 Open Access Article
Open Access ArticleStructured for success: conjugated polymer binders with tailored composition and architecture for lithium-ion batteries
Xiuyu
Jin
 a,
Bharathkumar H.
Javaregowda
b,
Jinhua
Sun
b and
Gao
Liu
a,
Bharathkumar H.
Javaregowda
b,
Jinhua
Sun
b and
Gao
Liu
 *a
*a
aThe Energy Storage and Distributed Resources Division (ESDR) Lawrence Berkeley National Laboratory, Berkeley, California 94720, USA. E-mail: gliu@lbl.gov
bDepartment of Industrial and Materials Science, Chalmers University of Technology, SE-412 96 Göteborg, Sweden
First published on 8th December 2025
Abstract
Lithium-ion batteries (LIBs) are the leading energy storage technology, yet enhancing their energy density and cycle life remains critical. Significant progress has been made in high-capacity anodes and high-voltage cathodes, but their performance is hindered by electrode degradation, where it is related to the behaviors of binders at the surface and interface. Conventional non-conductive binders like poly(vinylidene difluoride) (PVDF), combined with conductive additives, often fail to maintain electrical pathways under repeated volume changes. Alternatively, conjugated polymer binders have emerged as a superior alternative, simultaneously offering intrinsic conductivity, mechanical flexibility, and strong adhesion through π-conjugated backbones and functional groups. Their tunable molecular structure enables efficient electron/ion transport while mitigating electrode cracking. Additionally, the development of hierarchically ordered nanostructures in conjugated polymer binder can further enhance their electrochemical performance. This review examines the design principles of conjugated polymer binders, focusing on molecular engineering and nanostructural control to optimize their performance in high-loading electrodes, such as silicon-based anodes. By addressing key challenges in binder functionality, these advanced materials pave the way for next-generation high-energy-density LIBs.
Broader contextHere, we explore a crucial yet often underappreciated component in lithium-ion battery (LIB) development: electrode binders. While significant advancements have been made in high-capacity anodes (e.g., silicon) and high-voltage cathodes, electrode degradation continues to limit the realization of next-generation batteries with high energy densities, long-term cycling stability and stable calendar life. Traditional non-conductive binders often fail to accommodate the severe volume fluctuations and interface instabilities during cycling, leading to loss of electrical connectivity and to worsened parasitic reactions. In contrast, conjugated conductive polymer binders present a promising path forward. This review critically evaluates recent progress in their molecular design and nano-structural engineering. Distinct from broader polymer binder reviews, our minireview concentrates specifically on conjugated systems, with an emphasis on the development of ordered molecular architectures and well-defined nanostructures. These features are shown to play a pivotal role in improving electrochemical performance by forming robust and conductive polymer networks. |
1. Introduction
Lithium-ion batteries (LIBs) have become the predominant energy storage technology, powering a wide range of applications ranging from consumer electronics, electric vehicles to grid storage.1 To address the increasing need for sustainable energy storage, it is essential to improve the energy density of electrodes. In the past 20 years, substantial advancements have been achieved in the development of high-capacity anodes2 (e.g., silicon-based materials) and high-voltage cathodes3–5 (e.g., nickel-rich layered oxides (NMC) and lithium-rich layered oxides), offering promising solutions to LIBs with higher energy density. Nevertheless, the efficiency and cycle life of these batteries are strongly limited by the properties of electrode materials and their interfaces,6 where binders play an essential role in maintaining structural stability, materials integrity and electrical connectivity of electrodes.5,7,8In traditional electrode fabrication, insulating binders like poly(vinylidene difluoride) (PVDF) are commonly combined with conductive carbon additives, such as acetylene black.9 However, this configuration often struggles to preserve the required conductive pathways in the electrode, especially when subjected to substantial volume changes and pulverization during cycling.10 PVDF itself has negligible intrinsic conductivity, and it blocks ion/electron pathways in electrodes, particularly in thick or high-loading designs (e.g., silicon anodes).11 Meanwhile, acetylene black particles rely on physical contact for electron percolation, which could be easily disrupted by volume changes, therefore isolating active material particles. In this scenario, PVDF cannot rebuild the electrical contacts after fracture, due to its lack of self-healing or dynamic bonding.12 In addition, the expansion and contraction of the high-capacity alloying type anode particles generate immense mechanical stress, causing the typically brittle solid electrolyte interphase (SEI) to crack and rupture. This rupture exposes fresh, highly reactive lithiated silicon surfaces to the electrolyte, triggering a destructive and continuous SEI reformation. Studies have shown that with a passive binder like PVDF, this process results in the formation of a thick, non-uniform, and mechanically unstable SEI dominated by organic carbonate species, which is particularly prone to failure.13 As a result, the continuous parasitic reactions at the electrode–electrolyte interfaces reduced the calendar life of the anodes through two main degradation modes: the irreversible consumption of the finite lithium inventory and a gradual increase in cell impedance.14
Conjugated polymer binders have emerged as a promising alternative, combining intrinsic electronic conductivity, mechanical resilience, and superior adhesion to electrode materials. Their unique π-conjugated backbones (or side chains) allow electron delocalization, creating uninterrupted conductive networks that enable efficient electron transport across the entire electrode structure (Fig. 1A).15,16 The flexible molecular nature balances rigidity and elasticity, reducing mechanical stress and preventing electrode cracking or pulverization, thereby enhancing cycle life. Conjugated polymers also form strong intermolecular interactions (e.g., π–π stacking, hydrogen bonding, or electrostatic interactions) with active materials, preventing particle detachment during cycling.17 The conjugated polymer binder also function as a powerful coating, which limits the direct contact between the bulk electrolyte and the highly reactive anode surface, inherently suppressing the extent of parasitic side reactions.18 Additionally, functionalization with polar groups (e.g., hydroxyl, ether, carboxyl or (trifluoromethylsulfonyl)imide (TFSI) groups) can simultaneously enhance lithium-ion transport.19,20 It was reported that conjugated polymer binder preferentially interact with the electrolyte salt (e.g., LiPF6), which accelerates the decomposition of the salts to form SEI based on LiF and LixPFy.21,22 Those thinner, inorganic-rich SEI are highly desirable, known for its high interfacial stability, mechanical robustness, and excellent passivation properties.
To improve the durability and performance of high-loading electrodes (such as Si and SiOx), conjugated polymer binders must be engineered with appropriate organic moieties and functional groups within a carefully designed framework. Fine-tuning the polymer's molecular arrangement significantly affects key properties, including electronic structure, electrolyte absorption, mechanical response, and ease of processing.23,24 In addition to the chemical nature of individual polymer molecules, the macroscopic packing behavior of the polymers also plays a crucial role. For example, it has been found that hierarchically ordered structures (HOS) with well-defined nanocrystalline morphologies in the binder can enhance both electronic and ionic conductivity (Fig. 1B).22 This review will systematically explore the chemical design principles of conjugated polymer binders, focusing on the relationship between their molecular structures and their functionality in lithium-ion batteries. Special emphasis is placed on strategies for constructing well-defined nanostructures to enhance their electrochemical performance.
2. Structural design of conjugated polymer binders and manipulation of π-conjugation and π–π stacking
2.1. Electronic conductivity modulation
Adjusting the chemical composition of the polymer backbone is crucial for modifying its electronic structure to enable practical use as a multifunctional binder in lithium-ion batteries. The choice of the repeating monomer units that constitute the conjugated backbone is the most direct way to define the polymer's inherent electronic characteristics, including its highest occupied molecular orbital (HOMO), lowest unoccupied molecular orbital (LUMO) energy levels and band gap. Different aromatic or heteroaromatic systems (e.g., thiophenes, fluorenes, phenylenes, pyrroles, quinones) offer distinct electronic profiles. Importantly, undoped conjugated polymers typically have low conductivity (10−10 to 10−5 S cm−1), which can be substantially increased by magnitudes via electrochemical or chemical doping.25 This suggests that the intrinsic characteristics of the polymer backbone critically influence the level of conductivity attainable in the material.17A critical consideration, often overlooked in literature, is that this electronic conductivity is not universally persistent. It manifests only when the polymer is held within its specific electrochemical doping window. This necessitates a crucial alignment between the polymer's redox potential and the electrode's actual operating potential. For instance, a polymer designed for a cathode binder must be able to sustain a stable p-doped (oxidized) state at high operating potentials (e.g., 3.0–4.5 V vs. Li/Li+). Conversely, for a binder in a low-potential anode (such as silicon, operating <1.0 V vs. Li/Li+), the polymer is only functionally conductive if it can be electrochemically reduced (n-doped) and remain in that conductive state at these low potentials.
Towards p-doping, incorporation of protonic acid is one of the most widely used methods. Certain conjugated polymers such as polyaniline (PANI) allow protonation of the imine nitrogen, converting the emeraldine base (semiconducting) form to the emeraldine salt (conducting) form.26 This process involves quinoid–benzenoid resonance stabilization, making the charged state very stable. Different organic and inorganic acids such as polyacrylic acid,27 anthranilic acid,28 phytic acid29,30 and sulfuric acid31 5-sulfo isophthalic acid32 have been demonstrated as excellent dopants for polyaniline, enabling practical use as binder for various anodes and cathodes. Additionally, conjugated polymer binder could also undergo electrochemical doping during the battery operation, towards promoted conductivity.33 For example, the electron-donating nature of the oxygen atoms in the ethylenedioxy bridge of poly(3,4-ethylenedioxythiophene) (PEDOT) lowers the overall band gap of the polymer and, crucially, reduces its oxidation potential. This makes PEDOT easier to p-dope. Thompson et al. quantified the electronic conductivity evolution of a PEDOT derivative, dihexyl-substituted poly(3,4-propylenedioxythiophene) (PProDOT-Hx2) as a function of p-doping level. They observed that the conductivity increased from ∼10−5 S cm−1 to 10−1 S cm−1 at ∼3.6 V, coinciding with the complete conversion of polarons to bipolarons (Fig. 2A).25 Higgins et al., came up with an in situ secondary doping of PEDOT: polystyrene sulfonate (PEDOT:PSS) with small amounts of formic acid (5 and 10 wt%) for Si anodes. Upon doping the electrical conductivity close to 4.2 S cm−1 was observed with Si nanoparticles electrodes. The system delivered a specific capacity of 3865 mA h g−1 at 1 A g−1 and could retain 75% after 100 cycles. Furthermore, at higher electrode thickness of 1.5 mg cm−2, a high areal capacity of 3 mA h cm−2 was observed for the 10 wt% formic acid doped PEDOT-PSS/Si electrodes.34
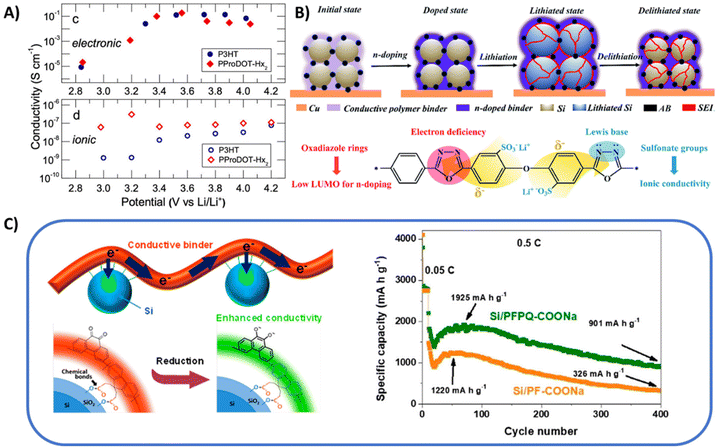 | ||
| Fig. 2 (A) Electronic and ionic conductivities of poly(3-hexylthiophene) (P3HT) and PProDOT-Hx2 at different p-doping state. Reprinted by permission from American Chemical Society, ref. 25, copyright 2020. (B) n-type conjugated polymer binder that maintains the electrical and mechanical integrity of the Si microparticle anode during charge and discharge cycles, as well as chemical structure of aromatic polyoxadiazole lithium sulfonate. Reprinted by permission from The Royal Society of Chemistry: ref. 39, copyright 2021. (C) Schematic illustration of the electrochemical behavior of PFPQ-COONa binder in Si anode, and cycling performance of Si/PF-COONa and Si/PFPQ-COONa at 0.5 C. For all measurements above, the areal loading of the active material was approximately 1.2 mg cm−2. Reprinted by permission from American Chemical Society, ref. 43, copyright 2018. | ||
Unlike p-doping, n-doping presents greater challenges because most π-conjugated systems are inherently electron-rich, leaving few classical conjugated polymers naturally suited for efficient n-doping.7 Effective n-doping generally requires polymers with low electron affinity to facilitate electron transfer from the dopant. Typical conjugated polymers based on polythiophene and polypyrrole derivatives have been applied as anode binder with some n-doping potential, such as poly[3-(potassium-4-butanoate)thiophene] (PPBT),35,36 poly[3-(2-ethylhexyl)thiophene-alt-2,5-terephthalic acid sodium salt] (PT1T).37 However, to enhance n-doping performance, introducing more electron-withdrawing heteroatoms (e.g., N, O, or S) into the backbone is suggested as it can help delocalize electron density and improve electron-accepting capability.38 Jiang et al. developed a highly conductive n-type polymer binder for silicon anode, using polyoxadiazoles (PODs).39,40 The strong electron-withdrawing nature of the oxadiazole ring endows PODs with low LUMO energy levels, facilitating n-doping and enabling superior electron transport properties (Fig. 2B). As a result, the capacity of silicon microparticles (4200 mAh g−1) can be almost entirely released during the half-cell testing. Polyisoindigo is another important class of n-type conducting polymer which works well at lower operating potentials and hence was employed as conducting additive cum binder for Si@C core shell anodes. The attractive mechanical and electrochemical properties of polyisoindigo makes it a promising n-type binder for Si anodes. The Si@C electrodes with polyisoindigo binder displayed high-capacity of 1400 mA h g−1 and maintains a stable performance over 500 cycles with average coulombic efficiency of 99.56%.41 Similarly, Matsumi et al. reported a bis(imino)acenaphthene quinone (BIAN)-type conjugated polymer, the delocalized lone pair of electrons on the nitrogen atoms enabled low-lying LUMO level of the binder (−3.69 eV), endowing its great potential to be n-doped.42
The same group designed and synthesized BIAN-fluorene copolymer binder for graphite anode to enhance the electronic conductivity and to prevent the continuous degradation of SEI. The copolymer has lower lying LUMO level than that of ethylene carbonate thus preventing continuous degradation of electrolyte during charge–discharge process. Furthermore, the HOMO and LUMO energy levels are in a position where the polymer gets doped well before the reduction of carbonate solvents of the electrolyte thus maintaining electronic conductivity. On the other hand, PVDF has LUMO energy level lying well above that of ethylene carbonate thus resulting in continuous degradation of EC and breakdown of SEI in each charge–discharge cycle. Thus, the BIAN-fluorene copolymer displayed better electrochemical performance compared to PVDF for graphite anode.44
Another effective strategy to enhance n-doping performance involves integrating donor/acceptor moieties, such as electron-withdrawing carbonyl groups, into the polymer backbone. This approach is particularly practical since polycondensation synthesis naturally allows for the incorporation of multiple monomer types. This is exemplified by the series work by Liu et al., as introducing fluorenone units into the polyfluorene backbone allows the polymer to undergo cathodic doping in the reducing lithium environment, enhancing its overall electrical conductivity by magnitudes.23,45,46 Pan et al. reported a similar design by introducing 10% of phenanthraquinone (PQ) into polyfluorene backbone (Fig. 2C). The produced copolymer (PFPQ-COONa) is successfully applied as binder for Si anode, delivering 76.1% capacity retention after 200 cycles (0.2 C).43
While the extended conjugated backbone primarily dictates the fundamental electronic characteristics of conjugated polymers, the moieties located on the polymer side chains also plays an important role in influencing the polymer's electrochemical characteristics. Sizable side chains can cause steric interference, hinder tight packing and weakening intermolecular interactions among polymer chains. This may prevent the development of planar backbone conformations, lowering crystallinity and, as a result, diminishing electrical conductivity. Additionally, the bulky side chains can induce torsional strain along the polymer backbone, disrupting its planarity.47 Such structural distortion may hinder π-electron delocalization, which is essential for effective charge transport in conjugated polymer binders.48 For example, poly(9,9-dioctylfluorene-co-fluorenone-co-methylbenzoic ester) (PFM), which features dioctyl side chains, exhibited an electronic conductivity of only 10−6 S cm−1.22 In contrast, a polyspirobifluorene-based binder with similar backbone but more compact side chain structure achieved a significantly higher conductivity of 4.7 × 10−3 S cm−1.49
Interestingly, bulky side chains do not always hinder the electronic conductivity of polymer binders, as they can also be conjugated and enhance long-range polaron mobility. For instance, Liu et al. developed a class of conductive polymers with π–π stacking side chains to facilitate electron transport (Fig. 3). By incorporating pyrene-based moieties via copolymerization, they achieved polymer binders with electron mobilities up to 8.5 × 10−4 cm2 V−1 s−1. These binders preserved electrode integrity and maintained stable Si interfaces over 1000 cycles, enabling high-capacity batteries with superior rate performance, thanks to their self-assembled nanostructures.
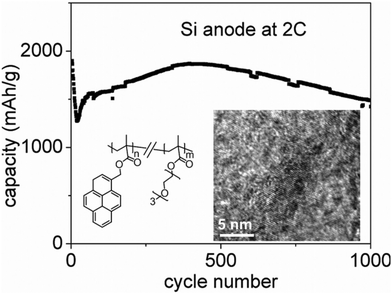 | ||
| Fig. 3 Structure of the side chain conducting poly(1-pyrenemethyl methacrylate-co-triethylene oxide methyl ether methacrylate) (PPyE), high-resolution transmission electron microscopy (HRTEM) image of PPyE polymer, and charge (delithiation) capacities of PPyE-based Si electrodes at 2 C rate. Reprinted by permission from American Chemical Society, ref. 50, copyright 2015. | ||
2.2. Polymer flexibility modulation
Rational design of conjugated polymer backbone is important for tuning their flexibility, as inherent rigidity of some classical conjugated polymers (e.g., polyacetylene) limits their use as binders, and they are not resistant to cycle-induced large strain. Incorporating flexible segments (e.g., introducing methyl benzoate ester blocks into the polyfluorene backbone23) addresses this brittleness, enabling stretchability and deformability. Despite these advantages, this modification comes at the cost of disrupted conjugation and hindered chain packing, ultimately compromising the polymer's crystallinity and electrical conductivity. Thompson et al. investigated conjugated break spacers (CBS) in PProDOT cathode binders for Li-ion batteries.51 Increased CBS content and spacer length reduced lamellar peak intensity, consequently lowering charge carrier mobility. These results aligned with rate capability tests of the lithium nickel cobalt aluminum oxide (NCA) cathodes with different PProDOT binder, where electronic conductivity (over mechanical properties) governed performance and polymers with higher conductivity retained more capacity (Fig. 4A).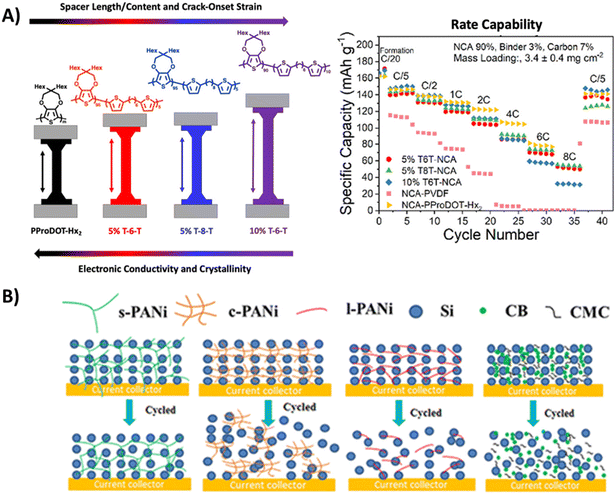 | ||
| Fig. 4 (A) Left: Relationship between the spacer length, crack-onset strain, electronic conductivity and crystallinity of the conjugated polymer with different conjugation break spacer. Right: Rate capability testing for the Li-NCA-5% T-6-T, Li-NCA-5% T-8-T, Li-NCA-10% T-6-T, Li-NCA-PProDOT-Hx2, and Li-NCA-PVDF cells with a cathode mass composition of 90% NCA, 3.5% Super P, 3.5% CNT, and 3% binder. Two formation cycles at C/20 were carried out before testing. Reprinted by permission from American Chemical Society, ref. 51, copyright 2024. (B) Schematic illustrations of cycle-induced structural changes of s-PANi/Si, c-PANi/Si, l-PANi/Si, and carboxymethyl cellulose (CMC) electrode systems. Reprinted by permission from American Chemical Society, ref. 52, copyright 2020. | ||
The ideal case is to establish 2D/3D-conjugated conductive network with favorable flexibility without compromising the continuous π-conjugation. In a systematic comparison of polyaniline-based binders with different architectures including star-like (s-PANI), cross-linked (c-PANI), and linear (l-PANI) (Fig. 4B), the silicon anode with s-PANI binder delivered a reversible capacity of 1776 mAh g−1 after 100 cycles at 500 mA g−1, significantly outperforming systems using c-PANI or l-PANI. It was found s-PANI/Si exhibited a much lower electrode thickness expansion (114%) than that of c-PANI/Si (286%).52 Similarly, Seferos et al. reported a 2D-conjugated polysulfonamide as binder for organic lithium-ion batteries.53 The findings indicate that the linear (1D) oligosulfonamide binder, lacking branching structures, exhibits weak mechanical properties and fails to ensure long-term stability in perylenetetracarboxylic dianhydride-based composite electrodes. In contrast, the 2D-conjugated polysulfonamide binder demonstrated no delamination or discoloration during cycling. Additionally, in rate performance tests, the electrode with the 2D-conjugated binder retained twice the capacity compared to the one with the 1D oligosulfonamide binder.
2.3. Polymer solvation modulation
A key requirement for scalable electrode manufacturing is satisfactory slurry processibility, which is governed by rheology. This presents a unique challenge for conjugated polymer binders. Without solubilizing side chains, their rigid conjugated backbones cause significant intermolecular aggregation, leading to dramatically high and uncontrolled viscosity.54 This problem cannot be solved by simply reducing the binder content, as is common in conventional non-conductive binder systems; here, the conjugated polymer dominates the conductive network, and maintaining its concentration is critical for electrode conductivity.Therefore, molecular engineering via “internal plasticization” is the preferred pathway. The side chains are the primary tool for tuning intermolecular interactions and solubility of conjugated polymers. The target is to achieve an ideal shear-thinning profile: a high viscosity or yield stress at low shear rates (at rest) to suspend components and prevent active material settling, followed by a sharp decrease in viscosity under the high shear rates of coating.55 These side chains achieve this dual function in two ways. First, long, bulky, or branched side chains provide crucial steric hindrance, shielding the rigid backbones and preventing them from stacking into aggregates, especially as they align under shear flow. Second, these same flexible side chains enhance the degree of polymer entanglement at rest. This entanglement creates a high initial (zero-shear) viscosity, which in turn leads to a more pronounced shear-thinning effect as these entanglements break and the chains align under shear.
So far, the most commonly applied side chain is still the alkyl groups based on hydrocarbons. Liu et al. inaugurally developed the polyfluorene-type polymers with tailored side chain structures as conductive binders for silicon anode applications.45 The polyfluorene polymers containing octyl side chains enhance the polymer's solvation in non-polar solvents, therefore the conductive polymer matrix is well-suited for the lithium-ion slurry manufacturing process. The cost-effective silicon/polymer composite anodes demonstrate exceptional cycling performance, delivering approximately 2100 mAh g−1 for silicon and 1400 mAh g−1 for the full electrode, even after 650 cycles—all without the need for conductive additives.
Compared to the hydrophobic side chains, the hydrophilic side chains (e.g., oligo ethylene glycol, carboxylic acid) could also enhance the polymer processability. More importantly, they also create ion-permeable pathways and improves ionic conductivity.23 It was demonstrated that the enhanced polarity of the PEFM polymer binder could result in a 3-fold electrolyte uptake improvement, compare to the PFM binder without oligo(ethylene glycol) side chains (Fig. 5A). Thompson et al. conducted a systematic study on the role of oligoether side chains in controlling solvent-induced swelling and electrolyte absorption.56 They designed a series of binders derived from dihexyl-substituted poly(3,4-propylenedioxythiophene) (PProDOT-Hx2), in which 5% to 35% of the hexyl groups were replaced with oligoether chains (Fig. 5B). Their results showed that as the oligoether content increased from 5% to 35%, the binder's swelling ratio rose from 41% to 97%. Simultaneously, the ionic conductivity improved fourfold, reaching a maximum value of 4 × 10−7 S cm−1. However, it should be noted that the combination of hexyl and oligoether side chains increased structural disorder in the binder, leading to a decline in electronic conductivity. The binder with 5% oligoether exhibited the highest electronic conductivity (1.1 S cm−1), while the 35% oligoether variant showed the lowest (0.14 S cm−1). Grazing-incidence wide-angle X-ray scattering (GIWAXS) analysis confirmed this trend, revealing reduced crystallinity in the doped polymer binders with higher oligoether fraction. As an optimal compromise, the (75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25) PProDOT binder that incorporating 25% oligo(ethylene glycol) side chains demonstrated the best rate capability and fastest charge/discharge kinetics in symmetric testing.
25) PProDOT binder that incorporating 25% oligo(ethylene glycol) side chains demonstrated the best rate capability and fastest charge/discharge kinetics in symmetric testing.
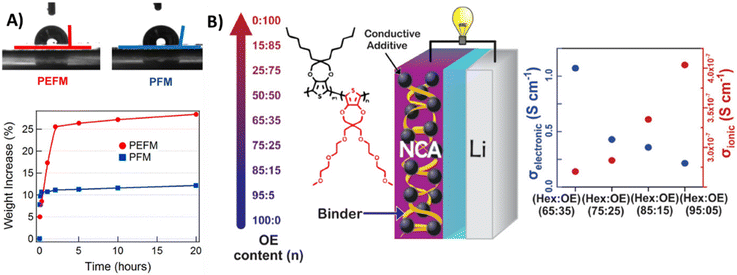 | ||
Fig. 5 (A) Water contact angles on the surfaces of PEFM and PFM films, and the swelling tests of PEFM and PFM polymer film in the 1 M LiPF6 ethylene carbonate (EC)/diethyl carbonate (DEC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) electrolyte. Reprinted by permission from American Chemical Society, ref. 23, copyright 2013. (B) Electronic and ionic conductivities of the PProDOT copolymer series with different amount of oligoether side chains. Reprinted by permission from American Chemical Society, ref. 56, copyright 2022. 1) electrolyte. Reprinted by permission from American Chemical Society, ref. 23, copyright 2013. (B) Electronic and ionic conductivities of the PProDOT copolymer series with different amount of oligoether side chains. Reprinted by permission from American Chemical Society, ref. 56, copyright 2022. | ||
3. Structural reordering to enhance conjugated polymer binder performance
3.1 Side chain cleavage
A more specialized and innovative strategy involves designing conjugated polymer binders with labile side chains. After slurry-based processing step, these specific side chains are intentionally cleaved and removed from the main conjugated backbone. The removal of these bulky solubilizing groups reduces steric hindrance and allows the conjugated backbones to pack more closely. This enhanced proximity facilitates stronger intermolecular π–π stacking, leading to a significant improvement in electronic conductivity.57 This method cleverly addresses the common trade-off where side chains necessary for good solubility (and thus processability) can impede optimal backbone packing for conductivity. For instance, Ponder et al. developed a carefully designed PProDOT-based polymer featuring cleavable 2-butyloctyl ester side chains.47 Following standard solution processing, the polymer films were treated with a basic solution to hydrolyze the side chains, converting them into an insoluble, solvent-resistant material with tight π–π stacking. This structural rearrangement led to a dramatic rise in electronic conductivity, from 6 S cm−1 to 60 S cm−1.However, the side chain cleavage process does not always require intricate molecular design. Liu et al. proposed that thermal-induced side chain cleavage could be a broadly applicable method, as demonstrated with polyfluorene-based polymer binder. Polyfluorene exhibits high charge mobility, but only in a well-ordered β-phase conformation.58,59 Unfortunately, this phase usually exists as isolated domains within a predominantly amorphous and insulating film. To optimize the chain packing for creating a percolating network of highly conductive phase, their study showed that heating poly(9,9-dioctylfluorene-co-fluorenone-co-methylbenzoic ester) (PFM) to 500 °C selectively removed its octyl side chains while preserving the polymer backbone (Fig. 6A and B).22 This transformation facilitated the development of hierarchically ordered structures (HOS) (Fig. 6C), significantly enhancing conductivity from 10−6 S cm−1 to 0.1 S cm−1. Notably, the HOS design also enabled rapid lithium-ion diffusion, comparable to that in graphite. Unlike conventional ion transport mechanisms, which rely on segmental polymer motion or solvated ions, the HOS conductive polymer couples lithium-ion movement with negative polaron (electron) diffusion, establishing it as a novel soft material for efficient ion transport. As a demonstration of its potential as a lithium-ion battery binder, the conductive HOS-PFM facilitated stable cycling and excellent capacity retention in high-loading silicon-based anodes (4.5 mAh cm−2).
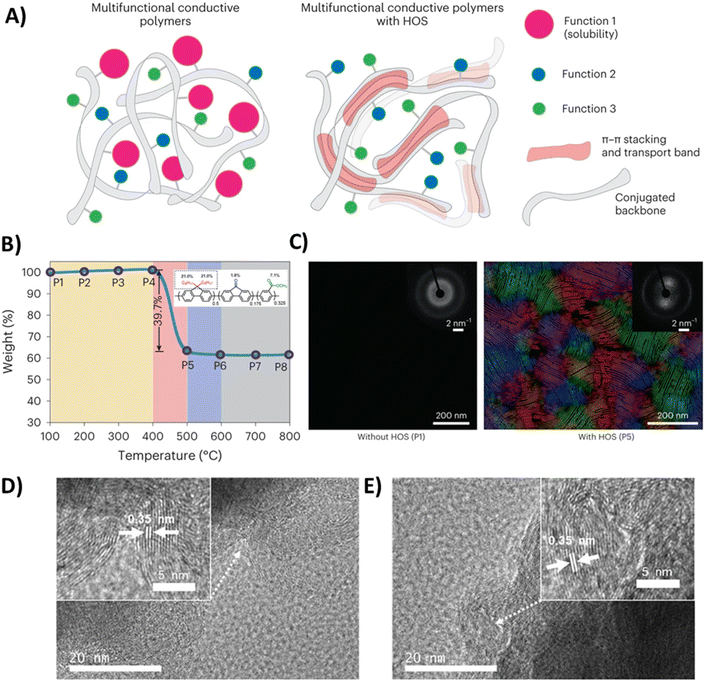 | ||
| Fig. 6 (A) Schematic illustration of polymer chain arrangements in multifunctional conductive polymers and multifunctional conductive polymers with HOS. Reprinted by permission from Springer Nature, ref. 22, copyright 2023. (B) Thermalgravimetric analysis of PFM. Samples are named P1–P8 based on the highest thermal processing temperature (100 to 800 °C). Tan shading: thermally stable region for PFM; pink shading: alkyl side-chain cleavage; purple shading: losing carbonyl groups; grey shading: carbonization region. Reprinted by permission from Springer Nature, ref. 22, copyright 2023. (C) Flow line maps tracking the polymer main chains and examples of diffraction patterns (insets, weighted averages over 3 × 3 pixels) of PFM and HOS-PFM acquired by 4D-STEM. The color wheel represents the orientation of the main chain of the polymer. Reprinted by permission from Springer Nature, ref. 22, copyright 2023. (D and E) High-resolution TEM image of SiOx-PFO-aq-400 before cycling (D) and after being cycled 100 times (E). Reprinted by permission from Wiley, ref. 60, copyright 2023. | ||
Subsequently, this approach was adapted for practical applications using the water-soluble polyfluorene binder poly(2,7-9,9(di(oxy-2,5,8-trioxadecane))fluorene) (PFO). Thermal treatment at 400 °C removed the oligo(ethylene glycol) side chains, and wide-angle X-ray scattering (WAXS) and X-ray diffraction (XRD) analyses revealed enhanced π–π stacking of the polymer backbone. The interchain spacing (d-spacing) decreased from 4.3 Å to 3.4 Å, which is close to double of the van der Waals radius of carbon and approaching ideal molecular packing. The resulting HOS-PFO displayed a six-order-of-magnitude increase in conductivity compared to pristine PFO, enabling stable cycling of high-loading SiOx anodes. Notably, the SiOx-HOS-PFO electrodes achieved ≈86% capacity retention over 200 cycles at 0.33 C, even without conductive carbon additives.60 Furthermore, HRTEM analysis demonstrated that the HOS-PFO maintained its crystallinity even after repeated cycling, highlighting the exceptional stability of the HOS frameworks (Fig. 6D and E).
3.2 Introduction of secondary polymer
Polymer blending can promote the reorganization of conjugated polymer, leading to well-ordered nanostructures with enhanced electrochemical performance. This approach has been extensively used in organic electronic device fabrication. For instance, combining conjugated polymer with secondary insulating polymer can greatly improve the π–π stacking of the conjugated polymers and therefore enhance the charge carrier mobility, owing to the nanoconfinement effect.61,62 This design principle also applies to the formulation of conjugated polymer-based binder for lithium-ion batteries. A well-studied case involves blending PEO (polyethylene oxide) with PEDOT:PSS as a polymer binder. Beyond boosting ionic conductivity, polyethylene glycol (PEG) also enhances PEDOT chain ordering, leading to improved electronic conductivity.63 Kang et al. demonstrated that the hydrogen bonding between PEG and PSS promotes phase separation, leading to a more linear and densely packed PEDOT conformation with increased crystallinity (Fig. 7A), as verified by GIWAXS analysis (Fig. 7B).64 At an optimal PEDOT:PSS/PEG ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3, the composite binder exhibited a 72-fold increase in electronic conductivity alongside improved mechanical properties. When applied in a silicon anode, this binder enabled a high-rate capacity of 500 mAh g−1 at 2 C and full capacity recovery to ∼2300 mAh g−1 at 0.1 C, showcasing excellent cycling stability. Compatible with PEDOT:PSS, CMC serves as an additional water-soluble polymer option beyond PEG. An et al. documented analogous conductivity enhancements using CMC, attributing them to charge-screening effects that cause phase separation and reorientation of PEDOT chains.65 Similarly, Song et al., developed a mixed ion/electron conducting binder for anodes by introducing ionically conducting PEO branches to an electronically conducting polythiophene with a redox active pendant. The ionically conductive branches of PEO were controlled to understand the contributions of electronic and ionic conductivity to LIB performance. This redox active binder when employed as a binder, the rate performance and the storage capacity was significantly improved when compared to that of PVDF. The polymers with simple octyl side chains were also prepared as a control which displayed poor electrolyte wettability and hence lower capacity than that of PEO comprising polythiophene binder.
0.3, the composite binder exhibited a 72-fold increase in electronic conductivity alongside improved mechanical properties. When applied in a silicon anode, this binder enabled a high-rate capacity of 500 mAh g−1 at 2 C and full capacity recovery to ∼2300 mAh g−1 at 0.1 C, showcasing excellent cycling stability. Compatible with PEDOT:PSS, CMC serves as an additional water-soluble polymer option beyond PEG. An et al. documented analogous conductivity enhancements using CMC, attributing them to charge-screening effects that cause phase separation and reorientation of PEDOT chains.65 Similarly, Song et al., developed a mixed ion/electron conducting binder for anodes by introducing ionically conducting PEO branches to an electronically conducting polythiophene with a redox active pendant. The ionically conductive branches of PEO were controlled to understand the contributions of electronic and ionic conductivity to LIB performance. This redox active binder when employed as a binder, the rate performance and the storage capacity was significantly improved when compared to that of PVDF. The polymers with simple octyl side chains were also prepared as a control which displayed poor electrolyte wettability and hence lower capacity than that of PEO comprising polythiophene binder.
 | ||
| Fig. 7 (A) Proposed mechanism of PEG in PEDOT:PSS. (B) 2D GIWAXS patterns of PEDOT:PSS films according to the PEG blending ratio. Reprinted by permission from Elsevier, ref. 64, copyright 2024. | ||
Building upon the concept of controlling phase separation through physical blending, a more delicate strategy involves engineering the nanostructure via chemical synthesis to create multifunctional composite binders. A prominent example is the core–shell aqueous conductive binder (PABS:PEDOT) for sulfur cathode, recently reported by Wang et al.66 They first synthesized a sulfonate-containing elastic copolymer (PABS) as the “core” via emulsion polymerization, followed by in situ polymerization to coat it with a conjugated polymer “shell” of PEDOT. The novelty of this binder architecture originates from the dual nature of the core–shell interaction, which combines electrostatic attraction with physical incompatibility. Consequently, during the drying process, the system undergoes bi-continuous mesoscopic phase separation towards highly controlled nanostructure with high conductivity (up to 32.5 S m−1). This approach stands in sharp contrast to simple physical blending of PABS and PSS:PEDOT, which, as their control experiment showed, leads to macroscopic agglomeration and poor conductivity (2.2 S m−1). Additionally, the PABS component provides chemical functionality, as its abundant ester and cyanide groups effectively capture lithium polysulfides. This suppression of the shuttle effect was validated in full-cell tests, where the battery with PABS:PEDOT maintained 81.6% of its specific capacity after 450 cycles at 0.5 C. Recently, a cross-scale stabilization binder based on electronically conductive PPy was used for µSi anodes to stabilize the SEI and electrode's stability against volume expansion. The cross scale stabilized binder comprised rigid polyacrylic acid (PAA), flexible poly(vinyl alcohol) (PVA) and conductive PPy with citric acid crosslinker. The rigid–flexible network of PAA–PVA with covalent and flexible hydrogen bonds provides pathway for the stress dissipation during cycling while the CA and PPy crosslinks direct the LiF rich growth of SEI by preferentially binding with PF6− ions. This multifunctional binder enabled stable cycling of µSi anodes showed high CE of 99.6% with excellent areal capacity and volumetric capacity of 5.9 mAh cm−2, 2458 mAh cm−3 respectively. Furthermore, full cells with NMC811 showed 81% capacity retention after 100 cycles with N&P ratio of 1.15.67
While conjugated polymers with a stacked architecture provide structural integrity, their limited elasticity can lead to electrode delamination under repeated volume changes. Introducing a functional secondary polymer represented a solution through mechanical reinforcement. This secondary polymer can form electrostatic interactions, hydrogen bonds, or covalent crosslinks with the primary conjugated polymer, creating a resilient and conductive network that maintains electrode stability during cycling. Matsumi et al. developed an n-type self-healing polymer composite (P-BIAN/PAA) for silicon anodes, where P-BIAN dynamically interacts with PAA via electrostatic hydrogen bonding.68 The nitrogen atoms in P-BIAN's di-imine backbone bond with PAA's carboxyl groups, creating a crosslinked, conductive network. This structure offers both mechanical flexibility to withstand silicon's volume expansion and sustained electronic conductivity across the electrode. Similarly, Taskin et al. developed a crosslinked polymer network by thermally inducing esterification between carboxylic acid-functionalized poly(fluorene phenylene) and PVA. When used as a binder for silicon anodes, this system demonstrated stable cycling performance, achieving a coulombic efficiency of 99.89% after 100 cycles.69
It is critical to note that these introduced charged moieties are also beneficial for efficient chelating the electrode active materials as well as current collector. Kang et al. recently engineered an adhesive-conductive (AC-polymer) interlayer by redesigning the polyanion dopant for PEDOT, towards the application for Li metal powders (LMP) anode.70 they synthesized a P(SS-co-AA) copolymer, chemically incorporating adhesive acrylic acid (AA) segments alongside the traditional styrene sulfonate (SS) units. This molecular-level integration achieved dual functionality, herein, the –COOH groups from the AA segment provided a 28% increase in adhesive strength, while the PEDOT chains maintained their highly ordered polymer stacking and high electrical conductivity (450 S cm−1). By securing the interface, this AC-polymer interlayer suppressed LMP delamination, reduced the Li stripping overpotential by 60%, and enabled stable cycling in high-rate full cells. Building on this concept of using functional interlayers for metal anodes, Mei et al. explored a similar strategy, but one based on nitrogen-based lithiophilic groups instead of carboxylic acids.71 They synthesized and compared two hexaazanonaphthalene (HATN)-based conjugated polymers, s-HATN (linked by flexible sigma bonds) and b-HATN (linked by sp2-carbon bonds), as a coating on the Cu current collector. The study found that tailoring the intramolecular conjugation was critical, as the more rigid, fully conjugated b-HATN exhibited higher electrical conductivity and a more favorable mesoporous structure compared to the flexible s-HATN. This superior combination of conductivity and lithiophilicity in b-HATN led to a dramatically reduced Li nucleation overpotential (down to 33.2 mV) and suppressed dendrite growth, enabling stable full-cell cycling.
This strategy of combining an elastic matrix with a conductive polymer was also demonstrated in earlier foundational work by Milroy and Manthiram.72 They identified that while conjugated polymers like polypyrrole (PPy) are conductive, their inherent brittleness prevents their direct use in sulfur cathodes with large volume changes. To solve this, they synthesized the nanocomposite by in situ emulsion polymerization of pyrrole within an elastomeric polyurethane (PU) continuous phase. This process created a distinct nanostructure where PPy nanoparticles formed an electrically percolating network within the insulating, but elastic PU matrix. This PPy-PU binder represents a nanoparticle-in-matrix percolation model, powerfully illustrating how nanostructural design can overcome the intrinsic flaws of single-component polymers.
Blending with an elastic secondary polymer is also an important strategy to improve ionic conductivity, compensating for the fact that the rigid conjugated chains of conductive polymers typically impede ion transport. Du et al. demonstrated this by fabricating a hybrid PEG/PANI binder for NMC811 cathodes.73 The ion-conductive PEG and electron-conductive PANI network enhanced Li+ transfer and provided a physical barrier to protect the cathode from HF corrosion. This blending approach has also been applied to PEDOT:PSS, where mixing it with PEO created a dual-conductor for LFP and LixNi0.5Mn1.5O4 cathodes, resulting in high electrical conductivity of 45 S cm−1 and ionic conductivity of 10−4 S cm−1.63 Along this line, Ryder et al. systematically studied the electrical conductivity and mechanical properties of variations in alginate–PANI blends with different (10–20 weight%) PANI compositions. This blend when employed as binder for graphite anode improved the rate performance and CE of the cells.
