Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A recyclable, reshapable and UV-curable polybenzoxazine vitrimer enabling closed-loop 3D printing applications†
Charles
Jehl
ab,
Antoine
Adjaoud
 a,
Ambre
Meyer
a,
Vincent
Boulic
ab,
Channya
Hesse
ab,
Laura
Puchot
a,
Ambre
Meyer
a,
Vincent
Boulic
ab,
Channya
Hesse
ab,
Laura
Puchot
 a,
Joamin
Gonzalez-Gutierrez
a,
Joamin
Gonzalez-Gutierrez
 a,
Alexander S.
Shaplov
a,
Alexander S.
Shaplov
 a,
Daniel F.
Schmidt
a,
Daniel F.
Schmidt
 a and
Pierre
Verge
a and
Pierre
Verge
 *a
*a
aLuxembourg Institute of Science and Technology (LIST), 5 avenue des Hauts-Fourneaux, Esch-sur-Alzette, L-4362, Luxembourg. E-mail: pierre.verge@list.lu
bDepartment of Physics and Materials Science, University of Luxembourg, 2 Avenue de l’Université, Esch-sur-Alzette, L-4365, Luxembourg
First published on 15th July 2025
Abstract
The additive manufacturing of a bio-based, UV active, recyclable and reshapable polybenzoxazine is demonstrated. A new ditelechelic benzoxazine monomer (CHDM-PA-mea) was prepared by esterification of phloretic acid (PA) and cyclohexane dimethanol (CHDM)-both chosen for sustainability-and then reacting the product with monoethanolamine (mea) and paraformaldehyde (PFA). This yielded a molecule with two benzoxazine rings, two ester groups and two aliphatic hydroxyls. UV-curability was obtained through partial acylation of the aliphatic hydroxyls to form methacrylates. The modified benzoxazine monomer was then 3D printed via UV-assisted materials extrusion, with thermal post-treatment yielding complex parts composed of a fully cured polybenzoxazine vitrimer. The influence of the degree of methacrylation on vitrimer characteristics was explored with the aim of balancing photopolymerisation and printability with vitrimeric properties, as assessed by reshaping and recycling abilities. This work identifies an optimal benzoxazine precursor enabling the production of complex 3D printed vitrimer parts that can be reshaped and recycled, with a Tα of 105 °C, a compressive modulus of 810 MPa and excellent dimensional stability. Ground 3D printed parts were used as viscosity modifiers for new prints, closing the successful recycling loop for this material.
1 Introduction
Approximately 12% of the global plastic production consists of thermosets.1 These materials possess high mechanical properties, good thermal stability, and chemical resistance due to their three-dimensional cross-linked structure. As a result, they are widely used in value-added applications, such as aviation and automotive.2 However, their permanent chemical structure prevents recycling and reshaping. As a result, they are usually landfilled or incinerated once out of service, leading to adverse environmental consequences such as air and soil pollution or the destruction of ecosystems.3 To address this issue, researchers have developed covalent adaptable networks (CANs), which feature dynamic bonds that can reshuffle under the influence of an external stimulus (temperature, pH, or UV radiation changes), allowing for the structural rearrangement of their network.4 Vitrimers, a type of associative CAN, have garnered significant interest given their recyclability and reshapability in the absence of depolymerisation.Among the various families of thermosets modified with dynamic bonds,5–8 polybenzoxazines provide an efficient combination of high performance and circularity.9,10 They possess characteristics similar to those of traditional phenolic or epoxy resins such as near-zero shrinkage,11,12 high glass transition temperatures (Tg> 100 °C),13,14 high thermal stability (Tonset> 200 °C),15,16 low water absorption,17 and high mechanical properties.18–20 The design flexibility and simplicity of the synthesis of benzoxazine monomers allow the use of natural or bio-based precursors21–27 and the incorporation of dynamic bonds within their structure. Furthermore, the dynamic exchanges can be internally catalysed9 and accelerated by an efficient mechanism of neighbouring group participation.28 Finally, benzoxazine vitrimers have been produced based on a wide range of bond exchange reactions: transesterification reactions (TERs) of esters,29–31 disulfide metathesis,32–34 boronic ester exchanges,35 transamination of vinylogous urethanes,36 imines,37 siloxane (silyl ethers)38 and acetal exchanges.39
Additive manufacturing has emerged as an attractive area of research due to its promise of design flexibility and waste minimization. Unlike subtractive manufacturing, which entails cutting a material from a block to create an object, additive manufacturing is a computer-controlled process that creates three-dimensional (3D) parts with complex geometries by adding the material layer by layer or section by section40,41 and finds applications in aerospace, automotive, medicine, electronics, sports equipment, construction and water purification.42–47 Among the various additive manufacturing techniques, material extrusion additive manufacturing (MEX) has gained significant interest from diverse industries due to its ease of use, cost-effectiveness and low energy consumption since it is possible to perform it at room temperature (RT).48,49 It also requires only small amounts of resin, making it a clear advantage for demonstrating proof of concept with materials prepared at the lab scale. Finally, MEX 3D printing, which involves depositing a flowable material layer by layer onto a build plate using an extrusion head that can move in three dimensions, is compatible with a broad range of materials, including viscous monomers. In this context, material extrusion proves to be well-suited for very viscous benzoxazine precursors, as opposed to vat photopolymerisation that requires low viscosity precursors. Nevertheless, MEX is highly sensitive to process parameters and monomer properties, which can result in various structural defects in the printed parts (bubbles, geometric deviations, bead width inconsistencies, bumps, voids, shrinkage and poor interlayer adhesion). Consequently, multiple tests are typically required to optimize the design of a part, leading to significant material waste. Thus, the processing of reusable, recyclable vitrimers via MEX not only addresses end-of-life concerns but also provides a convenient means of recycling waste generated during process optimization. This synergy represents significant progress with respect to waste reduction and supports the transition to more sustainable production of polymeric products.50,51
MEX 3D printing and additive manufacturing in general have not been widely reported in the context of benzoxazines and resultant thermoset materials.52–54 Similar to the 3D printing of other thermoset resins, the curing process of benzoxazines after printing typically leads to the collapse of the printed structures/parts due to a significant decrease in viscoelastic properties caused by the high temperatures needed for curing.55 To address this issue, one solution is to develop a benzoxazine-based precursor that can undergo room temperature radical polymerisation during the 3D printing process, allowing the shape of the part to be fixed prior to thermally initiated benzoxazine ring-opening polymerisation (ROP). In this context, a convenient approach is the attachment of (meth)acrylate groups to the benzoxazine-based precursor followed by room-temperature photopolymerisation. Ishida et al. were the first to report the methacrylation of the benzoxazine precursor by reacting (3-phenyl-3,4-dihydro-2H-benzo[e][1,3]oxazin-6-yl)methanol and methacryloyl chloride (mac).56 Next, Wang et al. designed a series of photosensitive benzoxazine oligomers using 2,2′-bis(trifluoromethyl)-4,4′-diaminobiphenyl and 2-isocyanatoethyl methacrylate for the printing of 3D objects by stereolithography.53 Similarly, Weigand et al. developed a UV active benzoxazine compound based on bisphenol-A, 2-(2-aminoethoxy) ethanol and mac for stereolithography.54
Inspired by the above-mentioned approach, this work began with the preparation of a new ditelechelic benzoxazine monomer (CHDM-PA-mea) by reacting phloretic acid (PA), which is obtained from the leaves of apple trees,57 with cyclohexane dimethanol (CHDM), a diol that can be derived from limonene or PET waste.58,59 This was followed by a reaction with mono-ethanolamine (mea) and paraformaldehyde (PFA), resulting in a molecule composed of two ester groups and containing two aliphatic hydroxyl groups, that can further react by tertiary-amine catalysed TERs following benzoxazine ROP. Sensitivity to photopolymerisation was introduced by partially converting the aliphatic –OH groups into methacrylate groups. It is noteworthy that the methacrylation results in a decrease of the number of –OH groups that are essential for bond exchange in this system.
