Open Access Article
Open Access ArticleA zero-dimensional hybrid halide with superior water resistance for high-efficiency X-ray scintillation and solid-state lighting†
Yu-Yin
Wang
 ab,
Yue
Wang
a,
Xin-Wei
Du
c,
Xue-Hui
Zhang
a,
Lin
Zhao
c,
Bing-Rong
Yan
c,
Ruo-Fan
Zhang
c,
De-Long
Liu
a,
Jing
Zhang
d and
Guoming
Lin
ab,
Yue
Wang
a,
Xin-Wei
Du
c,
Xue-Hui
Zhang
a,
Lin
Zhao
c,
Bing-Rong
Yan
c,
Ruo-Fan
Zhang
c,
De-Long
Liu
a,
Jing
Zhang
d and
Guoming
Lin
 *be
*be
aSchool of Chemistry, Chemical Engineering and Materials, Jining University, Qufu, Shandong 273155, P. R. China
bSchool of Future Membrane Technology, Fuzhou University, Fuzhou, 350108, China
cSchool of Resource & Environment and Safety Engineering, Jining University, Qufu, Shandong 273155, P. R. China
dSchool of Physics and Chemistry, Hunan First Normal University, Changsha City, Hunan 410205, P. R. China
eDepartment of Physics, National University of Singapore, Singapore 117551, Singapore. E-mail: lingmdbs@nus.edu.sg
First published on 17th March 2025
Abstract
In recent years, hybrid metal halides have gained considerable attention for optoelectronic applications due to their outstanding photophysical properties, despite challenges with stability. In this study, we present the design and synthesis of a highly stable and efficient zero-dimensional (0D) hybrid copper(I) halide, [FBZPA]4Cu5Br13 (FBZPA = protonated 1-(4-fluorobenzyl)piperazine)), for advanced scintillation and solid-state lighting applications. This material exhibits efficient yellow light emission with a photoluminescence quantum yield of 88.5%, driven by radiative recombination of self-trapped excitons, which is facilitated by structural deformation and strong electron–phonon coupling within the 0D structure. [FBZPA]4Cu5Br13 shows excellent scintillation properties, including a high light yield (∼39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 photons MeV−1), a low detection limit (0.102 μGyair s−1), and a high spatial resolution (15 lp mm−1), making it an ideal candidate for high quality X-ray imaging. Additionally, we fabricated a white light-emitting diode (WLED) by combining [FBZPA]4Cu5Br13 with a commercial blue phosphor on a UV chip. The WLED exhibited a high color rendering index of 90 with stable emission. It demonstrates remarkable stability, retaining its structure and optical properties after exposure to water, and intense light, without requiring encapsulation or chemical modifications. This study highlights [FBZPA]4Cu5Br13 as a promising material for next-generation scintillation and solid-state lighting applications.
100 photons MeV−1), a low detection limit (0.102 μGyair s−1), and a high spatial resolution (15 lp mm−1), making it an ideal candidate for high quality X-ray imaging. Additionally, we fabricated a white light-emitting diode (WLED) by combining [FBZPA]4Cu5Br13 with a commercial blue phosphor on a UV chip. The WLED exhibited a high color rendering index of 90 with stable emission. It demonstrates remarkable stability, retaining its structure and optical properties after exposure to water, and intense light, without requiring encapsulation or chemical modifications. This study highlights [FBZPA]4Cu5Br13 as a promising material for next-generation scintillation and solid-state lighting applications.
Introduction
Scintillator materials, which absorb high-energy radiation such as X-rays and gamma rays and emit light in response, are essential components in medical diagnostics, security screening, and industrial non-destructive testing.1–4 Organic scintillators are attractive due to their low cost and ease of customization, and they can be used to fabricate large, flexible devices.5,6 However, their primary composition of hydrocarbons leads to poor X-ray absorption, resulting in low light emission efficiency and reduced durability.7–9 Commercial inorganic scintillators like thallium-doped cesium iodide (CsI-Tl),10 terbium-doped gadolinium oxysulfide (Gd2O2S-Tb),11 and bismuth germanate (BGO)12 are widely used, but come with limitations such as complex preparation processes, toxicity concerns, and high costs. Recently, low-dimensional organic–inorganic hybrid halides have emerged as promising alternatives.13–19 These materials combine the advantages of organic components, such as flexibility and customization, with the superior X-ray absorption properties of heavy metal halides. This combination has the potential to overcome the limitations of both organic and conventional scintillators.Lead halide perovskites have garnered significant attention for their outstanding optical and electronic properties.20–26 However, their practical application is limited due to concerns over lead toxicity and material instability. To overcome these challenges, researchers have focused on lead-free, low-dimensional metal halides for light-emitting applications. These materials show great promise, offering high photoluminescence quantum yield (PLQY), tunable optical bandgaps, large Stokes shifts and improved stability.27–29 The combination of eco-friendliness, affordability, tunable properties, high PLQY, and strong light output positions Cu(I)-based zero-dimensional (0D) halides as promising candidates for next-generation X-ray scintillators, capable of delivering high precision and reliability in advanced imaging applications. Cu's abundance makes these materials affordable and suitable for large-scale production. Beyond their potential in full-color displays and solid-state lighting, lead-free metal halides have also been proven to be effective as X-ray scintillators.
