Single microbe photoelectrochemical device using scanning electrochemical microscopy†
Arun Kumar
Samuel
,
Shweta
Shinde
,
Alagammai
Palaniappan
,
Prerna
Bhalla
and
Aravind Kumar
Chandiran
 *
*
Department of Chemical Engineering Indian Institute of Technology Madras Adyar, Chennai, Tamil Nadu 600036, India. E-mail: aravindkumar@iitm.ac.in
First published on 10th October 2024
Abstract
Cyanobacteria are known for their efficient oxygenic photosynthesis, capturing solar energy to produce oxygen and organic compounds under various light conditions. In this work, we employed Synechocystis pevalekii cyanobacteria in a DSSC-based device and studied their photoelectrochemical (PEC) properties. The genome-scale metabolic model was simulated under dark and light conditions using MATLAB 2020b's COBRA Toolbox (Gurobi optimizer) to understand the light driven reactions. Guided by these simulations, the PEC devices were made in two different configurations, wherein one, the microbes are free-floating and in the second, they are anchored onto an anode as biofilm. The role of concentration of microbes, their age, conditioning and nutrient media on PEC performance is analysed. Finally, the photocurrent response of a single microbe on an agar-based biofilm was studied with a scanning electrochemical microscope (SECM) using a 1 μm Pt-tip as the working electrode, under both dark and light conditions. This single microbial device serves as a benchmark to identify or calculate the maximum possible PEC performance from a bulk microbial device.
Introduction
Nature has effectively utilized solar energy by employing bio-organisms to convert water and carbon-dioxide (CO2) into chemical products through a process involving the generation of electron–hole pairs from solar photons.1,2 This principle finds its exemplification in photosynthesis where the electrons reduce carbon dioxide to produce sugars, while positively charged holes oxidize water.3,4 Artificial photosynthetic devices have recently emerged for applications such as hydrogen production,5–7 carbon dioxide conversion into fuels,8 nitrogen fixation,9 and solar cells.10,11 These devices harness sunlight to generate electricity/fuels using oxygenic phototrophs, incorporating either components of organisms like chromophores or entire organisms.12 The first strategy involves extracting light-absorbing chromophores, particularly from the Photosystem I complex,13,14 and integrating them with electron donors and acceptors to facilitate charge separation.15 Another method integrates the living microorganisms into devices.16,17 Upon illumination, the photosynthetic reaction centre generates electron–hole pairs, which subsequently move through internal charge carriers.16 These carriers can be transported to external electrodes or mediators, or captured by lipid-soluble electron acceptors that permeate the cell membrane, releasing electrons at the anode. Simultaneously, holes oxidize water at a catalyst, completing the cycle as photogenerated electrons transfer through the external circuit, with oxygen reduced back to water at the cathode.18 Photo-microbial fuel cells follow a similar principle but utilize an organic substrate instead of water.19 Living photovoltaic (or biophotovoltaic) devices utilizing water and microbes, are promising for solar energy conversion due to their potential for self-repair and replication, potentially reducing production and maintenance costs.20Bio-photovoltaic (BPV) devices currently exhibit low conversion efficiencies, typically below 0.1%.21 A primary challenge lies in exporting photogenerated electrons to external contacts or anodes, hindered by the insulating properties of cell membranes. Research efforts have explored strategies such as microbial mutations,20 and immobilization of microbes,22 on diverse substrates to enhance power conversion efficiency. For instance, microbial electrolysis cells utilizing both wild-type and mutant strains of Synechocystis sp. PCC 6803 have shown efficacy in hydrogen production.23 Similarly, BPV devices employing Synechocystis mutants with enhanced electron export capabilities have achieved high anodic power densities.18 Immobilizing microorganisms on various substrates further enhances charge export efficiency. For example, immobilizing Chlorella vulgaris in alginate gel biofilms,22 or using nanoporous transparent conducting glass with Synechocystis sp. PCC 6803 cyanobacteria,24 has led to improved power conversion efficiencies due to enhanced microbe-electrode contacts.
In bio-photoelectrochemical systems, redox mediators play a crucial role in facilitating efficient charge extraction by enabling electron transfer between microorganisms and electrodes.25 Advancements involve optimizing mediators to match specific microbial metabolic pathways, such as using ferricyanide with cyanobacteria,26 or menadione with Escherichia coli.27 Nanostructured and surface-modified electrodes, like graphene-coated or conducting polymer-functionalized electrodes, enhance mediator interactions and electron transfer efficiency.28,29 Genetic engineering approaches enhance mediator production, exemplified by engineered Shewanella oneidensis producing increased riboflavin.30 Hybrid systems that combine artificial and natural mediators leverage both high redox potential and biocompatibility.31 Conductive hydrogels with metal nanoparticles further improve transfer efficiency and reduce overpotential.32 Among various redox-mediators, ferricyanide based complexes stands out as a promising redox mediator due to their high redox potential, facilitating efficient electron transfer from microorganisms to electrodes.33,34 Its chemical stability and reversible redox properties ensure long-term performance and compatibility with a wide range of microorganisms, including cyanobacteria. Its ease of integration, and proven effectiveness in enhancing the performance highlight its suitability for optimizing charge extraction efficiency in these systems.34,35
This work undertakes a systematic approach to understand the metabolic reactions of cyanobacterial strains. Various electrochemical techniques are employed to identify suitable electron mediators for transferring photogenerated electrons from microbes to the photoanode. Dye-sensitized solar cell (DSSC) architecture is utilized to investigate the charge transfer mechanism in Synechocystis pevalekii. This microbe is used because of biocompatibility, efficient light absorption under diverse conditions, cost-effectiveness, structural flexibility, and potential for integration with bio-hybrid systems.36–38 The DSSC architecture provides an efficient charge transfer pathway in microbial solar cells, enhancing their overall performance and energy conversion efficiency. Previous research demonstrated BPV responses in freely floating device configurations. This study employs an immobilization strategy to investigate the impact of metabolic reactions on BPV responses in these strains. Immobilization within biofilms is critical for enhancing stability, improving efficiency, and ensuring longevity in microbial solar cells.39 This approach optimizes microbial performance by leveraging the natural properties of biofilms, ensuring direct contact and efficient charge transfer with minimal energy loss. The performance of the latter is compared with the free-floating devices.
The electrochemistry of individual microbes in various redox mediators or electrolyte reveals fundamental insights into charge transfer mechanisms. Conventional electrochemistry with macroscopic electrodes provides an average perspective of electrochemical potential, but different surfaces of bio-organisms may exhibit varied redox properties.40 Scanning electrochemical microscopy (SECM) is utilized to explore localized charge carriers from immobilized single-cell microbes, a previously unexplored area. SECM serves as an excellent electroanalytical tool for examining the release of redox species under irradiation, accurately gauging local currents at the microelectrode tip precisely positioned above the specimen.41,42 This study transitions from a broad two-electrode device configuration to a single-cell device, offering valuable insights into the fundamental mechanisms of cyanobacterial bio-photoelectrochemical behaviour. Additionally, genome-scale metabolic modeling is employed to observe flux changes associated with terminal Ferredoxin NADP+ oxidoreductase (FNR)-catalyzed reactions in representative cyanobacteria species like Synechocystis.43 Complete experimental details are provided in ESI.†
Results and discussion
Metabolic modelling in Synechocystis strain
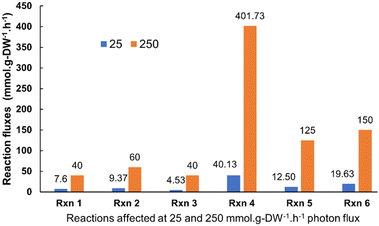
Synechocystis sp. 6803 is an extensively studied and robust model cyanobacterium, and numerous research studies have been undertaken to understand the intracellular electron pathways of photosynthetic organisms. It has a versatile carbon metabolism, and its entire genome has been sequenced. Modelling was conducted to understand the metabolic reaction pathways of Synechocystis strains.44–47 The objective of the modeling was to observe the changes in flux associated with the terminal Ferredoxin NADP+ oxidoreductase (FNR) catalysed reaction in a representative cyanobacteria (Synechocystis). The generated NADPH (Nicotinamide adenine dinucleotide phosphate) interacts with the exogenous redox mediators (such as potassium ferricyanide) and the latter facilitating exo-electrogenesis. Thus, any variation in flux through FNR catalysed reaction can assist in predicting the light-dependent electrogenic activity of the microbe. iSyn731, genome-scale metabolic (GSM) model for Synechocystis sp. 6803 was used in the study and the cyanobacterial metabolism was modelled under both dark and light regime by constraining the model appropriately. COBRA Toolbox (Gurobi optimizer) on MATLAB 2020b was used for simulation and analysis. The model's exchange reactions were constrained as per the artificial sea water nutrient (ASN III) medium composition to mimic experimental conditions.For simulating light-dependent reactions, the exchange reactions for photons that act upon Photosystems I and II were constrained to have an uptake rate of 200 μmol photons m−2 s−1. Moreover, the light-independent or carbon fixation reactions were not completely shut off, rather their rates (in mmol g-DW−1 h−1) were reduced to 10% of the initial values to simulate the process of photosynthesis. The light-independent/dark phase reactions were then modeled in the system by blocking the photon exchange reactions and reducing the flux through the light-dependent photosynthetic reactions to 10% of their original rates as defined in the model. Subsequently, the flux through biomass reaction and ferredoxin NADP+ oxidoreductase was examined using flux balance analysis (FBA). The maximum allowable flux through the biomass reaction was found to be 4.92 × 10−4 and 3.56 × 10−4 mmol g-DW−1 h−1 for light-dependent and independent conditions, respectively. This indicates that biomass generation is marginally increased under light regime when compared to dark. Furthermore, the flux for ferredoxin NADP+ reductase catalyzed reaction showed significant increase in the light phase of photosynthesis (140 mmol g-DW−1 h−1) compared to the dark phase (0.0262 mmol g-DW−1 h−1). The effect of light intensity on FNR flux is provided in Fig. S1 (ESI†). This modeling prediction justified the increased light-dependent electrogenic activity of cyanobacteria. The metabolic reactions (approximately 400 in number) belonging to key pathways like amino acids metabolism, fatty acid biosynthesis, glutathione metabolism and energy metabolism displayed higher flux values under increased light intensity. Of these, the reactions contributing to energy metabolism such as citric acid cycle, oxidative phosphorylation and glyoxylate metabolism were considered central to the electrogenic capabilities of the cyanobacterium as these reactions involve metabolites which participate in electron transfer process (Fig. 1 and Table S1, ESI†). The increased flux through these reactions of interest highlights the role of photon flux (light intensity) in modulating the cyanobacterial metabolism to influence current generation. Many of these reactions as captured through the modelling analysis, wherein it has been reported that a particular reaction of interest is shown to generate the electron transfer units (ferredoxin and plastoquinol). For instance, Reaction 1 (denoted as Rxn 1) is reported to generate reduced-ferredoxin, besides breaking down pyruvate. Therefore, an increased flux through this reaction contributes to increased reduced-ferredoxin generation, which may further impact electron generation through FNR. Similarly, Reaction 5 (denoted as Rxn 5) is known to generate plastoquinol, Moreover, Reaction 3 (Rxn 3) and Reaction 4 (Rxn 4) are involved in glyoxylate metabolism that has protective role against photorespiration, the latter being detrimental to cyanobacteria. The bio-photoelectrochemical devices were made with Synechocystis pevalekii. However, the modeling was done using Synechocystis sp. 6803 where the metabolic reactions are expected to be same for both the microbes.
Photo-electrochemical evaluation using Synechocystis pevalekii strain
Following the understanding of metabolic pathways of Synechocystis strain, DSSC type devices (Fig. S2, ESI†) were constructed to corroborate the outcome of the comprehensive modeling analysis. The initial bio-electrochemical device series was developed using mesoporous-TiO2 coated on fluorine doped tin oxide (FTO) as a photoanode and Pt-coated on FTO as the counter-electrode. A Nafion-117 membrane was employed to separate the anode and cathode. To facilitate full contact with the TiO2, Synechocystis pevalekii and potassium ferricyanide were employed at concentrations of 5 mM each. Before assessing the photoelectrochemical response of microbe, cyclic voltammetry (CV) was analysed for both the media and the redox-mediator as controls (Fig. S3, ESI†). While ASN-III media exhibited no oxidation or reduction current, the presence of the redox mediator resulted in both oxidation and reduction currents.48 Based on the CV experiment, a potential of 0.3 V was applied to extract the photogenerated charge carriers from the Synechocystis pevalekii microbe. Chronoamperometry (CA) experiments were carried out using Synechocystis pevalekii in conjunction with ASN-III media and ferricyanide as the electrolyte, lasting for 12 hours (Fig. S3(b), ESI†).Attempts to characterize the device using conventional J–V characterization, under AM1.5G light, failed or were not found suitable due to slow response of microbial device. So, throughout the study chronoamperometry was employed to understand the photoresponse of the device. From Fig. S3 (ESI†), it can be noticed that the utilisation of ASN-III media and the redox mediator in electrolyte exhibited a higher photoresponse compared to the microbe-based device, evident under both illuminated and dark conditions. This underscores the prominence of electrolytes in influencing the device performance. Previous studies have used this highly conductive ASN-III media as an electrolyte, demonstrating higher photocurrent.49–51 However, ASN-III media's high conductivity can overshadow the effects of microbes in an ideal bio-photoelectrochemical system.
To mitigate the impact of photocurrent contributions from the ASN-III media and redox mediator, a second set of the device was constructed with an identical configuration, replacing ASN-III media with NaCl while maintaining ferricyanide as the redox mediator. The photoelectrochemical characteristics of the microbes were evaluated in a two-electrode system. CV experiments were conducted on blank NaCl and NaCl with K4[Fe(CN)6] (Fig. S4, ESI†). The blank NaCl shows no redox signatures, however its combination with ferricyanide exhibited a redox potential at 0.3 V. This potential serves as an applied/monitoring potential for subsequent experiments.
Microbe conditioning experiments were conducted on the Synechocystis pevalekii strain using NaCl as the media in an incubator, alternating between light and dark conditions every two and four hours, and the results are provided in Fig. 2(a). The purpose of this conditioning experiment was to determine if there were any differences in photoresponse relative to the conditioning time. Specifically, the microbes were subjected to alternating 2-hour light and dark cycles within an incubator, and their photoresponse was measured. The same conditioning process was repeated with light and dark cycles alternating every 4-hour, and the photoresponse under these conditions was also recorded. Our results showed no significant difference in photocurrent between the 2-hour and 4-hour cycles, indicating that a minimum conditioning time is sufficient to elicit all relevant metabolic reactions from the microbes. This lack of variation suggests that the microbes exhibit a stable and adaptable photocurrent response across these cycle durations. Such robustness implies that either cycle length can be used interchangeably in experiments without impacting photocurrent measurements, simplifying experimental design and reducing the need for precise control over light–dark cycles. Additionally, this observation suggests that the microbes have developed a form of photoresistance or robust photoadaptation, overcoming potential stress responses from photodamage. After the conditioning experiment, the CA experiments were performed on the conditioned microbes in the presence of NaCl and redox mediator (NaCl + RM). The photoresponse current of the Synechocystis pevalekii strain is clearly higher that of the blank NaCl + RM electrolyte. Additionally, the optical density (O.D.) of the Synechocystis pevalekii was varied and their photocurrent response is studied (Fig. 2(b)). The O.D. of 2 showed an improved photocurrent response than the others, possibly due to higher light absorption.
 | ||
| Fig. 2 (a) Effect of microbe conditioning and (b) O.D concentration of Synechocystis pevalekii recorded at an applied potential of 0.3 V under dark and light (green shade). | ||
Effect of microbe ageing on the photoelectrochemical performance of Synechocystis pevalekii strain
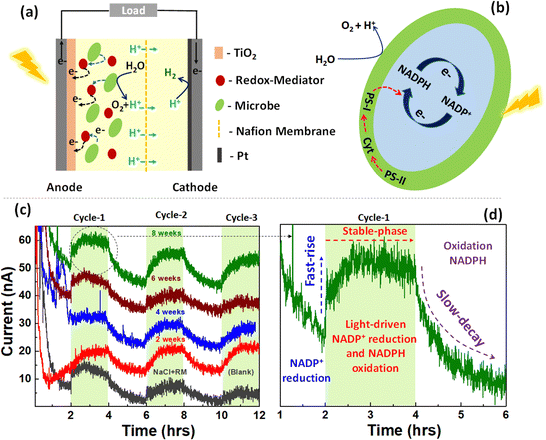
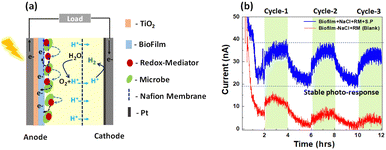
The impact of microbe ageing on photoelectrochemical performance was investigated (Fig. 3(a)), utilising Synechocystis pevalekii at different growth time. The conventional photoelectrochemical metabolic reactions are depicted in Fig. 3(b). In oxygenic photosynthesis process, light photons harvested by chlorophyll pigments (P700 and P680) at photosystem (PS) I and II reaction centers, embedded in the thylakoid membrane. This initiates electron extraction from water, releasing oxygen. These electrons traverse through PSII, cytochrome b6f, plastocyanin (PC)/Cytc6, and PSI to ferredoxin (Fd) in the photosynthetic electron transfer chain (PETC), producing NADPH and Adenosine triphosphate (ATP) through an electrochemical gradient.52,53 The redox mediator is expected to extract electron from NADPH and transfer it to anode. The complete mechanism of device operation is provided in Fig. 3(a and b). Photocurrent responses were measured over a 12-hour period under both light and dark conditions (Fig. 3(c)), employing NaCl and ferricyanide as the electrolyte. Synechocystis pevalekii microbes cultured for eight weeks exhibited good photocurrent performance, comparable to the initial period. The initial response within the first 1–2 hours of darkness indicated a rapid rise phase attributed to the reduction of NADP+. A stabilised photocurrent was observed in light conditions (2–4 hours), attributed to light-driven NADP+ reduction and NADPH oxidation. Subsequently, a gradual decay region was observed due to the oxidation of NADPH under dark conditions.54,55 The observed current is due to the extraction of electrons from NADPH by redox mediator and subsequently pumped at the anode. It has to be noted that the photocurrent values of 4- and 6-weeks old microbes are less than 2- and 8-weeks old microbes. The variation in photocurrent intensity across the different cultivation periods is closely tied to the physiological stages of microbial growth.56,57 At the 2-week mark, the culture is typically in the exponential growth phase, which is characterized by rapid cell division and high metabolic activity leading to peak NADPH production, which supports strong photosynthetic activity and, consequently, high photocurrent intensity. However, as the culture progresses to the 4- to 6-week period, it generally enters the stationary phase where the nutrient depletion and waste accumulation reduce metabolic activity and NADPH production.58,59 This decline in NADPH production possibly results in lower photocurrent intensity. As a result, photosynthetic activity slightly diminishes, leading to a reduced rate of electron transfer and a corresponding decrease in photocurrent intensity. Nutrient limitations during this phase further shift the metabolic focus from growth to maintenance, which reduces overall electron production. Additionally, extended exposure to light during this stationary phase can cause photo-damage to the photosynthetic apparatus, particularly the photosystems and chlorophyll, which further contributes to the decline in photocurrent.60Various methods exist for generating consistent electrical energy from living microorganisms, yet a notable challenge persists in maintaining a stable output of photocurrent response from them. Previous experiments have demonstrated photocurrent responses in free-floating device configurations. In this section, Synechocystis pevalekii immobilized on a biofilm is employed as photoanode. The immobilized device consists of a mesoporous-TiO2-coated FTO serving as the photoanode and a Pt-coated FTO acting as the counter-electrode (Fig. 4(a)). Nafion-117 membrane was used between the photoanode and cathode, separated by a gasket. A thin layer of agar-gel as biofilm was applied to the surface of the photoanode, serving as the substrate for immobilising highly diluted Synechocystis pevalekii microbes. Standard electrolytes, NaCl and ferricyanide, were utilized under both light and dark conditions. The immobilisation strategy proved effective, yielding a stable photocurrent response with a current extraction of ∼20 nA at an applied voltage of 0.3 V over 12-hour duration, a notable improvement compared to the outcomes observed with free-floating devices. This experiment revealed that immobilizing microbes is essential for sustaining a stable photo-current response over an extended period (Fig. 4(b)). The device employing agar-based biofilms embedded with microbes enhances the stability of photocurrent responses. This improvement might be due to direct electron transfer, offering physical protection to the cells, enhancing nutrient availability, influencing redox mediator dynamics, and ensuring long-term stability of cell attachment and activity.61,62
Although theoretically it is possible to make a comparison between the devices made with flooded and biofilm based electrodes, the differences in device operation like (i) charge conduction through the agar film or redox mediator and (ii) the effect on microbe growth or the metabolic processes due to difference in composition of agar/NaCl media, made comparison difficult.
Single microbe photoelectrochemical cell using SECM
Further, we aimed to assess the extent of photocurrent extraction from an individual cell of the Synechocystis pevalekii microbe. To achieve this, localized electrochemical technique called scanning electrochemical microscopy (SECM) was employed to probe the charge transfer under dark and light conditions in a constant height mode.63 The measurement employed a platinum (Pt) tip as the working electrode, utilizing ferricyanide and NaCl as a redox mediator in an electrolyte solution, with Synechocystis pevalekii as the microbial specimen of interest. Operating at a fixed distance between the Pt electrode tip and the surface of the microbe, a potential of 0.3 V was applied to facilitate reversible oxidation/reduction reactions that occur in the redox mediator. The Pt electrode measures the steady-state current flowing between the tip and the microbial surface, reflecting local electrochemical photocurrent activity at the microbe-electrode interface. The experimental configuration, depicted in Fig. 5(a), consisted of a single-microbe device with an agar biofilm. Optical microscope images of the immobilized Synechocystis pevalekii microbe on the agar biofilm were captured using optical microscope integrated with the SECM (Fig. 5(b)). Fig. 5(c) illustrates the experimental setup, wherein a thin layer of agar-gel was applied to a glass substrate. Subsequently, a highly diluted solution of Synechocystis pevalekii microbes was drop-casted onto it. This microbe-immobilized glass substrate was housed in a Petri dish and positioned on the sample stage. In the SECM experiment, a 1 μm Pt-tip served as the working electrode to probe the photoelectrochemical response, while a Nafion-ionomer-encapsulated Pt-wire functioned as the counter electrode, establishing a typical two-electrode device configuration.The Pt-tip was precisely positioned on the surface of a single Synechocystis pevalekii cell, and a potential of 0.3 V was applied. The redox-electrolyte, comprising NaCl and ferricyanide, exhibited no photo-current response. However, in the presence of Synechocystis pevalekii + NaCl + ferricyanide as a redox-electrolyte under both light and dark conditions, the SECM experiments demonstrated that a single cell of Synechocystis pevalekii could generate a photo-current of approximately of ∼100 pA under optimised conditions (Fig. 5(d)). Additional details on single microbe imaging is provided in the ESI† (Fig. S5 and related discussions). Under light conditions indicated an initial rise attributed to the reduction of NADP+. The light-driven NADP+ reduction and NADPH oxidation were observed, followed by NADPH oxidation under dark conditions, effectively distinguishing the metabolic photosynthetic and respiratory pathways under light and dark conditions, respectively.64 Therefore, by leveraging the localized SECM technique, we quantified the amount of photocurrent generated by a single cell of Synechocystis pevalekii. Assuming a microbe occupying a geometric area of few tens of micrometers on a biofilm, the devices is expected to produce few 100 s of μA per cm2. However, the devices shown in Fig. 3 and 4 shows photocurrent in few nA. This data shows that there is good scope of improving device performance by enabling close packing of microbes and improving charge transfer process. This approach provided insights into the significance of employing SECM as an electroanalytical tool to predict maximum expected device performance.
Conclusions
In conclusion, Synechocystis sp. proves to be a robust and extensively studied cyanobacterium, offering valuable insights into photosynthetic and electrogenic processes. The study utilized the iSyn731 genome-scale metabolic model to elucidate the impact of light and dark conditions on metabolic fluxes, particularly focusing on the ferredoxin NADP+ oxidoreductase-catalyzed reaction. Findings revealed an increased biomass production and significant electrogenic activity under light conditions, highlighting the photosynthetic efficiency of Synechocystis pevalekii strain. Further experiments involving the Synechocystis pevalekii strain in DSSC-based devices demonstrated the photoelectrochemical potential of these microbes, with immobilized configurations providing more stable photocurrent responses compared to free-floating systems. Additionally, SECM tool was used to quantify the photocurrent generation at the single-cell level, emphasizing the precision and utility of this localized electrochemical method. These comprehensive analyses underscore the potential of cyanobacteria in bio-photoelectrochemical applications and pave the way for further exploration of microbial electrogenesis and its practical implementations.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The project is funded by Department of Biotechnology, Government of India (Sanction no. BT/IN/Swiss/51/ACK/2018-19).Notes and references
- J. He and C. Janáky, ACS Energy Lett., 2020, 5, 1996–2014 CrossRef CAS.
- N. Kornienko, J. Z. Zhang, K. K. Sakimoto, P. Yang and E. Reisner, Nat. Nanotechnol., 2018, 13, 890–899 CrossRef CAS.
- M. Calvin, J. Chem. Educ., 1949, 26(12), 639 CrossRef CAS.
- D. Heineke and R. Scheibe, in Encyclopedia of Life Sciences, Wiley, 2009 Search PubMed.
- Y. Amao, ChemCatChem, 2011, 3, 458–474 CrossRef CAS.
- X. Ruan, D. Meng, C. Huang, M. Xu, D. Jiao, H. Cheng, Y. Cui, Z. Li, K. Ba, T. Xie, L. Zhang, W. Zhang, J. Leng, S. Jin, S. K. Ravi, Z. Jiang, W. Zheng, X. Cui and J. Yu, Adv. Mater., 2024, 36, 2309199 CrossRef CAS.
- Z. Li, Y. Zhou, Y. Zhou, K. Wang, Y. Yun, S. Chen, W. Jiao, L. Chen, B. Zou and M. Zhu, Nat. Commun., 2023, 14, 5742 CrossRef CAS PubMed.
- F. Gao, G. Liu, A. Chen, Y. Hu, H. Wang, J. Pan, J. Feng, H. Zhang, Y. Wang, Y. Min, C. Gao and Y. Xiong, Nat. Commun., 2023, 14, 6783 CrossRef CAS.
- S. L. Meng, X. B. Li, C. H. Tung and L. Z. Wu, Chem, 2021, 7, 1431–1450 CAS.
- E. C. Hann, S. Overa, M. Harland-Dunaway, A. F. Narvaez, D. N. Le, M. L. Orozco-Cárdenas, F. Jiao and R. E. Jinkerson, Nat. Food, 2022, 3, 461–471 CrossRef CAS PubMed.
- Z. Wang, Y. Hu, S. Zhang and Y. Sun, Chem. Soc. Rev., 2022, 51, 6704–6737 RSC.
- T. Cardona, Front. Plant Sci., 2016, 7, 257 Search PubMed.
- F. Akita, R. Nagao, K. Kato, Y. Nakajima, M. Yokono, Y. Ueno, T. Suzuki, N. Dohmae, J. R. Shen, S. Akimoto and N. Miyazaki, Commun. Biol., 2020, 3, 232 CrossRef CAS.
- J. Gao, H. Wang, Q. Yuan and Y. Feng, Front. Plant Sci., 2018, 9, 357 CrossRef.
- F. E. Poynton, S. A. Bright, S. Blasco, D. C. Williams, J. M. Kelly and T. Gunnlaugsson, Chem. Soc. Rev., 2017, 46, 7706–7756 RSC.
- E. Trampe, K. Koren, A. R. Akkineni, C. Senwitz, F. Krujatz, A. Lode, M. Gelinsky and M. Kühl, Adv. Funct. Mater., 2018, 28, 1804411 CrossRef.
- C. J. Howe and P. Bombelli, Electricity Production by Photosynthetic Microorganisms, Joule, 2020, 2065–2069 CrossRef.
- R. W. Bradley, P. Bombelli, D. J. Lea-Smith and C. J. Howe, Phys. Chem. Chem. Phys., 2013, 15, 13611–13618 RSC.
- X. Cao, X. Huang, P. Liang, N. Boon, M. Fan, L. Zhang and X. Zhang, Energy Environ. Sci., 2009, 2, 498–501 RSC.
- A. J. McCormick, P. Bombelli, R. W. Bradley, R. Thorne, T. Wenzel and C. J. Howe, Energy Environ. Sci., 2015, 8, 1092–1109 RSC.
- M. J. Kim, S. J. Bai, J. R. Youn and Y. S. Song, J. Power Sources, 2019, 412, 301–310 CrossRef CAS.
- F. L. Ng, S. M. Phang, V. Periasamy, K. Yunus and A. C. Fisher, Sci. Rep., 2017, 7, 16237 CrossRef PubMed.
- A. J. McCormick, P. Bombelli, D. J. Lea-Smith, R. W. Bradley, A. M. Scott, A. C. Fisher, A. G. Smith and C. J. Howe, Energy Environ. Sci., 2013, 6, 2682–2690 RSC.
- T. Wenzel, D. Härtter, P. Bombelli, C. J. Howe and U. Steiner, Nat. Commun., 2018, 9, 1299 CrossRef PubMed.
- A. Gemünde, B. Lai, L. Pause, J. Krömer and D. Holtmann, ChemElectroChem, 2022, 9, e202200216 CrossRef.
- A. C. Gonzalez-Aravena, K. Yunus, L. Zhang, B. Norling and A. C. Fisher, RSC Adv., 2018, 8, 20263–20274 RSC.
- T. O. Boynton, L. E. Daugherty, T. A. Dailey and H. A. Dailey, Biochemistry, 2009, 48, 6705–6711 CrossRef CAS PubMed.
- L. F. Chen, H. Yu, J. Zhang and H. Y. Qin, RSC Adv., 2022, 12, 22770–22782 RSC.
- A. Kisieliute, A. Popov, R. M. Apetrei, G. Cârâc, I. Morkvenaite-Vilkonciene, A. Ramanaviciene and A. Ramanavicius, Chem. Eng. J., 2019, 356, 1014–1021 CrossRef CAS.
- J. Zhao, F. Li, S. Kong, T. Chen, H. Song and Z. Wang, Adv. Sci., 2023, 10, 2206622 CrossRef CAS.
- W. Ji, J. Liu, C. Sha, Y.-C. Yong, Y. Jiang and Z. Fang, Green Carbon, 2024, 2, 322 CrossRef.
- H. Chen, J. Li, Q. Fan, T. Zheng, Y. Zhang, Y. C. Yong and Z. Fang, Chem. Eng. J., 2023, 460, 141863 CrossRef CAS.
- D. P. B. T. B. Strik, H. V. M. Hamelers and C. J. N. Buisman, Environ. Sci. Technol., 2010, 44, 532–537 CrossRef CAS.
- Y. Lee, J. Lee and S. Kim, J. Mater. Chem. A, 2023, 11, 19707–19717 RSC.
- D. Fang, G. Gao, Y. Yang, Y. Wang, L. Gao and J. Zhi, ChemElectroChem, 2020, 7, 2513–2526 CrossRef CAS.
- M. Kokkonen, P. Talebi, J. Zhou, S. Asgari, S. A. Soomro, F. Elsehrawy, J. Halme, S. Ahmad, A. Hagfeldt and S. G. Hashmi, J. Mater. Chem. A, 2021, 9, 10527–10545 RSC.
- M. Anam, H. I. Gomes, G. Rivers, R. L. Gomes and R. Wildman, Sustainable Energy Fuels, 2021, 5, 4209–4232 RSC.
- R. Chauhan, A. Srivastava, P. M. Shirage and K. Bala, Sol. Energy, 2024, 270, 112369 CrossRef CAS.
- T. Wenzel, D. Härtter, P. Bombelli, C. J. Howe and U. Steiner, Nat. Commun., 2018, 9, 1299 CrossRef.
- E. J. Zakem, M. F. Polz and M. J. Follows, Nat. Commun., 2020, 11, 5680 CrossRef CAS PubMed.
- C. Santana Santos, B. N. Jaato, I. Sanjuán, W. Schuhmann and C. Andronescu, Chem. Rev., 2023, 123, 4972–5019 CrossRef CAS PubMed.
- G. Bedendi, L. D. De Moura Torquato, S. Webb, C. Cadoux, A. Kulkarni, S. Sahin, P. Maroni, R. D. Milton and M. Grattieri, ACS Meas. Sci. Au, 2022, 2, 517–541 CrossRef CAS PubMed.
- H. Firoozabadi, M. M. Mardanpour and E. Motamedian, Sci. Rep., 2021, 11, 12294 CrossRef CAS.
- H. Knoop, M. Gründel, Y. Zilliges, R. Lehmann, S. Hoffmann, W. Lockau and R. Steuer, PLoS Comput. Biol., 2013, 9, e1003081 CrossRef CAS.
- J. I. Hendry, A. Bandyopadhyay, S. Srinivasan, H. B. Pakrasi and C. D. Maranas, Curr. Opin. Biotechnol, 2020, 64, 17–23 CrossRef CAS PubMed.
- T. R. Maarleveld, J. Boele, F. J. Bruggeman and B. Teusink, Plant Physiol., 2014, 164, 1111–1121 CrossRef CAS.
- H. Knoop, M. Gründel, Y. Zilliges, R. Lehmann, S. Hoffmann, W. Lockau and R. Steuer, PLoS Comput. Biol., 2013, 9, 1–15 CrossRef PubMed.
- J. Tschörtner, B. Lai and J. O. Krömer, Front. Microbiol., 2019, 10, 866 CrossRef.
- J. S. Foster, S. J. Green, S. R. Ahrendt, S. Golubic, R. P. Reid, K. L. Hetherington and L. Bebout, ISME J., 2009, 3, 573–587 CrossRef CAS PubMed.
- R. K. Stuart, X. Mayali, J. Z. Lee, R. Craig Everroad, M. Hwang, B. M. Bebout, P. K. Weber, J. Pett-Ridge and M. P. Thelen, ISME J., 2016, 10, 1240–1251 CrossRef CAS.
- A. D. Temraleeva, S. A. Dronova, S. V. Moskalenko and S. V. Didovich, Microbiology, 2016, 85, 389–399 CAS.
- L. T. Wey, P. Bombelli, X. Chen, J. M. Lawrence, C. M. Rabideau, S. J. L. Rowden, J. Z. Zhang and C. J. Howe, ChemElectroChem, 2019, 6, 5375–5386 CrossRef CAS.
- L. Nikkanen, D. Solymosi, M. Jokel and Y. Allahverdiyeva, Physiol. Plant., 2021, 173, 514–525 CrossRef CAS.
- F. Xu, S. Yuan and H. H. Lin, Plant Signaling Behav., 2011, 6, 55 CrossRef CAS.
- B. M. Berla, R. Saha, C. D. Maranas and H. B. Pakrasi, Sci. Rep., 2015, 5, 14894 CrossRef CAS PubMed.
- G. Saper, D. Kallmann, F. Conzuelo, F. Zhao, T. N. Tóth, V. Liveanu, S. Meir, J. Szymanski, A. Aharoni, W. Schuhmann, A. Rothschild, G. Schuster and N. Adir, Nat. Commun., 2018, 9, 2168 CrossRef.
- V. M. Luimstra, J. M. Schuurmans, A. M. Verschoor, K. J. Hellingwerf, J. Huisman and H. C. P. Matthijs, Photosynth. Res., 2018, 138, 177–189 CrossRef CAS.
- J. Chen, N. Gao, L. Li, M. Zhu, J. Yang, X. Lu and Y. Zhang, Environ. Sci. Pollut. Res., 2017, 24, 8469–8478 CrossRef CAS.
- T. P. Ramalho, G. Chopin, L. Salman, V. Baumgartner, C. Heinicke and C. Verseux, npj Microgravity, 2022, 8, 43 CrossRef CAS.
- C. M. Lewis, J. D. Flory, T. A. Moore, A. L. Moore, B. E. Rittmann, W. F. J. Vermaas, C. I. Torres and P. Fromme, J. Am. Chem. Soc., 2022, 144, 2933–2942 CrossRef CAS.
- J. C. Chin, W. H. Khor, W. W. F. Chong, Y. T. Wu and H. S. Kang, Mater. Today Proc., 2022, 65, 2970–2978 CrossRef CAS.
- S. Vajravel, S. Sirin, S. Kosourov and Y. Allahverdiyeva, Green Chem., 2020, 22, 6404–6414 RSC.
- K. McKelvey, M. A. Edwards and P. R. Unwin, Anal. Chem., 2010, 82, 6334–6337 CrossRef CAS PubMed.
- J. Hu, W. Meng, Y. Su, C. Qian and W. Fu, Front. Mar. Sci., 2023, 10, 1260709 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, electrochemical and simulation data are provided in ESI. See DOI: https://doi.org/10.1039/d4tc03265a |
| This journal is © The Royal Society of Chemistry 2025 |