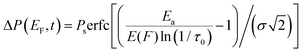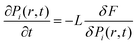Progress in chiral organic ferroelectrics
Yipeng
Zang
ab,
Bolin
Feng
c and
Xiaoqing
Gao
 *ac
*ac
aWenzhou Key Laboratory of Biophysics, Wenzhou Institute, University of Chinese Academy of Sciences, Wenzhou, 325000, China. E-mail: xqgao@ucas.ac.cn
bSchool of Material Science and Engineering, Harbin Institute of Technology, Harbin, 150001, China
cWenzhou Medical University, Wenzhou, 325035, China
First published on 26th November 2025
Abstract
Chiral organic ferroelectrics exhibit advantages including portability, flexibility, and biocompatibility, thereby offering novel solutions for emerging applications such as flexible electronics and implantable medical devices. With the fast development of materials science and technology, this field has advanced from “blind exploration” to the “precision design” of chiral organic ferroelectric materials, significantly accelerating the discovery of high-performance candidates. This review aims to systematically elucidate the dual roles of chirality in chiral organic ferroelectrics: polarity induced by symmetry breaking and dynamic response regulated by topological protection. It thoroughly analyzes the classification and synthesis strategies of various chiral organic ferroelectric systems along the entire “structure–mechanism–performance” chain, aiming to offer a whole picture of this new field.
1. Introduction
In 1848, Louis Pasteur observed that distinct optical activity—specifically optical rotation—was exhibited by different chiral tartaric acid crystals.1–3 This observation laid the foundation for a century of rapid advancement in chiral materials.4–8 Then, in 1920, American physicist Joseph Valasek discovered that potassium sodium tartrate crystals exhibited “anomalous” responses to external forces or electric fields, specifically permanent polarization and hysteresis effects.9,10 This principle established the foundation for the subsequent invention of ferroelectric devices.11–17 Consequently, tartaric acid crystals functioned as a pivotal link connecting two phenomena that initially appeared unrelated: chirality and ferroelectricity.18–22 This discovery further propelled the vigorous development of ferroelectric materials over the subsequent century.23–27 Thereafter, the research focus transitioned from the 1920 discovery of ferroelectricity in Rochelle salt (potassium sodium tartrate) to the contemporary advancement of chiral organic ferroelectrics.28–32Ferroelectric crystals must crystallize in one of the ten polar point groups—1 (C1), 2 (C2), m (Cs), mm2 (C2v), 3 (C3), 3m (C3v), 4 (C4), 4mm (C4v), 6 (C6), and 6mm (C6v). Notably, among the eleven chiral point groups in crystallography, five overlap with the aforementioned polar ferroelectric point groups; this overlap reflects a strong correlation between chiral structures and the formation of ferroelectric order. Chirality, in contrast, significantly enhances the likelihood of a material exhibiting ferroelectricity.33–37
Generally, ferroelectric phase transition mechanisms are categorized into two main types: ionic displacement-driven transitions38–40 and ionic order–disorder transitions.41–43 Organic ferroelectrics predominantly undergo order–disorder phase transitions.44–46
The systematic development of ferroelectrics has long been constrained by a paradigm centered on inorganic materials.47,48 While conventional inorganic ferroelectrics (e.g., BaTiO349,50 and Pb(Zr, Ti)O3 (PZT)51,52) exhibit high piezoelectric coefficients (d33 > 150 pC N−1) and Curie temperatures (Tc > 400 K), their rigid structures, high environmental toxicity (e.g., lead content), and high energy consumption during synthesis restrict their use in flexible electronics and biomedicine.53,54
To address these limitations, two pivotal theoretical advances emerged in 2020: (1) Xiong et al. proposed the “ferroelectrochemistry” framework, which provides systematic guidance for the rational design of molecular ferroelectrics via molecular symmetry control and crystal engineering strategies55,56 and (2) the field of chiral materials entered its “third design phase”—an application-driven stage focused on the precise design of chiral entities to meet practical requirements.57,58 Core strategies derived from these theories include the quasi-spherical theory-based chemical design approach, the homochirality-introducing chemical design approach, and the H/F substitution-based chemical design approach.55 This theoretical foundation has enabled several landmark advances.59–61 Obviously, these two frameworks complement each other, offering novel concepts to overcome the aforementioned limitations of inorganic ferroelectrics and collectively advancing the development of chiral organic ferroelectrics.28,31,62–64
Notably, chirality—an intrinsic feature of molecular asymmetry—has two key functions in ferroelectrics: (1) symmetry breaking-induced polarity: a single chiral molecule (e.g., those with sulfur chiral centers) drives crystal growth in polar space groups (P21), overcoming the symmetry constraints of conventional inorganic ferroelectrics, and (2) topologically regulated dynamic response: chiral helical domains (e.g., Liao's group discovered a multilevel response of chiral domains to a second harmonic generation-circular polarization degree via ferroelectric phase transitions in chiral organic ferroelectrics) enhance polarization-switching fatigue resistance to >107 cycles via a sigmoidal topological protection mechanism.65,66
This review focuses on the latest advances in the “structure–mechanism–performance” research chain of chiral organic ferroelectrics, with the goal of clarifying the significance of incorporating chirality into ferroelectrics and highlighting their unique advantages in addressing the limitations of traditional materials (Fig. 1). By identifying current challenges and open questions, we seek to help researchers develop a deeper, more comprehensive understanding of this cutting-edge field.
 | ||
| Fig. 1 From molecular design for structural diversity to performance tuning for ferroelectric device diversity, reproduced from ref. 79 with permission from [John Wiley and Sons Ltd.] [S. Sahoo, et al., A Chiral B–N Adduct as a New Frontier in Ferroelectrics and Piezoelectric Energy Harvesting, Angew. Chem., Int. Ed., 2024, 63, e202400366. https://doi.org/10.1002/anie.202400366], copyright 2024; ref. 80 with permission from [American Chemical Society] [H.-P. Lv, Y.-R. Li, X.-J. Song, N. Zhang, R.-G. Xiong and H.-Y. Zhang, A poling-free supramolecular crown ether compound with large piezoelectricity, J. Am. Chem. Soc., 2023, 145(5), 3187–3195. https://doi.org/10.1021/jacs.2c12951], copyright 2023; ref. 92 with permission from [American Physical Society.] [H.-Y. Zhang, et al., Ferroelectric phase transition driven by switchable covalent bonds, Phys. Rev. Lett., 2023, 130(17), 176802. https://doi.org/10.1103/PhysRevLett.130.176802], copyright 2023; ref. 104 with permission from [Springer Nature] [S. Bera, S. Guerin, H. Yuan, et al., Molecular engineering of piezoelectricity in collagen-mimicking peptide assemblies, Nat. Commun., 2021, 12, 2634. https://doi.org/10.1038/s41467-021-22895-6], copyright 2021; ref. 66 with permission from [American Chemical Society] [X.-J. Song, et al. Enantiomeric Ferroelectric Chiral Domains, J. Am. Chem. Soc., 2025, 147(19), 16568–16577. https://doi.org/10.1021/jacs.5c04038], copyright 2025; ref. 69 with permission from [National Academy of Sciences] [P. Li, W. Liao, Y. Tang, W. Qiao, D. Zhao, Y. Ai, Y. Yao, R. Xiong, Organic enantiomeric high-Tc ferroelectrics, Proc. Natl. Acad. Sci. U. S. A., 2019, 116(13), 5878–5885, https://doi.org/10.1073/pnas.1817866116], copyright 2019; ref. 125 with permission from [American Chemical Society] [C.-H. Ma, Y.-K. Liao, Y. Zheng, S. Zhuang, S.-C. Lu, P.-W. Shao, J.-W. Chen, Y.-H. Lai, P. Yu, J.-M. Hu, R. Huang and Y.-H. Chu, ACS Appl. Mater. Interfaces, 2022, 14(19), 22278–22286. https://doi.org/10.1021/acsami.2c02281.], copyright 2022; ref. 126 with permission from [Elsevier BV.] [ H. Wang, G. Gou and J. Li. Ruddlesden–Popper perovskite sulfides A3B2S7: A new family of ferroelectric photovoltaic materials for the visible spectrum. Nano Energy, 2016, 22, 507–513. https://doi.org/10.1016/j.nanoen.2016.02.036], copyright 2016; ref. 94 with permission from [John Wiley and Sons Ltd.] [H. Y. Zhang, et al. Ferroelectric lithography in single-component organic enantiomorphic ferroelectrics. Angew. Chem., Int. Ed., 2022, 134, e202200135.], copyright 2022. | ||
2. Structural diversity
This section will systematically analyze the structural diversity of chiral organic ferroelectric materials based on the theoretical framework of “ferroelectrochemistry”—a diversity primarily derived from the synergistic regulation of molecular symmetry breaking and assembly patterns. Meanwhile, it will delve into four main structural types: single-component chiral molecules, host–guest complexes, dynamic valent systems, and dynamic response systems, and deeply dissect the correlation mechanism between their design strategies and the resulting properties.2.1. Single-component chiral molecular systems
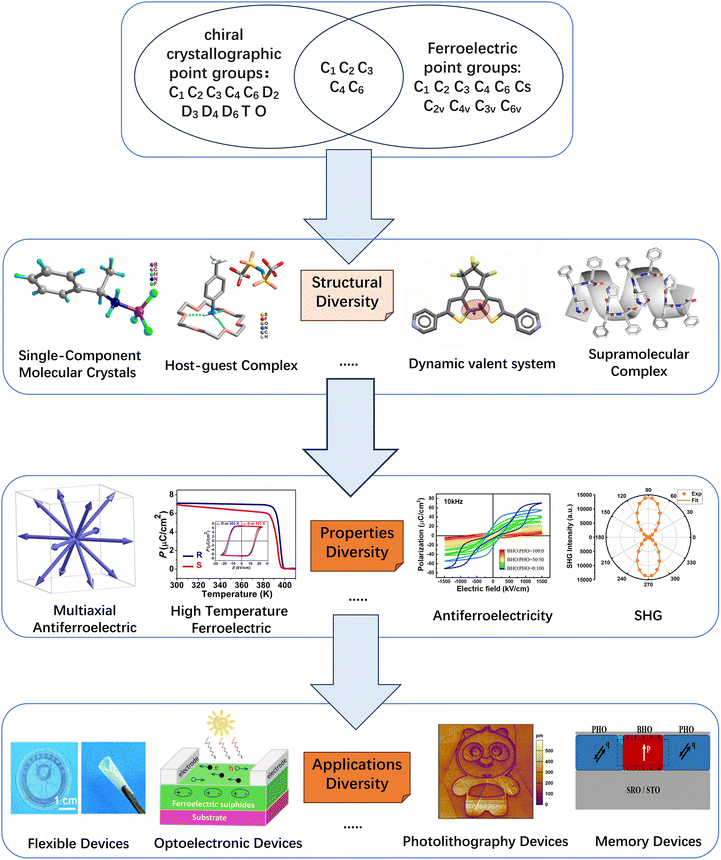
Single-component chiral molecular systems achieve ferroelectricity by introducing chiral centers into a single molecular component to induce non-centrosymmetric crystal structures. As typical representatives in chiral organic ferroelectric research, these materials include carbon-chiral photoisomer systems, nitrogen-chiral ferroelectrics and sulfur-chiral ferroelectrics.67–71Carbon chirality plays a pivotal role in chiral organic ferroelectrics. For instance, the organic molecular crystal (R/S)-3-quinuclidinol belongs to the P61/P65 space group (Fig. 2a), exhibiting a high Curie temperature (Tc) of 400 K and a saturation polarization (Ps) of 7 µC cm−2 (Fig. 2b).69 Another example is the flexible molecular crystal R-3-hydroxyquinuclidinium Cl, which crystallizes in the chiral P41 space group and exhibits a piezoelectric response with Tc = 340 K.70 Notably, carbon chirality is also prominent in amino acid and polysaccharide composite ferroelectrics, which offer excellent biocompatibility.72–76 For example, glycine-PVA composite films prepared via self-assembly exhibit a piezoelectric coefficient of 5.3 pC N−1 (Fig. 2b).73 Strong hydrogen bonds (O–H⋯O, bond length 2.7 Å) at the PVA–glycine interface induce the self-assembly of γ-glycine crystals throughout the membrane, forming a sandwich heterostructure—critical for achieving a strong macroscopic piezoelectric effect while maintaining exceptional flexibility. Similarly, glycine–PEO composite films (with polyethylene oxide) form a sandwich structure, reaching a piezoelectric coefficient of up to 8.2 pC N−1, attributed to hydrophobic interfaces (e.g., PTFE, polytetrafluoroethylene) inducing oriented alignment of PVA molecules (Fig. 2c).74 Further ultrasonic-assisted processing can elevate d33 to 10.4 pC N−1.77
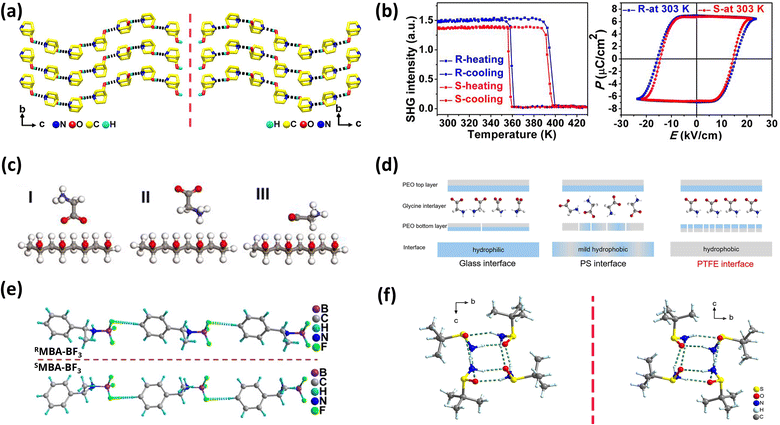 | ||
| Fig. 2 (a) The infinite hydrogen-bonded helical chains in (R)-3-quinuclidinol and (S)-3-quinuclidinol, showing a mirror-image relationship; (b) (R)- and (S)-3-quinuclidinol of SHG (second harmonic generation effect) response (middle) and P–E hysteresis loops recorded at 303 K, reproduced from ref. 69 with permission from [National Academy of Sciences] [P. Li, W. Liao, Y. Tang, W. Qiao, D. Zhao, Y. Ai, Y. Yao and R. Xiong, Organic enantiomeric high-Tc ferroelectrics, Proc. Natl. Acad. Sci. U. S. A., 2019, 116(13) 5878–5885, https://doi.org/10.1073/pnas.1817866116.], copyright 2019. (c) Three possible ways glycine bonds with the polyvinyl alcohol chain, reproduced from ref. 73 with permission from [American Association for the Advancement of Science] [F. Yang et al. Wafer-scale heterostructured piezoelectric bio-organic thin films. Science, 2021, 373, 337–342. https://doi.org/10.1126/science.abf2155], copyright 2021; (d) schematic of the bottom PEO layer induced by different interfaces and the orientation of γ-glycine crystals, reproduced from ref. 74 with permission from [Elsevier BV] [Q. Yu, Y. Bai, Z. Li, F. Jiang, R. Luo, Y. Gai, Z. Liu, L. Zhou, Y. Wang, C. Li, K. Ren, D. Luo, H. Meng and Z. Li, Interface-induced high piezoelectric γ-glycine-based flexible biodegradable films, Nano Energy, 2024, 121, 109196, https://doi.org/10.1016/j.nanoen.2023.109196.], copyright 2023. (e) H-bonding interactions present in R/SMBA-BF3, reproduced from ref. 79 with permission from [John Wiley and Sons Ltd.] [S. Sahoo, et al. A Chiral B–N Adduct as a New Frontier in Ferroelectrics and Piezoelectric Energy Harvesting. Angew. Chem., Int. Ed. 2024, 63, e202400366. https://doi.org/10.1002/anie.202400366], copyright 2024; (f) crystal structures of Rs-tBuSA, reproduced from ref. 59 with permission from [John Wiley and Sons Ltd.] [H. Peng, Z.-K. Xu, Y. Du, P.-F. Li, Z.-X. Wang, R.-G. Xiong, W.-Q. Liao, The First Enantiomeric Stereogenic Sulfur-Chiral Organic Ferroelectric Crystals. Angew. Chem., Int. Ed., 2023, 62, e202306732.], copyright 2023. | ||
Additionally, layered chitosan–PVA composite films can be prepared via solution diffusion or electrospinning. These films exhibit excellent acoustic impedance matching: their acoustic impedance ranges from 1.2 to 1.4 g cm−3, highly compatible with muscle tissue (1.04–1.06 g cm−3), making them ideal for biomedical ultrasound applications (Fig. 2d).75,78
Nitrogen-chiral systems also exhibit excellent ferroelectric properties. [R/SC6H5CH(CH3)NH2BF3](R/SMBA-BF3) crystals crystallize in the polar chiral monoclinic P21 space group (Fig. 2e), with a residual polarization (Pr) of 7.65 µC cm−2, a piezoelectric coefficient (d33) of 3.5 pC N−1, and characteristics of triple-axis reorientability and high-frequency response—making them promising for piezoelectric energy harvesting and flexible electronics.79
As a milestone achievement in the field of sulfur-chiral materials developed by Liao's group, Rs/Ss-tBuSA features a core design that incorporates a single chiral center and an H/F substitution strategy: its ingenious symmetry-breaking mechanism involves the inherent steric hindrance of the sulfur chiral center, which synergizes with the highly sterically hindered tert-butyl group to break molecular symmetry, while the intermolecular N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S hydrogen bond network further reduces crystal symmetry—ultimately leading to crystallization in the chiral polar space group P21 (Fig. 2f).59
S hydrogen bond network further reduces crystal symmetry—ultimately leading to crystallization in the chiral polar space group P21 (Fig. 2f).59
In terms of ferroelectric properties, Rs/Ss-tBuSA undergoes a 432F2-type high-temperature crystalline plastic ferroelectric phase transition at ∼348 K; it retains C2 point group symmetry post-transition and exhibits multi-axis ferroelectric properties featuring 12 equivalent polarization directions. Polarization reversal (with a polarization value of 0.3 µC cm−2) can be achieved by applying an external electric field, showcasing its potential for rapid response (Fig. 2f).59
Performance parameter differences (Tc, Ps, and d33) across chiral centers (carbon, nitrogen, and sulfur) indicate that the specific atoms serving as chiral centers, along with their local molecular environment and conformation, profoundly impact the final performance of chiral organic ferroelectrics. For example, (R/S)-3-quinuclidinol has a Tc of up to 400 K, compared to 348 K for Rs/Ss-tBuSA, suggesting that carbon chirality can sometimes provide higher thermal stability. Thus, chiral centers should be strategically selected based on specific application requirements to achieve optimal ferroelectric performance.
2.2. Host–guest complex systems
Host–guest complex systems leverage the confinement effect of macrocyclic molecules (hosts)—such as crown ethers and cyclodextrins—to realize the orderly arrangement and polarization enhancement of guest molecule dipoles. This strategy overcomes the limitations of traditional external field polarization, significantly improves molecular orderliness, and provides a versatile route to ferroelectricity.As a classic crown ether-confined example, [(CF3–C6H4–NH3)(18-crown-6)][TFSA] achieves ferroelectricity without external field polarization via precise host–guest engineering, relying on three synergistic mechanisms. Firstly, cavity confinement ensures structural order: the 18-crown-6 cavity (diameter 2.6–3.2 Å) precisely matches the guest cation CF3–C6H4–NH3+, fixing the cation's orientation through N–H⋯O hydrogen bonds to form a highly ordered polar supramolecular chain (Fig. 3a). Secondly, dynamic conformational regulation optimizes phase transition: driven by F⋯F halogen bonds (72.3 meV, ∼7 kJ mol−1), and the TFSA− anion (TFSA = bis(trifluoromethanesulfonyl)ammonium) undergoes a chair-to-boat transformation during phase transition. The C–F⋯O hydrogen bond (bonding energy 25 kJ mol−1) and π–π stacking synergistically enhance the stability of polarization, with the Curie temperature also increasing by 35 K. Thirdly, multi-level noncovalent interactions enhance performance: C–F⋯π interactions (≈3.4 Å) and π–π stacking (benzene spacing ≈4.2 Å) improve structural stability, which—combined with low elastic stiffness (C11 = 10.89 GPa and C22 = 8 GPa)—yields a high piezoelectric response (d33 = 42 pC N−1, Fig. 3a). This sophisticated supramolecular design (size matching, conformational dynamics, and multi-interactions) collectively drives dipole ordering and spontaneous polarization, demonstrating supramolecular engineering's potential in regulating material performance.80
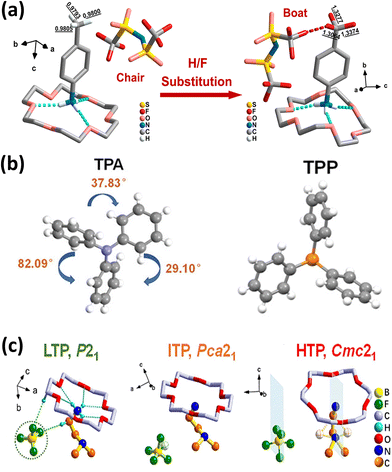 | ||
| Fig. 3 (a) The structural unit of [(CH3/CF3–C6H4–NH3)(18-crown-6)][TFSA] in the low-temperature phase exhibits a host–guest structure, reproduced from ref. 80 with permission from [American Chemical Society] [H.-P. Lv, Y.-R. Li, X.-J. Song, N. Zhang, R.-G. Xiong and H.-Y. Zhang. A poling-free supramolecular crown ether compound with large piezoelectricity. J. Am. Chem. Soc. 2023, 145(5), 3187–3195. https://doi.org/10.1021/jacs.2c12951], copyright 2023. (b) Crystal structures of TPA and TPP, reproduced from ref. 81 with permission from [Wiley-VCH Verlag.] [J. Yang, X. Wu, J. Shi, B. Tong, Y. Lei, Z. Cai and Y. Dong, Achieving Efficient Phosphorescence and Mechanoluminescence in Organic Host–Guest System by Energy Transfer. Adv. Funct. Mater., 2021, 31, 2108072. https://doi.org/10.1002/adfm.202108072], copyright 2021. (c) Asymmetric units of MCBF as LTP, ITP and HTP, respectively, reproduced from ref. 82 with permission from [Elsevier] [M.-M. Lun, J.-Q. Luo, Z.-X. Zhang, J. Li, L.-Y. Xie, H.-F. Lu, Y. Zhang and D.-W. Fu, Piezoelectric self-power supply driven by ferroelastic host–guest supramolecule with considerable electromechanical conversion capability, Chem. Eng. J., 2023, 475, 145969, https://doi.org/10.1016/j.cej.2023.145969.], copyright 2023. | ||
As a typical example of organic host–guest doping, the doped system featuring triphenylamine (TPA) as the host and compounds containing 2–4 TPA repeating units (2TPA, 3TPA, 4TPAB, and 4TPAL) as guests can form host–guest eutectics—belonging to the noncentrosymmetric polar space group C1c1 (Fig. 3b). C–H⋯π interactions (bond lengths 2.745–2.872 Å) form between TPA molecules, stabilizing the guest molecules while providing structural support for the crystal's piezoelectricity. The three benzene rings in TPA molecules adopt a highly twisted conformation, imparting intrinsic polarity to the molecule. The non-centrosymmetric space group (C1c1) of its crystal further amplifies this polarity, forming the structural basis for macroscopic piezoelectric response—the crystal structure readily deforms under external force, generating a significant piezoelectric response.81
Another host–guest supramolecular compound, [(N,N-dimethylethylene-di-ammonium)(18-crown-6)]BF4 (MCBF), features a crown ether as the host and an organic cation as the guest (Fig. 3c).82 By controlling the crown ether type and the monoprotonated state of the organic cation, this approach enables MCBF crystallization within the chiral polar space group P21, achieving a longitudinal piezoelectric coefficient d22 of 46.1 pC N−1 and a piezoelectric voltage coefficient g22 of 1000 × 10−3 V m N−1 at room temperature. The Curie temperature Tc = 336 K corresponds to an Aizu notation mm2F2-type ferroelastic phase transition. The exceptional piezoelectric response of MCBF stems from two key factors: first, intrinsic strain induced by ferroelastic properties (with piezoelectric coefficients markedly enhanced after 0.041 strain application) and second, the formation of a metastable intermediate-temperature phase under external stress, creating a quasi-isotropic phase boundary that further amplifies electromechanical conversion capabilities.
