Nitrogen-rich energetic salts of 5,5′-dinitramino-3,3′-methylene-1H-1,2,4-bistriazolate: powerful alliance towards good thermal stability and high performance
Yanna
Wang†
a,
Xiaoming
Yang†
 b,
Xinrui
Li
b,
Xinrui
Li
 bd,
Jun
Zhao
a,
Qi
Wang
a,
Tonglai
Zhang
bd,
Jun
Zhao
a,
Qi
Wang
a,
Tonglai
Zhang
 c and
Zhimin
Li
c and
Zhimin
Li
 *c
*c
aSchool of Chemistry and Chemical Engineering, Xingtai University, Xing tai 054001, China
bChina Safety Technology Research Academic of Ordnance Industry, Beijing 100053, China
cState Key Laboratory of Explosion Science and Safety Protection, Beijing Institute of Technology, Beijing 100081, China. E-mail: lizm@bit.edu.cn
dSchool of Chemical Engineering, Nanjing University of Science and Technology, Xiaolingwei 200, Nanjing, Jiangsu 210094, China
First published on 19th November 2025
Abstract
Two new nitrogen-rich energetic salts based on 5,5′-dinitramino-3,3′-methylene-1H-1,2,4-bistriazolate (DNAMT), namely, (DAG)2(DNAMT)·4H2O (1) and (NH3OH)(DNAMT) (2), were designed and synthesized, and they were characterized by IR spectroscopy, elemental analysis, and single-crystal X-ray diffraction. The thermal stability of the two salts were studied by differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). The decomposition temperatures of 1 and 2 were determined to be 221.2 °C and 214 °C, respectively. The apparent activation energies (Ea) of the thermal decomposition processes of 1 and 2 were 189.2 and 218.9 kJ mol−1, respectively. Compound 1 had appropriate impact sensitivity (11.5 J) and was friction-insensitive (>360 N). In contrast, compound 2 was an impact- and friction-sensitive explosive (IS: 3 J; FS: 56 N). The detonation properties of compounds 1 and 2 were calculated using EXPLO 5 (V6.04) based on their experimental densities and calculated heats of formation. The detonation properties of 1 (D: 7943 m s−1 and P: 21.20 GPa) and 2 (D: 8339 m s−1 and P: 26.75 GPa) dramatically exceeded those of TNT but were lower than those of RDX. Meanwhile, compounds 1 and 2 possessed higher nitrogen contents (53.83% and 50.82%, respectively). Both compounds displayed good thermal stability, high energetic properties and low sensitivities as high nitrogen content energetic materials.
Introduction
It has been a challenging target for a long time to design new energetic materials with high energy and low sensitivity for meeting the growing demands of military and civilian applications.1,2 However, the high performance and low sensitivity are often contradictory to each other.3–5 Nitrogen-rich heterocycles have been studied over the past years with growing interest due to their high performance and high positive heats of formation with a large number of N–N and C–N bonds, high ring strain and high level of environmental compatibility.6–8The nitrogen-rich heterocycle compounds usually refer to triazole, tetrazole, tetrazine, bi-triazole and bi-tetrazole. Compared with other nitrogen-rich heterocyclic compounds, bi-triazole ring compounds have the advantages of good thermal stability and low sensitivities. Therefore, the performance of bi-triazole rings can be improved by introducing a nitramino group, which improves the oxygen balance and nitrogen content and eventually results in a higher exothermicity of the combustion and detonation processes, thus increasing their further applications.9–12
Nitrogen-rich ionic salts are a class of important energetic materials due to their advantages such as lower vapor pressures and higher densities than non-ionic molecules.13–15 In the nitramino group-modified bitriazole ring compounds, the nitramino group presents an acidic proton that makes it possible to form corresponding energetic salts.16 In these energetic ionic salt molecules, a large number of intermolecular and intramolecular hydrogen bonds are formed through hydrogen rich groups, which can generally enhance the stability of the nitramino-containing compounds.17,18
The bridged heterocyclic compounds have produced numerous promising energetic ionic salts.19–21 The 5,5′-dinitramino-3,3′-methylene-1H-1,2,4-bistriaz-ole (DNAMT), a typical bridged bi-triazole heterocyclic compound, was first reported by Dippold.22 The application of DNAMT is limited due to its high sensitivity. However, DNAMT is able to form salts through mono- or double-deprotonated modes owing to the presence of two acidic hydrogen atoms.23,24
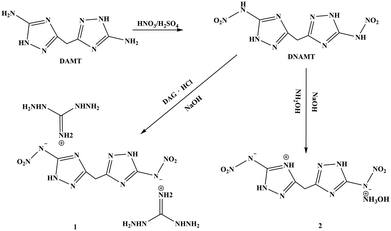
In a continuing effort to seek new high-performance nitrogen-rich energetic materials, two new ionic salts, namely, (DAG)2(DNAMT)·4H2O (1) and (NH3OH)(DNAMT) (2), were synthesized according to a straightforward method (Scheme 1). The two compounds were fully characterized by infrared spectroscopy and elemental analysis, and the molecular structures were confirmed by single crystal X-ray diffraction. Moreover, the performance and Hirshfeld surface analysis of these compounds were studied in detail. Compounds 1 and 2 showed high energetic properties and low sensitivities.
Results and discussion
Synthesis
DNAMT (1.4 g, 5 mmol) and NaOH (0.4 g, 10 mmol) were suspended in 20 mL of water and stirred for 10 min at 70 °C for obtaining a clear solution. Afterwards N,N′-diaminoguanidine hydrochloride (DAG·HCl, 1.3 g, 10 mmol) and hydroxyamine hydrochloride (NH2OH·HCl, 0.7 g, 10 mmol) were dissolved in 10 mL of water and added to the above solution dropwise. After continuously stirring for 30 min, the mixture was filtered after cooling to room temperature. Colourless needle crystals were obtained by evaporation of the mother liquor at room temperature for 2 days.X-ray crystallography
Crystals of compounds 1 and 2 suitable for X-ray diffraction measurements were collected by slow evaporation from the reaction solution at room temperature. The structures of these new compounds are illustrated in Fig. 1–4. The crystal data, structure refinement details and selected bond lengths (Å) and bond angles (°) of compounds 1 and 2 can be found in Tables S1–S9. The CCDC numbers of compounds 1 and 2 are 1939348 and 2422609, respectively.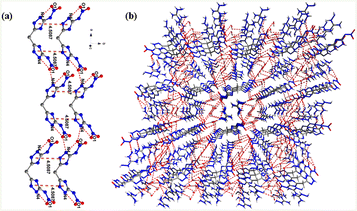 | ||
| Fig. 2 (a) Partial hydrogen bonds and π–π interaction of 1 viewed along the c axis; (b) Auger-type stacking assembly form of 1 viewed down the b axis. | ||
 | ||
| Fig. 4 (a) Two kinds of π–π stacking structure of 2 viewed along the c axis; (b) hydrogen bonds of 2 viewed along the a axis. | ||
Compound 1 crystallized in the monoclinic system with C2/c space group with a calculated crystal density of 1.582 g cm−3 at 163 K. As illustrated in Fig. 1, the molecule of compound 1 consisted of two DAG+ cations, one DNAMT2− anion and four crystal H2O molecules. Two triazole rings in DNAMT lost two hydrogen atoms in the two N2 positions to exist as a dianion DNAMT2−. The N6 position in DAG accepted the hydrogen atoms and formed a monovalent cation.
Interestingly, the C atom that connected the two triazole rings was nearly coplanar with each triazole ring, as confirmed by the torsion angles of N4–N5–C2–C3 (179.51°) and C3–C2–N3–C1 (−179.33°). Furthermore, there was a slight contortion between the nitramino group and the triazole plane, confirmed by the torsion angles of N1–N2–C1–C4 (−6°). Additionally, the structure of DAG cation was nearly coplanar, as confirmed by the torsion angles of N10–N9–C4–N6 (2.83°) and N6–C4–N7–N8 (179.93°).
As shown in Fig. 2(a), the corresponding triazoles in different molecules formed parallel face-to-face π–π interactions; the distance of the π–π interactions was 4.5087 Å, which was slightly longer than the typical aromatic π–π interactions, indicating that the crystal packing of compound 1 exhibited weak π–π interactions. The packing structures assembled via various hydrogen bonds. The distances of intramolecular hydrogen bonds and intermolecular hydrogen bonds (red dotted lines) were within the range of 2.574 Å to 3.039 Å. The dense packing of a large number of molecules finally formed a stable 3D supramolecular network (Fig. 2(b)). As a result, the hydrogen bonds and π–π stacking increased the stability of this molecule.
Compound 2 crystallized in the monoclinic system with P21/n space group and a calculated crystal density of 1.768 g cm−3 at 163 K. The density of compound 2 was slightly higher than that of compound 1. As illustrated in Fig. 3, an intramolecular transfer of hydrogen atoms occurred in compound 2, with the hydrogen on one of the amino nitrogen migrating to the nitrogen of one of the triazole rings; thus, the molecule of compound 2 consisted only one NH4O+ cation and one DNAMT2− anion.
As illustrated in Fig. 4(a), the crystal units were repeated in face-to-face weak π–π stacking, and the distances of π–π interactions were 4.279 Å, which was slightly longer than that of typical aromatic π–π interactions. This stacking interaction also contributed to the higher densities. Moreover, there were several kinds of strong intermolecular and intramolecular hydrogen bonds of O–H⋯N, N–H⋯N, and N–H⋯O, which were composed of O, N, and H atoms on the triazole ring, hydroxylamine and nitryl. The arrangements of π–π stacking, intermolecular hydrogen bonding and absence of water content increased the stability and density of this molecule.
Thermal decomposition
To assess the thermal stabilities of compounds 1 and 2, the samples were tested by the DSC method and TG technology under nitrogen gas at a heating rate of 10 °C min−1. The curves are shown in Fig. S3 and S4. Compounds 1 and 2 showed one endothermic peak prior to violent decomposition. The endothermic peak of compound 1 ranged from 35.0 °C to 74.9 °C, corresponding to the loss of water molecules, which can be confirmed by the TG curve with a reduced mass of 13.35% (calculated value, 13.83%). Compound 1 started to decompose at 214.6 °C, and the peak temperature was 221.2 °C, accompanied by an immediate and violent decomposition process. During the thermal decomposition process, the mass loss of the substance was 52.25%, corresponding to the decomposition of the DNAMT molecule (calculated value, 51.69%). Compound 2 exhibited an endothermic peak at 176.5 °C without a sudden mass loss, as evidenced by the TG curve. It was also observed that as the temperature increased, compound 2 slowly started to evaporate. It started to decompose at 194 °C, and the peak temperature of the violent decomposition process was 214 °C. During the thermal decomposition process, the mass loss of the substance was 68%, corresponding to the decomposition of the DNAMT molecule without the group of –NNO2 (calculated value, 68.9%). Due to the different formation mechanisms of these two compounds, their decomposition processes were also distinct. Notably, during the process of thermal decomposition, both compounds 1 and 2 exploded due to the violent decomposition. The values of apparent activation energies (Ea) of compounds 1 and 2, determined by the Kissinger's method25 and Ozawa's method,26 were 189.2 kJ mol−1 and 218.9 kJ mol−1, respectively (see Table S11). The Arrhenius equations were expressed using the Kissinger's method as follows: ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k = 41.84–189.2 × 10−3/RT for (1) and ln
k = 41.84–189.2 × 10−3/RT for (1) and ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k = 50.24–218.9 × 10−3/RT for (2). Results illustrated the moderate thermokinetic activity of these compounds. In addition, the corresponding critical temperature of thermal explosion (Tb) according to Zhang's equations, the values of the peak temperature while β → 0(Tp0), free energy of activation (ΔG), the entropy of activation(ΔS), and the enthalpy of activation (ΔH) were obtained and are listed in Table S12.
k = 50.24–218.9 × 10−3/RT for (2). Results illustrated the moderate thermokinetic activity of these compounds. In addition, the corresponding critical temperature of thermal explosion (Tb) according to Zhang's equations, the values of the peak temperature while β → 0(Tp0), free energy of activation (ΔG), the entropy of activation(ΔS), and the enthalpy of activation (ΔH) were obtained and are listed in Table S12.
Heat of formation
The energies of combustion of compounds 1 and 2 were measured by bomb calorimetry, a well-established experimental technique, and the standard enthalpies of formation of compounds 1 and 2 were calculated using the formula ΔcHθ = ΔcU + ΔnRT, the combustion reaction equations (eqn (1) and (2)) and the Hess thermochemical cycle. The measured constant volume combustion energies were −12.29 kJ g−1 (−6397 kJ mol−1) and −11.59 kJ g−1 (−3514 kJ mol−1) for compounds 1 and 2, respectively.| C7H28N20O8(s) + 10O2(g) → 7CO2(g) + 14H2O(l) + 10N2(g) | (1) |
| C5H9N11O5(s) + 4.75O2(g) → 5CO2(g) + 4.5H2O(l) + 5.5N2(g) | (2) |
As shown in Table 1, the calculated enthalpies of formation of compounds 1 and 2 were −376.5 kJ mol−1 and 248 kJ mol−1, respectively. The high water content affected the enthalpy of formation of compound 1.
| Sample | ΔcU (kJ g−1) | Q v (kJ mol−1) | Q p (kJ mol−1) | ΔfHθ (kJ mol−1) |
|---|---|---|---|---|
| ΔcU is the constant volume combustion energies. Qv is the constant volume combustion energies. Qp is the constant pressure combustion. ΔfHθ is the formation enthalpy. | ||||
| 1 | −12.29 | −6397 | −6380 | −376.5 |
| 2 | −11.59 | −3514 | −3500 | 248 |
Detonation parameters
With the data of room-temperature density and experimentally calculated heat of formations (ΔfHθ), the detonation properties of compounds 1 and 2 were predicted by the EXPLO5 program (version 6.04), and the results are listed in Table 2. As can be seen in Table 2, the calculated detonation velocity of compound 1 was 7943 m s−1, and the detonation pressure was 21.20 GPa. The calculated detonation velocity of compound 2 was 8339 m s−1, and the detonation pressure was 26.75 GPa. The detonation properties of these two compounds dramatically exceeded that of TNT (6881 m s−1) and were similar to that of RDX (8748 m s−1). Meanwhile, the nitrogen content of compounds 1 and 2 were higher than those of RDX and TNT. Obviously, the results showed that compounds 1 and 2 can be considered promising high-energy, high nitrogen explosives.| Com. | 1 | 2 | RDX | TNT |
|---|---|---|---|---|
| a Decomposition temperature (from DSC, β = 10 °C min−1). b Room-temperature densities calculated by the volume expansion equation ρ298 K = ρT/(1 + αV(298 − T)); αV = 1.5 × 10−4 K−1,27 163.15 K. c Nitrogen content. d Oxygen balance (oxygen balance for CaHbOcNd: 1600(c − 2a − b/2)/MW). e Calculated enthalpy of formation. f Impact sensitivity. g Friction sensitivity. h Heat of detonation. i Detonation temperature. j Detonation velocity. k Detonation pressure. | ||||
| Formula | C7H28N20O8 | C5H9N11O5 | C3H6N6O6 | C7H5N3O6 |
| M [g mol−1] | 520.4 | 303.2 | 222.1 | 227.1 |
| T dec (°C)a | 221.2 | 214.0 | 210.0 | 240.0 |
| ρ (g cm−3)b | 1.548 | 1.733 | 1.820 | 1.650 |
| N (%)c | 53.83 | 50.82 | 37.83 | 18.50 |
| Ω (%)d | −61.48 | −50.13 | −21.61 | −73.98 |
| ΔfHθ (kJ mol−1)e | −376.5 | 248.0 | 80.00 | −155.0 |
| IS (J)f | 11.5 | 3.0 | 7.5 | 15.0 |
| FS (N)g | >360 | 56 | 120 | 353 |
| −ΔExUθ (kJ kg−1)h | 3102 | 4246 | 6125 | 4226 |
| T det (K)i | 2206 | 2972 | 4236 | 3100 |
| D (m s−1)j | 7943 | 8339 | 8748 | 6881 |
| P (GPa)k | 21.20 | 26.75 | 34.90 | 19.50 |
Sensitivities
The sensitivities toward impact (IS) and friction (FS) were measured by the Bundesamt Für Materialforschung (BAM) standard method.28 As shown in Table 2, compound 1 can be classified as a friction insensitive (FS > 360 N) and impact sensitive (IS = 11.5 J) energetic material, while compound 2 is a substance sensitive to both impact and friction (IS = 3 J, FS = 5.6 N). It can be seen from the results that the sensitivities of DNAMT ionic salts were much lower than that of DNAMT (IS = 1.5 J, FS = 60 N).Hirshfeld surfaces analysis
To gain further insight into the physical properties of these new organic energetic salts, the Hirshfeld surfaces and the associated two-dimensional (2D) fingerprint plots as well as the percentage contributions of close contacts were analyzed and are presented in Fig. 5. In typical Hirshfeld surface analysis, the red and blue regions on the surfaces denote the high- and low-close contact populations, respectively. The white, blue, and red spots on the Hirshfeld surfaces represent the distance d equal to, exceeding, and less than the van der Waals distance, respectively. Although molecular crystals 1 and 2 show different shapes of fingerprint plots, their close contact distributions are basically consistent.As shown in Fig. 5, all the red dots in the crystals of compounds 1 and 2 are related to the intermolecular hydrogen bonds, including H⋯N, N⋯H, O⋯H and H⋯O interactions, corresponding to the appearance of two sharp spikes present in the bottom of the spectra. In contrast, the blue parts normally belong to π–π stacking, such as C⋯N, O⋯N and N⋯N interactions.29,30 The total percentage of hydrogen bonding interactions of compounds 1 and 2 are 56.9% and 66.8%, respectively. Additionally, the percentage of π–π stacking was 5.5% and 18.8%, respectively. Results showed that the hydrogen bonding and π–π interactions are clearly dominant in both the molecules. Besides, the population of the O⋯O interactions were 2.3% for 1 and 3% for 2, which are less than that of RDX (18.6%).31 The higher hydrogen bond content and π–π stacking interaction and lower O⋯O interactions suggest that compounds 1 and 2 have lower sensitivity compared with DNAMT and RDX, which can also be confirmed from the experimental results.
Experimental
Caution! Although these compounds were not deplored in the processes of this research, appropriate standard safety precautions should still be followed e.g., eye protection, face shields, leather gloves, and ear plugs.All the chemical reagents were obtained from commercial channels and used without further purification. Crystals suitable for X-ray diffraction measurements were obtained as described in the Experiment section. Data were collected on a Rigaku AFC-10/Saturn 724+ CCD diffractometer. All crystals were irradiated with graphite mono-chromated Mo Kα radiation (λ = 0.71073 Å) at 153(2) K via a multi-scan mode. All the structures were determined and refined using the SHELXL-2015 and Olex2 software. The crystallographic data and experimental details of all compounds are listed in Table S1. Elemental analyses were performed on a Flash EA 1112 fully automatic trace element analyzer. IR spectra were recorded using a Bruker Equinox 55 infrared spectrometer with KBr pellets from 400 to 4000 cm−1. Differential scanning calorimetry (DSC) and thermogravimetric analysis (TG) measurements were carried out with a Pyris-1 differential scanning calorimeter and Pyris-1 thermogravimetric analyzer (Perkin-Elmer, USA), respectively, at a heating rate of 10 °C min−1 in a dry nitrogen atmosphere with a flow rate of 20 mL min−1. The experimentally determined constant-volume energy of combustion was tested by a Parr-6200 bomb calorimeter (static jacket) with a 6510 water handling system.
DNAMT was synthesized according to the corresponding literature.22 Subsequently, compounds 1 and 2 were obtained by the reaction of DNAMT and DAG and NH2OH, respectively.
Synthesis of (DAG)2(DNAMT)·4H2O (1)
0.27 g of DNAMT (1.0 mmol) was put into a flask with 10 mL of deionized water. The mixture was stirred at 60 °C. Then, NaOH (0.08 g, 2.0 mmol) was added to the suspension solution. A 5 mL aqueous solution of DAG·HCl (0.26 g, 2.0 mmol) was dropwise added to the above mixture solution with stirring. The settled solution was obtained after 30 min and filtered after cooling to room temperature. The colorless acicular crystals were obtained from the filtrates. Yield: 0.3402 g, 73%. CCDC: 1939348. IR (KBr): 3431, 3350, 2868, 2365, 2031, 1685, 1522, 1436, 1381, 1243, 1180, 1133, 1093, 1003, 922, 856, 770, 720, 695, 663, 610, 522 cm−1. Elemental analysis for C7H28N20O8 (520.49): calculated: C 18.83%, H 5.38%, N 53.79%; found: C 18.45%, H 5.56%, N 53.40%.Synthesis of (NH3OH)(DNAMT) (2)
0.27 g of DNAMT (1.0 mmol) was placed into a flask with 10 mL of deionized water. The mixture was stirred at 60 °C. Then an aqueous solutions of hydroxylamine was dropwise added to the above mixture solution with continuous stirring until clear. The settled solution was obtained after 30 min and filtered after cooling to room temperature. The colorless short rod-like crystals were obtained from the filtrates. Yield: 0.2306 g, 65%. CCDC: 2422609. IR (KBr): 3263, 3077, 2975, 2710, 2103, 1616, 1519, 1452, 1392, 1332, 1302, 1233, 1132, 1091, 1038, 1008, 987, 928, 875, 773, 712, 476. Elemental analysis for C5H14N12O7 (303.2): calculated (%): C 19.81%, H 2.99%, N 50.82%; found: C 19.95%, H 2.98%, N 50.89%.Conclusions
In summary, two nitrogen-rich energetic organic salts based on 5,5′-dinitramino-3,3′-methylene-1H-1,2,4-bistriazolate (DNAMT) were synthesized and characterized. The structures of these two compounds were determined by single crystal X-ray diffraction, and the ionic salts showed remarkable intermolecular interactions. These compounds had a high nitrogen content (53.83% for 1 and 50.82% for 2) and heats of formation (−376.5 kJ mol−1 for 1, 248 kJ mol−1 for 2). Moreover, the experimental measurements and theoretical calculations confirmed that both compounds possessed promising combination properties including high thermal stability (Td: 221.2 °C for 1, 214 °C for 2), high detonation performances (D: 7943 m s−1 for 1, 8339 m s−1 for 2; P: 21.20 GPa for 1, 26.76 GPa for 2) and low mechanical sensitivities (IS: 11.5 J for 1, 3 J for 2; FS: >360 N for 1, 56 N for 2). Our results suggested that the synthesized energetic salts with hydrogen bonding and π-stacking can achieve a balance between high energy and good stability as energetic materials.Conflicts of interest
There are no conflicts to declare.Data availability
All relevant data are within the manuscript and its additional files.Supplementary information (SI): Fig. S1–S9 and Tables S1–S12. See DOI: https://doi.org/10.1039/d5ce01052g.
CCDC 1939348 and 2422609 contain the supplementary crystallographic data for this paper.32a,b
Acknowledgements
The authors acknowledge the financial support from the State Key Laboratory of Explosion Science and Safety Protection (Grant No. YBKT 22-03), the Fundamental Research Funds for the Central Universities (Grant No. 2017cx10001), the Innovation Capacity Enhancement Project of Xingtai (Grant No. 2023ZZ074) and the Young Talent Program of Xingtai (Grant No. 2020ZZ042).Notes and references
- S. Li, S. Wang, J. Wang, Z. Wang, H. Yin, Q. Ma and F. Chen, J. Mater. Chem. A, 2025, 13, 8337–8342 RSC.
- D. Kumar, G. H. Imler, D. A. Parrish and J. M. Shreeve, J. Mater. Chem. A, 2017, 5, 16767–16775 RSC.
- D. E. Chavez, J. C. Bottaro, M. Petrie and D. A. Parrish, Angew. Chem., Int. Ed., 2015, 54, 12973–12975 CrossRef CAS PubMed.
- T. Lu, C. Wang, G. Wang, S. Wang, J. Song, H. Yin, G. Fan and F. Chen, New J. Chem., 2019, 43, 13330–13333 RSC.
- M. A. Kettner and T. M. Klapötke, Chem. Commun., 2014, 50, 2268–2270 RSC.
- P. Yi, C. Lin, X. Yi, P. He, T. W. Wang and J. G. Zhang, ACS Appl. Mater. Interfaces, 2024, 16, 23426–23433 CAS.
- A. A. Dippold and T. M. Klapötke, J. Am. Chem. Soc., 2013, 135, 9931–9938 CrossRef CAS PubMed.
- G. F. Zhang, C. Xiao, Z. Dong, M. F. Lv, Y. X. Liu, X. Hao, Y. B. Zou, H. Q. Zhang and Z. W. Ye, Cryst. Growth Des., 2023, 23, 6756–6764 CrossRef CAS.
- J. Ma, A. K. Chinnam, G. Cheng, H. Yang, J. Zhang and J. M. Shreeve, Angew. Chem., Int. Ed., 2021, 60, 5497–5504 CrossRef CAS PubMed.
- J. H. Zhang, S. Dharavath, L. A. Mitchell, D. A. Parrish and J. M. Shreeve, J. Am. Chem. Soc., 2016, 138, 7500–7503 CrossRef CAS PubMed.
- Y. X. Tang, C. L. He, G. H. Imler, D. A. Parrish and J. M. Shreeve, J. Mater. Chem. A, 2017, 5, 6100–6105 RSC.
- X. M. Yang, Y. N. Wang, W. J. Zhang, Y. L. Yin, Z. M. Li and T. L. Zhang, CrystEngComm, 2020, 22, 4573–4579 RSC.
- L. Jiang, R. Lv, J. Wang, S. Song, Y. Chen, X. Ge, K. Wang and Q. Zhang, FirePhysChem, 2023, 3, 98–105 CrossRef CAS.
- J. T. Wu, J. G. Zhang, X. Yin, Z. Y. Cheng and C. X. Xu, New J. Chem., 2015, 39, 5265–5271 RSC.
- H. X. Gao, R. H. Wang, B. Twamley, M. Hiskey and J. M. Shreeve, Chem. Commun., 2006, 4007–4009 RSC.
- S. Chen, Y. Shang, J. Jiang, M. Huang, J. Ren, T. Guo, C. Yu, W. Zhang and X. Chen, Energ. Mater. Front., 2022, 3, 122–127 CrossRef CAS.
- Y. G. Xu, L. L. Tian, P. C. Wang, Q. H. Lin and M. Lu, Cryst. Growth Des., 2019, 19, 1853–1859 CrossRef CAS.
- Q. Lai, L. Pei, T. Fei, P. Yin, S. P. Pang and J. M. Shreeve, Nat. Commun., 2022, 13, 6937 CrossRef CAS PubMed.
- J. C. Zhang, H. Su, S. Guo, Y. L. Dong, S. W. Zhang, T. Zou, S. H. Li and S. P. Pang, Cryst. Growth Des., 2018, 18, 2217–2224 CrossRef CAS.
- Y. Wang, J. Ye, N. Yang, H. X. Ma, Y. Z. Zhang and Z. Q. Guo, CrystEngComm, 2021, 23, 7635–7642 RSC.
- Y. A. Feng, Y. G. Bi, W. Y. Zhao and T. L. Zhang, J. Mater. Chem. A, 2016, 4, 7596–7600 RSC.
- A. A. Dippold, M. Feller and T. M. Klapötke, Cent. Eur. J. Energ. Mater., 2011, 8, 261–278 CAS.
- X. Yin, J. Wang, Q. Ma and S.-M. Wang, J. Struct. Chem., 2018, 59, 1195–1199 CrossRef CAS.
- Y. N. Wang, X. M. Yang, W. J. Zhang, H. B. Li, Z. M. Li, L. Wang and T. L. Zhang, CrystEngComm, 2019, 21, 6452–6459 RSC.
- H. E. Kissinger, Anal. Chem., 1957, 29, 17021706 CrossRef.
- T. Ozawa, Bull. Chem. Soc. Jpn., 1965, 38, 1881–1886 CrossRef CAS.
- D. Fischer, T. M. Klapötke and J. Stierstorfer, Am. Ethnol., 2014, 53, 8172–8175 CAS.
- Y. H. Joo and J. M. Shreeve, Angew. Chem., Int. Ed., 2009, 48, 564–567 CrossRef CAS PubMed.
- J. H. Zhang, Q. H. Zhang, T. T. Vo, D. A. Parrish and J. M. Shreeve, J. Am. Chem. Soc., 2015, 137, 1697–1704 CrossRef CAS PubMed.
- W. Li, K. C. Wang, X. J. Qi, Y. H. Jin and Q. H. Zhang, Cryst. Growth Des., 2018, 18, 1896–1902 CrossRef CAS.
- S. T. Chen, Y. J. Liu, Y. A. Feng, X. J. Yang and Q. H. Zhang, Chem. Commun., 2020, 56, 1493–1496 RSC.
- (a) CCDC 1939348: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2331mx; (b) CCDC 2422609: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2m9xpk.
Footnote |
| † Co-first authors, these authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2026 |