Two semiconductive haloargentates with metal-complex cations: crystal structures, band gaps, photocurrent responses and theoretical investigations
Shu-Yue
Xie
a,
Ming-Hui
Liu
a,
Ning
Wang
a,
Xi-Meng
Zhang
a,
Shen-Hao
Wang
a,
Yan
Yang
 ab,
Jun
Li
ab,
Jun
Li
 *ab and
Bo
Zhang
*ab and
Bo
Zhang
 *ab
*ab
aCollege of Chemistry and Chemical Engineering, Liaocheng University, Liaocheng 252059, China. E-mail: junli@lcu.edu.cn; bzhang@lcu.edu.cn
bState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, China
First published on 21st November 2025
Abstract
The construction of new haloargentate hybrids with semiconductor nature and photoelectric performances is very fascinating, yet remains challenging. Herein, using in situ-generated [Co(2,2-bipy)3]2+ and [Ni(2,2-bipy)3]2+ metal-complex cations as structure-directing agents, we successfully fabricated two new members of the haloargentate family, namely [NH4][Co(2,2-bipy)3]2Ag6I11 (1) and [NH4][Ni(2,2-bipy)3]2Ag6Br11 (2), respectively. These two compounds are structurally isomorphic and contain two-dimensional [Ag6X11]n5n− (X = I and Br) anionic layers, which are perforated with large hexagonal windows. The characterization of the optical properties of compounds 1 and 2 shows that they exhibit optical band gaps of 1.75 and 2.84 eV, respectively, demonstrating visible light-responsive semiconductor behavior. Under alternating light irradiation, the two as-synthesized materials possess good photoelectric conversion abilities, with their photocurrent densities (0.20 and 0.22 μA cm−2 for 1 and 2, respectively) observed to be comparable with many excellent metal halide competitors. Further, density functional theory calculations disclose that these photosensitive metal complexes play key roles in charge transfer and carrier transport, ultimately resulting in satisfactory photoelectric performances. Additionally, Hirshfeld surface analysis, thermogravimetric studies, and X-ray photoelectron spectroscopy characterization of the title compounds were also conducted in this work.
Introduction
Over the past few decades, organic–inorganic hybrid haloargentates have aroused extensive research attention, mainly benefiting from their rich structural diversities and unique physicochemical properties.1,2 In particular, these materials show versatile potential in some advanced applications, such as white-light emission, photo/thermochromism, ferroelectricity, photostrictive effect, X-ray detection, and sewage purification.3–7 Structurally, Ag+ ions have flexible coordination adaptabilities, including [AgX2] dumbbell, [AgX3] triangle, [AgX4] tetrahedron, and [AgX6] octahedron (X = I, Br, and Cl). More attractively, these fundamental building blocks are easily condensed through sharing vertex/edge/face and short-range silver-silver interactions, which enable the construction of diverse secondary building units (e.g., [Ag5X7], [Ag4X7], [Ag6X10], [Ag5X9], [Ag2X4], [Ag7X13], [Ag10X18], [Ag3X7], [Ag8X14], and [Ag6X12]).1,2 As a result, many haloargentate-based hybrids ranging from discrete clusters to infinite chains, extended two-dimensional (2D) layers, and three-dimensional (3D) frameworks have been well documented, which provide abundant structural platforms for tuning the performances and functionalities of materials.Meanwhile, the advancement of haloargentate-based hybrids is linked to the progress in synthetic methods. Among the various structure-directing agents, metal-complex cations stand out due to their stable templating effects, where their introduction may endow the as-obtained products with novel architectures and unusual physicochemical functionalities. For example, three types building units of [AgI2] dumbbell, [AgI3] triangle and [AgI4] tetrahedron coexist in the interesting compound [Ni(5,5-dmbpy)3]2Ag4.9I8.9·4H2O, while [Co(2,2-bipy)3]Ag3I6 exhibits the characteristic hole-like configuration. More intriguingly, the [Co(phen)3]Ag2I4·3DMF, [Ni(2,2-bipy)3]2Ag13Br17, and [Mn(2,2-bipy)3]Ag3I5 materials can be effectively and efficiently applied in the photocatalytic degradation of organic pollutants.8–12 Furthermore, the rich coordination patterns between silver and halogens, as well as the complex Ag⋯Ag interactions may ultimately lead to diverse compositions in the haloargentate family, which can achieve regulation of their semiconductor properties.8,13–16 Meanwhile, the introduction of photosensitive cations may further enhance or change the optoelectronic properties of the as-obtained materials. To date, the elaborative design of similar haloargentate counterparts and in-depth understanding of their structure–function relationships are highly appealing but remain a great challenge.
Herein, by employing in situ-formed [Co(2,2-bipy)3]2+ and [Ni(2,2-bipy)3]2+ metal-complexes as charge-compensating agents, we fortunately isolate two new layered haloargentates, namely [NH4][Co(2,2-bipy)3]2Ag6I11 (1) and [NH4][Ni(2,2-bipy)3]2Ag6Br11 (2). Notably, the resulting hybrids exhibit visible light-responsive optical bandgaps, manifesting their significant photoelectric switching performances when exposed to alternating light illumination. Also, Hirshfeld surface analysis, thermal stability analysis, X-ray photoelectron spectroscopy, and theoretical studies are performed on the title compounds.
Experimental section
Materials and methods
AgI (Adamas, 98%), AgBr (Aladdin, 99.5%), CoCl2·6H2O (Adamas, 98%), NiCl2·6H2O (Sigma-Aldrich, 99.9%), NH4I (Adamas, 99%), 2,2-bipy (Aladdin, 99%), CH3CN (Kermel, 99.5%), HBr (Greagent, 40 wt.%), and HI (Adamas, 55–57 wt%). All starting reagents were purchased from commercial sources and used as received without any further purification.Powder X-ray diffraction (PXRD) data were collected in the 2θ range of 5−65° at room temperature on a SmartLab diffractometer. Energy-dispersive X-ray (EDX) spectra were recorded on an FIB-SEM-GX4 scanning electron microscope (Thermo Fisher Scientific). X-ray photoelectron spectral (XPS) analyses were performed using an ESCLAB 250Xi spectrometer (Thermo Fisher Scientific). The thermogravimetric behaviors of the title compounds were examined using an NETZSCH STA449C unit. Solid optical diffuse reflection measurements of compounds 1 and 2 were performed using a Hitachi UH4150 UV/vis spectrophotometer.
Synthesis of [NH4][Co(2,2-bipy)3]2Ag6I11 (1)
AgI (2.0 mmol, 0.469 g), CoCl2·6H2O (0.5 mmol, 0.119 g), 2,2-bipy (1.5 mmol, 0.234 g), NH4I (2.0 mmol, 0.290 g), CH3CN (2.0 mL), and HI (3.0 mL) were evenly mixed and transferred into a 20 mL Teflon-lined stainless steel reactor, which was heated at 413 K for 6 days. After natural cooling, the resulting product was washed with ethanol several times, and left to dry in the air. Dark-red block-shaped crystals of 1 were obtained by hand selection with a yield of 23% based on AgI.Synthesis of [NH4][Ni(2,2-bipy)3]2Ag6Br11 (2)
Compound 2 was prepared via the reaction of AgBr (3.0 mmol, 0.563 g), NiCl2·6H2O (0.5 mmol, 0.119 g), 2,2-bipy (1.5 mmol, 0.234 g), CH3CN (4.0 mL), and HBr (1.0 mL) at 413 K for 5 days. After similar treatment as mentioned above, pale-red block-shaped crystals in about 26% yield based on AgBr were acquired by manual separation.Photocurrent measurements
Photoelectrochemical studies of the title compounds were carried out on a CHI660E electrochemistry workstation with a three-electrode system. Herein, Ag/AgCl, Pt wire, and the designed device acted as the reference electrode, auxiliary electrode, and working electrode, respectively. The working electrode was fabricated using the typical solution coating method, as described in many reports in the literature. Firstly, 5.0 mg of microcrystalline powder was added to a mixed solution containing 475 μL of ethanol and 25 μL of Nafion, followed by ultrasonic treatment. After that, the acquired slurry was carefully dropped onto an ITO substrate, and then kept in the air. During the measurements, a KCl aqueous solution (0.1 mol L−1, 50.0 mL) was utilized as the electrolyte, and an Xe lamp (PLS-SXE300UV, PerfectLight) equipped with/without a 420 nm filter was employed to mimic sunlight illumination.X-ray crystallography
Crystallographic data collection of the title compounds was completed using a Bruker SMART CCD area detector using Mo Kα radiation (λ = 0.71073 Å) at 293(2) K. Both structures were solved by direct methods and further refined by full-matrix least-squares on F2 with the help of the SHELXL-2014 program.17 All non-hydrogen atoms were processed anisotropically, while the hydrogen atoms bonded to C and N were geometrically generated. The important crystallographic parameters are listed in Table 1, and the SI including bond lengths/angles and hydrogen bond interactions are summarized in Tables S1–S4.| Compound | 1 | 2 |
|---|---|---|
| R 1 = ∑‖Fo| − |Fc‖/∑|Fo|. wR2 = [∑w(Fo2 − Fc2)2/∑w(Fo2)2]1/2. | ||
| CCDC number | 2492134 | 2492135 |
| Empirical formula | C60H52Ag6Co2I11N13 | C60H52Ag6Ni2Br11N13 |
| Formula weight | 3116.12 | 2598.79 |
| Crystal system | Trigonal | Trigonal |
| Space group |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
| a/Å | 14.4809(13) | 14.0354(11) |
| b/Å | 14.4809(13) | 14.0354(11) |
| c/Å | 31.006(3) | 30.393(2) |
| γ/° | 120 | 120 |
| V/Å3 | 5630.8(11) | 5185.1(9) |
| Z | 3 | 3 |
| λ/Å | 0.71073 | 0.71073 |
| T/K | 293(2) | 293(2) |
| ρ calcd/g cm−3 | 2.757 | 2.497 |
| μ/mm−1 | 6.531 | 8.602 |
| F(000) | 4266 | 3678 |
| Measured reflections | 8986 | 9654 |
| Independent reflections | 2205 | 2366 |
| No. of parameters | 141 | 142 |
| R int | 0.0737 | 0.0530 |
| GOF | 1.007 | 1.031 |
| R 1, wR2 [I > 2σ(I)] | 0.0450, 0.0945 | 0.0362, 0.0821 |
| R 1, wR2 [all data] | 0.0812, 0.1073 | 0.0566, 0.0876 |
Calculation details
Due to their structural isomorphism, compound 2 was selected as a representative for conducting the electronic structure analyses. The theoretical investigations involving band structure and density of states (DOS) were performed using density functional theory (DFT), as implemented in the Vienna Ab initio Simulation Package (VASP). It should be noted that VASP is a core tool for atomic-scale electronic structure calculations and quantum mechanics simulations in the fields of chemistry and physics, which is widely used to study the electronic states, magnetic properties, and dynamic behavior of materials. The generalized gradient approximation of Perdew–Burke–Ernzerhof (PBE) parameterization was employed for the exchange–correlation potential.18,19 During the calculations, Ag 4d104s1, Br 4s24p5, Ni 3d84s2, C 2s22p3, N 2s22p4, and H 1s1 were adopted as the valence electrons. In the case of compound 2, the plane wave cutoff energy was expanded into 500 eV, with a 3 × 3 × 1 Monkhorst–Pack k-point grid. The other calculation parameters and convergence criteria were the default settings of the code, calling for no additional comments.Results and discussion
Structural descriptions
The single-crystal X-ray diffraction identification indicates that compounds 1 and 2 are isostructural phases. Hence, only compound 2 is chosen as an example to depict its structure in detail. It is determined that compound 2 crystallizes in a trigonal crystal system with the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) space group, which is characterized by a 2D anionic layer of [Ag6Br11]n5n− (Fig. 1). Its asymmetric unit includes 1/6 of a formula motif, that is, one crystallographically independent Ag(1)+ ion (18f site), one Br(1)− ion (18f site), half a Br(2)− ion (9e site with −1 symmetry), 1/3 of a Br(3)− ion (6c site with 3-point symmetry), 1/6 of an [NH4]+ ion, and 1/3 of an [Ni(2,2-bipy)3]2+ metal-complex (Fig. 1a). In compound 2, the Ag atom is four-coordinated, giving a slightly distorted tetrahedral configuration. The Br(1) and Br(2) atoms serve as the bridging sites, while Br(3) is a μ3-Br atom linking three Ag atoms. As observed in many similar metal halides, the Ni2+ ion is surrounded by six N atoms from three ligands, adopting an octahedral coordination geometry. The Ag–Br bond lengths and Br–Ag–Br bond angles lie in the range of 2.5805(5)–2.8144(8) Å and 101.94(2)–124.84(2)°, respectively. The interatomic distance of Ni–N and N–Ni–N bond angle vary from 2.054(4) to 2.078(4) Å and 78.29(14)° to 171.18(15)°, respectively. These values are appropriate and rival some related analogues such as K[Ni(2,2-bipy)3]2Ag6Br11, [Ni(phen)3]2Ag13Br17·2DMSO·3H2O, K[NH4][Ag4Br6(Hmta)], [Ni(2,2-bipy)3]AgBiBr6, and [Ni(phen)3]Pb2I6·CH3CN.11,14,20–22
space group, which is characterized by a 2D anionic layer of [Ag6Br11]n5n− (Fig. 1). Its asymmetric unit includes 1/6 of a formula motif, that is, one crystallographically independent Ag(1)+ ion (18f site), one Br(1)− ion (18f site), half a Br(2)− ion (9e site with −1 symmetry), 1/3 of a Br(3)− ion (6c site with 3-point symmetry), 1/6 of an [NH4]+ ion, and 1/3 of an [Ni(2,2-bipy)3]2+ metal-complex (Fig. 1a). In compound 2, the Ag atom is four-coordinated, giving a slightly distorted tetrahedral configuration. The Br(1) and Br(2) atoms serve as the bridging sites, while Br(3) is a μ3-Br atom linking three Ag atoms. As observed in many similar metal halides, the Ni2+ ion is surrounded by six N atoms from three ligands, adopting an octahedral coordination geometry. The Ag–Br bond lengths and Br–Ag–Br bond angles lie in the range of 2.5805(5)–2.8144(8) Å and 101.94(2)–124.84(2)°, respectively. The interatomic distance of Ni–N and N–Ni–N bond angle vary from 2.054(4) to 2.078(4) Å and 78.29(14)° to 171.18(15)°, respectively. These values are appropriate and rival some related analogues such as K[Ni(2,2-bipy)3]2Ag6Br11, [Ni(phen)3]2Ag13Br17·2DMSO·3H2O, K[NH4][Ag4Br6(Hmta)], [Ni(2,2-bipy)3]AgBiBr6, and [Ni(phen)3]Pb2I6·CH3CN.11,14,20–22
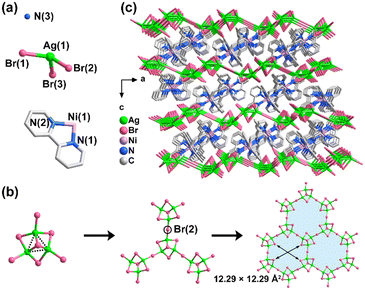 | ||
| Fig. 1 (a) The asymmetric unit of compound 2. (b) The trinuclear [Ag3Br7] moiety and the formation process of the [Ag6Br11]n5n− anionic layer. (c) Perspective view of compound 2 along the b axis. | ||
As illustrated in Fig. 1b, the secondary building unit of compound 2 is the trinuclear [Ag3Br7] motif, which is constructed by three [AgBr4] tetrahedra by sharing Br(1) and Br(3) atoms. Notably, the Ag⋯Ag separation in the [Ag3Br7] trimer reaches 3.0384(8) Å, suggesting the significant closed-shell d10–d10 argentophilic interactions.23 Then, these [Ag3Br7] units are further condensed via bridging Br(2) atoms to generate a 2D-[Ag6Br11]n5n− anionic layer (Fig. 1c). Regarding the [Ag3Br7] unit as a 3-connected node, the anionic layer of compound 2 can be classified as a {63} topology (Fig. S2). The [Ag6Br11]n5n− anionic slab perforated with large hexagonal windows is arranged parallel to the ab plane, exhibiting an ABC stacking mode along the c axis. Each window is defined by a 24-membered ring built up from six [Ag3Br7] units by vertex-sharing, with a cross section of 12.29 × 12.29 Å2 (Fig. 1b). The charge-balancing and space-compensating [Ni(2,2-bipy)3]2+ metal-complexes along with the [NH4]+ ions isolate the anionic [Ag6Br11]n5n− layers, resulting in a sandwich-like architecture (Fig. 1c). Then, a 3D supramolecular network is generated by the extensive hydrogen bonding contacts, with the C–H⋯Br and N–H⋯Br distances and angles changing from 3.5770(7)–3.702(5) Å and 114.4–159.5°, respectively (Fig. S4). Notably, the obvious C–H⋯π and π⋯π interactions are not found in compound 2. These structural features closely resemble that of the previously documented [NH4][Fe(2,2-bipy)3]2[Ag6Br11], K[Zn(2,2-bipy)3]2Ag6I11, and K[Ni(2,2-bipy)3]2Ag6Br11.11,16,24
Hirshfeld surface analysis
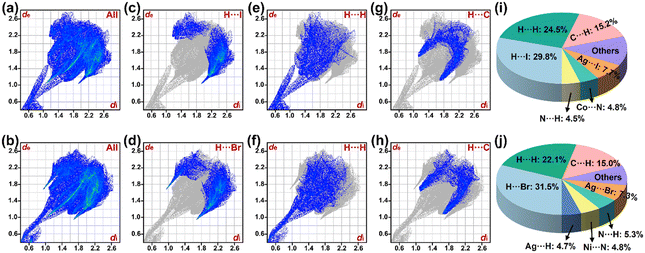
Hirshfeld surface analysis reveals the intricate intermolecular forces rooted in molecular crystals, offering a new perspective for understanding crystal structures.25 In contrast with the other techniques, the Hirshfeld surface method tends to be more intuitive and visual, quantifying the contributions from multiple intermolecular interactions.As depicted in Fig. 2a and b, the 2D fingerprint diagrams display the contact frequencies of various atom pairs in hybrid compounds 1 and 2, respectively. In these plots, the x-axis (de) and y-axis (di) represent the distances from the molecular surface to external and internal atoms, respectively. It has been found that the distributions of intermolecular interactions in title compounds are uniform and have similar characteristic shapes. In terms of their forms, the fingerprint images of both compounds appear like a guitar, which covered areas mainly scattered in the range of 2.4 Å ≤ de + di ≤ 4.8 Å. The narrower de + di ranges suggest that stronger intermolecular contacts exist in the title compounds. In both compounds, H⋯X/X⋯H (where X = I/Br) contacts like the wings of a bird dominate, occupying 29.8% and 31.5% of the total Hirshfeld surface (Fig. 2c and d), respectively. In compound 1, the percentage of the top left area is 5.4%, with the corresponding ratio of 24.4% in the bottom right. In the case of compound 2, the proportions in the top left and bottom right are 7.2% and 24.3%, respectively. A high occupying ratio manifests the key role and influence in the structural stability, which has also been witnessed in the metal halides of [Co(5,5-dmpy)3]Ag5I8, K[NH4][Ag4Br6(Hmta)], [Pb(MCP)2I]PbI3, [BPy]2[Bi2Cl8(bpym)], [TMSO]MnCl3, and [DAPEDA]InCl6·Cl·H2O.14,15,26–29 In title compounds, the second contributors are the H⋯H contacts, comprising 24.5% (1) and 22.1% (2) of the total interactions (Fig. 2e and f), respectively. Additionally, the shares of C⋯H/H⋯C contacts are also notable, which exhibit similarly and are nearly equal in proportion (15.2% for compound 1 and 15.0% for compound 2), as shown in Fig. 2g and h, respectively. It is worth noting that although the other interactions such as Ag⋯I and Ag⋯Br constitute a relatively small fraction they still cannot be ignored, highlighting the synergism in the respective molecular packing. The remaining interactions (e.g., Co⋯N, N⋯H, Ni⋯H, Ag⋯H, C⋯I, and Ni⋯N contacts) are given in Fig. S7 and S8, respectively. For convenience, the contribution percentage of the various interactions in compounds 1 and 2 is summarized in Fig. 2i and j, respectively. In summary, the Hirshfeld surface analysis clearly displays the distribution characteristics of the intermolecular forces in the two compounds, further elucidating their similarities and differences.
Physical characterization
As shown in Fig. 3a and b, good agreement is observed between the experimental PXRD patterns and the simulated ones, indicating the formation of a homogeneous phase. This also implies that the samples used for next characterization and measurements have high purity. Subsequently, we examined the thermogravimetric behaviors of the title compounds under a nitrogen atmosphere. In the case of compounds 1 and 2, it was found that obvious weight loss occurs at about 260 °C and 215 °C (Fig. 3c and d), respectively. In particular, these decomposition temperatures are impressive, which outperform or compete well with many thermally stable halide-based counterparts, including K[Ni(2,2-bipy)3]2Ag6I11, [NH4][Fe(2,2-bipy)3]2[Ag6Br11], [La(DMF)8]Bi2I9, [C13H14N]SbI4, [Hdabco]4Cu4I8, [Bubtz]2Pb3I8, [TMPDA]ZnBr4, and [DPA]3InCl6.16,24,29–34 Upon further heating, we noticed that both compounds display consecutive weight losses, finally reaching equilibrium at 800 °C. This phenomenon can be preliminarily attributed to the simultaneous evaporation and removal of both organic and inorganic components, ultimately leading to structural collapse, which has also frequently appeared in the cases of [NH4]2AgI3, [NH4][Ag5I6(Hmta)], [NH4][Co(phen)3]BiI6, [NH4][Fe(2,2-bipy) 3]2[Ag6Br11], and [NH4]2CuPbBr5.3,7,16,35,36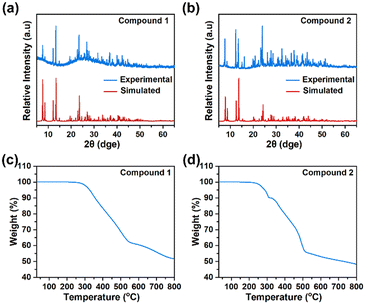 | ||
| Fig. 3 PXRD patterns of compounds 1 (a) and 2 (b). The thermogravimetric curves of compounds 1 (c) and 2 (d). | ||
EDX analyses verify the presence of the expected elements existing in the title compounds, i.e. Ag, I, Co, C, and N, for compound 1 (Fig. S9), and Ag, Br, Ni, C, and N for compound 2 (Fig. S10). These results are consistent with the X-ray crystallography studies. Their chemical compositions are further confirmed by the XPS survey results (Fig. 4a), which exhibit the distinct characteristic signals of the corresponding constituent elements. The high-resolution Ag-3d spectra of both materials are presented in Fig. 4b, which reveal characteristic peaks centered at 367.39/367.37 and 373.48/373.33 eV, corresponding to the 3d5/2 and 3d3/2 states of the Ag+ ions, respectively. In the case of halogens, the high-resolution I-3d spectrum exhibits two single peaks at 618.46 (3d5/2 orbit) and 629.90 eV (3d3/2 orbit), while the Br-3d spectrum is deconvoluted into two peaks at 67.48 (3d5/2 state) and 68.51 eV (3d3/2 state), as shown in Fig. 4c and d, respectively. Notably, these binding energy values coincide with the findings documented in the literature, such as [C12N2H14]Ag8I10, [Zn(phen)3]2[Ag2Bi2I12], [C10H17N3O]Ag3I5, K[NH4][Ag4Br6(Hmta)], [Co(2,2-bipy)3]2[AgBiBr7][Bi2Br9]·H2O, and Cu2[C24H18N4O2]Ag3Br5.13,14,37–39 The high-resolution spectra of Co-2p and Ni-2p are provided in Fig. S11 and S12, respectively, which indicate that the observed results are also normal and call for no further comment.
 | ||
| Fig. 4 (a) XPS survey spectra of compounds 1 and 2. (b) High-resolution Ag-3d peaks in compounds 1 and 2. (c) High-resolution I-3d peaks in compound 1. (d) High-resolution Br-3d peaks in compound 2. | ||
Optical properties
To evaluate the optoelectronic suitability of the as-synthesized materials, their solid-state UV-vis diffuse reflection spectra were further studied. As shown in Fig. 5a, the optical absorption edge of compound 1 is estimated to be 1.75 eV, which blue-shifts to 2.84 eV in the case of compound 2 (Fig. 5b). Tracing the source, this large discrepancy is mainly ascribed to the different halogens in their structures. It is worth noting that the band gaps of these two compounds lie in the visible light range, implying their visible light-responsive semiconductive behaviors with good light-harvesting abilities. These values correspond well with their respective crystal colors, and match well with that of some reported haloargentate analogues, such as K[Co(2,2-bipy)3]2Ag6I11 (1.75 eV), [Fe(2,2-bipy)3]AgBiBr6 (1.82 eV), [C3H9NI]4AgBiI8 (1.87 eV), [Co(2,2′-bipy)3]Ag3I6 (2.03 eV), [Zn(2,2-bipy)3]2Ag13Br17 (2.71 eV), [Co(phen)3]2Ag11I15·H2O (2.84 eV), [C4H10N]4AgBiBr8 (2.85 eV), and [Cu(phen)2Br]AgBr2 (2.87 eV).9,11,20,21,24,40–42 For convenience, a comparison of the bandgaps of some representative examples in the literature is summarized in Fig. 5c and Table S5.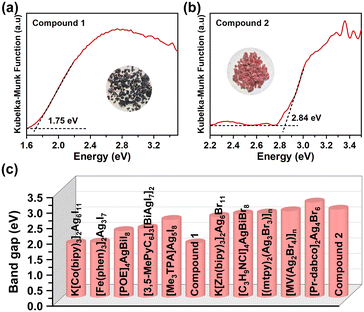 | ||
| Fig. 5 (a) The UV-vis diffuse reflection spectrum of compound 1. (b) The UV-vis diffuse reflection spectrum of compound 2. (c) The comparison of the bandgaps in this work and references. | ||
Photocurrent responses
Given their suitable optical band gaps, we further assessed the transient photocurrent responses of the title compounds, which are essential for predicting the solar conversion efficiencies in semiconductor devices. Fig. 6 presents the photocurrent-time curves of compounds 1 and 2. As can be clearly seen, both compounds demonstrate fast and distinct signal responses, maintaining good performance stabilities during the repeated light-switching cycles. As a necessary precondition for their practical application, this is unambiguously superior to most Pb-based metal halides, which routinely suffer from poor durability due to their inherent hydrolytic instability. Under periodic visible-light illumination in on/off mode, the samples exhibit similar response behaviors, achieving the photocurrent densities of approximately 0.20 and 0.22 μA cm−2 (Fig. 6a and b), respectively. These high values indicate that compounds 1 and 2 possess strong carrier transport capabilities, which exceed or are comparable with some excellent photoelectric candidates, such as [Ag2I2(phen)], [H2-4,4′-dpa]Ag6I8, [Co(5,5-dmpy)3]Ag5I8, [C6H14N]4CuBiI8·H2O, [NH4][Fe(2,2-bipy)3]2Ag6Br11, K[NH4][Ag4Br6(Hmta)], [Co(2,2-bipy)3]2Ag4Bi2Br6, [H2MPA]2AgBiBr8, [Pb2(bdc)1.5(aiz)], [Ni2(C17O2N2H16)Pb(μ-1,3-SCN)2(H2O)]2, and [(C17O2N2H16Cd)2(μ1,5-dca)2Cd].14–16,43–50 Furthermore, when the light source was switched to a full-spectrum environment, an enhanced photocurrent response was observed in the designed electrode (Fig. 6c and d), respectively. This may be ascribed to the improved light intensity, generating more photoelectron-hole pairs and resulting in significantly higher photocurrent values. Similar characteristics have also been witnessed in some well-known compounds such as [Zn(2,2-bipy)3]2Ag2BiI6(I)1.355(I3)1.645, [Pb(MCP)2I]PbI3, [Fe(phen)3]Ag2PbBr6, Cs[MCP]BiBr4, and [MCP]3Cs2Bi2I6Cl2.26,51–54 These performances underscore the considerable potential of both materials for highly efficient photosensitive applications.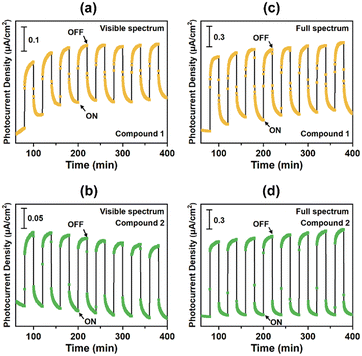 | ||
| Fig. 6 The photocurrent curves upon visible light illumination for compounds 1 (a) and 2 (b). The photocurrent curves of full-spectrum light irradiation for compounds 1 (c) and 2 (d). | ||
Theoretical studies
To gain deeper insight into the structure–activity relationships, especially the origin of the optical and optoelectronic properties, first-principles calculations based on density functional theory (DFT) were performed, including band structure, total density of states (DOS) and partial density of states (PDOS). The band structure of compound 2, as shown in Fig. 7a, reveals that its valence band maximum (VBM) and conduction band minimum (CBM) are both located at the Z points of the Brillouin zone, suggesting its direct bandgap semiconductor characteristic. It has been found that the band edges of compound 2 have a relatively flat distribution, indicating the strong electronic localizations and negligible coulomb interactions between the adjacent moieties in compound 2. This is normal and widely appears in the case of hybrid halides decorated by large organic templates. Further analyses disclosed that its theoretical band gap is estimated to be 1.85 eV, which is smaller than the experimental result obtained from the UV-vis diffuse reflection measurement. This discrepancy primarily arises from the inherent approximations in the DFT method when treating the exchange-correlation energy, a common systematic error in theoretical calculations.18,19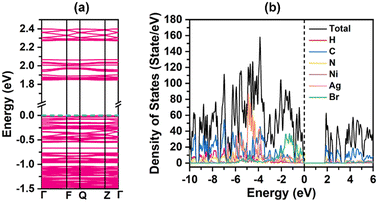 | ||
| Fig. 7 (a) The band structure diagram of compound 2. (b) The DOS and atom-resolved PDOS plots of compound 2. | ||
The DOS and PDOS plots are further shown in Fig. 7b and Fig. S14, respectively, which reflect the atomic contributions to the total electronic states. The CBM of compound 2 is predominantly composed of C-2p, N-2p, and Ni-3d states, with negligible contribution from the Br-4p orbital. Its VBM primarily originates from the Br-4p, N-2p, and Ni-3d states, with minor contributions from the C-2p and Ag-3d states. The regions in the range of −4 to −5 eV mostly stem from the Ag-4d orbital. The regions far from the Fermi level such as −6 to −10 eV are from the C-2p and N-2p states, while the regions in the range of −12 to −18 eV are dominated by the C-2s and Br-4s states. Consequently, the optical adsorption of compound 2 can be primarily attributed to the collaborative combination of photosensitive metal complexes and electron-rich inorganic units, especially the former. In other words, the optically active [Ni(2,2-bipy)3]2+ cations may play the key role in the charge transfer and carrier transport, which may be the main reason why it exhibits a satisfactory photoelectric performance. This resembles the cases of some documented metal halide counterparts, such as K[Co(bipy)3]2Ag6I11, [Co(5,5-dmpy)3]Ag5I8, [Fe(bipy)3]AgBiBr6, [NH4][Fe(bipy)3]2Ag6Br11, [Zn(bipy)3]2Ag13Br17, and [Co(bipy)3]2Ag4Bi2Br6.11,15,16,21,24,46
Conclusion
In summary, two new metal-complexes decorated by haloargentate hybrids characteristic of [Ag6X11]n5n− layers and abundant weak interactions were solvothermally obtained and structurally characterized. Attractively, the two materials feature visible light-responsive optical band gaps (1.75 eV for 1 and 2.84 eV for 2), resulting in good photoelectric conversion performances (0.20 μA cm−2 for 1 and 0.22 μA cm−2 for 2) that rival many promising photovoltaic candidates. This work may provide valuable insight for the development of new semiconductors with desirable optical properties. Our group is conducting further relevant exploration into the synthesis and gaining deeper insights into their structure–activity relationships.Author contributions
Shu-Yue Xie: investigation, data curation, writing - original draft. Ming-Hui Liu: investigation, formal analysis. Ning Wang: investigation. Xi-Meng Zhang: investigation. Shen-Hao Wang: investigation. Yan Yang: conceptualization. Jun Li: conceptualization, software, writing, review & editing, funding acquisition. Bo Zhang: methodology, writing, review & editing, supervision. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data presented in this study are available from the corresponding author on request. Supplementary information (SI): Additional crystallographic data, more structural representations, Hirshfeld surface analyses, physical characterizations, and DFT results. See DOI: https://doi.org/10.1039/d5ce00975h.CCDC 2492134 and 2492135 contain the supplementary crystallographic data for this paper.55a,b
Acknowledgements
Financial support provided by the Nature Science Foundation of Shandong Province (ZR2025MS204) is greatly acknowledged.Notes and references
- T. L. Yu, Y. M. Guo, G. X. Wu, X. F. Yang, M. Xue, Y. L. Fu and M. S. Wang, Coord. Chem. Rev., 2019, 397, 91–111 CrossRef CAS
.
- X. X. Li and S. T. Zheng, Coord. Chem. Rev., 2021, 430, 213663 CrossRef CAS
.
- T. D. Creason, H. Fattal, I. W. Gilley, T. M. McWhorter, M. H. Du and B. Saparov, ACS Mater. Au, 2021, 1, 62–68 CrossRef CAS PubMed
.
- P. F. Hao, W. P. Wang, L. F. Zhang, J. J. Shen and Y. L. Fu, Inorg. Chem. Front., 2019, 6, 287–292 RSC
.
- C. F. Wang, H. J. Li, M. G. Li, Y. Cui, X. Song, Q. W. Wang, J. Y. Jiang, M. M. Hua, Q. Xu, K. Zhao, H. Y. Ye and Y. Zhang, Adv. Funct. Mater., 2021, 31, 2009457 CrossRef CAS
.
- C. F. Wang, H. J. Li, Q. Ji, C. Ma, L. Liu, H. Y. Ye, B. Cao, G. L. Yuan, H. F. Lu, D. W. Fu, M. G. Ju, J. L. Wang, K. Zhao and Y. Zhang, Adv. Funct. Mater., 2022, 32, 2205918 CrossRef CAS
.
- Z. Z. Xue, X. D. Meng, X. Y. Li, J. Pan and G. M. Wang, Cryst. Growth Des., 2021, 21, 1055–1061 CrossRef CAS
.
- B. Zhang, W. A. Li, J. Li, Y. P. Xu, Y. R. Xu, W. H. Wang and G. D. Zou, Inorg. Chem. Commun., 2020, 121, 108219 CrossRef CAS
.
- C. Y. Tang, Y. W. Sun, J. B. Liu, Q. F. Xu and C. Y. Zhang, Dalton Trans., 2022, 51, 16784–16789 RSC
.
- C. Y. Tang, J. Yao, Y. Y. Li, Z. R. Xia, J. B. Liu and C. Y. Zhang, Inorg. Chem., 2020, 59, 13962–13971 CrossRef CAS PubMed
.
- C. Y. Yue, X. W. Lei, Y. F. Han, X. X. Lu, Y. W. Tian, J. Xu, X. F. Liu and X. Xu, Inorg. Chem., 2016, 55, 12193–12203 CrossRef CAS PubMed
.
- X. W. Lei, C. Y. Yue, J. Q. Zhao, Y. F. Han, Z. R. Ba, C. Wang, X. Y. Liu, Y. P. Gong and X. Y. Liu, Eur. J. Inorg. Chem., 2015, 2015, 4412–4419 CrossRef CAS
.
- Y. M. Tian, T. H. Ren, H. J. Zhu and D. X. Jia, CrystEngComm, 2024, 26, 3911–3919 RSC
.
- M. H. Liu, W. Y. Wen, H. Y. Shen, Y. Yang, J. Li and B. Zhang, Dalton Trans., 2024, 53, 2991–2997 RSC
.
- M. H. Liu, X. C. Ren, W. Y. Wen, B. H. Li, J. Q. Li, J. Li and B. Zhang, Molecules, 2023, 28, 6116 CrossRef CAS PubMed
.
- B. Zhang, J. Li, X. Chen, M. F. Yang, H. Y. Shen and J. C. Zhu, Inorg. Chem. Commun., 2022, 137, 109250 CrossRef CAS
.
- G. M. Sheldrick, Acta Crystallogr., Sect. A:Found. Adv., 2015, 71, 3–8 CrossRef
.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 17, 3865–3868 CrossRef PubMed
.
- G. Kresse and J. Furthmuller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS
.
- Y. L. Shen, L. M. Zhang, S. F. Li, P. P. Sun, W. Q. Jiang and D. X. Jia, Eur. J. Inorg. Chem., 2018, 2018, 826–834 CrossRef CAS
.
- B. Zhang, J. Li, M. Pang, Y. S. Wang, M. Z. Liu and H. M. Zhao, Inorg. Chem., 2022, 61, 406–413 CrossRef CAS PubMed
.
- B. Zhang, H. Y. Sun, J. Li, Y. R. Xu, Y. P. Xu, X. Yang and G. D. Zou, Dalton Trans., 2020, 49, 1803–1810 RSC
.
- M. Jansen, Angew. Chem., Int. Ed. Engl., 1987, 26, 1098–1110 CrossRef
.
- X. W. Lei, C. Y. Yue, J. Q. Zhao, Y. F. Han, J. T. Yang, R. R. Meng, C. S. Gao, H. Ding, C. Y. Wang, W. D. Chen and M. C. Hong, Inorg. Chem., 2015, 54, 10593–10603 CrossRef CAS PubMed
.
- A. V. Parwani, CrystEngComm, 2009, 2009, 93–95 Search PubMed
.
- B. Zhang, J. Li, L. Z. Li, X. C. Ren, M. Pang and Y. N. Shao, Cryst. Growth Des., 2021, 21, 5317–5324 CrossRef CAS
.
- J. C. Jin, N. N. Shen, Y. P. Lin, L. K. Gong, H. Y. Tong, K. Z. Du and X. Y. Huang, Inorg. Chem., 2020, 59, 13465–13472 CrossRef CAS PubMed
.
- X. W. Kong, L. X. Wu, X. Yang, D. Y. Wang, S. X. Wang, S. Y. Li, C. Y. Yue, F. Yu and X. W. Lei, Adv. Opt. Mater., 2024, 12, 2302710 CrossRef CAS
.
- C. Sun, J. P. Zang, Y. Q. Liu, Q. Q. Zhong, X. X. Xing, J. P. Li, C. Y. Yue and X. W. Lei, CCS Chem., 2021, 3, 3341–3356 Search PubMed
.
- J. H. Zhang, X. Yang, T. H. Ren and D. X. Jia, Dalton Trans., 2023, 52, 6804–6812 RSC
.
- G. N. Liu, R. Y. Zhao, R. D. Xu, C. C. Li and G. C. Guo, CCS Chem., 2022, 4, 1273–1283 CrossRef CAS
.
- K. Zhu, O. Reid, S. Rangan, L. Wang, J. B. Li, K. A. J. Durai, K. Zhou, N. Javed, L. Kasaei, C. Q. Yang, M. X. Li, Y. Sun, K. Tan, M. Cotlet, Y. Liu, L. C. Feldman, D. M. O'Carroll, K. Zhu and J. Li, Nature, 2025, 644, E36 CrossRef CAS PubMed
.
- X. N. Tang, C. C. Gao, J. S. Guo, M. Y. Yuan, S. H. Fan, C. C. Li and G. N. Liu, Cryst. Growth Des., 2023, 23, 7982–7991 CrossRef CAS
.
- Y. Y. Ma, Y. M. Sun, W. J. Xu, X. L. Liu, Q. Q. Zhong, Y. R. Song, H. Q. Fu, C. Y. Yue and X. W. Lei, Adv. Opt. Mater., 2022, 10, 2200386 CrossRef CAS
.
- X. C. Ren, J. Li, W. H. Wang, X. Chen, B. Zhang and L. Z. Li, Inorg. Chem. Commun., 2021, 130, 108714 CrossRef CAS
.
- C. Sun, Y. H. Guo, S. S. Han, J. Z. Li, K. Jiang, L. F. Dong, Q. L. Liu, C. Y. Yue and X. W. Lei, Angew. Chem., 2020, 59, 16465–16469 CrossRef CAS PubMed
.
- C. Y. Yue, B. Hu, X. W. Lei, R. Q. Li, F. Q. Mi, H. Gao, Y. Li, F. Wu, C. L. Wang and N. Lin, Inorg. Chem., 2017, 56, 10962–10970 CrossRef CAS PubMed
.
- B. Zhang, Y. N. Wang, J. Li, X. C. Ren, Y. Yang and X. R. Yang, Cryst. Growth Des., 2022, 22, 7434–7442 CrossRef CAS
.
- J. J. Shen, F. Wang, X. X. Li, T. L. Yu, P. F. Hao and Y. L. Fu, RSC Adv., 2016, 6, 98916–98920 RSC
.
- Y. P. Yao, B. Kou, Y. Peng, Z. Y. Wu, L. N. Li, S. S. Wang, X. Y. Zhang, X. T. Liu and J. H. Luo, Chem. Commun., 2020, 56, 3206–3209 RSC
.
- T. L. Yu, Y. B. Fu, Y. L. Wang, P. F. Hao, J. J. Shen and Y. L. Fu, CrystEngComm, 2015, 17, 8752–8761 RSC
.
- B. A. Connor, L. Leppert, M. D. Smith, J. B. Neaton and H. I. Karunadasa, J. Am. Chem. Soc., 2018, 140, 5235–5240 CrossRef CAS PubMed
.
- L. Huang and J. Zhou, Inorg. Chem., 2020, 59, 16814–16818 CrossRef CAS PubMed
.
- J. Li, S. Y. Xie, M. Pang, J. C. Zhu, J. T. Wu, Y. D. Zhang and B. Zhang, Molecules, 2023, 28, 8033 CrossRef CAS PubMed
.
- L. Y. Bi, T. L. Hu, M. Q. Li, B. K. Ling, M. S. Lassoued, Y. Q. Hu, Z. X. Wu, G. J. Zhou and Y. Z. Zheng, J. Mater. Chem. A, 2020, 8, 7288–7296 RSC
.
- B. Zhang, J. Li, M. Pang, X. Chen and M. Z. Liu, Inorg. Chem., 2022, 61, 9808–9815 CrossRef CAS PubMed
.
- M. S. Lassoued, T. B. Wang, Q. W. Li, X. Y. Liu, W. P. Chen, B. Jiao, Q. Y. Yang, Z. X. Wu, G. J. Zhou, S. J. Ding, Z. C. Zhang and Y. Z. Zheng, Mater. Chem. Front., 2022, 6, 2135–2142 RSC
.
- S. Dey, S. Sil, B. Dutta, K. Naskar, S. Maity, P. P. Ray and C. Sinha, ACS Omega, 2019, 4, 19959–19968 CrossRef CAS PubMed
.
- D. Majumdar, A. Dey, S. Roy, D. Sutradhar and S. Hazra, Inorg. Chem. Commun., 2024, 162, 112155 CrossRef CAS
.
- D. Majumdar, S. Roy, A. Dey and D. Sutradhar, J. Mol. Struct., 2023, 1294, 136438 CrossRef CAS
.
- B. Zhang, J. Li, Y. Yang, W. H. Wang, H. Y. Shen and Y. N. Shao, Dalton Trans., 2022, 51, 13361–13367 RSC
.
- X. C. Ren, J. Li, W. H. Wang, Y. N. Shao, B. Zhang and L. Z. Li, J. Solid State Chem., 2022, 308, 122912 CrossRef CAS
.
- S. Y. Xie, J. Q. Li, J. J. Dong, X. M. Zhang, Y. Yang, J. Li and B. Zhang, Inorg. Chem., 2025, 64, 15280–15291 CrossRef CAS PubMed
.
- J. Li, S. Y. Xie, J. J. Dong, H. H. Zhao, Y. Yang, Y. Wu and B. Zhang, Inorg. Chem., 2025, 64, 9790–9797 CrossRef CAS PubMed
.
-
(a)
CCDC 2492134: Experimental Crystal Structure Determination, 2025, DOI:10.25505/fiz.icsd.cc2pn8f2
; (b) CCDC 2492135: Experimental Crystal Structure Determination, 2025, DOI:10.25505/fiz.icsd.cc2pn8g3
.
| This journal is © The Royal Society of Chemistry 2026 |