3.3 Introduction of metal ions
The ability of metal ions to induce crosslinking is rooted in fundamental coordination chemistry. Polymer chains in advanced binders are often designed with specific functional groups that can act as Lewis bases (electron-pair donors). Metal cations, being positively charged, act as Lewis acids (electron-pair acceptors). Bonds like M2+–carboxylate or M3+–sulfonate can partially reform after fracture, enabling self-healing during electrode volume changes. A prime example is the crosslinking of the widely used conductive polymer PEDOT:PSS with multivalent cations such as Mg2+.74 The PEDOT:PSS aqueous dispersion contains a large excess of PSS chains with sulfonate (–SO3H) groups. The Mg2+ ion can coordinate with the deprotonated sulfonate anions (–SO3−) on adjacent PSS chains, forming a stable –SO3−⋯Mg2+⋯−O3S– ionic bridge. This process links the PSS chains, and by extension the PEDOT chains they are entangled with, into a robust 3D network.Meanwhile, it is important to consider the effects of metal ion dopants in electronic and ionic conductivity of the conjugated polymers. Jiang et al. conducted a systematic study on how crosslinking metal cations (Li+, Na+, Mg2+, Ca2+, and Sr2+) influence the conductivity of the n-type POD polymer binder (Fig. 8A).40 Their findings revealed that larger ionic radii induce greater conformational disorder in polymer chains, enhancing electrolyte wettability and ionic conductivity. However, increased chain entanglement strengthens mechanical strength at the expense of elasticity, which is a critical trade-off for long-term cycling stability. Among the tested ions, Ca2+-POD demonstrated an optimal balance, effectively mitigating electrode volume expansion while preserving structural integrity and conductive pathways (Fig. 8B). Cells employing the Ca-POD binder delivered outstanding performance, retaining 1770.1 mAh g−1 after 100 cycles at 0.2 C and achieving 1281.6 mAh g−1 at 1 C.
 | ||
| Fig. 8 (A) Synthesis procedure and structures of Mn+-PODs. (B) SEM images of the Si anodes with PAALi and Mn+-PODs binders before and after 100 cycles at 0.2 C. Reprinted by permission from American Chemical Society, ref. 40. Copyright 2023. | ||
This ion-cross-linking approach is also a powerful tool for modulating slurry viscosity, a critical parameter for fabricating high-loading electrodes. This was systematically investigated by Liu et al. for PEDOT:PSS binders in Si anodes.75 They found that the viscosity of the binder solution could be precisely tuned by the choice of multivalent cation dopant, with the observed trend directly correlating to the cations’ ionic potential (Φ) and softness parameters (Σ). Cations with a higher ionic potential, such as Sn4+, exerted stronger electrostatic forces with the PSS chains, leading to a dramatic increase in viscosity, from 5.28 mPa s for pristine PEDOT:PSS to 60.5 mPa s for Sn4+-cross-linked PEDOT:PSS. Importantly, the choice of ion also controlled the electrode morphology. The higher solubility of Sn4+ sulfite facilitated the formation of a smooth, homogeneously dispersed Sn4+-cross-linked PEDOT:PSS film. In contrast, group 2 ions (e.g., Sr2+, Ba2+) formed large, detrimental precipitates of insoluble sulfites. Specifically, a sheet structure with a diameter of ∼500 nm appeared on the Sr2+-cross-linked film, whereas square shaped particles appeared on the Ba2+/Mg2+/Ca2+-cross-linked binder film. This demonstrates that the rheological properties of conjugated polymer slurries can be rationally engineered via ion dopant selection to achieve the desired viscosity for high-loading processing. The optimized Sn4+-cross-linked binder, which combined high viscosity with uniformity, enabled Si anodes to retain 1876.4 mAh g−1 after 100 cycles.
3.4 Introduction of small molecules
Small molecules are highly effective additives for conjugated polymer binders in lithium-ion batteries, enabling precise crosslinking control and multifunctional enhancement. Typical example include glycerol, which leverages its trifunctional hydroxyl groups to form robust covalent networks via esterification with acid groups on polymers like PEDOT:PSS.76 Unreacted hydroxyl groups further establish dynamic hydrogen bonds, enabling self-healing during electrode volume changes. Simultaneously, glycerol plasticizes the binder by increasing chain mobility, effectively suppressing crack propagation while enhancing ionic transport and mechanical flexibility. Jiang et al. developed an innovative 3D crosslinked conductive polyoxadiazole (POD) binder using glycerol (GL), effectively dissipating mechanical stress and accommodating volume changes in silicon particles (Fig. 9A).19 Notably, similar to the effects of metal ions, incorporating small-molecule crosslinkers can increase structural disorder and reduce the crystallinity of conjugated polymers. This reduction in crystallinity here however benefits ionic conductivity: POD-c-GL demonstrates higher ionic conductivity than POD across all tested temperatures. At 30 °C, for instance, crosslinking boosts the Li+ conductivity of POD-c-GL to 2.61 × 10−4 S cm−1 from 2.24 × 10−4 S cm−1. Nevertheless, a trade-off emerges in electronic conductivity. POD-c-GL exhibits an electronic conductivity of 5.84 × 10−2 S cm−1, slightly lower than non-crosslinked POD (9.19 × 10−2 S cm−1). A further trade-off involves mechanical performance. Excessive crosslinking increases brittleness primarily due to elevated local stress, while insufficient crosslinking compromises the material's structural integrity. Therefore, optimizing the crosslinking degree is critical. | ||
| Fig. 9 (A) Schematic drawing of the crosslinked POD-c-GL binder formed by condensation between LPOD and GL. Reprinted by permission from Wiley, ref. 19, copyright 2024. (B) Architecting an elastic binding network to promote e−/Li+ transport via the proposed in situ photo-crosslinking strategy. (C) DMT modulus mappings of the above three binder electrodes. The scale bars represent 300 nm. Reprinted by permission from Cell Press, ref. 78, copyright 2025. | ||
When employing small molecules as crosslinkers for conjugated polymer binders, the processing strategy is a critical consideration. While many studies have directly utilized pre-crosslinked polymer binders in slurry-based electrode fabrication,77 the ‘in situ’ crosslinking approach—where the conjugated polymer binder is crosslinked after electrode coating—is often the preferred method. Yang et al. demonstrated this by employing an light-induced thiol–ene click reaction between a 3,6-dioxa-1,8-octanedithiol crosslinker and olefin-terminated side chains on a linear polyfluorene binder (PFDP-Li) to form a binder network (Fig. 9B).78In situ crosslinking was found to facilitate a homogeneous electron/ion transport network in SiOx electrodes. Compared to SiOx anodes with ex situ crosslinked PFDP-Li binders, those with in situ crosslinked PFDP-Li exhibited superior rate performance and lower charge transfer resistance, attributed to more uniform binder coverage. A more direct visualization was provided by Derjaguin–Muller–Toporov (DMT) modulus mapping. Both uncrosslinked PFDP-Li and ex situ crosslinked PFDP-Li electrodes displayed localized high-modulus regions (Fig. 9C), suggesting incomplete embedding of SiOx particles within the polymer network, which leads to uneven internal stress during electrode volume changes. In contrast, the in situ crosslinked electrode showed consistent DMT modulus values matching the binder, confirming the formation of a uniform and fully integrated 3D elastic network. Along this direction, Pan et al., developed an in situ crosslinking strategy with Si nanoparticles using PEDOT:PSS and citric acid crosslinker in IPA. This binder with multiple anchoring sites forms strong hydrogen bonding, chemical and physical crosslinks with Si nanoparticles. The linear PEDOT chains within the binder provides excellent electronic conductivity to the binder while the hydroxyl groups PSS promote Li ion conductivity. Furthermore, the citric acid crosslinker enables the formation of lithium citrate SEI layer on the Si anode which could buffer the volume changes during lithiation and delithiation process. This unique binder design could hold up to 90% Si particles and displayed excellent electrochemical performance metrics of 89% and 81% capacity retention after 2000 cycles at 0.1 and 0.2 A g−1.79
Similarly, Jiang et al., developed a dual crosslinking of Si anodes by an esterification reaction between –SO3H of PODs and –OH groups on Si particles and functionalized CNT-OH particles at 120 °C under vacuum. This crosslinked mixed ion/electron conducting binder provides mechanically robust network through multiple hydrogen bonds for the Si particles which help to maintain electrodes structural integrity and stress management during cycling. The Si anode with this binder showed a very high capacity of 3079 mA h g−1 at 0.6 A g−1 after 200 cycles and 2151 mA h g−1 at 2 A g−1 after 500 cycles.80
4. Conclusion and perspective
Conjugated polymer binders have emerged as a transformative solution for next-generation lithium-ion batteries, effectively addressing three critical challenges: sufficient electron conductivity, smooth ion transport, and mechanical integrity. By rationally tailoring the polymer backbone and side-chain functionalities, their π-conjugation characteristics and solid-state chain organization can be precisely modulated to optimize n-type or p-type doping performance, thereby enabling various applications in both anode and cathode systems. Moreover, the molecular architecture of these conjugated polymers critically determines their interfacial behavior within the electrode–electrolyte matrix, dictating their adhesion to active materials, accommodation of volumetric strain, as well as electrolyte solvation dynamics which is essential for enabling sufficient processability and facilitating efficient electron/ion transport.A critical advantage driving the development of conjugated polymer binders is their potential to significantly improve practical, full-cell energy density. Conventional electrodes always require two inactive components including an insulating binder (like PVDF) and a separate conductive additive (like carbon black). These “dead weight” materials dilute the cell's overall gravimetric and volumetric energy density. Because conjugated binders are multifunctional, they can partially or completely replace the conductive additive. Furthermore, these polymers are what enable the use of high-capacity materials like the Si anode. For example, a layered biphenyl-polyoxadiazole (CBPOD) binder enabled a high-performance Si anode, allowing an NMC811||Si full cell to exhibit a remarkable gravimetric energy density of 574 Wh kg−1.80
The performance of conjugated polymer binders is fundamentally governed by their molecular packing, particularly the π–π stacking that dictates both electrical and mechanical properties. As this review has detailed, strategies to tune this structural orderliness normally aim for a “Goldilocks” state, balancing processability, mechanical integrity, and mixed conductivity. However, this goal is frustrated by inherent trade-offs, such as sacrificing electronic conductivity for flexibility or impeding ion transport when optimizing π–π stacking. Consequently, the widely adapted design principle evolve from merely balancing these compromises to overcoming them. This generally necessitates the development of multifunctional polymers with hierarchical architectures, where nano structural control is leveraged to decouple conflicting properties. In such systems, mechanical robustness, electronic conductivity, and ionic transport can be engineered as distinct, yet synergistic, functions of the overall architecture. Various components, such as secondary polymers, small molecules, and ions, have demonstrated their ability to function as additives with conjugated polymer binders to achieve this goal.
While simplifying the design of binder systems remains an ideal goal, recent advances in understanding ion transport physics within polymer binders have opened new avenues for a paradigm shift. Notably, the discovery of ballistic ion transport in a densely packed, electron-conductive polyfluorene binder has also demonstrated Li+ conductivities one to two orders of magnitude higher than those of conventional PEO-based systems.81 This unique ion-conduction mechanism, being independent of segmental motion, breaks the fundamental trade-offs of conventional polymers. It enables a single material to be simultaneously mechanically robust, electron-conductive, and ion-conductive. This discovery moves binder design away from multi-component formulations and toward simpler, high-energy systems based on a single, multifunctional polymer. The redox activity of the polymer binders also becomes crucial in enhancing the ion transport and diffusion kinetics in LIBs. Thus, employing different redox active moieties in the polymer backbone or side chains becomes important in not only improving the ion transport but also improves the storage capacity, adhesion and overall stability of the batteries.