Recent studies have identified copper(I)-based halides as promising eco-friendly and efficient materials for X-ray scintillation applications.30–34 Researchers have developed various structures, including 0D and two-dimensional (2D) hybrids, which exhibit strong PL and tunable optical properties. Liu et al. reported broadband green emission in mononuclear Cu(I) halide complexes (PPh3)2(3-methylpyridine[CuX] (X = Cl, Br, I) for high-resolution X-ray imaging.35 Yang et al. presented a 2D Cu(I) iodide hybrid (cis-2,6-dimethylmorpholine)4[Cu4I8]·2H2O, which demonstrated excellent X-ray imaging performance, high light yield, moisture resistance, and a resolution of 108 line pairs per millimeter.36 Lian et al. introduced two 0D Cu(I) halide materials (tetrabutylammonium cation)CuX2 (X = Cl, Br), emitting bright green and blue light with high efficiency, making them promising for flexible X-ray imaging due to their strong luminescence and reabsorption-free properties.37 Wang et al. synthesized Cu(I) halide crystals, (C2H8N)3Cu2I5 and (C2H8N)4Cu2Br6, effective for X-ray imaging, capable of detecting very low radiation doses and achieving a resolution of 10 line pairs per millimeter.38 However, despite these advantages, Cu(I)-based halides still face challenges such as low X-ray light yield, and low moisture resistance that limit their practical applications. Therefore, further research is necessary to overcome these limitations and fully realize the potential of Cu-based halide scintillators in advanced X-ray imaging technologies.
In recent years, researchers have actively explored the incorporation of fluorinated organic cations to enhance the stability of organic–inorganic hybrid materials.39,40 Fluorinated salts have been widely utilized in functional materials due to their high thermal stability and excellent heat resistance.17,18 Additionally, the introduction of fluorinated organic molecules has been shown to improve the water resistance and thermal stability of hybrid membranes. In this work, we synthesized a Cu(I)-based metal halide hybrid, [FBZPA]4Cu5Br13 (FBZPA: protonated 1-(4-fluorobenzyl)piperazine), which exhibits a high PLQY of 88.49%, strong X-ray light yield, prolonged radiation stability, and excellent water resistance. In its structure, one [CuBr3]2− triangular unit is linked to one or two [CuBr4]3− tetrahedral units by their edges, forming CuBr clusters. FBZPA was selected from a wide range of commercial sources due to its desirable structural characteristics and its well-documented potential for enhancing stability in hybrid materials. These CuBr clusters are further surrounded by highly stable, hydrophobic, fluorinated aromatic nitrogen-based organic cations, contributing to the material's exceptional water resistance. The high PLQY and large Stokes shift make this halide a high-performance X-ray scintillator. Additionally, the broad excitation band and excellent water resistance make [FBZPA]4Cu5Br13 highly suitable for solid-state lighting applications. This work offers a valuable platform for exploring the relationship between the structure and properties of bulk crystalline materials, advancing research and applications for 0D Cu(I) halide luminescent materials.
Results and discussion
Composition and structure
Bulk single crystals of [FBZPA]4Cu5Br13 were synthesized using a solvent evaporation crystallization method, as described in the ESI.† Single-crystal X-ray diffraction analysis confirmed that [FBZPA]4Cu5Br13 crystallizes in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group and exhibits a 0D structure. The lattice parameters are a = 10.1218(5) Å, b = 10.2760(5) Å, c = 32.8442(16) Å, with angles α = 86.595(2)°, β = 84.229(2)°, and γ = 89.055(2)° (Fig. 1a and Table S1, ESI†). In this halide structure, Cu+ cations are coordinated by either three or four Br− anions, forming [CuBr3]2− trigonal planar units and [CuBr4]3− tetrahedral units, with Cu–Br bond lengths ranging from 2.3033(15) to 2.6347(16) Å (Fig. 1b). These triangular and tetrahedral units are edge-linked to form two types of clusters in 1
space group and exhibits a 0D structure. The lattice parameters are a = 10.1218(5) Å, b = 10.2760(5) Å, c = 32.8442(16) Å, with angles α = 86.595(2)°, β = 84.229(2)°, and γ = 89.055(2)° (Fig. 1a and Table S1, ESI†). In this halide structure, Cu+ cations are coordinated by either three or four Br− anions, forming [CuBr3]2− trigonal planar units and [CuBr4]3− tetrahedral units, with Cu–Br bond lengths ranging from 2.3033(15) to 2.6347(16) Å (Fig. 1b). These triangular and tetrahedral units are edge-linked to form two types of clusters in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratios, which are spatially separated by the organic [FBZPA]2+ cations (Fig. 1c). The presence of fluorine atoms within the [FBZPA]2+ cations enhances hydrophobicity and stability of the crystal structure. The [FBZPA]2+ cations further create a layered structure that offers additional protection to the [CuBr3]2− and [CuBr4]3− clusters against oxidation and moisture-induced degradation. Powder X-ray diffraction (PXRD) analysis of the ground crystals showed a close match with the simulated pattern, indicating high purity and uniformity of the synthesized material (Fig. 1d). Elemental mapping using energy-dispersive X-ray spectroscopy (EDS) showed a uniform distribution of Cu, F, Br, C, and N throughout the crystals (Fig. 1e). Additionally, X-ray photoelectron spectroscopy (XPS) confirmed the presence of Cu in the expected +1 oxidation state, with the high-resolution Cu 3d spectrum exhibiting spin–orbit splitting peaks consistent with previously reported CuBr powder41 (Fig. S1, ESI†).
