Open Access Article
Open Access ArticleA cross-scale collaborative design of copper current collectors for practical anode-free lithium metal batteries
Jiawang Feng
a,
Tianzhengyi Zhanga,
Ming Liua,
Guoxing Tiana,
Yuqian Fan a,
Ailing Song
a,
Ailing Song
 *a,
Guangjie Shao
*ab and
Zhipeng Ma
*a,
Guangjie Shao
*ab and
Zhipeng Ma
 *ab
*ab
aHebei Key Laboratory of Applied Chemistry, Hebei Key Laboratory of Heavy Metal Deep-Remediation in Water and Resource Reuse, School of Environmental and Chemical Engineering, Yanshan University, Qinhuangdao 066004, China. E-mail: ailing.song@ysu.edu.cn; shaoguangjie@ysu.edu.cn; mazp@ysu.edu.cn
bState Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao 066004, China
First published on 23rd December 2025
Abstract
Anode-free lithium metal batteries (AFLMBs) represent the pinnacle of next-generation energy storage, promising exceptional energy densities and seamless integration with existing manufacturing infrastructure. However, their commercialization is critically hindered by the uncontrollable lithium deposition behavior on the inherently lithiophobic copper current collector (Cu CC), leading to low coulombic efficiency, dendritic growth, and rapid failure. While extensive efforts have been dedicated to modifying the Cu CC, a fragmented view often prevails, lacking a unified design principle that bridges different scales. This review moves beyond conventional summaries by proposing a novel paradigm of cross-scale collaborative design. We construct a comprehensive strategic framework that systematically integrates modification strategies from the atomic/nano-scale (crystal facet engineering, artificial SEIs), through the micro-scale (3D porous, scaffold, and gradient structures), to the macro-scale (magnetic field introduction). A particular focus is placed on elucidating the synergistic mechanisms by which these multi-scale interventions collectively regulate the initial nucleation barrier, guide homogeneous Li+ flux, and accommodate deposition-induced volume changes. Crucially, our analysis is consistently guided by the stringent performance benchmarks of the ‘zero Li inventory’ anode-free configuration, serving as a critical lens to assess the practical viability of each strategy. We further provide a forward-looking perspective on the fundamental challenges and scalable manufacturing pathways for translating these advanced current collectors from laboratory prototypes to industrial applications. This review aims to establish foundational design principles and inspire innovative solutions for developing practical high-energy-density lithium metal batteries.
Broader contextThe global transition towards sustainable energy systems is critically dependent on the development of energy storage technologies with radically higher performance. While lithium-ion batteries have dominated the landscape, their energy density is approaching its theoretical limit, creating a pressing need for post-lithium-ion technologies. The anode-free lithium metal battery configuration, which maximizes energy density at the cell and system level by eliminating the initial anode host material, stands as the most promising candidate to power the next generation of electric vehicles and grid storage. However, the fundamental challenge of controlling lithium deposition on a bare current collector has hindered its practical realization, often leading to divergent research efforts focused on isolated solutions. This review provides a pivotal synthesis of these efforts by introducing a transformative cross-scale collaborative design philosophy. By constructing a unified framework that bridges atomic-level interactions, micro-scale architectures, and macro-scale physical fields, we move beyond incremental improvements to outline a coherent roadmap for engineering the ideal lithium metal host. Our work not only consolidates the state-of-the-art but also establishes a new paradigm for rational current collector design, aiming to accelerate the transition of anode-free batteries from a high-potential concept to a commercial reality that will underpin the future of electrified transportation and a resilient power grid. |
1. Introduction
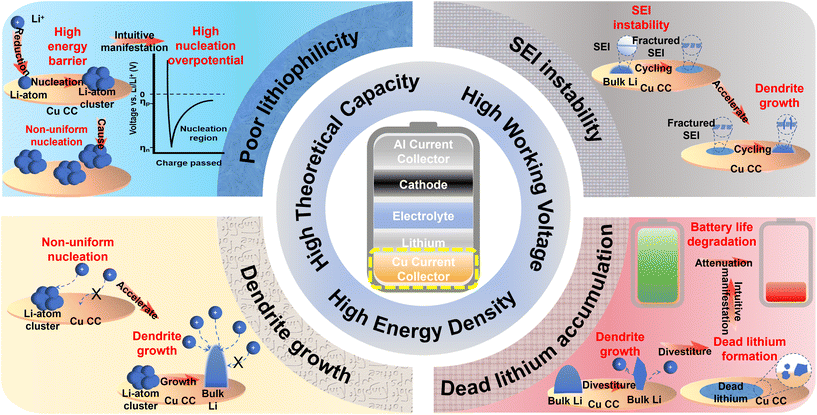
Fundamental shifts in the global energy architecture, propelled by the “dual-carbon” initiative, are intensifying performance requirements for next-generation storage technologies.1–5 Electric vehicles, low-altitude aviation, and smart grids demand electrochemical systems with drastically improved energy densities, pushing conventional graphite-anode lithium-ion batteries toward their intrinsic limitations.6–8 This impasse has renewed focus on lithium metal batteries (LMBs), recognized for their ultra-high theoretical energy density of ∼500 Wh kg−1. In particular, the anode-free lithium metal battery (AFLMB) configuration, which omits any initial anode lithium source, represents a critical advancement, achieving system-level energy densities exceeding 450 Wh kg−1 and positioning itself as a leading candidate for ultimate energy storage.9–11Translating lithium metal anodes from concept to practical implementation, however, confronts persistent scientific barriers. The inherent high reactivity of lithium and unstable electrodeposition kinetics lead to continuous electrolyte decomposition, dendritic lithium formation, and a chronically unstable solid electrolyte interphase (SEI). This cascade rapidly depletes active lithium and electrolyte, causing poor coulombic efficiency, rapid capacity decay, and substantial safety concerns.12–17
Functional modification of the current collector has emerged as a pivotal approach to addressing these issues. Moving beyond its traditional role as a passive electron conductor and mechanical support, the current collector in AFLMBs acts as an active substrate. It defines the initial nucleation behavior and spatially guides lithium deposition; its surface properties critically influence nucleation barriers, deposit morphology, and interfacial integrity.18 Commercial electrolytic copper foil, valued for its high conductivity, established processing technology, and robust mechanical properties, remains the most viable substrate. Yet its innate lithiophobicity, predominant (111) crystal faceting, and tendency to form a feeble native SEI contribute to a substantial nucleation overpotential (30–50 mV) and heterogeneous ion flux, initiating dendrite proliferation and accumulation of inactive lithium (Fig. 1).19–22
 | ||
| Fig. 1 Advantages of lithium metal batteries and issues when using electroplated copper foil as a lithium anode. | ||
While surface engineering and three-dimensional structuring of copper current collectors represent active research fronts, existing reviews often concentrate on isolated tactics without a unified, application-oriented framework.23–26 Many reported modifications are evaluated in cells with excess lithium, a condition starkly different from the “zero-lithium-inventory” reality in AFLMBs. Successful anode-free systems necessitate current collectors that achieve exceptional reversibility in the very first deposition/stripping cycle, demanding unprecedented precision and durability in their design.27–30
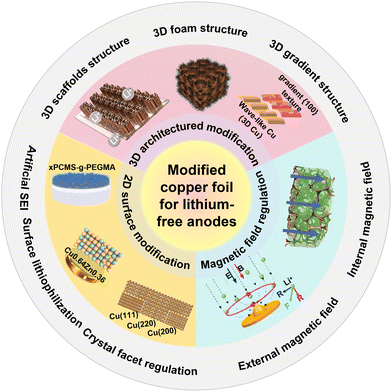
This review synthesizes prior research through an integrated “cross-scale” lens, examining functional copper collector design across atomic/nano (e.g., crystallographic orientation, lithium-philic modification), micro (e.g., artificial SEIs, porous scaffolds), and macro (e.g., field-assisted) regimes (Fig. 2 and Table 1). We elucidate the interconnections and synergies among these strategies, detailing how they collectively guide initial nucleation, homogenize ion transport, and accommodate deposition-induced strain. By critically assessing their applicability toward the anode-free configuration, this analysis aims to establish foundational principles for developing practical, high-energy-density lithium metal batteries characterized by extended cycle life and intrinsic safety.
| Scale | Strategies | Mechanism | Overpotential | CE (1st) | Dendrite | Stabilization |
|---|---|---|---|---|---|---|
| Atomic/nano | Crystal-facet engineering | Nucleation effect | Low | Medium | High | High |
| Lithium-philic modification | Nucleation effect; influences SEI | Low | Medium | Medium | Medium | |
| Micro-perspective | Artificial SEI | Regulation of Li flux; dense deposition | High | High | Low | High |
| 3D structural design | Regulation of Li flux; inhibition of expansion | High | Low | Low | High | |
| Macro-perspective | Magnetic-field introduction | Regulation of Li flux | Medium | Low | Medium | High |
2. Surface engineering in two dimensions
In lithium-ion batteries, current collectors function primarily as electron transporters and physical supports for active materials, operating without direct contact with Li+ ions or participation in faradaic reactions.31,32 The paradigm shifts fundamentally in lithium metal batteries, where the current collector evolves from a passive conductor into an electrochemically active substrate—directly mediating lithium nucleation, guiding deposition morphology, and influencing interfacial evolution.33,34 The initial nucleation behavior of lithium and the structure of the resulting solid electrolyte interphase (SEI) are decisive for deposition/stripping reversibility. Consequently, the nucleation barrier and microstructural characteristics of the collector surface become critical determinants of cycling stability.35,36 Plain commercial copper foil falls short of these requirements, making surface modification not merely beneficial but essential.372.1. Tailoring crystallographic orientation
According to classical heterogeneous nucleation theory, the nucleation size, morphology, and areal density of lithium on copper substrates are strongly influenced by the crystallographic orientation of the Cu surface.38,39 Assuming minimal surface roughness, a smaller lattice mismatch reduces the interfacial contact angle (θ) and consequently the nucleation barrier. Simultaneously, a higher surface energy enhances the adsorption of Li adatoms, thereby facilitating nucleation (Fig. 3a).40,41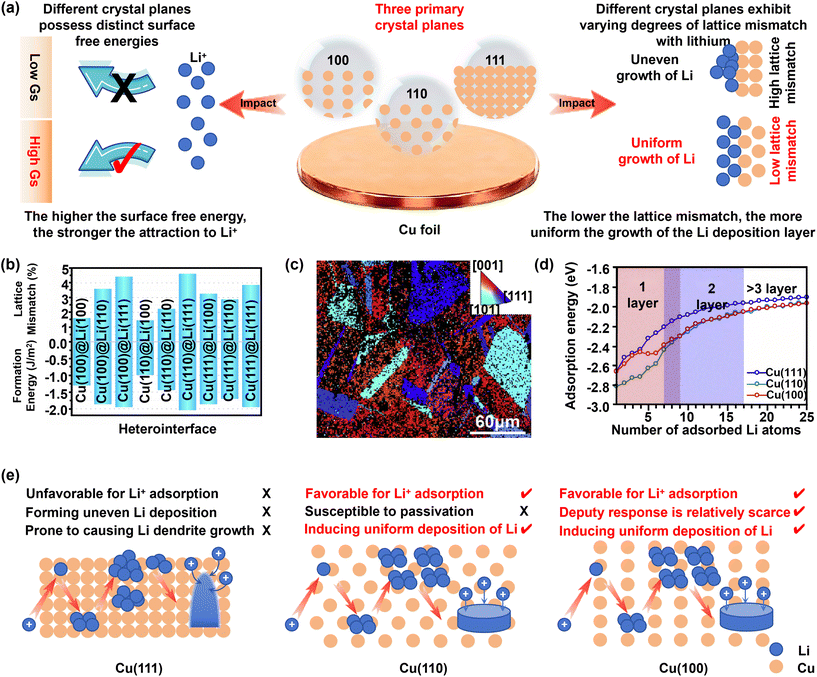 | ||
| Fig. 3 (a) Schematic diagram of the characteristics of three crystal planes. (b) Lattice mismatches and binding energies of nine heterointerfaces per unit area.44 Copyright © 2023, American Chemical Society. (c) Selective deposition of Li metal on the an-Cu current collector.45 Copyright © 2019, Elsevier. (d) Adsorption energy of Li atoms on the Cu (111), (110) and (100) planes.45 Copyright © 2019, Elsevier. (e) Schematic diagram of the effect of different crystal planes of copper foil on lithium deposition behavior. | ||
Conventional battery-grade copper foils are predominantly dominated by the (111) orientation. This facet exhibits a significant lattice mismatch with lithium and possesses the lowest surface energy among low-index Cu planes, impairing Li+ adsorption. These factors collectively contribute to a high heterogeneous nucleation barrier, often initiating uneven lithium deposition and accelerating dendrite formation.42,43
DFT and AIMD simulations by Li et al. revealed that the Cu(110)@Li(100) interface demonstrates the smallest lattice mismatch (1.46%) and the highest binding energy (−0.99 J m−2). The Cu(100)@Li(100) configuration also shows favorable characteristics, with a mismatch of 1.66% and a binding energy of −1.30 J m−2 (Fig. 3b).44
Controlling the preferential orientation of copper foil allows modulation of its surface free energy, enabling more controlled lithium deposition. Kim and colleagues discovered that Cu(100) single-crystal foil exhibits a lower Li nucleation overpotential compared to Cu(111) and Cu(110), with lithium preferentially adsorbing on Cu(100) regions (Fig. 3c). Their DFT calculations provided mechanistic insight: while Cu(110) shows the lowest Li adsorption barrier at low Li coverage, Cu(100) becomes more favorable under multi-layer Li adsorption conditions, with both significantly outperforming Cu(111) (Fig. 3d). Although a low adsorption barrier promotes lithium deposition, the Cu(110) surface is prone to passivation, which can impede subsequent lithium growth, leading to a higher observed nucleation overpotential compared to Cu(100).45 Building on this understanding, the team developed a copper current collector with a homogeneous (100) texture, achieving a tenfold increase in nucleation density and a twofold improvement in cycling stability.46 In a complementary approach, Zhen et al. demonstrated that the FSI− anion can direct the electrodeposition of copper ions along the (110) plane. The resulting highly (110)-oriented copper foil, employed in anode-free configurations, enabled uniform lithium deposition. When paired with an NCM811 cathode in a 350 Wh kg−1 pouch cell, the assembly retained 54.80% capacity after 100 cycles.47
Meanwhile, among the low-index Cu surfaces, the Cu(100) facet exhibits the strongest anion-binding capability. This tendency promotes substantial anion enrichment within the inner Helmholtz plane (IHP), which in turn facilitates the formation of an inorganic-rich SEI such as LiF and Li3N, while improving both its mechanical robustness and the ionic transport characteristics. Experimental studies have shown that anions including FSI− and TFSI− in ether-based electrolytes, as well as PF6− and ClO4− in ester-based systems, interact strongly with the Cu(100) surface, ultimately giving rise to a mechanically stiff and ionically conductive inorganic interphase.48 In electrolyte systems with inherently higher anion concentrations, including localized high-concentration electrolytes (LHCE) and ionic liquids, the influence of such facet-dependent interactions is expected to be even more significant according to theoretical predictions. Nevertheless, direct experimental confirmation in these systems is still limited, indicating that further systematic investigation is required to fully elucidate these effects.
Collectively, both Cu(100) and Cu(110) facets offer superior lithiophilicity over Cu(111), attributed to their reduced lattice mismatch and elevated surface energy, leading to more homogeneous nucleation and enhanced cycling stability. However, the excessively high surface energy of Cu(110) makes it susceptible to passivation via side reactions, thereby diminishing its lithiophilicity. Considering the overall balance of properties, Cu(100) emerges as the most promising orientation due to its optimal lattice matching and moderate surface energy (Fig. 3e).
However, it should be noted that the current mainstream methods for preparing single-crystal copper foils are still CVD and high-temperature degradation. For anode-free lithium metal batteries, these methods can neither meet the requirements of large-scale production nor reduce costs, making the facet control strategy difficult to implement. Developing single-crystal electroplating technology is an effective approach for achieving low-cost and large-scale production. The detailed parameters of the three production modes are listed in Table 2. In addition, for anode-free lithium metal batteries, electrodeposition provides a thickness-matched, crystallographically tailored, and manufacturing-compatible route that is inaccessible to both CVD and thermal recrystallization approaches. Electrodeposition uniquely enables direct fabrication of µm-thick single-crystal Cu foils under ambient conditions with high areal throughput and roll-to-roll compatibility, offering a collector-grade pathway intrinsically aligned with the manufacturing demands of anode-free lithium metal batteries. In contrast, CVD is fundamentally limited to thin-film regimes and serves primarily as a model platform, while high-temperature annealing suffers from stochastic grain evolution and low effective single-crystal yield.
| Metric | Electrodeposition | CVD | High-T annealing |
|---|---|---|---|
| Processing environment | Ambient pressure, wet process | High vacuum, gas-phase | High temperature, controlled atmosphere |
| Typical temperature | RT to 80 °C | 700–1000 °C | 900–1050 °C |
| Thickness suitability (10 µm) | Directly accessible | Fundamentally inefficient | Dependent on starting foil |
| Single-crystal scalability | Large-area, continuous | Limited by chamber size | Stochastic, non-uniform |
| Production mode | Roll-to-roll/continuous | Batch | Batch |
| Areal throughput | µm min−1 | nm to sub-µm min−1 | Hours per batch |
| Post-treatment process | No/optional mild annealing | Mandatory annealing | Mandatory annealing |
| Unit cost (10 µm) | 6000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 CNY m−2 (prediction) 000 CNY m−2 (prediction) |
≥30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 CNY m−2 000 CNY m−2 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–30 000–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 CNY m−2 000 CNY m−2 |
| Substrate transfer | Not required | Often required | Not required |
| Compatibility with battery manufacturing | High | Low | Medium |
| Relevance to anode-free LMBs | Intrinsic collector-grade foil | Model system only | Limited by grain boundaries |
| Overall industrial viability | High | Low | Medium–low |
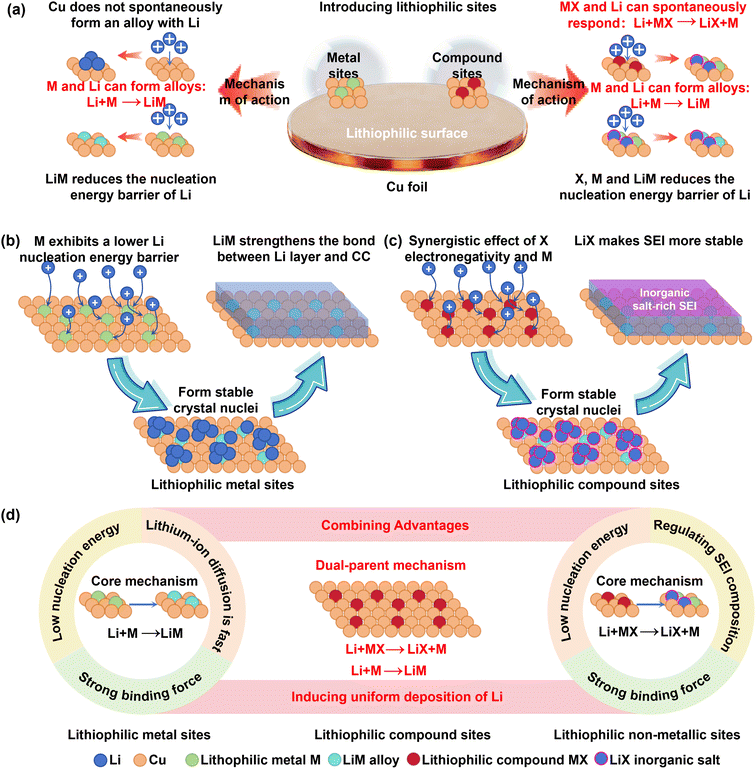
2.2. Enhancing surface lithiophilicity
Beyond crystallographic tuning, introducing lithiophilic sites on the collector surface is an effective strategy to reduce the lithium nucleation barrier.49 “Lithiophilicity” describes the affinity of a material or surface for lithium atoms, ions, or metal. Stronger lithiophilicity implies more facile and stable interactions with lithium. Lithiophilic sites are generally categorized into metallic sites and compound-based sites.50–52 Metallic sites operate through alloying reactions with lithium, while compound sites often leverage the electronegativity of anionic groups combined with cation replacement reactions to direct lithium deposition, thereby extending cycling life (Fig. 4a).During initial lithium deposition, the nucleation overpotential correlates with the solubility and lithiophilicity of the metal substrate. Metals like Au, Ag, Zn, and Mg, which exhibit significant solubility in lithium, can achieve near-zero nucleation overpotentials upon full lithiation. In contrast, metals with lower solubility, such as Al and Pt, show small but measurable overpotentials (5 mV and 8 mV, respectively).53 Introducing lithiophilic metals not only lowers the nucleation barrier, facilitating the formation of stable nuclei, but also helps homogenize the Li+ flux, preventing localized aggregation.7 For instance, decorating copper foil with Ag nanoparticles, a Cu0.64Zn0.36 alloy layer, or a Sn layer significantly reduces the nucleation overpotential. Deposition preferentially occurs at these lithiophilic sites, promoting uniform Li+ flux distribution. Notably, Sn(111) can induce the oriented growth of lithium along specific crystallographic directions (Li7Sn2(021) and Li22Sn5(822)), further enhancing deposition uniformity.58,60–63
Beyond nucleation, lithiophilic modifiers influence the subsequent growth stage by altering the dimensionality and migration rate of Li+ diffusion on the collector surface.64 For example, coating copper with liquid metal Ga forms an in situ CuGa2 alloy layer that anchors the Ga. This modification reduces the lateral diffusion resistance for lithium, favoring two-dimensional planar deposition over dendritic growth, thereby improving the quality and stability of the lithium layer.60
Strong adhesion between the lithium deposit and the collector is crucial for performance stability. Insufficient adhesion can lead to detachment during repeated cycling, generating “dead Li” and capacity fade.65–67 Thin layers of metals like Au, Ag, or Pt enhance adhesion by forming intermetallic compounds or solid solutions with lithium. This improved mechanical integrity helps maintain deposit stability even under high current densities and prolonged cycling.62,68,69 It is noteworthy, however, that Au and Ag can exhibit excessive diffusion into lithium over time, gradually reducing surface lithiophilicity. In contrast, Pt, with its lower solubility, maintains more consistent lithiophilic properties. In addition, Zn, Sn and other metals have good solubility in Cu, and the high stability of this alloy state (solid solution) can make such lithium-philic metals exist stably on the surface of the collector, thus ensuring the long-term stability of the lithium-philicity.59,70 Furthermore, despite their high cost, ultrathin Au and Ag layers are still used in specific research contexts due to their excellent lithiophilicity and well-defined interfacial energetics. They are particularly useful when highly uniform nucleation or isolation of intrinsic deposition mechanisms is required, such as in pilot-scale demonstrations, extreme fast-charging (XFC) performance evaluations, and model interfacial studies. In practical scenarios demanding cost-effective implementation, low-cost lithiophilic materials (e.g., Sn-based, Zn-based, and Ga-based coatings) serve as scalable alternatives. These materials reduce nucleation barriers through alloying or interfacial substitution reactions; however, compared with noble metals, they involve different trade-offs in mechanical and electrochemical properties.
In essence, leveraging alloying reactions enhances affinity, lowers nucleation barriers, and promotes stable nucleus formation. These modifications alter surface properties (roughness, adhesion), enrich active sites, and homogenize Li+ distribution. Furthermore, the formation of solid solutions (atomic diffusion) or intermetallic phases (chemical bonding) strengthens the metallurgical bond between the lithium layer and the copper collector, mitigating detachment and ensuring cycling stability.71–74
Copper surfaces can be directly functionalized with these elements via etching or thermal treatment.86–88 For example, selective etching creates a textured surface where formed CuCl structures act as “lithiophilic cages”, significantly increasing the binding energy with Li and fostering a homogeneous, stable LiCl-rich SEI. This leads to uniform Li+ flux and deposition, enabling an LFP∥etch-Cu full cell to cycle stably for 500 hours (0.5C, CE ≈ 96%).88 Alternatively, constructing an Ag@CuO heterostructure introduces oxygen.77 During lithium deposition, a displacement reaction with CuO generates lithiophilic Li2O, lowering the nucleation overpotential and promoting uniform nucleation. The in situ formed Li2O-rich SEI further enhances Li+ flux, guiding uniform deposition.
