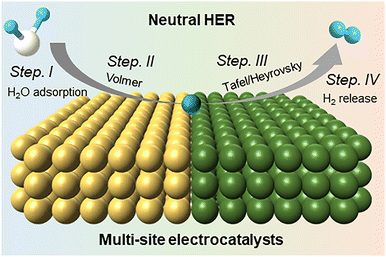
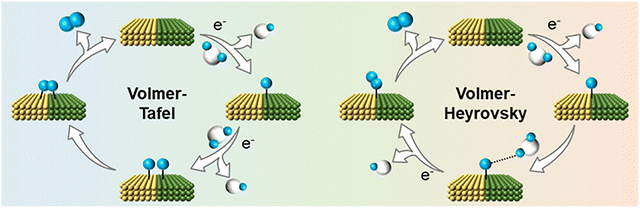
Multi-site electrocatalysts for hydrogen production under neutral conditions
Zhouzhou
Wang†
 a,
Jianqing
Zhou†
b,
Yaran
Shi
a,
Li
Luo
a,
Haoran
Li
a,
Qiancheng
Zhou
a,
Chunchun
Wang
a,
Zhuo
Xing
*a,
Ze
Yang
*a and
Ying
Yu
a,
Jianqing
Zhou†
b,
Yaran
Shi
a,
Li
Luo
a,
Haoran
Li
a,
Qiancheng
Zhou
a,
Chunchun
Wang
a,
Zhuo
Xing
*a,
Ze
Yang
*a and
Ying
Yu
 *a
*a
aInstitute of Nanoscience and Nanotechnology, College of Physical Science and Technology, Central China Normal University, Wuhan 430079, China. E-mail: xingzhuo@ccnu.edu.cn; yz@ccnu.edu.cn; yuying01@ccnu.edu.cn
bHubei Key Laboratory of Photoelectric Materials and Devices, School of Materials Science and Engineering, Hubei Normal University, Huangshi 435002, China
First published on 12th November 2025
Abstract
Electrochemical water splitting offers a sustainable pathway for hydrogen production, yet realizing high-efficiency operation at neutral pH remains a formidable challenge due to the sluggish interfacial kinetics, limited ionic conductivity, and complex proton transfer behavior. Recently, the emergence of multi-site electrocatalysts has provided a powerful strategy to decouple and optimize each elementary step of the neutral hydrogen evolution reaction (HER). This review presents a timely and in-depth analysis of the reaction mechanisms, electrolyte effects, and interfacial micro-environments that define the HER under neutral conditions. We highlight the recent progress in the performance metrics, design, synthesis, and structural engineering of multi-site catalytic systems, with an emphasis on their role in facilitating water dissociation and hydrogen evolution, along with critical discussions on advanced characterization techniques. Finally, we examine the prospects of translating laboratory-scale discoveries to practical neutral-pH water electrolysis systems. This review aims to offer a foundational understanding and forward-looking perspectives for developing next-generation electrocatalysts tailored to neutral water splitting.
1. Introduction
The global push toward a carbon-neutral economy has positioned hydrogen (H2) as a cornerstone in future sustainable energy systems.1–9 As a clean, high-energy-density fuel, hydrogen is uniquely expected to replace the intermittent renewable energy sources (e.g., solar, wind, and tidal energy) and facilitate decarbonization in hard-to-electrify sectors such as heavy industries and long-haul transport in the offshore and inland areas.10–17 In addition, hydrogen serves as a promising energy carrier for storing the abundant but unutilized resource in deep-sea and far-sea zones, where the cost of electricity is low, but the cost of transporting electricity to inland is high. Therefore, paving a new path for hydrogen production by electricity draws a fascinating blueprint for a future low-carbon world. However, for the two half-reactions of water splitting, namely, the reduction of water to H2 (hydrogen evolution reaction, HER) and the oxidation of water to O2 (oxygen evolution reaction, OER), strongly acidic and basic solutions have been widely used to provide high concentrations of H+ and OH−, which offers a high solution conductivity without addition of any supporting electrolyte and thus enhances the rate of the HER and OER.18–26Recently, the electrochemical method for reducing CO2 emissions has attracted considerable attention because it is a more sustainable and environment-friendly approach to high-value chemicals.27–32 Many of these processes occur in the presence of an electrolyte at a mild pH, rather than any strong acid/base, to hold the soluble reactants and products and/or to modulate the selectivity of the desired reaction.33–37 During these processes, the HER or OER becomes a counter-electrode reaction or a competing reaction. As the nearly most fundamental process in (electro)chemical reactions, the HER involves two-electron transfer, that is, the Volmer process and Tafel/Heyrovsky process. It is however not well understood that the mechanism and behaviors for neutral and near-neutral HERs demonstrated in many publications are pretty different from those for acidic and alkaline HERs.38–42
For instance, as suggested by the Nernst equation, the HER activity in different electrolyte follows the descending order from acidic media to neutral media to alkaline media. Nevertheless, this order is not displayed as the experimental observations, while the reactivity under alkaline media is distinctly superior to that under neutral media.43–47 For this reason, many reports have commented that mild media can alleviate the corrosion of the electrolyzer for hydrogen production through water electrolysis, compared to the acidic and alkaline environments;40,48,49 in reality, due to the extremely poor performance of neutral water splitting, the excessive high voltage required at the equal hydrogen production rate will cause serious damage to the electrolytic device. Thus, understanding the HER and developing efficient catalysts are critical to optimize the reaction under mild pH conditions.
An efficient electrocatalytic reaction needs fast reaction thermodynamics and kinetics, efficient charge and mass transport, fully exposed catalytic sites, and structural and (electro)chemical stability during the operation. In fundamentally challenging neutral media, the limited ionic conductivity and the absence of readily available protons or hydroxide ions in neutral media compared to acidic or alkaline environments necessitate advanced catalyst design to achieve high efficiency. This challenge arises primarily from the difficulty in facilitating simultaneous adsorption of reactants, transformation of intermediates, and desorption of products on the catalyst surface. Achieving multi-functions at a single site is often limited by the Sabatier principle, while the multi-site electrocatalysts have emerged as a powerful strategy to design synergistic multi-functionality catalytic sites.
A multi-site electrocatalyst refers to the catalyst that possesses two or more distinct and spatially coupled active sites (either chemically, or structurally, or electronically different) to participate in different steps of an electrochemical reaction with the synergistic interplay of diverse active centers. Here, it is assumed that one certain site is unable to fulfill all the needed functions. To date, such a catalyst has remained far from expectations. Given the accelerating progress and growing interest in this field, a timely and comprehensive review of multi-site electrocatalysts for the neutral HER, especially to explain the differences between the neutral HER and the acidic/alkaline HER from a fundamental perspective, is necessary to promote its development, showcase its advanced level, and envision its perspective applications, which is not yet available.
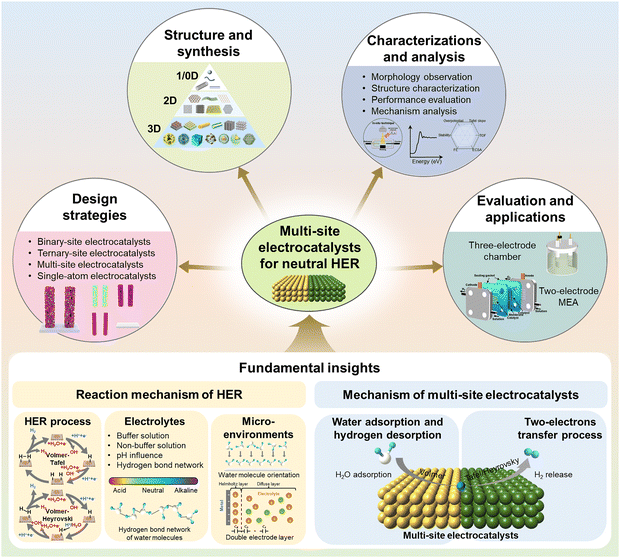
This review aims to provide a comprehensive and focused overview of multi-site electrocatalysts for hydrogen evolution under neutral conditions (Fig. 1). We begin by critically outlining the principles of neutral HER, followed by an elucidation of the underlying mechanisms specific to multi-site electrocatalysts. Subsequently, we discuss the recent advances in design strategies, synthetic methodologies, and advanced characterizations. We further examine the current efforts made to integrate these catalysts into membrane electrode assembly (MEA)-based electrolyzers operating with neutral electrolytes. Finally, we discuss the existing limitations and offer perspectives on future research directions under both theoretical insights and application-oriented considerations. We hope this review can provide sufficiently profound insights for those interested in this field, present rational catalyst design approaches that can meet the unique challenges of neutral HER, and ultimately contribute to bridging the gap between laboratory innovations and scalable, real-world hydrogen production technologies.
2. Fundamentals of the electrocatalytic neutral HER
2.1. Reaction mechanism of the HER under neutral conditions
In acidic media with abundant protons (H+), the HER proceeds primarily through the direct reduction of hydronium ions (H3O+):
| 2H3O+ + 2e− → H2 + 2H2O (Total reaction) | (1) |
| H3O+ + e− + * → *H + H2O (Volmer step) | (2) |
| *H + *H → H2 (Tafel step) | (3) |
| *H + H3O+ + e− → H2 + H2O (Heyrovsky step) | (4) |
| 2H2O + 2e− → H2 + 2OH− (Total reaction) | (5) |
| H2O + e− + * → *H + OH− (Volmer step) | (6) |
| *H + *H → H2 (Tafel step) | (7) |
| *H + H2O + e− → H2 + OH− (Heyrovsky step) | (8) |
It is well known that the HER activity in acidic media is generally superior to that in alkaline media, which can be understood by the Nernst equation and thermodynamics theory in principle.
The standard reduction potential (E0) for proton reduction (eqn (1)) in acidic media is as follows:
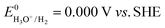 | (9) |
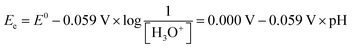 | (10) |
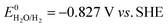 | (11) |
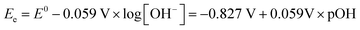 | (12) |
It can be found that the 0.827 V difference relative to the SHE (eqn (9) and (11)) reflects the greater thermodynamic barrier associated with the HER under alkaline conditions compared to that under acidic conditions. The fundamental reason lies in the difference in proton source. In acidic media, free protons are readily available and require minimal energy to be reduced. In alkaline media, the electrolysis must first overcome the high bond dissociation energy of water molecules (237.1 kJ mol−1) to generate surface-adsorbed hydrogen (*H), making the initial step of HER (Volmer step) more energetically demanding.
Furthermore, it can be inferred that the HER under neutral conditions is thermodynamically more favorable than under alkaline conditions. However, experimental studies have consistently shown that the catalytic activity of the HER in neutral media is generally lower than that in alkaline media. This discrepancy arises because the aforementioned discussion is based solely on thermodynamic considerations, without accounting for the aspects of reaction kinetics. In reality, the HER kinetics is strongly influenced by the nature of reactants, electrolyte composition, and catalyst surface properties, which will be discussed in later sections.
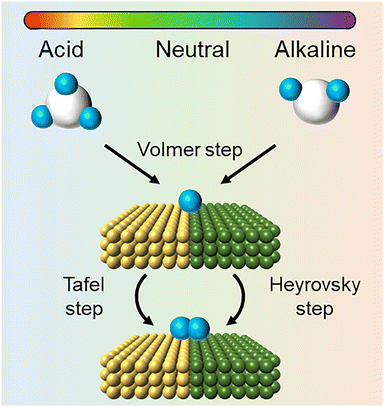
Understanding the mechanistic differences between the HERs in acidic and alkaline electrolytes is helpful for us to reconsider the unique behavior of HER in neutral media. Notably, eqn (10) and (12) imply that the equilibrium reduction potentials for the reaction of H3O+/H2 and H2O/H2 are numerically identical within a given solution, since pH + pOH = 14. However, this does not imply that H3O+ and H2O are interchangeable species when considering the HER. The above-mentioned thermodynamic analysis assumes that all steps (H3O+ or H2O reduction, water dissociation, and mass transport) occur with sufficiently fast kinetics, rendering eqn (10) and (12) indistinguishable in terms of equilibrium reduction potential. This simplification holds in strongly acidic or alkaline electrolytes, where H3O+ is either abundant or negligible. In such cases, focusing solely on either H3O+ or H2O as the reactant is sufficient to understand the HER pathway. However, in neutral or near-neutral environments where the concentration of H3O+ is neither abundant nor negligible and H2O serves as both the solvent and the reactant (Fig. 3), it becomes essential to carefully distinguish between H3O+ and H2O as proton sources for the HER. A proper understanding of the interplay between H3O+ reduction and H2O reduction is thus critical to accurately elucidate the HER mechanism under neutral conditions.
Consider the reaction rate constant (k) for H3O+ and H2O reduction according to the following Arrhenius equation:
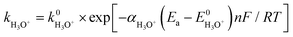 | (13) |
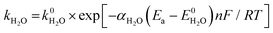 | (14) |
Eqn (13) and (14) show that k of H3O+ and H2O increases exponentially when Ea is shifted from positive to negative potential, but their E0 values are different (eqn (9) and (11)). The exponential term in eqn (13) is thus larger than that in eqn (14) at a given Ea value. Meanwhile, k0 for H3O+ is also known to be larger than k0 for H2O according to their different activation energies because the transfer of H+ from H3O+ to the electrode surface needed for the Volmer step and Heyrovsky step is much more facile than that from H2O.58–61 Therefore, at a given Ea value, the k value for H3O+ is larger than that for H2O, that is, the reaction for H3O+ reduction is faster than that for H2O reduction.
In contrast to alkaline media where the HER proceeds predominantly via H2O reduction, the HER in neutral environments is a mixed reaction, owing to the moderate concentration of H3O+. Numerous studies have revealed that under neutral conditions and at low overpotentials, H3O+ acts as the primary proton donor, while as the potential increases, the concentration of H3O+ becomes insufficient to sustain the reaction, and H2O gradually takes over as the dominant proton source.62–64 The specific reaction process is mainly described by eqn (5)–(8) and partly controlled by eqn (1)–(4). Notably, it remains a fundamental question: Why is the HER slower in neutral media than in alkaline media, even though both rely on water as the main reactant? The primary reason lies in the inherent disadvantages of neutral electrolytes compared to their alkaline counterparts. Neutral electrolytes typically exhibit: (i) lower ionic conductivity, which hampers efficient charge transport especially at high overpotentials; (ii) more diffuse electrical double layer (Edl) over the catalyst surface, which weakens the interfacial electric field;65–69 and (iii) poorer interfacial polarization and orientation of water molecules. These contribute to sluggish reaction kinetics despite the potentially favorable thermodynamics for the neutral HER compared to the alkaline HER, which highlights the functions of electrolytes and catalysts for the neutral HER, which will be discussed below.
While pH 7 is typically used as the benchmark for neutrality, the actual behavior of the HER around this value involves more complex mechanistic transitions. Several studies have revealed that a critical mechanistic shift occurs wherein the dominant proton donor changes from H3O+ (pH < 4) to H2O (pH > 4), which has been discussed above.59,70 Additionally, some work has shown that as soon as the pH exceeds 0 (i.e., [H+] < 1.0 M), the linear sweep voltammetry (LSV) curve begins to exhibit a distinct cathodic feature attributed to the mass transport limitation of H3O+.71,72 For instance, Yuan et al. used a platinum rotating disk electrode (RDE) to record the LSV curves across a series of pH values.72 Due to the convective mass transport introduced by electrode rotation, a plateau emerged in place of the distinct reduction peak, reflecting the formation of a diffusion-limited current density (JL). This JL arises because H3O+ near the electrode surface is rapidly consumed, and the rate of its replenishment from the bulk solution becomes the limiting factor. Importantly, JL is directly proportional to the H3O+ concentration in bulk solutions and increases with higher concentrations of the conjugate acid in buffer solutions, but is independent of the catalyst itself. This observation emphasizes the importance of comparing the HER performance under consistent reactant conditions (i.e., in both H3O+-dominant and H2O-dominant reduction reactions). Using overpotentials at a current density (j) of −10 mA cm−2 as a benchmark is commonly used to evaluate the performance of designed electrocatalysts for the neutral HER, but the comparisons with other reports must be made between HER catalysis reactions under the same dominant proton source.
We therefore advocate that when defining acidic, neutral, and alkaline HER conditions, researchers should not rely solely on pH as a label. Instead, one must consider the proton source species at that pH (e.g., H+ and conjugate acid). Generally, solutions with a pH ranging from 3.0 to 11.0 are classified as “neutral” solutions. However, for specific neutral HER studies, especially when comparing the performance of different catalysts under different pH conditions, it is necessary to clearly specify the measurement configuration and parameters (e.g., rotation speed and electrolyte stirring) and explicitly identify the dominant reactant species at the applied potential (H3O+ or H2O). Moreover, at low current densities, the reactant species may be H3O+ rather than H2O, which requires special attention when conducting mechanistic explanations.
Pure water (pH ca. 7.0) cannot serve as a practical electrolyte for neutral HER due to its ultralow ionic conductivity. Therefore, neutral HER systems must employ either buffered solutions consisting of a weak acid and its conjugate base (or vice versa), or non-buffered salt solutions containing metal cations and inert anions. Both must remain chemically and electrochemically stable across the potential window where water splitting occurs, and with distinct physicochemical properties and implications for catalytic behavior.
Neutral buffered electrolytes such as phosphate (e.g., KH2PO4/K2HPO4), borate, or bicarbonate buffers maintain a relatively constant pH by reversibly consuming or releasing protons.39 Their role goes beyond pH stabilization: (i) Buffering action. Buffered electrolyte maintains consistent proton activity at the electrode surface, which is especially important under dynamic electrolysis conditions where OH− is generated during water reduction; (ii) Proton mediation. Protic buffers can actively participate in the HER by mediating interfacial proton-coupled electron transfer (PCET), effectively acting as a bridge between bulk water and catalytic sites; (iii) Electrostatic stabilization. Buffer species can modify the interfacial field, stabilizing *H intermediates and electrode double layer.
Neutral non-buffered electrolytes such as Na2SO4, KCl, and NaNO3 do not regulate the pH but serve primarily to enhance the ionic conductivity and support the electric double layer. Simpler interfacial chemistry allows more direct interpretation of catalyst behavior and avoids the buffer-specific interactions that may confound mechanistic studies. However, it is highly susceptible to local pH gradients, especially at high current densities. In addition, the accumulation of OH− near the cathode can elevate the local pH, reduce water dissociation rates, and ultimately suppress HER kinetics. Additionally, poor proton replenishment due to the absence of a conjugate acid–base equilibrium reduces the HER kinetics. Therefore, while non-buffered systems are valuable for mechanistic insights, buffered electrolytes are often preferred for practical applications due to their stabilizing effects on the interfacial environment.
The concentration of (non-)buffering species must be sufficiently high to maintain its effective pH control during electrolysis.73,74 Increasing the concentration of the supporting electrolyte can reduce the solution resistance, thereby improving the HER current and lowering the overpotentials. However, excessively high concentrations can increase the viscosity, reduce the ion diffusivity, and suppress the HER activity due to mass transport limitations. A compromise electrolyte concentration is therefore suitable for reaction. In addition, some studies use natural water sources with low concentrations of electrolytes, such as natural waste waters or seawater, aiming to evaluate the feasibility of large-scale hydrogen production without pH regulation.75–77 However, these approaches encounter serious limitations by very low ionic conductivity, cation precipitation (e.g., Mg2+ and Ca2+) on cathode surface, and local pH instability, leading to limited proton diffusion and reduced efficiency.
Alkali metal cations can significantly modulate the interfacial microenvironment, influencing the water structure, intermediate stabilization, and even *H desorption energetics. Considering such cation effects in the electrocatalytic HER, we recommend using 1.0 M solutions of monovalent metal salts (e.g., K+ and Na+) as either buffered or non-buffered neutral electrolytes and avoiding multivalent cations that form insoluble hydroxides (e.g., Mg2+ and Ca2+). By standardizing the electrolyte composition, researchers can more reliably interpret and compare the HER activity data across studies and more accurately dissect the intrinsic behaviors of catalysts under neutral conditions.
The Edl is typically modelled with two regions, which were successively developed by Helmholtz,78 Gouy,79 Chapman,80 Stern,81 Grahame,82,83 Devanathan,84 Bockris,85 Bard-Faulkner,86 and others:87,88 the inner Helmholtz plane (IHP) that comprises specifically adsorbed ions (often buffer species, cations such as Na+/K+, or surface-bound reaction intermediates) directly interacting with the electrode surface and the outer Helmholtz plane (OHP) where solvated ions approach the surface without direct adsorption. Beyond the Edl is called the diffuse layer (Gouy–Chapman layer), extending from the OHP into the bulk solution, where the charge density gradually neutralizes due to thermal motion.
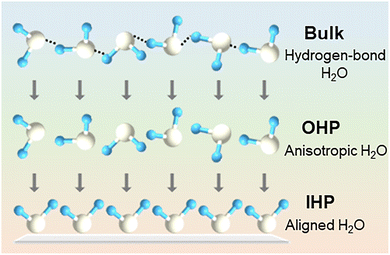
It is worth emphasizing that the journey of a water molecule from the bulk solution to its eventual reduction into hydrogen involves more than just the well-studied steps of adsorption, dissociation, and desorption over catalytic surface sites. Several subtle yet critical interfacial processes, often overlooked, also play pivotal roles in determining the overall HER kinetics (Fig. 4).
In bulk solutions, water molecules predominantly exist as H2O networks, stabilized by extensive hydrogen bonding interactions. However, on the catalytic surface, the HER typically proceeds via the reaction of individual and isolated water molecules. Thus, a hydrogen-bonded water network must first dissociate into free water molecules, which can migrate toward the electrode surface. This process entails the disruption of the hydrogen-bond network, requiring energy and being influenced by the local electrolyte composition, ionic strength, and hydration environment. Following this, water molecules entering the Edl undergo reorientation. As traversing from the OHP to the IHP, they must transition from an anisotropic configuration to one in which the H atom aligns toward the catalyst surface – a prerequisite for the HER process. This reorientation process is governed by the electric field strength, surface charge density, and interfacial solvation structure.89
These pre-adsorption steps, hydrogen-bond network dissociation and interfacial molecular reorientation together require additional energetic input that is strongly coupled to both the catalyst surface property and the structure of the Edl.90–99 More attention should be paid toward understanding and engineering these interfacial dynamics, as they represent an underexplored but important issue in the overall kinetics of HER under neutral conditions.
The Debye length (λD), which characterizes the thickness of the diffuse layer, can exceed several nanometers in low ionic strength solutions.89,100 As a result, the potential drop from the electrode into the bulk occurs over a much larger distance, weakening the interfacial electric field strength. In high-ionic-strength electrolytes (e.g., acidic or alkaline media), the Edl is thin and compact, typically on the scale of several nanometers. This configuration facilitates steep potential gradients, enhances charge separation, and supports strong electric fields that are highly effective for polarizing water molecules and driving interfacial charge transfer. In contrast, in neutral media, the Edl becomes thick and diffuse due to low concentrations of free ions (H3O+ and OH− are both ca. 10−7 M), weak electrostatic screening, and reduced ionic conductivity.
Therefore, the properties of the Edl affect the neutral HER in the following critical ways: (i) Attenuated interfacial electric field. A compact Edl concentrates the potential gradient near the electrode surface, aligning dipoles and polarizing reactants.101,102 In a diffuse Edl, this electric field is diluted, leading to weaker water molecule orientation and polarization, slower O–H bond weakening and dissociation, and reduced efficiency of the Volmer step (water dissociation to *H). Thus, the initial step of the HER becomes kinetically sluggish, even when thermodynamically favorable. (ii) Impaired proton transport. In a thick Edl, the region of potential gradient overlaps poorly with regions of ion accumulation. Since proton donors (H3O+ or OH−) are sparse in neutral media, this misalignment reduces the availability of reactive species at the precise location where electron transfer occurs. Additionally, the free energy for PCET increases, as the movement of protons or water molecules into the Edl requires overcoming both entropic and electrostatic barriers. (iii) Delayed ionic replenishment and local pH gradients. In the neutral HER, the Volmer step produces OH− based on eqn (6). In a well-structured Edl, these OH− ions can rapidly move into the bulk solution. However, in a diffuse Edl, the removal of OH− from the catalyst surface is slower, and the local accumulation of OH− near the surface elevates the interfacial pH, which leads to a mismatch between bulk and local pH, further destabilizing the reaction conditions and possibly inhibiting the catalyst activity. (iv) Influence on buffer adsorption and cation effects. The extended Edl region in neutral electrolytes allows buffer species and hydrated cations to penetrate deeper into the interfacial zone. These species can modulate the orientation of water molecules, affect the hydrogen-bonding network, and compete with water for adsorption sites. This dynamic leads to subtle but significant changes in the energetics of intermediate formation and desorption, which are not captured in bulk measurements.
2.2. Neutral HER mechanism of multi-site electrocatalysts
In general, the water reduction under neutral conditions involves four fundamental steps (Fig. 5). According to the Sabatier principle, single-site catalysts typically optimize only part of this multi-step process, often leading to suboptimal overall activity. In contrast, rationally designed multi-site electrocatalysts are expected to integrate distinct functional sites capable of promoting each individual step, thereby synergistically enabling a more efficient and concerted HER pathway under neutral conditions.Equally crucial is the desorption of the final surface *H2. If *H2 desorption is hindered, it not only reduces the overall hydrogen production efficiency but also leads to excessive bubble accumulation. These bubbles can physically block access between the active sites and water, thus may trigger catalyst deactivation under prolonged negative-bias operation. This issue is particularly pronounced at high current densities, where mass transport and gas diffusion become dominant factors limiting the catalytic activity. As a result, improving the bubble dynamics of H2 detachment under high-current operation has become a major focus in the field.
Multi-site catalysts offer a synergistic solution by enabling complementary roles across different active components. Some sites facilitate the adsorption of reactants, while others promote the desorption of products. For instance, Yan et al. designed a dual-site catalyst where Cu species were introduced to modify Co centers known for strong *H binding, thereby facilitating hydrogen release.103 The Co sites facilitate the H2O adsorption, while the Cu sites regulate the H2 desorption. Additionally, Guo and colleagues incorporated a TiO2 layer over a Ni3S2–MoS2 catalyst, enhancing its water adsorption capacity under neutral conditions and thus accelerating the HER activity.104
It is worth emphasizing that an ideal electrocatalyst for the neutral HER should exhibit both hydrophilicity and aerophobicity, a dual property confirmed by many recent studies. While often related, hydrophilicity and aerophobicity are not inherently equivalent. Typically, strongly hydrophilic surfaces resist bubble adhesion due to stronger solid–liquid interactions, which destabilize gas–solid contact. However, this does not imply that all hydrophilic surfaces are inherently aerophobic. In this regard, the aerophobicity of the material is influenced by multiple factors: (i) Surface micro/nanostructure: hydrophilic materials with micro-/nano-scale roughness can form “bubble traps” that promote gas accumulation. (ii) Surface energy heterogeneity: the presence of local hydrophobic regions on an otherwise hydrophilic surface can cause gas bubble pinning. (iii) Surface chemistry: the behaviors of different gases are inconsistent over one surface, so certain hydrophilic materials may chemically adsorb specific gases, affecting bubble dynamics. For example, TiO2, despite its strong hydrophilicity, can retain bubbles under rough surface conditions.105–110 Similarly, carbon materials with oxygen-containing groups (e.g., graphene oxide) are hydrophilic, but their gas interaction behavior strongly depends on their reduction state and surface morphology.111–114
Therefore, we advocate for systematic and detailed investigations of the interaction between water molecules and H2 bubbles on catalyst surfaces, especially for materials with complex microstructures or oxygen functional groups. Given that catalyst morphology and surface chemistry may evolve during long-term operation, it is essential to characterize these interactions not only under open-circuit conditions but also under applied bias during electrolysis.
However, this design increases the potential of excessive *OH accumulation on the catalyst surface when the *OH affinity is too strong to desorption. In such cases, the catalyst may evolve into hydroxide-rich phases (e.g., (oxy)hydroxides) during the HER, like the structural reconstruction observed during the OER. Once the OH-coordinating sites become saturated, they lose their strong capacity of *OH affinity to promote further water destabilization. In this context, excessive *H accumulation is not considered problematic, as *H intermediates upon the adsorption sites are theoretically consumed during the second electron transfer step during neutral HER operation. As discussed in the HER mechanism, the Tafel process involves the direct coupling of two *H species, typically located on adjacent active sites, whereas the Heyrovsky process involves one *H species reacting with a water molecule to generate H2.
In homogeneous single-site catalysts, two neighboring identical sites can provide comparable *H adsorption energies, allowing for efficient Tafel-type recombination, provided that the inter-site distance is sufficiently short to enable *H–*H coupling. Alternatively, a single site capable of adsorbing multiple hydrogen atoms can also facilitate the Tafel step within a localized region. While multi-site catalysts follow similar principles, the presence of distinct active centers offers additional tunability over *H adsorption and desorption energies, potentially improving the Tafel recombination kinetics.
In contrast, the Heyrovsky step poses a suppositional challenge for homogeneous single-site catalysts. During the Volmer step, the metal center (M*) forms an M–H1–OH intermediate following the O–H bond cleavage of H1–OH. To proceed through the Heyrovsky pathway, this intermediate must evolve into an M–H1–H2–OH configuration. However, because H1 and H2 do not share a bonding electron pair in this M–H1–H2–OH configuration, the adsorption of the incoming H2–OH molecule is weak. If the M* site exhibits a strong hydrogen binding affinity to hold the M–H1–H2–OH configuration, the desorption of H2 becomes energetically unfavorable, in accordance with the Sabatier principle.
This challenge highlights the importance of multi-site catalyst design in facilitating the Heyrovsky pathway. Introducing an additional component that can stabilize the H2–OH molecule in the M–H1–H2–OH transition state, or tuning the adsorption energies of both M–H1–OH (Volmer step) and M–H1–H2–OH (Heyrovsky step) intermediates, is expected to enhance the reaction kinetics. This multi-site designed strategy allows for synergistic effects that single-site catalysts inherently lack.
It is important to note, however, that while techniques to probe the Volmer step have made progress, the experimental differentiation of the Tafel and Heyrovsky pathways remains extremely challenging. This limitation underscores the urgent need for more advanced in situ and operando characterization tools. Nevertheless, the strategic design of multi-site catalysts offers a promising route to more precisely control the surface reaction energetics and optimize the HER activity under neutral conditions.
2.3. Neutral OER mechanism of multi-site electrocatalysts
While considerable efforts have been devoted to understanding the HER under neutral conditions, achieving efficient overall water splitting also requires overcoming the kinetic bottleneck imposed by the OER, which is a four-electron, multi-proton process with intrinsically sluggish kinetics. In neutral media, these challenges are further magnified by low ionic conductivity, weak proton-coupling capacity, and unstable intermediate adsorption, all of which contribute to high overpotentials and poor energy efficiency. Thus, the rational design of multi-site electrocatalysts offers a promising pathway to tailor the surface chemistry and micro-environments for improved OER activity under neutral conditions.In neutral media, water molecules (H2O) serve as the oxygen source:
| 2H2O → O2 + 4H+ + 4e− (Total reaction) | (15) |
| H2O + * → *OH2 (Water adsorption) | (16) |
| *OH2 → *OH + H+ + e− (Form *OH) | (17) |
| *OH → *O + H+ + e− (Form *O) | (18) |
| *O + H2O → *OOH + H+ + e− (Form *OOH) | (19) |
| *OOH → *OO + H+ + e− (Form *OO) | (20) |
| *OO → O2 (Oxygen release) | (21) |
The theoretical potential of the OER is 1.23 V (vs. RHE). The challenge lies in balancing the adsorption energies of *OH, *O, and *OOH intermediates. According to the Sabatier principle, overly strong or weak binding of these intermediates leads to poor catalytic activity. In addition, in neutral media, the low concentration of available hydroxyl (OH−) groups and weak buffering capacity slow the release of oxygen-containing species and make the PCET step sluggish. This contributes to higher kinetic barriers and larger overpotentials of the OER compared to acidic or alkaline conditions.
The neutral OER suffers from some issues: (i) Sluggish reaction kinetics. Each OER step involves multi-electron and multi-proton transfers, but the low mobility of H+ in neutral electrolytes slows PCET. In addition, intermediates such as *OH and *OOH are not efficiently stabilized due to weak electrostatic interaction at the interface. In addition, the thicker and diffuse electrical double layer at neutral pH reduces the interfacial field strength, hindering the orientation and polarization of water molecules needed for the O–H bond cleavage. (ii) High energy barriers. The formation of the *O and *OOH intermediates generally requires high potential, and the lack of optimal binding energy matching leads to large theoretical overpotentials. In neutral systems, additional overpotential is needed to drive the reaction due to poor ionic conductivity and mass transport resistance. (iii) Poor buffering and local pH drift. Accumulated H+ during the OER is not efficiently neutralized in unbuffered or weakly buffered systems, causing local acidification, which may alter the surface state or trigger catalyst degradation. Conversely, in some phosphate-buffered systems, anion adsorption (e.g., H2PO4−) can block active sites, suppressing the OER activity. (iv) Catalyst instability. Many high-performance OER catalysts in alkaline media (e.g., NiFe layered double hydroxides) undergo limited surface reconstruction or severe dissolution under neutral conditions owing to the absence of OH− as a stabilizing species.
Multi-site electrocatalysts – systems that integrate distinct functional sites on the same surface or within a hybrid nanostructure – thus offer a versatile strategy to decouple and optimize the sequential steps of the OER. Their advantages under neutral conditions include: (i) Tailored intermediate adsorption. Different catalytic sites can selectively stabilize different intermediates. For example, one site with high oxophilicity can favor *OH or *O adsorption, while a neighboring site with moderate binding strength can promote *OOH formation and desorption.117 This functional separation avoids the binding-energy scaling limitations of single-site catalysts. (ii) Accelerated proton-coupled electron transfer. Multi-site systems can incorporate proton relay centers (e.g., fluoride/borate anions,118 coordinated phosphate groups,119 and BF2(OH)2− anions120) near redox-active centers, facilitating PCET via internal hydrogen bonding or Grotthuss-like proton transfer. This improves the efficiency of O–H bond cleavage and reduces the overpotential. (iii) Electric field modulation and Edlstructuring. Engineering surface heterogeneity (e.g., metal-oxide junctions and defect-rich zones) can modulate local electric fields and compress the electrical double layer, enhancing water molecule polarization and OER rates. For instance, combining transition metal-based catalysts with conductive supports (e.g., RuIr/CoNC121 and (N, S)-RGO@CoN122) helps balance charge transport and intermediate stabilization. (iv) Cooperative redox and synergistic effects. Systems integrating redox-inert supports (e.g., TiO2123 and Cr2O376) with active metals can store and shuttle electrons, smoothing electron transfer fluctuations during the OER. In addition, adjacent sites with variable oxidation states (e.g., Ni3+/Ni2+ and Mn4+/Mn3+)124,125 can buffer redox transitions and resist deactivation. These effects are particularly valuable in neutral environments, where the reaction driving force is intrinsically weaker, and maintaining redox flexibility is crucial.
3. Design strategies of multi-site electrocatalysts for neutral water splitting
The development of multi-site electrocatalysts for neutral water splitting has been gaining momentum after years of painstaking research. In order to systematically elucidate the design strategies for neutral water splitting electrocatalysts, this section will provide a comprehensive discussion of several types of electrocatalysts (Table 1), including binary-site catalysts, ternary-site electrocatalysts, multi-site (≥quaternary) electrocatalysts, and single-phase electrocatalysts, respectively.| Catalyst | Method | Electrolyte | Activity (mV@mA cm−2) | Stability (h@mA cm−2) | Ref. |
|---|---|---|---|---|---|
| MoP2/MoP | Phosphidation | 1 M PBS | 196@10 | 4k cycles | 126 |
| MoS2/α-MoC | Calcination | 1 M PBS | 123@10 | 12@10 | 132 |
| Pt@Cu | Wet chemical reduction | 1 M PBS | 35@10 | 10@−1.25 V | 131 |
| Ru–VO2 | Hydrothermal | 1 M PBS | 81@10 | — | 133 |
| Ni2P–Ni12P5@Ni3S2 | Sulfidation and phosphidation | 1 M PBS | 34@10 | 24@−0.034 V | 134 |
| MoP/Mo2N | Polymerization and calcination | 1 M PBS | 91@10 | 48@∼20 | 135 |
| Am–Mo–NiS0.5Se0.5 | Hydrothermal | 1 M PBS | 48@10 | 300@10 | 136 |
| MoP–Ru2P | Calcination | 1 M PBS | 126@10 | 12@10 | 127 |
| Ni/α-Ni(OH)2 | Etching | 1 M PBS | 110@10 | 10@10 | 137 |
| V2O3@Ni | Hydrothermal and annealing | 1 M PBS | 100@10 | 10@10 | 138 |
| CoO/Co4N | Hydrothermal and ammonolysis | 1 M PBS | 145@10 | 2k cycles | 139 |
| Ni4Mo–V2O3 | Hydrothermal and annealing | 1 M PBS | 39.3@10 | 5.56@50 | 140 |
| Ni(S0.5Se0.5)2 | Hydrothermal and selenization | 1 M PBS | 124@10 | 20@10 | 141 |
| Ni3N-VN | Hydrothermal and nitridation | 1 M PBS | 85@10 | 40@20 | 142 |
| CuRu/CB | Wet chemical method | 1 M PBS | 91@10 | 3k cycles | 143 |
| CoP/Ni2P | Hydrothermal and phosphidation | 1 M PBS | 54@10 | 36@100 | 144 |
| Ni/NiMoN | Solvothermal and nitridation | 0.5 M Na2SO4 + 0.25 M KH2PO4 + 0.25 M K2HPO4 | 37@10 | 24@10 | 145 |
| Co(OH)2 UNA | Electrodeposition | 1 M PBS | 70@10 | 10@150 | 146 |
| Cu–Ni3S2 | Microwave irradiation | 1 M PBS | 218@10 | 2k cycles | 147 |
| Ni3N/Ni/NF | Electrodeposition and nitridation | 1 M KPi | 19@10 | 50@10 | 148 |
| CoNiP/CoxP | Electrodeposition and phosphidation | Natural seawater | 290@10 | 500@10 | 149 |
| Mn–Co–P | Electrodeposition and phosphidation | 1 M PBS | 86@10 | 1k cycles | 150 |
| N–Co2P/CC | Hydrothermal and phosphidation | 1 M PBS | 42@10 | 33.33 | 151 |
| CoP3/CoMoP/NF | Hydrothermal and phosphidation | 1 M PBS | 89@10 | 20@10 | 152 |
| F-CoP-Vp | Phosphidation | 1 M PBS | 108@10 | 20@10 | 153 |
| Os/OsS2 | Calcination | 1 M PBS | 58@10 | 14@10 | 154 |
| CoW(OH)x | Electrodeposition | 1 M PBS | 73.6@10 | 70@20 | 155 |
| Ru–CoP/Ni2P | Hydrothermal and phosphidation | 0.01 M PBS | 125@10 | 50@100 | 156 |
| NiCo2Se4 HUNSs | Hydrothermal, calcination, and hydrothermal selenization | 1 M PBS | 102@100 | 200@100 | 157 |
| Pt/Ni–Mo–N–O | Hydrothermal and nitridation | 1 M PBS | 101@100 | 50@30 | 158 |
| Ru/Mo2CTx | In situ reduction | 1 M PBS | 73@10 | 30@20 | 159 |
| NixB/Mo0.8B3 | Hydrothermal and boronization | 1 M PBS | 83@10 | 50@500 | 160 |
| Pt–IrO2/CC | Annealing and electrodeposition | 1 M PBS | 26@10 | 20@20 | 161 |
| Rh@Pt2L | Epitaxial growth | 1 M PBS | 19@10 | 5@10 | 162 |
| Pt/WO2 | Hydrothermal and decomposition | Natural seawater | 290@10 | 500@100 | 163 |
| Mn–Ni–S/NF | Hydrothermal and sulfidation | 1 M PBS | 84@10 | 24@60 | 164 |
| Ni4Mo–V2O3 | Hydrothermal and annealing | 1 M PBS | 39.3@10 | 5.56@50 | 165 |
| FeOx/FeP | Hydrothermal and phosphidation | 1 M PBS | 96@10 | 60@20–60 | 166 |
| Bi–B/BiB3O6@HC | Activation and electroless plating | 1 M PBS | 88.5@10 | 200@10 | 167 |
| Mn–NiO–Ni/Ni–F | Hydrothermal and pyrolysis | Natural seawater | 170@10 | 14@−0.14 V | 168 |
| Co2P–FeP | Coprecipitation and phosphidation | 1 M PBS | 109@10 | — | 169 |
| Ru–WO3−x | Hydrothermal and annealing | 1 M PBS | 19@10 | 30@20 | 170 |
| PtPdRhRuCu MMNs | Wet-chemical reduction | 1 M PBS | 28@10 | 100@10–100 | 45 |
| B–Os aerogels | NaBH4-induced gelation | 1 M PBS | 33@10 | 20@10 | 171 |
| RuSex–RuNC | Organic–inorganic multi-conversion | 1 M KPi | 29@10 | 100@10–30 | 172 |
| Ir–HxWO3 | Hydrogen intercalation | 1 M PBS | 20@10 | 100@10 | 173 |
| V0.8Mo0.2Se2−x | Calcination and wet chemical | 1 M PBS | 122.3@10 | 60@100 | 174 |
| CrOx/Ni–Cu | Thermodecomposition | 1 M KPi | 48@10 | 24@−0.1 V | 175 |
| Pt/np–Co0.85Se | Electrochemically selective etching | 1 M PBS | 55@10 | 40@−0.05 V | 176 |
3.1. Binary-site electrocatalysts
Among various multi-site neutral electrocatalysts, binary-site neutral electrocatalysts are famous due to their relatively simple structure and tunable composition, leading to extensive reports in the context of neutral water splitting. It is common to obtain a product with a two-phase structure of homogeneous atoms after treatment of raw materials, which can function as efficient binary-site electrocatalysts for neutral water splitting. For instance, Shao's group developed a facile phosphidation method to fabricate a two-phase structure of MoP700 (MoP2/MoP) catalyst for neutral water splitting, which owns dual active sites of MoP2 and MoP phase, as illustrated in Fig. 7a.126 A considerable electron delocalization effect between Mo and P occurred in the MoP2 component that had a larger atomic percentage of P, strengthening the Mo–P bond. Consequently, the MoP2 component demonstrates enhanced catalytic activity in water dissociation due to strong electrostatic interactions between Mo and OH− species, as well as P and H atoms. Meanwhile, the MoP phase within the MoP2/MoP heterostructure optimizes Mo site accessibility, facilitating hydrogen atom recombination during the Tafel step. Density functional theory (DFT) results (Fig. 7b and c) reveal reduced kinetic barriers for H2O dissociation on Mo–MoP2 surfaces compared to Mo–MoP interfaces, confirming superior hydrolytic capability. Conversely, the Mo–MoP surface exhibits a weaker hydrogen binding strength, enabling efficient H–H bond formation. This synergistic combination of two phases enables the MoP2/MoP catalyst to achieve an overpotential of 196 mV at 10 mA cm−2, which is even comparable with the noble metal Pt/C catalyst (181 mV), while demonstrating markedly superior performance to single-phase catalysts (276 mV for MoP2 and 327 mV for MoP). Furthermore, the catalytic system can be optimized with ruthenium phosphide (Ru2P), where the MoP phase serves as a hydrogen recombination center, while Ru2P provides water adsorption sites.127 This two-phase MoP–Ru2P structure achieves exceptional performance, only requiring a low overpotential of 126 mV at 10 mA cm−2, which is superior to its constituent phases of MoP and Ru2P. Inspired by the transition metal phosphides, transition metal carbides and sulfides with a two-phase structure were also investigated for neutral water splitting.128–130 For example, Xu's group fabricated two-phase WC–W2C nanocrystals embedded into hollow carbon dodecahedrons (WC–W2C/HCDs) by a simple template-engaged strategy, which showed excellent HER activity in a wide pH range.128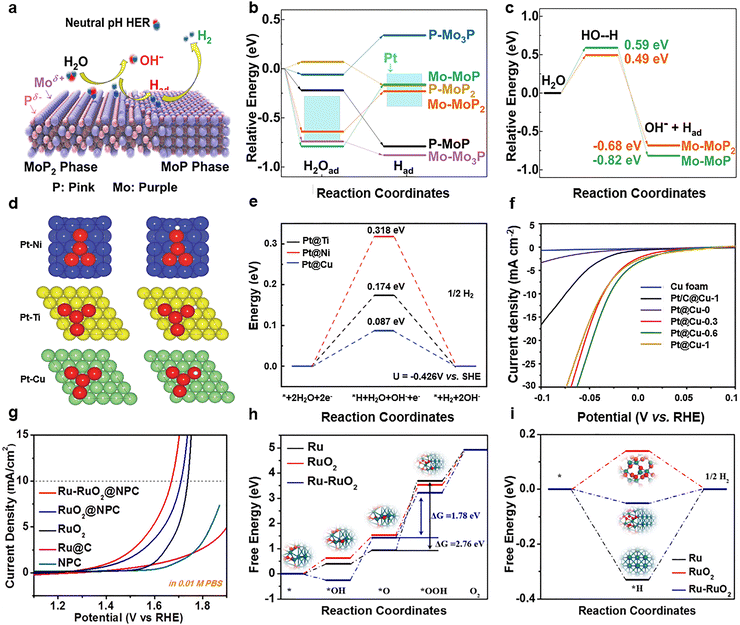 | ||
| Fig. 7 (a) Schematic diagram of the neutral HER over MoP2/MoP, (b) adsorption energies of *H and *H2O and (c) free energy profiles for water dissociation. Reprinted with permission from ref. 126. Copyright 2019, American Chemical Society. (d) Schematic of Pt–Ti, Pt–Ni, and Pt–Cu models, (e) corresponding Gibbs free energy of *H adsorption, and (f) the HER polarization curves of catalysts. Reprinted with permission from ref. 131. Copyright 2021, The Authors. (g) Neutral OER polarization curves, (h) free energy profiles for the neutral OER, and (i) adsorption energies of *H for Ru–RuO2@NPC and the compared catalysts. Reprinted with permission from ref. 178. Copyright 2022, Elsevier. | ||
Besides accelerated H2O dissociation in the Volmer step, optimizing the *H adsorption energy is also an effective strategy to design binary-site electrocatalysts for neutral water splitting. It is known to all that the noble metal of Pt exhibits excellent HER performance, but its scarcity and high cost severely impeded the industrialization of water electrolysis. Therefore, the binary-site composite electrocatalysts with low content of noble metal provide an affordable solution. For example, Parkin et al. conducted a systematic study on platinum-based binary catalytic systems comprising Pt and non-noble metallic elements including Ni, Ti, and Cu.131 As illustrated in Fig. 7d, the binary-site crystal models were established for the theoretical calculation. Fig. 7e presents the Gibbs free energy diagram for hydrogen adsorption, revealing that the Pt–Cu binary catalyst exhibits a near-thermoneutral |ΔG*H| value of 0.087 eV. This value is significantly lower than those observed for Pt–Ni (0.318 eV) and Pt–Ti (0.174 eV) binary-site systems, demonstrating the efficient regulation of *H adsorption energy by binary-site composite electrocatalysts. Subsequently, a variety of binary Pt–Cu catalysts with varying platinum contents were fabricated and examined. As the polarization curves showed in Fig. 7f, the Pt@Cu-0.6 catalyst with low Pt loading achieved a current density of 10 mA cm−2 at an exceptionally low overpotential of 28 mV, greatly surpassing traditional Pt/C catalysts supported on copper substrates, according to the electrochemical evaluations in a 1 M phosphate-buffered saline (PBS) solution. Furthermore, other binary-site composite catalysts that consist of noble metals and non-noble metals were explored, such as Ru–Cu binary-site composite catalysts, which demonstrated enhanced HER performance under neutral pH conditions.143 Meanwhile, non-noble metal Ni–Cu binary-site composite catalysts only require 284 mV to obtain a current density of 10 mA cm−2 in neutral media.177 Therefore, constructing the metal–metal (M–M) binary-site is a promising strategy for designing efficient electrocatalysts for neutral water splitting.
Additionally, metal oxides (MO)-138,161,166,179–182 and metal hydroxide (MOH)-based137,146,155,183 binary-site electrocatalysts were explored for neutral water splitting. Jiang et al. paired V2O3 with Ni4Mo to construct a binary NiMo4–V2O3 catalyst by employing a straightforward topotactic conversion approach.140 High-resolution transmission electron microscopic (HRTEM) analysis clearly reveals the (110) and (012) planes of V2O3, confirming that the supported matrix consists of V2O3, and Ni4Mo exists as dispersed nanoparticles. This catalyst exhibited exceptional HER activity, requiring a low overpotential of 39.3 mV to reach 10 mA cm−2 in neutral media, surpassing both Ni4Mo (60.5 mV) and Ni–V2O3 (85.0 mV). Computational studies revealed that the highly oxyphilic nature of V2O3 enhances water molecule binding and promotes OH− intermediate formation, while *H intermediates preferentially bind to Ni4Mo sites. As the DFT results illustrated, the activation barrier for water dissociation decreased significantly to 0.42 eV for Ni4Mo–V2O3, while Ni4Mo required a high value of 0.65 eV, validating the effective collaboration of MO-based binary-site electrocatalysts for boosting the neutral HER activity.
Besides various binary-site electrocatalysts for the neutral HER, the binary-site neutral OER electrocatalysts have been developed in recent years. Through a controlled pyrolysis synthesis, Wang et al. successfully fabricated binary-site Ru–RuO2 heterostructures embedded within N, P co-doped carbon matrices (Ru–RuO2).178 As the LSV results illustrated in Fig. 7g, the binary-site composite represented exceptional HER and OER bifunctional performance in a wide pH range (0–14). In detail, Ru–RuO2@NPC is capable of achieving 10 mA cm−2 at an overpotential of 440 mV for the neutral OER, significantly outperforming commercial Pt/C and other noble metal Ru-based electrocatalysts. Meanwhile, the binary-site Ru–RuO2 electrocatalyst shows a decent neutral HER activity, which yields a Tafel slope of 113 mV dec−1. After 10 hours of chronopotentiometry and 2000 cycles of cyclic voltammetry (CV) tests, the binary-site Ru–RuO2 electrocatalyst exhibits good durability. Furthermore, first-principles computations elucidated the electronic synergy at binary-site Ru–RuO2, where the electronic modulation between Ru and RuO2 reduced the key energy barriers of the OER (Fig. 7h). In detail, the adsorption of oxygen intermediates was optimized by the Ru site in Ru–RuO2, resulting in a reduced energy barrier of *OOH (1.78 eV), in sharp contrast to 1.91 eV for RuO2. As illustrated in Fig. 7i, this synergistic electronic optimization between binary-site metallic Ru and oxide phases underpins the optimized adsorption of *H intermediate, boosting the neutral water splitting catalytic activity. Additionally, Niu and co-workers133 fabricated a binary-site Ru–VO2 electrocatalyst, which consists of Ru-doped vanadium oxide substrates and Ru nanoparticles. Impressively, the binary-site Ru–VO2 electrocatalyst demands an ultralow overpotential of 269 mV to deliver 10 mA cm−2 for the neutral OER, and it possesses excellent mass activity, outperforming commercial RuO2-CC and Ru-CC by factors of 6.5 and 4.2, respectively. Furthermore, the assembled symmetrical electrolyzer exhibits good overall neutral water splitting, which only needs 1.63 V to output 10 mA cm−2 for overall water splitting, and can stably run over 125 hours. After theoretical calculation, it is found the *OH prefers to absorb on the Ru site due to the synergistic interaction of binary-site catalyst, which destabilizes the hydrogen-bonding and accelerates the reaction kinetics for boosting neutral water splitting. Moreover, binary-site catalysts have been extended to nitrides,135,139,142,145,148 phosphides,134,144,149–153,169,184 sulfides,132,147,154,164,185,186 carbides,159,187,188 selenides,136,141,157,174 borides,160,167,171 and alloys162 for efficient neutral water splitting.
3.2. Ternary-site electrocatalysts
As discussed in the fundamentals of the neutral HER, water adsorption and dissociation in the Volmer step usually require a coordination of the H affinity site and the O affinity site, while the hydrogen desorption in the Tafel/Heyrovsky step demands a site with weak hydrogen adsorption. Therefore, three kinds of catalytic sites with a specific function are fundamental requirements for good neutral water splitting catalysts. In this regard, ternary-site electrocatalysts have been widely investigated in recent years, especially for neutral and alkaline water splitting.Among various ternary-site electrocatalysts, transition metal-based ternary-site materials with earth-abundant nature have gained great attention in the field of neutral water splitting. Due to their low cost and tunable electronic structure, nickel (Ni)-based ternary-site electrocatalysts have been widely investigated. It is well known that metal Ni exhibits good hydrogen adsorption, which is beneficial for the Volmer step, but impedes the subsequent hydrogen desorption step. To overcome this limitation, functional sites with weak hydrogen adsorption should be incorporated to construct multi-site electrocatalysts. Taking advantage of sulfurization and phosphorization methods, Zhao and co-workers developed a novel NiS–Ni2P/Ni ternary-site electrocatalyst.189 In Fig. 8a, the HRTEM result shows lattice fringes of 0.171, 0.192 and 0.204 nm, which belongs to the (110) plane of NiS, the (210) plane of Ni2P, and the (111) plane of Ni, respectively. Consequently, the NiS–Ni2P/Ni ternary-site electrocatalyst only requires a low overpotential of 115 mV to output 10 mA cm−2 for the neutral HER, which is superior to that of Ni2P/Ni (175 mV) and NiS–Ni2P (221 mV), even comparable with the noble metal of Pt/C catalysts (Fig. 8b). In order to gain a better understanding of the synergistic mechanisms, DFT calculations were utilized to investigate the behavior of hydrogen intermediate. Fig. 8c illustrates that the absolute value of ΔG*H reached 0.425 eV for the Ni metal, resulting in a sluggish desorption process. Surprisingly, the ΔG*H value dropped to 0.006 eV for the ternary-site NiS–Ni2P/Ni catalyst, demonstrating that the hydrogen adsorption behavior was optimized by the synergy of multiple sites of the NiS–Ni2P/Ni catalyst. Besides non-metal elements, transition metals were incorporated into Ni-based materials to fabricate ternary-site electrocatalysts. Liu et al. reported a facile hydrothermal method to fabricate a ternary-site CoNi2S4–WS2–Co9S8 (NiCoWS) catalyst.190 As the polarization curves displayed, the NiCoWS catalyst demonstrates significantly superior neutral HER activity compared to NiS and NiCoS, necessitating an overpotential of only 146 mV to achieve 10 mA cm−2 in neutral media. According to theoretical analysis, the electron-accumulated WS2 site is beneficial for optimizing the *H adsorption behavior, and the hole-accumulated Co9S8 and CoNi2S4 sites accelerate the adsorption and dissociation of water molecules. As a result, the ternary-site NiCoWS exhibits an ideal H adsorption energy, which is almost equal to zero, in sharp contrast to the weak adsorption of WS2 catalysts. Moreover, the cooperation of multiple sites of NiCoWS effectively lowers the energy barrier of water dissociation, greatly speeding up the slow water dissociation process and boosting the neutral HER performance of Ni based ternary-site catalysts. In addition, other non-metal and metal elements were introduced into Ni-based catalysts to fabricate multi-site catalysts, thus achieving MnO–NiO–Ni/Ni–F,168 Ni3N@Ni–B,191 Ni/W–Ni(OH)2,192 (Ni3S2–MoS2)@TiO2,104 amorphous-Mo NiS0.5Se0.5 nanosheets@crystalline NiS0.5Se0.5 nanorods,136 and Fe–NiS/H193 ternary-site catalysts.
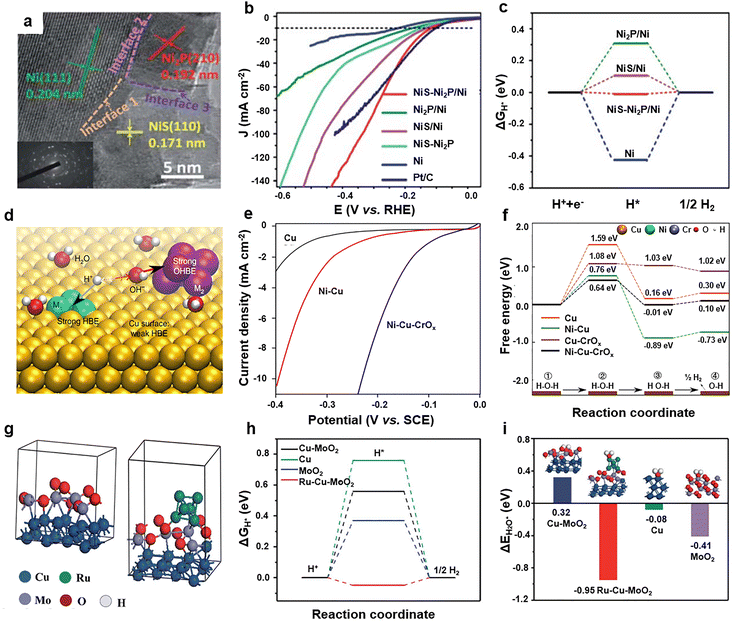 | ||
| Fig. 8 (a) HRTEM image, (b) polarization curves, and (c) adsorption energy of *H of different catalysts. Reprinted with permission from ref. 189. Copyright 2022, The Royal Society of Chemistry. (d) Schematic diagram of the design strategy, (e) neutral HER polarization curves, and (f) free energy profiles of water dissociation of Ni–Cu–CrOx catalysts. Reprinted with permission from ref. 175. Copyright 2018, The Authors. (g) Structure models, and adsorption energy of (h) *H and (i) *H2O of catalysts. Reprinted with permission from ref. 194. Copyright 2022, The Royal Society of Chemistry. | ||
Besides the Ni-based binary-site catalyst, copper (Cu)-based materials were widely investigated in the field of neutral water splitting. Different from the strong hydrogen adsorption of Ni, Cu shows a weak adsorption ability. Therefore, individual Cu catalysts and Ni catalysts exhibit poor neutral HER activity, while the binary site of Ni–Cu catalysts can balance the hydrogen adsorption behavior. For example, Sargent et al. have conducted a systematic investigation into the HER activity of single-site, binary-site, and ternary-site electrocatalysts under neutral conditions. The ternary-site electrocatalysts were designed by using Cu, Ni and CrOx (Fig. 8d).175 Compared with Cu and Ni–Cu, the ternary CrOx/Ni–Cu exhibits enhanced neutral HER activity, which is capable of achieving 7.7 mA cm−2 at a small overpotential of 155 mV (Fig. 8e). In addition, the Ni and CrOx sites of CrOx/Ni–Cu exhibit robust interactions with hydrogen and hydroxyl groups, respectively, as evidenced by the DFT calculations. This interaction is advantageous for the efficient dissociation of water molecules. As shown in Fig. 8f, the energy barrier of water dissociation of CrOx/Ni–Cu ternary-site catalysts is only 0.64 eV, which is much decreased compared to that of Cu (1.59 eV) and Cu-CrOx (1.08 eV). Additionally, Cu displays a weak hydrogen adsorption ability, which promotes the release of hydrogen, thus the CrOx/Ni–Cu electrocatalyst displayed superior HER performance in neutral media. By simple precipitation and annealing techniques, Mu et al. successfully constructed ternary-site Ru–Cu–MoO2 catalysts for neutral water splitting (Fig. 8g).194 The TEM image reveals that Ru–Cu–MoO2 catalysts present a unique hollow octahedron nanostructure after calcination. After the systematic investigation, it is found that the Ru–Cu–MoO2 electrocatalyst has outstanding HER performance in an environment of wide pH range. As the DFT results show in Fig. 8h, compared with the high hydrogen adsorption energy of Cu (0.78 eV) for weak interaction, a significant decrease in the hydrogen adsorption energy for Cu-MoO2 (0.58 eV) and ternary-site Ru–Cu–MoO2 (−0.08 eV) was observed, indicating an optimal hydrogen adsorption ability. Meanwhile, the Ru–Cu–MoO2 electrocatalyst demonstrated the lowest adsorption energy of water molecules, thus accelerating the sluggish water dissociation (Fig. 8i). As a result, the ternary-site Ru–Cu–MoO2 catalysts possess superior HER activity in acid, neutral, and alkaline media. Similarly, the Pt–Cu–CuOx ternary-site electrocatalyst also exhibit good performance for the neutral HER.195 It is found that the ternary-site electrocatalysts show similar components of metal and metal oxides/hydroxides, which collaborate to optimize the adsorption of various intermediates, resulting in notably enhanced performance. Furthermore, a variety of Co-based,196–200 Mo-based,158,165,201 Ru-based,156,202 and W-based203–205 ternary-site neutral electrocatalysts have been developed, which also exhibit enhanced neutral water splitting performance.
3.3. Multi-site (≥quaternary) electrocatalysts
Based on the mechanism of neutral HER and OER discussed above, except water and *H adsorption/desorption, there are several neutral OER intermediates such as *OH, *O and *OOH. Consequently, ternary-site electrocatalysts often struggle to effectively balance the adsorption behavior of those reaction intermediates. Therefore, multi-site (≥quaternary) electrocatalysts are desirable for efficient water splitting under neutral conditions.As discussed in the section of binary-site and ternary-site electrocatalysts, metal sites play a key role in the overall catalytic process of neutral water splitting. However, metal sites usually possess too strong or too weak interaction with reaction intermediates. Non-metal (N, P, etc.) sites own strong electronegativity and have the potential to optimize the electronic structure of metal sites. Therefore, the incorporation of both metal and non-metal sites is a common strategy for the design of multi-site (≥quaternary) electrocatalysts. Taking advantage of metal and non-metal sites, Liu and co-workers demonstrated a novel molybdenum-nickel bimetallic carbonitride quaternary-site electrocatalyst (Mo–Ni–N–C) for neutral water splitting.206 According to the elemental mapping results, the Mo, Ni, N, and C elements are uniformly distributed on the whole Mo–Ni–N–C quaternary-site electrocatalyst. Benefiting from the synergism of multiple sites, the Mo–Ni–N–C electrocatalyst exhibits a thermo-neutral ΔG*H value of 0.05 eV (Fig. 9a), while the original Mo site of MoC shows a too strong adsorption behavior (−0.48 eV) in Fig. 9b. As a result, the Mo–Ni–N–C quaternary-site electrocatalyst exhibits decent neutral HER activity with an overpotential of 135 mV at 10 mA cm−2 in a 1 M PBS electrolyte.206 Moreover, non-metal P and S were also investigated to be incorporated into quaternary-site electrocatalysts. Qu's group rationally designed cobalt/nickel phosphosulfide nanowires (NiCoPS) on carbon cloth by hydrothermal, sulfurization and phosphorization methods.207 The TEM mapping results validate the even distribution of four elements on the NiCoPS nanowires. Taking advantage of the synergy of non-metallic and metal sites, the NiCoPS quaternary-site electrocatalyst can output 10 mA cm−2 with a low overpotential of 55 mV for the neutral HER (Fig. 9c) and also demonstrate excellent HER performance in both alkaline and acidic electrolytes. Furthermore, similar quaternary multi-site electrocatalysts based on metal and non-metal elements have been developed for neutral water splitting.210,211
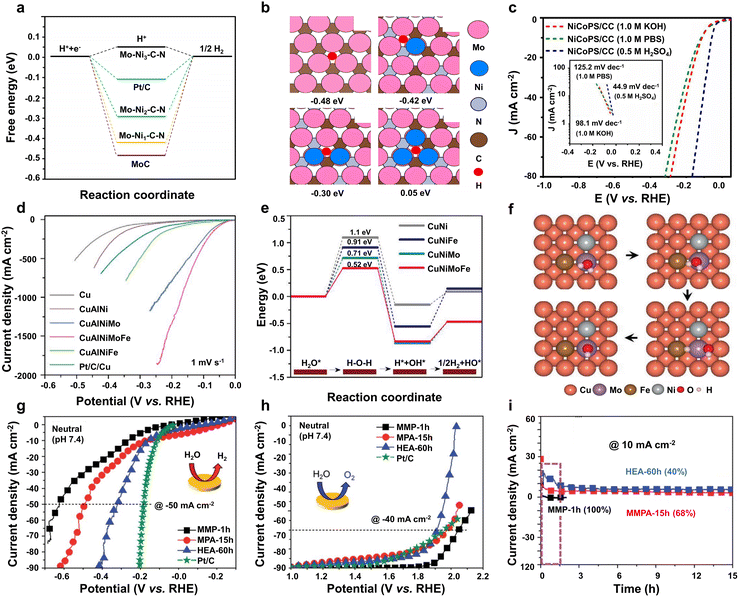 | ||
| Fig. 9 (a) Adsorption energy of *H and (b) structural models for catalysts. Reprinted with permission from ref. 206. Copyright 2017, Elsevier. (c) HER polarization curves of NiCoPS. Reprinted with permission from ref. 207. Copyright 2017, Tsinghua University Press and Springer-Verlag Berlin Heidelberg. (d) Neutral HER polarization curves, (e) free energy profiles of water dissociation, and (f) structural models for CuAlNiMoFe catalysts. Reprinted with permission from ref. 208. Copyright 2020, Wiley-VCH. (g) HER and (h) OER polarization curves, and (i) stability tests of the CuCoNiFeMn electrocatalyst. Reprinted with permission from ref. 209. Copyright 2021, The Royal Society of Chemistry. | ||
Apart from the metal and non-metal elements of multi-site (≥quaternary) electrocatalysts, the high-entropy alloy that only consists of multiple metal elements has also been widely investigated in the field of water splitting. Based on the designed alloying and dealloying technique, Jiang et al. fabricated a unique CuAlNiMoFe quinary-site electrocatalyst, which possesses a nanoporous structure.208 Benefiting from the synergism of multiple sites, the CuAlNiMoFe electrocatalyst demonstrates excellent neutral HER performance, which only demands an ultralow overpotential of 23 mV to drive an HER current density of 10 mA cm−2, while the overpotential of the CuAlNi catalyst as high as 202 mV is required to obtain the same current density (Fig. 9d), suggesting boosted HER performance of the CuAlNiMoFe electrocatalyst. From the DFT simulation results displayed in Fig. 9e, it can be observed that compared with the high energy barrier of H2O dissociation (1.1 eV) of CuNi binary-site catalysts, the energy barrier of the CuAlNiMoFe electrocatalyst is only 0.52 eV, confirming the optimized water dissociation behavior. In detail, as the structure models displayed in Fig. 9f, the hydrogen and hydroxyl groups are found to adsorb on the Mo and Ni(Fe) sites, respectively, and the sluggish water dissociation was optimized by their synergy. Additionally, the Mo site facilitates the hydrogen production by combining the H intermediate. Thus, the CuAlNiMoFe quinary-site electrocatalyst is capable of regulating different intermediates’ adsorption behavior, resulting in a significant increase in HER activity.
Furthermore, the cost-effective high-energy milling method was employed to fabricate multi-site (≥quaternary) electrocatalysts. For example, Cho et al. synthesized HEA-60 h (CuCoNiFeMn) quinary-site electrocatalysts by this method, and the electrocatalyst possesses uniform elemental distribution.209 Benefiting from the synergism of different sites, the designed CuCoNiFeMn quinary-site electrocatalyst can deliver a neutral HER current density of 50 mA cm−2 at an overpotential of 320 mV (Fig. 9g) and exhibit excellent HER activity in a wide pH range. According to the chronoamperometry test results, the electrocatalyst remains stable at −50 mA cm−2 over 20 hours. However, the OER activity of the CuCoNiFeMn electrocatalyst was systematically investigated by the test system in neutral media. From the OER polarization curves shown in Fig. 9h, it can be observed that the electrocatalyst exhibits good OER performance, which is capable of outputting 40 mA cm−2 at a low overpotential of 680 mV and surpassing the activity of noble metal benchmark RuO2 and other reported good electrocatalysts. Meanwhile, compared with the large Tafel slope of other catalysts, the CuCoNiFeMn electrocatalyst exhibits a smaller OER Tafel slope of 96.2 mV dec−1, which validates its favorable neutral OER reaction kinetics. The OER stability tests were conducted on a three-electrode system by a chronoamperometry method. As clearly presented in Fig. 9i, after the equilibrium process at the beginning, the CuCoNiFeMn electrocatalyst runs steady over 15 hours at a current density of 10 mA cm−2, indicating its good OER stability in neutral electrolytes. Furthermore, high-entropy metal oxides such as hollow-structured ZnFeNiCuCoRu–O212 and mesoporous-spherical PtPdRhRuCu45 demonstrate good OER activity and stability in a wide pH range. In addition, a series of quinary, quaternary and nonary non-noble metal high-entropy alloys were investigated as anode catalysts for neutral seawater splitting. It was found that the non-noble metal high-entropy alloys present decent OER activity and good stability in neutral seawater electrolytes.213
3.4. Single-atom electrocatalysts
The previously discussed binary-, ternary-, and multi-site electrocatalysts represent systems composed of multiple distinct active site components functioning cooperatively. Here, we wish to highlight a special class of nominally single-site catalysts such as single-atom catalysts (SACs), in which the isolated metal atom is anchored onto structurally and electronically tunable substrates. While these systems contain only one type of active site, the precise design of the metal coordination environment and the tailored metal-substrate interactions (MSIs) enable them to exhibit multi-site-like functionalities. Such catalysts offer atomic-level control over the active center, allowing it to be rationally engineered for the target reaction pathway. Meanwhile, the chemical nature and electronic properties of the support can be modulated to enhance activity, stability, or interfacial transport. Although formally composed of a single site, the functional heterogeneity introduced by the metal-support interface allows these materials to mimic the synergistic behavior observed in multi-site systems, making them highly attractive for neutral-pH water splitting applications.SACs feature isolated metal atoms dispersed on conductive or semiconducting supports. Despite being monoatomic in composition, their active site heterogeneity arises from variations in coordination environment, electronic structure, and local strain. Such heterogeneity enables the regulation of various functionalities, serving as the foundation for multi-site systems. Recent work by Wang et al.214 has emphasized SACs as promising platforms for hydrogen evolution due to their maximum atom efficiency and tunable active centers. For instance, Pt single atoms embedded in nanoporous Co0.85Se (Pt/np–Co0.85Se) have been shown to lower the energy barrier for water activation while facilitating *H adsorption with ideal binding energies under neutral pH.176 Similarly, Zhang et al.215 demonstrated that Pt SACs supported on CoP-based nanotube arrays exhibited the outperforming performance compared to commercial Pt/C in neutral PBS. A defining feature of SACs is their strong coupling with supports, often referred to as electronic MSIs. This interfacial synergy governs not only the stability of single atoms but also their catalytic reactivity. It influences charge redistribution, d-band center tuning, and local electric fields. Wang's team investigated Pt atoms anchored on defective layered double hydroxides and revealed that the strength of the MSIs directly determines the HER activity via the modulation of hydrogen binding energy (ΔG*H).216 This single-atom design strategy was also exhibited for Pt-,163,217,218 Ru-,170,172,219,220 and Ir173,221,222-SACs/clusters structure.
Single-phase electrocatalysts, particularly those based on SACs, represent an elegant solution to the challenge of HER under neutral pH-combining simplicity of structure with the complexity of function. Rational engineering of MSIs, control over local coordination, and spatial asymmetry via Janus configurations are proven to be essential strategies. With continued advances in in situ characterization and atomic-level synthesis, these materials are poised to rival and even surpass traditional multi-metallic systems in both activity and durability.
4. Structure and synthesis of multi-site electrocatalysts
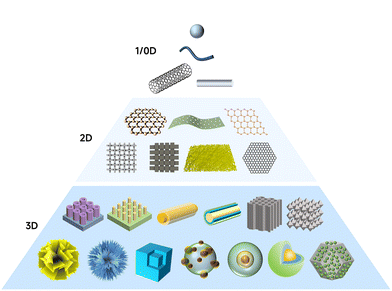
The structure of the material often determines its performance, while the synthesis method directly determines the formation of the final structure. Designing electrocatalysts with tailored architecture is crucial for optimizing the kinetics of the hydrogen evolution reaction, especially under neutral pH where proton concentration, interfacial charge transfer, and gas bubble detachment are limiting. Multi-site electrocatalysts benefit not only from compositional synergy but also from structural frameworks that expose active sites, facilitate ion and gas transport, and ensure long-term operation. The formation of multi-site catalysts can be understood as the designed generation of spatially coupled and functionally distinct active centers within a surface catalytic platform. This process generally involves the strategies that break the uniformity of single-site frameworks, giving rise to the materials for which diverse atomic or electronic configurations coexist in proximity. Such diversity may originate from variations in local coordination, bonding interactions, or electronic states, which collectively produce sites with different catalytic functions. Crucially, the proximity of these sites allows for dynamic electron transfer and joint participation in reaction pathways. In many cases, these multi-site environments emerge through thermodynamically or kinetically driven processes during synthesis. Thus, the general mechanism of multi-site catalyst formation is rooted in introducing and stabilizing heterogeneous functional components in a unified catalytic system, while ensuring that distinct sites are sufficiently coupled to enable synergistic reactions. Since the controllable growth of multi-site catalysts is challenging, understanding how a different dimensional (from zero-dimensional to three-dimensional, Fig. 10) structure influences the performance of multi-site catalysts, alongside corresponding synthetic approaches is of great guiding significance for the design of multi-site catalysts.4.1. Zero-dimension structure
Zero-dimensional (0D) nanostructures such as nanoparticles, SACs, and quantum dots offer discrete and highly exposed catalytic sites. Despite their limited size, these structures can provide chemically heterogeneous surfaces, supporting multiple types of active centers through heterometallic doping or surface functionalization. Under neutral pH, 0D catalysts shine by promoting fast turnover at localized domains. By embedding functional species into nanoscale carbon matrices or metal oxides, researchers can tune the adsorption energies of reaction intermediates with atomic precision. For instance, Zhang et al.215 developed a potential-cycling method in a three-electrode cell for preparing a platinum single-atom catalyst for the neutral HER. The catalyst comprises single Pt atoms anchored on cobalt phosphide (CoP)-based nanotube (NT) arrays grown on a nickel foam (NF) substrate, with each NT exhibiting a diameter of ∼140 nm and a wall thickness of ∼20 nm. The synthesis eliminates Pt nanoparticles/grains, ensuring atomically dispersed Pt centers without binders, which is critical for maximizing Pt utilization and optimizing electronic interactions between Pt atoms and the substrate. Similarly, the study by Tang et al.202 reported a 0D HER catalyst composed of Ru nanoparticles, Cr2O3, and N-doped graphene (NG) designed for the neutral HER. The catalyst leverages a multi-site strategy to accelerate water dissociation: Cr2O3 binds *OH groups strongly, NG provides strong *H binding, and Ru facilitates H2 coupling with weak H binding energy. In addition, this synergy dramatically enhances the HER kinetics, achieving a low overpotential of 53 mV at 10 mA cm−2 under neutral conditions, surpassing Ru/C. High-temperature calcination was conducted to obtain Ru-Cr2O3/NG by dispersing NG into the mixture solution containing Ru and Cr precursors, followed by drying in air at room temperature and annealing at 750 °C in an Ar atmosphere for 3 h. In addition, the high-temperature synthesis method has been used to prepare nanoparticles with high crystallinity. Sun and co-workers223 synthesized a series of noble-metal-based (Pt, Ru, Pd, and Ir) carbon-group (Si, Ge, and Sn) intermetallic phases by a molten-salt-assisted method heating the mixture of metal salts at 800 °C for 2 h under N2 flow (Fig. 11e). As an example, PtSi required a low overpotential of 66 mV at 10 mA cm−2 in neutral media with >97% faradaic efficiency. The Si incorporation into Pt lowers the binding energy between Pt sites and *H intermediates, boosting the hydrogen generation for the neutral HER.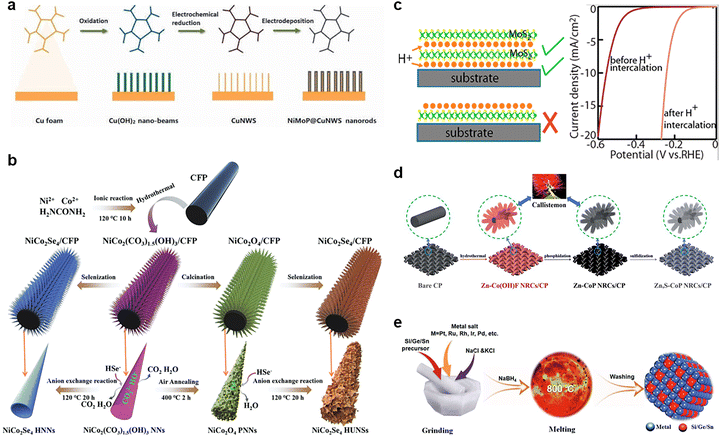 | ||
| Fig. 11 (a) Preparation process of 3D structure NiMoP@CuNWs/CF. Reprinted with permission from ref. 237. Copyright 2024, Dalian Institute of Chemical Physics, the Chinese Academy of Sciences. (b) Schematic of the synthetic process of 3D NiCo2Se4 HNNs/CFP and NiCo2Se4 HUNSs/CFP. Reprinted with permission from ref. 157. Copyright 2024, The Authors. (c) Schematic of the major role of proton intercalation between the 2D monolayer and underlying structures, as well as the promoted performance. Reprinted with permission from ref. 230. Copyright 2017, American Chemical Society. (d) Schematic of the preparation of 1D callistemon-like Zn,S–CoP NRCs/CP. Reprinted with permission from ref. 227. Copyright 2019, The Royal Society of Chemistry. (e) Schematic of the synthesis of a number of 0D noble-metal-based carbon-group intermetallic phases. Reprinted with permission from ref. 223. Copyright 2022, Wiley-VCH. | ||
Typical synthesis methods include colloidal synthesis, allowing size, shape, and composition control via ligand engineering;224 thermal decomposition of metal precursors, often yielding oxide-metal hybrids;225 encapsulation within conductive hosts (e.g., core–shell carbon spheres), preserving the high reactivity of the nanoscale core while preventing aggregation.226 These 0D systems are also widely used as building blocks in constructing hierarchical hybrids, where nanoparticle-doped 2D or 3D matrices act as a platform to combine multiple reaction sites.
4.2. One-dimension structure
One-dimensional (1D) nanostructures such as nanowires, nanorods, and nanotubes are known for their vectorial electron transport and minimal grain boundaries, making them ideal conduits for current collection in electrocatalytic systems. In the neutral HER, where the reaction kinetics is often constrained by the sluggish interfacial dynamics, 1D morphologies facilitate anisotropic diffusion of ions and help overcome gas bubble trapping. They also provide an excellent platform for establishing axial heterojunctions, which favors the study on reaction mechanism. For example, Zheng et al.173 designed an Ir–HxWO3 catalyst for the neutral HER. The catalyst is synthesized via hydrogen insertion into 1D aligned WO3 nanorod arrays on carbon fibers, followed by Ir nanoparticle deposition. The HxWO3 support functions as a proton sponge, creating a local acid-like microenvironment around Ir sites, which enhances the HER kinetics by enabling thermodynamically favorable Volmer–Tafel steps. Consequently, 1D Ir–HxWO3 demonstrated acid-like activity with a low overpotential of 20 mV at 10 mA cm−2 and a low Tafel slope of 28 mV dec−1, which are even comparable to those in an acidic environment. The construction of local acid-like environment for the neutral HER over 1D WOx was also designed by Qiao's work, where the in situ formed (HxWOy) phase via continuous hydrogen insertion from water acts as a proton reservoir.163 In addition, Yan et al.227 synthesized Zn,S-doped cobalt phosphide nanorod clusters grown on carbon paper (Zn,S-CoP NRCs/CP), emphasizing the interplay between material morphology, synthesis techniques, and catalytic efficiency (Fig. 11d). The obtained nanorods with a diameter of 50–160 nm were prepared by a hydrothermal method, followed by phosphorization and sulfuration by reacting with sodium hypophosphite and sulfur, respectively. Abundant defects expose more active sites, while the nanoscale morphology increases the surface area, both critical for improved catalysis. The resulting Zn,S-CoP NRCs/CP demonstrates exceptional HER activity (67 mV overpotential at 10 mA cm−2) and OER performance (391 mV overpotential at 10 mA cm−2) under neutral conditions. DFT calculations suggest that Zn/S doping optimizes the electronic structure of CoP, favoring the hydrogen adsorption/desorption kinetics for the HER.Typical 1D synthetic approaches include seed-mediated method162,228 (quickly synthesizing catalysts with adjustable components and core–shell distributions), solvothermal growth170 (enabling morphology tuning through precursor concentration and surfactant control), and catalyst etching159 (especially for forming hollow nanotubes that further increase surface accessibility). Furthermore, 1D materials are convenient for constructing hierarchical materials, which provides researchers with a favorable platform for designing high-performance catalysts and studying reaction mechanisms.
4.3. Two-dimensional structures
Two-dimensional (2D) materials such as nanosheets, layered hydroxides, and exfoliated dichalcogenides provide intimate contact of active sites with electrolytes, exposing maximal catalytic surface while enabling easy modification of electronic and chemical properties. The thin nature of 2D materials reduces the diffusion path length for charge carriers and facilitates fast proton coupling reactions. An exemplary system is Ni/Fe/Co-based layered hydroxide,46,153,192 which often shows a good 2D nanosheet morphology. For instance, Tang et al.192 developed a hetero-structure Ni/Ni(OH)2 nanosheet array via a hydrothermal method followed by plasma reduction, with tungstate intercalation into Ni(OH)2 to modulate the electronic structure. The ultrathin Ni(OH)2 nanosheets host metallic Ni nanoparticles on their surface, forming a stable Ni/Ni(OH)2 heterojunction. Tungstate intercalation optimizes the Ni/Ni(OH)2 interface by reducing the energy barrier for water dissociation (Volmer step) and enhancing the Ni–H bond strength, while plasma reduction preserves the nanosheet morphology with slight surface roughening, attributed to synergistic electronic modulation and structural robustness.Another typical two-dimensional material is molybdenum disulfide (MoS2) with structural flexibility.229 Li and co-workers focused on enhancing the neutral HER performance of MoS2 nanosheets through proton intercalation.230 The MoS2 catalysts were synthesized as 2D nanosheets, with proton intercalation occurring between monolayer MoS2 and substrates or within interlayers of thicker MoS2 structures. Characterization analysis shows proton-induced expansion of interlayer spacing without compromising the crystallinity or composition. The proton intercalation is stable even under prolonged electrochemical cycling. Synthesis methods include electrochemical polarization at negative potentials in acidic media or immersion in acidic solutions (e.g., TFSI), enabling protons to intercalate into MoS2 (Fig. 11c). HER performance improvements by proton intercalation arise from two key effects: electronic modulation via intercalated protons, which optimize hydrogen adsorption energy (via d-band center shifts) and enhance electrical conductivity, and structural stability due to proton retention even in alkaline media (hydroxide ions cannot easily displace protons in confined interlayers). This results in a low overpotential (∼73 mV at 10 mA cm−2 in neutral media) and remarkable stability (>2000 cycles with no degradation), bridging bare MoS2's performance gap across all pH conditions. Additionally, Liang's team reported a hybrid catalyst composed of 2D metallic 1T-MoS2 nanosheets hybridized with Ni2+δOδ(OH)2−δ nanoparticles, which exhibits enhanced HER performance in neutral media.185 The nanosheet morphology involves the in situ growth of Ni2+δOδ(OH)2−δ nanoparticles on 1T-MoS2 surfaces, forming a stable heterostructure with uniform elemental distribution. The synthesis combines interface engineering and tuning of the MoS2-to-Ni ratio, avoiding Ni doping into the MoS2 lattice. The hybridization enables a bifunctional mechanism: Ni2+δOδ(OH)2−δ nanoparticles facilitate water adsorption and dissociation to supply protons, while 1T-MoS2 efficiently catalyzes H2 generation. An overpotential of ≈153 mV is needed for the 1T-MoS2/Ni2+δOδ(OH)2−δ electrode to reach a cathodic current density of 10 mA cm−2 in 1 M PBS, about 133 mV less than that for the 1T-MoS2 catalyst electrode.
Synthesis strategies for 2D structures include hydrothermal growth of LDHs or oxyhydroxides on planar substrates,231,232 electrodeposition233 or atomic layer deposition (ALD)104 technique of thin films, plasma spraying for preparing nanoflats,234 chemical vapor deposition (CVD) for high-purity 2D chalcogenide or nitride films with controlled thickness and grain boundaries.235,236 In addition, defect engineering in 2D structures, such as introducing oxygen vacancies, sulfur vacancies, or heteroatoms like Se or P, often generates localized sites with unique electronic configurations, supporting different HER steps.
4.4. Three-dimensional structures
Three-dimensional (3D) structures such as mesoporous frameworks, self-supporting foams, and interconnected nanonetworks are essential for promoting mass transfer, reactant accessibility, and gas bubble release, all of which are rate-limiting under neutral conditions due to moderate ionic conductivity and sluggish proton diffusion.One exemplary system reported by Dinh et al.175 illustrated how a CrOx/Ni–Cu multi-site configuration supported on a 3D Cu foam promotes the neutral HER. Here, the Ni sites bind hydrogen, CrOx sites destabilize water molecules via strong hydroxyl affinity, and the Cu has a weak hydrogen binding energy, promoting hydride coupling. The 3D Cu foam substrate is used to anchor the multi-site catalyst to increase the active surface area. The synergistic spatial integration of distinct reaction sites within a 3D matrix enables ultralow overpotentials (48 mV at 10 mA cm−2) for the neutral HER, which is superior to the CrOx/Ni–Cu multi-site configuration supported on a polished Cu foil (7 mA cm−2 at −0.2 V vs. RHE.). The 3D composite structure was synthesized by depositing Ni and CrOx on the 3D CuO nanowire scaffold, while the 3D CuO nanowire was grown on a copper foam using a thermal treatment method. Another typical example is the construction of a three-dimensional core–shell structure. Our previous work demonstrated a 3D shell@core catalyst for the neutral HER (Fig. 11a), which was synthesized by growing an amorphous material composed of Ni, Mo, and P on Cu nanowires (NiMoP@CuNWs).237 The exceptional hydrogen evolution reaction (HER) activity is attributed to the unique amorphous rod-like nature of NiMoP@CuNWs with a special hydrophilic feature, and more exposed active sites contribute to enhanced mass transfer and promote effective contact between the electrode and the electrolyte solution. Consequently, this 3D NiMoP@CuNWs catalyst only requires an overpotential of 35 mV to reach a current density of 10 mA cm−2. Additionally, Zhu's team157 synthesized 3D hierarchical NiCo2Se4 nanosheets via selenization of porous NiCo2O4 nanoneedles on carbon fiber paper (CFP) (Fig. 11b). The nanosheets exhibit a “non-layered” ultrathin morphology with structural distortion, abundant dangling bonds, and unsaturated surface atoms, maximizing active site exposure and interfacial charge transfer. The three-step synthesis involves: hydrothermal growth of NiCo2(CO3)1.5(OH)3 nanoneedles on CFP, annealing to form porous NiCo2O4 precursors, and selenization to create hierarchical NiCo2Se4 nanosheets with Se vacancies and structural reconstruction. The unique architecture enhances both thermodynamics (Se vacancies modulate the electronic structure for optimal HER hydrogen adsorption) and kinetics (COMSOL simulations show that nanosheet edges promote charge aggregation, surpassing nanoneedle “tip effects”). As a result, for the 3D hierarchical NiCo2Se4, only 92 mV is required to reach 100 mA cm−2 for the neutral HER.
Typically, synthesis routes for 3D structures include: (i) Metal–organic frameworks (MOFs) and their derivatives.44,172,238–240 MOFs doped with Fe, Co, or Ni are pyrolyzed to produce porous carbon backbones embedded with metal or metal oxide nanostructures. (ii) Load on 3D substrates.241–246 Catalysts such as Ni–Fe, Co–P, and Mn–S are grown on conductive 3D scaffolds such as nickel/copper foam, nanorod/nanowire, or porous carbon spheres. (iii) Template-assisted methods.247,248 Soft or hard templates are used to sculpt hierarchical porosity into metal oxides or composites, later removed post-synthesis. Due to the large specific surface area provided by this 3D structure and its facilitation of material transport, this structure can be considered when designing catalysts for high current density. Beyond high surface area, this structure also relieves mechanical stress and is beneficial for practical use.
The structural design of multi-site electrocatalysts is deeply intertwined with their functional behavior under neutral water electrolysis conditions. Three-dimensional systems address bulk diffusion and electrode integration; two-dimensional sheets emphasize site exposure and interfacial tuning; one-dimensional nanostructures enhance directional conductivity and bubble release; zero-dimensional nanostructures facilitate precise control of the reaction sites. Each structural class comes with distinct synthetic strategies, advantages, and limitations. Increasingly, hybrid architectures that combine these dimensionalities, such as 0D particles on 2D supports or 1D arrays embedded in 3D scaffolds, are gaining attention for maximizing the HER performance under neutral pH. Looking forward, key challenges lie on the controllable preparation of catalysts, including scalable fabrication of hierarchical and hybrid structures, fast and low-cost synthetic methods for efficient catalysts, precise control of inter-site distances and spatial heterogeneity, and in situ monitoring of structural evolution under real electrolysis conditions. Developments in advanced synthetic chemistry and operando characterization will be essential to fully exploit the structural advantages of multi-site catalysts and translate them into efficient, durable, and economically viable solutions for green hydrogen production in neutral environments.
5. Characterizations and physicochemical property analysis of multi-site electrocatalysts
Characterizing the morphology and structure of the designed catalysts is pivotal for elucidating the synthesis–structure–performance relationships that dictate the activity. Such characterizations are also essential for unraveling reaction mechanisms and understanding active site interactions at the nano- and atomic scales. Additionally, the rational design and effective application of multi-site electrocatalysts which contain various catalytic active sites require a comprehensive understanding of their physicochemical properties. Advanced techniques, which are used to probe the morphology, structure, performance, and reaction mechanism of the obtained catalysts, form the foundation for linking structure to performance, guiding material optimization, and depicting reaction process. This section delves into these methodologies, emphasizing their role in the comprehensive understanding of multisite electrocatalysts (Fig. 12). | ||
| Fig. 12 Schematic of the characterization and analysis techniques for understanding electrocatalysts for the neutral HER. | ||
5.1. Morphology observation
Understanding the physical architecture of a catalyst is essential, particularly for multi-site systems where phase distribution and interface structure influence electrocatalytic behavior. Morphological features such as microstructure, surface roughness, porosity, and exposed facets affect both mass transport and electrochemical active surface area. Multi-site electrocatalysts, particularly those operating in neutral electrolyte water electrolysis systems, e.g., CoFe-LDH,249 IrOx/NiOOH,250 and Ru-based251 catalysts, often possess nanoscale heterostructures and distributed active centers that demand high-resolution observations. Tools such as scanning electron microscopy (SEM), transmission electron microscopy (TEM), scanning transmission electron microscopy (STEM), electron energy loss spectroscopy (EELS), and atomic force microscopy (AFM) are essential for understanding the spatial arrangement and interactions of multiple active sites that define catalytic performance.Typically, SEM is used as an initial diagnostic tool to visualize macro- and micro-scale morphology, including particle size distribution, nanostructure growth patterns, and coating uniformity.252 For instance, the work reported by Shi et al.253 demonstrated that scanning electron microscopic images during neutral seawater electrolysis showed that interwoven nanowires were formed in situ inside the phosphorylated Ni foam (Fig. 13a and b). For a more in-depth study, internal features such as crystallinity and grain boundaries were analyzed by TEM and HRTEM. It is obvious from the TEM images that PtSA–Ni6.6Fe0.4P3 is a framework of interwoven nanowires with a diameter of about 60 nm (Fig. 13c), and no apparent lattice stripes on the PtSA–Ni6.6Fe0.4P3 nanowires were observed (Fig. 13d). The HRTEM image of PtSA–Ni6.6Fe0.4P3 (inset is the corresponding fast Fourier transform (FFT) image) also shows diffuse central spotting, which also suggests that it maintains an amorphous phase. Additionally, STEM, especially in the high-angle annular dark field (HAADF) mode, further enables this by realizing z-contrast imaging and atomic-level resolution. Meanwhile, when STEM is combined with energy-dispersive X-ray spectroscopy (EDX), spatially resolved elemental mapping becomes possible. In a study on PtSA–Ni6.6Fe0.4P3, its HAADF-STEM image and the corresponding EDS elemental mapping analysis confirmed the presence and homogeneous distribution of Pt, Ni, Fe, and P on the nanowires (Fig. 13e). HRTEM and HADDF-STEM are also used to probe the exposed crystal planes of the material. It is worth noting that electron microscopic images are localized for this analysis; and for high-resolution electron microscopy techniques, the preparation process of the target sample influences the final result. Therefore, the sample preparation process used for high-resolution electron microscopy is encouraged to be described in detail in the Methods section.
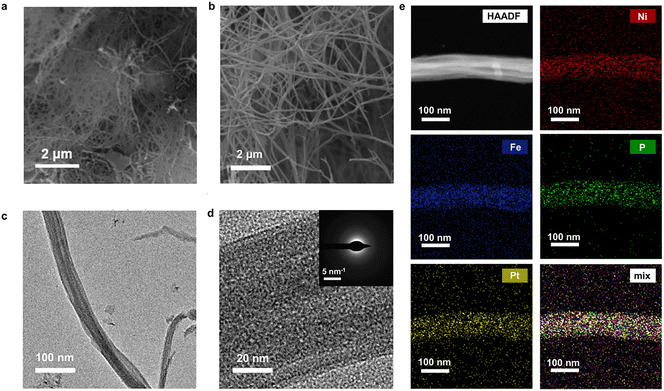 | ||
| Fig. 13 (a) SEM images of Ni6.6Fe0.4P3 and (b) PtSA–Ni6.6Fe0.4P3. (c) TEM image of PtSA–Ni6.6Fe0.4P3. (d) HRTEM image of PtSA–Ni6.6Fe0.4P3, with the inset showing the corresponding FFT diffraction. (e) HAADF-STEM image of PtSA–Ni6.6Fe0.4P3 with corresponding EDS elemental mapping analysis. Reprinted with permission from ref. 253. Copyright 2023, The Authors. | ||
In order to reveal the oxidation state and energy transformations, SEM/TEM alone is not enough to demonstrate the characterization, while EELS provides nanoscale electronic structure maps and fine structures that can differentiate between coordinated environments, and is one of the indispensable means of characterization of neutral electrolytic water. With EELS, for example, Huang et al.254 investigated the oxygen-doped chemical states and discovered that the Co-L fringes (L3 and L2) of Co9S8-O-300 shift significantly toward higher energy loss than that of pristine Co9S8, and the L3/L2 ratio is observed to increase from 3.4 to 4.5, which suggests that the Co atoms formed higher oxidation states because of the incorporation of oxygen. The O-K fringe spectrum at around 529.9 eV shows a distinct leading-edge peak attributed to the hybridization signals of the O 2p and Co 3d orbital electrons, confirming the presence of lattice oxygen atoms in Co9S8. This is crucial for the determination of the chemical state in neutral medium catalysis. Besides, AFM technique complements electron microscopy by providing true spatial and 3D surface profiles with nanometer resolution. It is used to characterize the physical properties of catalysts such as surface roughness and porosity that affect catalyst wetting, gas release, and exposure of active sites. The AFM provides an effective method for identifying atomic configurations on the surfaces of planar conductive samples and serves as an effective way to understand the model materials that actually support the catalyst system.
The integration of SEM, TEM, STEM, EELS, and AFM forms a powerful toolset for deciphering the physicochemical intricacies of multi-site electrocatalysts, particularly in neutral pH electrolytic environments where subtle interactions among active centers determine the overall performance. In practical studies of materials, these methods demonstrate how these techniques can be combined to produce a complete picture from macroscopic textures to atomic-scale functions. Such integrated approaches are essential for the rational design of high-performance catalysts that balance activity, durability, and selectivity. However, in situ morphology observation techniques are highly expected to be exploited for understanding material structure evolution and catalytic reaction processes. In addition to the aforementioned technologies, new nano-imaging technologies applied in other fields have come into view and are worthy of development, e.g., synchrotron radiation nano-imaging technology,255–258 fluorescence imaging technology,259,260 electrochemical imaging technology,261–263 ultrasonic imaging technology,264,265 and electron tomography technology.1,266–268
5.2. Structural characterization
Unlike imaging techniques, which primarily describe morphology and atomic arrangements, structural characterization provides essential information about the crystalline phase, amorphous features, defect distribution, coordination environment, and electronic configuration. This is particularly vital for establishing the structural framework of multi-site electrocatalysts, describing structure–performance relationships, and understanding how diverse active centers in multi-site electrocatalysts synergize under the dynamic conditions of neutral water electrolysis. In neutral water splitting systems, the catalytic activity is usually affected by subtle changes in valence state, localized defects, short-range (dis)ordering, and surface reaction environments. Therefore, a combination of various spectroscopic tools such as X-ray diffraction (XRD), pair distribution function (PDF) analysis, Mössbauer spectroscopy, X-ray photoelectron spectroscopy (XPS), X-ray absorption fine structure (XAFS) spectroscopy, time-of-flight secondary ion mass spectrometry (TOF-SIMS), Raman spectroscopy, electron paramagnetic resonance (EPR) spectroscopy, photoluminescence (PL) spectroscopy, UV-visible-near-infrared (UV-VIS-NIR) absorption spectroscopy, and nuclear magnetic resonance (NMR) spectroscopy are required. The combined use of these tools allows for a more comprehensive assessment of the physicochemical properties of the material.XRD is essential for determining the crystalline phase, strain and grain size in electrocatalysts. For example, in Ir–HxWO3 composites, the WO3, HxWO3 and Ir–HxWO3 electrocatalysts have well-defined hexagonal WO3 diffraction peaks (JCPDS 85-2460), but no diffraction peaks for HxWO3 (Fig. 14a).173 XRD spectra revealed the crystalline phases of the synthesis process, allowing researchers to relate structural transformations to electrocatalytic activity. However, many of the active materials in neutral electrolysis are with low crystallinity. In this case, PDF analysis becomes essential to address short-range atomic arrangements that cannot be resolved by XRD. PDF analysis, derived from total X-ray or neutron scattering, captures both crystalline and amorphous structures. It is uniquely valuable for characterizing disordered or amorphous catalysts in numerous systems where the active sites may lack long-range order. In addition to this, Mössbauer spectroscopy can reveal the short-term order of materials. For example, this spectroscopy provides a precise probe for iron-containing catalysts, which can distinguish the Fe2+/Fe3+ species, high-spin/low-spin states, and electronic environments.270–272 Thus, XRD reveals that the phase of the material, PDF analysis and Mössbauer spectroscopy can expose the hidden short-range ordering of the material, and the combination of all the three can play a complementary role in characterizing the phase of the material and further elucidating the structural information of the material. However, the main drawback of Mössbauer spectroscopy is that only a limited number of nuclei exhibit the Mössbauer effect, and many of them must be measured at low temperatures or in experimental environments with specific preparation conditions, which imposes significant limitations on its application. In fact, only a few nuclei such as 57Fe and 119Sn have been fully utilized to date. Despite huge limitations, Mössbauer spectroscopy remains one of the important methods in material analysis. With the further development of experimental techniques, it can be expected that it will continuously overcome its limitations and play a more important role in various research fields.
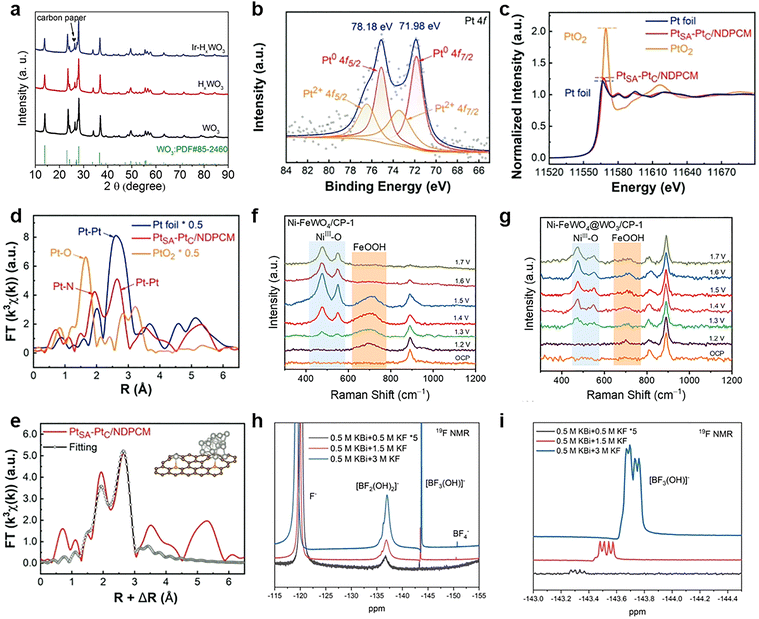 | ||
Fig. 14 (a) XRD patterns of as-obtained Ir–HxWO3 and compared samples. Reprinted with permission from ref. 173. Copyright 2023, The Authors. (b) High-resolution XPS spectra of Pt 4f for PtSAPtC/NDPCM. (c) XANES spectra and (d) corresponding k3-weight FT-EXAFS of the normalized Pt L3-edge of Pt foil, PtSA–PtC/NDPCM and PtO2. (e) EXAFS fitting curves of PtSA–PtC/NDPCM in the R space of the Pt L3-edge. Inset: The atomic models of PtSA–PtC/NDPCM. Reprinted with permission from ref. 269. Copyright 2023, Wiley-VCH. In situ Raman spectra of (f) Ni–FeWO4/CP-1 and (g) Ni–FeWO4@WO3/CP-1 under different operated potentials versus RHE. Reprinted with permission from ref. 117. Copyright 2023, Wiley-VCH. (h) 19F NMR spectra of mixed KBi and KF. (i) Zoom-in 19F NMR spectra of BF2(OH)2−, which shows the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 splitting due to B–F coupling. Reprinted with permission from ref. 120. Copyright 2023, Wiley-VCH. 1 splitting due to B–F coupling. Reprinted with permission from ref. 120. Copyright 2023, Wiley-VCH. | ||
The surface sensitivity of XPS makes it well suitable for analyzing oxidation states and elemental distributions at different catalytic sites. The variation in binding free energies provides evidence for charge transfer interactions between poly-metals (e.g., nickel and iron or cobalt and manganese) that are known to modulate the OER and HER activities in neutral environments. Complementarily, XAFS (both X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS)) spectroscopy extends this analysis to block and provide element-specific insights into coordination numbers and bond lengths for the overall catalyst. For example, combined XPS and XAFS studies of the PtSA–PtC/NDPCM system (Fig. 14b–e) have shown that catalytic valence transitions occur during the catalytic process, and that two different states of PtSA and PtC coexist in PtSA–PtC/NDPCM.269 In addition, the TOF-SIMS technique provides a powerful tool for detecting the components of the surface/bulk catalyst. For instance, by analyzing the species distribution of the NiCoP–Cr2O3 catalysts by TOF-SIMS, Sha et al.10 pointed out that the PO4− group was in situ formed after the shutdown of the intermittent power supply, which was conducive to resist the corrosion of Cl− in seawater.
Raman and EPR spectroscopy provide important insights into vibrational and magnetic properties. Raman spectroscopy can identify metal–oxygen (M–O) bonding modes in layered double hydroxides (Fig. 14f and g) and defect states in doped carbon.117 The manipulation of Raman can also reveal surface intermediates such as *OOH or *OH.250 However, EPR is very sensitive to unpaired electrons, which helps to identify radical species, verify the generation of radicals, or detect oxygen vacancies in the catalyst. These features often serve as fingerprint indicators of catalytic reactivity and defect-mediated pathways.273
UV-VIS-NIR spectroscopy and spotlight spectroscopy provide information on the electronic energy band structure, which is important for semiconductor or hybrid catalysts. For example, narrowing of the optical band gap indicates enhanced light absorption and charge carrier generation in MOF-derived or carbon nitride catalysts. Concentrated light quenching in nitrogen-doped systems reflects improved charge separation and recombination suppression critical for catalytic efficiency at applied potentials. These optical methods provide insights into how doping, structural disorder, or heterojunction formation tunes the charge transfer dynamics in neutral-pH systems. In addition, solid-state NMR can provide valuable structural insights into catalysts with organic ligands or MOF-derived components. 13C and 15N NMR can detect the chemical environment of heteroatoms in nitrogen-doped carbon carriers. In MOF-derived catalysts, the shift of the NMR peaks reveals changes in coordination between the metal center and the organic linker before and after electrolysis. 1H NMR can also be used to detect water or hydroxide species adsorbed on the catalyst surface, which will affect proton-coupled electron transfer reactions, especially under neutral conditions. For example, the key fluoroborate species (BF2(OH)2− anion) formed in mixed KBi/KF (KBi = potassium borate) electrolytes (Fig. 14h and i) increase the rate of OER under near-neutral pH conditions, leading to high catalytic activity of high-abundance electrocatalysts under near-neutral conditions.120
Spectroscopic techniques are indispensable in decoding the structure–function relationships of neutral water separation multi-site electrocatalysts. By integrating structural, surface, electronic, and magnetic/proton characterization, a multidimensional understanding of active sites, support interactions, and degradation mechanisms can be constructed, which will facilitate the rational design of next-generation catalysts with optimized activity, selectivity, and long-term durability under wild operating conditions. For example, the lattice structure and inner strain field of an electrocatalyst have a significant impact on its electronic configuration and reactivity. Typically, XRD, Raman spectroscopy, and HR-TEM with geometric phase analysis, and EXAFS analysis are commonly used to jointly probe lattice strain. In addition, the strain shifts the d-band center of the transition metal-based catalyst towards/away from the Fermi energy level. The upward or downward shift of the d-band center essentially serves to bring the hydrogen adsorption strength closer to the ideal value.274–276 The detailed cases are as follows: MoS2 was successfully converted from a semiconductor to a metal by tensile strain, accelerating the charge transfer between the catalyst and the reactants.277 Similarly, the structure and strain of PS–Cu after acid treatment showed lattice enlargement and tensile strain, which exhibited more excellent catalytic activity compared to commercial copper nanoparticles (Cu NPs) and copper foams.278
Nevertheless, in order to accurately capture the structural evolution of multi-site catalysts in the neutral HER, the development of spectroscopic techniques is expected to perform collaborative measurements in space and time: (i) pushing the limits of spatial resolution by moving towards the nanoscale or even atomic scale (e.g., nano-XAFS) to distinguish and resolve the unique behavior of different active sites and (ii) improved temporal resolution by moving towards millisecond, microsecond and femtosecond scales (e.g., ultrafast/energy-dispersive XAFS and transient absorption spectroscopy) to capture fast dynamic processes (intermediate adsorption/desorption, valence fluctuations, and catalyst reconstruction).
5.3. Performance evaluation
To assess the influence of structural design and synthesis strategy on the catalysts, a reliable and meaningful assessment of HER performance under neutral conditions is necessary, which calls for a rigorous application of quantitative electrochemical metrics. These metrics allow researchers to benchmark catalytic activity, elucidate mechanistic behavior, and guide the rational design of catalysts to overcome the intrinsic limitations of neutral-pH environments. To evaluate the electrochemical performance of a catalyst, a series of tests and calculations are required for the as-prepared catalyst to derive several evaluation metrics. The most used metrics include overpotential, Tafel slope, turnover frequency (TOF), electrochemical specific surface area (ECSA), faradaic efficiency (FE), and stability.CV and LSV curves are commonly collected on an electrochemical workstation to assess the overpotential of the sample; however, mass transport limitations and solution resistance may inflate this value, and thus iR correction (ohmic drop compensation) is essential for fair comparisons. Therefore, researchers should clearly specify which electrochemical workstation and method to be used for iR correction, such as manual compensation based on resistance values, or 60%–100% automatic compensation. Additionally, providing non-compensated polarization curves for the catalyst performance is important to the overall assessment of data reliability. Moreover, a moderate compensation value (80%–90%) is necessary because an excessively large compensation value will lead to over-correction of the actual performance, while a low compensation value is insufficient to reflect the actual performance. Another important matter is the sweep speed during the CV/LSV measurement. A fast sweep speed will significantly increase the charging current (non-Faraday current) of electrical double layer at the electrode/solution interface, resulting in the misjudgement of performance. When evaluating the performance, a sweep speed of less than 5 mV s−1 is necessary.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) j). The Tafel slope is derived from the Tafel equation: η = a + b*log
j). The Tafel slope is derived from the Tafel equation: η = a + b*log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) j, where b is the Tafel slope, j refers to the current density, and a is a constant. It is worth mentioning that the Tafel equation was an empirical formula proposed by Julius Tafel in 1905.280 Through studying the HER process, Tafel was the first to quantitatively describe the electrode kinetics process using the above-mentioned empirical formula. Later, Butler and Volmer assumed that the steps of gaining or losing electrons were elementary steps; and by applying the transition state theory and the Nernst equation in chemical kinetics, they derived a more fundamental equation of electrode process kinetics: Butler–Volmer equation (B–V equation). For the HER, when the cathode overpotential is large, the B–V equation can be simplified to the Tafel equation. A smaller Tafel slope indicates a more rapid increase in current density with a small increment in applied potential, reflecting superior kinetic efficiency in electrocatalytic process. Here, the charge transfer resistance (Rct) of catalyst-coated electrodes measured by electrochemical impedance spectroscopy (EIS) is often used as a supplement to assess the reaction kinetics. For example, IrOx/Ni(OH)2 was analyzed by EIS and found to increase the electron transfer at the IrOx/Ni(OH)2 interface, presenting a smaller charge transfer resistance in 0.5 M KHCO3 (Fig. 15a).250 Similarly, Co-based MOF-derived catalysts benefit from a highly conjugated framework that promotes electron delocalization and inter-site connectivity.281 Furthermore, the Tafel slope serves as a diagnostic tool for identifying the rate-determining step in the electrocatalytic reaction.282–286 When Tafel slope is ca. 30 mV dec−1, the Tafel step (chemical recombination) is rate-determining; when the Tafel slope is ca. 40–60 mV dec−1, the Heyrovsky step (electrochemical desorption) dominates; and when the Tafel slope is ca. 120 mV dec−1, the Volmer step (electrochemical water dissociation) is limiting. In neutral media, HER typically exhibits Tafel slopes >120 mV dec−1, indicating a Volmer-limited mechanism due to sluggish water dissociation.
j, where b is the Tafel slope, j refers to the current density, and a is a constant. It is worth mentioning that the Tafel equation was an empirical formula proposed by Julius Tafel in 1905.280 Through studying the HER process, Tafel was the first to quantitatively describe the electrode kinetics process using the above-mentioned empirical formula. Later, Butler and Volmer assumed that the steps of gaining or losing electrons were elementary steps; and by applying the transition state theory and the Nernst equation in chemical kinetics, they derived a more fundamental equation of electrode process kinetics: Butler–Volmer equation (B–V equation). For the HER, when the cathode overpotential is large, the B–V equation can be simplified to the Tafel equation. A smaller Tafel slope indicates a more rapid increase in current density with a small increment in applied potential, reflecting superior kinetic efficiency in electrocatalytic process. Here, the charge transfer resistance (Rct) of catalyst-coated electrodes measured by electrochemical impedance spectroscopy (EIS) is often used as a supplement to assess the reaction kinetics. For example, IrOx/Ni(OH)2 was analyzed by EIS and found to increase the electron transfer at the IrOx/Ni(OH)2 interface, presenting a smaller charge transfer resistance in 0.5 M KHCO3 (Fig. 15a).250 Similarly, Co-based MOF-derived catalysts benefit from a highly conjugated framework that promotes electron delocalization and inter-site connectivity.281 Furthermore, the Tafel slope serves as a diagnostic tool for identifying the rate-determining step in the electrocatalytic reaction.282–286 When Tafel slope is ca. 30 mV dec−1, the Tafel step (chemical recombination) is rate-determining; when the Tafel slope is ca. 40–60 mV dec−1, the Heyrovsky step (electrochemical desorption) dominates; and when the Tafel slope is ca. 120 mV dec−1, the Volmer step (electrochemical water dissociation) is limiting. In neutral media, HER typically exhibits Tafel slopes >120 mV dec−1, indicating a Volmer-limited mechanism due to sluggish water dissociation.
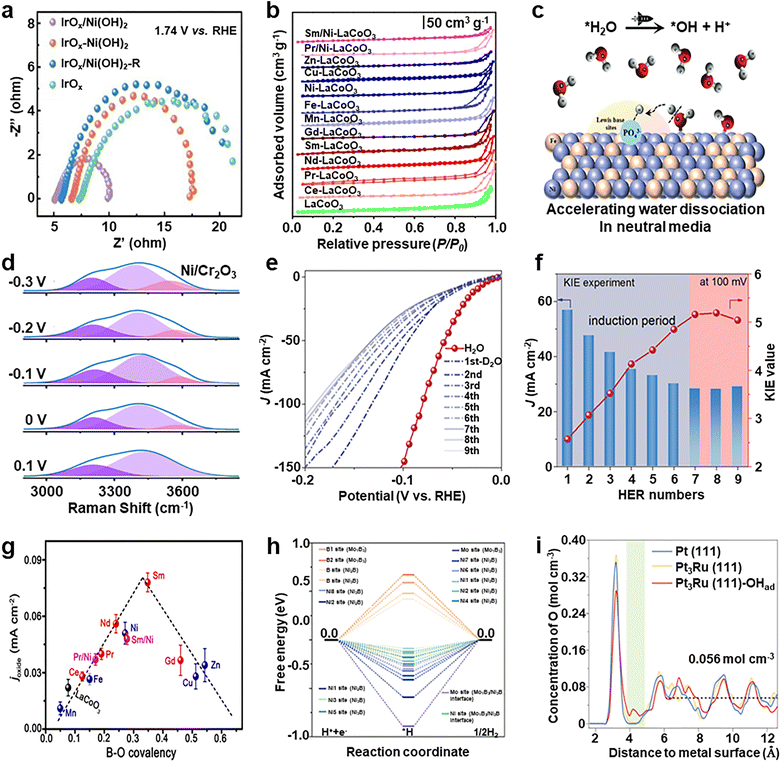 | ||
| Fig. 15 (a) Nyquist plots of different catalysts at 1.74 V vs. RHE in 0.5 M KHCO3. Reprinted with permission from ref. 250. Copyright 2023, Wiley-VCH. (b) BET surface areas of 13 TNPOs measured by nitrogen adsorption–desorption isotherms. Reprinted with permission from ref. 287. Copyright 2022, American Chemical Society. (c) Schematic of accelerating interface H2O dissociation induced by Lewis's base-PO43− in neutral media. Reprinted with permission from ref. 288. Copyright 2024, Wiley-VCH. (d) In situ Raman spectra for Ni/Cr2O3 catalysts at different cathodic potentials. Reprinted with permission from ref. 289. Copyright 2025, American Chemical Society. (e) LSV curves of Ir–HxWO3 in a 1.0 M PBS (H2O and D2O) electrolyte. (f) Calculated KIE values (JH2O/JD2O) under 100 mV overpotential. Reprinted with permission from ref. 173. Copyright 2023, The Authors. (g) Volcano-type relationship between the neutral-pH OER activity and the B–O covalency. Reprinted with permission from ref. 287. Copyright 2022, American Chemical Society. (h) Energy profiles to determine the HER active sites of different catalyst interfaces. Reprinted with permission from ref. 160. Copyright 2024, Wiley-VCH. (i) The concentration distribution profiles of O atoms of water along the surface normal direction resulting from the AIMD-simulated interface structures on different catalysts. Reprinted with permission from ref. 290. Copyright 2022, The Authors. | ||
It should be noted that the Tafel slope value can be affected by mass transport, Edl thickness, and bubble adhesion, especially at low buffer concentrations. Care must be taken to analyze Tafel slopes in the low overpotential region where kinetic control dominates. It is a relatively reliable method to obtain the Tafel slope near j = 10 mA cm−2 to evaluate the reaction kinetics. Furthermore, the reaction process can be analyzed by comparing the Tafel slopes at different current densities. In any case, standardizing the selection of the current density is necessary when using the Tafel slope to analyze reactions.
Large (electrochemical) specific surface area contributes to improved catalyst utilization, especially in atomically dispersed systems, where maximizing accessibility is critical. It is worth noting that by normalizing the polarization curves using ECSA, the intrinsic activity of individual catalyst sites can be obtained. An optimized catalyst should exhibit many catalytic active sites (related to the morphology and structure) and high intrinsic activity per site (related to chemical properties); therefore, we suggest researchers to present the normalized polarization curves of the catalysts based on their (electrochemical) specific surface area.
Ensuring complete bubble collection and accurate volumetric measurements is critical for reliable FE values. Notably, when using the water displacement method, special attention must be paid to eliminating the influence of the air column generated by the initial atmospheric pressure on the subsequent gas volume. More standard quantification methods such as GC, mass spectrometry, and hydrogen sensing are thus recommended for use. Generally, a higher FE indicates better catalytic selectivity of the electrocatalyst. However, when dealing with gas products, one should not merely focus on measuring the content of the target gas (H2 or O2). Instead, the contents of hydrogen in oxygen and oxygen in hydrogen should also be measured simultaneously. This is because both of these situations can pose safety hazards and lead to a rapid decline in product quality.
An increasingly important metric in the evaluation of electrocatalyst durability is S-number, or stability number. Originally proposed in the context of oxygen evolution catalysts, it is now being extended to HER systems, particularly under conditions where catalyst degradation or metal dissolution limits long-term performance. The S-number is defined as the molar amount of product generated per mole of active element lost: S-number = nH2/ndissolved![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) metal, where nH2 is the total moles of hydrogen evolved and ndissolved
metal, where nH2 is the total moles of hydrogen evolved and ndissolved![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) metal is the moles of catalyst component lost into the electrolyte, typically measured via ICP.296 Higher S-numbers indicate better stability, that is, more hydrogen is produced per unit of catalyst loss. For HER catalysts, especially those containing transition metals such as Ni, Co, Fe, and Mo, which are vulnerable to leaching in near-neutral electrolytes, the S-number serves as a powerful normalization tool for comparing stability across different systems and compositions. To accurately determine the S-number, quantitative H2 evolution must be measured (via gas chromatography or mass flow meters), electrolyte samples should be periodically collected and analyzed for dissolved catalyst elements using ICP techniques, and long-term tests should be conducted during chronoamperometry and chronovoltammetry operation.
metal is the moles of catalyst component lost into the electrolyte, typically measured via ICP.296 Higher S-numbers indicate better stability, that is, more hydrogen is produced per unit of catalyst loss. For HER catalysts, especially those containing transition metals such as Ni, Co, Fe, and Mo, which are vulnerable to leaching in near-neutral electrolytes, the S-number serves as a powerful normalization tool for comparing stability across different systems and compositions. To accurately determine the S-number, quantitative H2 evolution must be measured (via gas chromatography or mass flow meters), electrolyte samples should be periodically collected and analyzed for dissolved catalyst elements using ICP techniques, and long-term tests should be conducted during chronoamperometry and chronovoltammetry operation.
Stability in the neutral HER is often compromised by surface reconstruction or leaching in mildly corrosive environments, bubble-induced delamination or contact loss, salt precipitation (e.g., carbonate scaling in bicarbonate electrolytes), and ion contamination particularly from multivalent cations such as Mg2+ or Ca2+ in natural (sea)waters. Common catalyst degradation pathways include metal leaching, support decomposition, and active site poisoning. Post-reaction examination of the catalyst using several sensible characterization methods such as inductively coupled plasma-mass spectrometry (ICP-MS) and XPS depth profiling can be used to quantify durability. For example, NiFeP/NiS-A assemblies of neutral seawater in electrolyzer can be operationally stable at 1.8 V@1.0 A cm−2 (60 °C) for over 220 hours. ICP-MS and in situ electrochemical Raman spectra and FTIR spectrum were used to study the origin of stability. The adsorption of H2O intermediates by the Ni(Fe)OOH active phase and the synergistic interaction with the amphiphilic Lewis base PO43− facilitate the dissociation of water molecules (*H2O → *OH + H+ + e−) and ensure a timely supply of *OH to the neutral medium (Fig. 15c). Meanwhile, PO43− having shown excellent results in the seawater decomposition also exhibits an excellent ability to repel chloride ions, achieving an O2 faradaic efficiency of about 98.2% and excellent seawater corrosion resistance.288
For a complete picture, stability testing should be paired with faradaic efficiency tracking, operando surface analysis, and ideally extended to two-electrode MEA systems under near-practical conditions. Furthermore, to approach application, the stability of the catalyst under intermittent power supply needs to be urgently studied, where the insight and mechanism are known little.
5.4. Mechanism analysis
Understanding the catalytic mechanism of the neutral HER is critical for the rational design and optimization of multi-site electrocatalysts. The concrete content is discussed in Section 2. The complexity of the neutral HER makes mechanistic studies challenging. Yet, such understanding is indispensable for decoding how multi-site electrocatalysts achieve synergistic catalytic processes, e.g., by spatially separating water dissociation from hydrogen recombination, or stabilizing key reaction intermediates through multi-function active sites. To elucidate these mechanisms, several advanced tools must be employed, such as in situ/operando spectroscopies, electrochemical analysis, and computational modeling.Furthermore, operando XAFS, including XANES and EXAFS, enables real-time tracking of the oxidation states and coordination environments of catalytic centers under applied bias. This is especially valuable in multi-site systems where redox dynamics (e.g., Co2+/Co3+ or Ni2+/Ni3+ transitions) plays a role in active site formation or regeneration, especially for the OER process.38,241 For example, Fabbri and co-workers38 developed the time-resolved operando hard XANES to track the Co oxidation state in electrolytes with different pH values. The change in Co oxidation state is measured by the energy shift of the Co K edge between the initial state and another state at the operating time. Combined with other characterizations and analyses, it is proposed that alkaline environments provide a low flat band potential that yields a low-potential Co redox transformation, in favor of surface reconstruction. Neutral and acidic environments afford an anodic shift of the Co redox transformation, leading to the increased catalytic overpotential. The highest overpotential at neutral pH is attributable to poor Co atom polarizability and slow Co oxidation state changes.
Electrochemical TEM/STEM is helpful to directly observe the morphological evolution of catalysts. These observations are essential for confirming whether the active site remains intact, reconstructs into a more active phase, or undergoes dissolution during operation.298–300 The hydrophilicity of the catalyst during the reaction is also worthy imaging because the extremely low reactant concentration suppresses the activity of neutral HER. For example, the intrinsic wettability of graphene exhibits an extremely low water contact angle (mean value of ca. 30°) after excluding the interference of the substrates and contaminants, and this hydrophilicity is caused by charge transfer between graphene and water based on H–π interactions.301 Furthermore, differential electrochemical mass spectrometry (DEMS) coupled with isotope labeling experiments can precisely track gas evolution (e.g., H2, O2) with high sensitivity and time resolution, which provide insights into the reaction mechanism, especially for the OER process.49,241
Mechanistic analysis of multi-site electrocatalysts for the neutral HER requires a synergy of advanced spectroscopic, electrochemical, and computational techniques. These tools together reveal how individual active sites contribute to distinct reaction steps, how interfaces and support interactions modify local reaction environments, and how catalyst dynamics under operating conditions impact overall performance. Deep mechanistic understanding will be critical for the rational design of next-generation neutral HER catalysts with industrial application potential.
6. Toward applications of neutral water electrolysis from the laboratory chamber to the practical water electrolyzer
The design of the electrocatalyst is ultimately verified in the electrolyzer to determine its application prospects. However, most commercial electrolyzers operate in either acidic or alkaline media owing to the property of the ion-exchange membrane used. The development of neutral pH electrolyzers is considered a breakthrough for cost-effective and stable hydrogen production. Transitioning from laboratory-scale experiments to practical water electrolyzers requires careful integration of electrode materials, membranes, and electrolytes that balance the performance requirements of both the HER and the OER, especially under neutral conditions. At the extremely low concentrations of protons and hydroxide ions in neutral electrolytes, researchers have employed both the proton exchange membrane (PEM) and the anion exchange membrane (AEM). This section explores the performance testing, operational dynamics, and challenges of different membrane electrode assembly-based water electrolyzer (MEAWE) configurations, including PEM-based water electrolyzers (PEMWEs) and AEM-based water electrolyzers (AEMWEs) using neutral electrolytes.6.1. Performance test from the three-electrode laboratory chamber to the two-electrode MEAWE
The transition from three-electrode laboratory systems to two-electrode setups is essential for moving toward practical water electrolysis systems. The three-electrode system, typically employed for fundamental studies and catalyst screening, involves a working electrode, a counter electrode, and a reference electrode. In this configuration, the reference electrode provides a stable potential against which the working electrode's potential is measured, allowing precise tracking of CV, LSV, Tafel slopes, EIS, and faradaic efficiency for both the HER and the OER.However, in practical applications, considering the final power consumption, two-electrode systems more closely represent real electrolyzers, where the reference electrode is eliminated, and both the anode and cathode are connected to the power supply directly. This setup better reflects the overall system performance, as it accounts for voltage losses that occur during water splitting in real-world devices. In addition, it simplifies the measurement of cell voltage and current density, which are key metrics in electrolyzer performance. The transition to two-electrode testing is pivotal to simulate realistic operational conditions. In addition, a two-electrode MEAWE device allows a more direct evaluation of the catalyst's stability and overall efficiency when scaled up (Fig. 16).
Therefore, we strongly encourage the use of two-electrode systems rather than three-electrode systems when conducting electrolyzer tests, as the former more accurately simulates industrial operating conditions. In the following sections, the application of PEMWE and AEMWE devices is discussed for hydrogen production via neutral water electrolysis. It is important to note that we do not consider the already-commercialized alkaline water electrolyzers (AWE), which typically employ composite diaphragms based on polyphenylene sulfide (PPS) fabrics as separators. This exclusion is because traditional alkaline electrolyzers, which are generally designed with non-zero-gap architectures, tend to exacerbate the kinetic limitations associated with both the HER and the OER under neutral conditions, thus rendering them less promising for future neutral water electrolysis applications.
6.2. PEMWE devices with the supply of neutral electrolytes
PEMWE is one of the most commercially viable technologies for water electrolysis. These devices typically operate in acidic environments, where the proton-conducting polymer membrane (e.g., Nafion) facilitates proton transport between the anode and the cathode. The acidic environment is generally favorable for high conductivity and efficient PCET during both the HER and the OER. In typical PEMWE operation, only pure water is supplied to the anode without the addition of any electrolyte; the protons (H+) generated at the anode (eqn (15)) are transported through the PEM to the cathode for the HER. One of the foremost challenges in the development of PEMWE lies in that the commonly used non-noble metal-based electrocatalysts, which exhibit high water-splitting activity, are not stable under acidic conditions. This degradation significantly contributes to the high cost of PEMWE systems using noble metals (Ru/Ir/Pt) as the catalyst.Alternatively, the use of neutral electrolytes in PEMWE feeding both the anode and the cathode can maintain the neutral pH condition around the electrode. It shows great potential benefits to inhibit the intrinsic problem of non-noble metal dissolution, which probably lower the cost of PEMWE. However, a critical consideration when using neutral electrolytes (especially buffer solutions) instead of pure water in PEMWE is whether the significantly lower proton concentration leads to a substantial decrease in reactivity. That is, PEM designed for acidic conditions (e.g., Nafion) are not always suitable for neutral pH. The proton conductivity of the membrane tends to decrease in neutral electrolytes, leading to reduced efficiency. Efforts to optimize membrane materials are a promising area of research for neutral PEMWE operation. Thus, neutral PEMWE devices face the challenge of maintaining high current density, sufficient conductivity, and stability of catalysts while minimizing cost. For instance, Rossi and co-workers proposed an approach to improve PEMWE performance by using a humidified gas stream (no liquid electrolyte) for the anode and a liquid saltwater catholyte.311 The commercially available Nafion-212 membrane was used as the solid electrolyte. Chloride ion repulsion by the PEM led to low chlorine generation around the anode. In addition, the enhanced electric field-driven proton transport enabled more efficient pH control across the cell. The vapor-fed anode configuration showed a similar performance to a conventional PEM electrolyzer. Typically, the cell voltage of the liquid-anolyte electrolyzer fed with DI water was 1.64 V at 0.5 A cm−2, like that obtained in the vapor-fed anode configuration (1.65 V at 0.5 A cm−2) (Fig. 17a and b). Remarkably, this vapor-fed anode configuration allows for the direct use of saltwater in conventional PEMWE electrolyzers without additional water purification at high faradaic efficiencies.
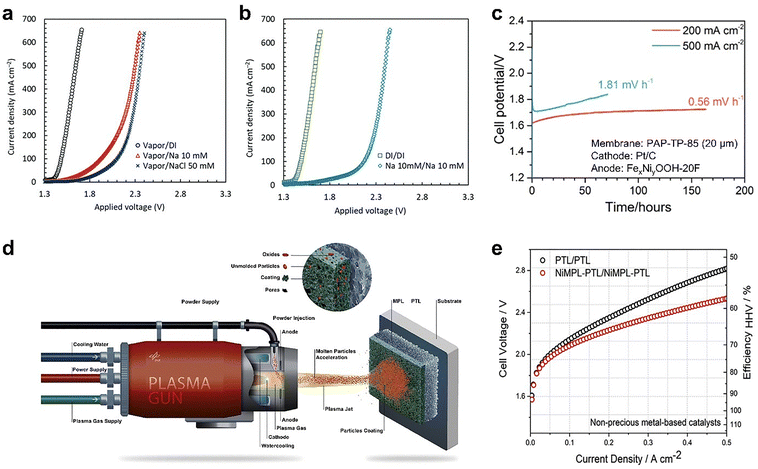 | ||
| Fig. 17 Performance test of the (a) vapor-fed anode and (b) liquid-anolyte configurations in different solutions. Reprinted with permission from ref. 311. Copyright 2021, Royal Society of Chemistry. (c) Stability test of the water-fed hydroxide exchange membrane electrolyzer at 200 and 500 mA cm−2 and 80 °C. The ionomer is PAP-TP-85 (20 µm) in the cathode with a loading of 30 wt% and is PAP-TP-85-MQN in the anode with a loading of 0.8 mg cm−2. Reprinted with permission from ref. 312. Copyright 2021, American Chemical Society. (d) Schematic of APS coating of Ni-MPL on PTL. (e) Polarization curves for the non-noble metal-based MEAs with different configurations: PTL/PTL and NiMPL-PTL/NiMPL-PTL. Reprinted with permission from ref. 313. Copyright 2021, The Authors. | ||
6.3. AEMWE devices with the supply of neutral electrolytes
In the past decade, AEMWE emerges as an advanced technology that operates in alkaline electrolytes feeding both the anode and the cathode, offering significant advantages over traditional PEM systems. AEMWE systems use anion-conducting polymer membranes that facilitate the transport of hydroxide ions (OH−) from the cathode to the anode. In this setup, the alkaline electrolytes are generally much less corrosive than acidic solutions, making AEMWE systems more attractive for large-scale applications using non-noble metal-based catalysts. Replacing alkaline electrolytes with neutral electrolytes will further reduce the electrolyte corrosion on the equipment. However, correspondingly, the extremely low concentration of OH− makes the activity and stability of neutral AEMWE often not as excellent as that of alkaline AEMWE. Therefore, it is important and necessary to develop neutral AEMWE with enhanced performance. This can be achieved not only by starting from the catalyst, but also from aspects such as membrane technology, electrolyzer structure design, external physical field regulation, and project management.The first attempt to operate a neutral AEMWE was reported by Zhuang's team in 2012.314 The AEMWE cell used a Ni–Fe anode prepared by solid-state electrochemical reduction and a Ni–Mo cathode fabricated directly on a stainless-steel skeleton, while a 70-mm-thick self-crosslinking quaternary ammonia polysulfone (xQAPS) AEM separated the anode/cathode and the device was fed with only pure water. At a current density of 0.4 A cm−2, the cell voltage is about 1.8–1.85 V at 70 °C, and remains stable during a test period of 8 hours. Apparently, feeding neutral pure water resulted in inferior AEMWE performance compared with an alkaline electrolyte feed, such as KOH.315–318 Apart from the activity, the stability of neutral AEMWE devices also falls far short of expectations. Xiao et al.312 constructed an AEMWE cell composed of Pt/C and self-supported FexNiyOOH-20F with a PAP-TP-85 membrane. The device with pure water feed showed a voltage rise of 0.56 mV h−1 for 160 hours operated at a current density of 200 mA cm−2. However, the same AEMWE device operating at 500 mA cm−2 showed a faster voltage increase (1.81 mV h−1) during 70 h (Fig. 17c). Since the intrinsic activity of neutral AEMWE is already quite poor, the bubble dynamics over the catalyst surface ought to be considered to overcome the limitation of mass transport in AEMWEs, particularly operating at a large current density. Razmjooei et al.313 formed a well-designed liquid/gas-diffusion layer (LGDL) to increase the performance of AEMWE feeding with pure water. The LGDL was developed by introducing nickel-based microporous layers (NiMPLs) on the top of a porous transport layer (PTL) by an air-plasma spraying method (Fig. 17d). The NiMPLs, with a wide range of Ni particle sizes, facilitated the quick release of gas bubbles, thereby improving the AEMWE performance. The low tortuosity of the NiMPL-PTL reduced the capillary pressure and bubble point, which led to lower transport polarization. In addition, the NiMPL-PTL decreased the interfacial contact resistance by increasing the contact area between the PTL and the MEA. As a result, using non-noble metal-based electrodes, the addition of the NiMPL layer to the PTL decreased the required cell potential from 2.82 to 2.53 V to reach 0.5 A cm−2 in pure water at 60 °C (Fig. 17e).
6.4. MEAWE devices with the supply of asymmetric electrolytes
To overcome the challenges of activity and stability for neutral MEAWE devices with the same electrolyte feed on both sides, as well as to expand the range of feeds and the application scenarios of electrolyzers, the asymmetric MEAWE with the supply of different electrolytes for the anode and cathode emerges as a promising configuration, especially when it comes to non-pure water electrolysis with complex ionic environments. That is, neutral electrolytes are supplied to the cathode (where the HER occurs), while alkaline or acidic electrolytes are used on the anode (where the OER occurs), or vice versa. The key challenge of MEAWE systems with asymmetric electrolytes is to maintain the correct ion gradients and ensure effective membrane separation between the two different electrolytes. Cross-leakage of ions from the anode to the cathode (and vice versa) can lead to performance degradation. Despite the big challenges, the asymmetric MEAWE allows for tailored electrolyte conditions to optimize both the HER and OER independently, thus it holds great promise in water electrolysis involving complex ionic environments. For this, system integration, including optimizing membrane stability, electrolyte flow, and electrode design, remains a key area of focus.For example, in the case of neutral natural seawater electrolysis, the two major challenges lie in the corrosion caused by the competing chlorine evolution reaction (ClER) at the anode, which poisons the catalytic sites, and the deposition of metal cations (e.g., Ca2+ and Mg2+) from seawater at the cathode, which covers the catalytic sites.319 Using MEAWE with symmetrical electrolytes is challenging to simultaneously solve these two problems. However, using the MEAWE with asymmetrical electrolytes will make the problem easier. For instance, neutral natural seawater is supplied to the cathode, while the acidic/alkaline electrolyte is introduced to the anode. This can avoid the challenge from ClER at the anode. Alternatively, neutral natural seawater is supplied to the anode, while acidic/alkaline electrolytes are introduced to the cathode. This can avoid the problem of Ca2+/Mg2+ precipitation at the cathode. Strasser's team320 operated various AEMWE devices using an independent and asymmetric electrolyte feed with different electrolyte compositions for the seawater electrolyzer, which enables direct feed of neutral seawater at the cathode and circulates pure KOH electrolytes at the anode (Fig. 18a). The assembled AEMWE devices consisted of NiFe-LDH on Au-sputtered Ti felt, Pt/C on a carbon-based gas diffusion electrode, and Tokuyama A201 as the anode, cathode, and membrane, respectively. Compared to commercial IrOx, the NiFe-LDH electrode limits the ClER and appears highly selective for the OER in alkalinized seawater even at cell potentials beyond 3.0 Vcell (Fig. 18b). The asymmetric AEMWE device ultimately showed higher stability for 100 h with additional repetitive cycles between the galvanostatic polarization curves. In addition, Shi et al.253 presented a asymmetric electrolyzer, which employed Pt single atoms supported on amorphous Ni6.6Fe0.4P3 nanowires (PtSA–Ni6.6Fe0.4P3) as the cathode and amorphous Ni5Fe2P3 nanowires as the anode, combined with a Na+ exchange membrane to prevent Cl− migration to the anode (Fig. 18c). The cathode chamber uses near-neutral seawater or a 4 M NaCl solution, while the anode chamber employs 4 M NaOH, creating a pH-asymmetric system that leverages chemical potential differences to reduce energy consumption. The electrolyzer achieves impressive performance, delivering 10 and 100 mA cm−2 at 1.31 and 1.46 V, respectively (Fig. 18d). This efficiency stems from the synergistic design. The super hydrophilic PtSA–Ni6.6Fe0.4P3 cathode enhances water dissociation kinetics and rapid H2 desorption, while the amorphous Ni5Fe2P3 anode provides fast OER kinetics. The design also mitigates Ca2+/Mg2+ precipitation via pH control and flow electrolyte dynamics, and low bubble adhesion on super hydrophilic surfaces accelerates mass transport.
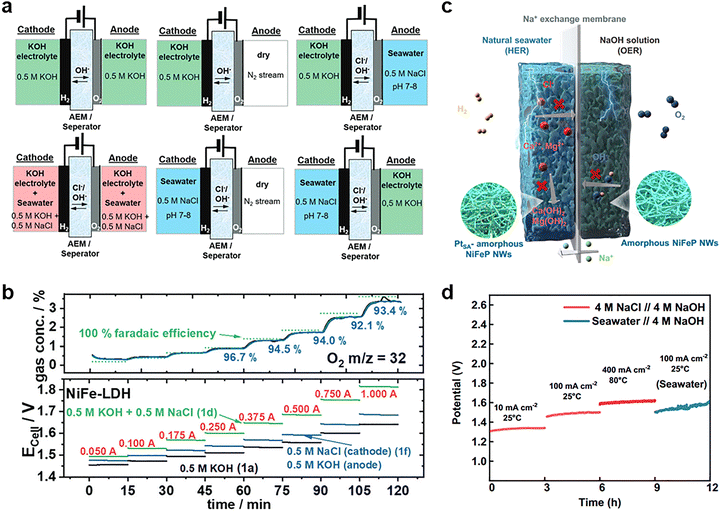 | ||
| Fig. 18 (a) Schemes for AEM electrolyzers using independent electrolyte feeds with different electrolyte compositions. (b) NiFe-LDH as the anode catalyst using the following electrolyte feed schemes: symmetric 0.5 M KOH (black), symmetric 0.5 M (KOH + NaCl) (green), and asymmetric 0.5 M KOH at the anode and 0.5 M NaCl at the cathode. Reprinted with permission from ref. 320. Copyright 2020, Royal Society of Chemistry. (c) Scheme for the asymmetric electrolyzer with the sodium ion exchange membrane. (d) Chronopotentiometry curves of asymmetric electrolyzers under different conditions. Reprinted with permission from ref. 253. Copyright 2023, The Authors. | ||
Recently, the MEAWE with an asymmetric reaction has also attracted more attention. The original intention of this technology is to replace the sluggish OER process with an oxidation reaction using less electrically driven organic substances, for reducing the cell voltage. It is also found that the replacement reaction at the anode can be designed to produce more valuable organic substances, even H2 (in this case, the faradaic efficiency of hydrogen reaches 200%). However, it requires an anode reaction with a low overpotential. Thus far, the commonly used reactants have included hydrazine,321–323 aldehyde,324,325 alcohol,326,327 and sulfion.328,329
The transition of multi-site electrocatalysts from laboratory chambers to practical electrolyzers remains a big challenge, primarily due to the significant differences between three-electrode testing conditions and the complex operating environment of two-electrode MEA-based devices. Neutral electrolytes with increased ohmic resistance and sluggish reaction kinetics necessitate higher applied voltages to drive the HER and OER, which in turn leads to energy inefficiencies and catalyst degradation. Beyond activity, stability emerges as the most key factor limiting practical deployment. Neutral HER/OER catalysts frequently undergo dissolution, agglomeration, and structural collapse under prolonged polarization, while membranes and supports in MEA devices face oxidative degradation, bubble-induced delamination, and metal ion poisoning. These degradation pathways, often masked in half-cell evaluations, may become dominant failure mechanisms in electrolyzer operations.
Another blank but important issue to consider is how multi-site catalysts influence the operation of MEA electrolyzers under practical high-current-density conditions. In such environments, device factors including local electric field, pressure buildup from gas evolution and electrolyzer assembly, temperature fluctuations, and distribution of flow field all significantly affect the performance and durability. While the factor regulations are often treated as engineering challenges, catalyst design itself can play an important regulatory role. Multi-site architectures, by virtue of combining distinct active centers and interfacial environments, will modulate local electric fields, facilitate bubble detachment, buffer thermal fluctuation, and promote ionic and mass transport. These properties not only enhance the intrinsic catalytic kinetics but also help to stabilize operation. However, systematic investigations directly linking designed sites to device-level responses remain scarce. Developing the multi-site catalysts that simultaneously address both molecular-scale reactivity and macroscopic electrolyzer operation represents a promising direction.
7. Conclusions and outlooks
In this review, a comprehensive and critical account of the recent progress in the field of neutral HER is presented, with particular focus on the development and mechanistic understanding of multi-site electrocatalysts. In contrast to the well-established acidic and alkaline HER systems, neutral water electrolysis offers advantages such as lower corrosivity, broader material compatibility, and potential integration with natural and waste water sources. However, it also imposes formidable thermodynamic and kinetic challenges due to the low proton concentration, slow water dissociation rates, and weak interfacial electric fields. Addressing these challenges demands innovative catalyst design principles that can promote multiple elementary steps of HER synergistically.Multi-site electrocatalysts – comprising spatially or chemically distinct active centres that each facilitate different stages of the HER process – have emerged as a promising class of materials capable of optimizing reactant adsorption, intermediate transformation, and product desorption in tandem. Through a combination of theoretical understanding and empirical advancements, it has been demonstrated that such catalysts can significantly enhance water dissociation, accelerate proton-coupled electron transfer, and improve H2 desorption kinetics under neutral pH conditions. The incorporation of diverse functional motifs including hydrophilic/hydrophobic interfaces, multi-metal centres, and atomically dispersed species enables finely tuned control over reaction pathways that would otherwise be inaccessible in single-site systems.
To demonstrate these advancements, in this review, the fundamental differences between HER mechanisms in acidic, alkaline and neutral media were first revisited, elucidating how electrolyte identity, electric double-layer structures, two-electron transfer, and adsorption/desorption of reactants/products influence catalytic behavior. Then multi-site catalyst strategies were systematically categorized according to compositional complexity, spanning binary, ternary, and quaternary systems, as well as single-atom catalysts with engineered multi-functionality. Furthermore, the synthetic methodologies and morphological architectures were discussed, ranging from zero-dimensional nanoparticles to three-dimensional porous frameworks that facilitate the dispersion and exposure of active sites. Characterization techniques including in situ and operando methods are highlighted for their ability to capture the dynamic evolution of catalysts during HER operation. Finally, the application of neutral HER research into practical water electrolysis systems was explored by evaluating the performance of catalysts in MEA-based devices such as PEMWE, AEMWE, and asymmetric-electrolyte MEAWE. To bridge the gap between fundamental breakthroughs and real-world applications, continued innovation is highly desired. Key directions for advancing the neutral HER toward scalable hydrogen production include enhancing intrinsic catalytic activity and applicability through rational design, expanding the capabilities of operando characterizations, and establishing standardized protocols for realistic performance evaluation under two-electrode and device-relevant conditions (Fig. 19).
7.1. Improving the intrinsic activity and applicability of catalysts
Why the performance of neutral water electrolysis is inferior to that of alkaline and acidic electrolysis? This fundamental issue has not been fully resolved. Understanding the characteristics of neutral water electrolysis can provide us with a basic and reasonable perspective to design excellent catalysts. While numerous multi-site electrocatalysts have demonstrated improved water adsorption, intermediate stabilization, and hydrogen evolution activity under neutral pH, the overall activity of most systems remains inferior to their acidic or alkaline counterparts. Enhancing the intrinsic activity of catalysts must therefore go beyond compositional tuning alone. Good designs should incorporate atomic-level engineering of active sites with complementary functions (e.g., water dissociation and *H coupling), electronic structure tailoring (via heteroatom doping, interfacial heterojunctions, surface strain, and others), and surface property regulation (such as hydrophilic/hydrophobic, bubble dynamics, and reactive microenvironment).Despite the remarkable progress in multi-site electrocatalysts for the neutral HER and OER, several nonnegligible challenges remain before their widespread adoption in practical electrolyzers. These limitations, often overshadowed by performance highlights, must be critically addressed to ensure the advances in laboratory design translate into real-world viability.
While multi-site catalysts represent an experimentally promising direction, their future hinges on the solution of the above-mentioned critical issues. This is a huge project that requires a great deal of effort. Fortunately, at present, artificial intelligence (AI) technology offers a more efficient approach for designing catalysts. In the past, DFT serves as a foundational tool for elucidating catalytic reaction mechanisms at the atomic level, which provides essential insights into adsorption energies, activation barriers, and electronic structure descriptors that govern catalytic performance. However, its high computational cost and limited scalability constrain its application to large-scale screening efforts, particularly under the complex and dynamic conditions relevant to neutral-pH water electrolysis. Recently, machine learning (ML) emerges as a powerful tool to overcome these limitations. ML models trained with high-quality DFT or experimental datasets can rapidly predict key thermodynamic and kinetic properties across vast chemical spaces, enabling high-throughput virtual screening of materials with minimal DFT calculations and experimental cost. To further advance the discovery of active, stable, and earth-abundant electrocatalysts for neutral-pH water splitting, the innovations in active learning, interpretable algorithms, advanced neural network architectures, and experimental-data integration have been demonstrated to be significantly potential.
Active learning strategies are expected to enable ML models to efficiently direct DFT calculations toward the most informative regions of material space, thereby enhancing predictive accuracy while minimizing the computational cost and human intervention. Concurrently, the integration of interpretable ML algorithms holds significant promise for uncovering the fundamental principles governing catalytic activity, providing mechanistic insights that can inform the rational design of tailored catalysts working in neutral-pH environments. Moreover, recent advancements in graph neural networks and transformer-based architectures have demonstrated superior capabilities in representing complex catalyst systems including those featuring mixed phases, lattice defects, and amorphous structures with the characteristics that are often encountered under near-neutral operating conditions. Finally, the incorporation of experimental data into DFT-ML workflows is anticipated to play a key role in mitigating the intrinsic limitations of DFT, such as systematic errors, and narrowing the gap between theoretical predictions and experimental observations, ultimately accelerating the discovery of efficient and robust electrocatalysts for water splitting under neutral-pH conditions.
7.2. Developing advanced operando and in situ characterization techniques
The precise revelation of the long-range and short-range structures of the designed materials is of great significance to uncover the “structure–activity relationship” of multi-site catalysts. Furthermore, understanding the dynamic nature of active sites during the neutral HER/OER is essential for designing high-performance catalysts. However, conventional post-reaction characterizations often fail to capture critical transient states or reconstruction phenomena that occur under real electrolysis conditions. This highlights the need for operando spectroscopies (e.g., XAFS, Raman, Mössbauer, NMR, and XRD) to track oxidation states, coordination environments, and bond evolution, in situ microscopic techniques (e.g., STEM, TEM, and AFM) to visualize morphological changes and interface formation, and electrochemical parameter variation (e.g., EIS and Cdl) to study kinetics and intermediate evolution. Furthermore, developing non-destructive ultra-fast spectroscopy technology under operando conditions can provide us with a profound perspective for understanding the reaction process. This requires the joint efforts of researchers from multiple disciplines, especially the investment and construction of advanced large-scale scientific facilities. For instance, the development of the 4th-generation synchrotron radiation source has promoted the wider and more precise application of XAFS technology. The brightness of its light source has increased by several hundred times compared to the 3rd-generation synchrotron radiation source, enabling real-time detection of higher-resolution material structures, especially in complex heterogeneous reaction systems for operando and in situ substance imaging, structural analysis, intermediate capture, etc. Ultimately, integrating multiple techniques in real-time electrolysis environments will unearth how local pH, electrolyte composition, and applied potential dynamically influence reaction pathways, enabling deeper mechanistic understanding of multi-site synergy.7.3. Building a two-electrode flow cell with advanced catalysts, membranes, and evaluation methods
In practical applications, the current density in two-electrode MEAWE systems must be high enough to achieve a current density of the order of amperes per square centimetre to compete with industrial standards. Designing high-efficiency multi-site catalysts is essential for reducing the overpotential and achieving high faradaic efficiency. Furthermore, if industrial application is to be achieved, a greater yet less-explored challenge lies in the large-scale production and stable operation of the catalysts: (i) Catalyst scalability. While laboratory systems often use small catalyst amounts, in real-world applications, catalyst loading must be optimized to ensure high active surface area while minimizing material costs. (ii). Long-term stability. Performance testing in a three-electrode system typically provides insights into short-term kinetics but lacks long-term operational data. MEAWE devices require durable catalysts and stable electrode/electrolyte interfaces to ensure longevity under continuous operation. (iii) Post-treatment of electrodes. For practical industrial operations, a significant concern is whether the electrodes are environmentally friendly, whether they can be recycled, and whether they can be reused after simple repair. These not only relate to the material cost, but also to the investment in manpower, space, time, and handling measures. However, there are currently few studies on this aspect, and it is generally assumed that when the electrodes fail, they are simply discarded. Conducting scientific research on post-treatment of materials in advance will provide technical reserves for subsequent industrial development.The neutral water electrolysis systems have made significant progress, but several challenges remain in terms of performance, stability, and economic viability for large-scale hydrogen production. The successful implementation of neutral HER and OER systems demands optimizing both electrolyte management and catalyst stability for real-world applications. As the transition to neutral pH systems becomes more feasible, the MEAWE systems with asymmetric electrolytes offer a flexible platform for optimizing reaction conditions, while AEMWE and PEMWE technologies focus on enhancing the catalyst durability. At this point, the development of membranes will become a significant constraint for the technical route. Besides, the life cycle assessment (LCA) and techno-economic analysis (TEA) of the neutral MEAWE merit further discussion, to evaluate whether it has the potential to rival traditional PEMWE and AEMWE in the future. Furthermore, the direct electrolysis of natural seawater is expected to be achieved through the development of a neutral MEAWE system. This represents a shining light in the future blueprint of water electrolysis for hydrogen production with abundant seawater resources.
To approach this target, the joint efforts of the entire integrated MEAWE system are essential, including catalysts, membranes, bipolar plates, flow channel structures, external physical field control and project management. Beyond the design of advanced multi-site catalysts, the successful translation of neutral HER/OER into practical electrolyzer systems demands attention to engineering challenges: How to ensure sealing during the design of the flow channel? How to make the electrolyte quickly remove the produced gas? If it cannot be made totally, what are the effects of local dehydration and overheating induced by insufficient removal of produced gas on the membrane? Is the electrolyzer designed in the atmosphere, or at one/both-side pressure? Will the residual voltage after the power cutting off cause the membrane to dissolve and become disabled? and so on.
These engineering issues have a direct impact on the actual application of electrolyzers, but as of now, there have been no complete solutions. Addressing these engineering barriers will require cross-disciplinary collaboration between materials chemists, electrochemists, and chemical engineers, as well as the employment of computational fluid dynamics and system-level modelling to optimize cell designs. Looking forward, the development of neutral water electrolysis demands matched co-engineering of catalysts, membranes, and cell architectures. Activity optimization must be tightly coupled with the strategies to improve catalyst stability. Ultimately, the path to scalable neutral water splitting does not lie in catalyst breakthroughs alone but in integrated design principles that merge material innovation with device-level engineering. By emphasizing both intrinsic activity and extrinsic durability in realistic operating environments, the field can begin to bridge the gap between laboratory demonstrations and industrially viable hydrogen production under neutral conditions.
As the energy landscape shifts toward distributed and sustainable hydrogen production, neutral water electrolysis stands as a promising avenue that balances safety, versatility, and scalability. Together, these insights and perspectives aim to stimulate further innovation at rationally engineered multi-site electrocatalysts, coupled with advanced characterizations and practical device architectures. Through interdisciplinary efforts spanning catalysis, materials science, electrochemistry, computer science, and system engineering, the vision of scalable and neutral-pH-driven hydrogen production can transition from laboratory innovation to industrial reality.
Author contributions
Y. Yu supervised this review. Z. Wang and Y. Yu conceived the topic and sorted out the manuscript. All authors collected data, wrote the manuscript, and have approved the final version of the manuscript.Conflicts of interest
There are no conflicts of interest to declare.Data availability
No primary research results, software or code has been included and no new data were generated or analyzed as part of this review.Acknowledgements
This work was supported by the National Key Research and Development Program of China (2022YFB3803600), the National Natural Science Foundation of China (52472205, 12275199, and 52301272), and the self-determined research funds of CCNU from the colleges’ basic research and operation of MOE (CCNU25ZH006 and CCNU22CJ018).Notes and references
- W. Shi, T. Shen, C. Xing, K. Sun, Q. Yan, W. Niu, X. Yang, J. Li, C. Wei, R. Wang, S. Fu, Y. Yang, L. Xue, J. Chen, S. Cui, X. Hu, K. Xie, X. Xu, S. Duan, Y. Xu and B. Zhang, Science, 2025, 387, 791–796 CrossRef CAS PubMed.
- R. Ram, L. Xia, H. Benzidi, A. Guha, V. Golovanova, A. Garzón Manjón, D. Llorens Rauret, P. Sanz Berman, M. Dimitropoulos, B. Mundet, E. Pastor, V. Celorrio, C. A. Mesa, A. M. Das, A. Pinilla-Sánchez, S. Giménez, J. Arbiol, N. López and F. P. García de Arquer, Science, 2024, 384, 1373–1380 CrossRef CAS.
- D. Guan, B. Wang, J. Zhang, R. Shi, K. Jiao, L. Li, Y. Wang, B. Xie, Q. Zhang, J. Yu, Y. Zhu, Z. Shao and M. Ni, Energy Environ. Sci., 2023, 16, 4926–4943 RSC.
- Z. Wang, Y. Chen, B. Sheng, J. Li, L. Yao, Y. Yu, J. Song, T. Yu, Y. Li, H. Pan, P. Wang, X. Wang, L. Zhu and B. Zhou, Angew. Chem., Int. Ed., 2024, 63, e202400011 CrossRef CAS.
- Z. Wang, Q. Wu, J. Wang and Y. Yu, Front. Energy, 2024, 18, 89–100 CrossRef CAS.
- H. Wang, Y. Yang, J. Liu, H. Wu, K. Wu, C. Lyu, J. Wu, W. Lau, Q. Wu and J. Zheng, Mater. Today Phys., 2023, 36, 101169 CrossRef CAS.
- Y. Zhang, Y. Li, X. Xin, Y. Wang, P. Guo, R. Wang, B. Wang, W. Huang, A. J. Sobrido and X. Li, Nat. Energy, 2023, 8, 504–514 CrossRef CAS.
- X. Xin, Y. Li, Y. Zhang, Y. Wang, X. Chi, Y. Wei, C. Diao, J. Su, R. Wang, P. Guo, J. Yu, J. Zhang, A. J. Sobrido, M.-M. Titirici and X. Li, Nat. Commun., 2024, 15, 337 CrossRef CAS PubMed.
- Z. Wang, B. Sheng, Y. Chen, S. M. Sadaf, J. Li, J. Yang, J. Song, L. Yao, Y. Yu, L. Zhu, X. Wang, Z. Huang and B. Zhou, Green Chem., 2023, 25, 288–295 RSC.
- Q. Sha, S. Wang, L. Yan, Y. Feng, Z. Zhang, S. Li, X. Guo, T. Li, H. Li, Z. Zhuang, D. Zhou, B. Liu and X. Sun, Nature, 2025, 639, 360–367 CrossRef CAS PubMed.
- Z. W. Seh, J. Kibsgaard, C. F. Dickens, I. Chorkendorff, J. K. Nørskov and T. F. Jaramillo, Science, 2017, 355, eaad4998 CrossRef.
- A. Odenweller and F. Ueckerdt, Nat. Energy, 2025, 10, 110–123 CrossRef.
- R. K. Balaji and F. You, Energy Environ. Sci., 2024, 17, 6138–6156 RSC.
- Q. Hassan, Int. J. Hydrogen Energy, 2023, 48, 34299–34315 CrossRef CAS.
- Y. Wang, Y. Zhang, X. Xin, J. Yang, M. Wang, R. Wang, P. Guo, W. Huang, A. J. Sobrido, B. Wei and X. Li, Science, 2023, 381, 291–296 CrossRef CAS.
- X. Xin, Y. Zhang, R. Wang, Y. Wang, P. Guo and X. Li, Nat. Commun., 2023, 14, 1759 CrossRef CAS PubMed.
- S. Guo, X. Li, J. Li and B. Wei, Nat. Commun., 2021, 12, 1343 CrossRef CAS PubMed.
- A. Li, S. Kong, K. Adachi, H. Ooka, K. Fushimi, Q. Jiang, H. Ofuchi, S. Hamamoto, M. Oura, K. Higashi, T. Kaneko, T. Uruga, N. Kawamura, D. Hashizume and R. Nakamura, Science, 2024, 384, 666–670 CrossRef CAS.
- L. Chong, G. Gao, J. Wen, H. Li, H. Xu, Z. Green, J. D. Sugar, A. J. Kropf, W. Xu, X.-M. Lin, H. Xu, L.-W. Wang and D.-J. Liu, Science, 2023, 380, 609–616 CrossRef CAS.
- W. Xu, Y. Wu, S. Xi, Y. Wang, Y. Wang, Y. Ke, L. Ding, X. Wang, J. Yang, W. Zhang, K. P. Loh, F. Ding, Z. Liu and M. Chhowalla, Nat. Synth., 2025, 1–9 Search PubMed.
- R. Wang, Y. Yang, J. Guo, Q. Zhang, F. Cao, Y. Wang, L. Han and T. Ling, Nat. Energy, 2025, 1–10 Search PubMed.
- Z. Huang, Z. Wang, Q. Zhou, H. Rabl, S. Naghdi, Z. Yang and D. Eder, Angew. Chem., Int. Ed., 2025, 64, e202419913 CrossRef CAS PubMed.
- J. Zhou, P. Li, X. Xia, Y. Zhao, Z. Hu, Y. Xie, L. Yang, Y. Liu, Y. Du, Q. Zhou, L. Yu and Y. Yu, Appl. Catal., B, 2024, 359, 124461 CrossRef CAS.
- Z. Huang, Z. Wang, H. Rabl, S. Naghdi, Q. Zhou, S. Schwarz, D. H. Apaydin, Y. Yu and D. Eder, Nat. Commun., 2024, 15, 9393 CrossRef CAS.
- C. Huang, Q. Zhou, D. Duan, L. Yu, W. Zhang, Z. Wang, J. Liu, B. Peng, P. An, J. Zhang, L. Li, J. Yu and Y. Yu, Energy Environ. Sci., 2022, 15, 4647–4658 RSC.
- L. Yu, Q. Zhu, S. Song, B. McElhenny, D. Wang, C. Wu, Z. Qin, J. Bao, Y. Yu, S. Chen and Z. Ren, Nat. Commun., 2019, 10, 5106 CrossRef PubMed.
- S. Nitopi, E. Bertheussen, S. B. Scott, X. Liu, A. K. Engstfeld, S. Horch, B. Seger, I. E. L. Stephens, K. Chan, C. Hahn, J. K. Nørskov, T. F. Jaramillo and I. Chorkendorff, Chem. Rev., 2019, 119, 7610–7672 CrossRef CAS PubMed.
- A. J. Martín and J. Pérez-Ramírez, Joule, 2019, 3, 2602–2621 CrossRef.
- R. Xia, S. Overa and F. Jiao, JACS Au, 2022, 2, 1054–1070 CrossRef CAS.
- W. Zhang, C. Huang, J. Zhu, Q. Zhou, R. Yu, Y. Wang, P. An, J. Zhang, M. Qiu, L. Zhou, L. Mai, Z. Yi and Y. Yu, Angew. Chem., Int. Ed., 2022, 61, e202112116 CrossRef CAS PubMed.
- Q. Zhou, X. Tang, S. Qiu, L. Wang, L. Hao and Y. Yu, Mater. Today Phys., 2023, 33, 101050 CrossRef CAS.
- C. Wang, Y. Yu and M. Qiu, Chem. Catal., 2023, 3, 100562 CAS.
- A. S. Varela, M. Kroschel, N. D. Leonard, W. Ju, J. Steinberg, A. Bagger, J. Rossmeisl and P. Strasser, ACS Energy Lett., 2018, 3, 812–817 CrossRef CAS.
- W. Zhang, C. Huang, Q. Xiao, L. Yu, L. Shuai, P. An, J. Zhang, M. Qiu, Z. Ren and Y. Yu, J. Am. Chem. Soc., 2020, 142, 11417–11427 CrossRef CAS PubMed.
- X. Yuan, K. Lee, J. B. Eisenberg, J. R. Schmidt and K.-S. Choi, Nat. Catal., 2024, 7, 43–54 CrossRef CAS.
- M. K. Goetz, M. T. Bender and K.-S. Choi, Nat. Commun., 2022, 13, 5848 CrossRef CAS.
- J. Xiao, J. J. Masana, M. Qiu and Y. Yu, Mater. Today Phys., 2024, 48, 101565 CrossRef CAS.
- J. Huang, A. H. Clark, N. Hales, K. Crossley, J. Guehl, R. Skoupy, T. J. Schmidt and E. Fabbri, Nat. Chem., 2025, 17, 856–864 CrossRef CAS PubMed.
- S. Anantharaj and V. Aravindan, Adv. Energy Mater., 2020, 10, 1902666 CrossRef CAS.
- Z. Zhou, Z. Pei, L. Wei, S. Zhao, X. Jian and Y. Chen, Energy Environ. Sci., 2020, 13, 3185–3206 RSC.
- X. Xiao, L. Yang, W. Sun, Y. Chen, H. Yu, K. Li, B. Jia, L. Zhang and T. Ma, Small, 2022, 18, 2105830 CrossRef CAS.
- F. Lai, H. Shang, Y. Jiao, X. Chen, T. Zhang and X. Liu, Interdiscip. Mater., 2024, 3, 492–529 CAS.
- Z. Yu, X. Rui and Y. Yu, EES Catal., 2023, 1, 695–703 RSC.
- Z.-F. Huang, J. Song, K. Li, M. Tahir, Y.-T. Wang, L. Pan, L. Wang, X. Zhang and J.-J. Zou, J. Am. Chem. Soc., 2016, 138, 1359–1365 CrossRef CAS PubMed.
- Y. Kang, O. Cretu, J. Kikkawa, K. Kimoto, H. Nara, A. S. Nugraha, H. Kawamoto, M. Eguchi, T. Liao, Z. Sun, T. Asahi and Y. Yamauchi, Nat. Commun., 2023, 14, 4182 CrossRef CAS PubMed.
- M. Zhang, J. Wang, Y. Zhang, L. Ye and Y. Gong, Dalton Trans., 2021, 50, 2973–2980 RSC.
- T. Feng, G. Yu, S. Tao, S. Zhu, R. Ku, R. Zhang, Q. Zeng, M. Yang, Y. Chen, W. Chen, W. Chen and B. Yang, J. Mater. Chem. A, 2020, 8, 9638–9645 RSC.
- N. Jiang, Z. Zhu, W. Xue, B. Y. Xia and B. You, Adv. Mater., 2022, 34, 2105852 CrossRef CAS.
- L. Zhang, L. Wang, Y. Wen, F. Ni, B. Zhang and H. Peng, Adv. Mater., 2020, 32, 2002297 CrossRef CAS.
- C. Huang, J. Zhou, D. Duan, Q. Zhou, J. Wang, B. Peng, L. Yu and Y. Yu, Chin. J. Catal., 2022, 43, 2091–2110 CrossRef CAS.
- C. Huang, Z. Wang, S. Cheng, Y. Liu, B. Deng, S. Xu, L. Yu and Y. Yu, Sci. China: Chem., 2024, 67, 3198–3208 CrossRef CAS.
- S. Chen, S. Shen, G. Liu, Y. Qi, F. Zhang and C. Li, Angew. Chem., Int. Ed., 2015, 54, 3047–3051 CrossRef CAS PubMed.
- Y. Qi, B. Zhang, G. Zhang, Z. Zheng, T. Xie, S. Chen, G. Ma, C. Li, K. Domen and F. Zhang, Joule, 2024, 8, 193–203 CrossRef CAS.
- N. Kousar, G. Patil, A. C. Kumbara, B. Nisty, G. H. Rajesh and L. K. Sannegowda, Dalton Trans., 2025, 54, 12714–12736 RSC.
- S. Aralekallu, K. Sannegowda Lokesh and V. Singh, Fuel, 2024, 357, 129753 CrossRef CAS.
- Q. Sun, N. J. Oliveira, S. Kwon, S. Tyukhtenko, J. J. Guo, N. Myrthil, S. A. Lopez, I. Kendrick, S. Mukerjee, L. Ma, S. N. Ehrlich, J. Li, W. A. Goddard, Y. Yan and Q. Jia, Nat. Energy, 2023, 8, 859–869 CrossRef CAS.
- R. Subbaraman, D. Tripkovic, D. Strmcnik, K.-C. Chang, M. Uchimura, A. P. Paulikas, V. Stamenkovic and N. M. Markovic, Science, 2011, 334, 1256–1260 CrossRef CAS PubMed.
- N. Govindarajan, A. Xu and K. Chan, Science, 2022, 375, 379–380 CrossRef CAS.
- L. Su, J. Chen, F. Yang, P. Li, Y. Jin, W. Luo and S. Chen, J. Am. Chem. Soc., 2023, 145, 12051–12058 CrossRef CAS.
- L. Liu, Y. Liu and C. Liu, J. Am. Chem. Soc., 2020, 142, 4985–4989 CrossRef CAS PubMed.
- O. Jung, M. N. Jackson, R. P. Bisbey, N. E. Kogan and Y. Surendranath, Joule, 2022, 6, 476–493 CrossRef CAS.
- J. Zhou, Y. Xie, L. Yang, Y. Liu, Y. Du, L. Yu and Y. Yu, Inorg. Chem. Front., 2023, 10, 2842–2859 RSC.
- A. P. Murthy, D. Govindarajan, J. Theerthagiri, J. Madhavan and K. Parasuraman, Electrochim. Acta, 2018, 283, 1525–1533 CrossRef CAS.
- Y. Park, M. Jun, T. Kwon, J. Y. Kim and K. Lee, Chem. Catal., 2023, 3, 100499 CAS.
- X. Cao, S. Cha and M. Gong, Acta Phys.-Chim. Sin., 2025, 41, 100041 CrossRef.
- C. Geng, X. Jiang, S. Hong, L. Wang, Y. Zhao, J. Qi, J. Shi, J. Wang, L. Peng, Z. Hu, Y. Guo, F.-M. Jin, Q.-H. Yang and W. Lv, Adv. Mater., 2024, 36, 2407741 CrossRef CAS.
- J. Yu, J. Yin, R. Li, Y. Ma and Z. Fan, Chem. Catal., 2022, 2, 2229–2252 CAS.
- P. Li, Y. Jiao, J. Huang and S. Chen, JACS Au, 2023, 3, 2640–2659 CrossRef CAS PubMed.
- S.-J. Shin, D. H. Kim, G. Bae, S. Ringe, H. Choi, H.-K. Lim, C. H. Choi and H. Kim, Nat. Commun., 2022, 13, 174 CrossRef CAS.
- T. Shinagawa, A. T. Garcia-Esparza and K. Takanabe, ChemElectroChem, 2014, 1, 1497–1507 CrossRef CAS.
- P. S. Lamoureux, A. R. Singh and K. Chan, ACS Catal., 2019, 9, 6194–6201 CrossRef CAS.
- X. Yuan, M. T. Bender, M. Ko and K.-S. Choi, Nat. Catal., 2025, 8, 495–506 CrossRef CAS.
- T. Shinagawa and K. Takanabe, J. Phys. Chem. C, 2015, 119, 20453–20458 CrossRef CAS.
- I. Katsounaros, J. C. Meier, S. O. Klemm, A. A. Topalov, P. U. Biedermann, M. Auinger and K. J. J. Mayrhofer, Electrochem. Commun., 2011, 13, 634–637 CrossRef CAS.
- R. Zhang, T. Zhai, H. Wang and S. Lu, Adv. Energy Mater., 2024, 5, 2400085 CAS.
- J. Guo, Y. Zheng, Z. Hu, C. Zheng, J. Mao, K. Du, M. Jaroniec, S. Qiao and T. Ling, Nat. Energy, 2023, 8, 264–272 CAS.
- M. Sarno, E. Ponticorvo and D. Scarpa, Electrochem. Commun., 2020, 111, 106647 CrossRef CAS.
- H. Helmholtz, Ann. Phys., 1853, 165, 211–233 CrossRef.
- M. Gouy, J. Phys. Theor. Appl., 1910, 9, 457–468 CrossRef.
- D. L. Chapman, Philos. Mag., 1913, 25, 475–481 Search PubMed.
- O. Stern, Angew. Phys. Chem., 1924, 30, 508–516 CAS.
- D. C. Grahame, Chem. Rev., 1947, 41, 441–501 CrossRef CAS.
- D. C. Grahame, J. Am. Chem. Soc., 1954, 76, 4819–4823 CrossRef CAS.
- M. a V. Devanathan, Trans. Faraday Soc., 1954, 50, 373–385 RSC.
- J. O. Bockris, M. a V. Devanathan, K. Müller and J. A. V. Butler, Proc. R. Soc. A, 1963, 274, 55–79 CAS.
- A. J. Bard, L. R. Faulkner and H. S. White, Electrochemical Methods: Fundamentals and Applications, John Wiley & Sons, 2022 Search PubMed.
- B. B. Damaskin and O. A. Petrii, J. Solid State Electrochem., 2011, 15, 1317–1334 CrossRef CAS.
- M. Dunwell, Y. Yan and B. Xu, Opin. Chem. Eng., 2018, 20, 151–158 CrossRef.
- C. M. Schott, P. M. Schneider, K.-T. Song, H. Yu, R. Götz, F. Haimerl, E. Gubanova, J. Zhou, T. O. Schmidt, Q. Zhang, V. Alexandrov and A. S. Bandarenka, Chem. Rev., 2024, 124, 12391–12462 CrossRef CAS PubMed.
- G. E. Walrafen, J. Chem. Phys., 1967, 47, 114–126 CrossRef CAS.
- F. H. Potter and R. G. Egdell, Surf. Sci., 1993, 297, 286–292 CrossRef CAS.
- K. J. Schweighofer, X. Xia and M. L. Berkowitz, Langmuir, 1996, 12, 3747–3752 CrossRef CAS.
- A. Hamelin, Electroanal. Chem., 1996, 407, 1–11 CrossRef.
- J. R. Errington and P. G. Debenedetti, Nature, 2001, 409, 318–321 CrossRef CAS PubMed.
- Y.-X. Jiang, J.-F. Li, D.-Y. Wu, Z.-L. Yang, B. Ren, J.-W. Hu, Y. L. Chow and Z.-Q. Tian, Chem. Commun., 2007, 4608–4610 RSC.
- I. Ledezma-Yanez, W. D. Z. Wallace, P. Sebastián-Pascual, V. Climent, J. M. Feliu and M. T. M. Koper, Nat. Energy, 2017, 2, 1–7 Search PubMed.
- T. Wang, Y. Zhang, B. Huang, B. Cai, R. R. Rao, L. Giordano, S.-G. Sun and Y. Shao-Horn, Nat. Catal., 2021, 4, 753–762 CrossRef CAS.
- A. H. Shah, Z. Zhang, C. Wan, S. Wang, A. Zhang, L. Wang, A. N. Alexandrova, Y. Huang and X. Duan, J. Am. Chem. Soc., 2024, 146, 9623–9630 CrossRef CAS PubMed.
- J.-X. Zhu, J. Cheng and K. Doblhoff-Dier, J. Chem. Phys., 2025, 162, 024702 CrossRef CAS PubMed.
- R. D. Armstrong and B. R. Horrocks, Solid State Ionics, 1997, 94, 181–187 CrossRef CAS.
- X. Ding, B. Garlyyev, S. A. Watzele, T. Kobina Sarpey and A. S. Bandarenka, Chem. – Eur. J., 2021, 27, 10016–10020 CrossRef CAS PubMed.
- T. K. Sarpey, A. V. Himmelreich, K.-T. Song, E. L. Gubanova and A. S. Bandarenka, Small Sci., 2024, 4, 2400042 CrossRef CAS PubMed.
- L. Yan, B. Zhang, J. Zhu, Y. Li, P. Tsiakaras and P. Kang Shen, Appl. Catal., B, 2020, 265, 118555 CrossRef CAS.
- D. Guo, Z. Wan, G. Fang, M. Zhu and B. Xi, Small, 2022, 18, 2201896 CrossRef CAS.
- M. Qu, G. Huang, X. Liu, X. Nie, C. Qi, H. Wang, J. Hu, H. Fang, Y. Gao, W.-T. Liu, J. S. Francisco and C. Wang, Chem. Sci., 2022, 13, 10546–10554 RSC.
- S. Banerjee, D. D. Dionysiou and S. C. Pillai, Appl. Catal., B, 2015, 176–177, 396–428 CrossRef CAS.
- B. Bharti, S. Kumar and R. Kumar, Appl. Surf. Sci., 2016, 364, 51–60 CrossRef CAS.
- B. J. Cha, S. Saqlain, H. O. Seo and Y. D. Kim, Appl. Surf. Sci., 2019, 479, 31–38 CrossRef CAS.
- J. Hu, T. Zhao, W. Geng, Y. Lu, X.-F. Zhao, Y.-Z. Li, Y.-Q. Tang, J.-W. Liu, L.-Y. Wang, C. Janiak, X.-Y. Yang and B.-L. Su, Chem. Commun., 2019, 55, 9275–9278 RSC.
- V. Bolis, C. Busco, M. Ciarletta, C. Distasi, J. Erriquez, I. Fenoglio, S. Livraghi and S. Morel, J. Colloid Interface Sci., 2012, 369, 28–39 CrossRef CAS PubMed.
- C. Zhang, Q. Fan, J. Xu, M. Huang, F. Luo, D. Wang and Z. Zheng, Chem. Eng. J., 2025, 505, 159162 CrossRef CAS.
- J. Zhou, P. Yang, P. A. Kots, M. Cohen, Y. Chen, C. M. Quinn, M. D. de Mello, J. Anibal Boscoboinik, W. J. Shaw, S. Caratzoulas, W. Zheng and D. G. Vlachos, Nat. Commun., 2023, 14, 2293 CrossRef CAS.
- Y. Peng, Z. Chen, R. Zhang, W. Zhou, P. Gao, J. Wu, H. Liu, J. Liu, A. Hu and X. Chen, Nano-Micro Lett., 2021, 13, 192 CrossRef CAS PubMed.
- F. Yang, X. Ma, W.-B. Cai, P. Song and W. Xu, J. Am. Chem. Soc., 2019, 141, 20451–20459 CrossRef CAS PubMed.
- S. Gong, T. Zhang, J. Meng, W. Sun and Y. Tian, Mater. Chem. Front., 2024, 8, 603–626 RSC.
- Z. Feng, C. Dai, P. Shi, X. Lei, R. Guo, B. Wang, X. Liu and J. You, Chem. Eng. J., 2024, 485, 149992 CrossRef CAS.
- S. Zhao, Y. Wang, Y. Hao, L. Yin, C. Kuo, H.-Y. Chen, L. Li and S. Peng, Adv. Mater., 2024, 36, 2308925 CrossRef CAS.
- Y. Hao, Y. Kang, S. Wang, Z. Chen, C. Lei, X. Cao, L. Chen, Y. Li, Z. Liu and M. Gong, Angew. Chem., Int. Ed., 2023, 62, e202303200 CrossRef CAS.
- Y. Shao, X. Xiao, Y. Zhu and T. Ma, Angew. Chem., Int. Ed., 2019, 58, 14599–14604 CrossRef CAS PubMed.
- K. Zhao, Y. Tao, L. Fu, C. Li and B. Xu, Angew. Chem., Int. Ed., 2023, 62, e202308335 CrossRef CAS PubMed.
- J. Xu, J. Li, Z. Lian, A. Araujo, Y. Li, B. Wei, Z. Yu, O. Bondarchuk, I. Amorim, V. Tileli, B. Li and L. Liu, ACS Catal., 2021, 11, 3402–3413 CrossRef CAS.
- D. Guo, Z. Zeng, Z. Wan, Y. Li, B. Xi and C. Wang, Adv. Funct. Mater., 2021, 31, 2101324 CrossRef CAS.
- S. Zhao, S.-F. Hung, L. Deng, W.-J. Zeng, T. Xiao, S. Li, C.-H. Kuo, H.-Y. Chen, F. Hu and S. Peng, Nat. Commun., 2024, 15, 2728 CrossRef CAS.
- M. Han, N. Wang, B. Zhang, Y. Xia, J. Li, J. Han, K. Yao, C. Gao, C. He, Y. Liu, Z. Wang, A. Seifitokaldani, X. Sun and H. Liang, ACS Catal., 2020, 10, 9725–9734 CrossRef CAS.
- K. H. Cho, S. Park, H. Seo, S. Choi, M. Y. Lee, C. Ko and K. T. Nam, Angew. Chem., Int. Ed., 2021, 60, 4673–4681 CrossRef CAS.
- X. Xie, M. Song, L. Wang, M. H. Engelhard, L. Luo, A. Miller, Y. Zhang, L. Du, H. Pan, Z. Nie, Y. Chu, L. Estevez, Z. Wei, H. Liu, C. Wang, D. Li and Y. Shao, ACS Catal., 2019, 9, 8712–8718 CrossRef CAS.
- Y. Gao, Z. Chen, Y. Zhao, W. Yu, X. Jiang, M. He, Z. Li, T. Ma, Z. Wu and L. Wang, Appl. Catal., B, 2022, 303, 120879 CrossRef CAS.
- S.-C. Sun, F.-X. Ma, H. Jiang, M.-X. Chen, P. Xu, L. Zhen, B. Song and C.-Y. Xu, Chem. Eng. J., 2023, 462, 142132 CrossRef CAS.
- Y. Chen, B. Gao, M. Wang, X. Xiao, A. Lv, S. Jiao and P. K. Chu, Nano Energy, 2021, 90, 106533 CrossRef CAS.
- N. M. Santhosh, S. Gupta, V. Shvalya, M. Košiček, J. Zavašnik and U. Cvelbar, Energy Fuels, 2025, 39, 1375–1383 CrossRef PubMed.
- Y. Tan, R. Xie, S. Zhao, X. Lu, L. Liu, F. Zhao, C. Li, H. Jiang, G. Chai, D. J. L. Brett, P. R. Shearing, G. He and I. P. Parkin, Adv. Funct. Mater., 2021, 31, 2105579 CrossRef CAS.
- Z. Cheng, Y. Xiao, W. Wu, X. Zhang, Q. Fu, Y. Zhao and L. Qu, ACS Nano, 2021, 15, 11417–11427 CrossRef CAS PubMed.
- Z. Niu, Z. Lu, Z. Qiao, S. Wang, X. Cao, X. Chen, J. Yun, L. Zheng and D. Cao, Adv. Mater., 2024, 36, 2310690 CrossRef CAS PubMed.
- H. Yang, P. Guo, R. Wang, Z. Chen, H. Xu, H. Pan, D. Sun, F. Fang and R. Wu, Adv. Mater., 2022, 34, 2107548 CrossRef CAS.
- Y. Gu, A. Wu, Y. Jiao, H. Zheng, X. Wang, Y. Xie, L. Wang, C. Tian and H. Fu, Angew. Chem., Int. Ed., 2021, 60, 6673–6681 CrossRef CAS PubMed.
- Y. Wang, X. Li, Z. Huang, H. Wang, Z. Chen, J. Zhang, X. Zheng, Y. Deng and W. Hu, Angew. Chem., Int. Ed., 2023, 62, e202215256 CrossRef CAS PubMed.
- X. Gao, Y. Chen, T. Sun, J. Huang, W. Zhang, Q. Wang and R. Cao, Energy Environ. Sci., 2020, 13, 174–182 RSC.
- M. Hu, J. Huang, S. Zhang, Z. Liu, Q. Li, M. Yang, H. Li, T. Goto and R. Tu, Int. J. Hydrogen Energy, 2021, 46, 9101–9109 CrossRef CAS.
- R.-Q. Li, P. Hu, M. Miao, Y. Li, X.-F. Jiang, Q. Wu, Z. Meng, Z. Hu, Y. Bando and X.-B. Wang, J. Mater. Chem. A, 2018, 6, 24767–24772 RSC.
- Z. Xie, W. Wang, D. Ding, Y. Zou, Y. Cui, L. Xu and J. Jiang, J. Mater. Chem. A, 2020, 8, 12169–12176 RSC.
- L. Zeng, K. Sun, Y. Chen, Z. Liu, Y. Chen, Y. Pan, R. Zhao, Y. Liu and C. Liu, J. Mater. Chem. A, 2019, 7, 16793–16802 RSC.
- Y. Ni, X. Ma, S. Wang, Y. Wang, F. Song, M. Cao and C. Hu, J. Power Sources, 2022, 521, 230934 CrossRef CAS.
- J. Zhao, T. Pan, J. Sun, H. Gao and J. Guo, Mater. Lett., 2020, 262, 127041 CrossRef.
- L. Liao, C. Cheng, H. Zhou, Y. Qi, D. Li, F. Cai, B. Yu, R. Long and F. Yu, Mater. Today Phys., 2022, 22, 100589 CrossRef CAS.
- L. Shang, Y. Zhao, X.-Y. Kong, R. Shi, G. I. N. Waterhouse, L. Wen and T. Zhang, Nano Energy, 2020, 78, 105375 CrossRef CAS.
- L. Zhang, B. Liu, N. Zhang and M. Ma, Nano Res., 2018, 11, 323–333 CrossRef CAS.
- L. Zhang, X. Gao, Y. Zhu, A. Liu, H. Dong, D. Wu, Z. Han, W. Wang, Y. Fang, J. Zhang, Z. Kou, B. Qian and T.-T. Wang, Nanoscale, 2021, 13, 2456–2464 RSC.
- F. Song, W. Li, J. Yang, G. Han, P. Liao and Y. Sun, Nat. Commun., 2018, 9, 4531 CrossRef PubMed.
- D. Liu, H. Ai, M. Chen, P. Zhou, B. Li, D. Liu, X. Du, K. H. Lo, K.-W. Ng, S.-P. Wang, S. Chen, G. Xing, J. Hu and H. Pan, Small, 2021, 17, 2007557 CrossRef CAS PubMed.
- T. Liu, X. Ma, D. Liu, S. Hao, G. Du, Y. Ma, A. M. Asiri, X. Sun and L. Chen, ACS Catal., 2017, 7, 98–102 CrossRef CAS.
- Y. Men, P. Li, J. Zhou, G. Cheng, S. Chen and W. Luo, ACS Catal., 2019, 9, 3744–3752 CrossRef CAS.
- D. Jiang, Y. Xu, R. Yang, D. Li, S. Meng and M. Chen, ACS Sustainable Chem. Eng., 2019, 7, 9309–9317 CrossRef CAS.
- K. Xu, Y. Sun, X. Li, Z. Zhao, Y. Zhang, C. Li and H. J. Fan, ACS Mater. Lett., 2020, 2, 736–743 CrossRef CAS.
- J. Zhu, F. Xia, Y. Guo, R. Lu, L. Gong, D. Chen, P. Wang, L. Chen, J. Yu, J. Wu and S. Mu, ACS Catal., 2022, 13312–13320 CrossRef.
- L. Zhang, P. F. Liu, Y. H. Li, C. W. Wang, M. Y. Zu, H. Q. Fu, X. H. Yang and H. G. Yang, ACS Catal., 2018, 8, 5200–5205 CrossRef CAS.
- H. Zhang, W. Liu, Z. Li, L. Qiao, K. Chi, X. Guo, D. Cao and D. Cheng, Adv. Sci., 2024, 11, 2401398 CrossRef CAS PubMed.
- H. Chen, Y. Xu, X. Li, Q. Ma, D. Xie, Y. Mei, G. Wang and Y. Zhu, Adv. Sci., 2024, 11, 2402889 CrossRef CAS PubMed.
- W. Yu, Z. Chen, Y. Fu, W. Xiao, B. Dong, Y. Chai, Z. Wu and L. Wang, Adv. Funct. Mater., 2023, 33, 2210855 CrossRef CAS.
- Y. Wu, L. Wang, T. Bo, Z. Chai, J. K. Gibson and W. Shi, Adv. Funct. Mater., 2023, 33, 2214375 CrossRef CAS.
- M. Rafiq, K. Harrath, M. Feng, R. Li, A. R. Woldu, P. K. Chu, L. Hu, F. Lu and X. Yao, Adv. Energy Mater., 2024, 14, 2402866 CrossRef CAS.
- L. Li, B. Wang, G. Zhang, G. Yang, T. Yang, S. Yang and S. Yang, Adv. Energy Mater., 2020, 10, 2001600 CrossRef CAS.
- Y. Guo, B. Hou, X. Cui, X. Liu, X. Tong and N. Yang, Adv. Energy Mater., 2022, 12, 2201548 CrossRef CAS.
- D. Bao, L. Huang, Y. Gao, K. Davey, Y. Zheng and S.-Z. Qiao, J. Am. Chem. Soc., 2024, 146, 34711–34719 CrossRef CAS PubMed.
- L. Zeng, Z. Liu, K. Sun, Y. Chen, J. Zhao, Y. Chen, Y. Pan, Y. Lu, Y. Liu and C. Liu, J. Mater. Chem. A, 2019, 7, 25628–25640 RSC.
- Z. Xie, W. Wang, D. Ding, Y. Zou, Y. Cui, L. Xu and J. Jiang, J. Mater. Chem. A, 2020, 8, 12169–12176 RSC.
- J. Huang, Y. Su, Y. Zhang, W. Wu, C. Wu, Y. Sun, R. Lu, G. Zou, Y. Li and J. Xiong, J. Mater. Chem. A, 2018, 6, 9467–9472 RSC.
- J. Fan, W. Hao, C. Fu, Z. Chen, R. Liang, C. Lian, Q. Zhang and G. Li, J. Mater. Chem. A, 2022, 10, 1535–1546 RSC.
- X. Lu, J. Pan, E. Lovell, T. H. Tan, Y. H. Ng and R. Amal, Energy Environ. Sci., 2018, 11, 1898–1910 RSC.
- J.-Z. Zhang, Z. Zhang, H.-B. Zhang, Y. Mei, F. Zhang, P.-X. Hou, C. Liu, H.-M. Cheng and J.-C. Li, Nano Lett., 2023, 23, 8331–8338 CrossRef CAS.
- J. Chen, C. Chen, M. Qin, B. Li, B. Lin, Q. Mao, H. Yang, B. Liu and Y. Wang, Nat. Commun., 2022, 13, 5382 CrossRef CAS.
- Y. Li, C.-K. Peng, H. Hu, S.-Y. Chen, J.-H. Choi, Y.-G. Lin and J.-M. Lee, Nat. Commun., 2022, 13, 1143 CrossRef CAS PubMed.
- K. Sun, X. Wu, Z. Zhuang, L. Liu, J. Fang, L. Zeng, J. Ma, S. Liu, J. Li, R. Dai, X. Tan, K. Yu, D. Liu, W.-C. Cheong, A. Huang, Y. Liu, Y. Pan, H. Xiao and C. Chen, Nat. Commun., 2022, 13, 1–11 Search PubMed.
- X. Zheng, X. Shi, H. Ning, R. Yang, B. Lu, Q. Luo, S. Mao, L. Xi and Y. Wang, Nat. Commun., 2023, 14, 4209 CrossRef CAS.
- J. Zhang, J. Li, H. Huang, W. Chen, Y. Cui, Y. Li, W. Mao, X. Zhu and X. Li, Small, 2022, 18, 2204557 CrossRef CAS PubMed.
- C.-T. Dinh, A. Jain, F. P. G. de Arquer, P. De Luna, J. Li, N. Wang, X. Zheng, J. Cai, B. Z. Gregory, O. Voznyy, B. Zhang, M. Liu, D. Sinton, E. J. Crumlin and E. H. Sargent, Nat. Energy, 2019, 4, 107–114 CrossRef CAS.
- K. Jiang, B. Liu, M. Luo, S. Ning, M. Peng, Y. Zhao, Y.-R. Lu, T.-S. Chan, F. M. F. de Groot and Y. Tan, Nat. Commun., 2019, 10, 1743 CrossRef PubMed.
- Z. Li, C. Yu, Y. Wen, Y. Gao, X. Xing, Z. Wei, H. Sun, Y.-W. Zhang and W. Song, ACS Catal., 2019, 9, 5084–5095 CrossRef CAS.
- N. Wang, S. Ning, X. Yu, D. Chen, Z. Li, J. Xu, H. Meng, D. Zhao, L. Li, Q. Liu, B. Lu and S. Chen, Appl. Catal., B, 2022, 302, 120838 CrossRef CAS.
- C. Peng, W. Zhao, Z. Kuang, J. T. Miller and H. Chen, Appl. Catal., A, 2021, 623, 118293 CrossRef CAS.
- Y. Yang, L. Liu, S. Chen, W. Yan, H. Zhou, X.-M. Zhang and X. Fan, Angew. Chem., Int. Ed., 2023, 62, e202306896 CrossRef CAS PubMed.
- N. Wang, P. Ou, S.-F. Hung, J. E. Huang, A. Ozden, J. Abed, I. Grigioni, C. Chen, R. K. Miao, Y. Yan, J. Zhang, Z. Wang, R. Dorakhan, A. Badreldin, A. Abdel-Wahab, D. Sinton, Y. Liu, H. Liang and E. H. Sargent, Adv. Mater., 2023, 35, 2210057 CrossRef CAS PubMed.
- Y. Li, W. Wang, M. Cheng, Y. Feng, X. Han, Q. Qian, Y. Zhu and G. Zhang, Adv. Mater., 2023, 35, 2206351 CrossRef CAS.
- R. Subbaraman, D. Tripkovic, D. Strmcnik, K.-C. Chang, M. Uchimura, A. P. Paulikas, V. Stamenkovic and N. M. Markovic, Science, 2011, 334, 1256–1260 CrossRef CAS PubMed.
- Y. Men, P. Li, F. Yang, G. Cheng, S. Chen and W. Luo, Appl. Catal., B, 2019, 253, 21–27 CrossRef CAS.
- X. Zhang and Y. Liang, Adv. Sci., 2018, 5, 1700644 CrossRef PubMed.
- S. Li, S. Sirisomboonchai, X. An, X. Ma, P. Li, L. Ling, X. Hao, A. Abudula and G. Guan, Nanoscale, 2020, 12, 6810–6820 RSC.
- M. Li, H. Wang, Y. Zhu, D. Tian, C. Wang and X. Lu, Appl. Surf. Sci., 2019, 496, 143672 CrossRef CAS.
- Z. Pu, M. Wang, Z. Kou, I. S. Amiinu and S. Mu, Chem. Commun., 2016, 52, 12753–12756 RSC.
- M. Liu, Z. Sun, C. Zhang, S. Li, C. He, Y. Liu and Z. Zhao, J. Mater. Chem. A, 2022, 10, 13410–13417 RSC.
- M. Ma, J. Xu, H. Wang, X. Zhang, S. Hu, W. Zhou and H. Liu, Appl. Catal., B, 2021, 297, 120455 CrossRef CAS.
- L. Xie, F. Qu, Z. Liu, X. Ren, S. Hao, R. Ge, G. Du, A. M. Asiri, X. Sun and L. Chen, J. Mater. Chem. A, 2017, 5, 7806–7810 RSC.
- Y. Tang, L. Dong, H. B. Wu and X.-Y. Yu, J. Mater. Chem. A, 2021, 9, 1456–1462 RSC.
- T. A. Kandiel, Appl. Catal., A, 2019, 586, 117226 CrossRef CAS.
- Q. Liu, C. Zhang, P. Wang, D. Chen, M. Xiao, L. Chen, S. Liu, J. Yu and S. Mu, J. Mater. Chem. A, 2022, 10, 12341–12349 RSC.
- P. Zhang, M. Wang, H. Chen, Y. Liang, J. Sun and L. Sun, Adv. Energy Mater., 2016, 6, 1502319 CrossRef.
- R. Boppella, J. Tan, W. Yang and J. Moon, Adv. Funct. Mater., 2019, 29, 1807976 CrossRef.
- W. Zhang, Y. Sun, Q. Liu, J. Guo and X. Zhang, J. Alloys Compd., 2019, 791, 1070–1078 CrossRef CAS.
- H. Liu, X. Peng, X. Liu, G. Qi and J. Luo, ChemSusChem, 2019, 12, 1334–1341 CrossRef CAS.
- R. Zhang, X. Wang, S. Yu, T. Wen, X. Zhu, F. Yang, X. Sun, X. Wang and W. Hu, Adv. Mater., 2017, 29, 1605502 CrossRef PubMed.
- M. J. Kenney, J. E. Huang, Y. Zhu, Y. Meng, M. Xu, G. Zhu, W.-H. Hung, Y. Kuang, M. Lin, X. Sun, W. Zhou and H. Dai, Nano Res., 2019, 12, 1431–1435 CrossRef CAS.
- L.-N. Zhang, S.-H. Li, H.-Q. Tan, S. U. Khan, Y.-Y. Ma, H.-Y. Zang, Y.-H. Wang and Y.-G. Li, ACS Appl. Mater. Interfaces, 2017, 9, 16270–16279 CrossRef CAS.
- L. Tang, J. Yu, Y. Zhang, Z. Tang and Y. Qin, RSC Adv., 2021, 11, 6107–6113 RSC.
- X.-D. Wang, Y.-F. Xu, H.-S. Rao, W.-J. Xu, H.-Y. Chen, W.-X. Zhang, D.-B. Kuang and C.-Y. Su, Energy Environ. Sci., 2016, 9, 1468–1475 RSC.
- G. Yan, C. Wu, H. Tan, X. Feng, L. Yan, H. Zang and Y. Li, J. Mater. Chem. A, 2017, 5, 765–772 RSC.
- T. Yu, Z. Wang, K. Tan, H. He and S. Yin, Mater. Today Phys., 2023, 35, 101138 CrossRef CAS.
- F. Wang, Y. Sun, Y. He, L. Liu, J. Xu, X. Zhao, G. Yin, L. Zhang, S. Li, Q. Mao, Y. Huang, T. Zhang and B. Liu, Nano Energy, 2017, 37, 1–6 Search PubMed.
- J. Li, Z. Xia, X. Zhou, Y. Qin, Y. Ma and Y. Qu, Nano Res., 2017, 10, 814–825 CrossRef CAS.
- R.-Q. Yao, Y.-T. Zhou, H. Shi, W.-B. Wan, Q.-H. Zhang, L. Gu, Y.-F. Zhu, Z. Wen, X.-Y. Lang and Q. Jiang, Adv. Funct. Mater., 2021, 31, 2009613 CrossRef CAS.
- A. Sivanantham, H. Lee, S. W. Hwang, B. Ahn and I. S. Cho, J. Mater. Chem. A, 2021, 9, 16841–16851 RSC.
- G. Meng, H. Tian, L. Peng, Z. Ma, Y. Chen, C. Chen, Z. Chang, X. Cui and J. Shi, Nano Energy, 2021, 80, 105531 CrossRef CAS.
- K. Wang, Y. Si, Z. Lv, T. Yu, X. Liu, G. Wang, G. Xie and L. Jiang, Int. J. Hydrogen Energy, 2020, 45, 2504–2512 CrossRef CAS.
- K. Miao, W. Jiang, Z. Chen, Y. Luo, D. Xiang, C. Wang and X. Kang, Adv. Mater., 2024, 36, 2308490 CrossRef CAS.
- F. Shiokawa, A. Asilah Haji Tajuddin, T. Ohto, Y. Yu, T. Fujita, H. Tanimoto, Z. Xi, S. Jeong and Y. Ito, Chem. Eng. J., 2024, 479, 147862 CrossRef CAS.
- Y. Wang, H. Su, Y. He, L. Li, S. Zhu, H. Shen, P. Xie, X. Fu, G. Zhou, C. Feng, D. Zhao, F. Xiao, X. Zhu, Y. Zeng, M. Shao, S. Chen, G. Wu, J. Zeng and C. Wang, Chem. Rev., 2020, 120, 12217–12314 CrossRef CAS.
- L. Zhang, L. Han, H. Liu, X. Liu and J. Luo, Angew. Chem., Int. Ed., 2017, 56, 13694–13698 CrossRef CAS PubMed.
- J. Li, R.-T. Gao, X. Liu, X. Zhang, L. Wu and L. Wang, ACS Appl. Mater. Interfaces, 2023, 15, 42501–42510 CrossRef CAS.
- C. Jin, L. Huo, J. Tang, S. Li, K. Jiang, Q. He, H. Dong, Y. Gong and Z. Hu, Small, 2024, 20, 2309509 CrossRef CAS.
- W. Wang, Y. Wu, Y. Lin, J. Yao, X. Wu, C. Wu, X. Zuo, Q. Yang, B. Ge, L. Yang, G. Li, S. Chou, W. Li and Y. Jiang, Adv. Funct. Mater., 2022, 32, 2108464 CrossRef CAS.
- R. Ge, Y. Wang, Z. Li, M. Xu, S.-M. Xu, H. Zhou, K. Ji, F. Chen, J. Zhou and H. Duan, Angew. Chem., Int. Ed., 2022, 61, e202200211 Search PubMed.
- D. Guo, X.-X. Xue, M. Jiao, J. Liu, T. Wu, X. Ma, D. Lu, R. Zhang, S. Zhang, G. Shao and Z. Zhou, Chem. Sci., 2024, 15, 16281–16290 RSC.
- W. Shao, Z. Xing, X. Xu, D. Ye, R. Yan, T. Ma, Y. Wang, Z. Zeng, B. Yin, C. Cheng and S. Li, J. Am. Chem. Soc., 2024, 146, 27486–27498 CrossRef CAS PubMed.
- Q. Wang, C. Xu, W. Liu, S. Hung, H. Bin Yang, J. Gao, W. Cai, H. M. Chen, J. Li and B. Liu, Nat. Commun., 2020, 11, 4246 CrossRef CAS PubMed.
- Z. Pu, T. Liu, G. Zhang, Z. Chen, D.-S. Li, N. Chen, W. Chen, Z. Chen and S. Sun, Adv. Energy Mater., 2022, 12, 2200293 CrossRef CAS.
- J. Liu, J. Yang, Y. Dou, X. Liu, S. Chen and D. Wang, Adv. Mater., 2025, 2420383 CrossRef CAS.
- Y. Yang, Y. Qian, H. Li, Z. Zhang, Y. Mu, D. Do, B. Zhou, J. Dong, W. Yan, Y. Qin, L. Fang, R. Feng, J. Zhou, P. Zhang, J. Dong, G. Yu, Y. Liu, X. Zhang and X. Fan, Sci. Adv., 2020, 6, eaba6586 CrossRef CAS PubMed.
- F. Zhang, Y. Zhu, Y. Chen, Y. Lu, Q. Lin, L. Zhang, S. Tao, X. Zhang and H. Wang, J. Mater. Chem. A, 2020, 8, 12810–12820 RSC.
- L. Yan, B. Zhang, J. Zhu, Z. Liu, H. Zhang and Y. Li, J. Mater. Chem. A, 2019, 7, 22453–22462 Search PubMed.
- J. Chen, Y. Ha, R. Wang, Y. Liu, H. Xu, B. Shang, R. Wu and H. Pan, Nano-Micro Lett., 2022, 14, 1–15 CrossRef.
- A. Fan, P. Zheng, C. Qin, X. Zhang, X. Dai, D. Ren, X. Fang, C. Luan and J. Yang, Electrochim. Acta, 2020, 358, 136927 CrossRef CAS.
- G. Li, D. Zhang, Y. Yu, S. Huang, W. Yang and L. Cao, J. Am. Chem. Soc., 2017, 139, 16194–16200 CrossRef CAS PubMed.
- G. Srividhya, T. Sangavi, C. Viswanathan and N. Ponpandian, ACS Appl. Energy Mater., 2024, 7, 154–164 CrossRef CAS.
- L. Zhang, B. Liu, N. Zhang and M. Ma, Nano Res., 2018, 11, 323–333 CrossRef CAS.
- Z. Lu and L. Sepunaru, Electrochim. Acta, 2020, 363, 137167 CrossRef CAS.
- W.-J. Kang, Y. Feng, Z. Li, W.-Q. Yang, C.-Q. Cheng, Z.-Z. Shi, P.-F. Yin, G.-R. Shen, J. Yang, C.-K. Dong, H. Liu, F.-X. Ye and X.-W. Du, Adv. Funct. Mater., 2022, 32, 2112367 CrossRef CAS.
- C.-H. Chiang, Y.-C. Yang, J.-W. Lin, Y.-C. Lin, P.-T. Chen, C.-L. Dong, H.-M. Lin, K. M. Chan, Y.-T. Kao, K. Suenaga, P.-W. Chiu and C.-W. Chen, ACS Nano, 2022, 16, 18274–18283 CrossRef CAS.
- H. Jin, X. Liu, A. Vasileff, Y. Jiao, Y. Zhao, Y. Zheng and S.-Z. Qiao, ACS Nano, 2018, 12, 12761–12769 CrossRef CAS PubMed.
- J. Xiao, C. Jiang, H. Zhang, Z. Xing, M. Qiu and Y. Yu, Chin. J. Catal., 2024, 63, 154–163 CrossRef CAS.
- S. Li, X. Qiu, X. An, E. Li, X. Li, G. Wang, P. Li, C. Shi, Y. Liu and G. Guan, Chem. Eng. J., 2024, 491, 151924 CrossRef CAS.
- L. Wang, L. Song, Z. Yang, Y.-M. Chang, F. Hu, L. Li, L. Li, H.-Y. Chen and S. Peng, Adv. Funct. Mater., 2023, 33, 2210322 CrossRef CAS.
- S. Yuan, Z. Pu, H. Zhou, J. Yu, I. S. Amiinu, J. Zhu, Q. Liang, J. Yang, D. He, Z. Hu, G. Van Tendeloo and S. Mu, Nano Energy, 2019, 59, 472–480 CrossRef CAS.
- L. Zhou, D. Guo, L. Wu, Z. Guan, C. Zou, H. Jin, G. Fang, X. Chen and S. Wang, Nat. Commun., 2024, 15, 2481 CrossRef CAS PubMed.
- L. Shang, Y. Zhao, X.-Y. Kong, R. Shi, G. I. N. Waterhouse, L. Wen and T. Zhang, Nano Energy, 2020, 78, 105375 CrossRef CAS.
- P. Kuang, Z. Ni, B. Zhu, Y. Lin and J. Yu, Adv. Mater., 2023, 35, 2303030 CrossRef CAS.
- W. Zhang, Y. Sun, Q. Liu, J. Guo and X. Zhang, J. Alloys Compd., 2019, 791, 1070–1078 CrossRef CAS.
- H. Yoon, H. J. Song, B. Ju and D.-W. Kim, Nano Res., 2020, 13, 2469–2477 CrossRef CAS.
- D. Y. Li, L. L. Liao, H. Q. Zhou, Y. Zhao, F. M. Cai, J. S. Zeng, F. Liu, H. Wu, D. S. Tang and F. Yu, Mater. Today Phys., 2021, 16, 100314 CrossRef CAS.
- R.-Q. Yao, Y.-T. Zhou, H. Shi, W.-B. Wan, Q.-H. Zhang, L. Gu, Y.-F. Zhu, Z. Wen, X.-Y. Lang and Q. Jiang, Adv. Funct. Mater., 2021, 31, 2009613 CrossRef CAS.
- G. Bahuguna, A. Cohen, B. Filanovsky and F. Patolsky, Adv. Sci., 2022, 9, 2203678 CrossRef CAS PubMed.
- J. M. Yu, J. Song, Y. K. Kim, J. Oh, K. Y. Kim, W. Y. Noh, W. J. Byun, J. U. Lee, C. Yang, J.-W. Jang, J. S. Lee and S. Cho, Nano Lett., 2023, 23, 5092–5100 CrossRef CAS.
- Y. Hao, Y. Kang, S. Wang, Z. Chen, C. Lei, X. Cao, L. Chen, Y. Li, Z. Liu and M. Gong, Angew. Chem., Int. Ed., 2023, 62, e202303200 CrossRef CAS.
- X. H. Chen, X. L. Li, T. Li, J. H. Jia, J. L. Lei, N. B. Li and H. Q. Luo, Energy Environ. Sci., 2024, 17, 5091–5101 RSC.
- J. (Jimmy) Liu, ChemCatChem, 2011, 3, 934–948 CrossRef.
- H. Shi, T. Wang, J. Liu, W. Chen, S. Li, J. Liang, S. Liu, X. Liu, Z. Cai, C. Wang, D. Su, Y. Huang, L. Elbaz and Q. Li, Nat. Commun., 2023, 14, 3934 CrossRef CAS PubMed.
- S. Huang, Y. Cao, C. Liang, M. Li, H. Yao, K.-H. Ye, Z. Huang, J. Meng and S. Zhang, J. Colloid Interface Sci., 2025, 690, 137382 CrossRef CAS.
- T. Li, B. He, X. Zhang, J. Fan, L. Gao, Z. Sun, J. Zhang, A. Guo, D. Pan, X. Yin, Y. Tong, C. Song, Y. Kohmura, M. Yabashi, T. Ishikawa, X. Gao and H. Jiang, Anal. Chem., 2022, 94, 13136–13144 CrossRef CAS PubMed.
- H. Kong, J. Zhang, J. Li, J. Wang, H.-J. Shin, R. Tai, Q. Yan, K. Xia, J. Hu, L. Wang, Y. Zhu and C. Fan, Natl. Sci. Rev., 2020, 7, 1218–1227 CrossRef CAS.
- Y. Xu, E. Hu, K. Zhang, X. Wang, V. Borzenets, Z. Sun, P. Pianetta, X. Yu, Y. Liu, X.-Q. Yang and H. Li, ACS Energy Lett., 2017, 2, 1240–1245 CrossRef CAS.
- K. Zhang, F. Ren, X. Wang, E. Hu, Y. Xu, X.-Q. Yang, H. Li, L. Chen, P. Pianetta, A. Mehta, X. Yu and Y. Liu, Nano Lett., 2017, 17, 7782–7788 CrossRef CAS.
- L. Gu, Y. Li, S. Zhang, M. Zhou, Y. Xue, W. Li, T. Xu and W. Ji, Nat. Methods, 2021, 18, 369–373 CrossRef CAS.
- C. Li, G. Chen, Y. Zhang, F. Wu and Q. Wang, J. Am. Chem. Soc., 2020, 142, 14789–14804 CrossRef CAS.
- C. H. Ryu, Y. Nam and H. S. Ahn, Chin. J. Catal., 2022, 43, 59–70 CrossRef CAS.
- Y. Liang, J. H. K. Pfisterer, D. McLaughlin, C. Csoklich, L. Seidl, A. S. Bandarenka and O. Schneider, Small Methods, 2019, 3, 1800387 CrossRef.
- L. K. S. Bonagiri, K. S. Panse, S. Zhou, H. Wu, N. R. Aluru and Y. Zhang, ACS Nano, 2022, 16, 19594–19604 CrossRef CAS.
- Z. Deng, Z. Huang, Y. Shen, Y. Huang, H. Ding, A. Luscombe, M. Johnson, J. E. Harlow, R. Gauthier and J. R. Dahn, Joule, 2020, 4, 2017–2029 CrossRef CAS.
- H. Huo, K. Huang, W. Luo, J. Meng, L. Zhou, Z. Deng, J. Wen, Y. Dai, Z. Huang, Y. Shen, X. Guo, X. Ji and Y. Huang, ACS Energy Lett., 2022, 7, 650–658 CrossRef CAS.
- J. Miao, P. Ercius and S. J. L. Billinge, Science, 2016, 353, aaf2157 CrossRef.
- Y. Yang, J. Zhou, F. Zhu, Y. Yuan, D. J. Chang, D. S. Kim, M. Pham, A. Rana, X. Tian, Y. Yao, S. J. Osher, A. K. Schmid, L. Hu, P. Ercius and J. Miao, Nature, 2021, 592, 60–64 CrossRef CAS PubMed.
- J. Schwartz, C. Harris, J. Pietryga, H. Zheng, P. Kumar, A. Visheratina, N. A. Kotov, B. Major, P. Avery, P. Ercius, U. Ayachit, B. Geveci, D. A. Muller, A. Genova, Y. Jiang, M. Hanwell and R. Hovden, Nat. Commun., 2022, 13, 1–7 Search PubMed.
- W. Yang, M. Li, B. Zhang, Y. Liu, J. Zi, H. Xiao, X. Liu, J. Lin, H. Zhang, J. Chen, Z. Wan, Z. Li, G. Li, H. Li and Z. Lian, Adv. Funct. Mater., 2023, 33, 2304852 CrossRef CAS.
- Y. Zeng, X. Li, J. Wang, M. T. Sougrati, Y. Huang, T. Zhang and B. Liu, Chem. Catal., 2021, 1, 1215–1233 CAS.
- J. Y. C. Chen, L. Dang, H. Liang, W. Bi, J. B. Gerken, S. Jin, E. E. Alp and S. S. Stahl, J. Am. Chem. Soc., 2015, 137, 15090–15093 CrossRef CAS.
- Y. Zeng, C. Li, B. Li, J. Liang, M. J. Zachman, D. A. Cullen, R. P. Hermann, E. E. Alp, B. Lavina, S. Karakalos, M. Lucero, B. Zhang, M. Wang, Z. Feng, G. Wang, J. Xie, D. J. Myers, J.-P. Dodelet and G. Wu, Nat. Catal., 2023, 6, 1215–1227 CrossRef CAS.
- X. Li, J. Cao, G. Chen, J. Xie, C. Gu, X. Li, F. C. Walsh, Y. Wang and W. Hu, ACS Nano, 2025, 19, 7851–7863 CrossRef CAS.
- T. He, W. Wang, F. Shi, X. Yang, X. Li, J. Wu, Y. Yin and M. Jin, Nature, 2021, 598, 76–81 CrossRef CAS PubMed.
- M. Luo and S. Guo, Nat. Rev. Mater., 2017, 2, 1–13 Search PubMed.
- Z. Xia and S. Guo, Chem. Soc. Rev., 2019, 48, 3265–3278 RSC.
- X. Mao, Z. Qin, S. Ge, C. Rong, B. Zhang and F. Xuan, Mater. Horiz., 2023, 10, 340–360 RSC.
- W.-J. Kang, Y. Feng, Z. Li, W.-Q. Yang, C.-Q. Cheng, Z.-Z. Shi, P.-F. Yin, G.-R. Shen, J. Yang, C.-K. Dong, H. Liu, F.-X. Ye and X.-W. Du, Adv. Funct. Mater., 2022, 32, 2112367 CrossRef CAS.
- C. Wei and Z. J. Xu, Small Methods, 2018, 2, 1800168 CrossRef.
- J. Tafel, Z. Phys. Chem., 1905, 50U, 641–712 CrossRef.
- S. Qian, F. Xu, Y. Fan, N. Cheng, H. Xue, Y. Yuan, R. Gautier, T. Jiang and J. Tian, Nat. Commun., 2024, 15, 2774 CrossRef CAS PubMed.
- O. van der Heijden, S. Park, R. E. Vos, J. J. J. Eggebeen and M. T. M. Koper, ACS Energy Lett., 2024, 9, 1871–1879 CrossRef CAS PubMed.
- F. Bao, E. Kemppainen, I. Dorbandt, R. Bors, F. Xi, R. Schlatmann, R. van de Krol and S. Calnan, ChemElectroChem, 2021, 8, 195–208 CrossRef CAS.
- S. Díaz-Coello, G. García, M. C. Arévalo and E. Pastor, Int. J. Hydrogen Energy, 2019, 44, 12576–12582 CrossRef.
- E. Sohouli, E. M. Khosrowshahi, P. Radi, E. Naghian, M. Rahimi-Nasrabadi and F. Ahmadi, Electroanal. Chem., 2020, 877, 114503 CrossRef CAS.
- T. Shinagawa, A. T. Garcia-Esparza and K. Takanabe, Sci. Rep., 2015, 5, 13801 CrossRef PubMed.
- Z.-Y. Yu, Y. Duan, Y. Kong, X.-L. Zhang, X.-Y. Feng, Y. Chen, H. Wang, X. Yu, T. Ma, X. Zheng, J. Zhu, M.-R. Gao and S.-H. Yu, J. Am. Chem. Soc., 2022, 144, 13163–13173 CrossRef CAS PubMed.
- M. Han, H. Wang, J. Zhou, K. Liu, N. Wang, X. Chen, Y. Liu and H. Liang, Adv. Funct. Mater., 2025, 35, 2415143 CrossRef CAS.
- L.-L. Wang, X.-R. Wang, H.-J. Wang, C. Zhang, J.-J. Li, G.-J. Feng, X.-X. Cheng, X.-R. Qin, Z.-Y. Yu and T.-B. Lu, J. Am. Chem. Soc., 2025, 147, 7555–7563 CrossRef CAS.
- P. Li, Y. Jiang, Y. Hu, Y. Men, Y. Liu, W. Cai and S. Chen, Nat. Catal., 2022, 5, 900–911 CrossRef CAS.
- Y.-T. Kim, P. P. Lopes, S.-A. Park, A.-Y. Lee, J. Lim, H. Lee, S. Back, Y. Jung, N. Danilovic, V. Stamenkovic, J. Erlebacher, J. Snyder and N. M. Markovic, Nat. Commun., 2017, 8, 1449 CrossRef PubMed.
- C. C. L. McCrory, S. Jung, J. C. Peters and T. F. Jaramillo, J. Am. Chem. Soc., 2013, 135, 16977–16987 CrossRef CAS PubMed.
- X. Liang, S. Wang, J. Feng, Z. Xu, Z. Guo, H. Luo, F. Zhang, C. Wen, L. Feng, C. Wan and M.-M. Titirici, Inorg. Chem. Front., 2023, 10, 2961–2977 RSC.
- S. Franz, H. Arab, G. L. Chiarello, M. Bestetti and E. Selli, Adv. Energy Mater., 2020, 10, 2000652 CrossRef CAS.
- C. Huang, Q. Zhou, L. Yu, D. Duan, T. Cao, S. Qiu, Z. Wang, J. Guo, Y. Xie, L. Li and Y. Yu, Adv. Energy Mater., 2023, 13, 2301475 CrossRef CAS.
- S. Geiger, O. Kasian, M. Ledendecker, E. Pizzutilo, A. M. Mingers, W. T. Fu, O. Diaz-Morales, Z. Li, T. Oellers, L. Fruchter, A. Ludwig, K. J. J. Mayrhofer, M. T. M. Koper and S. Cherevko, Nat. Catal., 2018, 1, 508–515 CrossRef CAS.
- Z. Wang, Q. Zhou, L. Luo, Y. Shi, H. Li, C. Wang, K. Lin, C. Wang, L. Zhu, L. Han, Z. Xing and Y. Yu, Chin. J. Catal., 2025, 76, 146–158 CrossRef CAS.
- M. Retuerto, L. Pascual, J. Torrero, M. A. Salam, Á. Tolosana-Moranchel, D. Gianolio, P. Ferrer, P. Kayser, V. Wilke, S. Stiber, V. Celorrio, M. Mokthar, D. G. Sanchez, A. S. Gago, K. A. Friedrich, M. A. Peña, J. A. Alonso and S. Rojas, Nat. Commun., 2022, 13, 7935 CrossRef CAS PubMed.
- C. W. Song, J. Lim, H. B. Bae and S.-Y. Chung, Energy Environ. Sci., 2020, 13, 4178–4188 RSC.
- C. W. Song, H. Suh, J. Bak, H. B. Bae and S.-Y. Chung, Chem, 2019, 5, 3243–3259 CAS.
- J. Zhang, K. Jia, Y. Huang, X. Liu, Q. Xu, W. Wang, R. Zhang, B. Liu, L. Zheng, H. Chen, P. Gao, S. Meng, L. Lin, H. Peng and Z. Liu, Adv. Mater., 2022, 34, 2103620 CrossRef CAS.
- B. Zhong, S. Wan, P. Kuang, B. Cheng, L. Yu and J. Yu, Appl. Catal., B, 2024, 340, 123195 CrossRef CAS.
- Z. Chen, W. Gong, J. Wang, S. Hou, G. Yang, C. Zhu, X. Fan, Y. Li, R. Gao and Y. Cui, Nat. Commun., 2023, 14, 5363 CrossRef CAS.
- X. Wang, C. Xu, M. Jaroniec, Y. Zheng and S. Qiao, Nat. Commun., 2019, 10, 4876 CrossRef CAS PubMed.
- H. Ren, Z. Zhang, Z. Geng, Z. Wang, F. Shen, X. Liang, Z. Cai, Y. Wang, D. Cheng, Y. Cao, X. Yang, M. Hu, X. Yao and K. Zhou, Adv. Energy Mater., 2024, 14, 2400777 CrossRef CAS.
- L. Karuppasamy, L. Gurusamy, S. Anandan, S. C. Barton, C.-H. Liu and J. J. Wu, Chem. Eng. J., 2024, 495, 153442 CrossRef CAS.
- Z. Duan, D. Zhao, Y. Sun, X. Tan and X. Wu, Nano Res., 2022, 15, 8865–8871 CrossRef CAS.
- P. Zhou, X. Lv, S. Tao, J. Wu, H. Wang, X. Wei, T. Wang, B. Zhou, Y. Lu, T. Frauenheim, X. Fu, S. Wang and Y. Zou, Adv. Mater., 2022, 34, 2204089 CrossRef CAS.
- J. Wang, Z. Wei, S. Mao, H. Li and Y. Wang, Energy Environ. Sci., 2018, 11, 800–806 RSC.
- S. Fan, X. Han, L. Li, Y. Xu, W. Gao, Y. Zhang, Z. Gao and C. Wang, Nano Res., 2025, 18, 94907305 CrossRef.
- R. Rossi, D. M. Hall, L. Shi, N. R. Cross, C. A. Gorski, M. A. Hickner and B. E. Logan, Energy Environ. Sci., 2021, 14, 6041–6049 RSC.
- J. Xiao, A. M. Oliveira, L. Wang, Y. Zhao, T. Wang, J. Wang, B. P. Setzler and Y. Yan, ACS Catal., 2021, 11, 264–270 CrossRef CAS.
- F. Razmjooei, T. Morawietz, E. Taghizadeh, E. Hadjixenophontos, L. Mues, M. Gerle, B. D. Wood, C. Harms, A. S. Gago, S. A. Ansar and K. A. Friedrich, Joule, 2021, 5, 1776–1799 CrossRef CAS.
- L. Xiao, S. Zhang, J. Pan, C. Yang, M. He, L. Zhuang and J. Lu, Energy Environ. Sci., 2012, 5, 7869–7871 RSC.
- L. Wan, J. Liu, D. Lin, Z. Xu, Y. Zhen, M. Pang, Q. Xu and B. Wang, Energy Environ. Sci., 2024, 17, 3396–3408 RSC.
- Z. Li, G. Lin, L. Wang, H. Lee, J. Du, T. Tang, G. Ding, R. Ren, W. Li, X. Cao, S. Ding, W. Ye, W. Yang and L. Sun, Nat. Catal., 2024, 7, 944–952 CrossRef CAS.
- X. Zhong, L. Sui, M. Yang, T. Koketsu, M. Klingenhof, S. Selve, K. G. Reeves, C. Ge, L. Zhuang, W. H. Kan, M. Avdeev, M. Shu, N. Alonso-Vante, J.-M. Chen, S.-C. Haw, C.-W. Pao, Y.-C. Chang, Y. Huang, Z. Hu, P. Strasser and J. Ma, Nat. Catal., 2024, 1–14 Search PubMed.
- M. Ning, F. Zhang, L. Wu, X. Xing, D. Wang, S. Song, Q. Zhou, L. Yu, J. Bao, S. Chen and Z. Ren, Energy Environ. Sci., 2022, 15, 3945–3957 RSC.
- W. Tong, M. Forster, F. Dionigi, S. Dresp, R. Sadeghi Erami, P. Strasser, A. J. Cowan and P. Farràs, Nat. Energy, 2020, 5, 367–377 CrossRef CAS.
- S. Dresp, T. N. Thanh, M. Klingenhof, S. Brückner, P. Hauke and P. Strasser, Energy Environ. Sci., 2020, 13, 1725–1729 RSC.
- J.-Y. Zhang, H. Wang, Y. Tian, Y. Yan, Q. Xue, T. He, H. Liu, C. Wang, Y. Chen and B. Y. Xia, Angew. Chem., Int. Ed., 2018, 57, 7649–7653 CrossRef CAS PubMed.
- Q. Qian, J. Zhang, J. Li, Y. Li, X. Jin, Y. Zhu, Y. Liu, Z. Li, A. El-Harairy, C. Xiao, G. Zhang and Y. Xie, Angew. Chem., Int. Ed., 2021, 60, 5984–5993 CrossRef CAS PubMed.
- F. Sun, D. He, K. Yang, J. Qiu and Z. Wang, Angew. Chem., Int. Ed., 2022, 61, e202203929 CrossRef CAS PubMed.
- T. Wang, L. Tao, X. Zhu, C. Chen, W. Chen, S. Du, Y. Zhou, B. Zhou, D. Wang, C. Xie, P. Long, W. Li, Y. Wang, R. Chen, Y. Zou, X.-Z. Fu, Y. Li, X. Duan and S. Wang, Nat. Catal., 2022, 5, 66–73 CrossRef CAS.
- G. Li, G. Han, L. Wang, X. Cui, N. K. Moehring, P. R. Kidambi, D. Jiang and Y. Sun, Nat. Commun., 2023, 14, 525 CrossRef CAS.
- R. Ge, Y. Wang, Z. Li, M. Xu, S.-M. Xu, H. Zhou, K. Ji, F. Chen, J. Zhou and H. Duan, Angew. Chem., Int. Ed., 2022, 61, e202200211 CrossRef CAS.
- Y. Wu, X. Zhu, S. Du, G. Huang, B. Zhou, Y. Lu, Y. Li, S. P. Jiang, L. Tao and S. Wang, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2300625120 CrossRef CAS.
- L. Zhang, Z. Wang and J. Qiu, Adv. Mater., 2022, 34, 2109321 CrossRef CAS PubMed.
- S. Zhang, Q. Zhou, Z. Shen, X. Jin, Y. Zhang, M. Shi, J. Zhou, J. Liu, Z. Lu, Y.-N. Zhou and H. Zhang, Adv. Funct. Mater., 2021, 31, 2101922 CrossRef CAS.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2026 |