Molecular interaction regulation by adding a third component with high miscibility suppresses the energetic disorder and reduces energy loss for efficient ternary solar cells†
Ruiying
Lin‡
a,
Shichu
Peng‡
a,
Zhenyu
Luo
a,
Jiaxin
Wu
a,
Yaocheng
Jin
 b,
Yanping
Huo
b,
Yanping
Huo
 b,
Liangang
Xiao
b,
Liangang
Xiao
 *ac and
Yonggang
Min
*ac and
Yonggang
Min
 *ac
*ac
aSchool of Materials and Energy, Guangdong University of Technology, Guangzhou 510006, China. E-mail: xiaolg@gdut.edu.cn; ygmin@gdut.edu.cn
bSchool of Chemical Engineering and Light Industry, Guangdong University of Technology, Guangzhou 510006, China
cGuangdong Provincial Laboratory of Chemistry and Fine Chemical Engineering Jieyang Center, Jieyang 515200, China
First published on 24th October 2024
Abstract
In the advancement of organic solar cells (OSCs), the ternary strategy has emerged as an effective approach for fabricating devices with high photovoltaic performance. In this contribution, we have introduced a novel wide bandgap donor, PBTz-Cl, into the D18:L8-BO binary system to address the excessive aggregation of D18. PBTz-Cl exhibits excellent miscibility with D18 in ternary films due to their similar building blocks. Our findings show that the addition of PBTz-Cl forms a molecular alloy within the amorphous regions of D18. This not only boosts additional exciton generation in D18 through Förster resonance energy transfer, but also suppresses the non-radiative recombination energy loss (ΔE3) due to the reduced crystallinity difference between D18 and L8-BO by enhancing the crystallinity of L8-BO. The optimized ternary blend film exhibits superior microstructure morphology and enhanced charge dynamics, leading to enhanced photovoltaic performance and remarkable stability. The resulting OSCs show a remarkable increase in performance with a JSC of 25.30 mA cm−2, a VOC of 0.911 V, and an FF of 78.33%. Our study highlights the effectiveness of the strategy, which derives from the synergistic effects of compatible polymer donors to precisely regulate molecular packing and optimize film morphology for improved photovoltaic performance.
Introduction
Organic solar cells (OSCs) have garnered considerable attention and hold promising prospects for applications due to their lightweight nature, flexibility, and enormous potential for high-throughput roll-to-roll manufacturing.1–4 Over the past three decades, various polymer donors, such as P3HT, PM6, and D18, along with non-fullerene electron acceptors like ITIC and Y6, have been successfully developed.4–13 Moreover, advancements in device and interface engineering enable the comprehensive exploration of new materials to enhance photovoltaic performance.14–18 In summary, the remarkable advancements in material design and device fabrication processes have resulted in the rapid progress of organic photovoltaic technology. Currently, state-of-the-art OSCs have achieved power conversion efficiencies (PCE) exceeding 19% in single-junction devices, gradually approaching the performance level of their inorganic counterparts.19–27The ternary strategy has been recognized as an effective way to boost the efficiency while maintaining a simple device fabrication process.28–34 Ternary solar cells (TSCs) typically consist of two acceptors and one donor or two donors and one acceptor in the active layer. By introducing a third component to the host binary blend, molecular packing, crystallinity, interface distribution of donors and acceptors, and light absorption spectra can be effectively manipulated,35–38 leading to enhanced exciton and charge dynamics, suppressed charge recombination, and thereby elevated short-circuit current (JSC) and fill factor (FF).39,40 For instance, adding a highly crystalline molecular donor, DRTB-T-C4, into the PM6:Y6 host binary system significantly improved charge transport, reduced recombination loss, and increased the FF to 81.3%.41 Similarly, adding a polymer acceptor, N2200, into the PBDB-T:IT-M binary blend effectively regulated vertical phase distribution for enhanced charge transport and collection and reduced trap states.42 Moreover, by constructing alloy-like components, the charge transfer state in ternary films could be finely tuned, resulting in reduced energy loss and elevated open-circuit voltage (VOC).43 Utilizing the fluorescence resonance energy transfer (FRET) of materials in ternary films has also been found to effectively convert high-energy excitons into charges, leading to a significant improvement in JSC. For example, the efficient FRET between the wide bandgap acceptor IDTT-M and Y6 provides a non-radiative pathway for IDTT-M in the ternary system as well as an additional pathway to facilitate exciton separation and charge collection.44 However, most studies have focused on the effects of energy and charge transfer in ternary blend films, with limited research on the miscibility and aggregation behavior, which are closely linked to the materials' compatibility in multi-component active layers and the energetic disorder of blend films.45–48 Recently reported studies have demonstrated that inhibiting the aggregation of small molecule electron acceptors can effectively enhance the photovoltaic performance.49 For example, by introducing the 3D-shaped guest acceptor TPA-4PDI into the PM6:Y6 binary system, the excessive aggregation behavior of Y6 was effectively suppressed. As a result, highly efficient ternary devices were achieved, demonstrating simultaneously increased open-circuit voltage (VOC), JSC and FF.50
D18 is a highly crystalline polymer and commonly used as a seeding agent in ternary systems to control the crystallinity and phase separation of blend films.51–53 However, its poor solubility and excessive aggregation often result in unfavorable morphology when processed from blend solutions. The key to elevating the photovoltaic performance of D18 based OSCs lies in alleviating the excessive aggregation of the D18 polymer at the molecular level. From the perspective of molecular thermodynamics and kinetics, molecular interactions play a significant role in crystallization, phase separation, and microstructure morphology. In conjugated organic materials, π–π interactions, dictated by their chemical structures, are key to the miscibility of materials. High miscibility within a ternary blend system is likely to form a mixed amorphous phase, which in turn improves solubility.54,55 Building on this understanding, by carefully designing a third component that mixes well with D18, it is feasible to obtain a blend film with a suitable microstructure for fabrication of high-efficiency D18 based TSCs. In this contribution, we introduced PBTz-Cl, a new wide bandgap donor with good chloroform solubility, and successfully used it as the third component in the D18:L8-BO binary system.56 The polymer PBTz-Cl has the same benzo[1,2-b:4,5-b′]dithiophene (BDT) electron donor units as D18 and when added in an appropriate amount, it significantly boosts the photovoltaic performance of the TSCs. The contact angle measurements and grazing-incidence wide-angle X ray scattering (GIWAXS) study suggested the outstanding miscibility and strong molecular interactions between PBTz-Cl and D18. This good compatibility leads to the formation of a mixed amorphous phase, which improves the solubility of D18. Consequently, the optimized ternary film displayed an advantageous microstructure morphology. In addition, the enhanced crystallinity of the L8-BO acceptor in the ternary blend also indicated the reduced crystallinity difference. As a result, the enhanced miscibility and reduced crystallinity difference synergistically contribute to the suppressed energetic disorder and reduced energy loss (ΔE3), resulting in a high VOC for ternary solar cells. Moreover, PBTz-Cl also functioned as a Förster resonance energy transfer (FRET) donor, generating additional D18 excitons for high photocurrent. This was conducive to markedly improved exciton dynamics and more balanced charge transfer, strongly corroborated by transient photovoltage (TPV), transient photocurrent (TPC), and femtosecond transient absorption spectroscopy (fs-TAS) investigations. Ultimately, the resultant ternary device exhibited a remarkable elevation in photovoltaic performance, with a significant increase in a JSC value of 25.30 mA cm−2, a VOC value of 0.911 V and an FF of 78.33%.
Results and discussion
The molecular structures of D18, PBTz-Cl, and acceptor L8-BO employed in this study are depicted in Fig. 1a. We determined the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels for D18, the D18:PBTz-Cl blend, PBTz-Cl and L8-BO using cyclic voltammetry (CV) measurements (Fig. S1, ESI†). The derived energy level diagram is showcased in Fig. 1b. The calculated HOMO/LUMO levels for D18 and L8-BO are –5.35/–3.39 eV and –5.67/–3.84 eV, respectively, whereas PBTz-Cl has HOMO/LUMO levels at –5.60 eV/–3.61 eV. Notably, PBTz-Cl exhibits a deeper HOMO level compared to D18, which is advantageous for promoting a higher VOC in TSCs. In the D18:PBTz-Cl blend, the HOMO level is reduced, aligning well with L8-BO, which might reduce voltage losses and enhance VOC in TSCs.We investigated the optical absorption of D18, PBTz-Cl, and their blend (weight ratio of D18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PBTz-Cl of 0.975
PBTz-Cl of 0.975![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.025). The UV-vis-NIR absorption spectra, shown in Fig. 1c, exhibit distinct optical absorption peaks at 545 nm and 580 nm for D18 and 510 nm for PBTz-Cl. The D18:PBTz-Cl blend film demonstrates an enhanced absorption coefficient across nearly the entire spectral region without peak shifts relative to the neat D18 film. As depicted in Fig. 1d, the binary and ternary blend films exhibit similar absorption trends to the characteristic peaks of D18. The absorption peak of L8-BO in the D18:L8-BO binary film is located at 795 nm, which experiences a slight blue-shift to 790 nm in the ternary blend. Importantly, the introduction of PBTz-Cl enhances the vibrational 0–1 peak of L8-BO, resulting in a lower I0–0/I0–1 ratio (1.52) in the ternary film compared to the binary film (1.60). This suggests a modification in the molecular packing and aggregation properties of L8-BO. Such changes are likely to foster more robust intermolecular interactions, enabling L8-BO to aggregate through face-to-face self-assembly. According to the reported study, the changes in molecular packing modes and molecular aggregation significantly impacted the non-radiative recombination of charge transfer states and VOC in OSCs.57 These changes in molecular packing not only enhance the films' optical properties but also their charge transport and collection capacity.58–60 Steady-state photoluminescence (PL) spectra for the neat D18 film, the D18:L8-BO binary film, and the D18:PBTz-Cl:L8-BO ternary film were recorded, with the corresponding spectra illustrated in Fig. S2 (ESI†). In these films, D18 was excited at 550 nm. Both binary and ternary films exhibit notable fluorescence quenching, especially in the ternary film, indicating more effective charge transfer. Additionally, the absorption spectrum of D18 and the PL emission spectrum of PBTz-Cl overlapped (Fig. 1e), leading to a complete quenching of PBTz-Cl's PL emission and an increase in theD18's emission as weight ratios of their blends changed (Fig. 1f). This suggests a strong FRET between D18 and PBTz-Cl, implying that they are closely packed (within 10 nm) and show strong intermolecular interactions.32,61 This non-radiative energy transfer from PBTz-Cl to D18 boosts the production of additional D18 excitons, potentially offering a new route to the increased photocurrent seen in ternary devices.
0.025). The UV-vis-NIR absorption spectra, shown in Fig. 1c, exhibit distinct optical absorption peaks at 545 nm and 580 nm for D18 and 510 nm for PBTz-Cl. The D18:PBTz-Cl blend film demonstrates an enhanced absorption coefficient across nearly the entire spectral region without peak shifts relative to the neat D18 film. As depicted in Fig. 1d, the binary and ternary blend films exhibit similar absorption trends to the characteristic peaks of D18. The absorption peak of L8-BO in the D18:L8-BO binary film is located at 795 nm, which experiences a slight blue-shift to 790 nm in the ternary blend. Importantly, the introduction of PBTz-Cl enhances the vibrational 0–1 peak of L8-BO, resulting in a lower I0–0/I0–1 ratio (1.52) in the ternary film compared to the binary film (1.60). This suggests a modification in the molecular packing and aggregation properties of L8-BO. Such changes are likely to foster more robust intermolecular interactions, enabling L8-BO to aggregate through face-to-face self-assembly. According to the reported study, the changes in molecular packing modes and molecular aggregation significantly impacted the non-radiative recombination of charge transfer states and VOC in OSCs.57 These changes in molecular packing not only enhance the films' optical properties but also their charge transport and collection capacity.58–60 Steady-state photoluminescence (PL) spectra for the neat D18 film, the D18:L8-BO binary film, and the D18:PBTz-Cl:L8-BO ternary film were recorded, with the corresponding spectra illustrated in Fig. S2 (ESI†). In these films, D18 was excited at 550 nm. Both binary and ternary films exhibit notable fluorescence quenching, especially in the ternary film, indicating more effective charge transfer. Additionally, the absorption spectrum of D18 and the PL emission spectrum of PBTz-Cl overlapped (Fig. 1e), leading to a complete quenching of PBTz-Cl's PL emission and an increase in theD18's emission as weight ratios of their blends changed (Fig. 1f). This suggests a strong FRET between D18 and PBTz-Cl, implying that they are closely packed (within 10 nm) and show strong intermolecular interactions.32,61 This non-radiative energy transfer from PBTz-Cl to D18 boosts the production of additional D18 excitons, potentially offering a new route to the increased photocurrent seen in ternary devices.
Then, we fabricated OSC devices with a conventional structure of ITO/PEDOT:PSS/active layer/PDIN/Ag. The photovoltaic performance of these devices, with various solution concentrations and the weight ratios of PBTz-Cl ranging from 0 to 20 wt%, is detailed in Fig. S3 and Tables S1, S2 (ESI†). Fig. 2a displays the representative current density–voltage (J–V) curves of D18:L8-BO, PBTz-Cl:L8-BO, and D18:PBTz-Cl:L8-BO binary and ternary OSCs, with photovoltaic parameters summarized in Table 1. As illustrated in Fig. S4 (ESI†), good reproducibility in photovoltaic performance was confirmed for 22 D18:L8-BO based binary and D18:PBTz-Cl:L8-BO based ternary devices. Specifically, D18:L8-BO based binary devices demonstrated a fill factor (FF) of 76.69%, a JSC of 24.37 mA cm−2, and a moderate PCE of 16.87%. Devices based on PBTz-Cl:L8-BO, however, exhibited a lower PCE of 10.19% due to a reduced FF of 58.17% and a JSC of 19.39 mA cm−2. Interestingly, adding 2.5 wt% PBTz-Cl into the D18:L8-BO enabled TSC device with an impressive PCE exceeding 18%, along with an improved FF of 78.33%, a JSC of 25.30 mA cm−2, and a VOC of 0.911 V. The increased JSC could be attributed to the enhanced absorption, more efficient charge transfer and the existence of non-radiative energy transfer. The optimized TSC device delivered a VOC of 0.911 V, exceeding that of the D18:L8-BO based binary device (VOC = 0.903 V) and even the PBTz-Cl:L8-BO based binary device (VOC = 0.904 V), despite its deep HOMO energy level of –5.60 eV. Fig. 2b presents the correlation between the device performance and the PBTz-Cl content, showing an enhancement in JSC and FF at 2.5 wt% PBTz-Cl. However, higher weight ratios of PBTz-Cl diminished the photovoltaic performance. Specifically, for 10 wt% PBTz-Cl, both the FF and JSC significantly reduced to 24.13 mA cm−2 and 75.61%, respectively, and the PCE dropped to 16.74%. Despite this, the VOC value in TSCs experienced a monotonic increase with the increase of PBTz-Cl. The observed enhancement in VOC suggests that the incorporation of PBTz-Cl might promote an alloy-like structure. This change is associated with a diminished energy offset and reduced energy loss.62,63 To comprehensively understand the superior JSC in TSCs, we performed external quantum efficiency (EQE) measurements, and the results are depicted in Fig. 2c. The D18:L8-BO-based binary device shows a significant photoresponse between 515–585 nm and 650–800 nm, which aligns with the absorption profiles of D18 and L8-BO, respectively. Conversely, the PBTz-Cl:L8-BO based binary device achieves a low EQE across the entire wavelength range due to the inferior morphology that hinders exciton dissociation and collection. However, the optimized ternary device demonstrates superior EQE values for almost the entire spectrum, with a notable peak of 86% at 550 nm. This indicates that excitons, especially those directly generated by D18 and the additional D18 exciton from FRET, are effectively separated and collected by the electrodes. The calculated current densities (Jcal) from EQE curves are 23.62 mA cm−2 for D18:L8-BO, 24.64 mA cm−2 for D18:PBTZ-Cl:L8-BO, and 19.30 mA cm−2 for PBTz-Cl:L8-BO based devices, consistent with the results obtained from J–V measurements.
| Active layer | V OC (V) | J SC/JCal (mA cm−2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| a Average PCE values were obtained from 22 devices. b Average PCE values were obtained from 10 devices. | ||||
| D18:L8-BO | 0.903 | 24.37/23.62 | 76.69 | 16.87 (16.33 ± 0.302)a |
| D18:PBTz-Cl:L8-BO | 0.911 | 25.30/24.64 | 78.33 | 18.05 (17.73 ± 0.271)a |
| PBTz-Cl:L8-BO | 0.904 | 19.39/19.30 | 58.17 | 10.19 (9.72 ± 0.325)b |
To further study the reason for a monotonic increase in VOC for TSCs, the total energy losses (Eloss) in TSCs with different ratios of PBTz-Cl were analyzed through highly sensitive EQE (s-EQE) and external electroluminescence quantum efficiency (EQEEL) measurements, which are depicted in Fig. 3a–e. The detail data of energy losses are listed in Table 2. The Eloss can be divided into three parts as follows:64
| Eloss = Eg − qVOC = (Eg − qVSQOC) + (qVSQOC − qVradOC) + (qVradOC − qVOC) = ΔE1 + ΔE2 + ΔE3 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) ln(EQEEL) (kB is Boltzmann's constant and T is the absolute temperature). When 2.5%wt PBTz-Cl was added to the devices, Eloss decreased from 0.556 eV to 0.547 eV, leading to an elevated VOC. This decline continued with more PBTz-Cl, dropping to 0.544 eV at 10%wt and reaching 0.522 eV at 20%wt. These reductions suggested that energy transfer between D18 and PBTz-Cl minimizes energy disorder and thus energy loss. The material-dependent ΔE1 and donor–acceptor ratio-dependent ΔE2 remained relatively stable (∼0.265 eV and ∼0.056 eV, respectively), indicating that non-radiative recombination energy loss ΔE3 is the main contributor to the total energy loss in TSCs. A decrease in ΔE3 from 0.236 eV to 0.205 eV was noted with increasing PBTz-Cl content, up to 20%wt. However, the highest PBTz-Cl content also led to a higher charge-separated state energy (ECS), suggesting a deeper HOMO level of the D18:PBTz-Cl donor and a reduced charge generation force (Eg − ECS), which could lower the photocurrent.65 Conversely, a 2.5%wt addition of PBTz-Cl achieved a balance between non-radiative recombination and charge generation, enhancing both JSC and VOC. To assess the impact of FRET between D18 and PBTz-Cl on energy disorder, we measured the Urbach energy (EU) to assess the density of states (DoS) distribution, which characterizes the width of the DoS tail.66 By fitting the edge of s-EQE with the equation αhv = α0
ln(EQEEL) (kB is Boltzmann's constant and T is the absolute temperature). When 2.5%wt PBTz-Cl was added to the devices, Eloss decreased from 0.556 eV to 0.547 eV, leading to an elevated VOC. This decline continued with more PBTz-Cl, dropping to 0.544 eV at 10%wt and reaching 0.522 eV at 20%wt. These reductions suggested that energy transfer between D18 and PBTz-Cl minimizes energy disorder and thus energy loss. The material-dependent ΔE1 and donor–acceptor ratio-dependent ΔE2 remained relatively stable (∼0.265 eV and ∼0.056 eV, respectively), indicating that non-radiative recombination energy loss ΔE3 is the main contributor to the total energy loss in TSCs. A decrease in ΔE3 from 0.236 eV to 0.205 eV was noted with increasing PBTz-Cl content, up to 20%wt. However, the highest PBTz-Cl content also led to a higher charge-separated state energy (ECS), suggesting a deeper HOMO level of the D18:PBTz-Cl donor and a reduced charge generation force (Eg − ECS), which could lower the photocurrent.65 Conversely, a 2.5%wt addition of PBTz-Cl achieved a balance between non-radiative recombination and charge generation, enhancing both JSC and VOC. To assess the impact of FRET between D18 and PBTz-Cl on energy disorder, we measured the Urbach energy (EU) to assess the density of states (DoS) distribution, which characterizes the width of the DoS tail.66 By fitting the edge of s-EQE with the equation αhv = α0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(hv/EU) (αhv is the absorption coefficient, h is Planck's constant, v is the incident photon frequency, and α0 is the absorption coefficient at Eg),67 we determined that ternary devices with PBTz-Cl exhibited a lower EU (25.97 meV compared to 26.71 meV in the D18:L8-BO based binary device), indicating a narrower DoS distribution, reduced energy disorder, and subsequently less non-radiative recombination and higher VOC.
exp(hv/EU) (αhv is the absorption coefficient, h is Planck's constant, v is the incident photon frequency, and α0 is the absorption coefficient at Eg),67 we determined that ternary devices with PBTz-Cl exhibited a lower EU (25.97 meV compared to 26.71 meV in the D18:L8-BO based binary device), indicating a narrower DoS distribution, reduced energy disorder, and subsequently less non-radiative recombination and higher VOC.
D18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PBTz-Cl PBTz-Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L8-BO L8-BO |
E g (eV) | qV OC (eV) | E loss (eV) | qV SQOC (eV) | qV radOC (eV) | ΔE1 (eV) | ΔE2 (eV) | ΔE3 (eV) | EQEEL (%) |
|---|---|---|---|---|---|---|---|---|---|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 1.4 |
1.461 | 0.905 | 0.556 | 1.196 | 1.141 | 0.265 | 0.055 | 0.236 | 0.345 |
0.975![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.025 0.025![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 1.4 |
1.461 | 0.914 | 0.547 | 1.196 | 1.139 | 0.264 | 0.057 | 0.226 | 10.894 |
0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 1.4 |
1.461 | 0.917 | 0.544 | 1.196 | 1.141 | 0.265 | 0.056 | 0.223 | 23.370 |
0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 1.4 |
1.457 | 0.931 | 0.526 | 1.192 | 1.136 | 0.265 | 0.056 | 0.205 | 3.506 |
Stability is also a very important indicator for evaluating the photovoltaic performance of OSCs. We measured the storage stability and thermal stability of the devices. Notably, the TSC device impressively retained more than 97% of its initial PCE after 1000 hours, showcasing remarkable storage stability. However, the D18:L8-BO based binary device only maintained 89% of its initial PCE (Fig. 2d). This reduction is mainly due to a drop in the FF. When subjected to 100 °C for an hour, the TSC device still held onto over 86% of its initial PCE, outperforming the D18:L8-BO device which only managed to maintain 80% of its initial PCE (Fig. S5, ESI†). This suggests that the D18:L8-BO binary device suffers from greater morphological degradation. Atomic force microscopy (AFM) images (Fig. S6a–f, ESI†) reveal that the root-mean-square (RMS) roughness of the D18:L8-BO film significantly decreased from 1.17 to 0.99 nm after thermal annealing, while the RMS roughness of both PBTz-Cl:L8-BO and D18:PBTz-Cl:L8-BO films remained stable, changing by an insignificant 0.05 nm. This indicates that the ternary films remain morphologically stable even after thermal annealing at high temperature.
To elucidate the mechanisms for the improved photovoltaic performance, hole-only and electron-only devices were fabricated to evaluate transport properties utilizing the space charge-limited current method. The corresponding J–V curves in the dark are illustrated in Fig. S7 and S8 (ESI†), with hole mobility (μh) and electron mobility (μe) values listed in Table S3 (ESI†). The D18:L8-BO film's μh (2.71 × 10−4 cm2 V−1 s−1) was much lower than its μe (4.39 × 10−4 cm2 V−1 s−1), leading to an imbalance in charge transport (μe/μh ratio of 1.61) that could induce charge accumulation and promote charge recombination. The D18:PBTz-Cl:L8-BO based ternary film, however, displayed a significantly enhanced μe of 8 × 10−4 cm2 V−1 s−1 and μh of 7.42 × 10−4 cm2 V−1 s−1, respectively. Moreover, the μe/μh ratio decreases to 1.33, indicating more balanced charge transport conducive to higher JSC and FF. Charge transfer between D18 and PBTz-Cl was further confirmed by the higher JSC in OSCs fabricated with a D18:PBTz-Cl mixture compared to neat D18, as shown in Fig. S9 (ESI†) and listed in Table S4 (ESI†).
For further understanding the factors that contribute to the enhanced photovoltaic performance in ternary devices, we examined the exciton dissociation and charge collection in both binary and ternary OSCs. We measured the photocurrent density as a function of effective applied voltage (Jph–Veff) under short-circuit conditions, as depicted in Fig. 4a. Under a high Veff, the Jph could be saturated (Jsat), reflecting the photon absorption capacity of the devices. As listed in Table S5 (ESI†), the increased Jsat suggests better light absorption in ternary films. The exciton dissociation efficiency (Pdiss) and charge collection efficiency (Pcoll) of D18:L8-BO based devices are estimated to be 95.84% and 87.32%, respectively. An optimized ternary device improved these efficiencies to 96.39% (Pdiss) and 89.09% (Pcoll), demonstrating that adding PBTz-Cl could facilitate exciton dissociation at the donor–acceptor interface and charge transport and charge collection, which consist with enhanced charge generation forces as mentioned above. Additionally, we investigated charge recombination behavior by examining J–V curves of devices under varying light intensity (Plight). The dependence of JSC on Plight can be expressed as JSC ∝ (Plight)S, where S is the exponential factor for evaluating the bimolecular recombination. The curves, presented in Fig. 4b, show S values of 0.98 for D18:L8-BO, 0.98 for D18:PBTz-Cl:L8-BO, and 0.96 for PBTz-Cl:L8-BO, indicating negligible bimolecular recombination in ternary systems and implying that adding 2.5 wt% PBTz-Cl would not create harmful recombination centers that are detrimental to device performance.
In addition to steady-state measurements, time-resolved experiments were also used to investigate the charge dynamics. The time-resolved photoluminescence (TRPL) spectra (Fig. S10, ESI†) showed that the ternary film with D18:PBTz-Cl:L8-BO had a fluorescence lifetime (τ) of 398 ps, shorter than the 404 ps of the binary D18:L8-BO film, indicating more efficient charge transfer. Additionally, transient photovoltage (TPV) and transient photocurrent (TPC) measurements revealed that the ternary device had a longer charge carrier lifetime (τTPV) of 5.57 μs (versus 5.51 μs for the binary device) (Fig. 4c) and a faster charge extraction time (τTPC) of 0.170 μs (versus 0.246 μs for the binary device) (Fig. 4d), suggesting reduced recombination and enhanced charge sweep-out in the ternary device. Evidently, adding PBTz-Cl actually has multiple advantages, including enhancing the charge transport and extraction, as well as suppressing the charge recombination, as confirmed by the highest JSC and FF observed in the ternary devices. Finally, femtosecond transient absorption (fs-TA) spectroscopy was further employed to investigate the excited-state dynamics process. Fig. 5a–c depict the fs-TA spectra of D18:L8-BO, D18:PBTz-Cl:L8-BO and PBTz-Cl:L8-BO blend films at selected time delays. A pump laser of 800 nm selectively excites the acceptor L8-BO in the blend films. Immediately after excitation, a strong positive excited state absorption (ESA) peak appeared around 900 nm while a negative ground state bleach (GSB) peak is observed at around 820 nm, with additional GSB peaks at ∼600 nm and ∼525 nm for D18 and PBTz-Cl, reaching their maximum within 100 ps and 4 ps, respectively. This suggests a rapid hole-transfer from L8-BO to the other components, generating a charge transfer state. The hole transfer kinetics was further analyzed using a biexponential model with a fast process (τ1) and a lower process (τ2), corresponding to the direct exciton dissociation of the charge transfer states at the donor/acceptor interfaces (exciton lifetime) and diffusion-mediated exciton dissociation at the donor/acceptor interfaces (charge carrier lifetime), respectively. As shown in Fig. 5d and Table S6 (ESI†), the PBTz-Cl:L8-BO blend showed the quickest charge transfer with a τ1 of only 0.8 ps, but also a fast decay (τ2 of 321 ps) to the ground state, leading to significant charge recombination and lower device performance. In contrast, the D18:L8-BO and D18:PBTz-Cl:L8-BO based blend films had longer τ1 of 9.4 ps and 8.9 ps, respectively. The D18 GSBs (524 nm) for the D18:L8-BO based binary film and the D18:PBTz-Cl:L8-BO ternary film continuously increase and reach a maximum after the initial rise. This indicates a diffusion-mediated charge process that occurs after the ultrafast charge transfer at the donor/acceptor interfaces. Therefore, these two films exhibited a longer τ2, 1763 ps for the ternary film and 1402 ps for the binary film. This result is also in accordance with the above TPV measurement. According to the reported study,68 the poor performance of the PBTz-Cl:L8-BO based device was attributed to the excessive mixing of the donor and acceptor, along with undersized donor/acceptor phase domains, causing short diffusion-mediated charge transfer and the fast charge recombination. Conversely, the D18:L8-BO based blend film benefitted from larger phase domains, resulting in slower charge transfer and recombination. The D18:PBTz-Cl:L8-BO based ternary blend film struck a balance between fast charge transfer and slow charge recombination, leading to a higher charge transfer state yield and a longer charge lifetime, aligning with their better device performance. This also agrees well with the energy loss analysis mentioned above, suggesting that the ternary blend manages to balance between charge generation and non-radiative recombination effectively.
To gain a better understanding of the enhanced exciton and charge dynamics, as well as the resulting boost in photovoltaic efficiency due to film morphology enhancements, we first examined the film surfaces using AFM. Fig. S11 (ESI†) illustrates the surface morphology of the pristine D18 film, which exhibits a fibrous network with a RMS roughness of 1.38 nm. This structure is advantageous for hole transport, but possibly hindering L8-BO permeation, resulting in unbalanced charge transport. The PBTz-Cl film, however, had a smoother and more uniform surface with a RMS of 0.85 nm, likely due to its high solubility and minimal aggregation leading to small phase domains. The D18:PBTz-Cl blend film maintained the fibrous structure with a slightly smoother RMS of 1.24 nm. Among three blend films, the D18:PBTz-Cl:L8-BO based ternary film had the roughest surface at 1.25 nm, compared to 1.17 nm for D18:L8-BO and 0.88 nm for PBTz-Cl:L8-BO (Fig. S6a–c, ESI†). This suggests that, when the donor blend (D18:PBTz-Cl) initially precipitates, L8-BO integrates more effectively into the forming ternary film during ternary film formation, promoting orderly packing and crystallinity, thereby enhancing charge transport, suppressing recombination and increasing photocurrent.
The fundamental origin of morphological evolution with the addition of PBTz-Cl was investigated by conducting the contact angle measurements to assess the compatibility between D18, PBTz-Cl, D18:PBTz-Cl, and L8-BO, and is shown in Fig. 6. The calculated surface energy (γ) and the Flory–Huggins interaction parameters (χ) between D18, PBTz-Cl and L8-BO are listed in Table 3. The γ values for D18, PBTz-Cl and L8-BO films are found to be 19.89, 20.29 and 23.22 mN m−1, respectively. The close γ values for D18 and PBTz-Cl imply favorable miscibility of these two polymers. Furthermore, the χ also supported this compatibility,69–71 with χ values of 0.002κ for D18 and PBTz-Cl and 0.037κ for PBTz-Cl and L8-BO, both of which are lower than 0.056κ for D18 and L8-BO. This suggested the excellent miscibility between D18/PBTz-Cl and PBTz-Cl/L8-BO, which would facilitate the formation of a D18:PBTz-Cl alloy-like phase and benefit L8-BO permeating into the polymer network for the orderly packing and crystallization during the film formation. According to the reported studies,62,72–77 such compatibility can lead to the formation of the alloy-like phase between two donors accounting for the monotonically elevated VOC of ternary devices and variations in the energy level of polymer blends as the PBTz-Cl content increases (Table S1 (ESI†) and Fig. 1).
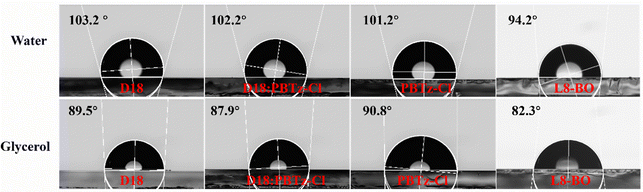 | ||
| Fig. 6 The contact angle images of D18, PBTz-Cl, D18:PBTz-Cl, and L8-BO films tested by using water and glycerol liquid drops. | ||
| Film | γ [mN m−1] | χ with D18 [mN m−1] | χ with L8-BO [mN m−1] |
|---|---|---|---|
| D18 | 19.89 | — | 0.056κ |
| PBTz-Cl | 20.29 | 0.002κ | 0.037κ |
| L8-BO | 22.07 | 0.056κ | — |
Finally, we performed grazing-incidence wide-angle X-ray scattering (GIWAXS) measurements to study the molecular crystallinity and orientation in neat, binary blend, and the optimized ternary films. Fig. 7a–f illustrate the 2D diffraction patterns and the extracted line-cut profiles in both in-plane (IP) and out-of-plane (OOP) directions. The accompanying data for d-spacing and the crystal coherence length (CCL) are found in Tables S7 and S8 (ESI†). The neat films of D18 and PBTz-Cl exhibit a preferred face-on orientation, with intense π–π stacking (010) diffraction peaks at 1.66 Å−1 and 1.56 Å−1 and lamellar packing (100) peaks at 0.31 Å−1 and 0.32 Å−1, respectively. A higher d-spacing of 4.03 Å for neat PBTz-Cl indicates a more loosely arranged π–π stacking in the OOP direction compared to that of 3.78 Å for the D18 film. In the D18:PBTz-Cl binary blend film, the π–π stacking (010) diffraction peak remains at 1.66 Å−1 in the OOP direction, but with a narrower full-width-half-maximum (FWHM) of 0.434 Å−1, signifying an increase in CCL. These findings demonstrate that the molecular packing of the D18:PBTz-Cl blend adopts the structure of D18, with PBTz-Cl well-integrated into the D18 framework without disrupting its molecular packing. This potentially forms a molecular alloy within the amorphous regions of the D18 polymer. The featured distributions of PBTz-Cl in ternary films effectively optimize the film morphology, leading to better charge transfer, lower energy losses, and consequently elevated VOC and PCE. All blend films displayed clear π–π stacking (010) diffraction peaks, contributed by the overlapping π–π stacking from the polymer donors (D18 or PBTz-Cl) and L8-BO. In binary blends, the π–π stacking (010) peak was at 1.73 Å−1, while in ternary blends, it appeared at 1.71 Å−1 with a d-spacing of 3.67 Å. More importantly, ternary blends also showed strong (100) peak scattering and a reduced FWHM for the L8-BO (100) peak from 0.314 Å−1 to 0.304 Å−1, with an increased CCL from 18.00 Å to 19.34 Å. These GIWAXS results implied the enhanced crystallinity of L8-BO in the ternary film, reducing the crystallinity difference between D18 and L8-BO that could inhibit the non-radiative recombination and achieve smaller ΔE3 for higher VOC in OSCs, which correlates well with the improved charge mobility, suppressed non-radiative recombination and decreased ΔE3 in TSCs mentioned above.
Conclusions
In this contribution, we introduced a new soluble polymer donor, PBTz-Cl, into the D18:L8-BO host system to fabricate high-efficiency TSCs. The structural synergy between PBTz-Cl and D18 led to an alloy-like structure, which could fine-tune the blend film morphology and subsequently enhanced the charge dynamics while reducing energy loss from non-radiative recombination. Additionally, the addition of PBTz-Cl facilitated the orderly packing of L8-BO molecules. As a result, we successfully developed a robust TSC device based on D18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PBTz-Cl
PBTz-Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L8-BO with a weight ratio of 0.975
L8-BO with a weight ratio of 0.975![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.025
0.025![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4. This device achieved an impressive PCE of 18.05%, with a VOC of 0.911 V, a JSC of 25.30 mA cm−2, and an FF of 78.33%. Our approach demonstrated the potential of combining compatible polymers to control the energy offset, regulate molecular packing, and optimize film morphology, which could effectively contribute to the suppressed energetic disorder and reduced non-radiative recombination energy loss. This approach holds great promise for the fabrication of high-performance TSCs and should promote the development of OSC technology.
1.4. This device achieved an impressive PCE of 18.05%, with a VOC of 0.911 V, a JSC of 25.30 mA cm−2, and an FF of 78.33%. Our approach demonstrated the potential of combining compatible polymers to control the energy offset, regulate molecular packing, and optimize film morphology, which could effectively contribute to the suppressed energetic disorder and reduced non-radiative recombination energy loss. This approach holds great promise for the fabrication of high-performance TSCs and should promote the development of OSC technology.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
L. X. acknowledges the financial support from the National Natural Science Foundation of China (51903057) and the Guangzhou Basic and Applied Basic Research Foundation (2023A04J0349). We also acknowledge the support from the National Key Research and Development Program of China (2020YFB0408100). L. X. would like to thank the Analysis and Test Center of the Guangdong University of technology for the UV-vis-NIR and PL spectroscopy measurements.References
- Y. Liang, D. Zhang, Z. Wu, T. Jia, L. Lüer, H. Tang, L. Hong, J. Zhang, K. Zhang, C. J. Brabec, N. Li and F. Huang, Nat. Energy, 2022, 7, 1180–1190 CrossRef CAS.
- M. Kaltenbrunner, M. S. White, E. D. Głowacki, T. Sekitani, T. Someya, N. S. Sariciftci and S. Bauer, Nat. Commun., 2012, 3, 770 CrossRef.
- K. Fukuda, K. Yu and T. Someya, Adv. Energy Mater., 2020, 10, 2000765 CrossRef CAS.
- X. Meng, L. Zhang, Y. Xie, X. Hu, Z. Xing, Z. Huang, C. Liu, L. Tan, W. Zhou, Y. Sun, W. Ma and Y. Chen, Adv. Mater., 2019, 31, 1903649 CrossRef CAS.
- K. Jin, Z. Xiao and L. Ding, J. Semicond., 2021, 42, 060502 CrossRef.
- G. Zhang, F. R. Lin, F. Qi, T. Heumüller, A. Distler, H.-J. Egelhaaf, N. Li, P. C. Y. Chow, C. J. Brabec, A. K. Y. Jen and H.-L. Yip, Chem. Rev., 2022, 122, 14180–14274 CrossRef CAS.
- J. Yuan and Y. Zou, Org. Electron., 2022, 102, 106436 CrossRef CAS.
- Z. Yao, X. Cao, X. Bi, T. He, Y. Li, X. Jia, H. Liang, Y. Guo, G. Long, B. Kan, C. Li, X. Wan and Y. Chen, Angew. Chem., Int. Ed., 2023, 62, e202312630 CrossRef CAS PubMed.
- J.-W. Lee, J. S. Park, H. Jeon, S. Lee, D. Jeong, C. Lee, Y.-H. Kim and B. J. Kim, Chem. Soc. Rev., 2024, 53, 4674–4706 RSC.
- A. Mishra, Energy Environ. Sci., 2020, 13, 4738–4793 RSC.
- P. Ding, D. Yang, S. Yang and Z. Ge, Chem. Soc. Rev., 2024, 53, 2350–2387 RSC.
- A. Mishra and G. D. Sharma, Angew. Chem., Int. Ed., 2023, 135, e202219245 CrossRef.
- B. Liu, C. Li, X. Gu, Y. Han, Z. Wei, Y. Cai, X. Zhang, H. Huang and Y. Huo, Chin. J Chem., 2024, 42, 485–490 CrossRef CAS.
- Z. He, B. Xiao, F. Liu, H. Wu, Y. Yang, S. Xiao, C. Wang, T. P. Russell and Y. Cao, Nat. Photonics, 2015, 9, 174–179 CrossRef CAS.
- K. Zhang, Z. Hu, C. Sun, Z. Wu, F. Huang and Y. Cao, Chem. Mater., 2017, 29, 141–148 CrossRef CAS.
- T. Liu, L. Huo, S. Chandrabose, K. Chen, G. Han, F. Qi, X. Meng, D. Xie, W. Ma, Y. Yi, J. M. Hodgkiss, F. Liu, J. Wang, C. Yang and Y. Sun, Adv. Mater., 2018, 30, 1707353 CrossRef.
- L. Guo, Q. Li, J. Ren, Y. Xu, J. Zhang, K. Zhang, Y. Cai, S. Liu and F. Huang, Energy Environ. Sci., 2022, 15, 5137–5148 RSC.
- X. Wu, Y. Wu, S. Peng, L. Xiao, Z. Xiao, W. Zhang, G. Ren, Y. Min and Y. Liu, Sol. RRL, 2023, 7, 2300136 CrossRef CAS.
- W. Gao, F. Qi, Z. Peng, F. R. Lin, K. Jiang, C. Zhong, W. Kaminsky, Z. Guan, C.-S. Lee, T. J. Marks, H. Ade and A. K. Y. Jen, Adv. Mater., 2022, 34, 2202089 CrossRef CAS PubMed.
- Y. Wei, Z. Chen, G. Lu, N. Yu, C. Li, J. Gao, X. Gu, X. Hao, G. Lu, Z. Tang, J. Zhang, Z. Wei, X. Zhang and H. Huang, Adv. Mater., 2022, 34, 2204718 CrossRef CAS.
- L. Kong, Z. Zhang, N. Zhao, Z. Cai, J. Zhang, M. Luo, X. Wang, M. Chen, W. Zhang, L. Zhang, Z. Wei and J. Chen, Adv. Energy Mater., 2023, 13, 2300763 CrossRef CAS.
- S. Luo, C. Li, J. Zhang, X. Zou, H. Zhao, K. Ding, H. Huang, J. Song, J. Yi, H. Yu, K. S. Wong, G. Zhang, H. Ade, W. Ma, H. Hu, Y. Sun and H. Yan, Nat. Commun., 2023, 14, 6964 CrossRef CAS PubMed.
- L. Zhu, M. Zhang, J. Xu, C. Li, J. Yan, G. Zhou, W. Zhong, T. Hao, J. Song, X. Xue, Z. Zhou, R. Zeng, H. Zhu, C.-C. Chen, R. C. I. MacKenzie, Y. Zou, J. Nelson, Y. Zhang, Y. Sun and F. Liu, Nat. Mater., 2022, 21, 656–663 CrossRef CAS PubMed.
- D. Li, N. Deng, Y. Fu, C. Guo, B. Zhou, L. Wang, J. Zhou, D. Liu, W. Li, K. Wang, Y. Sun and T. Wang, Adv. Mater., 2023, 35, 2208211 CrossRef CAS PubMed.
- L. Wang, C. Chen, Y. Fu, C. Guo, D. Li, J. Cheng, W. Sun, Z. Gan, Y. Sun, B. Zhou, C. Liu, D. Liu, W. Li and T. Wang, Nat. Energy, 2024, 9, 208–218 CrossRef CAS.
- Y. Sun, L. Wang, C. Guo, J. Xiao, C. Liu, C. Chen, W. Xia, Z. Gan, J. Cheng, J. Zhou, Z. Chen, J. Zhou, D. Liu, T. Wang and W. Li, J. Am. Chem. Soc., 2024, 146, 12011–12019 CrossRef CAS.
- Y. Jiang, S. Sun, R. Xu, F. Liu, X. Miao, G. Ran, K. Liu, Y. Yi, W. Zhang and X. Zhu, Nat. Energy, 2024, 9, 975–986 CrossRef CAS.
- D. Qiu, H. Zhang, C. Tian, J. Zhang, L. Zhu, Z. Wei and K. Lu, Adv. Mater., 2023, 35, 2307398 CrossRef CAS PubMed.
- T. N. Duan, W. Y. Feng, Y. L. Li, Z. X. Li, Z. Zhang, H. Z. Liang, H. B. Chen, C. Zhong, S. Jeong, C. D. Yang, S. S. Chen, S. R. Lu, O. A. Rakitin, C. X. Li, X. J. Wan, B. Kan and Y. S. Chen, Angew. Chem., Int. Ed., 2023, 62, e202308832 CrossRef CAS.
- Z. Chen, J. Zhu, D. Yang, W. Song, J. Shi, J. Ge, Y. Guo, X. Tong, F. Chen and Z. Ge, Energy Environ. Sci., 2023, 16, 3119–3127 RSC.
- C. Zhang, J. Li, W. Deng, J. Dai, J. Yu, G. Lu, H. Hu and K. Wang, Adv. Funct. Mater., 2023, 33, 2301108 CrossRef CAS.
- L. Xiao, B. He, Q. Hu, L. Maserati, Y. Zhao, B. Yang, M. A. Kolaczkowski, C. L. Anderson, N. J. Borys, L. M. Klivansky, T. L. Chen, A. M. Schwartzberg, T. P. Russell, Y. Cao, X. Peng and Y. Liu, Joule, 2018, 2, 2154–2166 CrossRef CAS.
- X. Ma, A. Zeng, J. Gao, Z. Hu, C. Xu, J. H. Son, S. Y. Jeong, C. Zhang, M. Li, K. Wang, H. Yan, Z. Ma, Y. Wang, H. Y. Woo and F. Zhang, Natl. Sci. Rev., 2021, 8, nwaa305 CrossRef.
- S. Cheng, J. Qiao, P. Lu, W. Qin and X. Hao, Chin. J Chem., 2024, 42, 1275–1283 CrossRef CAS.
- N. Zhang, K. Jiang, F. R. Lin, Y. An, G. Du, T. Xia, A. K. Y. Jen and H.-L. Yip, Mater. Today Energy, 2024, 42, 101548 CrossRef CAS.
- K. N'Konou, S. Y. Kim and N. Y. Doumon, Carbon Energy, 2024, e579 CrossRef.
- N. Y. Doumon, F. V. Houard, J. Dong, P. Christodoulis, M. V. Dryzhov, G. Portale and L. J. A. Koster, J. Mater. Chem. C, 2019, 7, 5104–5111 RSC.
- S. Jeong, A. Rana, J.-H. Kim, D. Qian, K. Park, J.-H. Jang, J. Luke, S. Kwon, J. Kim, P. S. Tuladhar, J.-S. Kim, K. Lee, J. R. Durrant and H. Kang, Adv. Sci., 2023, 10, 2206802 CrossRef CAS PubMed.
- N. Gasparini, A. Salleo, I. McCulloch and D. Baran, Nat. Rev. Mater., 2019, 4, 229–242 CrossRef.
- Q. An, F. Zhang, J. Zhang, W. Tang, Z. Deng and B. Hu, Energy Environ. Sci., 2016, 9, 281–322 RSC.
- R. Ma, T. Liu, Z. Luo, K. Gao, K. Chen, G. Zhang, W. Gao, Y. Xiao, T.-K. Lau, Q. Fan, Y. Chen, L.-K. Ma, H. Sun, G. Cai, T. Yang, X. Lu, E. Wang, C. Yang, A. K. Y. Jen and H. Yan, ACS Energy Lett., 2020, 5, 2711–2720 CrossRef CAS.
- H. Liu, Z. Chen, R. Peng, Y. Qiu, J. Shi, J. Zhu, Y. Meng, Z. Tang, J. Zhang, F. Chen and Z. Ge, Chem. Eng. J., 2023, 474, 145807 CrossRef CAS.
- W. Peng, Y. Lin, S. Y. Jeong, Z. Genene, A. Magomedov, H. Y. Woo, C. Chen, W. Wahyudi, Q. Tao, J. Deng, Y. Han, V. Getautis, W. Zhu, T. D. Anthopoulos and E. Wang, Nano Energy, 2022, 92, 106681 CrossRef CAS.
- D. Li, L. Wang, C. Guo, Y. Liu, B. Zhou, Y. Fu, J. Zhou, D. Liu, W. Li and T. Wang, ACS Mater. Lett., 2023, 5, 2065–2073 CrossRef CAS.
- D. Li, L. Zhu, X. Liu, W. Xiao, J. Yang, R. Ma, L. Ding, F. Liu, C. Duan, M. Fahlman and Q. Bao, Adv. Mater., 2020, 32, 2002344 CrossRef CAS PubMed.
- P. Bi, S. Zhang, T. Xiao, M. Cui, Z. Chen, J. Ren, C. Qin, G. Lu, X. Hao and J. Hou, Sci. China: Chem., 2021, 64, 599–607 CrossRef CAS.
- J. Gao, N. Yu, Z. Chen, Y. Wei, C. Li, T. Liu, X. Gu, J. Zhang, Z. Wei, Z. Tang, X. Hao, F. Zhang, X. Zhang and H. Huang, Adv. Sci., 2022, 9, 2203606 CrossRef CAS.
- L. Xiao, X. Wu, G. Ren, M. A. Kolaczkowski, G. Huang, W. Tan, L. Ma, Y. Liu, X. Peng, Y. Min and Y. Liu, Adv. Funct. Mater., 2021, 31, 2105304 CrossRef CAS.
- T. Wang, X. Wang, R. Yang and C. Li, Sol. RRL, 2021, 5, 2100496 CrossRef.
- D. Lee, H. Hwang, D. H. Sin, C. Park, S. G. Han, J. Mun, J. Noh, S. H. Kim, H. Kim, H. Lee, C. Lee, J. Rho, K. Cho and M. S. Jeong, ACS Energy Lett., 2021, 6, 2610–2618 CrossRef CAS.
- A. A. Mohapatra, V. Tiwari and S. Patil, Energy Environ. Sci., 2021, 14, 302–319 RSC.
- S. Peng, J. Luo, P. Li, L. Xiao, C. Yang, Y. Jin, R. Lin, Y. Huo, Y. Liu and Y. Min, Chem. Eng. J., 2023, 474, 145501 CrossRef CAS.
- H. Lu, G. Ran, Y. Liu, Z. Pei, W. Liu, Y. Liu, Z. Tang, W. Zhang and Z. Bo, Adv. Funct. Mater., 2023, 33, 2301866 CrossRef CAS.
- M. Liu, X. Ge, X. Jiang, F. Guo, S. Gao, Q. Peng, L. Zhao and Y. Zhang, Adv. Funct. Mater., 2023, 33, 2300214 CrossRef CAS.
- Q. Liu, Y. Jiang, K. Jin, J. Qin, J. Xu, W. Li, J. Xiong, J. Liu, Z. Xiao and K. Sun, Sci. Bull., 2020, 65, 272–275 CrossRef CAS PubMed.
- J. Luo, S. Peng, J. Zhang, Y. Jin, D. Chen, Y. Mu, L. Xiao, D. Hu, Q.-D. Yang, S. Ji, Y. Min and Y. Huo, Org. Electron., 2023, 120, 106860 CrossRef CAS.
- R. Xu, Y. Jiang, F. Liu, W. Su, W. Liu, S. Xu, H. Fan, C. Jiang, Q. Zong, W. Zhang and X. Zhu, Chem. Eng. J., 2023, 464, 142507 CrossRef CAS.
- N. Yi, Q. Ai, W. Zhou, L. Huang, L. Zhang, Z. Xing, X. Li, J. Zeng and Y. Chen, Chem. Mater., 2019, 31, 10211–10224 CrossRef CAS.
- X. Xu, Y. Li and Q. Peng, Adv. Mater., 2022, 34, 2107476 CrossRef CAS PubMed.
- J. Luo, S. Peng, J. Zhang, Y. Jin, D. Chen, Y. Mu, L. Xiao, D. Hu, Q.-D. Yang, S. Ji, Y. Min and Y. Huo, Org. Electron., 2023, 120, 106860 CrossRef CAS.
- X. K. Chen, M. K. Ravva, H. Li, S. M. Ryno and J. L. Brédas, Adv. Energy Mater., 2016, 6, 1601325 CrossRef.
- S. Cai, L. Chen, D. Zha and Y. Chen, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 624–634 CrossRef CAS.
- R. Ma, H. Li, T. A. D. Peña, X. Xie, P. W.-K. Fong, Q. Wei, C. Yan, J. Wu, P. Cheng, M. Li and G. Li, Adv. Mater., 2023, 35, 2304632 Search PubMed.
- Q. Zhao and F. He, J. Energy Chem., 2024, 93, 174–192 CrossRef CAS.
- A. R. Clapp, I. L. Medintz, J. M. Mauro, B. R. Fisher, M. G. Bawendi and H. Mattoussi, J. Am. Chem. Soc., 2004, 126, 301–310 CrossRef CAS PubMed.
- L. Xiao, T. Liang, K. Gao, T. Lai, X. Chen, F. Liu, T. P. Russell, F. Huang, X. Peng and Y. Cao, ACS Appl. Mater. Interfaces, 2017, 9, 29917–29923 CrossRef CAS PubMed.
- K. Yu, W. Song, Y. Li, Z. Chen, J. Ge, D. Yang, J. Zhang, L. Xie, C. Liu and Z. Ge, Small Struct., 2021, 2, 2100099 CrossRef CAS.
- Y. Yan, Y. Zhang, Y. Liu, Y. Shi, D. Qiu, D. Deng, J. Zhang, B. Wang, M. A. Adil and K. Amin, Adv. Energy Mater., 2022, 12, 2200129 CrossRef CAS.
- K. Nakano, Y. Chen, B. Xiao, W. Han, J. Huang, H. Yoshida, E. Zhou and K. Tajima, Nat. Commun., 2019, 10, 2520 CrossRef PubMed.
- T. Kirchartz, B. E. Pieters, J. Kirkpatrick, U. Rau and J. Nelson, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 115209 CrossRef.
- F. Urbach, Phys. Rev., 1953, 92, 1324 CrossRef CAS.
- H. Zhuo, X. Li, J. Zhang, C. Zhu, H. He, K. Ding, J. Li, L. Meng, H. Ade and Y. Li, Nat. Commun., 2023, 14, 7996 CrossRef CAS.
- X. Liao, Q. Xie, Y. Guo, Q. He, Z. Chen, N. Yu, P. Zhu, Y. Cui, Z. Ma, X. Xu, H. Zhu and Y. Chen, Energy Environ. Sci., 2022, 15, 384–394 RSC.
- Z. Zhang, D. Deng, Y. Li, J. Ding, Q. Wu, L. Zhang, G. Zhang, M. J. Iqbal, R. Wang, J. Zhang, X. Qiu and Z. Wei, Adv. Energy Mater., 2022, 12, 2102394 CrossRef CAS.
- H. Liang, Y. Wang, X. Guo, D. Yang, X. Xia, J. Wang, L. Zhang, Y. Shi, X. Lu and M. Zhang, J. Mater. Chem. A, 2022, 10, 10926–10934 RSC.
- F. Liu, L. Zhou, W. Liu, Z. Zhou, Q. Yue, W. Zheng, R. Sun, W. Liu, S. Xu, H. Fan, L. Feng, Y. Yi, W. Zhang and X. Zhu, Adv. Mater., 2021, 33, 2100830 CrossRef CAS.
- X. Wang, J. Wang, P. Wang, C. Han, F. Bi, J. Wang, N. Zheng, C. Sun, Y. Li and X. Bao, Adv. Mater., 2023, 35, 2305652 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tc03741c |
| ‡ S. P and R. L contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |






