A vinylene-linked porphyrin covalent organic framework for efficient sunlight-driven photocatalytic organic transformations
Xiao-Meng
Zhang
abc,
Jing-Lan
Kan
c,
Ying
Dong
c,
Fan
Yang
 *c,
Qi-Kui
Liu
*c and
Cheng
Liu
*c,
Qi-Kui
Liu
*c and
Cheng
Liu
 *ab
*ab
aState Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, School of Chemical Engineering, Dalian University of Technology, Dalian, 116024, China. E-mail: liuch1115@dlut.edu.cn
bDepartment of Polymer Science and Materials, School of Chemical Engineering, Dalian University of Technology, Dalian, 116024, China
cCollege of Chemistry, Chemical Engineering and Materials Science, Collaborative Innovation Center of Functionalized Probes for Chemical Imaging in Universities of Shandong, Key Laboratory of Molecular and Nano Probes, Ministry of Education, Shandong Normal University, Jinan 250014, P. R. China. E-mail: yangfan1180@sdnu.edu.cn; qikuiliu2004@163.com
First published on 27th November 2025
Abstract
A vinylene-linked porphyrin-based COF was synthesized via aldol condensation reaction. This COF demonstrates prominent exciton dissociation and charge transfer, exhibiting exceptional photoactivity in the photooxidation of sulfides and oxidative coupling of benzylamines under sunlight irradiation.
Covalent organic frameworks (COFs),1 featuring permanent porosity, tunable bandgaps and homogeneous catalytic sites, have emerged as particularly promising photocatalysts since a hydrazone-based COF was first introduced into the photocatalytic hydrogen evolution reaction (HER) in 2014.2 By precisely regulating the topological structures, building blocks and linkages, COFs acting as photocatalysts have been successfully applied in the fields of the HER,3 organic transformations,4 pollution degradation,5 CO2 reduction6 and H2O2 generation reactions.7 Nevertheless, it remains imperative to continuously improve the photocatalytic activity to meet the requirements of high quality industrial-scale production, even under the irradiation of natural sunlight.8
The incorporation of functional building blocks such as Bodipy units,9 diketopyrrolopyrrole (DPP) units10 or porphyrin units11 into COF materials is an effective strategy for regulating the light absorption properties and crystallinity, and modulating the charge transfer properties of materials. The porphyrin unit possesses an 18-electron π-conjugated system, which is a classical type of photosensitizer. Due to their unique electronic structure, porphyrin materials typically demonstrate large molar extinction coefficient and high reactive oxygen species (ROS) generation efficiency under the irradiation of natural sunlight. To date, porphyrin monomers have been extensively employed in the construction of COFs and significant research progress has been achieved in photocatalytic studies.12 Despite this, achieving optimal electronic coupling between the porphyrin and other building blocks in the conjugated skeletons, which is beneficial for charge transfer and exciton separation processes, remains a fundamental research bottleneck. The introduction of vinylene (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond) linkages13,14 in COFs enables efficient charge delocalization and high structural stability, which are widely adopted for constructing high-performance photocatalysts. Wang and coworkers synthesized a porphyrin-containing cyano-substituted vinylene linked Por-sp2c-COF via Knoevenagel condensation reactions, which demonstrates significantly higher photocatalytic activity for amine oxidation than its imine-linked counterpart.15 The Lang group also reported similar findings.16 However, the nonplanarity of cyano-substituted vinylene linkages may cause a decrease in conjugation degree and product purity of the COFs,17,18 which will degrade the photoactivity. Therefore, the exploration of other types of vinylene-linked porphyrin based COFs is of significant importance. Jiang and coworkers developed a non-cyano-substituted vinylene linked 3D porphyrin COF via aldol condensation, which exhibits prominent H2O2 production rate in the presence of guest molecules TTF.19 It is suggested that further modifications to parameters like topological structures or solubility of porphyrin monomers should enable the construction of COFs with high crystallinity and enhanced photocatalytic activity.