To enable the practical adoption of conjugated polymer binders, scalable and efficient synthesis methods must also be developed. Currently, conjugated polymer production relies on energy-intensive organic synthesis and polymerization techniques, such as polycondensation of specialized aromatic or heterocyclic monomers (e.g., fluorene, thiophene, or aniline).82 Processes like electrochemical polymerization or transition-metal-catalyzed coupling (e.g., Stille, Kumada reactions83) suffer from high energy demands and low yields. Furthermore, post-polymerization modifications, such as sulfonate group incorporation to balance ionic and electronic conductivity, add complexity to the synthesis. Future research should prioritize environmentally benign processing to facilitate commercialization. So far, the processing of most conjugated polymers require hazardous solvents (e.g., chlorobenzene), complicating handling and disposal.84 While traditional PVDF binders also use toxic solvents like NMP, efforts are underway to transition to aqueous alternatives. Additionally, optimizing the quality of slurry processing of conjugated polymer binder is critical, as conjugated polymers seldom display predictable rheology and they tend to aggregate in electrode formulations,85 often necessitating surfactants or additives to ensure uniformity. By addressing these synthesis and processing challenges, conjugated polymer binders can unlock their full potential for next-generation battery applications.
Looking forward, the design principles of multifunctional conjugated binders are proving critical not only for conventional liquid-electrolyte systems but also for next-generation high-energy-density platforms like solid-state batteries (SSBs). In SSBs, creating a stable, multifunctional interface to address the poor solid–solid contacts and sluggish Li+ transport in the composite electrodes is a primary challenge. Li et al. demonstrated a dual conductive polymer network for LiCoO2 cathode in SSB, formed by the molecular interaction of an ionic conductor (lithiated polyvinyl formal-derived single-ion conductor, LiPVFM), an electronic conductor (PEDOT:PSS), and a lithium salt (LiODFB). This composite network yielded both exceptionally high electronic conductivity (68.9 S cm−1) and high Li+ ionic conductivity (2.76 × 10−4 S cm−1).86 The HOS-PFM polymer binder serves as another key example. Its inherent electronic and ionic conductivity enabled the fabrication of “additive-free” silicon anodes (60% Si, 40% HOS-PFM) for use in SSBs. In a full cell test, this “additive-free” anode demonstrated a 114% improvement in cycle life compared to electrodes using a conventional PVDF/PEO/carbon black formulation. Ultimately, this evolution of the binder from a passive glue to a dominant, multifunctional, and active component is the key to unlocking the next generation of high-energy-density batteries.
Conflicts of interest
The authors declare no conflict of interest.Data availability
No primary research results, software or code have been included and no new data were generated or analyzed as part of this review.Acknowledgements
This work was funded by the Vehicle Technologies Office of the US Department of Energy under the Advanced Battery Materials Research (BMR) Program, Solid-State Battery Program, and by the Laboratory Directed Research and Development (LDRD) Program at Lawrence Berkeley National Laboratory (LBNL), which is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under contract no. DE-AC02-05CH11231. J.S. ackonwledges the Swedish Energy Agency (Energimyndigheten) for the funding (grant number P2023-01041), Vinnova Grant (2023-02551), Competence and Excellence for the Electrification of the Transport System (COMPEL), and Area of Advance - Production at Chalmers.References
- M. Li, J. Lu, Z. Chen and K. Amine, 30 Years of Lithium-Ion Batteries, Adv. Mater., 2018, e1800561, DOI:10.1002/adma.201800561.
- H. Zhao, X. Bo, H. Xu, L. Wang, W. A. Daoud and X. He, Advancing lithium-ion battery anodes towards a sustainable future: Approaches to achieve high specific capacity, rapid charging, and improved safety, Energy Storage Mater., 2024, 72, 103696, DOI:10.1016/j.ensm.2024.103696.
- Q. Li, D. Zhou, M. Chu, Z. Liu, L. Yang, W. Wu, D. Ning, W. Li, X. Liu and J. Li, et al., A comprehensive understanding on the anionic redox chemistry of high-voltage cathode materials for high-energy-density lithium-ion batteries, Chem. Soc. Rev., 2025, 54(7), 3441–3474, 10.1039/d4cs00797b.
- J. Xiang, Y. Wei, Y. Zhong, Y. Yang, H. Cheng, L. Yuan, H. Xu and Y. Huang, Building Practical High-Voltage Cathode Materials for Lithium-Ion Batteries, Adv. Mater., 2022, 34(52), e2200912, DOI:10.1002/adma.202200912.
- M. Okubo, S. Ko, D. Dwibedi and A. Yamada, Designing positive electrodes with high energy density for lithium-ion batteries, J. Mater. Chem. A, 2021, 9(12), 7407–7421, 10.1039/d0ta10252k.
- N. Kim, Y. Kim, J. Sung and J. Cho, Issues impeding the commercialization of laboratory innovations for energy-dense Si-containing lithium-ion batteries, Nat. Energy, 2023, 8(9), 921–933, DOI:10.1038/s41560-023-01333-5.
- Q. He, J. Ning, H. Chen, Z. Jiang, J. Wang, D. Chen, C. Zhao, Z. Liu, I. F. Perepichka and H. Meng, et al., Achievements, challenges, and perspectives in the design of polymer binders for advanced lithium-ion batteries, Chem. Soc. Rev., 2024, 53(13), 7091–7157, 10.1039/d4cs00366g.
- L. Yang, T. Meng, W. Zheng, J. Zhong, H. Cheng, Y. Tong and D. Shu, Advanced binder design for high-performance silicon anodes, Energy Storage Mater., 2024, 72, 103766, DOI:10.1016/j.ensm.2024.103766.
- L. Fransson, T. Eriksson, K. Edström, T. Gustafsson and J. O. Thomas, Influence of carbon black and binder on Li-ion batteries, J. Power Sources, 2001, 101(1), 1–9, DOI:10.1016/s0378-7753(01)00481-5.
- P. Das and B. C. Thompson, Development of design strategies for conjugated polymer binders in lithium-ion batteries, Polym. J., 2022, 55(4), 317–341, DOI:10.1038/s41428-022-00708-x.
- A. M. Battaglia, J. T. Liu, V. Lotocki, K. L. Perry and D. S. Seferos, Stable, high-rate, organic zinc-ion batteries accomplished using an ion-conducting marine-inspired binder, EES Batteries, 2025, 1, 1173–1183, 10.1039/d5eb00125k.
- Z. Chen, L. Christensen and J. R. Dahn, Comparison of PVDF and PVDF-TFE-P as Binders for Electrode Materials Showing Large Volume Changes in Lithium-Ion Batteries, J. Electrochem. Soc., 2003, 150(8), A1073, DOI:10.1149/1.1586922.
- C. C. Nguyen, T. Yoon, D. M. Seo, P. Guduru and B. L. Lucht, Systematic Investigation of Binders for Silicon Anodes: Interactions of Binder with Silicon Particles and Electrolytes and Effects of Binders on Solid Electrolyte Interphase Formation, ACS Appl. Mater. Interfaces, 2016, 8(19), 12211–12220, DOI:10.1021/acsami.6b03357.
- J. D. McBrayer, M.-T. F. Rodrigues, M. C. Schulze, D. P. Abraham, C. A. Apblett, I. Bloom, G. M. Carroll, A. M. Colclasure, C. Fang and K. L. Harrison, et al., Calendar aging of silicon-containing batteries, Nat. Energy, 2021, 6(9), 866–872, DOI:10.1038/s41560-021-00883-w.
- Y. Shi, L. Peng, Y. Ding, Y. Zhao and G. Yu, Nanostructured conductive polymers for advanced energy storage, Chem. Soc. Rev., 2015, 44(19), 6684–6696, 10.1039/c5cs00362h.
- Y. Shi, X. Zhou and G. Yu, Material and Structural Design of Novel Binder Systems for High-Energy, High-Power Lithium-Ion Batteries, Acc. Chem. Res., 2017, 50(11), 2642–2652, DOI:10.1021/acs.accounts.7b00402.
- S. Chen, Z. Song, L. Wang, H. Chen, S. Zhang, F. Pan and L. Yang, Establishing a Resilient Conductive Binding Network for Si-Based Anodes via Molecular Engineering, Acc. Chem. Res., 2022, 55(15), 2088–2102, DOI:10.1021/acs.accounts.2c00259.
- C. Song, J. Huang and Z. Wang, A conjugated conductive polymer coating towards a robust micro-Si anode for lithium storage, Chem. Commun., 2025, 61(65), 12135–12138, 10.1039/d5cc02737c.
- Y. Yu, C. Yang, J. Zhu, B. Xue, J. Zhang and M. Jiang, An Advanced 3D Crosslinked Conductive Binder for Silicon Anodes: Leveraging Glycerol Chemistry for Superior Lithium-Ion Battery Performance, Angew. Chem., Int. Ed., 2025, 64(6), e202418794, DOI:10.1002/anie.202418794.
- I. M. Nugraha, J. Olchowka, C. Brochon, D. Flahaut, M. Bousquet, B. Cabannes-Boue, R. B. Nuernberg, E. Cloutet and L. Croguennec, An Alternative Polymer Material to PVDF Binder and Carbon Additive in Li-Ion Battery Positive Electrode, Adv. Sci., 2024, 11(46), e2409403, DOI:10.1002/advs.202409403.
- H. Wang, H. Cheng, D. Li, F. Li, Y. Wei, K. Huang, B. Jiang, H. Xu and Y. Huang, Lithiated Copper Polyphthalocyanine with Extended π–Conjugation Induces LiF–Rich Solid Electrolyte Interphase toward Long–Life Solid–State Lithium–Metal Batteries, Adv. Energy Mater., 2023, 13(16), e2204425, DOI:10.1002/aenm.202204425.
- T. Zhu, H. Sternlicht, Y. Ha, C. Fang, D. Liu, B. H. Savitzky, X. Zhao, Y. Lu, Y. Fu and C. Ophus, et al., Formation of hierarchically ordered structures in conductive polymers to enhance the performances of lithium-ion batteries, Nat. Energy, 2023, 8(2), 129–137, DOI:10.1038/s41560-022-01176-6.
- M. Wu, X. Xiao, N. Vukmirovic, S. Xun, P. K. Das, X. Song, P. Olalde-Velasco, D. Wang, A. Z. Weber and L. W. Wang, et al., Toward an ideal polymer binder design for high-capacity battery anodes, J. Am. Chem. Soc., 2013, 135(32), 12048–12056, DOI:10.1021/ja4054465.
- K. Dai, H. Zhao, Z. Wang, X. Song, V. Battaglia and G. Liu, Toward high specific capacity and high cycling stability of pure tin nanoparticles with conductive polymer binder for sodium ion batteries, J. Power Sources, 2014, 263, 276–279, DOI:10.1016/j.jpowsour.2014.04.012.
