Impact of ligand-protected gold nanoclusters on liposomal morphology: fission to semi-gel formation in aqueous medium
Mallika
Mukherjee
a,
Asmita
Das
 b and
Pradipta
Purkayastha
b and
Pradipta
Purkayastha
 *a
*a
aDepartment of Chemical Sciences and Centre for Advanced Functional Materials, Indian Institute of Science Education and Research (IISER) Kolkata, Mohanpur 741246, WB, India. E-mail: ppurkayastha@iiserkol.ac.in
bDepartment of Chemistry, Rabindranath Tagore University, Hojai, Assam 782436, India
First published on 1st December 2025
Abstract
6-Aza-2-thiothymine (ATT)-protected gold nanoclusters (ATT–AuNCs) trigger controlled liposomal fission or transformation from fibrils to a semi-lipid gel depending on the presence or absence of cholesterol in the lipid membrane. Interactions between the ligand-protected AuNCs and the liposomes were found to be electrostatic or through hydrogen bonding or both, synchronized to the physical environment.
Ultrasmall metal nanoclusters (MNCs, ranging <2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm in size) have occupied a sizeable area in modern day research on nanoscience and nanotechnology. In particular, MNCs from noble metals, such as Au, Ag, Cu, and Pt, have been widely applied in fluorescence sensing and bioimaging because of their tuneable emission within the ultra-violet (UV) to near-infrared-II (NIR-II) region, considerable photoluminescence quantum yield (PLQY), large Stokes shift and excellent photostability.1–6 The unique aggregation or packing patterns of metal atoms and organic ligands largely determine the physicochemical properties of MNCs,7,8 which are distinctly different from plasmonic metal nanoparticles. MNCs possess discrete energy levels and hence show radiative emission due to electronic transitions.9,10 It is not surprising that the MNCs are broadly considered as credible competitors to standard fluorophores. As compared to organic dyes, they are more photostable with considerable PLQY11,12 and compared to quantum dots, much smaller, less toxic and less prone to blinking.13
nm in size) have occupied a sizeable area in modern day research on nanoscience and nanotechnology. In particular, MNCs from noble metals, such as Au, Ag, Cu, and Pt, have been widely applied in fluorescence sensing and bioimaging because of their tuneable emission within the ultra-violet (UV) to near-infrared-II (NIR-II) region, considerable photoluminescence quantum yield (PLQY), large Stokes shift and excellent photostability.1–6 The unique aggregation or packing patterns of metal atoms and organic ligands largely determine the physicochemical properties of MNCs,7,8 which are distinctly different from plasmonic metal nanoparticles. MNCs possess discrete energy levels and hence show radiative emission due to electronic transitions.9,10 It is not surprising that the MNCs are broadly considered as credible competitors to standard fluorophores. As compared to organic dyes, they are more photostable with considerable PLQY11,12 and compared to quantum dots, much smaller, less toxic and less prone to blinking.13
The practically non-toxic ligand-protected AuNCs have attracted much attention in a wide range of applications over the past several years and proved their applicability in biosensing, bio-imaging, cancer radiotherapy, antibacterial functions, and photodynamic therapy (PDT).14–19 Among the various categories of popular protecting ligands, 6-aza-2-thiothymine or ATT holds an important position. The present report is based on ATT-protected AuNCs, which exhibit strong PL,20 making them ideal for various applications in biocompatible conditions.21,22 ATT–AuNCs, functionalized with targeting ligands, were used for selective delivery of therapeutic agents to cancer cells.21,22 When combined with photosensitizers, these can also enhance PDT efficiency.23 Moreover, ATT–AuNCs are proven antibiotics against various pathogens.21
Although there are some reports on the action of metal nanoparticles on liposomes,24–26 we could hardly find any work on controlling liposomal fusion, fission and aggregation with the surface ligands of MNCs. Recently we found that L-glutathione (GSH)-coated AuNCs, intriguingly, control the vesicle morphology depending upon surface interactions through electrostatic charge and/or the hydrogen-bonding centres of GSH.27 Meanwhile, they fuse the unilamellar vesicles constructed with zwitterionic lipids or aggregate (without fusion) those having negative surface charge. Electrostatic attraction and hydrogen bonding were the key in these cases.
Classification based on lamellarity includes unilamellar vesicles (ULVs), multi-lamellar vesicles (MLVs) with more than one lipid bilayer and multi-vesicular vesicles (MVVs) that are constructed of vesicles inside one another.28,29 When size is accounted for, the ULVs are often divided into small unilamellar vesicles (SUVs, 20–100 nm), large unilamellar vesicles (LUVs, >100 nm), and giant unilamellar vesicles (GUVs, >1000 nm). Liposomal fusion is a promising method to construct complex drug delivery systems with desired structure and characteristics.29
ATT is a popular membrane disrupting agent and because of this property it is used as a MALDI-MSI matrix.30 Moreover, ATT functions similarly as the other liposome deforming substances, such as, surfactants,31 proteins32 and peptides (Fig. S1).33 Interestingly, the deformation did not lead to well-defined liposomal fission. In this work, we prepared ATT–AuNCs following the method described in one of our earlier studies.34 These AuNCs typically show shoulders/peaks in the absorption spectrum at around 400 and 490 nm attributed to the π → π* and the n → π* transitions in the ATT ligands (Fig. S2a), which are also reflected in their excitation spectrum (Fig. S2b) and emit at around 530 nm (Fig. S2c). The excited AuNCs decay in ∼7.3 ns (Fig. S2d) and show relatively good photostability (Fig. 2e and f). TEM analysis provides the size distribution averaging around 1 nm (Fig. S2g) dispersed well with negative surface charge (Fig. S2h). Detailed information on the elemental composition and chemical state of the ATT–AuNC surface was obtained from XPS analysis (Fig. S2i). Herein, we have demonstrated splitting (fission) of LUVs by ATT–AuNCs, producing daughter liposomes specifically for the cholesterol-containing ones. Cholesterol (Chol) plays a crucial role in lipid membrane formation by providing structural stability to it.35,36
In the absence of Chol, we noted disruption of the LUVs by the ATT–AuNCs to generate fibrils to semi-gel-like morphology depending upon the surface charge and H-bonding centres of the vesicles at 24 °C in aqueous buffer. Apparently, Chol restricts the accessibility of the ATT–AuNCs to the lipid bilayer. The structures of the ligands and the lipids are given in Scheme S1.
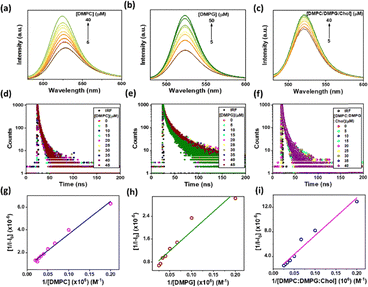
The ATT–AuNCs were interacted with the LUVs formed by zwitterionic (dimyristoylphosphatidylcholine, DMPC), anionic (dimyristoylphosphatidylglycerol, DMPG) and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DMPC
1 DMPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMPG
DMPG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol mixed lipids. The lipid head groups may influence ligand to metal charge transfer (LMCT) with the AuNCs, because the surface ligands contribute largely to enhancing the fluorescence of AuNCs.32 The AuNC fluorescence increases either by increasing the capability of the ligands as energy donors or by enhancing the electropositivity (or oxidation state) of the metal core. The PL intensity of the ATT–AuNCs progressively increased on gradual addition of the LUVs to 20 µM ATT–AuNCs in aqueous buffer solution (pH 7.4) (Fig. 1a–c) due to the adsorption of the AuNCs on the surface of the LUVs. The rate of the PL enhancement varied due to the varying strength of interaction. DMPC and DMPG interacted gradually with the LUVs, while the intensity quickly jumped higher with the first addition for the mixed lipid LUVs. This is supported by the time-resolved emission decay data where there is a gradual increase in the average lifetime (τav) of the ATT–AuNCs (Fig. 1d–f and Tables S1–S3) as the restricted movements of the fluorophore lower the non-radiative energy loss. While the electrostatic interaction between the ATT–AuNCs with negative surface charge and the DMPC LUVs is straight forward, that with the DMPG LUVs, bearing negative surface charge, is interesting. Nevertheless, the ATT–AuNCs were possibly adsorbed on the DMPG LUVs through H-bonding as was observed in our previous study.27,34 We noted before that ATT has good H-bonding propensity with the AuNC surface functional groups27 and other ligands (Arg).34 We also saw that the repulsive effect of the surface ligands was neutralized and overwhelmed in the excited state due to change in the prototropicity of the H-bond donors enabling favourable interaction with the DMPG vesicles.27 The ATT ligands over the Au core behave similarly.34 The ATT–AuNCs also interacted with the mixed lipid LUVs contributed both by electrostatic and H-bonding. The calculated binding constants (Kb) for the three cases (2.49 × 104, 3.63 × 104 and 2.12 × 104 M−1 for DMPC, DMPG and mixed lipid LUVs, respectively) from the well-known Benesi–Hildebrand double reciprocal plots (see SI) (Fig. 1g–i) confirmed the binding modes to be 1
Chol mixed lipids. The lipid head groups may influence ligand to metal charge transfer (LMCT) with the AuNCs, because the surface ligands contribute largely to enhancing the fluorescence of AuNCs.32 The AuNC fluorescence increases either by increasing the capability of the ligands as energy donors or by enhancing the electropositivity (or oxidation state) of the metal core. The PL intensity of the ATT–AuNCs progressively increased on gradual addition of the LUVs to 20 µM ATT–AuNCs in aqueous buffer solution (pH 7.4) (Fig. 1a–c) due to the adsorption of the AuNCs on the surface of the LUVs. The rate of the PL enhancement varied due to the varying strength of interaction. DMPC and DMPG interacted gradually with the LUVs, while the intensity quickly jumped higher with the first addition for the mixed lipid LUVs. This is supported by the time-resolved emission decay data where there is a gradual increase in the average lifetime (τav) of the ATT–AuNCs (Fig. 1d–f and Tables S1–S3) as the restricted movements of the fluorophore lower the non-radiative energy loss. While the electrostatic interaction between the ATT–AuNCs with negative surface charge and the DMPC LUVs is straight forward, that with the DMPG LUVs, bearing negative surface charge, is interesting. Nevertheless, the ATT–AuNCs were possibly adsorbed on the DMPG LUVs through H-bonding as was observed in our previous study.27,34 We noted before that ATT has good H-bonding propensity with the AuNC surface functional groups27 and other ligands (Arg).34 We also saw that the repulsive effect of the surface ligands was neutralized and overwhelmed in the excited state due to change in the prototropicity of the H-bond donors enabling favourable interaction with the DMPG vesicles.27 The ATT ligands over the Au core behave similarly.34 The ATT–AuNCs also interacted with the mixed lipid LUVs contributed both by electrostatic and H-bonding. The calculated binding constants (Kb) for the three cases (2.49 × 104, 3.63 × 104 and 2.12 × 104 M−1 for DMPC, DMPG and mixed lipid LUVs, respectively) from the well-known Benesi–Hildebrand double reciprocal plots (see SI) (Fig. 1g–i) confirmed the binding modes to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The DMPG LUVs possess surface hydroxyl groups, which interact with the N-atoms of the ATT ligands on the AuNC surface through H-bond interaction.
1. The DMPG LUVs possess surface hydroxyl groups, which interact with the N-atoms of the ATT ligands on the AuNC surface through H-bond interaction.
Cell division is a crucial biological phenomenon that plays a significant role in the overall growth processes in the living systems. To study in the same line, particularly those involving cellular fission, LUVs composed of DMPC, DMPG, and cholesterol in a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
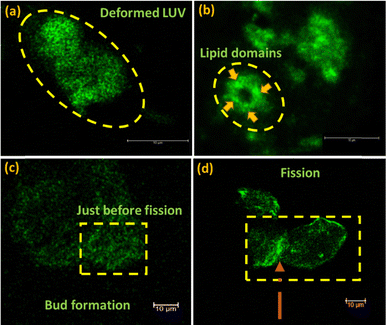
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of approximately 400 nm size, are considerably good cell mimics.37,38 The phosphocholine (PC) and phosphoglycerol (PG) engage in beneficial interactions with the hydroxyl oxygen of the ATT ligand on the AuNCs and form non-covalent H-bonds. Consequently, the green-emitting AuNCs, adhered to the liposomal surfaces, lead to the formation of buds as intermediates, which potentially destabilise the LUVs. Typically, liposome division happens via the formation of lipid rafts or lipid-specific domains. Our findings reveal that the adsorption of the ATT–AuNCs on the LUVs was greatly influenced by the polar head groups. Notably, the ATT–AuNCs bind better with the DMPG LUVs compared to the DMPC ones. As indicated by the higher binding constant. This suggests selective interaction of the ATT–AuNCs with saturated lipid molecules based on their polar functional groups. Hence, the ATT–AuNCs interact with the mixed (DMPC
1 of approximately 400 nm size, are considerably good cell mimics.37,38 The phosphocholine (PC) and phosphoglycerol (PG) engage in beneficial interactions with the hydroxyl oxygen of the ATT ligand on the AuNCs and form non-covalent H-bonds. Consequently, the green-emitting AuNCs, adhered to the liposomal surfaces, lead to the formation of buds as intermediates, which potentially destabilise the LUVs. Typically, liposome division happens via the formation of lipid rafts or lipid-specific domains. Our findings reveal that the adsorption of the ATT–AuNCs on the LUVs was greatly influenced by the polar head groups. Notably, the ATT–AuNCs bind better with the DMPG LUVs compared to the DMPC ones. As indicated by the higher binding constant. This suggests selective interaction of the ATT–AuNCs with saturated lipid molecules based on their polar functional groups. Hence, the ATT–AuNCs interact with the mixed (DMPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMPG
DMPG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol 2
Chol 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) LUVs, and deform them (Fig. 2a) with a binding preference towards the DMPG portions in the liposomes, resulting in the formation of distinct lipid domains (Fig. 2b) and buds (Fig. 2c) before complete fission (Fig. 2d). These sequences of events were observed randomly throughout the mixed LUV samples (lipid concentration is 1 mM in each set) added on with ATT–AuNCs. The H-bonding interactions provided better stabilisation than the electrostatic interactions, mitigating repulsive forces. Analysis of the lipid structures revealed that DMPG contains a phosphate and hydroxyl group, while DMPC has ammonium and carboxylate groups. H-bonding between the –NH of ATT and –OH of DMPG likely contributed to stabilising the LMCT state of the AuNCs. In contrast, the electrostatic attractions between DMPC and the ATT-AuNCs were relatively weak, leading to fewer interactions compared to DMPG.
1) LUVs, and deform them (Fig. 2a) with a binding preference towards the DMPG portions in the liposomes, resulting in the formation of distinct lipid domains (Fig. 2b) and buds (Fig. 2c) before complete fission (Fig. 2d). These sequences of events were observed randomly throughout the mixed LUV samples (lipid concentration is 1 mM in each set) added on with ATT–AuNCs. The H-bonding interactions provided better stabilisation than the electrostatic interactions, mitigating repulsive forces. Analysis of the lipid structures revealed that DMPG contains a phosphate and hydroxyl group, while DMPC has ammonium and carboxylate groups. H-bonding between the –NH of ATT and –OH of DMPG likely contributed to stabilising the LMCT state of the AuNCs. In contrast, the electrostatic attractions between DMPC and the ATT-AuNCs were relatively weak, leading to fewer interactions compared to DMPG.
Chol is essential to maintain the ordered membrane structure with a preference for saturated lipids.34 To investigate the role of Chol and the lipid polar groups, we prepared two sample sets with the LUVs: (i) DMPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol (3
Chol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and (ii) DMPG
1) and (ii) DMPG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol (3
Chol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). When presented with the ATT–AuNCs (25 µM), we noted significant deformation or twisting of the lipid bilayer in the DMPC/Chol LUVs, demonstrating formation of fission buds after a certain time (12 min) (Fig. 3a). This is attributed to the electrostatic interaction between the LUVs and the AuNCs. Conversely, we found profuse fission and formation of daughter liposomes when the ATT–AuNCs react with the DMPG LUVs, supposedly, due to the stronger H-bonding interactions (Fig. 3b). The calculated binding constants suggest that the association is lowest in the case of the Chol-doped mixed LUVs. The AuNCs also induced fission buds before complete fission in this case (Fig. 3c).
1). When presented with the ATT–AuNCs (25 µM), we noted significant deformation or twisting of the lipid bilayer in the DMPC/Chol LUVs, demonstrating formation of fission buds after a certain time (12 min) (Fig. 3a). This is attributed to the electrostatic interaction between the LUVs and the AuNCs. Conversely, we found profuse fission and formation of daughter liposomes when the ATT–AuNCs react with the DMPG LUVs, supposedly, due to the stronger H-bonding interactions (Fig. 3b). The calculated binding constants suggest that the association is lowest in the case of the Chol-doped mixed LUVs. The AuNCs also induced fission buds before complete fission in this case (Fig. 3c).
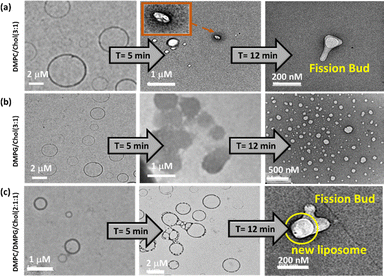 | ||
Fig. 3 TEM micrographs showing transformations of the Chol-containing LUVs on adding the ATT–AuNCs: (a) DMPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol (3 Chol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), (b) DMPG 1), (b) DMPG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol (3 Chol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and (c) DMPC 1) and (c) DMPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMPG DMPG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol (2 Chol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) LUVs. 1) LUVs. | ||
Conversion of liposomes into fibrils without any external stimulus, such as temperature or pH change, is quite rare. This transformation requires deformation and potential snapping of the lipid membrane. The extent of membrane shearing depends upon the amount of Chol in the bilayer, which provides stability to the membrane by forming lipid rafts. On adding ATT–AuNCs to the Chol-devoid DMPC and DMPG LUVs, the situation was quite different from the previous observations. Instead of LUV fission, the DMPC LUVs were converted to fibrous strands and the DMPG LUVs became a semi-gel. While this was intriguing, on a deeper analysis, we noted that the fibril formation occurred due to the electrostatic interaction between the ATT–AuNCs with negative surface charge and the zwitterionic DMPC LUVs triggered by the absence of Chol in the bilayers (Fig. 4a). The ATT aromatic rings interact with the hydrophobic lipid bilayer to snap off the membrane.30 As mentioned earlier, H-bonding between the aza N-atoms of ATT and the –OH groups of the DMPG liposomes overcomes the electrostatic repulsion, and rapid disruption of the lipid bilayer takes place initially through fibril formation followed by a semi-gel texture, probably, due to the linking through the H-bonds (Fig. 4b). The fibril and semi-gel formations were further supported by the acquired AFM images (Fig. S3). These formations might have biological implications because amyloid-β 40 fibril formation is known to occur due to the disruption of the liposome membrane.
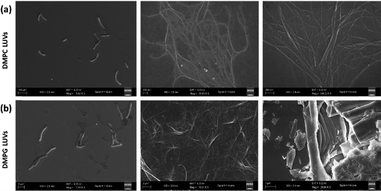 | ||
| Fig. 4 FESEM micrographs showing the conversion of the (a) DMPC and (b) DMPG LUVs into fibres and a semi-gel, respectively, by interaction with the ATT–AuNCs. | ||
In summary, we synthesized AuNCs protected with ATT (ATT–AuNCs) and interacted them with LUVs made from zwitterionic DMPC, anionic DMPG and mixed DMPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMPG lipids with and without a calculated amount of Chol. The intention was preparing LUVs, bearing lipid raft-induced stability provided by Chol and comparing the outcomes of the interactions with the ATT–AuNCs with vesicles in the absence of Chol. While the zwitterionic DMPC LUVs interact electrostatically with the negatively charged ATT–AuNCs, the anionic DMPG vesicles provide repulsion to them. However, ATT forms H-bonds with the head groups of DMPG, which overcomes the electrostatic repulsion and binds strongly to the AuNCs. The aromatic ATT rings intrude the hydrophobic lipid membranes and disrupt them to shear them either into SUVs in the presence of Chol or fibres in the absence of Chol. The Chol-imparted stability to the lipid membrane results in fission of the LUVs to generate SUVs. The fibres formed from the LUVs by the ATT–AuNCs in the absence of Chol showed differential texture characteristics with DMPC and DMPG. While the stable DMPC fibres do not undergo any further change after prolonged incubation, the DMPG ones interact through H-bonds and form a semi-gel. Similar phenomena were observed with the mixed LUVs in the presence and absence of Chol. However, due to the excess of DMPC in them, we found only fibre formation. The results can be generalized using other MNCs and with various surface charges on the LUVs. These in vitro studies with the cell-mimicking LUVs might open new avenues for applying these results in biological environments.
DMPG lipids with and without a calculated amount of Chol. The intention was preparing LUVs, bearing lipid raft-induced stability provided by Chol and comparing the outcomes of the interactions with the ATT–AuNCs with vesicles in the absence of Chol. While the zwitterionic DMPC LUVs interact electrostatically with the negatively charged ATT–AuNCs, the anionic DMPG vesicles provide repulsion to them. However, ATT forms H-bonds with the head groups of DMPG, which overcomes the electrostatic repulsion and binds strongly to the AuNCs. The aromatic ATT rings intrude the hydrophobic lipid membranes and disrupt them to shear them either into SUVs in the presence of Chol or fibres in the absence of Chol. The Chol-imparted stability to the lipid membrane results in fission of the LUVs to generate SUVs. The fibres formed from the LUVs by the ATT–AuNCs in the absence of Chol showed differential texture characteristics with DMPC and DMPG. While the stable DMPC fibres do not undergo any further change after prolonged incubation, the DMPG ones interact through H-bonds and form a semi-gel. Similar phenomena were observed with the mixed LUVs in the presence and absence of Chol. However, due to the excess of DMPC in them, we found only fibre formation. The results can be generalized using other MNCs and with various surface charges on the LUVs. These in vitro studies with the cell-mimicking LUVs might open new avenues for applying these results in biological environments.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: experimental details, supporting figures and tables. See DOI: https://doi.org/10.1039/d5cp04060d.Acknowledgements
Financial support from the Science and Engineering Research Board (SERB), Government of India through the project CRG/2022/000006 is gratefully acknowledged. M. M. thanks SERB for her fellowship.Notes and references
- H. Nasrollahpour, B. J. Sánchez, M. Sillanpää and R. Moradi, ACS Appl. Nano Mater., 2023, 6, 12609–12672 CrossRef CAS.
- V. Sethumadhavan and N. Nonappa, Dalton Trans., 2025, 54, 11770–11789 RSC.
- Y. Xiao, Z. Wu, Q. Yao and J. Xie, Aggregate, 2021, 2, 114–132 CrossRef CAS.
- Y. An, Y. Ren, M. Bick, A. Dudek, E. H.-W. Waworuntu, J. Tang, J. Chen and B. Chang, Biosens. Bioelectron., 2020, 154, 112078 CrossRef CAS.
- N. Nonappa, Beilstein J. Nanotechnol., 2020, 11, 533–546 CrossRef PubMed.
- L. Zhang and E. Wang, Nano Today, 2014, 9, 132–157 CrossRef CAS.
- H. Li, X. Kang and M. Zhu, Coord. Chem. Rev., 2025, 539, 216738 CrossRef CAS.
- M. S. Mathew, G. Krishnan, A. A. Mathews, K. Sunil, L. Mathew, R. Antoine and S. Thomas, Nanomaterials, 2023, 13, 1874 CrossRef CAS PubMed.
- S. Maity, S. Kolay, S. Chakraborty, A. Devi, Rashi and A. Patra, Chem. Soc. Rev., 2025, 54, 1785–1844 RSC.
- T. Q. Yang, B. Peng, B. Q. Shan, Y. X. Zong, J. G. Jiang, P. Wu and K. Zhang, Nanomaterials, 2020, 10, 261 CrossRef CAS PubMed.
- A. Moscatelli, Nat. Nanotech., 2018, 13, 620 CrossRef CAS.
- J. Li, J.-J. Zhu and K. Xu, Trends Anal. Chem., 2014, 58, 90–98 CrossRef CAS.
- J. M. Obliosca, C. Liu, R. A. Batson, M. C. Babin, J. H. Werner and H. C. Yeh, Biosensors, 2013, 3, 185–200 CrossRef CAS.
- C. Zhang, X. Gao, W. Chen, M. He, Y. Yu, G. Gao and T. Sun, iScience, 2022, 25, 105022 CrossRef CAS.
- Z. Abbaszadeh, M.-M. Mousavi and M. Mahmoudpour, Microchem. J., 2024, 207, 111988 CrossRef CAS.
- Y. Bai, T. Shu, L. Su and X. Zhang, Crystals, 2020, 10, 357 CrossRef.
- S. M. van de Looij, E. R. Hebels, M. Viola, M. Hembury, S. Oliveira and Y. Vermonden, Bioconjug. Chem., 2022, 33, 4–23 CrossRef CAS.
- H. Zhang, R. Peng, Y. Luo, Q. Cui, F. Gong and L. Li, ACS Appl. Bio Mater., 2022, 5, 3115–3125 CrossRef CAS.
- R. Han, M. Zhao, Z. Wang, H. Liu, S. Zhu, L. Huang, Y. Wang, L. Wang, Y. Hong, Y. Sha and Y. Jiang, ACS Nano, 2020, 14, 9532–9544 CrossRef CAS.
- H. Peng, Z. Huang, H. Deng, W. Wu, K. Huang, Z. Li, W. Chen and J. Liu, Angew. Chem., Int. Ed., 2020, 59, 9982–9985 CrossRef CAS PubMed.
- H.-Q. Jiang, L.-Y. Lu, Z.-M. Weng, K.-Y. Huang, Y. Yang, H.-H. Deng, Y.-Y. Xu, W. Chen and Q.-Q. Zhuang, ACS Omega, 2023, 8, 47123–47133 CrossRef CAS PubMed.
- H.-H. Deng, X.-Q. Shi, P. Balasubramanian, K.-Y. Huang, Y.-Y. Xu, Z.-N. Huang, H.-P. Peng and W. Chen, Nanomaterials, 2020, 10, 281 CrossRef CAS PubMed.
- S. M. van de Looij, E. R. Hebels, M. Viola, M. Hembury, S. Oliveira and T. Vermonden, Bioconjugate Chem., 2021, 33, 4–23 CrossRef PubMed.
- M. Musielak, J. Potoczny, A. Boś-Liedke and M. Kozak, Int. J. Mol. Sci., 2021, 22, 6229 CrossRef CAS PubMed.
- D. Pornpattananangkul, S. Olson, S. Aryal, M. Sartor, C.-M. Huang, K. Vecchio and L. Zhang, ACS Nano, 2010, 4, 1935–1942 CrossRef CAS.
- S. Pakian, A. Mirkani, H. Sadeghi-Abandansari and M.-R. Nabid, J. Drug Delivery Sci. Technol., 2024, 99, 105956 CrossRef CAS.
- M. Mukherjee, A. Chatterjee and P. Purkayastha, J. Phys. Chem. B, 2025, 129, 5207–5216 CrossRef CAS.
- H. Nsairat, D. Khater, U. Sayed, F. Odeh, A. Al Bawab and W. Alshaer, Heliyon, 2022, 8, e09394 CrossRef CAS.
- I. Mardešić, Z. Boban, W. K. Subczynski and M. Raguz, Membranes, 2023, 13, 320 CrossRef.
- N. S. Porto, S. Serrao, G. Bindi, N. Monza, C. Fumagalli, V. Denti, I. Piga and A. Smith, Metabolites, 2025, 15, 531 CrossRef CAS PubMed.
- T. P. Sudbrack, N. L. Archilha, R. Itri and K. A. Riske, J. Phys. Chem. B, 2011, 115, 269–277 CrossRef CAS.
- J. D. Cortese, B. Schwab 3rd, C. Frieden and E. L. Elson, Proc. Natl. Acad. Sci. U. S. A., 1989, 86, 5773–5777 CrossRef CAS.
- K. Izumi, C. Saito and R. Kawano, Micromachines, 2023, 14, 373 CrossRef PubMed.
- A. Chatterjee and P. Purkayastha, Mater. Adv., 2021, 2, 1343–1350 RSC.
- E. Khodadadi, E. Khodadadi, P. Chaturvedi and M. Moradi, Membranes, 2025, 15, 173 CrossRef CAS.
- M. Ahmed, J. Membrane Sci. Technol., 2024, 14, 1000408 Search PubMed.
- S. Deshpande, W. K. Spoelstra, M. van Doorn, J. Kerssemakers and C. Dekker, ACS Nano, 2018, 12, 2560–2568 CrossRef CAS.
- L. Olivi, M. Berger, R. N. P. Creyghton, N. De Franceschi, C. Dekker, B. M. Mulder, N. J. Claassens, P. Rian ten Wolde and J. van der Oost, Nat. Commun., 2021, 12, 4531 CrossRef CAS.
| This journal is © the Owner Societies 2026 |