 Open Access Article
Open Access ArticleOccurrence and distribution of selected pharmaceuticals in fresh fish along the Kenyan coast and assessment of potential human health risks†
Veronica Wayayi
Ogolla Wanjeri
ab,
Eric
Okuku
b,
Jane Catherine
Ngila
a,
Edward
Waiyaki
b,
Joseph Kamau
Nyingi
b and
Patrick Gathura
Ndungu
 *c
*c
aDepartment of Chemical Sciences, University of Johannesburg, Johannesburg, South Africa
bKenya Marine and Fisheries Research Institute, P. O Box 81651, Mombasa, Kenya
cDepartment of Chemistry, University of Pretoria, Hatfield, Pretoria, South Africa. E-mail: patrick.ndungu@up.ac.za
First published on 6th May 2025
Abstract
Fish consumption is known to have several health benefits. However, consuming fish contaminated with pharmaceuticals can potentially lead to long-term detrimental effects and other health risks for consumers. This study aimed to assess the potential human risks associated with fish consumption from Tudor creek. The creek is one of the peri-urban creeks near the East African coastal city of Mombasa, Kenya. A novel comprehensive analysis of 14 selected pharmaceuticals was conducted for the first time in different fish species from Tudor creek. The concentrations of pharmaceuticals in fish muscles and gills from Tudor creek ranged between detection limit (DL)–1623.98 ng g−1 and between DL–1785.60 ng g−1, respectively. High pharmaceutical concentrations were observed in fish species Platax pinnatus, Lethrinus mahsena, and Acanthurus blochii with total concentrations of ∑3870.80 ng g−1, ∑3435.57 ng g−1 and ∑3329.37 ng g−1, respectively. The estimated daily intake (EDI) of pharmaceuticals through consumption of fish ranged between 1.01–1441.70 ng kg−1 bw per day. The Target Hazard Quotients (THQs) for trimethoprim, the parent tetracycline compound, and caffeine exceeded 5%, suggesting that all three substances posed health risks. There is a need to create public awareness of the impact caused by pharmaceuticals discharged into aquatic/marine environments. Furthermore, there is a need for policies and legislation on the disposal of pharmaceuticals. Effective monitoring and enforcement will also be necessary to help prevent negative impacts on livelihood, sustainability of our marine environment, and human health.
Environmental significanceData on the occurrence and distribution of contaminants of emerging concern are limited, particularly on the African continent and especially along the coastlines of East Africa. This study combined household surveys on fish consumption with the determination of levels and distribution of 14 selected pharmaceuticals in fresh fish typically consumed by people living in the area. Various common pharmaceutical compounds were detected in fish, and consumption of the fish poses a potential human health risk. There is a need for new policies and regulations on the responsible disposal of pharmaceuticals, as well as the development of collaborative networks between relevant government structures, local industries, communities, and other stakeholders to address the issue and ensure the sustainable use of our oceans and coastal areas. |
Introduction
Seafood consumption rates have increased worldwide in recent years, mainly due to rising urbanization and awareness of its nutritional benefits.1,2 Seafood, such as fish, crustaceans, and mollusks, plays an important role in providing 17% of animal protein and 7% of all essential protein to over 3 billion people in developing countries.2 It is the most accessible and affordable source of animal protein, including micronutrients like vitamins (e.g. A, D, and B12) and essential minerals, and typically has low calorific values and fat content, which can be beneficial for human health.3–5 Furthermore, seafood is known for its positive influence on the health of young children and has been widely recognized as “nature's superfood”.3,6,7 Despite the multiple health benefits of seafood in addressing food and nutritional security among poor and vulnerable populations, even in small quantities, seafood from polluted water bodies can be hazardous to human health due to exposure to potentially harmful substances, such as emerging organic contaminants. This risk is especially significant for populations with high fish consumption rates.8,9In recent decades, there has been growing concern about the occurrence and level of pharmaceutical compounds and their residues in various environmental compartments (e.g. water, sediment and biota). Due to their extensive use and the potential harm they can cause to human and ecological health, pharmaceutical compounds and their residues are considered emerging organic contaminants.8,10 These compounds are consumed worldwide for the treatment and prevention of human diseases and in several veterinary applications.11,12 Pharmaceuticals are introduced into the aquatic environment via both treated and untreated wastewater, discharged from households, pharma manufacturing and packaging facilities, animal husbandry farms, aquaculture facilities, and hospitals. This leads to the ‘pseudo-persistence’ of pharmaceuticals in the aquatic environment, as the rate of input exceeds the rate of degradation.8,13,14 The main concern with the release of pharmaceutical residues into marine waters is their potential to bio-concentrate and bioaccumulate at significantly higher levels than in the surrounding water. This occurs through diffusion across the gills, digestive tract, and skin of aquatic organisms due to the polar nature of pharmaceuticals and their high bioavailability to filter-feeding organisms.13,15,16 Currently, the occurrence and bioaccumulation of pharmaceuticals in marine organisms have attracted a lot of attention, with several studies reporting their presence in marine fish.17–21 Some aquatic organisms bio-accumulate pharmaceuticals by direct partitioning from the abiotic environment through inhalational exposure (bio-concentration) or dietary sources (trophic transfer).22,23 Aquatic life faces potential risks from exposure to even low concentrations of pharmaceuticals in the environment.8,9,24 For instance, research has shown that non-steroidal anti-inflammatory drugs (NSAIDs), including diclofenac, can have detrimental effects on the kidneys of fish and disrupt the ovulation process within fish populations.25,26 The antidiabetic drug metformin has also been reported to cause the feminization of male fish in the aquatic environment.27 A recent study on South Asian clams (Corbicula fluminea) found that sulfamethoxazole at environmentally relevant concentrations had neurotoxic effects on the clams,28 and another study on Mediterranean mussels (Mytilus galloprovincialis), found moderate effects on the mussels when exposed to various non-steroidal anti-inflammatory drugs.29 Further information on studies examining the effects of pharmaceutical compounds on various aquatic organisms can be found in a number of reviews in the literature.30,31 The impacts of pharmaceuticals on human health range from direct effects, such as consuming seafood containing hazardous compounds, to indirect impacts, such as contributing to antimicrobial resistance.32 Therefore, there is a need to determine their concentration in seafood.
The peri-urban creeks along the Kenyan coast, particularly Tudor Creek, are among the largest in Kenya and serve as important nursery grounds for many fish species.33 Data on fish species and the quantities from different fish landing sites are available. However, information on the frequency and quantities of marine fishery products consumed by households along Tudor creek is limited. Tudor creek's fish community structure has changed due to pollution and climate change.33,34 The creek receives sewage discharge from non-functional treatment plants and wastewater leaking into storm drains, untreated effluent from informal settlements, as well as from other domestic and industrial sources due to inadequate wastewater management and sanitation facilities that have persisted for more than a decade.35,36 Previous studies have reported high concentrations of acetaminophen, trimethoprim, sulfamethoxazole, carbamazepine, and nevirapine in the surface water of Tudor creek.37 Hence, determining the concentration of pharmaceuticals in various fish and the average daily intake is required to assess the toxicological risk associated with fish consumption. The study aimed to determine the human health risks associated with consuming fish from Tudor creek. Fourteen pharmaceutical compounds in different fish species samples were accurately measured. The compounds were selected based on their high annual consumption38 and previous studies on pharmaceutical distribution in the surface seawater in Tudor creek37 as well as their occurrence in Kenyan rivers and wastewater samples.39–42
Materials and methods
Study area
Tudor creek is located on the eastern side of Mombasa Island (Fig. 1) and is separated from Makupa creek by a landfill. The fish were purchased dead for use in the study. Fish samples (Epinephelus coioides, Sillago sihama, Sphyraena flavicauda, Pomadasys multimaculatus, Acanthurus blochii, Platax pinnatus, and Lethrinus mahsena) were randomly purchased from local fishermen's daily catch in Tudor creek. | ||
| Fig. 1 A map highlighting the villages of Ganahola, Simitini, and Bandarini along Tudor Creek, where household surveys were conducted. | ||
Surveys were conducted in Ganahola (- 3°99′ 50.73′′ S, 39°63′ 15.23′′ E), Simitini (- 4°03′ 30.25′′ S, 39.65′ 72.79′′ E), and Bandarini (- 4°02′ 05.87′′ S, 39°39′ 14.50′′ E) along Tudor creek in Mombasa County to estimate the average daily fish intake rate. These three villages serve as major fish landing sites for the fish catch along Tudor creek. The Ganahola community engages in fishing activities, and relies on the creek's rich biodiversity for their livelihoods. Simitini is a coastal settlement within Mombasa County, characterized by its fishing activities whereas Bandarini contributes to the local fishing industry and offers insights into the community's interaction with the marine environment. Data on household fish consumption was collected using a semi-structured questionnaire. The household surveys were conducted systematically, with participants from every third house being interviewed. The criterion for participation was whether members of the household consumed fish as part of their diet.
Pharmaceutical analysis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v methanol/water mixture. For internal standard calibration, separate mixtures of isotopically labeled internal standards were prepared in methanol, and further dilutions were also prepared in a 50
50 v/v methanol/water mixture. For internal standard calibration, separate mixtures of isotopically labeled internal standards were prepared in methanol, and further dilutions were also prepared in a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v methanol/water mixture. Solid phase extraction (SPE) cartridges, specifically, Oasis hydrophilic–lipophilic balance (HLB, 6 cm3, 500 mg), were purchased from Waters (Milford, USA), and supplied by Microsep, South Africa.
50 v/v methanol/water mixture. Solid phase extraction (SPE) cartridges, specifically, Oasis hydrophilic–lipophilic balance (HLB, 6 cm3, 500 mg), were purchased from Waters (Milford, USA), and supplied by Microsep, South Africa.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in a 50 mL borosilicate glass vial. The resulting mixture was vortexed for 2 min and thereafter sonicated at 25 °C for 15 min. The samples were centrifuged (Hettich EBA) at 4500 rpm for 10 min, and then the supernatant was transferred to a borosilicate glass vial. This extraction procedure was repeated with methanol and acetonitrile, followed by phosphate buffer. The extracts were combined and evaporated using a Genevac EZ-2 system (Genevac SP Scientific). Immediately after concentration, the extract was diluted with 200 mL of ultrapure water (18.2 MΩ × cm at 25 °C), pH adjusted to 2, and 0.5 g of Na2EDTA was added to chelate the metal ions in the solution. The samples were then passed through SPE at a 1 mL min−1 flow rate. Once the entire sample had passed through the SPE (HLB; 6 cm3, 500 mg), 10 mL of reagent water was added to wash out the Na2EDTA.
1 in a 50 mL borosilicate glass vial. The resulting mixture was vortexed for 2 min and thereafter sonicated at 25 °C for 15 min. The samples were centrifuged (Hettich EBA) at 4500 rpm for 10 min, and then the supernatant was transferred to a borosilicate glass vial. This extraction procedure was repeated with methanol and acetonitrile, followed by phosphate buffer. The extracts were combined and evaporated using a Genevac EZ-2 system (Genevac SP Scientific). Immediately after concentration, the extract was diluted with 200 mL of ultrapure water (18.2 MΩ × cm at 25 °C), pH adjusted to 2, and 0.5 g of Na2EDTA was added to chelate the metal ions in the solution. The samples were then passed through SPE at a 1 mL min−1 flow rate. Once the entire sample had passed through the SPE (HLB; 6 cm3, 500 mg), 10 mL of reagent water was added to wash out the Na2EDTA.
Using a vacuum, the cartridges were dried for 30 min. The analytes were then eluted with 3 mL of acetonitrile/methanol (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v) solution. The solvent was subsequently evaporated to dryness using a Genevac EZ-2 system and reconstituted by adding 100 μL of HPLC grade water and methanol (50
50 v/v) solution. The solvent was subsequently evaporated to dryness using a Genevac EZ-2 system and reconstituted by adding 100 μL of HPLC grade water and methanol (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v). Finally, the sample was injected into an Ultra Performance Liquid Chromatography (UPLC) system hyphenated to a quadrupole-time-of-flight (QTOF) mass spectrometry instrument (Waters® Synapt G2). Procedures used for analysis using the UPLC-QTOF are provided in the ESI Section (S1).†
50 v/v). Finally, the sample was injected into an Ultra Performance Liquid Chromatography (UPLC) system hyphenated to a quadrupole-time-of-flight (QTOF) mass spectrometry instrument (Waters® Synapt G2). Procedures used for analysis using the UPLC-QTOF are provided in the ESI Section (S1).†
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 10
1 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively.
1, respectively.
| Analyte | Linearity (R2) | LOD (ng L−1) | LOQ (ng L−1) | Fish muscles | Fish gills | ||
|---|---|---|---|---|---|---|---|
| RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | ||||
| Acetaminophen | 0.9978 | 0.07 | 0.50 | 1.67 | 112 | 2.50 | 87 |
| Acetyl salicylic | 0.9949 | 0.23 | 1.67 | 7.39 | 91 | 4.19 | 109 |
| Diclofenac | 0.9986 | 0.07 | 0.52 | 16.33 | 82 | 14.56 | 81 |
| Ibuprofen | 0.9905 | 0.16 | 1.21 | 18.63 | 99 | 16.12 | 111 |
| Trimethoprim | 0.9958 | 0.10 | 0.73 | 2.76 | 98 | 4.63 | 108 |
| Sulfamethoxazole | 0.9975 | 0.08 | 0.61 | 2.43 | 82 | 3.35 | 76 |
| Tetracycline | 0.9958 | 0.11 | 0.80 | 12.98 | 74 | 12.46 | 104 |
| Erythromycin | 0.9930 | 0.20 | 1.46 | 1.46 | 83 | 2.00 | 95 |
| Carbamazepine | 0.9992 | 0.04 | 0.32 | 0.18 | 107 | 1.48 | 100 |
| Nevirapine | 0.9996 | 0.25 | 1.86 | 1.01 | 83 | 0.96 | 77 |
| Caffeine | 0.9997 | 0.02 | 0.18 | 0.42 | 109 | 0.56 | 114 |
| Cetirizine | 0.9991 | 0.06 | 0.42 | 0.45 | 107 | 0.33 | 95 |
| Lidocaine | 0.9983 | 0.05 | 0.39 | 1.33 | 73 | 1.71 | 70 |
| Bupivacaine | 0.9993 | 0.06 | 0.46 | 1.50 | 84 | 1.67 | 77 |
Survey on fish consumption along Tudor creek
A descriptive, cross-sectional study design was utilized to collect data for this survey. Participants in the cross-sectional study were chosen based on the inclusion and exclusion criteria.42 Households were systematically selected, with the participants from every third house being interviewed. The inclusion criterion for participation in the survey was the consumption of fish by the household as part of their diet. Data on household fish consumption was collected by administering a semi-structured questionnaire. The questionnaire was divided into demographics and household fish consumption (see ESI Material 1†). The demographic section comprised structured questions, i.e., answers were provided from which the respondent picked the most appropriate option. The section on the average quantity of fish consumption per person in a household per day for the residents of Ganahola, Simitini, and Bandarini in Tudor creek was calculated using eqn (1).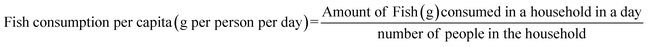 | (1) |
Human health risk assessment
The daily intake of pharmaceuticals was estimated based on the concentration detected in seafood (fish) and their daily consumption among a diverse group of people living along Tudor creek. The estimated daily intake (EDI; in ng kg−1 body weight/day bw/d) of pharmaceuticals for Tudor creek residents (Ganahola, Simitini, and Bandarini) through fish consumption in the average intake scenario was calculated using eqn (2).9,43 | (2) |
The acceptable daily intake (ADI) value refers to the amount of a substance that can be consumed daily over a lifetime without any negative impact on human health. However, a major challenge in conducting a risk assessment for human health is the lack of ADI values for all pharmaceuticals. Hence ADI values for pharmaceuticals used to treat humans were determined using eqn (3).