2 ratios, which are spatially separated by the organic [FBZPA]2+ cations (Fig. 1c). The presence of fluorine atoms within the [FBZPA]2+ cations enhances hydrophobicity and stability of the crystal structure. The [FBZPA]2+ cations further create a layered structure that offers additional protection to the [CuBr3]2− and [CuBr4]3− clusters against oxidation and moisture-induced degradation. Powder X-ray diffraction (PXRD) analysis of the ground crystals showed a close match with the simulated pattern, indicating high purity and uniformity of the synthesized material (Fig. 1d). Elemental mapping using energy-dispersive X-ray spectroscopy (EDS) showed a uniform distribution of Cu, F, Br, C, and N throughout the crystals (Fig. 1e). Additionally, X-ray photoelectron spectroscopy (XPS) confirmed the presence of Cu in the expected +1 oxidation state, with the high-resolution Cu 3d spectrum exhibiting spin–orbit splitting peaks consistent with previously reported CuBr powder41 (Fig. S1, ESI†).
Photoluminescence properties of [FBZPA]4Cu5Br13
The [FBZPA]4Cu5Br13 crystals are colorless under natural light but emit bright yellow light when exposed to a 365 nm ultraviolet (UV) lamp (Fig. 2a). When excited at 331 nm, the crystals exhibit broad-spectrum light emission centered at 564 nm, with a full width at half-maximum (FWHM) of 127 nm and a significant Stokes shift of 233 nm. The UV-vis absorption spectrum shows a sharp absorption onset, indicative of direct band-edge absorption (Fig. S2, ESI†), and a direct band gap of 3.30 eV was determined using a Tauc plot. The Commission Internationale de l'Éclairage (CIE) chromaticity coordinates of the crystals were found to be (0.43, 0.52), placing them in the yellow region of the CIE chromaticity diagram (Fig. 2b). The photoluminescence excitation (PLE) spectrum shows that [FBZPA]4Cu5Br13 can be excited by UV light within the 240–380 nm range, with an optimal excitation wavelength at 331 nm (Fig. 2c). The intense luminescence of the [FBZPA]4Cu5Br13 crystals is attributed to their remarkably high PLQY, reaching 88.49%. (Fig. 2d). To explore the mechanism of the broadband emission, excitation wavelength-dependent PLE spectra were analyzed. These spectra exhibited consistent shapes across emission wavelengths from 450 to 700 nm and excitation wavelengths from 270 to 370 nm, indicating that the broadband emission originates from the same excited states. This rules out the possibility of Cu+ ion luminescence arising from different excited-state levels. PL decay times at 80 K and 300 K were measured to be 107.6 μs and 88.3 μs, respectively, suggesting that the emission involves long-lived excited states, with non-radiative decay processes increasing at higher temperatures (Fig. 2e). Bulk and micrometer-scale crystals exhibit almost identical PL suggesting that the luminescence properties of the materials are primarily determined by their intrinsic structure, despite being significantly affected by size or surface effects (Fig. 2f). The broad FWHM, prolonged lifetime, and significant Stokes shift suggest that the broadband emission originates from the radiative recombination of self-trapped excitons (STEs), a behavior commonly observed in various 0D hybrid metal halide structures. This claim is further supported by the linear relationship between excitation power density and emission intensity (Fig. 2g). To further explore the PL mechanism, a temperature-dependent PL emission spectroscopy study was conducted across a range of 80 K to 300 K (Fig. 2h). A slight variation in emission wavelength with temperature is likely attributed to minor shifts in the bandgap and thermal expansion effects, both of which minimally affect the overall emission characteristics. Additionally, the emission intensity consistently increased as the temperature decreased from 300 K to 80 K, indicating a reduction in thermal quenching and non-radiative recombination processes. The temperature-dependent emission behavior is influenced by electron–phonon coupling, which can cause an enhancement of the emission at lower temperatures by suppressing non-radiative pathways.The thermal activation energy (Ea) was determined by fitting the integrated emission intensity as a function of temperature using the Arrhenius equation:
 | (1) |
Radioluminescence properties of [FBZPA]4Cu5Br13