The displacement reaction strategy (Li + MX → M + LiX) can be extended by replacing the non-lithiophilic Cu with other lithiophilic metals.89 These compounds significantly impact deposition behavior. Depositing a 50 nm ZnF2 layer on copper, for instance, drastically reduces the nucleation overpotential from 79 mV to 11 mV (at 1 mA cm−2), benefiting from the presence of lithiophilic Zn, LiF, and LiZn. This also increases the LiF content in the SEI, improving Li+ flux and mechanical stability, enabling stable cycling even under high areal capacity (6 mAh cm−2).90
Therefore, introducing lithiophilic metals addresses the high nucleation barrier on copper, promoting uniform deposition and extending battery life. Incorporating non-metallic lithiophilic elements reduces the nucleation barrier by enhancing binding strength and modifying SEI composition. Compound-based lithiophilic modifiers, leveraging a dual-affinity mechanism, combine the advantages of both metallic and non-metallic elements, exerting the most profound influence on lithium deposition (Fig. 4d). In addition, surface lithiophilic modification may alter the interfacial double-layer structure and the surface state of the deposited lithium. Such changes could influence the adsorption and decomposition behavior of anions in the electrolyte, thereby modifying the composition and functionality of the resulting SEI. These interfacial effects require further investigation to deepen the mechanistic understanding of anode-free systems and to support the practical advancement of AFLMBs. Notably, the processes for introducing elements like Zn, Sn, or ZnF2 are relatively straightforward and low cost, presenting promising prospects for industrial scaling and accelerating the practical application of lithium metal batteries.72–77
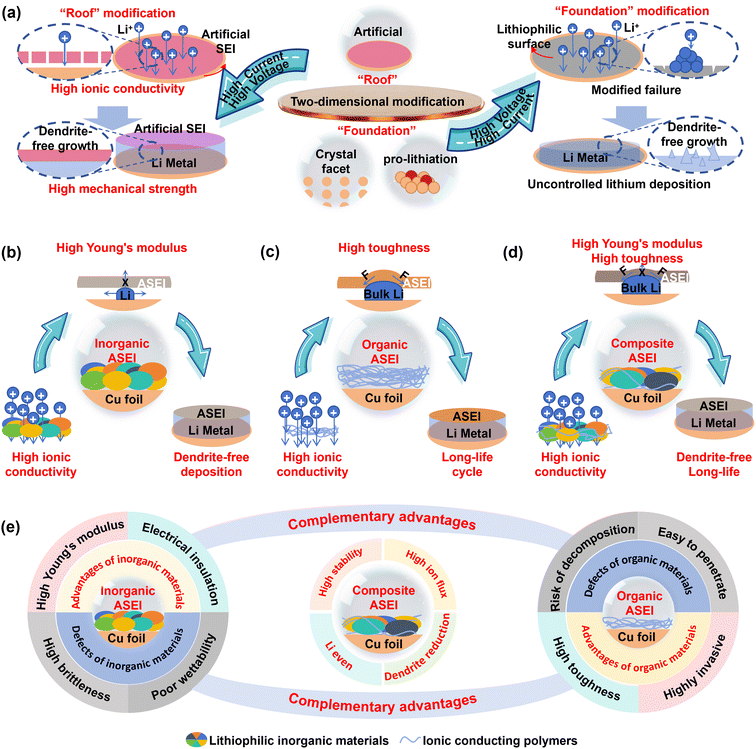
2.3. Constructing an artificial SEI (ASEI)
While surface lithiophilization effectively regulates lithium deposition in the nucleation and early growth stages, its efficacy can diminish at high currents (due to tip-enhanced dendrite growth) or with increasing deposit thickness (as lithium overburden covers the modified surface).63 To address these limitations, research focus has expanded from the “foundation” (the substrate) to the “roof”—the SEI.An ideal SEI should exhibit high Li+ conductivity, excellent electrolyte wettability, robust physicochemical stability, and high mechanical strength.91 Naturally formed SEIs, however, are often unstable, comprising irreversible and unstable electrolyte reduction products (e.g., Li2O, Li2CO3, ROCO2Li, ROLi). Their continuous decomposition during cycling leads to side reactions, thick SEI formation, and electrolyte depletion, exacerbating active Li loss and inhomogeneous Li+ flux, which in turn promotes dendrite growth and short circuits.92
Artificial solid electrolyte interphases (ASEIs) offer a “top-down” design strategy. By precisely controlling composition, ASEIs can isolate the lithium layer from the electrolyte, minimize parasitic reactions, and guide uniform lithium deposition—even under high current densities and capacities—acting like a protective “quilt” over the copper foil. As lithium deposits and accumulates, this “quilt” is pushed upward, remaining functional (Fig. 5a).93
ASEIs can be categorized into inorganic, organic, and composite types. Inorganic ASEIs, typically composed of lithiophilic inorganic materials (Li3N, LiF, ZrO2, Li3Sb, Li3Bi, etc.), offer extremely high Li+ flux (supporting high-current cycling) and a high Young's modulus (resisting dendrite penetration) (Fig. 5b).94 For instance, a dense, uniform inorganic ASEI comprising ZrO2, Li2O, Li3N, and LiNxOy was formed via spontaneous reaction between lithium and a ZrO(NO3)2 solution. The abundance of grain boundaries in this multi-component ASEI facilitates rapid Li+ diffusion, while its high modulus enables stable cycling for over 550 hours under demanding conditions (10 mA cm−2, 10 mAh cm−2).95
Hybridizing lithium salts with high modulus and lithium alloys with high diffusion coefficients can yield ASEIs with high ionic conductivity, electronic insulation, and thermodynamic stability. For example, a hybrid Li3Sb/LiF ASEI leverages the ultra-high Li+ diffusion coefficient of Li3Sb (2.0 × 10−4 cm2 s−1) to eliminate diffusion barriers at high currents (up to 20 mA cm−2), while LiF provides electronic insulation and mechanical strength.96 Similarly, a Li3Bi/LiF hybrid ASEI demonstrates high ionic conductivity (3.5 × 10−4 S cm−1), high resistivity (9.04 × 104 Ω cm), and a high modulus (1348 GPa), ensuring uniform deposition at 20 mA cm−2.97 However, the high modulus of inorganic ASEIs often comes with brittleness. The significant volume changes during cycling can cause fracture, allowing electrolyte penetration and dendrite growth. Furthermore, their poor electrolyte wettability can lead to high interfacial polarization.98,99
Organic ASEIs are primarily based on polymer matrices functionalized with ion-conducting groups. These polymers offer good ionic conductivity, high flexibility (accommodating volume changes), and superior electrolyte wettability due to their functional groups (Fig. 5c).100–102 For instance, incorporating porous xPCMS-g-PEGMA nanofillers into a lithiated single-ion conductive Nafion membrane enhances its mechanical strength via a hypercrosslinked core, while the PEGMA chains ensure homogeneous composite formation and facilitate efficient Li+ transport. This ASEI enables a 2800-hour cycle life under 10 mA cm−2 and 10 mAh cm−2.103 Using a dynamic imine-based gel formed from flexible telechelic PEG and rigid chitosan also achieves a 3200-hour cycle life under similar conditions.104 Drawbacks of organic ASEIs include generally lower mechanical strength (increasing dendrite penetration risk) and potential decomposition at high voltages.105
Composite ASEIs synergistically combine inorganic and organic components, aiming to achieve high ionic conductivity, thermodynamic stability, excellent wettability, dendrite suppression, and stress tolerance simultaneously (Fig. 5d).106 One design features a multicomponent gradient ASEI with an outer layer of lithiated fluorinated carboxylate and an inner layer of LiF, Li2S, and Li2SO3. This structure provides enhanced Li affinity and wettability, enables rapid Li+ transport (supporting 15 mA cm−2), suppresses dendrites, and ensures uniform deposition.107 Another approach uses a LiBr-HBU layer, creating a uniform, dense hybrid ASEI with excellent electrolyte wettability/stability and high ionic conductivity (2.75 × 10−4 S cm−1, ∼50 times that of native SEIs). Symmetric cells with this modification show significantly improved performance, especially at high current densities (1333 hours at 15 mA cm−2).108
Overall, the instability of native SEIs severely limits battery performance. ASEIs, through rational design and controlled composition, overcome these limitations, ensuring high ionic conduction, stability, and safety, making them a cornerstone technology for practical LMBs. Inorganic ASEIs excel in high-rate scenarios due to their high conductivity and modulus but suffer from brittleness. Organic ASEIs offer superior toughness for high-capacity cycling but face challenges in mechanical strength and voltage stability. Composite ASEIs merge these advantages, using organic phases to absorb stress and inorganic phases to provide strength and block decomposition pathways, ultimately delivering exceptional performance under both high-current and high-capacity conditions (Fig. 5e). Meanwhile, the ASEI can effectively suppress the occurrence of parasitic reactions by isolating the deposited lithium from direct contact with the electrolyte. Its high stability can mitigate corrosion-driven interfacial degradation and significantly reduce the risk of gas generation and explosion.
The path to widespread application, however, involves overcoming hurdles related to process complexity, scalability, and cost. Many current ASEI fabrication methods rely on sophisticated techniques like ALD or CVD, which are time-consuming and challenging to scale. Furthermore, the synergistic mechanisms within multi-component composite ASEIs require deeper understanding. Future efforts should focus on developing simple, low-cost manufacturing techniques and elucidating composition–structure–property relationships to accelerate the deployment of high-energy-density lithium metal batteries.109
3. Three-dimensional architecture design
While 2D copper current collector modifications effectively regulate lithium deposition under moderate conditions, their efficacy diminishes under high current densities or thick lithium plating. This limitation arises from the dynamic shift in the deposition interface—from the electrolyte–collector boundary to an electrolyte–lithium interface—which progressively attenuates the surface modification effects. Concurrently, substantial volumetric expansion and accumulated mechanical stress can induce structural failure.110 Under high current operation, the disparity in activity across surface sites is amplified, triggering heterogeneous lithium deposition and accelerating dendrite formation.111In contrast, rationally designed 3D copper architectures present a paradigm shift. Their high specific surface area provides abundant nucleation sites while enabling more homogeneous electric field distribution and Li+ flux redistribution, collectively guiding uniform, dendrite-free lithium deposition.112 Current 3D Cu CC designs are primarily categorized into scaffold structures, porous frameworks, and gradient architectures.
3.1. Scaffold-structured frameworks
3D scaffolds typically consist of nano/microscale fibrous networks integrated onto a 2D copper foil substrate (Fig. 6a).113 For instance, the etching and subsequent reduction of copper foil creates a submicron scaffold with nanoscale protrusions. This design significantly increases the specific surface area to disperse local current density, provides efficient electron conduction pathways, and ensures structural stability. The protruding tips on each fiber act as active nucleation sites, promoting uniform electric field distribution for dendrite-free deposition.114 Alternatively, a one-step coelectrodeposition method can fabricate a 3D copper-based scaffold by depositing carbon nanotubes (CNTs) and copper simultaneously onto a foil. The uniform copper coating on CNTs prevents excessive exposed carbon that could trigger additional SEI formation. After calendaring to optimize density, the Li∥3D Cu/CNT half-cell maintains a high coulombic efficiency of 99% over 800 cycles.115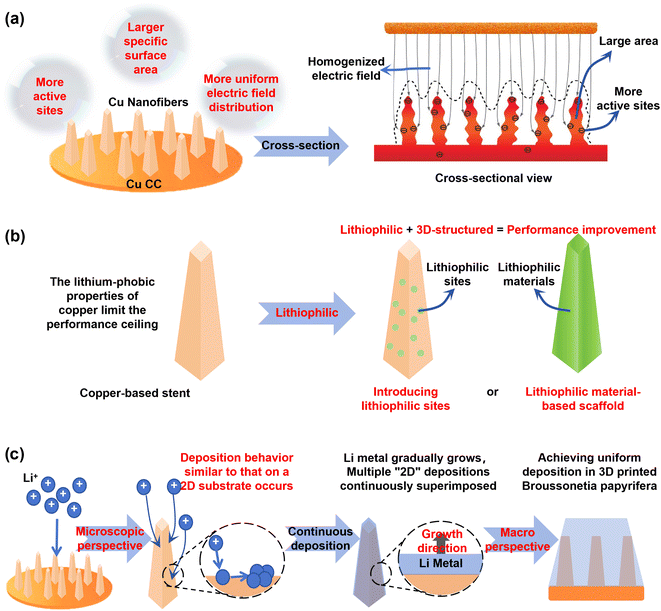 | ||
| Fig. 6 (a) Schematic diagrams of 3D scaffold structures, along with its cross-sectional view. Copyright © 2015, C. Yang et al.114 (b) Schematic diagram of lithiophilic scaffold construction. (c) Schematic diagram of uniform Li deposition induced by scaffold structure. | ||
Despite these improvements, the intrinsic lithiophobicity of copper ultimately limits the performance ceiling of 3D scaffolds. Integrating lithiophilic modifications has thus become a crucial strategy for enhancing their electrochemical performance (Fig. 6b).116 For example, magnetron sputtering was used to selectively decorate copper fibers with lithiophilic tin sites or an ultrathin zinc oxide layer. By enhancing electron conduction and Li+ diffusion rates, these modifications successfully guided uniform lithium deposition. The 3D scaffold's high surface area and reduced local current density, combined with uniformly distributed tin sites, created a rapid electron-ion transport network. This synergy enabled a symmetric cell with the Sn–Cu collector to achieve an extended cycle life of nearly 1500 hours at 3 mA cm−2 and 6 mAh cm−2.59 Similarly, a copper scaffold coated with an ultrathin Zn layer demonstrated exceptional stability in symmetric cells (4 mA cm−2, 500 h) and full cells (81% capacity retention after 1000 cycles).117
Beyond metallic coatings, scaffolds incorporating lithiophilic metals, carbon-based materials, and metal–organic frameworks (MOFs) have been widely explored.118–121 One design features a 3D Cu/CNT/Sn composite collector with a tin-coated CNT scaffold on copper foil.120 Another innovative approach synthesized a Co@Zn-CNT scaffold grown in situ on cobalt centers, with atomically dispersed zinc species on the outer sheath.121 The CNT matrix enhances structural stability, while the Sn layer or Zn species serve as lithiophilic sites to guide uniform Li deposition. The modified Co@Zn-CNT-Cu electrode enables dense, uniform lithium deposition even at high areal capacities (10 mAh cm−2) while maintaining excellent cycling stability and capacity retention.
In essence, the nanofibrous network partitions the lithium deposition process into numerous independent microdomains (Fig. 6c). Within each domain, deposition occurs on a stable fibrous substrate, mimicking the homogeneous mechanism on 2D foil. This structural segmentation achieves uniform electric field distribution, promotes homogeneous Li+ flux, suppresses dendrite growth, and ultimately induces uniform lithium deposition across the entire electrode.
3.2. Porous framework designs
Porous structures offer an even higher specific surface area than scaffolds, providing more active sites and more uniform electric field distribution.122 Their interconnected pore channels ensure efficient Li+ diffusion, further inhibiting dendrite growth.123,124 Unlike scaffolds that provide deposition guides, porous skeletons partition space into interconnected compartments. Li+ initially deposits onto the skeleton, and these compartments are gradually filled during plating, eventually forming a uniform lithium coating that encapsulates the structure (Fig. 7a).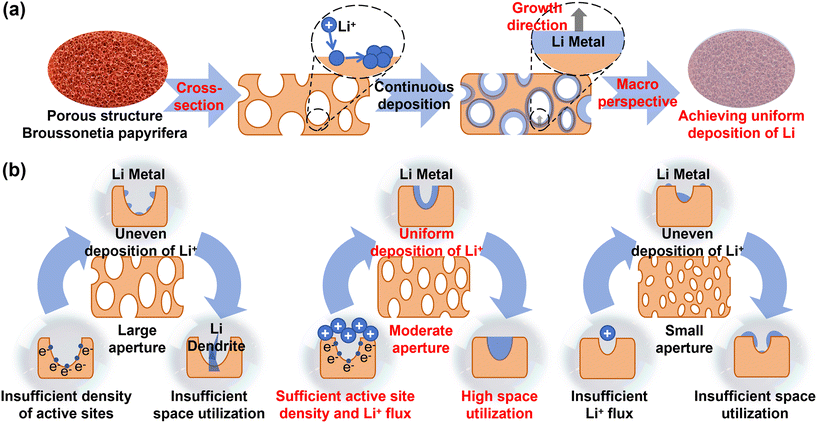 | ||
| Fig. 7 (a) Schematic diagram of uniform Li deposition induced by porous structure. (b) Schematic diagram of the effect of pore size on Li deposition. | ||
For instance, electrochemical dealloying of zinc from brass produces a 3D porous copper structure with an average thickness of ∼14 μm and ∼72% porosity. This collector effectively suppresses lithium dendrite growth even at high areal capacities (10 mAh cm−2) and current densities (10 mA cm−2).125 Alternatively, electrodeposited 3D interconnected copper foam with a “lithium dendrite cage” structure utilizes its internal pores to accommodate dendrites, inhibiting vertical growth and mitigating volume changes.126
Introducing lithiophilic sites remains an effective strategy to overcome copper's inherent lithiophobicity in 3D porous frameworks. Researchers have loaded Cu2Se/Cu2O heterostructured nanowires as kinetically enhanced nucleation sites. Leveraging the synergy between optimized pore structure and heterostructured nanowires, the Cu2Se/Cu2O@3D Cu composite collector exhibits exceptional dendrite-suppression capability and electrochemical performance: an ultra-long symmetric cell lifespan of 3000 hours at 1 mA cm−2, remaining stable for 300 h even at 20 mA cm−2.127
Pore size critically influences deposition behavior (Fig. 7b). Excessively large pores reduce active site density, while overly small pores impede Li+ diffusion.127 Commercial copper foam, with its large pore sizes (150–400 μm), often suffers from insufficient conductive contact and physical support within the pores, reducing deposition/stripping reversibility and inevitably leading to dendrites and failure.128 Using the hydrogen bubble template (HBT) method, Guo et al. prepared a series of 3D porous copper collectors with varying pore sizes and wall structures. The best performance was achieved with a 3D Cu structure featuring an average surface pore size of 25.18 μm and the lowest distribution probability (32.08%) of macropores (100–250 nm) in the pore walls.127
3.3. Gradient architectures
The performance gains from scaffold and porous designs confirm that 3D structures effectively regulate internal electric fields and Li+ flux.129,130 However, in practical application, homogenous 3D frameworks like standard copper foam suffer from current concentration effects. Lithium preferentially nucleates and grows on the top surface, progressively blocking the structure and hindering Li+ transport to the interior, thus underutilizing the 3D framework (Fig. 8a).131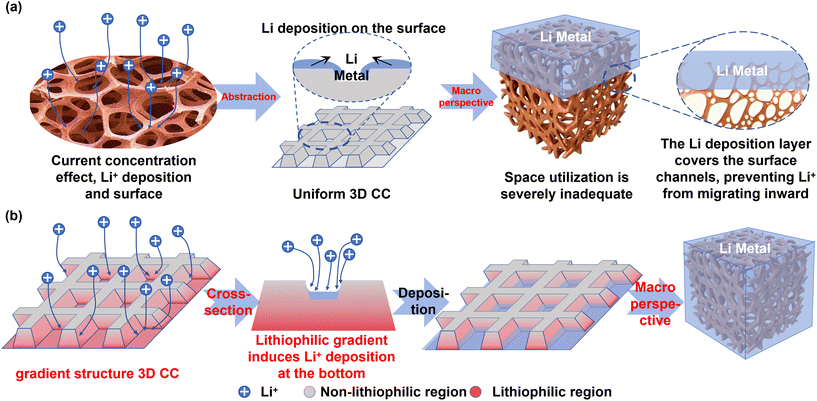 | ||
| Fig. 8 (a) Schematic diagram of the effect of current concentration on Li deposition behavior. (b) Schematic diagram of the effect of 3D gradient structure on Li deposition behavior. | ||
Constructing gradient structures by introducing varying lithiophilic properties from the interior to the surface can significantly improve spatial utilization and promote denser lithium deposition (Fig. 8b).132,133 As established, the Cu(100) facet exhibits superior lithiophilicity compared to Cu(111). Leveraging this difference, a gradient structure can be created. For instance, inducing a preferential Cu(100) orientation at the bottom and a Cu(111) orientation at the top of a wavy or grooved copper collector forms a lithiophilicity gradient. This design promotes preferential deposition at the bottom, alleviating current concentration at the top, homogenizing the electric field, suppressing dendrites, and directing more Li+ to deposit internally, thereby enhancing spatial utilization efficiency. A wavy copper substrate with a high, gradient Cu(100) texture (ranging from 96.80% in the valleys to 47.10% on the peaks) used as a current collector in AFLMBs demonstrated remarkable capacity retentions of 87% (120 cycles) with LiFePO4 and 77% (110 cycles) with LiNi0.8Co0.1Mn0.1O2 cathodes. This design successfully achieved the preferential deposition of Li at the bottom.134,135 Beyond crystallographic control, a dual-gradient structure was created by doctor-blading a liquid metal (GaInSn) layer onto copper foam as a gradient lithiophilic layer and depositing a nanoscale gold layer at the bottom as a conductive gradient. This design endowed the electrode with exceptional cycling performance: a half-cell lifespan exceeding 850 hours (5 mAh cm−2), a high pore utilization rate of 88.80%, and no dendrite formation. The corresponding full cell with LiFePO4 cathode sustained over 2000 cycles with 80% capacity retention at 1C.136
In conclusion, 3D current collector design demonstrates significant advantages in reducing local current density and promoting uniform lithium deposition, establishing itself as a potent strategy for optimizing lithium metal anodes. However, a critical consideration for their application in AFLMBs—which lack supplemental active lithium—is their high surface area, which necessitates extensive SEI formation. The continuous reformation and fracture of the SEI during cycling can drastically accelerate the irreversible consumption of the limited lithium inventory, severely limiting the cycle life of AFLMBs.137 Consequently, research on 3D copper current collectors for AFLMBs remains at a nascent but highly promising stage of exploration.
4. Magnetic field-enhanced current collectors
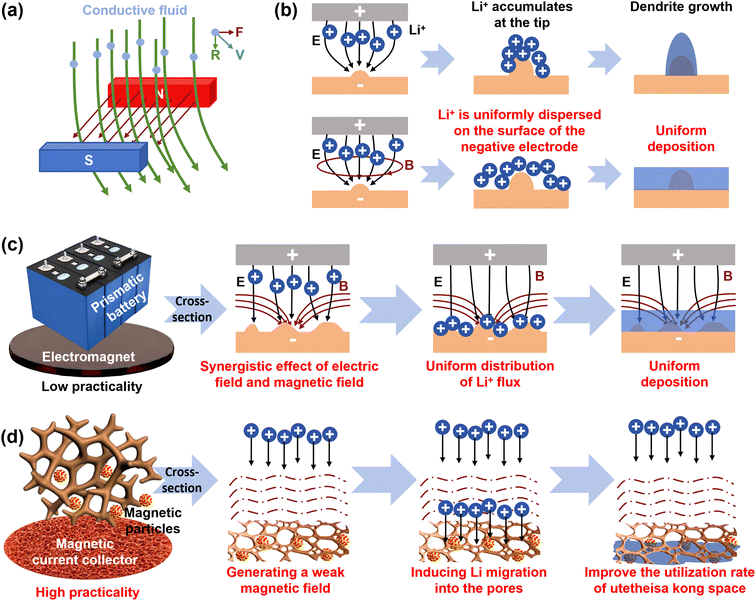
In industrial electroplating, magnetic fields have long been employed to refine grain structure, control texture, and direct dendrite growth in metal deposits.138,139 This approach leverages the magnetohydrodynamic (MHD) effect, where Lorentz forces act on conductive fluids (e.g., liquid metals, electrolytes) in a magnetic field, inducing convective flows that enhance mass transport and homogenize ion distribution (Fig. 9a). Inspired by this mature technology, introducing magnetic fields to regulate lithium deposition has emerged as a highly effective strategy.140–143 During battery operation, the steady migration of Li+ to the negative electrode resembles a micro-scale electroplating process.144,145 Under a sole electric field, inherent field inhomogeneities cause uneven Li+ concentration at the collector surface, accelerating dendritic growth. The incorporation of a magnetic field uniformly generates multiple MHD micro-vortices within the cell. This micro-circulation of the anode-side electrolyte enhances mass transport, homogenizes the Li+ concentration at the interface, and ultimately promotes uniform lithium deposition (Fig. 9b).146–148Magnetic field application is typically categorized into external and internal configurations. Applying an external magnetic field to the lithium metal anode, for instance, subjects Li+ to Lorentz forces during electrodeposition, inducing helical motion and activating the MHD effect. This results in markedly more uniform lithium deposition (Fig. 9c), significantly improving cycling lifespan and coulombic efficiency.148 However, external fields, often generated by electromagnets, can lead to bulky, heavy battery systems. Furthermore, in practical applications, external magnetic fields may cause electromagnetic interference, disrupting the operation of sensitive integrated electronics.149
To circumvent these issues, research has shifted focus to internally generated magnetic fields by decorating copper current collectors with magnetic materials, particularly ferromagnetic substances.150 Constructing a magnetic copper collector not only leverages the MHD effect for uniform plating but also addresses the challenge of insufficient spatial utilization in 3D frameworks (Fig. 9d).151,152 For example, a magnetic collector was designed by growing MOF-74(Ni) on a copper foam (CF) substrate.153 The introduced high-porosity magnetic MOF-74 effectively reduced local current density, provided abundant nucleation sites, and significantly enhanced Li+ mobility at the collector surface. This synergy suppressed dendrite growth, fostered a stable SEI, and enabled deep lithium deposition, with Li+ mobility increasing approximately sixfold compared to a non-magnetic environment.
Further enhancing this concept, Sheikholeslami et al. reported that smaller ferromagnetic particles (e.g., Fe–Co–Ni alloys) generate stronger driving forces under an applied field.154 The superimposed effect of the magnetic field and ferromagnetic particles can more effectively accelerate Li+ migration, facilitating dense lithium deposition deep within the CF scaffold. Building on this, a 3D copper-based magnetic and lithiophilic collector (CNZ) was constructed by integrating a Ni–Co alloy (internal micro-magnet) with ZnO (lithiophilic sites).155 The micro-magnetic field generated by the alloy boosted the depth of lithium deposition from 300 μm in plain CF to 1000 μm, while the ZnO sites reduced the nucleation overpotential, enabling a uniform and dense lithium metal layer.
Notably, in lithium–sulfur (Li–S) batteries, magnetic current collectors exert positive effects on both electrodes. Beyond the anode-side MHD effect, the magnetic catalytic effect (MCE) allows for tailoring the catalytic properties of ferromagnetic materials by modulating their electronic structure.156,157 For instance, a magnetic field can trigger a spin-state transition in Co atoms of a ferromagnetic CoSx catalyst from low-spin to high-spin, generating unpaired 3d electrons and lowering the free energy barrier for lithium polysulfide (LiPS) redox reactions.158 Moreover, under a magnetic field, unconventional d–p hybridized ferromagnetic Fe3M (M = Al, Si, Ga, Ge, Sn) materials exhibit significantly enhanced d–p hybridization near the Fermi level due to increased electron cloud overlap between M and Fe atoms, substantially boosting the overall kinetics of the Li–S reaction system.159 These findings demonstrate that magnetic collectors can holistically improve Li–S battery performance and provide a promising development pathway for future designs.
In conclusion, existing studies generally suggest that the introduction of internal magnetic fields can trigger MHD convection in the electrolyte, which effectively homogenizes lithium-ion flux near the electrode surface. As a result, lithium deposition under magnetic-field-assisted conditions tends to be more spatially uniform, with suppressed concentration gradients and the formation of relatively dense and compact lithium layers compared with magnet-free systems.147 Nevertheless, current evidence regarding the role of magnetic fields in preferential lithium deposition remains limited. Reports that directly correlate magnetic-field-induced ion transport with facet-selective growth, orientation preference, or intrinsic crystallization pathways of lithium are still scarce. The lack of systematic experimental data and quantitative analysis makes it challenging to decouple transport-driven effects from intrinsic deposition kinetics, thereby hindering the development of accurate and predictive theoretical models that describe magnetic-field-mediated lithium deposition. Addressing this knowledge gap will require coordinated experimental design and modeling efforts in future studies.
In addition, it is worth noting that despite the promising role of magnetic-field-assisted strategies in regulating lithium-ion flux and deposition behavior, it should be emphasized that current studies remain largely confined to coin-cell or symmetric-cell configurations. Existing experiments primarily focus on demonstrating the feasibility of magnetohydrodynamic (MHD)-induced convection in suppressing concentration polarization and regulating initial lithium nucleation at small scales. To date, there are no systematic reports on the implementation of embedded magnetic structures in stacked pouch-cell configurations, and consequently, quantitative evaluation of flux uniformity and long-term structural stability at the device-relevant scale remains elusive.
From a practical perspective, extending magnetic-field-assisted designs to stacked pouch cells introduces several unresolved challenges, including maintaining uniform magnetic field distribution across multilayer electrodes, ensuring sufficient penetration depth and the effectiveness of magnetic structures in thick electrodes, and understanding the coupled evolution of magnetic functionality and electrochemical stability during prolonged cycling. Addressing these issues will be essential for translating magnetically modulated lithium deposition from proof-of-concept demonstrations toward realistic anode-free lithium metal battery systems.
5. Summary and prospective
Lithium metal batteries represent a pivotal direction for next-generation high-energy-density storage, with the core challenge lying in controlling lithium deposition behavior on copper current collectors. This review has systematically examined modification strategies—spanning 2D surface engineering, 3D architectural design, and magnetic field regulation—demonstrating how optimized nucleation kinetics, suppressed dendrite growth, and stabilized SEI layers significantly enhance cycling stability and safety (summarizing the representative modification systems and their key electrochemical parameters in recent studies in Table 3). Despite remarkable progress, bridging the gap from laboratory prototypes to commercial deployment requires multi-faceted bottlenecks to be overcome, necessitating coordinated advances in theoretical understanding, material design, and engineering integration. Key challenges and future directions are outlined below.| Anode | Half-cell parameters | CE (%) | Cycle number | Full-cell parameters | Cathode | Energy density | Initial CE (%) | Cycle number | Capacity retention | E/C | N/P | Electrolyte volume | Temp. | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| An-Cu | 1 mA cm−2 | 97.7 | 60 | 1C | LFP | — | — | 240 | 57.1% | — | — | 100 μL | Room temp. | 45 |
| 1 mAh cm−2 | ||||||||||||||
| CuCC-SSIC | 0.5 mA cm−2 | 99.4 | 400 | 0.2C/0.3C | NCM811 | 350 Wh kg−1 | ∼60.0 | 100 | 54.8% | — | — | — | Room temp. | 47 |
| 1 mAh cm−2 | ||||||||||||||
| Cu|Ag@PDA-GO | 0.2 mA cm−2 | 98.1 | 45 | 0.5 mA cm−2 | NCM111 | — | 86.3 | 80 | 65.5% | — | — | 50 μL | Room temp. | 63 |
| 1.6 mAh cm−2 | ||||||||||||||
| Etch-Cu | 0.5 mA cm−2 | 98.0 | — | 0.5C | LFP | — | ∼80.0 | 500 | 53.7% | — | — | — | Room temp. | 88 |
| 0.5 mAh cm−2 | ||||||||||||||
| LiF-LiZn/Cu | 1 mA cm−2 | 99.2 | 200 | 1 mA cm−2 | NCM811 | — | ∼99.0 | 50 | 88.0% | — | 0.9 | — | Room temp. | 90 |
| 2 mAh cm−2 | ||||||||||||||
| LiZrO(NO3)2@Cu | 1 mA cm−2 | 96.2 | 100 | 0.5C | LCO | — | ∼90.0 | 100 | 72.4% | — | — | 50 μL | Room temp. | 95 |
| 1 mAh cm−2 | ||||||||||||||
| LiF/Li3Sb | — | — | — | 3C | S | 325.28 Wh kg | ∼100 | 60 | 91.5% | 3 μl mg−1 | — | ∼1.3 mL | Room temp. | 96 |
| Li3Bi/LiF | — | — | — | 0.2C | NCM811 | — | ∼81.0 | 100 | 79.2% | — | — | 100 μL | Room temp. | 97 |
| xPCMS-g-PEGMA/LN | 1 mA cm−2 | 99.2 | 300 h | 0.5C | LFP | — | ∼97.0 | 100 | 79.0% | — | — | 50 μL | Room temp. | 103 |
| 1 mAh cm−2 | ||||||||||||||
| DFFSA–Li | 1 mA cm−2 | 99.0 | 65 h | 0.2C/0.5C | NCM811 | 461.6 Wh kg−1 | ∼85.0 | 100 | 84.7% | 1.6 g Ah−1 | 1.28 | 8.78 g | Room temp. | 107 |
| 1 mAh cm−2 | ||||||||||||||
| 3D Cu-CNT | 0.5 mA cm−2 | 98.3 | 800 | 0.2C | LFP | — | — | 20 | 73.0% | — | — | — | Room temp. | 115 |
| 1 mAh cm−2 | ||||||||||||||
| Zn/ZnO@Li | 0.5 mA cm−2 | 98.0 | 160 | 2C | LTO | — | ∼95.0 | 1000 | 81.0% | — | — | 60 μL | Room temp. | 117 |
| 1 mAh cm−2 | ||||||||||||||
| 3D Cu | 1 mA cm−2 | 96.0 | 100 | 1C | LFP | — | ∼80.0 | 100 | 82.6% | — | — | — | Room temp. | 125 |
| 1 mAh cm−2 | ||||||||||||||
| Cu2Se/Cu2O@3D Cu | 1 mA cm−2 | 97.2 | 500 | 2C | LFP | — | ∼97.0 | 600 | 72.0% | — | — | 50 μL | Room temp. | 127 |
| 1 mAh cm−2 | ||||||||||||||
| LHC600 | 1 mA cm−2 | 97.4 | 350 | 1C | LFP | — | ∼87.0 | 400 | 81.2% | — | 2.79 | 40 μL | Room temp. | 134 |
| 1 mAh cm−2 | ||||||||||||||
| CNZ | 1 mA cm−2 | 95.0 | 590 | 1C | LFP | — | ∼99.0 | 300 | 80.0% | — | — | — | Room temp. | 155 |
| 1 mAh cm−2 |
5.1. Limitations of current modification strategies
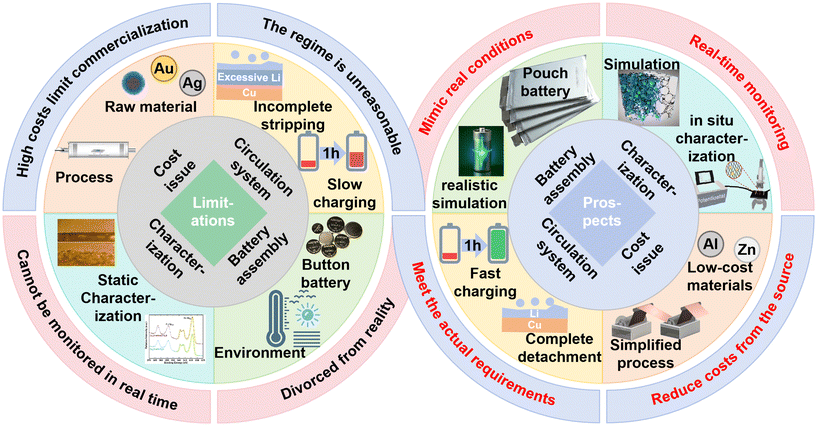
While 2D surface engineering improves deposition uniformity through crystallographic tuning, lithiophilic modification, and artificial SEIs, its efficacy diminishes under high current densities or large areal capacities due to lithium overburden or SEI fracture. Alloy-based lithiophilic layers effectively reduce nucleation barriers, yet the high cost of noble metals (e.g., Au, Ag) impedes scalable application. Artificial SEI layers often face trade-offs between chemical stability and mechanical strength, and complex fabrication routes (e.g., CVD, electrospinning) hinder industrial adoption. 3D frameworks mitigate local current density via their high surface area but exacerbate active lithium consumption through continuous SEI formation/fracture, ultimately impairing cycle life. Magnetic field regulation homogenizes Li+ distribution via MHD effects, yet external magnets introduce bulk and electromagnetic interference, while the long-term stability and field attenuation of embedded magnetic particles remain inadequately studied. Furthermore, compatibility between electrolytes and modified copper surfaces is often overlooked; certain lithiophilic layers may trigger side reactions, accelerating active lithium loss, which is a critical concern for anode-free configurations lacking lithium reserves.5.2. Performance degradation in practical scenarios
Laboratory protocols, optimized for fundamental insight, typically employ low current densities (0.1–0.5 mA cm−2) and excess lithium (e.g., 5 mAh pre-plated Li cycling 1 mAh). These conditions diverge sharply from real-world operation. In electric vehicles and grid storage, fast-charging demands (2–5C) induce deposition rates exceeding Li+ diffusion, leading to localized ion accumulation, dendritic growth, and internal short-circuit risks. Pre-plated lithium masks irreversible loss during initial SEI formation, inflates coulombic efficiency metrics, and reduces practical energy density. Moreover, small-format coin cells fail to replicate challenges in pouch cells—volume expansion, interfacial impedance accumulation, and rate capability decay are markedly exacerbated at larger scales. For instance, 3D copper structures may collapse under mechanical stress in pouch cells, and magnetic field uniformity becomes difficult to maintain in multi-layer stacks, collectively limiting energy density and cycle life.In addition to performance enhancements, the practical implementation of copper current collector engineering for anode-free lithium metal batteries necessitates careful consideration of risk assessment and quality-control issues. In such systems, rigorous verification of complete lithium stripping is particularly critical, as residual inactive lithium or partial reversibility may lead to inaccurate coulombic efficiency while obscuring progressive degradation processes. Moreover, operation under practical conditions—such as high current densities and fast-charging protocols—introduces elevated safety risks that remain insufficiently addressed in most laboratory-scale studies.
From a broader perspective, the lack of standardized evaluation methodologies represents a key barrier to the rational comparison of different copper current collector designs. Performance metrics commonly reported in the literature, especially coulombic efficiency, are often insufficient to fully capture failure modes associated with non-uniform lithium deposition, interfacial instability, or mechanical degradation. Therefore, future studies should emphasize the integration of operando and in situ diagnostic tools, including optical monitoring, stress or pressure analysis, and X-ray-based techniques, to enable more reliable assessment of reliability and reproducibility. Addressing these challenges will be essential for translating copper current collector design strategies from proof-of-concept demonstrations to practical anode-free battery systems (Fig. 10).
In summary, copper current collector modifications provide a cornerstone for practical high-energy-density batteries. The synergistic development of 2D surface, 3D structural, and magnetic strategies has markedly improved cycling stability and energy density. Future efforts must prioritize commercial viability. For example, single-crystal copper foils—currently produced via costly CVD processes—require low-cost, scalable alternatives like electroplating to enable large-format battery manufacturing. In anode-free LMBs, where no excess lithium compensates for irreversible loss, achieving high reversibility—especially during the first cycle where significant active lithium is consumed in SEI formation—is paramount. Enhancing first-cycle coulombic efficiency is a critical milestone for commercial viability.
Testing protocols must evolve to mirror real-world conditions: fast-charging capability should be emphasized, and discharge should ensure complete lithium stripping to maximize energy density. Current understanding of lithium deposition, largely derived from “static post-mortem analysis in coin cells”, lacks insights into dynamic processes under practical operation. Advanced in situ/operando characterization techniques are essential to observe deposition/stripping behavior in real time, guiding precision engineering of interfaces.
Through deeper integration of fundamental research and scalable engineering, modified copper current collectors are poised to accelerate the adoption of lithium metal and anode-free batteries in electric vehicles and grid storage, injecting renewed momentum into the development of advanced energy storage technologies.
Author contributions
Jiawang Feng: conceptualization, investigation, writing – original draft, writing – review and editing, visualization; Tianzhengyi Zhang, Ming Liu, Guoxing Tian: conceptualization, investigation, writing – review and editing, supervision; Yuqian Fan, Ailing Song, Guangjie Shao, Zhipeng Ma: conceptualization, investigation, supervision.Conflicts of interest
The authors declare no conflict of interest.Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (52074241, 52174281, 51674221 and 51704261), the Hebei Province Natural Science Foundation (B2025203050), the Science and Technology Project of Hebei Education Department (BJ2020038) and the Cultivation Project for Basic Research and Innovation of Yanshan University (2024LGQL003).References
- Y. Ding, Z. P. Cano, A. Yu, J. Lu and Z. Chen, Electrochem. Energy Rev., 2019, 2, 1–28 CrossRef.
- S. Yuan, T. Kong, Y. Zhang, P. Dong, Y. Zhang, X. Dong, Y. Wang and Y. Xia, Angew. Chem., Int. Ed., 2021, 60, 25624–25638 CrossRef PubMed.
- W. Ai, Z. Huang, L. Wu, Z. Du, C. Zou, Z. He, R. Shahbazian-Yassar, W. Huang and T. Yu, High-rate, Energy Storage Mater., 2018, 14, 169–178 CrossRef.
- R. Schmuch, R. Wagner, G. Hörpel, T. Placke and M. Winter, Nat. Energy, 2018, 3, 267–278 CrossRef.
- X. Chi, M. Li, J. Di, P. Bai, L. Song, X. Wang, F. Li, S. Liang, J. Xu and J. Yu, Nature, 2021, 592, 551–557 CrossRef PubMed.
- Y. Zhang, Y. Wu, H. Li, J. Chen, D. Lei and C. Wang, Nat. Commun., 2022, 13, 1297 CrossRef PubMed.
- S. Li, F. Wu, T. Chen, K. Kang, R. Guo, C. Liu, Y. Niu, A. Gao, R. Zhao, X. Wang, Y. Bai and C. Wu, Energy Mater. Adv., 2025, 6, 168 CrossRef.
- M. F. Roslan, P. R. Satpathy, T. Prasankumar, V. K. Ramachandaramurthy, M. Mansor and S. L. Walker, J. Energy Storage, 2025, 123, 116808 CrossRef.
- B. Zhang, J. Ma, Y. Zhao, T. Li, J. Yang, Z. Zhang, S. Wei and G. Zhou, Rare Met., 2024, 43, 942–970 CrossRef.
- K. Yoon, S. Lee, K. Oh and K. Kang, Adv. Mater., 2022, 34, e2104666 CrossRef PubMed.
- L. Li, W. Liu, H. Dong, Q. Gui, Z. Hu, Y. Li and J. Liu, Adv. Mater., 2021, 33, e2004959 CrossRef PubMed.
- S. Huo, L. Wang, B. Su, W. Xue, Y. Wang, H. Zhang, M. Li, J. Qiu, H. Xu and X. He, Adv. Mater., 2024, 36, 2411757 Search PubMed.
- M. Liu, A. Song, X. Zhang, J. Wang, Y. Fan, G. Wang, H. Tian, Z. Ma and G. Shao, Nano Energy, 2025, 136, 110749 CrossRef.
- A. Fu, C. Wang, J. Peng, M. Su, F. Pei, J. Cui, X. Fang, J. F. Li and N. Zheng, Adv. Funct. Mater., 2021, 31, 2009805 CrossRef.
- A. Pei, G. Zheng, F. Shi, Y. Li and Y. Cui, Nano Lett., 2017, 17, 1132–1139 CrossRef PubMed.
- Z. Guo, J. Wang, X. Li, Z. Wang, H. Guo, W. Peng, G. Yan, G. Li, X. Zhang, N. Wang, J. Yang and X. Huang, Electrochem. Energy Rev., 2025, 8, 8 CrossRef.
- X. Pu, S. Zhang, D. Zhao, Z. Xu, Z. Chen and Y. Cao, Electrochem. Energy Rev., 2024, 7, 21 CrossRef.
- X. Liang, Q. Pang, I. R. Kochetkov, M. S. Sempere, H. Huang, X. Sun and L. F. Nazar, Nat. Energy, 2017, 2, 17119 Search PubMed.
- Y. Liu, D. Gao, H. Xiang, X. Feng and Y. Yu, Energy Fuels, 2021, 35, 12921–12937 CrossRef.
- Z. L. Brown, S. Jurng and B. L. Lucht, J. Electrochem. Soc., 2017, 164, A2186–A2189 Search PubMed.
- J. Liu, Z. Bao, Y. Cui, E. J. Dufek, J. B. Goodenough, P. Khalifah, Q. Li, B. Y. Liaw, P. Liu, A. Manthiram, Y. S. Meng, V. R. Subramanian, M. F. Toney, V. V. Viswanathan, M. S. Whittingham, J. Xiao, W. Xu, J. Yang, X. Yang and J. Zhang, Nat. Energy, 2019, 4, 180–186 CrossRef.
- J. Lin, C. Ma, C. Li, X. Zou, W. Chen, J. Zhou and P. Lian, ACS Appl. Mater. Interfaces, 2025, 17, 33191–33198 CrossRef.
- W. Bao, R. Wang, B. Li, C. Qian, Z. Zhang, J. Li and F. Liu, J. Mater. Chem. A, 2021, 9, 2957–2984 Search PubMed.
- V. Pande and V. Viswanathan, ACS Energy Lett., 2019, 4, 2952–2959 CrossRef.
- X. Zhang, L. J. Huang, G. B. Yang, J. P. Song, G. H. Cong, S. S. Liu, Y. T. Huang, Z. Y. Liu and L. Geng, Electrochim. Acta, 2024, 498, 144633 CrossRef.
- Y. Xu, B. Yu, Y. Wang, F. Tan, G. Cheng, W. Yang, H. Gao and Z. Zhang, Electrochim. Acta, 2022, 435, 141337 CrossRef.
- X. Shen, R. Zhang, P. Shi, X. Chen and Q. Zhang, Adv. Energy Mater., 2021, 11, 2003416 CrossRef.
- A. A. Assegie, C. Chung, M. Tsai, W. Su, C. Chen and B. Hwang, Nanoscale, 2019, 11, 2710–2720 RSC.
- A. B. Gunnarsdóttir, C. V. Amanchukwu, S. Menkin and C. P. Grey, J. Am. Chem. Soc., 2020, 142, 20814–20827 CrossRef PubMed.
- D. Lin, Y. Liu, Y. Li, Y. Li, A. Pei, J. Xie, W. Huang and Y. Cui, Nat. Chem., 2019, 11, 382–389 Search PubMed.
- P. Zhu, D. Gastol, J. Marshall, R. Sommerville, V. Goodship and E. Kendrick, J. Power Sources, 2021, 485, 229321 Search PubMed.
- M. J. Lain, J. Brandon and E. Kendrick, Batteries, 2019, 5, 64 Search PubMed.
- B. A. Johnson and R. E. White, J. Power Sources, 1998, 70, 48–54 CrossRef CAS.
- I. T. Røe and S. K. Schnell, J. Mater. Chem. A, 2021, 9, 1142–1148 RSC.
- J. Lee, J. Kim, D. G. Lee, D. Son, J. Lee, S. Kim, S. Han, N. Choi, T. K. Lee and J. Lee, Chem. Eng. J., 2024, 499, 156296 CrossRef CAS.
- C. Park, J. Kim, W. Lim and J. Lee, Exploration, 2024, 4, 20210255 CrossRef CAS.
- T. Fuchs, C. G. Haslam, A. C. Moy, C. Lerch, T. Krauskopf, J. Sakamoto, F. H. Richter and J. Janek, Adv. Energy Mater., 2022, 12, 2201125 Search PubMed.
- L. Liu and J. Wang, Nano Lett., 2023, 23, 10251–10258 Search PubMed.
- Y. Li, Y. Li, A. Pei, K. Yan, Y. Sun, C. Wu, L. Joubert, R. Chin, A. L. Koh, Y. Yu, J. Perrino, B. Butz, S. Chu and Y. Cui, Science, 2017, 358, 506–510 CrossRef CAS.
- Y. Gu, H. Xu, X. Zhang, W. Wang, J. He, S. Tang, J. Yan, D. Wu, M. Zheng, Q. Dong and B. Mao, Angew. Chem., Int. Ed., 2019, 58, 3092–3096 Search PubMed.
- X. Cheng, R. Zhang, C. Zhao and Q. Zhang, Chem. Rev., 2017, 117, 10403–10473 CrossRef CAS PubMed.
- K. Ishikawa, S. Harada, M. Tagawa and T. Ujihara, ACS Appl. Mater. Interfaces, 2020, 12, 9341–9346 CrossRef CAS PubMed.
- Q. Zhao, Y. Deng, N. W. Utomo, J. Zheng, P. Biswal, J. Yin and L. A. Archer, Nat. Commun., 2021, 12, 6034 CrossRef CAS PubMed.
- G. Li, D. Shi, Z. Hao, Y. Lu, Q. Zhao, Z. Yan, W. Xie, X. Meng and J. Chen, J. Phys. Chem. C, 2023, 127, 16297–16303 CrossRef CAS.
- Y. Kim, S. H. Kwon, H. Noh, S. Yuk, H. Lee, H. S. Jin, J. Lee, J. Zhang, S. G. Lee, H. Guim and H. Kim, Energy Storage Mater., 2019, 19, 154–162 CrossRef.
- J. Y. Kim, O. B. Chae, M. Wu, E. Lim, G. Kim, Y. J. Hong, W. Jung, S. Choi, D. Y. Kim, I. Gereige, J. Suk, Y. Kang and H. Jung, Nano Energy, 2021, 82, 105736 CrossRef CAS.
- J. Zhan, L. Deng, Y. Liu, M. Hao, Z. Wang, L. T. Dong, Y. Yang, K. Song, D. Qi, J. Wang, S. Wang, H. Liu, W. Zhou and H. Chen, Adv. Mater., 2025, 37, 2413420 CrossRef CAS.
- Z. Hao, G. Li, C. Zheng, X. Liu, S. Wu, H. Li, K. Zhang, Z. Yan and J. Chen, Angew. Chem., Int. Ed., 2024, 63, e202407064 CrossRef CAS.
- Y. Liu, C. Sun, Y. Lu, X. Lin, M. Chen, Y. Xie, C. Lai and W. Yan, Chem. Eng. J., 2023, 451, 138570 Search PubMed.
- D. Li, Y. Gao, C. Xie and Z. Zheng, Appl. Phys. Rev., 2022, 9, 011424 CAS.
- C. Shan, Z. Qin, Y. Xie, X. Meng, J. Chen, Y. Chang, R. Zang, L. Wan and Y. Huang, Carbon, 2023, 204, 367–376 CrossRef CAS.
- J. Lu, Z. Ma, Y. Wang, W. Dai, X. Cheng, J. Zuo, H. Lei and Z. Fu, Small, 2024, 20, e2406359 CrossRef PubMed.
- K. Yan, Z. Lu, H. Lee, F. Xiong, P. Hsu, Y. Li, J. Zhao, S. Chu and Y. Cui, Nat. Energy, 2016, 1, 16010 CrossRef CAS.
- J. Du, W. Wang, M. Wan, X. Wang, G. Li, Y. Tan, C. Li, S. Tu and Y. Sun, Adv. Energy Mater., 2021, 11, 2102259 Search PubMed.
- J. Cao, Y. Shi, A. Gao, G. Du, M. Dilxat, Y. Zhang, M. Cai, G. Qian, X. Lu, F. Xie, Y. Sun and X. Lu, Nat. Commun., 2024, 15, 1354 Search PubMed.
- H. Jo, J. Lee, E. Kwon, S. Yu, B. G. Kim, S. Park, J. Moon, M. J. Ko and H. Lim, ACS Nano, 2024, 18, 35718–35728 Search PubMed.
- X. Duan, J. Sun, L. Shi, S. Dong and G. Cui, Interdiscip. Mater., 2025, 4, 217–234 Search PubMed.
- R. Guan, S. Liu, C. Wang, Y. Yang, D. Lu and X. Bian, Chem. Eng. J., 2021, 425, 130177 Search PubMed.
- H. Wang, Y. Mao, P. Xu, Y. Ding, H. Yang, J. Li, Y. Gu, J. Han, L. Zhang and B. Mao, Energy Environ. Sci., 2025, 18, 2622–2633 RSC.
- K. Wang, J. Hu, T. Chen, W. Zhang, Z. Deng, Q. Chen, K. Wang and J. Wu, J. Energy Storage, 2024, 89, 111879 CrossRef.
- B. Hubert, Y. Nikodimos, B. J. Hwang and J. P. Chu, J. Power Sources, 2024, 589, 233660 CrossRef.
- J. Seo, J. Lim, H. Chang, J. Lee, J. Woo, I. Jung, Y. Kim, B. Kim, J. Moon and H. Lee, Small, 2024, 20, e2402988 CrossRef PubMed.
- Z. T. Wondimkun, W. A. Tegegne, J. Shi-Kai, C. Huang, N. A. Sahalie, M. A. Weret, J. Hsu, P. Hsieh, Y. Huang, S. Wu, W. Su and B. J. Hwang, Energy Storage Mater., 2021, 35, 334–344 CrossRef.
- J. Zhu, Y. Wu, X. Huang, L. Huang, M. Cao, G. Song, X. Guo, X. Sui, R. Ren and J. Chen, Nano Energy, 2019, 62, 883–889 CrossRef.
- Y. Zhou, P. Wang, K. Wang, X. Fang, W. Li, J. Nai, Y. Liu, Y. Wang, S. Zou, H. Yuan, X. Tao and J. Luo, Adv. Funct. Mater., 2025, 35, 2424022 CrossRef.
- B. Zhou, A. Bonakdarpour, I. Stoševski, B. Fang and D. P. Wilkinson, Prog. Mater. Sci., 2022, 130, 100996 CrossRef.
- Y. Liu, Y. Li, J. Sun, Z. Du, X. Hu, J. Bi, C. Liu, W. Ai and Q. Yan, Nano Res. Energy, 2023, 2, e9120048 CrossRef.
- P. Du, C. Yuan, X. Cui, K. Zhang, Y. Yu, X. Ren, X. Zhan and S. Gao, J. Mater. Chem. A, 2022, 10, 8424–8431 RSC.
- J. Teng, X. Tang, H. Li, Q. Wu, D. Zhao and J. Li, J. Power Sources, 2022, 540, 231642 CrossRef.
- X. F. Tan, Q. Gu, D. Qu, A. X. B. Yong, W. Yang, S. D. McDonald, S. Matsumura and K. Nogita, Acta Mater., 2020, 201, 341–349 CrossRef.
- S. J. Liu, K. J. Jiao and J. H. Yan, Energy Storage Mater., 2023, 54, 689–712 CrossRef.
- X. Zhang, S. Huang, M. Xiao, S. J. Wang, Y. Z. Meng and D. M. Han, J. Power Sources, 2025, 631, 236200 Search PubMed.
- S. T. Wang, Y. Liu, J. R. Huang, S. Z. Liu, S. L. Li, M. R. Liu, Z. C. Ma, T. F. Yang, Y. J. Yang and S. Y. Gao, Nano Energy, 2025, 138, 110843 Search PubMed.
- J. Q. Cao, W. X. Chen, A. S. Gao, D. Muhtar, G. Y. Du, G. Y. Qian, X. Y. Lu, F. Y. Xie, Y. Sun and X. Lu, Angew. Chem., Int. Ed., 2025, 64, e202413065 Search PubMed.
- V. P. Nguyen, H. C. Shim, Y. Byeon, J. Kim and S. Lee, Adv. Sci., 2025, 12, e2416426 CrossRef.
- S. Qian, C. Xing, M. Zheng, Z. Su, H. Chen, Z. Wu, C. Lai and S. Zhang, Adv. Energy Mater., 2022, 12, 2103480 CrossRef.
- M. Gao, Q. Dong, M. Yao, X. Wang, J. Li, W. Zhang, H. Huang, H. Guo, Z. Sun, Q. Chen, X. Han and W. Hu, Adv. Funct. Mater., 2024, 34, 2401442 CrossRef.
- Z. Liu, L. Sun, X. Liu and Q. Lu, Chem. – Eur. J., 2024, 30, e202402032 CrossRef.
- B. Li, Y. Chao, M. Li, Y. Xiao, R. Li, K. Yang, X. Cui, G. Xu, L. Li, C. Yang, Y. Yu, D. P. Wilkinson and J. Zhang, Electrochem. Energy Rev., 2023, 6, 7 CrossRef.
- L. Song, D. Ning, Y. Chai, M. Ma, G. Zhang, A. Wang, H. Su, D. Hao, M. Zhu, J. Zhang, D. Zhou, J. Wang and Y. Li, Small Methods, 2023, 7, 2300168 CrossRef PubMed.
- B. Han, Y. Zou, G. Xu, S. Hu, Y. Kang, Y. Qian, J. Wu, X. Ma, J. Yao, T. Li, Z. Zhang, H. Meng, H. Wang, Y. Deng, J. Li and M. Gu, Energy Environ. Sci., 2021, 14, 4882–4889 RSC.
- B. Wu, C. Chen, L. H. J. Raijmakers, J. Liu, D. L. Danilov, R. Eichel and P. H. L. Notten, Energy Storage Mater., 2023, 57, 508–539 Search PubMed.
- C. Wang, R. Odstrcil, J. Liu and W. Zhong, J. Energy Chem., 2022, 73, 248–258 CrossRef.
- Y. Wu, C. Wang, C. Wang, Y. Zhang, J. Liu, Y. Jin, H. Wang and Q. Zhang, Mater. Horiz., 2024, 11, 388–407 RSC.
- N. Li, G. Kang, S. Huang, J. Biao, Y. Liu, F. Kang and Y. Cao, ACS Appl. Mater. Interfaces, 2025, 17, 36627–36638 CrossRef PubMed.
- S. Miao, Y. Jia, Z. Deng, Y. Deng, R. Chen, X. Zhang, C. Xu, M. Yao and W. Cai, Tungsten, 2024, 6, 212–229 Search PubMed.
- Q. Zhang, J. Luan, Y. Tang, X. Ji, S. Wang and H. Wang, J. Mater. Chem. A, 2018, 6, 18444–18448 Search PubMed.
- R. U. R. Sagar, M. W. Fazal, A. Durajski, M. W. Khan, N. Mahmood and Y. Chen, Nano Lett., 2025, 25, 10970–10976 CrossRef PubMed.
- S. T. Oyakhire, W. Zhang, A. Shin, R. Xu, D. T. Boyle, Z. Yu, Y. Ye, Y. Yang, J. A. Raiford, W. Huang, J. R. Schneider, Y. Cui and S. F. Bent, Nat. Commun., 2022, 13, 3986 CrossRef PubMed.
- V. P. Nguyen, H. C. Shim, Y. W. Byeon, J. H. Kim and S. M. Lee, Adv. Sci., 2025, 12, 2416426 CrossRef.
- Y. Liu, X. Meng, Y. Shi, J. Qiu and Z. Wang, Adv. Mater., 2023, 35, 2305386 CrossRef PubMed.
- K. Vishweswariah, N. G. Ningappa, M. D. Bouguern, A. Kumar M R, M. B. Armand and K. Zaghib, Adv. Energy Mater., 2025, 2501883 CrossRef.
- H. Song, J. Lee, M. Sagong, J. Jeon, Y. Han, J. Kim, H. G. Jung, J. S. Yu, J. Lee and I. D. Kim, Adv. Mater., 2024, 36, 2407381 CrossRef.
- Y. Liu, Y. Zhen, T. Li, F. Bettels, T. He, M. Peng, Y. Liang, F. Ding and L. Zhang, Small, 2020, 16, 2004770 CrossRef.
- C. Chen, Q. Liang, G. Wang, D. Liu and X. Xiong, Adv. Funct. Mater., 2022, 32, 2107249 CrossRef.
- A. Hu, W. Chen, X. Du, Y. Hu, T. Lei, H. Wang, L. Xue, Y. Li, H. Sun, Y. Yan, J. Long, C. Shu, J. Zhu, B. Li, X. Wang and J. Xiong, Energy Environ. Sci., 2021, 14, 4115–4124 RSC.
- Q. Ran, H. Zhao, J. Liu, L. Li, Q. Hu, J. Song, X. Liu and S. Kormarneni, J. Energy Chem., 2023, 83, 612–621 CrossRef.
- S. Chen, C. Pan, Q. Wang, J. L. Luo and X. Z. Fu, Adv. Funct. Mater., 2024, 34, 2409812 CrossRef CAS.
- Z. Cao, H. Chen, Z. Du, J. Gu, Q. Zhu, B. Li and S. Yang, Adv. Energy Mater., 2022, 12, 2201189 CrossRef CAS.
- C. Chang, Y. Yao, R. Li, Z. H. Guo, L. Li, C. Pan, W. Hu and X. Pu, Nano Energy, 2022, 93, 106871 CrossRef CAS.
- S. Gao, F. Sun, N. Liu, H. Yang and P. Cao, Mater. Today, 2020, 40, 140–159 CrossRef CAS.
- Y. Nikodimos, W. Su, K. N. Shitaw, S. Jiang, L. H. Abrha, M. A. Weret, S. K. Merso, T. M. Hagos, C. Huang, K. Lakshmanan, W. Huang, C. Chang, J. Lin, S. Wu, C. Yang and B. J. Hwang, Energy Storage Mater., 2023, 61, 102861 CrossRef.
- S. Li, J. Huang, Y. Cui, S. Liu, Z. Chen, W. Huang, C. Li, R. Liu, R. Fu and D. Wu, Nat. Nanotechnol., 2022, 17, 613–621 CrossRef CAS PubMed.
- C. Chen, J. Zhang, B. Hu, Q. Liang and X. Xiong, Nat. Commun., 2023, 14, 4018 CrossRef CAS PubMed.
- H. Wang, Y. Peng, R. Tamate, K. Nishikawa and S. Nakanishi, J. Power Sources, 2025, 641, 236801 CrossRef CAS.
- W. Lu, L. Sun, Y. Zhao, T. Wu, L. Cong, J. Liu, Y. Liu and H. Xie, Energy Storage Mater., 2021, 34, 241–249 CrossRef.
- M. Pang, Z. Jiang, C. Luo, Z. Yao, T. Fu, T. Pan, Q. Guo, Y. Li, S. Xiong, C. Zheng, W. Sun, G. Zhou and S. Liu, Energy Environ. Sci., 2024, 17, 7699–7711 RSC.
- D. Han, Z. Wang, S. Chen, J. Zhou, S. Chen, M. Wang, D. Wu, X. Meng, C. W. Bielawski and J. Geng, Small, 2024, 20, 2405453 CrossRef CAS PubMed.
- S. Zhou, Y. Zhang, S. Chai, I. Usman, Y. Qiao, S. Luo, X. Xie, J. Chen, S. Liang, A. Pan and H. Zhou, Chem. Eng. J., 2021, 404, 126508 CrossRef CAS.
- Y. Cheng, J. Chen, Y. Chen, X. Ke, J. Li, Y. Yang and Z. Shi, Energy Storage Mater., 2021, 38, 276–298 CrossRef.
- Y. Wang, J. Tan, Z. Li, L. Ma, Z. Liu, M. Ye and J. Shen, Energy Storage Mater., 2022, 53, 156–182 CrossRef.
- S. Park, H. J. Jin and Y. S. Yun, Adv. Mater., 2020, 32, 2002193 CrossRef CAS PubMed.
- C. Zhang, R. Lyu, W. Lv, H. Li, W. Jiang, J. Li, S. Gu, G. Zhou, Z. Huang, Y. Zhang, J. Wu, Q. H. Yang and F. Kang, Adv. Mater., 2019, 31, 1904991 CrossRef CAS.
- C. Yang, Y. Yin, S. Zhang, N. Li and Y. Guo, Nat. Commun., 2015, 6, 8058 CrossRef CAS PubMed.
- S. K. Park, D. Copic, T. Z. Zhao, A. Rutkowska, B. Wen, K. Sanders, R. He, H. Kim and M. De Volder, ACS Nano, 2023, 17, 14658–14666 CrossRef CAS PubMed.
- W. Shin and A. Manthiram, ACS Appl. Mater. Interfaces, 2022, 14, 17454–17460 CrossRef CAS PubMed.
- Q. Chen, Y. Yang, H. Zheng, Q. Xie, X. Yan, Y. Ma, L. Wang and D. Peng, J. Mater. Chem. A, 2019, 7, 11683–11689 RSC.
- W. Chen, S. Li, C. Wang, H. Dou and X. Zhang, Energy Environ. Mater., 2023, 6, e12412 CrossRef CAS.
- J. Kim, S. Lee, J. Kim, J. Park, H. Lee, J. Kwon, S. Sun, J. Choi, U. Paik and T. Song, Carbon Energy, 2024, 6, e610 CrossRef CAS.
- S. K. Park, S. Kim, R. He, K. Sanders, U. Hwang, Z. An, M. Hamidinejad, J. W. Kim and M. De Volder, Small, 2025, 21, 2501292 CrossRef CAS.
- T. Zhao, S. Li, F. Liu, Z. Wang, H. Wang, Y. Liu, X. Tang, M. Bai, M. Zhang and Y. Ma, Energy Storage Mater., 2022, 45, 796–804 CrossRef.
- H. Liu, E. Wang, Q. Zhang, Y. Ren, X. Guo, L. Wang, G. Li and H. Yu, Energy Storage Mater., 2019, 17, 253–259 CrossRef.
- F. Shen, F. Zhang, Y. Zheng, Z. Fan, Z. Li, Z. Sun, Y. Xuan, B. Zhao, Z. Lin, X. Gui, X. Han, Y. Cheng and C. Niu, Energy Storage Mater., 2018, 13, 323–328 CrossRef.
- N. Li, T. Jia, Y. Liu, Y. Ouyang, Y. Lv, G. Zhong, Y. Wang, B. Sun, S. Lu, S. Huang, F. Kang and Y. Cao, Mater. Today Energy, 2023, 36, 101341 CrossRef CAS.
- Y. Ma, X. Ma, J. Bai, W. Xu, H. Zhong, Z. Liu, S. Xiong, L. Yang and H. Chen, Small, 2023, 19, 2301731 CrossRef CAS PubMed.
- S. Kim, M. Lee, S. Oh and W. Ryu, Chem. Eng. J., 2023, 474, 145447 CrossRef CAS.
- G. Guo, K. Zhang, K. Zhu, P. Yang, Z. Shao, S. Liu, L. Lin, W. Zhuang, P. Xue, Q. Zhang and Y. Yao, Adv. Funct. Mater., 2024, 34, 2402490 CrossRef CAS.
- Q. Yun, Y. B. He, W. Lv, Y. Zhao, B. Li, F. Kang and Q. H. Yang, Adv. Mater., 2016, 28, 6932–6939 CrossRef CAS PubMed.
- W. Liu, D. Lin, A. Pei and Y. Cui, J. Am. Chem. Soc., 2016, 138, 15443–15450 CrossRef CAS PubMed.
- L. Qin, Y. Wu, M. Shen, B. Song, Y. Li, S. Sun, H. Zhang, C. Liu and J. Chen, Energy Storage Mater., 2022, 44, 278–284 CrossRef.
- S. H. Wang, Y. X. Yin, T. T. Zuo, W. Dong, J. Y. Li, J. L. Shi, C. H. Zhang, N. W. Li, C. J. Li and Y. G. Guo, Adv. Mater., 2017, 29, 1703721–1703729 Search PubMed.
- H. Shen, P. Tang, Q. Wei, Y. Zhang, T. Yu, H. Yang, R. Zhang, K. Tai, J. Tan, S. Bai and F. Li, Small, 2023, 19, 2206000 CrossRef CAS PubMed.
- Z. Li, X. Huang, L. Kong, N. Qin, Z. Wang, L. Yin, Y. Li, Q. Gan, K. Liao, S. Gu, T. Zhang, H. Huang, L. Wang, G. Luo, X. Cheng and Z. Lu, Energy Storage Mater., 2022, 45, 40–47 CrossRef.
- H. Li, G. Wang, J. Hu, J. Li, J. Huang and S. Xu, Energy Environ. Mater., 2024, 7, e12768 CrossRef CAS.
- J. Qi, Y. Feng, J. Yu, H. Wang, Z. Wu, J. Zhao, Y. Jiang, J. Liu, Y. Li, L. Zhou, K. Zhang and J. Chen, Energy Storage Mater., 2025, 77, 104176 CrossRef.
- W. Guan, Y. Lv, C. Ma, Q. Zhang, A. Wei and X. Liu, Mater. Chem. Front., 2023, 7, 315–324 RSC.
- X. He, D. Bresser, S. Passerini, F. Baakes, U. Krewer, J. Lopez, C. T. Mallia, Y. Shao-Horn, I. Cekic-Laskovic, S. Wiemers-Meyer, F. A. Soto, V. Ponce, J. M. Seminario, P. B. Balbuena, H. Jia, W. Xu, Y. Xu, C. Wang, B. Horstmann, R. Amine, C. Su, J. Shi, K. Amine, M. Winter, A. Latz and R. Kostecki, Nat. Rev. Mater., 2021, 6, 1036–1052 CrossRef CAS.
- Z. Yubo, C. Jianzhong, Z. Zhihao, Z. Haitao and Q. Ke, Mater. Sci. Eng., A, 2005, 406, 286–292 CrossRef.
- Y. Luo, Z. Zhang, B. Li, M. Gao, Y. Qiu and M. He, JOM, 2017, 69, 2640–2643 CrossRef CAS.
- J. Q. Wang and G. Xiao, J. Appl. Phys., 1996, 79, 5587–5589 CrossRef CAS.
- M. Miura, Y. Oshikiri, A. Sugiyama, R. Morimoto, I. Mogi, M. Miura, S. Takagi, Y. Yamauchi and R. Aogaki, Sci. Rep., 2017, 7, 45511 CrossRef CAS PubMed.
- Y. D. Yu, Z. L. Song, H. L. Ge and G. Y. Wei, Surf. Eng., 2014, 30, 83–86 CrossRef CAS.
- O. Aaboubi, A. Y. A. Omar, A. Franczak and K. Msellak, J. Electroanal. Chem., 2015, 737, 226–234 CrossRef CAS.
- L. M. A. Monzon and J. M. D. Coey, Electrochem. Commun., 2014, 42, 38–41 CrossRef CAS.
- D. Lin, Y. Liu and Y. Cui, Nat. Nanotechnol., 2017, 12, 194–206 CrossRef CAS.
- Q. Cheng, L. Wei, Z. Liu, N. Ni, Z. Sang, B. Zhu, W. Xu, M. Chen, Y. Miao, L. Chen, W. Min and Y. Yang, Nat. Commun., 2018, 9, 2942 CrossRef.
- L. Yu, J. Wang and Z. J. Xu, Small Struct., 2021, 2, 2000043 CrossRef CAS.
- K. Shen, Z. Wang, X. Bi, Y. Ying, D. Zhang, C. Jin, G. Hou, H. Cao, L. Wu, G. Zheng, Y. Tang, X. Tao and J. Lu, Adv. Energy Mater., 2019, 9, 1900260 CrossRef.
- M. Bae, M. Kang and Y. Piao, Adv. Funct. Mater., 2025, 35, 2421952 CrossRef CAS.
- Z. Gao, Y. Schwab, Y. Zhang, N. Song and X. Li, Adv. Funct. Mater., 2018, 28, 1800563 CrossRef.
- S. Zhou, X. Meng, C. Fu, J. Chen, Y. Chen, D. Xu, S. Lin, C. Han, Z. Chang and A. Pan, Small, 2023, 19, 2207764 CrossRef CAS PubMed.
- M. Liu, J. Ma, X. Zhang, J. Wang, Y. Fan, A. Song, G. Shao and Z. Ma, Adv. Funct. Mater., 2025, 35, 2416527 CrossRef CAS.
- H. Chen, J. Hu, H. Li, J. Zhang, Q. Chen, G. Hou and Y. Tang, Small, 2024, 20, 2307598 Search PubMed.
- M. Sheikholeslami and M. Gorji-Bandpy, Powder Technol., 2014, 256, 490–498 Search PubMed.
- J. Zhang, H. Chen, M. Wen, K. Shen, Q. Chen, G. Hou and Y. Tang, Adv. Funct. Mater., 2022, 32, 2110110 CrossRef CAS.
- F. A. Garcés-Pineda, M. Blasco-Ahicart, D. Nieto-Castro, N. López and J. R. Galán-Mascarós, Nat. Energy, 2019, 4, 519–525 Search PubMed.
- J. Feng, C. Shi, X. Zhao, Y. Zhang, S. Chen, X. Cheng and J. Song, Adv. Mater., 2024, 36, 2414047 CrossRef CAS PubMed.
- C. Y. Zhang, C. Zhang, G. W. Sun, J. L. Pan, L. Gong, G. Z. Sun, J. J. Biendicho, L. Balcells, X. L. Fan, J. R. Morante, J. Y. Zhou and A. Cabot, Angew. Chem., Int. Ed., 2022, 61, e202211570 Search PubMed.
- G. Sun, C. Y. Zhang, M. Jin, J. Li, X. Pan, A. Cabot, G. Sun and J. Y. Zhou, J. Am. Chem. Soc., 2025, 147, 27251–27264 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |