (Fig. 6i and j), in the first stage, the damage to the structure by cracks becomes progressively more severe with the increase of voltage because the creation and development of cracks are electrochemically driven. In the second stage, dislocations act as nucleation sites for intragranular crack hatching, initiating premature cracks. In the third stage, premature cracks gradually develop into real cracks with two parallel planes (classic cracks). The third type of intragranular cracks (fine-serried low angle grain boundary) is mainly formed by mechanical damage caused by the concentration of stress within the primary particles. The main causes of this mechanical degradation are (1) fatigue strain during cycling, (2) dislocations between primary particles and (3) “false” primary particles with grain boundaries.172
Additionally, inhomogeneous delithiation induces lattice strain alongside lattice rotation (refers to displacement of the momentum transfer in the 3D reciprocal space in directions orthogonal to the displacement caused by lattice strain). Moreover, the degree of lattice rotation intensifies as the voltage increases as shown in Fig. 6k–m, ultimately leading to mechanical degradation of Ni-rich cathodes in the form of partial irreversible plane gliding and intragranular cracking.173 Compared to polycrystalline particles, lithium diffusion paths are longer in larger single-crystal cathodes,174 making inhomogeneous delithiation more severe. Consequently, lattice rotation is more pronounced in single-crystal cathodes. This lattice rotation cannot be eliminated by lithium reinsertion like lattice strain, and it is largely unavoidable since it serves as one of the fundamental mechanisms for distorting the lattice to accommodate heterogeneous electrochemical reactions within single-crystal cathodes during lithium extraction–insertion cycles. Although lattice gliding along the (003) crystal plane is largely reversible, lattice gliding and microcracks were observed on the surface of the Ni-rich cathode in the charged state. These microcracks originated from within the crystal. Most intracrystalline cracks and lattice gliding disappeared in the fully discharged state, with only minor traces of the gliding process remaining (Fig. 6n–q). However, non-self-recovering lattice gliding and microcracks persist, as confirmed by COMSOL simulation results (Fig. 6r and s). The shear stress component along the yz direction can trigger gliding along the (003) plane. During charging/discharging, the sign of shear stress reverses while its absolute value is not equal, leading to incomplete reversibility of lattice gliding. This partially irreversible slip accumulates into crack openings after prolonged cycling, consequently causing post-cycling surface cracking in single crystals (Fig. 6t).65
Based on the formation and evolution mechanism of intragranular cracks, Morzy et al.170 concluded that intragranular cracks negatively affect the battery performance in two main ways, as shown in Fig. 6b–f. First, for intragranular cracks that are not completely sealed (i.e., fail to self-heal) during discharge and re-formed during subsequent charging, they will expand and reach the surface of the primary crystal after multiple cycles, leading to electrolyte flooding, surface reduction (Fig. 6c) and material detachment (Fig. 6d). Secondly, the repeated formation and closure of intragranular cracks can lead to (003) plane gliding (Fig. 6e), which may even lead to the fragmentation of primary grains and the formation of flakes after long-term cycling (Fig. 6f).
2.2.1.3. Detrimental effects of microcracks. The generation of microcracks is the main reason for the decrease in the cycling performance of Ni-rich and low cobalt cathodes. Intergranular cracks will make the particles fail to connect and thus reduce the conductivity of secondary particles,150 and also exacerbate the uneven SOC, which further reduces the activity of the material and makes the particles more susceptible to chalking and fragmentation. In addition, the formation of cracks will expose more of the inner surface of the active material, accelerate the penetration of the electrolyte into the interior of the particles and result in the dissolution of TMs and the loss of active substances.148,175 At the same time, the electrolyte reacts with unstable Ni4+ in the material to form a disordered phase.176 Due to the significant differences in the crystal lattice between the surface rock salt phase, the spinel-like phase, and the layered phase in the bulk phase, the cracking of secondary particles is further exacerbated.66,177 Ultimately, it will lead to hindered diffusion of Li+, increased impedance, and decreased electrochemical performance. This side reaction causes phase transition of the layered cathode, leading to structural collapse and O2 release.178–180
2.2.2.1. Causes of cation mixing. The reasons for the occurrence of Li/Ni mixing in Ni-rich cathodes are complex. The main reasons include (1) similar ionic radius, (2) low migration energy barriers of Ni ions, (3) magnetic interactions, and (4) thermodynamic instability. Generally, layered materials have the R
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m structure as shown in Fig. 7a.181 In this structure, oxygen anions form a cubic close-packed lattice, with lithium and transition metal (TM) cations orderly occupying alternating layers of octahedral sites along the crystallographic c-axis ([001] direction).182 Therefore, the TM sites (3a) and Li sites (3b) of the standard R
m structure as shown in Fig. 7a.181 In this structure, oxygen anions form a cubic close-packed lattice, with lithium and transition metal (TM) cations orderly occupying alternating layers of octahedral sites along the crystallographic c-axis ([001] direction).182 Therefore, the TM sites (3a) and Li sites (3b) of the standard R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m structure are clearly separated. However, during the sintering process of Ni-rich cathode materials, Ni2+ is difficult to be completely oxidized to Ni3+. Given the similar radius of Ni2+ (0.069 nm) and Li+ (0.076 nm), during high-temperature synthesis, the spontaneous occupation of Li sites by Ni2+ significantly increases the system's configurational entropy (ΔS > 0). Driven by high temperature (T), the entropy increase (−TΔS) dominates the system's Gibbs free energy (ΔG), resulting in ΔG < 0. Therefore, the formation of Li/Ni antisite defects constitutes a thermodynamically spontaneous process, primarily driven by the thermodynamic principle of entropy increase. This ultimately leads to Ni deviations from stoichiometric ratios in Ni-rich cathodes (as exemplified by LiNiO2, where the chemical formula changes from ideal LiNiO2 to non-stoichiometric Li1−xNi1+xO2).183,184 Therefore, excess lithium is often required during synthesis to counteract this thermodynamic tendency. The excess lithium increases the lithium chemical potential and, following the law of mass action, preferentially occupies lithium layer sites.185 As a result, Ni2+ is confined to the transition metal layer, effectively reducing cation mixing and ensuring the integrity of the layered structure.
m structure are clearly separated. However, during the sintering process of Ni-rich cathode materials, Ni2+ is difficult to be completely oxidized to Ni3+. Given the similar radius of Ni2+ (0.069 nm) and Li+ (0.076 nm), during high-temperature synthesis, the spontaneous occupation of Li sites by Ni2+ significantly increases the system's configurational entropy (ΔS > 0). Driven by high temperature (T), the entropy increase (−TΔS) dominates the system's Gibbs free energy (ΔG), resulting in ΔG < 0. Therefore, the formation of Li/Ni antisite defects constitutes a thermodynamically spontaneous process, primarily driven by the thermodynamic principle of entropy increase. This ultimately leads to Ni deviations from stoichiometric ratios in Ni-rich cathodes (as exemplified by LiNiO2, where the chemical formula changes from ideal LiNiO2 to non-stoichiometric Li1−xNi1+xO2).183,184 Therefore, excess lithium is often required during synthesis to counteract this thermodynamic tendency. The excess lithium increases the lithium chemical potential and, following the law of mass action, preferentially occupies lithium layer sites.185 As a result, Ni2+ is confined to the transition metal layer, effectively reducing cation mixing and ensuring the integrity of the layered structure.
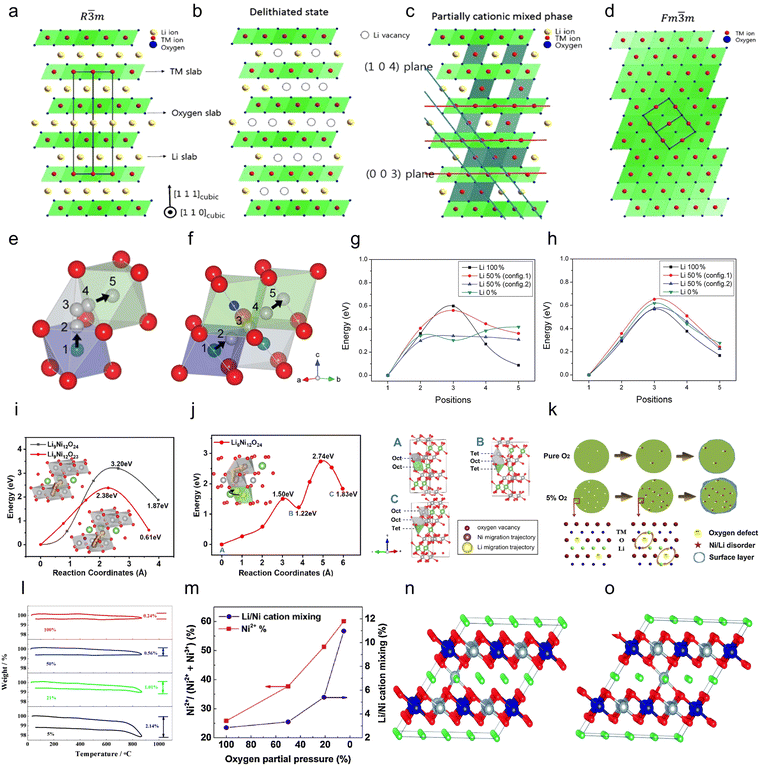 | ||
Fig. 7 (a) The illustration of the well-ordered R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m structure. (b) The illustration of the R m structure. (b) The illustration of the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m structure with Li vacancies in the highly charged state. (c) The illustration of the partially cation mixed phase with TM ions in the Li slab. (d) The illustration of the cation disorder or cation mixing phase with Fm m structure with Li vacancies in the highly charged state. (c) The illustration of the partially cation mixed phase with TM ions in the Li slab. (d) The illustration of the cation disorder or cation mixing phase with Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m structure.181 Copyright 2015, John Wiley and Sons. (e) and (f) Migration routes of Ni ions to Li sites in the layered structure: (e) Oh → Td → Oh route and (f) Oh → VO → Oh route. (g) and (h) Energy barriers calculated for migration of a Ni ion for (g) the Oh → Td → Oh route and (h) the Oh → VO → Oh route.69 Copyright 2011, American Chemical Society. (i) and (j) Nudged elastic band (NEB) calculations comparing the energy barriers for Ni migration in LiNiO2 at (i) 25% delithiation under pristine and oxygen-deficient conditions and (j) 50% delithiation (green, grey, and red spheres represent Ni, Li, and O ions, respectively).183 Copyright 2025, Elsevier. (k) Influence of oxygen defect on structural instability. (l) Thermogravimetric analysis demonstrating oxygen deficiency at low oxygen partial pressure.187 Copyright 2015, Elsevier. (m) Direct proportionality between oxygen defect ratio and cation mixing degree.188 Copyright 2016, Elsevier. (n) and (o) Two inequivalent positions in the Li layer: (n) configuration A and (o) configuration B. The grey spheres denote Ni, the blue spheres denote Co, the green spheres denote Li and the red spheres denote O.190 Copyright 2014, Royal Society of Chemistry. m structure.181 Copyright 2015, John Wiley and Sons. (e) and (f) Migration routes of Ni ions to Li sites in the layered structure: (e) Oh → Td → Oh route and (f) Oh → VO → Oh route. (g) and (h) Energy barriers calculated for migration of a Ni ion for (g) the Oh → Td → Oh route and (h) the Oh → VO → Oh route.69 Copyright 2011, American Chemical Society. (i) and (j) Nudged elastic band (NEB) calculations comparing the energy barriers for Ni migration in LiNiO2 at (i) 25% delithiation under pristine and oxygen-deficient conditions and (j) 50% delithiation (green, grey, and red spheres represent Ni, Li, and O ions, respectively).183 Copyright 2025, Elsevier. (k) Influence of oxygen defect on structural instability. (l) Thermogravimetric analysis demonstrating oxygen deficiency at low oxygen partial pressure.187 Copyright 2015, Elsevier. (m) Direct proportionality between oxygen defect ratio and cation mixing degree.188 Copyright 2016, Elsevier. (n) and (o) Two inequivalent positions in the Li layer: (n) configuration A and (o) configuration B. The grey spheres denote Ni, the blue spheres denote Co, the green spheres denote Li and the red spheres denote O.190 Copyright 2014, Royal Society of Chemistry. | ||
In addition, a drive for Li/Ni mixing is also generated during Ni-rich cathode cycling, which is attributed to the low migration barrier for Ni ions. In the charging process, as Li+ is released (Fig. 7b), the migration barrier for Ni ions is reduced and the TM ions occupy the Li sites (Fig. 7c). The material would gradually become a disordered spinel phase and collapse during charge and discharge, and eventually transition from the original layered phase to the rock salt phase (Fig. 7d).181 There are two migration paths for Ni during cycling.69,186 Path a (Fig. 7e): Ni ions migrate from one octahedral (Oh) site to another octahedral site (Oh–Td–Oh) via a tetrahedral (Td) site. This migration may occur within or between layers. Path b (Fig. 7f): Ni ions migrate from one octahedral (Oh) site to another octahedral site (Oh–VO–Oh) via vacancies (oxygen vacancy, VO). This migration occurs within the TM layer. Based on the calculations of the migration barriers for the two pathways, it is found that the energy barriers in the Oh–Td–Oh route are related to the cation configuration (Fig. 7g). In addition, by comparing the number of nearest cations and their distances from the Td and Oh (Li) sites, as well as the magnitude of the energy barriers at these positions, Kim et al.69 confirmed that the main factor determining the migration energy barriers is the coulomb repulsion from the surrounding cations. On the other hand, for the Oh–VO–Oh pathway, the migration energy barrier is very little affected by the configuration (Fig. 7h). In conclusion, the migration probability of both routes is the same in the Li 100% and Li 50% (configuration 1 containing more Li+) states, whereas Ni ions are more prone to migrate via the Oh (Ni)–Td–Oh (Li) route in the Li 50% (configuration 2 containing less Li+) and Li 0% states, which may lead to phase transition and performance degradation. In addition, according to the stabilization energy of octahedral sites, Ni ions tend to migrate from octahedral sites to tetrahedral sites. Li et al. further confirmed that the Ni migration barrier is influenced by Li vacancy concentration and oxygen vacancy concentration through density functional theory (DFT) calculations. When 25% and 50% of Li ions were extracted from a Li12Ni12O24 supercell without oxygen vacancies, the energy barrier for Ni ion migration to Li layer octahedral sites decreased from 3.20 eV to 2.74 eV (Fig. 7i and j) as Li vacancies formed, indicating that the presence of Li vacancies promotes Ni migration. Furthermore, the calculations indicate that oxygen vacancies exert a more pronounced effect on Ni migration in Ni-rich cathodes. The presence of oxygen vacancies reduces the Ni migration barrier (2.38 eV) and formation energy (0.61 eV), suggesting that oxygen vacancies also promote Ni migration.183 This result is also supported by Bi et al.,187 who found that oxygen vacancies also form in Ni-rich cathodes when the partial pressure of oxygen during sintering is too low, thereby promoting the occurrence of Li/Ni mixing (Fig. 7k–m).188 Based on the above findings, the researchers further found the relationship between cation mixing and oxygen release. With the release of oxygen during charging, more oxygen vacancies are generated in the Ni-rich cathode, which promote the migration of Ni ions via Oh–VO–Oh and continuously intensify Li/Ni mixing during long cycling.189
The arrangement of cations in the lattice is also related to the nature of the magnetic properties. Fig. 7n and o show two configurations of the atomic arrangement in the lattice: configuration A: six pairs of 180° Ni–O–Ni structures, and configuration B: six 90° Ni–O–Ni structures.190 According to the super-exchange interaction,191 Ni2+ (t62ge2g) and Ni3+ (t62ge1g) have fully filled t2g states and partially filled eg states, respectively. Therefore, the 180° Ni–O–Ni super-exchange interaction is stronger than the 90° Ni–O–Ni super-exchange interaction. That is, when Ni–O–Ni is in the 180° configuration, more energy is gained through orbital interactions than when it is in the 90° configuration. Therefore, 180° Ni–O–Ni can stabilize Ni in the Li layer. Further studies by Zheng et al.192 showed that Ni2+–O–Ni2+ has the strongest super-exchange interaction, and that the more Ni2+–O–Ni2+ there is, the lower the formation energy of Li/Ni mixed defects, and the easier Li/Ni mixed defects can be formed. In general, when there is no Li/Ni mixing, the Ni in the TM layer and the Ni in the neighboring layer form a 90° Ni–O–Ni structure. When Li/Ni mixing occurs, the randomly distributed Ni in the Li layer may form an 180° Ni–O–Ni super-exchange structure.
In addition, it is shown by studies that the occurrence of cation mixing in Ni-rich materials is essentially due to their poor thermal stability. Taking LiNiO2 as an example, its decomposition occurs during the heating process, which ultimately leads to Li/Ni mixing and the formation of the NiO rock salt phase. The thermal decomposition equations are eqn (1) and (2).
2.2.2.2. Effects of cation mixing. First, cation mixing would directly lead to lattice oxygen release. Li et al.183 confirmed through DFT calculations that during Li/Ni cation mixing, Ni ions migrate into the Li layer, leaving vacancies in the TM layer. These TM vacancies may be filled by Li+ from the Li layer to form a Li–O–Li structure, or may remain vacant to form a Li–O–VacTM structure. Both structures promote oxygen oxidation reactions.193,194 Furthermore, for Ni-rich cathode systems with different Li/Ni mixing ratios, the Bader charge, magnetic moment, and oxygen vacancy formation energy were calculated at different charge states (Fig. 8a–c). Fig. 8a and b reveal that oxygen atoms in the Li–O–VacTM configuration exhibit smaller overall Bader charges and higher magnetic moments, indicating a greater tendency to participate in charge compensation during lithium extraction and a higher oxidation state. Furthermore, regardless of the configuration, the oxygen vacancy formation energy in Ni-rich cathodes with Li/Ni mixing is lower than in the perfect layered structure (0% Li/Ni mixing; Fig. 8c). Ab initio molecular dynamics (AIMD) simulations reveal that in systems with Li/Ni mixing, oxygen atoms adjacent to TM vacancies form O–O dimers at lower delithiation concentrations, whereas this does not occur in the perfect layered structure. This indicates that cation mixing accelerates the extraction of lattice oxygen. In addition, refinement results from neutron diffraction provide further support for these simulation findings. As shown in Fig. 8k,195 during delithiation, the degree of Li/Ni mixing correlates with trends in the isotropic temperature factor of oxygen atoms (representing lattice oxygen instability) and lattice stress (leading to structural collapse). This indicates that the activation of anion redox reactions and the abrupt increase in stress are closely related to Li/Ni mixing, ultimately leading to structural degradation and reduced cycling life in high-voltage Ni-rich cathodes.
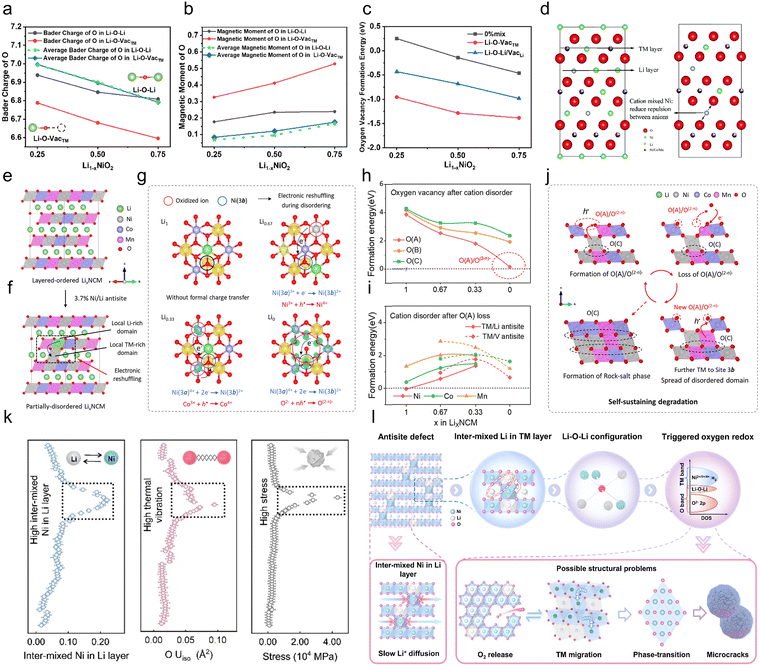 | ||
| Fig. 8 (a) Bader charge and (b) oxygen magnetic moment evolution with delithiation. (c) Li/Ni mixing dramatically lowers the oxygen vacancy formation energy, confirming its role in promoting oxygen release.183 Copyright 2025, Elsevier. (d) Schematics showing that cation-mixed Ni2+ contributes to structural integrity retention during discharge and charge, highlighting its role in stabilizing the crystal framework.197 Copyright 2017, The Royal Society of Chemistry. Schematic representation of (e) layered-ordered LixNCM and (f) the local Li-rich and TM-rich domain created after Li/Ni disordering associated with the potential spontaneous electronic reshuffling. (g) Spin density diagram of the local disordered domain, in which the Ni/Li disorder is in the form of NiLi–LiNi antisites with x = 1, 0.67 and 0.33, and NiV–VNi antisites with x = 0. The yellow and cyan isosurfaces represent the electrons with spin up (↑) and down (↓), respectively. The black arrow reflects the electronic reshuffling induced by cation disorder compared to that in the layered-ordered system. The formal charge reaction equations during disordering are marked under the diagrams. (h) Formation energy of VO after cation disorder as well as (i) the formation energy of cation disorder after oxygen loss. (j) Schematic illustration of the self-sustaining degradation process. (k) Evolution of Ni migration extent, oxygen isotropic displacement parameter (Uiso), and structural stress with cycling, as refined from operando neutron diffraction data. (l) Schematic of the dual impact of antisite defects on structural evolution.195 Copyright 2024, John Wiley and Sons. | ||
Chen et al.196 elucidated the mechanism of lattice oxygen release induced by cation mixing at the electronic scale under highly delithiated conditions. This mixing process induces electronic structure reorganization in the high delithiation state as shown in eqn (5) (Fig. 8e–g). The TM ions migrate to the Li layer and are reduced and leave behind electronic holes, while the electronic states near the Fermi energy level will then get holes. During deep delithiation, this electronic recombination excites lattice oxygen activity and increases the risk of lattice oxygen loss. Further thermodynamic calculations show that regardless of the coordination mode (O(A): Li–O–Li; O(B): Li–O–TM; O(C): TM–O–TM), there is a mutually inverse thermodynamic relationship between lattice oxygen stability and cation mixing (Fig. 8h and i). Cation mixing induces lattice oxygen instability and promotes oxygen loss and oxygen vacancy formation, a process that in turn exacerbates cation mixing and further electronic recombination. This “self-sustained” degradation pathway continues throughout the structural decline of the Ni-rich cathode, with cation mixing as the nucleation site and cation mixing and oxygen loss processes occurring alternately. O(A)/O(2−n)− ions in the lattice accumulate and eventually escape from the lattice, while residual O(C) ions and migrating TM ions accumulate to form the rock salt phase, which ultimately leads to an extensive irreversible phase transition (Fig. 8j). In other words, cation mixing is closely related to lattice oxygen stability.
| Ni(3a)x+ + (x − 2)e− → Ni(3b)2+, nAy+ + (x − 2)h˙ → nA(y+x−2)/n+ | (5) |
To further discuss the impact of Li/Ni mixing on Ni-rich cathodes, it is also necessary to understand how Li+ migrates. There are two main Li+ migration paths:198 the first type of migration path (Fig. 9a) occurs when the two Li sites immediately adjacent to the endpoints of the hop are simultaneously occupied by Li+. The diffusing Li+ then migrates along the shortest path connecting the initial site of the hop and the vacancy. This path passes through a dumbbell of the oxygen ion, called the oxygen dumbbell hop (ODH). The second type of migration path (Fig. 9b), called the tetrahedral site hop (TSH), is a situation where Li+ migrates along a curved path through a tetrahedral site when one or both sites immediately adjacent to the hop endpoints are vacant. Therefore, under the influence of spatial effects and electrostatic interactions, when Li/Ni mixing occurs, the activation energy of Li+ migration through the TSH pathway increases, and the spacing of Li layers decreases, which ultimately inhibits the migration of Li+.199 Zhao et al.200 have shown that the Li layer spacing decreases (2.66 Å, 2.65 Å, 2.63 Å) with the increasing Li/Ni mixing degree (0%, 3.7%, 7.4%). The calculated Li+ diffusion barriers were 0.496 eV and 0.608 eV for the models with 3.7% and 7.4% Li/Ni mixing degree, respectively. The migration coefficients of Li+ decreased with the increase of Li/Ni mixing (Fig. 9c). In addition, the migration barrier of Ni ions after the occurrence of Li/Ni mixing and dislocation was as high as 1.05 eV, indicating that it is difficult to migrate (Fig. 9d). Ni2+, located in the Li layer, has a higher valence state than Li+ and therefore generates greater electrostatic repulsion. As a result, Li+ migration along both the ODH and TSH paths will be inhibited, which means that Li/Ni mixing inhibits the migration of Li+.