The scintillation properties of the synthesized [FBZPA]4Cu5Br13 single crystals were thoroughly evaluated by analyzing factors such as steady-state scintillation yield, X-ray intensity-dependent luminescence, detection limits, and X-ray imaging. Under X-ray excitation, the optically transparent bulk crystals exhibited a yellow emission (Fig. 3a). The steady-state scintillation light yield of [FBZPA]4Cu5Br13 was determined by comparing the integrated radioluminescence (RL) intensity with that of LuAG:Ce, a reference scintillator with a known light yield of 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 photons MeV−1. Both samples were prepared with equal thicknesses of 3 mm and identical surface areas to ensure an accurate comparison. The light yield for the [FBZPA]4Cu5Br13 sample was then calculated using the following formula:
000 photons MeV−1. Both samples were prepared with equal thicknesses of 3 mm and identical surface areas to ensure an accurate comparison. The light yield for the [FBZPA]4Cu5Br13 sample was then calculated using the following formula: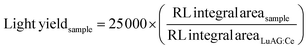 | (2) |
A scintillation yield of approximately 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 photons MeV−1 was obtained for [FBZPA]4Cu5Br13. The RL emission peak closely aligned with the PL emission, suggesting minimal contribution from defect luminescence during the scintillation process. Although the calculated X-ray absorption coefficient of [FBZPA]4Cu5Br13 is lower than that of LuAG:Ce (Fig. S3, ESI†), its higher RL light yield enables it to exhibit superior luminescence performance. Additionally, the RL intensity increased linearly with strong X-ray dose rates ranging from approximately 5000 to 55
100 photons MeV−1 was obtained for [FBZPA]4Cu5Br13. The RL emission peak closely aligned with the PL emission, suggesting minimal contribution from defect luminescence during the scintillation process. Although the calculated X-ray absorption coefficient of [FBZPA]4Cu5Br13 is lower than that of LuAG:Ce (Fig. S3, ESI†), its higher RL light yield enables it to exhibit superior luminescence performance. Additionally, the RL intensity increased linearly with strong X-ray dose rates ranging from approximately 5000 to 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μGyair s−1 (Fig. 3b), demonstrating good proportionality and making it suitable for quantitative X-ray detection. X-ray dose rates were calibrated with an ion chamber dosimeter to ensure accuracy. Detection limits were established by comparing the RL spectra obtained under dark conditions with those measured at specific, controlled radiation levels. Under low X-ray dose rates, the RL intensity remains linearly correlated with the dose rates (Fig. 3c). With a signal-to-noise ratio (SNR) of 3, the detection limit for [FBZPA]4Cu5Br13 was calculated to be 0.102 μGyair s−1. Remarkably, the detection limit is just 1/55th of the threshold (5.5 μGyair s−1) needed for X-ray medical diagnostics, highlighting its exceptional sensitivity. The spatial resolution assessment of the [FBZPA]4Cu5Br13 scintillation screen revealed a resolution of 15.0 lp mm−1 at a modulation transfer function (MTF) of 0.2 (Fig. 3d), which is comparable to those of other metal halide-based scintillators. By positioning a power conversion module and a communication interface module circuit board in the X-ray imaging platform and utilizing [FBZPA]4Cu5Br13 microparticles as a thin scintillation screen on transparent glass, we demonstrated its excellent X-ray imaging performance (Fig. 3e and f). These results highlight the potential of [FBZPA]4Cu5Br13 as a scintillator for radiography and advanced imaging applications.
000 μGyair s−1 (Fig. 3b), demonstrating good proportionality and making it suitable for quantitative X-ray detection. X-ray dose rates were calibrated with an ion chamber dosimeter to ensure accuracy. Detection limits were established by comparing the RL spectra obtained under dark conditions with those measured at specific, controlled radiation levels. Under low X-ray dose rates, the RL intensity remains linearly correlated with the dose rates (Fig. 3c). With a signal-to-noise ratio (SNR) of 3, the detection limit for [FBZPA]4Cu5Br13 was calculated to be 0.102 μGyair s−1. Remarkably, the detection limit is just 1/55th of the threshold (5.5 μGyair s−1) needed for X-ray medical diagnostics, highlighting its exceptional sensitivity. The spatial resolution assessment of the [FBZPA]4Cu5Br13 scintillation screen revealed a resolution of 15.0 lp mm−1 at a modulation transfer function (MTF) of 0.2 (Fig. 3d), which is comparable to those of other metal halide-based scintillators. By positioning a power conversion module and a communication interface module circuit board in the X-ray imaging platform and utilizing [FBZPA]4Cu5Br13 microparticles as a thin scintillation screen on transparent glass, we demonstrated its excellent X-ray imaging performance (Fig. 3e and f). These results highlight the potential of [FBZPA]4Cu5Br13 as a scintillator for radiography and advanced imaging applications.
Photophysical mechanism of [FBZPA]4Cu5Br13
To gain deeper insight into the photophysical properties of [FBZPA]4Cu5Br13, density functional theory (DFT) calculations were conducted. The electronic structure analysis revealed that the material has a direct band gap, consistent with the UV-vis analysis, with both the valence band maximum (VBM) and the conduction band minimum (CBM) located at the R-point in the Brillouin zone. The calculated band gap is 3.14 eV, slightly lower than the experimentally measured value of 3.30 eV (Fig. 4a). The total density of state (TDOS) and partial DOS calculations indicated that the VBM is mainly composed of Cu 3d, Br 4p, and the ligand's N 2p orbitals, while the CBM is primarily comprised of Cu 4s orbitals and contributions from the organic ligand (Fig. 4b). This suggests that both the inorganic Cu–Br clusters and the organic [FBZPA]2+ cations play a role in the electronic transitions. The charge density of the highest occupied molecular orbital (HOMO) revealed that the charge is primarily concentrated around the Br and Cu atoms as well as the ligand, whereas the charge density of the lowest unoccupied molecular orbital (LUMO) is mainly localized on the ligand (Fig. 4c and d). This charge distribution is consistent with the DOS analysis, indicating significant charge transfer between the inorganic clusters and organic cations upon excitation.Moreover, calculations of bond lengths and bond angles in both the ground and excited states reveal significant structural changes upon excitation (Fig. 4e and f). In the excited state, the longer Cu–Br bond lengths increase, while the shorter Cu–Br bond lengths decrease compared to the ground state. Additionally, the C–N bridging bond in the ligand shortens upon excitation. These changes suggest that the [CuBr4]3− tetrahedra undergo Jahn–Teller distortion, a phenomenon typically associated with d9 Cu(II) complexes, but also present in Cu(I) systems due to pseudo-Jahn–Teller effects. This distortion leads to the formation of STEs, which are responsible for the observed broadband emission, characterized by a large Stokes shift and extended lifetimes. The distortion localizes the excited state, reducing non-radiative recombination and enhancing the PL efficiency. These theoretical insights align well with experimental observations, such as high PLQY, significant Stokes shift, and temperature-dependent emission properties. The interaction of structural distortion and charge transfer between the inorganic clusters and organic cations plays a crucial role in the photophysical behavior of [FBZPA]4Cu5Br13, making it a promising candidate for X-ray scintillation and optoelectronic applications.
Stability properties of [FBZPA]4Cu5Br13
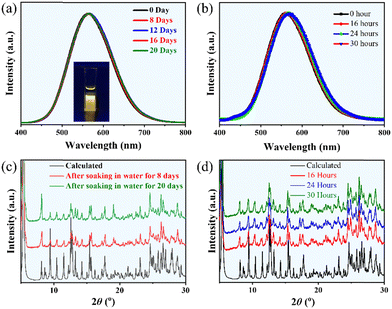
[FBZPA]4Cu5Br13 exhibits exceptional structural and spectral stability, maintaining its integrity after extended soaking in regular DI water for 20 days or subjecting to intense light irradiation (300 W Xe lamp) for 30 hours (Fig. 5a and b). Additionally, XPS analysis confirmed that Cu remains in the expected +1 oxidation state even after 20 days of water soaking (Fig. S1, ESI†). This remarkable stability is reflected in only minor shifts in emission bands, a slight reduction in PL intensity, and minimal changes in PXRD patterns (Fig. 5c and d). The exceptional water stability of [FBZPA]4Cu5Br13 can be attributed to its unique structural characteristics. Specifically, the CuBr clusters are encapsulated by organic [FBZPA]2+ cations, which not only enhance the overall structural integrity but also form hydrogen bonds that shield the Cu(I)-based core from hydrolysis. Furthermore, the hydrophobic nature of the fluorophenyl group in the [FBZPA]2+ cation significantly reduces water-induced degradation by limiting moisture penetration. In summary, [FBZPA]4Cu5Br13 maintains its structural and optical properties across various challenging environments, including water and intense light exposure, while consistently emitting yellow light.Performance of the WLED
The efficient and stable luminescence of [FBZPA]4Cu5Br13 prompted us to explore its potential in solid-state lighting applications. A WLED device was fabricated through coating a mixture of [FBZPA]4Cu5Br13 and a commercial blue phosphor, (BaMgAl10O17:Eu2+), onto a UV chip emitting at 365 nm (Fig. 6a). The resulting WLED emitted bright light with a correlated color temperature (CCT) of 6012 K, a high color rendering index (CRI) of 90, and CIE chromaticity coordinates of (0.32, 0.35), closely resembling those of natural sunlight (Fig. 6b). The CRI of this WLED significantly outperformed those of the commercially available YAG:Ce3+-coated blue chips (CRI < 80), highlighting the superior light quality. Both the emission peaks and the overall spectral shape remained stable as the drive current increased, further confirming the robustness of the WLED device (Fig. 6c). During continuous operation at 20 mA, no notable drop in luminous efficiency was observed, demonstrating its excellent emission and thermal stability (Fig. 6d). This performance, combined with the material's stability, positions [FBZPA]4Cu5Br13 as a promising candidate for advanced optoelectronic applications, including efficient and durable WLED devices.Conclusion
We successfully designed and synthesized a novel 0D hybrid Cu(I) halide, [FBZPA]4Cu5Br13, which functions as a highly efficient yellow light emitter with a PLQY of 88.5%. Both experimental characterization and theoretical analysis revealed that the yellow emission results from the radiative recombination of STEs, driven by structural deformation and strong EP coupling within the 0D structure. Remarkably, [FBZPA]4Cu5Br13 demonstrates exceptional chemical and physical stability under extreme conditions, including exposure to water, and intense light, without the need for encapsulation or chemical modifications. This robustness addresses the common weaknesses of organic–inorganic hybrid halides, particularly their poor water resistance. Additionally, [FBZPA]4Cu5Br13 exhibits outstanding scintillation properties making it highly suitable for advanced X-ray imaging applications. Its high light yield, low detection limit, and superior spatial resolution position [FBZPA]4Cu5Br13 as a strong candidate for such X-ray imaging applications. Additionally, we fabricated a WLED by combining [FBZPA]4Cu5Br13 with a commercial blue phosphor on a UV chip. The resulting WLED exhibited a high CRI of 90 and CIE chromaticity coordinates close to those of natural sunlight, with stable emission under varying drive currents and continuous operation. In summary, this work opens up new possibilities for eco-friendly, water-resistant materials with excellent scintillation performance, positioning [FBZPA]4Cu5Br13 as an ideal candidate for X-ray imaging and WLED applications. From the perspective of X-ray scintillation applications, iodide complexes with higher X-ray attenuation capabilities would be more suitable. Exploring such materials in future studies could be a promising direction.Data availability
All data supporting the findings of this study are available within the manuscript and its ESI† file. Additional datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work is financially supported by the Natural Science Foundation of China (Grant No. 22201099 and 22101282), Shandong Provincial Natural Science Foundation (Grant No. ZR2021QB204, ZR2023QB096), and Jining University 2019, 2020 “100 Outstanding Talents” support program cultivation project (2022ZYRC1). This work is also supported by University Feature Laboratory for Energy Conversion and Nanocatalysis of Shandong Province.References
- C. Greskovich and S. Duclos, Ceramic scintillators, Annu. Rev. Mater. Sci., 1997, 27, 69–88 CAS.
- K. Han, K. Sakhatskyi, J. Jin, Q. Zhang, M. V. Kovalenko and Z. Xia, Seed-crystal-induced cold sintering toward metal halide transparent ceramic scintillators, Adv. Mater., 2022, 34, 2110420 CAS.
- Y. He, I. Hadar and M. G. Kanatzidis, Detecting ionizing radiation using halide perovskite semiconductors processed through solution and alternative methods, Nat. Photonics, 2022, 16, 14–26 CAS.
- M. Nikl and A. Yoshikawa, Recent R&D trends in inorganic single-crystal scintillator materials for radiation detection, Adv. Opt. Mater., 2015, 3, 463–481 CAS.
- T. J. Hajagos, C. Liu, N. J. Cherepy and Q. Pei, High-Z sensitized plastic scintillators: a review, Adv. Mater., 2018, 30, 1706956 CrossRef PubMed.
- M. Koshimizu, Recent progress of organic scintillators, Jpn. J. Appl. Phys., 2022, 62, 010503 CrossRef.
- H. Chen, M. Lin, Y. Zhu, D. Zhang, J. Chen, Q. Wei, S. Yuan, Y. Liao, F. Chen and Y. Chen, Halogen-bonding boosting the high performance X-ray imaging of organic scintillators, Small, 2024, 20, 2307277 CrossRef CAS PubMed.
- X. Du, S. Zhao, L. Wang, H. Wu, F. Ye, K.-H. Xue, S. Peng, J. Xia, Z. Sang and D. Zhang, Efficient and ultrafast organic scintillators by hot exciton manipulation, Nat. Photonics, 2024, 18, 162–169 Search PubMed.
- M. Sénoville, F. Delaunay, M. Pârlog, N. Achouri and N. Orr, Neutron-γ discrimination with organic scintillators: Intrinsic pulse shape and light yield contributions, Nucl. Instrum. Methods Phys. Res., Sect. A, 2020, 971, 164080 Search PubMed.
- V. Nagarkar, T. Gupta, S. Miller, Y. Klugerman, M. Squillante and G. Entine, Structured CsI (Tl) scintillators for X-ray imaging applications, IEEE Trans. Nucl. Sci., 1998, 45, 492–496 CAS.
- W. Rossner, M. Ostertag and F. Jermann, Properties and applications of gadolinium oxysulfide based ceramic scintillators, Electrochem. Soc. Proc., 1999, 98, 94–187 Search PubMed.
- Z. Cho and M. Farukhi, Bismuth germanate as a potential scintillation detector in positron cameras, J. Nucl. Med., 1977, 18, 840–844 CAS.
- V. Morad, Y. Shynkarenko, S. Yakunin, A. Brumberg, R. D. Schaller and M. V. Kovalenko, Disphenoidal zero-dimensional lead, tin, and germanium halides: highly emissive singlet and triplet self-trapped excitons and X-ray scintillation, J. Am. Chem. Soc., 2019, 141, 9764–9768 CAS.
- S. Wu, B. Zhou and D. Yan, Low-dimensional organic metal halide hybrids with excitation-dependent optical waveguides from visible to near-infrared emission, ACS Appl. Mater. Interfaces, 2021, 13, 26451–26460 CAS.
- Y. Wang, G. Lin, B. Su, X. Wang, S. Wang, Z. Cheng, D. Li, X.-W. Lei and C.-Y. Yue, Centimeter-sized lead-free iodide-based hybrid double perovskite single crystals for efficient X-ray photoresponsivity, Dalton Trans., 2022, 51, 10234–10239 RSC.
- Y. Wang, S. Zhang, Y. Wang, J. Yan, X. Yao, M. Xu, X.-W. Lei, G. Lin and C.-Y. Yue, 0D triiodide hybrid halide perovskite for X-ray detection, Chem. Commun., 2023, 59, 9239–9242 CAS.
- Y.-Y. Wang, Y. Feng, X.-T. Liu, L.-Y. Cao, Q.-Y. Xu, H. Qu, T. Zhao, Y. Li and G. Lin, Organic–Inorganic Hybrid Halide X-ray Scintillator with High Antiwater Stability, Inorg. Chem., 2024, 63, 16224–16232 CAS.
- Y.-Y. Wang, G.-H. Jia, M.-R. Huo, M.-T. Cheng, X.-Y. Chen, A.-R. Chang, J. Zhang, Y.-Y. Li and G.-M. Lin, A lead-free zero-dimensional hybrid antimony halide perovskite X-ray scintillator with exceptional emission efficiency and excellent stability as a highly sensitive fluorescent probe, Inorg. Chem. Front., 2024, 11, 5034–5042 Search PubMed.
- Y. Y. Wang, W. T. Song, X. R. Yao, X. Y. Chen, M. T. Cheng, G. H. Jia, H. Z. Liu, X. T. Liu, H. H. Yang and C. H. Qi, A New Polar Lead-Free Hybrid Halide X-Ray Scintillator, Adv. Opt. Mater., 2024, 12, 2400190 CAS.
- J. Han, Y. Li, P. Shen, Q. Li, Y. Liu, Y. Wei, P. Zhang and Z. Quan, Pressure-Induced Free Exciton Emission in a Quasi-Zero-Dimensional Hybrid Lead Halide, Angew. Chem., 2024, 136, e202316348 Search PubMed.
- L. Liu, Y. Bai, X. Zhang, Y. Shang, C. Wang, H. Wang, C. Zhu, C. Hu, J. Wu and H. Zhou, Cation diffusion guides hybrid halide perovskite crystallization during the gel stage, Angew. Chem., 2020, 132, 6035–6043 Search PubMed.
- Y. Liu, Y. Zhang, Z. Yang, H. Ye, J. Feng, Z. Xu, X. Zhang, R. Munir, J. Liu and P. Zuo, Multi-inch single-crystalline perovskite membrane for high-detectivity flexible photosensors, Nat. Commun., 2018, 9, 5302 CAS.
- G. Xing, N. Mathews, S. Sun, S. S. Lim, Y. M. Lam, M. Grätzel, S. Mhaisalkar and T. C. Sum, Long-range balanced electron-and hole-transport lengths in organic-inorganic CH3NH3PbI3, Science, 2013, 342, 344–347 CrossRef CAS PubMed.
- Y. Wang, C. Sun, B. Su, X. Li, X. Meng, H. Lou, Z. Cheng, Y. Wang and G. Lin, Highly efficient broadband white-light emission in two-dimensional semi-conductive hybrid lead chlorides, Dalton Trans., 2022, 51, 14930–14936 RSC.
- G. Lin, Y. Lin, R. Cui, H. Huang, X. Guo, C. Li, J. Dong, X. Guo and B. Sun, An organic–inorganic hybrid perovskite logic gate for better computing, J. Mater. Chem. C, 2015, 3, 10793–10798 Search PubMed.
- G. Lin, Y. Lin, H. Huang, R. Cui, X. Guo, B. Liu, J. Dong, X. Guo and B. Sun, Novel exciton dissociation behavior in tin-lead organohalide perovskites, Nano Energy, 2016, 27, 638–646 Search PubMed.
- J. Jin, S. Geng, K. Han, Z. Xiao and Z. Xia, Blue-Light-Excitable Red-to-Near Infrared Photoluminescence in 0D Antimony (III) Bromide Hybrids for Supplemental Lighting, Adv. Opt. Mater., 2024, 2303178 CrossRef CAS.
- Z. Li, G. Song, Y. Li, L. Wang, T. Zhou, Z. Lin and R.-J. Xie, Realizing tunable white light emission in lead-free indium (III) bromine hybrid single crystals through antimony (III) cation doping, J. Phys. Chem. Lett., 2020, 11, 10164–10172 CrossRef CAS PubMed.
- F. Locardi, M. Cirignano, D. Baranov, Z. Dang, M. Prato, F. Drago, M. Ferretti, V. Pinchetti, M. Fanciulli and S. Brovelli, Colloidal synthesis of double perovskite Cs2AgInCl6 and Mn-doped Cs2AgInCl6 nanocrystals, J. Am. Chem. Soc., 2018, 140, 12989–12995 CrossRef CAS PubMed.
- J. H. Han, T. Samanta, Y. M. Park, H. J. Kim, N. S. Manikanta Viswanath, H. W. Kim, B. K. Cha, S. B. Cho and W. B. Im, Highly stable zero-dimensional lead-free metal halides for X-ray imaging, ACS Energy Lett., 2022, 8, 545–552 CrossRef.
- D.-Y. Li, J.-H. Wu, X.-Y. Wang, X.-Y. Zhang, C.-Y. Yue and X.-W. Lei, Reversible triple-mode photo-and radioluminescence and nonlinear optical switching in highly efficient 0D hybrid cuprous halides, Chem. Mater., 2023, 35, 6598–6611 CrossRef CAS.
- R. Li, Y. Zhou, X. Zhang, J. Lin, J. Chen, C. Chen, X. Pan, P. Wang, R. Chen and J. Yin, In/Bi-based Direct-and Indirect-Gap Hybrid Double-Perovskite-Derived 1D Halides with Near-Unity Quantum Yield via Sb3+ Doping, Chem. Mater., 2023, 35, 9362–9369 CrossRef CAS.
- Y.-H. Liu, N.-N. Wang, M.-P. Ren, X. Yan, Y.-F. Wu, C.-Y. Yue and X.-W. Lei, Zero-dimensional hybrid cuprous halide of [BAPMA] Cu2Br5 as a highly efficient light emitter and an X-ray scintillator, ACS Appl. Mater. Interfaces, 2023, 15, 20219–20227 CrossRef CAS PubMed.
- Y. H. Liu, X. Yan, L. Xiao, W. Jiang, Q. Liu, T. C. Liu, T. Y. Yan, C. Y. Yue and X. W. Lei, Water-Stable 0D Hybrid Manganese Halides with Adjustable Crystal Structure and Emission Color, Adv. Opt. Mater., 2023, 11, 2301010 Search PubMed.
- X. Liu, Y. Jiang, F. Li, X. Xu, R. Li, W. Zhu, J. Ni, C. Ding, S. Liu and Q. Zhao, Thermally activated delayed fluorescent scintillators based on mononuclear copper(I) halide complexes for high-resolution X-ray imaging, Adv. Opt. Mater., 2023, 11, 2202169 CrossRef CAS.
- H.-J. Yang, W. Xiang, X. Zhang, J.-Y. Wang, L.-J. Xu and Z.-N. Chen, High spatial resolution X-ray scintillators based on a 2D copper(I) iodide hybrid, J. Mater. Chem. C, 2024, 12, 438–442 RSC.
- L. Lian, X. Wang, P. Zhang, J. Zhu, X. Zhang, J. Gao, S. Wang, G. Liang, D. Zhang and L. Gao, Highly luminescent zero-dimensional organic copper halides for X-ray scintillation, J. Phys. Chem. Lett., 2021, 12, 6919–6926 CrossRef CAS PubMed.
- Z. Wang, Y. Du, C. Wang, L. Ma, C. Li, T. Lin, J. Xiao and Z. Yan, Dimethylamine Copper(I) Halide Single Crystals: Structure, Physical Properties, and Scintillation Performance, Inorg. Chem., 2024, 63, 13546–13557 CrossRef CAS PubMed.
- X. Wang, K. Rakstys, K. Jack, H. Jin, J. Lai, H. Li, C. S. K. Ranasinghe, J. Saghaei, G. Zhang, P. L. Burn, I. R. Gentle and P. E. Shaw, Engineering fluorinated-cation containing inverted perovskite solar cells with an efficiency of >21% and improved stability towards humidity, Nat. Commun., 2021, 12, 52 CrossRef CAS PubMed.
- B. Kim and S. I. Seok, Molecular aspects of organic cations affecting the humidity stability of perovskites, Energy Environ. Sci., 2020, 13, 805–820 RSC.
- R. P. Vasquez, CuBr by XPS, Surf. Sci. Spectra, 1993, 2, 144–148 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2369729. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5tc00788g |
| This journal is © The Royal Society of Chemistry 2025 |