Enhancing the NO2 detection ability of surface acoustic wave sensors with ZnO-decorated N-doped porous carbon nanosheets†
Xue
Li
a,
Yuan
Feng
 a,
Haifeng
Lv
a,
Junjie
Shi
b,
Yuanjun
Guo
a,
Haifeng
Lv
a,
Junjie
Shi
b,
Yuanjun
Guo
 *a,
Sean
Li
*a,
Sean
Li
 *b and
Xiaotao
Zu
*a
*b and
Xiaotao
Zu
*a
aKey School of Physics, University of Electronic Science and Technology of China, Chengdu, 610054, P. R. China. E-mail: xtzu@uestc.edu.cn; guoyuanjun@uestc.edu.cn
bIn School of Materials Science and Engineering, University of New South Wales, Sydney, NSW, Australia. E-mail: sean.li@unsw.edu.au
First published on 29th October 2024
Abstract
Effective detection of nitrogen dioxide (NO2) is crucial for environmental safety and human health. Gas sensors utilizing surface acoustic wave (SAW) technology hold significant promise for detecting hazardous gases, but their performance heavily depends on the intrinsic properties of the sensing layer materials. In this study, we elaborate on using ZnO nanoparticle-dispersed N-doped porous carbon nanosheets (ZnO@N-PCNs) for SAW sensors aimed at achieving rapid NO2 detection at room temperature. The resultant ZnO@N-PCNs SAW gas sensor exhibits a significant frequency shift of approximately −4.4 kHz, which is much higher than that of the pristine N-PCNs SAW sensor at a NO2 concentration of 20 ppm. It responds quickly when exposed to NO2 gas, and demonstrates specific selectivity and good reproducibility over both short and long terms. This work reveals the sensing properties of ZnO-optimized SAW sensors and provides valuable guidance for the development of high-sensitivity SAW sensors.
1. Introduction
Nitrogen dioxide (NO2), as one of the typical harmful gases polluting the air, has a negative impact on human health and environmental safety. Long-term exposure to high concentrations (ppm) of NO2 gas can readily lead to respiratory diseases such as asthma and lung cancer, and in some cases, even result in death.1,2 Even at low concentrations, NO2 gas contaminants can damage hemoglobin in the blood over time, leading to health crises such as respiratory irritation, headaches, and oxygen deficiency.3,4 Additionally, the emission of NO2 into the atmosphere contributes to air pollution, acid rain, and other environmental problems, including vegetation withering and land degradation, which cause irreversible damage to the environment. Hence, the development of advanced gas sensors for real-time monitoring of NO2 is urgently needed. These gas sensors should be simple to use, and have low detection limits, high sensitivity, precise selectivity, and high reproducibility.A variety of gas sensors, such as electrochemical sensors,5 quartz crystal sensors,6 semiconductor sensors,7 optics sensors,8 and surface acoustic wave (SAW) sensors,9,10 have been extensively utilized to detect NO2 gas. Most of these sensors are characterized by being relatively expensive, having low sensitivity and poor selectivity, and requiring harsh testing environments.10,11 In contrast, SAW sensors are considered a promising alternative for NO2 gas detection due to their affordability, portability, low detection limits, excellent sensitivity, and rapid response/recovery time at room temperature.12 As an acoustic wave resonator, the acoustic energy of a SAW device is concentrated on the surface area between the double interdigital transducers (IDTs). Disturbances from external stimuli can cause changes in the quality, resistance, and elasticity of the sensitive layer as shown in Fig. S1 (ESI†). These factors can influence the speed of acoustic wave propagation, typically resulting in changes in frequency and amplitude.12,13 Therefore, selecting appropriate sensing materials with optimal structures and compositions is crucial for engineering high-sensitivity SAW sensors.
In recent years, two-dimensional (2D) nanomaterials featuring various fine nanostructures, such as graphene and its derivative, g-C3N4, and MXene, have emerged as key players in enhancing the sensitivity of SAW sensors and significantly accelerating gas adsorption kinetics. Their multiple advantages,14 including excellent electrical conductivity, large specific surface areas, and fine pore structures, contribute to these improvments.15,16 However, recent studies have indicated that the lack of active adsorption sites is a major issue limiting the sensing performance of SAW sensors, often resulting in low sensitivity and poor selectivity to gases.
To address this, various modification methods, including doping with heteroatoms,17 decorating with metal oxide nanoparticles,18 constructing heterostructures,19 and more, have been proposed to optimize these 2D nanomaterials, further enhancing the sensing properties of the corresponding SAW sensors. Among these methods, decorating 2D materials with nanosized transition metal oxide particles such as TiO2, WO3, SnO2, and CuO is particularly advantageous due to their adjustable structures, small size and dimensions, and high electron transfer capabilities.20
These polar materials can also provide abundant adsorption sites, enhance the chemical affinity of 2D surfaces for harmful gas molecules, and thereby further improve sensing capabilities. For example, Sun et al. reported that immobilizing SnO2 nanocrystals on the GO surface with a porous structure resulted in a very low detection limit, outstanding sensitivity, and a rapid response time compared to the original materials.21 Kedharesara et al. reported a TiO2 nanoplate decorated 2D g-C3N4 composite, which has a large surface area, numerous oxygen vacancies, and functional groups that provide abundant active sites for the adsorption and diffusion of NO2 molecules.22 Similarly, Zhang et al. prepared a GO-MoS2/SnO2 ternary composite that achieved excellent sensing performance, primarily due to its large surface area, increased adsorption and reaction sites, and enhanced conductivity through the heterojunctions.23 ZnO offers numerous advantages, including chemical and thermal stability, high electron mobility, nontoxicity, a large exciton binding energy, and low cost. These merits are expected to enhance electron transport kinetics and provide abundant active sites, making ZnO an excellent candidate for 2D sensing materials in SAW sensors for NO2 detection.24–26 However, ZnO nanoparticles have not yet been adopted as a candidate to decorate 2D sensing materials, and their behavior in SAW sensors for NO2 detection has not been investigated.
In this study, ZnO nanoparticle decorated N-doped porous carbon nanosheets (ZnO@N-PCNs) are designed for the first time as sensing layer materials for NO2 detection using SAW sensors at room temperature. Experimental and theoretical results show that ZnO provides N-doped porous carbon nanosheets with abundant adsorption sites, significantly enhancing the NO2 detection capability of SAW sensors. The improved NO2 detection ability of ZnO@N-PCNs in SAW sensors is demonstrated by their high sensitivity, precise selectivity, and good reproducibility at room temperature. Additionally, we investigate the impact of various relative humidities on the sensing performance and elucidate the microscopic sensing mechanism of SAW sensors with ZnO@N-PCN sensing layers. Impressively, the theoretical calculations based on density functional theory (DFT) simulate the structure of different prepared samples from the atomic/electronic scale through the establishment of computational models. It simulated the complex reaction processes and calculated the adsorption energies of the sensing materials. This work not only elucidates the key properties of ZnO-decorated N-doped porous carbons in SAW sensors but also provides a new approach for designing highly efficient sensing materials for SAW sensors.
2. Results and discussion
2.1 Material design and characterization
ZnO@N-PCNs were derived through the impregnation method and high-temperature calcination, and are schematically depicted in Fig. 1a (see the ESI† for testing and preparative details). Fig. 1b shows the XRD patterns of N-PCNs and ZnO@N-PCNs. The N-PCNs display a broad diffraction peak at around 23°, corresponding to the lattice plane (002) of graphitic carbon, indicating a layered structure of carbon materials.27Fig. 1c indicates the fluffy morphology of the N-PCNs, which are composed of thin carbon nanosheets. They exhibit graphene-like microscopic morphology with wrinkles. In the XRD pattern of the ZnO@N-PCNs, strong characteristic peaks appear at 31.7°, 34.4°, and 36.2°, in addition to the peaks attributed to carbon. These emerging peaks correspond to (100), (002), and (101) lattice planes of ZnO (JCPDS NO. 36-1451), respectively. Fig. 1d clearly shows the distribution of ZnO nanoparticles, with an average size of 32.38 nm (Fig. S2, ESI†), on the surfaces of N-PCNs, resembling scattered pearls on plates. It can be observed that the growth of ZnO does not disrupt the overall morphology of N-PCNs. The TEM image in Fig. 1e further confirms that ZnO nanoparticles are uniformly distributed across the carbon nanosheets. Energy-dispersive X-ray (EDX) analysis was performed to identify the elements present in the ZnO@N-PCN composite (Fig. S3, ESI†). The EDX elemental mappings (Fig. 1f-i) clearly show the distribution of all elements in the ZnO@N-PCN composite, including C, N, O, and Zn.The chemical valence and orbital states of the synthesized sensing materials were further examined by XPS analysis. The distinct peaks in the survey spectrum (Fig. 2a) correspond to the elements of Zn, O, N, and C. In the XPS spectra of Zn 2p (Fig. 2b), two peaks attributed to Zn 2p3/2 and 2p1/2 are clearly visible, located at approximately 1022.7 and 1045.9 eV, respectively.28 This suggests the fully oxidized states of Zn species.28 The O 1s spectra show two peaks at 531.5 and 532.7 eV, respectively (Fig. 2c). The peak at 531.5 eV is attributed to oxygen vacancies or defects on the sample surface,21,22 while the peak at 532.7 eV is associated with absorbed active oxygens,28 and the peak at 530.2 eV is attributed to surface lattice oxygen combined with Zn2+. The high-resolution N 1s spectra of the sample in Fig. 2d show three distinct peaks corresponding to N (398.8 eV), pyrrolic N (399.7 eV), and graphitic N (400.6 eV).26,29 This result suggests that nitrogen has been successfully introduced into the sample, which is also supported by the EDX results. The presence of elemental N is beneficial for enhancing the electronic conductivity of carbon nanosheets. Fig. 2e depicts the XPS spectra of C 1s resolved into three distinct peaks at 284.8, 285.7, and 288.9 eV, respectively. These peaks are assigned to the C–C (epoxy and hydroxyl groups), C–N, and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds (carboxyl groups), respectively.23,27 FTIR analysis was conducted to further analyze the chemical structure and the surface functional groups of the ZnO@N-PCNs (Fig. S4, ESI†). The characteristic band located at 1646 cm−1 can be attributed to the stretching modes of C
O bonds (carboxyl groups), respectively.23,27 FTIR analysis was conducted to further analyze the chemical structure and the surface functional groups of the ZnO@N-PCNs (Fig. S4, ESI†). The characteristic band located at 1646 cm−1 can be attributed to the stretching modes of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C
C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, indicating that the deoxidation and hydroxylation reaction dominated the process of carbonation.30 The slight peak at 1054 cm−1 corresponds to C–O–C vibration.31
O, indicating that the deoxidation and hydroxylation reaction dominated the process of carbonation.30 The slight peak at 1054 cm−1 corresponds to C–O–C vibration.31
 | ||
| Fig. 2 (a) The XPS spectrum of the ZnO@N-PCNs; (b)–(e) XPS spectra of Zn 2p, O 1s, N 1s, and C 1s; (f) Raman spectra of the ZnO@N-PCNs and N-PCNs. | ||
There are two dominant Raman peaks positioned at 1352.9 and 1554.8 cm−1 in the spectra (Fig. 2f), both of which correspond to D and G bands of carbon materials, respectively.32 The D band usually represents the disordered structure of amorphous carbon and the G band suggests the sp2-hybridized graphitic structure of ones. The intensity ratio of the D band and G band (ID/IG) is a direct indicator of the graphitization degree of carbon. The calculated ID/IG value of N-PCNs is about 0.998, suggesting the partially graphitized carbon structure and further verifying the XRD results.33 Despite post-treatments via impregnation of ZnO, the ID/IG value of the sample material remains almost unchanged, demonstrating the structure/chemical stability of the N-PCNs. Meanwhile, the slight peaks around 379 and 575 cm−1 reflect the A1(TO) and A1(LO) phonon modes, which are usually represented by the oxygen vacancy and zinc interstitial defects of the ZnO sample.34 This phenomenon exactly validates the aforementioned-XPS analysis results.
The N-PCNs and ZnO@N-PCNs exhibit absorption in the UV-vis DRS spectra (Fig. S4a, ESI†), caused by the generation of electrons from the valence to the conduction band within the sensing material.35 At the same time, the ZnO@N-PCNs display a slight shift in the absorption edge (∼372 nm) in comparison with the N-PCNs (∼362 nm), which is attributed to the prominent light harvesting ability of the ZnO nanoparticles to enhance the periodic reflections of light (Fig. S5a, ESI†).35 Furthermore, the PL measurement explored the charge separation and recombination behaviors of the two samples. The ZnO@N-PCN composite clearly exhibits a lower intensity than the N-PCNs (Fig. S5b, ESI†), indicating the weaker recombination ability and excellent separation of photoinduced charge carriers in the ZnO@N-PCN composite, which is beneficial for light-assisted gas sensing reactions.35–37 Moreover, electrochemical impedance spectroscopy (EIS) was carried out on the two samples (Fig. S6, ESI†). The ZnO@N-PCN composite shows a smaller arc radius compared with the N-PCNs, demonstrating the smaller charge transfer resistance of the ZnO@N-PCNs. This implies faster interfacial charge transport of the change carriers and can assist in augmenting gas sensing fidelity.38 The N2 adsorption–desorption method was employed to analyze the specific surface area and porous structure of the as-prepared sensing materials. The ZnO@N-PCNs are characterized by a large BET-specific surface area of 541.24 m2 g−1 and an extensive pore size of 3.29 nm (Fig. S7a and b, ESI†). It may facilitate the adsorption of NO2 on the surface of the sensing layer and promote NO2 gas molecules to diffuse into the interior of the sensing film, further enhancing the large-area contact between the ZnO@N-PCNs and the target gas, thereby improving the sensing performance. To assess the intrinsic characteristic of the SAW sensor, the working frequency (WF) and the insertion loss (IL) of both samples were also measured, as shown in Fig. S8 (ESI†). The ZnO@N-PCNs-based SAW sensor possesses a WF of 201.16 MHz and an IL of −22.51 dB, the N-PCNs-based SAW sensor possesses a WF of 201.17 MHz and an IL of −20.49 dB, and the bare SAW sensor possesses a WF of 201.18 MHz and an IL of −16.88 dB. The discrepancy of WF results from the increased mass loading, causing a frequency shift of SAW devices to a lower level. The difference in the IL is attributed to the decrease in the SAW energy at the different interface sensing layers.22,39
2.2 DFT calculations
To further investigate the adsorption mechanism of NO2 molecules on the ZnO@N-PCN surface, the adsorption of optimal energy models of NO2 molecules on the ZnO, N-PCN, and ZnO@N-PCN surfaces was calculated using the first principles of density functional theory (DFT).40,41 Computational details are described in the ESI.† The adsorption energies of the optimal O2 adsorption configuration are shown in Fig. S9 (ESI†). The adsorption energies of O2 on the ZnO and N-PCN surfaces are 0.92 eV and 0.54 eV, respectively. The large adsorption energy indicates that ZnO can readily absorb more oxygen molecules to form additional O2−, which subsequently reacts with NO2 molecules to form NO3−. Additionally, the preparation of ZnO nanoparticles generates numerous oxygen vacancies, enabling the absorption of oxygen molecules from the air. These molecules then combine with electrons on the composite surface to form various oxygen species, thereby enhancing the adsorption of more target gases. Fig. 3a–c show the adsorption configurations and corresponding adsorption energies of NO2 molecules on the N-PCNs, ZnO, and ZnO@N-PCNs. The interaction between NO2 and carbon is sustained via strong covalent N–O bonds with a binding energy of 0.80 eV. ZnO also interacts with NO2 molecules via N–O bonds with a binding energy of dual bonding of 2.13 eV. Differently, a dual binding mechanism based on N–O and Zn–O bonds is observed between NO2 molecules and ZnO@N-PCNs, with a binding energy of 2.75 eV. Particularly, ZnO with adsorptive sites can provide more active sites to adsorb NO2 molecules, while N-PCNs provide a large specific area to support the attachment of more adsorptive sites to it. In short, the synergetic effect promotes the composite (ZnO@N-PCNs) to adsorb more NO2 on the composite (Fig. 3d). It is noteworthy that the adsorption energy of NO2 on the surface of ZnO@N-PCNs is greater than that of O2, indicating that the adsorption is exothermic. | ||
| Fig. 3 The favorable configurations of NO2 adsorption on (a) N-PCNs, (b) ZnO, and (c) ZnO@N-PCNs. (d) The adsorption energies of NO2 on different sensing materials. | ||
2.3 Sensing performance
The frequency shifts of different NO2 concentrations, ranging from 1 ppm to 100 ppm, were measured at room temperature (Fig. 4a and b). Clearly, the frequency shift of both types of SAW sensors gradually increases with the concentrations of the NO2 gas. Notably, a larger negative frequency shift of ∼9.14 kHz (nearly ∼1.88-fold increase) compared to that of the N-PCN SAW sensor (∼4.86 kHz) at a concentration of 100 ppm NO2 (Fig. 4c) is observed in the ZnO@N-PCNs based-SAW sensor. This can be attributed to the abundant adsorption sites dispersed onto carbon nanosheets with a large specific surface area and heterostructure-induced fast electron transport for NO2-associated surface reactions. Fig. 4d shows little change over 7 cycles towards a concentration of 10 ppm NO2, indicating the excellent short-time repeatability of the ZnO@N-PCN SAW sensor. The selectivity of ZnO@N-PCN and N-PCN SAW sensors was evaluated by exposing them to 20 ppm of interfering gases, including CH2Cl2, NH3, H2S, H2, C6H14 and NO2 (Fig. 4e). Notably, the frequency shift of the ZnO@N-PCN-based SAW sensor exhibits a higher negative shift at a concentration of 20 ppm NO2 gas. This result indicates that the ZnO@N-PCNs SAW sensor possesses excellent selectivity for NO2 gas. The enhanced selectivity of the ZnO@N-PCNs SAW sensor towards NO2 gas can be attributed to the abundant adsorption sites dispersed onto N-doped carbon nanosheets with a large specific surface area and fast electron transport. Meanwhile, NO2 has the highest affinity for electrons and the strongest ability to capture electrons compared with the aforementioned-gases, which favors promoting surface reactions with semiconductor oxides through O and N atoms.22 The ZnO@N-PCNs SAW sensor also exhibits long-term stability for every 7 days consecutively up to 63 days, as shown in Fig. 4f, with only slight fluctuations observed. Table S1 (ESI†) summarizes the sensing performance of various SAW sensors with different sensing layers for NO2 detection, covering sensitive materials, operating temperature, sensitivity, and response/recovery time. It clearly shows the advantages of the ZnO@N-PCNs SAW sensor in NO2 detection at room temperature.The effect of different humidity levels, including 20%, 32%, 66%, and 88%, on the frequency shifts was further measured at a concentration of 20 ppm NO2 (Fig. S10a, ESI†). The response shift of the ZnO@N-PCNs SAW sensor tends to increase with the humidity. This experimental phenomenon indicates that adsorbing NO2 plays a dominant role in the negative frequency shift when exposed to low humidity. As the humidity increases, the dominant factor affecting the sensor's response becomes the adsorption of H2O molecules, as the physically adsorbed water molecules add weight to the sensing layer.21,42 The high tolerance of the ZnO@N-PCNs SAW sensor was further demonstrated by repeated testing under high humidity conditions, as shown in Fig. S10b (ESI†).
2.4 Sensing mechanism
The speed of acoustic wave propagation over the sensing layer in the SAW gas sensor is mainly influenced by three factors: mass, conductivity, and elasticity, which manifests as changes in frequency.43,44 The relationship between the frequency shift and these parameters can be defined using the following equation (eqn (1))45 | (1) |
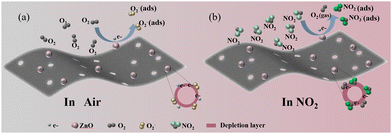
According to the above experimental results, both types of SAW sensors exhibit a negative shift when exposed to NO2 gas, indicating that the main factor for the augmented negative frequency shift should be ascribed to the mass loading or the electrical loading.22,23,35,39 Accordingly, the effect of elastic loading on frequency shifts can be excluded from the sensing mechanism. An impedance-type sensor was manufactured and measured under the same conditions to evaluate and demonstrate the main contribution of mass and conductivity to the frequency response. As depicted in Fig. S11a (ESI†), the resistance of ZnO@N-PCNs obviously increased when exposed to 100 ppm NO2 gas, whereas the resistance of N-PCNs slightly decreased. As can be observed from Fig. S11b (ESI†), the ZnO@N-PCNs sensor exhibits a sensitivity about 0.2%  with a response/recovery time of 84.6 s/100 s, respectively.22,35 Besides, the relevant data were substituted into the third term of eqn (1) to calculate the resonant frequency shift. It shows that the resonant frequency shift caused by resistance changes can be almost negligible at a concentration of 100 ppm NO2. These results imply that the frequency change caused by resistance accounts for a small, negligible proportion of the overall frequency shift, further clarifying that the mass loading effect is the dominant cause of the negative frequency shift in the ZnO@N-PCNs SAW sensor. This also provides a feasible method to quantitatively calculate the mass loading effect of the SAW gas sensor. The gas sensing mechanism schematic diagram of the ZnO@N-PCNs is depicted in Fig. 5. In an air atmosphere, O2 molecules are attached to the ZnO@N-PCN surface and are ionized into oxygen species (e.g., O2− and O2−) by accepting free electrons from the conduction bands. This process is accompanied by the generation of an electron depletion layer (eqn (2) and (3)), as shown in Fig. 5a. When the ZnO@N-PCNs are exposed to a NO2 gas atmosphere (Fig. 5b), the generated oxygen species chemically adsorb NO2 molecules to produce some of the NO2− and NO3− ions, which consume electrons to form a deeper depletion layer on the surface. This process is accompanied by the decay of electron concentration and the increase in sample resistance, as given in the following eqn (4)–(6). It should be noted that the negatively charged sites located on the pyridinic-N of N-PCNs contribute to the process of adsorbing NO2 gas molecules, and a number of active oxygens species adsorbed on the surface of the sensing material also provide desired sites for the rapid adsorption of NO2.22 Moreover, the large surface area and abundant defective oxygen vacancies of the ZnO@N-PCNs provide more adsorption sites that are beneficial for adsorbing more oxygen and target gas molecules, thus facilitating the fast diffusion kinetics of NO2via quick adsorption and desorption.46
with a response/recovery time of 84.6 s/100 s, respectively.22,35 Besides, the relevant data were substituted into the third term of eqn (1) to calculate the resonant frequency shift. It shows that the resonant frequency shift caused by resistance changes can be almost negligible at a concentration of 100 ppm NO2. These results imply that the frequency change caused by resistance accounts for a small, negligible proportion of the overall frequency shift, further clarifying that the mass loading effect is the dominant cause of the negative frequency shift in the ZnO@N-PCNs SAW sensor. This also provides a feasible method to quantitatively calculate the mass loading effect of the SAW gas sensor. The gas sensing mechanism schematic diagram of the ZnO@N-PCNs is depicted in Fig. 5. In an air atmosphere, O2 molecules are attached to the ZnO@N-PCN surface and are ionized into oxygen species (e.g., O2− and O2−) by accepting free electrons from the conduction bands. This process is accompanied by the generation of an electron depletion layer (eqn (2) and (3)), as shown in Fig. 5a. When the ZnO@N-PCNs are exposed to a NO2 gas atmosphere (Fig. 5b), the generated oxygen species chemically adsorb NO2 molecules to produce some of the NO2− and NO3− ions, which consume electrons to form a deeper depletion layer on the surface. This process is accompanied by the decay of electron concentration and the increase in sample resistance, as given in the following eqn (4)–(6). It should be noted that the negatively charged sites located on the pyridinic-N of N-PCNs contribute to the process of adsorbing NO2 gas molecules, and a number of active oxygens species adsorbed on the surface of the sensing material also provide desired sites for the rapid adsorption of NO2.22 Moreover, the large surface area and abundant defective oxygen vacancies of the ZnO@N-PCNs provide more adsorption sites that are beneficial for adsorbing more oxygen and target gas molecules, thus facilitating the fast diffusion kinetics of NO2via quick adsorption and desorption.46
| O2(gas) → O2(ads) | (2) |
| O2(ads) + e− → O2−(ads) | (3) |
| NO2 + e− → NO2−(ads) | (4) |
| NO2 + O2−(ads) → NO2 + O2 | (5) |
| 2NO2 + O2−(ads) + e− → 2NO3−(ads) | (6) |
3. Conclusions
In this work, ZnO@N-PCNs have been designed as a sensitive material for SAW devices to detect NO2 gas. The porous sensing material, with a large surface area, is deposited onto the SAW device using the drop coating method. It exhibits an outstanding frequency shift of approximately –4.4 kHz at a NO2 concentration of 20 ppm at room temperature. The ZnO@N-PCNs SAW sensor demonstrates good stability, a fast response time, outstanding selectivity, and excellent short- and long-term repeatability. Furthermore, increased humidity significantly enhances the sensor's performance, while the prepared SAW sensor maintains good stability even under high humidity conditions. The enhanced sensing performance can be attributed to the synergistic effects of the composition and structure of ZnO@N-PCNs, including an enlarged specific surface area, increased adsorption energy, and additional active adsorption sites. These factors facilitate the diffusion and adsorption of NO2 molecules on sensitive materials. This work is expected to inspire a novel design pathway for the sensing materials used in SAW gas sensors.Author contributions
Xue Li: conceptualization, methodology, investigation, experiments, data curation, writing – original draft, and writing – review & editing. Yuan Feng: computational analysis and modeling. Haifeng Lv: formal analysis and funding acquisition. Junjie Shi: writing – review & editing. Yuanjun Guo: discussion, resources, methodology, visualization, and writing – review & editing. Sean Li: writing – review & editing. Xiaotao Zu: resources and funding acquisition.Data availability
The authors declare that data will be made available on request.Conflicts of interest
The authors declare no competing interests.Acknowledgements
This work was financially supported by the Key Project of National Natural Science Foundation of China-China Academy of Engineering Physics joint Foundation (Grant No. U1830204) and the Natural Science Foundation of Sichuan Province (NSFSC, Grant No. 24NSFSC1271).References
- S. F. Camilleri, G. H. Kerr, S. C. Anenberg and D. E. Horton, All-Cause NO2-Attributable Mortality Burden and Associated Racial and Ethnic Disparities in the United States, Environ. Sci. Technol. Lett., 2023, 10, 1159–1164 CrossRef CAS PubMed.
- M. Sun, A. Hanif, T. Wang, Q. Gu and J. Shang, Ambient temperature NO2 removal by reversible NO2 adsorption on copper-based metal-organic frameworks (MOFs)-derived nanoporous adsorbents, Sep. Purif. Technol., 2023, 314, 123563 CrossRef CAS.
- H. Long, A. H. Trochimczyk, T. Pham, Z. Tang, T. Shi, A. Zettl, C. Carraro, M. A. Worsley and R. Maboudian, High Surface Area MoS2/Graphene Hybrid Aerogel for Ultrasensitive NO2 Detection, Adv. Funct. Mater., 2016, 26, 5158–5165 CrossRef CAS.
- H. J. Lee, T. Kuwayama and M. FitzGibbon, Simultaneous decreases in NO2 levels and disparities in California during the COVID-19 pandemic, Atmos. Environ., 2024, 318, 120214 CrossRef CAS.
- L. Zhu, L. Wei, K. Cheng, K. Li, Q. Rong, C. Zhang, Y. Yu, C. Wang and Y. Guo, Synthesize of Ni0.75Zn0.25Fe2O4 Spinel Oxide as a Working Electrode for Formaldehyde Electrochemical Gas Sensors, J. Electrochem. Soc., 2024, 171, 017507 CrossRef CAS.
- S. Chen, X. Duan, C. Li, S. Li, P. Li, D. Su, X. Sun, Y. Guo, W. Chen and Z. Wang, La-Ce-MOF nanocomposite coated quartz crystal microbalance gas sensor for the detection of amine gases and formaldehyde, J. Hazard. Mater., 2024, 467, 133672 CrossRef CAS.
- Z. Wang, J. Hu, J. Lu, X. Zhu, X. Zhou, L. Huang and L. Chi, Charge Transport Manipulation via Interface Doping: Achieving Ultrasensitive Organic Semiconductor Gas Sensors, ACS Appl. Mater. Interfaces, 2023, 15, 8355–8366 CrossRef CAS.
- C. Li, X. Han, F. Ma, X. Zhao, Z. Wang, H. Qi, M. Guo and K. Chen, Multiplexed fiber-optic photoacoustic sensors for simultaneous detection of multi-point gases, Sens. Actuators, B, 2024, 399, 134801 CrossRef CAS.
- J. Yang, T. Wang, C. Zhu, X. Yin, P. Dong and X. Wu, A Composite gas-phase explosives sensor with frequency-resistance dual-signal display based on surface acoustic wave, ACS Appl. Electron. Mater., 2023, 5, 1526–1535 CrossRef CAS.
- G. F. Fine, L. M. Cavanagh, A. Afonja and R. Binions, Metal oxide semi-conductor gas sensors in environmental monitoring, Sensors, 2010, 10, 5469–5502 CrossRef CAS.
- L. Wang, Y. Cheng, S. Gopalan, F. Luo, K. Amreen, R. K. Singh, S. Goel, Z. Lin and R. Naidu, Review and perspective: gas separation and discrimination technologies for current gas sensors in environmental applications, ACS Sens., 2023, 8, 1373–1390 CrossRef CAS PubMed.
- X. Li, W. Sun, W. Fu, H. Lv, X. Zu, Y. Guo, D. Gibson and Y. Q. Fu, Advances in sensing mechanisms and micro/nanostructured sensing layers for surface acoustic wave-based gas sensors, J. Mater. Chem. A, 2023, 11, 9216–9238 RSC.
- A. Mujahid and F. L. Dickert, Surface acoustic wave (SAW) for chemical sensing applications of recognition layers, Sensors, 2017, 17, 2716 CrossRef.
- D. Zhang, W. Pan, M. Tang, D. Wang, S. Yu, Q. Mi, Q. Pan and Y. Hu, Diversiform gas sensors based on two-dimensional nanomaterials, Nano Res., 2023, 16, 11959–11991 CrossRef.
- Q. L. Hao, L. Q. Yu, X. Q. Yang, R. T. Xu and Y. K. Lv, Two-Dimensional Nitrogen-Doped Carbon Nanosheets Derived from g-C3N4/ZIF-8 for Solid-Phase Microextraction in Exhalation of Esophageal Cancer Patients, ACS Appl. Mater. Interfaces, 2023, 15, 5990–5997 CrossRef CAS.
- S. Li, L. Yu, C. Zhang, S. Wang, R. Li, F. Zhao, M. Yin, H. Du, Y. Jia and X. Fan, Template Based Synthesis of Porous Graphdiyne Nanosheet for Reversible and Fast NO2 Detection by UV Irradiation, ChemPhysChem, 2023, 24, e2023000 Search PubMed.
- K. Nithyakalyani and M. C. J. Christ, ZnO quantum dots @ nitrogen and sulfur Co-doped porous carbon nanosheets for the detection of lung cancer biomarkers in exhaled breath, Mater. Sci. Semicond. Process., 2023, 167, 107797 CrossRef CAS.
- S. Absalan, S. Nasresfahani and M. H. Sheikhi, High-performance carbon monoxide gas sensor based on palladium/tin oxide/porous graphitic carbon nitride nanocomposite, J. Alloys Compd., 2019, 795, 79–90 CrossRef CAS.
- L. Jiang, J. Gao, J. Fan, F. Qin, H. Lv, M. Khan, J. Wang, Y. Fan, H. Wu and K. Shi, Spirally growing on porous C3N4 thin layer with BiOCl nanosheets to enhance NO2 gas sensitivity at room temperature, J. Alloys Compd., 2023, 938, 168644 CrossRef CAS.
- Y. Lu, D. Wang, W. Lv, Y. Xia, K. Ou, Y. Li, Z. Du, Y. He, J. Dai, S. Wu, J. Luo and Y. Tang, Surface acoustic wave hydrogen sulfide gas sensors based on porous SnO2-SiO2 composite films, Sens. Actuators, B, 2024, 417, 136117 CrossRef CAS.
- X. Sun, T. Chen, Y. Liang, C. Zhang, S. Zhai, J. Sun and W. Wang, Enhanced sensitivity of SAW based ammonia sensor employing GO-SnO2 nanocomposites, Sens. Actuators, B, 2023, 375, 132884 CrossRef CAS.
- K. S. Pasupuleti, M. Reddeppa, S. S. Chougule, N. Bak, D. J. Nam, N. Jung, H. D. Cho, S. G. Kim and N. D. Kim, High performance langasite based SAW NO2 gas sensor using 2D g-C3N4@TiO2 hybrid nanocomposite, J. Hazard. Mater., 2022, 427, 128174 CrossRef CAS.
- J. Zhang, J. Zhou, H. Chen, Y. Liu, D. Liang, Y. Guo, Y. Zhan, Y. Fu and H. Duan, Integration strategies of ternary hierarchical nanocomposite designs with activated ultraviolet lights and surface acoustic waves for enhancing NO2 sensing at room-temperature, Chem. Eng. J., 2024, 482, 149067 CrossRef CAS.
- E. Oh, H. Y. Choi, S. H. Jung, S. Cho, J. C. Kim, K. H. Lee, S. W. Kang, J. Kim, J. Y. Yun and S. H. Jeong, High-performance NO2 gas sensor based on ZnO nanorod grown by ultrasonic irradiation, Sens. Actuators, B, 2009, 141, 239–243 CrossRef CAS.
- S. Kim, J. E. Yang, Y. S. Park, M. Park, S. J. Kim and K. K. Kim, Convergence Gas Sensors with One-Dimensional Nanotubes and Pt Nanoparticles Based on Ultraviolet Photonic Energy for Room-Temperature NO2 Gas Sensing, Nanomaterials, 2023, 13, 2780 CrossRef CAS.
- Y. Nagarjuna and Y. J. Hsiao, TeO2 doped ZnO nanostructure for the enhanced NO2 gas sensing on MEMS sensor device, Sens. Actuators, B, 2024, 401, 134891 CrossRef CAS.
- G. Ren, B. Huang, C. Li, C. Lin and Y. Qian, Facile and template-free strategy to construct N, P co-doped porous carbon nanosheets as a highly efficient electrocatalyst towards oxygen reduction reaction, J. Electroanal. Chem., 2020, 877, 114732 CrossRef CAS.
- R. S. Ganesh, G. K. Mani, R. Elayaraja, E. Durgadevi, M. Navaneethan, S. Ponnusamy, K. Tsuchiya, C. Muthamizhchelvan and Y. Hayakawa, ZnO hierarchical 3D-flower like architectures and their gas sensing properties at room temperature, Appl. Surf. Sci., 2018, 449, 314–321 CrossRef CAS.
- H. Zhang, Z. Chen, Z. Sun, M. Cai, W. Liu, W. Ye, H. Gao, J. Han, Y. Cheng, Q. Zhang and M. S. Wang, Unraveling the origin of enhanced K+ storage of carbonaceous anodes enabled by nitrogen/sulfur co-doping, Adv. Funct. Mater., 2023, 33, 2300769 CrossRef CAS.
- Y. Zhang, G. Zhao, L. Gan, H. Lian and M. Pan, S-doped carbon nanosheets supported ZnO with enhanced visible-light photocatalytic performance for pollutants degradation, J. Cleaner Prod., 2021, 319, 128803 CrossRef CAS.
- Z. Zhang, L. Li, Y. Qing, X. Lu, Y. Wu, N. Yan and W. Yang, Hierarchically Interconnected N-Doped Carbon Aerogels Derived from Cellulose Nanofibrils as High Performance and Stable Electrodes for Supercapacitors, J. Phys. Chem. C, 2018, 122, 23852–23860 CrossRef CAS.
- X. L. Huang, X. Zhang, L. Zhou, Z. Guo, H. K. Liu, S. X. Dou and Z. Wang, Orthorhombic Nb2O5 Decorated Carbon Nanoreactors Enable Bidirectionally Regulated Redox Behaviors in Room-Temperature Na–S, Batt. Adv. Sci., 2022, 10, 2206558 CrossRef.
- T. Yang, W. Gao, B. Guo, R. Zhan, Q. Xu, H. He, S. J. Bao, X. Li, Y. Chen and M. Xu, A railway-like network electrode design for room temperature Na-S battery, J. Mater. Chem. A, 2019, 7, 150–156 RSC.
- C. Kumari, A. Pandey and A. Dixit, Zn interstitial defects and their contribution as efficient light blue emitters in Zn rich ZnO thin films, J. Alloys Compd., 2018, 735, 2318–2323 CrossRef CAS.
- K. S. Pasupuleti, D. Vidyasagar, L. N. Ambadi, N. Bak, S. G. Kim and M. D. Kim, UV light activated g-C3N4 nanoribbons coated surface acoustic wave sensor for high performance sub-ppb level NO2 detection at room temperature, Sens. Actuators, B, 2023, 394, 134471 CrossRef CAS.
- Z. You, Y. Su, Y. Yu, H. Wang, T. Qin, F. Zhang, Q. Shen and H. Yang, Preparation of g-C3N4 nanorod/InVO4 hollow sphere composite with enhanced visible-light photocatalytic activities, Appl. Catal., B, 2017, 213, 127–135 CrossRef CAS.
- B. Li, J. Guo, Z. Yuan, X. Xiang, X. Jiang, J. Nie, B. Zhang, X. Yuan and X. Zu, Microstructure evolution and performance degradation of sol-gel silica films induced by helium ion irradiation, Opt. Mater., 2024, 147, 114689 CrossRef CAS.
- W. Meng, S. Wu, X. Wang and D. Zhang, High-sensitivity resistive humidity sensor based on graphitic carbon nitride nanosheets and its application, Sens. Actuators, B, 2020, 315, 128058 CrossRef CAS.
- K. S. Pasupuleti, S. Ghosh, N. Jayababu, C. J. Kang, H. D. Cho, S. G. Kim and M. D. Kim, Boron doped g-C3N4 quantum dots based highly sensitive surface acoustic wave NO2 sensor with faster gas kinetics under UV light illumination, Sens. Actuators, B, 2023, 378, 133140 CrossRef CAS.
- Y. H. Zhang, Y. L. Li, F. L. Gong, K. F. Xie, M. Lin, H. L. Zhang and S. M. Fang, Al doped narcissus-like ZnO for enhanced NO2 sensing performance: An experimental and DFT investigation, Sens. Actuators, B, 2020, 305, 127489 CrossRef CAS.
- Y. H. Zhang, Y. L. Li, F. L. Gong, K. F. Xie, H. L. Zhang and S. M. Fang, Double-platelet Pd@ZnO microcrystals for NO2 chemical sensors: their facile synthesis and DFT investigation, Phys. Chem. Chem. Phys., 2019, 21, 22039–22047 RSC.
- D. N. Mahaseth and T. Islam, An Ultrafast Capacitive Humidity Sensor With Excellent Static and Dynamic Characteristics, IEEE Sens. J., 2024, 24, 8674–8681 CAS.
- Y. Li, Y. Li, R. Zhang, S. Li, Z. Liu, J. Zhang and Y. Fu, Progress in wearable, acoustical sensors for diagnostic applications, Biosens. Bioelectron., 2023, 237, 115509 CrossRef CAS.
- J. Zhou, Y. Guo, Y. Wang, Z. Ji, Q. Zhang, F. Zhuo, J. Luo, R. Tao, J. Xie, J. Reboud, G. McHale, S. Dong, J. Luo, H. Duan and Y. Fu, Flexible and wearable acoustic wave technologies, Appl. Phys. Rev., 2023, 10, 021311 CAS.
- X. Li, Y. Feng, J. Long, H. Lv, Y. Guo and X. Zu, MXene-activated graphene oxide enhancing NO2 capture and detection of surface acoustic wave sensors, Sens. Actuators, B, 2024, 401, 135006 CrossRef CAS.
- R. Kumar, O. A. Dossary, G. Kumar and A. Umar, Zinc Oxide Nanostructures for NO2 Gas-Sensor Applications: A Review, Nano-Micro Lett., 2015, 7, 97–120 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section and supplementary figures. See DOI: https://doi.org/10.1039/d4tc03690e |
| This journal is © The Royal Society of Chemistry 2025 |