Open Access Article
Open Access ArticleIsocyanide-functionalised phosphines: an uncharted field
Petr Štěpnička
Department of Inorganic Chemistry, Faculty of Science, Charles University, Hlavova 2030, 128 00 Prague, Czech Republic. E-mail: stepnic@natur.cuni.cz
First published on 10th December 2025
Abstract
While numerous functional phosphines have been reported, investigated, and applied to date, phosphines equipped with an additional isocyanide moiety have received only limited attention. This frontier article provides an overview of the chemistry of these compounds, which has not yet been comprehensively reviewed. In particular, different types of phosphinoisocyanides (viz., isocyanide-tethered phosphines as well as compounds featuring the direct P–NC bond) and routes towards them, in addition to the reactivity, coordination behaviour, and transformations of these molecules in both their native and coordinated forms, are discussed. In particular, this overview focuses on the synthesis, reactivity, and coordination properties of phosphines bearing the isocyanide moiety at the organic (aliphatic or aromatic) or organometallic (ferrocene) scaffold. Also discussed is the chemistry of isocyanophosphines of the R2PNC type that arise, in metal-coordinated form (as C-donors), via reactions between cyano complexes and chlorophosphines. All these compounds have remarkable synthetic potential that mainly stems from the specific reactivity of isocyanides, which readily undergo diverse addition and insertion reactions, thereby providing access to, e.g., complexes with monodentate and P,C-chelate or bridged phosphinoisocyanide ligands and structurally unique carbene complexes.
Introduction
Triorganophosphines1 are indispensable ligands for coordination chemistry and catalysis by transition metal complexes, useful reagents in organic synthesis, and efficient organocatalysts.2 The attractiveness of phosphines lies in an elaborate synthetic methodology that makes them broadly accessible, as well as the possibility to fine-tune their donor (basicity) and steric properties through organic substituents.3 Besides changing the hydrocarbyl substituents attached to the phosphorus atom, the family of phosphines can be significantly expanded by incorporating additional “functional” groups into their structures. This strategy broadens design possibilities for phosphine ligands because the introduced groups can, among others, act as additional donor sites, changing the overall coordination behaviour, alter the polarity and solubility of the compounds, and serve as reactive groups that provide access to other hybrid phosphines through their synthetic modifications. Numerous hybrid phosphines have been reported, featuring additional nitrogen-based, sulfonate, carboxylate, and other groups.4 However, despite the considerable progress, some areas remain less explored, including phosphines bearing an isocyanide moiety.Organic isocyanides are reactive molecules,5 whose unique chemistry reflects their partial carbene character (Fig. 1).6 Despite interesting and useful reactivity, however, the development of their chemistry and practical applications has been hindered by their repugnant penetrating odours (especially for volatile compounds).
Of particular relevance to coordination chemistry is the ability of isocyanides to coordinate transition metals as σ-donor/π-acceptor ligands,7 which are isoelectronic with CO. In addition, isocyanides readily add nucleophiles or insert into metal–carbon bonds, which can be advantageously applied in the preparation of metal carbene complexes.8
As stated above, phosphines bearing an isocyanide functional group remain rare. This can be partly attributed to difficulties related to their synthesis,9 mainly to the sensitivity of the phosphine groups, which can react with the agents typically used to prepare isocyanides or under the conditions used to obtain them. Although only a handful of compounds have been reported to date, rich and unconventional reactivity has already been reported. This frontier article highlights the unique chemistry of phosphinoisocyanides to encourage further research focused on these disregarded yet attractive compounds. Attention is paid to isocyanide-tethered triorganophosphines as well as to phosphinoisocyanides featuring the direct P–NC bond that arise by transformations of coordinated cyanide ligands in transition metal complexes (Fig. 2).
The chemistry of isocyanide-functionalised phosphines
Early developments
Very likely the first, intentionally prepared and explored phosphinoisocyanide was (2-isocyanoethyl)diphenylphosphine (1). This compound, reported in a communication in 197110 and a full report three years later,11 was obtained by base-catalysed addition of diphenylphosphine across the double bond in vinylisocyanide12 (Scheme 1). The reaction was performed in the presence of a catalytic amount of potassium tert-butoxide in refluxing benzene, and the product was isolated by vacuum distillation and obtained as a viscous liquid in 53% yield. An analogous route was previously used to prepare the isomeric nitrile, Ph2PCH2CH2CN (2), from acrylonitrile. This reaction, however, proceeded spontaneously without any catalyst.13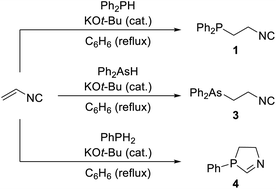 | ||
| Scheme 1 Synthesis of 1 and similar reactions that produce the corresponding arsine 3 and azaphosphole 4. | ||
A similar method was successfully applied for the preparation of the corresponding arsine, Ph2AsCH2CH2NC (3; 31% yield). In contrast, the reaction involving phenylphosphine resulted in an air-sensitive, oily product, analysed as C9H10NP, lacking the isocyanide group according to the IR spectra. This compound was formulated as 3-phenyl-4,5-dihydro-3H-1,3-azaphosphole (4), arising from the addition of a P–H bond across vinylisocyanide and subsequent intramolecular cyclisation by the addition of the second P–H bond.14
Compound 1 was characterised using 1H NMR and IR spectroscopy, mass spectrometry, elemental analysis, and molecular weight determination by osmometry. The diagnostic CN stretching vibration in the neat sample was detected at 2154 cm−1.
In both early reports,10,11 only preliminary coordination experiments with [Cr(nbd)(CO)4] (nbd = norbornadiene) were reported for 1. The reaction performed in benzene with equimolar amounts of these educts yielded a multinuclear complex tentatively formulated as [Cr3(CO)12(1)4], with three cis-Cr(CO)4 units bridged by two phosphinoisocyanide ligands and coordinated by additional monodentate 1 at both terminal Cr atoms, based on elemental analysis, IR spectra, and molecular weight determination. Arsine 3 reacted similarly.10,11
A decade later,15 isocyanide 1 emerged as part of a panel of donor-substituted nitriles tested in cycloaddition reactions with Rh(I), Ir(I), Pd(II), and Pt(II) azide complexes,16 particularly for comparison with the structurally related nitrile 2. Thus, the reaction of [Pd(N3)2(PPh3)2] with an excess of 1 in dichloromethane yielded phosphinotetrazolate complex 5 as a colourless precipitate (80% yield; Scheme 2). A similar reaction with nitrile 2 produced isomeric compound 6. However, while the reaction with 1 proceeded swiftly, affording 5 within a few minutes at ambient temperature, the cycloaddition of 2 required a longer reaction time and elevated temperature (refluxing THF for 3 days; yield of 6: 75%). The formulation of the tetrazolate complexes was supported only by elemental analysis; no additional characterisation data were provided.
