Open Access Article
Open Access ArticleUnprecedented 1,2-mesityl shift for the synthesis of iridafurans
Maria
Talavera
 *a,
Antonio
Gómez
a,
Nicolás
Otero
*a,
Antonio
Gómez
a,
Nicolás
Otero
 b,
Ángeles
Peña-Gallego
b,
Ángeles
Peña-Gallego
 b and
Sandra
Bolaño
b and
Sandra
Bolaño
 *a
*a
aCINTECX, Universidade de Vigo, Grupo de Novos Materiais, Departamento de Química Inorgánica, 36310 Vigo, Spain. E-mail: matalaveran@uvigo.es; bgs@uvigo.es
bUniversidade de Vigo, Departamento de Química Física, Campus Universitario, 36310, Vigo, Spain
First published on 4th December 2025
Abstract
The reactivity of tertiary propargylic alcohols bearing a mesityl substituent with [IrCp*Cl(NCMe)(PMe3)]PF6 has revealed a previously unreported 1,2-mesityl rearrangement, leading to the formation of α-iridafuran complexes. Comparative analysis with the rhodium analogue as well as different substituents at the propargylic alcohol highlights the unique reactivity of the iridium system, the key role of the mesityl group and the importance of substituent topology.
Introduction
Terminal propargyl alcohols are a particularly attractive class of compounds due to their structural duality which has been widely exploited in transformations involving the activation of C(sp3)–O or C(sp)–H bonds.1,2 In stoichiometric organometallic chemistry, the reactivity of these alcohols has traditionally been oriented towards the generation of allenylidene complexes,3–5 which serve as key intermediates of vinylidenes, acetylides, allenyl or alkoxycarbene complexes, among others. At Ir and Rh, a few examples are known.6–12 Thus, Werner and co-workers developed square-planar allenylidene complexes by activating the C(sp)–H bond, usually followed by acidification or Al2O3-assisted dehydration.6–9More recently, the [IrCp*Cl(phosphine)] system has been studied for its ability to generate methoxy(alkenyl)carbene intermediates, key precursors in orthometallation processes.13 In contrast, rhodium analogues tend to evolve into α-rhodafurans through the insertion of alkynes into Rh![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds.14
C bonds.14
In addition to this route, alternative methodologies have been described for obtaining α-metallafurans from alkynes, ketones, ynones or allenols.15,16 Noteworthy is the work of Xia et al. and Esteruelas and co-workers using secondary propargyl alcohols to form α-osmafurans through insertion of the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C moiety into the Os–H bond followed by β-H elimination.17,18 Also, Wong and co-workers described the formation of group 8 metallafurans from tertiary propargylic alcohols bearing picoline substituents via 5-exo-dig cyclization.19
C moiety into the Os–H bond followed by β-H elimination.17,18 Also, Wong and co-workers described the formation of group 8 metallafurans from tertiary propargylic alcohols bearing picoline substituents via 5-exo-dig cyclization.19
A particularly relevant feature in this context is the 1,2-migration of groups, a well-established pathway for hydrogen atoms but less common for bulky aryl groups. Moreover, the presence of electron-donating groups at the aryl substituent has been reported to reduce their migratory aptitude.20 Thus, even though aryl group migrations have been described in vinylidene systems,20–23 examples involving 1,2 displacements of mesityl groups are scarce and required the participation of heteroatoms.24
Herein, an unprecedented reactivity of tertiary propargyl alcohols towards [MCp*Cl(NCMe)(PMe3)]PF6 (M = Ir, Rh) complexes is presented. It gives rise to two divergent pathways: the classic formation of allenylidenes and, surprisingly, an unprecedented tautomerization through 1,2-shift of a mesityl group, leading to α-iridafuran complexes. This finding represents a new dimension in the reactivity of propargyl alcohols and is supported by a combined experimental and theoretical study that rationalizes the factors governing this transformation.
Results and discussion
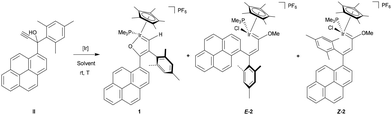
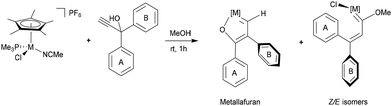
The propargylic alcohol II was synthesized from the corresponding ketone (see SI) and was reacted with [IrCp*Cl(NCMe)(PMe3)]PF6 in methanol at room temperature. The reaction led to the new iridafuran complex [IrCp*Cl{![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–C(Mes)
CH–C(Mes)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(1-pyrenyl)–O–}(PMe3)]PF6 (1) together with the expected methoxy(alkenyl)carbene iridium complexes [IrCp*Cl{
C(1-pyrenyl)–O–}(PMe3)]PF6 (1) together with the expected methoxy(alkenyl)carbene iridium complexes [IrCp*Cl{![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(OMe)–(Z/E)–CH
C(OMe)–(Z/E)–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Mes)(1-pyrenyl)}(PMe3)]PF6 (Z/E-2) in a 1.1
C(Mes)(1-pyrenyl)}(PMe3)]PF6 (Z/E-2) in a 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3
1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, respectively (Tables 1 and 2, entry 1). Unlike all previously reported transformations involving the acetonitrile complex and propargylic alcohols in methanol—where depending on the nature of the γ-carbon substituents, only the formation of one or two methoxy(alkenyl)carbene complexes was consistently observed—13 the present study reveals, for the first time, the emergence of an iridafuran complex. In addition, the steric hindrance of the pyrenyl substituent competes with the mesityl group and favours the presence of both Z/E-2 isomers, which did not occur when bearing 2-spirobifluorene instead of 1-pyrene.25 Notably, the formation of complex 1 implies not only a different reaction pathway for propargylic alcohols with organometallic complexes beyond the well-known formation of allenylidene complexes, but also, a 1,2-mesityl rearrangement has been identified which, to the best of our knowledge, has no precedent in the existing literature.
1 ratio, respectively (Tables 1 and 2, entry 1). Unlike all previously reported transformations involving the acetonitrile complex and propargylic alcohols in methanol—where depending on the nature of the γ-carbon substituents, only the formation of one or two methoxy(alkenyl)carbene complexes was consistently observed—13 the present study reveals, for the first time, the emergence of an iridafuran complex. In addition, the steric hindrance of the pyrenyl substituent competes with the mesityl group and favours the presence of both Z/E-2 isomers, which did not occur when bearing 2-spirobifluorene instead of 1-pyrene.25 Notably, the formation of complex 1 implies not only a different reaction pathway for propargylic alcohols with organometallic complexes beyond the well-known formation of allenylidene complexes, but also, a 1,2-mesityl rearrangement has been identified which, to the best of our knowledge, has no precedent in the existing literature.
| Entry | Solvent | T | Additive | % 1a | Z/E-2 ratioa |
|---|---|---|---|---|---|
| [Ir] = [IrCp*Cl(NCMe)(PMe3)]PF6.a Determined by 1H NMR spectra.b Possible allenylidene complex is formed. | |||||
| 1 | MeOH | rt | — | 32 | 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | MeOH | rt | 8 equiv. II | 35 | 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | CD2Cl2 | rt | NaPF6 | 45 | —b |
| 4 | MeOH | 213 K to rt | — | 19 | 1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | MeOH | 328 K | — | 30 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 6 | MeOH | rt | H2O | 32 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | MeOH | rt | Mol. sieves | 63 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | MeOH or THF | rt | Base | — | — |
| Entry | M | A | B | % Furana | Z/E ratioa |
|---|---|---|---|---|---|
[M] = [MCp*(PMe3)]PF6.a Determined by 1H NMR spectra.b After heating in CH3CN, E isomer completely evolves to Z isomer.c In 30 minutes, a Z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E ratio of 2 E ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is obtained. 1 is obtained. |
|||||
| 1 | Ir | 1-Pyrenyl | Mesityl | 32 | 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1b 1b |
| 2 | Ir | 1-Pyrenyl | Phenyl | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2b 2b |
| 3 | Ir | 2-Naphthyl | Mesityl | 15 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | Ir | 1-Naphthyl | Mesityl | 27 | 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 1.4 |
| 5 | Rh | 1-Pyrenyl | Mesityl | 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0c 0c |
| 6 | Rh | 1-Pyrenyl | Phenyl | — | 5.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
The formation of complex 1 was studied by modification of the reaction conditions of the original set-up. Initially, varying the molar ratio of iridium acetonitrile complex to propargylic alcohol yielded no significant changes in the outcome (Table 1, entry 2). Then, it was observed that complex 1 is also formed when the reaction is carried out in dichloromethane instead of methanol in the presence of NaPF6 (Table 1, entry 3). This suggests that methanol does not act as nucleophile but rather promotes the chloride ligand labilization. Therefore, both the hydrogen at Cα and the oxygen atom stem from the propargylic alcohol, contrary to methoxy(alkenyl)carbene complexes, where CβH and the oxygen atom derive from methanol. Note that absence of a NaPF6 in CH2Cl2 or THF leads to the slow formation and decomposition of allenylidene derivatives as previously observed with other systems.12 In addition, variable temperature experiments revealed no selective pathway preference (Table 1, entries 4 and 5). The reaction required a minimum temperature of 283 K to proceed, yielding complexes 1 and Z/E-2 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.7
2.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6 ratio. This ratio suggests the presence of a higher energy barrier for complex 1 than the methoxy(alkenyl)carbene complexes Z/E-2. When reaction was performed at 328 K, only complexes 1 and Z-2 were obtained in a 1
1.6 ratio. This ratio suggests the presence of a higher energy barrier for complex 1 than the methoxy(alkenyl)carbene complexes Z/E-2. When reaction was performed at 328 K, only complexes 1 and Z-2 were obtained in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3 ratio, along with minor impurities. This isomerization reaction was also observed by heating of the Z/E-2 mixture. Further heating to 343 K in CD3CN for 24 h led to the heterolytic cleavage of the C–O bond of complex Z-2 producing [IrCp*{(Z)–CH
2.3 ratio, along with minor impurities. This isomerization reaction was also observed by heating of the Z/E-2 mixture. Further heating to 343 K in CD3CN for 24 h led to the heterolytic cleavage of the C–O bond of complex Z-2 producing [IrCp*{(Z)–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Mes)(1-pyrenyl)}(CO)(PMe3)]PF6 and CH3Cl. Given that the formation of methoxy(alkenyl)carbene complexes proceeds via allenylidene intermediates with concomitant water release,26 the role of water was further examined (Table 1, entries 6 and 7). While direct water addition did not alter the outcome, performing the reaction in the presence of activated molecular sieves to scavenge water significantly increased the yield of complex 1. Under these conditions, a mixture of 1 and Z/E-2 was obtained in a 5.4
C(Mes)(1-pyrenyl)}(CO)(PMe3)]PF6 and CH3Cl. Given that the formation of methoxy(alkenyl)carbene complexes proceeds via allenylidene intermediates with concomitant water release,26 the role of water was further examined (Table 1, entries 6 and 7). While direct water addition did not alter the outcome, performing the reaction in the presence of activated molecular sieves to scavenge water significantly increased the yield of complex 1. Under these conditions, a mixture of 1 and Z/E-2 was obtained in a 5.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.1
2.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, respectively. Finally, global reaction to form complex 1 implies the elimination of HCl. While HCl could be absorbed by the molecular sieves added, explaining the higher amount of compound 1 under those conditions, further confirmation of its removal was attempted. Therefore, KtBuO was added in both THF and MeOH (Table 1, entry 8) trying to trap the acid and favour the reaction towards the formation of complex 1. However, the base deprotonates the propargylic alcohol and no reaction towards the iridium acetonitrile complex is observed.
1 ratio, respectively. Finally, global reaction to form complex 1 implies the elimination of HCl. While HCl could be absorbed by the molecular sieves added, explaining the higher amount of compound 1 under those conditions, further confirmation of its removal was attempted. Therefore, KtBuO was added in both THF and MeOH (Table 1, entry 8) trying to trap the acid and favour the reaction towards the formation of complex 1. However, the base deprotonates the propargylic alcohol and no reaction towards the iridium acetonitrile complex is observed.
Complexes Z/E-2 showed in NMR spectroscopy the typical resonances for this family of complexes.25,27,28 Regarding complex 1, in the 1H NMR spectrum, the CαH appeared as a doublet of 1 Hz due to the coupling to the phosphorus atom at 10.74 ppm while the corresponding carbon atom resonance decreased to 217.1 ppm also as a doublet of 9.5 Hz in the 13C{1H} NMR spectrum. Furthermore, Cβ and Cγ are displayed at 150.4 and 211.0 ppm where the latter is a doublet of 2 Hz. Finally, the correlation between the proton at the Cα and a methyl group of the mesityl ligand in the {1H, 1H} NOESY spectrum suggested that the mesityl group is now bonded to the Cβ instead of the Cγ. This 1,2-mesityl shift was further confirmed by X-ray diffraction analysis of suitable crystals of compound 1 (Fig. S59). There, the Ir atom, which is slightly disordered, is displaced from the furan ring plane formed by the three carbon atoms and the oxygen atom by only around 0.03 Å. Meanwhile, the Ir–Cα bond of around 2.04 Å as well as the C–C bonds of the rings are consistent with partial double-bond character as other iridafurans described in literature.29–32
Interestingly, when compound IV bearing a phenyl group instead of a mesityl group was used as starting material, the reaction with the acetonitrile complex only provided the expected mixture of methoxy(alkenyl)carbene iridium complexes [IrCp*Cl{![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(OMe)–(Z/E)–CH
C(OMe)–(Z/E)–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh(1-pyrenyl)}(PMe3)]PF6 (Z/E-3) in a 1
CPh(1-pyrenyl)}(PMe3)]PF6 (Z/E-3) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio (Table 2, entry 2). As before, the E-3 isomer evolves to the most thermodynamically favoured Z-3 isomer when the mixture of both complexes was refluxed in acetonitrile for 6 hours. Unlike Z-2 and all the previously reported methoxy(alkenyl)carbene complexes of this family, Z-3 present in 1H NMR spectrum a fluxional behaviour as the methoxy resonance split into two peaks at 3.70 and 3.65 ppm in CD2Cl2. These peaks coalesced at 3.68 ppm when heating up to 328 K in CD3CN and got sharpened when raising temperature up to 346 K. The 1H NMR spectrum of Z-3 in CDCl3, CD2Cl2, acetone-d6 and CD3CN showed the role of the solvent as the methoxy resonance splits in every case although it decreased as the polarity increased (Fig. S38).
2 ratio (Table 2, entry 2). As before, the E-3 isomer evolves to the most thermodynamically favoured Z-3 isomer when the mixture of both complexes was refluxed in acetonitrile for 6 hours. Unlike Z-2 and all the previously reported methoxy(alkenyl)carbene complexes of this family, Z-3 present in 1H NMR spectrum a fluxional behaviour as the methoxy resonance split into two peaks at 3.70 and 3.65 ppm in CD2Cl2. These peaks coalesced at 3.68 ppm when heating up to 328 K in CD3CN and got sharpened when raising temperature up to 346 K. The 1H NMR spectrum of Z-3 in CDCl3, CD2Cl2, acetone-d6 and CD3CN showed the role of the solvent as the methoxy resonance splits in every case although it decreased as the polarity increased (Fig. S38).
To better understand the influence of the pyrenyl group on the 1,2-shift leading to iridafuran complexes, propargylic alcohols 1-(2,4,6-trimethylphenyl)-1-(naphthalen-2-yl)prop-2-yn-1-ol (VI) and 1-(2,4,6-trimethylphenyl)-1-(naphthalen-1-yl)prop-2-yn-1-ol (VIII), where the pyrenyl group was replaced by a naphthyl moiety, were reacted towards [IrCp*Cl(NCMe)(PMe3)]PF6 in methanol. For compound VI (Table 2, entry 3) complex [IrCp*Cl{![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(OMe)–(E)–CH
C(OMe)–(E)–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh(2-naphthyl)}(PMe3)]PF6 (E-4) and around a 15% of the possible iridafuran complex were obtained. Thus, the formation of iridafuran was significantly reduced compared to reactions involving the pyrenyl group described above. For compound VIII, which resembles more closely to propargylic alcohol II due to the presence of a Cγ–C1 bond in both substituents, a mixture of [IrCp*Cl{
CPh(2-naphthyl)}(PMe3)]PF6 (E-4) and around a 15% of the possible iridafuran complex were obtained. Thus, the formation of iridafuran was significantly reduced compared to reactions involving the pyrenyl group described above. For compound VIII, which resembles more closely to propargylic alcohol II due to the presence of a Cγ–C1 bond in both substituents, a mixture of [IrCp*Cl{![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(OMe)–(Z/E)–CH
C(OMe)–(Z/E)–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh(1-naphthyl)}(PMe3)]PF6 (Z/E-5) isomers and the iridafuran [IrCp*Cl{
CPh(1-naphthyl)}(PMe3)]PF6 (Z/E-5) isomers and the iridafuran [IrCp*Cl{![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–C(Mes)
CH–C(Mes)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(1-naphthyl)–O–}(PMe3)]PF6 (6) in a 1.3
C(1-naphthyl)–O–}(PMe3)]PF6 (6) in a 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4
1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio was obtained (Table 2, entry 4). This result contrasts with the previously reported reaction with the propargylic alcohol bearing 1-naphthyl and phenyl groups where no iridafuran is obtained.27 The amount of iridafuran 6 is very similar to the obtained for the mixture of 1 and Z/E-2, suggesting that not only the mesityl group, but also the position of substitution on the naphthyl ring (position 1 vs. 2) significantly affects the steric environment and, consequently, the efficiency of the 1,2-mesityl shift. Note that bulky 2-spirobifluorene group did not promote the formation of iridafuran.25 Complex 6 displayed similar features than complex 1 in NMR spectroscopy with a doublet of 1 Hz in the 1H NMR spectrum at 10.66 ppm for CαH with its carbon as a doublet of 10.1 Hz at 217.5 ppm at 13C{1H} NMR spectrum. Also, CαH correlates in {1H, 1H} NOESY spectrum with the methyl groups of the mesityl substituent, confirming its shift to Cβ.
1 ratio was obtained (Table 2, entry 4). This result contrasts with the previously reported reaction with the propargylic alcohol bearing 1-naphthyl and phenyl groups where no iridafuran is obtained.27 The amount of iridafuran 6 is very similar to the obtained for the mixture of 1 and Z/E-2, suggesting that not only the mesityl group, but also the position of substitution on the naphthyl ring (position 1 vs. 2) significantly affects the steric environment and, consequently, the efficiency of the 1,2-mesityl shift. Note that bulky 2-spirobifluorene group did not promote the formation of iridafuran.25 Complex 6 displayed similar features than complex 1 in NMR spectroscopy with a doublet of 1 Hz in the 1H NMR spectrum at 10.66 ppm for CαH with its carbon as a doublet of 10.1 Hz at 217.5 ppm at 13C{1H} NMR spectrum. Also, CαH correlates in {1H, 1H} NOESY spectrum with the methyl groups of the mesityl substituent, confirming its shift to Cβ.
To evaluate the influence of the metal center on the reactivity pattern, treatment of [RhCp*Cl(NCMe)(PMe3)]PF6 with propargylic alcohols bearing a pyrenyl group and a mesityl group (II) or a phenyl group (IV) in methanol was studied. These reactions led in both cases to mixtures of Z/E methoxy(alkenyl)carbene rhodium isomers. Thus, for compound II, the reaction afforded [RhCp*Cl{![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(OMe)–(Z/E)–CH
C(OMe)–(Z/E)–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Mes)(1-pyrenyl)}(PMe3)]PF6 (Z/E-7) in a 2
C(Mes)(1-pyrenyl)}(PMe3)]PF6 (Z/E-7) in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio after 30 minutes in methanol (Table 2, entry 5). Upon prolonging the reaction time to 1 h, complete isomerization to the thermodynamically favoured Z-7 was observed. However, contrary to the reactivity of the iridium analogue, the rhodium system led to the formation of the possible rhodafuran complex in only ca. 5% yield.
1 ratio after 30 minutes in methanol (Table 2, entry 5). Upon prolonging the reaction time to 1 h, complete isomerization to the thermodynamically favoured Z-7 was observed. However, contrary to the reactivity of the iridium analogue, the rhodium system led to the formation of the possible rhodafuran complex in only ca. 5% yield.