- P. Das, B. Zayat, Q. Wei, C. Z. Salamat, I.-B. Magdău, R. Elizalde-Segovia, D. Rawlings, D. Lee, G. Pace and A. Irshad, et al., Dihexyl-Substituted Poly(3,4-Propylenedioxythiophene) as a Dual Ionic and Electronic Conductive Cathode Binder for Lithium-Ion Batteries, Chem. Mater., 2020, 32(21), 9176–9189, DOI:10.1021/acs.chemmater.0c02601.
- N. V. Blinova, J. Stejskal, M. Trchová and J. Prokeš, Control of polyaniline conductivity and contact angles by partial protonation, Polym. Int., 2007, 57(1), 66–69, DOI:10.1002/pi.2312.
- J. Tong, C. Han, X. Hao, X. Qin and B. Li, Conductive Polyacrylic Acid-Polyaniline as a Multifunctional Binder for Stable Organic Quinone Electrodes of Lithium-Ion Batteries, ACS Appl. Mater. Interfaces, 2020, 12(35), 39630–39638, DOI:10.1021/acsami.0c10347.
- F. Balqis, C. Eldona, B. T. Laksono, Q. Aini, F. H. Hamid, H. S. Wasisto and A. Sumboja, Conductive Polymer Frameworks in Silicon Anodes for Advanced Lithium-Ion Batteries, ACS Appl. Polym. Mater., 2023, 5(7), 4933–4952, DOI:10.1021/acsapm.3c00531.
- C. Zhang, Q. Chen, X. Ai, X. Li, Q. Xie, Y. Cheng, H. Kong, W. Xu, L. Wang and M.-S. Wang, et al., Conductive polyaniline doped with phytic acid as a binder and conductive additive for a commercial silicon anode with enhanced lithium storage properties, J. Mater. Chem. A, 2020, 8(32), 16323–16331, 10.1039/d0ta04389c.
- H. Wu, G. Yu, L. Pan, N. Liu, M. T. McDowell, Z. Bao and Y. Cui, Stable Li-ion battery anodes by in situ polymerization of conducting hydrogel to conformally coat silicon nanoparticles, Nat. Commun., 2013, 4, 1943, DOI:10.1038/ncomms2941.
- H. Gao, Q. Lu, Y. Yao, X. Wang and F. Wang, Significantly Raising the Cell Performance of Lithium Sulfur Battery via the Multifunctional Polyaniline Binder, Electrochim. Acta, 2017, 232, 414–421, DOI:10.1016/j.electacta.2017.02.160.
- H. Y. Lin, C. H. Li, D. Y. Wang and C. C. Chen, Chemical doping of a core-shell silicon nanoparticles@polyaniline nanocomposite for the performance enhancement of a lithium ion battery anode, Nanoscale, 2016, 8(3), 1280–1287, 10.1039/c5nr07152f , from NLM PubMed-not-MEDLINE.
- B. Zayat, P. Das, B. C. Thompson and S. R. Narayan, In Situ Measurement of Ionic and Electronic Conductivities of Conductive Polymers as a Function of Electrochemical Doping in Battery Electrolytes, J. Phys. Chem. C, 2021, 125(14), 7533–7541, DOI:10.1021/acs.jpcc.0c08934.
- T. M. Higgins, S. H. Park, P. J. King, C. J. Zhang, N. McEvoy, N. C. Berner, D. Daly, A. Shmeliov, U. Khan and G. Duesberg, et al., A Commercial Conducting Polymer as Both Binder and Conductive Additive for Silicon Nanoparticle-Based Lithium-Ion Battery Negative Electrodes, ACS Nano, 2016, 10(3), 3702–3713, DOI:10.1021/acsnano.6b00218 , from NLM PubMed-not-MEDLINE.
- Y. H. Kwon, K. Minnici, J. J. Park, S. R. Lee, G. Zhang, E. S. Takeuchi, K. J. Takeuchi, A. C. Marschilok and E. Reichmanis, SWNT Anchored with Carboxylated Polythiophene “Links” on High-Capacity Li-Ion Battery Anode Materials, J. Am. Chem. Soc., 2018, 140(17), 5666–5669, DOI:10.1021/jacs.8b00693.
- R. Na, K. Minnici, G. Zhang, N. Lu, M. A. Gonzalez, G. Wang and E. Reichmanis, Electrically Conductive Shell-Protective Layer Capping on the Silicon Surface as the Anode Material for High-Performance Lithium-Ion Batteries, ACS Appl. Mater. Interfaces, 2019, 11(43), 40034–40042, DOI:10.1021/acsami.9b13941.
- K.-L. Wang, K.-T. Chen, Y.-H. Yi, Y.-H. Hung, H.-Y. Tuan and M. Horie, High-Performance Lithium Ion Batteries Combining Submicron Silicon and Thiophene–Terephthalic Acid-Conjugated Polymer Binders, ACS Sustainable Chem. Eng., 2019, 8(2), 1043–1049, DOI:10.1021/acssuschemeng.9b05800.
- S.-M. Kim, M. H. Kim, S. Y. Choi, J. G. Lee, J. Jang, J. B. Lee, J. H. Ryu, S. S. Hwang, J.-H. Park and K. Shin, et al., Poly(phenanthrenequinone) as a conductive binder for nano-sized silicon negative electrodes, Energy Environ. Sci., 2015, 8(5), 1538–1543, 10.1039/c5ee00472a.
- Y. Yu, J. Zhu, K. Zeng and M. Jiang, Mechanically robust and superior conductive n-type polymer binders for high-performance micro-silicon anodes in lithium-ion batteries, J. Mater. Chem. A, 2021, 9(6), 3472–3481, 10.1039/d0ta10525b.
- Z. Sun, J. Zhu, C. Yang, Q. Xie, Y. Jiang, K. Wang and M. Jiang, N-Type, Polyoxadiazole Conductive Polymer Binders Derived High-Performance Silicon Anodes Enabled by Crosslinking Metal Cations, ACS Appl. Mater. Interfaces, 2023, 15(10), 12946–12956, DOI:10.1021/acsami.2c19587.
- A. Mery, P. Bernard, A. Valero, J. P. Alper, N. Herlin-Boime, C. Haon, F. Duclairoir and S. Sadki, A polyisoindigo derivative as novel n-type conductive binder inside Si@C nanoparticle electrodes for Li-ion battery applications, J. Power Sources, 2019, 420, 9–14, DOI:10.1016/j.jpowsour.2019.02.062.
- S. N. Mishra, S. Punyasloka, B. S. Mantripragada, A. Pradhan and N. Matsumi, Enabling Ultrafast Charging in Graphite Anodes Using BIAN-Based Conjugated Polymer/Lithium Polyacrylate as a Binder, ACS Appl. Energy Mater., 2023, 6(23), 11954–11962, DOI:10.1021/acsaem.3c02129.
- Y. Zhao, L. Yang, Y. Zuo, Z. Song, F. Liu, K. Li and F. Pan, Conductive Binder for Si Anode with Boosted Charge Transfer Capability via n-Type Doping, ACS Appl. Mater. Interfaces, 2018, 10(33), 27795–27800, DOI:10.1021/acsami.8b08843.
- S. G. Patnaik, R. Vedarajan and N. Matsumi, BIAN based functional diimine polymer binder for high performance Li ion batteries, J. Mater. Chem. A, 2017, 5(34), 17909–17919, 10.1039/c7ta03843g.
- G. Liu, S. Xun, N. Vukmirovic, X. Song, P. Olalde-Velasco, H. Zheng, V. S. Battaglia, L. Wang and W. Yang, Polymers with tailored electronic structure for high capacity lithium battery electrodes, Adv. Mater., 2011, 23(40), 4679–4683, DOI:10.1002/adma.201102421.
- H. Zhao, Z. Wang, P. Lu, M. Jiang, F. Shi, X. Song, Z. Zheng, X. Zhou, Y. Fu and G. Abdelbast, et al., Toward practical application of functional conductive polymer binder for a high-energy lithium-ion battery design, Nano Lett., 2014, 14(11), 6704–6710, DOI:10.1021/nl503490h.
- J. F. Ponder Jr, S. A. Gregory, A. Atassi, A. K. Menon, A. W. Lang, L. R. Savagian, J. R. Reynolds and S. K. Yee, Significant Enhancement of the Electrical Conductivity of Conjugated Polymers by Post-Processing Side Chain Removal, J. Am. Chem. Soc., 2022, 144(3), 1351–1360, DOI:10.1021/jacs.1c11558.
- F. C. Grozema, P. T. van Duijnen, Y. A. Berlin, M. A. Ratner and L. D. A. Siebbeles, Intramolecular Charge Transport along Isolated Chains of Conjugated Polymers: Effect of Torsional Disorder and Polymerization Defects, J. Phys. Chem. B, 2002, 106(32), 7791–7795, DOI:10.1021/jp021114v.
- A. C. Kıgılcım, M. E. Cetintasoglu, M. Tokur, O. S. Taskin, E. Bulut and E. Güzel, Versatile Spiro-Fluorene-Based Polymer Binder for Li-Ion Batteries, ACS Appl. Polym. Mater., 2025, 7(4), 2708–2715, DOI:10.1021/acsapm.5c00081.
- S. J. Park, H. Zhao, G. Ai, C. Wang, X. Song, N. Yuca, V. S. Battaglia, W. Yang and G. Liu, Side-chain conducting and phase-separated polymeric binders for high-performance silicon anodes in lithium-ion batteries, J. Am. Chem. Soc., 2015, 137(7), 2565–2571, DOI:10.1021/ja511181p.
- P. Das, C. Z. Salamat, B. Zayat, R. Elizalde-Segovia, Y. Wang, X. Gu, S. R. Narayan, S. H. Tolbert and B. C. Thompson, Evaluating the Impact of Conjugation Break Spacer Incorporation in Poly(3,4-propylenedioxythiophene)-Based Cathode Binders for Lithium-Ion Batteries, Chem. Mater., 2024, 36(3), 1413–1427, DOI:10.1021/acs.chemmater.3c02555.
- X. He, R. Han, P. Jiang, Y. Chen and W. Liu, Molecularly Engineered Conductive Polymer Binder Enables Stable Lithium Storage of Si, Ind. Eng. Chem. Res., 2020, 59(7), 2680–2688, DOI:10.1021/acs.iecr.9b05838.
- J. T. Liu, E. Grignon, A. M. Battaglia, M. Imran, C. Copeman, H. A. Mills, A. J. Howarth, E. H. Sargent and D. S. Seferos, An Investigation of Conjugated Sulfonamide Materials as Binders for Organic Lithium-Ion Batteries, Chem. Mater., 2023, 35(22), 9692–9701, DOI:10.1021/acs.chemmater.3c02105.
- B. Kuei and E. D. Gomez, Chain conformations and phase behavior of conjugated polymers, Soft Matter, 2016, 13(1), 49–67, 10.1039/c6sm00979d.
- C. D. Reynolds, H. Walker, A. Mahgoub, E. Adebayo and E. Kendrick, Battery electrode slurry rheology and its impact on manufacturing, Energy Adv., 2025, 4(1), 84–93, 10.1039/d4ya00380b.
- P. Das, R. Elizalde-Segovia, B. Zayat, C. Z. Salamat, G. Pace, K. Zhai, R. C. Vincent, B. S. Dunn, R. A. Segalman and S. H. Tolbert, et al., Enhancing the Ionic Conductivity of Poly(3,4-propylenedioxythiophenes) with Oligoether Side Chains for Use as Conductive Cathode Binders in Lithium-Ion Batteries, Chem. Mater., 2022, 34(6), 2672–2686, DOI:10.1021/acs.chemmater.1c03971.
- J. Shanahan, L. Yan, Y. Olanrewaju, S. Kashani, H. Ade, F. So and W. You, Acid-Triggered Side Chain Cleavage Leads to Doped Conjugated Polymers of High Conductivity, J. Am. Chem. Soc., 2024, 146(47), 32243–32248, DOI:10.1021/jacs.4c09843.
- L. Niu, Q. Yang, C. Li, Z. He, J. Xin and J. Liu, Insights into the β–phase of polyfluorene: characterization, regulation, and mechanism, J. Polym. Sci., 2023, 62(3), 463–479, DOI:10.1002/pol.20230679.
- P. Prins, F. C. Grozema, B. S. Nehls, T. Farrell, U. Scherf and L. D. A. Siebbeles, Enhanced charge-carrier mobility inβ-phase polyfluorene, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 74(11), 113203, DOI:10.1103/PhysRevB.74.113203.
- X. Jin, Z. Zhu, Q. Miao, C. Fang, D. Huang, R. Giovine, L. Chen, C. Dun, J. J. Urban and Y. Fu, et al., Green Electrode Processing Enabled by Fluoro-Free Multifunctional Binders for Lithium-Ion Batteries, Adv. Sci., 2025, e2416995, DOI:10.1002/advs.202416995.
- Y. Q. Zheng and Z. Bao, Molecularly Designed and Nanoconfined Polymer Electronic Materials for Skin-like Electronics, ACS Cent. Sci., 2024, 10(12), 2188–2199, DOI:10.1021/acscentsci.4c01541.
- S. Nikzad, H.-C. Wu, J. Kim, C. M. Mahoney, J. R. Matthews, W. Niu, Y. Li, H. Wang, W.-C. Chen and M. F. Toney, et al., Inducing Molecular Aggregation of Polymer Semiconductors in a Secondary Insulating Polymer Matrix to Enhance Charge Transport, Chem. Mater., 2020, 32(2), 897–905, DOI:10.1021/acs.chemmater.9b05228.
- M. B. McDonald and P. T. Hammond, Efficient Transport Networks in a Dual Electron/Lithium-Conducting Polymeric Composite for Electrochemical Applications, ACS Appl. Mater. Interfaces, 2018, 10(18), 15681–15690, DOI:10.1021/acsami.8b01519.
- N. J. Kong, M. S. Kim, J. H. Park, J. Kim, J. Jin, H.-W. Lee and S. J. Kang, Promoting homogeneous lithiation of silicon anodes via the application of bifunctional PEDOT:PSS/PEG composite binders, Energy Storage Mater., 2024, 64, 103074, DOI:10.1016/j.ensm.2023.103074.
- S. Wu, L. He, Y. Lu, J. Zheng, L. Li, X. Geng, C. Sun, H. Zhao, G. Jiang and F. Di, et al., Volumetric Stress Managements on Silicon Anode of Lithium–Ion Batteries by a Self–Adaptable Binder, Energy Environ. Mater., 2024, 8(3), e12859, DOI:10.1002/eem2.12859.
- W. Wang, L. Hua, Y. Zhang, G. Wang and C. Li, A Conductive Binder Based on Mesoscopic Interpenetration with Polysulfides Capturing Skeleton and Redox Intermediates Network for Lithium Sulfur Batteries, Angew. Chem., Int. Ed., 2024, 63(38), e202405920, DOI:10.1002/anie.202405920.
- K. J. Hu, J. X. Chen, J. H. Zhang, X. Y. Sang, T. Meng, Z. C. Wang and X. L. Hu, Binder-enabled cross-scale stabilization of high-areal-capacity micro-sized silicon anodes for high-voltage lithium-ion batteries, Energy Storage Mater., 2025, 75, 104029, DOI:10.1016/j.ensm.2025.104029.
- A. Gupta, R. Badam and N. Matsumi, Heavy-Duty Performance from Silicon Anodes Using Poly(BIAN)/Poly(acrylic acid)-Based Self-Healing Composite Binder in Lithium-Ion Secondary Batteries, ACS Appl. Energy Mater., 2022, 5(7), 7977–7987, DOI:10.1021/acsaem.2c00278.
- O. S. Taskin, N. Yuca, J. Papavasiliou and G. Avgouropoulos, Interconnected conductive gel binder for high capacity silicon anode for Li-ion batteries, Mater. Lett., 2020, 273, 127918, DOI:10.1016/j.matlet.2020.127918.
- D. Kang, M. Jeong, S. Kim, M. Song, C. B. Dzakpasu, S. H. Kim, J. Lim, S. Eom, S. Jung and J. Jang, et al., A Tailored Adhesive–Conductive Interlayer for Interface Stabilization of Large–Scale Lithium Metal Powder Electrodes for High–Energy–Density Batteries, Adv. Energy Mater., 2025, 15(38), e05780, DOI:10.1002/aenm.202405780.
- S. Mei, Z. Zheng, Y. Lian, Z. Wei, X. Chen, C. Peng, Y. Zhang, X. Zhang, L. Ding and Y. Peng, et al., Lithiophilic Polymers of High Conjugation and Mesoporosity Enable Lean and Dendrite-Free Lithium Anode, ACS Appl. Mater. Interfaces, 2024, 16(36), 47620–47630, DOI:10.1021/acsami.4c09372.
- C. Milroy and A. Manthiram, An Elastic, Conductive, Electroactive Nanocomposite Binder for Flexible Sulfur Cathodes in Lithium-Sulfur Batteries, Adv. Mater., 2016, 28(44), 9744–9751, DOI:10.1002/adma.201601665.
- Y. Cao, X. Qi, K. Hu, Y. Wang, Z. Gan, Y. Li, G. Hu, Z. Peng and K. Du, Conductive Polymers Encapsulation To Enhance Electrochemical Performance of Ni-Rich Cathode Materials for Li-Ion Batteries, ACS Appl. Mater. Interfaces, 2018, 10(21), 18270–18280, DOI:10.1021/acsami.8b02396.
- L. Yan, X. Gao, J. P. Thomas, J. Ngai, H. Altounian, K. T. Leung, Y. Meng and Y. Li, Ionically cross-linked PEDOT:PSS as a multi-functional conductive binder for high-performance lithium–sulfur batteries, Sustainable Energy Fuels, 2018, 2(7), 1574–1581, 10.1039/c8se00167g.
- X. Liu, A. Iqbal, N. Ali, R. Qi and X. Qian, Ion-Cross-Linking-Promoted High-Performance Si/PEDOT:PSS Electrodes: The Importance of Cations’ Ionic Potential and Softness Parameters, ACS Appl. Mater. Interfaces, 2020, 12(17), 19431–19438, DOI:10.1021/acsami.0c00755.
- X. Liu, J. Zai, A. Iqbal, M. Chen, N. Ali, R. Qi and X. Qian, Glycerol-crosslinked PEDOT:PSS as bifunctional binder for Si anodes: Improved interfacial compatibility and conductivity, J. Colloid Interface Sci., 2020, 565, 270–277, DOI:10.1016/j.jcis.2020.01.028.
- A. Gupta, R. Badam, B. S. Mantripragada, S. N. Mishra and N. Matsumi, Ultra–Durability and Reversible Capacity of Silicon Anodes with Crosslinked Poly–BIAN Binder in Lithium–Ion Secondary Batteries for Sturdy Performance, Adv. Sustainable Syst., 2024, 9(1), 2400263, DOI:10.1002/adsu.202400263.
- L. Wang, Z. Song, Y. Li, Y. Huang, H. Zhang, Z. Yin, J. Xiao, C. Zhu, Y. Zhao and M. Zhang, et al., Establishing an elastic electron/lithium-ion transport network via in situ crosslinking for stabilizing interphases in SiO electrodes, Matter, 2025, 8(5), 101952, DOI:10.1016/j.matt.2024.101952.
- B. Q. Chen, D. M. Xu, S. M. Chai, Z. Chang and A. Q. Pan, Enhanced Silicon Anodes with Robust SEI Formation Enabled by Functional Conductive Binder, Adv. Funct. Mater., 2024, 34(34), 2401794, DOI:10.1002/adfm.202401794.
- Y. Yu, C. Yang, Y. Jiang, Z. Shang, J. Zhu, J. Zhang and M. Jiang, Robust Nitrogen/Sulfur Co–Doped Carbon Frameworks as Multifunctional Coating Layer on Si Anodes Toward Superior Lithium Storage, Adv. Energy Mater., 2024, 15(5), 2403086, DOI:10.1002/aenm.202403086.
- D. Li, C. Fang, S. Thapa, H. Sternlicht, G.-H. Lee, F. Ahmed, X. Jin, Q. Miao, R. Giovine and W. Yang, et al., Ballistic ion transport through hierarchically-ordered-structure polymer binder, Energy Environ. Sci., 2025, 18(13), 6566–6576, 10.1039/d4ee06071g.
- T. Yamamoto, π-Conjugated Polymers with Electronic and Optical Functionalities: Preparation by Organometallic Polycondensation, Properties, and Applications, Macromol. Rapid Commun., 2002, 23(10–11), 583, DOI:10.1002/1521-3927(20020701)23:10/11<583::aid-marc583>3.0.co;2-i.
- S. Cheng, R. Zhao and D. S. Seferos, Precision Synthesis of Conjugated Polymers Using the Kumada Methodology, Acc. Chem. Res., 2021, 54(22), 4203–4214, DOI:10.1021/acs.accounts.1c00556.
- M. Mooney, A. Nyayachavadi and S. Rondeau-Gagné, Eco-friendly semiconducting polymers: from greener synthesis to greener processability, J. Mater. Chem. C, 2020, 8(42), 14645–14664, 10.1039/d0tc04085a.
- Z. Xu, K. S. Park, J. J. Kwok, O. Lin, B. B. Patel, P. Kafle, D. W. Davies, Q. Chen and Y. Diao, Not All Aggregates Are Made the Same: Distinct Structures of Solution Aggregates Drastically Modulate Assembly Pathways, Morphology, and Electronic Properties of Conjugated Polymers, Adv. Mater., 2022, 34(32), e2203055, DOI:10.1002/adma.202203055.
- H. Li, F. Lian, N. Meng, C. Xiong, N. Wu, B. Xu and Y. Li, Constructing Electronic and Ionic Dual Conductive Polymeric Interface in the Cathode for High–Energy–Density Solid–State Batteries, Adv. Funct. Mater., 2020, 31(13), 2008487, DOI:10.1002/adfm.202008487.
| This journal is © The Royal Society of Chemistry 2026 |