C bond) linkages13,14 in COFs enables efficient charge delocalization and high structural stability, which are widely adopted for constructing high-performance photocatalysts. Wang and coworkers synthesized a porphyrin-containing cyano-substituted vinylene linked Por-sp2c-COF via Knoevenagel condensation reactions, which demonstrates significantly higher photocatalytic activity for amine oxidation than its imine-linked counterpart.15 The Lang group also reported similar findings.16 However, the nonplanarity of cyano-substituted vinylene linkages may cause a decrease in conjugation degree and product purity of the COFs,17,18 which will degrade the photoactivity. Therefore, the exploration of other types of vinylene-linked porphyrin based COFs is of significant importance. Jiang and coworkers developed a non-cyano-substituted vinylene linked 3D porphyrin COF via aldol condensation, which exhibits prominent H2O2 production rate in the presence of guest molecules TTF.19 It is suggested that further modifications to parameters like topological structures or solubility of porphyrin monomers should enable the construction of COFs with high crystallinity and enhanced photocatalytic activity.
In this work, a porphyrin-based monomer, named 5,10,15,20-tetrakis(6-methylpyridin-3-yl)porphyrin (p-Por-CH3) with planar configuration and high solubility was designed and synthesized. This monomer features carbon atoms with active α-H, which was subsequently introduced into the synthesis of vinylene linked Por-vinyl-COF via aldol condensation reactions. An imine linked Por-imine-COF with identical molecular formula and an isostructural skeleton was synthesized via Schiff-base reaction for comparison. According to the photophysical characterization and theory calculations, the Por-vinyl-COF exhibits broader absorption range, more prominent charge transfer and exciton dissociation characteristics compared to the Por-imine-COF. Two porphyrin COFs were subsequently introduced into the photocatalytic oxidation of sulfides, and Por-vinyl-COF exhibits significantly superior photo activity compared to the Por-imine-COF. It is worth noting that the Por-vinyl-COF enables the high-efficiency photooxidation of sulfides and benzylamine coupling under ambient sunlight irradiation.
Monomers [1,1′-biphenyl]-4,4′-dicarbaldehyde (BPDA) and 4,4′,4″,4‴-(porphyrin-5,10,15,20-tetrayl)tetraaniline (p-Por-NH2) were obtained from commercial supplies. Monomer p-Por-CH3 (Fig. S1) was synthesized according to the procedure demonstrated in Scheme S1. Por-vinyl-COF was obtained based on aldol condensation with benzoic anhydride as a catalyst/solvent at 180 °C for 3 days. Por-imine-COF was synthesized via Schiff-base condensation by using acetic acid (3 M) as a catalyst and o-dichlorobenzene/n-butanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a solvent at 120 °C for 3 days (Fig. 1a). Both porphyrin COFs exhibit high crystallinity, and their well-defined structures can be confirmed by the comparison of the measured powder X-ray diffraction (PXRD) patterns with simulated PXRD patterns calculated from Materials Studio (ver. 2018). As indicated in Fig. 1b, the diffraction peaks of Por-vinyl-COF at 3.02°, 6.10° and 8.94° are individually ascribed to the (110), (220) and (330) planes. The broad peak at 20.74° is assigned to the (001) planes, which correspond to the π–π stacking. The diffraction peaks of Por-imine-COF at 2.22°, 3.02°, and 6.04° are attributed to the (100), (110) and (220) planes, respectively (Fig. 1c). Of note, the Pawley refinements of the two porphyrin COFs show negligible differences between the experimental and simulated PXRD patterns (Por-vinyl-COF: Rwp = 2.33% and Rp = 1.77%; Por-imine-COF: Rwp = 3.55% and Rp = 2.48%). According to the simulated crystal data, both porphyrin COFs are ascribed to space group P4 with AA stacking models. The detailed parameters of the two COFs are a = 42.07 Å, b = 42.07 Å, c = 4.43 Å, α = 90°, β = 90° and γ = 90° for Por-vinyl-COF and a = 41.46 Å, b = 41.46 Å, c = 4.12 Å, α = 90°, β = 90° and γ = 90° for Por-imine-COF.
1) as a solvent at 120 °C for 3 days (Fig. 1a). Both porphyrin COFs exhibit high crystallinity, and their well-defined structures can be confirmed by the comparison of the measured powder X-ray diffraction (PXRD) patterns with simulated PXRD patterns calculated from Materials Studio (ver. 2018). As indicated in Fig. 1b, the diffraction peaks of Por-vinyl-COF at 3.02°, 6.10° and 8.94° are individually ascribed to the (110), (220) and (330) planes. The broad peak at 20.74° is assigned to the (001) planes, which correspond to the π–π stacking. The diffraction peaks of Por-imine-COF at 2.22°, 3.02°, and 6.04° are attributed to the (100), (110) and (220) planes, respectively (Fig. 1c). Of note, the Pawley refinements of the two porphyrin COFs show negligible differences between the experimental and simulated PXRD patterns (Por-vinyl-COF: Rwp = 2.33% and Rp = 1.77%; Por-imine-COF: Rwp = 3.55% and Rp = 2.48%). According to the simulated crystal data, both porphyrin COFs are ascribed to space group P4 with AA stacking models. The detailed parameters of the two COFs are a = 42.07 Å, b = 42.07 Å, c = 4.43 Å, α = 90°, β = 90° and γ = 90° for Por-vinyl-COF and a = 41.46 Å, b = 41.46 Å, c = 4.12 Å, α = 90°, β = 90° and γ = 90° for Por-imine-COF.
Additional characterizations were conducted to confirm the successful construction of the COFs. According to Fig. S2a, the Fourier transform infrared (FT-IR) spectra of the two COFs indicate the disappearance of the C–H stretching band (3418 cm−1) of the p-Por-CH3 monomer and N–H stretching band (3360 and 3430 cm−1) of p-Por-NH2. Meanwhile, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching band (1690 cm−1) of the BPDA monomer dramatically decreased after condensation, accompanied by the formation of a new C
O stretching band (1690 cm−1) of the BPDA monomer dramatically decreased after condensation, accompanied by the formation of a new C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching band (1628 cm−1) in Por-vinyl-COF and C
C stretching band (1628 cm−1) in Por-vinyl-COF and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching band (1620 cm−1) in Por-imine-COF, respectively. The detailed structural information was also determined via solid-state 13C CP/MAS nuclear magnetic resonance (NMR) spectroscopy. As shown in Fig. S2b, in Por-vinyl-COF, the chemical shifts at 154 ppm and 146 ppm are individually ascribed to the pyridine and pyrrole carbons. In Por-imine-COF, the shifts at 157 ppm and 155 ppm are assigned to the carbon atoms adjacent to the nitrogen atom in the imine bond, and the peaks at 150 ppm and 146 ppm are attributed to pyrrole carbons. Broad peaks ranging from 110 ppm to 145 ppm in both COFs are assigned to aromatic carbons. The Brunner–Emmett–Teller (BET) surface areas of Por-vinyl-COF and Por-imine-COF were measured by N2 adsorption and desorption experiments at 77 K, which were determined to be 190.1 m2 g−1 for Por-vinyl-COF and 545.2 m2 g−1 for Por-imine-COF, respectively (Fig. S2c). The pore sizes of the two COFs were analyzed by non-local density functional theory (NLDFT), which centered at 2.1 nm for Por-vinyl-COF and 2.0 nm for Por-imine-COF (Fig. S2d). These results are in accordance with the simulated AA stacking models of the COFs. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were applied to investigate the morphology of the two porphyrin COFs. According to Fig. S3, Por-vinyl-COF exhibits a blocky structure, and Por-imine-COF presents a fibrous morphology. In order to evaluate the thermal stability of the COFs, thermogravimetry analysis (TGA) was performed. As exhibited in Fig. S4, both porphyrin COFs demonstrate remarkable thermal stability with less than 5% weight loss even at temperatures exceeding 450 °C.
N stretching band (1620 cm−1) in Por-imine-COF, respectively. The detailed structural information was also determined via solid-state 13C CP/MAS nuclear magnetic resonance (NMR) spectroscopy. As shown in Fig. S2b, in Por-vinyl-COF, the chemical shifts at 154 ppm and 146 ppm are individually ascribed to the pyridine and pyrrole carbons. In Por-imine-COF, the shifts at 157 ppm and 155 ppm are assigned to the carbon atoms adjacent to the nitrogen atom in the imine bond, and the peaks at 150 ppm and 146 ppm are attributed to pyrrole carbons. Broad peaks ranging from 110 ppm to 145 ppm in both COFs are assigned to aromatic carbons. The Brunner–Emmett–Teller (BET) surface areas of Por-vinyl-COF and Por-imine-COF were measured by N2 adsorption and desorption experiments at 77 K, which were determined to be 190.1 m2 g−1 for Por-vinyl-COF and 545.2 m2 g−1 for Por-imine-COF, respectively (Fig. S2c). The pore sizes of the two COFs were analyzed by non-local density functional theory (NLDFT), which centered at 2.1 nm for Por-vinyl-COF and 2.0 nm for Por-imine-COF (Fig. S2d). These results are in accordance with the simulated AA stacking models of the COFs. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were applied to investigate the morphology of the two porphyrin COFs. According to Fig. S3, Por-vinyl-COF exhibits a blocky structure, and Por-imine-COF presents a fibrous morphology. In order to evaluate the thermal stability of the COFs, thermogravimetry analysis (TGA) was performed. As exhibited in Fig. S4, both porphyrin COFs demonstrate remarkable thermal stability with less than 5% weight loss even at temperatures exceeding 450 °C.
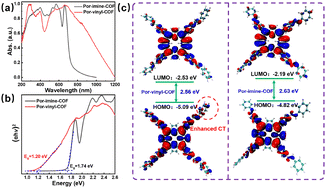
Solid-state UV-vis diffuse reflectance spectroscopy was performed to evaluate the light absorption properties of the two porphyrin COFs. As illustrated in Fig. 2a, Por-vinyl-COF exhibits a broader absorption range with the edge extending to 1000 nm and represents a bathochromic shift of 200 nm compared to Por-imine-COF. As calculated from the Tauc plots, the bandgaps of Por-vinyl-COF and Por-imine-COF are individually 1.20 eV and 1.74 eV (Fig. 2b). The narrower bandgap of Por-vinyl-COF may originate from the optimized charge transfer property in the conjugated skeleton. The reduction potentials of the COFs were measured by cyclic voltammetry (CV). According to Fig. S5, the reduction potentials of Por-vinyl-COF and Por-imine-COF are −0.98 eV and −0.75 eV, respectively. Combining these with the calculated bandgaps, the oxidative potentials were determined to be 0.22 eV for Por-vinyl-COF and 0.99 eV for Por-imine-COF. The segments of the COFs were calculated by density functional theory (DFT) computations at the B3LYP/6-311G(d,p) level. As indicated in Fig. 2c, Por-vinyl-COF exhibits a narrower calculated bandgap and more delocalized electronic character upon HOMO-to-LUMO excitation compared to Por-imine-COF, which aligns well with experimental observations. This electronic structure, favorable for charge separation, underpins its enhanced photocatalytic activity. As shown in Fig. 3a, the electrostatic potential map demonstrates that Por-vinyl-COF has a more distinct charge separation, with its C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C linker being more negative and its porphyrin unit more positive compared to the Por-imine-COF. This feature is consistent with the significantly weaker steady-state fluorescence intensity of Por-vinyl-COF (Fig. S6). The enhanced polarization translates to superior performance in photoelectrical tests: Por-vinyl-COF generates a significantly higher photocurrent density (Fig. 3b), unequivocally confirming its higher charge separation and transfer efficiencies. Electrochemical impedance spectroscopy (EIS) was tested to evaluate the charge resistance of the COFs. As shown in Fig. 3c, the Nyquist radius of Por-vinyl-COF is obviously smaller than that of the Por-imine-COF, which indicates enhanced charge migration ability of Por-vinyl-COF.
C linker being more negative and its porphyrin unit more positive compared to the Por-imine-COF. This feature is consistent with the significantly weaker steady-state fluorescence intensity of Por-vinyl-COF (Fig. S6). The enhanced polarization translates to superior performance in photoelectrical tests: Por-vinyl-COF generates a significantly higher photocurrent density (Fig. 3b), unequivocally confirming its higher charge separation and transfer efficiencies. Electrochemical impedance spectroscopy (EIS) was tested to evaluate the charge resistance of the COFs. As shown in Fig. 3c, the Nyquist radius of Por-vinyl-COF is obviously smaller than that of the Por-imine-COF, which indicates enhanced charge migration ability of Por-vinyl-COF.
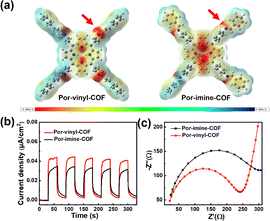 | ||
| Fig. 3 (a) Electrostatic potential map of two porphyrin COFs. (b) Photo-current response of two COFs. (c) EIS measurements of the COFs. | ||
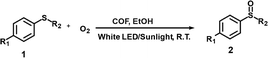
Por-vinyl-COF with exceptional photo-chemical and photo-electrical properties demonstrates intrinsic suitability for photocatalytic organic transformations. In this work, the photocatalytic oxidation of sulfides to sulfoxides and the benzylamine oxidative coupling reaction were conducted to evaluate the photo activity of Por-vinyl-COF. These reactions support common synthetic strategies for producing intermediates in pharmaceuticals and pesticides.20,21 Firstly, oxidation of sulfides was executed by placing 4-methoxybenzyl sulfide (0.3 mmol, 1a), ethanol (3 mL, solvent) and Por-vinyl-COF (10 mg, catalyst) into a quartz tube. The tube was charged with oxygen (1 bar) and irradiated under a white LED lamp (2000 W m−2). The reaction process was monitored via thin layer chromatography (TLC) measurement and 1H-NMR spectra with diphenylacetonitrile (DPAT) as an internal standard. As shown in Table 1 and Fig. S7, the oxidation reaction can be finished in only 1 hour with 99% 1H-NMR determined yield and 92% isolated yield. The control experiments were also conducted, and as demonstrated in Table S1 and Fig. S8, the light, oxygen and photocatalyst are indispensable for this photooxidation reaction, and ethanol is the best reaction medium.
| Entry | Substrate | Product | Yielda (%) [time]Por-vinyl-COF | Yielda (%) [time]Por-imine-COF | Yieldb (%) [time]Por-vinyl-COF |
|---|---|---|---|---|---|
| a Irradiated by a 10 W white LED lamp (2000 W m−2). b Irradiated by natural sunlight (700–1100 W m−2). c Yields were determined by 1H-NMR spectra. d Isolated yield. | |||||
| 1 |
1a: R1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) OCH3, R2 OCH3, R2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH3 CH3 |
2a | 99,c 92d [1 h] | 50,c 48d [1 h] | 97c [2 h] |
| 2 |
1b: R1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H, R2 H, R2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH3 CH3 |
2b | 97,c 91d [3 h] | 50,c 47d [3 h] | 91c [4.5 h] |
| 3 |
1c: R1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) F, R2 F, R2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH3 CH3 |
2c | 97,c 91d [3 h] | 29,c 26d [3 h] | 91c [3.5 h] |
| 4 |
1d: R1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Cl, R2 Cl, R2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH3 CH3 |
2d | 94,c 90d [3 h] | 12,c 12d [3 h] | 97c [6 h] |
| 5 |
1e: R1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH3, R2 CH3, R2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH3 CH3 |
2e | 99,c 98d [2.5 h] | 40,c 38d [2.5 h] | 98c [3.5 h] |
| 6 |
1f: R1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H, R2 H, R2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2CH3 CH2CH3 |
2f | 99,c 96d [3 h] | 27,c 25d [3 h] | 97c [7 h] |
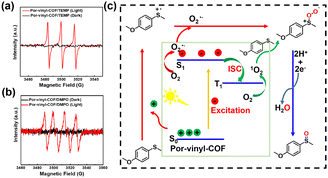
As a comparison, the Por-imine-COF exhibits poor photocatalytic activity in the oxidation of 1a with a yield of 50% (Table 1 and Fig. S9). This result demonstrates the superior photo activity of Por-vinyl-COF. To establish substrate diversity, a series of sulfides bearing electron-donating and electron-withdrawing substituents were introduced into the photocatalytic reactions. As demonstrated in Table 1 and Fig. S10–S19, Por-vinyl-COF shows higher photocatalytic activity with sulfoxide yields of 94–99% within 3 hours. Its catalytic performance is comparable to that of other high-performing COF materials (Table S2), and the apparent quantum yields (AQY) of products 2a–2f range from 9.47% to 27.10% (Table S3). Meanwhile, the yield of sulfoxides by using Por-imine-COF as a photocatalyst is only 12–50%. To verify the recyclability of Por-vinyl-COF, the material was introduced into the photocatalytic experiments for five cycles. As indicated in Fig. S20, the photo activity and the original crystalline structure of Por-vinyl-COF are well preserved after five catalytic cycles. This COF was subsequently introduced into window ledge photocatalytic experiments. The reaction tubes were directly placed under sunlight with power density in the range of 700 to 1100 W m−2, and the reactions can be completed within 7 hours with high yields of 91% to 98% (Table 1 and Fig. S21). The turn-over number (TON) and frequency (TOF) for the synthesis of 2a were determined to be 29.75 and 14.88 h−1, respectively. Furthermore, the large-scale synthesis was executed by using 1a (0.93 g, 6 mmol) in 30 mL ethanol with 50 mg Por-vinyl-COF, and the reaction can be finished in 5 hours with 0.98 g of isolated 2a (yield: 96.4%). Electron paramagnetic resonance (EPR) spectroscopy and active oxygen species quenching experiments were performed to elucidate the mechanism of the photocatalytic reaction. EPR measurements were conducted to investigate the active oxygen species by using 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) and 2,2,6,6-tetramethylpiperidine (TEMP) as trapping agents. As exhibited in Fig. 4a and b, both singlet oxygen 1O2 and superoxide radical anion O2˙− signals can be detected under light irradiation. The quenching experiments were conducted by adding different scavengers into the reaction system. In which, 0.3 equivalent of sodium azide (NaN3), p-benzoquinone (BQ) and t-butanol (t-BuOH) were introduced and served as scavengers of 1O2, O2˙− and ˙OH, respectively. As a result, both NaN3 and BQ significantly inhibited the reaction (Fig. S22), whereas the addition of t-BuOH has a negligible influence on this reaction. Therefore, a reaction mechanism is proposed in Fig. 4c. First, a persulfoxide intermediate is formed through two pathways: (1) 1a was oxidized by a h+ to generate a sulfur radical cation, which then reacts with O2˙−; (2) 1a directly reacts with the generated 1O2. Subsequently, product 2a is obtained from the persulfoxide intermediate with the aid of electrons and protons supplied by the reaction medium (CH3CH2OH). To validate the multifunctionality of Por-vinyl-COF, the material was subsequently introduced into the photo oxidative coupling of benzylamines under the irradiation of sunlight. As demonstrated in Table 2 and Fig. S23, the reaction can be finished in 3 hours with high yields of 86–93%.
In conclusion, a novel porphyrin monomer, p-Por-CH3, was synthesized and applied to construct the vinylene-linked Por-vinyl-COF. An imine-linked Por-imine-COF with an isostructural skeleton was also synthesized for comparison. Despite its lower BET surface area, Por-vinyl-COF exhibits significantly superior photocatalytic performance to Por-imine-COF in the photooxidation of sulfides, attributed to its superior exciton separation and charge transfer. Of note, Por-vinyl-COF facilitates the oxidation of sulfides and the coupling of benzylamine under natural sunlight. Overall, this work offers new insight for constructing a vinylene-linked porphyrin COF capable of mediating highly efficient organic transformations.
We are grateful for the financial support from the Fundamental Research Funds for the Central Universities (No. DUT22LAB605) and the National Natural Science Foundation of China (Grant No. 22201166).