In case of compound IV, complex [RhCp*Cl{![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(OMe)–(Z)–CH
C(OMe)–(Z)–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh(1-pyrenyl)}(PMe3)]PF6 (Z-8) with small amounts of possibly E-8, was present in the reaction mixture (Table 2, entry 6). As happened for complex Z-3, bearing iridium, the rhodium complex Z-8 also presented a fluxional behaviour. However, coalescence of the split resonances could not be achieved as compound Z-8 is not temperature stable.
CPh(1-pyrenyl)}(PMe3)]PF6 (Z-8) with small amounts of possibly E-8, was present in the reaction mixture (Table 2, entry 6). As happened for complex Z-3, bearing iridium, the rhodium complex Z-8 also presented a fluxional behaviour. However, coalescence of the split resonances could not be achieved as compound Z-8 is not temperature stable.
The contrasting reactivity of Ir and Rh complexes might arise from differences in metal–carbon bond strength and electronic stabilization. Iridium forms stronger M–C bonds and better stabilizes high-energy intermediates to form iridafurans. Additionally, the steric and electronic influence of the mesityl group is accommodated by Ir but not by Rh, explaining the negligible formation of rhodafurans under similar conditions.
As experimental studies did not provide further hints regarding the mechanism, DFT calculations were performed. They revealed that formation of complex 1 is energetically favoured with respect to complexes 2 by around 6.0 kcal mol−1, although both reactions are downhill by ΔG = −35.27 kcal mol−1 for complex 1 and ΔG = −28.92/−29.29 kcal mol−1 for complexes Z/E-2. Compared to the reaction with phenyl substituent in gamma carbon, DFT calculations supported that formation of the possible iridafuran complex is energetically favoured by only around 2.6 kcal mol−1, a quite lower value than the mesityl derivative. As before, both reactions are downhill by ΔG = −24.40/−22.77 kcal mol−1 for complexes Z/E-3 and ΔG = −27.00 kcal mol−1 for the hypothetical iridafuran.
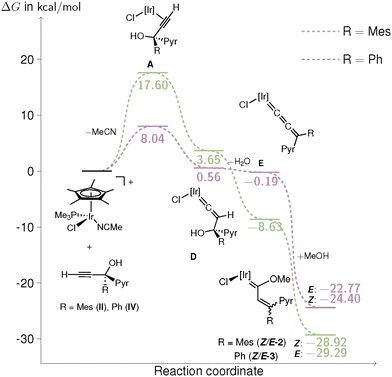
A reasonable mechanism for the formation of 1 would imply a two-step process of chloride elimination and 1,2-mesityl shift. A tentative proposal would take place through a keto–enol tautomerism (Fig. 1). This reaction may occur through an initial substitution of the acetonitrile ligand at [IrCp*Cl(NCMe)(PMe3)]PF6 by the propargylic alcohol to form intermediate A. From this species, a concerted process for the tautomerization and mesityl migration would afford intermediate B featuring an iridacyclopropane. Subsequent C–H bond activation through oxidative addition, followed by reductive elimination of HCl, would lead to intermediate C. The resulting vacant coordination site at the iridium centre would then facilitate cyclization to form the iridafuran complex 1.
Formation of complex A exhibits rather large free energy differences (8.04 kcal mol−1 for IV and 17.60 kcal mol−1 for II) being the thermodynamic-determining step (Table S4) as proposed by the lack of reactivity of the acetonitrile complex at low temperatures. However, intermediate A also corresponds to the initial step in the mechanism of the methoxy(alkenyl)carbene complexes Z/E-2 or Z/E-3. From this point, intermediate A evolves into a vinylidene complex D, which subsequently eliminates a water molecule to form the allenylidene derivative E. Intermediate E reacts with methanol, which acts not only as the solvent but also as a nucleophile, leading to the formation of complexes 2 or 3 (Fig. 2). All these steps are exergonic in both cases and follow the mechanism proposed by Selegue26 for the formation of allenylidene complexes. Therefore, the thermodynamic-determining step for this pathway corresponds to the formation of intermediate A, which is significantly more favored when the system bears a phenyl substituent rather than a mesityl group.
Nevertheless, in the case of the iridafuran pathway, following the formation of intermediate A, the tautomerization reaction to generate intermediate B requires a conformational rearrangement to A′. For the mesityl-substituted derivative, this transformation has an energy cost of 10.49 kcal mol−1 (Fig. 1, orange trace), whereas for the phenyl analogue, it increases substantially to 16.51 kcal mol−1 (Fig. 1, blue trace). From intermediate A′ onward, all subsequent steps in the reaction pathway are exergonic, leading to the formation of the iridafuran complex in both cases. However, the exclusive experimental observation of the iridafuran product from compound II, is likely governed by the more accessible energy profile of the A → A′ rearrangement in this system.
Conclusions
In conclusion, this paper shows a previously unreported 1,2-mesityl rearrangement between carbon atoms leading to the formation of iridafuran complexes. This transformation diverges from the well-established reactivity of propargylic alcohols towards methoxy(alkenyl)carbenes. The mesityl group has shown to play a key role in enabling this rearrangement while the phenyl derivatives, usually more prone to migrate, did not lead towards the formation of the iridafuran complex. Experimental comparative studies with rhodium analogues highlight the unique reactivity of the iridium system while the study with 1- and 2-naphthyl substituted propargylic alcohols remark the importance of the substitution position of the aryl group with position 1 favouring the formation of iridafuran complexes. These results not only broaden the possibilities for group migrations but also introduce a new way to build metallacyclic structures. The mechanistic insights presented here are expected to inspire further exploration of unconventional rearrangement pathways and their synthetic applications across organometallic and materials chemistry.Author contributions
Conceptualization, M. T. and S. B.; investigation, M. T., A. G., N. O. and A. P-G.; formal analysis, N. O. and A. P-G.; writing—original draft preparation, M. T.; writing—review and editing, all.; supervision and funding acquisition, M. T. and S. B.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI).Supplementary information: synthesis and analytics of all the compounds, crystallographic data and details of DFT calculations. See DOI: https://doi.org/10.1039/d5dt02755a.
CCDC 2481597 contains the supplementary crystallographic data for this paper.33
Acknowledgements
This work has been supported by Spanish Government and NextGenerationEU/PRTR funds (María Zambrano contract), the ERDF (0072_IBEROS_MAIS_1_E), Deputación de Pontevedra (INPO23) and Xunta de Galicia (GRC2024/27; ED431C 2021/49). All authors are thankful to the University of Vigo CACTI services for recording the X-ray data, NMR and mass spectra.References
- M. Liang, H. Ma, X.-R. Song and Q. Xiao, Adv. Synth. Catal., 2024, 366, 2659–2677 CrossRef CAS.
- F. Doraghi, A. M. Mahdavian, S. Karimian, B. Larijani and M. Mahdavi, Adv. Synth. Catal., 2023, 365, 2991–3019 CrossRef CAS.
- C. Coletti, A. Marrone and N. Re, Acc. Chem. Res., 2012, 45, 139–149 CrossRef CAS PubMed.
- M. I. Bruce, Chem. Rev., 1998, 98, 2797–2858 CrossRef CAS PubMed.
- V. Cadierno and S. E. García-Garrido, in Transition Metal Complexes of Neutral eta1-Carbon Ligands, ed. R. Chauvin and Y. Canac, Springer Berlin Heidelberg, Berlin, Heidelberg, 2010, ch. 6, pp. 151–218. DOI:10.1007/978-3-642-04722-0_6.
- H. Werner, Chem. Commun., 1997, 1997, 903–904 RSC.
- H. Werner, R. Wiedemann, M. Laubender, B. Windmüller, P. Steinert, O. Gevert and J. Wolf, J. Am. Chem. Soc., 2002, 124, 6966–6980 CrossRef CAS PubMed.
- K. Ilg and H. Werner, Organometallics, 2001, 20, 3782–3794 CrossRef CAS.
- H. Werner, R. W. Lass, O. Gevert and J. Wolf, Organometallics, 1997, 16, 4077–4088 CrossRef CAS.
- Y. Ishii, K.-i. Ogio, M. Nishio, M. Retbøll, S. Kuwata, H. Matsuzaka and M. Hidai, J. Organomet. Chem., 2000, 599, 221–231 CrossRef CAS.
- M. A. Esteruelas, L. A. Oro and J. Schrickel, Organometallics, 1997, 16, 796–799 CrossRef CAS.
- N. Soto-Durán, D. Gallego-García, M. Talavera and S. Bolaño, Inorg. Chim. Acta, 2025, 579, 122577 CrossRef.
- M. Talavera and S. Bolaño, Molecules, 2021, 26, 4655 CrossRef CAS PubMed.
- M. Talavera, R. Pereira-Cameselle and S. Bolaño, Dalton Trans., 2018, 47, 9064–9071 RSC.
- D. Chen, Y. Hua and H. Xia, Chem. Rev., 2020, 120, 12994–13086 CrossRef CAS PubMed.
- B. J. Frogley and L. J. Wright, Coord. Chem. Rev., 2014, 270–271, 151–166 CrossRef CAS.
- H. Zhang, R. Lin, M. Luo and H. Xia, Sci. China: Chem., 2010, 53, 1978–1981 CrossRef CAS.
- M. A. Esteruelas, F. J. Lahoz, E. Onate, L. A. Oro and B. Zeier, Organometallics, 1994, 13, 1662–1668 CrossRef CAS.
- C.-F. Yeung, H.-L. Shek, S.-M. Yiu, M.-K. Tse and C.-Y. Wong, Organometallics, 2021, 40, 2458–2466 CrossRef CAS.
- Y. Mutoh, K. Imai, Y. Kimura, Y. Ikeda and Y. Ishii, Organometallics, 2011, 30, 204–207 CrossRef CAS.
- T. Watanabe, H. Abe, Y. Mutoh and S. Saito, Chem. – Eur. J., 2018, 24, 11545–11549 CrossRef CAS PubMed.
- T. Watanabe, Y. Mutoh and S. Saito, J. Am. Chem. Soc., 2017, 139, 7749–7752 CrossRef CAS PubMed.
- M. Korb, S. M. B. H. Ghazvini and P. J. Low, Chem. – Eur. J., 2024, 30, e202400930 CrossRef CAS PubMed.
- H. Braunschweig, A. Damme, J. O. C. Jimenez-Halla, T. Kupfer and K. Radacki, Angew. Chem., Int. Ed., 2012, 51, 6267–6271 CrossRef CAS PubMed.
- V. C. Arias-Coronado, R. Pereira-Cameselle, A. Ozcelik, M. Talavera, Á. Peña-Gallego, J. L. Alonso-Gómez and S. Bolaño, Chem. – Eur. J., 2019, 25, 13496–13499 CrossRef CAS PubMed.
- J. P. Selegue, Organometallics, 1982, 1, 217–218 CrossRef CAS.
- M. Talavera, A. Peña-Gallego, J. L. Alonso-Gómez and S. Bolaño, Chem. Commun., 2018, 54, 10974–10976 RSC.
- M. Talavera, K. M. Cid-Seara, A. Peña-Gallego and S. Bolano, Dalton Trans., 2021, 50, 11216–11220 RSC.
- J. P. Morales-Cerón, V. Salazar-Pereda, D. Mendoza-Espinosa, J. G. Alvarado-Rodríguez, J. Cruz-Borbolla, N. Andrade-López and J. M. Vásquez-Pérez, Dalton Trans., 2015, 44, 13881–13889 RSC.
- M. A. Esteruelas, F. Leon, S. Moreno-Blázquez, M. Oliván and E. Oñate, Inorg. Chem., 2023, 62, 16810–16824 CrossRef CAS PubMed.
- K. N. Wong, K. H. G. Mak, W. Y. Fan, V. S. Sridevi and W. K. Leong, J. Organomet. Chem., 2013, 741–742, 40–46 CrossRef CAS.
- C. Cristóbal, S. García-Rubín, Y. A. Hernández, J. López-Serrano, M. Paneque, C. M. Posadas, M. L. Poveda, N. Rendón and E. Álvarez, Organometallics, 2010, 29, 5744–5747 CrossRef.
- CCDC 2481597: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2p99jv.
| This journal is © The Royal Society of Chemistry 2026 |