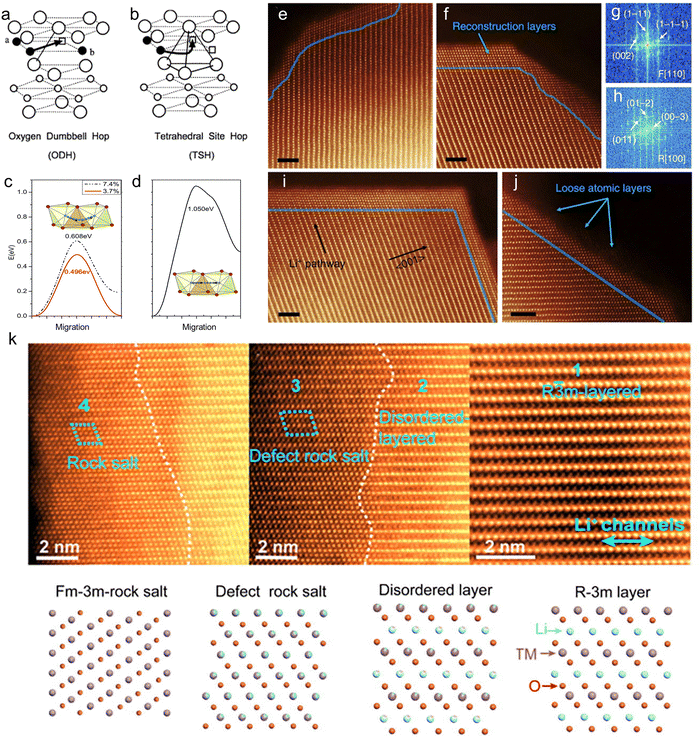 | ||
Fig. 9 The two lithium migration paths in layered LixCoO2. The filled circles are lithium ions, the empty squares are lithium vacancies, the large empty circles are oxygen ions, and the small empty circles are cobalt ions. (a) The ODH occurs when the sites a and b adjacent to the end points of the hop are simultaneously occupied by lithium ions. (b) The TSH occurs when one or both of the sites adjacent to the endpoints of the hop are vacant.198 Copyright 2001, Elsevier. (c) The energy curves of Li atom migration paths for different Li+/Ni2+ ion exchange rates. (d) The energy curve of the Ni atom migration path. The insets are illustrations of the migration path.200 Copyright 2017, Royal Society of Chemistry. (e) Surface morphology of NCM after 30 h of electrolyte exposure (equivalent to one full cycle duration in this study). (f) Surface structure of NCM after 1 cycle (2.0–4.7 V), with the blue arrow indicating the surface reconstruction layer. (g) Fast Fourier transform (FFT) pattern from the surface reconstruction layer (Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m [110] zone axis). (h) FFT pattern from the NCM bulk structure (R m [110] zone axis). (h) FFT pattern from the NCM bulk structure (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m [100] zone axis). (i) Orientation-dependent thickness variation of the surface reconstruction layer of NCM after 1 cycle (2.0–4.7 V). (j) Atomic-scale image showing loose surface layers of NCM, with blue lines demarcating the interface between bulk NCM and the reconstruction layer. The blue lines indicate the boundaries between the NCM layered structure and the surface reconstruction layer in all images. Scale bars: 2 nm in (e)–(j).201 Copyright 2014, Springer Nature. (k) HAADF STEM images at different regions from the bulk to the surface of Ni-rich cathodes and the corresponding structure models for explaining the structure transition process. The white dashed lines denote the surface reconstruction layer at different stages.202 Copyright 2018, Elsevier. m [100] zone axis). (i) Orientation-dependent thickness variation of the surface reconstruction layer of NCM after 1 cycle (2.0–4.7 V). (j) Atomic-scale image showing loose surface layers of NCM, with blue lines demarcating the interface between bulk NCM and the reconstruction layer. The blue lines indicate the boundaries between the NCM layered structure and the surface reconstruction layer in all images. Scale bars: 2 nm in (e)–(j).201 Copyright 2014, Springer Nature. (k) HAADF STEM images at different regions from the bulk to the surface of Ni-rich cathodes and the corresponding structure models for explaining the structure transition process. The white dashed lines denote the surface reconstruction layer at different stages.202 Copyright 2018, Elsevier. | ||
Lin et al.201 found that Li/Ni mixing was evident on the surface of the Ni-rich cathode (Fig. 9e) and thickened after cycling (Fig. 9f–h). Rock salt phases were formed in the diffusion path of Li+ (Fig. 9i), which would seriously hinder the migration of Li+. In addition, a loose atomic layer exists on the outer surface of the mixing layer (Fig. 9j). Lin et al.202 found that after cycling, the material is layered inside while the surface is in a rock salt phase, and the transition region exhibits a disordered layered phase and a defective rock salt phase. As shown in Fig. 9k, they similarly suggested that Ni migrates along the Li diffusion direction during cycling, which leads to the evolution of the structure and dissolution of Ni, ultimately leading to a decrease in capacity. In summary, Li/Ni mixing is believed to be the origin of the phase transition. This irreversible phase transition also exacerbates oxygen release, promotes interfacial reactions and contributes to the degradation of the electrochemical performance of Ni-rich cathodes.203
Although most of the studies showed that Li/Ni mixing is harmful to the overall performance of Ni-rich cathodes, some of them found that a moderate amount of Li/Ni mixing is beneficial to Ni-rich cathodes. Tang et al.204 investigated the relationship between Li/Ni mixing and Li+ diffusion barriers, and found that when Ni2+ ions occupy Li+ sites, the stronger interaction between Ni and O drives the nearest Li+ ions to diffuse into the neighboring vacancies. In other words, Li/Ni mixing will promote the diffusion of Li+ and enhance the rate performance of Ni-rich cathodes. Moderate cation mixing enhances Li+ diffusion kinetics and structural stability by facilitating concerted Li+ hopping through Ni2+-induced vacancy attraction and buffering interlayer repulsion during deep delithiation, thereby improving rate capability and high-voltage cycling retention.86,205 However, a large amount of Li/Ni mixing could lead to the collapse of the layered structure. Excessive mixing obstructs Li+ channels, traps lithium in transition metal layers, and accelerates oxygen loss, triggering irreversible layer-to-spinel/rock-salt phase collapse.206 This is similarly supported by the study conducted by Sun et al.,197 which found that proper cation mixing reduces repulsive forces and inhibits structural collapse, which is essential for supporting the layered structure of lithium transition metal oxide materials, especially in the deep delithiation state. The above-mentioned gain effect from cation mixing arises because moderate cation mixing enhances structural stability by supporting the Li layer, reducing repulsive forces between adjacent oxygen layers during delithiation, and suppressing transition metal ion migration and phase transitions.207 Ni2+ ions occupying Li sites generate a “pillar effect” through electrostatic repulsion,208 effectively preventing the continuous migration of transition metal ions toward lithium sites during charge–discharge cycles (Fig. 8d). In summary, as shown in Fig. 8l, Ni intercalated in the Li layer supports the layered structure but inhibits Li ion diffusion, while Li intercalated in the TM layer forms a Li–O–Li configuration. This configuration triggers anionic (O2−) redox activity and induces oxygen release, leading to electrochemical performance degradation and structural deterioration. The concurrently formed oxygen vacancies further promote Ni migration from the TM layer to the Li layer, exacerbating Li/Ni mixing. Notably, the Li–O–VacTM configuration can form during Ni migration, which also promotes anion redox activity and disrupts lattice oxygen stability.
3. Gas-generation mechanisms of Ni-rich cathodes
Gas generation in Ni-rich cathodes mainly occurs due to three aspects: (1) the decomposition reactions of surface RLCs, such as LiOH, Li2CO3 and LiHCO3, (2) the release of lattice oxygen at high voltage, and (3) oxidation reactions of the electrolyte with the cathode. The gas-generation mechanism of LIBs is complicated with many influencing factors. The mechanisms and solution strategies should be investigated clearly to reduce the damage caused by gas generation in batteries, such as battery expansion and even explosion.209,2103.1. Decomposition of surface residual lithium compounds
Li2CO3, which is the main component of RLCs, is not stable, and its decomposition mechanism is mainly divided into chemical decomposition211 and electrochemical decomposition.212,213 Freiberg et al.214 concluded that the decomposition of surface Li2CO3 is a purely chemical decomposition reaction. The decomposition of Li2CO3 was demonstrated to be a chemical process by on-line electrochemical mass spectrometry using an electrode consisting only of Li2CO3 and conducting carbon. This reaction occurs via protons formed during anodic oxidation of trace alcoholic electrolyte impurities and the electrolyte solvent or induced by high HF in the electrolyte (Fig. 10a), ultimately releasing CO2 gas. In the field of lithium-air batteries, Mahne et al.213 found that Li2CO3 decomposes to form singlet oxygen 1O2 at >3.8 V, which is an electrochemical decomposition. By 18O isotopic labelling of the small amount of surface Li2CO3 remaining after synthesis, Renfrew et al.215 found that the degradation of the carbonate electrolyte during the first charge process occurred with minimal gas evolution. Most of the CO/CO2 release during the first charge regardless of the voltage interval is due to Li2CO3 oxidation and not because of electrolyte oxidation. In addition, the decomposition of RLCs affects the release of lattice oxygen. When some of the Li2CO3 is washed off the surface of the transition metal oxide cathode, CO2 evolution decreases and lattice-oxygen release is suppressed, resulting in reduced O2 generation (Fig. 10b). Hatsukade et al.216 investigated the origin of CO2 during cycling of NCM cathodes using the 13C isotope labelling method and found that the decomposition of surface Li2CO3 at high voltage is one of the reasons for CO2 generation. However, it is not the main reason. After a long time of high potential cycling, Li2CO3 will be consumed continuously, and the CO2 produced by chemical oxidation and electrochemical oxidation of the electrolyte solvent at high voltage becomes the main reason, and this decomposition is mainly related to the release of lattice oxygen from the NCM (Fig. 10c). Kaufman et al.102 fabricated a cathode with lithium carbonate as the only active material, and confirmed that the source of CO2 is the electrochemical oxidation of Li2CO3 rather than chemical oxidation by an isotopic labeling method and carbon cathode control experiments. This process is accompanied by the production of reactive oxygen species, which react directly or indirectly with the electrolyte to further produce CO2 and the rest of the organic debris (Fig. 10d). Regarding the electrochemical decomposition mechanism, Cao et al.217 found that Li2CO3 would decompose electrochemically at about 4 V when a LiTFSI/LiFSI based electrolyte is used, releasing CO2 gas and highly reactive monoclinic oxygen 1O2. The 1O2 would in turn react with the carbon at the cathode and with the organic electrolyte to produce CO2 and CO gases (Fig. 10e).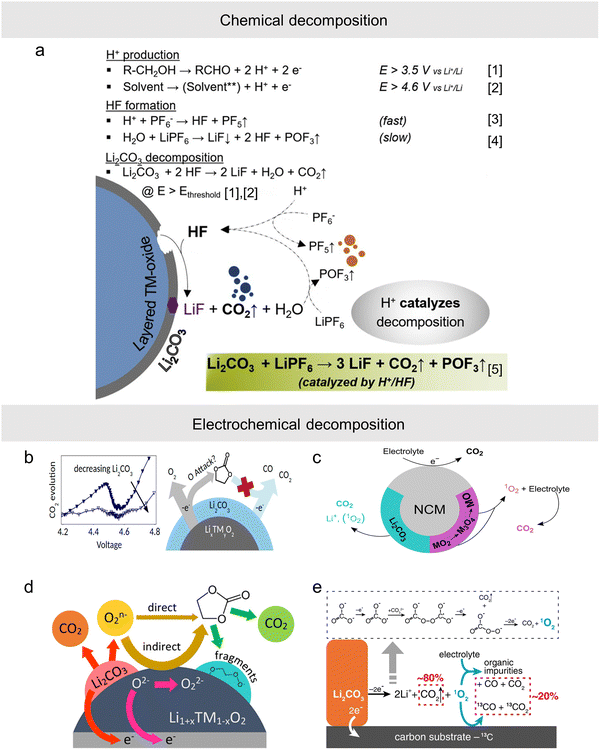 | ||
| Fig. 10 (a) Scheme of Li2CO3 decomposition in the LIB environment. Protons catalyze the decomposition of Li2CO3, whereas their formation strongly depends on the purity and kind of solvents used.214 Copyright 2020, Elsevier. (b) The main mechanisms of the CO/CO2 release during the first charge.215 Copyright 2017, American Chemical Society. (c) The mechanisms of CO2 production during cycling of NCM cathodes.216 Copyright 2018, American Chemical Society. (d) Reactive oxygen released from the NCM lattice is triggered by the oxidation of surface lithium carbonate.102 Copyright 2021, American Chemical Society. (e) Li2CO3 is electro-oxidized to form CO2 and singlet O2 (1O2). A possible pathway is proposed in the dashed line box. 1O2 is highly reactive and it attacks the electrolyte and the carbon substrate to form carbon monoxide and carbon dioxide, which contribute to ∼20% of the overall gas evolution.217 Copyright 2022, Springer Nature. | ||
3.2. The release of lattice oxygen at high voltage
Layered oxide cathodes show evident oxygen evolution at high SOC, where increased Ni content correlates with a reduced voltage threshold for lattice oxygen release.28,45,218,219 It ultimately triggers structural collapse, parasitic interfacial reactions, and catastrophic thermal runaway. Therefore, understanding the mechanisms that link oxygen activity to gas evolution is pivotal for the rational design of next-generation Ni-rich cathodes.The mechanism of lattice oxygen release can be explained at two levels: (1) during deep delithiation, overlapping of transition metal (TM) 3d and O 2p orbitals induces anion redox reactions. (2) The unstable layered structure accelerates lattice oxygen release.220 Among these, the anion redox in the deeply delithiated state constitutes both the thermodynamic driver and the fundamental mechanism for oxygen release from the Ni-rich cathode lattice. Specifically, this refers to the elevation of the Ni4+/Co4+ 3d energy levels under highly delithiated conditions, leading to progressive overlap with the O 2p band (as shown in Fig. 11a).221 This p–d orbital overlap enables the rapid absorption of electrons from O2− by the conduction bands of Co and Ni, leading to partial reduction of Ni4+/Co4+ and the formation of the oxidized species Oα− (α < 2). This process ultimately drives oxygen out of the lattice, generating reactive oxygen species (O2 or O−) and creating oxygen vacancies.222,223In situ X-ray absorption fine structure (XAFS) measurements at the Ni K-edge provide direct evidence for this mechanism. As shown in Fig. 11b, during charging, the Ni K-edge of the Ni-rich cathode shifts toward higher energies below 4.2 V, indicating an increase in the Ni valence state. Subsequently, charging to 4.6 V results in a decrease in the Ni valence state due to oxygen release. Furthermore, in the total electron yield mode of the O K-edge XAFS (Fig. 11c), a sharp increase in the Eg state at 4.6 V indicates anion redox participation in charge compensation. With continued lithium extraction, a distinct oxygen redox signature appears near 523.7 eV in the resonant inelastic X-ray scattering (RIXS) spectrum, indicating irreversible oxygen redox reactions within the Ni-rich cathode as shown in Fig. 11d.224
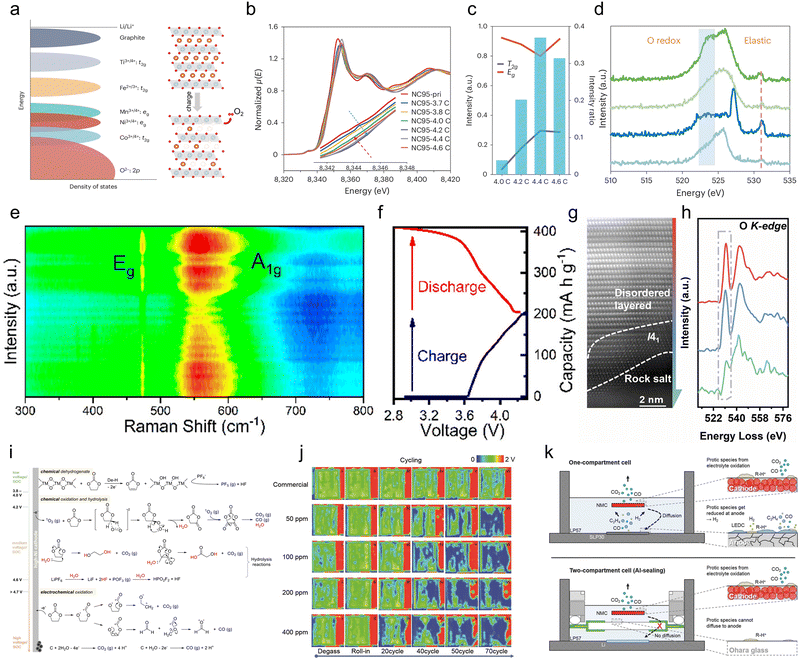 | ||
| Fig. 11 (a) Schematic illustration of transition metal levels and oxygen release from the Ni-rich cathode lattice.221 Copyright 2025, Springer Nature. (b) Ni K-edge XANES of charged LiNi0.95Co0.05O2 (NC95). (c) O K-edge XAFS during NC95 charging. (d) O K-edge RIXS of NC95.224 Copyright 2024, Springer Nature. (e) In situ Raman spectra and (f) the corresponding charge-discharge profile of NCM811 during the third cycle.76 Copyright 2020, American Chemical Society. (g) HAADF image showing the EELS scanning path from the particle interior (upper) to the surface (lower). (h) Corresponding O K-edge EELS spectra, revealing the progressive change in the oxygen oxidation state across different regions.226 Copyright 2022, Elsevier. (i) Schematic illustration of three electrolyte decomposition pathways on the surface of Ni-rich cathodes, including chemical dehydrogenation, chemical oxidation with singlet oxygen, and electrochemical oxidation.235 Copyright 2023, John Wiley and Sons. (j) Through-transmission ultrasonic scanning image of the formation process of pouch cells with different water content in the electrolyte.236 Copyright 2022, Chemical Industry Press. (k) Schematic illustration of gassing related processes for an SLP30 graphite/NCM full-cell in a one-compartment configuration and an NCM/Li half-cell in an Al-sealed two-compartment cell. In both cases, electrolyte oxidation generates CO2/CO and protic decomposition products abbreviated as R-H+; however, only in the one-compartment cell, these protic species can diffuse to the anode and get reduced under the release of H2.237 Copyright 2016, Institute of Physics. | ||
The instability of the layered structure constitutes the structural cause for lattice oxygen desorption. This instability triggers and exacerbates oxygen loss through multiple mechanisms under high voltage. Firstly, in a deeply delithiated state, highly oxidized TM ions attract electrons from the TM–O bond.225 Simultaneously, the electrostatic repulsion between TMO6 layers diminishes, weakening both TM–O and O–TM–O bond strengths. As shown in Fig. 11e and f, during charging, the Eg and A1g band intensities of NCM811 significantly decay, particularly in the voltage range of 4.15–4.3 V. It indicates that the TM–O and O–TM–O bond strengths weaken at high voltages, making surface oxygen atoms more prone to release from the surface structure.76 Furthermore, the crystal structure undergoes an irreversible phase transition from the pristine layered phase to a spinel-like phase and then to a completely inert rock salt phase in the deep delithiated state; this reconstruction process is accompanied by the rearrangement and release of lattice oxygen, as shown in Fig. 11g and h. Atomic-resolution electron energy loss spectroscopy (EELS) reveals the electronic structure of oxygen in different regions of the 4.8 V-charged sample. The pre-edge intensity of the O K-edge spectrum gradually diminishes from the material interior toward the surface, indicating an increase in oxygen vacancies and a decrease in oxygen atoms coordinated with TM ions.226 Meanwhile, as described in Section 2.2.2.2, cation mixing inherent to Ni-rich cathodes and exacerbated during charge/discharge processes can trigger electron rearrangement during deep delithiation. This process excites lattice oxygen activity, ultimately leading to lattice oxygen release, particularly in surface regions. This lattice oxygen release process, driven by the combined effects of weakened bond energy, lattice phase transitions, and cation mixing, ultimately manifests as a sustained degradation of both material structure and electrochemical performance.
It is noteworthy that the redox reaction of lattice oxygen requires sufficiently high charging voltages for activation. Typically, at low voltages, the capacity of Ni-rich cathodes is primarily contributed by the redox reaction of TM cations, with limited involvement of oxygen anion redox. At high voltages (4.5 V), both TM cation redox and oxygen anion redox contribute to capacity. However, this anion redox process is irreversible.196,227 After lattice oxygen is released, it forms reactive oxygen species such as O22− (peroxide), O2− (superoxide), O−, and molecular O2. These highly reactive oxygen species readily react with trace H2O and CO2 present in the electrolyte, forming hydroxyl and carbonate species and releasing molecular O2. Subsequently, these hydroxyl and carbonate species react with Li+ originating from the cathode, ultimately forming RLCs (mainly LiOH and Li2CO3). The reactions can be described as eqn (6)–(10).228 In addition, the Ni-rich cathode surface undergoes a transition from a layered structure to a rock salt phase after the release of lattice oxygen.229,230 This cascade is regarded as a key trigger of thermal runaway.45,122,231,232 Thermally activated oxygen species engage in highly exothermic reactions with the organic electrolyte. The attendant heat evolution rapidly catalyzes electrolyte decomposition, undermines the cathode framework, and inexorably propels the cell toward catastrophic thermal runaway. Although the precise pathway of oxygen-evolution reactions in Ni-rich layered oxides remains elusive, the foregoing analysis indicates that oxygen release originates from lattice-oxygen oxidation. The reactive oxygen species produced in this process, together with continued lattice-oxygen depletion, drive a structural cascade from the layered phase to spinel and, ultimately, to a NiO-type rock salt framework.233 It is worth mentioning that oxygen release is inherently a bulk process, though its kinetics are partly limited by the long diffusion pathways of lattice oxygen. Thus, surface coatings, compositional-gradient designs, and Ni-concentration-engineered core–shell architectures are widely adopted to further curb oxygen evolution.
| Ni3+ + O2− (lattice) → Ni2+ + O− | (6) |
| O− + O− → O2− (active) + O | (7) |
| O2− (active) + CO2 → CO32− | (8) |
| O2− (active) + H2O → 2OH− | (9) |
| O + O → O2 | (10) |
3.3. Electrolyte decomposition
Most of the gas generated is produced by the oxidative decomposition reaction of the electrolyte at high voltage which is divided into electrochemical oxidation and chemical oxidation. The electrolyte with ethyl carbonate (EC) as the solvent shows the following oxidative decomposition mechanism. Firstly, when the voltage is 3.8–4.0 V, the hydrogen atom on EC is attached to the oxygen atom of the layered oxide cathode.234 In the LiPF6-based electrolyte, the active hydroxyl groups on the cathode surface could further react with the PF6− anion to release gaseous PF5. PF5 is extremely unstable and reacts with residual moisture in the electrolyte to form HF. Secondly, when the voltage is higher than 4.2 V (80% SOC), the chemical oxidation reaction mainly occurs, which depends on the release of reactive species. For example, in addition to the triplet O2, the concentrated Ni4+ also induces the cathode to release singlet 1O2 from its layered structure. The singlet 1O2 tends to immediately nucleophilically attack EC to form a carbonyl group on EC, which is eventually decomposed into CO2, CO and H2O. CO is the concomitant product of CO2, and the amount of CO is much lower than that of CO2. The reaction is as shown in eqn (11):| 21O2 + EC → 2CO2 + CO + 2H2O | (11) |
Moreover, active proton-containing substances including acids and ethylene glycol, which originate from the hydrolysis of organic solvents and lithium salts, could react with LiPF6, resulting in the formation of LiF, HF, HPO2F2 and POF3 gases. Thirdly, under the overvoltage of >4.7 V, gas generation mainly comes from electrochemical oxidation. Electrochemical oxidation depends on the catalytic activity, specific surface area and voltage window of the cathode material. EC releases CO and CO2 through a ring-opening reaction under the catalysis of the oxide cathode. In addition to the solvent, the carbon additives in the electrode could also be decomposed to release CO and CO2. The whole reactions are represented in Fig. 11i.235
In addition, due to the presence of trace H2O in the actual electrolyte, the H2O in the electrolyte will decompose to produce H2 when the decomposition potential of H2O is reached (eqn (12)). According to the changes in ultrasonic transmission images during cycling of batteries assembled with electrolytes of different water contents (Fig. 11j), it can be seen that the higher the water content of the electrolyte, the earlier more gases appear inside the battery during cycling.236
| 2H2O + 2e− → H2 + 2OH− | (12) |
In fact, the water content in standard commercial electrolytes is very low. However, the amount of H2 detected in batteries far exceeds the upper limit of the amount of H2 that can be produced by the decomposition of trace amounts of water in the electrolyte. Metzger et al.237 found that a completely dry graphite/NCM full cell produces far more H2 gas than that produced by the reduction of trace H2O contaminants in the electrolyte and electrodes. They attributed this to the fact that solvents in the electrolyte oxidize to protonated solvents (denoted as R-H+) on the NCM surface, which subsequently diffuse to the anode surface to be reduced, especially at high temperatures and high voltages (Fig. 11k). The process is affected by the catalytic influence of TMs;238 therefore, Ni-rich cathodes tend to face more severe gas production phenomena due to the high Ni content and high catalytic activity of Ni.
4. Strategies to suppress the structural damage and gas emissions
To meet the rising demand for high-energy-density batteries, in-depth investigations have clarified the structural degradation and gas-evolution pathways of Ni-rich layered cathodes, thereby informing a suite of stabilization strategies. Common strategies include surface modification, element doping, electrolyte design and structural engineering.4.1. Surface modification
Surface coatings act as a physical barrier that isolate the contact between the electrolyte and the Ni-rich cathode surface, thus suppressing parasitic interfacial reactions and micro-crack formation.239–244 Appropriately engineered coating compositions could further stabilize surface lattice oxygen, inhibit gas evolution, and facilitate Li+ ion and electronic transport, thereby improving electrode kinetics. Some coatings directly convert RLCs into functional and well-ordered surface layers, markedly enhancing structural robustness. In addition, some surface coatings may form near-surface ionic doping synchronously during the coating process, further improving the structural stability of the material. In these coating strategies, coating materials are numerous, including metal oxide materials, lithium-ion conductors, and carbon materials.38,245–249Metal oxide coatings shield Ni-rich cathodes by physically separating them from the electrolyte and markedly mitigate particle fracture and cracking during repeated cycling. Currently, common metal oxide coating materials include Al2O3,250 WO3,251 ZrO2252,253 and others.254,255 However, such surface coatings are generally electrochemically inert and have poor electrical conductivity. What's worse, overcoating usually results in a decrease in the rate performance and discharge capacity of the cathode material. As shown in Fig. 12a–c, Cao et al.256 employed a wet chemical method to coat NCM surfaces with varying amounts of Al2O3. Although this physical barrier enhanced cycling performance, the electrochemically inert Al2O3 reduced the discharge specific capacity of NCM. Moreover, due to low ionic conductivity, the coating exhibits poor enhancement effects on rate performance. When the coating content is excessive, it may even lead to a deterioration in rate performance. Therefore, when selecting a metal oxide for coating, the selection of dosage is very important. To achieve more precise coating coverage, Zhao et al.257 employed atomic layer deposition (ALD) to realize thinner and more uniform Al2O3 coatings. However, coating individual NCM secondary particles with inert metal oxides still inhibits ion and electron migration. Based on this, Wang et al.258 employed ALD to coat composite NCM811 cathodes with Al2O3 as shown in Fig. 12d (designated as AO-n, where n corresponds to the number of ALD cycles for thickness control of the Al2O3 coating layer). By comparing the electrochemical performance of NCM with varying coating thicknesses, they observed that due to the low lithium-ion conductivity of Al2O3, the specific capacity and coulombic efficiency of the AO-5, AO-10, and AO-50 samples were lower than those of the pristine NCM811 (Fig. 12e). However, the moderately coated AO-10 sample effectively suppressed side reactions between the cathode and the electrolyte while enhancing structural stability. This achieved optimal cycling stability alongside a significant improvement in rate performance (Fig. 12f). However, early studies on traditional inert metal oxide coatings often focused solely on the coating's ability to suppress interfacial side reactions and were oriented toward performance enhancement while neglecting the coating's impact on the crystal lattice and its inhibitory effect on oxygen release.
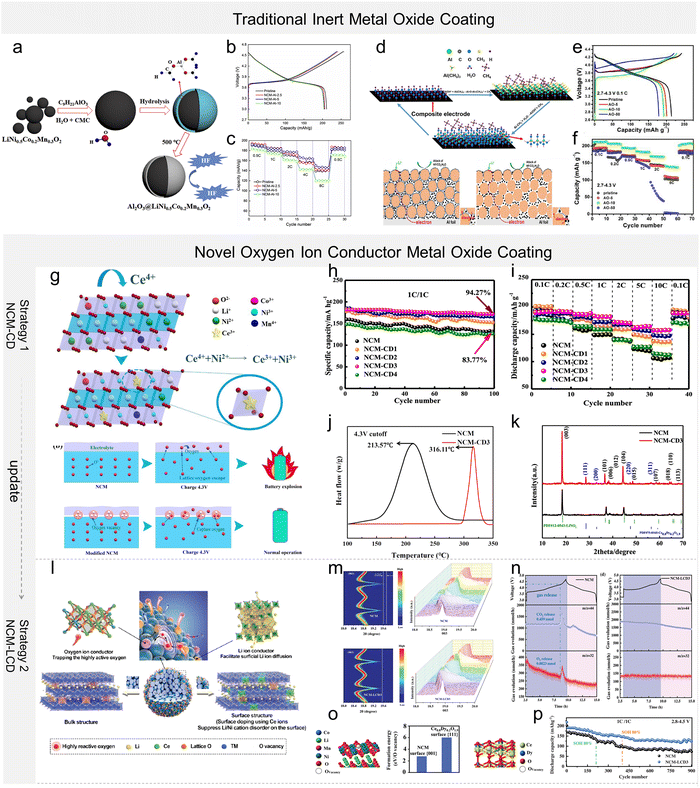 | ||
| Fig. 12 (a) Al2O3 coating process on NCM cathodes. (b) Initial 0.1 C charge/discharge profiles and (c) rate capability of pristine and Al2O3 coated NCM samples.256 Copyright 2020, Elsevier. (d) Fabrication process and Li+ transport schematics comparing coating on powder vs. electrode using the ALD method. (e) Initial 0.1 C charge/discharge profiles and (f) rate performance of AO-n samples.258 Copyright 2022, Elsevier. (g) Schematic diagram of cation disorder of NCM and Ce0.8Dy0.2O1.9-modified NCM (up) and schematic illustration of the oxygen vacancies in the Ce0.8Dy0.2O1.9-modified layer enhancing the structural stability of Ni-rich materials (bottom). (h) Cycling performance at 1 C and (i) rate capability of pristine NCM and Ce0.8Dy0.2O1.9-modified NCM. (j) Differential scanning calorimetry (DSC) traces showing the heat flow of NCM and NCM-CD3 charged to 4.3 V. (k) Ex situ X-ray diffraction (XRD) patterns of the pristine and NCM-CD3 electrodes after 100 cycles at 1 C.260 Copyright 2019, American Chemical Society. (l) Schematic diagram of effectively suppressing Li/Ni cation disorder via the nanoscale surface doping approach and reaction mechanisms of the oxygen ion conductor Ce0.8Dy0.2O1.9 and lithium-ion conductor Li8CeO6 multifunctional surficial modification layer to enhance interfacial stability and safety performance of Ni-rich materials. (m) Contour plots of in situ XRD patterns of NCM (top) and NCM-LCD3 (bottom) for the (003) peak. (n) Voltage profiles of NCM and NCM-LCD3 at 2.8–4.7 V and the corresponding gas evolution during the first cycle with a current density of 200 mA g−1 through in situ differential electrochemical mass spectroscopy measurements (DEMS). (o) Oxygen vacancy formation energy of NCM and oxygen ion conductor Ce0.8Dy0.2O1.9 on the particle surfaces. (p) Comparison of long-term cycling performance of pristine NCM and NCM-LCD3.261 Copyright 2023, John Wiley and Sons. | ||
With a deepening understanding of the degradation mechanisms in Ni-rich cathodes and the demand to expand their practical applications, researchers have recognized that lattice oxygen release and the resulting chain reactions are the root causes limiting high-voltage applications of Ni-rich cathodes and leading to thermal runaway safety issues.259 Consequently, the goal of coating design has expanded beyond conventional performance metrics to focus on enhancing lattice oxygen stability while suppressing surface side reactions, thereby inhibiting oxygen release and structural degradation under high-voltage conditions. This transformation has given rise to a new generation of metal oxide functional coatings represented by oxygen ion conductors. Beyond providing conventional physical isolation, these materials actively guide and dissipate reactive oxygen species escaping from the lattice under high voltage through their internal oxygen vacancies and rapid oxygen ion migration pathways. This effectively suppresses the accumulation and release of oxygen, mitigating oxygen-induced structural degradation from the source while significantly enhancing material thermal stability under extreme conditions. Wang et al.260 coated NCM811 with oxygen ion conductor Ce0.8Dy0.2O1.9 (NCM-CD) to achieve excellent electrochemical properties (Fig. 12g–i). The abundant oxygen vacancies in Ce0.8Dy0.2O1.9 inhibit the release of oxygen, which improves the thermal stability and safety of Ni-rich cathode materials, as shown in Fig. 12j. Moreover, during the coating process, Ce4+ doping into the material lattice inhibits the disorder of Li+/Ni2+ cations and improves the structural stability of the material, enabling the cathode material to maintain an ordered structure after cycling (Fig. 12k). Benefiting from the suppressed interfacial side reactions, reduced irreversible surface structure degradation, and inhibited oxygen release enabled by this coating strategy, NCM-CD ultimately achieved satisfactory cycling and rate performance. Dai et al.240 proposed a kind of inert phase (La2Mo2O9) with abundant oxygen vacancies, which is used as the surface coating of Ni-rich cathodes (L-NCM) and can act as both an oxygen anchor and an oxygen storage unit (Fig. 13a). With this modification scheme, the H2–H3 phase transition of the Ni-rich cathode is significantly suppressed (Fig. 13b), while the stability of lattice oxygen is enhanced due to the higher binding energies of interfacial La–O and Mo–O compared to Ni–O (Fig. 13c). In addition, the protective effect of the coating also leads to a weakened reaction of the electrolyte with oxygen. These favorable effects effectively inhibit the structural deterioration and improve the electrochemical performance of the Ni-rich cathode, resulting in a capacity retention rate of 94% for the full cell after 400 cycles (Fig. 13d).
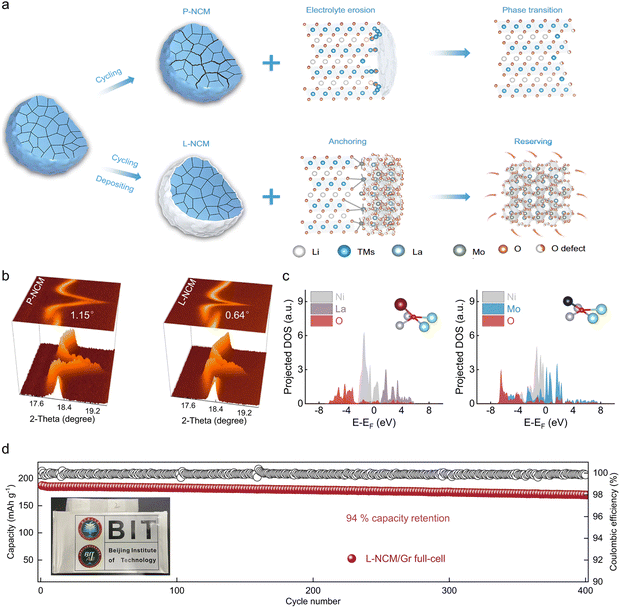 | ||
| Fig. 13 (a) Schematic illustrations of the operando oxygen anchoring and reserving strategy. (b) Selected (003) counter plot of in situ XRD patterns for pristine NCM (P-NCM) and L-NCM upon initial cycle. (c) Projected density of states (DOS) for surface oxygen coordinated by one La, two Li and two Ni (left), and surface oxygen coordinated by one Mo, two Li and two Ni (right). (d) Cycling performance of the L-NCM/Gr pouch-type full cell at 0.5 C under 2.7–4.2 V. Inset: Photo of the assembled pouch cell (size: 5 cm long and 8 cm wide).240 Copyright 2023, Springer Nature. | ||
To simultaneously stabilize the cathode lattice, accelerate Li+ transport and suppress Rct, lithium-ion-conductive surface coatings were used to modify the Ni-rich cathodes.262,263 Common lithium-ion conductor coatings include Li4Ti5O12,264 Li2WO4,265 and so on. Tang et al.266 used NASICON-type Li1.3Y0.3Zr1.7(PO4)3 (LYZP) to coat single-crystal LiNi0.83Co0.11Mn0.06O2 (SC-NCM83) to form SC-NCM83@Zr/LYZP, and introduced trace amounts of Zr doping in the formation of a coating layer (Fig. 14a). In situ XRD tests show that SC-NCM83@Zr/LYZP displays a reversible and weaker H2–H3 phase transition with a maximum c-axis shrinkage of 2.60%, an a-axis change of 1.88%, and a volume change of 4.89%. The lattice expansion/contraction during the first charge–discharge cycle is significantly lower than that of the pristine SC-NCM811 (Fig. 14b, c and g), enhancing the integrity of the layered structure. In addition, SC-NCM83@Zr/LYZP achieves more stable lattice oxygen and suppressed surface side reactions with significantly lower O2 and CO2 release, as shown in Fig. 14d and e. Compared with SC-NCM83, the exothermic peak of SC-NCM83@Zr/LYZP shifts backward and the exothermic amount is significantly reduced (Fig. 14h), demonstrating excellent thermal stability. Therefore, the capacity retention of SC-NCM83@Zr/LYZP in the extended voltage range of 2.75–4.5 V is 81.2%, and it also exhibits higher stability at a high current density of 1 C, retaining 77.3% capacity compared to 65.6% for the unmodified SC-NCM83 (Fig. 14f). Wang et al.267 coated LiNi0.9Co0.05Mn0.05O2 (NCM9) with the perovskite lithium/oxygen dual-ion conductor La4NiLiO8 (LNLO) (Fig. 14i). The stable oxygen vacancies/interstitials in LNLO can effectively stabilize the lattice oxygen on the surface of the Ni-rich cathode. The gas production problem of the Ni-rich cathode is fundamentally reduced (Fig. 14j), and the change in the lattice constant during cycling is increased as well, which improves phase transition reversibility and inhibits particle breakup after long cycling (Fig. 14k). As a result, the material shows excellent cycling stability, and the capacity retention rate of 66.33% is still achieved at 400 cycles at a high voltage of 4.5 V (Fig. 14l). At the same time, the good lithium-ion conductivity also allows a better rate performance. Moreover, on the basis of Ce0.8Dy0.2O1.9-coated NCM811, Wang et al.268 further designed a modification strategy based on combining the metal oxide Ce0.8Dy0.2O1.9 with the lithium-ion conductor Li8CeO6 as the surface coating (NCM-LCD3) as shown in Fig. 12l, which suppressed the H2–H3 phase transition (Fig. 12m) and reduced the deleterious gas production phenomenon (Fig. 12n). The inhibition of oxygen release is attributed to the high oxygen vacancy formation energy of Ce0.8Dy0.2O1.9 as shown in Fig. 12o. As a result, NCM-LCD3 demonstrated excellent high voltage cycling stability with a cycle life of over 400 cycles (Fig. 12p).
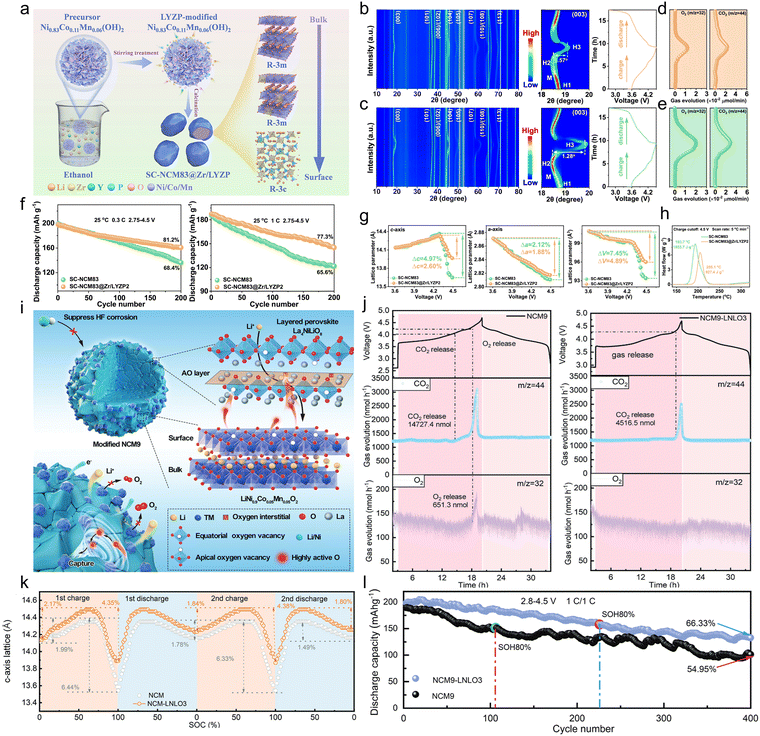 | ||
| Fig. 14 (a) Schematic of the SC-NCM83@Zr/LYZP synthesis process. In situ XRD tests of (b) SC-NCM83@Zr/LYZP2 and (c) pristine SC-NCM83 during initial cycling at 0.1 C between 2.75 and 4.5 V. The corresponding variations in the (g) c-axis parameter, a-axis parameter, and cell volume during the charging process. In situ DEMS data of (d) SC-NCM83@Zr/LYZP2 and (e) SC-NCM83 measured under the same electrochemical conditions as in situ XRD. (h) DSC curves of SC-NCM83@Zr/LYZP2 and SC-NCM83 electrodes after charging to 4.5 V. (f) Cycling performance of SC-NCM83@Zr/LYZP2 and SC-NCM83 at different current density. (h) DSC curves of SC-NCM83@Zr/LYZP2 and SC-NCM83 electrodes after charging to 4.5 V. 266 Copyright 2025, Elsevier. (i) Schematic diagram of effectively inhibiting the release of highly active oxygenates in the surficial lattice by oxygen vacancies and oxygen interstitials in the layered perovskite LNLO to improve battery safety performance and alleviate stability issues of ultrahigh-Ni layered oxide cathode material NCM9 (NCM9-LNLO). (j) Voltage profiles of as-prepared electrodes at 2.8–4.7 V and the corresponding gas evolution during the first cycle of NCM9 and NCM9-LNLO3 with a current density of 200 mA g−1 through in situ DEMS measurements. (k) Comparison of the changes of lattice parameter c obtained by fitting XRD patterns during the first two charge/discharge processes of NCM9 and NCM9-LNLO3. (l) Comparison of the long-term cycling performance of pristine NCM9 and NCM9-LNLO3 at 2.8–4.5 V.267 Copyright 2023, John Wiley and Sons. | ||
The carbon material could improve the electron and ion conductivity, prevent the structural collapse caused by the dissolution of TM ions, and effectively suppress the capacity decay. Guo et al.269 prepared carbon-coated LiNi0.92Co0.06Al0.02O2 nanosheets by sol–gel assisted high-temperature annealing. The carbon coating can protect the electrode from the influence of H2O and CO2, which enhances the electrode's corrosion resistance and makes it easier to store. The modified cathode exhibits excellent structural integrity, and the battery capacity is also improved. Liu et al.270 used sucrose and glucose as carbon materials to construct a nano-carbon coating on the surface. It is found that carbon coating, as a physical barrier between the cathode material and the electrolyte, can effectively prevent the formation of microcracks and inhibit the side reaction at the interface, and improve the rate and cycling performance of the cathode. In addition, the coating effect of sucrose is better than that of glucose. The reason is that the glucose coating is dense, while the sucrose coating is relatively loose and has a larger specific surface area. Therefore, the sucrose coating can inhibit the erosion of the electrolyte more effectively.
In addition to constructing coating layers on the surface, researchers have devised a variety of novel surface treatments in recent years. Sun et al.56 proposed a washing process using an aqueous solution of Co that simultaneously removes RLCs and forms a dual protective coating of an outer F-rich layer and an inner Co-rich layer on the Ni-rich layered cathode (Fig. 15a and b). The Co-rich layer formed during the washing process depletes the surface RLCs and prevents direct contact of the electrolyte with the surface of the Ni-rich cathode, improving the lattice oxygen stability. The additional fluorine coating further reacts with the surface RLCs to generate a prefabricated LiF coating (Fig. 15c), which effectively mitigates the decomposition of electrolytes and impurities, improves the interfacial stability, and suppresses gas generation (Fig. 15d). The synergistic effect of the two modifications together improves the long-term cycling stability of the Ni-rich cathode (Fig. 15e). Cai et al.271 proposed a surface lanthanide strategy for stabilizing the high-voltage cathode based on the general principles of high-voltage cathode near-surface design using LCO as a substrate, which modulates the near-surface structure of the material (La-LCO). An implanted La/Ca gradient is formed by the exchange of La3+/Ca2+ with Li+ in aqueous solution. After the annealing treatment, a perovskite protective layer La1−wCawCoO3−δ was formed on the surface of LCO, while the near surface showed a gradient doping of La3+/Ca2+ (Fig. 15f). The nanoscale La1−wCawCoO3−δ surface perovskite phase functions as an oxygen buffer that reversibly stores oxygen while elevating the lattice-oxygen release potential, as proved by DEMS tests, thereby effectively suppressing oxygen and other derivative gas release. After 100 cycles at 1 C and 3.0–4.5 V, the pouch cell of La-LCO exhibits nearly imperceptible swelling, in stark contrast to the pronounced bulging observed in the pristine LCO (P-LCO) pouch cell (Fig. 15g and h). Concurrently, it mitigates electrochemical degradation caused by interfacial side reactions and structural decay. And it was generalized to Ni-rich layered cathodes (La-NCM) with high energy density (Fig. 15i). In order to solve the limitation that the current coating means are only limited to the secondary particle surface coating but cannot inhibit the side reactions at the root of the microscopic solid–liquid interface between primary particles, Yoon et al.242 chose to select a cobalt boride (CoxB) for the simultaneous coating of both the primary particle interface and the secondary particle surface of NCM811 (Fig. 15j). There is a strong reactivity between CoxB and the surface oxygen of NCM811 and a passivation film can be formed without consuming too much lattice oxygen. Eventually, primary particle cracking and surface transition were suppressed (Fig. 15k), and gas escape was reduced (Fig. 15l), thus effectively improving rate performance and high temperature cycling stability (Fig. 15m and n). Sun et al.272 applied thermal quenching (140 °C min−1) to high-Ni LiNixMnyCozO2 (x ≥ 0.8) immediately after calcination. The non-equilibrium cooling expels Li+ toward the surface, creating a nano-crystalline Li2CO3 overlayer that passivates against electrolyte oxidation and stabilizes lattice oxygen. Quenched NMC-Q exhibits 195 mAh g−1 at 100 mA g−1 and retains 90% capacity after 300 cycles at 200 mA g−1. Wang et al.273 performed rapid deionized water treatment on NCM811 followed by subsequent heat treatment. The treatment leaches a few surface Li+ layers and triggers in-plane Ni–O rearrangement, yielding a 2–4 nm rocksalt NiO-like phase that is coherent with the underlying layered lattice.This surface reconstruction phase passivates the material surface and forms stable self-anchored Ni–O clusters, thereby suppressing electrochemical cycling degradation in Ni-rich materials. The modified NCM maintains 86% retention after 300 cycles at 1 C without external dopants or coatings. Lu et al.274 developed a surface solid reaction strategy to stabilize LiNi0.83Co0.07Mn0.1O2 cathodes by constructing a conformal La(OH)3 nanocoating via an anhydrous wet-chemical method. Using ethanol and hexamethylenetediamine, this decomposition-induced precipitation technique achieved nanoscale-precision coating without damaging the moisture-sensitive structure. Following high-temperature sintering, this coating undergoes a controlled solid reaction with the NCM surface. This process introduces moderate Li/Ni disorder into the cathode surface layer, which synergistically interacts with the generated LiLaO2 to enhance the cathode's cycling performance. After 400 cycles at 1 C, capacity retention jumps from 35.5% to 80.8%, while 5 C discharge remains at 144 mAh g−1. Notably, the anhydrous medium avoids proton or carbonate contamination that typically plagues aqueous coating processes.
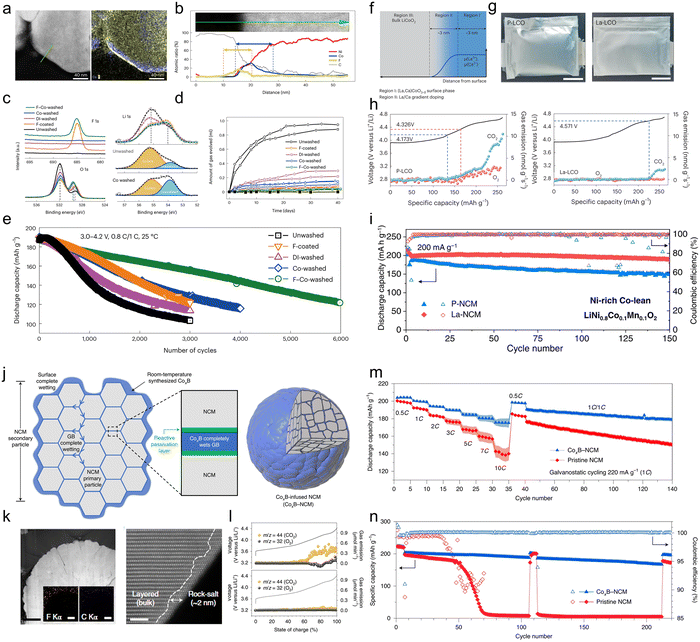 | ||
| Fig. 15 (a) and (b) A TEM image and the corresponding energy dispersive spectrometer (EDS) overlay map showing Co and F distribution (a) and EDS line scan results of the surface of the F-Co-washed cathode (b) along a green arrow in panel a. The vertical dashed lines in panel b indicate the thickness of each coating layer. (c) Comparison of F 1s, O 1s and Li 1s X-ray photoelectron spectroscopy (XPS) spectra of the unwashed, F-coated, deionized water-washed (DI-washed), Co-washed and F-Co-washed cathodes. The vertical dashed lines in panel c indicate the binding energy of XPS peaks for each cathode, and the dashed curves indicate the fitted profiles by peaks from the residual lithium compounds and intercalated lithium on the surface. (d) Gas evolution from pouches featuring a 4.3 V charged cathode and the electrolyte during storage at 60 °C. In panel (d) black filled-square data represent the volume changes of the pouch containing the electrolyte and the uncharged unwashed cathode. (e) Long-term cycling performance using pouch-type full cells.56 Copyright 2023, Springer Nature. (f) Schematic of the treatment process, the resulting surface architecture with a La/Ca gradient and perovskite layer, and the corresponding chemical potential (µ) profile of La3+/Ca2+. (g) Optical photos of P-LCO and La-LCO pouch cells after 100 cycles at 1 C (3.0–4.5 V), demonstrating the enhanced physical integrity. (h) In situ DEMS profiles during the first charge to 4.7 V, confirming the suppression of oxygen-related gas release in the La-LCO half-cell. (i) Cycling performance of P-NCM and La-NCM in coin-type half-cells at 1 C at 2.8–4.4 V vs. Li+/Li.271 Copyright 2023, Springer Nature. (j) Schematic coating-plus-infusion microstructure in which CoxB uniformly coats the surface of NCM secondary particles and infuses into the grain boundaries between the NCM primary particles. (k) TEM image and EDS mapping (inset) of CoxB-NCM after 200 cycles at a 7 C discharge rate at 45 °C and HR-TEM of a secondary particle surface in cycled CoxB-NCM. (l) In situ DEMS data of pristine NCM (top) and CoxB-NCM (bottom) during the first charge at 0.2 C in the voltage range of 3.0–4.4 V versus Li/Li+ at 25 °C. (m) Rate tests and 1 C cycling of CoxB-NCM and pristine NCM in the range of 3.0–4.4 V versus Li/Li+ at 25 °C. The shading shows the standard deviations calculated from five cells. (n) 7 C discharge cycling tests in the range of 3.0–4.4 V versus Li/Li+ at 45 °C, with 6 intermittent cycles with 0.2 C charge/discharge conducted after every 100 cycles.242 Copyright 2021, Springer Nature. | ||
4.2. Element doping
Element doping is also an effective strategy to improve the electrochemical performance of Ni-rich layered oxide cathodes.275–277 This simple modification improves the stability of the lattice structure at the atomic level, increases the bonding strength between the metal ions and the oxygen atoms, which reduces the degree of cation mixing and irreversible phase transitions, and prevents microcracking. In addition, proper doping can promote the migration of Li+ and refine the microstructure of the material.278–281 So far, it has been reported that strategies such as single cation doping, single anion doping and mixed ion doping can be used to improve the comprehensive properties of Ni-rich cathode materials.Cation doping can effectively improve the cycling performance and internal structure stability of Ni-rich cathodes.224,282 The different doping sites depend on the radius of the doped cation. Some small radius cations, such as K+, Na+, and Mg2+, are more inclined to occupy the position of lithium ions, inhibit cation mixing and play a columnar effect. The columnar effect can significantly inhibit the formation and expansion of microcracks, so as to obtain better cyclic performance.283 However, this type of doping may also lead to capacity degradation. Using a simple mixed molten salt sintering process, Jian et al.284 synthesized a Na-doped single crystal LiNi0.82Co0.125Mn0.055O2 (NCM-Na) Ni-rich cathode. In this case, Na+ uniformly occupies the Li sites, and Na+ can selectively stabilize the surrounding Li+ by regulating the orientation of the Jahn-Teller effect of Ni3+. These stabilized Li ions provide preferential channels that enable high-speed movement of Li ions. Benefiting from this, the Li inhomogeneity and phase separation during charging and discharging of NCM-Na are greatly reduced, thus reducing the risk of structural defects and mechanical degradation and improving cycling stability and rate performance. Qiu et al.285 pioneered a novel doping process and proposed the concept of a Mg2+ releasing membrane for the first time. That is, the doping of Mg2+ is realized during the cycling process. A self-supporting MgV2O4 membrane was synthesized by incorporating mobile Mg2+ into the MgV2O4 structure, which releases Mg2+ during electrochemical processes and acts as a “pillar” within the lattice to provide structural reinforcement to the cathode surface.
Cations with large ionic radius, such as Ti4+, Ta5+, Mo6+, etc., often occupy the position of the TM layer and enhance the strength of the metal–O bond in the TM layer, and thus improve the lattice oxygen stability of the Ni-rich cathode.81 Park et al.286 systematically investigated the effect of doping of various elements, including Co, Al, Ti, Ni, Ta, W, Mo, etc., on the LiNi0.9Mn0.1O2 (NM90) cathode. It was found that doping of high valence elements could not only inhibit the H2–H3 phase transition more effectively but also refine the primary grain size (Fig. 16a). As a result, better cycling performance is presented (Fig. 16b and c). After matching the fluorine-containing electrolyte with Mo-doped NM90, the capacity retention rate of the full cell reached 85.6% after 1000 cycles at 4.3 V. Further, Ryu et al.287 studied in detail the effect of Mo doping amount and synthesis temperature on a Ni/Co/Mn/Al quaternary Ni-rich cathode (NCMA94) containing 94% Ni. The results show that the electrochemical properties of the Mo doped Ni-rich layered cathode are largely determined by the primary particle size and morphology of the cathode material and the crystallinity of the material. The higher the crystallinity and the finer the primary particles, the better the capacity and cycling performance of the Ni-rich cathode. In addition, the primary particles become finer with the increase of Mo content (Fig. 16d), while the strength of the material tends to stabilize at 1% Mo content (Fig. 16e). The electrochemical performance of the Ni-rich cathode is improved after Mo element doping (Fig. 16f). Yang et al.224 constructed LiNi0.94Co0.05Te0.01O2 (NC95T) using Te6+ doping, and the introduction of Te6+ led to the formation of a highly thermodynamically stable Ni–Te ordered structure within the TM layer and improved particle morphology. This micromorphology and ordered atomic arrangement resulted in a reduction of lattice strain during cycling for NC95T (Fig. 16g and h), and significantly improved the lattice oxygen stability. Further calculations show that for the NC95T cathode, especially in the fully delithiated state, a localized O 2p state near the oxygen Fermi energy level appears in the ordered Ni–Te structure, which makes the electrons in the lattice oxygen more stable (Fig. 16i). The Bader charge of the NC95T cathode has a discrete nature, which suggests the passivation of the lattice oxygen (Fig. 16j). In addition, the introduction of the ordered Ni–Te structure results in a high oxygen vacancy formation energy for NC95T (Fig. 16k), which also confirms the excellent stability of lattice oxygen.
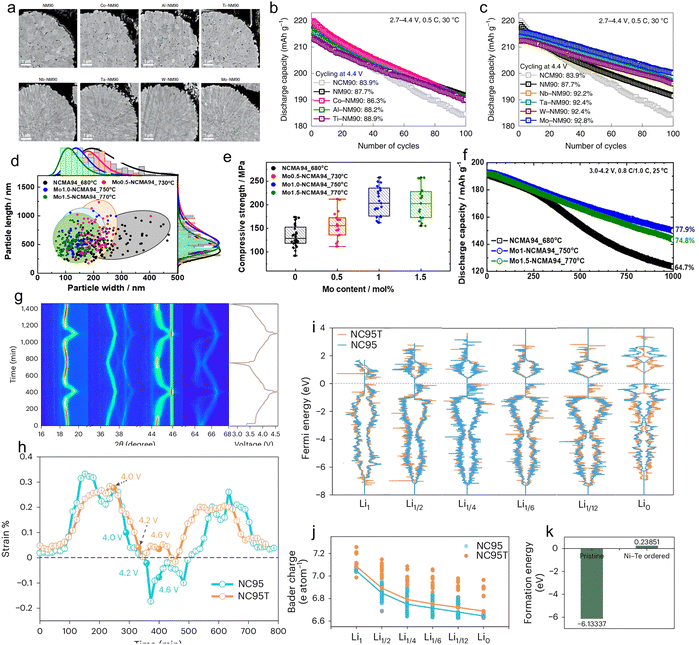 | ||
| Fig. 16 (a) Comparison of primary particle morphologies of undoped and doped NM90 cathodes. (b) and (c) Comparison of NM90 cycling performance after doping with (b) low valence cations and (c) high valence cations.286 Copyright 2022, Springer Nature. (d) Distributions of the primary particle size. (e) Secondary particle strength of the optimized NCMA94 and Mo-doped NCMA94 cathodes. (f) Long-term cycling performance of optimized NCMA94, Mo1-NCMA94, and Mo1.5-NCMA cathodes in full cells.287 Copyright 2023, Elsevier. (g) In situ XRD of NC95T of the first two cycles and (h) corresponding calculated lattice strain. (i) The density of states of oxygen. (j) Bader charge on oxygen and average Bader charge difference between the pristine and Ni–Te ordered sample. (k) Oxygen vacancy formation energy (pristine means NC95 and Ni–Te ordered represents NC95T).224 Copyright 2024, Springer Nature. | ||
Anion doping, when compared with cation doping, is suggested to provide stronger solubility resistance in acid for Ni-rich materials.288 It can also prevent Li or TM ions from occupying incorrect sites and improve the energy density of the electrode.289 When the oxygen atom in the layered cathode part is replaced by a halogen atom (X), the formed X-TM (Ni, Co, Mn) has a higher bonding strength than the original O–TM (Ni, Co, Mn), thus enhancing the structural strength of the Ni-rich cathode. Zhang et al.290 found that the F− doping strategy can reduce the Li/Ni mixing, and accelerating rate calorimetry studies suggest that samples with higher F content in the cathode will have safety advantages. Similarly, F-doped NCM811 was prepared by a solid-state reaction method. The modified samples show better cycling performance due to the strong bonding of TM and F as well as improved Li+ transport behavior.291 In addition, Park et al.292 found that the capacity retention of sulfur-doped LiNiO2 cathode materials was significantly improved. Since the electronegativity of sulfur is lower than that of oxygen, sulfur doping can prevent interlayer elongation and structural decomposition caused by lithiation/delithiation during the cycle by increasing the transport of Li+. Cl− doping has a good inhibitory effect on Li/Ni disorder and could increase the diffusion rate of Li+ as well.289
To combine the advantages of different ions, some researchers have adopted the strategy of mixed ion doping. The synergistic effect between dopants is utilized to maximize the performance of Ni-rich cathodes. Lee et al.80 improved the stability of the LiNi0.92Co0.04Mn0.04O2 (NCM92) cathode based on a double doping strategy by employing Al3+ and Nb5+ (AlNb-NCM92). Al3+ doping enhances the stability of the crystal structure, while Nb5+ doping optimizes the morphology of primary particles. Under the synergistic action of the two elements, the lattice ordering of the Ni-rich cathode increases and the lattice strain decreases (Fig. 17a–c). Microcracks are almost absent in AlNb-NCM92 after 1000 cycles, which is attributed to the fine and radially aligned primary particles effectively dispersing the strains imposed on the particles during the charge/discharge process, thus suppressing the generation of microcracks (Fig. 17e–g). The cathode can still maintain 88.3% of the initial capacity after 1000 cycles. Zhang et al.293 found through experiments that Cr–Mg co-doping can lead to a synergistic reaction to form a complementary structure. Cr occupies the Ni layer and Mg occupies the Li layer. The synergistic reaction can reduce the cation mixing degree and improve the stability of the structure, thus enhancing the cycle performance of the cathode and showing a higher discharge capacity. Zhou et al.294 mitigated the structural degradation and oxygen release problems of Ni-rich cathode after prolonged cycling by employing Mg2+/F− co-doping (Mg1+F2) (Fig. 17d). The co-doping strategy results in a high Ni2+ migration barrier and high oxygen vacancy formation energy (Fig. 17h–j). In situ XRD tests reveal a more stable and reversible structure of Mg1+F2, which exhibits lower Li/Ni mixing and suppressed H2–H3 phase transition (Fig. 17k–m and p). Electron paramagnetic resonance (EPR) results indicate (Fig. 17n and o) that the oxygen vacancy concentration of Mg1+F2 in the charged state is almost unchanged compared with that in the pristine state, whereas the oxygen vacancy concentration of the unmodified Ni-rich cathode increased significantly. The results of in situ DEMS show that the CO2 release from Mg1+F2 is reduced, and the onset voltage of the release is delayed, indicating that the cathode/electrolyte interfacial side reactions are suppressed. Notably, there is no O2 release observed in the test, indicating that the co-doping of Mg2+ and F− can effectively stabilize the lattice oxygen and thus inhibit the O2 release (Fig. 17q and r). For LiNi0.96Co0.03Mn0.01O2, Xu et al.295 combined trace W-doping (microstructural refinement) with a conformal LiF layer (grain-boundary sealing). The W-doped matrix forms slender primary particles that relieve inter-granular stress; the LiF layer blocks HF attack and electrolyte penetration.
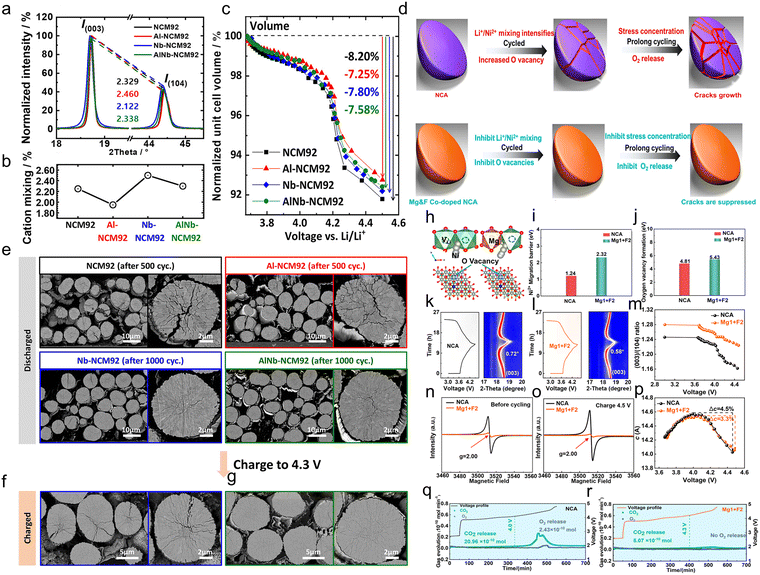 | ||
| Fig. 17 Magnified XRD patterns highlighting (003) and (104) peaks (a) and (b) cation mixing of Ni2+ in the Li layer of the as-prepared NCM92, Al-NCM92, Nb-NCM92 and AlNb-NCM92. (c) Corresponding normalized lattice volume variations of the cathodes measured by in situ XRD.80 Copyright 2024, American Chemical Society. (d) Schematic diagram of the structural evolution of NCA and Mg1+F2.294 Copyright 2023, American Chemical Society. Cross-sectional scanning electron microscope (SEM) images of (e) discharged NCM92, Al-NCM92, Nb-NCM92 and AlNb-NCM92 cathode particles and charged (f) Nb-NCM92 and (g) AlNb-NCM92 cathode particles after long-term cycles.80 Copyright 2024, American Chemical Society. (h) Simplified model of Ni migration to the Li site and simplified model of lattice oxygen escape. (i) Migration barrier of Ni2+ in NCA and Mg1+F2 to the Li layer. (j) Escape barrier of lattice oxygen in NCA and Mg1+F2. In situ XRD patterns of the (003) peak in the initial cycle for (k) NCA and (l) Mg1+F2. (m) Evolution of the (003)/(104) ratio for NCA and Mg1+F2. EPR of NCA and Mg1+F2 before (n) and after (o) cycling. (p) Evolution of lattice parameter c for NCA and Mg1 F2. In situ DEMS measurements for (q) NCA and (r) Mg1+F2.294 Copyright 2023, American Chemical Society. | ||
4.3. Electrolyte design
Electrolyte engineering is one of the strategies that can significantly and simultaneously improve the cycle life and safety of Ni-rich cathodes. In high voltage cycles, the electrolyte would oxidize and decompose, corrode the surface of the cathode and form a CEI layer on the surface of the material, resulting in increased interface impedance and thus affecting the cycle performance of the battery.245,248,296–299 In addition to surface engineering, appropriate electrolyte additives could also be selected to improve the high voltage and high temperature stability of the electrolyte, thereby reducing the damage from the interface side reaction to the cathode material and forming a stable CEI on its surface.88,243,245,300–304 Common additives can be divided into solvents and lithium salts,296,305–318 such as fluoroethylene carbonate (FEC), vinyl carbonate (VC), LiNO3, LiBF4 and so on. In addition to a single additive, the simultaneous use of multiple additives can also produce synergistic effects. Zhang et al.319 proposed a trimethyl borate (TMB) electrolyte additive with low oxidation potential that forms a stable and dense CEI, suppresses the dissolution of TMs, and eventually enables stable cycling of NCM90/Li batteries at high voltages of 4.7 V and 4.5 V. The NCM90/Li battery with 1 wt% TMB addition has a capacity retention rate of 77.8% after 200 cycles at 4.5 V and can still reach 70.7% after 100 cycles at an ultra-high voltage of 4.7 V (Fig. 18a–c). Zou et al.320 inhibited EC dehydrogenation on the cathode surface by introducing the 4-fluoro-N,N-dimethylbenzenesulfonamide electrolyte additive (FBSN) and formed a dense, rigid substance with high ionic conductivity and a self-healing CEI on the Ni-rich cathode surface. Therefore, the cycle stability of the Ni-rich cathode is obviously improved. Cheng et al. proposed to promote preferential deposition of anions by constructing dissolved sheaths enriched with multiple anions and to form solid electrolyte interfaces enriched with inorganics. Therefore, the performance of the Ni-rich cathode under high voltage and high temperature is effectively improved. At the same time, the existence of the weak solvation structure greatly reduces the Rct at low temperature and inhibits the growth of lithium dendrites.297,321–323 This high-chaos electrolyte (HC-E) design strategy enables NCM811/Li to exhibit excellent electrochemical performance in the temperature range of −30 to 45 °C at 4.7 V(Fig. 18d–f).324 Chen et al.325 proposed a strategy involving the engineering of an insoluble CEI to address interface-induced degradation in Ni-rich cathodes. A series of siloxanes containing unsaturated units (propargyloxytrimethylsilane (PMSL), allyloxytrimethylsilane (AMSL), and trimethylmethoxysilane (TMSL)) were used as electrolyte additives (Fig. 18g). These siloxane compounds, featuring high unsaturation with numerous reactive sites, undergo increased polymerization reactions via 3D topological pathways, forming insoluble CEI materials. Thus, the side reactions between the cathode and the electrolyte, transition metal dissolution/crosstalk effects, as well as stress corrosion cracking and impedance increase in the active material are suppressed, as shown in Fig. 18h. Owing to the stable CEI, the NCM811/graphite pouch cell maintains a capacity retention rate exceeding 85% after approximately 300 cycles at 60 °C. The higher the unsaturation level of unsaturated units in the electrolyte additives, the lower the gas evolution in the pouch cell after prolonged cycling as shown in Fig. 18i. | ||
| Fig. 18 (a) Schematic diagram of the action mechanism of TMB on the NCM90 cathode surface. (b) and (c) Electrochemical performance of NCM90/Li cells with/without TMB.319 Copyright 2024, Elsevier. Cycling performance of the NCM811/Li cells using HC-E and baseline electrolytes at (d) 45 °C and (e) −30 °C, and (f) first discharge specific capacity at different temperatures.324 Copyright 2023, American Chemical Society. (g) Molecular structures of TMSL, AMSL, and PMSL (with increasing unsaturation) and their proposed function in forming a passivation layer. (h) Origin of multifunctionality illustrating the stabilization of both electrode interphase and bulk electrolyte via possible electrochemical polymerization pathways. (i) Enhanced cycling stability of commercial NCM811/graphite pouch cells in base and additive-containing electrolytes at elevated temperatures.325 Copyright 2022, John Wiley and Sons. | ||
With the shift in electrolyte design from relying on single functional components to developing synergistic electrolyte systems, locally high-concentration electrolytes (LHCEs) have emerged. LHCEs successfully addresses the bottlenecks of traditional high-concentration electrolytes (high viscosity, poor wetting, and high cost) by introducing inert diluents. This approach preserves the inherent advantages of high-concentration lithium salts, such as a rich inorganic interface and suppressed solvent side reactions, while effectively enhancing the interface stability of Ni-rich cathodes. For example, Jia et al.326 designed a non-flammable LHCE based on a mixed solvent of tetramethylene sulfone and trimethyl phosphate, combined with the 1H, 1H, 5H-octafluoropentyl 1,1,2,2-tetrafluoroethyl ether high flash point diluent (SEO-M1). Mechanistic studies indicate that compared to the baseline electrolyte (E-Baseline), the LHCE effectively mitigates irreversible phase transformations on the surface of Ni-rich cathodes. As shown in Fig. 19a and b, after cycling in the baseline electrolyte, NMC811 exhibits two thicker degradation phases: the rock salt phase and the cation mixed phase. In contrast, NMC811 cycled in SEO-M1 formed only a thin cation-mixed layer. Furthermore, NMC811 cycled in SEO-M1 exhibits a more inorganic-rich CEI with less organic carbonate and more LiF, confirming that this LHCE suppresses surface side reactions on NCM811 (Fig. 19c). Consequently, the optimal LHCE significantly enhances the cycling stability of graphite/NCM811 batteries after 500 cycles compared to the baseline electrolyte (Fig. 19d). Li et al.327 employed multifunctional flame-retardant diluent ethoxy (pentafluoro) cyclopropanecarbonitrile (PFPN) to regulate a locally high-concentration electrolyte (LHCE-PFPN) based on triethyl phosphate (TEP). This approach achieved outstanding flame retardancy while maintaining excellent compatibility with high-voltage cathodes and lithium metal anodes. PFPN preferentially reduces on the lithium metal surface to form LiF and PFPN derivatives. Subsequently, PFPN derivatives migrate to the NCM811 surface, undergoing ring-opening polymerization or oxidative decomposition to generate inorganic compounds, ultimately concurrently constructing an inorganic-rich SEI and CEI as shown in Fig. 19e. This stable interfacial structure maintains lithium metal anode stability while suppressing electrolyte decomposition on the NCM811 surface and reducing TM dissolution (Fig. 19f). Consequently, it significantly enhances the cycling performance of Li/NCM811 batteries under harsh high-temperature and high-voltage conditions, as demonstrated in Fig. 19g and h.
 | ||
| Fig. 19 (a) HAADF and (b) bright field STEM images of pristine NCM811, and after cycling with E-baseline and SEO-M1 at 45 °C, showing suppressed cation mixing and microcracking. (c) XPS spectra of C 1s, O 1s, and F 1s for graphite anodes cycled with E-Baseline and SEO-M1, indicating a superior CEI composition. (d) Average specific discharge capacity and coulombic efficiency of Gr/NMC811 full cells with different electrolytes at 25 °C and C/3 rate.326 Copyright 2025, American Chemical Society. (e) Reaction mechanism of PFPN in forming a robust SEI/CEI. (f) Schematic diagram comparing the solvation structures, interphase formation, and Li deposition behaviors in LHCE-PFPN and LHCE-1,1,2,2-tetrafluoroethyl-2,2,3,3-tetrafluoropropylether (TTE). (g) and (h) Cycle performance of Li/NCM811 cells demonstrating the superiority of the LHCE-PFPN electrolyte at 60 °C (g) and under a high cut-off voltage of 4.5 V (h).327 Copyright 2024, Elsevier. | ||
4.4. Structural engineering
Structural engineering plays a key role in improving the structural stability of Ni-rich layered cathode materials.56,92,328–330 By precisely controlling the microstructure, structural damage and gas emission during charging and discharging can be effectively controlled.331 Common structural engineering approaches include single-crystallization and concentration gradient design.275,332–334The secondary particles of polycrystalline Ni-rich cathode materials are aggregated from primary particles at the nanometer scale, with grain boundaries between the primary particles. Due to lithiation/delithiation during the charge/discharge process, the crystals continuously undergo anisotropic contraction/expansion, resulting in a high volume change rate and stress accumulation, which ultimately leads to contact failure between the primary particles and consequently rupture of the secondary particles. The electrolyte penetrates the material along the cracks eventually.70,335 In contrast, single-crystal Ni-rich cathode particles do not have grain boundaries and thus can inhibit the penetration of the electrolyte. Moreover, single-crystal particles can eliminate primary intergranular stresses and effectively reduce the drastic changes in lattice parameters during the cycling process, thus reducing intergranular cracks and particle cracking and comminution caused by irreversible phase transitions (Fig. 20a).336 The single-crystal structure also has a high bulk density and is non-porous to withstand greater pressure.337 Nam et al.338 synthesized single-crystal Ni-rich (DSNCM) cathodes using a pellet-assisted mechanical densification process. As shown in Fig. 20b and c, after 150 cycles at high voltage, the single-crystal material can still maintain 85% capacity retention which is higher than that of the poly-crystal sample (PNCM). Moreover, the formation of intergranular cracks is inhibited, which is helpful to improve the mechanical stability of single-crystal materials. Liu et al.339 synthesized Ni-rich single-crystal materials using a binary molten salt method. Compared to the poly-crystal sample, the single-crystal sample shows a lower degree of cation mixing and larger lithium layer spacing, and the RLC is effectively removed from the surface of single-crystal particles.
 | ||
| Fig. 20 (a) Schematic diagram of structural changes during the cycle of single-crystal and poly-crystal Ni-rich cathodes.336 Copyright 2020, Elsevier. (b) Comparison of electrochemical properties of DSNCN and PNCM. (c) SEM of DSNCM and PNCM after cycling.338 Copyright 2023, Elsevier. (d) Rate capabilities and (e) chemical diffusion coefficients of lithium ions in P-NCM90 and three S-NCM cathodes. (f) TEM image of the S-NCM90 cathode particle charged to 4.3 V at 0.5 C and part of electron diffraction patterns from the regions marked (i) and (ii). The yellow arrows are parallel to the lithium-ion diffusion path in the particle. The dot-line diagram displays the c-axis lattice parameters at locations along the dashed yellow line. Overlays and deconvolution of the (003) reflection peaks recorded by in situ XRD in the voltage range of 4.15–4.5 V: (g) and (k) P-NCM90 at 0.025 C and (i) and (m) P-NCM90 at 0.5 C, (h) and (l) S-NCM90 at 0.025 C and (j) and (n) S-NCM90 at 0.5 C, (o) S-NCM70 at 0.5 C, and (p) S-NCM80 at 0.5 C.341 Copyright 2021, American Chemical Society. | ||
However, the structural characteristics of single-crystal Ni-rich cathodes also pose some problems, leading to controversial results in current single-crystal designs. The large size (∼µm) of the single-crystal particles leads to a long Li+ diffusion path and there is a lack of fast diffusion paths along the boundaries of the single-crystal particles, which results in slower Li+ diffusion kinetics and reduced rate performance.340 This will also lead to an inhomogeneous Li+ distribution, generating inhomogeneous structural stresses and inducing the formation of intragranular cracks. For example, Ryu et al.341 systematically investigated the performance degradation and fading mechanisms of single-crystal NCM cathodes (S-NCM70, S-NCM80, S-NCM90) and poly-crystal NCM cathodes (P-NCM70, P-NCM80, P-NCM90) with the same particle size and Ni content of 70, 80, and 90%, respectively. They found that adopting a single-crystal cathode design exacerbated capacity fade and markedly impaired rate capability (Fig. 20d), while the Li diffusion coefficients did not differ significantly (Fig. 20e), suggesting that the decrease in rate performance is mainly due to the difference in the morphology of the single-crystal and poly-crystal cathodes. The lithium ions in single-crystal NCM cathode particles migrate by overall diffusion mainly through a two-dimensional path along the layer planes, whereas the poly-crystal NCM cathode has a three-dimensional fast diffusion path through the grain boundary network. In addition, they found that the capacity decay mechanism of the single-crystal NCM cathode is different from that of the poly-crystal NCM cathode. In situ XRD tests show that the single-crystal cathode experiences more severe structural inhomogeneity at a high rate due to long diffusion paths, with the presence of two-phase coexistence, and this phenomenon is exacerbated with increasing Ni content (Fig. 20g–p). It is further found that different regions of the charged S-NCM90 cathode particles have different lattice parameters, and the inhomogeneous structure induces inhomogeneous stress (Fig. 20f). This stress will lead to planar gliding and particle rupture of the cathode during the cycling process, which ultimately leads to capacity degradation. Therefore, the modification of the Ni-rich cathode by single-crystallization still needs to be assisted by other modification means to realize the comprehensive improvement of battery performance.
Another structural engineering strategy is core shell structure and concentration gradient design.334 On the surface of Ni-rich materials, high activity and high valence Ni3+/Ni4+ is easy to react with the electrolyte, resulting in the degradation of the surface structure of the material. Therefore, the design of a high to low concentration gradient of Ni ions from the core to the particle surface can effectively enhance the interface stability. The design principle is shown in Fig. 21a.342 There are three generations of models (Fig. 21b–d). The first-generation concentration gradient design model uses NCM811 as the core, and the external shell is an NCM material with low Ni content and concentration gradient (Fig. 21b).343 The second-generation model is a full concentration gradient Ni-rich cathode material with LiNi0.86Co0.10Mn0.04O2 inside and LiNi0.70Co0.10Mn0.20O2 outside (Fig. 21c).344 This model solves the defect that the common core–shell structure is too thin to maintain stability at high temperature. The concentration gradient of Ni, Co and Mn is extended over the entire particle length. The third-generation model is a two-sloped full concentration gradient (Fig. 21d)345 LiNi0.84Co0.06Mn0.09Al0.01O2 material with a double-oblique concentration gradient slope presenting different element content change rates from the inside to the outside, and the internal change is relatively gentle, while the external change is relatively fast. Recently, Kim et al.346 coated Co(OH)2 on the surface of Ni0.91Co0.06Mn0.03(OH)2 (NCM91), and obtained a cathode material with rich Ni inside and poor Ni outside after sintering with lithium (Co-NCM91). In full cell tests, Co-NCM91 achieved a capacity retention rate of up to 85% after 300 cycles. Li et al.347 synthesized a NCM811 (CS) cathode material with a spinel shell by co-precipitation. The spinel shell has a three-dimensional Li+ transport channel, which can enhance electrochemical performance. The CS-003 sample with 3% spinel Li–Mn–O as the shell is charged and discharged at a high rate of 10 C, and the capacity retention is 81.6% after 200 cycles, which is much higher than the capacity retention of NCM811 which is 73.1%.
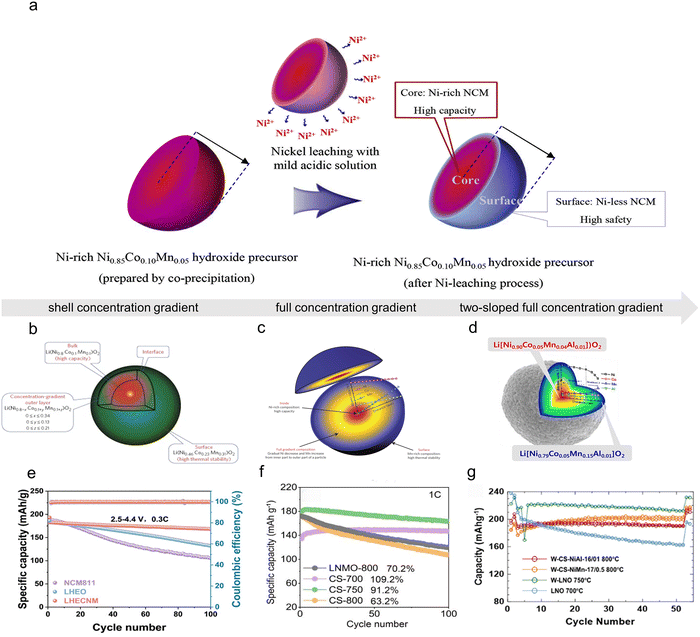 | ||
| Fig. 21 (a) Design model of the Ni-rich cathode concentration gradient structure.342 Copyright 2018, Elsevier. (b) First generation concentration gradient design.343 Copyright 2009, Springer Nature. (c) Second generation concentration gradient design.344 Copyright 2012, Springer Nature. (d) Third generation concentration gradient design.345 Copyright 2016, American Chemical Society. (e) Comparison of electrochemical properties of NCM811, LHEO and LHECNM.348 Copyright 2025, John Wiley and Sons. (f) Comparison of electrochemical properties of LNMO-800, CS-700, CS-750 and CS-800.349 Copyright 2023, Elsevier. (g) Comparison of cycling performance of W-containing core–shell materials with pristine and W-containing LiNiO2.350 Copyright 2022, American Chemical Society. | ||
Zhao et al.348 constructed a high-entropy gradient core–shell architecture (LHECNM) consisting of a Ni-rich, Co-free LiNi0.89Mn0.11O2 core enveloped by a LiNi1/6Mn1/6Al1/6Ti1/6Mo1/6Ta1/6O2 shell. The six-metal high-entropy shell exploits the cocktail effect of multiple cations to create a robust, concentration-gradient interface that passivates the surface against electrolyte attack and arrests TM migration. Compared with NCM811 and high entropy-doped Ni-rich cobalt-free LiNi0.8Mn0.12Al0.02Ti0.02Mo0.02Ta0.02O2 cathode material (LHEO), this dual modification yields a discharge capacity of 201.6 mAh g−1 at 0.3 C, retains ≥92% of its capacity after long-term cycling, and sustains stable high-rate performance up to 5 C, demonstrating the potency of combining compositional and gradient engineering (Fig. 21e). Jing et al.349 adopted a different but equally instructive route, first synthesizing a core–shell hydroxide precursor Ni(OH)2@Mn(OH)2 with a Ni-rich core and a Mn-rich shell. After lithiation at 700–800 °C, the Mn surface layer gradually diffused inward, producing a graded Li1.08Ni0.9Mn0.1O2 cathode whose gradient sharpness could be tuned by calcination temperature. At 700 °C (CS-700) and 750 °C (CS-750), a distinct Mn-rich outer layer persists, effectively buffering the high-Ni core from electrolyte contact; at 800 °C (CS-800), complete Mn/Ni homogenization erodes this protective gradient and accelerates degradation. Electrochemical data corroborate this trend: CS-750 delivers 187 mAh g−1 at 0.1 C, and retains 91% after 100 cycles at 1 C and 79% after 500 cycles, whereas CS-800 suffers severe capacity fade linked to irreversible phase transitions near 4.2 V (Fig. 21f). The dQ/dV profiles further reveal that gradient-preserved samples (CS-700 and CS-750) maintain sharp, stable redox peaks, underscoring the critical role of a persistent Mn-rich gradient in suppressing structural degradation under high-voltage cycling.
In order to prevent elements such as Al, Mg, or Mn located in the shell from diffusing into the core during the heat treatment process of the core–shell structure, Rathore et al.350 used dry-coating of W to arrest inter-diffusion and stabilize thin shells. It was found that merely 1 wt% W, dry-coated onto hydroxide precursors, forms immobile WOx clusters that physically block Mn (or Al) migration across core–shell boundaries during 800 °C calcination. In W-CS-NiMn-17/0.5 sample, this “pinning” effect preserves the intended Mn-rich outer shell; after 100 cycles, capacity retention is ∼80% higher than in the W-free analogue (Fig. 21g). The result demonstrates that sub-monolayer pinning agents can substitute for thicker, heavier coatings while still enforcing compositional gradients.
5. Summary and outlook
Degradation of the Ni-rich cathode structure (including the surface, interface and bulk phase) is mainly categorized into five distinct categories. (1) The formation of RLCs. This is due to the excessive lithium precursor introduced during synthesis or surface remodeling during cooling and storage stages. (2) Interfacial reactions. During cycling, the TM ion is constantly dissolved, and TM fluoride fragments form irreversibly. Surface RLCs also undergo side reactions with the electrolyte, producing organic and inorganic by-products that ultimately accelerate the growth of a CEI layer on Ni-rich particle surfaces. (3) Irreversible phase transition. The causes of irreversible phase transition on the surface of Ni-rich cathodes are complicated, which are related to cation mixing, oxygen release, charge/discharge rate, and SOC. (4) Microcrack generation. This is mainly caused by the anisotropic expansion/contraction of the Ni-rich cathode due to the irreversible phase transition, and is closely related to the SOC, pores and oxygen vacancies. (5) Cation mixing. The reasons for cation mixing include the similarity of Li/Ni ion radius, the low migration barrier of Ni, the effect of magnetic interaction and the poor thermal stability of Ni-rich cathodes.The gas release of Ni-rich cathodes is mainly due to the decomposition of RLCs, degradation of the electrolyte on the surface, and the release of lattice oxygen under high voltage. As the voltage continues to rise, the surface RLCs formed during the preparation and storage process and the organic electrolyte will gradually decompose on the Ni-rich cathode surface to produce gas (e.g., O2, CO and CO2). In addition, at high SOC, the oxygen evolution potential is reduced, and the lattice oxygen is unstable and would eventually release in the form of O2 gas. The above problems could be solved by different modification methods, including surface modification, element doping, electrolyte design, structural engineering, etc.
To further improve the energy density and stability of Ni-rich layered oxides and promote their large-scale application, future work should focus on the following aspects. Firstly, the selection of dopants and coatings should be assisted by further theoretical research. Secondly, it is necessary to achieve the controllable preparation of high-performance Ni-rich layered oxides with high lattice oxygen stability and high structural stability through structural engineering. Finally, it is important to implement composite strategies (particle engineering and surface/bulk stabilization techniques) to achieve synergies. Through continuous ingenious design and mechanism research, Ni-rich cathodes will have a wider application market in the future.
Conflicts of interest
There are no conflicts to declare.Data availability
Data availability is not applicable to this article as no new data were created or analyzed in this study.Acknowledgements
The authors acknowledge financial support from the National Natural Science Foundation of China (22408239) and the Fundamental Research Funds for the Central Universities. S. Jiang was supported by the Postdoctoral Innovation Talent Support Program (BX20240038).References
- S. Chu, Y. Cui and N. Liu, Nat. Mater., 2016, 16, 16–22 CrossRef PubMed.
- C. Y. Wang, T. Liu, X. G. Yang, S. Ge, N. V. Stanley, E. S. Rountree, Y. Leng and B. D. McCarthy, Nature, 2022, 611, 485–490 CrossRef CAS PubMed.
- V. Viswanathan, A. H. Epstein, Y. M. Chiang, E. Takeuchi, M. Bradley, J. Langford and M. Winter, Nature, 2022, 601, 519–525 CrossRef CAS PubMed.
- G. Luderer, S. Madeddu, L. Merfort, F. Ueckerdt, M. Pehl, R. Pietzcker, M. Rottoli, F. Schreyer, N. Bauer, L. Baumstark, C. Bertram, A. Dirnaichner, F. Humpenöder, A. Levesque, A. Popp, R. Rodrigues, J. Strefler and E. Kriegler, Nat. Energy, 2021, 7, 32–42 CrossRef.
- S. Pan, W. Fang, J. Yan, S. Zhang and H. Zhang, Energy Environ. Sci., 2025, 18, 5868–5896 RSC.
- T. Jiang, D. Shen, Z. Zhang, H. Liu, G. Zhao, Y. Wang, S. Tan, R. Luo and W. Chen, Nat. Rev. Clean Technol., 2025, 1, 474–492 CrossRef.
- A. Yoshino, Angew. Chem., Int. Ed., 2012, 51, 5798–5800 CrossRef CAS PubMed.
- M. Armand and J.-M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS.
- Global EV Outlook 2019, International Energy Agency, Paris, 2019.
- A. Kwade, W. Haselrieder, R. Leithoff, A. Modlinger, F. Dietrich and K. Droeder, Nat. Energy, 2018, 3, 290–300 CrossRef.
- Y. Dong and J. Li, Chem. Rev., 2023, 123, 811–833 CrossRef CAS PubMed.
- M. M. Thackeray, W. I. F. David, P. G. Bruce and J. B. Goodenough, Mater. Res. Bull., 1983, 18, 461–472 CrossRef CAS.
- W. Ou, S. D. Marks, R. F. de Menezes, R. He, Z. Zhang, C. Sindt, J. Thurston, C. Jaye, B. Cowie, L. Thomsen, Z. Zhuo, J. Guo, W. Yang, Z. Dong, R. Tenent, K. G. Sprenger and M. F. Toney, Adv. Energy Mater., 2025, 15, 2404652 CrossRef CAS.
- A. K. Padhi, K. S. Nanjundaswamy and J. B. Goodenough, J. Electrochem. Soc., 1997, 144, 1188–1194 CrossRef CAS.
- H. Liu, F. C. Strobridge, O. J. Borkiewicz, K. M. Wiaderek, K. W. Chapman, P. J. Chupas and C. P. Grey, Science, 2014, 344, 1252817 CrossRef PubMed.
- K. Mizushima, P. C. Jones, P. J. Wiseman and J. B. Goodenough, Mater. Res. Bull., 1980, 15, 783–789 CrossRef CAS.
- C. Lin, J. Li, Z.-W. Yin, W. Huang, Q. Zhao, Q. Weng, Q. Liu, J. Sun, G. Chen and F. Pan, Adv. Mater., 2024, 36, 2307404 CrossRef CAS.
- T. Ohzuku and Y. Makimura, Chem. Lett., 2001, 30, 642–643 CrossRef.
- S. W. Oh, S. H. Park, C.-W. Park and Y.-K. Sun, Solid State Ionics, 2004, 171, 167–172 CrossRef CAS.
- P. Y. Liao, J. G. Duh and S. R. Sheen, J. Power Sources, 2005, 143, 212–218 CrossRef CAS.
- M. Zheng, X. Zhu, H. Zheng, Z. Bo and J. Lu, Nat. Energy, 2025, 10, 789–792 CrossRef.
- Y. S. Meng, V. Srinivasan and K. Xu, Science, 2022, 378, eabq3750 CrossRef CAS PubMed.
- M. Li, J. Lu, Z. Chen and K. Amine, Adv. Mater., 2018, 30, 1800561 CrossRef.
- J. U. Choi, N. Voronina, Y.-K. Sun and S.-T. Myung, Adv. Energy Mater., 2020, 10, 2002027 CrossRef CAS.
- Z. Wu, C. Zhang, F. Yuan, M. Lyu, P. Yang, L. Zhang, M. Zhou, L. Wang, S. Zhang and L. Wang, Nano Energy, 2024, 126, 109620 CrossRef CAS.
- A. Manthiram, ACS Cent. Sci., 2017, 3, 1063–1069 CrossRef CAS PubMed.
- G.-T. Park, N.-Y. Park, H.-H. Ryu, H. H. Sun, J.-Y. Hwang and Y.-K. Sun, Chem. Soc. Rev., 2024, 53, 11462–11518 RSC.
- F. Liu, S. Li, C. Leung, X. Jiang, H. Liu, T. Li, Q. Liu, G. Sun, Z. Wang, Z. Zhang, Y. Lai, Y. Ren and J. Yang, Adv. Mater., 2025, 37, 2419856 CrossRef CAS.
- W. Li, E. M. Erickson and A. Manthiram, Nat. Energy, 2020, 5, 26–34 CrossRef CAS.
- M. Sun, Y. Xie, C. Zhong, Y. Huang, H. Chen, H. Huang, P. Dai, S. Liu, W. Zheng, C. Liu, S. Liao, L. Huang, S. Sun and X. Wang, Energy Storage Mater., 2024, 65, 103166 CrossRef.
- W. Xue, M. Huang, Y. Li, Y. G. Zhu, R. Gao, X. Xiao, W. Zhang, S. Li, G. Xu, Y. Yu, P. Li, J. Lopez, D. Yu, Y. Dong, W. Fan, Z. Shi, R. Xiong, C.-J. Sun, I. Hwang, W.-K. Lee, Y. Shao-Horn, J. A. Johnson and J. Li, Nat. Energy, 2021, 6, 495–505 CrossRef CAS.
- L. Li, L. Fu, M. Li, C. Wang, Z. Zhao, S. Xie, H. Lin, X. Wu, H. Liu, L. Zhang, Q. Zhang and L. Tan, J. Energy Chem., 2022, 71, 588–594 CrossRef CAS.
- R. Zhang, C. Wang, P. Zou, R. Lin, L. Ma, T. Li, I.-H. Hwang, W. Xu, C. Sun, S. Trask and H. L. Xin, Nat. Energy, 2023, 8, 695–702 CrossRef CAS.
- H. Zhao, W.-Y. A. Lam, L. Sheng, L. Wang, P. Bai, Y. Yang, D. Ren, H. Xu and X. He, Adv. Energy Mater., 2022, 12, 2103894 CrossRef CAS.
- Y. Liu, Y. Xin, B. He, F. Zhang, C. Wang and H. Tian, Adv. Mater., 2025, 37, 2417353 CrossRef CAS PubMed.
- L. Liang, M. Su, Z. Sun, L. Wang, L. Hou, H. Liu, Q. Zhang and C. Yuan, Sci. Adv., 2024, 10, eado4472 CrossRef CAS.
- A. Aishova, G.-T. Park, C. S. Yoon and Y.-K. Sun, Adv. Energy Mater., 2020, 10, 1903179 CrossRef CAS.
- X. Wang, Y.-L. Ding, Y.-P. Deng and Z. Chen, Adv. Energy Mater., 2020, 10, 1903864 CrossRef CAS.
- L. Yu, A. Dai, T. Zhou, W. Huang, J. Wang, T. Li, X. He, L. Ma, X. Xiao, M. Ge, R. Amine, S. N. Ehrlich, X. Ou, J. Wen, T. Liu and K. Amine, Nat. Commun., 2025, 16, 434 CrossRef CAS PubMed.
- W. E. Gent, G. M. Busse and K. Z. House, Nat. Energy, 2022, 7, 1132–1143 CrossRef CAS.
- I. Martens, N. Vostrov, M. Mirolo, S. J. Leake, E. Zatterin, X. Zhu, L. Wang, J. Drnec, M.-I. Richard and T. U. Schulli, Nat. Commun., 2023, 14, 6975 CrossRef CAS PubMed.
- Y. Zhang, Z. Chen, X. Shi, C. Meng, P. Das, S. Zheng, F. Pan and Z.-S. Wu, Adv. Energy Mater., 2023, 13, 2203045 CrossRef CAS.
- Y.-K. Sun, ACS Energy Lett., 2022, 7, 1774–1775 CrossRef CAS.
- H. Yang, L. Wang, Y. Li, Z. Zhuo, T. Wu, J. Liu, L. Xu, H. Du, S. Liu, L. Wu, S. Zhao, M. Tang, W. Yang and H. Yu, Proc. Natl. Acad. Sci. U. S. A., 2024, 121, e2412460121 CrossRef CAS.
- S. Li, G. Qian, X. He, X. Huang, S.-J. Lee, Z. Jiang, Y. Yang, W.-N. Wang, D. Meng, C. Yu, J.-S. Lee, Y. S. Chu, Z.-F. Ma, P. Pianetta, J. Qiu, L. Li, K. Zhao and Y. Liu, Nat. Commun., 2022, 13, 704 CrossRef CAS PubMed.
- U.-H. Kim, G.-T. Park, B.-K. Son, G. W. Nam, J. Liu, L.-Y. Kuo, P. Kaghazchi, C. S. Yoon and Y.-K. Sun, Nat. Energy, 2020, 5, 860–869 CrossRef CAS.
- B. Cui, Z. Xiao, S. Cui, S. Liu, X. Gao and G. Li, Electrochem. Energy Rev., 2024, 7, 27 CrossRef CAS.
- L. Yu, J. Wang, T. Zhou, W. Huang, T. Li, L. Ma, X. Xiao, S.-B. Son, S. N. Ehrlich, J. Wen, K. Amine and T. Liu, Nat. Commun., 2025, 16, 6519 CrossRef CAS.
- L. Zou, Y. He, Z. Liu, H. Jia, J. Zhu, J. Zheng, G. Wang, X. Li, J. Xiao, J. Liu, J.-G. Zhang, G. Chen and C. Wang, Nat. Commun., 2020, 11, 3024 CrossRef.
- W. Jiang, X. Wang, X. Yang, Y. Zhao, J. Yao, X. Yang, W. Luo, L. Luo, J. Duan, P. Dong, Y. Zhang, B. Li and D. Wang, Energy Environ. Sci., 2025, 18, 6154–6167 RSC.
- J. Dutta, S. Ghosh and S. K. Martha, ACS Appl. Mater. Interfaces, 2024, 16, 19720–19729 CrossRef CAS.
- J. Yang, P. Yang, L. Zheng, H. Wang and H. Liu, ACS Appl. Energy Mater., 2025, 8, 7929–7938 CrossRef CAS.
- J. Li, H. Liu, J. Xia, A. R. Cameron, M. Nie, G. A. Botton and J. R. Dahn, J. Electrochem. Soc., 2017, 164, A655–A665 CrossRef CAS.
- S. Lee, L. Su, A. Mesnier, Z. Cui and A. Manthiram, Joule, 2023, 7, 2430–2444 CrossRef CAS.
- W. Yuan, W. Peng, C. Wu, N. Liu, C. Shen, Z. Xiao, J. Liu, C. Li, Y. Guo, Q. Huang, P. Zhang, H. Pan, L. Wen, L. Shi, L. Lu, D. Ren, K. Wu, M. Ouyang and X. Liu, Adv. Energy Mater., 2025, 15, 2405907 CrossRef CAS.
- H.-H. Ryu, H.-W. Lim, S. G. Lee and Y.-K. Sun, Nat. Energy, 2024, 9, 47–56 CrossRef CAS.
- H. Liu, X. Liu, Z. Wang, L. Zhu and X. Zhang, ACS Appl. Mater. Interfaces, 2024, 16, 943–956 CrossRef CAS.
- D. Kong, M. Zhang, Y. Xiao, J. Hu, W. Zhao, L. Han and F. Pan, Nano Energy, 2019, 59, 327–335 CrossRef CAS.
- L. Zou, Z. Liu, W. Zhao, H. Jia, J. Zheng, Y. Yang, G. Wang, J.-G. Zhang and C. Wang, Chem. Mater., 2018, 30, 7016–7026 CrossRef CAS.
- F. Zhang, S. Lou, S. Li, Z. Yu, Q. Liu, A. Dai, C. Cao, M. F. Toney, M. Ge, X. Xiao, W.-K. Lee, Y. Yao, J. Deng, T. Liu, Y. Tang, G. Yin, J. Lu, D. Su and J. Wang, Nat. Commun., 2020, 11, 3050 CrossRef CAS PubMed.
- X.-H. Meng, D. Xiao, Z.-Y. Zhou, W.-Z. Liu, J.-L. Shi, L.-J. Wan and Y.-G. Guo, J. Am. Chem. Soc., 2024, 146, 14889–14897 CrossRef CAS.
- M. Jiang, D. L. Danilov, R.-A. Eichel and P. H. L. Notten, Adv. Energy Mater., 2021, 11, 2103005 CrossRef CAS.
- P. Yan, J. Zheng, T. Chen, L. Luo, Y. Jiang, K. Wang, M. Sui, J.-G. Zhang, S. Zhang and C. Wang, Nat. Commun., 2018, 9, 2437 CrossRef.
- T. Liu, L. Yu, J. Lu, T. Zhou, X. Huang, Z. Cai, A. Dai, J. Gim, Y. Ren, X. Xiao, M. V. Holt, Y. S. Chu, I. Arslan, J. Wen and K. Amine, Nat. Commun., 2021, 12, 6024 CrossRef CAS PubMed.
- Y. Bi, J. Tao, Y. Wu, L. Li, Y. Xu, E. Hu, B. Wu, J. Hu, C. Wang, J.-G. Zhang, Y. Qi and J. Xiao, Science, 2020, 370, 1313–1317 CrossRef CAS PubMed.
- S. Yin, W. Deng, J. Chen, X. Gao, G. Zou, H. Hou and X. Ji, Nano Energy, 2021, 83, 105854 CrossRef CAS.
- C. Delmas, J. P. Pérès, A. Rougier, A. Demourgues, F. Weill, A. Chadwick, M. Broussely, F. Perton, P. Biensan and P. Willmann, J. Power Sources, 1997, 68, 120–125 CrossRef CAS.
- F. Wu, J. Tian, N. Liu, Y. Lu, Y. Su, J. Wang, R. Chen, X. Ma, L. Bao and S. Chen, Energy Storage Mater., 2017, 8, 134–140 CrossRef.
- Y. Kim, D. Kim and S. Kang, Chem. Mater., 2011, 23, 5388–5397 CrossRef CAS.
- W. Li, H. Y. Asl, Q. Xie and A. Manthiram, J. Am. Chem. Soc., 2019, 141, 5097–5101 CrossRef CAS PubMed.
- P. Liu, L. Yang, B. Xiao, H. Wang, L. Li, S. Ye, Y. Li, X. Ren, X. Ouyang, J. Hu, F. Pan, Q. Zhang and J. Liu, Adv. Funct. Mater., 2022, 32, 2208586 CrossRef CAS.
- K. Min, C. Jung, D.-S. Ko, K. Kim, J. Jang, K. Park and E. Cho, ACS Appl. Mater. Interfaces, 2018, 10, 20599–20610 CrossRef CAS PubMed.
- M. Yang, S. Zhao, P. Guo, M. Cui, H. Li, M. Wang, J. Wang, F. Wu and G. Tan, Energy Storage Mater., 2025, 78, 104272 CrossRef.
- C. Liu, Q. Luo, L. Li, C. Wei, S. Li, X. Li, W. Li, Z. Zhang, Z. Wu, Z. Jiang, H. Yang, L. Zhang, L. Lv, X. Chen, S. Cheng and C. Yu, Chem. Eng. J., 2024, 500, 156866 CrossRef CAS.
- J. H. Choi, T. J. Embleton, K. Ko, H. Jang, Y. Son, J. Park, S. Lee and P. Oh, ChemElectroChem, 2024, 11, e202300705 CrossRef CAS.
- Q. Gan, N. Qin, Z. Wang, Z. Li, Y. Zhu, Y. Li, S. Gu, H. Yuan, W. Luo, L. Lu, Z. Xu and Z. Lu, ACS Appl. Energy Mater., 2020, 3, 7445–7455 CrossRef CAS.
- G. Ko, S. Jeong, S. Park, J. Lee, S. Kim, Y. Shin, W. Kim and K. Kwon, Energy Storage Mater., 2023, 60, 102840 CrossRef.
- H. Yu, Y. Cao, L. Chen, Y. Hu, X. Duan, S. Dai, C. Li and H. Jiang, Nat. Commun., 2021, 12, 4564 CrossRef CAS.
- J. P. Singh, H. Devnani, A. Sharma, W. C. Lim, A. Dhyani, K. H. Chae and S. Lee, Energy Adv., 2024, 3, 1869–1893 RSC.
- S.-B. Lee, N.-Y. Park, G.-T. Park, U.-H. Kim, S.-J. Sohn, M.-S. Kang, R. M. Ribas, R. S. Monteiro and Y.-K. Sun, ACS Energy Lett., 2024, 9, 740–747 CrossRef CAS.
- H. H. Sun, U.-H. Kim, J.-H. Park, S.-W. Park, D.-H. Seo, A. Heller, C. B. Mullins, C. S. Yoon and Y.-K. Sun, Nat. Commun., 2021, 12, 6552 CrossRef CAS PubMed.
- B. Wang, K. Li, G. Xu, Z. Zhang, X. Wang, J. Sun, Y. Song, X. Zhang, Y. Liang, D. Kong, Y. Qiu, Q. Teng, X. Cui, J. Chen, J. Zhao, J. Wang, H. Yang, J. Huang and Y. Tang, Angew. Chem., Int. Ed., 2025, 64, e202502725 CrossRef CAS PubMed.
- F. Fan, R. Zheng, C. Zeng, H. Xu, X. Wang, G. Tian, S. Wang, C. Wang, P. Liu and C. Shu, J. Energy Chem., 2025, 105, 24–34 CrossRef CAS.
- J.-K. Liu, X.-R. Yang, C.-W. Wang, Z.-W. Yin, Y.-Y. Hu, L. Deng, Z. Wang, Y. Zhou and J.-T. Li, J. Energy Chem., 2024, 98, 67–76 CrossRef CAS.
- N.-Y. Park, G. Cho, S.-B. Kim and Y.-K. Sun, Adv. Energy Mater., 2023, 13, 2204291 CrossRef CAS.
- C. Li, J. Liu, Y. Su, J. Dong, H. Zhang, M. Wang, Y. Guan, K. Yan, N. Liu, Y. Lu, N. Li, Y. Su, F. Wu and L. Chen, Energy Storage Mater., 2025, 74, 103893 CrossRef.
- S. Chen, R. Yang, G. Wu, Z. Zheng, W. Wang, S. Wang and Y. Gao, Energy Storage Mater., 2025, 78, 104276 CrossRef.
- Y. Zhang, Y. Wu, H. Li, J. Chen, D. Lei and C. Wang, Nat. Commun., 2022, 13, 1297 CrossRef CAS.
- T. Dong, S. Zhang, Z. Ren, L. Huang, G. Xu, T. Liu, S. Wang and G. Cui, Adv. Sci., 2024, 11, 2305753 CrossRef CAS.
- B. Wang, K. Li, G. Xu, Z. Zhang, X. Wang, J. Sun, Y. Song, X. Zhang, Y. Liang, D. Kong, Y. Qiu, Q. Teng, X. Cui, J. Chen, J. Zhao, J. Wang, H. Yang, J. Huang and Y. Tang, Angew. Chem., Int. Ed., 2025, 64, e202502725 CrossRef CAS PubMed.
- F. Cheng, X. Zhang, Y. Qiu, J. Zhang, Y. Liu, P. Wei, M. Ou, S. Sun, Y. Xu, Q. Li, C. Fang, J. Han and Y. Huang, Nano Energy, 2021, 88, 106301 CrossRef CAS.
- Z. Li, Y. Wang, J. Wang, C. Wu, W. Wang, Y. Chen, C. Hu, K. Mo, T. Gao, Y.-S. He, Z. Ren, Y. Zhang, X. Liu, N. Liu, L. Chen, K. Wu, C. Shen, Z.-F. Ma and L. Li, Nat. Commun., 2024, 15, 10216 CrossRef CAS PubMed.
- S. H. Song, S. Hong, M. Cho, J.-G. Yoo, H. Min Jin, S.-H. Lee, M. Avdeev, K. Ikeda, J. Kim, S. C. Nam, S.-H. Yu, I. Park and H. Kim, Chem. Eng. J., 2022, 448, 137685 CrossRef CAS.
- X. Xiao, L. Wang, J. Li, B. Zhang, Q. Hu, J. Liu, Y. Wu, J. Gao, Y. Chen, S. Song, X. Zhang, Z. Chen and X. He, Nano Energy, 2023, 113, 108528 CrossRef CAS.
- X. Li, Q. Gu, B. Qiu, C. Yin, Z. Wei, W. Wen, Y. Zhang, Y. Zhou, H. Gao, H. Liang, Z. He, M. Zhang, Y. S. Meng and Z. Liu, Mater. Today, 2022, 61, 91–103 CrossRef CAS.
- B. Aktekin, A. E. Sedykh, K. Müller-Buschbaum, A. Henss and J. Janek, Adv. Funct. Mater., 2024, 34, 2313252 CrossRef CAS.
- H.-J. Noh, Z. Chen, C. S. Yoon, J. Lu, K. Amine and Y.-K. Sun, Chem. Mater., 2013, 25, 2109–2115 CrossRef CAS.
- X. Xu, H. Huo, J. Jian, L. Wang, H. Zhu, S. Xu, X. He, G. Yin, C. Du and X. Sun, Adv. Energy Mater., 2019, 9, 1803963 CrossRef.
- H. S. Liu, Z. R. Zhang and A. Y. Y. Z. L. Gong, Electrochem. Solid-State Lett., 2004, 7, A190–A193 CrossRef CAS.
- H. Liu, Y. Yang and J. Zhang, J. Power Sources, 2006, 162, 644–650 CrossRef CAS.
- D. P. Abraham, R. D. Twesten, M. Balasubramanian, I. Petrov, J. McBreen and K. Amine, Electrochem. Commun., 2002, 4, 620–625 CrossRef CAS.
- L. A. Kaufman and B. D. McCloskey, Chem. Mater., 2021, 33, 4170–4176 CrossRef CAS.
- N. Mijung, Y. Lee and J. Cho, J. Electrochem. Soc., 2006, 153, A935 CrossRef CAS.
- J. Eom, M. G. Kim and J. Cho, J. Electrochem. Soc., 2008, 155, A239 CrossRef CAS.
- N. V. Faenza, L. Bruce, Z. W. Lebens-Higgins, I. Plitz, N. Pereira, L. F. J. Piper and G. G. Amatucci, J. Electrochem. Soc., 2017, 164, A3727 CrossRef CAS.
- I. A. Shkrob, J. A. Gilbert, P. J. Phillips, R. Klie, R. T. Haasch, J. Bareño and D. P. Abraham, J. Electrochem. Soc., 2017, 164, A1489 CrossRef CAS.
- W. M. Seong, K.-H. Cho, J.-W. Park, H. Park, D. Eum, M. H. Lee, I.-S. S. Kim, J. Lim and K. Kang, Angew. Chem., Int. Ed., 2020, 59, 18662–18669 CrossRef CAS PubMed.
- W. M. Seong, Y. Kim and A. Manthiram, Chem. Mater., 2020, 32, 9479–9489 CrossRef CAS.
- P. Tan, S. Diao, T. Huang, T. Zhang, Z. Yang, Y. Zhang and H. Zhou, Chem. Eng. Sci., 2020, 222, 115716 CrossRef CAS.
- D. Bresser, D. Buchholz, A. Moretti, A. Varzi and S. Passerini, Energy Environ. Sci., 2018, 11, 3096–3127 RSC.
- F. Liu, N. A. Hashim, Y. Liu, M. R. M. Abed and K. Li, J. Membr. Sci., 2011, 375, 1–27 CrossRef CAS.
- L. Xiao, D. M. Davenport, L. Ormsbee and D. Bhattacharyya, Ind. Eng. Chem. Res., 2015, 54, 4174–4182 CrossRef CAS PubMed.
- S. S. Zhang, X. Fan and C. Wang, ChemElectroChem, 2019, 6, 1536–1541 CrossRef CAS.
- G. J. Ross, J. F. Watts, M. P. Hill and P. Morrissey, Polymer, 2000, 41, 1685–1696 CrossRef CAS.
- Y. Bi, Q. Li, R. Yi and J. Xiao, J. Electrochem. Soc., 2022, 169, 020521 CrossRef CAS.
- L. Ouyang, Z. Wu, J. Wang, X. Qi, Q. Li, J. Wang and S. Lu, RSC Adv., 2020, 10, 19360–19370 RSC.
- H. Sheng, X. H. Meng, D. D. Xiao, M. Fan, W. P. Chen, J. Wan, J. Tang, Y. G. Zou, F. Wang, R. Wen, J. L. Shi and Y. G. Guo, Adv. Mater., 2022, 34, 2108947 CrossRef CAS PubMed.
- D. Aurbach, K. Gamolsky, B. Markovsky, G. Salitra, Y. Gofer, U. Heider, R. Oesten and M. Schmidt, J. Electrochem. Soc., 2000, 147, 1322 CrossRef CAS.
- I. Takahashi, H. Kiuchi, A. Ohma, T. Fukunaga and E. Matsubara, J. Phys. Chem. C, 2020, 124, 9243–9248 CrossRef CAS.
- D. Li, H. Li, D. Danilov, L. Gao, J. Zhou, R.-A. Eichel, Y. Yang and P. H. L. Notten, J. Power Sources, 2018, 396, 444–452 CrossRef CAS.
- S. Klein, P. Bärmann, T. Beuse, K. Borzutzki, J. E. Frerichs, J. Kasnatscheew, M. Winter and T. Placke, ChemSusChem, 2021, 14, 595–613 CrossRef CAS.
- J. Hou, X. Feng, L. Wang, X. Liu, A. Ohma, L. Lu, D. Ren, W. Huang, Y. Li, M. Yi, Y. Wang, J. Ren, Z. Meng, Z. Chu, G.-L. Xu, K. Amine, X. He, H. Wang, Y. Nitta and M. Ouyang, Energy Storage Mater., 2021, 39, 395–402 CrossRef.
- H. Luo, B. Zhang, H. Zhang, Q. Zheng, X. Wu, Y. Yan, Z. Li, Y. Tang, W. Hao, G. Liu, Y.-H. Hong, J. Ye, Y. Qiao and S.-G. Sun, J. Phys. Chem. Lett., 2023, 14, 4565–4574 CrossRef CAS PubMed.
- G. Zampardi and F. La Mantia, Batteries Supercaps, 2020, 3, 672–697 CrossRef CAS.
- J.-N. Zhang, Q. Li, Y. Wang, J. Zheng, X. Yu and H. Li, Energy Storage Mater., 2018, 14, 1–7 CrossRef.
- Y. Jiang, C. Qin, P. Yan and M. Sui, J. Mater. Chem. A, 2019, 7, 20824–20831 RSC.
- W. Xue, R. Gao, Z. Shi, X. Xiao, W. Zhang, Y. Zhang, Y. G. Zhu, I. Waluyo, Y. Li, M. R. Hill, Z. Zhu, S. Li, O. Kuznetsov, Y. Zhang, W.-K. Lee, A. Hunt, A. Harutyunyan, Y. Shao-Horn, J. A. Johnson and J. Li, Energy Environ. Sci., 2021, 14, 6030–6040 RSC.
- W. Gu, G. Xue, Q. Dong, R. Yi, Y. Mao, L. Zheng, H. Zhang, X. Fan, Y. Shen and L. Chen, eScience, 2022, 2, 486–493 CrossRef CAS.
- J. Zhang, S. Ma, J. Zhang, J. Zhang, X. Wang, L. Wen, G. Tang, M. Hu, E. Wang and W. Chen, Nano Energy, 2024, 128, 109814 CrossRef CAS.
- S. Hwang, W. Chang, S. M. Kim, D. Su, D. H. Kim, J. Y. Lee, K. Y. Chung and E. A. Stach, Chem. Mater., 2014, 26, 1084–1092 CrossRef CAS.
- E. Jo, S. Hwang, S. M. Kim and W. Chang, Chem. Mater., 2017, 29, 2708–2716 CrossRef CAS.
- S. S. Zhang, Energy Storage Mater., 2020, 24, 247–254 CrossRef.
- S.-M. Bak, K.-W. Nam, W. Chang, X. Yu, E. Hu, S. Hwang, E. A. Stach, K.-B. Kim, K. Y. Chung and X.-Q. Yang, Chem. Mater., 2013, 25, 337–351 CrossRef CAS.
- S. Hwang, S. M. Kim, S.-M. Bak, S. Y. Kim, B.-W. Cho, K. Y. Chung, J. Y. Lee, E. A. Stach and W. Chang, Chem. Mater., 2015, 27, 3927–3935 CrossRef CAS.
- I. Belharouak, D. Vissers and K. Amine, J. Electrochem. Soc., 2006, 153, A2030–A2035 CrossRef CAS.
- H. J. Bang, H. Joachin, H. Yang, K. Amine and J. Prakash, J. Electrochem. Soc., 2006, 153, A731–A737 CrossRef CAS.
- K. Chen, W. Cai, Z. Hu, Q. Huang, A. Wang, Z. Zeng, J. Song, Y. Sun, Q. Kong, W. Feng, T. Chen, Z. Wu, Y. Song and X. Guo, Electron, 2024, 2, e27 CrossRef CAS.
- K. Aranda and A. Manthiram, Adv. Energy Mater., 2025, e02617 CrossRef.
- L. Karger, S. Korneychuk, S. Sicolo, H. Li, W. van den Bergh, R. Zhang, S. Indris, A. Kondrakov, J. Janek and T. Brezesinski, Adv. Funct. Mater., 2024, 34, 2402444 CrossRef CAS.
- H. Yu, Q. Han, L. Chen, L. Chen, H. Jiang and C. Li, Adv. Funct. Mater., 2024, 34, 2410384 CrossRef CAS.
- Z. W. Li, F. Lin, X. D. Zhang, X. S. Zhang, R. Wen, X. Li and Z. J. Zheng, Adv. Funct. Mater., 2024, 35, 2415035 CrossRef.
- H. Xie, H. Peng, D. Jiang, Z. Xiao, X. Liu, H. Liang, M. Wu, D. Liu, Y. Li, Y. Sun, S. Zhong, Z. Qian and R. Wang, Chem. Eng. J., 2023, 470, 144051 CrossRef CAS.
- V. Tvergaard and J. W. Hutchinson, J. Am. Ceram. Soc., 2005, 71, 157–166 CrossRef.
- A. O. Kondrakov, A. Schmidt, J. Xu, H. Geßwein, R. Mönig, P. Hartmann, H. Sommer, T. Brezesinski and J. Janek, J. Phys. Chem. C, 2017, 121, 3286–3294 CrossRef CAS.
- J.-M. Lim, T. Hwang, D. Kim, M.-S. Park, K. Cho and M. Cho, Sci. Rep., 2017, 7, 39669 CrossRef CAS.
- Q. Lin, W. Guan, J. Zhou, J. Meng, W. Huang, T. Chen, Q. Gao, X. Wei, Y. Zeng, J. Li and Z. Zhang, Nano Energy, 2020, 76, 105021 CrossRef CAS.
- P. Yan, J. Zheng, M. Gu, J. Xiao, J.-G. Zhang and C.-M. Wang, Nat. Commun., 2017, 8, 14101 CrossRef CAS PubMed.
- S. Lee, G. Song, B. Yun, T. Kim, S. H. Choi, H. Kim, S. W. Doo and K. T. Lee, ACS Nano, 2024, 18, 10566–10581 CrossRef CAS PubMed.
- Q.-T. Liao, S.-J. Guo, M.-Y. Qi, S.-D. Zhang, P.-Z. Ma, J.-Y. Li, A.-M. Cao and L.-J. Wan, Sustainable Energy Fuels, 2023, 7, 4805–4824 RSC.
- H. Liu, M. Wolfman, K. Karki, Y.-S. Yu, E. A. Stach, J. Cabana, K. W. Chapman and P. J. Chupas, Nano Lett., 2017, 17, 3452–3457 CrossRef CAS PubMed.
- S. Watanabe, M. Kinoshita, T. Hosokawa, K. Morigaki and K. Nakura, J. Power Sources, 2014, 258, 210–217 CrossRef CAS.
- H.-H. Ryu, K.-J. Park, C. S. Yoon and Y.-K. Sun, Chem. Mater., 2018, 30, 1155–1163 CrossRef CAS.
- C. S. Yoon, H.-H. Ryu, G.-T. Park, J.-H. Kim, K.-H. Kim and Y.-K. Sun, J. Mater. Chem. A, 2018, 6, 4126–4132 RSC.
- C. S. Yoon, D.-W. Jun, S.-T. Myung and Y.-K. Sun, ACS Energy Lett., 2017, 2, 1150–1155 CrossRef CAS.
- F. Wu, N. Liu, L. Chen, Y. Su, G. Tan, L. Bao, Q. Zhang, Y. Lu, J. Wang, S. Chen and J. Tan, Nano Energy, 2019, 59, 50–57 CrossRef CAS.
- H.-H. Ryu, G.-T. Park, C. S. Yoon and Y.-K. Sun, J. Mater. Chem. A, 2019, 7, 18580–18588 RSC.
- H.-H. Ryu, N.-Y. Park, D. R. Yoon, U.-H. Kim, C. S. Yoon and Y.-K. Sun, Adv. Energy Mater., 2020, 10, 2000495 CrossRef CAS.
- F. Lin, D. Nordlund, T.-C. Weng, Y. Zhu, C. Ban, R. M. Richards and H. L. Xin, Nat. Commun., 2014, 5, 3358 CrossRef PubMed.
- M. M. Besli, S. Xia, S. Kuppan, Y. Huang, M. Metzger, A. K. Shukla, G. Schneider, S. Hellstrom, J. Christensen, M. M. Doeff and Y. Liu, Chem. Mater., 2019, 31, 491–501 CrossRef CAS.
- Y. Mao, X. Wang, S. Xia, K. Zhang, C. Wei, S. Bak, Z. Shadike, X. Liu, Y. Yang, R. Xu, P. Pianetta, S. Ermon, E. Stavitski, K. Zhao, Z. Xu, F. Lin, X.-Q. Yang, E. Hu and Y. Liu, Adv. Funct. Mater., 2019, 29, 1900247 CrossRef.
- W.-S. Yoon, M. Balasubramanian, K. Y. Chung, X.-Q. Yang, J. McBreen, C. P. Grey and D. A. Fischer, J. Am. Chem. Soc., 2005, 127, 17479–17487 CrossRef CAS PubMed.
- W.-S. Yoon, K. Y. Chung, J. McBreen, D. A. Fischer and X.-Q. Yang, J. Power Sources, 2006, 163, 234–237 CrossRef CAS.
- H. M. Hollmark, L.-C. Duda, M. Dahbi, I. Saadoune, T. Gustafsson and K. Edström, J. Electrochem. Soc., 2010, 157, A962–A966 CrossRef CAS.
- U.-H. Kim, E.-J. Lee, C. S. Yoon, S.-T. Myung and Y.-K. Sun, Adv. Energy Mater., 2016, 6, 1601417 CrossRef.
- X. Wu, B. Song, P.-H. Chien, S. M. Everett, K. Zhao, J. Liu and Z. Du, Adv. Sci., 2021, 8, 2102318 CrossRef CAS PubMed.
- A. O. Kondrakov, H. Geßwein, K. Galdina, L. de Biasi, V. Meded, E. O. Filatova, G. Schumacher, W. Wenzel, P. Hartmann, T. Brezesinski and J. Janek, J. Phys. Chem. C, 2017, 121, 24381–24388 CrossRef CAS.
- S. Ahmed, A. Pokle, S. Schweidler, A. Beyer, M. Bianchini, F. Walther, A. Mazilkin, P. Hartmann, T. Brezesinski, J. Janek and K. Volz, ACS Nano, 2019, 13, 10694–10704 CrossRef CAS PubMed.
- Y. Nomura, K. Yamamoto, Y. Yamagishi and E. Igaki, ACS Nano, 2021, 15, 19806–19814 CrossRef CAS PubMed.
- H. Wu, C. Qin, K. Wang, X. Han, M. Sui and P. Yan, J. Power Sources, 2021, 503, 230066 CrossRef CAS.
- J. K. Morzy, W. M. Dose, P. E. Vullum, M. C. Lai, A. Mahadevegowda, M. F. L. De Volder and C. Ducati, ACS Appl. Energy Mater., 2024, 7, 3945–3956 CrossRef CAS PubMed.
- F. Guo, Y. Chen, Y. Song, Y. Deng, W. Hua, W. Yang, T. Chen, Z. Wu, L. Qiu and X. Guo, Small, 2024, 20, 2310321 CrossRef CAS.
- S.-Y. Lee, G.-S. Park, C. Jung, D.-S. Ko, S.-Y. Park, H. G. Kim, S.-H. Hong, Y. Zhu and M. Kim, Adv. Sci., 2019, 6, 1800843 CrossRef PubMed.
- W. Huang, T. Liu, L. Yu, J. Wang, T. Zhou, J. Liu, T. Li, R. Amine, X. Xiao, M. Ge, L. Ma, S. N. Ehrlich, M. V. Holt, J. Wen and K. Amine, Science, 2024, 384, 912–919 CrossRef CAS.
- Q. Zhang, J. Wang, Y. Chu, W. Huang, X. Huang, X. Xiao, L. Ma, T. Liu, K. Amine, J. Lu and C. Yang, Nat. Energy, 2025, 10, 1001–1012 CrossRef CAS.
- H.-H. Sun and A. Manthiram, Chem. Mater., 2017, 29, 8486–8493 CrossRef CAS.
- S. Lee, W. Li, A. Dolocan, H. Celio, H. Park, J. H. Warner and A. Manthiram, Adv. Energy Mater., 2021, 11, 2100858 CrossRef CAS.
- C. Xu, K. Märker, J. Lee, A. Mahadevegowda, P. J. Reeves, S. J. Day, M. F. Groh, S. P. Emge, C. Ducati, B. Layla Mehdi, C. C. Tang and C. P. Grey, Nat. Mater., 2021, 20, 84–92 CrossRef CAS.
- S. Li, Z. Jiang, J. Han, Z. Xu, C. Wang, H. Huang, C. Yu, S.-J. Lee, P. Pianetta, H. Ohldag, J. Qiu, J.-S. Lee, F. Lin, K. Zhao and Y. Liu, Nat. Commun., 2020, 11, 4433 CrossRef CAS PubMed.
- H.-H. Ryu, G.-T. Park, C. S. Yoon and Y.-K. Sun, Small, 2018, 14, 1803179 CrossRef PubMed.
- C. S. Yoon, H.-H. Ryu, G.-T. Park, J.-H. Kim, K.-H. Kim and Y.-K. Sun, J. Mater. Chem. A, 2018, 6, 4126–4132 RSC.
- W. Liu, P. Oh, X. Liu, M.-J. Lee, W. Cho, S. Chae, Y. Kim and J. Cho, Angew. Chem., Int. Ed., 2015, 54, 4440–4457 CrossRef CAS PubMed.
- Y. Hinuma, Y. S. Meng, K. Kang and G. Ceder, Chem. Mater., 2007, 19, 1790–1800 CrossRef CAS.
- S. Li, Y. Li, Z. Zhang, X. Shen, H. Ji, Z. Liu, Z. Hu, H. Wang, H. Yu, Z. Hu, S.-C. Haw, C.-T. Chen, Q. Kong, Y. Gao, X. Wang, R. Yu, Z. Wang and L. Chen, Energy Storage Mater., 2025, 79, 104337 CrossRef.
- J. Li, G. Liang, W. Zheng, S. Zhang, K. Davey, W. K. Pang and Z. Guo, Nano Mater. Sci., 2023, 5, 404–420 CrossRef CAS.
- D. Lee, C. Nam, J. Kim, S. Hwang, B. Koo, H. Hyun, J. Chung, S. Seo, M. Song, J. Song, M. Kim, D. H. Alsem, N. J. Salmon, S. Lee, Y. Cho, N. Kim, D. A. Shapiro and J. Lim, Nat. Commun., 2025, 16, 9018 CrossRef CAS.
- X. Ma, B. Kang and G. Ceder, J. Electrochem. Soc., 2010, 157, A925 CrossRef CAS.
- Y. Bi, W. Yang, R. Du, J. Zhou, M. Liu, Y. Liu and D. Wang, J. Power Sources, 2015, 283, 211–218 CrossRef CAS.
- J. Zheng, J. Xiao and J.-G. Zhang, Nano Today, 2016, 11, 678–694 CrossRef CAS.
- K. Min, S.-W. Seo, Y. Y. Song, H. S. Lee and E. Cho, Phys. Chem. Chem. Phys., 2017, 19, 1762–1769 RSC.
- H. Chen, J. A. Dawson and J. H. Harding, J. Mater. Chem. A, 2014, 2, 7988–7996 RSC.
- J. Kanamori, J. Phys. Chem. Solids, 1959, 10, 87–98 CrossRef CAS.
- J. Zheng, G. Teng, C. Xin, Z. Zhuo, J. Liu, Q. Li, Z. Hu, M. Xu, S. Yan, W. Yang and F. Pan, J. Phys. Chem. Lett., 2017, 8, 5537–5542 CrossRef CAS PubMed.
- X. Cao, H. Li, Y. Qiao, P. He, Y. Qian, X. Yue, M. Jia, J. Cabana and H. Zhou, Joule, 2022, 6, 1290–1303 CrossRef CAS.
- D.-H. Seo, J. Lee, A. Urban, R. Malik, S. Kang and G. Ceder, Nat. Chem., 2016, 8, 692–697 CrossRef CAS PubMed.
- K. Wu, P. Ran, W. Yin, L. He, B. Wang, F. Wang, E. Zhao and J. Zhao, Angew. Chem., Int. Ed., 2024, 63, e202410326 CAS.
- W. Chen, D. Muhtar, K. Li, G. Xiao, J. Cao, Y. Tang, G. Qian, X. Lu, Y. Sun and X. Lu, Chem. Mater., 2024, 36, 1249–1261 CrossRef CAS.
- G. Sun, X. Yin, W. Yang, A. Song, C. Jia, W. Yang, Q. Du, Z. Ma and G. Shao, Phys. Chem. Chem. Phys., 2017, 19, 29886–29894 RSC.
- A. Van der Ven and G. Ceder, J. Power Sources, 2001, 97–98, 529–531 CrossRef CAS.
- R. J. Clément, Z. Lun and G. Ceder, Energy Environ. Sci., 2020, 13, 345–373 RSC.
- E. Zhao, L. Fang, M. Chen, D. Chen, Q. Huang, Z. Hu, Q.-B. Yan, M. Wu and X. Xiao, J. Mater. Chem. A, 2017, 5, 1679–1686 RSC.
- F. Lin, I. M. Markus, D. Nordlund, T.-C. Weng, M. D. Asta, H. L. Xin and M. M. Doeff, Nat. Commun., 2014, 5, 3529 CrossRef PubMed.
- Q. Lin, W. Guan, J. Meng, W. Huang, X. Wei, Y. Zeng, J. Li and Z. Zhang, Nano Energy, 2018, 54, 313–321 CrossRef CAS.
- M. Dixit, B. Markovsky, F. Schipper, D. Aurbach and D. T. Major, J. Phys. Chem. C, 2017, 121, 22628–22636 CrossRef CAS.
- Z. Tang, S. Wang, J. Liao, S. Wang, X. He, B. Pan, H. He and C. Chen, Research, 2019, 2019, 2198906 CAS.
- S. Liu, Z. Liu, X. Shen, X. Wang, S.-C. Liao, R. Yu, Z. Wang, Z. Hu, C.-T. Chen, X. Yu, X. Yang and L. Chen, Adv. Energy Mater., 2019, 9, 1901530 CrossRef.
- Y. Gao, X. Wang, J. Geng, F. Liang, M. Chen and Z. Zou, J. Electron. Mater., 2023, 52, 72–95 CrossRef CAS.
- C. Yang, Y. Su, W. Su, S. Ma, X. Zhu, S. Wu, Y. Li, L. Chen, D. Cao, M. Wang, Q. Huang, Y. Guan, F. Wu and N. Li, Energy Storage Mater., 2025, 75, 104019 CrossRef.
- G. Qu, X. Chen, F. Yang, Z. Yang, G. Liu, J. Lee, R. Fong, J. Tian, R. Wen, C. Wang and Y. Huang, Small, 2025, 21, 2502609 CrossRef CAS PubMed.
- R. Jung, M. Metzger, F. Maglia, C. Stinner and H. A. Gasteiger, J. Electrochem. Soc., 2017, 164, A1361 CrossRef CAS.
- A. C. Martinez, S. Grugeon, D. Cailleu, M. Courty, P. Tran-Van, B. Delobel and S. Laruelle, J. Power Sources, 2020, 468, 228204 CrossRef CAS.
- Y. Bi, T. Wang, M. Liu, R. Du, W. Yang, Z. Liu, Z. Peng, Y. Liu, D. Wang and X. Sun, RSC Adv., 2016, 6, 19233–19237 RSC.
- S. Yang, P. He and H. Zhou, Energy Environ. Sci., 2016, 9, 1650–1654 RSC.
- N. Mahne, S. E. Renfrew, B. D. McCloskey and S. A. Freunberger, Angew. Chem., Int. Ed., 2018, 57, 5529–5533 CrossRef CAS PubMed.
- A. T. S. Freiberg, J. Sicklinger, S. Solchenbach and H. A. Gasteiger, Electrochim. Acta, 2020, 346, 136271 CrossRef CAS.
- S. E. Renfrew and B. D. McCloskey, J. Am. Chem. Soc., 2017, 139, 17853–17860 CrossRef CAS PubMed.
- T. Hatsukade, A. Schiele, P. Hartmann, T. Brezesinski and J. Janek, ACS Appl. Mater. Interfaces, 2018, 10, 38892–38899 CrossRef CAS PubMed.
- D. Cao, C. Tan and Y. Chen, Nat. Commun., 2022, 13, 4908 CrossRef CAS PubMed.
- X. Liu, G.-L. Xu, V. S. C. Kolluru, C. Zhao, Q. Li, X. Zhou, Y. Liu, L. Yin, Z. Zhuo, A. Daali, J.-J. Fan, W. Liu, Y. Ren, W. Xu, J. Deng, I. Hwang, D. Ren, X. Feng, C. Sun, L. Huang, T. Zhou, M. Du, Z. Chen, S.-G. Sun, M. K. Y. Chan, W. Yang, M. Ouyang and K. Amine, Nat. Energy, 2022, 7, 808–817 CrossRef CAS.
- F. Kong, C. Liang, L. Wang, Y. Zheng, S. Perananthan, R. C. Longo, J. P. Ferraris, M. Kim and K. Cho, Adv. Energy Mater., 2018, 9, 1802586 CrossRef.
- J. Shen, Z. Cao, Z. Li, L. Yang, S. Ji, Y. Qin, C. Fu, W. Yang, C. Ding, W. Ji, Y. Huang and N. Zhang, Adv. Funct. Mater., 2025, 2502419 CrossRef CAS.
- M. Zheng, Y. You and J. Lu, Nat. Rev. Mater., 2025, 10, 355–368 CrossRef CAS.
- W. Kong, J. Zhang, D. Wong, W. Yang, J. Yang, C. Schulz and X. Liu, Angew. Chem., Int. Ed., 2021, 60, 27102–27112 CrossRef CAS PubMed.
- A. Manthiram, A. Vadivel Murugan, A. Sarkar and T. Muraliganth, Energy Environ. Sci., 2008, 1, 621–638 RSC.
- T. Yang, K. Zhang, Y. Zuo, J. Song, Y. Yang, C. Gao, T. Chen, H. Wang, W. Xiao, Z. Jiang and D. Xia, Nat. Sustainability, 2024, 7, 1204–1214 CrossRef.
- J. Zhang, Z. Hua, Z. Wu, X. Cao, W. Yang, R. Shao, Y. Bai, Z. Wang and K. Sun, J. Energy Chem., 2025, 107, 183–193 CrossRef CAS.
- S. Li, Z. Liu, L. Yang, X. Shen, Q. Liu, Z. Hu, Q. Kong, J. Ma, J. Li, H.-J. Lin, C.-T. Chen, X. Wang, R. Yu, Z. Wang and L. Chen, Nano Energy, 2022, 98, 107335 CrossRef CAS.
- Q. Li, D. Zhou, M. Chu, Z. Liu, L. Yang, W. Wu, D. Ning, W. Li, X. Liu, J. Li, S. Passerini and J. Wang, Chem. Soc. Rev., 2025, 54, 3441–3474 RSC.
- A. Chen, K. Wang, J. Li, Q. Mao, Z. Xiao, D. Zhu, G. Wang, P. Liao, J. He, Y. You and Y. Xia, Front. Energy Res., 2020, 8, 593009 CrossRef.
- H. Zhang, H. Liu, L. F. J. Piper, M. S. Whittingham and G. Zhou, Chem. Rev., 2022, 122, 5641–5681 CrossRef CAS PubMed.
- J. Wandt, A. T. S. Freiberg, A. Ogrodnik and H. A. Gasteiger, Mater. Today, 2018, 21, 825–833 CrossRef CAS.
- P. M. Attia, E. Moch and P. K. Herring, Nat. Commun., 2025, 16, 611 CrossRef CAS PubMed.
- Z. Cui, C. Liu, F. Wang and A. Manthiram, Nat. Energy, 2025, 10, 490–501 CrossRef CAS.
- N. Liu, L. Chen, H. Wang, J. Zhao, F. Gao, J. Liu, J. Dong, Y. Lu, N. Li, Q. Shi, Y. Su and F. Wu, Chem. Eng. J., 2023, 472, 145113 CrossRef CAS.
- C. Leau, Y. Wang, C. Gervillie-Mouravieff, S. T. Boles, X. H. Zhang, S. Coudray, C. Boussard-Pledel and J. M. Tarascon, Nat. Commun., 2025, 16, 757 CrossRef CAS PubMed.
- Z. Cui and A. Manthiram, Angew. Chem., Int. Ed., 2023, 62, e202307243 CrossRef CAS PubMed.
- H. Xie, K. Huang, J. Du, Y. Han and Y. Shen, Energy Storage Sci. Technol., 2022, 11, 4030–4037 CAS.
- M. Metzger, B. Strehle, S. Solchenbach and H. A. Gasteiger, J. Electrochem. Soc., 2016, 163, A798 CrossRef CAS.
- X. Wang, D. Ren, H. Liang, Y. Song, H. Huo, A. Wang, Y. Gao, J. Liu, Y. Gao, L. Wang and X. He, Energy Environ. Sci., 2023, 16, 1200–1209 RSC.
- Y. F. Liu, H. X. Liu, Y. F. Zhu, H. R. Wang, J. Y. Li, Y. C. Li, H. Y. Hu, Z. G. Wu, X. D. Guo and Y. Xiao, Adv. Mater., 2025, 37, 2417540 CrossRef CAS PubMed.
- Z. Dai, Z. Li, R. Chen, F. Wu and L. Li, Nat. Commun., 2023, 14, 8087 CrossRef CAS PubMed.
- C. Liu, C. Miao, M. He, J. Wang, Q. Chen, S. Nie and W. Xiao, J. Power Sources, 2023, 566, 232961 CrossRef CAS.
- M. Yoon, Y. Dong, J. Hwang, J. Sung, H. Cha, K. Ahn, Y. Huang, S. J. Kang, J. Li and J. Cho, Nat. Energy, 2021, 6, 362–371 CrossRef CAS.
- T. Meng, S. Yang, Y. Peng, P. Li, S. Ren, X. Yun and X. Hu, Adv. Energy Mater., 2025, 15, 2404009 CrossRef CAS.
- K. Wu, Z. Li and X. Chen, Adv. Funct. Mater., 2024, 34, 2315327 CrossRef CAS.
- F. Wu, J. Dong, L. Chen, L. Bao, N. Li, D. Cao, Y. Lu, R. Xue, N. Liu, L. Wei, Z. Wang, S. Chen and Y. Su, Energy Storage Mater., 2021, 41, 495–504 CrossRef.
- C. Liu, C. Miao, M. He, J. Wang, Q. Chen, S. Nie and W. Xiao, J. Power Sources, 2023, 566, 232961 CrossRef CAS.
- K.-W. Nam, S.-M. Bak, E. Hu, X. Yu, Y. Zhou, X. Wang, L. Wu, Y. Zhu, K.-Y. Chung and X.-Q. Yang, Adv. Funct. Mater., 2013, 23, 1047–1063 CrossRef CAS.
- A. Butt, G. Ali, K. Tul Kubra, R. Sharif, A. Salman, M. Bashir and S. Jamil, Energy Technol., 2022, 10, 2100775 CrossRef CAS.
- Y.-K. Sun, S.-T. Myung, M.-H. Kim, J. Prakash and K. Amine, J. Am. Chem. Soc., 2005, 127, 13411–13418 CrossRef CAS PubMed.
- P. Karayaylali, R. Tatara, Y. Zhang, K.-L. Chan, Y. Yu, L. Giordano, F. Maglia, R. Jung, I. Lund and Y. Shao-Horn, J. Electrochem. Soc., 2019, 166, A1022 CrossRef CAS.
- T.-Y. Shim, Y.-W. Yoo, D.-Y. Hwang and S.-H. Lee, Ceram. Int., 2023, 49, 12138–12143 CrossRef CAS.
- V.-C. Ho, S. Jeong, T. Yim and J. Mun, J. Power Sources, 2020, 450, 227625 CrossRef CAS.
- F. Schipper, H. Bouzaglo, M. Dixit, E. M. Erickson, T. Weigel, M. Talianker, J. Grinblat, L. Burstein, M. Schmidt, J. Lampert, C. Erk, B. Markovsky, D. T. Major and D. Aurbach, Adv. Energy Mater., 2018, 8, 1701682 CrossRef.
- S. Wang, K. Liang, H. Zhao, M. Wu, J. He, P. Wei, Z. Ding, J. Li, X. Huang and Y. Ren, Nat. Commun., 2025, 16, 1 CrossRef PubMed.
- X. Ping, X. Pei, D. Zou, C. Wang, X. Li, C. Cao, H. Lei, C. Yang, Q. Cheng, W. Liu, X. Cao, M. Liu and Y. Wang, Electrochim. Acta, 2025, 530, 146297 CrossRef CAS.
- G. Cao, Z. Jin, J. Zhu, Y. Li, B. Xu, Y. Xiong and J. Yang, J. Alloys Compd., 2020, 832, 153788 CrossRef CAS.
- L. Zhao, G. Chen, Y. Weng, T. Yan, L. Shi, Z. An and D. Zhang, Chem. Eng. J., 2020, 401, 126138 CrossRef CAS.
- L. Wang, Q. Su, W. Shi, C. Wang, H. Li, Y. Wang, G. Du, M. Zhang, W. Zhao, S. Ding and B. Xu, Electrochim. Acta, 2022, 435, 141411 CrossRef CAS.
- Y. Huang, D. Su, L. Zheng, G. Yang, K. Li, J. Jiang, Q. Pan, S. Hu, Y. Li, Q. Li, H. Wang, F. Zheng and X. Ou, Energy Storage Mater., 2024, 71, 103678 CrossRef.
- L. Wang, G. Liu, X. Ding, C. Zhan and X. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 33901–33912 CrossRef CAS PubMed.
- L. Wang, G. Liu, R. Xu, X. Wang, L. Wang, Z. Yao, C. Zhan and J. Lu, Adv. Energy Mater., 2023, 13, 2203999 CrossRef CAS.
- X.-M. Fan, Y.-D. Huang, H.-X. Wei, L.-B. Tang, Z.-J. He, C. Yan, J. Mao, K.-H. Dai and J.-C. Zheng, Adv. Funct. Mater., 2022, 32, 2109421 CrossRef CAS.
- H. Zheng, Z. Wang, L. Chen, H. Jiang and C. Li, Particuology, 2023, 80, 74–80 CrossRef CAS.
- Y.-D. Xu, W. Xiang, Z.-G. Wu, C.-L. Xu, Y.-C. Li, X.-D. Guo, G.-P. Lv, X. Peng and B.-H. Zhong, Electrochim. Acta, 2018, 268, 358–365 CrossRef CAS.
- S.-J. Sim, S.-H. Lee, B.-S. Jin and H.-S. Kim, J. Power Sources, 2021, 481, 229037 CrossRef CAS.
- W. Tang, Z. Shu, A. Li, X. Huang and W. Li, Energy Storage Mater., 2025, 77, 104185 CrossRef.
- L. Wang, G. Liu, R. Wang, X. Wang, L. Wang, Z. Yao, C. Zhan and J. Lu, Adv. Mater., 2023, 35, 2209483 CrossRef CAS PubMed.
- K. Wang, J. Qiu, F. Hou, M. Yang, K. Nie, J. Wang, Y. Hou, W. Huang, W. Zhao, P. Zhang, J. Lin, J. Hu, F. Pan and M. Zhang, Adv. Energy Mater., 2023, 13, 2301216 CrossRef CAS.
- J. Guo, R. Ding, Y. Wu and P. Zheng, Appl. Phys. A:Mater. Sci. Process., 2023, 129, 617 CrossRef CAS.
- Z. Liu, Z. Wang, T. Lu, P. Dai, P. Gao and Y. Zhu, J. Alloys Compd., 2018, 763, 701–710 CrossRef CAS.
- M. Cai, Y. Dong, M. Xie, W. Dong, C. Dong, P. Dai, H. Zhang, X. Wang, X. Sun, S. Zhang, M. Yoon, H. Xu, Y. Ge, J. Li and F. Huang, Nat. Energy, 2023, 8, 159–168 CrossRef CAS.
- H. Sun, Z. Yang, R. Ghosh, S. Hwang, A. Hu, Y. Zhang, J. Liu, C. Sun, S. Sainio, D. Nordlund, X. Xiao and F. Lin, Nat. Commun., 2025, 16, 1478 CrossRef CAS PubMed.
- H. Wang, Q. Shi, J. Dong, M. Wang, Y. Lu, Y. Liu, J. Liu, N. Li, Q. Huang, Y. Su, F. Wu and L. Chen, Adv. Funct. Mater., 2025, 35, 2422806 CrossRef CAS.
- S.-Q. Lu, Q. Zhang, F. Meng, Y.-N. Liu, J. Mao, S. Guo, M.-Y. Qi, Y.-S. Xu, Y. Qiao, S.-D. Zhang, K. Jiang, L. Gu, Y. Xia, S. Chen, G. Chen, A.-M. Cao and L.-J. Wan, J. Am. Chem. Soc., 2023, 145, 7397–7407 CrossRef CAS PubMed.
- H. Li, H. Liu, S. Luo, J. Arbiol, E. Suard, T. Bergfeldt, A. Missyul, V. Baran, S. Mangold, Y. Zhang, W. Hua, M. Knapp, H. Ehrenberg, F. Pan and S. Indris, Nat. Commun., 2025, 16, 2203 CrossRef CAS PubMed.
- Z. Yao, T. Fu, T. Pan, C. Luo, M. Pang, S. Xiong, Q. Guo, Y. Li, S. Liu, C. Zheng and W. Sun, Nat. Commun., 2025, 16, 2791 CrossRef CAS PubMed.
- Y. Liu, Y. Xin, B. He, F. Zhang, C. Wang and H. Tian, Adv. Mater., 2025, 37, e2417353 CrossRef PubMed.
- X. Wang, Y. Bai, X. Wang and C. Wu, Chin. J. Chem., 2020, 38, 1847–1869 CrossRef CAS.
- X. Ping, X. Pei, D. Zou, C. Wang, X. Li, C. Cao, H. Lei, C. Yang, Q. Cheng, W. Liu, X. Cao, M. Liu and Y. Wang, Electrochim. Acta, 2025, 530, 146297 CrossRef CAS.
- J. O. Herrera-Robles, M. Azami-Ghadkolai, R. K. Bordia, H. Camacho-Montes, C. A. Rodríguez-Gonzalez, Y. Espinosa-Almeyda and P. G. Mani-Gonzalez, Mater. Chem. Phys., 2025, 332, 130188 CrossRef CAS.
- Y. Lv, S. Huang, J. Zhang, G. Kang, Y. Liu, N. Li, Y. Liang, X. Zhong, T. Jia, Y. Ouyang, P. Qin, F. Kang, J. Zhang and Y. Cao, Adv. Funct. Mater., 2024, 34, 2312284 CrossRef CAS.
- N. Liu, L. Chen, H. Wang, J. Zhao, F. Gao, J. Liu, J. Dong, Y. Lu, N. Li, Q. Shi, Y. Su and F. Wu, Chem. Eng. J., 2023, 472, 145113 CrossRef CAS.
- H. Li, P. Zhou, F. Liu, H. Li, F. Cheng and J. Chen, Chem. Sci., 2019, 10, 1374–1379 RSC.
- J. Jian, X. Xu, X. Pan, G. Han, R. Xiao, Z. Liu, D. Sun, X. Zhang, Q. Zhou, H. Zhu, G. Yin, H. Huo, Y. Ma, P. Zuo, X. Cheng and C. Du, Chem. Eng. J., 2024, 496, 154344 CrossRef CAS.
- Q. Qiu, J. Ruan, W. Zhou, W. Hu, W. Wu, J. Zhang, F. Fang, D. Sun, J. Zang and Y. Song, Small, 2025, 21, 2503589 CrossRef CAS PubMed.
- G.-T. Park, B. Namkoong, S.-B. Kim, J. Liu, C. S. Yoon and Y.-K. Sun, Nat. Energy, 2022, 7, 946–954 CrossRef CAS.
- H.-H. Ryu, H.-W. Lim, S. G. Lee and Y.-K. Sun, Energy Storage Mater., 2023, 59, 102771 CrossRef.
- W. Choi and A. Manthiram, J. Electrochem. Soc., 2006, 153, A1760 CrossRef CAS.
- F. Kong, C. Liang, R. C. Longo, D.-H. Yeon, Y. Zheng, J.-H. Park, S.-G. Doo and K. Cho, Chem. Mater., 2016, 28, 6942–6952 CrossRef CAS.
- N. Zhang, J. Stark, H. Li, A. Liu, Y. Li, I. Hamam and J. R. Dahn, J. Electrochem. Soc., 2020, 167, 080518 CrossRef CAS.
- S.-B. Kim, H. Kim, D.-H. Park, J.-H. Kim, J.-H. Shin, J.-S. Jang, S.-H. Moon, J.-H. Choi and K.-W. Park, J. Power Sources, 2021, 506, 230219 CrossRef CAS.
- S. H. Park, Y. K. Sun, K. S. Park, K. S. Nahm, Y. S. Lee and Masaki Yoshio, Electrochim. Acta, 2002, 47, 1721–1726 CrossRef CAS.
- B. Zhang, L. Li and J. Zheng, J. Alloys Compd., 2012, 520, 190–194 CrossRef CAS.
- Y. Zhou, H. Zhang, Y. Wang, T. Wan, P. Guan, X. Zhou, X. Wang, Y. Chen, H. Shi, A. Dou, M. Su, R. Guo, Y. Liu, L. Dai and D. Chu, ACS Nano, 2023, 17, 20621–20633 CrossRef CAS PubMed.
- J. Xu, J. You, Y. Wu, R. Zheng, H. Sun, Y. Liu, S. Liu and Z. Wang, J. Energy Chem., 2025, 106, 699–709 CrossRef CAS.
- S. Tan, Z. Shadike, J. Li, X. Wang, Y. Yang, R. Lin, A. Cresce, J. Hu, A. Hunt, I. Waluyo, L. Ma, F. Monaco, P. Cloetens, J. Xiao, Y. Liu, X.-Q. Yang, K. Xu and E. Hu, Nat. Energy, 2022, 7, 484–494 CrossRef CAS.
- S. Liu, W. Tian, J. Shen, Z. Wang, H. Pan, X. Kuang, C. Yang, S. Chen, X. Han, H. Quan and S. Zhu, Nat. Commun., 2025, 16, 2474 CrossRef CAS PubMed.
- Z. Xu, X. Zhang, J. Yang, X. Cui, Y. Nuli and J. Wang, Nat. Commun., 2024, 15, 9856 CrossRef CAS PubMed.
- Y. Jie, S. Wang, S. Weng, Y. Liu, M. Yang, C. Tang, X. Li, Z. Zhang, Y. Zhang, Y. Chen, F. Huang, Y. Xu, W. Li, Y. Guo, Z. He, X. Ren, Y. Lu, K. Yang, S. Cao, H. Lin, R. Cao, P. Yan, T. Cheng, X. Wang, S. Jiao and D. Xu, Nat. Energy, 2024, 9, 987–998 CrossRef CAS.
- Y. Zhang, Y. Zhang, X. Wang, H. Gong, Y. Cao, K. Ma, S. Zhang, S. Wang, W. Yang, L. Wang and J. Sun, Adv. Energy Mater., 2024, 15, 2403751 CrossRef.
- Z. Li, Y. Liao, H. Ji, X. Lin, Y. Wei, S. Hao, X. Hu, L. Yuan, Z. Huang and Y. Huang, Adv. Energy Mater., 2024, 15, 2404120 CrossRef.
- Y. Gao, Y. Yang, T. Yang, Z. Zhang, L. Tang, Z. Mao, Y. Zhang, D. Luo and Z. Chen, Adv. Energy Mater., 2024, 15, 2403063 CrossRef.
- H. Kim, J. M. Kim, G. T. Park, Y. J. Ahn, J. Y. Hwang, D. Aurbach and Y. K. Sun, Adv. Energy Mater., 2024, 15, 2403386 CrossRef.
- Y. Yang, N. Yao, Y. X. Yao, L. Xu, X. F. Wen, Z. Li, Z. L. Yang, C. Yan and J. Q. Huang, Adv. Energy Mater., 2024, 15, 2403183 CrossRef.
- J. Li, H. Hu, J. Zhu, X. Ma, Y. Hu, H. Zhang, F. Liu, S. Zhang and X. Ji, Adv. Mater., 2025, 37, 2501659 CrossRef CAS PubMed.
- R. Gu, D. Zhang, S. Xu, X. Guo, Y. Xiao, Z. Sheng, Q. Xu, J. Xu, S. Zhu, K. Liao, S. Gong, P. Shi and Y. Min, Nat. Commun., 2025, 16, 5474 CrossRef CAS PubMed.
- Y. Song, J. Ju, J. Wang, K. Li, X. Wang, R. Gao, H. Lu, D. Chao and Y. Wang, Adv. Mater., 2025, 37, 2500941 CrossRef CAS PubMed.
- H. Wang, D. Yan, H. Liu, S. Li, X. Niu, C. Ouyang, H. Li and L. Wang, Adv. Mater., 2025, 37, 2509760 CrossRef CAS PubMed.
- Y. Lu, Q. Cao, W. Zhang, T. Zeng, Y. Ou, S. Yan, H. Liu, X. Song, H. Zhou, W. Hou, P. Zhou, N. Hu, Q. Feng, Y. Li and K. Liu, Nat. Energy, 2025, 10, 191–204 CAS.
- S. Jiang, R. Li, L. Chen, C. Sun, J. Wang, J. Zheng, L. Chen, T. Deng and X. Fan, Adv. Mater., 2025, 37, 2417285 CrossRef CAS PubMed.
- C. Song, S. H. Han, Y. Choi, H. R. Shin, M. K. Kim, C. Gong, D. Chen, J. W. Lee, S. Hong and N. S. Choi, Adv. Mater., 2025, 37, 2418773 CrossRef CAS PubMed.
- M. Wang, M. Li, J. Wu, Y. Meng, J. Hao, D. Zhou, C. Han and B. Li, Adv. Mater., 2025, 37, 2502076 CrossRef PubMed.
- Y. Shen, T. Li, K. Ren, S. Yuan, K. Ding, K. Xia, J. L. Bao and Y. Wang, Adv. Mater., 2025, 37, 2501654 CrossRef CAS PubMed.
- J. Li, J. Chen, X. Xu, J. Shen, Z. Wang, Z. Guo, P. Lin, J. Sun, B. Huang and T. Zhao, Adv. Mater., 2025, 37, 2501006 CrossRef CAS PubMed.
- Z. Han, L. Chen, G. Zheng, D. Zhang, K. Yang, G. Xiao, H. Xu, Y. Li, X. An, Y. Ma, S. Guo, Y. Chen, T. Hou, Y. Cao, C. Zhang, Y. B. He and M. Liu, Adv. Mater., 2025, 37, 2416668 CrossRef CAS PubMed.
- W. Yang, J. Cai, C. Xu, A. Chen, Y. Wang, Y. Shi, P. He and H. Zhou, Adv. Mater., 2025, 37, 2505285 CrossRef CAS PubMed.
- S. Li, H. Hong, X. Yang, D. Li, Q. Xiong, D. Zhang, S. Wang, Z. Huang, H. Lv and C. Zhi, Adv. Mater., 2025, 37, 2504333 CrossRef CAS PubMed.
- Y. Li, Q. Qu, L. Lv, J. Shao and H. Zheng, Adv. Funct. Mater., 2024, 34, 2314100 CrossRef CAS.
- Y. Zhang, Y. Chen, Q. He, J. Ke, W. Wang, J.-F. Wu, P. Gao, Y. Li and J. Liu, J. Energy Chem., 2024, 92, 639–647 CrossRef CAS.
- Y. Zou, Q. Zheng, Y. Tang, Y. Yan, S. Zhou, H.-G. Liao, Y. Qiao, J. Bao and S.-G. Sun, Chem. Eng. J., 2024, 488, 151153 CrossRef CAS.
- C. Li, Y. Zhong, R. Liao, T. Yi, M. Zhou, R. Liu, S. Liu and D. Wu, Adv. Mater., 2025, 37, 2500142 CrossRef CAS PubMed.
- W. Yang, A. Chen, P. He and H. Zhou, Nat. Commun., 2025, 16, 4229 CrossRef CAS PubMed.
- H. Kwon, H. J. Choi, J. K. Jang, J. Lee, J. Jung, W. Lee, Y. Roh, J. Baek, D. J. Shin, J. H. Lee, N. S. Choi, Y. S. Meng and H. T. Kim, Nat. Commun., 2023, 14, 4047 CrossRef CAS PubMed.
- F. Cheng, W. Zhang, Q. Li, C. Fang, J. Han and Y. Huang, ACS Nano, 2023, 17, 24259–24267 CrossRef CAS PubMed.
- Y. Chen, Q. He, Y. Mo, W. Zhou, Y. Zhao, N. Piao, C. Liu, P. Xiao, H. Liu, B. Li, S. Chen, L. Wang, X. He, L. Xing and J. Liu, Adv. Energy Mater., 2022, 12, 2201631 CrossRef CAS.
- H. Jia, B. Broekhuis, Y. Xu, Z. Yang, D. Kautz, L. Zhong, M. H. Engelhard, Q. Zhao, M. E. Bowden, B. E. Matthews, C. Connor, F. Lin, C. Wang and W. Xu, ACS Appl. Mater. Interfaces, 2025, 17, 6260–6270 CrossRef CAS PubMed.
- M. Li, L. Xu, X. Wang, D. Mo, Y. Hu, N. Wang, K. Yang and K. Deng, Chem. Eng. J., 2025, 504, 158923 CrossRef CAS.
- L. Bai, Y. Xu, Y. Liu, D. Zhang, S. Zhang, W. Yang, Z. Chang and H. Zhou, Nat. Commun., 2025, 16, 3484 CrossRef CAS PubMed.
- P. Xu, X. Guo, B. Jiao, J. Chen, M. Zhang, H. Liu, X. Yu, M. Appleberry, Z. Yang, H. Gao, F. Yang, X. Weng, Y. Shen, J. Gu, Y. S. Meng, C. Brooks, S. P. Ong and Z. Chen, Nat. Commun., 2024, 15, 9842 CrossRef CAS PubMed.
- X. Gao, B. Li, G. Rousse, A. V. Morozov, M. Deschamps, E. Elkaïm, L. Zhang, K. Kummer, A. M. Abakumov and J. M. Tarascon, Adv. Energy Mater., 2024, 15, 2402793 CrossRef.
- L. Wang, T. Liu, T. Wu and J. Lu, Nature, 2022, 611, 61–67 CrossRef CAS PubMed.
- A. Manthiram, J. C. Knight, S.-T. Myung, S.-M. Oh and Y.-K. Sun, Adv. Energy Mater., 2016, 6, 1501010 CrossRef.
- F. Kong, C. Liang, L. Wang, Y. Zheng, S. Perananthan, R. C. Longo, J. P. Ferraris, M. Kim and K. Cho, Adv. Energy Mater., 2019, 9, 1802586 CrossRef.
- Z. Huang, J. Yan, Z. Liu, W. Wang, Y. Tang, Z. Zhang, T. Yang, X. Wang, X. Li, Q. Kong, S. Lan, H. Zhu, Y. Ren and Q. Liu, Adv. Funct. Mater., 2024, 34, 2400956 CrossRef CAS.
- H. H. Sun, H.-H. Ryu, U.-H. Kim, J. A. Weeks, A. Heller, Y.-K. Sun and C. B. Mullins, ACS Energy Lett., 2020, 5, 1136–1146 CrossRef CAS.
- X. Fan, G. Hu, B. Zhang, X. Ou, J. Zhang, W. Zhao, H. Jia, L. Zou, P. Li and Y. Yang, Nano Energy, 2020, 70, 104450 CrossRef CAS.
- J. Langdon and A. Manthiram, Energy Storage Mater., 2021, 37, 143–160 CrossRef.
- G. Nam, J. Hwang, D. Kang, S. Oh, S. Chae, M. Yoon and M. Ko, J. Energy Chem., 2023, 79, 562–568 CrossRef CAS.
- Z. Liu, R. Tang, F. Xiao, L. Zeng, Y. Wang and J. Liu, Appl. Surf. Sci., 2023, 640, 158437 CrossRef CAS.
- Y. Zou, D. Yu, Y. Tang, Y. Yan, Y. Qiao, J. Bao and S.-G. Sun, Chem. Eng. J., 2025, 504, 158800 CrossRef CAS.
- H.-H. Ryu, B. Namkoong, J.-H. Kim, I. Belharouak, C. S. Yoon and Y.-K. Sun, ACS Energy Lett., 2021, 6, 2726–2734 CrossRef CAS.
- Y. Lee, H. Kim, T. Yim, K.-Y. Lee and W. Choi, J. Power Sources, 2018, 400, 87–95 CrossRef CAS.
- Y.-K. Sun, S.-T. Myung, B.-C. Park, J. Prakash, I. Belharouak and K. Amine, Nat. Mater., 2009, 8, 320–324 CrossRef CAS PubMed.
- Y.-K. Sun, Z. Chen, H.-J. Noh, D.-J. Lee, H.-G. Jung, Y. Ren, S. Wang, C. S. Yoon, S.-T. Myung and K. Amine, Nat. Mater., 2012, 11, 942–947 CrossRef CAS PubMed.
- B.-B. Lim, S.-T. Myung, C. S. Yoon and Y.-K. Sun, ACS Energy Lett., 2016, 1, 283–289 CrossRef CAS.
- Y. Kim, H. Park, K. Shin, G. Henkelman, J. H. Warner and A. Manthiram, Adv. Energy Mater., 2021, 11, 2101112 CrossRef CAS.
- Q. Li, R. Dang, M. Chen, Y. Lee, Z. Hu and X. Xiao, ACS Appl. Mater. Interfaces, 2018, 10, 17850–17860 CrossRef CAS PubMed.
- B. Zhao, X. Sun, H. Bi, T. Yang, H. Li, D. Luo, Y. Zhang and Z. Chen, Adv. Funct. Mater., 2025, 35, 2423717 CrossRef CAS.
- Z. Jing, S. Wang, Q. Fu, V. Baran, A. Tayal, N. P. M. Casati, A. Missyul, L. Simonelli, M. Knapp, F. Li, H. Ehrenberg, S. Indris, C. Shan and W. Hua, Energy Storage Mater., 2023, 59, 102775 CrossRef.
- D. Rathore, M. Garayt, Y. Liu, C. Geng, M. Johnson, J. R. Dahn and C. Yang, ACS Energy Lett., 2022, 7, 2189–2195 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2026 |




![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 citations and an h-index of 167 (Google Scholar), and has been recognized as a Highly Cited Researcher (Materials Science and Chemistry) by Web of Science/Clarivate Analytics.
000 citations and an h-index of 167 (Google Scholar), and has been recognized as a Highly Cited Researcher (Materials Science and Chemistry) by Web of Science/Clarivate Analytics.