Improving voltage and quantum efficiency in blade-coated ITO-free organic solar cells processed with a non-halogenated solvent†
Cuifen
Zhang
a,
Zheng
Li
a,
Yi
Lin
a,
Zhibo
Wang
b,
Huawei
Hu
 b,
Ming
Wang
b,
Ming
Wang
 *a,
Zheng
Tang
*a,
Zheng
Tang
 *a and
Zaifei
Ma
*a and
Zaifei
Ma
 *a
*a
aState Key Laboratory for Modification of Chemical Fibers and Polymer Materials, Center for Advanced Low-dimension Materials, College of Materials Science and Engineering, Donghua University, Shanghai, 201620, P. R. China. E-mail: mwang@dhu.edu.cn; ztang@dhu.edu.cn; mazaifei@dhu.edu.cn
bCollege of Materials Science and Engineering, Donghua University, Shanghai, 201620, P. R. China
First published on 28th October 2024
Abstract
Developing ITO-free device structures, industrially compatible, and environmentally friendly fabrication processes is crucial for advancing organic solar cells (OSCs) technology toward commercialization. This study investigates the performance differences of ITO-free OSCs based on PM6:Y6, fabricated using three methods: spin-coating with chloroform (CF) solution, spin-coating with o-xylene (OXY) solution, and blade-coating with OXY solution. The findings reveal that the active layer prepared by blade-coating with OXY solution exhibits greater donor–acceptor phase separation and poorer molecular packing compared to the spin-coated CF solution, resulting in reduced exciton dissociation efficiency and inferior charge carrier transport. Additionally, the active layer blade-coated from OXY solution presents a higher density of trap states, leading to increased trap-assisted recombination, lower electroluminescence quantum efficiency (EQEEL), and higher non-radiative voltage losses, which restrict the open-circuit voltage (VOC) of the devices. To mitigate these performance losses, we introduce an acceptor side chain modification strategy to improve the morphology and structural order of the active layer, thereby enhancing exciton dissociation efficiency and reducing the density of trap states. This results in significant improvements in both short-circuit current density (JSC) and VOC. Using the Y6DT acceptor as an example, the performance of ITO-free OSCs fabricated by blade-coating with OXY solution deliver a power conversion efficiency (PCE) of 13.5%.
1. Introduction
Organic solar cells (OSCs) offer several advantages, including low cost, lightweight, and the potential for roll-to-roll mass production and large-area fabrication, which have attracted significant attention from both academia and industry over the past few decades.1–5 Thanks to the continuous development in organic photovoltaic materials, device fabrication processes, and a deeper understanding of device operation mechanisms, the power conversion efficiency (PCE) of OSCs under standard AM1.5 illumination has now surpassed 20%, meeting the requirements for commercial applications.6,7 However, most high-efficiency OSCs are currently fabricated using active layer solutions prepared with halogenated solvents and spin-coated onto indium tin oxide (ITO) coated glass substrates.6–8 The high cost of ITO electrodes, the limitations of spin-coating for large-scale production, and the environmental pollution caused by the extensive use of halogenated solvents restrict the industrial development of organic photovoltaic technology.9,10 Therefore, developing ITO-free device structures, industrially compatible, and environmentally friendly OSC fabrication processes is crucial for advancing OSC technology toward commercialization.9–13Currently, several film-forming techniques compatible with large-scale and large-area production of OSCs, including spray coating, slot–die coating, and blade coating, have been applied to construct OSCs. In 2022, Chen et al. used inkjet printing with a high-temperature layer-by-layer deposition strategy to prepare OSCs with a PCE over 13.0%.14 The devices were based on a PM6:BTP-BO-4Cl donor–acceptor system. In 2023, Liu et al. employed slot–die coating technique, and ZR1-C3 and L8BO as active materials, to construct OSCs, achieving a PCE of 13.4%.15 In 2023, Huang et al. successfully fabricated flexible OSCs with a PCE of close to 9.0%, using roll-to-roll slot–die coating with PBDB-T:ITIC as the active material system.16 Despite the critical importance of roll-to-roll slot–die coating for the industrial development of OSCs, the high initial material investments pose a barrier to the development of this technology.
Given the similarity in principles between blade coating and slot–die coating, blade coating is considered an important technique for achieving large-scale and large-area fabrication of OSC devices under laboratory conditions.17,18 In 2006, Schilinsky et al. pioneered the use of blade coating to fabricate OSCs with a PCE exceeding 4.0% on ITO electrodes using P3HT and PCBM.19 In a subsequent study in 2021, Zhang et al. utilized blade coating and sequential deposition strategies to fabricate OSCs with a PCE exceeding 16.0% based on the PM6/BTP-eC9 active material system.20 In 2024, Liu et al. employed a solid additive strategy for blade-coated solar cells, and realized a PCE of 18.4% using PM6:BTP-eC9 as the active materials and ITO as the substrate electrode.21 Although blade coating technology has made significant progress in the organic photovoltaic field in recent years, the performance of blade-coated devices still lags those prepared by spin-coating with the same active materials.22 For example, Zhao et al. reported a 30% reduction in PCE for PBTA-TF:IT-M devices prepared with blade coating using o-xylene (OXY) solvent compared to those made with spin-coating.23 This performance degradation is primarily due to changes in the active layer drying dynamics leading to altered active layer morphology, reduced internal quantum efficiency (IQE), limited short-circuit current density (JSC) and fill factor (FF), and increased voltage losses (Vloss), ultimately resulting in a significant decline in device performance.
Developing processing methods using non-halogenated, low-toxicity solvents is also critical for the industrial development of OSC technology.24,25 In 2017, Farahat et al. used cyclopentyl methyl ether mixed with toluene as the solvent, employing SMPV1:PCBM as the active material system, to fabricate OSCs with a PCE of 8.1% via spin-coating.26 In a later study in 2021, Liu et al. used OXY as the solvent and PM6:L14 as the active layer material system to prepare OSCs with a PCE approaching 15.0% on ITO electrodes through spin-coating.27 In 2020, Xu et al. fabricated OSCs with a PCE of 17.3% using toluene as a solvent and PM6:BTP-BO-4Cl as the active material system.28 In 2022, Sun et al. constructed OSCs with a high PCE of 17.8% using p-xylene as the solvent and PM6:PM7:Y6 ternary blend active layer via spin-coating.29 In 2024, Zhang et al. constructed OSCs based on the ternary blend of PM6:D10:L8BO, by blade coating from toluene solution, and achieved a PCE of 18.2% via a rapid solidification strategy.30 Notably, these OSCs fabricated with non-halogenated solvents all employed ITO electrodes and involved complex fabrication processes such as high-temperature annealing or the use of additives, complicating the mass and large-area industrial production of OSCs.31,32
In this study, we focus on the performance loss issues of ITO-free OSCs fabricated using non-halogenated solvents and blade coating. Through systematic device physics analysis, we elucidate the effects of non-halogenated solvents and blade coating processes on the morphology and molecular packing properties of the active layer. Then, we identify the reasons for the low quantum efficiency and high voltage loss in blade-coated OSCs prepared with non-halogenated solvents. Ultimately, by employing acceptor side-chain modification strategies, we optimize the morphology and molecular packing properties of the blade-coated active layers prepared with non-halogenated solvents. This allows us to realize increased exciton dissociation efficiency, reduced density of trap states, and consequently improving the photovoltaic and electroluminescence external quantum efficiency (EQEEL) of the devices, leading to synergistic enhancements in JSC and open-circuit voltage (VOC) for blade-coated ITO-free OSC devices fabricated with non-halogenated solvents.
2. Results and discussion
2.1. Performance limit in blade-coated ITO-free solar cells processed with a non-halogenated solvent
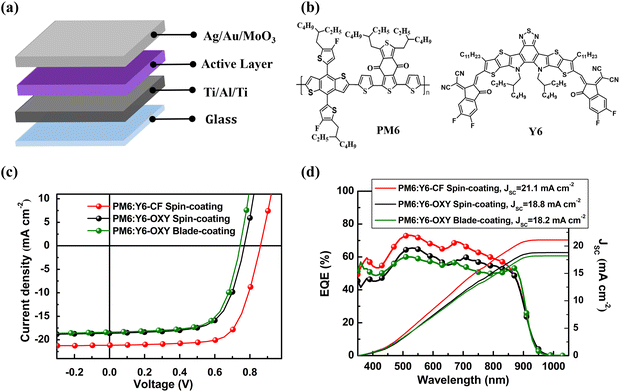
In this work, we constructed inverted ITO-free OSCs using the classical donor/acceptor combination PM6:Y6 as the BHJ active layer.33–35 The device structure is glass/Ti (2 nm)/Al (80 nm)/Ti (2 nm)/active layer (107 ± 8 nm)/MoO3 (10 nm)/Au (1 nm)/Ag (5 nm), where the multilayer metal Ti/Al/Ti serves as the bottom reflective cathode, and the ultrathin MoO3/Au/Ag serves as the top transparent anode.36 The schematic of the device structure is shown in Fig. 1a, and the chemical structures of the active layer donor and acceptor materials PM6 and Y6 are shown in Fig. 1b.Based on the optimal processing conditions for the PM6:Y6 active material system reported in the literature, we first fabricated reference devices by spin-coating chloroform (CF) solution (with 0.5 vol% CN) to deposit the active layer.37,38 Subsequently, we constructed another set of ITO-free OSCs by spin-coating the active layer using the non-halogenated solvent OXY as the solvent. Finally, we prepared a set of OSCs using a blade-coating technique with OXY. The current density–voltage (J–V) curves and external quantum efficiency (EQE) spectra of these three sets of ITO-free OSC devices are shown in Fig. 1c and d, respectively, and the photovoltaic performance parameters of the devices are listed in Table 1.
| Processing condition | J SC (mA cm−2) | V OC (V) | FF (%) | PCE (%) | E g (eV) | V loss (V) | ΔVr (V) | ΔVnr (V) |
|---|---|---|---|---|---|---|---|---|
| CF spin-coating | 21.2 (21.9 ± 0.7) | 0.86 (0.85 ± 0.01) | 69.6 (67.5 ± 2.1) | 12.7 (12.4 ± 0.3) | 1.42 | 0.56 | 0.35 | 0.25 |
| OXY spin-coating | 18.6 (18.2 ± 0.5) | 0.77 (0.74 ± 0.03) | 66.5 (65.6 ± 1.5) | 9.5 (9.3 ± 0.2) | 1.41 | 0.64 | 0.34 | 0.30 |
| OXY blade-coating | 18.4 (18.3 ± 0.6) | 0.74 (0.70 ± 0.04) | 67.5 (66.7 ± 1.1) | 9.2 (8.9 ± 0.3) | 1.41 | 0.67 | 0.34 | 0.34 |
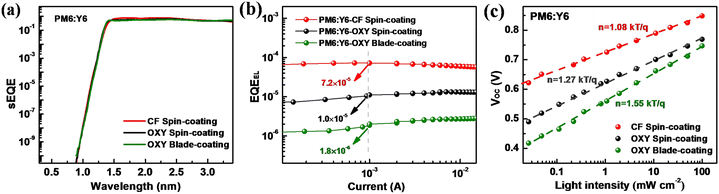
From the J–V curves, it can be found that the PCE of the PM6:Y6 device constructed by spin-coating CF solution is 12.7%, with a JSC of 21.2 mA cm−2, VOC of 0.86 V, and FF of 69.6%. Notably, this result indicates that the PCE of the ITO-free OSC is slightly lower than that of the PM6:Y6 device with an ITO-based electrode reported in the literature.38 This is mainly due to the lower reflectance of the Ti/Al/Ti electrode and the lower transmittance of the MoO3/Au/Ag electrode compared to the Ag reflective electrode and the ITO transparent electrode in OSC devices based on ITO electrodes (Fig. S1, ESI†), which limits the optical absorption efficiency of the active layer in the ITO-free OSCs.36
We also note that the ITO-free OSCs constructed using OXY solution, whether by spin-coating or blade-coating, exhibited significantly lower PCE than the ITO-free OSCs prepared by spin-coating CF solution: the PCE of the devices constructed by spin-coating OXY solution was 9.5%, and the PCE of the devices prepared by blade-coating OXY solution was 9.2%. The main reason for this is that the devices prepared using OXY solution had lower JSC and VOC: the JSC of the devices constructed by spin-coating OXY solution was 18.6 mA cm−2, while the JSC of the devices prepared by blade-coating OXY solution was 18.4 mA cm−2; the VOC of the devices constructed by spin-coating OXY solution was 0.77 V, while the VOC of the devices prepared by blade-coating OXY solution further decreased to 0.74 V.
2.2. Origin of quantum efficiency losses in blade-coated solar cells processed with a non-halogenated solvent
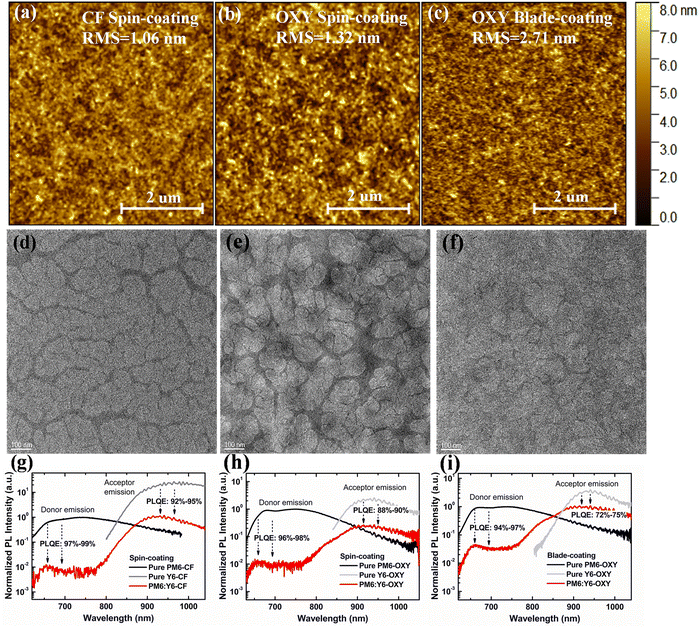
First, we investigated the reasons for the differences in JSC of the ITO-free OSCs fabricated by spin-coating with CF solution, spin-coating with OXY solution, and blade-coating with OXY solution. For an OSC, JSC is primarily determined by the optical properties and the IQE of the device. Given that the device architectures of these three ITO-free solar cells are identical, these devices are expected to have the same optical interference conditions. Therefore, the optical properties of these devices are determined by the absorption of the active layers. From UV-visible spectroscopic measurements, we observed that the absorption coefficient spectra of the active layers prepared using spin-coating with CF solution, spin-coating with OXY solution, and blade-coating with OXY solution exhibited slight differences (Fig. S1, ESI†), suggesting that the optical properties of the ITO-free OSCs fabricated using different solvents and deposition processes differ slightly. Therefore, the difference in JSC should be attributed to differences in the IQEs of the devices. To validate this, we determined the optical constants of the non-active and active layers processed with different solvents and coating methods, and conducted optical transfer matrix simulations (TMM) for the ITO-free OSCs. The TMM results (Fig. S2, ESI†) indicate that the IQEs of devices processed by spin-coating with CF solution, spin-coating with OXY solution, and blade-coating with OXY solution are 77%, 65%, and 58%, respectively.In OSCs, IQE is mainly determined by the exciton dissociation efficiency and the transport of charge carriers within the active layer.39,40 The former is primarily influenced by the degree of phase separation between the donor and acceptor materials in the active layer. To elucidate the reasons for different IQEs in ITO-free devices prepared using different solvents and deposition processes, we first characterized the PM6:Y6 active layers using atomic force microscopy (AFM). As shown in Fig. 2a–c, the root mean square (RMS) surface roughness of the active layer prepared by spin-coating with CF solution is 1.06 nm, this is increased to 1.32 nm for the active layer prepared by spin-coating with OXY solution. The RMS surface roughness is further increased to 2.71 nm for the active layer prepared by blade-coating with OXY solution. Based on the AFM results, we can confirm that changes in solvent and deposition process can indeed alter the morphology of the active layer in ITO-free OSCs, affecting the degree of phase separation between donor and acceptor materials. This, in turn, impacts the exciton dissociation efficiency of the active layer and consequently the IQE and JSC of the device.
Then, we conducted transmission electron microscope (TEM) measurements to analyze the morphology of the active layers processed under various conditions, as depicted in Fig. 2d–f. For the PM6:Y6 active layer processed by spin-coating from CF solution, distinct dark fibrillar structures of PM6, with a diameter ranging from 10 to 20 nm, can be clearly observed. The brighter regions are likely to represent amorphous Y6 and the PM6:Y6 blend phases. The morphology of the PM6:Y6 active layer processed by spin-coating from OXY solution differs from that processed with CF, exhibiting less pronounced fibrillar structures and larger amorphous regions. Moreover, the fibrillar characteristics are even more challenging to discern in the active layer processed by blade-coating from OXY solution. However, certain similarities can be discerned in the TEM images of the active layers processed by blade-coating and spin-coating from OXY solution.
To further understand the impact of solvent and deposition process variations on the exciton dissociation efficiency in the PM6:Y6 active layer, we performed photoluminescence (PL) quenching measurements on PM6:Y6 active layers prepared using different solvents and deposition processes. The results are shown in Fig. 2g–i. By comparing the PL spectra of the bulk heterojunction (BHJ) active layers, pure donor PM6, and pure acceptor Y6 films, we calculated the PL quenching efficiencies of PM6 and Y6 in the BHJ active layer prepared by spin-coating with CF solution to be 97–99% and 92–95%, respectively. In the PM6:Y6 active layer prepared by spin-coating with OXY solution, the PL quenching efficiency of PM6 remains at 96–98%, while that of Y6 drops to 84–90%. This suggests that the phase separation in the active layer prepared using the OXY solution is greater than that in the active layer prepared with the CF solution, thereby limiting the exciton dissociation efficiency of the acceptor. In the PM6:Y6 active layer prepared by blade-coating with OXY solution, the PL quenching efficiency of PM6 is 94–97%, but that of Y6 further decreases to 72–75%. This shows that the domain size of Y6 acceptor in the active layer prepared by blade-coating with OXY solution is even larger than that in the active layer prepared by spin-coating with OXY solution, further limiting the exciton dissociation efficiency of the acceptor. Therefore, the lower IQE and JSC of OSCs prepared by spin-coating or blade-coating with OXY solution compared to those prepared by spin-coating with CF solution can indeed be attributed to the low exciton dissociation efficiency, caused by suboptimal active layer morphology.
We then further studied the effects of different solvents and deposition processes on the charge carrier transport properties in ITO-free OSCs and their impact on device IQE and JSC. In OSCs, carrier transport is related to the structural properties of the active layer.41 Therefore, we performed grazing-incidence wide-angle X-ray scattering (GIWAXS) measurements on PM6:Y6 active layers prepared using different solvents and deposition processes. As shown in Fig. 3a–c, on the molecular level, the GIWAXS pattern of the active layer prepared by spin-coating with CF solution exhibits strong aromatic stacking signals at qz = 1.85 Å−1, corresponding to a face-on orientation with a π–π stacking distance of 3.4 Å. Additionally, on the mesoscopic level, diffraction signals at qxy = 0.3 Å−1 can be observed, which is assigned to the lamellar stacking with a distance of 20.9 Å. More importantly, higher order diffraction peaks are observed for the PM6:Y6 active layer, processed by spin-coating from CF solution, suggesting a high structural order. In contrast, the GIWAXS pattern of the active layer prepared by spin-coating with OXY solution shows weaker π–π stacking signals and lamellar packing signals, and the higher order diffraction peaks disappear, which indicates that the structural order of the active layer prepared by spin-coating with OXY solution is reduced, compared to that processed with CF. The GIWAXS scattering signals further weaken, and the structural order decreases even more in the active layer prepared by blade-coating with OXY solution. This phenomenon indicates that the change from spin-coating with CF solution to blade-coating with OXY solution affects the structural order of donor and acceptor molecules in the active layer. Given the weaker structural order in the active layer prepared by blade-coating with OXY solution, we predict poorer carrier transport in OSCs fabricated with blade-coating using OXY solution, which further limits JSC.
 | ||
| Fig. 3 GIWAXS patterns of the PM6:Y6 active layers processed by (a) spin-coating CF solution, (b) spin-coating OXY solution, and (c) blade-coating OXY solution. | ||
To verify the above hypothesis, we constructed electron-only devices with the architecture of ITO/ZnO/active layer/PFN-Br/Ag and hole-only devices with the architecture of ITO/PEDOT:PSS 4083/active layer/MoO3/Ag using spin-coating with CF solution, spin-coating with OXY solution, and blade-coating with OXY solution, and performed J–V characterizations. The results are shown in Fig. S3 (ESI†). By fitting the space-charge-limited-current (SCLC) region, we determined the electron mobility (μe) and hole mobility (μh) of these devices. Specifically, we found that the ITO-free OSCs constructed by spin-coating with CF solution have higher electron and hole mobilities of 4.3 (±0.9) × 10−4 and 6.4 (±2.3) × 10−4 cm2 V−1 s−1, respectively. The devices constructed by spin-coating with OXY solution show reduced electron and hole mobilities of 2.4 (±0.7) × 10−4 and 4.3 (±1.6) × 10−4 cm2 V−1 s−1, respectively. The devices constructed by blade-coating with OXY solution exhibit even lower electron and hole mobilities of 1.8 (±0.8) × 10−4 and 3.1 (±1.0) × 10−4 cm2 V−1 s−1, respectively. Combined with the GIWAXS analysis, we conclude that the weaker structural order in the active layer prepared by blade-coating with OXY solution compared to that prepared by spin-coating leads to reduced charge carrier mobilities, which is another reason for the limited IQE and JSC of the device. Furthermore, the decreased charge carrier mobilities also result in reduced FF. Therefore, the performance of ITO-free OSCs constructed by blade-coating with OXY solution is lower compared to those constructed by spin-coating with CF solution.
2.3. Voltage losses in blade-coated solar cells processed with a non-halogenated solvent
Next, we systematically investigated the reasons for the differences in VOC of ITO-free OSCs prepared with different solvents and deposition techniques. The VOC of OSCs is determined by the energy bandgap (Eg) of the active layer and the voltage losses (Vloss) of the device. The specific expression for VOC is as follows:42 | (1) |
Given the known Eg of the active layers, eqn (1) allows us to calculate that the Vloss for devices constructed by spin-coating CF solution is 0.56 V (Table 1), for those constructed by spin-coating OXY solution is 0.64 V, and for those constructed by blade-coating OXY solution is even higher at 0.67 V. This indicates that changes in solvents and deposition techniques lead to variations in Vloss of the ITO-free OSCs, which is the primary reason for the differences in VOC.
To clarify why different solvents and deposition techniques result in varying Vloss in ITO-free OSCs, we systematically analyzed ΔVr and ΔVnr. First, through highly sensitive external quantum efficiency (sEQE) and electroluminescence (EL) measurements of the ITO-free OSCs, we obtained sEQE spectra covering eight orders of magnitude, as shown in Fig. 4a. Using eqn (2):42,43
 | (2) |
 | (3) |
 | (4) |
| ΔVnr = VOC,rad − VOC | (5) |
 | ||
| Fig. 4 (a) sEQE, (b) EQEEL, and (c) VOCvs. light intensity curves for the PM6:Y6 ITO-free OSCs processed by spin-coating CF solution, spin-coating OXY solution, and blade-coating OXY solution. | ||
As listed in Table 1, we found that the ΔVr values of the ITO-free OSCs prepared with different solvents and deposition techniques are similar, approximately 0.34–0.35 V, but their ΔVnr values differ: 0.25 V for devices prepared by spin-coating CF solution, 0.30 V for devices prepared by spin-coating OXY solution, and higher at 0.34 V for devices prepared by blade-coating OXY solution.
The ΔVnr of OSC devices is determined by the EQEEL: the higher the EQEEL, the lower the ΔVnr.42 Therefore, EQEEL measurements were conducted (Fig. 4b), which revealed that the devices constructed by spin-coating CF solution have an EQEEL of approximately 7.2 × 10−5, higher than those constructed by spin-coating OXY solution (1.0 × 10−5) and blade-coating OXY solution (1.8 × 10−6). Based on these results, we determine that the excessively low EQEEL of ITO-free OSCs prepared by blade-coating OXY solution leads to excessively high ΔVnr, which limits the device VOC.
The EQEEL of OSCs is determined by the recombination dynamics of charge carriers. Rapid charge transfer (CT) state recombination and significant trap-assisted recombination are common causes of low EQEEL.42,43 To identify the reasons for the low EQEEL and high ΔVnr in ITO-free OSCs constructed by blade-coating OXY solution, we further investigated the recombination mechanisms of charge carriers in devices prepared with different solvents and deposition techniques. First, by analyzing the sEQE spectra (Fig. 4a), we found that the CT state absorption characteristics in PM6:Y6 based ITO-free OSCs did not change with solvent or deposition technique. Therefore, the energetic properties of the CT states in these devices are similar, and we can infer that the CT state recombination rates are similar regardless of the solvent or deposition technique. Therefore, rapid CT state recombination is not the cause of the low EQEEL in ITO-free OSCs constructed by blade-coating OXY solution.
Next, we conducted light intensity-dependent VOC measurements on ITO-free OSCs constructed using different solvents and deposition techniques to study the impact of solvent selection and deposition technique on trap-assisted recombination of charge carriers.44,45 As shown in Fig. 4c, the slope of the VOCvs. light intensity curve for devices prepared by spin-coating CF solution is 1.08 kT/q, indicating an ideality factor of 1.08, which means the recombination mechanism is primarily bimolecular recombination under open-circuit conditions.46 The ideality factor for devices prepared by spin-coating OXY solution is 1.27, higher than that of devices prepared by spin-coating CF solution, indicating the presence of trap-assisted recombination under open-circuit conditions. Trap-assisted recombination is non-radiative, hence the lower EQEEL in devices constructed by spin-coating OXY solution. The ideality factor for devices prepared by blade-coating OXY solution is even higher at 1.55, indicating more severe trap-assisted recombination, which results in a higher recombination rate and severely limited EQEEL.43
The above results are confirmed by transient photovoltage (TPV) measurements (Fig. S5, ESI†), which show that the lifetime of charge carriers in OSCs constructed by spin-coating CF solution is higher compared to those constructed by spin-coating OXY solution and blade-coating OXY solution. Based on these results, we infer that the low EQEEL in OSCs prepared by blade-coating OXY solution is caused by severe trap-assisted recombination.
To understand the impact of solvent selection and deposition technique on trap-assisted recombination in ITO-free OSCs, we conducted a more in-depth analysis of the J–V curves of electron-only devices prepared by spin-coating CF solution, spin-coating OXY solution, and blade-coating OXY solution (Fig. S6, ESI†). Specifically, using eqn (6):47
 | (6) |
The density of trap states in the active layer of OSCs is mainly related to the molecular structure of the donor and acceptor materials, material purity, the structural order and morphology of the active layer. In this work, although we used the same donor and acceptor materials, we did observe that the morphology and structural order of the active layer were influenced by solvent selection and deposition technique. Based on these observations, we conclude that the non-ideal morphology and structural order of the active layer in devices constructed by blade-coating OXY solution leading to a high density of trap states, low exciton dissociation efficiency, severe trap-assisted recombination of charge carriers, limited EQEEL, high ΔVnr, and low IQE, ultimately resulting in restricted VOC and JSC of the devices.
2.4. Improving performance of blade-coated ITO-free solar cells processed with a non-halogenated solvent
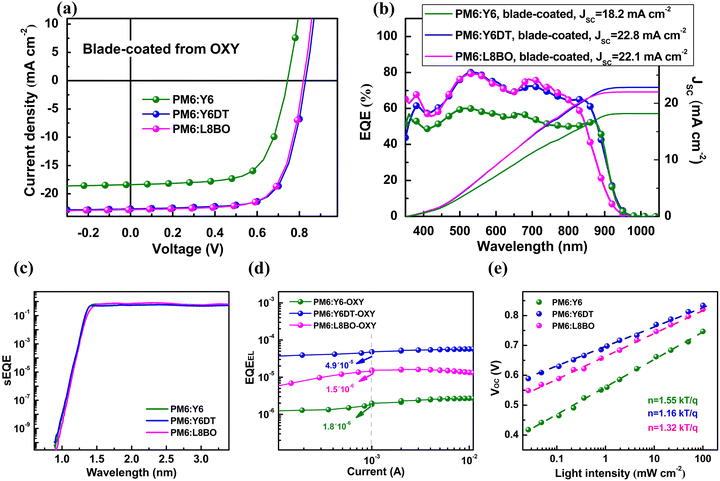
The key to improve the performance of ITO-free OSCs prepared by blade-coating OXY solution lies in optimizing the morphology and structural order of the active layer. Common methods for controlling active layer morphology include post-annealing and the use of solvent additives.48,49 However, these methods often complicate the fabrication process, hindering the industrialization of OSCs. Modifying the side chains of acceptor materials is an effective approach to regulating the morphology and structural order of the active layer without complicating the fabrication process of OSCs. In fact, for the ITO-free OSCs based on PM6:Y6, constructed by blade-coating from OXY solution, we observed that post-annealing and the utilization of solvent additives had either minimal or negative impacts on the device performance (Fig. S7, ESI†). Therefore, in this study, we focus on the impact of acceptor side chain modifications on the performance of ITO-free OSCs prepared by different solvents and deposition techniques.Here, we employed Y6DT and L8BO as the acceptors for constructing ITO-free OSCs. Compared to Y6, Y6DT has a significantly increased number of methylene groups on the side chains attached to the nitrogen atoms in its core unit, while the side chains of the core unit of L8BO are altered from linear to branched. The chemical structures of the Y6DT and L8BO are shown in Fig. 5.
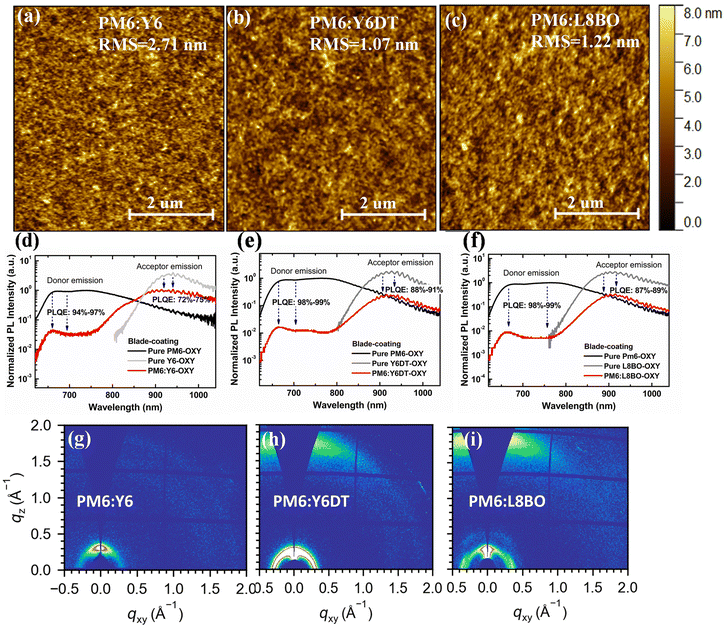
First, AFM measurements were conducted on the PM6:Y6, PM6:Y6DT, and PM6:L8BO active layers prepared by blade-coating OXY solution. As shown in Fig. 6a–c, the RMS surface roughness of the PM6:Y6DT and PM6:L8BO active layers is 1.07 nm and 1.22 nm, respectively, which is lower than that of the PM6:Y6 active layer (2.71 nm), indicating that acceptor side chain modifications can indeed alter the morphology of the active layer.
Subsequently, PL quenching measurements were performed on the PM6:Y6, PM6:Y6DT, and PM6:L8BO active layers prepared by blade-coating OXY solution. As shown in Fig. 6d–f, the acceptor PL quenching efficiencies of the PM6-Y6DT and PM6:L8BO active layers are 88–91% and 87–89%, respectively, significantly higher than that of the PM6:Y6 active layer (72–75%), demonstrating that acceptor side chain modifications can enhance exciton dissociation efficiency by optimizing the morphology of the active layer.
Additionally, GIWAXS measurements were conducted on the PM6:Y6, PM6:Y6DT, and PM6:L8BO active layers prepared by blade-coating OXY solution. The GIWAXS patterns (Fig. 6g–i) reveal that the PM6:Y6DT and PM6:L8BO active layers exhibit stronger scattering signals and a higher structural order, compared to the PM6-Y6 active layer. This suggests that acceptor side chain modifications also improve the molecular packing properties of the active layer, which is beneficial for enhancing the JSC and FF of OSCs.
We then fabricated ITO-free OSCs based on PM6:Y6DT and PM6:L8BO by blade-coating OXY solution in a glove box. J–V and EQE measurements (Fig. 7a and b) show that the JSC of devices based on PM6:Y6DT and PM6:L8BO are 22.9 and 22.0 mA cm−2, respectively, significantly higher than that of PM6:Y6 devices (Table 2). Moreover, the VOC of devices based on PM6:Y6DT and PM6:L8BO are 0.83 and 0.82 V, respectively, also showing substantial improvement compared to PM6:Y6 devices. Consequently, the PCE of ITO-free devices constructed by blade-coating OXY solution is significantly enhanced through acceptor side chain modifications, with the PCE of PM6:Y6DT devices reaching 13.5%. Note that we have also constructed ITO-free OSCs based on PM6:Y6DT and PM6:L8BO by blade-coating OXY solution in air, and ITO-free OSCs with a large area of 2.0 cm−2. In both cases, we realized improved device performance with the acceptor side chain modification strategy. The results are provided in Fig. S8 and S9 (ESI†).
| Active Layer | J SC (mA cm−2) | V OC (V) | FF (%) | PCE (%) | E g (eV) | V loss (V) | ΔVr (V) | ΔVnr (V) |
|---|---|---|---|---|---|---|---|---|
| PM6:Y6 | 18.4 (18.3 ± 0.6) | 0.74 (0.70 ± 0.04) | 67.5 (66.7 ± 1.1) | 9.2 (8.9 ± 0.3) | 1.41 | 0.67 | 0.34 | 0.34 |
| PM6:Y6DT | 22.9 (22.1 ± 1.1) | 0.83 (0.80 ± 0.03) | 71.1 (70.1 ± 1.8) | 13.5 (13.1 ± 0.5) | 1.41 | 0.58 | 0.33 | 0.25 |
| PM6:L8BO | 22.0 (22.2 ± 0.6) | 0.82 (0.81 ± 0.01) | 70.4 (68.2 ± 2.4) | 12.7 (12.1 ± 0.6) | 1.43 | 0.61 | 0.34 | 0.28 |
To understand the reasons for the improved VOC in devices with acceptor side chain modifications, we first determined the Eg of the PM6:Y6DT and PM6:L8BO active layers prepared by blade-coating OXY solution through PL and absorption tests (Fig. S10, ESI†), which are 1.41 and 1.43 eV, respectively. Using eqn (1), we calculated that the Vloss of PM6:Y6DT and PM6P:L8BO devices are 0.58 and 0.61 V (Table 2), respectively, lower than that of PM6:Y6 devices (0.67 V). Thus, the lower Vloss accounts for the increased VOC in devices with acceptor side chain modifications. Notably, although the Eg of the PM6:L8BO active layer is higher than that of the PM6:Y6DT active layer, the Vloss of PM6:L8BO devices is also higher, resulting in a slightly lower VOC (0.82 V) compared to PM6:Y6DT devices (0.83 V). Additionally, the higher Eg of the PM6:L8BO active layer leads to a shorter onset wavelength in the EQE spectrum compared to PM6:Y6DT devices, limiting the JSC of PM6:L8BO devices. Consequently, the PCE of PM6:L8BO devices is lower than that of PM6:Y6DT devices.
To understand the origin of the impact of acceptor side chain modifications on the Vloss of ITO-free OSCs prepared by blade-coating OXY solution, we measured the sEQE of PM6:Y6DT and PM6:L8BO devices (Fig. 7c). From the sEQE, we find that the VOC,rad of PM6:Y6DT and PM6:L8BO devices is similar to that of PM6:Y6 devices, indicating that their ΔVr values are close. Using eqn (5), we calculated the ΔVnr values (Table 2), finding that PM6:Y6DT devices have the lowest ΔVnr at 0.25 V, while PM6:L8BO devices have a ΔVnr of 0.28 V, lower than that of PM6:Y6 devices but higher than that of PM6:Y6DT devices. EQEEL measurements (Fig. 7d) show that PM6:Y6DT devices have the highest EQEEL, and PM6:L8BO devices also exhibit an improvement compared to PM6:Y6 devices. These results indicate that modifying the side chains of Y6 molecules effectively reduces ΔVnr and increases VOC in ITO-free OSCs prepared by blade-coating OXY solution. Furthermore, increasing the size of the branched side chains on the nitrogen atoms of the Y6 core is more effective in reducing ΔVnr and increasing VOC compared to changing the linear side chains to branched side chains.
To understand why acceptor side-chain modification improve EQEEL in ITO-free OSCs prepared by blade-coating OXY solution, we conducted light intensity-dependent VOC measurements on PM6:Y6DT and PM6:L8BO devices. As shown in Fig. 7e, the ideality factors of PM6:Y6DT and PM6:L8BO devices are 1.16 and 1.32, respectively, both lower than that of PM6:Y6 devices (1.55). This suggests that the side chain modification strategy effectively suppresses trap-assisted recombination of charge carriers, leading to higher EQEEL in PM6:Y6DT and PM6:L8BO devices compared to PM6:Y6 devices.
Finally, to further understand the origin of the impact of acceptor side chain modifications on trap-assisted recombination in ITO-free OSCs constructed by blade-coating OXY solution, we prepared electron-only devices based on PM6:Y6DT and PM6:L8BO and conducted J–V measurements. As shown in Fig. S11 (ESI†), by fitting the trap-filled region of the J–V curves, we determined that the densities of trap states in the active layers of PM6:Y6DT and PM6:L8BO devices are 5.4 (±1.9) × 1017 and 1.1 (±0.2) × 1018 cm−3, respectively, both lower than the density of trap states in PM6:Y6 devices (2.6 (±0.7) × 1018 cm−3). Based on these observations, we confirm that modifying the side chains of Y6 can indeed improve the morphology and structural order of the active layer in ITO-free OSCs prepared by blade-coating OXY solution, thereby enhancing exciton dissociation efficiency, reducing density of trap states, and ultimately improving JSC, VOC, and overall device performance (Fig. 7a and b). Notably, increasing the size of the branched side chains on the nitrogen atoms of the Y6 core unit is more effective in enhancing device performance than changing the linear side chains to branched side chains.
3. Conclusion
In summary, by comparing the performance differences of ITO-free OSCs based on PM6:Y6 prepared using spin-coated CF solution, spin-coated OXY solution, and blade-coated OXY solution, we demonstrated the impact of solvents and deposition techniques on the morphology and structural order of the active layer. We found that the active layer prepared by blade-coating OXY solution exhibited greater donor–acceptor phase separation and poorer molecular packing properties compared to the active layer prepared by spin-coating CF solution. This resulted in reduced exciton dissociation efficiency and poorer charge carrier transport, thereby limiting the quantum efficiency and JSC of OSCs prepared by blade-coating OXY solution. Additionally, we showed that the active layer prepared by blade-coating OXY solution has a higher density of trap states compared to the active layer prepared by spin-coating CF solution, leading to more severe trap-assisted recombination in the OSCs. This resulted in lower EQEEL and higher non-radiative voltage losses, which limited the VOC of the devices. To address the performance loss in ITO-free OSCs prepared by blade-coating OXY solution, we proposed an acceptor side chain modification strategy. This approach improved the morphology and structural order of the active layer, thereby enhancing exciton dissociation efficiency and reducing density of trap states. As a result, both JSC and VOC of the devices were significantly improved. Using Y6DT as the acceptor, the performance of ITO-free OSCs constructed by blade-coating OXY solution reached 13.5%. To the best of our knowledge, this is the highest PCE reported for blade-coated ITO-free OSCs fabricated with a non-halogenated solvent.Author contributions
C. Z., T. Z., M. W. and Z. M. conceived the project. C. Z. fabricated and characterized the OPV devices. Y. L. performed PL measurements. Z. W. performed GIWAX measurements under supervision of W. H., Z. L. synthesized PM6 donor material. C. Z., T. Z., M. W., and Z. M. wrote the manuscript. All authors contributed to the finalizing of the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Fundamental Research Funds for the Central Universities (2232022A13), and the Natural Science Foundation of Shanghai (Grant No. 22ZR1401900).References
- N. S. Sariciftci, L. Smilowitz, A. J. Heeger and F. Wudl, Science, 1992, 258, 1474–1476 CrossRef CAS PubMed.
- S. H. Park, A. Roy, S. Beaupré, S. Cho, N. Coates, J. S. Moon, D. Moses, M. Leclerc, K. Lee and A. J. Heeger, Nat. Photonics, 2009, 3, 297–302 CrossRef CAS.
- J. Hou, O. Inganäs, R. H. Friend and F. Gao, Nat. Mater., 2018, 17, 119–128 CrossRef CAS PubMed.
- Y. Xiao, C. Zuo, J. Zhong, W. Wu, L. Shen and L. Ding, Adv. Energy Mater., 2021, 11, 2100378 CrossRef CAS.
- R. Søndergaard, M. Hösel, D. Angmo, T. T. Larsen-Olsen and F. C. Krebs, Mater. Today, 2012, 15, 36–49 CrossRef.
- S. Guan, Y. Li, C. Xu, N. Yin, C. Xu, C. Wang, M. Wang, Y. Xu, Q. Chen, D. Wang, L. Zuo and H. Chen, Adv. Mater., 2024, 36, 2400342 CrossRef CAS.
- Y. Jiang, S. Sun, R. Xu, F. Liu, X. Miao, G. Ran, K. Liu, Y. Yi, W. Zhang and X. Zhu, Nat. Energy, 2024, 9, 975–986 CrossRef CAS.
- L. Zhu, M. Zhang, J. Xu, C. Li, J. Yan, G. Zhou, W. Zhong, T. Hao, J. Song, X. Xue, Z. Zhou, R. Zeng, H. Zhu, C.-C. Chen, R. C. I. MacKenzie, Y. Zou, J. Nelson, Y. Zhang, Y. Sun and F. Liu, Nat. Mater., 2022, 21, 656–663 CrossRef CAS.
- Y. Li, G. Xu, C. Cui and Y. Li, Adv. Energy Mater., 2018, 8, 1701791 CrossRef.
- X. Chen, G. Xu, G. Zeng, H. Gu, H. Chen, H. Xu, H. Yao, Y. Li, J. Hou and Y. Li, Adv. Mater., 2020, 32, 1908478 CrossRef CAS.
- X. Zheng, L. Zuo, F. Zhao, Y. Li, T. Chen, S. Shan, K. Yan, Y. Pan, B. Xu, C. Li, M. Shi, J. Hou and H. Chen, Adv. Mater., 2022, 34, 2200044 CrossRef CAS PubMed.
- L. Mao, J. Tong, S. Xiong, F. Jiang, F. Qin, W. Meng, B. Luo, Y. Liu, Z. Li, Y. Jiang, C. Fuentes-Hernandez, B. Kippelen and Y. Zhou, J. Mater. Chem. A, 2017, 5, 3186–3192 RSC.
- Q. Fan, Q. Xiao, H. Zhang, J. Heng, M. Xie, Z. Wei, X. Jia, X. Liu, Z. Kang, C. Li, S. Li, T. Zhang, Y. Zhou, J. Huang and Z. Li, Adv. Mater., 2024, 36, 2307920 CrossRef CAS.
- X. Chen, R. Huang, Y. Han, W. Zha, J. Fang, J. Lin, Q. Luo, Z. Chen and C. Ma, Adv. Energy Mater., 2022, 12, 2200044 CrossRef CAS.
- Y. Liu, J. Zhang, C. Tian, Y. Shen, T. Wang, H. Zhang, C. He, D. Qiu, Y. Shi and Z. Wei, Adv. Funct. Mater., 2023, 33, 2300778 CrossRef CAS.
- Y.-C. Huang, H.-C. Cha, S.-H. Huang, C.-F. Li, S. R. M. Santiago and C.-S. Tsao, Polymers, 2023, 15, 4005 CrossRef CAS.
- Y. Xiao, C. Zuo, J. Zhong, W. Wu, L. Shen and L. Ding, Adv. Energy Mater., 2021, 11, 2100378 CrossRef CAS.
- L. Zhang, B. Lin, B. Hu, X. Xu and W. Ma, Adv. Mater., 2018, 30, 1800343 CrossRef PubMed.
- P. Schilinsky, C. Waldauf and C. J. Brabec, Adv. Funct. Mater., 2006, 16, 1669–1672 CrossRef CAS.
- Y. Zhang, K. Liu, J. Huang, X. Xia, J. Cao, G. Zhao, P. W. K. Fong, Y. Zhu, F. Yan, Y. Yang, X. Lu and G. Li, Nat. Commun., 2021, 12, 4815 CrossRef CAS.
- Z. Liu, Y. Fu, J. Wu, X. Yi, M. Zhao, M. Huang, J. Liu and Z. Xie, Adv. Funct. Mater., 2024, 401558 Search PubMed.
- S. Dong, K. Zhang, B. Xie, J. Xiao, H. Yip, H. Yan, F. Huang and Y. Cao, Adv. Energy Mater., 2019, 9, 1802832 CrossRef.
- W. Zhao, S. Zhang, Y. Zhang, S. Li, X. Liu, C. He, Z. Zheng and J. Hou, Adv. Mater., 2018, 30, 1704837 CrossRef.
- S. Lee, D. Jeong, C. Kim, C. Lee, H. Kang, H. Y. Woo and B. J. Kim, ACS Nano, 2020, 14, 14493–14527 CrossRef CAS PubMed.
- S. Li, H. Zhang, S. Yue, X. Yu and H. Zhou, Nanotechnology, 2022, 33, 072002 CrossRef CAS.
- M. E. Farahat, P. Perumal, W. Budiawan, Y.-F. Chen, C.-H. Lee and C.-W. Chu, J. Mater. Chem. A, 2017, 5, 571–582 RSC.
- B. Liu, H. Sun, J.-W. Lee, J. Yang, J. Wang, Y. Li, B. Li, M. Xu, Q. Liao, W. Zhang, D. Han, L. Niu, H. Meng, B. J. Kim and X. Guo, Energy Environ. Sci., 2021, 14, 4499–4507 RSC.
- X. Xu, L. Yu, H. Yan, R. Li and Q. Peng, Energy Environ. Sci., 2020, 13, 4381–4388 RSC.
- W. Sun, H. Chen, B. Zhang, Q. Cheng, H. Yang, Z. Chen, G. Zeng, J. Ding, W. Chen and Y. Li, Chin. J. Chem., 2022, 40, 2963–2972 CrossRef CAS.
- B. Zhang, W. Chen, H. Chen, G. Zeng, R. Zhang, H. Li, Y. Wang, X. Gu, W. Sun, H. Gu, F. Gao, Y. Li and Y. Li, Energy Environ. Sci., 2024, 17, 2935 RSC.
- Z. Jia, J. Pan, X. Chen, Y. Li, T. Liu, H. Zhu, J. Yao, B. Yan and Y. (Michael) Yang, Energy Environ. Sci., 2024, 17, 3908–3916 RSC.
- D. Wang, G. Zhou, Y. Li, K. Yan, L. Zhan, H. Zhu, X. Lu, H. Chen and C. Li, Adv. Funct. Mater., 2022, 32, 2107827 CrossRef CAS.
- S. Alam, H. Aldosari, C. E. Petoukhoff, T. Váry, W. Althobaiti, M. Alqurashi, H. Tang, J. I. Khan, V. Nádaždy, P. Müller-Buschbaum, G. C. Welch and F. Laquai, Adv. Funct. Mater., 2024, 34, 2308076 CrossRef CAS.
- M. Liu, X. Ge, X. Jiang, D. Chen, F. Guo, S. Gao, Q. Peng, L. Zhao and Y. Zhang, Nano Energy, 2023, 112, 108501 CrossRef CAS.
- J. Zhao, S. Chung, H. Li, Z. Zhao, C. Zhu, J. Yin, K. Cho and Z. Kan, Adv. Funct. Mater., 2023, 33, 2307355 CrossRef CAS.
- Z. Tang, L. M. Andersson, Z. George, K. Vandewal, K. Tvingstedt, P. Heriksson, R. Kroon, M. R. Andersson and O. Inganäs, Adv. Mater., 2012, 24, 554–558 CrossRef CAS.
- J. Wang, C. Zhang, Y. Lin, H. Wu, X. Feng and Z. Ma, ACS Appl. Energy Mater., 2023, 6, 10163–10171 CrossRef CAS.
- J. Yuan, Y. Zhang, L. Zhou, G. Zhang, H.-L. Yip, T.-K. Lau, X. Lu, C. Zhu, H. Peng, P. A. Johnson, M. Leclerc, Y. Cao, J. Ulanski, Y. Li and Y. Zou, Joule, 2019, 3, 1140–1151 CrossRef CAS.
- W. Ma, J. R. Tumbleston, L. Ye, C. Wang, J. Hou and H. Ade, Adv. Mater., 2014, 26, 4234–4241 CrossRef CAS PubMed.
- J. A. Bartelt, Z. M. Beiley, E. T. Hoke, W. R. Mateker, J. D. Douglas, B. A. Collins, J. R. Tumbleston, K. R. Graham, A. Amassian, H. Ade, J. M. J. Fréchet, M. F. Toney and M. D. McGehee, Adv. Energy Mater., 2013, 3, 364–374 CrossRef CAS.
- T. Wang, G. Kupgan and J.-L. Brédas, Trends Chem., 2020, 2, 535–554 CrossRef CAS.
- K. Vandewal, K. Tvingstedt, A. Gadisa, O. Inganäs and J. V. Manca, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 125204 CrossRef.
- J. Wang, X. Jiang, H. Wu, G. Feng, H. Wu, J. Li, Y. Yi, X. Feng, Z. Ma, W. Li, K. Vandewal and Z. Tang, Nat. Commun., 2021, 12, 6679 CrossRef CAS PubMed.
- T. Kirchartz, F. Deledalle, P. S. Tuladhar, J. R. Durrant and J. Nelson, J. Phys. Chem. Lett., 2013, 4, 2371–2376 CrossRef CAS.
- Y. Cui, H. Yao, L. Hong, T. Zhang, Y. Tang, B. Lin, K. Xian, B. Gao, C. An, P. Bi, W. Ma and J. Hou, Natl. Sci. Rev., 2020, 7, 1239–1246 CrossRef CAS PubMed.
- L. J. A. Koster, V. D. Mihailetchi, R. Ramaker and P. W. M. Blom, Appl. Phys. Lett., 2005, 86, 123509 CrossRef.
- S. C. Jain, Ashok K. Kapoor, W. Geens, J. Poortmans, R. Mertens and M. Willander, J. Appl. Phys., 2002, 92, 3752–3754 CrossRef CAS.
- X. He, C. C. S. Chan, J. Kim, H. Liu, C. Su, U. Jeng, H. Su, X. Lu, K. S. Wong and W. C. H. Choy, Small Methods, 2022, 6, 2101475 CrossRef CAS.
- T. Zhang, H. Han, Y. Zou, Y.-C. Lee, H. Oshima, K.-T. Wong and R. J. Holmes, ACS Appl. Mater. Interfaces, 2017, 9, 25418–25425 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tc02821j |
| This journal is © The Royal Society of Chemistry 2025 |