 Open Access Article
Open Access ArticleAir–water exchange fluxes of phthalates and phenolics xenoestrogens on Xietang River and East Taihu Lake in Suzhou and the potential health risks†
Minhao
Wang
 ab,
Dongling
Li
ab,
Dongling
Li
 a,
Ting
Tong
a,
Ziyu
Zhang
c,
Yuwei
Xia
d,
Xinhui
Shi
a,
Haifei
Zhang
a,
Ting
Tong
a,
Ziyu
Zhang
c,
Yuwei
Xia
d,
Xinhui
Shi
a,
Haifei
Zhang
 b,
Kui
Chen
e,
Fang
Wang
e,
Xiaowei
Tie
e and
Lei
Han
b,
Kui
Chen
e,
Fang
Wang
e,
Xiaowei
Tie
e and
Lei
Han
 *a
*a
aDepartment of Health and Environmental Sciences, School of Science, Xi'an Jiaotong–Liverpool University, Suzhou, China. E-mail: Lei.Han@xjtlu.edu.cn
bDepartment of Chemistry, School of Physical Science, University of Liverpool, Liverpool, UK
cDepartment of Biosciences and Bioinformatics, School of Science, Xi'an Jiaotong–Liverpool University, Suzhou, China
dDepartment of Applied Mathematics, School of Mathematics and Physics, Xi'an Jiaotong–Liverpool University, Suzhou, China
eEurofins Technology Service (Suzhou) Co., Ltd. Suzhou, 215100, China
First published on 13th March 2025
Abstract
As endocrine disruptors, xenoestrogens are prevalent in inland lakes and are mainly attributed to atmospheric transportation. There is limited research on the air–water exchange of xenoestrogens in inland waters and the consequent health effects. This study investigated the air–water exchange process of selected xenoestrogens and associated health risks in adults by examining water and PM2.5 samples from the East Taihu Lake and Xietang River in Suzhou. The mean concentration of total xenoestrogens in surface water and PM2.5 were 40.30 ± 7.19 ng L−1 and 460.13 ± 31.87 ng m−3 (East Taihu Lake), 536.69 ± 99.62 ng L−1 and 63.93 ± 3.82 ng m−3 (Xietang River), respectively. Utilising a two-film model, the air–water exchange flux was calculated, with BPA exhibiting the most comprehensive range among all xenoestrogens from −8.88 × 109 to −1.01 × 1010 near East Taihu Lake and −1.32 × 1010 to −1.13 × 1010 (ng m−2 d−1) near Xietang River. Different xenoestrogens displayed various air–water exchange directions. DBP shows the highest dry deposition fluxes of 9373.26 ± 611.59 near East Taihu Lake and 478.97 ± 48.00 (ng m−2 d−1) near Xietang River. The study also assessed the non-dietary exposure risk of six xenoestrogens in PM2.5 for adults, concluding that no non-cancer risks were identified, with a hazard index below 1. DEHP concentration is within an acceptable level of carcinogenic risk (incremental lifetime cancer risk <10−6). Results from this study underscore the importance of developing and implementing xenoestrogen management policies in the Taihu Lake Basin.
Environmental significanceThis research investigates the occurrence of xenoestrogens in freshwater systems in Suzhou, with a specific focus on the environmental mechanisms of air–water exchange. It also evaluates the potential health risks associated with inhalation of these substances, providing information on the effects of human actions on ecosystems and human health. The results emphasize the importance of implementing policies for the management of xenoestrogens, particularly in the Taihu Lake Basin, to reduce hazards and protect both the environment and public health. |
1. Introduction
Xenoestrogens, such as phthalates (PAEs), bisphenols (BPs) and alkylphenols (APs), share structural similarities to estrogen and are commonly found in plastics, pesticides, chemicals, and water resources, contributing to pollution in the atmosphere and surface water.1–4 These compounds are widely distributed in the environment and food, posing a global exposure risk to populations.5 Even at low concentrations in water, they disrupt the endocrine system in wildlife.6 Laboratory tests have revealed that certain xenoestrogens exhibit estrogen-like activity.7 Concerns regarding their impact on infants and children's endocrine function have prompted Bisphenol A (BPA) prohibition in baby bottle production in Europe, Canada and the United States.7 Exposure to xenoestrogens can lead to obesity, reproductive disorders, and genetic effects on sperm, which ultimately affects sperm quality and spermatogenesis.8,9Xenoestrogens primarily originate from human activities, including industry, transportation, commercial enterprises, and volatilisation from water sources.10–12 They enter water bodies through atmospheric deposition, direct releases, and urban runoff, making it essential to have a comprehensive understanding of their transport dynamics between the atmosphere and surface water, which significantly impacts the distribution and circulation of these compounds.13–15 Studying the air–water exchange of xenoestrogens is important as it plays a crucial role in comprehending and managing their transmission, distribution, and ultimate fate in the environment. As an emerging environmental pollutant, xenoestrogens can infiltrate the water system through various pathways, such as atmospheric deposition and river transport.16 These pollutants accumulate in water bodies and can be re-released into the atmosphere, impacting global biogeochemical cycles and ecosystem health.17
Xenoestrogen can be transported over vast distances through long-range atmospheric transport and water body flow, and studying air–water exchange aids in understanding the dispersion and redistribution of these pollutants globally.18 In the context of urban rivers, Chandra and Chakraborty.19 indicated that air–water exchange fluxes play an essential role in determining the fate of PAEs in a tropical urban riverine catchment. Moreover, atmospheric dry deposition should not be overlooked when studying the air–water transport in xenoestrogens.16 Historical data reveal that dry deposition is the primary source of organic pollutants in significant water bodies such as the Great Lakes, with dry deposition occurring 1.5 to 5.0 times higher than wet deposition.20 Most studies examining the exchange of xenoestrogens at the air–water interface have focused on the ocean, with limited research on this process in inland surface water. Mi et al.21 demonstrated that seasonal meteorological variations influence the exchange process at the air-sea interface, leading to the long-distance migration of PAEs from land to the ocean, and different PAEs exhibit unique directions in air–sea exchange. Xie et al.12 conducted a study of APs on the North Sea, highlighting the deposition, especially during winter, and the subsequent re-volatilisation into the atmosphere in warmer seasons with increasing temperatures and humidity.
Inland surface waters, such as rivers, lakes and wetlands, are inevitably impacted by human activities, including industrial discharges, agricultural water use, urban development and wastewater discharges.22 The input of pollutants from these anthropogenic activities directly affects water quality and disrupts the structure and function of aquatic ecosystems, exposing them to significant environmental stresses.23,24 In Suzhou, over 40% of the city's total urban area is covered by surface water, but the slow flow of these water bodies hinders the migration of non-point source pollutants in surface water and thus causes high concentrations of local pollutants.25,26 The Xietang River, located within a densely populated region, was chosen as the monitoring site for this study. Previous investigations by Wu et al.27 and Yuan et al.28 have detected the presence of organochlorine pesticides (OCPs) and polycyclic aromatic hydrocarbons (PAHs) in the sediments of this river. As a comparison, Taihu Lake, which is classified as a class IV drinking water source for Suzhou, was selected as the reference sampling site.29
Previous studies have demonstrated that toxic pollutants entering surface water or the atmosphere through air–water exchange pose a significant threat to human health and the environment in urban areas, thereby increasing health risks for residents.30,31 Kim et al.32 revealed that, particularly in non-metropolitan areas, the risk of cognitive decline associated with air pollution exposure is more pronounced in women. While there has been considerable epidemiological and toxicological research on xenoestrogens in China, many studies have predominantly concentrated on indoor air pollution.33–35 Moreover, existing literature has primarily targeted specific urban areas, such as Beijing, Nanjing, Tianjin, Shenzhen and Tibet, leaving cities such as Suzhou underexplored.36–40 Additionally, previous toxicological investigations have focused mainly on vulnerable and occupationally exposed populations, which may not adequately represent the exposure levels of the broader urban population.38,40,41
In this study, we aim to explore the exchange flux of phthalates and phenolics xenoestrogens on Xietang River and East Taihu Lake in Suzhou, to understand the impact factors and evaluate the potential health risks to human. The main research objectives of this study were: (1) to monitor the concentration of xenoestrogens in atmospheric aerosols at the research site; (2) to identify the critical environmental factors which had impact on the exchange flux; (3) to assess the potential health risks to the population of different genders at the research site. This study fills the knowledge gap regarding the environmental behaviour and transport mechanisms of these xenoestrogens in inland surface waters, providing insights into pollution management and mitigation strategies. We also highlight the need for gender-specific risk assessments in environmental health studies. These findings are expected to promote theoretical research and provide references for practical applications in atmospheric pollution monitoring, environmental risk assessment, and policy making.
The main research contents of this study focused on (1) monitoring xenoestrogen concentration in air and water samples; (2) calculating the air–water exchange fluxes using the two-film model, with discussion on impact factors; (3) conducting health risk assessments and evaluating non-carcinogenic and cancer risks.
2. Experimental
2.1 Water and aerosol sampling
Two sampling areas in Suzhou were selected for this study: The Xietang River (A densely populated area) and the East Taihu Lake. The sampling points distribution is shown in Fig. 1 (detailed explanation is provided in ESI†). The East Taihu Lake represents the conditions of extensive inland shallow lakes influenced by both natural and anthropogenic factors42,43 In contrast, the Xietang River reflects the environmental changes occurring in urban aquatic ecosystems under significant human pressure.44 Additionally, xenoestrogens are more prevalent in the atmosphere during summer, a pattern linked to the release of various materials, indoor ventilation, industrial activities, and atmospheric chemical processes, all of which significantly impact air–water exchange dynamics.45,46 Therefore, the surface water was sampled on the 22nd July and 5th August in the Xietang River and 26th June and 3rd July in the East Taihu Lake in 2022, respectively. Atmospheric particulate matter (PM2.5, particles with an aerodynamic diameter of ≤2.5 μm) was sampled from 22nd June to 10th August in Xietang River and from 21st June to 5th July 2022 in the East Taihu Lake, respectively (Detailed site information is provided in ESI, Table S1†). The method for collecting water samples is closely similar to the methodology employed in our previous research.47 A portable water quality analyser (YSI ProPlus, USA) was taken to measure in situ physical–chemical parameters, including dissolved oxygen (DO), conductivity, ammonia nitrogen (NH4+–N), nitrate nitrogen (NO3+–N) and pH.48 Three parallel water samples were obtained from the near-shore zone and filtrated with microporous filtration membranes (Jinteng, China) by solvent filtration/degassing systems (Jinteng, China).High-volume air samplers (TH1000CII, Tianhong, China) were applied to collect PM2.5 aerosol samples with a sampling time of 24 hours. Aerosol was collected on preheated (450 °C for six hours) quartz fibre filters (203 mm × 254 mm, Whatman) at a 1.05 m3 min flow rate. All the filters were wrapped in preheated aluminium foil and stored in a dark freezing room at −20 °C before the analysis.
2.4 Sample pretreatment and instrumental analysis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 77
77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 135
135![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 194), diethyl phthalate (DEP) (149; 149
194), diethyl phthalate (DEP) (149; 149![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 177
177![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 121
121![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 222), dibutyl phthalate (DBP) (149; 149
222), dibutyl phthalate (DBP) (149; 149![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 223
223![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 205
205![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 121), benzyl butyl phthalate (BBP) (149; 149
121), benzyl butyl phthalate (BBP) (149; 149![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 91
91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 206
206![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 238), di(2-ethylhexyl)phthalate (DEHP) (149; 149
238), di(2-ethylhexyl)phthalate (DEHP) (149; 149![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 167
167![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 219
219![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 113). As for detecting PAEs in atmospheric particulate matter samples, the Thermo Trace 1300 Series Gas Chromatograph with ISQ MS was used with the same scanning mode and selected ion, and the primary parameter setting is on ESI, Table S2.†
113). As for detecting PAEs in atmospheric particulate matter samples, the Thermo Trace 1300 Series Gas Chromatograph with ISQ MS was used with the same scanning mode and selected ion, and the primary parameter setting is on ESI, Table S2.†
HPLC was used for the analysis of BPA in water using an ODS C18 column (250 mm × 4.5 mm, 5 μm) with a column temperature set to 25 °C and an injection volume of 10 μL. The mobile phase was water–methanol (3 + 1), and the flow rate was set to 0.8 mL min−1. A fluorescence detector was used, with an absorption wavelength of 227 nm and an emission wavelength of 313 nm. As for the BPA and APs in the particulate matter sample, LC-MS with acclaim polar Advantage II (50 mm × 3 mm, 3 μm) column was used for xenoestrogens analysis. Parameters are shown in ESI, Table S3.† The standard curves of all phenolic compounds reached R2 > 0.9999.
2.5 Quality assurance/quality control
The target contaminants in this research are usually used as plasticisers, commonly present in most plastic products. Consequently, it is necessary to utilise glassware for all experimental procedures and to employ ultrapure water and n-hexane for ultrasonic cleaning before each use of experimental supplies. Three replicates of each water sample and atmosphere particulate matter sample were set up to ensure the accuracy of the results. Furthermore, the accuracy of the experiment is ensured by the recovery test and the blank test (including samples blank and solvents blank). The assay results showed that all solvents and samples blank were below the detection limits, and the sample recovery rate was within acceptable limits (73.8–123.0%). The Limits of Detection (LODs) ranges from 0.88–2.17 ng L−1 for PAEs and 0.09–0.12 ng L−1 for phenolics. The Limit of Quantitation (LOQ) values are 2.94–7.22 ng L−1 for PAEs and 0.30–0.40 ng L−1 for phenolics. Detailed data for each target pollutants are shown in the ESI, Table S4.†2.6 Air–water exchange fluxes
A two-film model was conducted to calculate plasticisers' air–water particulate exchange flux (Faw, ng m−2 d−1). The exchange flux was defined by Whitman (1962).49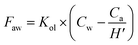 | (1) |
When applied the ff for air–water exchange analysis, the uncertainty (u) of ff was also calculated, and ff values in the range of 0.5 × (1 ± u) may be considered equilibrium.50 In this study, fugacity fraction values falling below 0.26 indicate volatilisation, values between 0.26 and 0.74 signify air–water equilibrium, and values exceeding 0.74 represent deposition. Detailed formulas are shown in the ESI, Section 2.†
The dry deposition fluxes (Fdd, ng m−2 d−1) were calculated according to the following equation:51
| Fdd = Cp × Vd | (2) |
2.7 Health impact assessment
After determining the concentration of plasticisers in the atmosphere particulate matter, the probabilistic exposure model is used to evaluate the average daily dose (ADD) in mg per kg per day and incremental lifetime cancer risk (ILCR) of DEHP via inhalation and dermal contact.55,56 The formula for ADD of inhalation and dermal contact exposure was shown in the ESI, Section 3.†The hazard index (HI) for non-dietary intake was determined by following the formula.56 RfD is each pollutant's chronic exposure reference dose with the mg per kg per day unit. Humans are exposed to non-cancer risks if the values of HI are greater than 1.
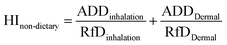 | (3) |
ILCR is the incremental lifetime cancer risk associated with inhalation and dermal contact, which was calculated by:55,57
 | (4) |
CSF is the cancer slope factor 0.014 per mg per kg per day for DEHP via inhalation or dermal contact.58
2.8 Statistical analysis
Statistical analyses were performed utilising R Software Version 4.1.3. EPIWEB v4.1 was employed to model the parameters of target plasticisers. By quantifying the levels of xenoestrogens in particle phases and estimating absorbed fractions in airborne particulates (Φ), it becomes feasible to predict gaseous concentrations (ng m−3) and subsequently determine the overall transfer of xenoestrogens between air and water in the Taihu basin region. The relationship between the particle-bound fraction, gaseous concentrations, and particle-bound concentrations of xenoestrogens is explained by the subsequent eqn (5):59 | (5) |
3. Results and discussion
3.1 Concentration of xenoestrogen in surface water and atmosphere
The concentrations of specific xenoestrogens in the surface water are shown in Fig. 2. Among the six xenoestrogens analysed, DBP, DEHP and BPA were present in all samples from East Taihu Lake, as illustrated in Fig. 2a. The detection rates for DEP, DMP and BBP in East Taihu Lake were approximately 25%, 83% and 67%, respectively. Xenoestrogens typically exhibit concentrations ranging from several to several tens of nanograms per litre, with a total concentration between 12.75 and 93.86 ng L−1 and an average concentration of 40.30 ± 7.19 ng L−1. In the water bodies of East Taihu Lake, the mean levels of DBP and DEHP exceeded those of three other PAEs (DEP, DMP, BBP) during these two days. This finding is consistent with a previous study conducted by Gao et al.61 in Taihu Lake, where DBP and DEHP were identified as the predominant PAEs in surface water in 2014, with average concentrations exceeding 1000 ng L−1, suggesting a positive trend towards decreased xenoestrogen levels in the Taihu Lake over time. In Fig. 2b, all samples collected from Xietang River showed the presence of six targeted xenoestrogens, with the total xenoestrogens levels ranging from 231.82 to 1030.90 ng L−1 and averaging at 536.69 ± 99.62 ng L−1 in Xietang River. DEHP and DBP were also identified as the primary xenoestrogens. The combined concentrations of the five PAEs were similar to those observed in the Yangtze River (Jiangsu section) in research by Ren et al.,62 ranging from around 300 to 1000 ng L−1. Zhao et al.63 conducted a study that revealed a higher mean concentration of total PAEs in the Lanzhou Section of the Yellow River, measuring approximately 1900 ng L−1. Yuan et al.64 reported that BPA levels at the river in Suzhou were approximately 100 ng L−1, predominantly originating from industrial discharges and higher than the concentrations observed in the present study. This difference indicates a decline in BPA concentration in surface water in Suzhou. Furthermore, the average concentration of BPA in the Xietang River is comparatively lower than that in the river Ganga (over 100 ng L−1) and Xiangjiang River (around 37 ng L−1) in 2022, which is resulted from the more stringent monitoring and regulation of domestic and industrial wastewater discharge in the Xietang River.65,66This study also conducted a comparative analysis of contamination levels of neighbouring compounds, specifically DBP, DEHP and BPA, in East Taihu Lake and Xietang River in China, with data from other inland surface waters globally (see ESI, Table S5†). The results indicated that the concentrations of xenoestrogens in East Taihu Lake were relatively low. Conversely, the Xietang River exhibited elevated levels of DBP, ranging from 163 to 298 ng L−1, and DEHP, ranging from 111 to 163 ng L−1, which were comparable to concentrations observed in certain water bodies in Africa, specifically Nigeria and Uganda. In contrast, regions in Europe, such as the Netherlands and Italy, reported significantly higher levels of BPA pollution, with concentrations reaching up to 3620 ng L−1, while the BPA concentrations in the study area were comparatively low, ranging from 12 to 39 ng L−1. Overall, the pollution levels in the areas examined in this study are considered moderately low on a global scale. However, the contamination observed in the Xietang River necessitates further investigation. Therefore, it is recommended that future analysis focus on pollutant source identification and ecological risk assessments in the Xietang River to optimise pollution control strategies.
The presence of DEHP, di-isobutyl phthalate (DIBP), DBP, BPA, nonylphenol (NP), and 4-tert-octylphenol (4 t-OP) have been detected in all PM2.5 samples collected from East Taihu Lake and Xietang River (Fig. 2). The concentrations of total xenoestrogen (sum of DEHP, DIBP, DBP, BPA, NP and 4 t-OP) bound to PM2.5 ranged from 54.45 to 863.40 ng m−3 (mean = 460.13 ± 31.87 ng m−3) near the East Taihu Lake and 11.13 to 127.84 ng m−3 (mean = 63.93 ± 3.82 ng m−3) near Xietang River. Higher concentrations of DBP (ng m−3) were observed at East Taihu Lake (range: 51.16–858.17) compared to DIBP, DEHP, BPA, 4 t-OP and NP, which exhibited ranges of 0.70–3.49, 1.16–5.47, 0.09–1.19, below the limit of quantification (<LOQ)-0.07 and 0.02–0.18, respectively. Salgueiro-González et al.67 revealed that DBP exhibits the most elevated levels within PM2.5, particularly in suburban and industrial regions, surpassing other xenoestrogens by several orders of magnitude, which may be linked to industrial processes such as plastic production. Temporally, the Xietang River exhibited higher levels of DBP (range: 7.73–14.30 ng m−3), DIBP (range: 21.05–48.05 ng m−3) and DEHP (range: 4.56–9.10 ng m−3) concentrations compared to BPA (range: 0.07–0.14 ng m−3), 4 t-OP (<LOQ) and NP (0.01–0.02 ng m−3). A study conducted in Guangdong Province by X. Liu et al.68 observed a similar concentration distribution trend in PM2.5 with DBP ranging from 0.78 to 11.20 ng m−3, DIBP from 0.86 to 9.92 ng m−3, DEHP from 1.21 to 82.60 ng m−3 and BPA from 0.01 to 1.65 ng m−3.
The levels of xenoestrogens in PM2.5 and water samples from both sampling exhibit an observable opposite trend (Fig. 2). Specifically, the concentration of xenoestrogens on PM2.5 above East Taihu Lake was higher, whereas Xietang River showed a higher concentration in water samples of these compounds. Previous research by Liu et al.69 indicated that urban rivers tend to have elevated concentrations of PAEs due to human activities, while suburban surface water shows significantly lower levels compared to urban and industrial areas. Similarly, Ma et al.70 observed that the average concentration of PM2.5-bound PAEs was higher in suburban than urban areas. Furthermore, the average wind speed during the sampling period was 5.09 m s−1 for Xietang River and 1.10 m s−1 for East Taihu Lake in this study. Consequently, the high wind speeds were conducive to the dispersion and dilution of particulate matter and its associated xenoestrogens.71
3.2 Air–water exchange fluxes of xenoestrogen
DBP, DEHP and BPA were detected in water and aerosol samples in East Taihu Lake and Xietang River, and their air–water exchange fluxes were calculated. Eqn (5) was used to calculate the gaseous concentration of xenoestrogens based on the measured concentration of PM2.5 particles (Table 1). The findings in Table 1 indicate that the estimated concentrations of DBP and BPA in the gaseous phase are higher than in the particulate phase, while DEHP shows an opposite trend. According to the findings of Teil et al.,72 most xenoestrogens exhibit a preference for the gaseous phase, particularly during summer months characterised by elevated outdoor temperature and reduced concentrations of PMs. In this context, DEHP, classified as a long-chain PAEs, possesses a higher octanol-air partition coefficient (KOA), which enhances its distribution in the particulate phase. Conversely, DBP, classified as a short-chain PAE, is characterised by low KOA values.73,74 Furthermore, Lee et al.75 conducted a study investigating the presence of PAEs in both the water and atmosphere of Asan Lake in Korea. Their results indicated that the concentration of DBP in the water and atmosphere was measured at 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng m−3 and 3.62 ng m−3, respectively. In comparison, DEHP concentrations were found to be 110
000 ng m−3 and 3.62 ng m−3, respectively. In comparison, DEHP concentrations were found to be 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng m−3 in water and 6.52 ng m−3 in the atmosphere. Lee et al.75 also noted that DBP predominantly exists in the gaseous phase, while DEHP is primarily associated with the particulate phase, which is similar with our results. It is noteworthy that, as an inland lake, the environmental concentrations of these compounds in East Taihu Lake are generally lower than those of Asan Lake, with the exception of the atmospheric concentration of DBP.
000 ng m−3 in water and 6.52 ng m−3 in the atmosphere. Lee et al.75 also noted that DBP predominantly exists in the gaseous phase, while DEHP is primarily associated with the particulate phase, which is similar with our results. It is noteworthy that, as an inland lake, the environmental concentrations of these compounds in East Taihu Lake are generally lower than those of Asan Lake, with the exception of the atmospheric concentration of DBP.
| C w (ng m−3) | C a (ng m−3) | |||
|---|---|---|---|---|
| Particulate | Gaseous | |||
| DBP | ||||
| East Taihu lake | 26th June | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 085 085 |
290.25 | 7171.16 |
| 3rd July | 8243 | 542.43 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 401.88 401.88 |
|
| Xietang river | 22nd July | 297![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 849 849 |
2.33 | 57.52 |
| 5th August | 163![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 196 196 |
27.72 | 684.83 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| DEHP | ||||
| East Taihu lake | 26th June | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 235 235 |
2.97 | 0.52 |
| 3rd July | 4266 | 1.16 | 0.20 | |
| Xietang river | 22nd July | 163![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485 485 |
7.57 | 1.32 |
| 5th August | 111![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 176 176 |
5.82 | 1.02 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| BPA | ||||
| East Taihu lake | 26th June | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 572 572 |
0.09 | 0.49 |
| 3rd July | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.10 | 0.56 | |
| Xietang river | 22nd July | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 497 497 |
0.13 | 0.73 |
| 5th August | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 255 255 |
0.11 | 0.63 | |
The analysis further demonstrates that the net flux of air–water exchange for gaseous xenoestrogens is approximately 1.1 to 35.6 times greater than that of PM2.5-bound xenoestrogens. This indicates that gaseous xenoestrogens have a more substantial influence on the total net air–water exchange process. The distribution of pollutants between gaseous and particle phases is crucial in determining their environmental fate and long-distance transportation.76 Gaseous xenoestrogens significantly affect air–water exchange because high ambient temperatures and solar radiation in summer will promote the volatilisation of xenoestrogen from particles to the gas phase.77 As PAEs with high vapour pressure and Henry's Law Constant, DBP tends to be distributed in the gas phase rather than bound to particles.78,79
Fig. 3 and 4 illustrate the air–water exchange fluxes of DBP, DEHP, and BPA in East Taihu Lake and Xietang River, which all showed day-to-day variations, possibly because the difference in atmosphere concentration for DBP (R = −0.99, p < 0.05), DEHP (R = 0.97, p < 0.05) and BPA (R = −0.99, p < 0.05), and the concentration discrepancy of DEHP in water are also significant impact the daily air–water exchange fluxes (R = 0.99, p < 0.05).
 | ||
| Fig. 3 Air–water exchange fluxes for DBP (a), DEHP (b) and BPA (c), their dry deposition fluxes (d) and meteorological condition (e and f) in East Taihu Lake. | ||
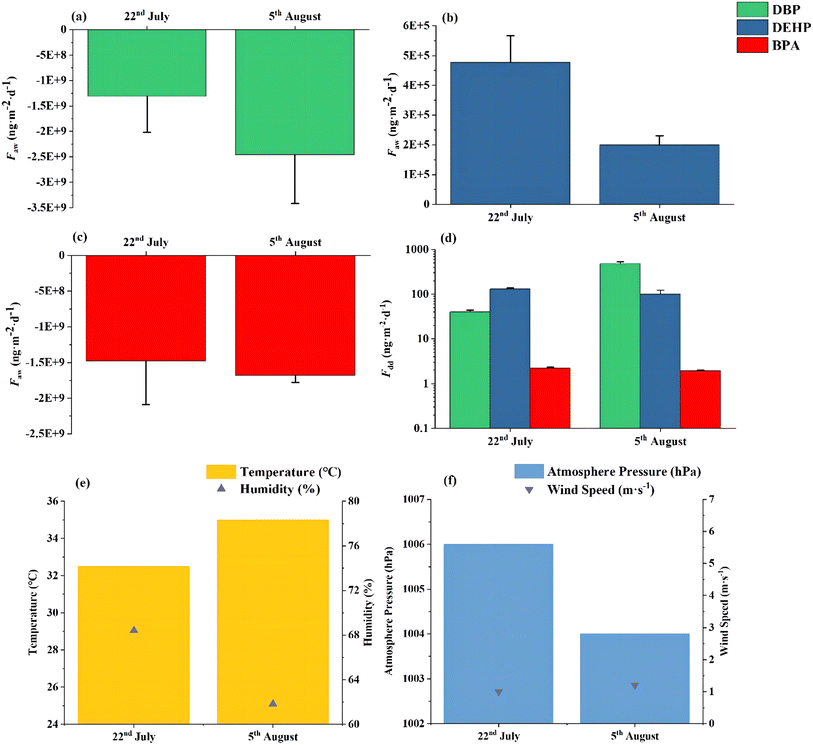 | ||
| Fig. 4 Air–water exchange fluxes for DBP (a), DEHP (b) and BPA (c), their dry deposition fluxes (d) and meteorological condition (e and f) in Xietang River. | ||
The air–water exchange flux (ng m−2 d−1) of individual xenoestrogens on 26th June and 3rd July in East Taihu Lake around −7.89 × 109 and −1.48 × 1010 for DBP (Fig. 3a) ranged from 1.84 × 104 to 9.93 × 105 and 9.64 × 104 to 3.39 × 105 for DEHP (Fig. 3b), around −8.88 × 109 and −1.01 × 1010 for BPA (Fig. 3c), respectively. The net flux (ng m−2 d−1) of air–water exchange in Xietang River for DBP varied from −5.74 × 107 to −1.44 × 107 and −7.50 × 108 to −7.22 × 108 on 22nd July and 5th August, respectively (Fig. 4a). Furthermore, a net exchange flux range of 1.70 × 105 to 1.30 × 107 ng m−2 d−1 and 1.65 × 106 to 1.92 × 107 ng m−2 d−1 was observed for DEHP on 22nd July and 5th August (Fig. 4b). Regarding the exchange flux of BPA on the Xietang River (Fig. 4c), the flux is around −1.32 × 1010 ng m−2 d−1 and −1.13 × 1010 ng m−2 d−1. The air–water exchange primarily involves deposition from air to water and volatilisation from water to air. Generally, BPA exhibited net deposition from air to water (ff < 0.26) at both sites and during various sampling times. While there are currently no publicly available data for the air–water exchange flux of BPA, Mukhopadhyay and Chakraborty (2021).80 reported the exchange of BPA in water and sediment, which indicated that the high solubility of BPA in water could result in its predominant presence in the dissolved phase, with similar trends for DBP in all sites and sampling times.
There has been limited research focus on the air–water exchange flux of DBP and DEHP in surface waters globally. Xie et al.50 concluded that net deposition was the primary mechanism for the air–water exchange of DBP in the North Sea, while volatilisation was dominant for DEHP. This aligns with our current findings, which indicate that the primary exchange process for DEHP in East Taihu Lake and Xietang River is net volatilisation from water to the atmosphere, whereas DBP experience net deposition. A study conducted in the South China Sea by Mi et al.21 also found that DEHP predominately volatilises into the atmosphere, with average fluxes consistent with our observations. This phenomenon may be attributed to the elevated temperatures of the lake waters, which enhance the likelihood of DEHP migrating from water to air due to its high hydrophobicity and low water solubility.81 Furthermore, research in the Arctic by Xie et al.82 indicated that DEHP primarily performed net deposition for the air-sea gas exchange, which might resulted from long-distance transport from land-based sources in European countries.
In India, the air–water exchange of DEHP exhibited spatial variations, with volatilisation occurring from surface water in areas with open burning dumps, while deposition was observed in industrial and residential zones, possibly related to using DEHP in masks during the COVID-19 pandemic.19 Consequently, regulatory authorities should consider developing appropriate standards for the incorporation of DEHP in masks.83 Furthermore, considering the sustained demand for mask usage even in the post-pandemic era, it is essential to prioritise centralised and environmentally safe disposal methods for masks rather than relying on direct landfill or incineration practices.84–86
Dry deposition fluxes (ng m−2 d−1) of xenoestrogens were calculated, with the trend indicating Fdd (DBP) > Fdd (DEHP) > Fdd (BPA) for both measurements in East Taihu Lake (Fig. 3d). The mean flux values were 5015.50 ± 450.87 and 9373.26 ± 611.59 for DBP, 51.29 ± 4.27 and 20.11 ± 0.02 for DEHP, and 1.51 ± 0.10 and 1.71 ± 0.02 for BPA, respectively. C. Wang et al.87 and Guo et al.88 both found that the dry deposition fluxes of DBP were lower than DEHP in the lake at Tianjin according to the model simulation results, identifying the atmosphere to be the primary source of DEHP. Furthermore, the deposition fluxes of DEHP measured in this study are significantly lower than the reported value from Zeng et al.89 in Guangzhou's urban and suburban areas, possibly proving that DEHP is not the primary pollutant in PM2.5 of Suzhou. The average dry deposition flux (ng m−2 d−1) of xenoestrogens on the Xietang River were 40.23 ± 3.56 and 478.97 ± 48.00 for DBP, 130.74 ± 7.12 and 100.57 ± 21.34 for DEHP, 2.24 ± 0.10 and 1.93 ± 0.09 for BPA, respectively. The trend of the average flux of DBP, DEHP and BPA was inconsistent in Xietang River compared to East Taihu Lake, with a disparity of DBP and DEHP dry deposition fluxes at different monitoring dates (Fig. 4d). The atmospheric concentration of PAEs is a significant factor influencing the direction and magnitude of air–water exchange processes, as evidenced by variations in the net air–water exchange and dry deposition flux of DBP on different days.21 This discrepancy in flux can be attributed to the differing concentrations of DBP adsorbed onto PM2.5 at Xietang River (2.33 ng m−3 and 27.72 ng m−3 shown in Fig. 2d).
Meteorological elements, such as wind speed, temperature, humidity, and atmospheric pressure, could impact the exchange processes occurring at the interface of the atmosphere.90 Changes in meteorological conditions could influence the distribution of xenoestrogens between the gaseous and particulate phases, thereby affecting their interface exchange processes.91 The partitioning of SVOCs between particle and gas is particularly sensitive to fluctuations in temperature.92 Furthermore, seasonal variations have also been shown to affect the particle/gas partitioning of SVOCs. However, these effects can vary among different compounds due to the distinct atmospheric reactions of various SVOCs and particle size distribution of their particle states, resulting in diverse behavior characteristics in the atmosphere.93 No significant correlation has been observed between humidity and the partition coefficient of SVOCs.94 Results of the Pearson correlation show that the air–water exchange flux of DEHP was significantly negatively correlated with the wind speed (R = −0.94, p < 0.05) (ESI, Fig. S1†). Zhu et al.95 have found that wind speed is also negatively correlated with the dry deposition flux of pollutants because the wind speed influences the diffusion of pollutants, which will decrease the concentration of pollutants and then decrease the dry deposition flux.
Significant connections have been noted between the chemical properties of pollutants and the rates of interface exchange in previous research.96 Disparities in dry deposition fluxes within the research area may be attributed to variations in the chemical properties of xenoestrogens. For example, in the Xietang River, air–water exchange fluxes were negatively correlated with water solubility at 25 °C of xenoestrogens in July and August (R = −0.99, p < 0.05) (ESI, Fig. S2†). The Henry's Law Constant is greatly affected by the water solubility, thus affecting the flux from water to the atmosphere.97 Other parameters, such as log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow, molecular weight, Henry's law constant, and vapour pressure, may also have an impact on the dry deposition fluxes (For detailed information in ESI, Section 5†).98–100
Kow, molecular weight, Henry's law constant, and vapour pressure, may also have an impact on the dry deposition fluxes (For detailed information in ESI, Section 5†).98–100
3.3 Health risk assessment
According to the Seventh Census in 2020, the resident population in Suzhou is about 12.75 billion people with a 1:1.09 sex ratio (Female/Male).101 Analysis of the HI for non-carcinogenic health risks associated with xenoestrogens in male and female adults are presented in Fig. 5. The results suggest negligible chronic risks to the population in the study area from non-dietary exposure. Specifically, the non-cancer risk assessment on Xietang River (Fig. 5a) indicates that DIBP exhibits the highest non-cancer risks in the region, with maximum values of 0.08 for males and 0.07 for females. This finding suggests that DIBP poses the most significant non-cancer risks in the Xietang River, potentially due to variations in bioavailability and reference doses. According to Li et al.,102 although DIBP is found at lower concentrations in the air, it exhibits greater bioavailability compared to DBP and DEHP, resulting in a higher daily intake. Additionally, as an industrial substitute for DBP, DIBP is required to have a lower RfD than DBP, which consequently contributes to an elevated HI.103 Conversely, DBP in the samples from East Taihu Lake shows higher HI values compared to other xenoestrogens for both genders, with peak values of 0.1 for males and 0.08 for females (Fig. 5b). This suggests that DBP is a more significant contributor to non-cancer risks in East Taihu Lake, as concentration is a critical factor in evaluating the HI associated with non-dietary exposure. The concentration of DBP in East Taihu Lake is orders of magnitude greater than that of other xenoestrogens.104 While the trend of HI values in both Fig. 5a and b suggests that females in the study area may be less susceptible than males, these differences were not found to be statistically significant (p > 0.05). Results from Zhang et al.105 have indicated that non-dietary exposure to outdoor dust would not induce non-cancer risk in adults on the Tibetan Plateau. Additionally, the HI resulting from gaseous and particle-phase xenoestrogen in Fig. 5 reveals that the gas-phase DBP, DIBP, and 4 t-OP are the primary contributors to the HI values in the Xietang River (Fig. 5a) and East Taihu Lake (Fig. 5b). Furthermore, the particle-phase DEHP, BPA, and NP in the two study regions constitute a significant portion of the non-cancer risk associated with total-phase (gas + particle phase) xenoestrogens.Non-dietary cancer risk associated with exposure to DEHP is considered an acceptable threshold in health risk assessment at an ILCRDEHP of 10−6. This study presents a summary of ILCR values for males and females across various phases of DEHP, as summarised in Fig. 6. The calculated ILCRDEHP were found to be below 1 × 10−11 for both times and across various sites, indicating very low cancer risks. The research suggests that gaseous DEHP in the study area poses a lower cancer risk than particle phase DEHP. They exhibited a trend of higher values in males than females, although this gender difference was not statistically significant (p > 0.05). Maceira et al.106 found a low cancer risk from outdoor respiratory exposure to DEHP, from a respiratory exposure study in Tarragona in Southern Spain, which showed similar conclusions to this study. When assessing the carcinogenic risk of DEHP, the DEHP concentration, exposure frequency, and inhalation rate will significantly determine the evaluation results, while body weight will also affect the evaluation results to a certain extent.107
 | ||
| Fig. 6 Carcinogenic risk assessment for non-dietary exposure to DEHP. The risk control line is drawn in red (ILCRDEHP = 10−6). | ||
4. Conclusion
This research has identified several key findings critical to understanding the behaviour of xenoestrogen in the exchange of air–water interfaces and their potential implications for public health. On the air–water interface, the exchange direction was deposition for DBP and BPA and volatilisation for DEHP. Additionally, the trend of dry deposition generally followed the order of DBP > DEHP > BPA, suggesting significant variations in the behaviour and fate of different xenoestrogens. This study also emphasises the significant impact of wind speed and specific chemical properties on the exchange of xenoestrogens between the atmosphere and water. There were non-carcinogenic and carcinogenic health risks of non-dietary intake routes.5. Limitations and implications
Several limitations exist in the present study. The estimation of gaseous xenoestrogen concentrations was conducted using the gas/particle partitioning model. However, several sources of uncertainty are associated with the estimation of gaseous pollutant concentrations through the gas-particle partitioning method. One significant assumption of the gas/particle partitioning model is that the gas and particle system is in a state of equilibrium. In field monitoring, this equilibrium may not be fully reached, which can result in either an overestimation or underestimation of gaseous pollutant concentrations.108 Additionally, the partitioning of SVOCs between gas and particulate phases is highly sensitive to variations in meteorological conditions, which can further contribute to fluctuations in the estimates of gaseous concentrations.109 Therefore, future research is essential to refer to actual monitoring data for a more comprehensive analysis of air–water exchange dynamics. The impact of gaseous pollutants on air–water interface exchange should also be emphasised in future, as they could significantly influence the transport and fate of xenoestrogens.The partitioning of particles and gases is significantly influenced by temperature and seasonal variations. This study lacked seasonal monitoring data of air–water exchange fluxes. Therefore, the dynamic characteristics of those parameters could not be adequately captured, particularly given the differences in reaction characteristics and particle size distribution among various compounds. This may lead to a limited understanding of the behaviour of xenoestrogens under diverse environmental conditions. Furthermore, the lack of long-term monitoring and comparative studies regarding the effects of humidity constrains the interpretation of the results, as the relationship between other meteorological factors and the distribution coefficient of xenoestrogen remains ambiguous. Future research should aim to conduct long-term monitoring at different seasons and evaluate the impacts of various meteorological indicators. The expected results will help reveal the key factors affecting the allocation of xenoestrogens and their interactions. Although current assessments indicate that the health risks associated with inhalation of outdoor air and direct skin contact are below established risk thresholds for adults, it is necessary to consider the cumulative risks for diverse demographic groups and exposure pathways. Additionally, the existing research does not address the collective toxicity of various xenoestrogens present in the atmosphere, highlighting a critical gap that requires further investigation.
Data availability
The data of concentrations presented in the current study are available on reasonable request from the corresponding author. Other data are included in the ESI.†Author contributions
Minhao Wang: writing – original draft, writing – review and editing, conceptualisation, data curation, formal analysis, investigation, methodology, software, validation, visualisation. Lei Han: writing – review and editing, conceptualisation, funding acquisition, methodology, project administration, resources, supervision, validation. Dongling Li and Ting Tong: data curation, investigation, validation. Ziyu Zhang, Yuwei Xia and Xinhui Shi: investigation, validation. Haifei Zhang: writing – review and editing, supervision. Kui Chen: resource, validation. Fang Wang and Xiaowei Tie: validation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study is funded by the Research Development Fund (RDF 20_02_20) and the Postgraduate Research Scholarship (PGRS2012022) from Xi'an Jiaotong–Liverpool University.References
- J. Chmielewski, G. Nowak-Starz and J. Łuszczki, et al., Environmental exposition to xenoestrogens (XEs) and related health effects, J. Elem., 2021, 26(3), 717–730 Search PubMed.
- T. Oyelowo, Chapter 2 - Estrogen Concepts, in. Mosby’s Guide to Women's Health, ed. T. Oyelowo, Saint Louis: Mosby, pp. , pp. 8–10 Search PubMed.
- I. A. Sheikh, I. A. Tayubi and E. Ahmad, et al., Computational insights into the molecular interactions of environmental xenoestrogens 4-tert-octylphenol, 4-nonylphenol, bisphenol A (BPA), and BPA metabolite, 4-methyl-2, 4-bis (4-hydroxyphenyl) pent-1-ene (MBP) with human sex hormone-binding globulin, Ecotoxicol. Environ. Saf., 2017, 135, 284–291 CrossRef CAS PubMed.
- X. Q. Wang, D. Ha and R. Yoshitake, et al., Exploring the Biological Activity and Mechanism of Xenoestrogens and Phytoestrogens in Cancers: Emerging Methods and Concepts, Int. J. Mol. Sci., 2021, 22, 8798 CrossRef CAS PubMed.
- I. Paterni, C. Granchi and F. Minutolo, Risks and benefits related to alimentary exposure to xenoestrogens, Crit. Rev. Food Sci. Nutr., 2017, 57, 3384–3404 CrossRef CAS PubMed.
- Y. Akkam, D. Omari and H. Alhmoud, et al., Assessment of Xenoestrogens in Jordanian Water System: Activity and Identification, Toxics, 2023, 11, 63 CrossRef CAS PubMed.
- P. D. Darbre, Environmental Contaminants: Environmental Estrogens – Hazard Characterization, in. Encyclopedia of Food Safety, ed. Motarjemi Y., Academic Press, Waltham, pp. , pp. 323–331 Search PubMed.
- A. F. Aissa, L. M. G. Antunes. Chapter 9 - Epigenetics of Personalized Toxicology, in, Personalized Epigeneticsed. Tollefsbol T. O., Academic Press, Boston, pp. , pp. 245–282 Search PubMed.
- F. H. Comhaire, W. A. E. Decleer. Chapter 8 - The Effects of Environmental Hormone Disrupters on Fertility, and a Strategy to Reverse their Impact, in. Handbook of Fertility, ed. Watson R. R., Academic Press, San Diego, pp. , pp. 89–97 Search PubMed.
- C. A. Staples, P. B. Dome and G. M. Klecka, et al., A review of the environmental fate, effects, and exposures of bisphenol A, Chemosphere, 1998, 36, 2149–2173 CrossRef CAS PubMed.
- H. Tuan Tran, C. Lin and X.-T. Bui, et al., Phthalates in the environment: characteristics, fate and transport, and advanced wastewater treatment technologies, Bioresour. Technol., 2022, 344, 126249 CrossRef CAS PubMed.
- Z. Xie, S. Lakaschus and R. Ebinghaus, et al., Atmospheric concentrations and air–sea exchanges of nonylphenol, tertiary octylphenol and nonylphenol monoethoxylate in the North Sea, Environ. Pollut., 2006, 142, 170–180 CrossRef CAS PubMed.
- J. Liu, L. Zhang and G. Lu, et al., Occurrence, toxicity and ecological risk of Bisphenol A analogues in aquatic environment – A review, Ecotoxicol. Environ. Saf., 2021, 208, 111481 CrossRef CAS PubMed.
- R. M. Meenu and U. Shanker, Occurrence, Distribution, and Removal of Phthalates by Nanomaterials, in. Handbook of Green and Sustainable Nanotechnology: Fundamentals, Developments and Applications, ed. Shanker U., Hussain C. M., Rani M., Springer International Publishing, Cham, pp. , pp. 729–762 Search PubMed.
- V. K. Sharma, G. A. K. Anquandah and R. A. Yngard, et al., Nonylphenol, octylphenol, and bisphenol-A in the aquatic environment: A review on occurrence, fate, and treatment, J. Environ. Sci. Health, Part A, 2009, 44, 423–442 CrossRef CAS PubMed.
- Y. Wang, X. Wu and Q. Zhang, et al., Occurrence, distribution, and air-water exchange of organophosphorus flame retardants in a typical coastal area of China, Chemosphere, 2018, 211, 335–344 CrossRef CAS PubMed.
- R. Balasubramanian, Air-sea interactions of semi-volatile organic compounds in the tropical environment of Southeast Asia, EPJ Web Conf., 2010, 9, 43–72 CrossRef CAS.
- T. Luarte, F. Tucca and J. Nimptsch, et al., Occurrence and air-water diffusive exchange legacy persistent organic pollutants in an oligotrophic north Patagonian lake, Environ. Res., 2022, 204, 112042 CrossRef CAS PubMed.
- S. Chandra and P. Chakraborty, Air–water exchange and risk assessment of phthalic acid esters during the early phase of COVID-19 pandemic in tropical riverine catchments of India, Chemosphere, 2023, 341, 140013 CrossRef CAS PubMed.
- S. J. Eisenreich, B. B. Looney and J. D. Thornton, Airborne organic contaminants in the Great Lakes ecosystem, Environ. Sci. Technol., 1981, 15, 30–38 CrossRef CAS.
- L. Mi, Z. Xie and W. Xu, et al., Air–Sea Exchange and Atmospheric Deposition of Phthalate Esters in the South China Sea, Environ. Sci. Technol., 2023, 57, 11195–11205 CrossRef CAS PubMed.
- T. T. Kondraju and K. S. Rajan, Water Quality in Inland Water Bodies: Hostage to the Intensification of Anthropogenic Land Uses, J. Indian. Soc. Remote Sens., 2019, 47, 1865–1874 CrossRef.
- K. R. Echols, J. C. Meadows and C. E. Orazio, Pollution of Aquatic Ecosystems II: Hydrocarbons, Synthetic Organics, Radionuclides, Heavy Metals, Acids, and Thermal Pollution, in. Encyclopedia of Inland Waters, ed. Likens G. E., Academic Press, Oxford, pp. , pp. 120–128 Search PubMed.
- S. Kosten, A. J. Veraart and V. Dakos, Regime Shifts and Tipping Points, in. Encyclopedia of Inland Waters, ed. Mehner T., Tockner K., 2nd edn, Elsevier, Oxford, pp. , pp. 352–361 Search PubMed.
- Z.-G. Ji, River Fate and Transportrivertransport, in. Encyclopedia of Sustainability Science and Technology, ed. Meyers R. A., Springer, New York, NY, pp. , pp. 9049–9062 Search PubMed.
- C. Zhu and Z. Hao, ANN-based Surface Water Quality Evaluation Model and its Application in Suzhou River, in International Joint Conference on Artificial Intelligence, 2009, pp. 159–162 Search PubMed.
- X. Wu, Q. Wang and Z. Yuan, et al., Concentrations, Sources and Ecological Risk of Organochlorine Pesticides in Urban Stream Sediments of Suzhou Industrial Park, China, Soil Sediment Contam., 2022, 31, 655–667 CrossRef CAS.
- Z. Yuan, B. He and X. Wu, et al., Polycyclic aromatic hydrocarbons (PAHs) in urban stream sediments of Suzhou Industrial Park, an emerging eco-industrial park in China: Occurrence, sources and potential risk, Ecotoxicol. Environ. Saf., 2021, 214, 112095 CAS.
- J. S. Kim, P. W. J. Batey and Y. Fan, et al., Embracing integrated watershed revitalization in Suzhou, China: learning from global case studies, Asia-Pac J. Reg. Sci., 2021, 5, 565–595 CrossRef.
- L. G. Tidwell, L. Blair Paulik and K. A. Anderson, Air–water exchange of PAHs and OPAHs at a superfund mega-site, Sci. Total Environ., 2017, 603–604, 676–686 CrossRef CAS PubMed.
- C. Wang, W. Wang and W. Deng, et al., Distribution characteristics, air-water exchange, ozone formation potential and health risk assessments of VOCs emitted from typical coking wastewater treatment process, Sci. Total Environ., 2023, 862, 160845 CrossRef CAS PubMed.
- H. Kim, J. Noh and Y. Noh, et al., Gender Difference in the Effects of Outdoor Air Pollution on Cognitive Function Among Elderly in Korea, Front. Public Health, 2019, 7, 375 CrossRef PubMed.
- S. Bu, Y. Wang and H. Wang, et al., Analysis of global commonly-used phthalates and non-dietary exposure assessment in indoor environment, Build. Environ., 2020, 177, 106853 CrossRef.
- Y. Li, J. Hou and Z. Wang, et al., Phthalate levels in Chinese residences: Seasonal and regional variations and the implication on human exposure, NSO, 2023, 2, 20230011 CrossRef.
- W. Liu, Y. Sun and N. Liu, et al., Indoor exposure to phthalates and its burden of disease in China, Indoor Air, 2022, 32, e13030 CAS.
- D. Fan, et al., Exposure of preschool-aged children to highly-concerned bisphenol analogues in Nanjing, East China, Ecotoxicol. Environ. Saf., 2022, 234, 113397 CrossRef CAS PubMed.
- C. Li, et al., The internal exposure of bisphenol analogues in South China adults and the associated health risks, Sci. Total Environ., 2021, 795(15), 148796 CrossRef CAS PubMed.
- L. Wang, et al., Non-dietary exposure to phthalates for pre-school children in kindergarten in Beijing, China, Build. Environ., 2020, 167, 106438 CrossRef.
- Y. Zhang, X. Li and H. Zhang, et al., Distribution, source apportionment and health risk assessment of phthalate esters in outdoor dust samples on Tibetan Plateau, China, Sci. Total Environ., 2022, 834, 155103 CrossRef CAS PubMed.
- C. Zhu, et al., Associations between Children’s Asthma and Allergic Symptoms and Phthalates in Dust in Metropolitan Tianjin, China, Chemosphere, 2022, 302, 134786 CrossRef CAS PubMed.
- Y. Pan, L. Han and X. Chen, et al., Occurrence of emerging bisphenol S analogues in urine from five occupational populations in South China, Environ. Int., 2023, 172, 107773 CrossRef CAS PubMed.
- J. Liu, L. Cheng and Q. Liu, et al., Sedimentary macrophyte δ13Ccellulose record of environmental evolution over the past century in East Taihu Lake, China, Ecol. Indic., 2023, 154, 110716 CrossRef CAS.
- C. Yang, X. Shen and J. Wu, et al., Driving forces and recovery potential of the macrophyte decline in East Taihu Lake, J. Environ. Manage., 2023, 342, 118154 CrossRef PubMed.
- Q. Zhao, Y. Zhou and J. Zhai, Bridging beauty and biodiversity: Coupling diversity and aesthetics through optimized plant communities in urban riverfront landscapes, Sci. Total Environ., 2024, 950, 175278 CrossRef CAS PubMed.
- Y. Li, F. Zhan and C. Shunthirasingham, et al., Seasonal air concentration variability, gas–particle partitioning, precipitation scavenging, and air–water equilibrium of organophosphate esters in southern Canada, Atmos. Chem. Phys., 2025, 25, 459–472 CrossRef CAS.
- N. Marlina, F. Hassan and H.-R. Chao, et al., Organophosphate esters in water and air: A minireview of their sources, occurrence, and air–water exchange, Chemosphere, 2024, 356, 141874 CrossRef CAS PubMed.
- M. Wang, H. Ding and G. Liang, et al., Occurrence, spatial distribution, risk assessment, and management of environmental estrogens in surface waters of the Taihu basin, Environ. Chem., 2024, 20, 339–353 CrossRef.
- YSI Incorporated, YSI ProPlus User Manual, 2009, https://www.ysi.com/File%20Library/Documents/Manuals/605596-YSI-ProPlus-User-Manual-RevD.pdf.
- W. G. Whitman, The two film theory of gas absorption, Int. J. Heat Mass Transfer, 1962, 5, 429–433 CrossRef.
- Z. Xie, R. Ebinghaus and C. Temme, et al., Atmospheric concentrations and air–sea exchanges of phthalates in the North Sea (German Bight), Atmos. Environ., 2005, 39, 3209–3219 CrossRef CAS.
- Z. Wu, L. Hu and T. Guo, et al., Aeolian transport and deposition of carbonaceous aerosols over the Northwest Pacific Ocean in spring, Atmos. Environ., 2020, 223, 117209 CrossRef CAS.
- Y. Chen, T. Lin and J. Tang, et al., Exchange of polycyclic aromatic hydrocarbons across the air-water interface in the Bohai and Yellow Seas, Atmos. Environ., 2016, 141, 153–160 CrossRef CAS.
- R. M. Hoff, W. M. J. Strachan and C. W. Sweet, et al., Atmospheric deposition of toxic chemicals to the Great Lakes: A review of data through 1994, Atmos. Environ., 1996, 30, 3505–3527 CrossRef CAS.
- Y. Zhang, G. Liang and Z. Liu, et al., Gas–particle partitioning and dry deposition of atmospheric parent, alkylated, nitrated and hydroxyl polycyclic aromatic hydrocarbons over the Bohai sea and northern Yellow sea in autumn, Atmos. Environ., 2024, 318, 120247 CrossRef CAS.
- S.-C. Chen and C.-M. Liao, Health risk assessment on human exposed to environmental polycyclic aromatic hydrocarbons pollution sources, Sci. Total Environ., 2006, 366, 112–123 CrossRef CAS PubMed.
- L. Wang, M. Liu and W. Tao, et al., Pollution characteristics and health risk assessment of phthalate esters in urban soil in the typical semi-arid city of Xi’an, Northwest China, Chemosphere, 2018, 191, 467–476 CrossRef CAS PubMed.
- Z. Zhao, X. Gong and Q. Ding, et al., Environmental implications from the priority pollutants screening in impoundment reservoir along the eastern route of China's South-to-North Water Diversion Project, Sci. Total Environ., 2021, 794, 148700 CrossRef CAS PubMed.
- United States Environmental Protection Agency (U.S. EPA), Guidelines for Carcinogen Risk Assessment, https://www.epa.gov/risk/guidelines-carcinogen-risk-assessment, 2013, accessed 5 April 2024.
- F. Wang, D. Zhao and S. Zhang, et al., Gas-particle partitioning and air-water exchange of polycyclic aromatic hydrocarbons in the Three Gorges Reservoir, southwest China, Atmos. Environ., 2023, 299, 119646 CrossRef CAS.
- R. S. Boethling, P. H. Howard and W. M. Meylan, Finding and estimating chemical property data for environmental assessment, Environ. Toxicol. Chem., 2004, 23, 2290–2308 CrossRef CAS PubMed.
- X. Gao, J. Li and X. Wang, et al., Exposure and ecological risk of phthalate esters in the Taihu Lake basin, China, Ecotoxicol. Environ. Saf., 2019, 171, 564–570 CrossRef CAS PubMed.
- J.-N. Ren, N.-Z. Zhu and X.-Z. Meng, et al., Occurrence and ecological risk assessment of 16 phthalates in surface water of the mainstream of the Yangtze River, China, Environ. Sci. Pollut. Res., 2023, 30, 66936–66946 CrossRef CAS PubMed.
- X. Zhao, J. Shen and H. Zhang, et al., The occurrence and spatial distribution of phthalate esters (PAEs) in the Lanzhou section of the Yellow River, Environ. Sci. Pollut. Res., 2020, 27, 19724–19735 CrossRef CAS PubMed.
- X. Yuan, T. Li and L. Zhou, et al., Characteristics and Risk Assessment of Estrogenic Compounds in Rivers of Southern Jiangsu Province, China, IERI Procedia, 2014, 9, 176–184 CrossRef.
- S. Kundu, A. Biswas and A. Ray, et al., Bisphenol A contamination in Hilsa shad and assessment of potential health hazard: A pioneering investigation in the national river Ganga, India, J. Hazard. Mater., 2024, 461, 132532 CrossRef CAS PubMed.
- Z. Liao, Y. Jian and J. Lu, et al., Distribution, migration patterns, and food chain human health risks of endocrine-disrupting chemicals in water, sediments, and fish in the Xiangjiang River, Sci. Total Environ., 2024, 172484 CrossRef CAS PubMed.
- N. Salgueiro-González, M. López de Alda and S. Muniategui-Lorenzo, et al., Determination of 13 estrogenic endocrine disrupting compounds in atmospheric particulate matter by pressurised liquid extraction and liquid chromatography-tandem mass spectrometry, Anal. Bioanal. Chem., 2013, 405, 8913–8923 CrossRef PubMed.
- X. Liu, X. Zeng and G. Dong, et al., Plastic Additives in Ambient Fine Particulate Matter in the Pearl River Delta, China: High-Throughput Characterization and Health Implications, Environ. Sci. Technol., 2021, 55, 4474–4482 CrossRef CAS PubMed.
- Y. Liu, Y. Tang and Y. He, et al., Riverine inputs, spatiotemporal variations, and potential sources of phthalate esters transported into the Bohai Sea from an urban river in northern China, Sci. Total Environ., 2023, 878, 163253 CrossRef CAS PubMed.
- B. Ma, L. Wang and W. Tao, et al., Phthalate esters in atmospheric PM2.5 and PM10 in the semi-arid city of Xi’an, Northwest China: Pollution characteristics, sources, health risks, and relationships with meteorological factors, Chemosphere, 2020, 242, 125226 CrossRef CAS PubMed.
- K. H. Kim, S.-B. Lee and D. Woo, et al., Influence of wind direction and speed on the transport of particle-bound PAHs in a roadway environment, Atmos. Pollut. Res., 2015, 6, 1024–1034 CrossRef.
- M.-J. Teil, E. Moreau-Guigon and M. Blanchard, et al., Endocrine disrupting compounds in gaseous and particulate outdoor air phases according to environmental factors, Chemosphere, 2016, 146, 94–104 CrossRef CAS PubMed.
- C. J. Weschler, T. Salthammer and H. Fromme, Partitioning of phthalates among the gas phase, airborne particles and settled dust in indoor environments, Atmos. Environ., 2008, 42, 1449–1460 CrossRef CAS.
- I. Cousins and D. Mackay, Correlating the physical–chemical properties of phthalate esters using the `three solubility’ approach, Chemosphere, 2000, 41, 1389–1399 CrossRef CAS PubMed.
- Y.-M. Lee, J.-E. Lee and W. Choe, et al., Distribution of phthalate esters in air, water, sediments, and fish in the Asan Lake of Korea, Environ. Int., 2019, 126, 635–643 CrossRef CAS PubMed.
- H. Lu, D. Chen and Z. Zhu, et al., Atmospheric phthalate esters in a multi-function area of Hangzhou: Temporal variation, gas/particle phase distribution, and population exposure risk, Sci. Total Environ., 2023, 894, 163987 CrossRef CAS PubMed.
- J. Jia, L. Deng and C. Bi, et al., Seasonal variations, gas-PM2.5 partitioning and long-distance input of PM2.5-bound and gas-phase polycyclic aromatic hydrocarbons in Shanghai, China, Atmos. Environ., 2021, 252, 118335 CrossRef CAS.
- A. I. Barrado, S. García and E. Barrado, et al., PM2.5-bound PAHs and hydroxy-PAHs in atmospheric aerosol samples: Correlations with season and with physical and chemical factors, Atmos. Environ., 2012, 49, 224–232 CrossRef CAS.
- F. W. Gaspar, R. Castorina and R. L. Maddalena, et al., Phthalate Exposure and Risk Assessment in California Child Care Facilities, Environ. Sci. Technol., 2014, 48, 7593–7601 CrossRef CAS PubMed.
- M. Mukhopadhyay and P. Chakraborty, Plasticizers and bisphenol A: Emerging organic pollutants along the lower stretch of River Ganga, north-east coast of the Bay of Bengal, Environ. Pollut., 2021, 276, 116697 CrossRef CAS PubMed.
- X. Li, Q. Wang and N. Jiang, et al., Occurrence, source, ecological risk, and mitigation of phthalates (PAEs) in agricultural soils and the environment: A review, Environ. Res., 2023, 220, 115196 CrossRef CAS PubMed.
- Z. Xie, R. Ebinghaus and C. Temme, et al., Occurrence and Air-Sea Exchange of Phthalates in the Arctic, Environ. Sci. Technol., 2007, 41, 4555–4560 CrossRef CAS PubMed.
- N. Shende, G. Hippargi and S. Gurjar, et al., Occurrence of phthalates in facemasks used in India and its implications for human exposure, Int. J. Environ. Health Res., 2024, 34, 166–182 CrossRef CAS PubMed.
- M. T. Mehran, N. S. Raza and H. M. Ali, et al., Global plastic waste management strategies (Technical and behavioral) during and after COVID-19 pandemic for cleaner global urban life, Energy Sources, Part A, 2025, 47, 4472–4481 CrossRef CAS.
- L. Lyu, M. Bagchi and N. Markoglou, et al., Towards environmentally sustainable management: A review on the generation, degradation, and recycling of polypropylene face mask waste, J. Hazard. Mater., 2024, 461, 132566 CrossRef CAS PubMed.
- J. Park, C. Lee and M. Lee, et al., Evaluation of hazardous substances emitted during mask use, Environ. Int., 2025, 196, 109296 CrossRef CAS PubMed.
- C. Wang, J. Li and C. Qiu, et al., Multimedia fates and ecological risk control strategies of phthalic acid esters in a lake recharged by reclaimed water using the QWASI fugacity model, Ecol. Modell., 2023, 475, 110222 CrossRef CAS.
- Y. Guo, C. Wang and P. Huang, et al., A method for simulating spatial fates of chemicals in flowing lake systems: Application to phthalates in a lake, Water Res., 2023, 232, 119715 CrossRef CAS PubMed.
- F. Zeng, Y. Lin and K. Cui, et al., Atmospheric deposition of phthalate esters in a subtropical city, Atmos. Environ., 2010, 44, 834–840 CrossRef CAS.
- L. Chen, S. Peng and J. Liu, et al., Dry deposition velocity of total suspended particles and meteorological influence in four locations in Guangzhou, China, J. Environ. Sci., 2012, 24, 632–639 CrossRef PubMed.
- Y.-M. Lee, J.-E. Lee and W. Choe, et al., Distribution of phthalate esters in air, water, sediments, and fish in the Asan Lake of Korea, Environ. Int., 2019, 126, 635–643 CrossRef CAS PubMed.
- F.-J. Zhu, W.-L. Ma and L.-Y. Liu, et al., Temporal trends of atmospheric PAHs: Implications for the gas-particle partition, Atmos. Environ., 2021, 261, 118595 CrossRef CAS.
- Q. T. Vuong, P. Q. Thang and T. N. T. Nguyen, et al., Seasonal variation and gas/particle partitioning of atmospheric halogenated polycyclic aromatic hydrocarbons and the effects of meteorological conditions in Ulsan, South Korea, Environ. Pollut., 2020, 263, 114592 CrossRef CAS PubMed.
- X. Zhou, J. Lian and Y. Cheng, et al., The gas/particle partitioning behavior of phthalate esters in indoor environment: Effects of temperature and humidity, Environ. Res., 2021, 194, 110681 CrossRef CAS PubMed.
- L. Zhu, J. Liu and L. Cong, et al., Spatiotemporal Characteristics of Particulate Matter and Dry Deposition Flux in the Cuihu Wetland of Beijing, PLoS One, 2016, 11, e0158616 CrossRef PubMed.
- N. Qin, W. He and X.-Z. Kong, et al., Atmospheric partitioning and the air–water exchange of polycyclic aromatic hydrocarbons in a large shallow Chinese lake (Lake Chaohu), Chemosphere, 2013, 93, 1685–1693 CrossRef CAS PubMed.
- D. Mackay, S. Paterson and W. H. Schroeder, Model describing the rates of transfer processes of organic chemicals between atmosphere and water, Environ. Sci. Technol., 1986, 20, 810–816 CrossRef CAS PubMed.
- S. Lu, L. Kang and S. Liao, et al., Phthalates in PM2.5 from Shenzhen, China and human exposure assessment factored their bioaccessibility in lung, Chemosphere, 2018, 202, 726–732 CrossRef CAS PubMed.
- M. MacLeod and D. Mackay, Modeling transport and deposition of contaminants to ecosystems of concern: a case study for the Laurentian Great Lakes, Environ. Pollut., 2004, 128, 241–250 CrossRef CAS PubMed.
- M. J. Teil, M. Blanchard and M. Chevreuil, Atmospheric fate of phthalate esters in an urban area (Paris-France), Sci. Total Environ., 2006, 354, 212–223 CrossRef CAS PubMed.
- Suzhou Municipal Bureau of Statistics, Survey Office of the National Bureau of Statistics in Suzhou, Suzhou Statistical Yearbook 2023, 2023rd edn, China Statistics Press, https://tjj.suzhou.gov.cn/sztjj/tjnj/2023/zk/indexeh.htm, 2023, accessed 17 September 2024 Search PubMed.
- X. Li, N. Zheng and W. Zhang, et al., Comprehensive assessment of phthalates in indoor dust across China between 2007 and 2019: Benefits from regulatory restrictions, Environ. Pollut., 2024, 342, 123147 CrossRef CAS PubMed.
- A. Kortenkamp and H. M. Koch, Refined reference doses and new procedures for phthalate mixture risk assessment focused on male developmental toxicity, Int. J. Hyg. Environ. Health, 2020, 224, 113428 CrossRef CAS PubMed.
- Y. Wang, L. Wang and Z. Jiang, et al., Non-dietary exposure to phthalates in primary school children: Risk and correlation with anthropometric indices, cardiovascular and respiratory diseases, Ecotoxicol. Environ. Saf., 2024, 286, 117203 CrossRef CAS PubMed.
- Y. Zhang, X. Li and H. Zhang, et al., Distribution, source apportionment and health risk assessment of phthalate esters in outdoor dust samples on Tibetan Plateau, China, Sci. Total Environ., 2022, 834, 155103 CrossRef CAS PubMed.
- A. Maceira, I. Pecikoza and R. M. Marcé, et al., Multi-residue analysis of several high-production-volume chemicals present in the particulate matter from outdoor air. A preliminary human exposure estimation, Chemosphere, 2020, 252, 126514 CrossRef CAS PubMed.
- J. Ma, L. Chen and Y. Guo, et al., Phthalate diesters in Airborne PM 2.5 and PM 10 in a suburban area of Shanghai: Seasonal distribution and risk assessment, Sci. Total Environ., 2014, 497–498, 467–474 CrossRef CAS PubMed.
- T. Anttila, K. E. J. Lehtinen and M. Dal Maso, Analytical expression for gas-particle equilibration time scale and its numerical evaluation, Atmos. Environ., 2016, 133, 34–40 CrossRef CAS.
- Y.-F. Li, L.-N. Qiao and N.-Q. Ren, et al., Gas/particle partitioning of semi-volatile organic compounds in the atmosphere: Transition from unsteady to steady state, Sci. Total Environ., 2020, 710, 136394 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5va00001g |
| This journal is © The Royal Society of Chemistry 2025 |



