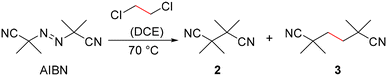Supramolecular assemblies involving triaryltelluronium cations: combining chalcogen bonding, hydrogen bonding and lone pair–π interactions†
Loic
Groslambert
a,
Andres
Padilla Hernandez
a,
Avantika
Hasija
bc,
Emmanuel
Aubert
b,
Patrick
Pale
 a and
Victor
Mamane
a and
Victor
Mamane
 *a
*a
aUMR 7177, Université de Strasbourg and CNRS, 1 Rue Blaise Pascal, 67000 Strasbourg, France. E-mail: vmamane@unistra.fr
bUniversité de Lorraine, CNRS, CRM2, F-54000 Nancy, France
cDepartment of Chemistry, The University of Manchester, Manchester, England, UK
First published on 25th November 2025
Abstract
In this manuscript, we report the synthesis and X-ray characterization of a series of solvates and co-crystals involving triaryltelluronium salts and different Lewis bases. In some cases, the triaryltelluronium cation is able to interact with acceptor atoms simultaneously through its three σ-holes and through a “pocket” formed by the three electron-deficient aromatic rings linked to tellurium. The latter interaction involves an acceptor atom that shows close contacts with both the ortho-hydrogens of two aromatic rings and the electron-deficient π-system of the third ring. Interestingly, the co-crystal formed between the triaryltelluronium cation and ditopic nitrile ligands provides three-dimentional supramolecular networks that combine Te⋯N chalcogen bonds, CAr–H⋯N hydrogen bonds and N lone pair⋯π interactions.
Introduction
Despite being much less studied compared to their lighter congeners based on sulphur and selenium, organotellurium compounds have attracted strong interest in various fields,1,2 including medicinal chemistry,3 organic synthesis,4,5 redox catalysis,6–8 and coordination chemistry.9 Recently, new applications based on chalcogen bonding (ChB) have emerged, such as catalysis,10–12 crystal engineering,13–15 and supramolecular chemistry.16–18 Analogous to halogen bonding (XB), ChB is a noncovalent interaction occurring between “electrophilic” chalcogen atoms and electron-rich sites such as lone pairs, anions or π-electrons.19 This “electrophilicity” results from the anisotropy of the electronic distribution on the chalcogen atom and is materialized on its electrostatic electron surface (ESP) map by regions of lower electron density called σ-holes. These regions are located along the extension of the σ bonds formed by the chalcogen atom and therefore coincide with empty σ* orbitals (Fig. 1A).20 Consequently, electrostatics, charge transfer, and a non-negligible contribution of dispersion forces all play a role in these interactions.21 Since tellurium is the least electronegative and the most polarizable atom in the chalcogen series, tellurium species exhibit the strongest ChB compared to their sulphur and selenium analogues.22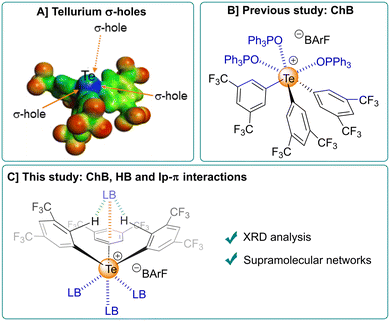 | ||
| Fig. 1 A] ESP map of telluronium cation 1+ showing the location of σ-holes (drawn with an isosurface cutoff for the electron density of 0.05 a0−3); reproduced with permission from ref. 38. Copyright 2025, American Chemical Society; B] structure of the tris-adduct between triaryltelluronium 1BArF and OPPh3; C] supramolecular association of 1BArF with Lewis bases (LB) through the combination of ChBs (blue hashed bonds) HBs (green hashed bonds) and lp–π interaction (red hashed bond). | ||
Being involved in recent years in the synthesis and study of the ChB properties of various organotellurium derivatives,23–25 our group has developed tetravalent telluronium salts R3Te+,X− as powerful ChB catalysts.26–28 The good catalytic performance of these telluronium salts and their hexavalent analogues R5Te+,X− was further demonstrated by other groups.29–36 Interestingly, telluroniums R3Te+,X− and particularly those based on the BArF anion (BArF: tetrakis[3,5-bis(trifluoromethyl)phenyl]borate) were shown to form up to three ChBs with Lewis bases in solution and in the solid state.37,38 Thus, the three possible ChB adducts involving telluronium 1BArF and triphenylphosphine oxide (OPPh3), namely [1·(OPPh3)n]BArF (n = 1, 2 and 3), were isolated and characterized by X-ray diffraction (XRD) (see Fig. 1B for the tris-adduct).38
Herein, the noncovalent interaction ability of 1BArF with Lewis bases was extended beyond ChB. Indeed, the three aryl groups of 1BArF were shown to cooperate through a combination of two hydrogen bonds (HB) and one lone pair (lp)–π interaction to host a fourth Lewis base, opening the possibility of building new supramolecular networks (Fig. 1C). Unlike HB, lp–π interactions between nonbonding electron pair and electron-deficient aromatic π-systems was much less explored in supramolecular chemistry.39 However, their involvement in biological contexts has been recognized since 1995.40–42 Today, the nature of lp–π interactions is better understood,43 and their importance has been highlighted in other fields such as organic synthesis.44 In the present work, the lp–π interaction was evidenced in several adducts of telluronium 1BArF with different Lewis bases in the solid state through X-ray diffraction (XRD) analysis. Building on this, and on the ability of 1BArF to strongly bind Lewis bases through ChB, we report here the first supramolecular networks that combine ChBs, HBs and lp–π interactions.
Experimental
Materials and methods
Telluronium salts 1BArF and 1OTf were prepared as previously described.27,38Crystal growth
The crystallizations were performed under argon and by using anhydrous solvents.Crystals of [{1⊃DMSO}·(DMSO)3]BArF (polymorph I) were obtained by slow diffusion of pentane into a solution of 1BArF (10 mg) in DMSO (2 mL) at 0 °C.
Crystals of [{1⊃DMSO}·(DMSO)3]BArF (polymorph II) were obtained by dissolving 1BArF in minimal amount of DMSO at 50 °C followed by evaporation at room temperature.
Crystals of [{1⊃THF}·(THF)3]BArF were obtained by slow diffusion of heptane into a solution of 1BArF (10 mg) in THF (2 mL) at −30 °C.
Crystals of [{1⊃acetone}·(acetone)]OTf were obtained by slow diffusion of heptane into a solution of 1OTf (10 mg) in DCE/acetone 1/1 (2 mL) at −30 °C.
Crystals of [{1⊃(DCE)1/2}·(NMO)3]BArF were obtained by slow diffusion of heptane into a solution of 1BArF (10 mg) and N-methylmorpholine N-oxide (NMO) (15 mg) in DCE (2 mL) at −30 °C.
Crystals of [(1BArF)(Mal)(DCE)] were obtained by slow diffusion of heptane into a solution of 1BArF (10 mg) and malononitrile (Mal) (5 mg) in DCE (2 mL) at −30 °C.
Crystals of [(1BArF)(Suc)(DCE)] were obtained by slow diffusion of heptane into a solution of 1BArF (10 mg) and succinonitrile (Suc) (6 mg) in DCE (2 mL) at −30 °C.
Crystals of [(1BArF)(Fum)2(DCE)2] were obtained by slow diffusion of heptane into a solution of 1BArF (10 mg) and fumaronitrile (Fum) (6 mg) in DCE (2 mL) at −30 °C.
Crystals of [1·(AIBN)]BArF were obtained by slow diffusion of heptane into a solution of 1BArF (10 mg) and AIBN (12 mg) in DCE (2 mL) at −30 °C.
