First-principles insights into atomic oxygen protection coatings composed of scale-like layered double hydroxide nanosheets
Denghang
Tang
ab,
Rui
Sun
 ab,
Jiayu
Zheng
ab,
Mengyun
Xu
a,
Haogeng
Li
a,
Hongyu
Gu
ab,
Jiayu
Zheng
ab,
Mengyun
Xu
a,
Haogeng
Li
a,
Hongyu
Gu
 *abc,
Yuzhi
Zhang
*ab,
Yi-Yang
Sun
*abc,
Yuzhi
Zhang
*ab,
Yi-Yang
Sun
 bc and
Lixin
Song
ab
bc and
Lixin
Song
ab
aResearch Center of Inorganic Coating Materials, Shanghai Institute of Ceramics, Chinese Academy of Sciences, Shanghai 201899, P. R. China. E-mail: guhongyu@mail.sic.ac.cn
bCenter of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences, Beijing 100049, P. R. China
cState Key Laboratory of High Performance Ceramics, Shanghai Institute of Ceramics, Chinese Academy of Sciences, Shanghai 201899, P. R. China
First published on 26th November 2025
Abstract
With human activities in outer space becoming increasingly frequent, including the construction of space stations and satellite constellations such as Starlink, OneWeb, and G60 Qianfan Constellation, the protection of spacecraft against atomic oxygen (AO) in the low-Earth orbit (LEO) has become critical. AO, characterized by its strong oxidative capability and high collisional energy, has been considered the most serious hazard to the LEO spacecraft. Recently, it was found that scale-like Mg–Al layered double hydroxide (LDH) coatings are highly effective in protecting flexible spacecraft parts from AO erosion. However, the AO protection mechanism of the scale-like coatings remains unclear, which limited the further development of this AO protection technology. The erosion process of AO takes place at the atomic scale and on the femtosecond time scale, making it difficult to reveal the microscopic mechanism solely by experimental and characterization studies. To address this, we investigated the interactions between AO and the coating materials using first-principles based calculations and simulations. By simulating the AO impact and calculating the AO adsorption energy on polysiloxane and LDH nanosheets, a possible AO protection mechanism was proposed using experimental and characterization studies. With the special scale-like structure, the coating mainly inhibits the AO erosion through the barrier effect on the coating surface and the obstruction effect inside coatings with long erosion paths. This work shows that the non-dense micro–nanostructure holds potential for AO protection, which will guide and expand the application of assembled nanomaterials in space exploration.
Introduction
Layered double hydroxides (LDHs) have been discovered and explored for applications in medicine, environment, energy and other fields owing to their special layered structure and a variety of chemical compositions.1–3 Layer-structured LDHs are used as a source of two-dimensional materials due to their low cost, easy preparation method and high quality.4–6 The potential of applying LDH nanosheets in space missions has been recently explored.7,8 In contrast to conventional dense inorganic coatings such as SiO2, Al2O3, and TiO2, which, while exhibiting excellent inertness to AO erosion,9–12,24 often suffer from brittleness leading to cracking under stress,13,14 the scale-like LDH coatings possess a unique non-dense micro–nanostructure composed of oriented nanosheets. This structure provides exceptional flexibility and stress release capabilities, allowing the coatings to maintain integrity under mechanical strain without cracking—a critical advantage for flexible spacecraft components. Additionally, this structure allows for longer erosion paths and enhanced obstruction effects. Because of the superior mechanical and antioxidant properties, scale-like Mg–Al LDH nanosheet coating has been studied as flexible protective coatings against AO, which may be applied in solar panels and structural and thermal control systems on spacecraft.15,16AO, one of the most abundant particles on the LEO, is considered the most serious threat to LEO spacecraft.17–19 A growing number of countries are turning to the Low Earth Orbit (LEO) for research and applications in satellite communications such as Starlink, OneWeb, and G60 Qianfan Constellation. This preference is due to LEO's closer proximity to Earth, which results in lower transmission latency, reduced path loss, and overall lower construction costs. Additionally, this orbital regime is also where spacecraft and space stations primarily operate. Hence, the protection of spacecraft against AO in the LEO has become critical. AO is also a kind of strong oxidant. During collision with a high-speed spacecraft, the kinetic energy of AO is up to 5 eV, which is strong enough to break the chemical bonds of most polymer materials.20–22 It is easy for highly active AO with large kinetic energy to oxidize and degrade polymer parts on a spacecraft, resulting in structural damage, functional failure and shortened service life.23–26 As a protective layer, scale-like Mg–Al LDH nanosheet coatings provide efficient protection on flexible polymer parts against AO.27 With a non-dense scale-like structure, these coatings exhibit outstanding AO protection performance among existing strategies.28–32 So far, the protection mechanism is unclear. Hence, it is necessary to explore the impact interaction mechanism between the Mg–Al LDH coating and high-speed AO to further guide the design of nanosheet materials for application in space missions. But the conventional methods through experiments can only characterize some macroscopic observations.33–36 It was difficult to investigate the microscopic interaction process of AO at an atomic scale. This undoubtedly limits the study of the mechanism. Hence, calculations, combined with experiments, are needed to explore the microscopic interaction process and reveal the mechanism.
Computational methods based on first-principles calculations focusing on changes in the electronic structure between atoms can accurately probe the interactions between AO and protection materials at the nanoscale from the most fundamental aspects. First-principles calculations and simulations can provide more perspectives for the interpretation of experimental interaction laws and have greater potential in the analysis of interaction mechanisms. Among them, ab initio molecular dynamics (AIMD) combined with classical molecular dynamics and density functional theory (DFT) is used to calculate the interaction force between atoms by solving the electronic structure from first principles and then Newtonian mechanics is adopted for the trajectory evolution.37–40 The AIMD simulation meets the current needs for researching the mechanism of impact interactions between AO and protection materials at the atomic scale.33,35
In this work, we used AIMD simulations based on actual conditions in space to investigate the impact interaction mechanisms between high-speed AO and Mg–Al LDH coatings. Polysiloxane modified on the nanosheet surface was firstly investigated for AO impact processes, followed by collision between AO and Mg–Al LDH nanosheets. Some representative impact sites on polysiloxane and Mg–Al LDH were selected for AIMD simulations. Combined with the previous studies,35 the interactions between static AO and the two materials were also investigated from the adsorption energy perspective. Finally, combined with the experimental test and characterization, a possible protection mechanism based on scale-like structures was proposed. This work provides important insights into the interaction of the coatings with high-speed and static AO and hopefully provides guidance for applications of materials with micro–nanostructures in space exploration.
Experimental and computational details
Experimental and characterization studies
The scale-like LDH coatings were prepared by spin-coating a slurry of Mg–Al LDH nanosheets on a polyimide (PI) substrate (Kapton HN, 50 µm, purchased from DuPont Co., Ltd). The nanosheets were prepared from a solution of magnesium chloride hexahydrate (MgCl2·6H2O; AR, ≥98%, Sinopharm Chemical Reagent Co., Ltd) and aluminum chloride hexahydrate (AlCl3·6H2O; AR, ≥98%, Sinopharm Chemical Reagent Co., Ltd) co-precipitated by ammonia and then subjected to a hydrothermal treatment. And (3-aminopropyl)triethoxysilane (APTES; 98%, Sigma-Aldrich) was used as a binder and an adhesion enhancer in the coating slurry. Finally, the wet coatings were heat-treated and samples denoted as Lx (x = 80, 110, 130, and 150), corresponding to different hydrothermal temperatures, were obtained. These reagents and solvents were all obtained from Sinopharm Chemical Reagent Co., Ltd and used as received without further purification. The detailed preparation is described in our work reported previously.27The morphology of the surface and cross-section was investigated by scanning electron microscopy (SEM, SU9000) and atomic force microscopy (AFM, Dimension Icon). X-ray photoelectron spectroscopy (XPS) analysis was performed on an ESCALAB 250Xi instrument using Al Kα radiation. Peak fitting was conducted using Avantage software, with binding energies calibrated to C 1s at 284.8 eV. In order to simulate the erosion of AO in the LEO environment, the ground-based space AO impact test was conducted in an electron cyclotron resonance facility. This system utilizes microwave plasma discharge and magnetic confinement to produce high-density oxygen plasma, which is then neutralized to form a beam of neutral atomic oxygen for material testing. The experimental AO flux was 5.22 × 1021 atoms per cm2 with an impact kinetic energy (Ek) of 5 eV. Finally, the mass loss (Δm; mg cm−2) was used as a parameter for evaluating resistance to AO. The erosion yield (E) was derived from the following equation: E = Δm/(ρ·F). In the equation, ρ is the density of the PI substrate (Kapton HN; ρ = 1.42 g cm−3).
Computational details
The AO model was generated by first constructing an isolated oxygen atom and performing structural optimization until convergence was achieved. The optimized atomic structure was then precisely positioned directly above the targeted bombardment site on the Mg–Al LDH or polysiloxane model, ensuring no initial bond formation. An initial velocity of −0.0760000 Å fs−1 was assigned along the –c direction to simulate a kinetic energy of 5 eV, which represents the typical kinetic energy of AO in the LEO. This rigorous parameter setup ensures consistent initial impact conditions and aligns with established computational protocols for simulating AO interactions with material surfaces. All the first-principles calculations were performed using the planewave basis set in Vienna Ab-initio Simulation Package (VASP),41–44 employing the Perdew, Burke and Ernzerhof (PBE) exchange–correlation functional and the projector-augmented wave (PAW) potential.45 A large enough cutoff kinetic energy of 400 eV was used in all calculations. The AIMD simulations were performed using the NVE microcanonical ensemble for 1000 steps (200 fs, polysiloxane) or 5000 steps (2500 fs, LDH). The Γ-point was only used for sampling the Brillouin zone. The adsorption energy (Eads) was defined by the energy difference between the adsorbent (Eadsorbent), adsorbates (Eadsorbates) and the adsorption product (Eadsorbates/adsorbent), derived from the following equation:| Eads = Eadsorbates/adsorbent − Eadsorbent − Eadsorbates | (1) |
Results and discussion
Characterization
From the XRD pattern (PDF#14-0109), it can be inferred that the nanosheets have a typical LDH structure with a hexagonal system structure and a special d value relationship (d003 = 2d006 = 3d009). A combination of surface and cross-sectional SEM images (Fig. 1b and c) shows that the nanosheets have an approximate ortho-hexagonal shape. The nanosheets assembled in a highly consistent orientation to form a scale-like micro–nanostructure similar to skin of animals. | ||
| Fig. 1 (a) XRD pattern of the Mg–Al LDH nanosheets. (b) Surface and (c) cross-sectional SEM images of the L110 sample. | ||
Impact on the polysiloxane sites
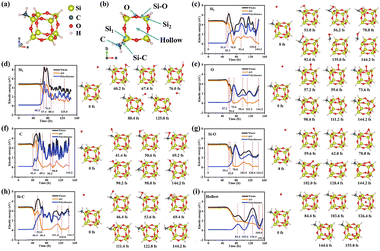
The coatings are obtained by oriented assembly of polysiloxane-modified nanosheets. Thus, AO interacts directly with the polysiloxane on the surface of nanosheets. And the interaction between high-speed AO and polysiloxane is worth further exploration. AIMD was used to investigate this fast process at the atomic scale. APTES upon hydrolysis and condensation would form polysiloxane with three-dimensional and organo-inorganic hybrid silicon-oxygen networks, which has been reported in some previous work. In order to match this real structure and computational effort, a simplified model of polysiloxane was referred to in reported models,34,35 as presented in Fig. 2a. And the AO bombardment sites on the polysiloxane along the –c direction are illustrated in Fig. 2b. Fig. 2c–i show the kinetic energy curves of AO, polysiloxane and the whole system, respectively, and the state diagrams at some special time nodes on some special sites. The influence of the kinetic energy has connection to the corresponding transformation of potential energy, which could reflect some special states during the interaction (such as bond formation, bond breaking, vibration, etc.). During the AO bombardment of these sites, the action of AO could be classified into two categories: scattering (Fig. 2c and d) and adsorption (Fig. 2e–i). Among these, the AO was scattered away only when it impacted the Si site, while in other simulations, the AO was adsorbed. The scattered AO, due to the strong oxidative properties and high reactivity, tended to take away some elements (C and H), while the adsorption of AO leads to increased oxidation of polysiloxane formation.Impact on the LDH sites
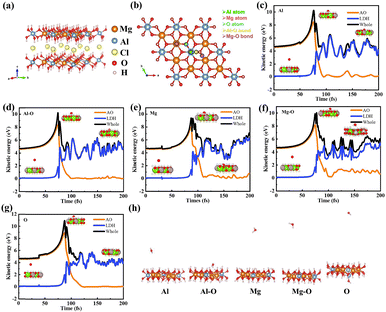
High-speed AO also has the potential to bombard the surface of nanosheets not covered with polysiloxane. A typical model of a Mg–Al layered double hydroxide is shown in Fig. 3a (molar ratio Mg/Al = 2). And the same AIMD method was applied to investigate the interaction between AO and Mg–Al LDH nanosheets. The AO bombardment sites on the Mg–Al LDH along the –c direction are displayed in Fig. 3b. And Fig. 3c–g shows the kinetic energy curves of AO, LDH and the whole system, respectively, and the state diagrams at some special time nodes on some special sites. In the pre-200 fs, the AO impacted the Mg–O site as scattering, while bombardment on all other sites presented as adsorption. With the interaction time increasing, all AO would form escaping small molecules (H2O or –OH in Fig. 3h), which is not exactly the same as the polysiloxane model.Multi-impact of massive AO
The real interaction was based on the continuous erosion of AO. Therefore, the multi-impact of massive AO deserved further study. The time for a single AO impact to form a steady state is much less than the period of the secondary AO bombardment under the fluence of 5.22 × 1021 atoms per cm2. And based on the previous results, only polysiloxane adsorbed AO to form stable intermediates. Therefore, it is essential to further investigate the formation of the final product. With continuous enrichment, the adsorbed AO on the structure of Si–O–O–Si was unstable and easy to form O2 with another AO impact (Fig. 4). Hence, high-speed AO would be scattered or adsorbed to increase the oxidation of the coatings, and the adsorbed AO might finally form stable O2.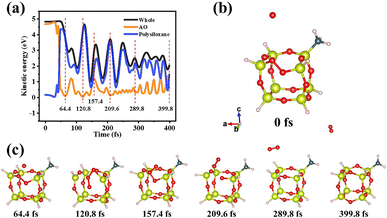 | ||
| Fig. 4 (a) Kinetic energy curves and (b) the snapshots at some special time nodes after continuous AO bombardment on the intermediate structure of Si–O–O–Si. | ||
Adsorption energy of AO
Strong reactivity is another characteristic of AO threatening to LEO spacecraft. Therefore, in this work, the adsorption energy of AO is calculated and discussed. Previous work explored the adsorption energy of AO on polysiloxane sites as shown in Fig. S1 and Table S1. As a complement to the LDH scale-like coatings, the adsorption of AO onto Mg–Al LDH sites is demonstrated in Fig. 5 and Table 1. The adsorption energy at each site is negative, which indicates that the adsorption of AO is a spontaneous process. From an energy perspective, AO is more likely to capture H on polysiloxanes and LDH to form hydroxyl groups and H2O molecules, or to oxidize carbon-containing groups, rather than forming oxide structures (Si–O/Mg–O–Al) in coatings. And AO adsorbed onto the Si–O network of polysiloxane would easily form O2 with another AO and escape from the coatings.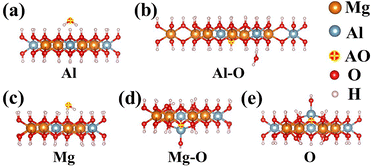 | ||
| Fig. 5 Adsorption sites of AO on the sites of the LDH model: (a) Al atom, (b) Al–O bond, (c) Mg atom, (d) Mg–O, and (e) O atom. | ||
| Adsorption site | Adsorption product | Adsorption energy (eV) |
|---|---|---|
| Al | H2O | −7.839 |
| Al–O | Mg–O–Al + H2O | −8.085 |
| Mg | H2O | −8.118 |
| Mg–O | Mg–O–Al | −7.817 |
| O | Mg–O–Al | −7.717 |
AO protection mechanism of the LDH scale-like coatings
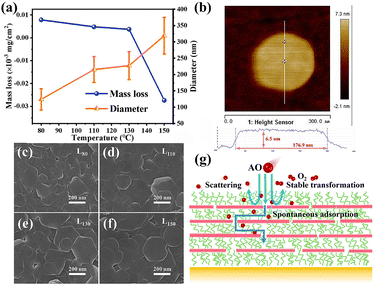
Non-dense coatings tended to provide migration paths for AO and it was difficult to obstruct the intrusion of AO at the atomic scale.28,46,47 However, the LDH nanosheet coatings reported in this work showed excellent AO protection performance. In comparison with the existing literature, the erosion yield of the coatings in this work has been improved by several orders of magnitude (to 4.6 × 10−28 cm3 per atom or negative). This seemed to be different from conventional knowledge. Therefore, it was necessary and valuable to clarify the underlying AO protection mechanism of the LDH nanosheet coatings. According to existing studies28,34,48 and the calculations in the previous section, the interaction between AO and materials was various, including scattering, adsorption, oxidation, etc. And for the LDH coatings with special scale-like structures in this study, the micro–nanostructure should also be explored in detail to reveal the AO protection mechanism.The micro–nano structure was an important factor affecting the interaction results between the coatings and AO. The SEM images after the AO exposure illustrated that the nanosheet coatings still maintained their original micro–nanostructure (Fig. S2). Therefore, the interaction between the micro–nanostructure of the coatings and AO should be further discussed. Fig. 6c–f show the SEM images of the LDH nanosheet coatings under different hydrothermal temperature conditions. All coatings had the same laminated-oriented nanosheets, and the difference in their micro–nanostructures was only reflected in the different sizes of nanosheets. The ideal Mg–Al LDH nanosheets possessed a regular ortho-hexagonal shape. Hence, the transverse dimensions of the nanosheets are shown in Fig. 6a by the diameter of their external circles, while the thickness information is given in Fig. 6b and Table 2. The Mg–Al LDH nanosheets, as quasi-two-dimensional materials, had a much larger diameter which was a more dominant factor than the thickness in the structure of the nanosheet coatings (diameter/thickness: 16.6–43.9). Therefore, the relationship between the nanosheet diameter and AO action needed to be further investigated. The variation of mass loss (F = 5.22 × 1021 atoms per cm2) and the nanosheet diameter of the LDH nanosheet coatings at different hydrothermal synthesis temperatures is presented in Fig. 6a. For LDH nanosheet coatings, the internal nanosheet diameter exhibits a strong negative correlation with the mass loss. The larger the nanosheet diameter, the lower the mass loss and the better the AO protection performance of the coatings. This could be explained as follows. Within the laminated nanosheet coatings, the AO can readily migrate through the interspace between the nanosheets, and the larger the nanosheet diameter, the longer the path of AO intrusion, and hence the greater the obstruction to AO erosion.
| Sample | Diameter | Thickness | D/t | ||
|---|---|---|---|---|---|
| Average (D; nm) | Standard deviation (nm) | Average (t; nm) | Standard deviation (nm) | ||
| L80 | 124.51 | 32.41 | 7.49 | 2.70 | 16.6 |
| L110 | 215.31 | 39.49 | 6.60 | 1.80 | 32.6 |
| L130 | 227.36 | 42.62 | 7.19 | 1.60 | 31.6 |
| L150 | 318.80 | 56.03 | 7.26 | 1.75 | 43.9 |
After the interactions, AO would eventually reach a steady state. For such a high AO dose of 5.22 × 1021 atoms per cm2, the mass gain of the samples was much less than this amount, and the interior of the coatings was only slightly oxidized. Therefore, it was inferred that there was a large amount of AO scattering on the coating surface without entering the coatings. Under the bombardment of a large amount of AO, the polysiloxane on the coating surface was oxidized to SiO2. According to the AIMD simulation and the adsorption energy calculations, polysiloxane and LDH are able to adsorb AO on, e.g., the Si–O bond or the nanosheet surface. Hence, part of this adsorbed AO may be converted into O2, –OH and H2O to escape from coatings, while another part would gradually diffuse and migrate along the nanosheet layer to the inside of the coatings.
Combining the above discussion on the experimental and computational aspects of the scale-like Mg–Al LDH coatings, the schematic diagram of the possible AO protection mechanism of the coatings is shown in Fig. 6g and explained as follows: (a) barrier effect: a large amount of AO bombarding on the surface of the coating was directly scattered away or was converted into stable products such as O2, –OH and H2O after being adsorbed and eventually escaped from the coating surface; (b) obstruction effect: a part of AO adsorbed onto the surface of the coating would diffuse and migrate to the interior, but the long erosion path provided by the laminated nanosheets and the adsorption of AO by the nanosheets on the path all significantly improved the erosion obstruction ability of this part of AO. And the adsorbed AO finally reached a steady state (O2, –OH, H2O or other oxide), thus preventing AO from diffusing to the protected substrates. These two effects jointly contributed to the excellent AO protection properties of the scale-like Mg–Al LDH coatings.
Conclusions
In summary, with the aim of exploring scale-like LDH nanosheets as an AO-resistant coating for spacecraft, first-principles calculations combined with experimental techniques were applied to explore the process of AO action at the atomic scale and investigate the AO protection mechanism within the femtosecond time scale. The calculations revealed that static AO was spontaneously adsorbed by the coatings, while high speed AO would be scattered or adsorbed. The adsorbed AO increased the oxidation of the coatings and finally reached a steady state (O2, –OH, H2O or other oxides). It was also experimentally demonstrated that the nanosheet size directly and significantly affected the resistance to AO erosion. Based on the calculation and experimental results, a possible AO protection mechanism of scale-like LDH coating was proposed. The coating mainly inhibits the AO erosion through the barrier effect on the coating surface and the obstruction effect inside coatings with long erosion paths. Our study provides insights into the impact and adsorption interaction between AO and scale-like LDH coating at the atomic level and demonstrates the capability of first-principles calculations in studying AO-related microprocesses. It also identifies the potential of nanosheet materials assembled into a special micro–nanostructure in future aerospace applications, promising to support the development of highly reliable flexible materials for low-Earth orbit constellations. As a perspective for future work, we plan to extend our first-principles calculations and experimental validations to include the effects of other critical space environment factors, such as thermal cycling and ultraviolet irradiation, to develop multifunctional coatings that address multiple space environment threats.Author contributions
Denghang Tang: writing – original draft, investigation, and visualization. Rui Sun: methodology and data curation. Jiayu Zheng: methodology and data curation. Mengyun Xu: formal analysis and methodology. Haogeng Li: formal analysis and methodology. Hongyu Gu: writing – review and editing and conceptualization. Yuzhi Zhang: resources and supervision. Yiyang Sun: writing – review and editing. Lixin Song: resources and supervision.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5cp03344f.Acknowledgements
This work was sponsored by the National Natural Science Foundation of China (nos. U22B20128, 51802332), the Youth Innovation Promotion Association CAS (no. 2022248), the Natural Science Foundation of Shanghai (no. 25ZR1402535), the Strategic Priority Research Program of the Chinese Academy of Sciences (no. XDB1520103) and the National program (2024-017). The authors thank the Integrated Computational Materials Research Center of SICCAS for computing time. The AI-driven experiments, simulations and model training were performed on the robotic AI-Scientist platform of Chinese Academy of Sciences.Notes and references
- Y. Yang, T. T. Hu, Y. X. Bian, F. Q. Meng, S. L. Yu, H. Li, Q. H. Zhang, L. Gu, X. S. Weng, C. L. Tan and R. Z. Liang, Adv. Mater., 2023, 35, 23 Search PubMed.
- T. He, Q. Li, T. Lin, J. X. Li, S. Bai, S. An, X. G. Kong and Y. F. Song, Chem. Eng., 2023, 462, 142041 CrossRef CAS.
- F. Q. Wang, P. C. Zou, Y. Y. Zhang, W. L. Pan, Y. Li, L. M. Liang, C. Chen, H. Liu and S. J. Zheng, Nat. Commun., 2023, 14, 6019 CrossRef CAS PubMed.
- Z. H. Zhou, X. F. Zhou, D. Lan, Y. Zhang, Z. R. Jia, G. L. Wu and P. F. Yin, Small, 2024, 20, 2305849 CrossRef CAS PubMed.
- A. Karmakar, A. V. Krishnan, R. Jayan, R. Madhu, M. M. Islam and S. Kundu, J. Mater. Chem. A, 2023, 11, 10684–10698 RSC.
- C. Y. Li, X. K. Wang, D. G. Ma, Y. Yan, P. W. Huo and Q. J. Yang, Adv. Sci., 2023, 10, 2301398 CrossRef CAS PubMed.
- S. N. Shi, S. J. Feng, C. H. Liu, M. He, X. H. Bu, Z. W. Zhang, Y. M. Zhou and Q. Z. Yao, Fibers Polym., 2023, 24, 1951–1961 CrossRef CAS.
- Z. T. Zhang, R. Y. Dong, D. S. Qiao and B. Y. Cao, Nanotechnology, 2020, 31, 465403 CrossRef CAS PubMed.
- T. K. Minton, T. E. Schwartzentruber and C. Xu, ACS Appl. Mater. Interfaces, 2021, 13, 51673–51684 CrossRef CAS PubMed.
- I. Gouzman, O. Girshevitz, E. Grossman, N. Eliaz and C. N. Sukenik, ACS Appl. Mater. Interfaces, 2010, 2, 1835–1843 CrossRef CAS.
- N. Shimosako, Y. Hara, K. Shimazaki, E. Miyazaki and H. Sakama, Acta Astronaut., 2018, 146, 1–6 CrossRef CAS.
- Y. F. Zhang, S. N. Chen, W. Q. Yan, Q. Li, L. Chen, Y. X. Ou and B. Liao, Surf. Coat. Technol., 2021, 423, 127582–127593 CrossRef CAS.
- I. Gouzman, E. Grossman, R. Verker, N. Atar, A. Bolker and N. Eliaz, Adv. Mater., 2019, 31, 1807738 CrossRef PubMed.
- M. Hołyńska, A. Tighe and C. Semprimoschnig, Adv. Mater. Interfaces, 2018, 5, 1701644 CrossRef.
- M. Chafiq, A. Chaouiki, T. Suhartono, F. Hazmatulhaq and Y. G. Ko, Chem. Eng. J., 2023, 462, 142239 CrossRef CAS.
- W. L. Qu, W. F. Wang, C. X. Zhang, X. W. Chen, J. Wang, W. H. Xue, J. X. Zhu and H. Wu, Chem. Eng. J., 2023, 477, 146862 CrossRef CAS.
- A. R. Kirmani, D. P. Ostrowski, K. T. VanSant, T. A. Byers, R. C. Bramante, K. N. Heinselman, J. H. Tong, B. Stevens, W. Nemeth, K. Zhu, I. R. Sellers, B. Rout and J. M. Luther, Nat. Energy, 2023, 8, 191–202 CrossRef CAS.
- N. Y. Nia, Nat. Energy, 2023, 8, 117–118 CrossRef.
- K. Wang, J. S. Ma, Y. Li, Y. H. Ding, N. L. Chen, H. Q. Shao and J. H. Jiang, Surf. Coat. Technol., 2024, 482, 130702 CrossRef CAS.
- J. Weerasinghe, K. Prasad, J. Mathew, E. Trifoni, O. Baranov, I. Levchenko and K. Bazaka, Nanomaterials, 2023, 13, 1763 CrossRef CAS PubMed.
- Y. P. Wu, L. Chen, H. Y. Xu, H. D. Zhou and J. M. Chen, Mater. Lett., 2023, 351, 135017 CrossRef CAS.
- R. Lian, X. Lei, S. Xue, Y. Chen and Q. Zhang, Appl. Surf. Sci., 2021, 535, 147654 CrossRef CAS.
- N. H. Crisp, P. C. E. Roberts, S. Livadiotti, V. T. A. Oiko, S. Edmondson, S. J. Haigh, C. Huyton, L. A. Sinpetru, K. L. Smith, S. D. Worrall, J. Becedas, R. M. Domínguez, D. González, V. Hanessian, A. Mølgaard, J. Nielsen, M. Bisgaard, Y. A. Chan, S. Fasoulas, G. H. Herdrich, F. Romano, C. Traub, D. García-Almiñana, S. Rodríguez-Donaire, M. Sureda, D. Kataria, R. Outlaw, B. Belkouchi, A. Conte, J. S. Perez, R. Villain, B. Heißerer and A. Schwalber, Prog. Aeronaut. Sci., 2020, 117, 100619 CrossRef.
- X. K. Song, H. Gong, H. C. Li, M. Y. Zhang, L. Jiang, C. Wang, P. P. Jiang, H. F. Wang, K. L. Cao, G. Liu, Q. B. Zhao and T. X. Fan, Adv. Funct. Mater., 2025, 35, 2413191 CrossRef CAS.
- M. Nishida, D. Kimura, K. Ashida, N. Furuta, Y. Iwase and Y. Ishida, Composites, Part B, 2025, 288, 111877 CrossRef CAS.
- C. J. Huang, J. Liu, L. B. Zhao, N. Hu and Q. Wei, Composites, Part A, 2023, 168, 107459 CrossRef CAS.
- D. H. Tang, H. Y. Gu, M. Y. Xu, R. Sun, H. G. Li, Y. Y. Sun, Y. Z. Zhang and L. X. Song, Surf. Coat. Technol., 2024, 483, 130757 CrossRef CAS.
- X. F. Pan, B. Wu, H. L. Gao, S. M. Chen, Y. Zhu, L. Zhou, H. Wu and S. H. Yu, Adv. Mater., 2022, 34, 2105299 CrossRef CAS PubMed.
- D. Wang, J. Ma, P. Li, L. Fan, Y. Wu, Z. Zhang, C. Xu and L. Jiang, ACS Appl. Mater. Interfaces, 2021, 13, 46003–46014 CrossRef CAS PubMed.
- Y. Zhang, Q. Li, H. Yuan, W. Yan, S. Chen, M. Qiu, B. Liao, L. Chen, X. Ouyang, X. Zhang and M. Ying, ACS Appl. Mater. Interfaces, 2022, 14, 21461–21473 CrossRef CAS PubMed.
- C. J. Huang, R. Z. Huang, Y. H. Cheng, L. B. Zhao, N. Hu and Q. Wei, Compos. Sci. Technol., 2024, 253, 110665 CrossRef CAS.
- B. Earp, J. Hubbard, A. Tracy, D. Sakoda and C. Luhrs, Composites, Part B, 2021, 219, 108874 CrossRef CAS.
- H. G. Li, H. Y. Gu, C. Ming, Y. Y. Sun, Y. Z. Zhang and L. X. Song, Appl. Surf. Sci., 2023, 609, 155274 CrossRef CAS.
- H. G. Li, H. Y. Gu, Y. Z. Zhang, L. N. Wu and L. X. Song, Comput. Mater. Sci., 2021, 186, 110007 CrossRef CAS.
- D. H. Tang, H. G. Li, H. Y. Gu, S. B. Lv, J. Y. Ma, Y. Z. Zhang and L. X. Song, Surf. Coat. Technol., 2022, 447, 128840 CrossRef CAS.
- D. Z. Chen, C. B. Xu, I. Gouzman and T. K. Minton, ACS Appl. Polym. Mater., 2022, 4, 3627–3635 CrossRef CAS.
- J. C. Du and J. M. Rimsza, npj Mater. Degrad., 2017, 1, 16 CrossRef.
- A. A. Hassanali, J. Cuny, V. Verdolino and M. Parrinello, Philos. Trans. R. Soc., A, 2014, 372, 20120482 CrossRef PubMed.
- S. Kansara, H. Kang, S. Ryu, H. H. Sun and J. Y. Hwang, J. Mater. Chem. A, 2023, 11, 24482–24518 RSC.
- B. Kirchner, P. J. di Dio and J. Hutter, Top. Curr. Chem., 2012, 307, 109–153 CrossRef CAS PubMed.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- G. Kresse and J. Furthmüller, Phys. Rev. B:Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed.
- G. Kresse and D. Joubert, Phys. Rev. B:Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef CAS.
- G. Kresse and J. Hafner, Phys. Rev. B:Condens. Matter Mater. Phys., 1993, 47, 558–561 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- Y. Z. Liu, Y. Sun, F. L. Zeng, Q. H. Zhang and L. Geng, Appl. Surf. Sci., 2014, 320, 908–913 CrossRef CAS.
- H. Zhang, S. Ren, J. Pu and Q. Xue, Appl. Surf. Sci., 2018, 444, 28–35 CrossRef CAS.
- X. Wang, Y. Li, Y. Qian, H. Qi, J. Li and J. Sun, Adv. Mater., 2018, 30, 1803854 CrossRef PubMed.
| This journal is © the Owner Societies 2026 |