 | ||
| Scheme 2 Reactions of isomeric phoshinoisocyanide 1 and phosphinonitrile 2 with a Pd(II)-azide complex. | ||
Analogous reactions between 1 and [Pt(N3)2(PPh3)2], [Pd(N3)(CN)(PPh3)2], and [Pd(N3)(CH2CN)(PPh3)2] resulted in product mixtures. According to spectroscopic analysis, the authors reported that cycloaddition occurred even in these cases (as determined from a decreased intensity of the azide vibrations in the IR spectra), but the species formed contained “isocyanide ligands”.15
Complexes with phosphinoisocyanides formed from phosphinoiminophosphorane proligands
The first defined complexes with phosphinoisocyanide ligands were obtained by a rather unusual, indirect route from phosphine-iminophosphorane 7, which is accessible by standard functional group manipulation.17 In a thermally induced reaction with Et4N[MBr(CO)5] (M = Mo, W), this compound yielded the expected P,N-chelate complexes [M(CO)4(7-κ2P,N)] (8-M, M = Mo, W; Scheme 3). However, similar reactions with homoleptic carbonyl complexes, [M(CO)6] (M = Cr, Mo, W), afforded products with C-bound phosphinoisocyanide ligands, [M(CO)5(Ph2P(CH2)3NC-κC)] (9-M, M = Cr, Mo, W). The presence of the coordinated isocyanide moiety in 9-M was inferred from the spectroscopic data (νNC ≈ 2174 cm−1 and δC(NC) 163, 153, and 143 ppm for M = Cr, Mo, and W, respectively), whereas the 31P NMR spectra exhibited resonance due to the uncoordinated phosphine moiety (δP ≈ −18 ppm). The conversion of 7 to the phosphinoisocyanide ligand was explained by nucleophilic attack of the iminophosphorane moiety on the metal-bound CO and subsequent elimination of triphenylphosphine oxide (formally an aza-Wittig reaction18).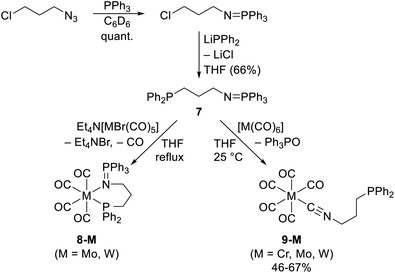 | ||
| Scheme 3 Synthesis of iminophosphorane 7 and its reactions, which produce iminophosphorane and phosphinoisocyanide complexes. | ||
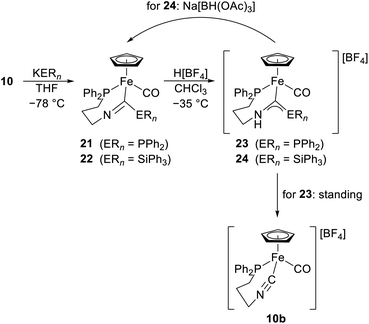
The reaction of 7 with [(η5-C5H5)FeI(CO)2] took a similar course, resulting in a cationic P,C-chelate complex [(η5-C5H5)Fe(CO)(Ph2P(CH2)3NC-κ2C,P)]I (10; νNC 2089 cm−1, δC(NC) 184, δP 54) and triphenylphosphine oxide. Complex 10 underwent smooth anion exchange with NH4[PF6] to yield [(η5-C5H5)Fe(CO)(Ph2P(CH2)3NC-κ2C,P)][PF6] (10a; see the molecular structure in Fig. 3) and, more significantly, reacted with n-propylamine under amine addition across the coordinated isocyanide group to selectively produce P-chelating diaminocarbene complex 11 (Scheme 4).17
 | ||
| Scheme 4 Synthesis of P,C-chelating phosphinoisocyanide complex 10 and its conversion to diaminocarbene complex 11. | ||
The fundamental role of the carbonyl ligands in transformation of the phosphorane moiety of 7 was proven via reactions with isoelectronic (η5-cyclopentadienyl)ruthenium(II) complexes (Scheme 5). While the reaction of 7 with [(η5-C5H5)RuI(PPh3)2] resulted in displacement of one PPh3 ligand to afford 12, that with [(η5-C5H5)RuCl(CO)2] produced isocyanide complex 13. Compounds 12 and 13 could be transformed into the P,C-bridged dinuclear complex 14 after reaction with the “other” Ru precursor (Scheme 5).17
Similar proligand transformations were observed during the reaction of 7 with [ReBr(CO)5], which produced isocyanide complexes 15 (νNC 2222 cm−1) and 16 (νNC 2137 cm−1), depending on the reaction stoichiometry (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 2
1 or 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Scheme 6). Upon heating in benzene, complex 15 underwent thermally induced intramolecular displacement of one CO ligand, producing P,C-chelate complex 17 (νNC 2219 cm−1).
1) (Scheme 6). Upon heating in benzene, complex 15 underwent thermally induced intramolecular displacement of one CO ligand, producing P,C-chelate complex 17 (νNC 2219 cm−1).
 | ||
| Scheme 6 Synthesis and reactions of Re(I)-carbonyl complexes with phosphinoisocyanide ligands formed from iminophosphorane 7. | ||
In a reaction with [Re2(CO)10], compound 7 also yielded isocyanide complex 18, which transformed into P,C-bridged complex 19 after irradiation with a medium-pressure mercury lamp (Scheme 7). Subsequent oxidative cleavage of the Re–Re bond with bromine produced “ligand-bridged” dinuclear compound 20.17
 | ||
| Scheme 7 Dinuclear Re(I) complexes with a phosphinoisocyanide ligand coordinated in different modes. | ||
More recently,19 the reaction of an iminophosphorane proligand with [(η5-C5H5)FeI(CO)2] followed by nucleophilic addition across the Fe-bound isocyanide group was utilised to prepare a pair of phosphino-amino and silyl-amino carbene complexes (Scheme 8). In particular, the addition of KPPh2 or KSiPh3 to complex 10 in THF at −78 °C generated imidoyl complexes 21 and 22 in 80% and 28% yields (δC(imidoyl) = 214.3 and 234.4 ppm), respectively. Subsequent protonation with H[BF4] at −35 °C produced the respective carbene complexes 23 and 24 in essentially quantitative yield (δC(carbene) = 276.4 and 299.4 ppm, respectively). However, while silylcarbene 24 could be isolated as a stable yellow solid, the phosphinocarbene 23 decomposed even at −35 °C under the liberation of Ph2PH and regeneration of the parent isocyanide complex (now as a [BF4]− salt 10b) within several hours. Silylcarbene 24 converted slowly back to 22 after the action of Na[BH(OAc)3] under vacuum.20
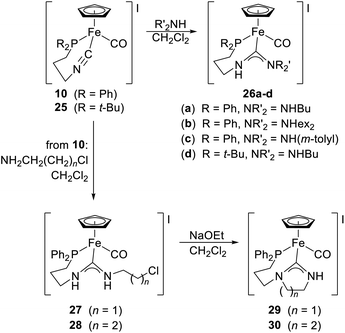
In subsequent work,20 complex 10 and an analogous compound bearing a di-tert-butylphosphino substituent (25) were used to prepare a series of protic diaminocarbene complexes 26a–d by the addition of amines (Scheme 9, yields ≈90%). Compounds with cyclic carbene groups (29 and 30) were obtained similarly from suitable ω-chloroalkyl amines and subsequent base-induced cyclisation.
The family of reported compounds was expanded to include additional oxy-amino carbenes 32a–c, resulting in high yields via the addition of KOR and subsequent protonation with H[BF4], similar to the preparation of silyl- and phosphino-substituted carbenes. Compounds with cyclic carbene moieties 33 and 34 were obtained in one step using ω-chloroalkyl alkoxides as the reagents (Scheme 10). In addition to the standard spectroscopic and analytical characterisation, the representative compounds were structurally authenticated using single-crystal X-ray diffraction analysis and further analysed computationally with emphasis on changes in their electronic structure.
Isocyanide-tagged triarylphosphines
In 2015,21 Duan and Mathey reported the preparation of isocyanide-substituted triarylphosphines 35a–c (Scheme 11). These compounds were obtained from substrates with a preinstalled isocyanide moiety, namely, by lithiation of 2-bromoaryl isocyanides 36a–c and subsequent quenching of the nonisolated lithio intermediate with chlorophosphines. DFT calculations of 35a have shown that the frontier molecular orbitals were delocalised. However, while the HOMO included the phosphorus lone pair and no contribution from the isocyanide moiety, the LUMO included the isocyanide π* orbital and no orbitals localised at the phosphorus atom. This finding suggested complementary reactivity for the two functional groups. | ||
| Scheme 11 Synthesis and reactions of isocyanide-substituted triarylphosphines [R/Ar = H/Ph (a), Me/Ph (b), and H/p-tolyl (c)]. | ||
When they were treated with lithium metal in THF, compounds 35a–c cyclised into azaphospholides 37a–c. These intermediates were hydrolysed to 2-aryl-1H-1,3-benzazaphospholes 38a–c or, alternatively, alkylated with reactive alkyl halides (PhCH2Br and MeI) and thionated to yield 3-alkyl-2-aryl-3H-1,3-benzazaphosphole sulfides 39a–c and 40a–c. Methylation of 35a–b with methyl triflate produced phosphonium salts, which reacted successively with potassium tert-butoxide and with trifluoroacetic acid to afford phosphorinium salts 41a–b (Scheme 11).
The reaction of 35a–b with 2 equiv. of [AuCl(tht)] (tht = tetrahydrothiophene) produced bis(chlorogold) complexes 42a–b (Scheme 12). In the crystal state, complexes 42a–b assembled in centrosymmetric dimeric arrays interconnected by pairs of weak intermolecular aurophilic contacts22 (3.46 Å for 42a and 3.21 Å for 42b). Chloride removal from 42a with AgOTf in dichloromethane produced dimeric gold(I) phosphinoamine complex 43, whose structure was stabilised by intramolecular aurophilic interactions according to X-ray diffraction analysis (Au⋯Au = 3.00 Å). The transformation of the ligand molecule was explained by hydrolysis of the isocyanide group to formamide, followed by further hydrolysis or, alternatively, a decarbonylation step, ultimately producing a phosphinoamine.
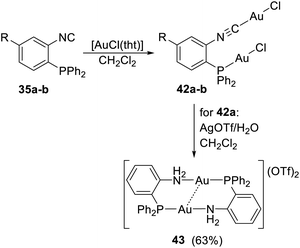 | ||
| Scheme 12 Synthesis of Au(I) complexes from 35a and 35b [R = H (a), Me (b)]. Intramolecular Au⋯Au interaction in the molecule or 43 are indicated by a dashed line. | ||
The reaction of 35a with PdCl2 in CH2Cl2 resulted in carbene complex 44 (Scheme 13; the molecular structure in shown in Fig. 4), very likely via accidental hydrolysis of the isocyanide group to an amine in one ligand and subsequent addition of the amine to the isocyanide moiety of a second ligand, either free or coordinated to Pd. A different reaction was observed with NiCl2 in THF, producing insoluble dibenzazaphosphorinium salt 45, albeit in a low yield.
 | ||
| Fig. 4 View of the complex cation in the structure of 44·CH2Cl2. The diagram was generated from the data deposited at the CCDC (deposition number: 1063708). | ||
In this context, the peculiar reactivity of phosphonium salts with isocyanoaryl substituents investigated by Michelin and coworkers is also worth mentioning (Scheme 14). The phosphonium salts were obtained by the standard alkylation of tertiary phosphines with 1-(chloromethyl)-2-isocyanobenzene (46)23 (a similar reaction in the presence of LiBr produced the corresponding bromide salts) and were converted to the less hygroscopic tetrafluoroborate salts 47-BF4 by subsequent anion exchange. These salts were used to prepare a series of standard Pt(II)-isocyanide complexes 48, which underwent smooth cyclisation into ylide-substituted indol-2-ylidene complexes 49 in the presence of triethylamine as a mild base.24
 | ||
| Scheme 14 Synthesis of isocyanides with phosphonium methyl substituents and their conversion to Pt(II) isocyanide and carbene complexes. | ||
Ferrocene-based phosphinoisocyanide
Ferrocene-based phosphinoisocyanide 50 was reported in 2017.25 Its preparation (Scheme 15) was based on the standard formylation of 1′-(diphenylphosphino)-1-aminoferrocene (51) and dehydration of the intermediate formamide. First, however, it was necessary to find a reliable route towards the phosphinoamine precursor.26 Notably, ferrocene cannot be directly nitrated because of facile oxidation to the cationic ferrocenium. Hence, the standard procedure for the preparation of anilines, which consists of nitration and reduction, could not be applied.27 For the preparation of 51, a method based on sequential lithiation/fuctionalisation of 1,1′-dibromoferrocene (52)28 and azide intermediates was devised. This route, however, required additional protection of the already present phosphine moiety to avoid unwanted Staudinger reaction.29 The approach applied initially employed phosphine sulfide intermediates (route A in Scheme 15). In the first step, bromide 52 was lithiated and phosphinylated to produce phosphine bromide 5328b and subsequently thionated with elemental sulfur to afford phosphine sulfide 53S. An analogous lithiation/functionalisation step was used to introduce an azide group to give 54S. The reaction of this rather unstable intermediate with Raney® nickel proceeded under the reduction of both the phosphine moiety and the azide group to yield an intermediate amine, which was converted in situ to formamide 55. Unfortunately, the transformation of 54S into 55 typically proceeded in low yields (<30%) and was accompanied by the reductive removal of the phosphorus substituent30 to produce FcNHCHO as a side product (Fc = ferrocenyl).To alleviate these problems, an alternative procedure (route B) was designed involving BH3-protected intermediates.31 In this case, adduct 53B was converted to P-protected azide 54B and, subsequently, to formamide 55B. Deprotection with 1,4-diazabicyclo[2.2.2]octane (dabco),32 ultimately produced formamide 55. Albeit longer, the “borane route” was better yielding and provided a reliable access to amine 51 and its P-protected form 51B, which could also be converted to 55.
Dehydration of 55 into the targeted isocyanide 50 was achieved in good yield (71%) using Castro's reagent (viz., (benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate, BOP)33 and 1,8-diazabicyclo(5.4.0)undec-7-ene (DBU) as a base. The use of conventional reagents such as POCl3/NEt3 or COCl2/i-Pr2NH resulted in decomposition.
Compounds 50 and 51 were completely characterised by spectroscopic and electrochemical methods, and their molecular structures were determined by X-ray diffraction analysis. Phosphinoisocyanide 50 was subsequently examined as a hybrid phosphine ligand for group 11 metal complexes.25 Unfortunately, reactions of 50 with the Cu(I) precursors CuCl and [Cu(MeCN)4][BF4] did not yield any defined products. The reaction with AgCl (AgCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 = 1
50 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in chloroform yielded insoluble coordination polymer 56 built up from dimeric Ag2(μ-Cl)2 units interlinked by P,C-bridging phosphinoisocyanide ligands into infinite linear ribbons (Scheme 16). Complexes with P,C-bridging 50 were also obtained from reactions with Ag[SbF6], which produced disilver(I) complexes 57 and 58 depending on the metal-to-ligand ratio. Complex 57, obtained with 1 equiv. of ligand per silver atom, was a symmetrical dimer containing an additional side-on-bonded acetone ligand. When 2 equiv. of 50 were employed, the reaction produced symmetrical, quadruply P,C-bridged disilver(I) complex 58, wherein the Ag(I) ions had identical distorted tetrahedral P2C2 donor sets. The molecular structures of 57 and 58 are presented in Fig. 5.
1) in chloroform yielded insoluble coordination polymer 56 built up from dimeric Ag2(μ-Cl)2 units interlinked by P,C-bridging phosphinoisocyanide ligands into infinite linear ribbons (Scheme 16). Complexes with P,C-bridging 50 were also obtained from reactions with Ag[SbF6], which produced disilver(I) complexes 57 and 58 depending on the metal-to-ligand ratio. Complex 57, obtained with 1 equiv. of ligand per silver atom, was a symmetrical dimer containing an additional side-on-bonded acetone ligand. When 2 equiv. of 50 were employed, the reaction produced symmetrical, quadruply P,C-bridged disilver(I) complex 58, wherein the Ag(I) ions had identical distorted tetrahedral P2C2 donor sets. The molecular structures of 57 and 58 are presented in Fig. 5.
The interaction of 50 with [AuCl(tht)] as an AuCl surrogate also produced two different complexes depending on the ligand amount (Scheme 17), viz. the “phosphine” complex 59 and the digold(I) complex 60. Subsequent chloride removal with AgNTf2 converted 59 to another digold(I) complex 61a, featuring equivalent, linear dicoordinate gold(I) centres. Similar compound 61b with a different counterion was obtained directly from 50 and [Au(tht)2][SbF6]. The Au⋯Au separation in the structurally characterised cation of 61b (5.44 Å) suggested the absence of intramolecular aurophilic interactions.
 | ||
| Scheme 17 Preparation of Au(I) complexes featuring 50 as a ligand (NTf2− = bis(trifluoromethanesulfonyl)imide anion, tht = tetrahydrothiophene). | ||
Because analogous dimers resulting from the isomeric phosphinonitrile ligand,34 [Au2(μ(P,N)-Ph2PfcCN)2]X2 (fc = ferrocene-1,1′-diyl, X = SbF6, N(SO2CF3)2), yielded highly active gold(I) catalysts,35 the catalytic properties of complex 61 were evaluated in the model gold-mediated cyclisation of (Z)-2-methylbut-1-en-3-yn-1-ol into 2,3-dimethylfuran (Scheme 18).36 Unlike the nitrile complexes, however, compounds 61a–b did not exhibit appreciable catalytic activity under otherwise similar reaction conditions. This difference was attributed to a hindered dissociation of the dimeric cation, very likely representing the catalyst activation step. Indeed, DFT calculations performed for the isomeric species [Au2(μ(P,C)-Ph2PfcNC)2]2+ and [Au2(μ(P,N)-Ph2PfcCN)2]2+ suggested that the dissociation of the isocyanide complex was approximately 12 kcal mol−1 greater than that of the nitrile complex (39 vs. 27 kcal mol−1).
Subsequent work37 focused on the reactivity of the dimeric Au(I) complexes towards azides to determine whether the coordinated isocyanide group will enter into cycloaddition reactions to possibly produce any tetrazolate species (vide supra). Unexpectedly, the reaction with (trimethylsilyl)azide (TMSN3) in dichloromethane/methanol converted 61b to another dinuclear complex 62 containing a pair of equivalent, P,N-bridging 1-(1′-(diphenylphosphino)ferrocen-1-yl)-5-aminotetrazole-κ2P,N4 ligands (Scheme 19). Apparently, the Au-bound isocyanide group underwent a twofold addition of HN3 in situ formed from TMSN3 and methanol, which was originally added to dissolve the poorly soluble precursor 61b.
This serendipitous discovery prompted a systematic study to determine whether the conversion of isocyanides to difficult-to-access 5-aminotetrazoles can be achieved with other substrates and, mainly, in a catalytic manner. Reaction tests revealed that as little as 0.1 mol% of the commonly used Au(I) complex [Au(PPh3)(MeCN)][SbF6] can convert a range of aliphatic and aromatic isocyanides (RNC) and TMSN3 (5 equiv.) to the corresponding 1-R-1H-tetrazol-5-amines rapidly and in high yields (usually >90%; Scheme 20). When the amount of TMSN3 was reduced to 1 equiv., the reaction produced only the respective cyanamide RNHCH, whereas the reaction without any catalyst yielded 1-R-1H-tetrazoles via a known cycloaddition reaction.38 In other words, three different products could be obtained selectively and in high yields from the same starting materials depending on the presence or absence of the gold catalyst and the amount of TMSN3 added to the reaction mixture (Scheme 20).
Another study39 compared the coordination behaviours of isocyanide 50 and the abovementioned isomeric phosphinonitrile Ph2PfcCN (63) in Pd(II) complexes. The compounds were already differentiated via reactions with [PdCl2(cod)] (cod = cycloocta-1,5-diene), representing a PdCl2 source. While 63 reacted cleanly to provide the bis(phosphine) complex [PdCl2(63-κP)2], a similar reaction with isocyanide 50 produced an intractable mixture, consistent with the complicated reactivity of phosphinoisocyanide 35a (vide supra).
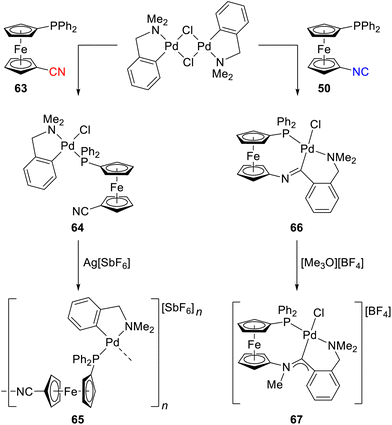
Dissimilar behaviour of 50 and 63 was also noted in the reactions of these compounds with [(LNC)Pd(μ-Cl)]2, where LNC is the cyclometalated 2-[(dimethylamino-κN)methyl]phenyl-κC1 ligand. Interaction of 63 with this dimer resulted in cleavage of the chloride bridges to produce the phosphine complex [(LNC)PdCl(63-κP)] (64), which converted to linear coordination polymer 65 propagating via P,N-bridging 63 after halogen removal (Scheme 21). In contrast, the reaction with 50 proceeded under the insertion of the isocyanide group into the Pd–C bond to afford imidoyl complex 66. Its subsequent methylation with Meerwein salt yielded the cationic, P-chelating aminocarbene complex 67.40
While compounds 66 and 67 were clearly differentiated by their NMR signatures (e.g., by the 13C NMR shifts of the Pd-bound carbon atoms; δC 199.5 and 232.0, respectively; both signals were observed as doublets because of coupling with the phosphine moiety), their molecular structures were markedly similar (Fig. 6). Still, however, small differences in the bond lengths suggested a stronger Pd–C bond and a weaker C–N bond in the carbene complex.
The experimental geometries of 66 and 67 were well reproduced by theoretical calculations, which also revealed that the Pd–C bond in both complexes could be described as a single bond. The conversion of 66 into 67 resulted in electron density accumulation in the Pd–C bond region, suggesting strengthening of the Pd–C bond in the carbene complex, consistent with the structural data. Furthermore, the analysis revealed that the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond retained a double character during the conversion, but its polarisation towards N increased after carbene formation.
C bond retained a double character during the conversion, but its polarisation towards N increased after carbene formation.
In cyclic voltammetry, the complexes underwent reversible oxidation assigned to the ferrocene/ferrocenium redox couple. Nonetheless, the redox potential determined for 66 was lower than that of 67, which is consistent with the cationic nature of the carbene complex (0.20 vs. 0.69 V vs. the ferrocene reference). In addition, carbene complex 67 exhibited an irreversible multielectron reduction, presumably corresponding to the Pd(II) → Pd(0) redox transition.
Similar reactions were accomplished with complexes possessing nontethered σ-methyl ligands. Two types of products were obtained, viz. the chloride-bridged dimer 68a and the monopalladium complex 69a, depending on the presence of the phosphine co-ligand (Scheme 22). The monopalladium complex 69a was smoothly transformed into the carbene complex 70a by methylation. Analogous reactions with η3-allyl precursors were successful only in the first step, producing imidoyl complexes 68b and 69b under concomitant η3-to-η1 haptotropic isomerisation of the allyl ligand. The subsequent methylation was unselective, resulting in an inseparable product mixture.
The dimeric imidoyl complexes served as convenient entries for bis-chelating complexes 71a–b via reactions with thallium(I) acetylacetonate, Tl(acac). The subsequent reaction with (diphenylphosphino)acetic acid proceeded under proton transfer and chelate coordination of the formed acetate41 to afford 72a–b; for 72b, this reaction was accompanied by double bond isomerisation at the allyl substituent.
Notably, whereas complexes 68a–b resulted as a mixture of chemically similar isomers (presumably cis and trans), only one species was obtained after dimer cleavage with PPh3 (for the Pd-methyl complex also during the carbene formation). This observation corresponds to the notion that groups with a strong trans effect42 become destabilised when they occupy mutually trans positions.43 In 69a–b and 70a, the C- and P-ligands exerting the largest trans influence are indeed found in the cis position. Even so, the imidoyl groups in 72a–b are located trans to the carboxylate oxygen.
The reaction of 50 with [PdCl2(cod)] proceeded under the formation of an ill-defined insoluble material (cf. the behaviour of 63). In the presence of primary or secondary amines, however, the reaction yielded P-chelate diaminocarbene Pd(II) complexes 73a–d (Scheme 23),44 which were isolated as air-stable solids in moderate to good yields (≈35–65%) by column chromatography. With suitably substituted amines, the reaction led to cyclic diaminocarbene complexes 74 and 76a–b (Scheme 23).
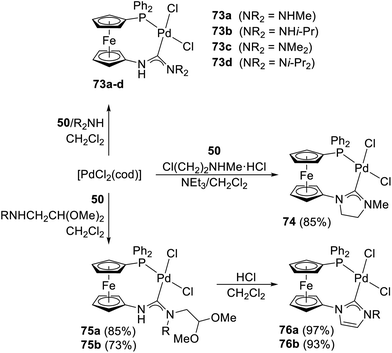 | ||
| Scheme 23 Synthesis of P-chelating Pd(II) diaminocarbene complexes [for 75 and 76: R = Me (a), i-Pr (b)]. | ||
All compounds were completely characterised, including structure determination for 73b (Fig. 7), 73c, 74, and 76a. In all the structures, the ferrocene cyclopentadienyls remained parallel (tilt angles < 6°), but the pivotal P–C(C5H4) and N–C(C5H4) bonds were mutually rotated by 5–13° from an eclipsed conformation to create a donor pocket suitable for the PdCl2 moiety. The coordination was further aided by rotation of the carbene NCN unit from the plane of its parent cyclopentadienyl ring by ≈40–45°.
 | ||
| Fig. 7 Molecular structure of 73b·CHCl3 (only one of the two structurally independent molecules is shown). CCDC reference number: 1922546. | ||
Cyclic voltammetry measurements performed for model compounds 73c, 74, and 76a revealed reversible (quasireversible for 73c) oxidations attributable to ferrocene-centred redox transitions. This assignment was corroborated by DFT calculations showing that electron removal occurs exclusively at the ferrocene unit, despite the conjugated nature of the compounds. Notably, the redox potentials of the first oxidation were lower (E°′ = 0.28 V for 73c, 0.35 V for 74, and 0.48 V for 76) than the potential determined for [PdCl2(dppf-κ2P,P′)] (E°′ = 0.58 V) under similar conditions, indicating a higher electron density at the ferrocene unit in the carbene complexes (the potentials are expressed relative to the ferrocene/ferrocenium reference; dppf = 1,1′-bis(diphenylphosphino)ferrocene).
An additional complex with a chiral carbene moiety, compound 77 (Scheme 24), was obtained by the reaction with (S)-2-(chloromethyl)pyrrolidine, accessible from (S)-proline. The compound was obtained as a mixture of two diastereoisomers differing in axial chirality at the ferrocene unit, which has a fixed conformation because of stable and rigid chelate coordination. The stereoisomers were separated by chromatography and characterised using electronic circular dichroism spectroscopy, and the dominant product was structurally authenticated as an (S,Rax) isomer.
 | ||
| Scheme 24 Synthesis of carbene complex 77 with a chiral carbene ligand (triethylamine is used to convert the chiral amine hydrochloride into the free base). | ||
The Pd(II)-carbene complexes were further applied as stable and defined precatalysts for Pd-mediated Miyaura borylation45 of aryl bromides with bis(pinacolato)diboron (Scheme 25). In this reaction, the imidazole-based carbene complexes performed best, achieving virtually complete conversion of 4-bromotoluene as the model substrate into the corresponding pinacol ester without unwanted biaryl coupling (1 mol% Pd, 85 °C, 1 h reaction time). Reaction scope tests performed with the easily accessible complex 74 confirmed its favourable catalytic properties and wide applicability of this catalytic system.
Phosphinoisocyanides with a P–NC bond
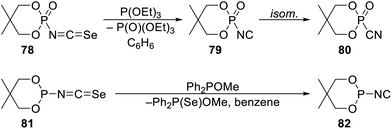
Compounds comprising a direct P–NC linkage also remain scarce,46 partly due to facile isomerisation of isocyanides R2PNC into their thermodynamically favoured “nitrile isomers” R2PCN. To prepare compounds with a P–NC bond, Stec and coworkers performed deselenylation of 2-isoselenocyanato-5,5-dimethyl-1,3,2-dioxaphosphorinane-2-oxide (78) with triethyl phosphite (Scheme 26).47 The reaction produced an unstable P-isocyanide 79, showing the CN stretching vibration at 2080 cm−1 and the 31P NMR resonance at δP 34.5, detected as a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 triplet due to interaction with 14N. However, this isocyanide was found to rapidly isomerise to the cyano derivative 80 (IR: 2210 cm−1, δP 28.5 in benzene).
1 triplet due to interaction with 14N. However, this isocyanide was found to rapidly isomerise to the cyano derivative 80 (IR: 2210 cm−1, δP 28.5 in benzene).
In an attempt to prepare the P(III) analogue (Scheme 26),48 crude 2-isoselenothiocyanato-1,3,2-dioxaphosphorinane 81 was similarly treated with methyl P,P-diphenylphosphinite (Ph2POMe) in benzene. In this case, however, the reaction led to Ph2P(Se)OMe and nitrile 82 (IR: 2170 cm−1, δP: −100.7), most likely due to a rapid isomerisation following the deselenylation step. The nitrile was isolated in a 60% yield.
Three decades later, Verkade et al.49 reported an analogous transformation in the molecules of azaphosphatranes (Scheme 27). Specifically, the reactions of P–Br derivatives 83 and 84 with (trimethylsilyl)cyanide were shown to produce P–NC products 85 and 86. While the less sterically encumbered compound 85 was isolated only as a mixture with the isomeric cyanide 87 (the 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 87 ratio was 90
87 ratio was 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), isocyanophosphine 86 could be isolated in pure form and characterised by spectroscopic methods (IR: νNC 2088 cm−1; δC(NC): 176.5, doublet with 2JPC = 27 Hz) and single-crystal X-ray crystallography. The compound was stable as a solid when stored under an inert atmosphere. Nevertheless, upon heating, it also transformed to the isomeric cyanide 88. A quantitative isomerisation of the sterically stabilised 86 was achieved upon heating the sample at 80 °C in acetonitrile for 120 h; considerably faster reactions were observed in the presence of a Lewis acid.
10), isocyanophosphine 86 could be isolated in pure form and characterised by spectroscopic methods (IR: νNC 2088 cm−1; δC(NC): 176.5, doublet with 2JPC = 27 Hz) and single-crystal X-ray crystallography. The compound was stable as a solid when stored under an inert atmosphere. Nevertheless, upon heating, it also transformed to the isomeric cyanide 88. A quantitative isomerisation of the sterically stabilised 86 was achieved upon heating the sample at 80 °C in acetonitrile for 120 h; considerably faster reactions were observed in the presence of a Lewis acid.
The first complexes featuring phosphinoisocyanides R2PNC as ligands were obtained by electrophilic functionalisation of (cyano)carbonylmetallate complexes. In 1979, Behrens et al.50 investigated the reactivity of the carbonylate complex Na[Mn2(CO)9(CN)] (89) towards a panel of electrophiles. The reaction involving this educt and Ph2PCl was shown to produce isocyanophosphine complex [Mn2(CO)9(CNPPh2-κC)] (90).
Independently and practically simultaneously, Höfler and Kemp described51 that cyanometallate K[(η5-C5H5)Mn(CO)2(CN)] (91), accessible from cymantrene (92) and KCN,52 reacts with chlorophosphines to produce isocyanide complexes 93a–c in good yields (49–73%; Scheme 28). The compound with the terminal diphenylphosphino group (93b) reacted with [(η5-C5H5)Mn(CO)2(THF)] to give the dinuclear complex 94, which was alternatively obtained from 91 and the chlorophosphine complex [(η5-C5H5)Mn(CO)2(PClPPh2)]. All compounds and the related isocyanoarsine complex [(η5-C5H5)Mn(CO)2(CNAsPh2-κC)] (95), obtained similarly from 91 and Ph2AsCl, were studied by spectroscopic methods (IR and NMR spectroscopy, mass spectrometry).
A follow-up study by Behrens and coworkers53 focused on compound 93b, and the related isocyanoarsine complexes [(η5-C5H5)Mn(CO)2(CNAsMe2-κC)] (95-Mn) and [(η5-C5H5)Re(CO)2(CNAsMe2-κC)] (95-Re). Attempts to convert these compounds into cationic nitrosyl complexes with NO[PF6] failed, leading to decomposition. For 93b, characteristic 13C NMR resonances were observed for Mn-bound CO (δC 227.4) and the isocyanide ligand (δC 212.4); an IR band attributed to the CN stretching mode was observed at 2020 cm−1 (in a dichloromethane solution).
Later on, an analogous transformation was described with the neutral (cyclopentadienyl)cobalt cyanide complex 96, which converted into isocyanide complex 97 upon reacting with ClPPh2 and subsequent anion exchange (Scheme 29).54 In a similar vein, the Mn(I) complex [(η5-C5H5)Mn(CO)2{CNP(Ph)N(SiMe3)2-κC}] (98) was prepared from Na[(η5-C5H5)Mn(CO)2(CN)] (89a) and ((Me3Si)2N)PhPCl. This complex was shown to spontaneously (albeit only partly) convert into P,C-bridged dimer [{μ(P,C)-Ph(N(SiMe3)2)PNC}{(η5-C5H5)Mn(CO)2}2] (99) under liberation of CO.55 The molecular structure of 99 is shown in Fig. 8. The formation of the dimeric complex was manifested by a shift of the νCN band from 2015 cm−1 (98, in cyclohexane) to 2040 cm−1 (99, in dichloromethane).
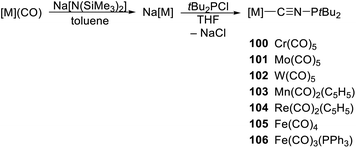
Very recently, this chemistry was revived by Kirk and Hill,56 who prepared a series of isocyanophosphine complexes using a generally similar route (Scheme 30). The starting carbonylates were generated from the corresponding neutral carbonyl complexes ([M(CO)6], M = Cr, Mo, W; [Fe(CO)5], [Fe(CO)4(PPh3)] and [(η5-C5H5)M(CO)3], M = Mn, Re) and Na[N(SiMe3)2] in toluene. Their subsequent reaction with t-Bu2PCl produced the isocyanophosphine complexes 100–106 in good yields (over 70%). Similar reactions between Na[W(CO)5] and heavier pnictines t-Bu2ECl (E = As and Sb) afforded the respective complexes with homologous isocyanopnictine ligands, [W(CO)5(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) NEtBu2-κC)]; attempts to prepare the Bi congener failed.
NEtBu2-κC)]; attempts to prepare the Bi congener failed.
The complexes were studied by spectroscopic methods, X-ray diffraction analysis, and DFT calculations. In their IR spectra, they exhibited diagnostic bands due to the carbonyl ligands and the isocyanide unit (νCN ≈ 2030–2110 cm−1); 13C NMR signals of the isocyanide groups were observed at δC 172–206. Structure diagrams for the representative compounds 102 and 106 are presented in Fig. 9.
 | ||
| Fig. 9 Views of the complex molecules in the structures of 102 and 106·C6H14. The diagrams were drawn using the data deposited with the CCDC (deposition no. 2403979 and 2403974). | ||
Compounds 100–104 were found57 to undergo [3 + 2] cycloaddition reactions with acetylenedicarboxylic acid dimethyl ester, an electron-poor alkyne, forming carbene complexes with 2,3-azaphospholyl-1-idene ligands (Scheme 31). A similar reaction was observed with CF3C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCO2Et, while methyl propiolate, ethyl 2-butynoate, and common dienophiles such as maleic anhydride, tetracyanoethylene, and in situ-generated benzyne did not react.
CCO2Et, while methyl propiolate, ethyl 2-butynoate, and common dienophiles such as maleic anhydride, tetracyanoethylene, and in situ-generated benzyne did not react.
 | ||
| Scheme 31 Cycloadditions reactions of isocyanophosphine complexes 100–104 and transmetalation of the group 6 carbene complexes to Au(I) (tht = tetrahydrothiophene). | ||
The carbene complexes displayed 13C NMR signals at δC 289.7, 281.5, and 266.5 for the group 6 metal complexes 107, 108, and 109, respectively, and at δC 289.6 and 247.5 for the (η5-C5H5)M complexes 110 and 111. Together with the results of DFT calculations performed on model species, this indicated that the carbene ligands in the newly prepared compounds are more π-acidic than the conventional N-heterocyclic carbenes (NHCs) and cyclic aminoalkyl carbenes (CAACs). Complexes 107–111 exhibited solvatochromism, which was more pronounced for the group 6 metal complexes. This phenomenon was also theoretically investigated. Analysis of the frontier molecular orbitals has shown that while the three highest occupied molecular orbitals are predominantly metal-centred, the LUMO is based on the heterocyclic carbene ligand with contributions from all ring atoms.
Compounds 107–109 transmetalated to gold (Scheme 31). The Mo-complex 108 was the most efficient for carbene transfer due to a rapid reaction, which was explained by a larger localisation of one of the higher-lying occupied molecular orbitals (HOMO−3) on the carbene carbon atom as compared to the Cr and W analogues. Attempts to isolate products of transmetalation from 109 to Rh(I), Rh(III), Pd(II) or Pt(II) were not successful.
Summary and outlook
This article highlights the multifaceted chemistry of hybrid phosphines equipped with an additional isocyanide substituent attached either to the organic backbone of the phosphine molecule or directly to the phosphorus atom, which have received only limited attention thus far. Nevertheless, even the few phosphinoisocyanides reported to date exert remarkable reactivities and coordination behaviour, stemming from the particular combination of the two chemically distinct functional parts.As hybrid ligands, phosphinoisocyanides combine two soft, σ-donor and π-acceptor groups, and can thus coordinate as unidentate P- and C-donors or P,C-chelating and bridging ligands. However, the two groups differ substantially in their steric profiles (viz. ψ-tetrahedral phosphine group vs. the linear isocyanide unit), which results in the peculiar coordination behaviour (e.g., reduced tendency towards the formation of chelate complexes, which can only occur if the organic part is sufficiently flexible and the donor groups are distant). Apart from the formation of simple and P,C-bridged complexes, the facile insertion and (cyclo)addition reactions involving the (coordinated) isocyanide group provide convenient access to structurally unique carbene complexes in which the carbene unit is tethered to the metal centre by the phosphine moiety. Of note are also the recent studies focused on compounds with the direct P–NC bond, which remained long elusive. All these aspects render phosphinoisocyanides appealing for further studies and applications.
Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software, or code were included, and no new data were generated as part of this review.Acknowledgements
The author acknowledges financial support from the Czech Science Foundation (project no. 19-09334S and 23-06718S).References
- According to IUPAC recommendation, hydrocarbyl derivatives of PH3 should be called phosphanes. This contribution, however, adheres to the older nomenclature (phosphines), which is still in use, e.g., by Chemical Abstracts and is recognised as an alternative to the IUPAC recommendation.
- (a) The chemistry of organophosphorus compounds, vol. 1, Primary, secondary and tertiary phosphines, polyphosphines and heterocyclic organophosphorus(III) compounds, ed. F. R. Hartley, Wiley, Chichester, 1990 Search PubMed; (b) C. A. McAuliffe, in Comprehensive Coordination Chemistry, ed. G. Wilkinson, R. D. Gillard and J. A. McCleverty, Pergamon Press, Oxford, 1987, vol. 2, ch. 14, pp. 989–1066 Search PubMed; (c) Phosphorus(III) Ligands in Homogeneous Catalysis: Design and Synthesis, ed. P. C. J. Kamer and P. W. N. M. van Leeuwen, Wiley, Chichester, 2012 Search PubMed; (d) D. H. Valentine and J. H. Hillhouse, Synthesis, 2003, 317 Search PubMed; (e) J. L. Methot and W. R. Roush, Adv. Synth. Catal., 2004, 346, 1035 CrossRef; (f) H. Guo, Y. C. Fan, Z. Sun, Y. Wu and O. Kwon, Chem. Rev., 2018, 118, 10049 CrossRef.
- C. A. Tolman, Chem. Rev., 1977, 77, 313 CrossRef.
- A detailed overview of the chemistry of functional phosphines is clearly beyond the scope of this article. The following review articles can served as an introduction to this area: (a) T. B. Rauchfuss, in Organometallic Coordination Chemistry and Catalysis, ed. L. H. Pignolet, Springer, Boston, 1983, ch. 7, pp. 239–256 Search PubMed; (b) A. Bader and E. Lindner, Coord. Chem. Rev., 1991, 108, 27 CrossRef; (c) C. S. Slone, D. A. Weinberger and C. A. Mirkin, Prog. Inorg. Chem., 1999, 48, 233 CAS; (d) P. Braunstein and F. Naud, Angew. Chem., Int. Ed., 2001, 40, 680 CrossRef CAS.
- Selected reviews: (a) I. Ryu, N. Sonoda and D. P. Curran, Chem. Rev., 1996, 96, 177 CrossRef CAS PubMed; (b) A. Dömling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168 CrossRef; (c) A. Dömling, Chem. Rev., 2006, 106, 17 CrossRef; (d) G. Qiu, Q. Ding and J. Wu, Chem. Soc. Rev., 2013, 42, 5257 RSC; (e) M. Suginome and Y. Ito, Sci. Synth., 2004, 19, 446 Search PubMed. For an overview of the medicinal applications of isocyanides, see: (f) A. Massarotti, F. Brunelli, S. Aprile, M. Giustiniano and G. C. Tron, Chem. Rev., 2021, 121, 10742 CrossRef PubMed.
- R. Ramozzi, N. Chéron, B. Braïda, P. C. Hiberty and P. Fleurat-Lessard, New J. Chem., 2012, 36, 1137 RSC.
- J. A. S. Howell, J.-Y. Saillard, A. Le Beuze and G. Jaouen, J. Chem. Soc., Dalton Trans., 1982, 2533 RSC.
- (a) L. Malatesta, in Progr. Inorg. Chem, ed. F. A. Cotton, Interscience Publishers, London, UK, 1959, vol. 1, pp. 283–379 Search PubMed; (b) F. Bonati and G. Minghetti, Inorg. Chim. Acta, 1974, 9, 95 CrossRef; (c) F. E. Hahn, Angew. Chem., Int. Ed. Engl., 1993, 32, 650 CrossRef; (d) R. A. Michelin, A. J. L. Pombeiro and M. F. C. Guedes de Silva, Coord. Chem. Rev., 2001, 218, 75 CrossRef; V. P. Boyarskiy, N. A. Bokach, K. V. Luzyanin and V. Y. Kukushkin, Chem. Rev., 2015, 115, 2698 Search PubMed; (e) S. Mukhopadhyay, A. G. Patro, R. S. Vadavi and S. Nembenna, Eur. J. Inorg. Chem., 2022, 31, e202200469 CrossRef.
- (a) I. Ugi, U. Fetzer, U. Eholzer, H. Knupfer and K. Offermann, Angew. Chem., Int. Ed. Engl., 1965, 4, 472 CrossRef; (b) S. A. Salami, D. Abdissa, S. Nogqala, O. E. Adeyanju, S. Sharma, X. Siwe-Noundou and R. W. M. Krause, ChemistrySelect, 2025, 10, e202405514 CrossRef.
- R. B. King and A. Efraty, J. Am. Chem. Soc., 1971, 93, 564 CrossRef.
- R. B. King and A. Efraty, J. Chem. Soc., Perkin Trans. 1, 1974, 1371 RSC.
- D. S. Matteson and R. A. Bailey, J. Am. Chem. Soc., 1968, 90, 3761 CrossRef.
- (a) F. G. Mann and I. T. Millar, J. Chem. Soc., 1952, 4453 RSC; (b) G. P. Schiemenz, Chem. Ber., 1966, 99, 514 CrossRef.
- J. Heinicke and A. Tzschach, Z. Chem., 1986, 26, 407 CrossRef.
- J. Erbe and W. Beck, Chem. Ber., 1983, 116, 3876 CrossRef.
- (a) Z. Dori and R. F. Ziolo, Chem. Rev., 1973, 73, 247 CrossRef; (b) W. P. Fehlhammer and W. Beck, Z. Anorg. Allg. Chem., 2015, 641, 1599 CrossRef.
- C.-Y. Liu, D.-Y. Chen, M.-C. Cheng, S.-M. Peng and S.-T. Liu, Organometallics, 1995, 14, 1983 CrossRef.
- (a) S. Shah and J. D. Protasiewics, Coord. Chem. Rev., 2000, 210, 181 CrossRef; (b) F. Palacios, C. Alonso, D. Aparicio, G. Rubiales and J. M. de los Santos, Tetrahedron, 2007, 63, 522 Search PubMed.
- I. Yu, C. J. Wallis, B. O. Patrick and P. Mehrkhodavandi, Organometallics, 2009, 28, 6370 CrossRef.
- I. Yu, C. J. Wallis, B. O. Patrick, P. L. Diaconescu and P. Mehrkhodavandi, Organometallics, 2010, 29, 6065 CrossRef.
- L. Zhang, W. Yu, C. Liu, Y. Xu, Z. Duan and F. Mathey, Organometallics, 2015, 34, 5697 CrossRef.
- (a) H. Schmidbaur and A. Schier, Chem. Soc. Rev., 2008, 37, 1931 RSC; (b) H. Schmidbaur and A. Schier, Chem. Soc. Rev., 2012, 41, 370 RSC.
- R. A. Michelin, G. Facchin and P. Uguagliati, Inorg. Chem., 1984, 23, 961 CrossRef.
- (a) R. A. Michelin, G. Facchin, D. Braga and P. Sabatino, Organometallics, 1986, 5, 2265 CrossRef CAS; (b) R. A. Michelin, M. Mozzon, G. Facchin, D. Braga and P. Sabatino, J. Chem. Soc., Dalton Trans., 1988, 1803 RSC.
- K. Škoch, I. Císařová, J. Schulz, U. Siemeling and P. Štěpnička, Dalton Trans., 2017, 46, 10339 RSC.
- I. R. Butler and S. C. Quayle, J. Organomet. Chem., 1998, 552, 63 CrossRef CAS.
- S. Sethi, P. K. Das and N. Behera, J. Organomet. Chem., 2016, 824, 140 CrossRef CAS.
- (a) L.-L. Lai and T.-Y. Dong, J. Chem. Soc., Chem. Commun., 1994, 2347 RSC; (b) I. R. Butler and R. L. Davies, Synthesis, 1996, 1350 CrossRef CAS.
- (a) Y. G. Gololobov, Tetrahedron, 1981, 37, 437 Search PubMed; (b) Y. G. Gololobov and L. F. Kasukhin, Tetrahedron, 1992, 48, 1353 CrossRef CAS.
- Similar reaction has been observed in: P. Štěpnička, B. Schneiderová, J. Schulz and I. Císařová, Organometallics, 2013, 32, 5754 CrossRef.
- J. M. Brunel, B. Faure and M. Maffei, Coord. Chem. Rev., 1998, 178–180, 665 CrossRef CAS.
- H. Brisset, Y. Gourdel, P. Pellon and M. Le Corre, Tetrahedron Lett., 1993, 34, 4523 CrossRef CAS.
- (a) B. Castro, J. R. Dormoy, G. Evin and C. Selve, Tetrahedron Lett., 1975, 16, 1219 CrossRef; (b) T. S. Mansour, S. Bardhan and Z.-K. Wan, Synlett, 2010, 1143 Search PubMed.
- (a) K. Škoch, I. Císařová and P. Štěpnička, Inorg. Chem., 2014, 53, 568 CrossRef PubMed; (b) K. Škoch, F. Uhlík, I. Císařová and P. Štěpnička, Dalton Trans., 2016, 45, 10655 RSC.
- K. Škoch, I. Císařová and P. Štěpnička, Chem. – Eur. J., 2015, 21, 15998 CrossRef PubMed.
- (a) P. Belmont and E. Parker, Eur. J. Org. Chem., 2009, 6075 CrossRef CAS; (b) A. S. K. Hashmi, L. Schwarz, J.-H. Choi and T. M. Frost, Angew. Chem., Int. Ed., 2000, 39, 2285 CrossRef CAS.
- K. Škoch, I. Císařová and P. Štěpnička, Chem. – Eur. J., 2018, 24, 13788 CrossRef.
- T. Jin, S. Kamijo and Y. Yamamoto, Tetrahedron Lett., 2004, 45, 9435 Search PubMed.
- K. Škoch, I. Císařová, F. Uhlík and P. Štěpnička, Dalton Trans., 2018, 47, 16082 Search PubMed.
- For similar compounds lacking the phosphinyl substituent at the ferrocene unit, see: M. Franc, P. Vosáhlo, J. Schulz, I. Císařová and P. Štěpnička, Dalton Trans., 2023, 52, 17701 Search PubMed.
- (a) A. Jegorov, B. Kratochvíl, V. Langer and J. Podlahová, Inorg. Chem., 1984, 23, 4288 CrossRef CAS; (b) A. Jegorov, J. Podlaha, J. Podlahová and F. Tureček, J. Chem. Soc., Dalton Trans., 1990, 3259 Search PubMed; (c) M. Zábranský, J. Soellner, F. Horký, I. Císařová, P. Štěpnička and T. Strassner, Eur. J. Inorg. Chem., 2019, 2284 CrossRef.
- (a) T. G. Appleton, H. C. Clark and L. E. Manzer, Coord. Chem. Rev., 1973, 10, 335 CrossRef CAS; (b) F. R. Hartley, Chem. Soc. Rev., 1973, 2, 163 RSC.
- R. G. Pearson, Inorg. Chem., 1973, 12, 712 CrossRef CAS.
- K. Škoch, J. Schulz, I. Císařová and P. Štěpnička, Organometallics, 2019, 38, 3060 Search PubMed.
- (a) T. Ishiyama, M. Murata and N. Miyaura, J. Org. Chem., 1995, 60, 7508 CrossRef CAS; (b) W. K. Chow, O. Y. Yuen, P. Y. Choy, C. M. So, C. P. Lau, W. T. Wong and F. Y. Kwong, RSC Adv., 2013, 3, 12518 RSC.
- For a review dealing with isocyanides substituents bonded via nitrogen (N–NC), see: D. Moderhack, Tetrahedron, 2012, 68, 5949 CrossRef CAS.
- W. J. Stec, A. Konopka and B. Uznański, J. Chem. Soc., Chem. Commun., 1974, 923 RSC.
- W. J. Stec, T. Sudoł and B. Uznański, J. Chem. Soc., Chem. Commun., 1975, 467 RSC.
- J. V. Kingston, A. Ellern and J. G. Verkade, Angew. Chem., Int. Ed., 2005, 44, 4960 Search PubMed.
- H. Behrens, P. Würstl, P. Merbach and M. Moll, Z. Anorg. Allg. Chem., 1979, 456, 16 CrossRef CAS.
- M. Höfler and W. Kemp, Chem. Ber., 1979, 112, 1934 Search PubMed.
- E. O. Fischer and R. J. J. Schneider, J. Organomet. Chem., 1968, 12, P27 CrossRef CAS.
- H. Behrens, G. Landgraf, P. Merbach, M. Moll and K.-H. Trummer, J. Organomet. Chem., 1983, 253, 217 CrossRef CAS.
- T. Avilés, F. Barroso and P. Royo, J. Organomet. Chem., 1987, 326, 423 CrossRef.
- W. F. McNamara, E. N. Duesler and R. T. Paine, Organometallics, 1988, 7, 384 CrossRef CAS.
- R. M. Kirk and A. F. Hill, Dalton Trans., 2025, 54, 8881 RSC.
- R. M. Kirk and A. F. Hill, Angew. Chem., Int. Ed., 2025, 64, e202504620 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |