Ethane-selective metal–organic frameworks for one-step purification of ethylene
Chunpu
Duan
a,
Rundao
Chen
 *a,
Jiaqi
Li
a,
Fang
Zheng
b,
Zhiguo
Zhang
*a,
Jiaqi
Li
a,
Fang
Zheng
b,
Zhiguo
Zhang
 ab,
Qiwei
Yang
ab,
Qilong
Ren
ab and
Zongbi
Bao
ab,
Qiwei
Yang
ab,
Qilong
Ren
ab and
Zongbi
Bao
 *ab
*ab
aKey Laboratory of Biomass Chemical Engineering of the Ministry of Education, College of Chemical and Biological Engineering, Zhejiang University, 866 Yuhangtang Road, Hangzhou 310058, China. E-mail: rdchen@zju.edu.cn; baozb@zju.edu.cn
bInstitute of Zhejiang University-Quzhou, Zhejiang University, 99 Zheda Road, Quzhou 324000, China
First published on 19th November 2025
Abstract
Ethylene/ethane separations remain among the most energy-intensive operations in the petrochemical value chain, owing to their close molecular sizes, polarizabilities, and volatilities. While cryogenic distillation is industrially entrenched, adsorption-based processes promise substantial energy savings and simpler flowsheets, particularly when impurity-targeted adsorbents are employed. This comprehensive review systematically examines recent advancements in ethane-selective MOF adsorbents that enable one-step purification of polymer-grade ethylene directly from cracked-gas mixtures. We delineate three fundamental separation mechanisms that govern selective ethane capture: (i) strategic incorporation of electronegative binding sites for enhanced C–H⋯site interactions, (ii) engineering of low-polarity hydrophobic pore environments to leverage different van der Waals interactions, and (iii) utilization of framework flexibility and gate-opening phenomena that preferentially accommodate ethane molecules. This review critically evaluates representative MOF systems across key performance metrics including equilibrium uptake capacities, IAST-predicted selectivity values, and experimental breakthrough behaviors under mixed-gas conditions. By establishing clear structure–property relationships and identifying emerging design principles, this work provides valuable insights for the rational development of next-generation ethane-selective adsorbents with optimized separation performance.
1 Introduction
Ethylene (C2H4) is a cornerstone feedstock for the global chemical industry, underpinning the manufacture of polymers, solvents, and myriad downstream intermediates.1 Industrial ethylene is predominantly produced via steam cracking of naphtha and other hydrocarbons, yielding a complex effluent in which ethylene and ethane (C2H6) typically dominate with an ethylene/ethane ratio that can reach approximately 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 To meet the stringent purity specifications of polymer-grade ethylene (≥99.95%), the cracked gas must be subjected to deep purification to remove residual paraffins as well as trace olefinic and polar impurities.3 Among these, the separation of ethylene from ethane remains one of the most challenging and energy-intensive tasks in large-scale chemical processing.4
1.2 To meet the stringent purity specifications of polymer-grade ethylene (≥99.95%), the cracked gas must be subjected to deep purification to remove residual paraffins as well as trace olefinic and polar impurities.3 Among these, the separation of ethylene from ethane remains one of the most challenging and energy-intensive tasks in large-scale chemical processing.4
Conventional ethylene/ethane purification is achieved through cryogenic distillation at low temperatures and high reflux ratios,5 exploiting small differences in volatility. While technologically mature, this approach is capital- and energy-intensive due to the nearly overlapping boiling points and kinetic diameters of the two C2 molecules (Table 1).6 In contrast, adsorption-based separations on solid porous media offer an attractive pathway to lower energy consumption, modular operation, and potential retrofit in existing plants.7 However, the close similarity in size, polarizability, and basic intermolecular interactions between ethane and ethylene complicates the design of adsorbents that can deliver both high selectivity and capacity under realistic conditions.8,9 Although activated carbons (AC) and zeolites could achieve ethylene-selective adsorption through mechanisms such as π-complexation and molecular sieving,10,11 reports on ethane-selective AC and zeolites are scarce due to their limited designability and tunability. In contrast, covalent organic frameworks (COFs), owing to their generally weak polarity, exhibit a certain degree of ethane-preferential adsorption ability.12,13 However, compared with metal–organic frameworks (MOFs), COFs typically exhibit larger pore sizes, fewer designable adsorption sites, and less pronounced flexibility, which to some extent restricts further improvement in their ethane selectivity.
| Compounds | Dimensions | Kinetic diameter (Å) | Boiling point (K) | Dipole moment × 1018 (esu cm) | Quadrupole moment × 1026 (esu cm2) | Polarizability × 1025 (cm3) |
|---|---|---|---|---|---|---|
| C2H6 | 3.81 × 4.08 × 4.82 | 4.4 | 184.6 | 0 | 0.65 | 44.3–44.7 |
| C2H4 | 3.28 × 4.18 × 4.84 | 4.2 | 169.4 | 0 | 1.50 | 42.5 |
Therefore, MOFs have emerged as particularly promising candidates for addressing these limitations.14–17 MOFs are porous crystalline materials formed by coordinating metal nodes with organic linkers, enabling atomistic precision in tailoring pore architectures, chemical environments, and framework by dynamics.18–21 Their high specific surface areas, tunable apertures, heterogeneous binding sites, and flexible response to guest molecules provide a rich landscape for engineering host–guest interactions that can be leveraged for challenging gas separations.22–25 Notably, MOFs allow separation mechanisms beyond size exclusion or simple physisorption,26,27 including specific electrostatic interactions,28 induced fit via framework flexibility,29 and modulation of hydrophobic/hydrophilic balance to steer selectivity.30,31
A key conceptual advance in adsorption-based purification of ethylene has been the pivot from ethylene-selective to ethane-selective adsorbents. Ethylene-selective materials often capture the desired product, necessitating additional steps to recover high-purity ethylene and regenerate the adsorbent, which can entail multiple adsorption–desorption cycles and concomitant energy penalties.32–35 In contrast, ethane-selective adsorbents remove the impurity in a single pass, enriching ethylene directly in the effluent and thereby simplifying the flowsheet.36 This one-step purification strategy can reduce energy consumption, minimize cycle complexity, and improve throughput, provided that the adsorbent combines sufficient ethane capacity with robust selectivity under mixed-gas conditions and in the presence of co-impurities.37
Recent research has delineated three principal microscopic mechanisms by which ethane-selective MOFs can discriminate between ethane and ethylene (Scheme 1): (i) electronegative binding sites, (ii) low-polarity hydrophobic pore environments, and (iii) structural flexibility that enables preferential accommodation of ethane. Electronegative sites such as polarized functional groups, or halogenated linkers, can engage ethane through favorable dispersive and weak electrostatic interactions while mitigating overly strong binding to the π-bond of ethylene, thus avoiding the classical pitfall of π-complexation that tends to favor ethylene. Hydrophobic, low-polarity pores can enhance van der Waals interactions with ethane, whose saturated nature can result in stronger non-specific adsorption than ethylene when the framework does not present π-affinitive sites. Finally, flexible frameworks can undergo subtle conformational changes upon guest uptake; by matching ethane's shape and dynamic profile, such MOFs can create induced-fit environments that stabilize ethane more than ethylene, even when static pore metrics suggest otherwise.
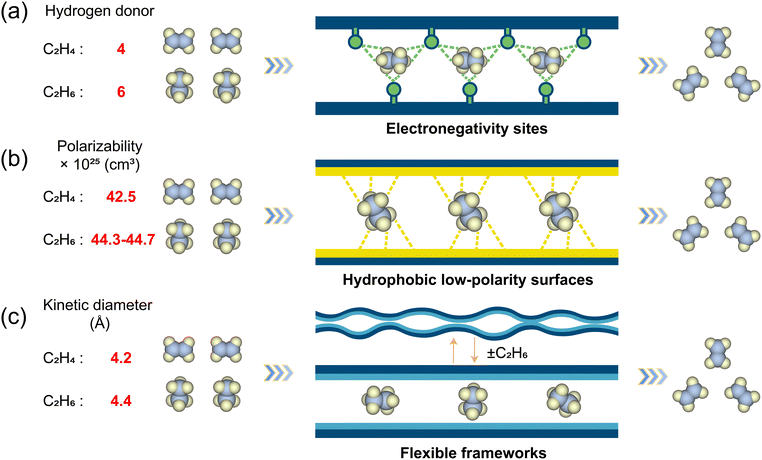 | ||
| Scheme 1 Three separation mechanisms of C2H6-selective MOFs: (a) electronegative sites, (b) hydrophobic low-polarity surfaces, and (c) flexible frameworks. | ||
An illustrative milestone in this field was the identification of ethane-selective behavior in zeolitic imidazolate frameworks such as ZIF-7,38 which catalyzed broader exploration of design rules for ethane capture. Building on this foundation, diverse MOF families have been engineered to incorporate electronegative nodes and linkers, fluorinated or otherwise low-polarity pore surfaces, and responsive topologies that amplify selectivity through framework breathing or gate-opening. The interplay between host–guest chemistry and pore morphology is routinely probed using a suite of techniques, including single-crystal and powder diffraction under gas loadings, in situ spectroscopies, and density functional theory, to localize binding sites, quantify interaction energies, and rationalize uptake trends.
This review synthesizes recent advances in ethane-selective MOF adsorbents aimed at one-step purification of ethylene. We organize the discussion around the three dominant separation mechanisms including electronegative sites, hydrophobic low-polarity pores, and flexible frameworks, emphasizing the underlying host–guest interactions, structure–property relationships, and strategies for targeted functionalization. We compare representative materials on key performance indicators, including equilibrium uptake, IAST-predicted selectivity, and breakthrough behavior. By distilling design principles across these classes, we outline opportunities for rational synthesis of next-generation ethane-selective MOFs and identify research directions such as multi-component competitive adsorption studies, operando structure characterization, and process-level techno-economic analyses that will accelerate the transition from laboratory discovery to industrial implementation. In doing so, we aim to provide a framework for the continued development of adsorbents that can materially reduce the energy footprint of ethylene production while meeting the demands of modern, low-carbon chemical manufacturing.
2 Ethane-selective MOFs featuring electronegative atom sites
Harnessing electronegative atom sites within MOF pores is a powerful strategy to bias competitive adsorption in favor of ethane. Although neither ethane nor ethylene forms classical hydrogen bonds, six C–H bonds of ethane can engage in multiple weak C–H⋯X interactions (X = N, O, and F) with polarized framework atoms, cumulatively strengthening its physisorption relative to ethylene, which presents fewer C–H donors and a π bond that can be deliberately disfavored. By engineering localized electrostatic fields and polarizable contact points on the pore surface without introducing π-complexation sites that preferentially bind ethylene, MOFs can amplify dispersive and weak electrostatic interactions with saturated C2 hydrocarbons, thereby achieving ethane-selective capture under mixed-gas conditions.A central design lever is the number, identity, and spatial arrangement of electronegative substituents grafted onto linkers or near metal nodes. Increasing the density of N/O/F functionalities generally raises the probability and strength of multipoint C–H⋯X contacts, but the impact on selectivity is highly sensitive to topology and site positioning. Yu and co-workers demonstrated this principle in a family of microporous fluorinated MOFs (TKL-104–107) derived from phthalate linkers.39 Strategic fluorination profoundly modulates the hydrocarbon adsorption while preserving the underlying pore geometry, enabling a clear readout of site-specific effects. Notably, frameworks that place fluorine ortho to the carboxylate group exhibit superior structural robustness, a prerequisite for reproducible separation performance. Within the same topology, tuning fluorine content reveals a non-monotonic dependence of ethane/ethylene selectivity on the degree of fluorination: the difluorinated TKL-106 outperforms both the monofluorinated TKL-105 and the tetrafluorinated TKL-107. This inversion arises because excessive fluorine loading strengthens interactions with both ethane and ethylene, thereby compressing the energetic gap that underpins selectivity. Interaction energy profiles and distributions corroborate that incremental fluorination simultaneously intensifies framework affinity for each C2 component. Optimal selectivity therefore emerges at intermediate substitution where C–H⋯F contacts enhance ethane binding, while π-affinitive environments for ethylene remain suppressed.
A particularly effective route to amplify ethane–MOF interactions is the deliberate use of organic linkers bearing a high density of electronegative atoms, which multiplicatively increases the number of weak, cooperative C–H⋯X (X = N and O) contacts available per adsorbed molecule. The prototypical example is the ethane-selective framework, MAF-49,36 synthesized by reaction between Zn(OH)2 and bis(5-amino-1H-1,2,4-triazol-3-yl)methane (H2batz) in dilute aqueous ammonia. In this structure, each Zn2+ center adopts tetrahedral coordination to triazolate nitrogen atoms from four distinct H2batz linkers, producing a three-dimensional network threaded by narrow, one-dimensional zigzag channels (Fig. 1a). The channel surfaces are densely decorated with electron-rich nitrogen atoms from the triazolate motifs (Fig. 1b and c), creating a quasi-continuous array of polarized contact points that act as “nano-traps” for ethane. Within these channels, each C2H6 guest engages in multiple C–H⋯N contacts via its two methyl groups, whereas the corresponding interactions with C2H4 are appreciably weaker due to fewer available C–H donors and a less favorable electrostatic complementarity. The resulting cross-sectional and electronic matching between the channel lining and ethane underpins the exceptional ethane selectivity observed for MAF-49 (Fig. 1d–f). Fixed-bed breakthrough experiments further validate this mechanistic picture: the material delivers polymer-grade ethylene under relevant mixture conditions, and even at low ethane partial pressure (C2H6/C2H4 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, v/v), it affords 1.68 mmol g−1 of 99.95%-pure C2H4, indicating efficient scavenging of trace ethane.
15, v/v), it affords 1.68 mmol g−1 of 99.95%-pure C2H4, indicating efficient scavenging of trace ethane.
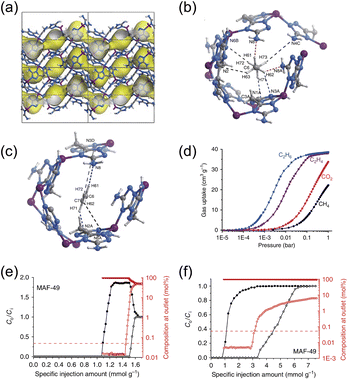 | ||
Fig. 1 (a) X-ray crystal structure of MAF-49·H2O. Preferential adsorption sites for (b) C2H6 and (c) C2H4 in MAF-49 revealed by computational simulations. (d) Gas adsorption isotherms for C2H6, C2H4, CO2 and CH4 in MAF-49 at 316 K. (e) C2H4/C2H6 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture breakthrough curves of MAF-49 at 313 K and 1 bar. (f) C2H4/C2H6 (15 1) mixture breakthrough curves of MAF-49 at 313 K and 1 bar. (f) C2H4/C2H6 (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture breakthrough curves of MAF-49 at 313 K and 1 bar. Reproduced from ref. 36 under the terms of the CC BY 4.0 license.36 1) mixture breakthrough curves of MAF-49 at 313 K and 1 bar. Reproduced from ref. 36 under the terms of the CC BY 4.0 license.36 | ||
An analogous design logic is realized in Zn(ad)(int) (ad = adenine; int = isonicotinate), reported by Ding and co-workers,40 which integrates two linkers rich in heteroatoms to furnish a high density of electronegative sites within well-defined pores (Fig. 2a). The frameworks exhibit strong ethane affinity at low pressures (Fig. 2b), consistent with multivalent host–guest interactions that discriminate against ethylene (Fig. 2c). Distinct from C2H4, adsorbed C2H6 molecules can establish multiple C–H⋯π contacts with three different heteroaromatic rings positioned around the binding pocket (Fig. 2d). In parallel, ethane forms two hydrogen-bond-like interactions: one with a non-coordinated carbonyl oxygen of the int ligand and another with a pyridinic nitrogen on adenine. The cooperative action of C–H⋯π and C–H⋯X interactions confers precise molecular recognition of ethane and stabilizes its adsorption under competitive conditions.
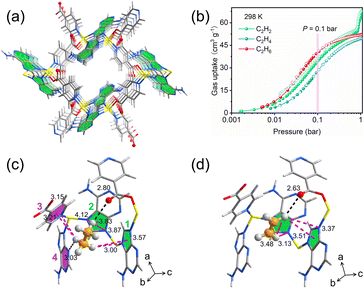 | ||
| Fig. 2 (a) 3D structure of Zn(ad)(int) viewed from the c axis. (b) Adsorption and desorption isotherms of C2 hydrocarbons on Zn(ad)(int) at 298K. (c) and (d) Local environment showing the binding modes of C2H6 and C2H4 in Zn(ad)(int). Reproduced from ref. 40 with permission from John Wiley and Sons. Copyright 2022.40 | ||
Another approach to amplify ethane–MOF interactions is to reduce the distance between electronegative atoms and ethane molecules, thereby enhancing the recognition capability for ethane. Zeng and co-workers designed an ethane-selective MOF material, JNU-2, with an xae topology (Fig. 3a and b).41 The cage-like cavities are interconnected through small apertures with a limiting diameter of approximately 3.7 Å, forming dumbbell-shaped channels. The adsorption kinetic profiles (Fig. 3c) indicate that the diffusion of C2H6 and C2H4 molecules in the MOFs is restricted by the narrow pores, and thus the surface properties at these narrow pores play a critical role in the selective adsorption of ethane. Breakthrough experiments (Fig. 3d) demonstrate that JNU-2 can effectively capture ethane from ethane/ethylene mixtures, achieving a high-purity ethylene productivity (99.99%) of 21.2 L kg−1. During adsorption, C2H6/C2H4 molecules pass through the narrow pores one by one, where a large number of electronegative oxygen atoms point toward the center. The spatial confinement enables the four carboxylate oxygen atoms to form more interactions with ethane, while ethylene only forms two weak hydrogen bonds (Fig. 3e), leading JNU-2 to preferentially adsorb ethane over ethylene.
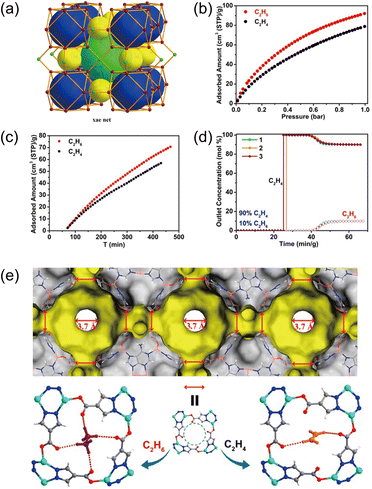 | ||
| Fig. 3 (a) Three different cages in JNU-2 arranged in an xae topology. (b) C2H6 and C2H4 single component adsorption isotherms of JNU-2 at 298 K. (c) Kinetic adsorption profiles of C2H6 and C2H4 on JNU-2 at 298 K. (d) Three cycles of dynamic column breakthrough curves for a C2H6/C2H4 (10/90) mixture through a packed bed of JNU-2 under dry conditions. (e) Schematic illustration of the multistage apertures in JNU-2 (Connolly surface). Comparison of the host–guest interactions of C2H6 and C2H4 with JNU-2 at the aperture by DFT calculations; weak hydrogen bonding (2.3 Å < H⋯O < 2.8 Å) is displayed in pink. Reproduced from ref. 41 with permission from American Chemical Society. Copyright 2019.41 | ||
Electronegative atom-rich linkers can markedly increase the multiplicity of ethane–framework contacts and thereby boost C2H6 selectivity. Yet, in many architectures, these heteroatoms compete with metal clusters for coordination, complicating synthetic control and narrowing the accessible design space. A pragmatic and widely adoptable alternative is post-synthetic or pre-synthetic appending of additional electronegative functional groups onto established linkers, which preserves the parent topology while selectively tuning the pore's electronic landscape. Gu et al.42 exemplified this approach by introducing Lewis-basic sites into prototypical zirconium MOFs: amino functionalization of UiO-67 to furnish UiO-67-(NH2)2 creates localized, polarized contact points without perturbing the robust Zr6 node connectivity (Fig. 4a). Under equimolar C2H6/C2H4 (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v) at 296 K and 1 bar, UiO-67-(NH2)2 exhibits an IAST selectivity of 1.7 (Fig. 4b and c), exceeding that of the unmodified UiO-67 (1.49). Grand canonical Monte Carlo (GCMC) simulations and dispersion-corrected density functional theory calculations attribute this gain to the emergence of three additional C–H⋯N interactions per ethane molecule, which cumulatively strengthen ethane binding while minimally affecting ethylene, thereby widening the difference in host–guest interaction energies. A similar functional-site augmentation was reported by Wang et al.,43 who grafted –NH2 groups into Tb-MOF-76 to yield Tb-MOF-76(NH2), an ethane-selective variant that leverages amino-induced weak contacts within an otherwise unchanged pore scaffold. In both systems, appending Lewis-basic substituents creates a multivalent, polarized adsorption environment that favors saturated C2 hydrocarbons through cooperative C–H⋯N interactions, without introducing π-acidic sites that could invert selectivity toward ethylene. Beyond improving selectivity, this strategy retains the mechanical integrity, thermal stability, and defect chemistry of the parent MOFs, facilitating scale-up and shaping.
50 v/v) at 296 K and 1 bar, UiO-67-(NH2)2 exhibits an IAST selectivity of 1.7 (Fig. 4b and c), exceeding that of the unmodified UiO-67 (1.49). Grand canonical Monte Carlo (GCMC) simulations and dispersion-corrected density functional theory calculations attribute this gain to the emergence of three additional C–H⋯N interactions per ethane molecule, which cumulatively strengthen ethane binding while minimally affecting ethylene, thereby widening the difference in host–guest interaction energies. A similar functional-site augmentation was reported by Wang et al.,43 who grafted –NH2 groups into Tb-MOF-76 to yield Tb-MOF-76(NH2), an ethane-selective variant that leverages amino-induced weak contacts within an otherwise unchanged pore scaffold. In both systems, appending Lewis-basic substituents creates a multivalent, polarized adsorption environment that favors saturated C2 hydrocarbons through cooperative C–H⋯N interactions, without introducing π-acidic sites that could invert selectivity toward ethylene. Beyond improving selectivity, this strategy retains the mechanical integrity, thermal stability, and defect chemistry of the parent MOFs, facilitating scale-up and shaping.
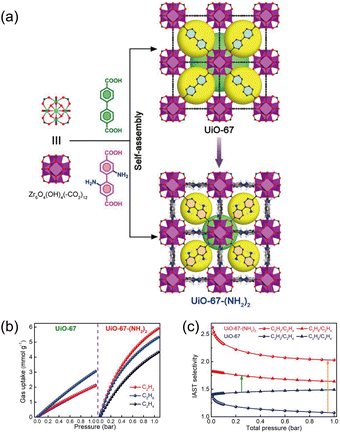 | ||
| Fig. 4 (a) Comparison of the crystal structures of UiO-67 and UiO-67-(NH2)2. (b) Gas adsorption isotherms of UiO-67-(NH2)2 at 296 K. (c) Predicted IAST selectivity curves for 50/50 C2H6/C2H4 and 1/99 C2H2/C2H4 mixtures at 296 K. Reproduced from ref. 42 with permission from American Chemical Society. Copyright 2022.42 | ||
To further amplify the multiplicity and strength of host–guest contacts with ethane, researchers have increasingly adopted highly electronegative fluorinated substituents. Trifluoromethyl groups, in particular, simultaneously enhance dispersive and weak electrostatic interactions without introducing π-acidic character that would favor ethylene. João and co-workers44 exemplified this approach by grafting –CF3 groups onto the archetypal UiO-66 framework to yield the ethane-selective phase UiO-66-2CF3. The incorporation of –CF3 substituents constrains local linker conformations and rotational freedom about node-linker dihedrals while increasing the electron density and polarizability of the channel lining. This modification strengthens non-specific contacts with ethane across a broad pressure window and produces a substantial increase in ethane/ethylene selectivity relative to the parent UiO-66, all while preserving the robust Zr6 inorganic node and overall topology. The result is an electronically tuned, mechanically resilient scaffold that couples high working capacity with enhanced mixed-gas discrimination.
Independent work by Jiang et al.45 further corroborates the efficacy of –CF3 functionalization as a design handle for ethane preference. They synthesized isostructural ethane-selective frameworks M(BTFM)(DABCO)0.5 (M = Zn and Cu; BTFM = 1,3-bis(trifluoromethyl)benzene-derived dicarboxylate), which feature one-dimensional rhombic channels lined with protruding trifluoromethyl groups (Fig. 5a). The –CF3 moieties generate a strongly corrugated, electronegative surface that furnishes a high density of adsorption sites for ethane. At 298 K and 1 bar, both Zn- and Cu-analogues preferentially adsorb ethane, with IAST selectivities of 1.88 and 1.83, respectively, for an equimolar C2H6/C2H4 mixture (Fig. 5b and c). GCMC simulations locate the primary binding regions in proximity to the –CF3 substituents and adjacent phenylene rings within the rhombic pores (Fig. 5d and e), indicating that cooperative dispersive contacts with fluorinated groups and aromatic surfaces govern uptake. Importantly, ethane's non-planar, pseudo-tetrahedral geometry meshes more effectively with the rhombic windows than the planar ethylene molecule, enabling a greater number of stronger van der Waals and weak electrostatic contacts and thereby driving predominant ethane adsorption under competitive conditions.
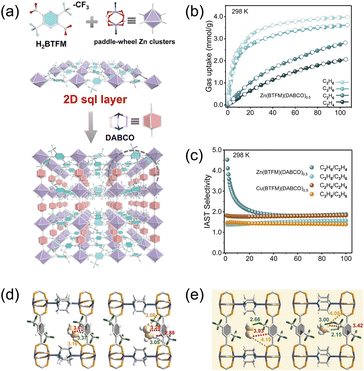 | ||
| Fig. 5 (a) Description of the crystallographic structure of Zn(BTFM)(DABCO)0.5. (b) The C2H4, C2H6, and adsorption isotherms of Zn(BTFM)(DABCO)0.5 at 298 K. (c) The IAST selectivity of C2H6/C2H4 (50/50, v/v) and C3H8/C3H6 (50/50, v/v) at 298 K for Zn(BTFM)(DABCO)0.5 and Cu(BTFM)(DABCO)0.5. (d) DFT calculated adsorption binding sites of C2H4@Zn(BTFM)(DABCO)0.5 and C2H6@Zn(BTFM)(DABCO)0.5. (e) Single-crystal structure of C2H4@Zn(BTFM)(DABCO)0.5 and C2H6@Zn(BTFM)(DABCO)0.5. Reproduced from ref. 45 with permission from Royal Society of Chemistry. Copyright 2022.45 | ||
In certain architectures, electronegative sites introduced to favor ethane binding can be sterically “shielded,” diminishing their accessibility and blunting the intended selectivity. A common source of shielding is coordinated solvent within the pores, which occludes approach pathways and perturbs the local adsorption landscape. Ma et al.46 demonstrated a general remedy by exposing latent electronegative sites through removal of coordinated water, thereby reversing the C2H6/C2H4 selectivity. Crystallization of Y(NO3)3·6H2O with H4TCHB afforded HIAM-317; activation with dichloromethane yielded HIAM-317a, in which water molecules remained coordinated to the metal chains. HIAM-317a presents a narrow pore aperture of 4.8 Å and exhibits kinetic exclusion of ethane, with markedly slower ethane uptake than ethylene at 298 K. Subsequent high-vacuum activation at 300 °C produced HIAM-317b, in which coordinated water was removed, enlarging the pore size to 7.4 Å (Fig. 6a) and transforming the adsorption behavior. DFT calculations and molecular simulations (Fig. 6b and c) reveal that, in HIAM-317b, the dominant binding sites for C2 hydrocarbons reside near carboxylate oxygen atoms and neighboring benzene rings. In HIAM-317a, coordinated water sterically hindered ethane from engaging these oxygen sites; upon water removal, the carboxylate oxygens become accessible, substantially increasing both the number and strength of ethane–framework interactions (Fig. 6d). Breakthrough measurements with an equimolar C2H6/C2H4 feed at 298 K confirm that HIAM-317b selectively captures ethane, enabling the production of high-purity ethylene (>99.99%).
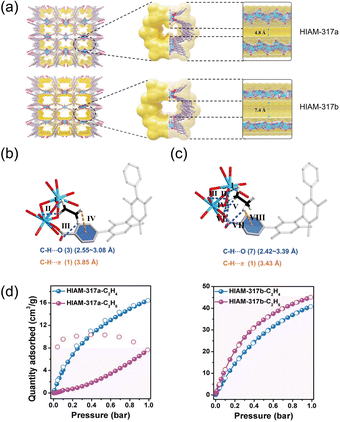 | ||
| Fig. 6 (a) The 3D framework structure and channel environment of HIAM-317a and HIAM-317b (view from a direction). (b) The preferential adsorption sites of HIAM-317b for C2H4 and C2H6; the C–H/p and C–H/O interactions are presented by orange and blue dashed lines, respectively. (d) C2H4/C2H6 isotherms of HIAM-317a and HIAM-317b at 298 K. Reproduced from ref. 46 with permission from Royal Society of Chemistry. Copyright 2024.46 | ||
Beyond increasing the multiplicity of contacts, enhancing the intrinsic strength of ethane–framework interactions is pivotal for increasing C2H6 selectivity. A representative advance is the introduction of metal-peroxo sites, as reported by Li et al.,47 in the synthesis of Fe2(O)2(dobdc) (Fig. 7a). At 298 K and 1 bar, this material exhibits a high equilibrium uptake for ethane (3.32 mmol g−1, Fig. 7b) and an exceptional IAST selectivity (∼4.4) for an equimolar C2H6/C2H4 mixture, enabling efficient discrimination between the two C2 components. High-resolution neutron powder diffraction, complemented by dispersion-corrected DFT calculations, reveals the mechanistic origin of this performance: isotopically labeled C2D6 engages the peroxo functionalities through multiple C–D⋯O contacts, with interaction distances of 2.17–2.22 Å, indicative of strong, directional weak bonds at the adsorption sites. In parallel, the non-planar, pseudo-tetrahedral geometry of ethane conforms better to the non-uniform pore surface of Fe2(O)2(dobdc) than does planar ethylene, thereby increasing the density and magnitude of van der Waals contacts. Together, these effects produce a pronounced energetic bias toward ethane. Process-relevant testing corroborates the thermodynamic selectivity: in a single fixed-bed breakthrough experiment at 298 K and 1.01 bar with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 C2H6/C2H4 feed, Fe2(O)2(dobdc) recovered 0.79 mmol g−1 of C2H4 at >99.99% purity (Fig. 7c).
1 C2H6/C2H4 feed, Fe2(O)2(dobdc) recovered 0.79 mmol g−1 of C2H4 at >99.99% purity (Fig. 7c).
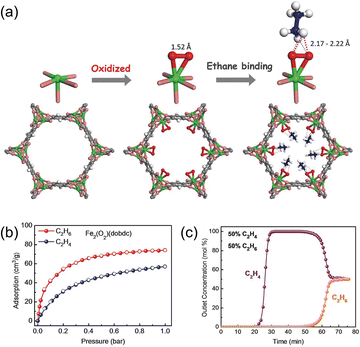 | ||
| Fig. 7 (a) Structures determined from NPD studies. Shown are structures of Fe2(dobdc), Fe2(O2)(dobdc), and Fe2(O2)(dobdc)⊃C2D6 at 7 K. (b) Adsorption (solid) and desorption (open) isotherms of C2H6 (red circles) and C2H4 (blue circles) in Fe2(O2)(dobdc) at 298 K. (c) Experimental column breakthrough curves for a C2H6/C2H4 (50/50) mixture in an absorber bed packed with Fe2(O2)(dobdc) at 298 K and 1.01 bar. Reproduced from ref. 47 with permission from The American Association for the Advancement of Science. Copyright 2018.47 | ||
The synergistic effect of multiple sites with suitable spatial distribution can also enhance the C2H6 affinity of materials. Song et al.48 advanced this concept by tuning the spatial distribution of nitrogen atoms within heteroaromatic traps to intensify cooperative interactions with C2H6. Using post-synthetic modification (PSM) of MOF-808, they grafted indole-5-carboxylic acid (Ind), benzimidazole-5-carboxylic acid (Bzz), and 1H-indazole-5-carboxylic acid (Izo) as targeted molecular traps (Fig. 8a). Among these, MOF-808-Bzz delivered the best ethane/ethylene separation, achieving an IAST selectivity of 1.9 at 298 K and 1 bar for an equimolar C2H6/C2H4 mixture (Fig. 8b). GCMC simulations and DFT calculations pinpoint the governing design rule: both the number and relative placement of nitrogen atoms in the benzofused five-membered rings govern the magnitude of cooperative binding. In MOF-808-Bzz, the benzodiazole motif enables a three-point binding geometry in which ethane simultaneously engages in C–H⋯π contacts with the benzene ring and dual C–H⋯N interactions with the imidazole ring. This spatially complementary arrangement enhances additivity and directionality of weak interactions, yielding a significantly higher ethane binding energy than in the Izo- or Ind-functionalized analogues (Fig. 8a). Fixed-bed breakthrough experiments with an equimolar C2H6/C2H4 feed at 298 K corroborate the dynamic separation advantage of MOF-808-Bzz, evidencing preferential C2H6 capture (Fig. 8c).
 | ||
| Fig. 8 (a) Schematic illustration of the configurations of three gas molecules and varying numbers and locations of functional sites on C2H6–host interactions. (b) Experimental adsorption isotherms of C2H2 (orange circles), C2H4 (cyan circles) and C2H6 (purple circles) on MOF-808-Bzz. (c) Dynamic breakthrough curves for C2H6/C2H4 (50/50, v/v) on MOF-808-Bzz at 298 K with a flow rate of 1 mL min−1, respectively. Reproduced from ref. 48 with permission from John Wiley and Sons. Copyright 2023.48 | ||
These results underscore a broader principle: achieving ethane selectivity via polar site introduction is most effective when sites are organized to enable synergistic, multipoint interactions rather than acting in isolation. In contrast, isolated polar functionalities can interact strongly with ethylene owing to its π electrons and larger quadrupole moment. Thus, precise spatial programming of heteroatom positions to favor multivalent engagement with the nonplanar ethane geometry is critical for maximizing ethane preference while suppressing unintended ethylene stabilization.
3 Ethane-selective MOFs featuring hydrophobic, low-polarity pore surfaces
In frameworks whose internal surfaces are dominated by hydrophobic, weakly polar groups (aromatic or aliphatic), adsorption is governed primarily by dispersion and induction forces that scale with the polarizability of the adsorbate. This provides a clear design lever: engineering low-polarity pore environments enhances noncovalent contacts with the more polarizable ethane (Table 1),6,49 while simultaneously diminishing the framework's affinity for ethylene by avoiding open metal sites and highly polar binding centers that could engage the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond. Notably, this strategy does not demand tight geometric commensurability between the pore shape and cross-section of ethane; instead, selectivity emerges from polarizability-driven interactions across intact surfaces.
C bond. Notably, this strategy does not demand tight geometric commensurability between the pore shape and cross-section of ethane; instead, selectivity emerges from polarizability-driven interactions across intact surfaces.
Pires et al.50 demonstrated this concept with IRMOF-8 (Zn4O(ndc)3; ndc = naphthalene-2,6-dicarboxylate), which displays a clear preference for C2H6 over C2H4. Electrostatic potential (ESP) mapping attributes this difference to the low-polarity naphthalene walls, which engage ethane more strongly than ethylene via enhanced dispersive contacts. Building on this principle, Lin et al.49 realized a highly ethane-selective, low-polarity MOF, Cu(Qc)2, by employing a shorter ligand (HQc = quinoline-5-carboxylic acid) to increase the effective framework–guest contact area. Hydrothermal synthesis from Cu(BF4)2·6H2O and HQc affords a twofold-interpenetrated dia network featuring 1D channels of 3.3 Å (Fig. 9a), where the narrow apertures intensify guest–wall interactions. At 298 K and 1 bar, Cu(Qc)2 adsorbs substantially more ethane (1.85 mmol g−1) than ethylene (0.78 mmol g−1) (Fig. 9b), corresponding to an IAST selectivity of 3.4. DFT-D calculations in conjunction with neutron powder diffraction (Fig. 9c and d) identify multiple C–D⋯π contacts between C2D6 and the internal aromatic rings as the dominant binding motif. These cooperative C–H⋯π interactions, supplemented by other weak supramolecular forces, underpin the preferential uptake of ethane and reinforce a generalizable guideline: contiguous, low-polarity aromatic surfaces can selectively stabilize C2H6 relative to C2H4 through cumulative dispersive and induction interactions.
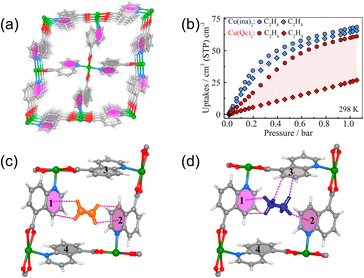 | ||
| Fig. 9 (a) Comparison of crystal structures and channels in Cu(Qc)2. (b) C2H6 and C2H4 sorption isotherms for Cu(ina)2 and Cu(Qc)2 at 298 K. Neutron diffraction crystal structures of [Cu(Qc)2]·0.41C2D6 (c) and [Cu(Qc)2]·0.16C2D4 (d). Reproduced from ref. 49 with permission from American Chemical Society. Copyright 2018.49 | ||
Beyond large, planar π-surfaces such as naphthalene and quinoline, assembling multiple six- and five-membered aromatic rings into the pore lining is a widely adopted route to construct hydrophobic, low-polarity channels. Jiang et al.51 reported a scandium-based framework, ScBPDC, synthesized from Sc(NO3)3·3H2O and biphenyl-4,4′-dicarboxylic acid (H2BPDC). ScBPDC exhibits uniform pores of approximately 8 Å whose walls are densely populated by benzene rings, forming a non-polar environment. Consistent with a polarizability-driven mechanism, ScBPDC exhibits promising C2H6/C2H4 separation with IAST selectivities of 1.5–1.7 at 298 K and 1 bar for an equimolar feed. In subsequent work, Jiang et al.52 corroborated the synergistic contribution of multiple low-polarity aromatic rings using MOF-841, constructed from 4,4′,4″,4‴-methane-tetrayltetrabenzoic acid (H4MTB), whose higher aromatic ring count relative to BPDC further enhances ethane uptake.
The role of preserving uninterrupted low-polarity surfaces was clarified by Ye et al.,53 who examined anion-shielding effects in two Ni-MOFs (Fig. 10a) assembled from NiCl2·6H2O with btz and bdp linkers (btz = 1,4-bis(4H-1,2,4-triazol-4-yl)-benzene; bdp = 1,4-benzene-dipyrazole). In Ni-MOF 1, coordinated Cl− anions protrude into the channels and partially occlude the pore space; Ni-MOF 2 lacks Cl− and thus exposes an uninterrupted aromatic surface of inherently low polarity. The chloride not only perturbs pore geometry but also introduces polar binding sites that compromise ethane selectivity. Accordingly, Ni-MOF 1 displays nearly indistinguishable isotherms for C2H6 and C2H4 (Fig. 10b), whereas Ni-MOF 2 shows clear ethane preference, achieving a C2H6 uptake of 5.94 mmol g−1 and an IAST selectivity of 1.9 at 298 K and 1 bar for an equimolar mixture. Computational site mapping indicates that primary adsorption regions reside at pore corners in both materials. In Ni-MOF 1, chloride ions occupy these corners, sterically masking portions of the five- and six-membered rings and impeding guest access to the ring planes; moreover, the polar Cl− sites interact with both C2H6 and C2H4, with a stronger propensity for ethylene, further eroding selectivity. In contrast, Ni-MOF 2 affords unhindered contact between ethane and the aromatic ring surfaces, enabling C2H6-selective adsorption. Fixed-bed breakthrough experiments with an equimolar feed confirm that Ni-MOF 2 delivers effective dynamic separation with favorable productivity (Fig. 10c).
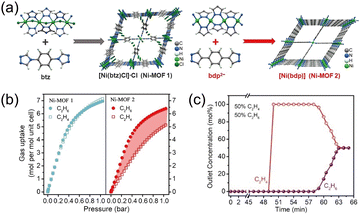 | ||
| Fig. 10 (a) Schematic diagram depicting the starting materials and the three-dimensional open framework of synthesized Ni-MOF 1 and Ni-MOF 2. (b) C2H4 and C2H6 single-component adsorption isotherms of Ni-MOF 1 and Ni-MOF 2 at 298 K. (c) Dynamic breakthrough curves of equimolar C2H6/C2H4 gas mixtures through a separation column filling with the Ni-MOF 2 sample at 298 K and 1 bar. Reproduced from ref. 53 with permission from John Wiley and Sons. Copyright 2023.53 | ||
Besides rigid, planar aromatic surfaces, which inherently limit contact with C2H6 and C2H4 due to their conjugation and resistance to distortion, incorporating aliphatic functionality offers a complementary route to ethane selectivity. Low-polarity alkyl groups (e.g., methyl and methylene) increase the density of van der Waals contact sites and introduce conformational flexibility, allowing local rearrangement within pores to better accommodate the nonplanar geometry of C2H6. Illustratively, Liang et al.54 reported Ni(bdc)(ted)0.5 (bdc = terephthalate; ted = 1,4-diazabicyclo[2.2.2]octane), an aliphatic MOF that, at 298 K and 1 bar, adsorbs 5.0 mmol g−1 of C2H6 with an IAST selectivity of 2 for an equimolar C2H6/C2H4 mixture. Building on this concept, Jiang et al.55 developed a fully aliphatic framework, Ni-MOF-BD, constructed from BODA (bicyclo[2.2.2]octane-1,4-dicarboxylic acid) and ted (Fig. 11a). The intrinsically low polarity of the alkyl-rich pore surface favors ethane binding (Fig. 11b and c). GCMC simulations show that C2H6 preferentially populates regions around the alkyl groups and engages the framework via multiple C–H⋯C dispersive contacts. Similarly, Zhou et al.56 synthesized Cu(bpdc)(ted)0.5 (ZUL-C3) and Ni(bpdc)(ted)0.5 (ZUL-C4) using fully aliphatic H2bpdc (bicyclo[1.1.1]-pentane-1,3-dicarboxylic acid) and ted; methylene-lined channels in both materials exhibit strong attractive interactions with ethane.
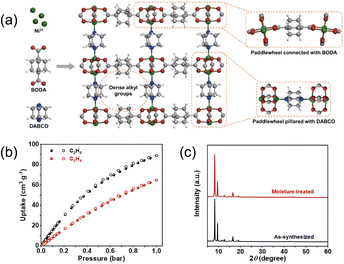 | ||
| Fig. 11 (a) Schematic diagram for the synthesis of Ni-MOF-BD. (b) Adsorption/desorption isotherms of C2H6 and C2H4 on Ni-MOF-BD at 298 K. (c) PXRD patterns of as-synthesized and moisture treated Ni-MOF-BD. Reproduced from ref. 55 with permission from Elsevier. Copyright 2022.55 | ||
Hybrid pore environments that combine conjugated aromatics with aliphatic segments can further enhance ethane discrimination by simultaneously maximizing dispersive contact density and geometric complementarity. Pei et al.57 demonstrated this using ZJU-120 and ZJU-121, where introduction of conjugated aromatic rings narrows the pores to 4.4 Å and 3.7 Å, respectively, while enhancing C2H6 selectivity. Within these confined channels, each C2H6 molecule can establish up to six C–H⋯π contacts with four aromatic rings from two opposing naphthalene units, whereas C2H4 forms only four interactions, yielding a clear energetic advantage for C2H6.
Due to the rigidity of ethylene, it is prone to be restricted by alkyl groups in ultramicropores. Wang et al.58 designed a methyl-rich MOF [Zn(BDC)(H2BPZ)]·4H2O (BPZ = 3,3′,5,5′-tetramethyl-4,4′-bipyrazole), which can effectively achieve ethane selectivity (Fig. 12a and b). Nonpolar pores decorated with benzene rings and methyl groups can effectively generate affinity for ethane and, notably, exert a repulsive interaction with C2H4 owing to the over-close distance between the H atoms of the rigid C2H4 molecule and the methyl groups (Fig. 12c). These two opposing effects—attraction toward ethane and repulsion toward ethylene—simultaneously achieve ethane selectivity.
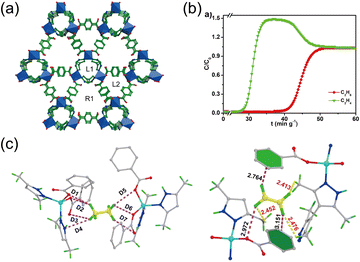 | ||
Fig. 12 (a) 3D structure of [Zn(BDC)(H2BPZ)]·4H2O viewed along the c axis. (b) Breakthrough curves of 1a for C2H6/C2H4(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 298 K. (c) Preferential adsorption sites for C2H6 and C2H4; the C–H⋯π and C–H⋯O/N interactions are displayed as pink lines, and the repulsive interactions are displayed as yellow lines. Reproduced from ref. 58 with permission from John Wiley and Sons. Copyright 2022.58 1) at 298 K. (c) Preferential adsorption sites for C2H6 and C2H4; the C–H⋯π and C–H⋯O/N interactions are displayed as pink lines, and the repulsive interactions are displayed as yellow lines. Reproduced from ref. 58 with permission from John Wiley and Sons. Copyright 2022.58 | ||
Introducing alkyl side chains into isoreticular frameworks provides a convenient route to hydrophobic pore construction. Using a machine learning-assisted workflow, Wang et al.59 designed a methyl-functionalized derivative of MOF-5, designated as A-66. The framework comprises [Zn4(μ4-O)N8O4] nodes, 3,3′,5,5′-tetramethyl-4,4′-bipyrazole (H2bpz-4CH3), and H2bdc (Fig. 13a–c). The resulting low-polarity, hydrophobic pore surfaces preferentially stabilize C2H6, yielding higher ethane uptake than ethylene (Fig. 13d and e). Benefiting from hydrophobicity, A-66 also exhibits notable robustness, retaining crystallinity after ambient exposure or water immersion.
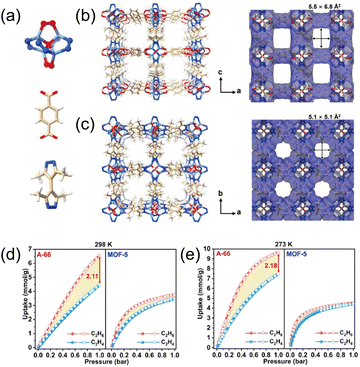 | ||
| Fig. 13 (a) AB-type SBU ([Zn4(μ4-O)N8O4]) and organic linkers (bdc2− and bpz-4CH32−) of A-66. (b) 3D structure and Connolly surface analysis of A-66 viewed along the b axis. (c) 3D structure and Connolly surface analysis of A-66 viewed along the c axis. (d) C2H6 and C2H4 adsorption–desorption isotherms of A-66 and MOF-5 at 298 K. (e) C2H6 and C2H4 adsorption–desorption isotherms of A-66 and MOF-5 at 273 K. Reproduced from ref. 59 with permission from John Wiley and Sons. Copyright 2025.59 | ||
In addition to fully aliphatic channels, sparsely packed perfluoroalkyl groups can also engineer low-polarity, water-stable pores. Yang et al.60 reported a distinctive MOF, Zn-FBA, built from a V-shaped linker, H2FBA, bearing two trifluoromethyl substituents that coordinate to zinc to form a framework with perfluoromethyl-lined channels (Fig. 14a–c). The pore walls are decorated with symmetrically oriented, electronegative fluorine atoms that generate a uniform electrostatic potential and impart low polarity. Zn-FBA maintains structural integrity after three months in air and one week in water (Fig. 14d), with the –CF3 groups contributing to ideal stability, and exhibits high C2H6 affinity (Fig. 14e–g).
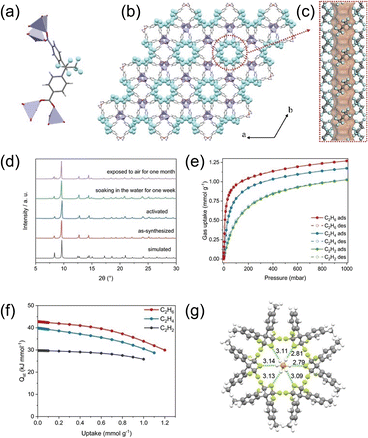 | ||
| Fig. 14 (a) The coordination environment of ZnO4 and FBA2− linkers. (b) Orthographic views down the c axis of Zn-FBA. (c) The hexagonal pore structure in Zn-FBA along the c axis, illustrated by the Connolly surface in orange. (d) PXRD patterns of Zn-FBA with different treatments. (e) C2H2, C2H4, and C2H6 single-component adsorption–desorption isotherms of Zn-FBA at 298 K. (f) Heat of adsorption of C2H6/C2H4/C2H2 on Zn-FBA. (g) DFT-D calculated C2H6 adsorption locations in Zn-FBA. Reproduced from ref. 60 with permission from John Wiley and Sons. Copyright 2022.60 | ||
These examples demonstrated that hydrophobic, low-polarity channels favor C2H6 over C2H4 predominantly via van der Waals (dispersion and induction) interactions. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of ethylene confers lower polarizability than ethane, attenuating its dispersive/inductive stabilization and thereby underpinning ethane selectivity. A practical constraint, however, is that sufficiently strong ethane–surface contacts typically require confined pores and narrow channels, which can limit capacity. Thus, achieving simultaneous high selectivity and high uptake entails careful balancing of the pore size, topology, and surface functionality to maximize the contact density without over-constraining the accessible volume.
C bond of ethylene confers lower polarizability than ethane, attenuating its dispersive/inductive stabilization and thereby underpinning ethane selectivity. A practical constraint, however, is that sufficiently strong ethane–surface contacts typically require confined pores and narrow channels, which can limit capacity. Thus, achieving simultaneous high selectivity and high uptake entails careful balancing of the pore size, topology, and surface functionality to maximize the contact density without over-constraining the accessible volume.
4 Ethane-selective MOFs featuring structural flexibility
Flexible MOFs can undergo stimulus-responsive structural transformations, a feature widely exploited in gas storage and separations. Upon activation, such frameworks may transition from an open to a pore-free state; subsequently, exposure to specific guest molecules can trigger progressive gate opening, allowing uptake as the structure evolves through a characteristic sequence of phases: pore-free → narrow-pore → open-pore. This gate-opening is typically guest-specific, leading to selective recognition and adsorption of probe molecules. Leveraging this behavior for C2H6/C2H4 separations is promising, as first demonstrated by Gücüyener et al. in ZIF-7 in 2010,38 which catalyzed continuing exploration of flexible MOFs for ethane-selective separations.Temperature modulation, which monotonically reduces uptake in rigid MOFs, exerts a more pronounced effect in flexible systems. Yang et al.61,62 reported a globally flexible framework, X-dia-1-Ni, formed by coordinating Ni2+ with 4-(4-pyridyl)-biphenyl-4-carboxylic acid (HL). Solvent removal induces a porous-to-nonporous transition via torsional reconfiguration of the HL linker. To tune the gate response, a bimetallic analogue, X-dia-1-Ni0.89Co0.11, was prepared; both materials are isostructural and exhibit global flexibility (Fig. 15a), showing preferential adsorption of C2H6 over C2H4 (Fig. 15b and c). The selectivity arises because, in the closed-pore state at low pressure, the more conformationally adaptable C2H6 better matches the local pore geometry, yielding stronger host–guest contacts (Fig. 15d and e). Gate-opening pressures for ethane depend on the metal composition, and the flexible uptake is highly temperature-dependent: isotherms at 263–273 K display pronounced gate opening favoring C2H6 in both frameworks, whereas at 298 K, the gate response is suppressed and capacities for both gases decline markedly. These observations highlight strong thermal sensitivity and indicate that effective C2H6/C2H4 separation occurs within a narrow, low-temperature window. Mechanistically, small temperature changes modulate rotation of the HL linker, thereby tuning the global flexibility and gate dynamics in X-dia-1-Ni and its Co-doped counterpart.
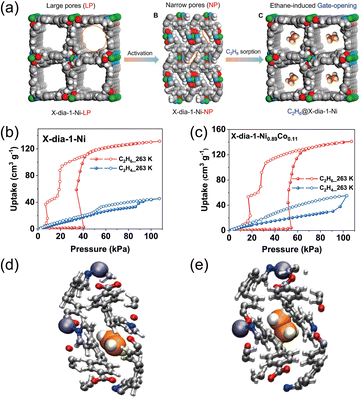 | ||
| Fig. 15 (a) Schematic diagrams of the pore structures of the LP (low pressure) and NP (high pressure) phases of X-dia-1-Ni and C2H6 adsorption induced structural transformation of X-dia-1-Ni. C2H4 and C2H6 adsorption isotherms for (b) X-dia-1-Ni at 263 K and (c) X-dia-1-Ni0.89Co0.11 at 263 K. Orthographic c-axis view of the optimized unit cell of X-dia-1-Ni-NP with (d) C2H6 and (e) C2H4 localized in the material, as obtained by using CP2K. Reproduced from ref. 62 with permission from American Chemical Society. Copyright 2024.62 | ||
To translate flexible, gate-responsive behavior into practical ethane–ethylene separations, the energetic cost of reopening narrow pores must be minimized; otherwise, gate opening remains strongly favored only at low temperature, limiting performance near ambient conditions. Rational control of framework flexibility is therefore essential to trigger structural transformations under mild operating conditions. Yang et al.63 addressed this by designing two isoreticular, ethane-selective flexible MOFs, NTU-101 and NTU-101-NH2, whose key differences reside in the constricted channels formed by interpenetrated networks (Fig. 16a). Introducing –NH2 replaces the three interframework hydrogen bonds present in NTU-101 with a single N–H⋯O interaction, which relaxes the interpenetration constraints and expands the narrow channels in NTU-101-NH2 to 3.9 Å. This pore enlargement improves geometric compatibility with C2H6/C2H4 and lowers the barrier to gate opening at elevated temperatures. Isothermal measurements confirm ethane selectivity in NTU-101-NH2, with the gate-opening pressure shifting to higher values as temperature increases (Fig. 16b), consistent with the enthalpy–entropy balance of the flexible transition. In situ PXRD shows that adsorption-induced expansion accompanies gate activation: the (1 2 1) reflection of NTU-101 and the (0 2 1) reflection of NTU-101-NH2 both shift to lower angles upon dosing, evidencing channel dilation. However, for NTU-101, the smaller constrictions prevent gate opening at 323–333 K, whereas NTU-101-NH2 remains responsive. Complementary in situ IR spectroscopy shows that C2H6/C2H4 primarily engages with the pyrimidine ring, providing the local stimulus for channel expansion. Operationally, NTU-101-NH2 delivers efficient C2H6/C2H4 separation at relatively high temperature (328 K). Under an equimolar feed (1/1, v/v; 2 mL min−1), the breakthrough separation time reaches 9.5 min g−1 (Fig. 16c), affording high-purity C2H4 at 15.7 mL g−1. These results highlight that judicious tuning of interframework interactions via targeted hydrogen-bond reconfiguration can lower the gate-opening energy, enabling ethane-selective, flexible MOFs to function under practical, near-ambient conditions.
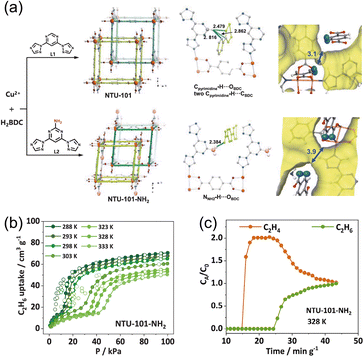 | ||
| Fig. 16 (a) The structures of the two MOFs show the view of the interpenetrated pcu frameworks, the formed H-bonds between the adjacent frameworks, and the narrow necks in NTU-101 and NTU-101-NH2, respectively. The probe radius for inner surface calculation is 1.4 Å. (b) C2H6 adsorption isotherms of NTU-101-NH2. (c) Experimental breakthrough curves of NTU-101-NH2 for C2H6/C2H4 (1/1, v/v, 2 mL min−1) mixtures. Reproduced from ref. 63 with permission from John Wiley and Sons. Copyright 2025.63 | ||
In addition to globally flexible frameworks, MOFs exhibiting local flexibility have garnered increasing interest. In these materials, external stimuli trigger structural adjustments confined to specific regions of the pore architecture. A key advantage is that, even in a constricted state, locally flexible MOFs preserve partial porosity characteristic of rigid frameworks, allowing uptake of C2H6 and C2H4 rather than collapsing into a fully nonporous phase. This attribute is particularly advantageous for C2H6/C2H4 separations under low-pressure conditions. An illustrative example is TYUT-17 reported by Zhang et al. (Fig. 17a),64 which achieves ethane selectivity at ambient temperature through the synergistic interplay of cross-sectional matching within the channels and multiple supramolecular contacts. Single-crystal diffraction reveals that flexibility originates from a gate-like swinging motion of the 3-methylisonicotinate ligands. Notably, C2H6 and C2H4 occupy distinct adsorption sites (Fig. 17b and c) and both engage in multiple C–H⋯N/π interactions with the framework. However, C2H6 resides closer to the channel center, whereas C2H4 is preferentially located near the channel corners. This spatial differentiation enables superior geometric complementarity between C2H6 and the methyl groups on the 3-methylisonicotinate ligands, enhancing cross-sectional matching. In addition, C2H6 molecules situated in the central cavity experience stronger C–H⋯N hydrogen-bonding contacts due to their proximity to the heteroaromatic rings, collectively favoring preferential gate opening for C2H6. At 298 K and 1 bar, TYUT-17 adsorbs 3.0 mmol g−1 of C2H6 (Fig. 17d) and displays IAST selectivities of 6.41 for an equimolar C2H6/C2H4 mixture (50/50, v/v) and 8.92 for a lean-ethane feed (10/90, v/v) (Fig. 17e). Remarkably, it achieves a record C2H6/C2H4 uptake ratio of 3.34 at 0.1 bar. Breakthrough experiments further confirm robust dynamics: at 298 K under an equimolar feed (50/50, v/v), the maximum separation time reaches 22 min g−1, indicating a significantly stronger binding affinity for C2H6 relative to C2H4. Preferential ethane adsorption is retained in the low-pressure regime even at elevated temperatures.
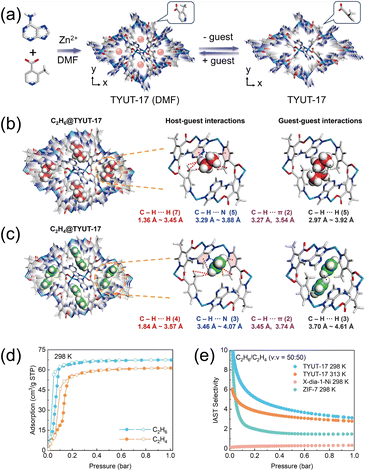 | ||
| Fig. 17 (a) The structural transformation of TYUT-17 between the guest-loaded and guest-free phases revealed by single-crystal X-ray diffraction, in which the 3-methylisonicotinic acid ligand undergoes rotation. Preferential binding sites for (b) C2H6 and (c) C2H4. (d) Single component gas adsorption isotherms for TYUT-17 at 298 K. (e) Comparison of the IAST selectivity with flexible MOFs for C2H6/C2H4 separation. Reproduced from ref. 64 with permission from John Wiley and Sons. Copyright 2025.64 | ||
More broadly, ethane-selective flexible MOFs exploit guest-triggered gate opening to achieve C2H6/C2H4 separation (Table 2). The gate-opening pressure is governed by factors including temperature, total pressure, and the identity/composition of the metal nodes, providing levers to modulate effective pore size and topology and thereby tune selectivity and productivity. Nevertheless, mechanistic understanding of the gate-opening phenomenon remains incomplete, complicating precise control of the activation pressure and temperature windows. This limitation hinders truly controllable separations and raises practical concerns for capturing trace levels of C2H6, where the threshold for gate activation may not be met. Continued advances will hinge on elucidating the energetics and kinetics of local structural responses to guide rational tuning of flexible MOFs for process-relevant conditions.
| Material | C2H6/C2H4 selectivity (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
T (K) | P (bar) | C2H6 uptake (mmol g−1) | High-purity (99.99%) C2H4 yield (L kg−1) | Ref. |
|---|---|---|---|---|---|---|
| a The production temperature of high-purity C2H4 is 293 K. b High-purity C2H4 has a purity of 99.9%. c High-purity C2H4 has a purity of 99.95%. | ||||||
| MAF-49 | 2.73 | 298 | 1 | 1.73 | 6.3 | 36 |
| SNNU-40 | 1.57 | 298 | 1 | 7.54 | — | 37 |
| ZIF-7 | 1.5 | 298 | 1 | 1.83 | 2.4 | 38 |
| TKL-105 | 1.37 | 298 | 1 | 5.62 | — | 39 |
| TKL-106 | 1.5 | 298 | 1 | 5.61 | — | 39 |
| TKL-107 | 1.36 | 298 | 1 | 6.00 | — | 39 |
| Zn(ad)(int) | 2.4 | 298 | 1 | 2.31 | 8.2 | 40 |
| JNU-2 | 1.5 | 298 | 1 | 4.19 | 21.2 | 41 |
| UIO-67-(NH2)2 | 1.7 | 296 | 1 | 5.32 | 12.5 | 42 |
| Tb-MOF-76(NH2) | 2.1 | 298 | 1 | 3.27 | 6.2c | 43 |
| UiO-66-2CF3 | 2.54 | 298 | 1 | 0.88 | — | 44 |
| Zn(BTFM)(DABCO)0.5 | 1.88 | 298 | 1 | 2.74 | 0.55 | 45 |
| HIAM-317b | 1.51 | 298 | 1 | 2.01 | 0.0138 | 46 |
| Fe2(O2)(dobdc) | 4.4 | 298 | 1 | 3.32 | 17.7 | 47 |
| MOF-808-Bzz | 1.9 | 298 | 1 | 2.2 | 2.8 | 48 |
| MOF-808-Ind | 1.23 | 298 | 1 | 1.68 | — | 48 |
| MOF-808-Izo | 1.22 | 298 | 1 | 1.74 | — | 48 |
| Cu(Qc)2 | 1.85 | 298 | 1 | 3.4 | 4.3 | 49 |
| IRMOF-8 | 1.75 | 298 | 1 | 4 | 2.5 | 50 |
| ScBPDC | 1.57 | 298 | 1 | 3.42 | 8.96 | 51 |
| MOF-841 | 1.6 | 298 | 1 | 4.66 | — | 52 |
| Ni-MOF 2 | 1.9 | 298 | 1 | 5.94 | 12c | 53 |
| Ni(bdc)(ted)0.5 | 1.85 | 298 | 1 | 5 | 3.9 | 54 |
| Ni-MOF-BD | 2 | 298 | 1 | 3.97 | — | 55 |
| ZUL-C3 | 2.04 | 298 | 1 | 2.3 | 10.1 | 56 |
| ZUL-C4 | 2.21 | 298 | 1 | 2.4 | 14.6 | 56 |
| ZJU-120a | 2.74 | 296 | 1 | 4.91 | 8.39 | 57 |
| [Zn(BDC)(H2BPZ)]·4H2O | 2.15 | 298 | 1 | 3.75 | — | 58 |
| A-66 | — | 298 | 1 | 4.45 | — | 59 |
| Zn-FBA | 2.9 | 298 | 1 | 1.25 | — | 60 |
| X-dia-1-Ni | 3.72 | 273 | 1 | 5.54 | 2.5 | 62 |
| X-dia-1-Ni0.89Co0.11 | 5.47 | 273 | 1 | 4.96 | — | 62 |
| NTU-101-NH2 | — | 328 | 0.5 | 1.79 | 15.7a | 63 |
| TYUT-17 | 6.41 | 298 | 0.1 | 2.79 | 35.2 | 64 |
| PCN-250 | 1.9 | 298 | 1 | 5.21 | 10.2 | 65 |
| SNNU-181-Mn3+6 | 1.6 | 298 | 1 | 5.49 | 39.6b | 66 |
| MAC-4 | 2.3 | 298 | 1 | 4.78 | 23.6b | 67 |
| LIFM-61 | 1.3 | 298 | 1 | 1.5 | — | 68 |
| LIFM-62 | 1.41 | 298 | 1 | 2.6 | — | 68 |
| LIFM-63 | 1.55 | 298 | 1 | 3.1 | — | 68 |
| ZnFPCP | 2.93 | 298 | 1 | 1.19 | — | 69 |
| NKMOF-Br | 2.65 | 298 | 1 | 4.22 | — | 70 |
| NKMOF-Me | 1.88 | 298 | 1 | 4.82 | — | 70 |
| Ni(IN)2 | 2.45 | 298 | 1 | 3.05 | — | 71 |
| MUF-15 | 1.96 | 298 | 1 | 4.69 | 6.6 | 72 |
| CPM-223-tpbz | 1.51 | 298 | 1 | 6.88 | — | 73 |
| CPM-233 | 1.64 | 298 | 1 | 7.45 | 13.4c | 73 |
| CPM-723 | 1.5 | 298 | 1 | 6.91 | — | 73 |
| CPM-733 | 1.75 | 298 | 1 | 7.13 | 19.7c | 73 |
| MIL-53(Al) | 1.3 | 298 | 1 | 2.05 | — | 74 |
5 Conclusions
This review comprehensively surveys the rapidly evolving field of ethane-selective MOFs for one-step ethylene purification, highlighting significant progress made in understanding and engineering the fundamental mechanisms governing ethane-selective adsorption. The separation strategies are systematically categorized into three primary types: electronegative binding sites, hydrophobic pore environments, and flexible frameworks, providing a robust theoretical framework for the rational design of adsorbents. Meanwhile, the clarification of key structure–property relationships underscores that exceptional ethane/ethylene selectivity can be achieved through precise regulation of pore chemistry, topology, and framework dynamic properties.Guided by these three separation strategies and corresponding structure–property relationships, low-polarity MOFs with local flexibility would exhibit the strongest application potential, as they combine hydrophobic pore environments for structural stability, flexible frameworks for tunable selectivity, and rigid segments for mechanical robustness and industrial shaping. Their separation efficiency is fundamentally governed by the precise regulation of gate-opening pressure, which can be modulated through temperature, mechanical stress, particle morphology, crystal size, and internal structural design. Among these, temperature adjustment provides a practical route for process adaptability, while targeted modifications of metal clusters, topology, and linker functionalization offer molecular-level control.
Looking forward, several promising research directions emerge that warrant further investigation. Future efforts should focus on developing multifunctional MOFs capable of simultaneously addressing multiple separation challenges, including the selective removal of trace ethane impurities while maintaining performance in the presence of co-existing gases such as acetylene and carbon dioxide and moisture. The exploration of dynamic framework behavior under realistic process conditions, combined with advanced computational modelling and operando characterization techniques, will provide deeper insights into the molecular-level mechanisms governing adsorption selectivity.
Additionally, scalability considerations, long-term stability assessments, and economic feasibility analyses will be crucial for transitioning laboratory-scale discoveries to industrial implementation. Current progress highlights the importance of developing green and scalable synthesis routes, particularly through the replacement of toxic amide-based solvents with environmentally benign alternatives. At the same time, reducing production costs by using readily available ligands will be essential for commercialization. Moreover, enhancing water stability and recyclability remains a decisive factor, as many MOFs are vulnerable to moisture-induced degradation. Strategies such as minimizing exposed open metal sites and incorporating hydrophobic ligands provide promising design directions. Overall, while substantial hurdles remain, the convergence of green synthesis, cost-effective ligand selection, and structural stabilization strategies is expected to accelerate the transition of MOFs from laboratory research to practical, industrial-scale ethane/ethylene separation.
The continued advancement of ethane-selective MOF technology holds tremendous potential for transforming ethylene purification processes, offering substantial energy savings, reduced environmental impact, and simplified process configurations compared to conventional cryogenic distillation. As synthetic methodologies continue to evolve and our fundamental understanding of host–guest interactions deepens, the rational design of next-generation MOF adsorbents with tailored separation properties will undoubtedly play a pivotal role in advancing sustainable chemical manufacturing practices.
Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (no. 22225802, 22421004, 22288102, and 223B2803).References
- H. Zimmermann and R. Walzl, Ethylene, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2009, vol. 13, pp. 465–529 Search PubMed.
- L. Zhang, Y. Wang, Y. Chen, Z. Wu, J. Tan, J. Li, B. Chen and L. Li, Coord. Chem. Rev., 2025, 544, 216974 CrossRef CAS.
- H.-G. Hao, Y.-F. Zhao, D.-M. Chen, J.-M. Yu, K. Tan, S. Ma, Y. Chabal, Z.-M. Zhang, J.-M. Dou, Z.-H. Xiao, G. Day, H.-C. Zhou and T.-B. Lu, Angew. Chem., Int. Ed., 2018, 57, 16067–16071 CrossRef CAS.
- D. S. Sholl and R. P. Lively, Nature, 2016, 532, 435–437 CrossRef.
- T. Ren, M. Patel and K. Blok, Energy, 2006, 31, 425–451 CrossRef CAS.
- J.-R. Li, R. J. Kuppler and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC.
- Y. Wang, S. B. Peh and D. Zhao, Small, 2019, 15, 1900058 CrossRef.
- P. J. Bereciartua, Á. Cantín, A. Corma, J. L. Jordá, M. Palomino, F. Rey, S. Valencia, E. W. Corcoran, P. Kortunov, P. I. Ravikovitch, A. Burton, C. Yoon, Y. Wang, C. Paur, J. Guzman, A. R. Bishop and G. L. Casty, Science, 2017, 358, 1068–1071 CrossRef CAS.
- B.-U. Choi, D.-K. Choi, Y.-W. Lee and B.-K. Lee, J. Chem. Eng. Data, 2003, 48, 603–607 CrossRef CAS.
- F. Gao, Y. Wang, X. Wang and S. Wang, Adsorption, 2016, 22, 1013–1022 CrossRef CAS.
- S. Aguado, G. Bergeret, C. Daniel and D. Farrusseng, J. Am. Chem. Soc., 2012, 134, 14635–14637 CrossRef CAS.
- Y. Xie, W. Wang, Z. Zhang, J. Li, B. Gui, J. Sun, D. Yuan and C. Wang, Nat. Commun., 2024, 15, 3008 CrossRef CAS.
- F. Jin, E. Lin, T. Wang, S. Geng, T. Wang, W. Liu, F. Xiong, Z. Wang, Y. Chen, P. Cheng and Z. Zhang, J. Am. Chem. Soc., 2022, 144, 5643–5652 CrossRef CAS.
- S. Kitagawa, R. Kitaura and S. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334–2375 CrossRef CAS.
- Z. Ji, H. Wang, S. Canossa, S. Wuttke and O. M. Yaghi, Adv. Funct. Mater., 2020, 30, 2000238 CrossRef CAS.
- Y. Han, L. Wang, Y. Zhang and B. Chen, Coord. Chem. Rev., 2025, 523, 216291 CrossRef CAS.
- Z. Zhang and D. Zhao, Chem Bio Eng., 2024, 1, 366–380 CrossRef CAS.
- S. Horike, S. Shimomura and S. Kitagawa, Nat. Chem., 2009, 1, 695–704 CrossRef CAS.
- R. Kitaura, K. Seki, G. Akiyama and S. Kitagawa, Angew. Chem., Int. Ed., 2003, 42, 428–431 CrossRef CAS PubMed.
- J. A. Mason, J. Oktawiec, M. K. Taylor, M. R. Hudson, J. Rodriguez, J. E. Bachman, M. I. Gonzalez, A. Cervellino, A. Guagliardi, C. M. Brown, P. L. Llewellyn, N. Masciocchi and J. R. Long, Nature, 2015, 527, 357–361 CrossRef CAS PubMed.
- L. Hamon, P. L. Llewellyn, T. Devic, A. Ghoufi, G. Clet, V. Guillerm, G. D. Pirngruber, G. Maurin, C. Serre, G. Driver, W. van Beek, E. Jolimaître, A. Vimont, M. Daturi and G. Férey, J. Am. Chem. Soc., 2009, 131, 17490–17499 CrossRef CAS.
- Z. Di, C. Liu, J. Pang, S. Zou, Z. Ji, F. Hu, C. Chen, D. Yuan, M. Hong and M. Wu, Angew. Chem., Int. Ed., 2022, 61, e202210343 CrossRef CAS.
- X. Zhang, Z. Chen, X. Liu, S. L. Hanna, X. Wang, R. Taheri-Ledari, A. Maleki, P. Li and O. K. Farha, Chem. Soc. Rev., 2020, 49, 7406–7427 RSC.
- J.-X. Wang, C.-C. Liang, X.-W. Gu, H.-M. Wen, C. Jiang, B. Li, G. Qian and B. Chen, J. Mater. Chem. A, 2022, 10, 17878–17916 RSC.
- B. Zhu, J. W. Cao, S. Mukherjee, T. Pham, T. Zhang, T. Wang, X. Jiang, K. A. Forrest, M. J. Zaworotko and K. J. Chen, J. Am. Chem. Soc., 2021, 143, 1485–1492 CrossRef CAS.
- J. Li, X. Han, X. Kang, Y. Chen, S. Xu, G. L. Smith, E. Tillotson, Y. Cheng, L. J. McCormick McPherson, S. J. Teat, S. Rudic, A. J. Ramirez-Cuesta, S. J. Haigh, M. Schroder and S. Yang, Angew. Chem., Int. Ed., 2021, 60, 15541–15547 CrossRef CAS.
- Z. Zhang, S. B. Peh, Y. Wang, C. Kang, W. Fan and D. Zhao, Angew. Chem., Int. Ed., 2020, 59, 18927–18932 CrossRef CAS.
- M. Zhang, J. Jiang, H. Zhao, Y. Wang, X. He, M. Chen, W. Wang, S. Wang, S. Wang, M. Wang, T. Sun, G. Qin, Y. Tang and H. Cui, Inorg. Chem., 2024, 63, 1507–1512 CrossRef CAS.
- K. Kishida, S. Horike, Y. Watanabe, M. Tahara, Y. Inubushi and S. Kitagawa, Chem. – Asian J., 2014, 9, 1643–1647 CrossRef CAS.
- G.-D. Wang, Y.-Z. Li, W.-J. Shi, L. Hou, Y.-Y. Wang and Z. Zhu, Angew. Chem., Int. Ed., 2022, 61, e202205427 CrossRef CAS PubMed.
- Z. Bao, J. Wang, Z. Zhang, H. Xing, Q. Yang, Y. Yang, H. Wu, R. Krishna, W. Zhou, B. Chen and Q. Ren, Angew. Chem., Int. Ed., 2018, 57, 16020–16025 CrossRef CAS PubMed.
- Z. Bao, S. Alnemrat, L. Yu, I. Vasiliev, Q. Ren, X. Lu and S. Deng, Langmuir, 2011, 27, 13554–13562 CrossRef CAS PubMed.
- H. M. Wen, C. Yu, M. Liu, C. Lin, B. Zhao, H. Wu, W. Zhou, B. Chen and J. Hu, Angew. Chem., Int. Ed., 2023, 62, e202309108 CrossRef CAS.
- R.-B. Lin, L. Li, H.-L. Zhou, H. Wu, C. He, S. Li, R. Krishna, J. Li, W. Zhou and B. Chen, Nat. Mater., 2018, 17, 1128–1133 CrossRef CAS PubMed.
- Y. Sun, S. Hu, J. Yan, T. Ji, L. Liu, M. Wu, X. Guo and Y. Liu, Angew. Chem., Int. Ed., 2023, 62, e202311336 CrossRef CAS.
- P.-Q. Liao, W.-X. Zhang, J.-P. Zhang and X.-M. Chen, Nat. Commun., 2015, 6, 8697 CrossRef PubMed.
- Y.-P. Li, Y.-N. Zhao, S.-N. Li, D.-Q. Yuan, Y.-C. Jiang, X. Bu, M.-C. Hu and Q.-G. Zhai, Adv. Sci., 2021, 8, 2003141 CrossRef CAS PubMed.
- C. Gücüyener, J. van den Bergh, J. Gascon and F. Kapteijn, J. Am. Chem. Soc., 2010, 132, 17704–17706 CrossRef PubMed.
- M.-H. Yu, H. Fang, H.-L. Huang, M. Zhao, Z.-Y. Su, H.-X. Nie, Z. Chang and T.-L. Hu, Small, 2023, 19, 2300821 CrossRef CAS.
- Q. Ding, Z. Zhang, Y. Liu, K. Chai, R. Krishna and S. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202208134 CrossRef CAS.
- H. Zeng, X.-J. Xie, M. Xie, Y.-L. Huang, W. Lu and D. Li, J. Am. Chem. Soc., 2019, 141, 20390–20396 CrossRef CAS PubMed.
- X.-W. Gu, J.-X. Wang, E. Wu, H. Wu, W. Zhou, G. Qian, B. Chen and B. Li, J. Am. Chem. Soc., 2022, 144, 2614–2623 CrossRef CAS.
- G.-D. Wang, R. Krishna, Y.-Z. Li, W.-J. Shi, L. Hou, Y.-Y. Wang and Z. Zhu, Angew. Chem., Int. Ed., 2022, 61, e202213015 CrossRef CAS PubMed.
- J. Pires, J. Fernandes, K. Dedecker, J. R. B. Gomes, G. Perez-Sanchez, F. Nouar, C. Serre and M. L. Pinto, ACS Appl. Mater. Interfaces, 2019, 11, 27410–27421 CrossRef CAS PubMed.
- S. Jiang, J. Li, M. Feng, R. Chen, L. Guo, Q. Xu, L. Chen, F. Shen, Z. Zhang, Y. Yang, Q. Ren, Q. Yang and Z. Bao, J. Mater. Chem. A, 2022, 10, 24127–24136 RSC.
- L.-L. Ma, P. N. Zolotarev, K. Zhou, X. Zhou, J. Liu, J. Miao, S. Li, G. P. Yang, Y.-Y. Wang, D. M. Proserpio, J. Li and H. Wang, Chem. Sci., 2024, 15, 19556–19563 RSC.
- L. Li, R.-B. Lin, R. Krishna, H. Li, S. Xiang, H. Wu, J. Li, W. Zhou and B. Chen, Science, 2018, 362, 443–446 CrossRef CAS PubMed.
- C. Song, F. Zheng, Y. Liu, Q. Yang, Z. Zhang, Q. Ren and Z. Bao, Angew. Chem., Int. Ed., 2023, 62, e202313855 CrossRef CAS.
- R.-B. Lin, H. Wu, L. Li, X.-L. Tang, Z. Li, J. Gao, H. Cui, W. Zhou and B. Chen, J. Am. Chem. Soc., 2018, 140, 12940–12946 CrossRef CAS PubMed.
- J. Pires, M. L. Pinto and V. K. Saini, ACS Appl. Mater. Interfaces, 2014, 6, 12093–12099 CrossRef CAS PubMed.
- S. Jiang, L. Li, L. Guo, C. Song, Q. Yang, Z. Zhang, Y. Yang, Q. Ren and Z. Bao, Sci. China: Chem., 2021, 64, 666–672 CrossRef CAS.
- S. Jiang, L. Guo, L. Chen, C. Song, B. Liu, Q. Yang, Z. Zhang, Y. Yang, Q. Ren and Z. Bao, Chem. Eng. J., 2022, 442, 136152 CrossRef CAS.
- Y. Ye, Y. Xie, Y. Shi, L. Gong, J. Phipps, A. M. Al-Enizi, A. Nafady, B. Chen and S. Ma, Angew. Chem., Int. Ed., 2023, 62, e202302564 CrossRef CAS.
- W. Liang, F. Xu, X. Zhou, J. Xiao, Q. Xia, Y. Li and Z. Li, Chem. Eng. Sci., 2016, 148, 275–281 CrossRef CAS.
- Y. Jiang, S. Jia, X.-Q. Liu, P. Cui and L.-B. Sun, Sep. Purif. Technol., 2022, 295, 121330 CrossRef CAS.
- J. Zhou, T. Ke, X. Zhu, B. Jin, Z. Bao, Z. Zhang, Y. Yang, Q. Ren and Q. Yang, ACS Appl. Mater. Interfaces, 2023, 15, 3387–3394 CrossRef CAS.
- J. Pei, J.-X. Wang, K. Shao, Y. Yang, Y. Cui, H. Wu, W. Zhou, B. Li and G. Qian, J. Mater. Chem. A, 2020, 8, 3613–3620 RSC.
- G.-D. Wang, Y.-Z. Li, W.-J. Shi, L. Hou, Y.-Y. Wang and Z. Zhu, Angew. Chem., Int. Ed., 2022, 61, e202205427 CrossRef CAS PubMed.
- Y. Wang, Z. J. Jiang, W. Lu and D. Li, Angew. Chem., Int. Ed., 2025, 64, e202500783 CrossRef CAS.
- L. Yang, L. Yan, W. Niu, Y. Feng, Q. Fu, S. Zhang, Y. Zhang, L. Li, X. Gu, P. Dai, D. Liu, Q. Zheng and X. Zhao, Angew. Chem., Int. Ed., 2022, 61, e202204046 CrossRef CAS PubMed.
- Q.-Y. Yang, P. Lama, S. Sen, M. Lusi, K.-J. Chen, W.-Y. Gao, M. Shivanna, T. Pham, N. Hosono, S. Kusaka, J. J. Perry IV, S. Ma, B. Space, L. J. Barbour, S. Kitagawa and M. J. Zaworotko, Angew. Chem., Int. Ed., 2018, 57, 5684–5689 CrossRef CAS.
- S.-M. Wang, M. Shivanna, S.-T. Zheng, T. Pham, K. A. Forrest, Q.-Y. Yang, Q. Guan, B. Space, S. Kitagawa and M. J. Zaworotko, J. Am. Chem. Soc., 2024, 146, 4153–4161 CrossRef CAS.
- W. Yang, J. Wang, K. Tan, H. L. Zhou, M. Zhang, R. Krishna, J. Duan and L. Huang, Angew. Chem., Int. Ed., 2025, 64, e202425638 CrossRef CAS.
- L. Zhang, B. Yu, M. Wang, Y. Chen, Y. Wang, L.-B. Sun, Y.-B. Zhang, Z. Zhang, J. Li and L. Li, Angew. Chem., Int. Ed., 2025, 64, e202418853 CrossRef CAS.
- Y. Chen, Z. Qiao, H. Wu, D. Lv, R. Shi, Q. Xia, J. Zhou and Z. Li, Chem. Eng. Sci., 2018, 175, 110–117 CrossRef CAS.
- S.-Y. Li, S.-C. Fan, P. Zhang, W.-Y. Yuan, Y. Wang and Q.-G. Zhai, Chem, 2024, 10, 2761–2775 CAS.
- G.-D. Wang, Y.-Z. Li, R. Krishna, W.-Y. Zhang, L. Hou, Y.-Y. Wang and Z. Zhu, Angew. Chem., Int. Ed., 2024, 63, e202319978 CrossRef CAS PubMed.
- C.-X. Chen, Z.-W. Wei, T. Pham, P.-C. Lan, L. Zhang, K. A. Forrest, S. Chen, A. M. Al-Enizi, A. Nafady, C.-Y. Su and S. Ma, Angew. Chem., Int. Ed., 2021, 60, 9680–9685 CrossRef CAS.
- F. Chen, N. Prasetyo, S. Sakaki, K. I. Otake and S. Kitagawa, Angew. Chem., Int. Ed., 2025, 64, e202423371 CrossRef CAS PubMed.
- S. Geng, E. Lin, X. Li, W. Liu, T. Wang, Z. Wang, D. Sensharma, S. Darwish, Y. H. Andaloussi, T. Pham, P. Cheng, M. J. Zaworotko, Y. Chen and Z. Zhang, J. Am. Chem. Soc., 2021, 143, 8654–8660 CrossRef CAS PubMed.
- M. Kang, S. Yoon, S. Ga, D. W. Kang, S. Han, J. H. Choe, H. Kim, D. W. Kim, Y. G. Chung and C. S. Hong, Adv. Sci., 2021, 8, 2004940 CrossRef CAS.
- O. T. Qazvini, R. Babarao, Z.-L. Shi, Y.-B. Zhang and S. G. Telfer, J. Am. Chem. Soc., 2019, 141, 5014–5020 CrossRef CAS.
- H. Yang, Y. Wang, R. Krishna, X. Jia, Y. Wang, A. N. Hong, C. Dang, H. E. Castillo, X. Bu and P. Feng, J. Am. Chem. Soc., 2020, 142, 2222–2227 CrossRef CAS.
- R. P. P. L. Ribeiro, B. C. R. Camacho, A. Lyubchyk, I. A. A. C. Esteves, F. J. A. L. Cruz and J. P. B. Mota, Microporous Mesoporous Mater., 2016, 230, 154–165 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |




