A theoretical study on the mechanism of C2H3–5 oxidation by N2O
Huanhuan
Wang
a,
Long
Qin
a,
Bingzhi
Liu
b,
Shaohua
Zhu
ac,
Zhandong
Wang
b and
Ran
Sui
 *a
*a
aCenter for Combustion Energy and Department of Energy and Power Engineering, Tsinghua University, Beijing 100084, China. E-mail: sui@tsinghua.edu.cn
bNational Synchrotron Radiation Laboratory, University of Science and Technology of China, Hefei 230029, China
cNational Key Laboratory of Solid Propulsion, Northwestern Polytechnical University, Xi'an 710072, China
First published on 18th November 2025
Abstract
Nitrous oxide fuel blend (NOFBX), consisting of nitrous oxide and small hydrocarbons like ethane and ethene, offers potential for green propellants and optimized propulsion systems. This study investigates the oxidation reaction pathways of N2O with C2H3–5 using G4 and CCSD(T)/cc-pVQZ//M06-2X(D3)/def2-TZVP methods. The oxidation typically begins with addition reactions at N or O atomic sites, or the H-abstraction reaction by N2O, followed by hydrogen transfer and bond dissociation, leading to stable molecules and free radicals. Additionally, RRKM/ME theory was applied to calculate the reaction rate constants and branching ratios for N2O + C2H3–5 over a temperature range of 300–3000 K and pressures of 1–100 atm. A comparative analysis identified the dominant reaction pathways for N2O with CH components. Simulations incorporating detailed reaction mechanisms and kinetic data improved predictions of ignition delay times for both N2O/C2H4 and N2O/C2H6 systems. The new model also shows higher/lower fuel conversion below/above ∼1000 K. Sensitivity analysis indicates that direct reactions between N2O and CH components have minimal impact on model accuracy at temperatures above the ignition point, becoming stronger near ∼1200 K before weakening at higher temperatures. The results provide essential data for refining kinetic models of N2O/C2H4 and N2O/C2H6 systems and offer a theoretical basis for designing and optimizing NOFBX propellants.
1. Introduction
Conventional hydrazine-based propellants – such as hydrazine, methylhydrazine, and unsymmetrical dimethylhydrazine – are expected to face increasingly strict restrictions due to their high toxicity, corrosiveness, and complex safety handling requirements.1 As such, the development of green alternatives has become a key concern. Among the candidates, nitrous oxide fuel blend (NOFBX), a single-component propellant composed of nitrous oxide (N2O) and small hydrocarbons,2 and particularly C2-based fuels,3,4 has attracted growing attention due to its non-toxic nature, high performance and low cost. However, the use of premixed NOFBX brings challenges, such as high flame temperature and increased risk of backfire, necessitating more precise control and understanding of the combustion process. In this context, in-depth studies on the combustion kinetics of NOFBX propellants are crucial for their practical applications.Kinetic studies of NOFBX generally fall into three categories: (1) the conventional chemistry of small hydrocarbons, (2) the decomposition of the oxidant N2O, and (3) the direct reactions between N2O and the hydrocarbon components. N2O easily forms complex couplings with free radicals in hydrocarbon fuels, increasing the difficulty of kinetic research on such processes.5–8 In the emission control of NOx pollutants, the interaction between N2O and the fuel also warrants consideration.9,10 Numerous detailed mechanisms for small hydrocarbons have been developed, including GRI-Mech 3.0,11 Aramco-Mech 2.0,12 USC-II,13 and the latest NUIG-Mech 1.3 mechanism.14 The thermal decomposition of N2O into N2 and O2 has also been investigated.15,16 In these mechanism models, C2 species act not only as core intermediates in the oxidative decomposition of hydrocarbon fuels (especially acetylene, ethylene-based fuels, and cracking products of higher carbon number fuels) but also as key participants in the formation of soot precursors (e.g., polycyclic aromatic hydrocarbons, PAHs). Therefore, research on the relevant kinetics of their reaction with N2O is of great significance for model optimization. Wang et al.17 studied laminar flames and developed a chemical kinetic model of N2O/C2H4 based on the USC-II mechanism, refining the kinetic parameters of eight key elementary reactions. However, they did not delve into the direct reactions between N2O and the hydrocarbon components. Zheng et al.18 proposed a kinetic model for the N2O–C2 system, validated in the range of 110–1700 K, 0.1–1.6 MPa, and equivalence ratios of 0.5–2.0. In this model, four new elementary reactions for the interactions between N2O and C2H3/C2H4 were incorporated. These pathways were derived by analogy with known O radical reactions with C2H3/C2H4 in the USC-II model13 and N2O reactions with C2H3/C2H2 from the Konnov model.19 Zhang et al.20 conducted autoignition experiments in a rapid compression machine (RCM) with N2O/C2H4 over a temperature range of 885–940 K, pressures of 2.5–4.3 MPa and equivalence ratios of 1.05 and 1.35. They found that the decomposition of N2O dominated at high temperatures, while its direct interaction with hydrocarbons became more significant at low temperatures. They further emphasized the need for more accurate low-temperature oxidation mechanisms and high-level theoretical data to improve combustion modeling.
From an atmospheric chemistry perspective, N2O is also a potent greenhouse gas,21 and its reactions with small organic radicals are of significant interest. Moreover, the oxidation of C2H3–5 radicals by N2O is mechanistically relevant to coal combustion because such reactions govern whether N2O, formed as an intermediate during fuel-nitrogen conversion, decomposes benignly to N2 or further oxidizes to NO, thus directly influencing NOx emissions. As early as 1959, Kenwright et al.22 reported rate constants for reactions between ethane radical (C2H5) and N2O at temperatures of 826–861 K. Tang et al.23 reviewed the combustion chemistry of unsaturated and oxygenated hydrocarbons in the presence of NO, NO2 and N2O, summarizing earlier findings of RH–N2O reactions since 1950s. Only a few studies, such as that by Trenwith et al.,24 provided experimental rate constants of the reaction of C2H4 + N2O → CH3CHO + N2 at 828–863 K. Li et al.25 used the B3LYP/6-31++G** method to investigate the oxidation mechanism of the N2O/C2H4 system, and obtained the reaction pathways of N2O and C2H4 that generated acetaldehyde and N2, with the former further decomposed into CH3 and CHO. They also estimated the activation energies and energetics of the subsequent steps leading to CO2 and H2O. Despite the above efforts, a comprehensive kinetic analysis of the N2O + C2H4 reaction has not been conducted.
To sum up, the direct reactions of N2O with C2H3, C2H4 and C2H5 radicals still lack systematic study, despite their relevance to both NOFBX combustion modeling and atmospheric chemistry, and hence require further investigation. To address this gap, the present study has two main objectives: (1) to elucidate the reaction mechanisms between N2O with C2H3–5 components and provide accurate kinetic data using high-precision quantum methods, and (2) to evaluate the impact of these reactions on the overall N2O–C2 model through comparisons of ignition delay times and sensitivity analyses, thereby providing a solid foundation for future kinetic model development.
2. Computational methods
2.1. Quantum chemistry calculations
Geometrical optimizations and frequency analyses of all reactants, intermediates (IMs), transition states (TSs), and products on the potential energy surfaces (PESs) of the reactions between N2O and C2H3, and C2H4 and C2H5 were carried out using the M06-2X(D3)/def2-TZVP method.26–29 The validity of each transition state was confirmed by verifying the presence of a single imaginary frequency and ensuring that the corresponding vibrational mode connects the intended reactants and products. Additionally, intrinsic reaction coordinate (IRC) calculations were performed to further verify that the transition states correctly connect the associated minima on the PESs. Single-point energy for each species at 0 K were calculated using the CCSD(T)/cc-pVQZ method, with errors less than ± 1 kcal mol−1. However, for the reaction path of N2O + C2H3, the CCSD(T)/cc-pVQZ//M06-2X(D3)/def2-TZVP method produced unreasonable energy values. To address this issue and enable comparison, the reactions of N2O + C2H3–5 series were also computed using the G4 method.30 All electronic structure calculations for the PESs were conducted using the Gaussian 16 program.31 Additionally, T1 diagnostics was performed using the CCSD(T)/cc-pVQZ method to assess the potentially strong influence of a strong electron correlation. For reaction pathways with T1 values greater than 0.045, a multi-reference method was employed. Consequently, based on the geometries optimized using the G4 method, single-point energies were recalculated using the multi-reference CASPT2(7e,7o)/cc-pVQZ method,32 implemented via the Mokit interface33 with the Molpro software.34 For the system N2O + C2H3, the main types of orbitals considered for the activation space include the π and π* orbitals of the N–N bond, the π and π* orbitals of the C–C bond, the π and π* orbitals of the entire structure, and the free radical orbitals of the CH part. For the system N2O + C2H5, 7e and 7o were selected. These mainly come from the bonding and non-bonding orbitals of the methyl part in C2H5, the π and π* orbitals of the N–N bond in the bonding direction, the π and π* orbitals in the p direction, and the free radical orbitals of the methylene part in C2H5.2.2. Kinetic methods
The rate constants for the different reaction pathways of N2O + C2H3–5 at 1–100 atm and 300–3000 K were calculated using the RRKM/master equation method,35 implemented via the kinetic calculation program MESS.36 Argon (Ar) was used as the bath gas, and the calculated intermediate IM1 was taken as the reactant. The critical temperature Tc and critical pressure Pc of IM1 were calculated using the Joback formula.37 The Lennard-Jones (L-J) parameters required calculating the collisional deactivation rate constants and were estimated from empirical equations:38σ = 2.44(Tc/Pc)1/3 and ε/kb = 0.77Tc. The obtained L-J parameters were as follows: σ = 5.68 Å and ε/kb = 441.57 K for the N2O + C2H3 system; σ = 5.87 Å and ε/kb = 508.35 K for N2O + C2H4; σ = 5.63 Å and ε/kb = 440.88 K for N2O + C2H5; and σ = 3.55 Å and ε/kb = 116.16 K for Ar. The average energy transferred per deactivating collision, which determines the collisional efficiency and plays an important role in the attenuation effect, was expressed by a simple power law: ΔEdown = Θ(T/300)0.80 cm−1,39 where the empirical parameter Θ was estimated to be 250 cm−1 in this work. Due to the small molecular weight of the investigated system and the presence of multiple unsaturated bonds in the molecules, the anharmonic effect of the low-frequency torsion was not considered. The effect of one-dimensional (1-D) asymmetric Eckart tunneling40 is also taken into account in the MESS software.36 All vibrational modes were treated as resonances, and their frequencies were scaled by a factor of 0.984, as appropriate for the M06-2X(D3)/def2-TZVP method.413. Results and discussion
The reactions of N2O with three CH species–C2H3, C2H4 and C2H5–are illustrated in Fig. 1–4 through their PESs, where intermediates and transition states are distinguished by subscript labels (a)–(c) for each system. For the N2O + C2H3 system, all energy values are provided at the G4 level. For the N2O + C2H4 and N2O + C2H5 systems, energies are given at both the G4 level and the CCSD(T)/cc-pVQZ//M06-2X(D3)/def2-TZVP level, with the latter shown in parentheses. Species with T1 diagnostic values exceeding 0.045 at the CCSD(T)/cc-pVQZ level are highlighted in red to indicate possible multi-reference characters. Detailed structural parameters and vibrational frequency data for the PES are provided in Tables S1 and S2 of SM1. Fig. 5–7 present the calculated rate constants for the three reaction systems, identifying the dominant reaction pathways between N2O and each CH species. The corresponding fitted rate constants are provided in Tables S4–S6. The PESs for the N2O + C2H3/N2O + C2H5 calculated using the CASPT2/cc-pVQZ//B3LYP/6-31G(2df,p) (G4 optimization method) method are provided in Fig. S1 and S2. The herein obtained kinetic data are incorporated into a reaction model, which was used to simulate ignition delay times (IDTs) and perform sensitivity analyses for the N2O/C2H4 system (Fig. 8 and 9), with additional IDT data for N2O/C2H6 provided in Fig. S3.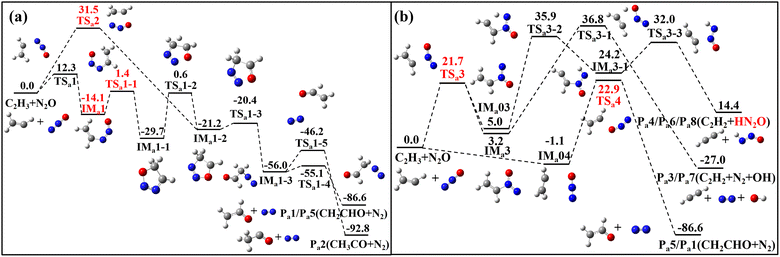 | ||
| Fig. 1 PES of the addition reaction channel for the N2O + C2H3 at 0 K calculated at the G4 level (in kcal mol−1). | ||
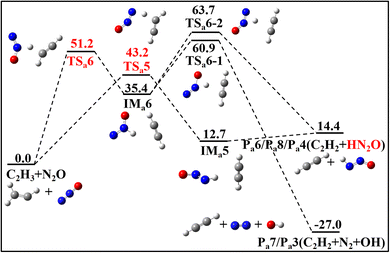 | ||
| Fig. 2 PES of the H-abstraction reaction channel for the N2O + C2H3 at 0 K calculated at the G4 level (in kcal mol−1). | ||
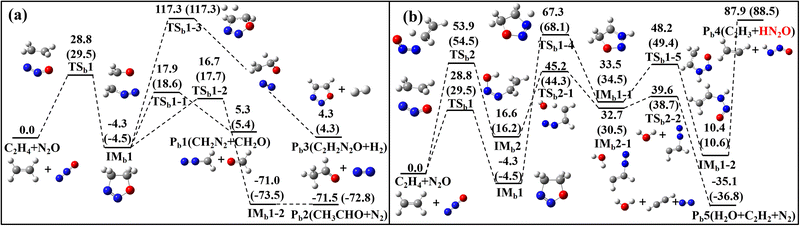 | ||
| Fig. 3 PES for the N2O + C2H4 reaction channels at 0 K, calculated at the G4 level, with CCSD(T)/cc-pVQZ//M06-2X(D3)/def2-TZVP values provided in parentheses (in kcal mol−1) | ||
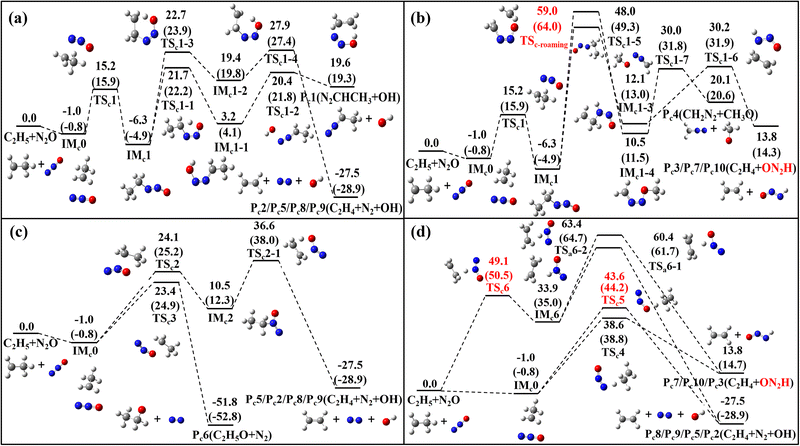 | ||
| Fig. 4 PES for the N2O + C2H5 reaction channels at 0 K, calculated at the G4 level, with CCSD(T)/cc-pVQZ//M06-2X(D3)/def2-TZVP values provided in parentheses (in kcal mol−1). | ||
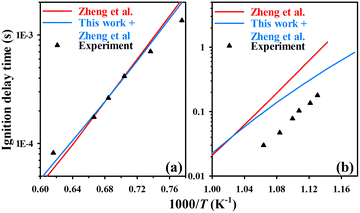 | ||
| Fig. 8 Predicted (lines, using the original model of ref. 18 with and without the N2O + C2H3–5 reactions of this work) and experimental (symbols) results of ignition delay times for the N2O/C2H4/Ar mixture of (a) equivalence ratio 0.5 and at 4 atm pressure42 and (b) equivalence ratio 1.05 and at 30 bar pressure.18 | ||
3.1. Potential energy surfaces
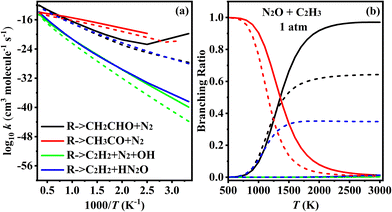
The PES for the reaction between N2O and C2H3 is depicted in Fig. 1 and 2. This reaction proceeds via five addition pathways and three hydrogen abstraction pathways, ultimately yielding four products: Pa1/Pa5 (CH2CHO + N2), Pa2 (CH3CO + N2), Pa3/Pa7 (CH2CH2 + N2 + OH), and Pa4/Pa6/Pa8 (C2H2 + HN2O). As illustrated in Fig. 1a, the C2H3 adds directly to the terminal nitrogen atom of N2O, forming a C-N bond. This adduct undergoes torsional rotation to form a five-membered ring intermediate, IMa1-1, which can then experience hydrogen migration between C-C bonds to form intermediate IMa1-2. Alternatively, C2H3 and N2O may form IMa1-2 directly through the transition state TSa2. Subsequent cleavage of the N–O bond leads to ring-opening and the formation of IMa1-3, which has two possible bond-breaking channels: in the first, direct C–N bond cleavage yields CH2CHO and N2; in the second, the C–N bond cleaves while hydrogen transfer occurs between the C–C bonds, producing CH3CO and N2. Comparison reveals that the second product is more exothermic. The C2H3 radical may also add to the central nitrogen atom (via TSa3) or to the terminal oxygen atom (via TSa4), as shown in Fig. 1b. Addition to the central nitrogen yields two distinct intermediates, differing by 1.8 kcal mol−1 in energy. These intermediates subsequently undergo intramolecular hydrogen transfer, in which a hydrogen atom from the CH2 group migrates through a five-membered ring transition state to the O or N atom, respectively, leading to decomposition and the formation of products Pa3 and Pa4. Addition of the C2H3 radical to the terminal oxygen atom results in the formation of Pa5 (CH2CHO + N2).In addition, N2O can abstract a hydrogen atom directly from the C2H3 radical to generate C2H2, as shown in Fig. 2. When the terminal nitrogen abstracts hydrogen from the CH2 group, HN2O is formed. Abstraction by the central nitrogen followed by hydrogen transfer can lead to either HN2O or N2 + OH. However, the transition states for these hydrogen transfers are associated with high energy barriers, with transition state energies of 60.9 and 63.7 kcal mol−1 for migration to the O and N atoms, respectively. Species with T1 diagnostic values greater than 0.045 are marked in red in Fig. 1 and 2. Since these species appear in both reaction channels, single-point energy calculations were performed using the CASPT2/cc-pVQZ method via Mokit software,33 based on G4-optimized structures. An active space of 7 electrons in 7 orbitals was used for all TSs and intermediates. The CASPT2 energies are provided in Fig. S1 of the SM1.
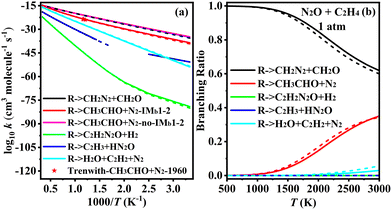
The PES for the reaction of N2O with C2H4 is shown in Fig. 3, comprising five distinct reaction channels. In Fig. 3a, the first two, the two carbon atoms of C2H4 were added to the nitrogen and oxygen atoms of N2O (via TSb1), forming a five-membered ring intermediate, IMb1. IMb1 can either undergo concerted cleavage of the C–C and N–O bonds to form Pb1 (CH2N2 + CH2O), or experience hydrogen transfer between the C–C bonds to form Pb2 (CH3CHO + N2). The latter channel corresponds to the reaction route proposed by Li et al. using the B3LYP/6-31++G** method.25 At the G4 level, the energy barriers for TSb1-1 and TSb1-2 differ by only 1.2 kcal mol−1, indicating both are kinetically accessible. The third channel involves a much higher energy barrier via TSb1-3 (117.3 kcal mol−1) and leads to the less favorable formation of Pb3 (C2H2N2O and H2). The IMb1 can also undergo intramolecular hydrogen transfer, as shown in Fig. 3b, where a hydrogen atom on a CH2 group migrates to the nitrogen atom, followed by bond cleavage to form Pb4. However, this pathway has a high product relative energy of 87.9 kcal mol−1. In another reaction channel, C2H4 adds via its carbon atom to the terminal nitrogen of N2O, while a hydrogen atom on the same carbon simultaneously transfers to the oxygen atom of N2O, forming a five-membered ring transition state TSb2. Subsequently, another hydrogen atom from the remaining carbon transfers to the oxygen atom, leading to the formation of H2O + C2H2 + N2 (Pb5). Compared to the first two channels, the last three pathways involve significantly higher energy barriers and are therefore less favorable under typical reaction conditions.
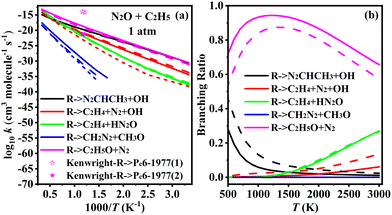
The PES for the N2O + C2H5 reactions are illustrated in Fig. 4, encompassing ten distinct reaction channels. Four of these channels originate from the addition of the C2H5 radical to the terminal nitrogen atom of N2O, as shown in Fig. 4(a) and (b). This addition forms the intermediate IMc1 via a newly generated C–N bond. IMc1 can undergo intra-molecular hydrogen transfer: either the H on the methylene group or the methyl group mitigates to the O atoms, forming IMc1-1 and IMc1-2, respectively. IMc1-1 proceeds via N–O bond breaking to yield the product Pc1 (N2CHCH3 + OH), while IMc1-2 undergoes C–N bond-breaking to form Pc2 (C2H4 + N2 + OH). An alternative pathway involves transfer of a hydrogen atom from the methyl group to the adjacent nitrogen atom, followed by C–N bond breaking to form Pc3 (C2H4 + ON2H). The transition state for this process, TSc1-5, involves a four-membered ring and features a higher transition state energy of 48.0 kcal mol−1. In addition, the possibility of methyl (CH3) group transfer (the roaming process) via 1Mc1 is considered. This pathway entails an even higher transition state energy (59.0 kcal mol−1), where the CH3 group transfers to the oxygen atom, followed by N–O bond cleavage to form Pc4 (CH2N2 + CH3O). The T1 diagnostic value for the roaming transition state (TSc-roaming) is notably high (0.061), indicating a significant multireference characteristic, and thus single-point energy calculations were performed using the CASPT2/cc-pVQZ//B3LYP/6-31G(2df,p) method. Similarly, since the product ON2H in Pc3 also has a T1 diagnostic value greater than 0.045, a multireference treatment was also applied to that pathway. For both channels, the transition states and intermediates were calculated with an active space of 7 electrons in 7 orbitals.
As shown in Fig. 4c, the C2H5 radical can also be added to either the central nitrogen or the terminal oxygen atom of N2O. Addition at the central nitrogen forms the intermediate IMc2, which subsequently undergoes hydrogen transfer between the methyl carbon and the oxygen atom, leading to the same product as Pc2, namely Pc5 (C2H4 + N2 + OH). In contrast, addition to the oxygen atom follows a more straightforward pathway, directly forming Pc6 (C2H5O + N2). Similar to the hydrogen abstraction reactions between N2O and the C2H3 radical, N2O can also abstract a hydrogen atom from the methyl group of the C2H5 radical to produce C2H4, as shown in Fig. 4d. All three atoms in N2O are capable of abstracting a hydrogen atom. Among these, abstraction by the terminal nitrogen has the lowest energy barrier, followed by abstraction by the terminal oxygen. Abstraction by the central nitrogen, along with the subsequent hydrogen transfer process, exhibits the highest energy barrier.
3.2. Rate constants for N2O + C2H3–5
Based on the above PESs studies, a further kinetic analysis of the reaction pathways for N2O + C2H3–5 was performed to obtain the rate constants over the temperature range of 300 to 3000 K at 1–100 atm.3.3. Ignition delay time and sensitivity analysis
Next, the N2O + C2H3–5 reaction rates developed in this work using the G4 method were incorporated with the N2O–C2 mechanism proposed by Zheng et al.18 The reactions C2H3 + N2O → CH3CO + N2 and N2O + C2H4 → C2H3 + HN2O in ref. 18 were replaced with the 14 calculated direct N2O–C2H3–5 reactions in this work. Ignition delay time (IDT) was selected to verify the optimized mechanism. That is because, for NOFBX rocket propellants, ignition reliability and ignition response speed are key indicators that determine the performance of the propulsion system, and IDT serves as a direct quantitative parameter to characterize these two indicators.IDT of N2O/C2H4 (diluted with Ar, equivalence ratio 0.5 and 4 atm pressure) were predicted using this combined model, which was compared with experimental results,18,42 obtained from shock tube measurements) and simulations obtained using the original model in Fig. 8. The inclusion of the 14 calculated direct N2O–C2H3–5 reactions improved the predictions of IDT, especially under low-temperature ranges. Similar improvement is also observed for the N2O/C2H6 mixture (see Fig. S3 of the SM1) and other pressures. Moreover, there is a more pronounced change in the predicted fuel conversion rates with temperature when the 14 reactions are added, albeit no experimental data is available for comparison. As shown in Fig. S4 of SM1, the new model results in higher conversions by up to 1.8% at temperatures of ∼1050 K and 3.22% at ∼1200 K.
Finally, sensitivity analysis (SA) was performed using the new kinetic model comprising the 14 N2O–C2H3–5 reactions and Zheng's model.18Fig. 9 shows the normalized sensitivity analysis coefficients of the top 5 most important reactions in the N2O–C2H3–5 system for C2H4 in the temperature range of 850–1550 K. At all investigated temperatures, the reactions C2H4 + N2O → CH2N2 + CH2O and C2H4 + N2O → CH3CHO + N2 are the most important among the 14 steps. At 850 K the system is under a weakly reacting state (i.e., before practical ignition, see Fig. S4), and hence no one among the 14 reactions are significant, as reflected by the small SA coefficients less than 0.1%. At this stage, C2H5 + N2O → C2H5O + N2 and C2H5 + N2O → C2H4 + HN2O have positive SA coefficients, indicating they are against the conversion of C2H4. Moreover, the SA coefficients gradually increase until ∼1200 K and then decrease with further increased temperatures. C2H5 + N2O → C2H5O + N2 also changes its SA to negative at 1200 K. This is consistent with the discrepancies in C2H4 conversion rates predicted by the new and original models, which is greatest at ∼1200 K, and suggests that the investigated N2O–C2H3–5 reactions are the most significant in the medium temperature regime.
4. Conclusions
This work investigates the reactions of N2O with C2H3, C2H4, and C2H5, using the G4 and CCSD(T)/cc-pVQZ//M06-2X(D3)/def2-TZVP methods, and provides a detailed study of the PESs and kinetics of these reactions. In the process of N2O oxidizing CH components, the reaction typically proceeds through the addition of the CH component to either the N or O site, followed by subsequent reactions. Alternatively, the hydrogen abstraction reaction from the CH component by N2O may occur first. The reaction paths of N2O + C2H3 and N2O + C2H5 are similar, where the radicals can add to any of the three atoms in the N2O molecule, or undergo hydrogen abstraction from the radical to form stable CH-containing products. In contrast, the N2O + C2H4 reaction primarily proceeds via the initial formation of a five-membered ring transition state, followed by subsequent reactions. Through kinetic studies, the rate constants and branching ratios of the N2O + CH species are discussed in detail. In the N2O + C2H3 reaction, the formation of CH3CO + N2 and CH2CHO + N2 corresponds to the dominant reaction pathways at low and high temperatures, respectively. In the N2O + C2H4 reaction, the formation of CH2N2 + CH2O dominates across the entire temperature range. In the N2O + C2H5 reaction, the primary product is C2H5O + N2. Comprehensive calculations of the rate constants updated the corresponding rate constant data, and 2 elementary reactions in a literature kinetic model were replaced with the herein theoretically calculated fourteen N2O–C2H3–5 steps. By incorporating the current theoretical findings into the N2O/C2H4 and N2O/C2H6 models and comparing with experimental data, an improvement in the ignition delay times is observed. The new model also yields higher/lower fuel conversion rates at temperatures below/above 1000 K. Sensitivity analysis further revealed that upon ignition the herein calculated N2O–C2H3–5 steps, which were missing from previous literature models, play a nonnegligible role promoting fuel conversion, which have a non-monotonic temperature dependence with the most pronounced impact at medium temperatures ∼1200 K. This study is hence beneficial for advancing the combustion mechanisms of N2O and small hydrocarbons and models of green rocket propellants from a quantitative perspective.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5cp03435c.Acknowledgements
This work was supported by the National Key R&D Program of China (2024YFB410480101), the National Natural Science Foundation of China (52506001, 52576126), and the Dushi Project of Tsinghua University. The numerical calculations in this paper were carried out at the Hefei Advanced Computing Center.References
- A. S. Gohardani, J. Stanojev, A. Demairé, K. Anflo, M. Persson, N. Wingborg and C. Nilsson, Prog. Aeronaut. Sci., 2014, 71, 128–149 CrossRef.
- V. Zakirov, A. Perera-Webb, R. Osborne, C. Milyaev, J. Anderson, S. Gwozdz and P. Ganapathiperumal, J. Br. Interplanet. Soc., 2021, 74, 223–233 Search PubMed.
- L. Werling and T. Hörger, Acta Astronaut., 2021, 189, 437–451 CrossRef CAS.
- L. Werling and P. Bätz, J. Propul. Power, 2022, 38, 254–266 CrossRef CAS.
- R. Ma, H. Zhang and W. Fan, Energy, 2024, 297, 131245 CrossRef CAS.
- W. Wang and H. Zhang, Combust. Flame, 2019, 202, 362–375 CrossRef CAS.
- C. Naumann, T. Kick, T. Methling, M. Braun-Unkhoff and U. Riedel, Int. J. Energetic Mater. Chem. Prop., 2019, 19, 65–71 CrossRef.
- J. Koop and O. Deutschmann, Appl. Catal., B, 2009, 91, 47–58 CrossRef CAS.
- F. Normann, K. Andersson, B. Leckner and F. Johnsson, Prog. Energy Combust. Sci., 2009, 35, 385–397 CrossRef CAS.
- A. N. Hayhurst and A. D. Lawrence, Prog. Energy Combust. Sci., 1992, 18, 529–552 CrossRef CAS.
- S. Skeen, G. Yablonsky and R. Axelbaum, Combust. Flame, 2010, 157, 1745–1752 CrossRef CAS.
- W. K. Metcalfe, S. M. Burke, S. S. Ahmed and H. J. Curran, Int. J. Chem. Kinet., 2013, 45, 638–675 CrossRef CAS.
- H. Wang, X. You, A. V. Joshi, S. G. Davis, A. Laskin, F. Egolfopoulos and C. K. Law, URL: http://ignis.usc.edu/USC_Mech_II.htm, 2007.
- S. Panigrahy, A. A. E.-S. Mohamed, P. Wang, G. Bourque and H. J. Curran, Proc. Combust. Inst., 2023, 39, 253–263 CrossRef.
- N. E. Meagher and W. R. Anderson, J. Phys. Chem. A, 2000, 104, 6013–6031 CrossRef CAS.
- S. Liu, W. Fan, X. Wang, J. Chen and H. Guo, Energy, 2022, 247, 123445 CrossRef CAS.
- W. L. Wang and H. Q. Zhang, Combust. Flame, 2019, 202, 362–375 CrossRef CAS.
- D. Zheng, P. F. Xiong and B. J. Zhong, Acta Phys.-Chim. Sin., 2019, 35, 1241–1247 CAS.
- A. A. Konnov, Combust. Flame, 2009, 156, 2093–2105 CrossRef CAS.
- F. Zhang, H. Y. Chen, J. C. Feng and D. Zheng, Fuel, 2021, 288, 119688 CrossRef CAS.
- A. R. Ravishankara, J. S. Daniel and R. W. Portmann, Science, 2009, 326, 123–125 CrossRef CAS PubMed.
- R. Kenwright, P. L. Robinson and A. B. Trenwith, J. Chem. Soc., 1959, 2079–2082 RSC.
- R. Tang and S. Cheng, Energies, 2023, 16, 4967 CrossRef CAS.
- A. Trenwith, J. Chem. Soc., 1960, 3722–3726 RSC.
- Y. Y. Li, R. P. Jiang, S. Xu and X. D. Gong, Propellants, Explos., Pyrotech., 2022, 47, e202200082 CrossRef CAS.
- Y. Zhao and D. G. Truhlar, Acc. Chem. Res., 2008, 41, 157–167 CrossRef CAS PubMed.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215–241 Search PubMed.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- H. Wang, C. Cao, S. Zheng and R. Sui, Combust. Sci. Technol., 2025, 197 DOI:10.1080/00102202.2025.2500503.
- B. Chan, M. L. Coote and L. Radom, J. Chem. Theory Comput., 2010, 6, 2647–2653 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian, Inc., Wallingford CT, 2016.
- H.-T. Guo, L. Yang, Z. Serinyel and C.-W. Zhou, Phys. Chem. Chem. Phys., 2025 10.1039/D5CP03515E.
- J. Zou, Molecular Orbital Kit (MOKIT), https://gitlab.com/jxzou/mokit, 2024 Search PubMed.
- H. Werner, P. Knowles, G. Knizia, F. Manby, M. Schutz, P. Celani, W. Gyorffy, D. Kats, T. Korona and R. Lindh, MOLPRO, a package of ab initio programs, version 2015.1, University of Stuttgart, 2015 Search PubMed.
- K. Kohse-Höinghaus, J. Troe, J.-U. Grabow, M. Olzmann, G. Friedrichs and K.-D. Hungenberg, Phys. Chem. Chem. Phys., 2018, 20, 10561–10568 RSC.
- Y. Georgievskii, J. A. Miller, M. P. Burke and S. J. Klippenstein, J. Phys. Chem. A, 2013, 117, 12146–12154 CrossRef CAS PubMed.
- B. E. Poling, J. M. Prausnitz and J. P. O’connell, Properties of gases and liquids, McGraw-Hill Education, 2001 Search PubMed.
- J. Welty, G. L. Rorrer and D. G. Foster, Fundamentals of momentum, heat, and mass transfer, John Wiley & Sons, 2014 Search PubMed.
- L. Zhang, Q. Chen and P. Zhang, Proc. Combust. Inst., 2015, 35, 481–489 CrossRef CAS.
- F. Zhang and T. S. Dibble, Phys. Chem. Chem. Phys., 2011, 13, 17969–17977 RSC.
- J. Bao, J. Zheng, I. Alecu, B. Lynch, Y. Zhao and D. Truhlar, https://comp.chem.umn.edu/freqscale/index.html, 2019.
- F. Deng, Y. Pan, W. Sun, F. Yang and Y. Zhang, Energy Fuels, 2017, 31, 14116–14128 CrossRef CAS.
| This journal is © the Owner Societies 2026 |