Template-induced crystallization of crown ether-confined complexes represents a highly precise supramolecular engineering method, in which host–guest interactions serve as nanoscale templates to guide molecular orientation and stacking. This approach enhances crystallinity, improves polarization stability, and increases the Curie temperature, typically without the need for an external polarization field, highlighting the powerful capabilities of self-assembly guided by molecular recognition.80–84
2.3. Dynamic valent system
Dynamic valent systems utilize the reversible rearrangement properties of dynamic valent bonds (such as covalent and hydrogen bonds) to achieve in situ control of the material structure and performance, endowing materials with self-healing and self-adaptive capabilities.85–90The interlocking covalent adaptive networks (ICANs) jointly developed by Zhang and Chen's groups achieve synergistic regulation of dual dynamic bonds through the parallel cross-linking of imine bonds (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) and disulfide bonds (Fig. 4a). The core design feature lies in pH-responsive reversible bonding: imine bonds selectively hydrolyze and break under organic alcohol or acidic conditions (pH < 4 is optimal), while they reform under neutral or alkaline conditions.91 Moreover, the dynamic disulfide bond crosslinking strategy developed by Hu's group introduces dynamic disulfide bonds into the polymer network to construct covalent adaptive networks (CANs) (Fig. 4b). They found that unidirectional multi-effect compression of foam at 130 °C induces a transformation of the foam's cell structure from its initial circular open-cell structure to an inward-concave cell structure. This structural transformation is key to unlocking negative Poisson's ratio (NPR) behavior.85
N) and disulfide bonds (Fig. 4a). The core design feature lies in pH-responsive reversible bonding: imine bonds selectively hydrolyze and break under organic alcohol or acidic conditions (pH < 4 is optimal), while they reform under neutral or alkaline conditions.91 Moreover, the dynamic disulfide bond crosslinking strategy developed by Hu's group introduces dynamic disulfide bonds into the polymer network to construct covalent adaptive networks (CANs) (Fig. 4b). They found that unidirectional multi-effect compression of foam at 130 °C induces a transformation of the foam's cell structure from its initial circular open-cell structure to an inward-concave cell structure. This structural transformation is key to unlocking negative Poisson's ratio (NPR) behavior.85
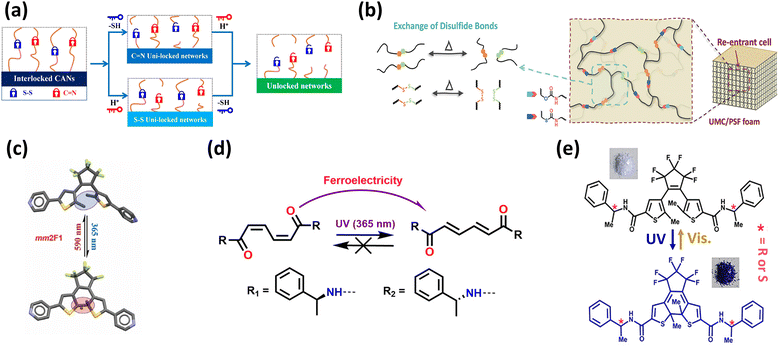 | ||
| Fig. 4 (a) Interlocking and unlocking mechanism of CANs, reproduced from ref. 91 with permission from [American Chemical Society] [S. Xiang, L. Zhou, R. Chen, K. Zhang and M. Chen. Interlocked covalent adaptable networks and composites relying on parallel connection of aromatic disulfide and aromatic imine cross-links in epoxy. Macromolecules, 2022, 55(23), 10276–10284. https://doi.org/10.1021/acs.macromol.2c01912], copyright 2022. (b) Convert them into auxiliary foam (UMC/PSF) through dynamic disulfide bond exchange, reproduced from ref. 85 with permission from [Wiley-VCH Verlag] [Z. Guo, Z. Dong, J. Gao, H. Zhang, B. Zhang, M. H. Li and J. Hu, Unlocking Auxetic Behavior in Recyclable Thermosetting Foams Enabled by Dynamic Disulfide Cross-linking Strategy. Small, 2024, 20, 2406876. https://doi.org/10.1002/smll.202406876], copyright 2024. (c) The ferroelectric phase transition in DTh-Py is driven by light-induced switchable covalent bonds, reproduced from ref. 92 with permission from [American Physical Society.] [H.-Y. Zhang, et al. Ferroelectric phase transition driven by switchable covalent bonds. Phys. Rev. Lett., 2023, 130, 176802. https://doi.org/10.1103/PhysRevLett.130.176802], copyright 2023. (d) ZE light isomerization process, reproduced from ref. 94 with permission from [John Wiley and Sons Ltd.] [H. Y. Zhang, et al. Ferroelectric lithography in single-component organic enantiomorphic ferroelectrics. Angew. Chem., Int. Ed., 2022, 134 e202200135. https://doi.org/10.1002/ange.202200135], copyright 2022. (e) Configuration changes and photochromism of RR and SS chemical structures under light exposure, reproduced from ref. 95 with permission from [American Chemical Society] [Y.-Y. Tang, Y.-L. Zeng and R.-G. Xiong., Contactless manipulation of write–read–erase data storage in diarylethene ferroelectric crystals. J. Am. Chem. Soc., 2022, 144(19), 8633–8640. https://doi.org/10.1021/jacs.2c01069], copyright 2022. | ||
Another notable dynamic valent ferroelectric, R/SMBA-BF3, crystallizes in the polar chiral monoclinic space group P21 and shows potential for piezoelectric energy harvesting (Fig. 2e). Its innovation lies in synergizing polar bonds with hydrogen bond networks, featuring three key strengths. For polar chain construction, BF3 receptors (host-like moieties) form highly polar B–N bonds (ΔEN = 1.0) via coordination with chiral amine donors (guest-like moieties); these bonds synergize with C–H⋯F hydrogen bonds to build a 1D polar chain, enabling long-range dipole ordering. The material exhibits ferroelectric (Pr = 7.65 µC cm−2), piezoelectric (d33 = 3.5 pC N−1), and nonlinear optical properties (SHG = 0.41 × KDP).79 This synergy yields robust, multifunctional, non-toxic ferroelectrics, paving the way for environmentally friendly ferroelectric development.
Zhang's group developed a photochromic ferroelectric, DTh-Py, which undergoes a light-driven ferroelectric phase transition through π–σ bond photoisomerization (Fig. 4c). Exposure to 365 nm UV light triggers an mm2 F1-type ferroelectric phase transition, transforming the crystal from a closed-loop to an open-loop state. This process involves the breaking of crystal symmetry via intramolecular covalent bond reorganization, leading to a polar-to-nonpolar transition and a flip of the polarization axis—a mechanism fundamentally distinct from traditional temperature/electric field-driven processes. During synthesis, a multi-step organic reaction was employed to construct the thiophene–pyridine photoresponsive matrix, followed by single crystal growth via solvent evaporation. X-ray diffraction confirmed the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C to C–C bonding transition in the thiophene ring and the reconfiguration of the hydrogen-bonding network. This system represents a breakthrough in ferroelectric mechanisms, as the phase transition is driven by covalent bond breaking and reorganization rather than mere molecular rearrangement. It provides a high-precision, reversible, light-controlled ferroelectric switching mode and substantially expands our understanding of ferroelectric phase transition types.92
C to C–C bonding transition in the thiophene ring and the reconfiguration of the hydrogen-bonding network. This system represents a breakthrough in ferroelectric mechanisms, as the phase transition is driven by covalent bond breaking and reorganization rather than mere molecular rearrangement. It provides a high-precision, reversible, light-controlled ferroelectric switching mode and substantially expands our understanding of ferroelectric phase transition types.92
Dynamic bonds offer a novel perspective for synthesizing and expanding the applications of chiral organic ferroelectric compounds, liberating ferroelectric phase transitions from the constraints of ion displacement and order–disorder models. Instead, they introduce an entirely new bonding paradigm—the formation and breaking of chemical bonds. From a microscopic intrinsic viewpoint, the reversible rearrangement of dynamic valence bonds (such as covalent bonds and hydrogen bonds) explains the differences in extrinsic properties (such as self-healing and piezoelectric response). This further demonstrates the guiding role of ferroelectric chemistry theory—crystal engineering—in the development of chiral organic ferroelectrics.
2.4. Dynamic response system
The dynamic response system of chiral organic ferroelectrics, particularly the light- and electric field-driven polarization modulation and phase transition behaviors, has emerged as a research focus in the field of advanced materials due to its promising applications in optoelectronic devices, data storage, and high-temperature sensors.85,87–89,93Certain valeric acid derivatives, such as (R,R)-(E,E)-1, undergo (Z,Z)-to-(E,E) isomerization upon irradiation with 365 nm ultraviolet light, accompanied by a molecular conformation transition from a benzene ring reverse-bent state to a collinear extended state with a benzene ring dihedral angle of 22.9°. This isomerization induces the formation of the polar chiral space group P21, driving the molecules to align along the b-axis in a polar manner (Fig. 4d).94
Tang's group demonstrated that fluorinated diaryl ethylene derivatives can achieve polarization switching via photoisomerization, thereby realizing bistable storage (from the white bicluster to the blue monocluster) under visible/UV light irradiation (Fig. 4e). This phenomenon is attributed to a symmetry transition from the chiral P21 to P41 space group.95
Additionally, the novel organic ferroelectric (TFTBSA-1, 3,4,5-trifluoro-N-(3,5-di-tert-butylsalicylidene)aniline) achieves non-destructive light-controlled polarization reversal through enol–ketone photoisomerization mediated by intramolecular hydrogen bonding. Its dielectric constant and spontaneous polarization can be reversibly switched via light-induced phase transitions.96–98 For example, Xiong's group reported the reaction between 3,5-di-tert-butylsalicylaldehyde and 3,4,5-trifluoroaniline. Under different reaction conditions, two crystalline phases are formed: the polar phase TFTBSA-1 (space group Pna21, exhibiting ferroelectricity) and the nonpolar phase TFTBSA-2 (space group P21/n, lacking ferroelectricity). At room temperature, TFTBSA-1 exists in the enol form, stabilized by intramolecular O–H⋯N hydrogen bonding, with a dipole moment of 3.47 D. Upon exposure to 365 nm UV light, it converts to the trans-ketone form, where the dipole moment increases to 4.42 D, the crystal color changes from yellow to orange, and the dielectric constant decreases from 4.65–4.70 to 4.25–4.30. Irradiation with 488 nm visible light enables reversible recovery to the enol form, verifying the feasibility of a light-controlled ferroelectric switch (Fig. 5a).98
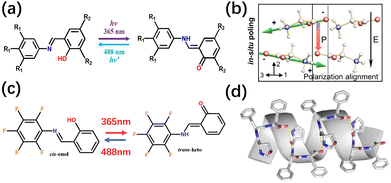 | ||
| Fig. 5 (a) TFTBSA-1 undergoes enol–keto photoisomerization under ultraviolet irradiation, reproduced from ref. 98 with permission from [American Chemical Society] [Y.-Y. Tang, J.-C. Liu, Y.-L. Zeng, H. Peng, X.-Q. Huang, M.-J. Yang and R.-G. Xiong. Optical Control of Polarization Switching in a Single-Component Organic Ferroelectric Crystal, J. Am. Chem. Soc., 2021, 143(34), 13816–13823. https://doi.org/10.1021/jacs.1c06108], copyright 2021. (b) Glycine molecules nucleate and orient themselves, accumulating their molecular dipole moments along the 2-axis direction parallel to the electric field to form spontaneous polarization (red arrow P), reproduced from ref. 100 with permission from [Springer Nature] [Z. Zhang, et al. Active self-assembly of piezoelectric biomolecular films via synergistic nanoconfinement and in situ poling. Nat. Commun., 2023, 14, 4094. https://doi.org/10.1038/s41467-023-39692-y], copyright 2023. (c) Schematic diagram showing the reversible structural photoisomerization of SA-PFA between the cis-enol form and the trans-keto form, reproduced from ref. 101 with permission from [Wiley-VCH Verlag] [W. Q. Liao, B. B. Deng, Z. X. Wang, T. T. Cheng, Y. T. Hu, S. P. Cheng and R. G. Xiong, Optically Induced Ferroelectric Polarization Switching in a Molecular Ferroelectric with Reversible Photoisomerization. Adv. Sci., 2021, 8, 2102614. https://doi.org/10.1002/advs.202102614], copyright 2021. (d) Schematic diagram of the zipper-like stacking structure of Hyp–Phe–Phe, reproduced from ref. 104 with permission from [Springer Nature] [S. Bera, S. Guerin, H. Yuan et al. Molecular engineering of piezoelectricity in collagen-mimicking peptide assemblies. Nat. Commun. 2021 12, 2634., https://doi.org/10.1038/s41467-021-22895-6], copyright 2021. | ||
Based on self-assembly and in situ polarization strategies, combined with electrospray deposition and nano-confinement effects, β-glycine crystals can be arranged in a specific orientation, significantly enhancing their piezoelectric properties and thermal stability. Mechanistically, nano-confinement (nano-droplets <100 nm) reduces the β-phase nucleation energy barrier according to the Ostwald step rule, promoting preferential nucleation (Fig. 5b). A 3–5 kV in situ electric field drives molecules to align along the [020] axis, forming a (020) net polarization structure. The combination of the two results in a piezoelectric strain coefficient d33 of 11.2 pm V−1 and a voltage coefficient g33 of 252 × 10−3 V m N−1, which remains stable up to 192 °C, making it suitable for high-temperature sensors.99,100
Moreover, organic ferroelectric SA-PFA (Fig. 5c) and TFTBSA achieve light-controlled polarization switching through cis-enol and trans-keto photoisomerization. The molecular dipole moment of SA-PFA increases from 2.70 D to 4.18 D. The mechanism can be explained by molecular orbital theory and dynamic hydrogen bond breaking and recombination.98,101
In addition, the piezoelectric effect of RMBA-BF3 originates from stress-induced lattice distortion and charge separation (Fig. 2e). One-dimensional hydrogen bond chains synergistically slip, causing charge center displacement, resulting in d33 = 3.5 pC N−1. Theoretically, this can be equivalent to a hydrogen bond-supported polar chain array, combined with an elastic continuous medium model to describe the stress-polarization response.79
Furthermore, Rs-tBuSA undergoes a 432F2-type plastic crystal phase transition (348 K, ΔS = 45.7 J mol−1 K−1), with high-temperature phase molecules undergoing a transition from ordered monoclinic to disordered cubic structures. The rotation of sulfur chiral centers disrupts the hydrogen bond network, leading to polarization switching. The O–H⋯O hydrogen bonds in PFND (2,2,3,3,4,4-hexafluoropentane-1,5-diol) dynamically reorganize at 37 °C. Thermal activation reduces bond energy, enhancing piezoelectric stability.59,102,103
Besides, using a collagen-mimicking peptide sequence (Hyp–Phe–Phe), Gazit's group has designed high-performance, biocompatible piezoelectric materials (Fig. 5d). It is found that the Hyp hydroxyl enhances intermolecular polarization through a hydrogen bond network, the low symmetry of the triclinic crystal system causes the dipole moment to be distributed along the b axis, and Phe's π–π stacking stabilizes the structure and optimizes charge transfer. Applying shear stress along the c-axis causes molecular chain slippage, leading to deformation of the hydrogen bond network and generating a shear piezoelectric coefficient d35 = −27 pm V−1.104
The dynamic response system represents the intuitive response of organic chiral molecules to macroscopic conditions such as temperature, pressure, and light exposure, manifesting in phenomena like photochromism and second harmonic generation (SHG). This reflects the macroscopic–microscopic correspondence in chiral organic ferroelectrics, aligning with the third developmental stage of chiral materials—transitioning from performance requirements to material design—and thereby guiding material design.
3. Physical mechanisms
The physical mechanisms of chiral organic ferroelectric materials encompass multiple dimensions, including topological domain evolution, multiphase coupling effects, theoretical simulations, and so on. This section will systematically analyze the relationship between their microscopic mechanisms and macroscopic properties through a combination of experiments and simulations.3.1. Ferroelectric domain wall dynamics and theoretical modeles
Ferroelectric domain wall dynamics encompasses multiscale theoretical models ranging from continuous media to discrete lattices, with chiral topologies and non-uniform field effects extending the traditional ferroelectric mechanism. Together, these models reveal the physical nature of polarization reversal and lay the foundation for the development of high-performance ferroelectric devices.105–108The wide-field wall can be described by a continuous medium model in defect-free infinite samples, whose motion equations combine kinetic energy density and thermodynamic potential density, yielding the limiting velocity 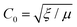 and effective mass m0 = γ0/C02, where C0 is the limiting velocity of the domain wall, u is the displacement of a ferroactive particle, m0 is the so-called effective mass of the unit square of the domain wall which at small velocities is equal to its ultimate value, and γ0 is the energy of the static domain wall. Under dissipative effects, the domain wall velocity is linearly related to the electric field v =
and effective mass m0 = γ0/C02, where C0 is the limiting velocity of the domain wall, u is the displacement of a ferroactive particle, m0 is the so-called effective mass of the unit square of the domain wall which at small velocities is equal to its ultimate value, and γ0 is the energy of the static domain wall. Under dissipative effects, the domain wall velocity is linearly related to the electric field v = ![[small mu, Greek, circumflex]](https://www.rsc.org/images/entities/i_char_e0b3.gif) E, and the mobility
E, and the mobility ![[small mu, Greek, circumflex]](https://www.rsc.org/images/entities/i_char_e0b3.gif) increases with the domain wall width, indicating that viscosity is the primary controlling factor.
increases with the domain wall width, indicating that viscosity is the primary controlling factor.
The narrow domain wall is governed by the lattice periodic potential barrier (Peierls barrier), with the surface energy density γ(U) exhibiting a cosine periodic distribution (Fig. 6a), and the barrier height V0 ∝ (δ/a)3exp(−π2δ/a), where V0 is the barrier value and δ/a is the width of the domain wall. The critical electric field 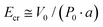 (where P0 means boundary conditions as the number of atomic planes approaches infinity and a is the interplane distance) determines the reversal mechanism: below Ecr, motion is achieved through the formation of extended domains via reverse domain nucleation, consistent with activation-type dynamics v ∝ exp(−
(where P0 means boundary conditions as the number of atomic planes approaches infinity and a is the interplane distance) determines the reversal mechanism: below Ecr, motion is achieved through the formation of extended domains via reverse domain nucleation, consistent with activation-type dynamics v ∝ exp(−![[small delta, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0c5.gif) /E), where v is the velocity of lateral motion of the domain wall,
/E), where v is the velocity of lateral motion of the domain wall, ![[small delta, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0c5.gif) is the activation field, and E is the non-critical electric field.106
is the activation field, and E is the non-critical electric field.106
 | ||
| Fig. 6 (a) Variation of polarization vectors in different narrow domain wall configurations (I and II). Periodic dependence of surface energy density on the domain wall surface when considering the discrete lattice time domain (III), reproduced from ref. 106 with permission from [Taylor and Francis Ltd.] [A. Sidorkin, Dynamics of domain walls in ferroelectrics and ferroelastics. Ferroelectrics, 1997, 191, 109–128. https://doi.org/10.1080/00150199708015628], copyright 1997. (b) Time-dependent switched polarization [ΔP(t)] as a function of the external voltage (Vext) at room temperature for (I) preset and (II) woken-up states, reproduced from ref. 109 with permission from [AIP Publishing LLC] [D. H. Lee, Y. Lee, K. Yang, J. Y. Park, S. H. Kim, P. R. S. Reddy, M. Materano, H. Mulaosmanovic, T. Mikolajick, J. L. Jones, U. Schroeder and M. H. Park, Domains and domain dynamics in fluorite-structured ferroelectrics. Appl. Phys. Rev., 2021, 8(2), 021312. https://doi.org/10.1063/5.0047977], copyright 2021. (c) Illustration of the machine learning-assisted multi-scale phase field model, reproduced from ref. 115 with permission from [American Institute of Physics] [C. Wu, J. Zhang, Y. Wang, T. Qian, C. Liu, H. Zhang, J. Wang and T. Xu, Artificial intelligence-assisted multi-scale phase field simulations for ferroelectrics: Cases for solid solution BaxSr1−xTiO3 and 2D ferroelectric In2Se3. J. Appl. Phys., 2025, 137(12), 124102. https://doi.org/10.1063/5.0256260], copyright 2025. | ||
The difference between wide- and narrow-domain wall dynamics highlights how ferroelectric materials behave based on the size. Wide domain walls are affected by viscous forces, while narrow ones are affected by atomic-scale Peierls barriers. This means that different strategies must be used to create the right domain walls, which can improve speed and energy efficiency.
Typically, ferroelectrics exhibit two types of nucleation and growth mechanisms: single crystal and polycrystalline. The KAI model (ΔP(t) = 2Ps[1 − exp(−(t/τ)n)]) is applicable to single crystals (here, Ps, τ, and n are the spontaneous polarization, switching time, and dimensionality factor, respectively), but polycrystalline thin films require the nucleation-limited switching (NLS) model due to uneven defect distribution (Fig. 6b). The Lorentz distribution must be introduced to describe the randomness of the switching time and reflect the non-uniformity of the local field. The inhomogeneous field model (IFM) and parasitic effects must consider circuit parasitic parameters (resistance Rs and capacitance Cp) and the local field Gaussian distribution. The polarization reversal dynamics satisfy
The nucleation of ferroelectric crystals embodies the size effect, which is closely related to domain wall dynamics. Theoretical studies have linked the growth of ferroelectric crystals to ferroelectric domain walls, implying that different domain wall motions under the size effect can achieve polarization reversal, thereby providing a theoretical foundation for ferroelectric polarization phenomena.
3.2. Theoretical simulation and computational design
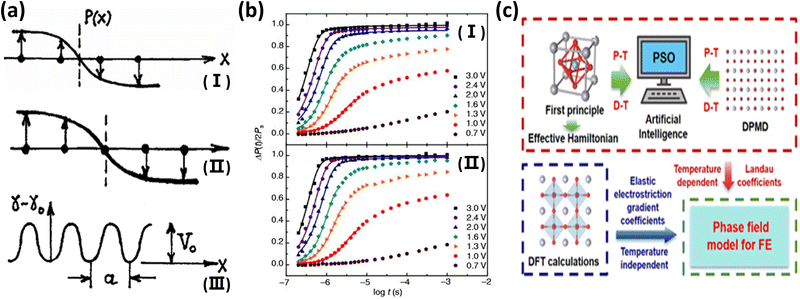
Computational methods based on density-functional theory (DFT), phase-field simulation and molecular dynamics (MD) provide theoretical support for material design.98,110–112Phase field simulation framework (Fig. 6c): (1) theoretical basis: based on the Landau–Ginzburg free energy generalized function, the total energy consists of Landau energy (describing the polarization–temperature relationship), gradient energy (controlling the width of the domain wall), elastic energy (coupling strain and polarization) and electric energy and is given by
| ftot = fLandau + fgrad + felas + felec |
| fLandau = α(T − Tc)P2 + βP4 + γP6 |
| fgrad = 1/2GijklPi,jPk,l, |
| felas = 1/2cijkl(εij − QijklPiPj)(εkl − QijklPkPl) |
| felec = −1/2κγEiPi. |
And the gradient coefficient, G, determines domain wall stability.113,114 (2) Kinetic equations: the polarization evolution is described by the time-dependent Ginzburg–Landau equation:
Research into the physical mechanisms of chiral organic ferroelectric materials has evolved from macroscopic phenomenological models to microscopic quantum-scale analysis. Symmetry breaking, topological domain protection, and multi-field coupling effects provide novel insights for the design of high-performance materials.
4. Applications
By virtue of their unique dynamic tunability, excellent biocompatibility, and multifunctional coupling properties, chiral organic ferroelectric materials have demonstrated significant application potential in several frontier fields of science and technology, including flexible electronics, biomedicine, energy conversion and storage, and smart sensing.116–121 These materials not only offer new options for developing next-generation high-performance devices but also open up new avenues for addressing technological challenges in specific domains.Flexible electronic devices require materials that exhibit both excellent mechanical toughness and stable electrical properties. Chiral organic ferroelectric materials exhibit outstanding performance in this regard. For instance, the fluorinated supramolecular crown ether compound reported by Zhang 's group has not only a high piezoelectric coefficient (d33) of 42 pC N−1 but also an elastic modulus of 5.04 GPa, enabling it to sensitively monitor human joint movements with a voltage sensitivity of 0.1 mV (Fig. 7a).80 Similarly, chiral myo-inositol organosilicon crystals, with a measured hardness of 72.8 MPa, are considered potential candidates for constructing flexible electrodes due to this mechanical characteristic.121,122 Furthermore, the HFPD–PVA composite film developed by Zhang 's group can maintain over 85% of its piezoelectric performance (d33 = 34.3 pC N−1) even when bent, demonstrating its value in wearable energy harvesting applications (Fig. 7b).103
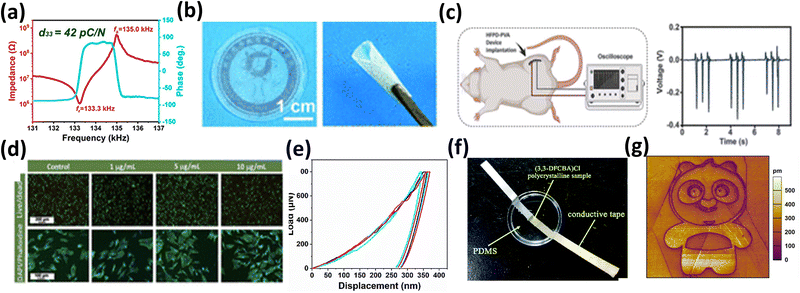 | ||
| Fig. 7 (a) Frequency dependence of the longitudinal impedance modulus |Z| and phase angle θ for [(CF3–C6H4–NH3)(18-crown-6)][TFSA] crystal rods, reproduced from ref. 80 with permission from [American Chemical Society] [H.-P. Lv, Y.-R. Li, X.-J. Song, N. Zhang, R.-G. Xiong and H.-Y. Zhang. A poling-free supramolecular crown ether compound with large piezoelectricity. J. Am. Chem. Soc., 2023 145(5), 3187–3195. https://doi.org/10.1021/jacs.2c12951], copyright 2023. (b) Solution evaporation thin film preparation and curling diagram of the tough film. (c) Piezoelectric measurements of PLA-encapsulated HFPD-PVA in SD rats and voltage generated by HFPD-PVA. (d) Live/dead staining (green indicates live cells) image of HFPD crystals, reproduced from ref. 103 with permission from [American Association for the Advancement of Science] [H.-Y. Zhang et al., Biodegradable ferroelectric molecular crystal with large piezoelectric response. Science, 2024, 383, 1492–1498. https://doi.org/10.1126/science.adj 1946], copyright 2024. (e) (S)-BINOL–DIPASi films measured by the nanoindentation method, reproduced from ref. 3 with permission from [American Chemical Society] [Y.-L. Zeng, Y. Ai, S.-Y. Tang, X.-J. Song, X.-G. Chen, Y.-Y. Tang, Z.-X. Zhang, Y.-M. You, R.-G. Xiong and H.-Y. Zhang. Axial-chiral BINOL multiferroic crystals with the coexistence of ferroelectricity and ferroelasticity. J. Am. Chem. Soc., 2022, 144(42), 19559–19566. https://doi.org/10.1021/jacs.2c08667], copyright 2022. (f) Pulsed device based on (3,3-DFCBA)Cl, reproduced from ref. 123 with permission from [Royal Society of Chemistry] [H.-Y. Zhang. A small-molecule organic ferroelectric with piezoelectric voltage coefficient larger than that of lead zirconate titanate and polyvinylidene difluoride. Chem. Sci., 2022, 13, 5006–5013. https://doi.org/10.1039/D1SC06909H], copyright 2022. (g) PFM panda-like pattern, reproduced from ref. 94 with permission from [John Wiley and Sons Ltd.] [H.-Y. Zhang, et al. Ferroelectric lithography in single-component organic enantiomorphic ferroelectrics. Angew. Chem., Int. Ed., 2022, 134, 22, e202200135. https://doi.org/10.1002/ange.202200135], copyright 2022. | ||
Besides, biocompatibility and biodegradability are critical indicators for biomedical materials. HFPD crystals show tremendous advantages in this area, featuring a high d33 value of approximately 138 pC N−1, coupled with excellent biocompatibility (cell viability >95%) and a controllable degradation cycle. These properties allow them to be used as implantable sensors for real-time monitoring of mechanical signals during tissue healing, followed by safe degradation after their mission is complete (Fig. 7c and d). Moreover, the HFPD material can control drug release via pH response, achieving an integrated “sensing-treatment-degradation” functionality.103 In the realm of neural stimulation and drug delivery, axially chiral BINOL crystals exhibit both ferroelectric and ferroelastic domains with a spontaneous strain of 2.7 × 10−2, making them suitable for precise neural stimulation applications (Fig. 7e).28
In addition, the core of energy harvesting lies in converting ambient mechanical or light energy into electrical energy. Orth-I-OA crystals can generate a voltage of 6.2 V at a vibration frequency of 1 Hz, with a power density of 25 µW cm−2, showcasing their potential in miniature piezoelectric energy harvesters (Fig. 7). For energy storage, the dielectric constant of (R/S)-BINOL-DIPASi increases by 2.5 times during its phase transition, which can be utilized in capacitors.28 (3,3-DFCBA)Cl exhibits an electrostrictive coefficient (Q33) of 4.29 m4 C−2, indicating its application prospects in pulse power systems and other fields requiring rapid energy release (Fig. 7f).123
Moreover, modulating the ferroelectric phase of materials with light signals is key to realizing light-controlled logic and high-density storage. Xiong's group has indicated that diarylethylene derivatives can be used to construct self-powered, light-controlled switches through a photo-induced ferroelectric phase transition.95 Such crystals can achieve a repeatable “write–read–erase” cycle. The (R,R)-(E,E)-1 material can achieve a storage density of 1 Tb per in2 with over 107 light-controlled switching cycles. It also enables patterning with a 20 nm resolution, offering possibilities for next-generation non-volatile memory and nanolithography technologies (Fig. 7g).94 Additionally, orth-I-OA crystals exhibit a second-harmonic generation (SHG) intensity that is 0.63 times that of KDP, also indicating their value in optoelectronic devices.124
A high piezoelectric voltage coefficient (g33) is a core metric for high-sensitivity sensors. The g33 value of (3,3-DFCBA)Cl is as high as 437.2 × 10−3 V m N−1, making it highly suitable for developing high-resolution pressure sensors.123 Concurrently, materials like chiral inositol crystals show significant application potential in ultrasonic probes and underwater sensors due to their excellent acoustic impedance matching properties (matching degree >90%).124
These applications demonstrate that the field is shifting from optimizing single properties toward integrating multiferroic and multifunctional coupling.125,126 For instance, (P(VDF-TrFE)) materials prepared via microcrosslinking exhibit not only ferroelectricity but also ferroelasticity.127 Furthermore, certain materials can respond to multiple external stimuli such as heat, stress, light, and electric fields. The emergence of luminescence ferroelectrics and photocatalytic molecular ferroelectrics further substantiates this trend. Concurrently, the advent of biocompatible and biodegradable organic molecular ferroelectrics has propelled the application of ferroelectric materials in biomedical fields. This marks the field's progression toward highly integrated, multifunctional materials to meet the demands of increasingly complex devices.
5. Perspective
The industrialization and clinical application of molecular piezoelectric materials face multiple challenges. In terms of performance, while the piezoelectric coefficient approaches that of traditional ceramics, there are significant gaps in high-temperature stability, frequency response, and mechanical durability. Balancing degradation and biocompatibility is essential, such as verifying the metabolic pathways of HFPD degradation products and developing breakthroughs in encapsulation technology for rapidly degrading materials. Large-scale production is constrained by the cost of high-purity raw materials, film uniformity (error must be <5%), and encapsulation yield (<70%). Interdisciplinary integration faces technical gaps, such as the wireless power supply efficiency of implantable devices (<15%) and signal transmission impedance matching (error >20%) issues that need to be resolved.99,103,104To address these challenges, material preparation can draw inspiration from skeletal structures to develop gradient modulus molecules, while integrating machine learning-assisted high-throughput screening.128–131 This approach combines first-principles calculations (e.g., predicting piezoelectric coefficients in Ma2Z4 monolayers) with neural network models to realize the inverse design of material properties.132–134 Application areas are also expanding: in healthcare, they are used for biodegradable pacemakers and bone repair scaffolds and in flexible electronics, they are used for electronic skin and beyond. Additionally, efforts are being made to develop green processes (such as solvent-free synthesis) and establish standards for lifespan assessment and degradation certification.135,136
The rise of these new materials will reshape the industrial landscape: in environmental protection, replacing lead-containing ceramics will reduce electronic waste by 5000 tons annually and cut carbon emissions by 40%.103,137 In healthcare, the implantable transient device market is projected to reach $100 billion by 2035, driving the evolution of precision medicine.103 In industry, they will empower smart factories (with fault prediction accuracy exceeding 95%) and smart cities (with energy self-sufficiency rates increasing by 20%).138–140
In summary, the path of chiral organic ferroelectrics from laboratory to industrialization is fraught with challenges, but the refinement of ferroelectrochemistry theories and breakthroughs in degradable materials have injected strong momentum into this field. Over the next decade, with the deepening of interdisciplinary collaboration and the iterative advancement of manufacturing technologies, chiral organic ferroelectrics are poised to usher in a new paradigm of “material–device–system” integration across healthcare, environmental sustainability, and energy sectors.
Author contributions
This manuscript was primarily authored by Yipeng Zang and Xiaoqing Gao. All authors have approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
This review does not provide original data. All data can be found in the original literature.Acknowledgements
The authors acknowledge financial support from the National Natural Science Foundation of China (22275042) and the Engineering Research Center of Clinical Functional Materials and Diagnosis & Treatment Devices of Zhejiang Province (WIUCASK19008).References
- M. Freemantle, Chem. Eng. News, 2003, 81, 27 CrossRef.
- J. Gal, Helv. Chim. Acta, 2013, 96, 1617–1657 CrossRef CAS.
- Y.-L. Zeng, Y. Ai, S.-Y. Tang, X.-J. Song, X.-G. Chen, Y.-Y. Tang, Z.-X. Zhang, Y.-M. You, R.-G. Xiong and H.-Y. Zhang, J. Am. Chem. Soc., 2022, 144, 19559–19566 CAS.
- L. D. Barron, Molecular light scattering and optical activity, Cambridge University Press, 2009 Search PubMed.
- X. Gao, B. Han, X. Yang and Z. Tang, J. Am. Chem. Soc., 2019, 141, 13700–13707 CrossRef CAS.
- P. Lodahl, S. Mahmoodian, S. Stobbe, A. Rauschenbeutel, P. Schneeweiss, J. Volz, H. Pichler and P. Zoller, Nature, 2017, 541, 473–480 CrossRef CAS.
- Y. Zhang, S. Yu, B. Han, Y. Zhou, X. Zhang, X. Gao and Z. Tang, Matter, 2022, 5, 837–875 CrossRef CAS.
- H. Zhu, J. Yi, M.-Y. Li, J. Xiao, L. Zhang, C.-W. Yang, R. A. Kaindl, L.-J. Li, Y. Wang and X. Zhang, Science, 2018, 359, 579–582 CrossRef CAS.
- J. Valasek, Phys. Rev., 1924, 24, 560 CrossRef CAS.
- M. Wu, Nat. Rev. Phys., 2021, 3, 726 CrossRef.
- R. L. Bunde, E. J. Jarvi and J. J. Rosentreter, Talanta, 1998, 46, 1223–1236 CrossRef CAS.
- C. s Lu, J. Vac. Sci. Technol., 1975, 12, 578–583 CrossRef CAS.
- L. E. MacKenzie and R. Pal, Nat. Rev. Chem., 2021, 5, 109–124 CrossRef CAS.
- W. Eerenstein, N. Mathur and J. F. Scott, Nature, 2006, 442, 759–765 CrossRef CAS.
- Q. Pan, Z.-X. Gu, R.-J. Zhou, Z.-J. Feng, Y.-A. Xiong, T.-T. Sha, Y.-M. You and R.-G. Xiong, Chem. Soc. Rev., 2024, 53, 5781–5861 RSC.
- H. Schmid, Ferroelectrics, 1994, 162, 317–338 CrossRef.
- J. Valasek, Phys. Rev., 1921, 17, 475 CrossRef CAS.
- X. Gao, X. Zhang, K. Deng, B. Han, L. Zhao, M. Wu, L. Shi, J. Lv and Z. Tang, J. Am. Chem. Soc., 2017, 139, 8734–8739 CrossRef CAS.
- X. Gao, X. Zhang, L. Zhao, P. Huang, B. Han, J. Lv, X. Qiu, S.-H. Wei and Z. Tang, Nano Lett., 2018, 18, 6665–6671 CrossRef CAS PubMed.
- H.-E. Lee, H.-Y. Ahn, J. Mun, Y. Y. Lee, M. Kim, N. H. Cho, K. Chang, W. S. Kim, J. Rho and K. T. Nam, Nature, 2018, 556, 360–365 CrossRef CAS.
- N. Shukla and A. J. Gellman, Nat. Mater., 2020, 19, 939–945 CrossRef CAS.
- M. Sun, L. Xu, A. Qu, P. Zhao, T. Hao, W. Ma, C. Hao, X. Wen, F. M. Colombari and A. F. de Moura, Nat. Chem., 2018, 10, 821–830 CrossRef CAS PubMed.
- R. W. Whatmore, Y.-M. You, R.-G. Xiong and C.-B. Eom, APL Mater., 2021, 9, 070401 CrossRef CAS.
- S. Das, Z. Hong, M. McCarter, P. Shafer, Y.-T. Shao, D. A. Muller, L. W. Martin and R. Ramesh, APL Mater., 2020, 8, 120902 CrossRef CAS.
- S. Troiler-McKinstry, Am. Ceram. Soc. Bull., 2020, 99, 22–23 Search PubMed.
- Y. Chen, W. Du, Q. Zhang, O. Ávalos-Ovando, J. Wu, Q.-H. Xu, N. Liu, H. Okamoto, A. O. Govorov and Q. Xiong, Nat. Rev. Phys., 2022, 4, 113–124 CrossRef.
- J. Lu, Y. Xue, K. Bernardino, N.-N. Zhang, W. R. Gomes, N. S. Ramesar, S. Liu, Z. Hu, T. Sun and A. F. de Moura, Science, 2021, 371, 1368–1374 CrossRef CAS PubMed.
- H. Peng, Y. Qin, X. G. Chen, X. J. Song, R. G. Xiong and W. Q. Liao, Angew. Chem., Int. Ed., 2025, 64, e202500285 CrossRef CAS PubMed.
- X. J. Song, Y. Qin, Y. Ai, X. G. Chen, Y. R. Weng, H. Peng, H. P. Lv, R. G. Xiong and W. Q. Liao, Angew. Chem., Int. Ed., 2025, 137, e202507554 CrossRef.
- Y. R. Weng, Y. Qin, Y. Ai, X. G. Chen, X. J. Song, H. Y. Zhang and W. Q. Liao, Angew. Chem., Int. Ed., 2025, 137, e202515806 CrossRef.
- H. Peng, J. C. Qi, Y. S. Liu, J. M. Zhang, W. Q. Liao and R. G. Xiong, Chin. J. Chem., 2024, 42, 1133–1144 CrossRef CAS.
- R. Wang, L. Li, J. Li, C. Wang, S. Cong, G. Zhao, X. Du, C.-R. Huang, H. Cao, W. Cheng, Y. Ye, C. Liu, B. Li, W.-Q. Liao, Z. Lu, R. Tang, R.-G. Xiong and G. Zou, Adv. Mater., 2025, 37, 2409251 CrossRef CAS PubMed.
- S. Horiuchi, Y. Tokunaga, G. Giovannetti, S. Picozzi, H. Itoh, R. Shimano, R. Kumai and Y. Tokura, Nature, 2010, 463, 789–792 CrossRef CAS.
- P.-P. Shi, Y.-Y. Tang, P.-F. Li, W.-Q. Liao, Z.-X. Wang, Q. Ye and R.-G. Xiong, Chem. Soc. Rev., 2016, 45, 3811–3827 RSC.
- K. Aizu, Rev. Mod. Phys., 1962, 34, 550 CrossRef CAS.
- S. Dong, J.-M. Liu, S.-W. Cheong and Z. Ren, Adv. Phys., 2015, 64, 519–626 CrossRef CAS.
- Z. Pajak, P. Czarnecki, B. Szafrańska, H. Małuszyńska and Z. Fojud, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 69, 132102 CrossRef.
- D. Lu, D. Sheptyakov, Y. Cao, H. Zhao, J. Zhang, M. Pi, X. Ye, Z. Liu, X. Zhang and Z. Pan, Adv. Funct. Mater., 2024, 34, 2411133 CrossRef CAS.
- R. Blinc, Ferroelectrics, 2004, 301, 3–8 CrossRef CAS.
- J. Harada, N. Yoneyama, S. Yokokura, Y. Takahashi, A. Miura, N. Kitamura and T. Inabe, J. Am. Chem. Soc., 2018, 140, 346–354 CrossRef CAS.
- D. Li, X. M. Zhao, H. X. Zhao, X. W. Dong, L. S. Long and L. S. Zheng, Adv. Mater., 2018, 30, 1803716 CrossRef.
- J. Zhao, J. Gao, W. Li, Y. Qian, X. Shen, X. Wang, X. Shen, Z. Hu, C. Dong and Q. Huang, Nat. Commun., 2021, 12, 747 CrossRef CAS PubMed.
- L. He, K. Xu, P. P. Shi, Q. Ye and W. Zhang, Adv. Electron. Mater., 2022, 8, 2100635 CrossRef CAS.
- S. Horiuchi and Y. Tokura, Nat. Mater., 2008, 7, 357–366 Search PubMed.
- M. Owczarek, K. A. Hujsak, D. P. Ferris, A. Prokofjevs, I. Majerz, P. Szklarz, H. Zhang, A. A. Sarjeant, C. L. Stern and R. Jakubas, Nat. Commun., 2016, 7, 13108 CrossRef CAS PubMed.
- S. Barman, A. Pal, A. Mukherjee, S. Paul, A. Datta and S. Ghosh, Chem. – Eur. J., 2024, 30, e202303120 CrossRef CAS PubMed.
- H. Han, W. Li, Q. Zhang, S. Tang, Y. Wang, Z. Xu, Y. Liu, H. Chen, J. Gu and J. Wang, Adv. Mater., 2024, 36, 2408400 CrossRef CAS.
- H. Hu, Z. Y. Jing, Q. Pan, T. T. Sha, H. R. Ji, X. X. Cao, X. J. Song, Z. J. Feng, J. Yao and R. J. Zhou, Adv. Mater., 2024, 36, 2413547 CrossRef CAS.
- M. Acosta, N. Novak, V. Rojas, S. Patel, R. Vaish, J. Koruza, G. A. Rossetti and J. Rödel, Appl. Phys. Rev., 2017, 4, 041305 Search PubMed.
- J. He, C. Yu, Y. Hou, X. Su, J. Li, C. Liu, D. Xue, J. Cao, Y. Su and L. Qiao, Nano Energ., 2022, 97, 107218 CrossRef CAS.
- Z. Chen, R. Liang, C. Zhang, Z. Zhou, Y. Li, Z. Liu and X. Dong, J. Mater. Sci. Technol., 2022, 116, 238–245 CrossRef CAS.
- J. Li, W. Qu, J. Daniels, H. Wu, L. Liu, J. Wu, M. Wang, S. Checchia, S. Yang and H. Lei, Science, 2023, 380, 87–93 CrossRef CAS.
- H. Dong, Z. Zhu, Z. Li, M. Li and J. Chen, JOM, 2024, 76, 340–352 CrossRef CAS.
- M. Habib, I. Lantgios and K. Hornbostel, J. Phys. D: Appl. Phys., 2022, 55, 423002 CrossRef CAS.
- H.-Y. Liu, H.-Y. Zhang, X.-G. Chen and R.-G. Xiong, J. Am. Chem. Soc., 2020, 142, 15205–15218 CrossRef CAS PubMed.
- A. V. Bune, V. M. Fridkin, S. Ducharme, L. M. Blinov, S. P. Palto, A. V. Sorokin, S. G. Yudin and A. Zlatkin, Nature, 1998, 391, 874–877 CrossRef CAS.
- Z.-B. Hu, X. Yang, J. Zhang, L.-A. Gui, Y.-F. Zhang, X.-D. Liu, Z.-H. Zhou, Y. Jiang, Y. Zhang and S. Dong, Nat. Commun., 2024, 15, 4702 CrossRef CAS.
- J. Lv, X. Gao, B. Han, Y. Zhu, K. Hou and Z. Tang, Nat. Rev. Chem., 2022, 6, 125–145 CrossRef PubMed.
- H. Peng, Z. K. Xu, Y. Du, P. F. Li, Z. X. Wang, R. G. Xiong and W. Q. Liao, Angew. Chem., Int. Ed., 2023, 62, e202306732 CrossRef.
- A. Laudari, J. Barron, A. Pickett and S. Guha, ACS Appl. Mater. Interfaces, 2020, 12, 26757–26775 CrossRef CAS.
- W. Gao, L. Chang, H. Ma, L. You, J. Yin, J. Liu, Z. Liu, J. Wang and G. Yuan, NPG Asia Mater., 2015, 7, e189–e189 CrossRef CAS.
- E. Pardo, C. Train, H. Liu, L. M. Chamoreau, B. Dkhil, K. Boubekeur, F. Lloret, K. Nakatani, H. Tokoro and S. I. Ohkoshi, Angew. Chem., Int. Ed., 2012, 51, 8356–8360 CrossRef CAS PubMed.
- L. Tang, X. Zhu, Y. Ma, H. Xu, S. Han, Y. Liu, Y. Chen, D. Wang, J. Luo and Z. Sun, InfoMat, 2024, 6, e12593 CrossRef CAS.
- H.-Y. Zhang, Y.-Y. Tang, P.-P. Shi and R.-G. Xiong, Acc. Chem. Res., 2019, 52, 1928–1938 CrossRef CAS.
- W. He, C. Chen, S. Wu, W. P. Wong, Z. Wu, K. Chang, J. Wang, H. Gao and K. P. Loh, J. Am. Chem. Soc., 2024, 147, 811–820 CrossRef PubMed.
- X.-J. Song, Y. Ai, X.-G. Chen, Y. Qin, Y.-Y. Tang, H.-P. Lv, P.-F. Li, H. Peng, Y.-R. Weng and H.-H. Chen, J. Am. Chem. Soc., 2025, 147, 16568–16577 CrossRef CAS PubMed.
- P. Deepthi and J. Shanthi, RSC Adv., 2016, 6, 33686–33694 RSC.
- A. Heredia, V. Meunier, I. K. Bdikin, J. Gracio, N. Balke, S. Jesse, A. Tselev, P. K. Agarwal, B. G. Sumpter and S. V. Kalinin, Adv. Funct. Mater., 2012, 22, 2996–3003 CrossRef CAS.
- P.-F. Li, W.-Q. Liao, Y.-Y. Tang, W. Qiao, D. Zhao, Y. Ai, Y.-F. Yao and R.-G. Xiong, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 5878–5885 CrossRef CAS.
- P. F. Li, Y. Y. Tang, Z. X. Wang, H. Y. Ye, Y. M. You and R. G. Xiong, Nat. Commun., 2016, 7, 13635 CrossRef CAS.
- H. Okamoto, T. Mitani, Y. Tokura, S. Koshihara, T. Komatsu, Y. Iwasa, T. Koda and G. Saito, Phys. Rev. B: Condens. Matter Mater. Phys., 1991, 43, 8224 CrossRef CAS PubMed.
- S. Wan, X. Li, Y. Chen, N. Liu, Y. Du, S. Dou, L. Jiang and Q. Cheng, Science, 2021, 374, 96–99 CrossRef CAS PubMed.
- F. Yang, J. Li, Y. Long, Z. Zhang, L. Wang, J. Sui, Y. Dong, Y. Wang, R. Taylor and D. Ni, Science, 2021, 373, 337–342 CrossRef CAS PubMed.
- Q. Yu, Y. Bai, Z. Li, F. Jiang, R. Luo, Y. Gai, Z. Liu, L. Zhou, Y. Wang, C. Li, K. Ren, D. Luo, H. Meng and Z. Li, Nano Energy, 2024, 121, 109196 CrossRef CAS.
- G. de Marzo, V. M. Mastronardi, L. Algieri, F. Vergari, F. Pisano, L. Fachechi, S. Marras, L. Natta, B. Spagnolo and V. Brunetti, Adv. Electron. Mater., 2023, 9, 2200069 CrossRef CAS.
- A. Islam, T. Yasin, M. J. Akhtar, Z. Imran, A. Sabir, M. Sultan, S. M. Khan and T. Jamil, J. Solid State Electrochem., 2016, 20, 571–578 CrossRef CAS.
- H. Xue, J. Jin, Z. Tan, K. Chen, G. Lu, Y. Zeng, X. Hu, X. Peng, L. Jiang and J. Wu, Sci. Adv., 2024, 10, eadn0260 CrossRef CAS PubMed.
- A. E. Kamanyi, E. T. A. Mohamed, W. Ngwa, et al., Determination of sound velocity and acoustic impedance of thin chitosan films by phase-sensitive acoustic microscopy, Health Monitoring of Structural and Biological Systems 2010, SPIE, 2010, vol. 7650, pp. 731–738 Search PubMed.
- S. Sahoo, S. Mukherjee, V. B. Sharma, W. I. Hernández, A. C. Garcia-Castro, J. K. Zaręba, D. Kabra, G. Vaitheeswaran and R. Boomishankar, Angew. Chem., Int. Ed., 2024, 63, e202400366 CrossRef CAS.
- H.-P. Lv, Y.-R. Li, X.-J. Song, N. Zhang, R.-G. Xiong and H.-Y. Zhang, J. Am. Chem. Soc., 2023, 145, 3187–3195 CrossRef CAS.
- J. Yang, X. Wu, J. Shi, B. Tong, Y. Lei, Z. Cai and Y. Dong, Adv. Funct. Mater., 2021, 31, 2108072 CrossRef CAS.
- M.-M. Lun, J.-Q. Luo, Z.-X. Zhang, J. Li, L.-Y. Xie, H.-F. Lu, Y. Zhang and D.-W. Fu, Chem. Eng. J., 2023, 475, 145969 CrossRef CAS.
- X.-J. Song, T. Zhang, Z.-X. Gu, Z.-X. Zhang, D.-W. Fu, X.-G. Chen, H.-Y. Zhang and R.-G. Xiong, J. Am. Chem. Soc., 2021, 143, 5091–5098 CrossRef CAS.
- Y.-K. Li, Y.-H. Tan, Y.-Z. Tang, X.-W. Fan, S.-F. Wang, T.-T. Ying and H. Zhang, Inorg. Chem. Front., 2022, 9, 3702–3708 RSC.
- Z. Guo, Z. Dong, J. Gao, H. Zhang, B. Zhang, M. H. Li and J. Hu, Small, 2024, 20, 2406876 CrossRef CAS PubMed.
- Z. Lei, H. Chen, S. Huang, L. J. Wayment, Q. Xu and W. Zhang, Chem. Rev., 2024, 124(12), 7829–7906 CrossRef CAS PubMed.
- L. Li, X. Peng, D. Zhu, J. Zhang and P. Xiao, Macromol. Chem. Phys., 2023, 224, 2300224 CrossRef CAS.
- L. Li, C. Wang, C.-R. Huang, W.-Q. Liao, X. Xu, L. Xiao, R. Wang, W. Cheng, T. He, S. Cong, Z. Kang, R.-G. Xiong and G. Zou, J. Am. Chem. Soc., 2025, 147(15), 12635–12643 CrossRef CAS.
- C. Y. Shi, D. D. He, B. S. Wang, Q. Zhang, H. Tian and D. H. Qu, Angew. Chem., Int. Ed., 2023, 62, e202214422 CrossRef CAS.
- P. Tan, X. Huang, Y. Wang, B. Xing, J. Zhang, C. Hu, X. Meng, X. Xu, D. Li, X. Wang, X. Zhou, N. Zhang, Q. Wang, F. Li, S. Zhang and H. Tian, Nat. Commun., 2024, 15, 10619 CrossRef CAS.
- S. Xiang, L. Zhou, R. Chen, K. Zhang and M. Chen, Macromolecules, 2022, 55, 10276–10284 CrossRef CAS.
- H.-Y. Zhang, N. Zhang, Y. Zhang, H.-H. Jiang, Y.-L. Zeng, S.-Y. Tang, P.-F. Li, Y.-Y. Tang and R.-G. Xiong, Phys. Rev. Lett., 2023, 130, 176802 CrossRef CAS PubMed.
- Z. Lei, H. Chen, S. Huang, L. J. Wayment, Q. Xu and W. Zhang, Chem. Rev., 2024, 124, 7829–7906 CrossRef CAS.
- H. Y. Zhang, H. H. Jiang, Y. Zhang, N. Zhang and R. G. Xiong, Angew. Chem., Int. Ed., 2022, 134, e202200135 CrossRef.
- Y.-Y. Tang, Y.-L. Zeng and R.-G. Xiong, J. Am. Chem. Soc., 2022, 144, 8633–8640 CrossRef CAS PubMed.
- J. Guo, W. Chen, H. Chen, Y. Zhao and Y. Zhang, Adv. Opt. Mater., 2021, 9, 2002146 CrossRef CAS.
- Y. Du, C.-R. Huang, Z.-K. Xu, W. Hu, P.-F. Li, R.-G. Xiong and Z.-X. Wang, JACS Au, 2023, 3, 1464–1471 CrossRef CAS PubMed.
- Y.-Y. Tang, J.-C. Liu, Y.-L. Zeng, H. Peng, X.-Q. Huang, M.-J. Yang and R.-G. Xiong, J. Am. Chem. Soc., 2021, 143, 13816–13823 CrossRef CAS.
- M. T. Chorsi, T. T. Le, F. Lin, T. Vinikoor, R. Das, J. F. Stevens, C. Mundrane, J. Park, K. T. M. Tran and Y. Liu, Sci. Adv., 2023, 9, 18 Search PubMed.
- Z. Zhang, X. Li, Z. Peng, X. Yan, S. Liu, Y. Hong, Y. Shan, X. Xu, L. Jin and B. Liu, Nat. Commun., 2023, 14, 4094 CrossRef CAS.
- W. Q. Liao, B. B. Deng, Z. X. Wang, T. T. Cheng, Y. T. Hu, S. P. Cheng and R. G. Xiong, Adv. Sci., 2021, 8, 2102614 CrossRef CAS.
- Y. Ai, Z. X. Gu, P. Wang, Y. Y. Tang, X. G. Chen, H. P. Lv, P. F. Li, Q. Jiang, R. G. Xiong and J. J. Zhang, Adv. Mater., 2024, 36, 2405981 CrossRef CAS.
- H.-Y. Zhang, Y.-Y. Tang, Z.-X. Gu, P. Wang, X.-G. Chen, H.-P. Lv, P.-F. Li, Q. Jiang, N. Gu and S. Ren, Science, 2024, 383, 1492–1498 CrossRef CAS.
- S. Bera, S. Guerin, H. Yuan, J. O’Donnell, N. P. Reynolds, O. Maraba, W. Ji, L. J. Shimon, P.-A. Cazade and S. A. Tofail, Nat. Commun., 2021, 12, 2634 CrossRef CAS.
- S. Yi, Z. Hong, Z. Ma, C. Zhou, M. Jiang, X. Huang, M. Huang, S. Aya, R. Zhang and Q.-H. Wei, Proc. Natl. Acad. Sci. U. S. A., 2024, 121, e2413879121 CrossRef CAS PubMed.
- A. Sidorkin, Ferroelectrics, 1997, 191, 109–128 CrossRef CAS.
- Y. Tikhonov, S. Kondovych, J. Mangeri, M. Pavlenko, L. Baudry, A. Sené, A. Galda, S. Nakhmanson, O. Heinonen and A. Razumnaya, Sci. Rep., 2020, 10, 8657 CrossRef CAS PubMed.
- V. Yadav, S. Pal and S. Uma, J. Phys. Chem. C, 2025, 129, 5666–5673 CrossRef CAS.
- D. H. Lee, Y. Lee, K. Yang, J. Y. Park, S. H. Kim, P. R. S. Reddy, M. Materano, H. Mulaosmanovic, T. Mikolajick, J. L. Jones, U. Schroeder and M. H. Park, Appl. Phys. Rev., 2021, 8, 021312 CAS.
- B. Li, L. Wang, L. Gao, T. Xu, D. Zhang, F. Li, J. Lyu, R. Zhu, X. Gao and H. Zhang, Angew. Chem., Int. Ed., 2024, 136, e202400511 CrossRef.
- W.-Q. Liao, Y.-L. Zeng, Y.-Y. Tang, H. Peng, J.-C. Liu and R.-G. Xiong, J. Am. Chem. Soc., 2021, 143, 21685–21693 CrossRef CAS.
- J. C. Liu, Y. Ai, Q. Liu, Y. P. Zeng, X. G. Chen, H. P. Lv, R. G. Xiong and W. Q. Liao, Adv. Mater., 2023, 35, 2302436 CrossRef CAS.
- P. Kumar, M. Hoffmann, A. Nonaka, S. Salahuddin and Z. Yao, Adv. Electron. Mater., 2024, 10, 2400085 CrossRef CAS.
- Y. Zhang, Q. Li, H. Huang, J. Hong and X. Wang, Phys. Rev. B, 2022, 105, 224101 CrossRef CAS.
- C. Wu, J. Zhang, Y. Wang, T. Qian, C. Liu, H. Zhang, J. Wang and T. Xu, Appl. Phys., 2025, 137, 124102 CAS.
- S. R. Berrow, J. Hobbs and C. J. Gibb, Small, 2025, 21, 2501724 CrossRef PubMed.
- G. Feng, X. Zhao, X. Huang, X. Zhang, Y. Wang, W. Li, L. Chen, S. Hao, Q. Zhu and Y. Ivry, Nat. Commun., 2025, 16, 3027 CrossRef CAS PubMed.
- C. Li, Y. Liu, B. Li, Z. Yuan, T. Yang, Y. Liu, H. Gao, L. Xu, X. Yu and Q. Luo, Nat. Mater., 2025, 24, 1066–1073 CrossRef CAS PubMed.
- W. Sun, H. Y. Zhang, X. Liu and R. G. Xiong, Adv. Mater., 2025, 37, 2417073 CrossRef CAS.
- H. Zhang, M. C. Nagashree, R. F. Webster, Z. Wang, X. Zheng, S. D. Kulkarni, R. B. Venkataramana, J. Seidel and P. Sharma, Adv. Funct. Mater., 2025, 35, 2502700 CrossRef CAS.
- C. Zhao, Z. Gao, Z. Hong, H. Guo, Z. Cheng, Y. Li, L. Shang, L. Zhu, J. Zhang and Z. Hu, Adv. Sci., 2025, 12, 2413808 CrossRef CAS PubMed.
- Z. X. Zhang, X. J. Song, Y. R. Li, X. G. Chen, Y. Zhang, H. P. Lv, Y. Y. Tang, R. G. Xiong and H. Y. Zhang, Angew. Chem., Int. Ed., 2022, 134, e202210809 CrossRef.
- H.-Y. Zhang, Chem. Sci., 2022, 13, 5006–5013 RSC.
- L. Xu, Y. Zhang, H. H. Jiang, N. Zhang, R. G. Xiong and H. Y. Zhang, Adv. Sci., 2022, 9, 2201702 CrossRef CAS PubMed.
- C.-H. Ma, Y.-K. Liao, Y. Zheng, S. Zhuang, S.-C. Lu, P.-W. Shao, J.-W. Chen, Y.-H. Lai, P. Yu, J.-M. Hu, R. Huang and Y.-H. Chu, ACS Appl. Mater. Interfaces, 2022, 14, 22278–22286 CrossRef CAS PubMed.
- H. Wang, G. Gou and J. Li, Nano Energy, 2016, 22, 507–513 CrossRef CAS.
- L. Gao, B.-L. Hu, L. Wang, J. Cao, R. He, F. Zhang, Z. Wang, W. Xue, H. Yang and R.-W. Li, Science, 2023, 381, 540–544 CrossRef CAS PubMed.
- K. Hu, Y. Jiang, W. Xiong, H. Li, P.-Y. Zhang, F. Yin, Q. Zhang, H. Geng, F. Jiang and Z. Li, Sci. Adv., 2018, 4, eaar5907 CrossRef PubMed.
- C. S. Manohar, B. S. Kumar, S. P. P. Sadhu, S. K. Srimadh, V. S. Muthukumar, S. Venketesh and K. Varma, Nanotechnol. Rev., 2019, 8, 61–78 CAS.
- Y. Shen, Y. Wang, I. W. Hamley, W. Qi, R. Su and Z. He, Prog. Polym. Sci., 2021, 123, 101469 CrossRef CAS.
- W. Yang, W. Ni, C. Yu, T. Gu, L. Ye, R. Sun, X. Ying, J. H. Yik, D. R. Haudenschild and S. Yao, ACS Biomater. Sci. Eng., 2024, 10, 2385–2397 CrossRef CAS PubMed.
- Y. Y. Tang, Y. Xie, Y. L. Zeng, J. C. Liu, W. H. He, X. Q. Huang and R. G. Xiong, Adv. Mater., 2020, 32, 2003530 CrossRef CAS.
- J. Luo, G. Tian, D.-G. Zhang, X.-C. Zhang, Z.-N. Lu, Z.-D. Zhang, J.-W. Cai, Y.-N. Zhong, J.-L. Xu and X. Gao, ACS Appl. Mater. Interfaces, 2023, 15, 48452–48461 CrossRef CAS PubMed.
- X. Chen, H. Qin, X. Qian, W. Zhu, B. Li, B. Zhang, W. Lu, R. Li, S. Zhang and L. Zhu, Science, 2022, 375, 1418–1422 CrossRef CAS.
- H. Gong, Y. Zhang, J. Ye, X. Zhou, X. Zhou, Y. Zhao, K. Feng, H. Luo, D. Zhang and C. Bowen, Adv. Funct. Mater., 2024, 34, 2311091 CrossRef CAS.
- Y.-J. Wu, J.-X. Guo, X. Zhao, C.-Y. Tang, T. Gong, Q. Jing, K. Ke, Y. Wang, R.-Y. Bao and K. Zhang, Prog. Mater. Sci., 2024, 150, 101422 CrossRef.
- X. Ma, S. Zhukov, H. von Seggern, G. M. Sessler, O. Ben Dali, M. Kupnik, Y. Dai, P. He and X. Zhang, Adv. Electron. Mater., 2023, 9, 2201070 CrossRef CAS.
- Y. Hu, J. Yang and S. Liu, Phys. Rev. Lett., 2024, 133, 046802 CrossRef CAS PubMed.
- J. Chang, J. Li, J. Ye, B. Zhang, J. Chen, Y. Xia, J. Lei, T. Carlson, R. Loureiro and A. M. Korsunsky, Nano-Micro Lett., 2025, 17, 1–20 CrossRef.
- J. Park, K. Shin and C. Lee, Int. J. Precis. Eng. Manuf., 2016, 17, 537–550 CrossRef.
| This journal is © The Royal Society of Chemistry 2026 |