Initial stage of O2 adsorption on Ag(100): vacancies and local oxide formation
B. V.
Andryushechkin
 *ab,
T. V.
Pavlova
*ab,
T. V.
Pavlova
 a,
V. M.
Shevlyuga
a,
V. M.
Shevlyuga
 a,
A. V.
Nartova
a,
A. V.
Nartova
 c and
V. I.
Bukhtiyarov
c and
V. I.
Bukhtiyarov
 c
c
aProkhorov General Physics Institute of the Russian Academy of Sciences, Vavilov str. 38, 119991, Moscow, Russia. E-mail: andrush@kapella.gpi.ru
bHSE University, Myasnitskaya str. 20, 101000, Moscow, Russia
cBoreskov Institute of Catalysis, Pr. Lavrentieva 5, 630090, Novosibirsk, Russia
First published on 1st December 2025
Abstract
The early stage of molecular oxygen adsorption on the Ag(100) surface was reexamined with scanning tunneling microscopy (STM) and density functional theory. Using an oxygen-functionalized STM tip, we demonstrated that the simple interpretation of the individual objects randomly distributed over the surface as chemisorbed oxygen atoms is incompatible with the cross-shaped structures observed in atomic-resolution images in the current work. In our model, each feature is an oxide-like cross formed by two intersecting O–Ag–O–Ag–O chains, with oxygen atoms in vacancies in the Ag(100) layer. Furthermore, we found numerous oxygen-free vacancies randomly diffusing over the surface under the STM probe even at 77 K.
The interaction of oxygen with Ag surfaces has attracted considerable attention due to the unique role of silver as a highly selective catalyst in the industrial ethylene epoxidation reaction.1 Although highly sophisticated studies have been conducted over the past five decades with O/Ag systems, which are thus quite well characterized, the reaction has not been reproduced under controlled conditions, and the nature of the active oxygen remains unclear.
It should be noted that most studies have focused on the Ag(110) and Ag(111) faces, leaving Ag(100) aside as a less interesting case, which was considered to have intermediate properties between open and close-packed surfaces. Nevertheless, the Ag(100) surface deserves detailed study, since, according to recent data,2,3 the selectivity of the epoxidation reaction on this face is highest compared to the (111) and (110) faces.
The structural properties of the oxidized Ag(100) surface were studied using high-resolution electron energy loss spectroscopy (HREELS),4 X-ray photoelectron diffraction (XPD),5 low-energy electron diffraction (LEED),5 and scanning tunneling microscopy.6–8 It was found that the O2 adsorption at 120 K leads to the adsorption of oxygen molecules at positions between four silver atoms, forming a c(2 × 4) structure.9 Further heating leads to dissociation of the oxygen molecules (already at 130 K), and a c(2 × 2) structure appears around 190 K. Based on XPD, a missing-row 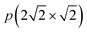 structure was proposed in which the oxygen atoms form a c(2 × 2) structure.5In situ surface X-ray diffraction (SXRD) experiments demonstrate the local presence of the missing-row structure at elevated temperatures and near-atmospheric pressures.10 This is supported by density functional theory (DFT) calculations, which showed that the reconstructed missing-row
structure was proposed in which the oxygen atoms form a c(2 × 2) structure.5In situ surface X-ray diffraction (SXRD) experiments demonstrate the local presence of the missing-row structure at elevated temperatures and near-atmospheric pressures.10 This is supported by density functional theory (DFT) calculations, which showed that the reconstructed missing-row 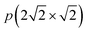 structure is thermodynamically more stable than the c(2 × 2) adatom structure.11 Application of STM to the O/Ag(100) system allows a previously unknown diversity of surface structures to be revealed. A disordered phase was formed at low coverages and temperatures of 130–200 K.6–8 It was visualized in a scanning tunneling microscope as an array of circular depressions or sombrero-like objects, depending on the bias voltage, and was attributed to an array of individual oxygen atoms chemisorbed in the hollow sites on Ag(100).6–8 In addition, STM revealed new well ordered c(4 × 6) and (6 × 6) reconstructions, which had not been previously reported.8
structure is thermodynamically more stable than the c(2 × 2) adatom structure.11 Application of STM to the O/Ag(100) system allows a previously unknown diversity of surface structures to be revealed. A disordered phase was formed at low coverages and temperatures of 130–200 K.6–8 It was visualized in a scanning tunneling microscope as an array of circular depressions or sombrero-like objects, depending on the bias voltage, and was attributed to an array of individual oxygen atoms chemisorbed in the hollow sites on Ag(100).6–8 In addition, STM revealed new well ordered c(4 × 6) and (6 × 6) reconstructions, which had not been previously reported.8
In this work, we investigated the disordered phase formed at the initial stage of the interaction of oxygen with the Ag(100) surface at higher temperatures of 300–423 K. It turned out that its STM images obtained for a clean tip are similar to those obtained in previous studies at lower temperatures.6–8 However, high-resolution STM measurement performed with the oxygen functionalized tip and DFT calculations, allows us to conclude that each object previously assigned to a chemisorbed oxygen atom is either an oxide-like cross (Ag4O5) or a vacancy in the upper Ag(100) layer. The resulting coverage for the disordered phase was found to be larger than in the simple chemisorbed oxygen model.
All experiments were carried out in an UHV setup with a base pressure 2 × 10−11 Torr containing LT-STM GPI CRYO12 operating at 77 K. The Ag(100) sample was cleaned by several cycles of Ar+ sputtering (600 eV) and annealing (750 K) and characterized by STM, LEED, and Auger electron spectroscopy (AES). A high-pressure reactor attached to the main setup was used to oxidize the Ag(100) sample by O2 with pressure of 1 × 10−6–1 × 10−4 Torr at the temperature range of 300–423 K. To acquire STM images, we used clean and oxygen-functionalized PtRh tips.
All DFT calculations were performed by using the VASP package13,14 with the PBE exchange–correlation functional.15 The silver (100) substrate was modeled by four- and six-layer slabs with an (8 × 8) surface unit cell. During structure optimization, the bottom two layers of Ag were held fixed, while the upper layers of Ag atoms as well as the oxygen atoms were allowed to relax.
The plane wave cut-off energy of 400 eV was applied. The reciprocal space was described with a Monkhorst–Pack grid16 of (4 × 4 × 1) k-points. The vacuum region of 15 Å was used between two neighboring slabs. STM simulations were performed within the Tersoff–Hamann approximation17 using the Hive program.18
Fig. 1a shows an STM image of the Ag(100) surface obtained after exposure to O2 (2.5 × 10−6 Torr for 6 min) at 373 K using a pure PtRh probe. A number of similar dark features forming a disordered phase are visible. Typically, these features are visualized in a scanning tunneling microscope as dark depressions (Fig. 1a and b); however, at low bias voltages, light protrusions appeared at their centers (see Fig. 1c). Similar STM images were observed for oxygen adsorption temperatures in the range of 300–473 K. The change in visualization of the disordered phase is consistent with STM data obtained in early studies of low-temperature (140–200 K) oxygen adsorption on Ag(100),6–8 where similar objects were associated with individual oxygen atoms. It is noteworthy that no direct evidence for such a simple interpretation was obtained in the studies,6–8 primarily due to the lack of atomic resolution on the substrate, which complicates the determination of adsorption sites.
It is known that the special state of the probe (functionalized with an oxygen atom) allows for obtaining STM images with higher resolution.19,20 In the previous studies using such a tip, we obtained high-resolution STM images of the disordered phase in the O/Ag(111) system21 and resolved the atomic structure of the Ag(111)-p(4 × 4)-O reconstruction.22
In the present work, we also used a functionalized STM probe. We found that prolonged scanning of the oxidized Ag(100) surface at negative bias voltages in the range of −2.5 V to −0.56 V leads to switching to the atomic-resolution mode, which we attribute to the formation of the oxygen-terminated tip. Fig. 2a shows an STM image obtained with this probe. It shows good atomic resolution, corresponding to the Ag(100) lattice, and cross-shaped structures instead of dark depressions or sombrero-like structures. Remarkably, this atomic resolution mode is stable while maintaining bias voltages in the above-mentioned range. Increasing the voltage to positive values immediately results in a switch to the normal scanning mode, as is evident from the STM image in Fig. 2b, which shows the same surface area as the image in Fig. 2a. An analysis of Fig. 2a allows us to conclude that there are two types of crosses: large (‘1’) and small (‘2’). Enlarged images of the large and small crosses are shown in Fig. 2c and Fig. 2d, respectively. Analysis of the STM images of both crosses suggests that their centers correspond to the positions of silver atoms in the Ag(100) lattice. We associate the depressions observed at the centers of each cross with the formation of vacancies in the upper silver layer.
Fig. 3 presents STM images demonstrating diffusion of crosses ‘1’ and ‘2’ during the scanning. We see that large crosses are not mobile, while small crosses can move over the surface. We specifically used two values of tunneling current to determine whether this motion is caused by the tip influence or by an intrinsic property of the system at 77 K. No principal difference in the speed of small crosses movement was detected; therefore, we can not exclude the noticeable diffusion rates of vacancies on the oxidized Ag(100) surface at low temperature. Notably, in normal scanning mode, in which both crosses are visualized as black spots, movement of some spots and stability of others can also be observed.
Further analysis was performed using DFT calculations. To find a suitable structural model of the large cross, we tested many configurations (see Fig. S1 in the SI). Fig. 4a shows the structural model for which the theoretical STM image (Fig. 4b) is in the best agreement with the experiment (Fig. 4c). It turned out that the large cross is a complex object in which five silver atoms in the top layer are removed, four oxygen atoms are located in corner vacancies, and the central oxygen atom is located between the second and third silver layers. This leads to the formation of a cross-shaped local oxide consisting of crossed O–Ag–O–Ag–O chains (see Fig. 4a). We also collected STM images obtained with the functionalized tip in the range from −2.5 V to −0.56 V and presented them in Fig. S2. It is clear that the shape of the objects remains virtually unchanged within the experimental noise level. The theoretical STM images of cross ‘1’ also show no principal changes in the mentioned range of Us. These facts suggest that the apparent shape of the cross is due to actual atomic geometry and not to an electronic effect.
Occasionally, in the STM images, we see connected crosses forming larger two-dimensional objects (see Fig. S3) with fragments of the c(2 × 2) structure inside. In this connection, object ‘1’ can be considered as an elemental building block for the construction of ordered 2D oxygen structures on Ag(100). Thus, there is preliminary evidence that the combination of crosses into a two-dimensional structure can lead to the formation of c(2 × 2) islands, embedded one layer deep into the silver surface.
Further DFT calculations showed that the experimental image of the small cross best corresponds to a simple vacancy in the upper silver layer (see Fig. 4d–f). Given the high mobility of small crosses at 77 K, this interpretation seems most likely. Sometimes two more types of cross-shaped objects (‘3’ and ‘4’) containing a central protrusion can be detected in the STM images (Fig. 4i and l). In this connection, we also considered models in which the oxygen atom is located in a vacancy. Indeed, two stable configurations were found in which the oxygen atom is located either between the first and second silver layers (Fig. 4g) or inside the first silver layer (Fig. 4j). In both cases, the theoretical STM images obtained at Us in the range of −1.5 to −2 V show protrusions at the centers (see Fig. 4h and k). It should be noted here that for a deep location of the oxygen atom, the height of the protrusion in the theoretical STM image shown in Fig. 4h is somewhat lower than the surrounding silver atoms, which is similar to the experimental STM image of object ‘3’ (Fig. 4i). For a top position of the oxygen atom in a vacancy, the height of the central protrusion measured on the theoretical STM image is about 0.4 Å, which is consistent with the height of the protrusion measured in the experimental STM image of object ‘4’ (Fig. 4l). Thus, the obtained results indicate the possibility of filling vacancies with oxygen atoms. It is noteworthy that filled vacancies are immobile or, at least, their diffusion rate is significantly lower than for empty vacancies.
In the next stage of the work, we estimated the energetics of three systems containing a vacancy in the upper Ag(100) layer and a single oxygen atom (see Fig. 4g, j and m). The first two configurations correspond to different positions of the oxygen atom in the vacancy (Fig. 4g and j). The third configuration contains a vacancy and one oxygen atom adsorbed in a hollow site on the terrace (see Fig. 4m). DFT calculations show that the latter configuration is more than 0.63 eV more favorable than configurations in which the vacancy is occupied by oxygen. In other words, at equilibrium, filling vacancies is unfavorable. However, we have a system that exhibits highly dynamic vacancy motion. Therefore, we see that oxygen atoms can temporarily occupy positions inside vacancies. Although the theoretical STM image in Fig. 4n shows the oxygen atom as a protrusion, we did see chemisorbed oxygen in our STM images. It is likely that oxygen on the terraces is highly mobile. Moreover, it is possible that oxygen adsorption on Ag(100) can lead to the formation of mobile silver adatoms, which can form Ag–O complexes moving along the surface. This explanation is consistent with the observations of a mobile O–Ag–O complex presented in ref. 23 and 24 for the O/Ag(110) system, in which vacancy formation occurs at temperatures below 200 K.
For completeness, two vacancy-free configurations were considered. In the first configuration, the oxygen atom was located above the silver atom, and in the second, it was located below the silver atom between the first and second silver layers. In both cases, the theoretical STM images show bright protrusions, unlike the experimental data.
The key point of this study is the direct evidence of the formation of oxygen-induced vacancies on the Ag(100) surface in the temperature range of 300–423 K. A similar effect was obtained for the O/Ag(111) and O/Ag(110) systems.21,23–25 We cannot definitively attribute all the features observed in previous studies, where oxygen adsorption was performed at low temperatures (130–200 K), to local oxide or vacancies. However, the similarity of the STM images obtained in these studies to the images of the disordered phase shown in Fig. 1 may indicate the possibility of vacancy formation at low temperatures.
It should be noted that Rocca et al.5 detected a phase transition in the O/Ag(100) system between two oxygen states characterized by an O 1s energy of 530 eV below room temperature and 528 eV above room temperature. We believe that in our case STM indeed reveals structures formed at the adsorption temperature (>300 K), since the characteristic cooling time to 246 K in our STM (<5 min) is significantly shorter than the phase transition time (50–333 min) reported in ref. 5 Since long-term scanning did not reveal any fundamental changes in the surface structures, we believe that the phase transition is blocked at 77 K. In this regard, taking into account the data of previous work,5 our structures should correspond to O 1s 528 eV.
In summary, STM and DFT methods were used to study the disordered oxygen phase forming on the Ag(100) surface at low coverages in the range of 300–473 K. Based on atomic-resolution STM images, we demonstrated that the dark depressions typically attributed to individual oxygen atoms are complex objects that can be described by oxide-like crosses formed by five vacancies and five oxygen atoms. We also detected the formation of single vacancies in the Ag(100) layer, which are highly mobile at 77 K. The formation of local oxide and vacancies leading to the generation of adatoms is important for understanding the catalytic properties of silver in particular and noble metals in general, as vacancies and adatoms are undercoordinated and therefore inherently reactive.
Author contributions
B. V. Andryushechkin: writing – review and editing, writing – original draft, and supervision. T. V. Pavlova: software, methodology, and investigation. V. M. Shevlyuga: formal analysis and data curation. A. V. Nartova: visualization, software and investigation. V. I. Bukhtiyarov: project administration and funding acquisition.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included in the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5cp04099j.Acknowledgements
This work was supported by the Russian Science Foundation (grant no. 24-63-00037; https://rscf.ru/project/24-63-00037/). This research was supported in part through computational resources of the HPC facilities at HSE University.Notes and references
- J. Serafin, A. Liu and S. Seyedmonir, J. Mol. Catal. A: Chem., 1998, 131, 157–168 CrossRef CAS.
- P. Christopher and S. Linic, J. Am. Chem. Soc., 2008, 130, 11264–11265 CrossRef CAS PubMed.
- M. Huš and A. Hellman, ACS Catal., 2019, 9, 1183–1196 CrossRef.
- D. Loffreda, A. Dal Corso, S. Baroni, L. Savio, L. Vattuone and M. Rocca, Surf. Sci., 2003, 530, 26–36 CrossRef CAS.
- M. Rocca, L. Savio, L. Vattuone, U. Burghaus, V. Palomba, N. Novelli, F. Buatier de Mongeot, U. Valbusa, R. Gunnella, G. Comelli, A. Baraldi, S. Lizzit and G. Paolucci, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 213–227 CrossRef CAS.
- S. Schintke, S. Messerli, K. Morgenstern, J. Nieminen and W.-D. Schneider, J. Chem. Phys., 2001, 114, 4206–4209 CrossRef CAS.
- M.-F. Hsieh, D.-S. Lin, H. Gawronski and K. Morgenstern, J. Chem. Phys., 2009, 131, 174709 CrossRef PubMed.
- I. Costina, M. Schmid, H. Schiechl, M. Gajdoš, A. Stierle, S. Kumaragurubaran, J. Hafner, H. Dosch and P. Varga, Surf. Sci., 2006, 600, 617–624 CrossRef CAS.
- S. Messerli, S. Schintke, K. Morgenstern, J. Nieminen and W.-D. Schneider, Chem. Phys. Lett., 2000, 328, 330–336 CrossRef CAS.
- A. Stierle, I. Costina, S. Kumaragurubaran and H. Dosch, J. Phys. Chem. C, 2007, 111, 10998–11002 CrossRef CAS.
- M. Gajdoš, A. Eichler and J. Hafner, Surf. Sci., 2003, 531, 272–286 CrossRef.
- https://www.sigmascan.ru .
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558–561 CrossRef CAS PubMed.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B: Solid State, 1976, 13, 5188–5192 CrossRef.
- J. Tersoff and D. R. Hamann, Phys. Rev. B: Condens. Matter Mater. Phys., 1985, 31, 805–813 CrossRef CAS PubMed.
- D. E. P. Vanpoucke and G. Brocks, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 241308(R) CrossRef.
- H. Mönig, M. Todorović, M. Z. Baykara, T. C. Schwendemann, L. Rodrigo, E. I. Altman, R. Pérez and U. D. Schwarz, ACS Nano, 2013, 7, 10233–10244 CrossRef PubMed.
- J. Bamidele, Y. Kinoshita, R. Turanský, S. H. Lee, Y. Naitoh, Y. J. Li, Y. Sugawara, I. S. Štich and L. Kantorovich, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 86, 155422 CrossRef.
- B. V. Andryushechkin, V. M. Shevlyuga, T. V. Pavlova, G. M. Zhidomirov and K. N. Eltsov, Phys. Rev. Lett., 2016, 117, 056101 CrossRef CAS PubMed.
- B. V. Andryushechkin, T. V. Pavlova and V. M. Shevlyuga, Phys. Chem. Chem. Phys., 2024, 26, 1322–1327 RSC.
- J. Pal, T. B. Rawal, M. Smerieri, S. Hong, M. Alatalo, L. Savio, L. Vattuone, T. S. Rahman and M. Rocca, Phys. Rev. Lett., 2017, 118, 226101 CrossRef PubMed.
- T. B. Rawal, M. Smerieri, J. Pal, S. Hong, M. Alatalo, L. Savio, L. Vattuone, T. S. Rahman and M. Rocca, Phys. Rev. B, 2018, 98, 035405 CrossRef CAS.
- T. E. Jones, T. C. R. Rocha, A. Knop-Gericke, C. Stampfl, R. Schlögl and S. Piccinin, Phys. Chem. Chem. Phys., 2014, 16, 9002–9014 RSC.
| This journal is © the Owner Societies 2026 |




