Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Multicycle operando Raman spectroscopy reveals reversible and irreversible transitions in LiNiO2 electrodes
Eva
del Campo Ortiz
 a,
Alex R.
Neale
a,
Alex R.
Neale
 b,
Manel
Sonni
b,
Manel
Sonni
 cd,
Luke M.
Daniels
cd,
Luke M.
Daniels
 c,
Grazyna Z.
Zukowska
a,
Matthew J.
Rosseinsky
c,
Grazyna Z.
Zukowska
a,
Matthew J.
Rosseinsky
 cd,
Marek
Marcinek
*a and
Laurence J.
Hardwick
cd,
Marek
Marcinek
*a and
Laurence J.
Hardwick
 *bd
*bd
aFaculty of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664 Warsaw, Poland. E-mail: marek.marcinek@pw.edu.pl
bStephenson Institute for Renewable Energy, Department of Chemistry, University of Liverpool, Liverpool L69 7ZD, UK. E-mail: hardwick@liverpool.ac.uk
cMaterials Innovation Factory, Department of Chemistry, University of Liverpool, Liverpool L69 7ZD, UK
dThe Faraday Institution, Quad One, Harwell Science and Innovation Campus, Didcot OX11 0RA, UK
First published on 31st October 2025
Abstract
Operando Raman spectroscopy of the delithiation and lithiation of LiNiO2 positive electrodes is reported over the first three cycles. Optimisation of the operando electrochemical Raman cell configuration facilitated the characterisation of composite electrodes across multiple cycles with high relevance for practical Li-ion cell chemistry. Tracking the Eg and A1g Raman modes highlighted localised irreversible changes, particularly correlated to first-cycle inefficiencies in LiNiO2, followed by reversible band shifts and intensity changes upon subsequent cycles. Furthermore, trends in both the band position and intensities were related to the state of lithiation (x(Li) in LixNiO2) and the phase transitions through hexagonal and monoclinic phases (H1, M, H2 and H3).
1. Introduction
The challenge of operando spectroscopy is to recreate measurement conditions as close as possible to the actual system of investigation.1 In the case of operando measurements on battery materials, the motivation is to follow the electrochemical reactions under potential regimes similar to those run in practical cells using commercially relevant electrode formulations, over multiple cycles.1 This will enable more precise understanding on how the charge storage mechanism and degradation processes evolve over time.Raman spectroscopy is one such technique that can be readily utilised as an operando method through access into an electrochemical cell via an optical window.2–6Operando Raman can offer detailed insights about battery components during cycling7,8 and provides information about crystal structure, phase compositions, and electronic properties by measuring the frequencies of chemical bond vibrations.9 Many developments have been reported on improving cell design and electrode configuration so that material capacities and properties can be more closely replicated at both low and high current rates.10,11
The Li-ion positive electrode material LiNiO2 has received renewed attention due to the requirement for increased material performance in next-generation Li-ion batteries,12 and the drive to move away from cobalt-containing active materials. LiNiO2 has a theoretical capacity of 275 Ah kg−1 based on full delithiation, but suffers from sustained capacity fade due to irreversible first-order structural phase transitions and large volume changes during cycling.12 Several Raman studies have been reported for LiNiO2; however, detailed structural characterisation and information on the source of the material is not always provided, making comparable analysis challenging when material synthesis plays a crucial role in the resulting electrochemical performance.13–16
Herein, operando Raman spectroscopic measurements of electrodes based on structurally well-characterised LiNiO2 are reported over three charge/discharge cycles utilising an improved composite electrode Raman cell configuration. Rapid processing, visualisation, and analysis of the large spectral data sets were aided by the use of the open-source tool PRISMA,17 highlighting key changes to main spectral bands as a function of lithium (de)intercalation and their relation to reversible and irreversible structural changes during the first cycles.
2. Experimental section
2.1. Synthesis and characterisation of LiNiO2
LiNiO2 was synthesised, as described in our previous study,18 by ball-milling stoichiometric amounts of dried LiOH and Ni(OH)2 in 45 mL sealed zirconia jars (using seven 10-mm-diameter zirconia balls). Ball milling was performed at 350 rpm for 150 minutes (10 minutes milling, 15 minutes rest) under an argon atmosphere (Fritsch Pulverisette 7 premium line Planetary Ball Mill). The powders were then placed in an alumina crucible inside a quartz tube, which was sealed at both ends using Swagelok end caps. The final black powders were obtained by annealing the ball-milled mixtures at 700 °C for 20 h under flowing dry O2 gas with a flow rate of 100 mL min−1. The powder was then handled inside an argon-filled glovebox (O2 < 0.1 ppm, H2O < 0.1 ppm).Solutions, measured by inductively coupled plasma mass spectrometry (ICP-MS) in triplicate, were prepared by dissolving 10 mg of powder into 2 mL of concentrated HCl inside an autoclave heated to 100 °C for 12 hours before diluting to 50 mL with ultra-pure water. Measurements were collected on a PerkinElmer ICP-MS NexION 2000 instrument (Table S1).
2.2. Magnetic measurements
Powders of LiNiO2 were pressed into a pellet. A portion of ca. 2 mg was loaded into a custom-made quartz tube and sealed under high vacuum (∼2 × 10−5 Torr). Magnetisation measurements were carried out using a commercial superconducting quantum interference device magnetometer MPMS3 (Quantum Design, USA). The contribution of the quartz tube to the magnetization was confirmed to be negligible prior to measuring the samples. Zero-field-cooled (ZFC) and field-cooled (FC) measurements at 100 Oe and a subsequent field-cooled measurement at 45 kOe were performed from 2 to 300 K.A freezing temperature (Tf) of 14 K was determined from the maxima of the 100 Oe zero-field-cooled (ZFC) data for LiNiO2 (Fig. S1a) and the inverse susceptibility of the 45 kOe data show linear Curie–Weiss behaviour (Fig. S1b). Both these observations confirm a near-stoichiometric sample of LiNiO2 is obtained.12,19,20 The extracted effective moment of 1.731(2) μB agrees perfectly with the S = ½ value expected (1.73 μB) for Ni3+.
2.3. Structural analysis of LiNiO2
Structural analysis of LiNiO2 was conducted on a 7Li-enriched sample by combining powder X-ray diffraction measured on an X-ray diffractometer (D8 ADVANCE, Bruker) using a Cu-Kα radiation source (λ = 1.5406 Å) and time-of-flight neutron powder diffraction measured at room temperature on the nanoscale-ordered materials diffractometer (NOMAD) at Oak Ridge National Laboratory. The 7Li-enriched samples were synthesised as described above using 7LiOH·H2O (lithium-7-enriched hydroxide monohydrate) dried overnight under dynamic vacuum (<10−4 mbar) at 170 °C. Combined Rietveld refinements against SPXRD and NPD data were carried out using Topas (Version 6) software.2.4. Operando Raman spectroscopy cell
The spectroelectrochemical half-cell used for the experiments was the ECC-Opto-Std test cell (EL-Cell), which enables a face-to-face arrangement of the electrodes. The assembly within the Raman cell follows the spacer configuration employed by Rosser et al.,10 including placing a glass fibre (GF) separator above and below the working electrode strip for full electrolyte wetting of the electrode. A small hole (2 mm) was made in the outer separator to allow optical access. The cell was sealed with a Raman-grade CaF2 window (Crystran), chosen since it only presents a single Raman band at 321 cm−1, which does not interfere with LiNiO2 characteristic bands. The schematic of the cell arrangement is depicted in Fig. S2.2.5. Electrode preparation
LiNiO2-containing composite electrodes were prepared on 0.05-mm-thick aluminium foil as the current collector. The mass proportions of active material, Super C65 carbon black (CB) and polyvinylidene fluoride (PVDF) binder in the tape-cast composite electrode were 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, respectively. These materials were dispersed in N-methyl pyrrolidone, and the resulting slurry was centrifugated in Thinky Mixer twice for 10 minutes. Afterwards, the aluminium foil was coated with the slurry using a doctor blade to give a cathode layer with a wet thickness of 150 µm, and this was then dried at 50–60 °C on the heated coating bed. All electrodes were prepared outside the glovebox and further dried for 5 h at 120 °C under vacuum in a Buchi oven. Finally, the electrodes were introduced into an Ar-filled (O2, H2O < 1 ppm) glovebox within the oven to avoid contact with air.
10, respectively. These materials were dispersed in N-methyl pyrrolidone, and the resulting slurry was centrifugated in Thinky Mixer twice for 10 minutes. Afterwards, the aluminium foil was coated with the slurry using a doctor blade to give a cathode layer with a wet thickness of 150 µm, and this was then dried at 50–60 °C on the heated coating bed. All electrodes were prepared outside the glovebox and further dried for 5 h at 120 °C under vacuum in a Buchi oven. Finally, the electrodes were introduced into an Ar-filled (O2, H2O < 1 ppm) glovebox within the oven to avoid contact with air.
Before the final drying step, working electrodes for coin cell and Raman cell measurements were prepared from the tape cast. Regarding coin cells, LiNiO2 electrode discs were cut with a diameter of 12 mm. For the operando Raman cell, electrodes were cut in strips of 12 × 3 mm following the optimised studies of Rosser et al.10 The Li metal counter/reference electrode was cut from a ribbon (Sigma-Aldrich, 99%; 0.38 mm thickness) with diameters of 14 mm and 9 mm for coin cells and the Raman cell, respectively. The electrolyte solution used for all the electrochemical measurements was 1 M Li[PF6] in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volumetric mixture of ethylene carbonate/dimethyl carbonate (EC
1 volumetric mixture of ethylene carbonate/dimethyl carbonate (EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC) (Solvionic). Both cell assemblies were carried out within an argon-containing glovebox (O2, H2O < 1 ppm). Schematics showing the free-standing and strip-electrode preparation and photographic images of the three types of electrodes used are shown in Fig. S3 and S4, respectively.
DMC) (Solvionic). Both cell assemblies were carried out within an argon-containing glovebox (O2, H2O < 1 ppm). Schematics showing the free-standing and strip-electrode preparation and photographic images of the three types of electrodes used are shown in Fig. S3 and S4, respectively.
2.6. Electrochemical characterisation
Galvanostatic charge–discharge tests within the Raman and coin cells were performed using Biologic VMP-150 potentiostat. The potentials were applied vs. the Li-metal counter/reference electrode from 3.0 to 4.3 V vs. Li+/Li to observe the full structural variation.21 The cell rested for 10 minutes at OCP (ca. 3 V) and then 3 cycles were recorded at a C/10 rate (where 1C was defined as 200 mAh g−1 herein, based on the approximate practical discharge capacity of LiNiO2). The measurements were completed at room temperature (ca. 23 °C) for the operando Raman measurements, while the coin cell electrochemical measurements were conducted inside a climate chamber held at 30 °C.2.7. Raman characterisation
An InVia Renishaw Raman spectrometer was used for the measurement of LiNiO2 tape-cast electrodes situated within the operando cell. A 633 nm wavelength laser was focussed through a microscope (Leica) via a 50× objective (Leica) onto the electrode with a spatial resolution at the surface of ca. 2–3 µm2 and with a power of 4 mW, which corresponded to a 50% energy attenuation. All Raman measurements for every experiment were performed in a single spot, therefore 3 × 25 s acquisitions per spectrum were selected to avoid sample burning. Consequently, each scan lasted 6 minutes and we therefore obtained around 100 spectra per cycle.During the operando experiments, the optical focus of the microscope on the sample was periodically checked and refocused, if needed, to compensate for minor shifts in focus within the cell during cycling.
2.8. PRISMA data treatment
Before processing the spectral data using PRISMA, the electrochemical and spectroscopic datasets were correlated according to collection times. Once the cycling of the cell had finished, a series of Raman spectra were obtained containing information corresponding to the specific times at which each spectrum was recorded. In parallel, for the electrochemical technique used, the relationship between time and all the independent variables, such as potential, capacity, state of lithiation (x(Li) in LixNiO2) and current, among others, was also logged. Using this information, each spectrum was then correlated to a specific variable before data treatment on PRISMA.173. Results and discussion
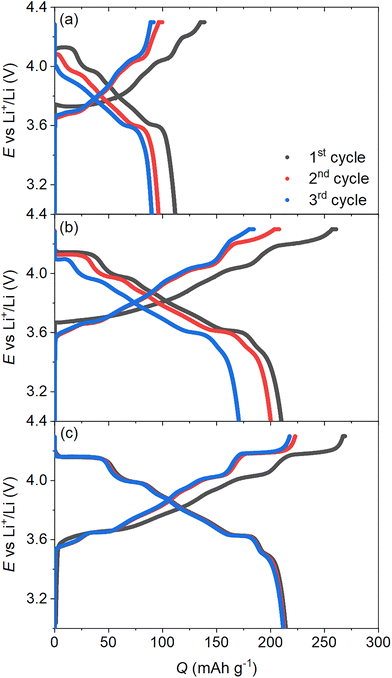
Free-standing electrodes with optical access by a pinhole in the current collector generally deliver expected electrochemical performance at low current rates when graphite is used as the active material.8,22–24 However, for positive electrode materials such as LiNiO2, analogous methodologies for preparing the free-standing electrode configuration is unsuitable and the observed capacities approach only half the expected capacity values (Fig. 1(a)).The performance loss in the bespoke free-standing LiNiO2 electrodes severely limits the practical relevance of any correlative in situ/operando spectroelectrochemical measurements and, instead, direct measurement of composite electrodes more representative of true cell conditions should be targeted. Following the approach by Rosser et al.,10 a rectangular electrode (12 × 3 mm), designated as an electrode strip, was punched from a conventional tape-cast composite electrode containing LiNiO2, conductive carbon black, and polyvinylidene fluoride (Kynar flex) binder. A glass-fibre separator was placed in between the electrode strip and lithium metal with an additional separator, containing a small hole for optical access, located between the window and the working electrode to allow facile electrolyte access (Fig. S2). Compared to the free-standing electrode, the electrochemical performance of the electrode strip is much improved (Fig. 1(b)), approaching the performance of the same composite LiNiO2 electrode material measured within a coin cell (Fig. 1(c)).
The LiNiO2 investigated within the optical cell was synthesised via the solid-state method reported previously, from LiOH and Ni(OH)2 reagents,18 and is further described within the SI. This method resulted in a powder sample with a stoichiometric composition of Li1.019(20)Ni1.000(27)O2, measured via inductively coupled plasma mass spectrometry (ICP-MS) (Table S1). This exact stoichiometry is further supported by assessment of the electronic state of nickel via measurement of magnetisation data which accurately confirm the oxidation state of Ni3+ through a measured effective moment of 1.731(2)µB (expected value of 1.73µB for S = ½) (Fig. S1).
Structural characterisation was performed via Rietveld refinement against powder X-ray and neutron diffraction data utilising a multidomain hexagonal R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m model reported previously,25 which accounts for both ordered R
m model reported previously,25 which accounts for both ordered R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m and disordered Fm
m and disordered Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m domains of differing size that might arise from Li/Ni mixing within the bulk powder (Fig. S5 and Table S2). A chemical order parameter, defined as the difference in Li content between the two distinct cation (Li and Ni) layers (i.e., η = |occLi–occNi|), of 0.95(3) is extracted from the refinement, indicating a highly ordered structure, with a low degree of cation mixing.
m domains of differing size that might arise from Li/Ni mixing within the bulk powder (Fig. S5 and Table S2). A chemical order parameter, defined as the difference in Li content between the two distinct cation (Li and Ni) layers (i.e., η = |occLi–occNi|), of 0.95(3) is extracted from the refinement, indicating a highly ordered structure, with a low degree of cation mixing.
The complete set of operando Raman spectra for the first three cycles are shown as stacked plots within Fig. 2(a)–(c), to correlate with the potential/capacity profiles in Fig. 2(d) for cycles 1–3. The data is also displayed as a spectral band intensity heat map in Fig. S6 that visibly shows the changing bands intensity trends as a function of capacity. Two characteristic bands for LiNiO2 are observed at 485 and 545 cm−1, which correspond to the Eg and A1g Raman active modes, respectively.26,27 The Eg mode is the result of the displacement of oxygen atoms along the oxygen layers, whereas the A1g mode originates from the oxygen displacement parallel to the c axis of the structure.15 The band shape, ratio, and positions of LiNiO2, before cycling, are broadly congruent with spectra reported in previous studies.13,15,16,27 However, slight variations in the specific band shapes and intensities do arise from the source and quality of the LiNiO2 active material, highlighting the importance of the structural and compositional characterisation of the material reported herein.
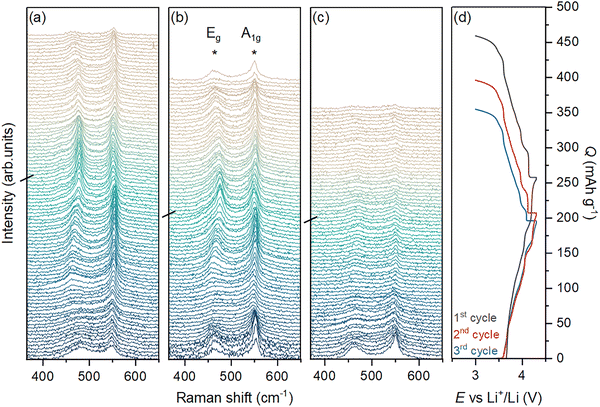 | ||
Fig. 2
Operando Raman spectra series for (a) cycle 1, (b) cycle 2, and (c) cycle 3 of LiNiO2 tape-cast electrode strip cycled vs. Li metal between 3–4.3 V vs. Li+/Li at a C/10 rate with 1 M Li[PF6] in ethylene carbonate/dimethyl carbonate (EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC) (1 DMC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vol). The stacking between spectra was correlated with the (d) potential profiles of LiNiO2vs. Li+/Li, represented as cumulative capacity per individual cycle. The two characteristic bands, Eg and A1g, for LiNiO2 were observed at 485 cm−1 and 545 cm−1, respectively.16,27 The breaks on the y axis in (a)–(c) represent the end of the charge and the beginning of discharge for each cycle. 1 vol). The stacking between spectra was correlated with the (d) potential profiles of LiNiO2vs. Li+/Li, represented as cumulative capacity per individual cycle. The two characteristic bands, Eg and A1g, for LiNiO2 were observed at 485 cm−1 and 545 cm−1, respectively.16,27 The breaks on the y axis in (a)–(c) represent the end of the charge and the beginning of discharge for each cycle. | ||
During the initial charging step, both the Eg and A1g bands undergo positional shifts and the broad band shapes sharpen as lithium is removed from the structure at increasing potentials. The band sharpening has been attributed to a decrease in the Ni–O bond distance distribution as Li is deintercalated.16 Additionally, band intensities generally increase as a function of LiNiO2 delithiation as reported previously. Upon first discharge, the spectral bands exhibit partial reversibility in the processes, showing reduced intensity and re-broadening, while the reversibility of the band positions is less clear to the eye. Generally, comparable trends in the band intensity and width are observed during second and third cycles.
However, to extract quantitative information on band changes during cycling and better understand the reversibility of these mechanisms, accurate spectral fitting of the data is critical to obtain specific band positions and intensities as a function of the cell cycling. To achieve this, approximately 100 Raman spectra per cycle (300 total spectra) were fitted using PRISMA as a high-throughput semi-automated spectra processing application and correlated with the cell capacity (i.e., experimental time for a galvanostatic experiment). As a result, the trends in vibrational modes during charge and discharge can be tracked and thereby correlate the observed changes with respect to the various potential, capacity, and, importantly, the state of lithiation (x(Li) in LixNiO2) properties of the LiNiO2 electrode. The full details for the methodology of spectral fitting are presented in the SI (Fig. S7) and the derived spectral information is in good agreement with manual data processing/fitting methods (Fig. S8).
The derived band positions and intensities for the Eg and A1g modes of the LiNiO2 electrode strip during the first three cycles as a function of the cumulative cycling capacity are presented in Fig. 3. The individual band intensities were normalised between 0 to 1 (i.e., Inorm(Eg) and Inorm(A1g)). The pre-normalised intensity data for this plot is shown in Fig. S9.
The positions of Raman bands vary with the strength of the interatomic forces within the lattice; therefore, the stronger these forces, the higher the frequencies and, consequently, the higher the wavenumber values.28,29 Regarding the first-cycle results shown in Fig. 2, trends in the band position shifts matched the operando study for LiNiO2 carried out by Jacquet et al.,16 wherein the, Eg and A1g Raman peaks shifted in opposite directions, with both presenting a U-shaped trend during the first half-cycle. However, during this cycle, the band shift range (ca. 15 cm−1 and 8 cm−1 for Eg and A1g, respectively) is more pronounced in this study. Importantly, the cell configuration and electrode design enabled the recording of successive cycles where the measured shift ranges become less pronounced. The Eg mode shifted position between 465–475 cm−1 and 461–472 cm−1 and the A1g mode shifted between 546–552 cm−1 and 544–550 cm−1 during the second and third cycles, respectively. The variation in the pattern of the Eg and A1g peak shifts as a function of xLi in LixNiO2, observed between cycle 1 and cycles 2/3, suggests a significant local structural change at the surface within the measured potential range that occurs during the first cycle, and is retained on the subsequent cycles.21
The data presented in Fig. 3 highlight a key irreversibility in the Eg mode as a function of cycling. Critically, the initial Eg band position in the early stages before charging (ca. 480–482 cm−1) is never recovered during the first cycle. Upon delithiation of LiNiO2 to ca. Li0.1NiO2 at 4.3 V vs. Li+/Li on the first charge, the U-shape trend is observed, but the band only recovers to ca. 475 cm−1 and drops to even lower wavenumber values upon re-lithiation (discharge) to 470 cm−1 at 3.0 V vs. Li+/Li. Conversely, the observed irreversibility in band position before and after the first cycle is not replicated in subsequent cycles, whereby the observed shifts in the Eg band during the second cycle are broadly replicated within the third cycle. This aligns with the poor first-cycle Coulombic efficiencies of LiNiO2, which improve considerably in the latter two cycles (Fig. 1). Upon initial charging to 4.3 V vs. Li+/Li, LiNiO2 transforms into the H3 structure via several intermediate phases, undergoing a substantial 9.4% reduction in unit cell volume, contributing not only to a capacity fade but also to structural disorder.30 The observed irreversibility in the Eg mode in the Raman spectra likely indicates that the monoclinic structure of LiNiO2 persists locally, even after the first charge to 4.3 V and subsequent lithiation to 3.0 V.31,32
To further correlate the observed spectral changes with structural transformations in the material, data was compared with the differential capacity (dQ/dE) response of the electrode (Fig. S12). The dQ/dE response as a function of potential was related to the state of x(Li) in LixNiO2 (Fig. S13), enabling comparison between different phase regions with the changes in Raman bands as a function of the state of lithiation. The Raman band shifts (Fig. S12) and intensities (Fig. S13) are compared with differential capacities as a function of x(Li) in LixNiO2 for the three charging half-cycles.
LiNiO2 presents the electrochemical signatures of the well-established structural phase transformations from the initial hexagonal H1 structure, through monoclinic M, and hexagonal H2 and H3 structures,33 observed as plateaus in the potential profile and peaks in the dQ/dE plots.12 The fcc ABCABC stacking sequence of O2− anions in the pristine R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m LiNiO2 structure is retained throughout each of these transitions. The H1 phase has been found to persist via a solid-solution reaction until x ≈ 0.8 (i.e., Li0.8NiO2), followed by the M phase until x = 0.4–0.36. The H2 phase is the dominant phase down to x ≈ 0.26, with the H2 to H3 transition occurring between x = 0.26–0.16.33
m LiNiO2 structure is retained throughout each of these transitions. The H1 phase has been found to persist via a solid-solution reaction until x ≈ 0.8 (i.e., Li0.8NiO2), followed by the M phase until x = 0.4–0.36. The H2 phase is the dominant phase down to x ≈ 0.26, with the H2 to H3 transition occurring between x = 0.26–0.16.33
The Eg peak positions for the first cycle are depicted in Fig. S13, and the shift towards lower wavenumbers was the result of the softening of the metal–oxygen–metal bonds during delithiation (charging), associated with the hexagonal to monoclinic phase transition beginning from x < 0.85 up to the turning point around x < 0.60 where the band position begins to rise.16 The second and third cycles also experienced this event until the turning points of x < 0.40 and x < 0.35, respectively. The state of lithiation (x(Li) in LixNiO2) ranges for each phase are depicted in Table 1, which, for cycle 1, follows the phase changes described above.33
| Structure | Potential range/V vs. Li+/Li | x(Li) in LixNiO2 | ||
|---|---|---|---|---|
| 1st cycle | 2nd cycle | 3rd cycle | ||
| H1 | 3–3.65 | 1–0.85 | 0.8–0.75 | 0.75–0.65 |
| H1 + M | 3.65–3.73 | 0.85–0.80 | 0.75–0.65 | 0.65–0.55 |
| M | 3.73–4.02 | 0.80–0.45 | 0.65–0.35 | 0.55–0.25 |
| M + H2 | 4.02–4.08 | 0.45–0.35 | 0.35–0.25 | 0.25–0.15 |
| H2 | 4.08–4.18 | 0.35–0.25 | 0.25–0.20 | 0.15–0.1 |
| H2 + H3 | 4.18–4.25 | 0.25–0.1 | 0.2–0.1 | 0.1–0.05 |
| H3 | >4.25 | 0.1–0 | 0.1–0 | 0.05–0 |
The further phase transitions from monoclinic LiNiO2 to H2 and then H3 are driven by the migration of Ni ions into the lithium layers, which mainly happens during the first cycle.34 These transitions are correlated with an increase in band position for the Eg mode (Fig. S12) related to the hardening of the metal–oxygen bond as Ni3+ is oxidised to Ni4+.35,36
Upon re-lithiation, the Eg band did not return to its initial position of 485 cm−1, instead finishing at ∼465 cm−1 which indicates that LiNiO2 did not return to its original structure (Fig. 3).37,38 This suggests that Raman spectroscopy is sensitive to local structural irreversibility, highlighting that the bonding within LiNiO2 does not fully revert locally to the initial structure after cycle 1. Despite full discharge to 3 V vs. Li+/Li, the stoichiometry of only ca. Li0.8NiO2 is recovered, corresponding approximately with the range of the monoclinic phase (Fig. S11) wherein the terminal Eg band position aligns reasonably well with this position (x = 0.8 for LixNiO2) during charging (Fig. S6). Overall, Raman spectroscopy appears to be more sensitive to these local changes than X-ray powder diffraction, whereby after the 1st cycle, the H1 structure remains the only phase detected.18 Previously, ex situ synchrotron X-ray powder diffraction of LiNiO2 (with low Li/Ni intermixing) revealed that, after 50 charge–discharge cycles, the reflections corresponding to the monoclinic phase began to persist in the diffraction pattern of x = 0 (LiNiO2), indicating that the M–H1 phase transition is not fully reversible upon discharge.18
In the second and third cycles, the Eg mode shift values for H3 after charging (ca. 470–474 cm−1) are greater than those for the H1 phase (ca. 465 cm−1, Fig. S12). The shifting of the A1g peak position to lower Raman shift values (from ca. 552 cm−1 [cycle 2] and 550 cm−1 [cycle 3] to ca. 548 and 546 cm−1 for cycles 2 and 3, respectively) indicates the elongation of the Ni–O bond along the c-axis and a reduction in the intensity of both active modes, which is consistent with the experimental data from the second and third cycles (Fig. S12 and S13).
In general, the discharge (lithiation) trends for the Eg and A1g active modes (Fig. 3) differed from the trends observed during charging, indicating that the phase transitions are not completely reversible for LiNiO2 during discharge for the potential range chosen, as expected, especially for the first cycle.
The data extracted on band intensities for both modes across the first three charge cycles are presented in Fig. 3 and Fig. S13. Band intensity in Raman spectroscopy is related to several parameters, and the general increase in band intensity as a function of lithiation (charging) is rationalised in depth for one cycle by Jacquet et al.16 Overall, the spectral intensity increased during charge and decreased during discharge reversibly for both active modes during all 3 cycles in our study.
The relative intensity ratio (I(Eg)/I(A1g)) was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at (x(Li) in LixNiO2 = 1) during the first delithiation (Fig. S14). Throughout the initial stages of delithiation, the Eg mode decreased in intensity, while the A1g mode intensity increased during the H1 → M transition. For cycles 2 and 3, and in agreement with the Raman shift results, both intensities decreased until arriving in the monoclinic structure region. During the presence of the M phase, the Eg and A1g intensities increased for all three cycles until reaching the H2 phase, where the A1g intensity experienced a maximum before decreasing at the end of the delithiation.
1 at (x(Li) in LixNiO2 = 1) during the first delithiation (Fig. S14). Throughout the initial stages of delithiation, the Eg mode decreased in intensity, while the A1g mode intensity increased during the H1 → M transition. For cycles 2 and 3, and in agreement with the Raman shift results, both intensities decreased until arriving in the monoclinic structure region. During the presence of the M phase, the Eg and A1g intensities increased for all three cycles until reaching the H2 phase, where the A1g intensity experienced a maximum before decreasing at the end of the delithiation.
For the H2 phase, the relative intensity ratio (I(Eg)/I(A1g)) consistently remained 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for the first, second and third cycles at x(Li) in LixNiO2 = 0.35, 0.25 and 0.15, respectively (corresponding to E vs. Li+/Li = 4.08, 4.11, and 4.15 V, respectively, Fig. S14). Across all cycles, the Eg intensity remains constant while the A1g intensity reduces for the H2 phase. Subsequently, at higher potentials, the intensity ratio reverses towards a maximum of I(Eg)/I(A1g) > 1.2, associated with the H3 phase transition causing a final increase in Eg intensity. Similar trends were observed in all three charge profiles in Fig. S13, highlighting that changes in band intensities were generally reversible during further cycles for LiNiO2.
2 for the first, second and third cycles at x(Li) in LixNiO2 = 0.35, 0.25 and 0.15, respectively (corresponding to E vs. Li+/Li = 4.08, 4.11, and 4.15 V, respectively, Fig. S14). Across all cycles, the Eg intensity remains constant while the A1g intensity reduces for the H2 phase. Subsequently, at higher potentials, the intensity ratio reverses towards a maximum of I(Eg)/I(A1g) > 1.2, associated with the H3 phase transition causing a final increase in Eg intensity. Similar trends were observed in all three charge profiles in Fig. S13, highlighting that changes in band intensities were generally reversible during further cycles for LiNiO2.
4. Conclusions
LiNiO2, with low Li/Ni intermixing, was analysed using operando Raman spectroscopy, revealing LiNiO2 structural evolution during the initial three cycles. By using an optimised Raman cell configuration, operando electrochemical Raman spectra could be collected for a conventional tape-cast composite electrode with practically representative electrochemical performance for more than one cycle. The fast deconvolution of many spectra across multiple cycles using PRISMA enabled a detailed analysis of the key Eg and A1g modes of LiNiO2. Trends in both the band position and intensities were related to the state of lithiation (x(Li) in LixNiO2) and the phase transitions through hexagonal and monoclinic phases (H1, M, H2 and H3). Tracking of the two key Raman modes (Eg and A1g) across multiple cycles highlighted the sensitivity of the method to irreversible local structural changes, particularly correlated to first cycle inefficiencies in LiNiO2 that account for capacity fade and structural irreversibility, whereby LiNiO2 does not revert to the initial spectra. Subsequent cycle analyses showed greater reversibility in the Raman shifts and intensity variations, highlighting that localised irreversible structural transitions occur predominantly during the first charge of LiNiO2 to 4.3 V vs. Li+/Li.Author contributions
E. C. O. performed the experiments and analyzed the data. A. R. N. supported the methodology development and data analysis. M. S. and L. D. conducted the PXRD measurements and contributed to data processing. L. J. H. and M. M. provided supervision, resources, and project administration. All authors discussed the results and contributed to writing – review and editing.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: schematic representation of the operando Raman cell configuration, LiNiO2 characterisation data (ICP-MS, magnetic susceptibility, powder XRD and refinements and the derived atomic coordinates and isotropic displacement parameters), supplementary operando Raman intensity plots, peak deconvolution information, non-normalised band intensity data trends, differential capacity data, and signal intensity ratio data. See DOI: https://doi.org/10.1039/d5cp03622d.Acknowledgements
As a part of the DESTINY PhD program, this publication acknowledges funding from the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie Actions COFUND (Grant Agreement No. 945357). LJH, MJR, and MS acknowledge the financial support from the Faraday Institution CATMAT (EP/S003053/1, FIRG016) and ARN acknowledges funding from the European Union's Horizon 2020 research and innovation programme under grant agreement no 957189 (BIG-MAP). The project is part of BATTERY 2030+, the large-scale European research initiative for inventing the sustainable batteries of the future. Authors acknowledge the support and guidance from Dr Eibar Flores, SINTEF, Norway, on the use of PRISMA.References
- L. Meyer, N. Saqib and J. Porter, J. Electrochem. Soc., 2021, 168, 090561 CrossRef.
- T. Lenk and U. Schröder, J. Solid State Electrochem., 2024, 28, 965–979 CrossRef CAS.
- R. Baddour-Hadjean and J. P. Pereira-Ramos, AIP Conf. Proc., 2010, 1267, 1137–1138 CrossRef.
- P. Novák, D. Goers, L. Hardwick, M. Holzapfel, W. Scheifele, J. Ufheil and A. Würsig, J. Power Sources, 2005, 146, 15–20 CrossRef.
- L. J. Hardwick, M. Hahn, P. Ruch, M. Holzapfel, W. Scheifele, H. Buqa, F. Krumeich, P. Novák and R. Kötz, Electrochim. Acta, 2006, 52, 675–680 CrossRef CAS.
- J. C. Panitz, F. Joho and P. Novák, Appl. Spectrosc., 1999, 53, 1188–1199 CrossRef CAS.
- T. J. Leckie, S. D. Robertson and E. Brightman, Energy Adv., 2024, 3, 2479–2502 RSC.
- A. R. Neale, D. C. Milan, F. Braga, I. V. Sazanovich and L. J. Hardwick, ACS Energy Lett., 2022, 7, 2611–2618 CrossRef CAS PubMed.
- R. E. Ruther, A. F. Callender, H. Zhou, S. K. Martha and J. Nanda, J. Electrochem. Soc., 2015, 162, A98–A102 CrossRef CAS.
- T. E. Rosser, E. J. F. Dickinson, R. Raccichini, K. Hunter, A. D. Searle, C. M. Kavanagh, P. J. Curran, G. Hinds, J. Park and A. J. Wain, J. Electrochem. Soc., 2021, 168, 070541 CrossRef CAS.
- J. S. Weaving, A. Lim, J. Millichamp, T. P. Neville, D. Ledwoch, E. Kendrick, P. F. McMillan, P. R. Shearing, C. A. Howard and D. J. L. Brett, ACS Appl. Energy Mater., 2020, 3, 7474–7484 CrossRef CAS.
- M. Bianchini, M. Roca-Ayats, P. Hartmann, T. Brezesinski and J. Janek, Angew. Chem., Int. Ed., 2019, 58, 10434–10458 CrossRef CAS PubMed.
- C. Lee, Y. Yokoyama, Y. Kondo, Y. Miyahara, T. Abe and K. Miyazaki, ACS Appl. Mater. Interfaces, 2020, 12, 56076–56085 CrossRef CAS PubMed.
- E. Flores, N. Vonrüti, P. Novák, U. Aschauer and E. J. Berg, Chem. Mater., 2018, 30, 4694–4703 CrossRef CAS.
- E. Flores, P. Novák and E. J. Berg, Front. Energy Res., 2018, 6, 1–16 CrossRef.
- Q. Jacquet, N. Mozhzhukhina, P. N. O. Gillespie, G. Wittmann, L. Perez, F. G. Capone, J. Rueff, S. Belin, R. Dedryvère, L. Stievano, A. Matic, E. Suard, N. B. Brookes, A. Longo, D. Prezzi, S. Lyonnard and A. Iadecola, Adv. Energy Mater., 2024, 14, 2401413 CrossRef CAS.
- E. Flores, N. Mozhzhukhina, X. Li, P. Norby, A. Matic and T. Vegge, Chem. Res., 2022, 2, 1–9 Search PubMed.
- J. Lim, M. Sonni, L. M. Daniels, M. Bahri, M. Zanella, R. Chen, Z. Li, A. R. Neale, H. Niu, N. D. Browning, M. S. Dyer, J. B. Claridge, L. J. Hardwick and M. J. Rossiensky, Adv. Mater., 2025, 37, 2417899 CrossRef CAS PubMed.
- A. Rougier, P. Gravereau and C. Delmas, J. Electrochem. Soc., 1996, 143, 1168–1175 CrossRef CAS.
- D. Goonetilleke, B. Schwarz, H. Li, F. Fauth, E. Suard, S. Mangold, S. Indris, T. Brezesinski, M. Bianchini and D. Weber, J. Mater. Chem. A, 2023, 11, 13468–13482 RSC.
- C. S. Yoon, D. W. Jun, S. T. Myung and Y. K. Sun, ACS Energy Lett., 2017, 2, 1150–1155 CrossRef CAS.
- C. Sole, N. E. Drewett and L. J. Hardwick, Faraday Discuss., 2014, 172, 223–237 RSC.
- J. Zou, C. Sole, N. E. Drewett, M. Velický and L. J. Hardwick, J. Phys. Chem. Lett., 2016, 7, 4291–4296 CrossRef CAS PubMed.
- L. J. Hardwick, H. Buqa and P. Novák, Solid State Ionics, 2006, 177, 2801–2806 CrossRef CAS.
- P. T. Barton, Y. D. Premchand, P. A. Chater, R. Seshadri and M. J. Rosseinsky, Chem. – Eur. J., 2013, 19, 14521–14531 CrossRef CAS PubMed.
- C. Ghanty, B. Markovsky, E. M. Erickson, M. Talianker, O. Haik, Y. Tal-Yossef, A. Mor, D. Aurbach, J. Lampert, A. Volkov, J.-Y. Shin, A. Garsuch, F. F. Chesneau and C. Erk, ChemElectroChem, 2015, 2, 1479–1486 CrossRef CAS.
- C. Julien and M. Massot, Solid State Ionics, 2002, 148, 53–59 CrossRef CAS.
- J. Molenda, P. Wilk and J. Marzec, Solid State Ionics, 2002, 146, 73–79 CrossRef CAS.
- P. Vandenabeele, Practical Raman Spectroscopy, John Wiley & Sons, Incorporated, Belgium, 1st edn, 2013 Search PubMed.
- M. Bianchini, A. Schiele, S. Schweidler, S. Sicolo, F. Fauth, E. Suard, S. Indris, A. Mazilkin, P. Nagel, S. Schuppler, M. Merz, P. Hartmann, T. Brezesinski and J. Janek, Chem. Mater., 2020, 32, 9211–9227 CrossRef CAS.
- L. de Biasi, A. Schiele, M. Roca-Ayats, G. Garcia, T. Brezesinski, P. Hartmann and J. Janek, ChemSusChem, 2019, 12, 2240–2250 CrossRef CAS PubMed.
- W. Li, J. N. Reimers and J. R. Dahn, Solid State Ionics, 1993, 67, 123–130 CrossRef CAS.
- C. Xu, P. J. Reeves, Q. Jacquet and C. P. Grey, Adv. Energy Mater., 2021, 11, 1–12 Search PubMed.
- I. Konuma, N. Ikeda, B. D. L. Campéon, H. Fujimura, J. Kikkawa, H. D. Luong, Y. Tateyama, Y. Ugata, M. Yonemura, T. Ishigaki, T. Aida and N. Yabuuchi, Energy Storage Mater., 2024, 66, 103200 CrossRef.
- M. E. Arroyo y de Dompablo, A. Van der Ven and G. Ceder, Phys. Rev. B:Condens. Matter Mater. Phys., 2002, 66, 1–9 CrossRef.
- J. P. Peres, F. Weill and C. Delmas, Solid State Ionics, 1999, 116, 19–27 CrossRef CAS.
- A. R. Genreith-Schriever, A. Alexiu, G. S. Phillips, C. S. Coates, L. A. V. Nagle-Cocco, J. D. Bocarsly, F. N. Sayed, S. E. Dutton and C. P. Grey, Chem. Mater., 2024, 36, 2289–2303 CrossRef CAS PubMed.
- S. Sicolo, M. Mock, M. Bianchini and K. Albe, Chem. Mater., 2020, 32, 10096–10103 CrossRef CAS.
| This journal is © the Owner Societies 2026 |