A spiropyran-based colorimetric probe for the highly selective and sensitive detection of copper(II) ions
Wangen
Miao
 *,
Limei
Xu
,
Jingli
Liu
and
Xiaoqin
Zhou
*
*,
Limei
Xu
,
Jingli
Liu
and
Xiaoqin
Zhou
*
School of Chemistry and Chemical Engineering, Key Laboratory of Clean Energy Materials Chemistry of Guangdong Higher Education Institutes, Lingnan Normal University, Zhanjiang 524048, China. E-mail: miaowangen@iccas.ac.cn; xqzhou@lingnan.edu.cn
First published on 25th November 2025
Abstract
A highly selective, sensitive and convenient probe, based on a photochromic and acidochromic benzoxazole-spiropyran derivative, was developed for visual Cu2+ detection. The detection remained unaffected by competing ions and achieved a detection limit of 10−7 M in both solution and test strip formats.
Trace copper ions, particularly the divalent Cu2+ are crucial for biological systems. Copper serves as an essential catalytic and structural cofactor in numerous life processes,1 including cellular respiration,2 antioxidant defense,3 neurotransmitter synthesis,4 connective tissue formation,5 and iron metabolism.6 The human body maintains copper homeostasis through sophisticated import, export, and storage mechanisms. Disruptions in this balance can be catastrophic, as demonstrated by genetic disorders such as Wilson's disease (copper overload)7 and Menkes disease (copper deficiency).8
On the other hand, widespread copper applications, including industrial catalysts and agricultural fungicides, cause environmental pollution. This results in high toxicity towards aquatic life (particularly fish and invertebrates) even at low concentrations.9 Thus, highly sensitive analytical methods are needed.
Traditional copper analysis techniques, including atomic absorption spectroscopy (AAS),10 high-performance liquid chromatography (HPLC),11 and inductively coupled plasma mass spectrometry (ICP-MS),12 provide highly sensitive detection capabilities for multiple metal elements. However, these methods are cost-prohibitive and require sophisticated laboratory infrastructure as well as skilled personnel. In contrast, the development of sensors with highly selective and sensitive responses to specific metal ions represents a promising alternative. Similar to pH test strips used for measuring solution acidity, such sensors offer simplicity and ease of operation.
Spiropyrans and their derivatives, which are among the most widely studied photochromic compounds, have been extensively applied in fields such as responsive materials,13 drug delivery,14 and cell adhesion.15 Upon exposure to UV light or binding with metal ions, the colorless spiropyran (SP) form isomerizes into the colored merocyanine (MC) form. This ring-opening reaction alters the electronic structure of the molecule, causing a distinct color change that is visible to the naked eye.16 Consequently, numerous spiropyran-based sensing platforms have been developed.17
Peng et al.18 recently reviewed spiropyrans functionalized with chelating agents (amines, carboxylic acids, macrocycles, heterocycles, organic dyes, copolymers, and nanoparticles), demonstrating their utility as fluorescent/colorimetric sensors for metal ions and anions in aqueous and biological systems. However, key limitations persist: (1) most sensors require improvement in metal ion selectivity; (2) many reported systems lack practical applicability; (3) SP–metal complex structures and mechanisms remain ambiguous. To address these challenges, we successfully synthesized a colorimetric spiropyran-based probe via a condensation reaction of 5-carboxy-1,2,3,3-tetramethyl-3H-indol and 3-benzoxazolyl-5-methyl-salicylaldehyde (Fig. 1 and Scheme S1). Interestingly, this probe exhibited high selectivity toward Cu2+ and strong anti-interference capability against other metal ions, achieving a detection limit of 10−7 M in both solution and test strip formats, thereby enabling convenient naked-eye detection of Cu2+.
 | ||
| Fig. 1 The schematic benzoxazole-spiropyran probe for highly selective, sensitive and convenient detection of Cu2+ by naked-eye. | ||
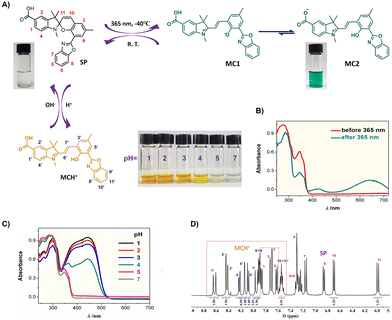
The benzoxazole-spiropyran probe exhibited excellent photochromic response at low temperatures. As shown in Fig. 2A, the colorless SP methanolic solution readily turned cyan upon 365 nm irradiation at −40 °C, indicating that the closed SP convert to the open MC. When the UV light was removed, the MC structure would recover its original closed SP gradually. In this process, if exposed in natural light or heated to room temperature, the recovery would be accelerated. The UV-vis spectra (Fig. 2B) further confirmed such photochromic conversion. Before 365 nm irradiation, two absorption bands appeared at 280 and 346 nm, respectively. The former was ascribed to the π–π* transition of the benzoindoline ring in the closed spiropyran, while the latter was attributed to the π–π* transition of the benzopyran moiety.19 After 365 nm irradiation, two new absorption bands appeared at 426 and 645 nm, which were attributed to the π–π* transitions of two types of trans-MC forms (MC1 and MC2, the cis- or trans- was identified by the double bond between benzoindoline and phenoxide ring),20 as illustrated in Fig. 2A. We speculated that initially formed MC1 readily isomerized to MC2 due to the higher acidity of the carboxyl group than the benzothiazole phenol. However, when the irradiation was at room temperature, no significant photochromic response occurred.
The introduction of H+ ions, which can protonate the MC benzofuran anions, was conducive to investigation of the SP → MC isomerization. Fig. 2A showed the acidochromic response of the benzoxazole-SP probe. The results indicated that the colorless SP solution turned to orange upon addition of H+ ions. And the UV-vis spectra (Fig. 2C) displayed that a distinct absorption at 458 nm appeared as pH < 3,21 confirming the formation of trans-MCH+ (Fig. 2A) in such isomerization. Compared to the absorption of the MC2 isomers (Fig. 2B), the absorption at 645 nm disappeared completely, revealing that only one isomer, MCH+, formed.
To further confirm the structure of the SP and MC isomers, 1H NMR was employed. As shown in Fig. 2D, the 1H NMR spectra of SP in DMSO-d6 (5 mg in 0.5 mL of DMSO-d6) with addition of DCl (3 µL of 20 wt% in D2O) indicated two sets of proton signals. One set was assigned to the SP isomer, where eleven aromatic protons were identified explicitly, including the characteristic benzopyran proton (H11) at δ 6.04 ppm. Four methyl groups appeared at δ 2.74, 2.34, 1.28, and 1.22 (Fig. S5). The other set was attributed to the single MC isomer. 13C NMR analysis (Fig. S7) also revealed the formation of a single MC isomer. According to a review by W. R. Browne et al.,19 there are eight possible MC conformers resulting from Z/E configurations around the benzopyran-benzoindoline linkage, as well as potential quinoidal forms. Under normal conditions, the trans-TTC or TTT isomers are predominant. Therefore, we proposed the observed MC species were the TTC isomer (Fig. 2A) due to the following three reasons: (1) rapid conversion of the initially formed cis-CCC isomer (from Cspiro–O cleavage) to the trans-TTC form due to steric strain; (2) the formed MC isomers are zwitterionic, the N in benzoindoline ring is positive while the O in phenoxide ring is negative. The electrostatic interaction prohibits the TTC → TTT conversion. And (3) the characteristic C signal at 184 ppm (Fig. S7), assigned to C2 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+), which is distinct from quinoidal carbonyl signals (ca. 195 ppm).
N+), which is distinct from quinoidal carbonyl signals (ca. 195 ppm).
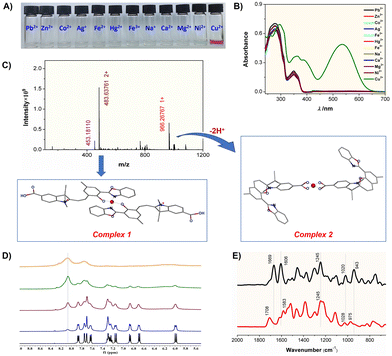
The sensing behavior was investigated by adding various metal ions (Pb2+, Zn2+, Co2+, Ag+, Fe2+/3+, Hg2+, Na+, Ca2+, Mg2+, Ni2+ and Cu2+) to the SP solution. Detailed procedures were provided in the experimental section of the SI. In all tests, the concentration of SP maintained constant at 0.1 mM. With the exception of Cu2+, no significant color change was observed upon addition of other metal ions (0.001 mM) to the SP solution (Fig. 3A). Notably, the introduction of Cu2+ ions resulted in a distinct color change from colorless to wine-red, readily visible to the naked eye. The color of the solution remains basically unchanged for more than a month, indicating that the formed complex is very stable. UV-vis spectra of the SP solution with various metal cations (Fig. 3B) showed negligible changes in absorption curves except for Cu2+, suggesting that SP neither forms stable complexes with other metal ions nor undergoes significant structural alteration. Upon adding Cu2+ ions, a new absorption peak at 536 nm increased significantly, indicating a transition from the ring-closed to the ring-open form upon coordination with Cu2+.
To confirm the structure of the complex of SP with Cu2+, we acquired high-resolution mass spectra (HRMS) of SP with Cu2+ in MeOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). As shown in Fig. 3C, two prominent peaks were observed at m/z 996.26767 (singly charged) and 483.63761 (doubly charged), confirming the presence of two distinct types of complexes. The former was corresponded to the carboxylate-bridged complex, [(SP-COO−)2Cu2+ + H+]+ (calculated 996.26899), designated as complex 2 (Fig. 3C). The latter was assigned to the ring-opened merocyanine–Cu2+ chelate (complex 1, calculated 483.63841).
1, v/v). As shown in Fig. 3C, two prominent peaks were observed at m/z 996.26767 (singly charged) and 483.63761 (doubly charged), confirming the presence of two distinct types of complexes. The former was corresponded to the carboxylate-bridged complex, [(SP-COO−)2Cu2+ + H+]+ (calculated 996.26899), designated as complex 2 (Fig. 3C). The latter was assigned to the ring-opened merocyanine–Cu2+ chelate (complex 1, calculated 483.63841).
Fig. 3D showed the 1H NMR titration of SP in DMSO-d6 with incremental Cu2+ equivalents (in D2O) at room temperature. Upon addition of Cu2+, new proton peaks, attributed to ring-opened merocyanine, emerged and intensified proportionally with increasing Cu2+ concentration. At 0.5 equivalents, the integration of these new signals was almost equal to that of the original SP protons, indicating approximately equal concentrations of both isomers. Concurrently, paramagnetic line broadening induced by Cu2+ resulted in progressive signal attenuation. This paramagnetic effect was even more pronounced in the 13C NMR spectra (Fig. S13), where all carbon peaks disappeared completely at 0.5 equivalents Cu2+.
Fig. 3E compared the FT-IR spectra of SP with and without Cu2+ ions. For pure SP (Fig. 3E, top), the carbonyl stretching of the –COOH group appeared at 1669 cm−1, indicating the formation of dimer through intermolecular hydrogen bonding. Characteristic vibrations included: C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching (oxazole ring) at 1606 cm−1, C–O stretching (oxazole ring) at 1245 cm−1, benzopyran C–O stretching at 1020 cm−1 and Cspiro–O stretching at 943 cm−1.22,23 Upon adding 0.5 equiv. of Cu2+ (Fig. 3E, bottom), significant spectral changes occurred: (1) The C
N stretching (oxazole ring) at 1606 cm−1, C–O stretching (oxazole ring) at 1245 cm−1, benzopyran C–O stretching at 1020 cm−1 and Cspiro–O stretching at 943 cm−1.22,23 Upon adding 0.5 equiv. of Cu2+ (Fig. 3E, bottom), significant spectral changes occurred: (1) The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching shifted to 1708 cm−1, signifying conversion from dimers to monomers; (2) The C
O stretching shifted to 1708 cm−1, signifying conversion from dimers to monomers; (2) The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching (oxazole ring) took a red-shift to 1583 cm−1, indicating coordination through the nitrogen atom to Cu2+; (3) the oxazole C–O stretching (1245 cm−1) remained unchanged, confirming that the oxazole oxygen did not participate in coordination; (4) the Cspiro–O band (943 cm−1) decreased markedly, indicating cleavage of the C–O bond. Together with 1H NMR data, these results confirmed the coexistence of two SP–Cu2+ complexes: a carboxylate-bridged dimer and a ring-opened chelate.
N stretching (oxazole ring) took a red-shift to 1583 cm−1, indicating coordination through the nitrogen atom to Cu2+; (3) the oxazole C–O stretching (1245 cm−1) remained unchanged, confirming that the oxazole oxygen did not participate in coordination; (4) the Cspiro–O band (943 cm−1) decreased markedly, indicating cleavage of the C–O bond. Together with 1H NMR data, these results confirmed the coexistence of two SP–Cu2+ complexes: a carboxylate-bridged dimer and a ring-opened chelate.
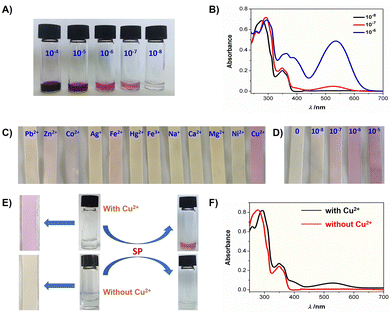
To further evaluate the sensitivity of SP toward Cu2+, SP solutions containing Cu2+ at concentrations ranging from 10−4 to 10−8 M were tested (Fig. 4A). A distinct color change from colorless to wine-red was observed even at Cu2+ concentrations as low as 10−7 M. Corresponding UV-vis spectra (Fig. 4B) exhibited a pronounced absorption band at 533 nm for the solution with 10−7 M Cu2+, confirming the high sensitivity of the visual detection method.
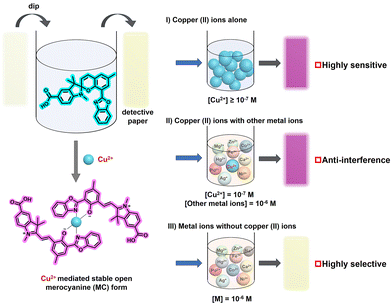
Test strips were prepared to facilitate SP-based visual detection of Cu2+ with enhanced convenience and selectivity, using the following procedure: Filter paper was immersed in a THF solution of SP (20 mM) and air-dried. The resulting colorless test strips were immersed in aqueous solutions containing 0.001 mM various cations. Strips immersed in Cu2+ solutions exhibited a distinct color change from colorless to wine-red (Fig. 4C), readily visible to the naked eye, while those exposed to other cations showed minimal color change. Additionally, test strips were immersed in Cu2+ solutions across a concentration range (Fig. 4D). A faint red coloration was observed even at Cu2+ concentrations as low as 10−7 M.
To further evaluate interference from competing ions during the Cu2+ detection, we prepared solutions containing various metal ions (Pb2+, Zn2+, Co2+, Fe3+, Hg2+, Ag+, Na+, Ca2+, Mg2+, Ni2+) with and without 10−7 M Cu2+. The concentration of all non-copper cations was maintained at 10−6 M. Upon addition of SP (final concentration: 0.1 mM), the solutions containing Cu2+ turned wine-red immediately (Fig. 4E), whereas solutions without Cu2+ remained colorless. Test strip analysis further confirmed exceptional selectivity toward Cu2+, with strong anti-interference capability against other competing ions. The high sensitivity was additionally verified by UV-vis spectra (Fig. 4F).
To examine the actual application, we determined the concentration of copper ions in the nearshore seawater of Jinsha Bay, Zhanjiang with the benzoxazole-spiropyran probe. The calibration curve was illustrated in Fig. S14. The determined absorbance of seawater was ca. 0.162, indicating [Cu2+] is ca. 3.3 × 10−7 M. The slope of calibration curve is the molar absorption coefficient (ε), which is ca. 4.9 × 105 L mol−1 cm−1. Such high ε value accounted for the exceptional sensitivity of Cu2+ detection. The qualitative naked-eye detection (Fig. S15) also revealed that the [Cu2+] was about in the range of 10−6–10−7 M. To verify our method, the standard method, atomic absorption spectroscopy, was compared to detect the same seawater sample. The corresponded calibration curve was showed in Fig. S16. And the absorbance of seawater was 0.227. Thus, the [Cu2+] is ca. 0.015 mg L−1, that is 2.3 × 10−7 M. The results indicated that our method is generally accurate and reliable.
In summary, we have successfully synthesized a benzoxazole-spiropyran probe and demonstrated exceptional selectivity and sensitivity for Cu2+ detection. This spiropyran enabled naked-eye identification of Cu2+ at concentrations as low as 10−7 M in both solution and test strip formats, exhibiting a distinct color change to wine-red. The high molar absorption coefficient (ε = 4.9 × 105 L mol−1 cm−1 at λmax = 535 nm) and strong anti-interference capability promote its convenient utility as a practical, efficient, and reliable sensor for Cu2+ detection.
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (21773103 and 21902070).
Conflicts of interest
There are no conflicts of interest to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). The synthesis of benzoxazole-spiropyran probe (SP), 1H NMR spectra of the intermediates and the final SP, the experimental methods of photochromic and acid-chromic test, high-resolution mass spectrum (HRMS) of SP with and without Cu2+, images of various metal ions (10−4 and 10−5 M) with the SP probe, visual detection to Cu2+ in the nearshore seawater and comparison with standard method, Atomic Absorption Spectroscopy (AAS) are available of supplementary information. See DOI: https://doi.org/10.1039/d5cc05144d.Notes and references
- Q. Xue, R. Kang, D. J. Klionsky, D. L. Tang, J. B. Liu and X. Chen, Autophagy, 2023, 19, 2175–2195 CAS.
- R. Paduvari, R. Arekal and D. M. Somashekara, Int. J. Biol. Macromol., 2025, 309, 142773 CAS.
- Y. Y. Wang, D. D. Li, K. F. Xu, G. Q. Wang and F. Zhang, Neural Regener. Res., 2025, 20, 3124–3143 CAS.
- I. F. Scheiber, J. F. B. Mercer and R. Dringen, Prog. Neurobiol., 2014, 116, 33–57 CrossRef CAS PubMed.
- Y. Liu, R. Niu, H. Zhao, Y. H. Wang, S. Y. Song, H. J. Zhang and Y. L. Zhao, J. Am. Chem. Soc., 2024, 146, 3675–3688 CrossRef CAS PubMed.
- M. T. Jeena, J. Link, J. Zhang, I. Harley, P. Turunen, R. Graf, M. Wagner, L. A. Baptista, H. R. A. Jonker, L. Y. Cui, I. Lieberwirth, K. Landfester, J. Rao, D. Y. W. Ng and T. Weil, Angew. Chem., Int. Ed., 2024, 63, 202412477 CrossRef.
- D. Strausak, J. F. B. Mercer, H. H. Dieter, W. Stremmel and G. Multhaup, Brain Res. Bull., 2001, 55, 175–185 CrossRef CAS PubMed.
- R. A. Pufahl, C. P. Singer, K. L. Peariso, S. J. Lin, P. J. Schmidt, C. J. Fahrni, V. C. Culotta, J. E. PennerHahn and T. V. OHalloran, Science, 1997, 278, 853–856 CrossRef CAS PubMed.
- W. Zhang, Y. N. Miao, K. F. Lin, L. Chen, Q. X. Dong and C. J. Huang, Environ. Pollut., 2013, 176, 158–164 CrossRef CAS.
- (a) L. S. Laxmeshwar, M. S. Jadhav, J. F. Akki, P. Raikar, J. Kumar, O. prakash and U. S. Raikar, Opt. Laser Technol., 2017, 91, 27–31 CrossRef CAS; (b) P. Hashemi and A. Rahimi, Spectrochim. Acta, Part B, 2007, 62, 423–428 CrossRef.
- (a) S. Saito, Y. Sato, T. Haraga, Y. Nakano, S. Asai, Y. Kameo, K. Takahashi and M. Shibukawa, J. Chromatogr. A, 2012, 1232, 152–157 CrossRef CAS PubMed; (b) K. A. High, J. S. Blais, B. A. J. Methven and J. W. McLaren, Analyst, 1995, 120, 629–634 RSC.
- (a) H. Huang, Y. Wei, Y. Xia, L. Wei, X. Chen, R. Zhang, L. Su, M. L. Rahman, M. Rahman, Q. Qamruzzaman, W. Guo, H. Shen, Z. Hu, D. C. Christiani and F. Chen, J. Exposure Sci. Environ. Epidemiol., 2021, 31, 571–580 CrossRef CAS; (b) F. Vanhaecke and M. Costas-Rodríguez, View, 2021, 2, 20200094 CrossRef CAS.
- (a) L. W. Ma, G. H. Wang, B. B. Ding and X. Ma, CCS Chem., 2022, 4, 2080–2089 CrossRef CAS; (b) X. F. Hu, B. Fang, P. Y. Li, J. X. Wang, J. Li, Y. H. Dai and M. Z. Yin, Adv. Funct. Mater., 2025, 35, 202423793 Search PubMed.
- (a) S. Song, H. Zhang and Y. Liu, Acc. Mater. Res., 2024, 5, 1109–1120 CrossRef CAS; (b) C. Su, G. Liu, Y. Zou, S. Ji and J. Gao, Int. J. Biol. Macromol., 2024, 280, 135857 CrossRef CAS.
- (a) Y. Hao, H. Cui, J. Meng and S. Wang, J. Photochem. Photobiol., A, 2018, 355, 202–211 CrossRef CAS; (b) J. Li, Z. Ma, A. Li, S. Huang, Y. Zhang, Y. Xue, X. Song, Y. Zhang, S. Hong, M. Wang, Z. Wu and X. Zhang, J. Mater. Chem. B, 2023, 11, 9525–9531 RSC.
- (a) Z. Sun, Y. Li, M. Wu, W. He, Y. Yuan, Y. Cao and Y. Chen, Angew. Chem., Int. Ed., 2024, 63, 202411629 CrossRef; (b) S. Wang, Y. Fan, Y. Wang, L. Gui, S. Huang, T. Wu and X. Tian, Adv. Funct. Mater., 2024, 34, 202311036 Search PubMed.
- (a) D. Zhang, Y. Sun, Z. Wang, F. Liu and X. Zhang, Nat. Commun., 2023, 14, 1901 CrossRef PubMed; (b) H. Zhang, L. Wang, J. Li and Q. Xu, Sens. Actuators, B, 2025, 423, 136734 CrossRef CAS.
- S. Chatterjee, B. Liu and H. S. Peng, Coord. Chem. Rev., 2024, 508, 215779 CrossRef CAS.
- L. Kortekaas and W. Browne, Chem. Soc. Rev., 2019, 48, 3406–3424 RSC.
- R. Klajn, Chem. Soc. Rev., 2014, 43, 148–184 RSC.
- J. Sunamoto, K. Iwamoto, M. Akutagawa, M. Nagase and H. Kondo, J. Am. Chem. Soc., 1982, 104, 4904–4907 CrossRef CAS.
- B. Mistry, R. Patel and V. Patel, J. Appl. Polym. Sci., 1997, 64, 841–848 CrossRef CAS.
- K. Fries, G. Sheppard, J. Bilbrey and J. Locklin, Polym. Chem., 2014, 5, 2094–2102 RSC.
| This journal is © The Royal Society of Chemistry 2026 |