[(1BArF)(2)1/2(3)(DCE)]: in a tube equipped with a screw cap were introduced 1BArF (41 mg), AIBN (30 mg) and freshly distilled DCE (3 mL). The mixture was heated under reflux for 30 min. After cooling to room temperature, the tube was placed in a freezer at −30 °C until formation of crystals.
Single crystal X-ray diffraction
The crystals were placed in oil, and a single crystal was selected, mounted on a glass fibre and placed in a low-temperature N2 stream.X-ray diffraction data collection was carried out on a Bruker APEX II DUO Kappa-CCD diffractometer equipped with an Oxford Cryosystem liquid N2 device, using Mo-Kα radiation (λ = 0.71073 Å). The crystal-detector distance was 38 mm. The cell parameters were determined (APEX3 software)45 from reflections taken from 3 set of 6 frames at 10 s exposure. The structure was solved using the program SHELXT-2018.46 The refinement and all further calculations were carried out using SHELXL-2019.47 The H-atoms were included in calculated positions and treated as riding atoms using SHELXL default parameters. The non-H atoms were refined anisotropically, using weighted full-matrix least-squares on F2. A semi-empirical absorption correction was applied using SADABS in APEX3.48
Results and discussion,
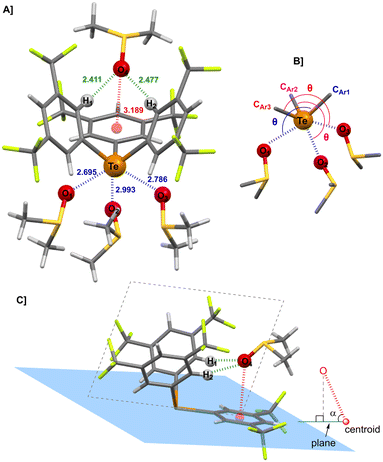
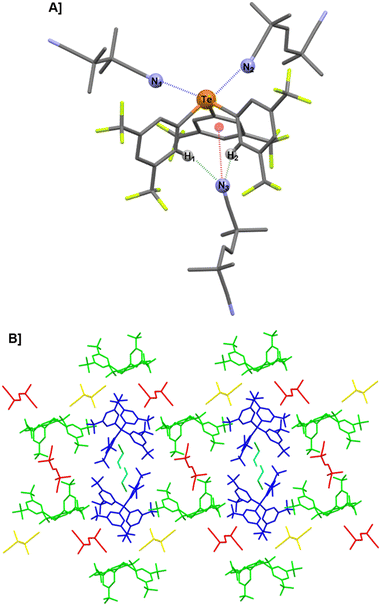
Telluronium 1BArF was recently shown to readily form co-crystals with OPPh3.38 In particular, the tris-adduct [1·(OPPh3)3]BArF (Fig. 1B) was obtained when an excess of Lewis base (more than six equivalents), was used. While triethylphosphine oxide (OPEt3) behaved similarly, a different situation occurred with DMSO. Indeed, the tris-adduct [1·(DMSO)3]BArF could be obtained only by strictly limiting the amount of DMSO to three equivalents relative to 1BArF. Using more than three equivalents of DMSO invariably resulted in the incorporation of a fourth DMSO molecule in the solvate, yielding a tetra-adduct.X-ray diffraction (XRD) analysis of the tetra-adduct between 1BArF and DMSO
Among the several co-crystallisation tests between 1BArF and excess DMSO, two crystal polymorphs, I and II, were obtained and analysed by XRD (see SI. For details, Tables S1 and S2, Fig. S1 and S2). Both crystal structures, with the general formula [{1⊃DMSO}·(DMSO)3]BArF, showed telluronium cation 1+ weakly coordinated to the BArF anion and surrounded by four molecules of DMSO. Three DMSO molecules directly interacted with the Te σ-holes whereas the fourth DMSO molecule was located in the “pocket” formed by the three electron-deficient aromatic groups of 1+.‡ Although belonging to different space groups, the structures of the two polymorphs exhibited similar geometrical parameters regarding the interactions of DMSO molecules with 1+. Therefore, only polymorph I, of space group P1 is presented here (Fig. 2A), while the data for polymorph II, in space group P21/n can be found in the SI (Fig. S3 and Table S3).The structure of the tetra-adduct [{1⊃DMSO}·(DMSO)3]BArF (polymorph I) is composed of two parts: one centred on the tellurium atom (Fig. 2B) and the other located within the “pocket” formed by the three aromatic groups (Fig. 2C). As revealed by the interatomic distances (d) below the sum of the van der Waals radii, and by the angles (θ) (Table 1),§ three Te⋯O ChBs are observed, each positioned opposite to a CAr–Te bond and nearly aligned with the Te σ-holes of telluronium 1+ (Fig. 1A). The reduction ratio (RR), defined as d(Te⋯O)/(rvdw(Te) + rvdw(O)), well below 1, and the θ values in the range 169–172° indicate strong ChBs between DMSO and 1+ in the solid state. The inclusion of the fourth DMSO molecule inside “pocket” of the telluronium cation 1+ results from the combination of two HBs and one lp–π interaction involving the O atom of DMSO and the three aromatic rings. This arrangement likely arises from the symmetric conformation of 1+, in which two aromatic groups lie on each side of the plane orthogonal (dashed plane, Fig. 2C) to the plane defined by Te and the third aromatic ring (Te–Ar blue plane, Fig. 2C). Indeed, the DMSO molecule is positioned within this orthogonal plane, nearly equidistant between the two aromatic groups, as shown by the similar distances and angles for O⋯H1 and O⋯H2 HBs.
| Interaction | d (Å) | θ (°) | α (°) | RR |
|---|---|---|---|---|
| a van der Waals radii (Å): r(vdw)Te = 2.06; r(vdw)O = 1.52; r(vdw)H = 1.20; r(vdw)C = 1.70.49 | ||||
| Te⋯O1 | 2.69(3) | 170.73 | — | 0.75 |
| Te⋯O2 | 2.99(1) | 169.10 | — | 0.84 |
| Te⋯O3 | 2.79(2) | 172.19 | — | 0.78 |
| O4⋯H1 | 2.41 | 154.21 | — | 0.88 |
| O4⋯H2 | 2.48 | 162.02 | — | 0.91 |
| O4⋯centroid | 3.19 | — | 84.26 | 0.99 (ref. 39) |
| O4⋯plane | 3.17 | — | — | — |
Although these HBs are relatively weak, the overall interaction between DMSO and the “pocket” of 1+ is reinforced by a strong lp–π interaction between O lone pair and the aromatic ring lying in the Te–Ar plane, as reflected by the angle α close to 90° and the lp–π distance below the sum of van der Waals radii of oxygen and carbon.39
Other tetra-adducts involving 1BArF and different Lewis bases
Based on the geometrical requirements for the interaction of LBs with the “pocket” formed by the three aromatic groups of telluronium cation 1+, additional structures containing such interaction were identified. In particular, small solvent molecule such as tetrahydrofuran (THF), acetone, dichloromethane (DCM) and 1,2-dichloroethane (DCE) could be incorporated into the Ar3Te+ “pocket”. The XRD-derived structures showed either the oxygen or the chlorine atom interacting through a combination of two HBs and one lp–π interaction, with RR values close to 1 (see SI, Table S7 and details for structures [{1⊃THF}·(THF)3]BArF, [{1⊃DCM}·(DMSO)3]BArF and [{1⊃acetone}·(acetone)]OTf).Interestingly, the ChB and pocket ligands could be varied, as shown by the complex [{1⊃(DCE)1/2}·(NMO)3]BArF (see Fig. S9 and S10 and Table S7). Furthermore, in this structure, the DCE ligand acts as a bridge between two Ar3Te+ units. These results clearly demonstrate the possibility of designing supramolecular networks.
Supramolecular networks
Since dinitriles have been identified as supramolecular synthons,50 several co-crystallizations with dinitrile derivatives were attempted with the aim of obtaining new supramolecular assemblies. Both malononitrile and succinonitrile afforded the same dimeric organization, involving two cations 1+ and two bridging dinitrile molecules interacting through Te⋯N ChB. The third Te σ-hole is occupied by one DCE chloride (see SI for details & Fig. S11–S14). Pleasingly, the more rigid fumaronitrile (Fum) yielded the supramolecular network [(1BArF)(Fum)2(DCE)2] combining both ChB and interaction through the “pocket”. In this structure, each telluronium cation 1+ is surrounded by four Fum molecules: three of them interact with the Te σ-holes through ChB, and the fourth interacts via the “pocket” formed by the three aromatic groups (Fig. 3A and S16), giving rise to a bilayer organization (Fig. 3B). Within each layer, the telluronium cation 1+ (in green) interacts with Fum (in blue) alternatively through ChB and through the “pocket” in a head-to-tail arrangement. The two layers are then linked by ChBs between 1+ and two Fum molecules (in pink) in a head-to-head arrangement to provide the bilayer structure. The different bilayers interact with one another and with the BArF anions through very weak F⋯F interactions, forming a more compact 3D assembly. Interestingly, the BArF anions are organized into chains alternating one anion and one DCE molecule, which interact through H⋯F HBs (Fig. S17).Since the absence of a network with malononitrile and succinonitrile could be due to their short chain length and/or flexibility, co-crystallization experiments between 1BArF and longer dinitriles were carried out. With radical reactivity in mind, the longer but still rigid azobisisobutyronitrile (AIBN) was selected.
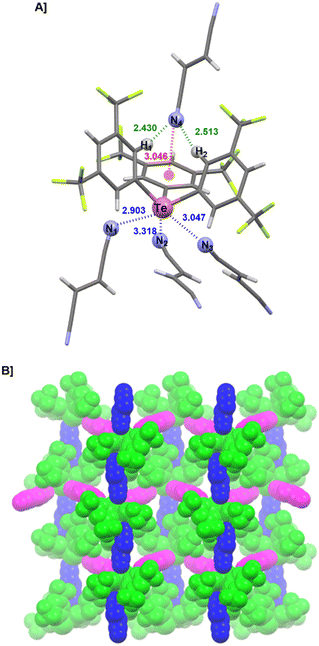
Rewardingly, when a DCE solution of 1BArF and AIBN (approx. 10 equiv.) was diffused in heptane at −30 °C, crystals corresponding to the formula [1·(AIBN)]BArF were obtained. Their XRD-derived structure exhibits a 1D organization of 1+ and AIBN molecules (Fig. 4). In this structure, AIBN acts as a ditopic ligand bridging two telluronium 1+via ChB, leading to a zig-zag chain. The third σ-hole is occupied by a fluorine atom from the BArF anion, while no interaction through the “pocket” is observed in this case.
With the aim of trapping the radical formed by the decomposition of AIBN, the solution of 1BArF and AIBN in DCE was heated at 70 °C for 30 min and then left at −30 °C until crystals formed. Surprisingly, the resulting crystal structure contained a mixture of 1BArF and two different dinitrile products derived from AIBN. Both originate from reactions of the expected isobutyronitrile radical: one is the recombination product, 2,2,3,3-tetramethylsuccinonitrile 2, while the other clearly results from double substitution of DCE chlorine atoms by the isobutyronitrile group to give 2,2,5,5-tetramethylhexanedinitrile 3 (Scheme 1).
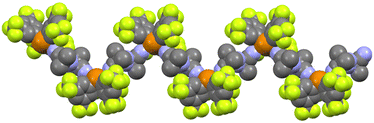
In this structure, with the general formula [(1BArF)(2)1/2(3)(DCE)], each telluronium cation interacts simultaneously through its “pocket” with dinitrile 3 and through ChB with both dinitriles 2 and 3 (Fig. 5A). Repetition of this motif along the two directions defined by these interactions generates a 2D structure. First, each telluronium cation 1+ (green) interacts with both dinitriles 2 (yellow) and 3 (red) via ChB to form a 1D zigzag chain. These chains are then interconnected through the interaction of dinitrile 3 with the “pocket” of two different telluronium cation 1+ in a tail-to-tail arrangement, producing a 2D layer. Within this layer (Fig. 5B), the telluronium cations are arranged in hexagons whose central cavities are occupied by BArF anions (blue) and DCE molecules (cyan), with one chlorine atom engaging in a weak interaction with the third Te σ-hole (RR = 1.01). The different layers further interact through multiple H⋯F and F⋯F contacts to generate a 3D network (not shown).
Conclusions
In this study, we show that triaryltelluronium cation 1+ is not only a strong ChB donor through the tellurium σ-holes, but is also able to concomitantly interact with different Lewis bases through HBs and lp–π interactions involving the three electron-deficient aromatic groups linked to tellurium. This dual behaviour of 1+ towards Lewis bases has, for the first time, allowed the isolation and characterization of supramolecular structures combining ChBs, HBs and lp–π interactions.The preliminary results with dinitriles described here reveal that chain length, as well as size and flexibility, appear to play key roles in determining the formation of supramolecular networks involving ChB and the “pocket” interactions. To better understand the factors governing such supramolecular assemblies, co-crystallizations of polytopic ligands with functional groups other than nitrile, along with other triaryltelluronium salts, are currently under investigation in our laboratory with the aim of obtaining new supramolecular networks.
Author contributions
L. G., H. A.: data curation, investigation, validation and visualization; A. P. H.: data curation and investigation; E. A.: data curation, investigation, validation, visualization, funding acquisition, writing (review and editing); P. P.: validation, visualization, writing (review and editing); V. M.: conceptualization, data curation, investigation, validation, visualization, funding acquisition, writing (original draft preparation), writing (review and editing), and project administration.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: SI contains XRD data and additional figures. See DOI: https://doi.org/10.1039/d5ce00866b.CCDC 2482975 ([{1⊃acetone}·(acetone)]OTf), 2482976 ([(1BArF)(Suc)(DCE)]), 2482977 ([{1⊃DMSO}·(DMSO)3]BArF (polymorph I)), 2482978 ([{1⊃DMSO}·(DMSO)3]BArF (polymorph II), 2482979 ([1·(AIBN)]BArF), 2482980 ([(1BArF)(2)1/2(3)(DCE)]), 2482981 ([(1BArF)(Fum)2(DCE)2]), 2482982 ([{1⊃ (DCE)1/2}·(NMO)3]BArF), 2482983 ([(1BArF)(Mal)(DCE)]) and 2482984 ([{1⊃THF}·(THF)3]BArF) contain the supplementary crystallographic data for this paper.51a–j
Acknowledgements
The authors gratefully thank the University of Strasbourg and the Centre National de la Recherche Scientifique (CNRS) for their financial support. The Agence Nationale de la Recherche (ANR) is acknowledged for funding this research through a collaborative grant (ANR-21-CE07-0014). The authors acknowledge the initiative of Excellence IDEX-Unistra (ANR-10-IDEX-0002-02) from the French national program “Investment for the Future”.Notes and references
- T. Chivers and R. S. Laitinen, Chem. Soc. Rev., 2015, 44, 1725 RSC.
- J. Ibers, Nat. Chem., 2009, 1, 508 CrossRef CAS PubMed.
- L. A. Ba, M. Döring, V. Jamier and C. Jacob, Org. Biomol. Chem., 2010, 8, 4203 RSC.
- N. Petragnani and H. A. Stefani, Tetrahedron, 2005, 61, 1613 CrossRef CAS.
- K. Grollier, A. Taponard and T. Billard, Eur. J. Org. Chem., 2020, 2020, 6943 CrossRef CAS.
- M. R. Detty and S. L. Gibson, Organometallics, 1992, 11, 2147 CrossRef CAS.
- M. R. Detty, F. Zhou and A. E. Friedman, J. Am. Chem. Soc., 1996, 118, 313 CrossRef CAS.
- C. Cremer and F. W. Patureau, JACS Au, 2022, 2, 1318 CrossRef CAS PubMed.
- S. Singh, N. Yadav, S. Mahala, J. Yadav, K. Behera, G. K. Rao, H. Joshi and K. N. Sharma, Dalton Trans., 2025, 54, 7970 RSC.
- S. Benz, A. I. Poblador-Bahamonde, N. Low-Ders and S. Matile, Angew. Chem., Int. Ed., 2018, 57, 5408 CrossRef CAS PubMed.
- P. Pale and V. Mamane, Chem. – Eur. J., 2023, 29, e202302755 CrossRef CAS PubMed.
- D. Jovanovic, M. Poliyodath Mohanan and S. M. Huber, Angew. Chem., Int. Ed., 2024, 63, e202404823 CrossRef CAS.
- T. Chivers, X. Gao and M. Parvez, Inorg. Chem., 1996, 35, 9 CrossRef CAS.
- E. R. T. Tiekink, Coord. Chem. Rev., 2022, 457, 214397 CrossRef CAS.
- A. Dhaka, I.-R. Jeon and M. Fourmigué, Acc. Chem. Res., 2024, 57, 362 CrossRef CAS.
- P. C. Ho, P. Szydlowski, J. Sinclair, P. J. W. Elder, J. Kübel, C. Gendy, L. M. Lee, H. Jenkins, J. F. Britten, D. R. Morim and I. Vargas-Baca, Nat. Chem., 2016, 7, 11299 CAS.
- R. Gleiter, G. Haberhauer, D. B. Werz, F. Rominger and C. Bleiholder, Chem. Rev., 2018, 118, 2010 CrossRef CAS PubMed.
- M. S. Taylor, Coord. Chem. Rev., 2020, 413, 213270 CrossRef CAS.
- C. B. Aakeroy, D. L. Bryce, G. R. Desiraju, A. Frontera, A. C. Legon, F. Nicotra, K. Rissanen, S. Scheiner, G. Terraneo, P. Metrangolo and G. Resnati, Pure Appl. Chem., 2019, 91, 1889 CrossRef CAS.
- P. Politzer, J. S. Murray and T. Clark, Phys. Chem. Chem. Phys., 2013, 15, 11178 RSC.
- S. Scheiner, Int. J. Quantum Chem., 2013, 113, 1609 CrossRef CAS.
- P. Pale and V. Mamane, ChemPhysChem, 2023, 24, e202200481 CrossRef CAS.
- R. Weiss, E. Aubert, L. Groslambert, P. Pale and V. Mamane, Chem. Sci., 2023, 14, 7221 RSC.
- R. Weiss, E. Aubert, L. Groslambert, P. Pale and V. Mamane, Chem. – Eur. J., 2022, 28, e202200395 CrossRef CAS PubMed.
- R. Weiss, Y. Cornaton, H. Khartabil, L. Groslambert, E. Hénon, P. Pale, J.-P. Djukic and V. Mamane, ChemPlusChem, 2022, 87, e202100518 CrossRef CAS.
- R. Weiss, E. Aubert, P. Pale and V. Mamane, Angew. Chem., Int. Ed., 2021, 60, 19281 CrossRef CAS.
- L. Groslambert, A. Padilla-Hernandez, R. Weiss, P. Pale and V. Mamane, Chem. – Eur. J., 2023, 29, e202203372 CrossRef CAS.
- L. Groslambert, P. Pale and V. Mamane, Chem. – Eur. J., 2024, 30, e202401650 CrossRef CAS.
- B. Zhou and F. P. Gabbaï, J. Am. Chem. Soc., 2021, 143, 8625 CrossRef CAS.
- B. Zhou and F. P. Gabbaï, Organometallics, 2021, 40, 2371 CrossRef CAS.
- K. Takagi, S. Hayashi and S. Sugie, Chem. Commun., 2024, 60, 11786 RSC.
- M. V. Il'in, Y. V. Safinskaya, D. A. Polonnikov, A. S. Novikov and D. S. Bolotin, J. Org. Chem., 2024, 89, 2916 CrossRef PubMed.
- I. O. Putnin, A. A. Sysoeva, M. V. Il'in and D. S. Bolotin, ChemCatChem, 2024, 16, e202400672 CrossRef CAS.
- N. Lohmann, K. Fengler, A. C. Keuper and O. García Mancheño, ChemCatChem, 2025, 17, e202401683 CrossRef CAS.
- X. Li, Y. Liu, W. Wang and Y. Wang, J. Am. Chem. Soc., 2025, 147, 3233 CrossRef CAS.
- A. A. Sysoeva, Y. V. Safinskaya, M. V. Il'in, A. S. Novikov and D. S. Bolotin, Org. Biomol. Chem., 2025, 23, 1970 RSC.
- L. Groslambert, Y. Cornaton, M. Ditte, E. Aubert, P. Pale, A. Tkatchenko, J.-P. Djukic and V. Mamane, Chem. – Eur. J., 2024, 30, e202302933 CrossRef CAS.
- L. Groslambert, Y. Cornaton, P. Pale, M. Ditte, A. Tkatchenko, J.-P. Djukic and V. Mamane, J. Org. Chem., 2025, 90, 8254 CrossRef CAS.
- T. J. Mooibroek, P. Gamez and J. Reedijk, CrystEngComm, 2008, 10, 1501 RSC.
- M. Egli and R. V. Gessner, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 180 CrossRef CAS.
- M. Egli and S. Sarkhel, Acc. Chem. Res., 2007, 40, 197 CrossRef CAS.
- G. J. Bartlett, A. Choudhary, R. T. Raines and D. N. Woolfson, Nat. Chem. Biol., 2010, 6, 615 CrossRef CAS.
- P. Panwaria and A. Das, Phys. Chem. Chem. Phys., 2022, 24, 22371 RSC.
- Y. Chen, Q. Zhen, F.-J. Meng, P. Yu and C. Xu, Chem. Rev., 2024, 124, 13370 CrossRef CAS PubMed.
- M86-EXX229V1 APEX3 User Manual, Bruker AXS Inc., Madison, USA, 2016 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A:Found. Adv., 2015, 71, 3–8 CrossRef.
- G. M. Sheldrick, Acta Crystallogr., Sect. C:Struct. Chem., 2015, 71, 3–8 Search PubMed.
- A. L. Spek, J. Appl. Crystallogr., 2003, 36, 7–13 CrossRef CAS.
- A. Bondi, J. Phys. Chem., 1964, 68, 441 CrossRef CAS.
- G. R. Desiraju, Angew. Chem., Int. Ed. Engl., 1995, 34, 2311 CrossRef CAS.
- (a) CCDC 2482975: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pbqzr; (b) CCDC 2482976: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pbr0t; (c) CCDC 2482977: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pbr1v; (d) CCDC 2482978: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pbr2w; (e) CCDC 2482979: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pbr3x; (f) CCDC 2482980: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pbr4y; (g) CCDC 2482981: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pbr5z; (h) CCDC 2482982: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pbr60; (i) CCDC 2482983: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pbr71; (j) CCDC 2482984: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pbr82.
Footnotes |
| † Dedicated to Professor Resnati, celebrating a career in fluorine and noncovalent chemistry on the occasion of his 70th birthday. |
| ‡ For sake of clarity, the tetra-adducts are represented by the general formula [{1⊃LB}·(LB)3]BArF where the signs ⊃ and · represent respectively the interaction through the “pocket” and the ChB. |
| § Two geometrical parameters are generally used as ChB indicators in the solid state: the CAr–Te-Y angle (θ) in the range 160–180° and Te-Y distance (d) below the sum of van der Waals radii of the interacting atoms (Σrvdw) (Y represents the acceptor atom). |
| This journal is © The Royal Society of Chemistry 2026 |