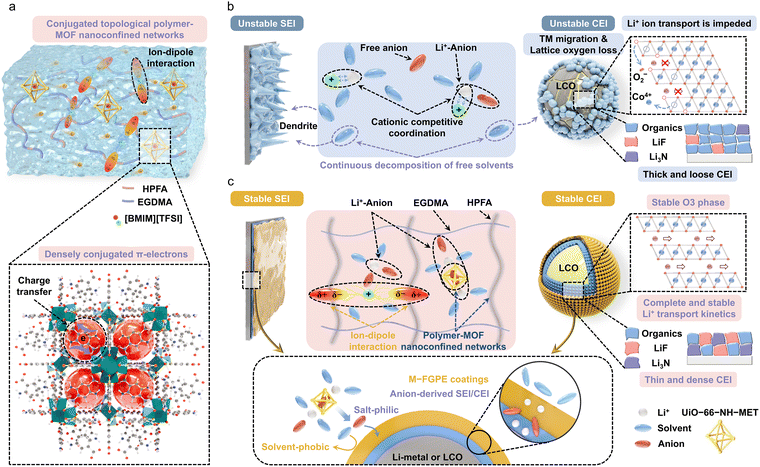
Conjugated topologically confined composite electrolytes for robust high-voltage and high-temperature semi-solid-state lithium metal batteries
Wang
Xu†
a,
Yongbiao
Mu†
 c,
Yaoyu
Yin†
d,
Anjun
Hu
c,
Yaoyu
Yin†
d,
Anjun
Hu
 *a,
Yuanjian
Li
e,
Jian
Wang
*a,
Qi
Liu
*a,
Yuanjian
Li
e,
Jian
Wang
*a,
Qi
Liu
 a,
Jianping
Long
a,
Jianping
Long
 a,
Lin
Zeng
a,
Lin
Zeng
 *c and
Shimou
Chen
*c and
Shimou
Chen
 *b
*b
aCollege of Materials and Chemistry & Chemical Engineering (College of Lithium Resources and Lithium Battery Industry), Chengdu University of Technology, Chengdu, 610059, China. E-mail: anjunhu@cdut.edu.cn; wangjian17@cdut.edu.cn
bState Key Laboratory of Chemical Resource Engineering, Beijing Key Laboratory of Electrochemical Process and Technology of Materials, Beijing University of Chemical Technology, Beijing, 10029, China. E-mail: chensm@mail.buct.edu.cn
cDepartment of Mechanical and Energy Engineering, Southern University of Science and Technology, Shenzhen, 518055, China. E-mail: zengl3@sustech.edu.cn
dSchool of Chemistry, University of Chinese Academy of Sciences, Beijing, 100049, China
eInstitute of Materials Research and Engineering (IMRE), Agency for Science, Technology and Research (A*STAR) 2 Fusionopolis Way, Innovis #08-03, Singapore, 138634, Republic of Singapore
First published on 28th November 2025
Abstract
High-voltage lithium metal batteries (LMBs) face severe cathode–electrolyte interfacial degradation at elevated temperatures. To address this issue, we develop a conjugated topologically confined composite electrolyte (M-FGPE) by in situ incorporating π-conjugated metal–organic frameworks (MOFs) into a fluorinated polymer matrix, using an imidazolium-based ionic liquid ([BMIM][TFSI]) as a safe yet dynamic platform for active solvation-structure engineering. The densely distributed π-conjugated electrons in MOFs strongly couple with TFSI− anions via orbital coupling, creating a nanoconfined environment that restricts anion migration. Concurrently, the C–F groups of the fluorinated polymer establish ion–dipole interactions with [BMIM]+ cations. This dual-interaction mechanism effectively disrupts the intrinsic Coulombic ordering of the ionic liquid, fostering an anion-dominated solvation structure and enhancing stability under high-voltage and high-temperature conditions. Furthermore, the unique electrolyte architecture facilitates the formation of a robust LiF/Li3N-rich electrode–electrolyte interface, which simultaneously suppresses lithium dendrites, transition metal dissolution, and lattice oxygen release. As a result of these synergistic effects, Li‖LiCoO2 cells deliver 76.2% capacity retention after 250 cycles at 4.60 V and 60 °C, while 5.4 Ah pouch cells (435 Wh kg−1) maintain 95.1% capacity retention after 30 cycles with a lean electrolyte dosage of 2.0 g Ah−1. This work pioneers a conjugated topological confinement strategy for high-energy LMBs.
Broader contextThe development of high-energy-density lithium metal batteries is hindered by the lack of electrolytes capable of enduring high-voltage and high-temperature conditions without undergoing interfacial degradation. While ionic liquid-based gel electrolytes offer good thermal stability, they suffer from strong ion pairing and aggregation, which restrict Li+ transport and intensify interfacial side reactions. This study introduces a paradigm-shifting design of conjugated topologically confined composite electrolytes that synergistically combine molecular-scale orbital coupling and ion–dipole interactions to reconfigure the solvation structure of ionic liquids. By in situ embedding π-conjugated metal–organic frameworks into a fluorinated polymer network, we establish a dual nanoconfinement mechanism that immobilizes anions through orbital coupling and dissociates cation aggregates via fluorinated dipole interactions. This creates an anion-dominated solvation environment with exceptional interfacial stability. Our strategy effectively suppresses dendrite growth and cathode degradation while facilitating the formation of robust LiF/Li3N-rich interphases, enabling long-term cycling under high voltage and temperature. The practical relevance is demonstrated by the stable operation of 5.4 Ah pouch cells with an energy density of 435 Wh kg−1 under a lean electrolyte of 2 g Ah−1. This work leverages conjugated topological control over ion coordination to provide a generalized strategy for high-performance lithium metal batteries. |
Introduction
The development of high-energy-density lithium metal batteries (LMBs) holds great promise for addressing the growing demand for advanced energy storage systems.1–3 Utilizing LiCoO2 (LCO) cathodes with elevated operating voltages (>4.50 V vs. Li+/Li) significantly improves energy density but introduces critical challenges related to material stability and electrolyte compatibility.4–6 Conventional LiPF6-carbonate electrolytes suffer from severe safety risks and rapid performance degradation under high-voltage (>4.40 V) and high-temperature (>55 °C) conditions. At the cathode, highly reactive Co4+ species and lattice oxygen (On−, 0 < n < 2), generated on the high-voltage LCO surface, catalyze electrolyte oxidation, producing COx gases.7 This reaction is accompanied by LiPF6 hydrolysis, which forms corrosive HF, leading to the deterioration of the cathode electrolyte interphase (CEI), transition metal (TM) dissolution, and structural degradation of the cathode.8 On the anode side, carbonate-based electrolytes form an unstable solid electrolyte interphases (SEI) on lithium metal, which promote dendritic lithium growth.9,10 Furthermore, the high flammability and volatility of carbonate solvents exacerbate safety concerns. Collectively, these issues have significantly limited the advancement of high-voltage LMBs.11To address these limitations, researchers have explored novel electrolyte systems.12,13 Gel polymer electrolytes (GPEs) combine the mechanical flexibility of polymers with the high ionic conductivity of liquid electrolytes.14–16 Their three-dimensional networks suppress lithium dendrite formation, while optimized polymer–ion interactions regulate Li+ solvation, enhancing interfacial stability under high-voltage conditions.17 Recently, ionic liquid electrolyte (ILE)-based GPEs have attracted attention owing to their non-flammability, low volatility, wide electrochemical windows, and superior thermal stability.18,19 Unlike flame-retardant additives or flame-retardant polymers, which often compromise conductivity or interfacial compatibility, ionic liquids offer inherent thermal stability and non-flammability. This allows them to function as dynamic media for solvation structure engineering, enabling molecular-level control to simultaneously optimize safety, conductivity, and interfacial stability. However, ILEs are hindered by strong Coulombic interactions arising from intense electrostatic attraction between bulky cations (e.g., [BMIM]+) and anions (e.g., [TFSI]−), which promotes ion pairing and aggregation, thereby increasing ionic clustering and reducing Li+ mobility.20 The introduction of organic co-solvents (e.g., carbonates) can weaken Coulombic forces via dielectric screening, suppressing ion aggregation and improving ion transport.19 Nonetheless, these co-solvents reintroduce safety hazards due to their flammability and reactivity. More critically, they can participate in interfacial side reactions that generate unstable SEI/CEI layers, ultimately compromising battery performance and life span.21
Electrolyte performance fundamentally depends on the Li+ solvation structure. While carbonate-based electrolytes exhibit high conductivity due to strong Li+–solvent coordination, they are prone to oxidative decomposition at high voltages.22 In contrast, ILEs employ anion-dominated solvation (e.g., by TFSI−), which enhances oxidative stability, but suffer from poor conductivity due to Li+–[BMIM]+ competition and ionic aggregation.23 Therefore, optimizing cation–anion coordination is critical to achieving both high-voltage stability and efficient Li+ transport.20 Metal–organic frameworks (MOFs) have emerged as promising candidates owing to their porous structures and tunable surface functionalities.24 Their nanoscale pores sterically hinder [BMIM]+–TFSI− pairing, while functional groups (e.g., –NH2) modulate Li+–anion interactions through Lewis acid–base effects, synergistically enhancing ionic conductivity and interfacial stability.25,26 The incorporation of MOFs not only mitigates Li+–[BMIM]+ competition and ion aggregation, but more importantly enables active engineering of the solvation structure at the molecular level. However, physically blended MOF composites often suffer from particle agglomeration and poor compatibility with the polymer matrix, which disrupts ion transport pathways.27,28 Thus, integrating the structural advantages of MOFs with polymer flexibility and ionic liquid stability through rational topological design remains a key challenge in advancing high-performance LMBs.29
In this work, we fabricate a conjugated topologically confined composite electrolyte (M-FGPE) to address interfacial failure in high-voltage LCO systems. Using 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([BMIM][TFSI]) as the solvent, we in situ integrate methacrylic anhydride (MET)-functionalized UiO-66-NH2 (UiO-66-NH-MET) MOFs into a copolymer network composed of 2,2,3,3,4,4,4-heptafluorobutyl acrylate (HPFA) and ethylene glycol dimethacrylate (EGDMA), forming a conjugated topological polymer–MOF nanoconfined structure (Fig. 1). This design enables three synergistic mechanisms: (i) densely conjugated π-electrons in UiO-66-NH-MET spatially confine TFSI− anions via molecular orbital coupling, creating an anion-confining microenvironment; (ii) highly electronegative C–F groups in HPFA establish ion–dipole interactions with [BMIM]+ cations, disrupting Coulombic ordering and promoting anion-dominated solvation; and (iii) the conjugated framework facilitates the formation of LiF/Li3N-rich SEI/CEI layers that effectively suppress TM dissolution, lattice oxygen evolution, and lithium dendrite growth. As a result, the M-FGPE exhibits a high room-temperature ionic conductivity of 1.84 mS cm−1 and a Li+ transference number of 0.62. Li‖LCO cells deliver 76.2% capacity retention after 250 cycles at 4.60 V and 60 °C. A 5.4 Ah pouch cell (435 Wh kg−1) maintains 95.1% capacity over 30 cycles with a lean electrolyte dosage of 2.0 g Ah−1 and a constrained N/P ratio of 2.5. This work presents a synergistic design paradigm for advancing high-energy-density and high-safety LMBs.
Results and discussion
Design and structure of M-FGPE
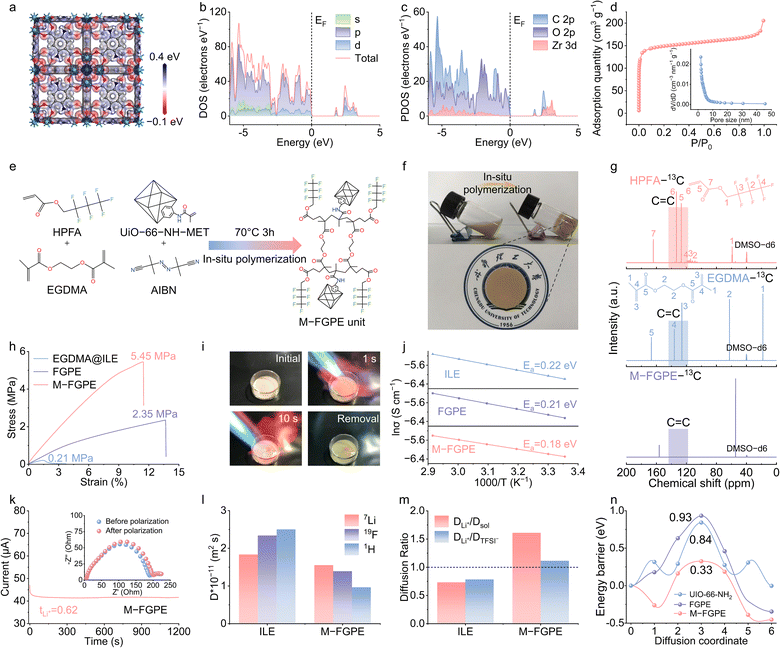
The three-dimensional porous architecture and tunable surface chemistry of metal–organic frameworks (MOFs) enable effective anion confinement in ionic liquid electrolytes (ILEs), particularly when combined with fluorinated polymers. In this work, UiO-66-NH2 was functionalized with methacryloyl groups (–NH–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–C(CH3)
O)–C(CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) (Fig. S1 and S2, SI) to allow covalent integration into the HPFA-EGDMA copolymer network via in situ polymerization, thereby achieving uniform molecular dispersion and eliminating interfacial incompatibility. The successful functionalization of UiO-66-NH-MET is confirmed through a combination of Fourier transform infrared (FTIR) spectroscopy, solid-state nuclear magnetic resonance (NMR), and X-ray photoelectron spectroscopy (XPS) analyses (Fig. S3, SI). These techniques consistently show the conversion of –NH2 to –NH– and the appearance of additional C
CH2) (Fig. S1 and S2, SI) to allow covalent integration into the HPFA-EGDMA copolymer network via in situ polymerization, thereby achieving uniform molecular dispersion and eliminating interfacial incompatibility. The successful functionalization of UiO-66-NH-MET is confirmed through a combination of Fourier transform infrared (FTIR) spectroscopy, solid-state nuclear magnetic resonance (NMR), and X-ray photoelectron spectroscopy (XPS) analyses (Fig. S3, SI). These techniques consistently show the conversion of –NH2 to –NH– and the appearance of additional C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations, providing definitive evidence of successful isopropenyl functionalization. Electrostatic potential (ESP) mapping reveals the dual functional role of UiO-66-NH-MET in anion regulation (Fig. 2a). The conjugated π-electrons donate charge to terminal oxygen atoms (regions of most negative ESP), while zirconium (Zr) sites exhibit a strong positive potential,30 enabling Lewis acidic coordination with TFSI− anions and complementary interactions with C–F groups in the polymer, effectively disrupting Coulombic ordering in the ILE. Density of states (DOS) analysis (Fig. 2b) shows that UiO-66-NH-MET exhibits a wide-bandgap and enables charge transfer between C 2p and O 2p orbitals (partial DOS, Fig. 2c), which prevents electronic conduction while supporting efficient ion transport.31 With a pore size of 2.25 nm and a specific surface area of 562.09 m2 g−1 (Fig. 2d), UiO-66-NH-MET effectively confines anions while preserving continuous ion conduction pathways.
C stretching vibrations, providing definitive evidence of successful isopropenyl functionalization. Electrostatic potential (ESP) mapping reveals the dual functional role of UiO-66-NH-MET in anion regulation (Fig. 2a). The conjugated π-electrons donate charge to terminal oxygen atoms (regions of most negative ESP), while zirconium (Zr) sites exhibit a strong positive potential,30 enabling Lewis acidic coordination with TFSI− anions and complementary interactions with C–F groups in the polymer, effectively disrupting Coulombic ordering in the ILE. Density of states (DOS) analysis (Fig. 2b) shows that UiO-66-NH-MET exhibits a wide-bandgap and enables charge transfer between C 2p and O 2p orbitals (partial DOS, Fig. 2c), which prevents electronic conduction while supporting efficient ion transport.31 With a pore size of 2.25 nm and a specific surface area of 562.09 m2 g−1 (Fig. 2d), UiO-66-NH-MET effectively confines anions while preserving continuous ion conduction pathways.
The M-FGPE was synthesized via in situ polymerization of UiO-66-NH-MET with HPFA–EGDMA in ILE (1 M LiTFSI in [BMIM][TFSI]), using a polypropylene separator as the structural scaffold (Fig. 2e and f). For comparison, a copolymerized fluorinated gel polymer electrolyte (FGPE) and ILE were prepared as control samples (Fig. S4, SI). Complete polymerization was confirmed by both 13C NMR and FTIR spectroscopy (Fig. 2g and Fig. S5, SI). Scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDS) mapping of both the surface and cross-section of M-FGPE confirmed the uniform dispersion of MOFs within the polymer matrix, with no observable agglomeration (Fig. S6, SI). X-ray diffraction (XRD) patterns showed that the characteristic MOF crystallinity (111/002 peaks) was retained,27,28 while the surrounding polymer domains remained amorphous (Fig. S7, SI).
M-FGPE exhibits significantly enhanced tensile strength (5.45 MPa) compared to the FGPE (2.35 MPa) and EGDMA@ILE (0.21 MPa) (Fig. 2h), suggesting sufficient mechanical integrity for suppressing lithium dendrites. Thermogravimetric analysis (TGA) reveals excellent thermal stability with a negligible mass loss below 400 °C (Fig. S8, SI), while flame tests confirmed its non-flammable nature (Fig. 2i). This outstanding thermal stability and flame resistance originate from the synergistic interactions among the [BMIM][TFSI] ionic liquid, π-conjugated MOFs, and the fluorinated polymer matrix, which together enhance the electrolyte's overall safety profile. Differential scanning calorimetry (DSC) analysis indicates broad melting peaks in all samples due to disordered crosslinked polymer accumulation (Fig. S9, SI); notably, M-FGPE exhibited a lower glass transition temperature (Tg) than FGPE, suggesting higher chain mobility and reduced activation energy (Ea) for ion transport. This Tg reduction arises from the conjugated topological structure, which modulates network connectivity and enhances chain dynamics through nanoscale interfacial interactions and diminished crosslinking density.
Electrochemical impedance spectroscopy (EIS) (Fig. S10 and Table S1, SI) showed that M-FGPE achieved a high room-temperature ionic conductivity of 1.84 mS cm−1, comparable to that of the ILE (2.05 mS cm−1). Temperature-dependent EIS measurements (Fig. S11, SI) and corresponding Arrhenius fitting (Fig. 2j) yielded an ion conduction value Ea of 0.18 eV for M-FGPE, which is lower than those of FGPE (0.21 eV) and ILE (0.22 eV). The Li+ transference number (tLi+) measurements increased significantly from 0.39 in the ILE to 0.62 in M-FGPE (Fig. 2k and Fig. S12, SI).
To further elucidate the Li+ transport mechanism, pulsed-field gradient nuclear magnetic resonance (PFG-NMR) was employed to measure the self-diffusion coefficients of Li+ (DLi+), TFSI− (DTFSI−), and the ionic liquid cation [BMIM]+ (Dsol) (Fig. S13, SI). These measurements provide critical insights into the migration dynamics and interionic interactions within the electrolyte systems. As shown in Fig. 2l, the diffusion coefficients in the ILE follow the order: Dsol > DTFSI− > DLi+, indicating that Li+ exhibits the slowest diffusion among the ionic species. This is attributed to the strong electrostatic coupling of Li+ within the viscous ionic matrix, which limits its independent motion and leads to a relatively low tLi+. In contrast, although the absolute diffusion coefficients of all species in M-FGPE are slightly reduced due to the presence of the solid polymer–MOF framework, fundamental inversion in transport behavior is observed. Notably, the diffusion order becomes: DLi+ > DTFSI− > Dsol, with both DLi+/Dsol and DLi+/DTFSI− > 1 (Fig. 2m). This inversion clearly demonstrates that in the M-FGPE system, Li+ becomes the fastest-moving species, indicating the restructuring of the ion transport paradigm that underlies the significantly enhanced tLi+.
To elucidate the Li+ transport mechanism in M-FGPE, we systematically investigated Li+ migration pathways and corresponding energy barriers. Density functional theory (DFT) calculations show that Li+ migration through the NH2–Zr4+–TFSI−–NH2 surface in UiO-66-NH2 presents a relatively low energy barrier compared to the segmental motion mechanism in FGPE chains (Fig. 2n and Fig. S14, SI). More significantly, the conjugated topological framework in M-FGPE actively participates in ion regulation via MOF-fluorinated polymer cooperative networks, enabling faster Li+ transport with lower energy barriers than those of individual UiO-66-NH2 or fluorinated polymer electrolytes. This enhancement arises from the dual interaction mechanism, where π-conjugated electrons coordinate with TFSI− anions through orbital coupling, while C–F groups engage in ion–dipole interactions with [BMIM]+ cations. This synergistic action creates superior Li+ migration pathways that transcend conventional transport mechanisms (Table S2, SI). Furthermore, linear sweep voltammetry (LSV) revealed an expanded electrochemical stability window of up to 4.85 V (Fig. S15, SI), attributed to MOF-enabled anion confinement and the resulting optimized solvation structure.
Li+ desolvation behaviors and solvation structures
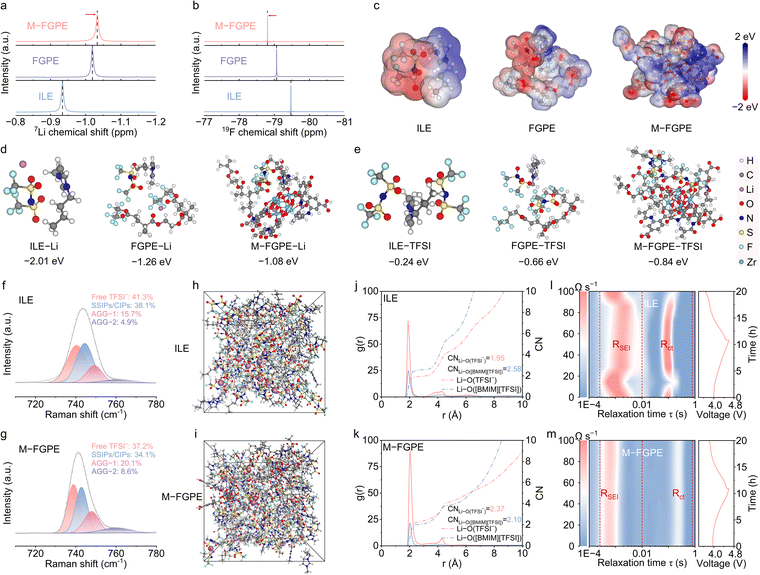
Solid-state NMR spectroscopy was employed to elucidate the Li+ solvation structures in various electrolyte systems. As shown in Fig. 3a, M-FGPE exhibits a more negative chemical shift for Li+ (−1.0327 ppm) compared to FGPE (−1.0191 ppm) and ILE (−0.9338 ppm), indicating an increased electron density around the Li+ nucleus and stronger shielding effects. To further clarify the Li+ coordination environment,32,33 complementary 19F NMR spectroscopy was conducted (Fig. 3b). M-FGPE displays the most upfield shift (−78.8049 ppm), relative to FGPE (−79.0659 ppm) and ILE (−79.4724 ppm), indicating decreased shielding of the fluorine nucleus and confirming enhanced Li+–TFSI− interaction in the M-FGPE.DFT calculations provide further insights into the coordination mechanisms through analysis of electrostatic potential (ESP) distributions and binding energies (Fig. 3c). The electronic structure leads to two key effects (Fig. 3d and e): significantly weakened Li+–polymer interactions and strengthened TFSI−–polymer binding. These results suggest that the synergistic effect of MOF-mediated anion confinement and C–F···[BMIM]+ ion–dipole interactions not only reduces Li+–polymer affinity but also enhances anion–polymer coordination.
Raman spectroscopy was used to probe the coordination states of TFSI− in different electrolytes. As shown in Fig. 3f and g, four coordination modes are observed in M-FGPE: free TFSI− (739 cm−1), solvent-separated/contact ion pairs (SSIPs/CIPs, 743 cm−1), and two types of aggregate clusters (AGG-1 at 748 cm−1 and AGG-2 at 760 cm−1).34,35 Quantitative analysis reveals that M-FGPE contains significantly higher fractions of aggregated species (AGG-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20.1%; AGG-2
20.1%; AGG-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.6%) compared to FGPE (16.5% and 5.4%, respectively) and ILE (15.7% and 4.9%, respectively) (Fig. S16, SI), indicating that the conjugated polymer–MOF network promotes anion aggregation. This increased aggregation is correlated with the enhanced formation of inorganic-rich SEI/CEI layers, contributing to improved interfacial stability.
8.6%) compared to FGPE (16.5% and 5.4%, respectively) and ILE (15.7% and 4.9%, respectively) (Fig. S16, SI), indicating that the conjugated polymer–MOF network promotes anion aggregation. This increased aggregation is correlated with the enhanced formation of inorganic-rich SEI/CEI layers, contributing to improved interfacial stability.
Molecular dynamics (MD) simulations further provided atomic-scale insights into solvation behavior (Fig. 3h and i and Fig. S17, SI). The results show that M-FGPE exhibits stronger Li+–TFSI− coordination and reduced solvent interactions compared to ILE. Radial distribution functions (g(r), solid lines) and coordination numbers (CN, dashed lines) indicate distinct solvation structures: in the ILE, Li+ shows predominant coordination with O atoms from [BMIM][TFSI] (strongest peak at ∼2 Å), while in M-FGPE, Li+–TFSI− interactions dominate with a higher peak intensity and CN values (Fig. 3j and k). These findings confirm the formation of anion-rich solvation sheaths in M-FGPE, where MOF confinement and polymer interactions synergistically stabilize the coordination environment.
Distribution of relaxation time (DRT) analysis was employed to investigate the Li+ desolvation kinetics in Li‖LCO cells. DRT spectra identify charge transfer resistance (Rct) in the low-frequency domain (100 > τ > 10−2 s), corresponding to Li+ solvation/desolvation dynamics, while the mid-frequency region (10−2 > τ > 10−4 s) reflects SEI resistance (RSEI) (Fig. 3l and m and Fig. S18, SI).36,37In situ electrochemical impedance spectroscopy (EIS) with DRT mapping at various states of charge (SOCs) tracks the evolution of Rct and RSEI. M-FGPE-based cells exhibit more reversible RSEI behavior than ILE-based cells, indicating stable SEI formation during cycling. The markedly reduced Rct observed in M-FGPE-based cells directly reflects the enhanced Li+ desolvation kinetics, which originate from an anion-dominated solvation structure that lowers the desolvation energy barrier. Simultaneously, the highly stable and reversible evolution of the RSEI unequivocally correlates with the formation of a robust, inorganic-rich LiF/Li3N interphase, ensuring exceptional interfacial stability throughout cycling.
Li anode deposition/stripping behaviours
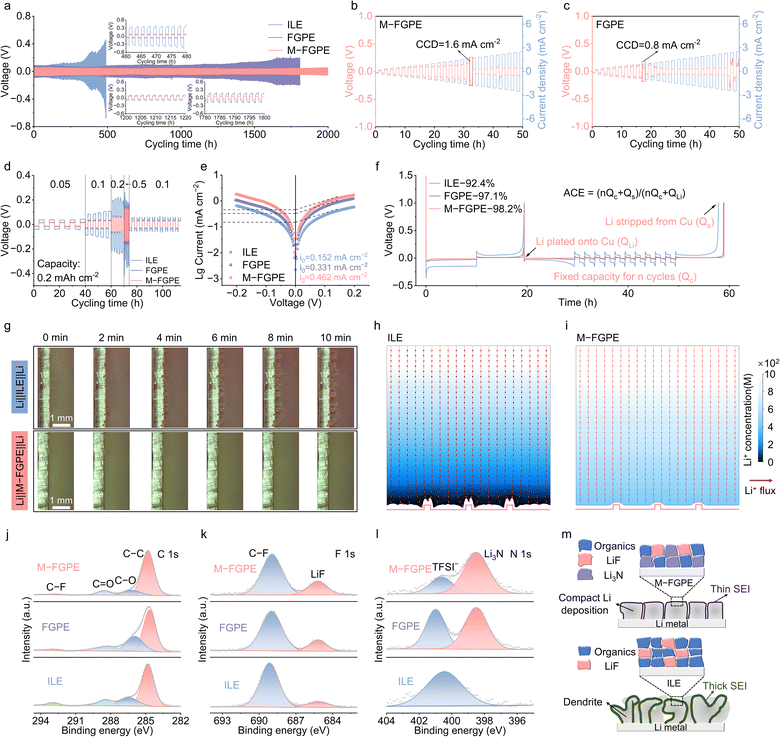
The interfacial stability of M-FGPE with lithium metal anodes was systematically evaluated using Li‖Li symmetric cells. As shown in Fig. 4a, Li‖M-FGPE‖Li cells exhibit remarkable cycling stability exceeding 2000 hours, whereas Li‖ILE‖Li cells display pronounced polarization after only 480 hours. Critical current density (CCD) measurements reveal that M-FGPE enables stable lithium stripping/plating up to 1.6 mA cm−2, twice the limit sustained by FGPE-based cells (0.8 mA cm−2) (Fig. 4b, c). Moreover, M-FGPE maintains stable voltage profiles across a wide current density range (0.05 to 0.5 mA cm−2), with significantly lower overpotentials compared to the ILE (Fig. 4d). Tafel analysis further confirms the superior charge transfer kinetics of M-FGPE, which exhibits a high exchange current density (I0) of 0.462 mA cm−2, markedly outperforming FGPE (0.331 mA cm−2) and ILE (0.152 mA cm−2) (Fig. 4e and Fig. S19, SI). In Li‖Cu half-cell tests, M-FGPE achieves an exceptional average Coulombic efficiency (ACE) of 98.2%, substantially exceeding that of the ILE (92.4%) (Fig. 4f).In situ optical microscopy was employed to directly visualize lithium deposition at 0.5 mA cm−2 (Fig. 4g and Fig. S20, SI). The ILE-based system exhibits irregular dendritic growth evolving into heterogeneous mossy domains, while M-FGPE enables smooth, dendrite-free lithium plating. These results were corroborated by post-cycling SEM analysis after 100 cycles (Fig. S21 and S22, SI), where lithium anodes from ILE cells displayed extensive mossy dendrites, in contrast to the uniform, compact deposits observed in M-FGPE-treated electrodes. Atomic force microscopy (AFM) measurements (Fig. S23, SI) confirmed reduced surface roughness in M-FGPE-treated lithium (height variation: 31.3 nm), verifying the polymer–MOF network's ability to regulate Li+ deposition kinetics and interfacial uniformity.
Finite element simulations (Fig. 4h) offer mechanistic insight into these observations. In ILEs, localized Li+ depletion layers form near the electrode surface, driving preferential deposition at protrusions and promoting dendrite growth.38 In contrast, M-FGPE supports rapid Li+ transport, eliminating such depletion zones and facilitating uniform deposition across the entire electrode (Fig. 4i). This homogeneous plating behavior arises from the synergistic effects of MOF-mediated anion confinement and polymer-enhanced Li+ mobility, which together establish efficient charge–transfer channels and underscore the importance of a stable, Li+-conductive SEI for dendrite suppression.
X-ray photoelectron spectroscopy (XPS) analysis revealed distinct differences in the SEI composition between the electrolyte systems (Fig. 4j–l). The C 1s spectra show that M-FGPE significantly suppresses the formation of organic species (C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and C–F) compared to the ILE (Fig. 4j and Table S3, SI), indicating reduced solvent decomposition due to optimized Coulombic interactions in the polymer–MOF confined environment. More importantly, the F 1s and N 1s spectra confirm the formation of inorganic LiF (685.2 eV) and Li3N (398.5 eV) in M-FGPE (Fig. 4k and l), attributed to preferential TFSI− decomposition within the anion-enriched solvation sheath. This distinctive SEI composition results from the combined effects of MOF-induced TFSI− confinement and polymer-facilitated ion–dipole interactions.
O, and C–F) compared to the ILE (Fig. 4j and Table S3, SI), indicating reduced solvent decomposition due to optimized Coulombic interactions in the polymer–MOF confined environment. More importantly, the F 1s and N 1s spectra confirm the formation of inorganic LiF (685.2 eV) and Li3N (398.5 eV) in M-FGPE (Fig. 4k and l), attributed to preferential TFSI− decomposition within the anion-enriched solvation sheath. This distinctive SEI composition results from the combined effects of MOF-induced TFSI− confinement and polymer-facilitated ion–dipole interactions.
As illustrated schematically in Fig. 4m, the LiF/Li3N-rich interphase formed in M-FGPE contributes to dendrite suppression through LiF's high interfacial energy and Li3N's low Li+ diffusion barrier (0.05 eV), which collectively reduce polarization, prevent electrolyte degradation, and enable uniform lithium deposition. In contrast, ILE systems form SEI layers dominated by organic species due to competitive Li+–[BMIM]+ coordination and excessive ion aggregation, which fail to stabilize the interface and prevent dendritic growth and electrolyte consumption.
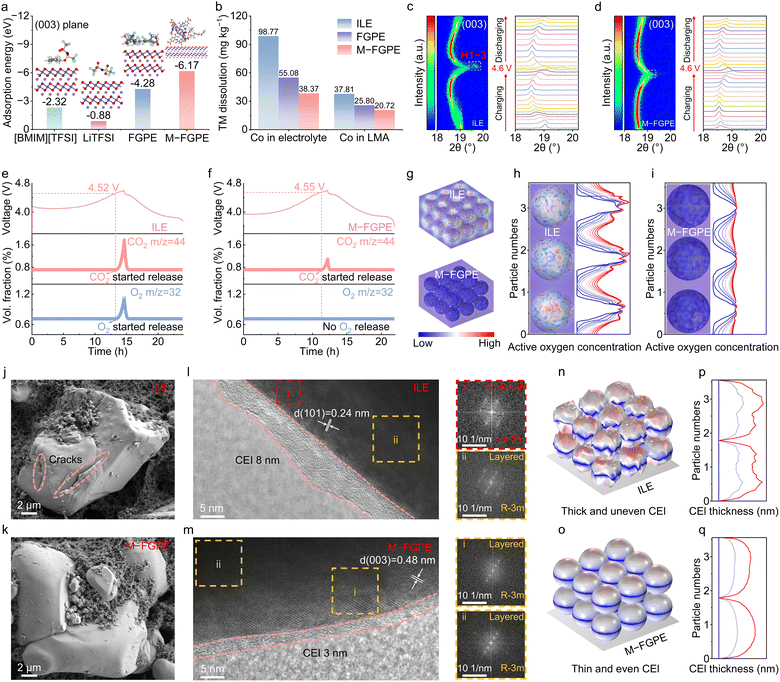
Structural evolution of the LCO and CEI
Density functional theory (DFT) calculations were performed to evaluate the adsorption energies of electrolyte components on the (003) surface of LCO cathodes. As shown in Fig. 5a, M-FGPE exhibits a significantly lower adsorption energy (−6.17 eV) compared to FGPE (−4.28 eV), TFSI− (−0.88 eV), and [BMIM][TFSI] (−2.32 eV), indicating strong preferential adsorption of the conjugated topological network at Co transition metal sites. This adsorption behavior facilitates the formation of an antioxidant protective layer at the cathode–electrolyte interface, effectively shielding reactive TM sites from solvent decomposition. Inductively coupled plasma optical emission spectroscopy (ICP-OES) analysis confirms that M-FGPE reduces cobalt dissolution by 61.2% and mitigates anode-side deposition by 45.2% relative to the ILE after 50 cycles (Fig. 5b). Thermal analysis further highlights the enhanced interfacial stability of M-FGPE, which generates only 39.2% of the exothermic heat observed in ILE systems (32.44 vs. 82.71 J g−1) after charging to 4.60 V (Fig. S24, SI), emphasizing the stabilizing effect of the anion-dominated solvation structure.Structural characterization using both ex situ and in situ X-ray diffraction (XRD) demonstrates the ability of M-FGPE to stabilize the layered structure of LCO during high-voltage cycling. In contrast to the ILE, which induces irreversible O3-to-H1-3 phase transitions after 50 cycles, M-FGPE preserves the original layered phase (Fig. S25, SI). Differential capacity (dQ/dV) analysis reveals improved structural reversibility in M-FGPE-based cells, as evidenced by minimal shifts in peak positions and intensities (Fig. S26, SI). In situ XRD during charging above 4.60 V further confirms this stabilization, with significantly reduced (003) peak splitting and diminished phase transition severity in M-FGPE relative to the ILE (Fig. 5c and d). These findings indicate that the anion-enriched solvation sheath in M-FGPE plays a vital role in preserving cathode structural integrity under high-voltage conditions.39
Lattice oxygen release, a critical degradation pathway for high-voltage LCO cathodes, was investigated using in situ differential electrochemical mass spectrometry (DEMS). ILE-based cells show pronounced CO2 and O2 evolution beginning at 4.52 V (Fig. 5e), whereas M-FGPE exhibits only delayed CO2 emission and complete suppression of oxygen evolution up to 4.55 V (Fig. 5f). Finite element method (FEM) simulations further reveal that M-FGPE's conjugated structure strongly adsorbs reactive oxygen species, effectively preventing their migration into the bulk electrolyte (Fig. 5g–i and Fig. S27, SI).40 In contrast, ILE's weaker molecular interactions permit oxygen diffusion and interfacial degradation. These results demonstrate that M-FGPE simultaneously suppresses lattice oxygen escape and interfacial side reactions by leveraging orbital coupling in MOFs and anion-dominated solvation for enhanced oxidative stability.
The mechanical integrity of LCO cathodes was examined through electron microscopy. Scanning electron microscopy (SEM) reveals that ILE cycling induces extensive surface cracking in LCO particles (Fig. 5j), promoting electrolyte penetration and secondary CEI formation, which accelerate TM dissolution. In contrast, M-FGPE maintains a crack-free morphology (Fig. 5k). High-resolution transmission electron microscopy (HRTEM) shows that the ILE leads to a thick (8 nm), heterogeneous CEI containing disordered rock-salt phases that hinder Li+ transport (Fig. 5l), whereas M-FGPE forms a uniform, thin (3 nm) CEI that retains the layered structure (Fig. 5m).
Simulations of CEI growth and morphology evolution over cycling further support these observations (Fig. 5n–q and Fig. S28, SI). In ILE systems, excessive reactive oxygen species trigger parasitic interfacial reactions, leading to thick, uneven CEI layers (Fig. 5n and p) and structural cracking. In contrast, M-FGPE enables thin and homogeneous CEI formation (Fig. 5o and q), consistent with experimental TEM results. This superior interfacial behavior is attributed to the formation of a LiF/Li3N-rich CEI, in which LiF passivates oxygen activity, and Li3N catalyzes its conversion, thus preventing uncontrolled CEI growth and maintaining cathode performance.
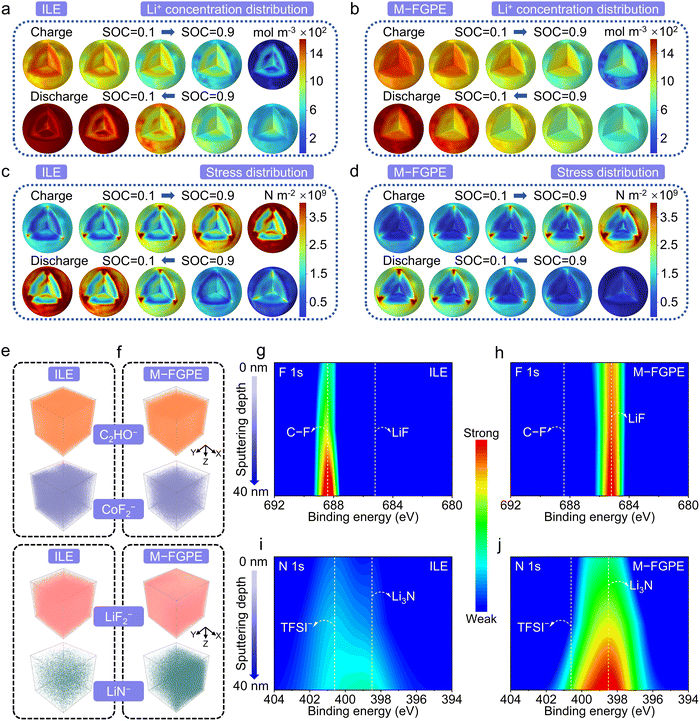
Finite element simulations were also conducted to compare Li+ transport and stress distributions in LCO cathodes under high-voltage operation. As illustrated in Fig. 6a and b, ILE-based systems suffer from severe Li+ concentration polarization, promoting phase transitions from layered to rock-salt structures41 and significantly reducing Li+ diffusivity. In contrast, M-FGPE maintains uniform Li+ concentration gradients due to its stable interphase. Stress distribution maps (Fig. 6c and d) further reveal that the ILE induces localized stress concentrations from uneven Li+ flux and strain mismatch, while M-FGPE promotes homogeneous Li+ ion flux and mechanical relaxation, thus preventing crack propagation and oxygen loss. These results highlight how M-FGPE's optimized interface design simultaneously alleviates electrochemical and mechanical degradation in high-voltage LCO cathodes.
Time-of-flight secondary ion mass spectrometry (TOF-SIMS) depth profiling reveals clear compositional differences in CEI layers. An ILE-derived CEI exhibits strong signals C2HO− and CoF2− fragments, indicative of organic solvent decomposition and TM dissolution under crowded solvation environments (Fig. 6e and f). In contrast, an M-FGPE-derived CEI displays enhanced signals for LiF2− and LiN− species (Fig. S29, SI), along with suppressed organic byproducts, confirming the efficacy of its anion-dominated solvation structure. Depth-resolved X-ray photoelectron spectroscopy (XPS) on cycled cathodes corroborates these findings: ILE-cycled LCO exhibits strong C–O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O signals (C 1s, Fig. S30, SI) and weak LiF/Li3N signatures (Fig. 6g and i), while M-FGPE significantly enhances LiF/Li3N formation and minimizes decomposition residues (Fig. 6h and j), especially in deeper CEI layers (Fig. S31, SI). These results confirm that M-FGPE's conjugated topological design enables stable 4.60 V operation of Li‖LCO cells by synergistically stabilizing the cathode bulk via π-electron orbital coupling and optimizing the CEI composition through anion-enriched solvation.
O signals (C 1s, Fig. S30, SI) and weak LiF/Li3N signatures (Fig. 6g and i), while M-FGPE significantly enhances LiF/Li3N formation and minimizes decomposition residues (Fig. 6h and j), especially in deeper CEI layers (Fig. S31, SI). These results confirm that M-FGPE's conjugated topological design enables stable 4.60 V operation of Li‖LCO cells by synergistically stabilizing the cathode bulk via π-electron orbital coupling and optimizing the CEI composition through anion-enriched solvation.
Electrolyte oxidative stability and electrochemical performance
To elucidate the critical role of M-FGPE in stabilizing LCO cathodes, interfacial compatibility was evaluated via electrochemical float analysis (EFA). As shown in Fig. 7a, Li‖ILE‖LCO cells exhibit continuously increasing leakage currents at 4.60 V, indicative of progressive electrolyte decomposition. In contrast, M-FGPE-based cells maintain stable, low leakage currents across a wide voltage window from 4.00 to 4.70 V (Fig. 7b and Fig. S32, SI), demonstrating significantly enhanced oxidative stability. Cyclic voltammetry (CV) analyses (Fig. 7c and Fig. S33, SI) further reveal improved reaction kinetics in M-FGPE-based systems, as evidenced by narrower half-peak widths and reduced voltage hysteresis relative to ILE cells, indicative of more efficient Li+ transport. The superior rate performance of M-FGPE is also reflected in its excellent cycling reversibility under high-voltage operation (Fig. 7d), underscoring its promise for advanced energy storage applications.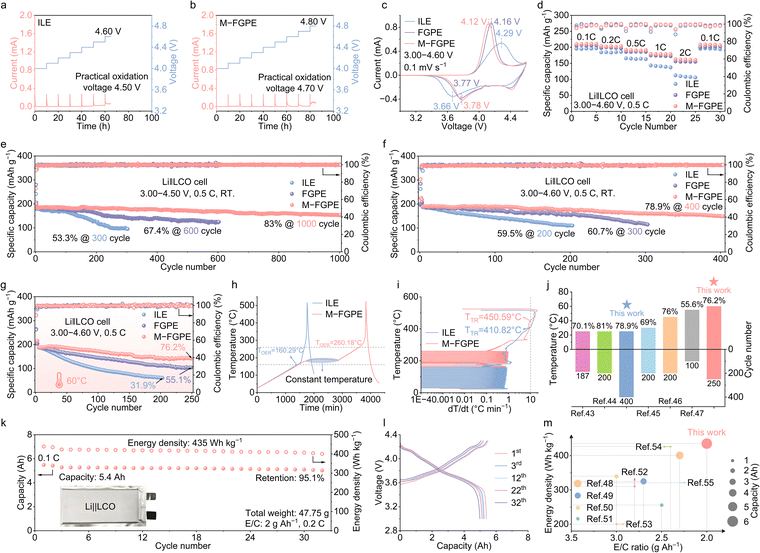 | ||
| Fig. 7 Electrochemical floating analysis of (a) ILE and (b) M-FGPE. (c) CV curves using the ILE, FGPE and M-FGPE at 0.1 mV s−1 between 3.00 and 4.60 V. (d) Rate performance of the Li‖LCO cells with the ILE, FGPE and M-FGPE. Cycling performance of Li‖LCO cells with cutoff voltages of (e) 4.50 V and (f) 4.60 V using the ILE, FGPE and M-FGPE charging and discharging at 0.5 C. (g) Cycling performance of the Li‖LCO cells of the ILE, FGPE and M-FGPE at 4.60 V and 60 °C. (h) ARC tests of fully charged Li‖ILE‖LCO and Li‖M-FGPE‖LCO coin cells in the HWS mode and (i) corresponding dT/dt vs. temperature curves. (j) Comparison of 4.50 V Li‖LCO cells prepared using M-FGPE in this study with other high-voltage LMB studies in terms of cycle numbers, capacity retention, and various temperatures.43–47 (k) Cycling performance of the 5.4 Ah Li‖LCO pouch cell over the voltage range of 3.00–4.30 V. The inset exhibits the optical image and (l) the corresponding capacity–voltage curves. (m) Comparison of Li-metal pouch cells in previous reports and this work.48–55 | ||
The electrochemical performance of M-FGPE in Li‖LCO cells was investigated under a range of practical conditions. At a cutoff voltage of 4.50 V, M-FGPE-based cells retain 83% of their initial capacity after 1000 cycles, in sharp contrast to ILE cells, which experience rapid capacity degradation due to parasitic reactions (Fig. 7e). When cycled at a high cutoff voltage of 4.60 V, M-FGPE enables a high specific capacity of 190.6 mAh g−1 with 78.9% capacity retention over 400 cycles (Fig. 7f). Even under demanding conditions of 4.60 V and 60 °C, which were deliberately selected to rigorously evaluate interfacial stability through accelerated aging while simulating real-world operational scenarios, M-FGPE-based cells achieve 76.2% retention after 250 cycles (Fig. 7g), outperforming previously reported electrolyte systems for LCO cathodes (Fig. 7j). Notably, M-FGPE also improves the cycling stability of high-Ni NCM811 cathodes (Fig. S34, SI), further supporting its versatility for high-voltage battery systems.
Thermal safety was rigorously assessed using accelerating rate calorimetry (ARC) in the heat-wait-search (HWS) mode, simulating adiabatic conditions to evaluate heat generation in fully charged cells. The onset temperature of exothermic reactions (TOER) and thermal runaway temperature (TTR) were defined at heating rates of 0.02 °C min−1 and 10 °C min−1, respectively.42 As shown in Fig. 7h and i, M-FGPE-based Li‖LCO cells exhibit a markedly higher TOER value of 260.18 °C compared to 160.29 °C for ILE-based cells, and remain thermally stable for over 20 hours at 170 °C without triggering failure. Additionally, M-FGPE increases TTR to 450.59 °C, significantly higher than ILE's 410.82 °C. These improvements are attributed to the conjugated topological confinement, which suppresses TFSI− pyrolysis and inhibits [BMIM]+ oxidation via stable C–F bonding. These results underscore M-FGPE's outstanding thermal stability and safety under abuse conditions (Table S4, SI).
The practical applicability of M-FGPE was demonstrated in large-format Li‖LCO pouch cells (5.4 Ah, approximately 12.8 × 5.8 × 0.4 cm3) assembled with a high cathode loading (4 mAh cm−2), a constrained N/P ratio of 2.5, and lean electrolyte usage (2 g Ah−1). Accounting for the total component mass (Fig. S35 and Table S5, SI), 4.30 V Li‖LCO pouch cells deliver a gravimetric energy density of 435 Wh kg−1 (Fig. 7k). These cells maintain 95.1% capacity retention over 30 cycles with stable voltage profiles (Fig. 7l). This excellent performance arises from the combined benefits of MOF-mediated solvent confinement, minimizing free solvent content and suppressing side reactions, and the formation of a dense, inorganic-rich LiF/Li3N SEI that ensures high Coulombic efficiency under lean electrolyte and constrained N/P ratio conditions.
Furthermore, benchmarking against recently reported pouch cells under similar electrolyte-lean and high-capacity regimes (Fig. 7m) highlights M-FGPE's clear advantage in realizing practical high-energy-density lithium metal batteries. Overall, these results confirm that the conjugated topological design of M-FGPE enables robust cathode protection through π-electron orbital coupling, while its anion-enriched solvation structure optimizes both CEI and SEI chemistries, collectively ensuring stable cell operation under high-voltage and high-temperature conditions.
Conclusions
In conclusion, this work presents a novel electrolyte design paradigm by introducing a conjugated topologically confined polymer–MOF composite electrolyte (M-FGPE). This innovative architecture synergistically integrates π-conjugated metal–organic frameworks with fluorinated polymer networks, effectively addressing the multifaceted challenges associated with high-voltage lithium metal batteries. The M-FGPE enables the formation of anion-dominated solvation structures through a dual mechanism of π-electron confinement and ion–dipole interactions, while simultaneously promoting the development of LiF/Li3N-rich interphases that suppress transition metal dissolution, lattice oxygen release, and lithium dendrite growth. The rational design of M-FGPE results in outstanding electrochemical and thermal performance, including high ionic conductivity (1.84 mS cm−1 at 25 °C), a superior Li+ transference number (0.62), and excellent thermal stability (TOER = 260.18 °C). These features enable the stable cycling of Li‖LCO cells at 4.60 V and 60 °C, achieving 76.2% capacity retention after 250 cycles, as well as reliable performance in practical 5.4 Ah pouch cells with a high energy density (435 Wh kg−1), maintaining 95.1% capacity retention over 30 cycles under stringent conditions (2 g Ah−1 electrolyte, N/P ratio = 2.5). This conjugated topological confinement strategy offers a versatile and scalable design principle for high-energy-density, high-safety, and long-term cycling lithium metal batteries.Author contributions
Wang Xu: conceptualization, visualization, methodology, and writing – original draft. Yongbiao Mu: conceptualization and visualization. Yaoyu Yin: conceptualization and visualization. Anjun Hu: investigation and writing – review & editing. Yuanjian Li: formal analysis and validation. Jian Wang: investigation and writing – review & editing. Qi Liu: validation. Jianping Long: validation. Lin Zeng: writing – review & editing, funding acquisition, project administration, and supervision. Shimou Chen: writing – review & editing, funding acquisition, project administration, and supervision.Conflicts of interest
The authors declare no conflicts of interest.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5ee04892c.
Acknowledgements
This work was supported by the Lithium Resources and Lithium Materials Key Laboratory of Sichuan Province (LRMKF202405), the National Natural Science Foundation of China (52402226 and 52425207), the Guangdong Major Project of Basic Research (No. 2023B0303000002), the Natural Science Foundation of Sichuan Province (2024NSFSC1016) and the High Level of Special Funds (No. G03034K001). W. Xu acknowledges support from Scientific Compass (https://www.shiyanjia.com/) for XPS analysis, SCI-GO (https://www.sci-go.com/) for NMR analysis, eceshi (https://www.eceshi.com/) for XRD analysis, and Ceshigo Research Service (https://www.ceshigo.com/) for providing testing services. Additionally, the author thanks Shenzhen Kejing Star Technology Company for providing the Vacuum Sealing Machine (MSK-115A-L) and Phadcalc (https://www.phadcalc.com/) for conducting molecular dynamics simulations.References
- D. Lu, R. Li, P. Yu, L. Lv, S. Yang, Y. Huang, C. Sun, S. Zhang, H. Zhang, J. Zhang, X. Xiao, T. Deng, L. Fan, L. Chen, J. Wang, E. Hu, C. Wang and X. Fan, Nature, 2024, 627, 101–107 CrossRef CAS PubMed.
- C. Lu, H. Jiang, X. Cheng, J. He, Y. Long, Y. Chang, X. Gong, K. Zhang, J. Li, Z. Zhu, J. Wu, J. Wang, Y. Zheng, X. Shi, L. Ye, M. Liao, X. Sun, B. Wang, P. Chen, Y. Wang and H. Peng, Nature, 2024, 629, 86–91 CrossRef CAS PubMed.
- Y. Chen, T. Xue, C. Chen, S. Jang, P. V. Braun, J. Cheng and C. M. Evans, Nat. Mater., 2024, 23, 1539–1546 CrossRef CAS PubMed.
- S. Zhang, R. Li, T. Deng, Q. Ma, X. Hong, H. Zhang, R. Zhang, S. Ding, Y. Wu, H. Zhu, M. Li, H. Zhang, D. Lu, B. Ma, L. Lv, Y. Li, L. Chen, Y. Shen, R. Guo and X. Fan, Nat. Energy, 2024, 9, 1285–1296 CrossRef CAS.
- Z. Cui, Z. Jia, D. Ruan, Q. Nian, J. Fan, S. Chen, Z. He, D. Wang, J. Jiang, J. Ma, X. Ou, S. Jiao, Q. Wang and X. Ren, Nat. Commun., 2024, 15, 2033 CrossRef CAS PubMed.
- S. Xia, C. Li, J. A. Yuwono, Y. Wang, C. Wang, M. Li, X. Zhang, J. Yang, J. Mao, S. Zheng and Z. Guo, Angew. Chem., Int. Ed., 2024, 63, e202409327 CrossRef CAS.
- H. Ren, G. Zheng, Y. Li, S. Chen, X. Wang, M. Zhang, W. Zhao, H. Yi, W. Huang, J. Fang, T. Liu, L. Yang, M. Liu, Q. Zhao and F. Pan, Energy Environ. Sci., 2024, 17, 7944–7957 RSC.
- B. Yang, Y. Wang, R. Zheng, W. Yang, Y. Li, T. Li, K. Li, A. Hu, J. Long and S. Ding, Angew. Chem., Int. Ed., 2025, 64, e202508486 CrossRef CAS PubMed.
- J. Chen, D. Zhang, L. Zhu, M. Liu, T. Zheng, J. Xu, J. Li, F. Wang, Y. Wang, X. Dong and Y. Xia, Nat. Commun., 2024, 15, 3217 CrossRef CAS PubMed.
- L. Wang, S. Xu, Z. Song, W. Jiang, S. Zhang, X. Jian and F. Hu, InfoMat, 2024, 6, e12551 CrossRef CAS.
- X. Zhang, P. Xu, J. Duan, X. Lin, J. Sun, W. Shi, H. Xu, W. Dou, Q. Zheng, R. Yuan, J. Wang, Y. Zhang, S. Yu, Z. Chen, M. Zheng, J.-F. Gohy, Q. Dong and A. Vlad, Nat. Commun., 2024, 15, 536 CrossRef CAS PubMed.
- K. Chen, A. Hu, G.-R. Zhu, Y. Li, J. Jiang, B. Yang, T. Li, K. Li, J. Chen, W. Xu, Z. Wang, R. Xu, W. Yang, J. Wang, G. Wu, J. Long and Z. W. Seh, ACS Nano, 2025, 19, 14284–14298 CrossRef CAS PubMed.
- S. Xia, X. Zhang, Z. Jiang, X. Wu, J. A. Yuwono, C. Li, C. Wang, G. Liang, M. Li, F. Zhang, Y. Yu, Y. Jiang, J. Mao, S. Zheng and Z. Guo, Adv. Mater., 2025, 37, 2510376 CrossRef CAS PubMed.
- M. J. Lee, J. Han, K. Lee, Y. J. Lee, B. G. Kim, K.-N. Jung, B. J. Kim and S. W. Lee, Nature, 2022, 601, 217–222 CrossRef CAS PubMed.
- K. Li, A. Hu, R. Xu, W. Xu, B. Yang, T. Li, Y. Li, Z. W. Seh, J. Long and S. Chen, Adv. Energy Mater., 2025, 15, 2501236 CrossRef CAS.
- T. Li, A. Hu, Y. Li, B. Yang, K. Li, K. Chen, J. Jiang, F. Li, Z. W. Seh, J. Wang and J. Long, Adv. Funct. Mater., 2025, 35, 2507310 CrossRef CAS.
- X. Liu, J. Zhang, X. Yun, J. Li, H. Yu, L. Peng, Z. Xi, R. Wang, L. Yang, W. Xie, J. Chen and Q. Zhao, Angew. Chem., Int. Ed., 2024, 63, e202406596 CrossRef CAS PubMed.
- J. Liu, Z. Wu, F. J. Stadler and Y. Huang, Angew. Chem., Int. Ed., 2023, 62, e202300243 CrossRef CAS PubMed.
- Y. Li, F. Ding, Y. Shao, H. Wang, X. Guo, C. Liu, X. Sui, G. Sun, J. Zhou and Z. Wang, Angew. Chem., Int. Ed., 2024, 63, e202317148 CrossRef CAS PubMed.
- W. Zou, J. Zhang, M. Liu, J. Li, Z. Ren, W. Zhao, Y. Zhang, Y. Shen and Y. Tang, Adv. Mater., 2024, 36, 2400537 CrossRef CAS.
- Y. Yin, Y. Peng, M. Zhou, P. Zhang, Y. Cheng, P. Chen, X. Xing, X. Ma, Q. Zhu, X. Sun, Q. Qian, X. Kang and B. Han, Sci. Bull., 2023, 68, 2362–2369 CrossRef CAS.
- Z. Cui, D. Wang, J. Guo, Q. Nian, D. Ruan, J. Fan, J. Ma, L. Li, Q. Dong, X. Luo, Z. Wang, X. Ou, R. Cao, S. Jiao and X. Ren, J. Am. Chem. Soc., 2024, 146, 27644–27654 CrossRef CAS PubMed.
- J. Li, J. Chen, X. Xu, J. Shen, Z. Wang, Z. Guo, P. Lin, J. Sun, B. Huang and T. Zhao, Adv. Mater., 2025, 37, 2501006 CrossRef CAS PubMed.
- X.-Y. Ma, X.-X. Wang, D.-H. Guan, C.-L. Miao, H.-F. Wang, Q.-Y. Zhu and J.-J. Xu, Angew. Chem., Int. Ed., 2025, 64, e202504767 CrossRef CAS PubMed.
- T. Di, Y. Yoshida and H. Kitagawa, J. Am. Chem. Soc., 2025, 147, 13948–13952 CrossRef CAS.
- C. Zhao, Z. Yan, B. Zhou, Y. Pan, A. Hu, M. He, J. Liu and J. Long, Angew. Chem., Int. Ed., 2023, 62, e202302746 CrossRef CAS PubMed.
- Y. Fan, Z. Chang, Z. Wu, Y. Feng, X. Du, M. Che, J. Tian, W. Xie and K. Zhang, Adv. Funct. Mater., 2024, 34, 2314288 CrossRef CAS.
- J. Mao, J. Iocozzia, J. Huang, K. Meng, Y. Lai and Z. Lin, Energy Environ. Sci., 2018, 11, 772–799 RSC.
- L. He, Y. Shao, S. Li, Y. Nie, Y. Chu, G. Feng, X. Liu, Q. Li, D. Luo, X. Wang and Z. Chen, Angew. Chem., Int. Ed., 2025, 64, e202507222 CrossRef CAS PubMed.
- R. Lin, Y. Jin, Y. Li, M. Fu, Y. Gong, L. Lei, Y. Zhang, J. Xu and Y. Xiong, Adv. Funct. Mater., 2024, 35, 2421880 CrossRef.
- Q. Liu, L. Yang, Z. Mei, Q. An, K. Zeng, W. Huang, S. Wang, Y. Sun and H. Guo, Energy Environ. Sci., 2024, 17, 780–790 RSC.
- Y. Liu, Z. Jin, Z. Liu, H. Xu, F. Sun, X. Zhang, T. Chen and C. Wang, Angew. Chem., Int. Ed., 2024, 63, e202405802 CrossRef CAS PubMed.
- H. Zhang, J. Deng, H. Xu, H. Xu, Z. Xiao, F. Fei, W. Peng, L. Xu, Y. Cheng, Q. Liu, G. Hu and L. Mai, Adv. Mater., 2024, 36, 2403848 CrossRef CAS PubMed.
- W. Xu, A. Hu, R. Zheng, Y. Li, K. Chen, J. Chen, Z. Wang, R. Xu, J. Wang, F. Li, J. Long and F. Wu, Adv. Funct. Mater., 2025, 35, e11135 CrossRef.
- C. Guo, K. Du, R. Tao, Y. Guo, S. Yao, J. Wang, D. Wang, J. Liang and S. Lu, Adv. Funct. Mater., 2023, 33, 2301111 CrossRef CAS.
- B. Ma, H. Zhang, R. Li, S. Zhang, L. Chen, T. Zhou, J. Wang, R. Zhang, S. Ding, X. Xiao, T. Deng, L. Chen and X. Fan, Nat. Chem., 2024, 16, 1427–1435 CrossRef CAS PubMed.
- P. Wang, Y. Liu, J. Cui, L. Zhao, D. Li, Y. Du and H. Li, Adv. Funct. Mater., 2024, 35, 2414430 CrossRef.
- Y. Cai, Y. Hou, Y. Lu, Q. Zhang, Z. Yan and J. Chen, Angew. Chem., Int. Ed., 2023, 62, e202218014 CrossRef CAS PubMed.
- F. Zhang, N. Qin, Y. Li, H. Guo, Q. Gan, C. Zeng, Z. Li, Z. Wang, R. Wang, G. Liu, S. Gu, H. Huang, Z. Yang, J. Wang, Y. Deng and Z. Lu, Energy Environ. Sci., 2023, 16, 4345–4355 RSC.
- X. Wu, Z. Piao, M. Zhang, G. Lu, C. Li, K. Jia, Z. Zhuang, R. Gao and G. Zhou, J. Am. Chem. Soc., 2024, 146, 14036–14047 CrossRef CAS PubMed.
- Y. Chu, Y. Mu, H. Gu, Y. Hu, X. Wei, L. Zou, C. Yu, X. Xu, S. Kang, K. Li, M. Han, Q. Zhang and L. Zeng, Adv. Mater., 2024, 36, 2405628 CrossRef CAS.
- G.-R. Zhu, Q. Zhang, Q.-S. Liu, Q.-Y. Bai, Y.-Z. Quan, Y. Gao, G. Wu and Y.-Z. Wang, Nat. Commun., 2023, 14, 4617 CrossRef CAS PubMed.
- C. Li, Y. Zhong, R. Liao, T. Yi, M. Zhou, R. Liu, S. Liu and D. Wu, Adv. Mater., 2025, 37, 2500142 CrossRef CAS PubMed.
- J. Yang, J. Yu, X. Ma, X. Zou, M. Yang, S. Sun, M. Wu, Y. Hu and F. Yan, Adv. Energy Mater., 2024, 14, 2404107 Search PubMed.
- Z. Sun, F. Li, J. Ding, Z. Lin, M. Xu, M. Zhu and J. Liu, ACS Energy Lett., 2023, 8, 2478–2487 CrossRef CAS.
- A. Zhang, Z. Bi, G. Wang, S. Liao, P. Das, H. Lin, M. Li, Y. Yu, X. Feng, X. Bao and Z.-S. Wu, Energy Environ. Sci., 2024, 17, 3021–3031 RSC.
- S. Yang, T. Meng, Z. Wang and X. Hu, Energy Storage Mater., 2024, 65, 103177 CrossRef.
- M. Zhang, K. Liu, Y. Gan, H. Wang, F. Liu, M. Bai, X. Tang, Z. Wang, S. Li, A. Shao, K. Zhou, T. Wang, Z. Wang, S. Yuan and Y. Ma, Adv. Energy Mater., 2022, 12, 2201390 CrossRef CAS.
- Y. Gao, F. Qiao, J. You, Z. Ren, N. Li, K. Zhang, C. Shen, T. Jin and K. Xie, Nat. Commun., 2022, 13, 5 CrossRef CAS PubMed.
- C. Zhu, C. Sun, R. Li, S. Weng, L. Fan, X. Wang, L. Chen, M. Noked and X. Fan, ACS Energy Lett., 2022, 7, 1338–1347 CrossRef CAS.
- W. Huang, J. Li, Q. Zhao, S. Li, M. Ge, J. Fang, Z. Chen, L. Yu, X. Huang, W. Zhao, X. Huang, G. Ren, N. Zhang, L. He, J. Wen, W. Yang, M. Zhang, T. Liu, K. Amine and F. Pan, Adv. Mater., 2024, 36, 2405519 CrossRef CAS PubMed.
- Y. Xia, P. Zhou, X. Kong, J. Tian, W. Zhang, S. Yan, W. Hou, H. Zhou, H. Dong, X. Chen, P. Wang, Z. Xu, L. Wan, B. Wang and K. Liu, Nat. Energy, 2023, 8, 934–945 CrossRef CAS.
- K. Long, S. Huang, H. Wang, A. Wang, Y. Chen, Z. Liu, Y. Zhang, Z. Wu, W. Wang and L. Chen, Energy Environ. Sci., 2024, 17, 260–273 RSC.
- G. Zhang, J. Chang, L. Wang, J. Li, C. Wang, R. Wang, G. Shi, K. Yu, W. Huang, H. Zheng, T. Wu, Y. Deng and J. Lu, Nat. Commun., 2023, 14, 1081 CrossRef CAS PubMed.
- J.-H. Kim, J.-M. Kim, S.-K. Cho, N.-Y. Kim and S.-Y. Lee, Nat. Commun., 2022, 13, 2541 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2026 |