X-ray-irradiation-induced photoluminescence and photochromic LiNbO3 phosphors for anti-counterfeiting and X-ray imaging†
Yueteng
Zhang
a,
Xue
Bai
a,
Heping
Zhao
a,
Jianbei
Qiu
 a,
Zhiguo
Song
a,
Zhiguo
Song
 a,
Jiayan
Liao
a,
Jiayan
Liao
 *b and
Zhengwen
Yang
*b and
Zhengwen
Yang
 *a
*a
aCollege of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, 650093, P. R. China. E-mail: yangzw@kust.edu.cn
bInstitute for Biomedical Materials and Devices (IBMD), Faculty of Science, University of Technology Sydney, NSW 2007, Australia. E-mail: jiayan.liao@uts.edu.au
First published on 22nd October 2024
Abstract
This study explored the innovative photochromic and photoluminescence properties of Eu3+-doped LiNbO3 phosphors under X-ray irradiation, demonstrating their great potential for anti-counterfeiting, X-ray detection and imaging. The photochromic phosphor LiNbO3:Eu3+ is synthesized, which undergoes a color change from white to brown under bright field conditions and displays red photoluminescence under dark field conditions after X-ray exposure due to defect formation. The addition of Eu3+ enhanced the photochromic reaction and rapid self-bleaching ability. The study delved into the mechanisms of photochromic and photoluminescence behavior, focusing on electron–hole pair separation and defect formation, which are central to the observed phenomena. In addition, the integration of these phosphors into polydimethylsiloxane can create multifunctional anti-counterfeiting labels and can also be used for “dual-mode” 3D X-ray imaging and detection, showing practical application potential.
Introduction
Photochromic (PC) materials, capable of altering their color through structural or configurational changes under specific light wavelengths, play a pivotal role in applications like optical storage and anti-counterfeiting. Photoluminescence (PL) is a type of cold luminescence, where a material absorbs photons (or electromagnetic waves) and re-emits them. PL materials have a wide range of potential applications, including pressure sensing, optical thermometers, LEDs, anti-counterfeiting, and imaging.1–3 Recent research predominantly utilizes semiconductor laser light sources for inscribing or erasing information within these media.4 The advent of X-ray induced PC and PL opens new avenues for multifunctional applications, including optical memory, anti-counterfeiting, and X-ray detection and imaging.5–7 Traditional scintillators like Bi4Ge3O12 and CsI:Tl offer high X-ray to light conversion efficiency but are hindered by their high synthesis temperatures (1700 °C) and cost.8,9 Additionally, despite the promising X-ray absorption and luminescence of metal halide materials, their instability and propensity for decomposition pose significant challenges.10–13The study of rare earth-doped oxide PC materials has gained momentum, with the PC mechanism largely attributed to cation vacancies from alkali metal ion volatilization or oxygen vacancies from oxygen atom volatilization during high-temperature sintering, creating color centers.14–17 These vacancy-related defects typically form deep traps (1.05–1.15 eV) under light stimulation, making it challenging for excited electrons to escape at room temperature without high energy. Consequently, many PC materials depend on external stimuli, such as heat, pressure, and light, to exhibit bleaching properties,18,19 which restricts the development of anti-counterfeiting technologies. Current anti-counterfeiting research efforts aim to enhance pattern complexity from two-dimensional to three-dimensional to increase the complexity and mitigate forgery risks.20 Additionally, an innovative idea is to introduce a time dimension to photoluminescence materials, utilizing their fast self-bleaching characteristics as a novel approach for enhanced functionality.21,22
LiNbO3 (LNO) is renowned for its exceptional photorefractive, electro-optic, and nonlinear optical properties, complemented by a low synthesis temperature.23–27 It has shown high X-ray absorption efficiency under X-ray irradiation.28 Our research reveals that Eu3+-doped LNO phosphors exhibit outstanding PC and PL characteristics. The incorporation of Eu3+ introduces new defects, which not only enhances the color contrast during discoloration but also significantly improves the self-bleaching efficiency of LNO.29 Upon X-ray exposure, LNO:0.01Eu3+ demonstrates rapid response times and significant reflectivity variations between colored and bleached states, with reflectivity changes dependent on the X-ray dose. Notably, discolored LNO:0.01Eu3+ phosphors possess self-bleaching capabilities. The material's bleaching extent, influenced by exposure duration, adds a dynamic dimension to anti-counterfeiting efforts.
We conducted a comprehensive investigation of the X-ray-induced discoloration, luminescence, self-bleaching, and UV-induced bleaching properties of LNO:0.01Eu3+ (Fig. 1). This study covers the impact of various X-ray doses and exposure durations on the luminescence intensity and color transitions of LNO:0.01Eu3+, as well as the color modifications' stability, repeatability, and fatigue resistance over repeated cycles. The self-bleaching curves of both pure LNO and LNO:0.01Eu3+ were analyzed, leading to the determination of the self-bleaching time coefficient. The study extends to the mechanisms of photochromism and luminescence under X-ray irradiation, exploring self-bleaching properties and mechanisms, as illustrated in our imaging analyses. Furthermore, we developed flexible LNO:0.01Eu3+ thin films, showcasing their potential in anti-counterfeiting applications through discoloration and self-bleaching. These findings underscore the vast potential of LNO:0.01Eu3+ in dynamic anti-counterfeiting and X-ray imaging applications. In practice, the spatial resolution of X-ray imaging is primarily influenced by the X-ray dose and the thickness of the scintillator film. Although thicker layers absorb more X-rays, they also induce significant self-absorption and multiple light scattering, which degrade spatial resolution.
Experimental
Material synthesis
LNO:xEu3+(x = 0.005, 0.01, 0.015, 0.02, 0.025, and 0.03) samples were synthesized using high purity LiCO3(99.99%), Nb2O5(99.95%) and Eu2O3(99.99%). The raw materials were weighed according to stoichiometry, mixed in an agate mortar for 15 minutes to ensure thorough blending, and then calcined in an alumina crucible at 1200 °C for 6 hours at a heating rate of 4 °C per minute. After natural cooling to room temperature, the samples were ground into a fine powder for performance testing and characterization.Polydimethylsiloxane (PDMS) served as the matrix for the prepared material. We combined 2 g of PDMS base resin (Sylgard184, Dow Corning, Part A), 0.2 g of curing agent (Sylgard184, Dow Corning, Part B), and 1.3 g of LNO:0.01Eu3+ phosphor. The base resin and curing agent were mechanically stirred for 5 minutes, after which the prepared sample was added and stirred for an additional 5 minutes. The mixture was then slowly poured onto a glass template and cured in a drying oven for 12 hours to form a PDMS-based photoluminescent film. Using PDMS as the matrix, anti-counterfeiting patterns were fabricated using a similar template method.
Structural characterization
X-ray diffraction (XRD) patterns were recorded using a Bruker D8 ADVANCE diffractometer. UV-visible diffuse reflectance spectroscopy (DRS) was conducted with a HITACHI U4100 spectrophotometer using BaSO4 as a reference. The morphology and elemental composition of the target samples were analyzed by scanning electron microscopy (SEM, JSM-7000F), and chemical states were characterized by X-ray photoelectron spectroscopy (XPS, AXIS ULTRA, Kratos Analytical Ltd), with all binding energies referenced to the C 1s peak at 284.8 eV. Room temperature photoluminescence (RT-PL) and photoluminescence excitation (PLE) spectra were recorded with an Edinburgh FLS980 instrument, which also measured decay lifetimes using a pulsed xenon lamp. X-ray detection utilized a D8 Focus diffractometer (Bruker) with Cu-Kα radiation (λ = 0.15405 nm) and a miniaturized X-ray tube (Amptek) with a maximum output of 1200 W. For thermoluminescence (TL) measurements, samples were mounted on a hot stage with a heating rate of 1 K s−1 (30–340 °C, LTTL-3DS) and irradiated under X-ray for 15 minutes prior to measurements.The applied X-ray dose rate ranged from 50 nGy s−1 to 2 mGy s−1, with voltage and current settings of 40 kV and 30 mA, respectively, during radiation stability measurements. The X-ray dose was measured in real time using a Smach RG1000 Geiger counter.
Self-bleaching rate fitting
To evaluate the self-bleaching performance of LNO fluorescent materials, we have defined the following: | (1) |
 | (2) |
The relationship between ΔRD and time for different samples is plotted in Fig. 4c, d and f, with all data well fitted by double exponential function as shown below:
 | (3) |
 | (4) |
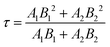 | (5) |
Results and discussion
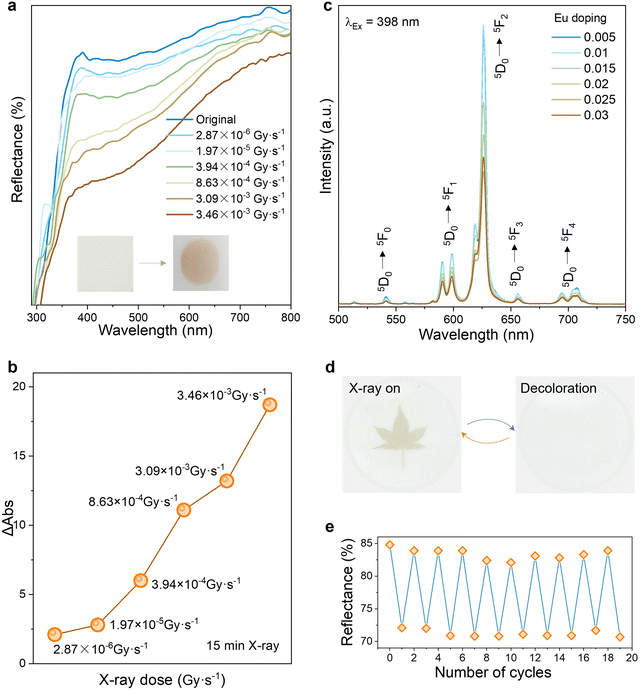
The luminescence and discoloration properties of inorganic materials are significantly influenced by the matrix type and phase structure, which in turn affects the formation of specific lattice defects related to discoloration. The X-ray diffraction (XRD) patterns of the LNO matrix samples, synthesized via a high-temperature solid-state method at different sintering temperatures, are depicted in Fig. S1 (ESI†). A minor impure phase of EuNbO4 was observed when sintered at 1050 °C, and the sample melted at 1250 °C. The sample annealed at 1200 °C for 6 hours exhibited the strongest luminescence response under 398 nm UV irradiation, as illustrated in Fig. S2 (ESI†). The optimized reaction condition for achieving good phase purity was found to be 1200 °C for 6 hours, aligning well with the JCPDS (20-0631) card. At the optimal sintering temperature, the XRD patterns of the LNO:0.01Eu3+ series phosphors, obtained by varying the doping amount of Eu3+, showed slight peak shifts, indicating successful doping of Eu3+ into the LNO lattice (Fig. 2a and Fig. S3, ESI†). Based on the general structural analysis system software, the Rietveld refined XRD pattern and refined data of the sample are shown in Fig. 2b and Table S1 (ESI†). It can be seen that the doping of Eu3+ causes the cell expansion, which corresponds to the peak shift of XRD, proving that Eu3+ is successfully doped into the lattice. Given the ionic radii and valence states—Li+(r = 0.76 Å CN = 6), Nb5+(r = 0.64 Å CN = 6), Eu3+(r = 0.95 Å CN = 6) —Eu3+ is expected to replace Li+ ions, as depicted in Fig. 2c. The room temperature crystal structure of stoichiometric lithium niobate, belonging to the space group R3c, can be conceptualized as derived from corundum. It features a hexagonal close-packed arrangement of oxygen atoms, where 2/3 of the octahedral sites are filled by cations. The SEM image in Fig. 2d shows that the particle size range of the LNO:0.01Eu3+ sample is between 5 and 15 μm. The uniform distribution of all elements in the element mapping image, including Nb, O, and Eu, was observed in the LNO:0.01Eu3+ material (Fig. 2e).We discovered that LNO:0.01Eu3+ exhibited a PC reaction under X-ray irradiation. To quantitatively assess the PC ability, we measured the reflection spectrum of LNO:0.01Eu3+ after exposure to varying doses of X-rays, as depicted in Fig. 3a. Following X-ray irradiation, the reflectivity of LNO:0.01Eu3+ significantly decreased due to the PC reaction, resulting in an absorption band spanning 400 to 800 nm. The dose intensity of 3.46 × 10−3 Gy s−1 was sufficient to induce the most substantial color change. With the extension of irradiation time, the diffuse reflection intensity decreased gradually (Fig. S4, ESI†). The PC degree ΔAbs, defined as the difference in reflectivity before and after irradiation, is commonly used to evaluate PC performance:
| ΔAbs = R0 − R1 | (6) |
The photoluminescence (PL) spectra of LNO doped with different concentrations of Eu were investigated. LNO:0.01Eu3+ demonstrates strong red emission under 398 nm excitation, with four emission peaks at approximately 593, 616, 650, and 698 nm, attributed to the 5D0 → 7Fj transitions (j = 1, 2, 3, and 4). The most intense emission peak occurs at a 1% Eu3+ doping level (Fig. 3c). Fig. S5 (ESI†) shows a series of narrow peaks in the wavelength range of 350–500 nm. The sharp absorption peaks appear at 363 nm (7F0 → 5D4), 380 nm (7F0 → 5L6), 398 nm (7F0 → 5D3), and 467 nm (7F0 → 5D2). We observed a significant decrease in the PL intensity of all samples following X-ray irradiation (Fig. S6a, ESI†). As the X-ray dose increases, there is a linear decrease in emission intensity. Consequently, the incorporation of PL introduces an additional mode of decoding by enabling the observation or detection of fluorescence changes under 398 nm excitation. This enhancement in anti-counterfeiting security offers more channels and information for readout. The PL spectra of LNO:0.01Eu3+ were measured at various times under a consistent X-ray dose, revealing that the PL intensity reached its minimum approximately 15 minutes after irradiation (Fig. S7, ESI†). Luminescence modulation (ΔR) is defined as:
 | (7) |
The bleaching process is illustrated in Fig. 3d, where the orange photochromic phosphor completely reverts to its original white color within 10 seconds after 395 nm irradiation. Fig. S9 (ESI†) shows the color change of pure LNO before and after X-ray irradiation. Following X-ray irradiation, distinct patterns emerge on the phosphor film. Subsequent exposure to a 395 nm ultraviolet lamp results in rapid fading until the film is completely bleached. Fig. 3e presents the reversible diffuse reflectance spectrum of LNO:0.01Eu3+ with alternating X-ray (15 min) and 395 nm light (10s) irradiation, demonstrating good reproducibility, reversibility, and stable discoloration after multiple cycles, indicating potential applications in anti-counterfeiting and optical storage media.
Most traditional fluorescent materials require external stimulation to fade. In contrast, LNO phosphors exhibit self-bleaching PC characteristics. Fig. 4a–d showcase the time-dependent self-bleaching spectra of pure LNO and LNO:0.01Eu3+, along with the corresponding data fitting diagrams, following the cessation of X-ray irradiation. Although the self-bleaching does not revert the diffuse reflectance spectrum to the initial curve due to the high sensitivity of the instruments, the sample surface's decolorization nearly returns to the initial level after a 3-hour delay, as visible to the naked eye. This diminished interaction between PL also facilitated the self-bleaching of luminescence over time, as illustrated in Fig. 4e. The self-bleaching curve was also fitted, as shown in Fig. 4f. After 3 h, the ΔRDR of LNO is 85.80%, the ΔRDR of LNO:0.01Eu3+ is 95.01%, and the ΔRDPL of LNO:0.01Eu3+ is 87.11%, indicating that Eu3+ doping enhances the self-bleaching performance of LNO (Table S4, ESI†). According to the data in Tables S5 and S6 (ESI†), Eu3+ doping significantly reduces the average recovery time τ, thereby improving the efficiency of self-bleaching. Moreover, the PL spectrum also exhibits characteristics of self-bleaching. Fast self-bleaching, approaching an infinite cycle photochromic effect, is beneficial for producing optical anti-counterfeiting materials.
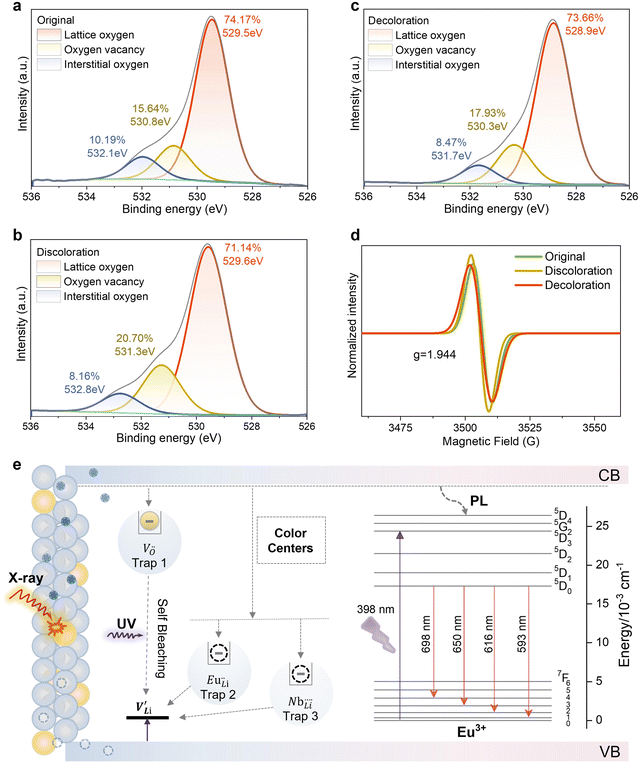
There are two primary photochromic mechanisms: the formation of oxygen vacancy defects and changes in ion valence.30,31Fig. 5a–c and Fig. S10, S11 (ESI†) display the XPS spectra of LNO:0.01Eu3+ before and after 15 minutes of X-ray irradiation, and subsequent bleaching with a 395 nm UV lamp. In Fig. 5a–c, the O spectra across the three figures all feature three peaks at 531.9/532.8/531.7, 530.8/531.3/530.3, and 529.4/529.6/528.9 eV, corresponding to interstitial oxygen, oxygen vacancies, and lattice oxygen, respectively. The high-temperature sintering process, which causes the volatilization of lithium ions, leads to charge imbalance and results in oxygen vacancies. A comparison of the integral area ratios before and after irradiation reveals a decrease in lattice oxygen content from 74.17% to 71.14% and an increase in oxygen vacancy from 15.64% to 20.70%, with interstitial oxygen decreasing from 10.19% to 8.16%. Irradiation with a 395 nm UV lamp reverses these changes, increasing lattice oxygen content from 71.14% to 73.66%, decreasing oxygen vacancies from 20.70% to 17.93%, and increasing interstitial oxygen from 8.16% to 8.47%. These findings demonstrate that X-ray irradiation enhances oxygen vacancy formation, while 395 nm UV lamp irradiation aids in lattice oxygen recovery. Electron paramagnetic resonance (EPR) spectra, indicative of near-magnetic and single-ionized oxygen vacancies, are employed to assess oxygen vacancy formation and content during coloration.32–34 The presence of oxygen vacancies can be further demonstrated by the EPR results. In Fig. 5d, the EPR signal of the oxygen vacancy can be clearly observed when g = 1.944 before irradiation. After irradiation, the EPR signal intensity increased, indicating the presence of oxygen vacancies. The Nb 3d spectrum (Fig. S11a–c, ESI†), with two peaks at 206.5/209.2, 205.6/208.6 eV, and 206.6/209.3 corresponding to Nb 3d5/2 and Nb 3d3/2, and the presence of a peak (205.6 eV) of Nb (3d5/2) indicates that X-ray irradiated LNO has undergone a reduction of Nb5+ to Nb4+.
We analyzed the thermoluminescence (TL) spectra of LNO:0.01Eu3+ post 15-minute X-ray irradiation at a 3.46 × 10−3 Gy s−1 dose. The broad temperature distribution from 50 °C to 250 °C in the thermoluminescence spectrum suggests defects within the LNO:0.01Eu3+ phosphor.35 TL peaks in all samples under various X-ray doses indicate dose-dependent enhancement, reaching a maximum at 3.46 × 10−3 Gy s−1 (Fig. S12a, ESI†). Post-irradiation TL spectra, measured over time, show a decreasing TL peak, aligning with the self-bleaching characteristics (Fig. S12b, ESI†). Fig. S13 and Table S7 (ESI†), through Gaussian fitting, reveal three TL peaks, with peak temperatures correlating to trap depths (E) calculated as:
 | (8) |
LNO typically exhibits lithium deficiency and is susceptible to various intrinsic defects, such as antisite defects  and lithium vacancies
and lithium vacancies 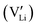 . Following Wilkinson et al.'s model, the lithium vacancy
. Following Wilkinson et al.'s model, the lithium vacancy 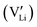 emerges as the predominant intrinsic defect in LNO, attributed to lithium's volatility during synthesis. To maintain charge balance, some Nb5+ ions may occupy Li+ sites, forming antisite defects
emerges as the predominant intrinsic defect in LNO, attributed to lithium's volatility during synthesis. To maintain charge balance, some Nb5+ ions may occupy Li+ sites, forming antisite defects  .36–38 Comparing the TL spectra of pure LNO (Gaussian fitting identified two peak positions for pure LNO samples at 91.94 °C (0.72 eV) and 177.61 °C (0.90 eV) in Fig. S14 and Table S8, ESI†) with LNO:0.01Eu3+, the addition of Eu3+ introduces new defects. It is speculated that Eu3+ can enter
.36–38 Comparing the TL spectra of pure LNO (Gaussian fitting identified two peak positions for pure LNO samples at 91.94 °C (0.72 eV) and 177.61 °C (0.90 eV) in Fig. S14 and Table S8, ESI†) with LNO:0.01Eu3+, the addition of Eu3+ introduces new defects. It is speculated that Eu3+ can enter  sites to form
sites to form  (supported by microscopic modeling in Fig. 2c). Therefore, LNO:0.01Eu3+ may contain
(supported by microscopic modeling in Fig. 2c). Therefore, LNO:0.01Eu3+ may contain  ,
, 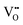 ,
, 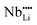 and
and  vacancies as color centers. Doping with Eu3+ enhances the concentration and depth of defects within the matrix, making the color change in LNO:0.01Eu3+ more pronounced than in pure LNO under the same X-ray dose (Fig. S15, ESI†). This effect leads to a reduced self-bleaching time coefficient, thereby accelerating the self-bleaching process. The shallow trap (trap 1) is usually caused by
vacancies as color centers. Doping with Eu3+ enhances the concentration and depth of defects within the matrix, making the color change in LNO:0.01Eu3+ more pronounced than in pure LNO under the same X-ray dose (Fig. S15, ESI†). This effect leads to a reduced self-bleaching time coefficient, thereby accelerating the self-bleaching process. The shallow trap (trap 1) is usually caused by  .2 In some reports,39 the ionization energy (IE) of cations can be used as a suitable parameter to indicate the trap depth energy. The IEs of Nb5+ and Eu3+ ions are 4877 and 2404 kJ mol−1, respectively. Considering that the ionization energy of Nb5+ is higher than that of Eu3+, the second trap is attributed to
.2 In some reports,39 the ionization energy (IE) of cations can be used as a suitable parameter to indicate the trap depth energy. The IEs of Nb5+ and Eu3+ ions are 4877 and 2404 kJ mol−1, respectively. Considering that the ionization energy of Nb5+ is higher than that of Eu3+, the second trap is attributed to  . These traps capture holes or electrons, acting as color centers with strong absorption in the visible spectrum, leading to the photochromic phenomenon. Fig. S16 (ESI†), displaying the XRD patterns of the phosphor before and after X-ray irradiation and upon fading, confirms the absence of phase transitions during the discoloration and fading process. Based on the analysis, we propose a potential mechanism for PC and PL processes, as illustrated in Fig. 5e. In this study, LNO:0.01Eu3+ phosphors have a large number of intrinsic defects during high temperature sintering (
. These traps capture holes or electrons, acting as color centers with strong absorption in the visible spectrum, leading to the photochromic phenomenon. Fig. S16 (ESI†), displaying the XRD patterns of the phosphor before and after X-ray irradiation and upon fading, confirms the absence of phase transitions during the discoloration and fading process. Based on the analysis, we propose a potential mechanism for PC and PL processes, as illustrated in Fig. 5e. In this study, LNO:0.01Eu3+ phosphors have a large number of intrinsic defects during high temperature sintering (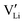 ,
, 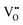 ,
,  and
and  ). These defects are located between the conduction band (CB) and valence band (VB), forming traps at different depths. Under X-ray excitation, some electrons in the valence band are captured by defects during the transition to the conduction band, forming a color center. Another part of the valence band electrons is excited to the conduction band to produce electron–hole pairs. The electrons are captured by Nb5+ to generate Nb4+. The charge transfer occurs between Nb5+ and Nb4+, and the holes may be captured by lithium vacancies, thus the electron–hole pairs are separated. Photochromism is a reversible process, and its self-bleaching behavior affects the capture depth and density of color centers. It is worth noting that the first and second trap depths of all samples are lower, and the corresponding density and area are greater than three (Table S7, ESI†). Therefore, most of the carriers are trapped by the defects of trap 1 and trap 2. The trap depths in all samples show a wide distribution between 0.68 eV and 0.98 eV. In addition, the depths of traps 1 and 2 (0.68–0.73 eV) are closer to the CB. At room temperature, the electrons trapped by these traps can easily escape from the control of the color center without any external stimulation and bind to the holes. With the delay of time, the color center gradually disappears. This fading process will be accelerated under the irradiation of a 395 nm ultraviolet lamp. As the delay time increases, the TL intensity decreases, indicating that these carriers near the CB gradually escape the limitation of the color center.
). These defects are located between the conduction band (CB) and valence band (VB), forming traps at different depths. Under X-ray excitation, some electrons in the valence band are captured by defects during the transition to the conduction band, forming a color center. Another part of the valence band electrons is excited to the conduction band to produce electron–hole pairs. The electrons are captured by Nb5+ to generate Nb4+. The charge transfer occurs between Nb5+ and Nb4+, and the holes may be captured by lithium vacancies, thus the electron–hole pairs are separated. Photochromism is a reversible process, and its self-bleaching behavior affects the capture depth and density of color centers. It is worth noting that the first and second trap depths of all samples are lower, and the corresponding density and area are greater than three (Table S7, ESI†). Therefore, most of the carriers are trapped by the defects of trap 1 and trap 2. The trap depths in all samples show a wide distribution between 0.68 eV and 0.98 eV. In addition, the depths of traps 1 and 2 (0.68–0.73 eV) are closer to the CB. At room temperature, the electrons trapped by these traps can easily escape from the control of the color center without any external stimulation and bind to the holes. With the delay of time, the color center gradually disappears. This fading process will be accelerated under the irradiation of a 395 nm ultraviolet lamp. As the delay time increases, the TL intensity decreases, indicating that these carriers near the CB gradually escape the limitation of the color center.
Leveraging the photochromic and self-bleaching properties, we adapted LNO:0.01Eu3+ as an anti-counterfeiting solution. The powder was combined with PDMS glue and poured into a square mold, creating a flexible film suitable for anti-counterfeiting labels and imaging. An X-ray imaging system (Fig. 6a) was established, placing the capsule sample or template (Fig. 6b) between the X-ray source and the flexible LNO:0.01Eu3+ film. A mask template was placed on the film's surface, and X-ray irradiation was used to embed the security pattern. The pattern can be quickly erased using 395 nm laser irradiation or allowed to self-bleach. The soft anti-counterfeiting labels can be easily shaped for various applications, ensuring adaptability. Fig. S17 (ESI†) displays the distinct pattern colors at different X-ray doses, illustrating LNO's potential for X-ray dose detection. Fig. 6c shows reversible writing and erasing of optical and color information with X-rays and 395 nm light or self-bleaching. High-resolution patterns are retained after multiple cycles, demonstrating excellent reproducibility of LNO:0.01Eu3+ as a dual-mode imaging material, significantly enhancing the security of inorganic photochromic materials in anti-counterfeiting applications. Fig. S18 (ESI†) captures the color transitions of the LNO:0.01Eu3+ phosphor film over time post-X-ray irradiation, highlighting the dynamic anti-counterfeiting features and high security standards possible with time-sensitive encryption.
Fig. 6d provides a schematic of the imaging process for a three-dimensional curved object. Fig. 6e displays the headset's physical image and its 3D imaging under X-rays, revealing both its internal structure and external contour. Fig. 6f shows the imaging pattern after X-ray irradiation in the bright field and the photoluminescence imaging pattern in the dark field, respectively. This confirms that X-rays, like 398 nm ultraviolet light, can excite the PL of Eu, indicating the potential of LNO:0.01Eu3+ phosphors for X-ray imaging applications. Using the phosphor film's flexibility, it is bent into a cylinder for 3D imaging. The mask template is applied to the curved surface, followed by X-ray irradiation. The flexible film maintains high resolution even after bending (Fig. 6f and Fig. S19, ESI†). Furthermore, Fig. S20 (ESI†) shows that the flexible film retains good flexibility and clear discoloration after stopping X-ray irradiation. LNO:0.01Eu3+ remains flexible even after four bends, demonstrating its advantages in imaging irregular and flexible objects.
Conclusions
This research showcases the development of innovative LNO:0.01Eu3+ oxide phosphors that exhibit photochromic behaviors under X-ray irradiation, transitioning from white to red with rapid fading under 395 nm UV light. Notably, Eu3+ doping enhances the self-bleaching efficiency of these phosphors, representing an advancement in anti-counterfeiting technologies. By incorporating these phosphors into flexible PDMS-based labels, their utility in security applications is demonstrated, leveraging dynamic color-changing and self-bleaching properties. The dual-mode functionality of these materials, driven by complex electron–hole and defect dynamics, not only improves encrypted information security but also expands capabilities in X-ray dose detection, establishing a versatile platform for enhanced security measures. Moreover, the phosphors' photoluminescence under X-ray irradiation and their ability to perform dual-mode imaging and display highlight their potential in multimodal display encryption technology, information security detection, intelligent anti-counterfeiting, and photoelectric applications.Author contributions
Z. Y. and J. L. conceptualized, designed, and oversaw the project. Y. Z. executed the experiments with support from J. L. and Z. Y. Manuscript preparation and revisions were collaboratively undertaken by Y. Z. and J. L. The entire team engaged in discussions and provided input for the manuscript.Data availability
The data supporting this study have been included within the article and the corresponding ESI.†Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the Key Project of the National Natural Science Foundation of China-Yunnan Joint Fund (U2102215), the National Natural Science Foundation (52472002), the National Natural Science Foundation of High End Foreign Expert Introduction Plan (G2022039008L), the Academician Workstation of Cherkasova Tatiana in Yunnan Province (202305AF150099), the Yunnan Province Major Science and Technology Special Plan (202302AB080005), and UTS Chancellor's Research Fellowship Program (J.L., PRO22-15457), and the National Health and Medical Research Council (J.L., 2025442).References
- X. Bai, Y. Cun and Zan Xu, et al., Multiple anti-Counterfeiting and optical storage of reersible dual-mode luminescence modification in photochromic CaWO4: Yb3+, Er3+, Bi3+ phosphor, Chem. Eng. J., 2022, 429, 132333 CrossRef CAS.
- J. Tang, P. Du and W. Li, et al., The integration of diverse fluorescence performances of Sr2−xSnO4:xSm3+ ceramics with an infinite luminescence modulation ratio, Chem. Eng. J., 2021, 410, 128287 CrossRef CAS.
- Z. Zhang, L. Guo and H. Sun, et al., Rare earth orthoniobate photochromics with self-activated upconversion emissions for high-performance optical storage applications, J. Mater. Chem. C, 2021, 9, 13841–13850 RSC.
- Y. Zhu, H. Sun and Q. Jia, et al., Site-Selective Occupancy of Eu2+ toward High Luminescence Switching Contrast in BaMgSiO4-Based Photochromic Materials, Adv. Opt. Mater., 2021, 9, 2001626 CrossRef CAS.
- Y. Song, H. Zhao and Y. Zi, et al., X-ray-Irradiation-Induced Discoloration and Persistent Radioluminescence for Reversible Dual-Mode Imaging and Detection Applications, ACS Energy Lett., 2023, 8, 2232–2240 CrossRef CAS.
- W. Xu, H. Xu and S. Wang, et al., Scintillation ma-terials based on metal iodates by rare earth doping modifications for use in radiolumi-nescence and X-ray imaging, CrystEngComm, 2021, 23, 4103–4108 RSC.
- Y. Zhang, X. Shan and X. Lv, et al., Multimoda-l luminescence in Pr3+ single-doped Li2CaSiO4 phosphor for optical information storag-e and anti-counterfeiting applications, Chem. Eng. J., 2023, 474, 145886 CrossRef CAS.
- C. Zaldo and E. Moya, Low-temperature X-ray-induced optical properties of Bi4Ge3O12 scintillators, J. Phys.: Condens. Matter, 1993, 5, 4935 CrossRef CAS.
- V. V. Nagarkar, T. K. Gupta and S. R. Miller, et al., Structured CsI (Tl) scintillators for X-ray imaging applications, IEEE Trans. Nucl. Sci., 1998, 45, 492–496 CAS.
- Q. S. Chen, J. Wu and X. Y. Ou, et al., All-inorganic perovskite nanocrystal scintillators, Nature, 2018, 561, 88–93 CrossRef CAS PubMed.
- J. Y. Liu, B. Shabbir and C. J. Wang, et al., Flexible, Printable Soft-X-Ray Detectors Base-d on All-Inorganic Perovskite Quantum Dots, Adv. Mater., 2019, 31, 1901644 CrossRef.
- M. Kunitski, N. Eicke and P. Huber, et al., Double-slit photoelectron interference in strong-field ionization of the neon dimer, Nat. Commun., 2019, 10, 1 CrossRef CAS.
- T. Leijtens, T. Giovenzana and S. N. Habisreutinger, et al., H.J. Hydrophobic Organic Hole Transporters for Improved Moisture Resistance in Metal Halide Perovskite Solar Cells, ACS Appl. Mater. Interfaces, 2016, 8, 5981–5989 CrossRef CAS.
- H. Wang, J. Lin and B. Deng, et al., Reversible multi-mode modulations of optical behavior in photochromic-translucent Nd-doped K0.5Na0.5NbO3 ceramics, J. Mater. Chem. C, 2020, 8, 2343–2352 RSC.
- H. Sun, Y. Zhang and J. Liu, et al., Reversible upconversion switching for Ho/Yb codoped (K, Na) NbO3 ceramics with excellent luminescence readout capability, J. Am. Ceram. Soc., 2018, 101, 5659–5674 CrossRef CAS.
- K. Li, L. Luo and Y. Zhang, et al., Tunable Luminescence Contrast in Photochromic Ceramics (1 - x) Na0.5Bi0.5TiO3- xNa0.5K0.5NbO3:0.002Er by an Electric Fie-ld Poling, ACS Appl. Mater. Interfaces, 2018, 10, 41525–41534 CrossRef CAS PubMed.
- Q. Zhang, Y. Zhang and H. Sun, et al., Tunable Luminescence Contrast of Na0.5Bi4.5Ti4O15: Re (Re = Sm, Pr, Er) Photochromics by Controlling the Excitation Energy of Luminescent Centers, ACS Appl. Mater. Interfaces, 2016, 8, 34581–34589 CrossRef CAS.
- T. Wei, B. Jia and L. Shen, et al., Reversible upconversion modulation in new photochromic SrBi2Nb2O9 based ceramics for optical storage and anti-counterfeiting applications, J. Eur. Ceram. Soc., 2020, 40, 4153–4163 CrossRef CAS.
- H. Cheng, W. Sun and Y. Lu, et al., Hot electrons in carbon nitride with ultralong lifetime and their application in reversible dynamic color displays. Cell Reports Physical, Science, 2021, 2, 100516 CAS.
- H. Zhao, Y. Cun and X. Bai, et al., Entir-ely Reversible Photochromic Glass with High Coloration and Luminescence Contrast f-or 3D Optical Storage, ACS Energy Lett., 2022, 7, 2060–2069 CrossRef CAS.
- P. Shao, P. Xiong and Y. Xiao, et al., Self-recoverable NIR mechanoluminescence from Cr3+ doped perovskite type aluminate, Adv. Powder Mater., 2024, 3, 100165 CrossRef.
- P. Li, Z. Zhang and X. Gao, et al., Fast self-bleaching Nb2O5-based photochromics for high security dynamic anti-counterfeiting and optical storage applications, Chem. Eng. J., 2022, 435, 134801 CrossRef CAS.
- L. Li, Y. Fan and Y. Li, et al., Double-centers of V, Ce–Codoped LiNbO3 from Hybrid Density Functional Theory Calculations: Electron Trapping and Excitation between the Defect Levels, Cryst. Growth Des., 2020, 20, 2774–2780 CrossRef CAS.
- Y. Fan, L. Li and Y. Li, et al., Hybrid density functional theory study of vanadium doping in stoichiometric and congruent LiNbO3, Phys. Rev. B, 2019, 99, 035147 CrossRef CAS.
- S. Wang, Y. Shan and W. Wang, et al., Lone-pair electron effect induced a rapid photorefractive response in site-controlled LiNbO3: Bi,M (M = Zn, In, Zr) crystals, Appl. Phys. Lett., 2021, 118, 191902 CrossRef CAS.
- D. Tu, C. N. Xu and A. Yoshida, et al., LiNbO3:Pr3+: A Multipiezo Material with Simultaneous Piezoelectricity and Sensitive Piezoluminescence, Adv. Mater., 2017, 29, 1606914 CrossRef PubMed.
- S. Lin, C. Xiong and D. Ma, et al., Persistent luminescence found in Mg2+ and Pr3+ co-doped LiNbO3 single crystal, J. Mater. Chem. C, 2018, 6, 10067–10072 RSC.
- D. Berben, B. Andreas and K. Buse, X-ray-induced photochromic effects in copper-doped lithium niobate crystals, Appl. Phys. B, 2014, 72, 729–732 CrossRef.
- X. Li, H. Lin and S. Lin, et al., Rare-Earth-Ion Doped Bi1.5ZnNb1.5O7 Photochromics: A Fast Self-Recoverable Optical Storage Medium for Dynamic Anti-Counterfeiting with High Security, Laser Photonics Rev., 2023, 17, 2200734 CrossRef CAS.
- X. Bai, Z. W. Yang and Y. H. Zhan, et al., Novel Strategy for Designing Photochromic Ceramic: Reversible Upconversion Luminescence Modification and Optical Information Storage Application in the PbWO4: Yb3+, Er3+ Photochromic Ceramic, ACS Appl. Mater. Interfaces, 2020, 12, 21936–21943 CrossRef CAS.
- Y. T. Ren, Z. W. Yang and Y. H. Wang, et al., Reversible multiplexing for optical information recording, erasing, and reading-out in photochromic BaMgSiO4:Bi3+ luminescence ceramics, Sci. China Mater., 2020, 63, 582–592 CrossRef CAS.
- S. Vuori, P. Colinet and I. Norrbo, et al., Detection of X-Ray Doses with Color-Changing Hackmanites: Mechanism and Application, Adv. Opt. Mater., 2021, 9, 2100762 CrossRef CAS.
- Q. W. Zhang, H. G. Sun and X. S. Wang, et al., Reversible Luminescence Modulation upon Photochromic Reactions in Rare-Earth Doped Ferroelectric Oxides by in Situ Photoluminescence Spectroscopy, ACS Appl. Mater. Interfaces, 2015, 7, 25289–25297 CrossRef CAS.
- T. Jiang, Y. F. Zhu and J. C. Zhang, et al., Multistimuli-Responsive Display Materials to Encrypt Differentiated Information in Bright and Dark Fields, Adv. Funct. Mater., 2019, 29, 1906068 CrossRef CAS.
- G. Qiu, H. Fang and X. Wang, et al., Largely enhanced mechano luminescence properties in Pr3+/Gd3+ co-doped LiNbO3 phosphors, Ceram. Int., 2018, 44, 15411–15417 CrossRef CAS.
- H.-L. Wang, Y. Hang and J. Xu, et al., Near-stoichiometric LiNbO3 crystal grown using the Czochralski method from Li-rich melt, Mater. Lett., 2004, 58, 3119–3121 CrossRef CAS.
- A. P. Wilkinson, A. K. Cheetham and R. H. Jarman, The defect structure of congruently melting lithium niobate, J. Appl. Phys., 1993, 74, 3080–3083 CrossRef CAS.
- S. Erdei and V. T. Gabrieljan, The twisting of LiNbO3 single crystals grown by the Czochralski method, Cryst. Res. Technol., 2006, 24, 987–990 CrossRef.
- Z. Zou, X. Tang and C. Wu, et al., How to tune trap properties of persistent phosphor: Photostimulated persistent luminescence of NaLuGeO4:Bi3+, Cr3+ tailored by trap engineering, Mater. Res. Bull., 2018, 97, 251–259 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tc03606a |
| This journal is © The Royal Society of Chemistry 2025 |