 | (3) |
To assess the target hazard quotient (THQ), EDI values were compared with the acceptable daily intake (ADI) values eqn (4). THQ ˂ 1% indicates a negligible risk and considerable risk when the ratio is 1–5%, while THQ > 5% indicates a potential risk to human health47,48
 | (4) |
Data analysis
Descriptive statistics, specifically, the sum, count and mean of the samples were used to summarize the results of pharmaceutical concentrations in fish muscle and gills samples. Pearson correlations tests were used to determine if there were any significant associations between the pharmaceuticals investigated. Principal Component Analysis (PCA) was performed using R statistical software, and statistical significance was defined at p < 0.05. Raw household survey data collected from three different communities along Tudor creek was entered and cleaned in Microsoft Excel. Missing values in household income were imputed from the available data to ensure a complete dataset. Descriptive statistics were generated to compute mean household sizes, incomes, and (daily) fish consumption quantities.Results and discussion
Demographics of the respondents
A total of 93 respondents were interviewed during the survey (Table 2), including fishermen, fish dealers, food vendors, small-scale business owners, and casual laborers. The survey respondents comprised 71% (n = 66) men and 29% women (n = 27). The male respondents predominated over the female respondents, which could be because fishing has traditionally been considered as a male occupation. The mean household size in the survey was seven, with the maximum size recorded as twenty and the minimum as one.| N = 93 | Site | Percentage of respondents |
|---|---|---|
| N = 28 | Ganahola | 30 |
| N = 32 | Simitini | 34 |
| N = 33 | Bandarini | 35 |
The ages of the respondents ranged from 18 to over 80 years, with a mean of 40 years. Regarding education levels, 51% of the respondents had little or no formal education (e.g., only madrassa or incomplete primary school), whereas 36% had completed only primary school, 8% had completed secondary school, and 5% had tertiary education. In terms of employment, 69% of the people surveyed were self-employed, 18% were formally employed, and 13% were unemployed (Fig. S1a†).
Small-scale businesses (42%) and fishing activities (27%) were the main sources of livelihood among the self-employed category for the households of Ganahola, Simitini, and Bandarini (Fig. S1b†). Among the respondents in Ganahola (47%) and Simitini (36%), it was observed that small-scale business was the main source of livelihood. In contrast, most respondents in Bandarini were involved in fishing activities (55%).
Fish consumption was common among the households, with 94% of the respondents having consumed fish within the week before this survey. Most respondents (78%) consumed fish frequently throughout the year, while 22% consumed fish mainly during the Northeast Monsoon, from November to March/April, when fish were more abundant. Among the respondents, 25% consumed fish ranging from 0.5 to 1 kg per day per household. The majority, which accounted for 68%, consumed less than 0.5 kg of fish daily per household. Only a small percentage, specifically 7%, reported consuming more than 1 kg of fish per day per household (Fig. S2a†). Compared to Simitini and Bandarini, a higher percentage of fish consumption was observed in Ganahola (Fig. S2b†). Low fish consumption was observed in Bandarini with 11% consuming <0.5 kg and 13% consuming 0.501–1 kg, despite fishing being the main source of livelihood. This is because household financial resources heavily influence fish consumption.
From the survey, the four most preferred fish species were the Gempylidae, Siganidae, Lethrinidae, and Gerreidae (Fig. 2) due to their taste, nutritional value, fewer bones, and more steak. However, these species of fish are rarely found in Tudor creek, and the most commonly fished and consumed fish species found in high numbers in Tudor creek were Serranidae, Sillaginidae, Sphyraenidae, Pomacentridae, Acanthuridae, and Pinguipedidae. The average quantity of fish consumption per person in a household per day for the residents of Ganahola, Simitini, and Bandarini was 170 g per person per day as per a questionnaire based on fish consumption survey conducted in Tudor creek.
It was noted that 65% of the respondents acquired fish by either fishing or purchasing directly from the landing sites. In contrast, 28% obtained fish from the local market, and 8% sourced it from local fish shops. Regarding the preferences for fish, 66% of the respondents bought fresh fish, 29% opted for smoked fish, and 6% preferred frozen fish.
Pharmaceutical concentration in fish muscles and gills
The concentrations of pharmaceuticals in fish muscle from Tudor creek ranged between DL–1624.0 ng g−1. These values were consistent with those reported in Kalk Bay harbor (not detected (nd)–1812.0 ng g−1) in South Africa20 but higher than those reported in fish samples (nd–231.1 ng g−1) from Coastal waters of the Saudi Red Sea,49 the Mar Menor lagoon (nd–3.5 ng g−1) in the Mediterranean Sea, SE Spain,19 and Laizhou Bay, (40–110 ng g−1) in North China.21 In fish muscle, the concentrations of lidocaine, caffeine, carbamazepine, and trimethoprim were high, with total concentrations of ∑295 ng g−1, ∑299 ng g−1, ∑302 ng g−1, and ∑3608 ng g−1, respectively (Fig. 3). On the other hand, fish gill samples showed high concentrations of tetracycline, ibuprofen, acetylsalicylic acid, and nevirapine, with total concentrations of ∑3297.76 ng g−1, ∑537.15 ng g−1, ∑808.73 ng g−1, and ∑10.19 ng g−1, respectively. | ||
| Fig. 3 Sum concentration of (a) NSAIDs, (b) selected pharmaceuticals, and (c) antibiotics in fish muscles and gills. | ||
Pharmaceutical concentrations at various coastal sites worldwide are summarized in Table 3. Analgesics (acetaminophen and acetylsalicylic acid) were detected in almost all the fish muscle samples (Fig. 4a), with the highest concentration of acetaminophen observed in Pomadasys multimaculatus (54.4 ng g−1) and acetylsalicylic acid in Platax pinnatus (193.8 ng g−1). Acetaminophen concentrations in fish muscles (5.4–54.4 ng g−1) were consistent with concentrations detected in Al Arbaeen lagoon, Saudi Red Sea49 but higher than those detected in Kalk Bay harbour, South Africa.20 On the other hand, the gill samples of Acanthurus blochii had a higher concentration of acetaminophen (58.04 ng g−1) compared to other fish species (Fig. 4b). These values were higher than the concentration found in Pterogymnus laniarius gill samples (33.26 ng g−1) from Kalk Bay harbour, South Africa.20 At the same time, the gills samples of Lethrinus mahsena showed the highest concentration of acetylsalicylic acid (431.8 ng g−1) as compared to Epinephelus coioides and Pomadasys multimaculatus, whose concentrations were below the detection limit (Fig. 4b).
| Pharmaceutical | Site | Country | Concentration in muscles | Concentration in gills | References |
|---|---|---|---|---|---|
| Acetaminophen | Al Arbaeen lagoon | Saudi Red Sea | nd–62.7 | 50 | |
| Acetaminophen | Kalk Bay harbor | South Africa | nd–17.95 | nd–33.26 | 21 |
| Acetaminophen | Tudor creek | Kenya | 5.41–54.39 | DL–58.04 | This study |
| Acetyl Salicylic | Tudor creek | Kenya | DL–223.06 | DL–4311.84 | This study |
| Ibuprofen | Al Arbaeen lagoon | Saudi Red Sea | nd–231.1 | 50 | |
| Ibuprofen | Sepetiba Bay and Parnaiba Delta | Brazilian coastal | nd–14.5 | 51 | |
| Ibuprofen | Tudor creek | Kenya | 0.49–242.13 | 3.29–296.64 | This study |
| Diclofenac | Kalk Bay harbor | South Africa | nd–1812 | nd–1089 | 21 |
| Diclofenac | Mar Menor lagoon | Mediterranean Sea (SE Spain) | nd–1.3 | 20 | |
| Diclofenac | Sepetiba Bay and Parnaiba Delta | Brazilian coastal | nd–5.6 | 51 | |
| Diclofenac | Tudor creek | Kenya | 2.02–44.77 | DL–31.69 | This study |
| Trimethoprim | Laizhou Bay | North China | nd–18 | nd–61 | 22 |
| Trimethoprim | Al Arbaeen lagoon | Saudi red Sea | nd–44.9 | 50 | |
| Trimethoprim | Tudor creek | Kenya | 5.74–1623.98 | 17.95–1427.43 | This study |
| Sulfamethoxazole | Laizhou Bay | North China | 11–40 | nd–110 | 22 |
| Sulfamethoxazole | Kalk Bay harbor | South Africa | nd–385.2 | nd–75.25 | 21 |
| Sulfamethoxazole | Southern Sea Korea | Southern Korea | nd | 19 | |
| Sulfamethoxazole | Al Arbaeen lagoon | Saudi Red Sea | nd–11.2 | 50 | |
| Sulfamethoxazole | Tudor creek | Kenya | 0.06–3.11 | DL–1.27 | This study |
| Erythromycin | Laizhou Bay | North China | nd–1.4 | nd–20 | 22 |
| Erythromycin | Southern Sea Korea | Southern Korea | nd–48.1 | 19 | |
| Erythromycin | Tudor creek | Kenya | DL–0.74 | 0.02–1.26 | This study |
| Tetracycline | Tudor creek | Kenya | DL–1114.47 | DL–1785.60 | This study |
| Carbamazepine | Kalk Bay harbor | South Africa | nd–22.9 | nd–22.83 | 21 |
| Carbamazepine | Mar Menor lagoon | Mediterranean Sea (SE Spain) | bdl −6.3 | 20 | |
| Carbamazepine | Sepetiba Bay and Parnaiba Delta | Brazilian coastal | nd–3.8 | 51 | |
| Carbamazepine | Al Arbaeen lagoon | Saudi Red Sea | 1.7–33.8.9 | 50 | |
| Carbamazepine | Tudor creek | Kenya | 0.44–122.66 | 0.02–3.90 | This study |
| Caffeine | Kalk Bay harbor | South Africa | nd–50.49 | nd–2.030 | 21 |
| Caffeine | Southern Sea of Korea | Southern Korea | nd–1.37 | 19 | |
| Caffeine | Tudor creek | Kenya | 2.71–203.86 | 2.28–8.03 | This study |
| Nevirapine | Tudor creek | Kenya | DL–1.09 | DL–5.03 | This study |
| Cetirizine | Tudor creek | Kenya | DL–4.25 | DL–2.65 | This study |
| Lidocaine | Tudor creek | Kenya | 7.90–167.06 | 0.84–15.74 | This study |
| Bupivacaine | Tudor creek | Kenya | DL–1.65 | 0.31–1.12 | This study |
For the non-steroidal anti-inflammatory drugs (NSAIDs), diclofenac and ibuprofen were detected in all the fish muscles (Fig. 4a), with the highest concentration of diclofenac (44.77 ng g−1) detected in Epinephelus coioides which was higher than those reported in Sepetiba Bay and Parnaiba Delta, Brazilian coastal waters, nd–14.5 ng g−1 (ref. 52) but lower in fish muscle samples from Al Arbaeen lagoon, Saudi Red Sea, nd–231.1 ng g−1.49 However, the concentration of ibuprofen in the muscle tissue of Acanthurus blochii was 242.13 ng g−1, which was higher than the values detected in Mar Menor lagoon, Mediterranean Sea, SE Spain, nd–1.3 ng g−1 (ref. 19) and Sepetiba Bay and Parnaiba Delta, Brazilian coastal waters, nd–5.6 ng g−1 (ref. 52) but lower than findings from Kalk Bay harbour, South Africa, nd–1812 ng g−1.20 In fish gill samples, the concentration of diclofenac and ibuprofen ranged between DL–31.7 ng g−1 and 3.29–296.6 ng g−1, respectively (Fig. 4b). The concentration of diclofenac in fish gills in this study was nd–1089 ng g−1, and this was lower than the values determined in Kalk Bay harbour, South Africa.20
The antibiotics in fish muscles and gills (trimethoprim, sulfamethoxazole, tetracycline, and erythromycin) were detected in most of the fish samples (Fig. 4c and d) and ranged between DL–1624.0 ng g−1 and DL–1785.6 ng g−1 respectively. Trimethoprim and tetracycline in fish muscles were highest in Platax pinnatus with concentrations of 1624.0 ng g−1 and 1114.5 ng g−1, respectively. Trimethoprim concentrations in our study were higher than those reported in Laizhou Bay, North China, nd–18 ng g−1 (ref. 21) and Al Arbaeen lagoon, Saudi Red Sea, nd–44.9 ng g−1.49 At the same time, trimethoprim and tetracycline in gills ranged between 17.95–1427.43 ng g−1 and DL–1785.60 ng g−1, respectively. Similarly, trimethoprim in gill samples (Table 3) reported in Laizhou Bay, North China21 were lower than the findings in our study. Sulfamethoxazole concentration in fish muscles and gills ranged between 0.06–3.11 ng g−1 and DL–1.27 ng g−1, respectively, with the highest concentration observed in Acanthurus blochii (in muscles) and Lethrinus mahsena (in gills). Liu et al.21 and Ojemaye and Petrik20 reported higher sulfamethoxazole concentrations (Table 3) compared to our study. Erythromycin concentrations in gills were higher compared to muscles, with sum concentrations of ∑2.72 ng g−1 and ∑1.99 ng g−1, respectively. Sillago sihama showed a high concentration of erythromycin in fish muscle (0.74 ng g−1) while Pomadasys multimaculatus showed in fish gills (1.26 ng g−1). Liu et al.21 detected higher concentrations of erythromycin (nd–20 ng g−1) in fish gills in Laizhou Bay, North China, compared to the one obtained in this study.
Carbamazepine and nevirapine concentrations in fish muscle ranged between 0.44–122.66 ng g−1 and DL–1.02 ng g−1, respectively, with the highest concentration observed in Sillago sihama species (Fig. 5).
 | ||
| Fig. 5 Comparison of pharmaceutical concentrations in the (a) fish muscles and (b) gills of different fish species. | ||
Carbamazepine values in our study were higher than those reported in Sepetiba Bay and Parnaiba Delta, Brazilian coastal waters52 and in Al Arbaeen Lagoon, Saudi Red Sea49 but lower than those reported by Ojemaye and Petrik, (2019)20 in Kalk Bay harbor, South Africa (Table 3). In fish gills, carbamazepine and nevirapine raged between 0.02–3.90 ng g−1 and DL–5.03 ng g−1, respectively. It's worth noting that the concentration of carbamazepine was high in fish muscle as compared to fish gills.
On the other hand, the concentration of caffeine and cetirizine in fish muscles ranged between 2.7–203.9 ng g−1 and DL–4.25 ng g−1, respectively, with a high concentration of caffeine observed in Sillago sihama and cetirizine in Pomadasys multimaculatus (Fig. 5b). However, the concentration of caffeine in fish muscle in Kalk Bay harbor, South Africa (nd–64.78 ng g−1)20 and Korea's Southern Sea (nd–64.78 ng g−1)18 was lower than the one obtained in this study. In comparison, caffeine and cetirizine were found at higher concentrations in fish muscle (298.7 ng g−1 and 7.6 ng g−1, respectively) than in fish gills (29.7 ng g−1 and 5.7 ng g−1, respectively).
Bupivacaine and lidocaine are the commonly used local analgesic anesthetics to improve postoperative pain control and reduce the required postoperative narcotics.53 Bupivacaine concentrations in fish muscle and gills ranged between DL–1.65 ng g−1 and 0.31–1.12 ng g−1, respectively. Acanthurus blochii exhibited notably high bupivacaine in fish muscle and gills (Fig. 5a and b). In comparison, lidocaine levels in fish muscle and gills ranged between 7.90–167.06 ng g−1 and 0.84–15.74 ng g−1, respectively, with a high concentration detected in Platax pinnatus (fish muscle) and Platax pinnatus (fish gills).
Relationships between pharmaceutical concentrations in fish
The correlation analysis of pharmaceutical concentrations in fish species is illustrated in Fig. 6a. Acetaminophen (ACN) showed a significant strong positive correlation (p < 0.05) with erythromycin (ERM) and bupivacaine (BCN), indicating similar pollution sources such as wastewater discharge, hospital effluents, or agricultural runoff. This correlation also suggests that these pharmaceuticals share similar bioaccumulation and uptake mechanisms that influence their absorption, metabolism, or retention in fish tissues.54 In contrast, carbamazepine (CBZ) showed a significant (p < 0.05) negative correlation with trimethoprim (TMP) and tetracycline (TC), suggesting selective bioaccumulation influenced by species-specific metabolism, feeding habits, differences in metabolic pathways, or degradation interactions within the aquatic environment.Principle component analysis (PCA) was also applied to further explore associations among pharmaceuticals in the fish species. As shown in Fig. 6b, DIM1 and DIM 2 accounted for 31.1% and 21.5% of the total variance, respectively, with DIM1 being highly associated with TC, CBZ, and NVP, while DIM 2 being highly associated with ACN and ERP suggesting that the fish species share similar pharmaceutical accumulation patterns based on their feeding habits and habitat preferences. NVP concentrations were high in Lethrinus mahsena and Platax pinnatus, whereas ERP was high in Acanthurus blochii and Pomadasys multimaculatus, indicating that these species have a higher affinity for absorbing or retaining these pharmaceuticals, possibly due to differences in metabolism, and feeding ecology.55 It's worth noting that the pharmaceutical concentrations in Pomadasys multimaculatus showed a statistically significant strong positive correlation (p < 0.05) with Sphyraena flavicauda, Acanthurus blochii and Lethrinus mahsena. Similarly, Platax pinnatus exhibited significant correlation with Pomadasys multimaculatus, Lethrinus mahsena and Acanthurus blochii. These correlations may be attributed to shared pollution sources, such as wastewater discharge, hospital effluents, or agricultural runoff, which likely increase the susceptibility of these fish species to pharmaceutical bioaccumulation due to their proximity to contamination sites and feeding behaviours.
No specific trends were observed in the concentrations of pharmaceutical compounds in fish muscle and gills of all the fish species examined. However, trimethoprim and tetracycline had the highest concentrations in fish muscle and gills among all the pharmaceutical compounds. This could be due to the high concentrations of trimethoprim and tetracycline in sediment and macroalgae species in Tudor creek.56 In addition, the acid dissociation constant (pKa) may influence the bioaccumulation of trimethoprim and tetracycline in organisms.
Human health risk assessment
Risk assessment was conducted based on the amount of pharmaceuticals bioaccumulated by fish along Tudor creek. Table 4 shows the EDI, ADI, and THQ values for the average exposure scenarios to the studied pharmaceuticals in the communities (Ganahola, Simitini, and Bandarini) along Tudor creek, who depend on fish as a source of protein. The EDI of pharmaceuticals through fish consumption ranged between 1.01–1441.7 ng kg−1 bw per day. Ganahola, Simitini and Bandarini residents were exposed to trimethoprim (1441.7 ng kg−1 bw per day) and tetracycline (979.26 ng kg−1 bw per day). The THQ values of trimethoprim, tetracycline, and caffeine were greater than 5%, which potentially signifies that consumption of these fish could pose a potential human health risk. In contrast, the risk associated with fish consumption containing acetaminophen, sulfamethoxazole, and erythromycin was negligible. Shaaban and Mostafa51 also observed that the antibiotics detected in the fish species from Saudi Arabia did not pose a potential human health risk from fish intake. In contrast, the findings of Mello, Cunha52 in the Brazilian coastal area show that adult Brazilian fish consumers are unlikely to experience adverse health effects from pharmaceuticals through the consumption of different fish species. It should be noted that guidelines or standards that provide crucial information on the risk assessment of pharmaceuticals are only available for a few types of pharmaceuticals. Several uncontrollable factors, such as variations in metabolism, personal health habits and underlying health conditions, and dietary behaviours influence human health risks. Additionally, pharmaceuticals may also be inadvertently consumed through other food sources.| Target compound | Mean concentration (ng g−1 dw) | EDI (adult) mean concentrations | ADI (μg per (kg per day)) | THQ (adult) |
|---|---|---|---|---|
| a Lowest therapeutic dose (mg per day) for the pharmaceuticals were obtained from Schwab et al., 2005.57 b Lowest therapeutic dose (mg per day) for the pharmaceuticals were obtained from Prosser and Sibley, 2015.58 c Lowest therapeutic dose (mg per day) for the pharmaceuticals were obtained from Sengar and Vijayanandan, 2022.50 d Not available. | ||||
| Acetaminophen | 20.9 | 58.58 | 10.71a | 0.55 |
| Acetyl salicylic | 93.9 | 262.62 | 7.00b | 3.75 |
| Diclofenac | 20.4 | 56.95 | 1.65c | 3.46 |
| Ibuprofen | 72.2 | 202.03 | 13.18c | 1.53 |
| Trimethoprim | 515.4 | 1441.73 | 1.65a | 87.51 |
| Sulfamethoxazole | 0.9 | 2.57 | 6.59c | 0.04 |
| Tetracycline | 350.1 | 979.26 | 6.59c | 14.86 |
| Erythromycin | 0.3 | 0.80 | 5.07 | 0.02 |
| Carbamazepine | 43.1 | 120.60 | 3.29c | 3.66 |
| Nevirapine | 0.4 | 1.01 | —d | —d |
| Caffeine | 42.7 | 119.35 | 1.20c | 9.95 |
| Cetirizine | 1.1 | 3.02 | 0.08c | 3.67 |
| Lidocaine | 42.1 | 117.83 | —d | —d |
| Bupivacaine | 0.6 | 1.75 | —d | —d |
A household survey conducted in the villages of Ganahola, Simitini, and Bandarini along Tudor Creek revealed that fish consumption is prevalent among residents. Notably, pharmaceutical contaminants, particularly trimethoprim and tetracycline, were detected at elevated concentrations in the muscle and gill tissues of various fish species from Tudor creek. Regular consumption of such contaminated fish could lead to the bioaccumulation of these substances in humans, posing significant health risks over time. Exposure to tetracycline has been associated with various adverse health effects, including oxidative stress, alterations in behavior, and disruptions to the digestive, nervous, and immune systems in aquatic organisms. These effects may also extend to humans consuming contaminated fish, potentially leading to similar health issues.59 Previous studies have reported that pharmaceuticals in fish can reach significantly higher concentrations in plasma than in ambient water.20,60,61 This can be attributed to the fact that the pharmaceuticals can be taken up by fish from the gills, skin or food, leading to enhanced levels of the pollutant in fish tissue.17,18,62
Conclusion
The results of the present study provide new information on the concentrations of pharmaceuticals in the muscles and gills of different fish species from Tudor creek for the first time. This study shows that diverse commonly prescribed pharmaceutical compounds are present at detectable, and even quantifiable, concentrations in fish. This is attributed to anthropogenic activities, including direct and indirect sewage discharges into Tudor creek, which contribute to the introduction of pharmaceuticals into the oceanic environment and their accumulation in various edible fish species.Fish consumption is common among households in Ganahola, Simitini, and Bandarini in Tudor creek, with most residents consuming less than 0.5 kg per day. These households may be at risk of pharmaceutical exposure through fish consumption. Risk assessment based on the calculated THQ indicated that a high THQ value (>5%) suggests potential human health risks from consuming fish from Tudor Creek, particularly due to exposure to trimethoprim, tetracycline, and caffeine. Pharmaceuticals can remain bioavailable to aquatic organisms for extended periods due to pseudo-persistence and may even re-enter the food web over time. Given these potential risks, it is crucial to conduct comprehensive risk assessments and implement strategies to mitigate pharmaceutical contamination in aquatic environments. The exposure to these pharmaceuticals through fish consumption by the households in Ganahola, Simitini, and Bandarini in Tudor creek is a cause of concern, highlighting the need for in-depth investigations, future monitoring and the promotion of proper disposal and management of pharmaceuticals to ensure the safety of local fish consumers. Additionally, a detailed ecological understanding is necessary to comprehend the accumulation and dispersion of pharmaceuticals within aquatic food webs.
There is a necessity to increase public awareness regarding the impact of pharmaceutical discharges into aquatic and marine environments. Additionally, it is imperative to develop and enforce policies and regulations for pharmaceutical disposal to prevent adverse effects on the sustainability of our marine ecosystems and the livelihoods and well-being of people who depend on these environments. Addressing the issue responsibly requires collaboration among governments, industries, communities, and individuals to ensure the long-term health of our oceans and coastal areas.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
VWO Wanjeri: Conceptualization, methodology, validation, formal analysis, writing original draft. E Okuku: Conceptualization, formal analysis, resources, review & editing, supervision. JC Ngila: Writing – review & editing, supervision. E Waiyaki: Methodology, validation, investigation. JK Nyingi: Methodology, validation, investigation, resources. PG Ndungu: Formal analysis, resources, writing – review & editing, supervision, project administration.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work received financial support from the Kenya Marine and Fisheries Research Institute (KMFRI) and the Western Indian Ocean Marine Science Association (WIOMSA) MARG I-(Grant No. 2020-CO29) and (WIOMSA) MARG II-(2022). VWOW, JCN, and PGN acknowledge the Faculty of Science at the University of Johannesburg for financial support. The authors acknowledge the University of Pretoria for research support. In addition, we would like to thank all respondents who spared their time to participate in this survey. Last but not least, we extend our gratitude to our field guides at all sites, without whom this work would not have been possible.References
-
J. L. Anderson, F. Asche, T. Garlock, et al., Aquaculture: its role in the future of food, in World Agricultural Resources and Food Security, Emerald Publishing Limited. 2017 Search PubMed
.
- K. Obiero, P. Meulenbroek and S. Drexler,
et al., The contribution of fish to food and nutrition security in Eastern Africa: emerging trends and future outlooks, Sustainability, 2019, 11(6), 1636 CrossRef
.
- L. Chiesa, F. Ceriani and M. Caligara,
et al., Mussels and clams from the italian fish market. is there a human exposition risk to metals and arsenic?, Chemosphere, 2018, 194, 644–649 CrossRef CAS PubMed
.
- Q. Liu, Y. Liao and L. Shou, Concentration and potential health risk of heavy metals in seafoods collected from Sanmen Bay and its adjacent areas, China, Mar. Pollut. Bull., 2018, 131, 356–364 CrossRef CAS PubMed
.
-
A. Gordon, C. Finegold, C. C. Crissman and A. Pulis, Fish production, consumption and trade in sub-Saharan Africa: a review analysis, Internal Working Paper, World Bank Group, Washington DC, 2013 Search PubMed
.
- A. Bennett, X. Basurto and J. Virdin,
et al., Recognize fish as food in policy discourse and development funding, Ambio, 2021, 50(5), 981–989 CrossRef PubMed
.
-
FAO, FAO Working for SDG14: Healthy Oceans for Food Security, Nutrition and Resilient Communities. 2017, pp. 10–11 Search PubMed
.
- H. Xie, H. Hao and N. Xu,
et al., Pharmaceuticals and personal care products in water, sediments, aquatic organisms, and fish feeds in the Pearl River Delta: occurrence, distribution, potential sources, and health risk assessment, Science of the total Environment, 2019, 659, 230–239 CrossRef CAS PubMed
.
- M. Ashfaq, K. Nawaz Khan and M. Saif Ur Rehman,
et al., Ecological risk assessment of pharmaceuticals in the receiving environment of pharmaceutical wastewater in Pakistan, Ecotoxicol. Environ. Saf., 2017, 136, 31–39 CrossRef CAS PubMed
.
- B. Shi, Y. Jiang and J. Yang,
et al., Ecological risks induced by consumption and emission of Pharmaceutical and personal care products in Qinghai-Tibet Plateau: insights from the polar regions, Environ. Int., 2023, 178, 108125 CrossRef CAS PubMed
.
- R. Moreno-González, S. Rodríguez-Mozaz and M. Gros,
et al., Input of pharmaceuticals through coastal surface watercourses into a Mediterranean lagoon (Mar Menor, SE Spain): sources and seasonal variations, Sci. Total Environ., 2014, 490, 59–72 CrossRef PubMed
.
- M. Biel-Maeso, C. Corada-Fernández and P. A. Lara-Martín, Determining the distribution of pharmaceutically active compounds (PhACs) in soils and sediments by pressurized hot water extraction (PHWE), Chemosphere, 2017, 185, 1001–1010 CrossRef CAS PubMed
.
- Â. Almeida, M. Solé and A. M. Soares,
et al., Anti-inflammatory drugs in the marine environment: bioconcentration, metabolism and sub-lethal effects in marine bivalves, Environ. Pollut., 2020, 263, 114442 CrossRef PubMed
.
- L. Yang, Y. Zhou and B. Shi,
et al., Anthropogenic impacts on the contamination of pharmaceuticals and personal care products (PPCPs) in the coastal environments of the Yellow and Bohai seas, Environ. Int., 2020, 135, 105306 CrossRef CAS PubMed
.
- G. McEneff, L. Barron and B. Kelleher,
et al., A year-long study of the spatial occurrence and relative distribution of pharmaceutical residues in sewage effluent, receiving marine waters and marine bivalves, Sci. Total Environ., 2014, 476–477, 317–326 CrossRef CAS PubMed
.
- C. R. Ohoro, A. O. Adeniji and L. Semerjian,
et al., Occurrence and distribution of pharmaceuticals in surface water and sediment of Buffalo and Sundays River estuaries, South Africa and their ecological risk assessment, Emerging Contam., 2021, 7, 187–195 CrossRef CAS
.
- D. Álvarez-Muñoz, S. Rodríguez-Mozaz and A. L. Maulvault,
et al., Occurrence of pharmaceuticals and endocrine disrupting compounds in macroalgaes, bivalves, and fish from coastal areas in Europe, Environ. Res., 2015, 143, 56–64 CrossRef PubMed
.
- H.-Y. Kim, I.-S. Lee and J.-E. Oh, Human and veterinary pharmaceuticals in the marine environment including fish farms in Korea, Sci. Total Environ., 2017, 579, 940–949 CrossRef CAS PubMed
.
- R. Moreno-González, S. Rodríguez-Mozaz and B. Huerta,
et al., Do pharmaceuticals bioaccumulate in marine molluscs and fish from a coastal lagoon?, Environ. Res., 2016, 146, 282–298 CrossRef PubMed
.
- C. Y. Ojemaye and L. Petrik, Occurrences, levels and risk assessment studies of emerging pollutants (pharmaceuticals, perfluoroalkyl and endocrine disrupting compounds) in fish samples from Kalk Bay harbour, South Africa, Environ. Pollut., 2019, 252, 562–572 CrossRef CAS PubMed
.
- S. Liu, T.-G. Bekele and H. Zhao,
et al., Bioaccumulation and tissue distribution of antibiotics in wild marine fish from Laizhou Bay, North China, Sci. Total Environ., 2018, 631–632, 1398–1405 CrossRef CAS PubMed
.
- A. Ruhí, V. Acuña and D. Barceló,
et al., Bioaccumulation and trophic magnification of pharmaceuticals and endocrine disruptors in a Mediterranean river food web, Sci. Total Environ., 2016, 540, 250–259 CrossRef PubMed
.
- Y. Ruan, H. Lin and X. Zhang,
et al., Enantiomer-specific bioaccumulation and distribution of chiral pharmaceuticals in a subtropical marine food web, J. Hazard. Mater., 2020, 394, 122589 CrossRef CAS PubMed
.
- D. Cerveny, R. Grabic and K. Grabicová,
et al., Neuroactive drugs and other pharmaceuticals found in blood plasma of wild European fish, Environ. Int., 2021, 146, 106188 CrossRef CAS PubMed
.
- L. K. Bickley, R. van Aerle and A. R. Brown,
et al., Bioavailability and Kidney Responses to Diclofenac in the Fathead Minnow (Pimephales promelas), Environ. Sci. Technol., 2017, 51(3), 1764–1774 CrossRef CAS PubMed
.
- H. Yokota, S. Eguchi and S. Hasegawa,
et al., Assessment of in vitro antiovulatory activities of nonsteroidal anti-inflammatory drugs and comparison with in vivo reproductive toxicities of medaka (Oryzias latipes), Environ. Toxicol., 2016, 31(12), 1710–1719 CrossRef CAS PubMed
.
- E. P. Ambrosio-Albuquerque, L. F. Cusioli and R. Bergamasco,
et al., Metformin environmental exposure: a systematic review, Environ. Toxicol. Pharmacol., 2021, 83, 103588 CrossRef CAS PubMed
.
- S. Liu, H. Zhao and M. Zheng,
et al., The physiological, biochemical and transcriptional responses to sulfamethoxazole in the Asian clam, Corbicula fluminea (O. F. Müller, 1774), Comp. Biochem. Physiol., Part C:Toxicol. Pharmacol., 2022, 260, 109406 CAS
.
- M. Mezzelani, S. Gorbi and Z. Da Ros,
et al., Ecotoxicological potential of non-steroidal anti-inflammatory drugs (NSAIDs) in marine organisms: bioavailability, biomarkers and natural occurrence in Mytilus galloprovincialis, Mar. Environ. Res., 2016, 121, 31–39 CrossRef CAS PubMed
.
- L. M. Madikizela and S. Ncube, Health effects and risks associated with the occurrence of pharmaceuticals and their metabolites in marine organisms and seafood, Sci. Total Environ., 2022, 837, 155780 CrossRef CAS PubMed
.
- M. d. J. S. Chaves, J. Kulzer and P. d. R. Pujol de Lima,
et al., Updated knowledge, partitioning and ecological risk of pharmaceuticals and personal care products in global aquatic environments, Environ. Sci.: Processes Impacts, 2022, 24(11), 1982–2008 RSC
.
- B. H. Maskrey, K. Dean and N. Morrell,
et al., A Simple and Rapid Ultra–High-Performance Liquid Chromatography–Tandem Mass Spectrometry Method for the Quantitation of Pharmaceuticals and Related Compounds in Mussels and Oysters, Environ. Toxicol. Chem., 2021, 40(12), 3263–3274 CrossRef CAS PubMed
.
-
M. Wainaina, Fish Community Structure of a Mangrove Creek, Tudor, Kenya, Moi University. 2010 Search PubMed
.
- J. Kerubo, A. Muthumbi and J. Onyari,
et al., Microplastics pollution in the sediments of creeks and estuaries of Kenya, western Indian Ocean, Afr. J. Mar. Sci, 2021, 43(3), 337–352 CrossRef
.
-
J. N. Kamau, Heavy Metal Distribution and Enrichment at Port-Reitz Creek, Mombasa. 2002 Search PubMed
.
- J. N. Kamau, J. C. Ngila and B. Kirui,
et al., Spatial variability of the rate of organic carbon mineralization in a sewage-impacted mangrove forest , Mikindani , Kenya, J. Soils Sediments, 2015, 2466–2475 CrossRef CAS
.
- V. O. W. Wanjeri, E. Okuku and A. Gachanja,
et al., Occurrence, distribution, and environmental risk of pharmaceutical residues in Mombasa peri-urban creeks, Kenya, Chemosphere, 2023, 311, 137144 CrossRef CAS PubMed
.
-
KEML, Kenya Essential Medicines List 2019, Published by the Ministry of Health, Ministry of Health. Report, 2019 Search PubMed
.
- P. Kairigo, E. Ngumba and L.-R. Sundberg,
et al., Occurrence of antibiotics and risk of antibiotic resistance evolution in selected Kenyan wastewaters, surface waters and sediments, Sci. Total Environ., 2020, 720, 137580 CrossRef CAS PubMed
.
- E. Ngumba, A. Gachanja and T. Tuhkanen, Occurrence of selected antibiotics and antiretroviral drugs in Nairobi River Basin, Kenya, Sci.
Total Environ., 2016, 539, 206–213 CrossRef CAS PubMed
.
- K. K'oreje, L. Vergeynst and D. Ombaka,
et al., Occurrence patterns of pharmaceutical residues in wastewater, surface water and groundwater of Nairobi and Kisumu city, Kenya, Chemosphere, 2016, 149, 238–244 CrossRef PubMed
.
- K. O. K'oreje, K. Demeestere and P. De Wispelaere,
et al., From multi-residue screening to target analysis of pharmaceuticals in water: development of a new approach based on magnetic sector mass spectrometry and application in the Nairobi River basin, Kenya, Sci. Total Environ., 2012, 437, 153–164 CrossRef PubMed
.
- S. L. Klosterhaus, R. Grace and M. C. Hamilton,
et al., Method validation and reconnaissance of pharmaceuticals, personal care products, and alkylphenols in surface waters, sediments, and mussels in an urban estuary, Environ Int, 2013, 54, 92–99 CrossRef CAS PubMed
.
-
USEPA, Method 1694: Pharmaceuticals and Personal Care Products in Water, Soil, Sediment, and Biosolids by HPLC/MS/MS: U.S. Environmental Protection Agency. 2007 Search PubMed
.
-
C. N. Ihudiebube-Splendor and P. C. Chikeme, A Descriptive Cross-Sectional Study: Practical and Feasible Design in Investigating Health Care–Seeking Behaviors of Undergraduates: SAGE Publications Ltd. 2020 Search PubMed
.
- G. W. Fehrenbach, R. Pogue and F. Carter,
et al., Implications for the seafood industry, consumers and the environment arising from contamination of shellfish with pharmaceuticals, plastics and potentially toxic elements: a case study from Irish waters with a global orientation, Sci. Total Environ., 2022, 157067 CrossRef CAS PubMed
.
- E. O. Otachi, W. Körner and A. Avenant-Oldewage,
et al., Trace elements in sediments, blue spotted tilapia Oreochromis leucostictus (Trewavas, 1933) and its parasite Contracaecum multipapillatum from Lake Naivasha, Kenya, including a comprehensive health risk analysis, Environ. Sci. Pollut. Res., 2014, 21(12), 7339–7349 CrossRef CAS PubMed
.
- N. Vragović, D. Bažulić and B. Njari, Risk assessment of streptomycin and tetracycline residues in meat and milk on Croatian market, Food Chem. Toxicol., 2011, 49(2), 352–355 CrossRef PubMed
.
- A. M. Ali, H. T. Rønning and L. K. Sydnes,
et al., Detection of PPCPs in marine organisms from contaminated coastal waters of the Saudi Red Sea, Sci. Total Environ., 2018, 621, 654–662 CrossRef CAS PubMed
.
- A. Sengar and A. Vijayanandan, Human health and ecological risk assessment of 98 pharmaceuticals and personal care products (PPCPs) detected in Indian surface and wastewaters, Sci. Total Environ., 2022, 807, 150677 CrossRef CAS PubMed
.
- H. Shaaban and A. Mostafa, Simultaneous determination of antibiotics residues in edible fish muscle using eco-friendly SPE-UPLC-MS/MS: occurrence, human dietary exposure and health risk assessment for consumer safety, Toxicol Rep, 2023, 10, 1–10 CrossRef CAS PubMed
.
- F. V. Mello, S. C. Cunha and F. H. Fogaça,
et al., Occurrence of pharmaceuticals in seafood from two Brazilian coastal areas: implication for human risk assessment, Sci. Total Environ., 2022, 803, 149744 Search PubMed
.
- J. B. Collins, J. Song and R. C. Mahabir, Onset and duration of intradermal mixtures of bupivacaine and lidocaine with epinephrine, Scand. J. Plast. Reconstr. Surg., 2013, 21(1), 51–53 Search PubMed
.
- A. J. Ebele, M. Abou-Elwafa Abdallah and S. Harrad, Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment, Emerging Contam., 2017, 3(1), 1–16 CrossRef
.
- C. Matthee, A. R. Brown and A. Lange,
et al., Factors Determining the Susceptibility of Fish to Effects of Human Pharmaceuticals, Environ. Sci. Technol., 2023, 57(24), 8845–8862 CrossRef CAS PubMed
.
- V. W. O. Wanjeri, E. Okuku and J. C. Ngila,
et al., Distribution of pharmaceuticals in marine surface sediment and macroalgae (ulvophyceae) around Mombasa peri-urban creeks and Gazi Bay, Kenya, Environ. Sci. Pollut. Res., 2025, 4103–4123 CrossRef CAS PubMed
.
- B. W. Schwab, E. P. Hayes and J. M. Fiori,
et al., Human pharmaceuticals in US surface waters: a human health risk assessment, Regul. Toxicol. Pharmacol., 2005, 42(3), 296–312 CrossRef CAS PubMed
.
- R. S. Prosser and P. K. Sibley, Human health risk assessment of pharmaceuticals and personal care products in plant tissue due to biosolids and manure amendments, and wastewater irrigation, Environ. Int., 2015, 75, 223–233 CrossRef CAS PubMed
.
- Y. Amangelsin, Y. Semenova and M. Dadar,
et al., The impact of tetracycline pollution on the aquatic environment and removal strategies, Antibiotics, 2023, 12(3), 440 Search PubMed
.
- D. Muir, D. Simmons and X. Wang,
et al., Bioaccumulation of pharmaceuticals and personal care product chemicals in fish exposed to wastewater effluent in an urban wetland, Sci. Rep., 2017, 7(1), 16999 CrossRef PubMed
.
- J. O. Osuoha, B. O. Anyanwu and C. Ejileugha, Pharmaceuticals and personal care products as emerging contaminants: need for combined treatment strategy, J. Hazard. Mater. Adv., 2023, 9, 100206 CAS
.
- R. Moreno-González, S. Rodriguez-Mozaz and M. Gros,
et al., Seasonal distribution of pharmaceuticals in marine water and sediment from a mediterranean coastal lagoon (SE Spain), Environ. Res., 2015, 138, 326–344 CrossRef PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4va00392f |
| This journal is © The Royal Society of Chemistry 2025 |