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: the characterization of COFs and the photocatalytic performance of COFs are supported. See DOI: https://doi.org/10.1039/d5cc05728k.Notes and references
- T. Irie, S. Das, Q. Fang and Y. Negishi, J. Am. Chem. Soc., 2025, 147, 1367–1380 CrossRef CAS PubMed.
- L. Stegbauer, K. Schwinghammer and B. V. Lotsch, Chem. Sci., 2014, 5, 2789–2793 RSC.
- Z. Luo, S. Zhu, H. Xue, W. Yang, F. Zhang, F. Xu, W. Lin, H. Wang and X. Chen, Angew. Chem., Int. Ed., 2025, 64, e202420217 CrossRef CAS.
- J.-R. Wang, K. Song, T.-X. Luan, K. Cheng, Q. Wang, Y. Wang, W. W. Yu, P.-Z. Li and Y. Zhao, Nat. Commun., 2024, 15, 1267 CAS.
- Z. Xu, Q. Duan and X. Cui, Chem. Eng. J., 2025, 511, 162241 CAS.
- Y. Zhang, X. Guan, Z. Meng and H.-L. Jiang, J. Am. Chem. Soc., 2025, 147, 3776–3785 CrossRef CAS.
- G. Liu, M. Lin, Y. Xing, S. Cheng, H. Wang, D. Li, X. Long and Q. Fang, Angew. Chem., Int. Ed., 2025, 64, e202500945 CrossRef CAS.
- Y. Chen and D. Jiang, Acc. Chem. Res., 2024, 57, 3182–3193 CrossRef CAS PubMed.
- R. Sánchez-Naya and F. Beuerle, Angew. Chem., Int. Ed., 2025, 64, e202423676 CrossRef.
- W. Zhao, J.-L. Kan, C. Zhao, Y. Guo, F. Yang and Y.-B. Dong, Chem. Commun., 2025, 61, 2810–2813 RSC.
- L.-B. Xing, K. Cheng, H. Li, K. Niu, T.-X. Luan, S. Kong, W. W. Yu, P.-Z. Li and Y. Zhao, Angew. Chem., Int. Ed., 2025, 64, e202425668 CrossRef CAS.
- Z. Zhou, Z. Wang, A. L. Blenko, J. Li and W. Lin, J. Am. Chem. Soc., 2025, 147, 10846–10852 CrossRef CAS.
- Y. Wang, X. Zhao, G. Han, J. Bi and Y. Zhao, Adv. Funct. Mater., 2025, 2508550, DOI:10.1002/adfm.202508550 n/a.
- H. Liu, D. Wang, Z. Yu, Y. Chen, X. Li, R. Zhang, X. Chen, L. Wu, N. Ding, Y. Wang and Y. Zhao, Sci. China Mater., 2023, 66, 2283–2289 CrossRef CAS.
- R. Chen, J.-L. Shi, Y. Ma, G. Lin, X. Lang and C. Wang, Angew. Chem., Int. Ed., 2019, 58, 6430–6434 CrossRef CAS.
- J.-L. Shi, R. Chen, H. Hao, C. Wang and X. Lang, Angew. Chem., Int. Ed., 2020, 59, 9088–9093 CrossRef CAS PubMed.
- S. Xu, M. Richter and X. Feng, Acc. Mater. Res., 2021, 2, 252–265 CrossRef CAS.
- B. Qi, R. Shen, Z. Ke, S. Wang, Y. Li, P. Zhang, D. Xu and X. Li, Rare Met., 2025 DOI:10.1007/s12598-025-03604-4.
- Z. Gong, Y. Gao, J. Li, Z. Cai, N. Liu and J. Jiang, Angew. Chem., Int. Ed., 2025, 64, e202423205 CrossRef CAS PubMed.
- S. Zhang, F. Zhang, B. Zeng, K. Zhang, Y. Wang, J. Shang and X. Lang, Appl. Catal., B, 2025, 369, 125147 CrossRef CAS.
- S.-I. Murahashi, Angew. Chem., Int. Ed. Engl., 1995, 34, 2443–2465 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |