W-incorporated high-performance layered cathode materials for advanced lithium-ion batteries
Xiaoyi
Hou
 *,
Haozhe
Wu
*,
Haozhe
Wu
 ,
Qirongxin
Shen
,
Dengdeng
Ai
and
Xi
Wu
,
Qirongxin
Shen
,
Dengdeng
Ai
and
Xi
Wu

Physics and Electronic Information Engineering, Qinghai Normal University, Xining, 810008, China
First published on 12th November 2025
Abstract
High-nickel cobalt-free cathode materials are regarded as some of the promising candidates for high-energy-density lithium-ion batteries due to their excellent attributes of high capacity and cost-effectiveness. However, increasing the nickel content and removing cobalt lead to degradation of the electrode–electrolyte interfacial environment and other bulk-phase layered structure issues, which significantly compromise the battery's cycle life. In this work, a W-doped LiNi0.90Mn0.07Al0.03O2 cathode material was synthesized by introducing a small amount (2 mol%) of tungsten (W) during the lithiation procedure. The W-doping enhanced the cyclic stability of the LiNi0.90Mn0.07Al0.03O2 cathode (capacity retention after 100 cycles: 90.82% vs. 88.99% for the pristine material at 4.3 V) remarkably. These improvements are attributed to the high-valence W6+ passivating the cathode material surface activity, thereby stabilizing the bulk structure and electrode–electrolyte interface. These findings provide valuable strategic insights for developing high-nickel, cobalt-free cathode materials in lithium-ion batteries.
1. Introduction
Due to the high operating voltage, low production cost, and superior reversible capacity, high-nickel ternary layered cathode materials (LiNixCoyMn1−x−yO2, LiNixCoyAl1−x−yO2, x ≥ 0.6) have been widely used in new energy vehicles, electric bicycles, and smart wearable devices.1–5 Extensive studies have demonstrated that the specific capacity of the layered cathode materials exhibits a positive relationship with the nickel content. Consequently, ultrahigh-nickel (Ni ≥ 0.9) layered oxides have emerged as a predominant research focus to achieve considerable energy density (>220 mAh g−1).6–8 Simultaneously, the continuously rising cobalt price, driven by geopolitical instability and inherent resource scarcity in main production regions, has accelerated the development of cobalt-free cathode materials. This strategic shift, however, introduces significant challenges: the absence of cobalt not only degrades electronic conductivity and reaction kinetics due to the loss of the Co3+/Co4+ conductive pathway but also exacerbates structural collapse and cation mixing by weakening the Co–O bond stability, while simultaneously compromising thermal stability and introducing greater safety risks.9 Consequently, the combination of ultra-high nickel content and cobalt absence generally delivers adverse effects on the overall electrochemical performance.10,11In such cobalt-free systems, the inherent issues of ultra-high nickel content are further aggravated. The ultra-high nickel content inevitably causes unstable Ni4+ species during deep charge/discharge cycles. These metastable Ni4+ ions are reduced to the stable Ni2+ ions through parasitic reactions with electrolytes.12–16 This process triggers severe interfacial side reactions and leads to excessive cathode electrolyte interphase (CEI) growth. Furthermore, the accumulated Ni2+ ions (ionic radius: 0.69 Å) preferentially migrate into Li layers (Li+ = 0.76 Å) due to their similar ionic radii and exacerbate cation disorder.17 This structural defect hinders the transmission of lithium ions as well as the reversible intercalation/de-intercalation reaction, deteriorating rate capability and cycling stability.18,19
To address these inherent challenges of high-nickel layered cathode materials, researchers have developed comprehensive modification strategies including ion doping,20 surface coating,21 core–shell architecture,22 and concentration gradient design.23,24 Based on the above discussions, strategies such as multifunctionalization,25 interface engineering,26 and all-solid-state thin-film microbatteries27,28 have been proposed for battery systems. Among these approaches, ion doping emerges as the most straightforward and effective method for mitigating Li+/Ni2+ cation mixing and stabilizing the layered structure.29,30 The doping mechanism involves incorporating elements into the crystal lattice to form the robust transition metal–oxygen bonds (TM–O) and expand the interlayer spacing, which could suppress lattice oxygen release and transition metal dissolution during deep cycling.31,32 Different dopants occupy specific crystallographic sites according to their ionic characteristics, with high-valence cations (W6+, Al3+, Zr4+, and Ta5+)33–36 preferentially substituting transition metal sites to enhance structural integrity, anions (F− and Cl−)37,38 stabilizing the oxygen sublattice, and divalent cations (Mg2+ and Zn2+)39,40 occupying lithium sites to block Ni2+ migration. Notably, W6+ doping demonstrates exceptional effectiveness due to its high valence state that facilitates charge compensation while simultaneously modifying the microstructure to inhibit microcrack formation and strengthen interfacial stability through the formation of a thin protective CEI layer. These multifunctional improvements collectively enhance the structural, thermal, and electrochemical stability of the cathode material, with W6+-doped systems exhibiting superior performance in suppressing cation disorder and maintaining capacity retention during high-rate cycling. Building upon these findings, researchers Zhao et al.41 successfully synthesized a 1 mol% W6+-doped NCW-2 cathode material using WO3 as the tungsten source and Ni0.9Co0.1(OH)2 as the precursor through high-energy ball milling. The resulting NCA-2 cathode demonstrated exceptional electrochemical performance, delivering an initial discharge capacity of 204.44 mAh g−1 at 1C with 93.25% capacity retention after 100 cycles. In a parallel development, researchers Yang et al.42 employed a solid-state reaction method with LiOH, NiO, Co3O4, MnO2, Al2O3, and WO3 precursors to fabricate LiNi0.895Co0.04Mn0.03Al0.03W0.005O2 (W-NCMA), which achieved 95% capacity retention after 100 cycles at 0.5C. While these studies conclusively demonstrate W6+ doping efficacy in Co-containing high-nickel cathodes, the research landscape remains notably sparse regarding its application in cobalt-free systems. This gap underscores the critical need for systematic investigation of W6+ doping effects in high-nickel cobalt-free layered cathodes to establish comprehensive structure–property relationships.
In this study, we synthesized LiNi0.90Mn0.07Al0.03O2 (NMA) cathode materials with varying W6+ doping concentrations (0–0.03 mol) using Ni0.90Mn0.07Al0.03(OH)2 as the precursor and WO3 as the dopant source. Systematic characterization revealed that the optimal 0.02 mol W6+ doping concentration effectively suppressed secondary particle growth, creating shorter Li+ diffusion pathways that significantly enhanced rate capability. The refined microstructure simultaneously provided increased electroactive surface area for improved reaction kinetics, ultimately delivering exceptional cycling stability with 90.82% capacity retention after 100 cycles at 1C under high current density conditions.
2. Experimental section
2.1. Materials preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratio) were precisely weighed and dissolved with moderation PEG 6000 and PEG 200 to form a homogeneous mixed metal solution (0.05 mol L−1 total concentration). The temperature was then elevated to 83 °C under constant stirring.
3 molar ratio) were precisely weighed and dissolved with moderation PEG 6000 and PEG 200 to form a homogeneous mixed metal solution (0.05 mol L−1 total concentration). The temperature was then elevated to 83 °C under constant stirring.
For the precipitation reaction, di-n-butylamine (C8H19N, Aladdin, analytical reagent grade) was added dropwise at a controlled rate when the temperature stabilized at 83 °C. The reaction proceeded for 5 h under isothermal conditions (83 ± 1 °C) with persistent nitrogen flow to ensure an oxygen-free environment. The resulting mixed hydroxide suspension was aged for 12 h under static conditions, followed by filtration, washing with deionized water, and vacuum drying at 80 °C for 12 h to obtain the precursor.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.04
1.04![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x (where x represents 0, 0.01, 0.02, and 0.03 molar ratios of W doping). The precisely weighed components were homogenized via planetary ball milling (zirconia media, 500 rpm, 2 h) to ensure a homogeneous distribution. The mixed powder was loaded into a tube furnace under flowing oxygen atmosphere (100 mL min−1) and subjected to a controlled thermal treatment protocol: first heated to 500 °C at 5 °C min−1 and maintained for 5 h, subsequently raised to 750 °C at 5 °C min−1 and maintained for 20 h, followed by naturally cooling to room temperature, yielding W6+-doped LiNi0.90Mn0.07Al0.03O2 cathode materials. The resulting products were designated as NMA (undoped), W-0.01 (1 mol% W), W-0.02 (2 mol%), and W-0.03 (3 mol%) based on their nominal tungsten content.
x (where x represents 0, 0.01, 0.02, and 0.03 molar ratios of W doping). The precisely weighed components were homogenized via planetary ball milling (zirconia media, 500 rpm, 2 h) to ensure a homogeneous distribution. The mixed powder was loaded into a tube furnace under flowing oxygen atmosphere (100 mL min−1) and subjected to a controlled thermal treatment protocol: first heated to 500 °C at 5 °C min−1 and maintained for 5 h, subsequently raised to 750 °C at 5 °C min−1 and maintained for 20 h, followed by naturally cooling to room temperature, yielding W6+-doped LiNi0.90Mn0.07Al0.03O2 cathode materials. The resulting products were designated as NMA (undoped), W-0.01 (1 mol% W), W-0.02 (2 mol%), and W-0.03 (3 mol%) based on their nominal tungsten content.
2.2. Material characterization
The crystal structure of the cathode material was characterized by powder X-ray diffraction (XRD) using a SmartLab diffractometer (Rigaku, Japan) with Cu Kα radiation operated at 40 kV and 150 mA. Data were collected over a 2θ range of 10–80° with a scan rate of 10° min−1. The morphology of the cathode material was observed using scanning electron microscopy (SEM, Zeiss Sigma 300) and transmission electron microscopy (TEM, Thermo Scientific Talos F200i S/TEM, 200 kV). High-resolution TEM imaging was performed for lattice spacing analysis. The surface elemental composition of the cathode material was analyzed by X-ray photoelectron spectroscopy (XPS; Thermo Fisher Scientific K-Alpha). All binding energies were calibrated using the C 1s peak at 284.8 eV as a reference.2.3. Electrochemical characterization
The cathode material, conductive carbon black, and polyvinylidene fluoride (PVDF) were homogeneously mixed in a weight ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in a beaker. N-Methyl-2-pyrrolidone (NMP) was added dropwise under stirring until a uniform mixture was formed. Magnetic stirring was applied at 450 rpm for 4 h. The slurry was coated onto aluminum foil and vacuum-dried at 80 °C for 12 h. The dried electrodes were roll-pressed and cut into 16-mm-diameter disks. For electrochemical testing, coin-type half-cells (CR2032) were assembled in an argon-filled glove box (<0.01 ppm H2O/O2) using 1 M LiPF6 in ethylene carbonate (EC)/diethyl carbonate (DEC)/dimethyl carbonate (DMC) (1
1 in a beaker. N-Methyl-2-pyrrolidone (NMP) was added dropwise under stirring until a uniform mixture was formed. Magnetic stirring was applied at 450 rpm for 4 h. The slurry was coated onto aluminum foil and vacuum-dried at 80 °C for 12 h. The dried electrodes were roll-pressed and cut into 16-mm-diameter disks. For electrochemical testing, coin-type half-cells (CR2032) were assembled in an argon-filled glove box (<0.01 ppm H2O/O2) using 1 M LiPF6 in ethylene carbonate (EC)/diethyl carbonate (DEC)/dimethyl carbonate (DMC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume) as the electrolyte and lithium metal as the counter electrode. Charge/discharge tests were performed in constant-current mode on a Neware system (CT-4008T-5V6A, China) at 2.5–4.3 V and 25 °C. Rate capability was evaluated at 0.1–5C (1C = 180 mA g−1). Cyclic voltammetry (CV) was conducted at 0.1 mV s−1 (2.5–4.3 V) using a CHI660e workstation. Electrochemical impedance spectroscopy (EIS) measurements were performed from 0.01 Hz to 100 kHz with a 10 mV amplitude. All tests were maintained at room temperature (25 °C).
1 by volume) as the electrolyte and lithium metal as the counter electrode. Charge/discharge tests were performed in constant-current mode on a Neware system (CT-4008T-5V6A, China) at 2.5–4.3 V and 25 °C. Rate capability was evaluated at 0.1–5C (1C = 180 mA g−1). Cyclic voltammetry (CV) was conducted at 0.1 mV s−1 (2.5–4.3 V) using a CHI660e workstation. Electrochemical impedance spectroscopy (EIS) measurements were performed from 0.01 Hz to 100 kHz with a 10 mV amplitude. All tests were maintained at room temperature (25 °C).
3. Results and discussion
The synthesized cathode materials were characterized by X-ray diffraction (XRD) to investigate the evolution of their diffraction peaks. As shown in Fig. 1a, the W6+-modified W-0.01, W-0.02 and W-0.03 samples exhibit similar diffraction patterns to the pristine NMA, all demonstrating a typical α-NaFeO2 hexagonal layered structure with the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group, which matches well with the standard PDF card (PDF #09-0063).43,44 This indicates that W6+ doping does not introduce any impurity phases in the cathode materials. As shown in Fig. 1b and c, the clear splitting of the (006)/(012) and (011)/(018) peak doublets further confirms that all four cathode materials maintain well-ordered layered structures.45
m space group, which matches well with the standard PDF card (PDF #09-0063).43,44 This indicates that W6+ doping does not introduce any impurity phases in the cathode materials. As shown in Fig. 1b and c, the clear splitting of the (006)/(012) and (011)/(018) peak doublets further confirms that all four cathode materials maintain well-ordered layered structures.45
To systematically investigate the effects of varying W6+ doping concentrations on structural parameters, Rietveld refinement of XRD patterns was performed using Fullprof software, with refinement profiles shown in Fig. 1d–g (panels d–g corresponding to NMA, W-0.01, W-0.02, and W-0.03, respectively). As summarized in Table 1, all refined patterns exhibit satisfactory reliability factors (Rp and Rwp < 10%), confirming the credibility of refinement results.
| a (Å) | c (Å) | V (Å3) | c/a | I (003)/I(104) | Li+/Ni2+ (%) | R p (%) | R wp (%) | |
|---|---|---|---|---|---|---|---|---|
| NMA | 2.8791 | 14.1984 | 102.100 | 4.931 | 1.548 | 4.72 | 2.43 | 3.08 |
| W-0.01 | 2.8705 | 14.1944 | 101.599 | 4.945 | 1.462 | 4.02 | 2.55 | 3.29 |
| W-0.02 | 2.8709 | 14.1980 | 101.344 | 4.945 | 1.727 | 2.63 | 2.58 | 3.32 |
| W-0.03 | 2.8762 | 14.2120 | 101.822 | 4.941 | 1.327 | 3.97 | 2.62 | 3.33 |
The refinement data reveal a gradual expansion along the c-axis with increasing W6+ content. This anisotropic lattice expansion is attributed to the larger ionic radius of W6+ (0.60 Å) compared to Ni3+ (0.56 Å).46 Since the c-axis corresponds to the (003) crystallographic plane, this expansion indicates that W6+ doping effectively enlarges the interplanar spacing, which is expected to provide wider Li+ diffusion channels and could potentially enhance the rate capability.
Notably, the refined unit cell volumes (V) demonstrate a non-monotonic variation trend – initially decreasing then increasing with W content – suggesting that the 0.02 mol W6+ doping optimally suppresses grain growth through solid-solution strengthening effects induced by W6+ incorporation. The c/a ratios, critical indicators of layered structure integrity, maintain values exceeding 4.9 for all samples (NMA and W-doped variants), unambiguously confirming their well-ordered layered configurations.47,48
Complementary to the structural parameters, the cation mixing behavior was quantitatively analyzed through XRD refinement. The results reveal a non-monotonic variation in Li+/Ni2+ cation mixing with increasing W6+ doping content, showing an initial decrease followed by an increase compared to the pristine material. Fundamentally, this phenomenon originates from charge compensation effects – as excessive incorporation (>0.02 mol) of a high-valence cation (W6+) inevitably induces the reduction of Ni3+ to Ni2+ to maintain charge balance, thereby promoting Li+/Ni2+ mixing.49–51
Among all doped cathodes, the 0.02 mol W6+-modified sample exhibits optimal electrochemical performance, demonstrating both the minimal cation mixing degree (2.63%) and the highest I(003)/I(104) intensity ratio (1.727), which collectively indicate superior structural ordering and reduced Li+/Ni2+ disorder compared to other doping concentrations.52 These values are superior to both the pristine material and other doping concentrations (Table 1), providing compelling evidence that appropriate W6+ doping can effectively minimize Li+/Ni2+ cation disorder in layered cathode materials.
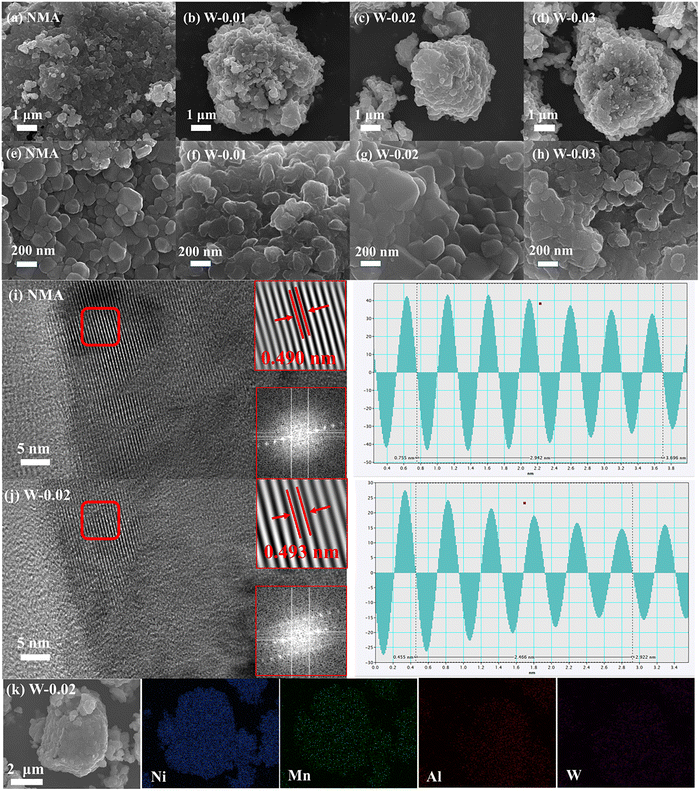
The morphological characteristics of four cathode materials (pristine NMA, W-0.01, W-0.02, and W-0.03) were systematically investigated through scanning electron microscopy (SEM) to elucidate the influence of varying W6+ doping concentrations on their morphological evolution, as shown in Fig. 2a–d. All samples remain largely unchanged, with the materials retaining a spherical shape resulting from the compact stacking of primary particles. Moreover, with increasing W6+ content, the secondary particle size demonstrates an initial decrease followed by an increase, a phenomenon that confirms that an appropriate amount of W6+ can modify the primary particle size.
The particle size plays a pivotal role in influencing the electrochemical performance as a larger specific surface area and smaller particle size facilitate lithium ion transport.53 The W-0.02 sample exhibits the smallest particle size, which may positively influence the Li-ion diffusion kinetics. To further investigate the microstructure, higher-magnification observations reveal that W-0.02 exhibits more densely packed primary particles and a smoother surface morphology (Fig. 2e–h). This clearly demonstrates that W6+ doping effectively regulates the microstructural evolution, leading to compact secondary particles. The enhanced particle compactness and reduced size in W-0.02 not only reinforce the crystal structure integrity but also increase the specific surface area, providing abundant electrochemically active sites that ultimately maximize the performance of this high-nickel layered cathode material. As evidenced by TEM and corresponding FFT patterns (Fig. 2i and j), both pristine NMA and W-0.02 exhibit well-defined (003) crystallographic planes, confirming layered structure preservation and the absence of W6+ induced impurities. IFFT analysis further reveals interlayer spacing expansion from 0.490 nm to 0.493 nm upon doping, attributed to a larger ionic radius of W. This lattice expansion facilitates Li+ migration while alleviating volumetric variations during charge/discharge processes.54,55
Finally, EDS analysis confirms successful W doping with uniform distribution in both bulk and surface regions (Fig. 2k), as evidenced by the co-existence of Ni, Mn, Al, and W signals.
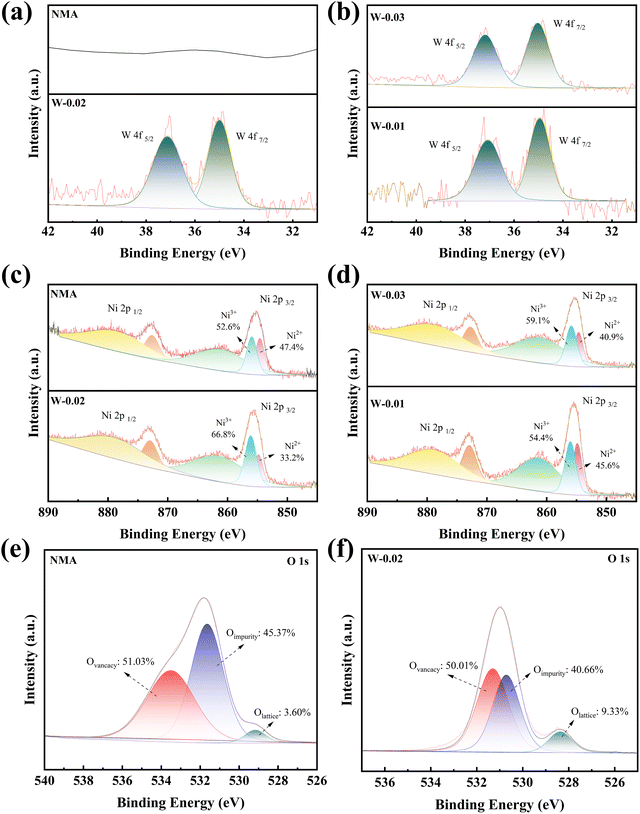
X-ray photoelectron spectroscopy (XPS) was employed to characterize the valence states of W and Ni in the cathode materials, and shown in Fig. 3. As expected, pristine NMA shows no detectable W signals (Fig. 3a). The W 4f5/2 and W 4f7/2 doublet peaks are clearly observed in W-0.02, confirming the successful incorporation of W6+ at the 0.02 mol doping level. Identical W 4f spectral features were observed in W-0.01 and W-0.03 samples (Fig. 3b). This demonstrates that the W 4f characteristic peaks can be detected in samples with different tungsten doping ratios. From Ni 2p spectra, the peaks at 873 and 855.2 eV are assigned to Ni 2p1/2 and Ni 2p3/2 orbitals, respectively. The Ni 2p3/2 peak can be further deconvoluted into two components at 854.6 (Ni2+) and 856.0 eV (Ni3+)56 (Fig. 3c and d). Quantitative analysis reveals that the Ni3+/Ni2+ ratios in the four cathodes exhibit a non-monotonic dependence on W6+ doping concentration, following the sequence: 1.11 (NMA), 1.20 (W-0.01), 2.00 (W-0.02), and 1.44 (W-0.03). To further probe the interfacial stability, the O 1s spectra of NMA and W-0.02 were analyzed, as shown in Fig. 3(e and f). The deconvolution results show that the oxygen impurity content in W-0.02 was 40.66%, lower than that in NMA (45.37%), which indicates a reduced presence of carbonate/bicarbonate ions and suppressed interfacial side reactions. Moreover, the lattice oxygen content in W-0.02 was 9.33%, higher than that in NMA (3.60%). This collective evidence confirms that W6+ doping effectively inhibits the release of lattice oxygen and improves the overall interfacial stability.57,58
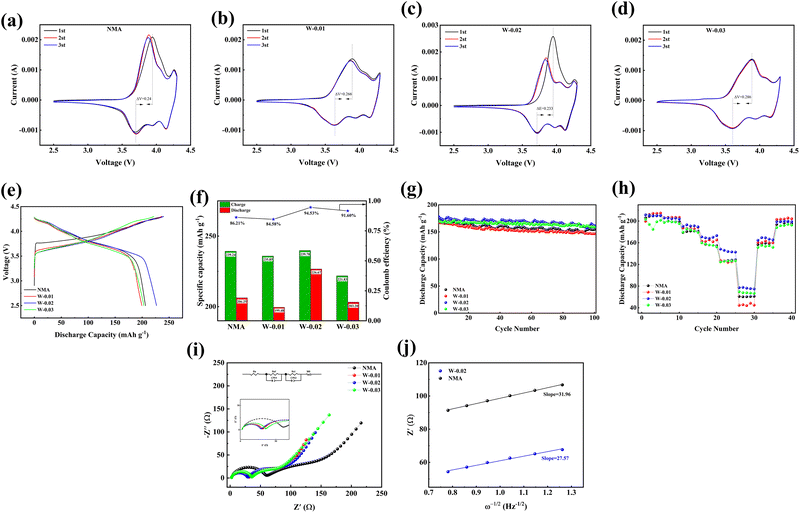
To further investigate the electrode reversibility of cathode materials, cyclic voltammetry (CV) measurements were conducted on four samples (pristine NMA, W-0.01, W-0.02, and W-0.03) between 2.5 and 4.3 V vs. Li/Li+ at a scan rate of 0.2 mV s−1 as shown in Fig. 4a–d. All CV curves exhibit three distinct redox couples: the oxidation peaks correspond to phase transitions from hexagonal (H1) to monoclinic (M), monoclinic (M) to hexagonal (H2), and hexagonal (H2) to hexagonal (H3), while the reduction peaks represent the reverse transitions (H3 → H2 → M → H1).59 The potential interval (ΔV) between the first redox couple serves as a critical indicator of electrode reversibility. NMA, W-0.01, W-0.02, and W-0.03 are 0.24, 0.266, 0.233, and 0.286 eV, respectively. Notably, W-0.02 demonstrates the smallest potential difference (0.233 eV), indicating superior electrode reversibility among all samples. This result correlates well with electrochemical performance tests, demonstrating that a small potential gap contributes to superior cycling stability by improving reaction kinetics.60
To investigate the effect of W6+ doping on electrochemical performance, coin-type CR2032 half-cells containing pristine NMA and modified samples were assembled and tested using a Neware electrochemical workstation. Fig. 4e and f displays the initial charge/discharge profiles of the cathode materials measured at 0.1C rate within a voltage window of 2.5–4.3 V. The pristine NMA, W-0.01, W-0.02, and W-0.03 samples deliver initial charge/discharge capacities of 239.24/206.25, 235.85/199.48, 239.78/226.67, and 221.83/203.20 mAh g−1, respectively, with corresponding initial Coulombic efficiencies of 86.21%, 84.58%, 94.53%, and 91.60%. The high ICE is more likely due to the W-doping stabilizing the crystal structure and reducing irreversible cation mixing or phase transitions during the first cycle, thereby minimizing irreversible capacity loss.
The cycling stability of all cathode materials was further evaluated at room temperature under a current density of 1C (180 mAh g−1) within a voltage window of 2.5–4.3V. As shown in Fig. 4g, the pristine NMA delivered initial and final (after 100 cycles) discharge capacities of 173.60 and 154.50 mAh g−1, respectively, corresponding to a capacity retention of 88.99%. In comparison, all W-doped cathodes showed improved performance. Among them, the W-0.02 cathode exhibited the most promising combination of high capacity and cycling stability, with capacities of 178.13 and 161.78 mAh g−1 (90.82% retention), outperforming both W-0.01 (167.27/146.56 mAh g−1, 87.62%) and W-0.03, which showed the highest retention (94.02%) but a lower initial capacity (169.13 mAh g−1). Notably, although W-0.03 showed higher capacity retention, its absolute cycled capacity (159.02 mAh g−1) remained lower than W-0.02, demonstrating that optimal W6+ doping (0.02 mol) effectively enhances high-current cycling performance.
Compared with flexible electrode systems, lithium-ion transport within the constrained crystalline framework of cathode oxides often encounters significant Li+ diffusion limitations, resulting in relatively sluggish kinetics. Consequently, rate capability becomes a critical performance metric for practical applications of Ni-rich cathode materials. The rate performance was evaluated using half-cells cycled at 0.1C for activation, followed by sequential cycling at 0.1, 0.2, 0.5, 1, 2, and 5C (5 cycles each rate), before finally returning to 0.2C as shown in Fig. 4h. The W-0.02 cathode delivered superior discharge capacities of 211.58, 205.66, 190.22, 170.80, 148.14, and 77.08 mAh g−1 at respective rates, maintaining 96.72% capacity recovery upon returning to 0.2C. In contrast, the pristine NMA cathode exhibited lower capacities (206.25, 197.42, 178.90, 160.12, 126.10, and 60.73 mAh g−1) with only 93.88% capacity retention, demonstrating the W-0.02 sample enhanced electrochemical performance through improved Li+ diffusion kinetics. The superior discharge capacity of W-0.01 at low current rates may be attributed to the moderate lattice expansion induced by limited W6+ doping, which facilitates Li+ transport through widened diffusion channels. However, this doping level proves insufficient to stabilize the layered structure against severe structural degradation under high current densities, as evidenced by the rapid capacity fading. Conversely, the consistently inferior capacity of W-0.03 across all rates stems from excessive W6+ incorporation, which not only introduces redox-inactive species but also reduction in redox-active sites consequently diminishes the specific discharge capacity of the cathode material.61
The superior electrochemical performance of W-0.02 originates from its optimal doping concentration that simultaneously modifies the cathode morphology, reduces primary particle size, and enhances Li+ diffusion kinetics. These structural advantages provide abundant electrochemically active sites while the strong W–O bonding stabilizes the crystal lattice, suppressing microcrack formation and reinforcing the cathode–electrolyte interphase.62
The impedance evolution is closely associated with interfacial stability and Li+ transport kinetics. Thus, EIS was employed to investigate the post-activation impedance of four cathode materials, as depicted in Fig. 4i. The equivalent circuit model is illustrated alongside, with detailed fitting parameters summarized in Table 2. All four cathode materials exhibited two distinct semicircles in their Nyquist plots. The high-frequency intercept with the real axis corresponds to the ohmic resistance (Rs), representing intrinsic bulk resistance. The high-frequency semicircle originates from the solid–electrolyte interphase (SEI) film resistance (Rsf), formed through vigorous electrolyte oxidation/reduction reactions during initial cycling. The medium-frequency semicircle reflects the charge transfer resistance (Rct), constituting the dominant component of electrochemical impedance, the slope in the low-frequency region corresponds to the Warburg impedance (σ) associated with Li+ diffusion.63,64
| Sample | R s (Ω) | R sf (Ω) | R ct (Ω) |
|---|---|---|---|
| NMA | 2.22 | 54.29 | 95.15 |
| W-0.01 | 2.15 | 27.82 | 28.13 |
| W-0.02 | 1.96 | 25.55 | 27.40 |
| W-0.03 | 2.21 | 32.86 | 45.62 |
After initial activation, the Rs values for NMA, W-0.01, W-0.02, and W-0.03 measured 2.22, 2.15, 1.96, and 2.21 Ω, respectively, demonstrating negligible variations that exclude Rs as a critical performance determinant. Notably, the Rsf values followed the sequence: 54.29 Ω (NMA) > 32.86 Ω (W-0.03) > 27.82 Ω (W-0.01) > 25.55 Ω (W-0.02). This is attributed to the 0.02 mol W6+ doping, which induces the formation of a more stable and ion-conductive interface phase in the W-0.02 cathode material. Furthermore, W-0.02 exhibited the lowest Rct (27.40 Ω) among all samples after activation. Fig. 4j depicted the linear fitting of Z′ vs. ω−1/2 for NMA and W-0.02 cathode materials, where the slope represents the Warburg impedance, showing an inverse linear relationship with Li+ diffusion coefficient. A larger slope corresponds to a smaller Li+ diffusion coefficient. The slope of W-0.02 (27.57) is lower than that of NMA (31.96), indicating faster Li+ diffusion in W-0.02. The Li+ diffusion coefficient (DLi+) can be calculated using the following equation:65,66
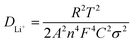 | (1) |
 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485C mol−1), C is the bulk Li+ concentration (4.99 × 10−2 mol cm−2), and σ is the Warburg coefficient derived from the linear fitting of Z′ versus ω−1/2. The DLi+ of NMA and W-0.02 cathode materials were calculated to be 3.51 × 10−15 and 4.72 × 10−15 cm2 s−1, respectively, using the aforementioned equation. These values are consistent with the prior analysis results.
485C mol−1), C is the bulk Li+ concentration (4.99 × 10−2 mol cm−2), and σ is the Warburg coefficient derived from the linear fitting of Z′ versus ω−1/2. The DLi+ of NMA and W-0.02 cathode materials were calculated to be 3.51 × 10−15 and 4.72 × 10−15 cm2 s−1, respectively, using the aforementioned equation. These values are consistent with the prior analysis results.
The superior electrochemical performance and enhanced Li+ diffusion kinetics of W-0.02 compared to NMA can be attributed to (i) refined primary particle size via 0.02 mol W6+ doping facilitating Li+ diffusion, (ii) inhibited irreversible phase transitions in the layered structure.
4. Conclusions
In summary, W6+-doped LiNi0.90Mn0.07Al0.03O2 (NMA) cathode materials with controlled doping concentrations (0–0.03 mol) were successfully synthesized through a coprecipitation method combined with a high-temperature solid-state reaction using WO3 as the tungsten precursor. Structural characterization reveals that the optimal W-0.02 effectively reduces primary particle size while enhancing structural stability, with Rietveld refinement and XPS analysis confirming significant suppression of Ni3+ to Ni2+ reduction (Ni3+/Ni2+ ratio increasing from 1.1 to 2.0) and decreased Li+/Ni2+ cation mixing from 4.72% to 2.63%. Electrochemical evaluation demonstrates outstanding performance with an initial discharge capacity of 178.13 mAh g−1 at 1C (2.5–4.3 V vs. Li+/Li), capacity retention of 90.8% after 100 cycles (161.78 mAh g−1), and 96.72% capacity recovery when returning to 0.2C after rate testing, attributed to strengthened W–O bonding (653 kJ mol−1) and optimized charge compensation. This work provides fundamental insights into cation doping strategies for high-nickel layered oxide cathodes in next-generation lithium-ion batteries.Conflicts of interest
There are no conflicts to declare.Data availability
All data generated or analyzed during this study are included in this published article.Acknowledgements
This project was financially supported by the Nature Science Foundation of Qinghai (2023-ZJ-983Q).References
- J. Xiao, F. Shi, T. Glossmann, C. Burnett and Z. Liu, Nat. Energy, 2023, 8(4), 329–339 CrossRef.
- Q. Shi, F. Wu, H. Wang, Y. Lu, J. Dong, J. Zhao and L. Chen, Energy Storage Mater., 2024, 67, 103264 CrossRef.
- A. Manthiram, Nat. Commun., 2020, 11(1), 1550 CrossRef CAS PubMed.
- L. Wang, T. Liu, T. Wu and J. Lu, Nature, 2022, 611(7934), 61–67 CrossRef CAS.
- L. Dong, Y. Liu, K. Wen, D. Chen, D. Rao, J. Liu and W. He, Adv. Sci., 2022, 9(5), 2104699 CrossRef CAS.
- X. Ou, T. Liu, W. Zhong, X. Fan, X. Guo, X. Huang and J. Lu, Nat. Commun., 2022, 13(1), 2319 CrossRef CAS PubMed.
- Z. Tan, Y. Li, X. Xi, S. Jiang, X. Li, X. Shen and J. Zheng, J. Energy Chem., 2022, 72, 570–580 CrossRef CAS.
- K. Yang, Y. Yi, Z. Yi, C. Yang, F. Liu, K. Wang and Z. Chen, Chem. Eng. J., 2023, 474, 145554 CrossRef CAS.
- F. Wu, Q. Li, L. Chen, W. Zi, Z. R. Chen, L. Y. Bao, Y. Lu, S. Chen and Y. F. Su, Acta Phys.-Chim. Sin., 2022, 38, 2007017 Search PubMed.
- W. Li, E. M. Erickson and A. Manthiram, Nat. Energy, 2020, 5(1), 26–34 CrossRef CAS.
- M. Li and J. Lu, Science, 2020, 367(6481), 979–980 CrossRef CAS PubMed.
- Y. H. Luo, H. X. Wei, L. B. Tang, Y. D. Huang, Z. Y. Wang, Z. J. He and J. C. Zheng, Energy Storage Mater., 2022, 50, 274–307 CrossRef.
- E. Hu, X. Wang, X. Yu and X. Q. Yang, Acc. Chem. Res., 2022, 51(2), 290–298 CrossRef.
- L. De Biasi, A. Schiele, M. Roca-Ayats, G. Garcia, T. Brezesinski, P. Hartmann and J. Janek, ChemSusChem, 2019, 12(10), 2240–2250 CrossRef CAS.
- Y. D. Huang, H. X. Wei, P. Y. Li, Y. H. Luo, Q. Wen, D. H. Le and J. C. Zheng, J. Energy Chem., 2022, 75, 301–309 CrossRef CAS.
- S. Vadivel, N. Phattharasupakun, J. Wutthiprom, S. Duangdangchote and M. Sawangphruk, ACS Appl. Mater. Interfaces, 2019, 11(34), 30719–30727 CrossRef CAS.
- H. J. Noh, S. Youn, C. S. Yoon and Y. K. Sun, J. Power Sources, 2013, 233, 121–130 CrossRef CAS.
- L. Zhang, E. A. Müller Gubler, C. W. Tai, Ł. Kondracki, H. Sommer, P. Novák and S. Trabesinger, ACS Appl. Mater. Interfaces, 2022, 14(11), 13240–13249 CrossRef CAS PubMed.
- F. Wu, N. Liu, L. Chen, Y. Su, G. Tan, L. Bao and J. Tan, Nano Energy, 2019, 59, 50–57 CrossRef CAS.
- Z. Sun, L. Xu, C. Dong, H. Zhang, M. Zhang, Y. Liu and Y. Chen, J. Mater. Chem. A, 2019, 7(7), 3375–3383 RSC.
- C. Sun, B. Zhao, J. Mao, K. H. Dai, Z. Y. Wang, L. B. Tang and J. C. Zheng, Adv. Funct. Mater., 2023, 33(30), 2300589 CrossRef CAS.
- Z. Wang, L. Li, H. Heo, L. Ren, Y. Wei, K. Lee and H. H. Park, J. Colloid Interface Sci., 2024, 666, 424–433 CrossRef CAS PubMed.
- H. Chen, H. Yuan, Z. Dai, S. Feng, M. Zheng, C. Zheng and Z. Wen, Adv. Mater., 2024, 36(33), 2401052 CrossRef CAS.
- J. Jeyakumar, M. Seenivasan, Y. S. Wu, S. H. Wu, J. K. Chang, R. Jose and C. C. Yang, J. Colloid Interface Sci., 2023, 639, 145–159 CrossRef CAS PubMed.
- Y. H. Xie, W. R. Zheng, J. Ao, Y. Q. Shao, X. Huang, H. Li, S. Y. Chen and X. H. Wang, Energy Storage Mater., 2023, 62, 102925 CrossRef.
- J. Q. Cao, Y. H. Xie, Y. Yang, X. H. Wang, W. Y. Li, Q. L. Zhang, S. Ma, S. Y. Chen and B. G. Liu, Adv. Sci., 2022, 9(9), 2104689 CrossRef CAS.
- B. Y. Ke, S. L. Cheng, C. C. Zhang, W. Y. Li, J. Zhang, R. M. Deng, J. Lin, Q. S. Xie, B. H. Qu, L. Qiao, D. L. Peng and X. H. Wang, Adv. Energy Mater., 2024, 14(12), 2303757 CrossRef CAS.
- B. Y. Ke and X. H. Wang, Proc. Natl. Acad. Sci. U. S. A., 2025, 122(16), e2415693122 CrossRef CAS.
- M. D. Radin, S. Hy, M. Sina, C. Fang, H. Liu, J. Vinckeviciute and A. Van der Ven, Adv. Energy Mater., 2017, 7(20), 1602888 CrossRef.
- B. Dong, A. D. Poletayev, J. P. Cottom, J. Castells-Gil, B. F. Spencer, C. Li and P. R. Slater, J. Mater. Chem. A, 2024, 12(19), 11390–11402 RSC.
- H. H. Ryu, K. J. Park, D. R. Yoon, A. Aishova, C. S. Yoon and Y. K. Sun, Adv. Energy Mater., 2019, 9(44), 1902698 CrossRef CAS.
- Y. Zhai, W. Yang, D. Ning, J. Yang, L. Sun, G. Schuck and X. Liu, J. Mater. Chem. A, 2020, 8(10), 5234–5245 RSC.
- H. H. Ryu, K. J. Park, D. R. Yoon, A. Aishova, C. S. Yoon and Y. K. Sun, Adv. Energy Mater., 2019, 9(44), 1902698 CrossRef CAS.
- W. Li, S. Lee and A. Manthiram, Adv. Mater., 2020, 32(33), 2002718 CrossRef CAS.
- N. Y. Park, G. Cho, S. B. Kim and Y. K. Sun, Adv. Energy Mater., 2023, 13(14), 2204291 CrossRef CAS.
- U. H. Kim, G. T. Park, B. K. Son, G. W. Nam, J. Liu, L. Y. Kuo and Y. K. Sun, Nat. Energy, 2020, 5(11), 860–869 CrossRef CAS.
- B. Wang, S. Hou, Y. Zhang, Y. Zhu and T. Zhang, J. Alloys Compd., 2024, 1003, 175561 CrossRef CAS.
- L. Azhari, B. Sousa, R. Ahmed, R. Wang, Z. Yang, G. Gao and Y. Wang, ACS Appl. Mater. Interfaces, 2022, 14(41), 46523–46536 CrossRef CAS.
- J. Tao, A. Mu, S. Geng, H. Xiao, L. Zhang and Q. Huang, J. Solid State Electrochem., 2021, 25(7), 1959–1974 CrossRef CAS.
- C. Xu, W. Xiang, Z. Wu, L. Qiu, Y. Ming, W. Yang and Y. Liu, Chem. Eng. J., 2021, 403, 126314 CrossRef CAS.
- S. Zhao, H. L. Ren, Y. Su, C. W. Li and X. M. Wang, Ceram. Int., 2024, 50(22), 44983–44992 CrossRef CAS.
- J. Yang, D. Gao, D. Zhang and C. Chang, J. Energy Storage, 2023, 63, 107088 CrossRef.
- W. Lee, S. Lee, E. Lee, M. Choi, R. Thangavel, Y. Lee and W. S. Yoon, Energy Storage Mater., 2022, 44, 441–451 CrossRef.
- Y. Lei, J. Ai, S. Yang, H. Jiang, C. Lai and Q. Xu, J. Alloys Compd., 2019, 797, 421–431 CrossRef CAS.
- H. Dong, G. Liu, S. Li, S. Deng, Y. Cui, H. Liu and X. Sun, ACS Appl. Mater. Interfaces, 2018, 11(2), 2500–2506 CrossRef.
- C. Li, J. Liu, Y. Su, J. Dong, H. Zhan, M. Wang and L. Chen, Energy Storage Mater., 2025, 74, 103893 CrossRef.
- L. Qiu, W. Xiang, W. Tian, C. L. Xu, Y. C. Li, Z. G. Wu and X. D. Guo, Nano Energy, 2019, 63, 103818 CrossRef CAS.
- P. Zhou, H. Meng, Z. Zhang, C. Chen, Y. Lu, J. Cao and J. Chen, J. Mater. Chem. A, 2017, 5(6), 2724–2731 RSC.
- H. X. Wei, L. B. Tang, Y. D. Huang, Z. Y. Wang, Y. H. Luo, Z. J. He and J. C. Zheng, Mater. Today, 2021, 51, 365–392 CrossRef CAS.
- Y. Lv, S. Huang, S. Lu, W. Ding, X. Yu, G. Liang and Y. Cao, J. Power Sources, 2022, 536, 231510 CrossRef CAS.
- L. Shen, Y. Gu, T. Xu, Q. Zhou, P. Peng, Y. Chen and J. Zheng, J. Colloid Interface Sci., 2024, 662, 505–515 CrossRef CAS PubMed.
- A. Sati, P. Pokhriyal, A. Kumar, S. Anwar, A. Sagdeo, N. P. Lalla and P. R. Sagdeo, J. Phys.: Condens. Matter, 2021, 33(16), 165403 CrossRef CAS.
- H. Kim, Y. Kong, W. M. Seong and A. Manthiram, ACS Appl. Mater. Interfaces, 2023, 15(22), 26585–26592 CrossRef CAS.
- W. Li, H. Y. Asl, Q. Xie and A. Manthiram, J. Am. Chem. Soc., 2019, 141(13), 5097–5101 CrossRef CAS PubMed.
- H. H. Sun, U. H. Kim, J. H. Park, S. W. Park, D. H. Seo, A. Heller and Y. K. Sun, Nat. Commun., 2021, 12(1), 6552 CrossRef CAS.
- G. E. McGuire, G. K. Schweitzer and T. A. Carlson, Inorg. Chem., 1973, 12(10), 2450–2453 CrossRef CAS.
- Y. H. Chu, J. W. Zhou, W. X. Liu, F. L. Chu, J. H. Li and F. X. Wu, Small Sci., 2023, 3(7), 2300023 CrossRef CAS.
- W. Song, A. Gao, Y. Liu, A. Liu, H. Peng, M. Li and F. Wang, J. Alloys Compd., 2025, 1010, 177611 CrossRef CAS.
- S. Lee, C. Li and A. Manthiram, Adv. Energy Mater., 2024, 14(24), 2400662 CrossRef CAS.
- H. Shin, J. Park and W. Choi, J. Alloys Compd., 2024, 988, 174265 CrossRef CAS.
- B. Li, F. Zhang, C. Li, X. Cui, S. Li, C. Gao and N. Zhang, J. Colloid Interface Sci., 2024, 672, 776–786 CrossRef CAS.
- G. T. Park, S. M. Han, J. H. Ryu, M. C. Kim, D. H. Kim, M. S. Kim and Y. K. Sun, ACS Energy Lett., 2023, 8(9), 3784–3792 CrossRef CAS.
- A. C. Lazanas and M. I. Prodromidis, ACS Meas. Sci. Au, 2023, 3(3), 162–193 CrossRef CAS PubMed.
- D. Zhang, Y. Liu, L. Wu, L. Feng, S. Jin, R. Zhang and M. Jin, Electrochim. Acta, 2019, 328, 135086 CrossRef CAS.
- X. Ping, X. Pei, D. Zou, C. Wang, X. Li, C. Cao and Y. Wang, Electrochim. Acta, 2025, 146297 CrossRef CAS.
- H. Zhang, X. Wang, A. Naveed, T. Zeng, X. Zhang, H. Shi and Y. Liu, Appl. Surf. Sci., 2022, 599, 153933 CrossRef CAS.
| This journal is © the Owner Societies 2026 |