In this context, the purpose of this study was to develop a dual curing benzoxazine precursor for 3D printing as shown in Fig. 1. In particular, the influence of methacrylation on the characteristics of the vitrimer is explored and the optimal –OH to methacrylate ratio is determined with the goal of balancing photopolymerisability and printability with vitrimeric properties. While the 3D-printed vitrimer parts exhibited excellent reshapability and recyclability, their reuse was successfully demonstrated by grinding them into powder and repeatedly utilizing it as a viscosity modifier for new prints. The finds of these efforts highlight the potential for producing long-lasting thermoset specimens combining additive manufacturing and closed-loop recycling.
 | ||
| Fig. 1 Strategy suggested for MEX 3D printing of a benzoxazine vitrimer using TERs for dynamic exchanges. | ||
2 Experimental section
2.1 Materials
3-(4-Hydroxyphenyl) propionic acid (TCI Europe, > 98.0%, phloretic acid, PA) and 2-aminoethanol (TCI Europe, > 99.0%, mono-ethanolamine, mea), 1,4-cyclohexanedimethanol (Aldrich, a mixture of cis and trans, 99%, CHDM), para-toluene sulfonic acid monohydrate (Aldrich, ≥ 98.5%, p-TSA), paraformaldehyde (Aldrich, 95%, PFA), 2,2-dimethoxy-2-phenylacetophenone (Aldrich, 99%, DMPA), fumed silica (Aldrich, amorphous silica powder with an average particle size of 0.2 μm, SiO2), triethylamine (Carl Roth, ≥ 99.5%, NEt3), sodium chloride (Carl Roth, ≥99.5%, NaCl), sodium hydroxide (Fisher Scientific, 98%, NaOH) and chloroform (Fisher Scientific, ≥ 99%, CHCl3) were used without purification. Anhydrous dichloromethane (Fisher Scientific, > 99%, DCM) and toluene (Fisher Scientific, > 99%, PhCH3) were obtained from an MBraun SPS-800 solvent purification system. Ultrapure deionized water was obtained using a Sartorius Arium® Comfort Smart Station. Methacryloyl chloride (Aldrich, ≥ 97.0%, mac) was purified by distillation. The distillation was done at atmospheric pressure under a flow of nitrogen while the crude reagent was heated to 110 °C.2.2 Synthetic procedures
2.3 Characterisation methods
 | (1) |
Other characterisation techniques are reported in the ESI.†
2.4 Recycling
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 40 s. To prepare materials for reuse in 3D printing, specimens were first cooled with liquid nitrogen and then ground into a fine powder with a Retsch ZM 200 centrifugal mill using an 80 μm sieve at 18
000 rpm for 40 s. To prepare materials for reuse in 3D printing, specimens were first cooled with liquid nitrogen and then ground into a fine powder with a Retsch ZM 200 centrifugal mill using an 80 μm sieve at 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 s.
000 rpm for 20 s.
Reprocessing was also done via MEX; here, ground powder was added to fresh resin and the MEX process was repeated, as described in 2.2.5.
3 Results and discussion
3.1 Structural characterisation of monomers
The synthesis of benzoxazine monomers with methacrylic functional groups is illustrated in Fig. 2a. The structure and purity of all products were estimated via NMR and FTIR analyses (Fig. S6–S8, S10–S12 and S15–S20†). During Fischer esterification, the clear disappearance of the peaks associated with the aliphatic hydroxyl groups of CHDM (δ = 3.15–3.30 ppm, CH2*–OH [2trans/cis], Fig. S1†) in the 1H spectrum of CHDM-PA (Fig. S6†) highlights the almost quantitative conversion of the diol to the desired diester despite the existence of two CHDM isomers. This process was accompanied by the appearance of a strong band at 1702 cm−1 attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration of the newly formed esters, several bands at 1480–1510 cm−1 assigned to the C
O vibration of the newly formed esters, several bands at 1480–1510 cm−1 assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations of the aromatic rings, a band at 825 cm−1 designated to out-of-plane C–H vibration in the para-substituted aromatic ring, and a shift of the broad band at ∼3300 to ∼3400 cm−1 associated with the substitution of aliphatic O–H stretches to the aromatic ones (Fig. S8†).
C stretching vibrations of the aromatic rings, a band at 825 cm−1 designated to out-of-plane C–H vibration in the para-substituted aromatic ring, and a shift of the broad band at ∼3300 to ∼3400 cm−1 associated with the substitution of aliphatic O–H stretches to the aromatic ones (Fig. S8†).
 | ||
| Fig. 2 (a) Synthetic route for photopolymerisable benzoxazine precursors; (b) 1H NMR spectrum of the main structure of CHDM-PA-mea/mac2 precursor and related substructures; (c) FTIR spectra of CHDM-PA-mea and CHDM-PA-mea/mac precursors highlighting characteristic peaks (simplified spectra; see full spectra in Fig. S20†); (d) focus on regions of 1H NMR spectra of CHDM-PA-mea and CHDM-PA-mea/mac precursors (simplified spectra; see full spectra in Fig. S18†). | ||
After the Mannich-like condensation reaction, the 1H NMR spectrum of the CHDM-PA-mea product (Fig. S10†) reveals the characteristic peaks of the benzoxazine rings, at δ = 3.91 and δ = 4.77 ppm, corresponding to N–CH2*–Ar [5] and N–CH2*–O [4], respectively. The peaks at δ = 6.65 ppm and δ = 6.99 ppm are attributed to the phenolic protons of CHDM-PA, indicating the presence of some residual reactant. The experimental integration of the methylene protons of the N–CH2*–O signal counted for 2.88H (4.00H theoretical), indicating that 72% of the oxazine rings were closed. As reported in other work, the remaining 28% consists of the starting ester (10%) and an oxazolidine substructure (18%) (Fig. S9, [a–f] in S10†).28
In the IR spectrum of the CHDM-PA-mea product in comparison with the CHDM-PA precursor, the decrease in the intensity of the O–H stretching vibrations at 3446 cm−1, the shift of the characteristic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band from 1702 to 1728 cm−1, the increase in the aliphatic C–H stretching at 2720–3060 cm−1 and the appearance of several bands at 1228 and 968–696 cm−1 attributed to the C–O–C vibration of the oxazine ring and C–H stretching of the tri-substituted aromatic rings were observed (Fig. S12†).
O band from 1702 to 1728 cm−1, the increase in the aliphatic C–H stretching at 2720–3060 cm−1 and the appearance of several bands at 1228 and 968–696 cm−1 attributed to the C–O–C vibration of the oxazine ring and C–H stretching of the tri-substituted aromatic rings were observed (Fig. S12†).
Regarding the third step of the synthesis, it was concluded from 1H NMR spectra of the monomers (Fig. S15 and S18†) that the experimental conditions used for the methacrylation favoured the formation of oxazolidine substructures. Indeed, the experimental integrations of N–CH2*–O [4, 4′] decrease from 2.88 to 2.10 between the starting benzoxazine and the benzoxazine produced using large amounts of mac and NEt3 (Fig. S18†). Furthermore, the presence of several peaks in the ranges of δ = 5.60–6.30 ppm and δ = 1.85–2.00 ppm, corresponding to CH2* = C(CH3)–CO [16, y, y′] and CH2 = C(CH3*)–CO [17, z, z′] (Fig. 2b and S15†), respectively, tends to indicate that methacrylate groups were grafted on different sites. The signals corresponding to the oxazolidine structure and the starting ester are all shifted between the 1H NMR spectrum of CHDM-PA-mea ([a–f], Fig. S10†) and the 1H NMR spectrum of CHDM-PA-mea/mac2 ([a–f], Fig. 2b and S15†), indicating a change in their chemical environment. This implies that mac reacted both with the aliphatic hydroxyl groups from mea counterparts and the phenolic hydroxyl groups coming either from oxazolidines or from unreacted CHDM-PA. Furthermore, the 1H NMR and FTIR analyses of CHDM-PA-mea/mac monomers demonstrated the impact of mac concentration on the chemical modification and acylation degree (AD of 31, 60 and 94% for CHDM-PA-mea/mac1, CHDM-PA-mea/mac2 and CHDM-PA-mea/mac3, respectively, Table S2†). Specifically, the bands at 3446 and 1030 cm−1 attributed to the –OH vibrations progressively disappeared, while the intensity of the bands at 1735 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and 1635 (aliphatic C
O) and 1635 (aliphatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) cm−1 associated with the formation of methacrylate groups increased with the increase of the amount of mac (Fig. 2c). The same trend can be observed in the NMR spectra as well (Fig. 2d).
C) cm−1 associated with the formation of methacrylate groups increased with the increase of the amount of mac (Fig. 2c). The same trend can be observed in the NMR spectra as well (Fig. 2d).
3.2 Thermal behaviour of precursors
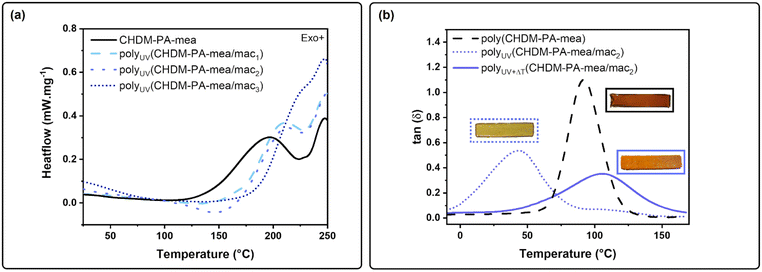
The differential scanning calorimetry (DSC) curves of all monomers are presented in Fig. 3a. The first exothermic peak (Texo,1 = 105–223 °C) is observed for CHDM-PA-mea corresponding to the ring opening of the benzoxazine rings. This peak is in the same range as the one observed in benzoxazine vitrimers with a similar structure.29–31 The second exothermic peak centred around 250 °C (Texo,2) is attributed to thermal degradation, as confirmed by thermogravimetric analysis (TGA, Fig. S21†). The presence of exothermic peaks for CHDM-PA-mea/mac1 and CHDM-PA-mea/mac2 in the range of 125–228 °C shows that the ring opening of benzoxazine still occurs, even in the presence of methacrylate groups. However, their Tonset values are shifted to higher temperatures compared to CHDM-PA-mea. This may be explained by the fact that these monomers contain fewer aliphatic hydroxyl groups, which are known to catalyse the ring opening of benzoxazine.60–62 The exothermic peak attributed to the ring opening reaction of CHDM-PA-mea/mac3 is slightly shifted towards higher temperatures, but a new, lower temperature exotherm (Texo,0 = 102–164 °C) is also observed. This is attributed to the thermally induced polymerisation of the highly reactive methacrylate functions prior to benzoxazine ring opening.The precursors were also subjected to rheokinetic measurements, with results shown in Fig. 3b. The data present the evolution of the complex viscosity of each monomer with increasing temperature. The curves show that the CHDM-PA-mea/mac systems display similar curing behaviour to the CHDM-PA-mea precursors upon heating. Gelation occurs more slowly as the extent of methacrylation increases, underlining the impact of –OH functions on the ROP of benzoxazines. However, the rapid increase in the complex viscosity of CHDM-PA-mea/mac3 at around 828 s is likely due to the polymerisation of the methacrylate functions, which form a preliminary acrylate network, confirming the conclusion drawn from DSC measurements.
3.3 Photopolymerisation of functionalized monomers
The UV curability of the precursors was investigated following the addition of DMPA as a radical initiator (0.5 phr) by performing rheokinetic measurements at room temperature under UV exposure. The graph shows the evolution of the complex viscosity of each resin as a function of time when exposed to UV irradiation (Fig. 4a).A significantly lower initial viscosity is observed in the functionalized resins compared to CHDM-PA-mea. This reduction can be attributed to the introduction of methacrylate functions, which decrease the number of hydroxyl groups and, consequently, the number of intermolecular hydrogen bonding interactions. Then, the curves show that the complex viscosity of each mac-functionalized monomer increases once UV irradiation starts. The curves showing the evolution of storage and loss moduli were used to determine the gelation time (Fig. S22†). As expected, it decreases with the amount of acrylate functionalities, from 84 to 16 to 4 s for CHDM-PA-mea/mac1 to CHDM-PA-mea/mac3.
PolyUV(CHDM-PA-mea/mac), referring to specimens that have only been UV cured, were subjected to dynamic mechanical thermal analyses to assess their α-transition temperature (Tα) as illustrated in Fig. 4b. Tα increases with the amount of mac, ranging from 19 °C for polyUV(CHDM-PA-mea/mac1) to 70 °C for polyUV(CHDM-PA-mea/mac3), showing that with more methacrylate functions, a higher cross-linking density is reached for polyUV(CHDM-PA-mea/mac) systems. Thus, a better shape retention can be expected when they are exposed to heat. The curves of the storage modulus confirm these observations, showing an increase in the rubbery plateau with increasing mac content (Fig. S23†).
The evolution of cross-linking density was also checked by swelling experiments of polyUV(CHDM-PA-mea/mac) in water and toluene (Fig. S24†). In water, swelling ratios are 7.1%, 3.1% and 1.8% for polyUV(CHDM-PA-mea/mac1), polyUV(CHDM-PA-mea/mac2) and polyUV(CHDM-PA-mea/mac3) respectively. In toluene, polyUV(CHDM-PA-mea/mac3) remained cohesive after more than 800 h of immersion, while polyUV(CHDM-PA-mea/mac2) broke into small pieces after 170 h and polyUV(CHDM-PA-mea/mac1) after only 3 h.
Insets of Fig. 4b capture the ability of 2 mm thick bar of each polyUV(CHDM-PA-mea/mac) to resist bending when a 50 g weight is applied to its extremity, illustrating an increase in the modulus with the mac content.
3.4 Characterisation of dual-cured materials
DSC measurements were performed on polyUV(CHDM-PA-mea/mac). As expected, the first exothermic peak observed in the thermogram of CHDM-PA-mea/mac3 and associated with methacrylate polymerisation is not visible anymore (Fig. 5a). Interestingly, the exothermic peak (Texo,1) centred around 215 °C indicates that the opening of the benzoxazine rings still occurs after the reaction of the methacrylate functions.Dynamic TGA analyses of polyUV(CHDM-PA-mea/mac) materials have been carried out and show that no degradation occurs until 230 °C (Table 1, column 3, Fig. S25†). In addition, static TGA analyses of these specimens reveal a mass loss of 4.2%, 2.5% and 3.9% for polyUV(CHDM-PA-mea/mac1), polyUV(CHDM-PA-mea/mac2) and polyUV(CHDM-PA-mea/mac3), respectively, after 3 h at 170 °C (Fig. S26†). As the observed values are all significantly lower than what would be expected in the case of loss of the methacrylate groups (∼6 wt%, ∼12 wt% and ∼17 wt%), this can be attributed to the departure of volatiles such as moisture rather than thermal degradation, providing evidence for stability upon thermal curing.
| Network | UV cured | UV + thermal cured | ||||||
|---|---|---|---|---|---|---|---|---|
| T α [°C] | T 5% [°C] | W toluene [%] | W water [%] | T α [°C] | T 5% [°C] | W toluene [%] | W water [%] | |
| a Determined from the maximum of the tan δ curve in rheology temperature sweep measurement in torsion mode. b Temperature of 5% of weight loss determined by TGA. c Determined just before breaking after 3 hours of immersion in toluene. d Determined just before breaking after 170 hours of immersion in toluene. e Determined after 200 hours of immersion in solvent. | ||||||||
| CHDM-PA-mea | — | — | — | — | 92 | 236 | 0.1 ± 0.0 | 1.7 ± 0.1 |
| CHDM-PA-mea/mac1 | 19 | 231 | 8.3 ± 1.1c | 7.1 ± 0.1 | 85 | 275 | 0.4 ± 0.4 | 2.7 ± 0.5 |
| CHDM-PA-mea/mac2 | 43 | 266 | 9.8 ± 0.2d | 3.1 ± 0.1 | 105 | 275 | 0.4 ± 0.4 | 2.6 ± 0.0 |
| CHDM-PA-mea/mac3 | 70 | 253 | 1.3 ± 0.2e | 1.8 ± 0.3 | 124 | 278 | 0.5 ± 0.3 | 2.3 ± 0.4 |
Dual-cured polyUV+ΔT(CHDM-PA-mea/mac) systems were then obtained after a thermal treatment of single cured polyUV(CHDM-PA-mea/mac) samples at 170 °C for 1 h. DSC data, reported in Fig. S27,† show that the treatment is enough to fully open the benzoxazine rings.
TGA measurements were also performed to assess the thermal stability of each dual-cured polymer (Fig. S28†). The results indicate that the stability increases with the introduction of mac, with a 5% weight loss reached at 236 °C for poly(CHDM-PA-mea) and around 270 °C for polyUV+ΔT(CHDM-PA-mea/mac) materials (Table 1, column 7). Presumably, the substitution of dangling hydroxyl groups by polymerised methacrylate functions enhances the thermal stability of the resulting network.
Dynamic mechanical thermal analyses were also performed on dual-cured systems. The additional thermal treatment leads to a significant increase in Tα, for instance, from 43 to 105 °C in the case of polyUV+ΔT(CHDM-PA-mea/mac2) as depicted in Fig. 5b, as a consequence of higher cross-linking density resulting from the ROP of benzoxazines (Fig. S29†). The same trend is observed for the other compositions (Table 1, columns 2 and 6). Moreover, the completion of the thermal curing results in an increase of the rubbery plateau of polyUV+ΔT(CHDM-PA-mea/mac) materials compared with their single UV cured counterparts (Fig. S30†). Interestingly, the rubbery plateau modulus of dual-cured samples also increases with the extent of methacrylation, which suggests that the methacrylates are not degraded during thermal curing.
The evolution of the cross-linking density, monitored by swelling experiments of polyUV+ΔT(CHDM-PA-mea/mac) in water and toluene (Fig. S31†), is also explained by this behaviour. Unlike single cured materials, dually cured samples no longer swell in toluene as a result of the ROP of benzoxazine (Table 1, columns 4 and 8). In water, thermally cured samples all have a similar swelling ratio, independent of mac content and slightly higher than that of the poly(CHDM-PA-mea) reference. This may be explained by a balance between increased crosslink density and the polarity variations arising from the changes in the content of polymerised methacrylate groups and phenolic rings. Interestingly, the crosslink density increases without a corresponding increase in Tg, which can be explained by that the methacrylate crosslinks are more flexible than benzoxazine crosslinks, indicating partial retention of the methacrylates.
3.5 Vitrimeric behaviour
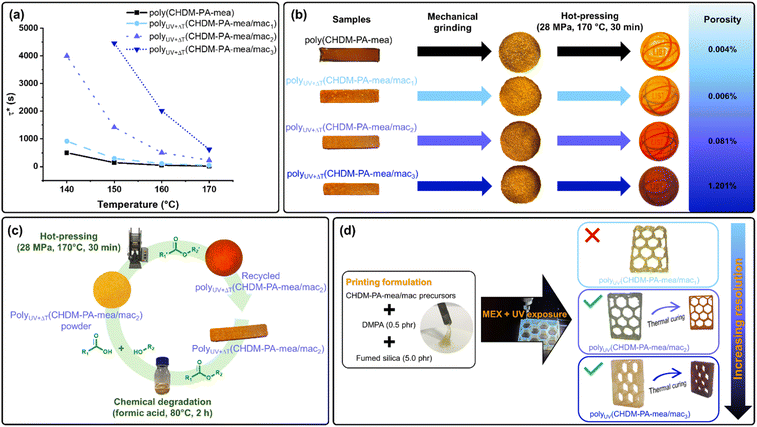
TERs govern the vitrimeric behaviour of poly(CHDM-PA-mea) and polyUV+ΔT(CHDM-PA-mea/mac). The TER is a mechanism that requires both ester bonds and –OH groups, usually in the presence of a catalyst that promotes faster dynamic exchange. In the case of benzoxazine-based vitrimers relying on the TER, tertiary amines generated from benzoxazine ROP play the catalytic role,28,63 but the excess of available aliphatic –OH over ester bonds groups also plays a major role.30,64As a result, it can be foreseen that methacrylation, which substitutes aliphatic –OH groups, will impact the efficiency of the dynamic process and impede the vitrimeric behaviour. The effect of methacrylation was assessed by stress relaxation measurements at different temperatures (Fig. S32†). The relaxation time (τ*), which corresponds to the time needed for a material to reach 1/e (0.37) of its original modulus,4 is a good representation of the ability of the material to behave as a vitrimer. It was determined for each polymer and plotted in Fig. 6a as a function of the temperature. Clearly, the relaxation time becomes significantly longer with increasing mac content as a result of the decreased number of –OH groups involved in TERs. The activation energies (Ea) of polyUV+ΔT(CHDM-PA-mea/mac) systems were determined and are all in the same range (Fig. S33†).
Both chemical and mechanical recycling were performed on dual-cured materials as illustrated in Fig. 6b and c, respectively. Micro-computed tomography (μCT) analyses performed on mechanically recycled discs revealed that the internal porosity increases with the rate of mac functionalization, from 0.006% and 0.081% to 1.201% for polyUV+ΔT(CHDM-PA-mea/mac1), polyUV+ΔT(CHDM-PA-mea/mac2) and polyUV+ΔT(CHDM-PA-mea/mac3), respectively, illustrating in all cases a good reconsolidation of the material even if for polyUV+ΔT(CHDM-PA-mea/mac3) the level of porosity could be of concern (Fig. S34†). PolyUV+ΔT(CHDM-PA-mea/mac2) was reprocessed in a similar way after targeted chemical degradation in formic acid. This first step is presumed to involve acid hydrolysis of the esters, resulting in the formation of the corresponding carboxylic acids and alcohols (Fig. S35†). In this scheme, the hot-pressing of the degraded material facilitates reformation of the esters. μCT analyses showed evidence of effective reconsolidation of the recovered material (<2% internal porosity). Dynamic mechanical thermal experiments and swelling tests were conducted to assess the effect of recycling on the properties of the reprocessed materials with various mac contents. Recycling processes—both mechanical and chemical—were found to increase the Tα of the materials, indicating altered mobility of the polymer segments (Table 2, column 3, Table S3 and Fig. S36 and S37†). Notably, this shift occurred without significant changes in the rubbery modulus or swelling behaviour (Table 2, columns 5–7), suggesting that the crosslinking density remains largely unaffected. In the case of polyUV+ΔT(CHDM-PA-mea/mac2), Tα increased from 105 °C to 116 °C after mechanical recycling and up to 126 °C after chemical recycling, likely due to degradation of the polymethacrylate segments. These findings demonstrate that recycled materials retain comparable network integrity while exhibiting changes in chain mobility, highlighting the effectiveness of the recycling approaches.
| Network | T α [°C] | T g [°C] | G′e [GPa] | W water [%] | W toluene [%] | |
|---|---|---|---|---|---|---|
a Mechanical recycling.
b Chemical recycling.
c Determined from the maximum of the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ curve in rheology temperature sweep measurement in torsion mode.
d Determined from the maximum of the loss modulus curve in rheology temperature sweep measurement in torsion mode.
e Determined within the rubbery plateau region (Tα + 40 °C) in rheology temperature sweep measurement in torsion mode.
f Determined after 200 hours of immersion in solvent.
g Determined after 150 hours of immersion in solvent. δ curve in rheology temperature sweep measurement in torsion mode.
d Determined from the maximum of the loss modulus curve in rheology temperature sweep measurement in torsion mode.
e Determined within the rubbery plateau region (Tα + 40 °C) in rheology temperature sweep measurement in torsion mode.
f Determined after 200 hours of immersion in solvent.
g Determined after 150 hours of immersion in solvent.
|
||||||
| polyUV+ΔT(CHDM-PA-mea/mac1) | Pristine | 85 | 59 | 0.02 | 2.7 ± 0.5f | 0.4 ± 0.4f |
| MRa | 103 | 75 | 0.02 | 1.1 ± 0.1g | 0.1 ± 0.0g | |
| polyUV+ΔT(CHDM-PA-mea/mac2) | Pristine | 105 | 64 | 0.03 | 2.6 ± 0.0f | 0.4 ± 0.4f |
| MR | 116 | 76 | 0.04 | 1.0 ± 0.1g | 0.0 ± 0.5g | |
| CRb | 126 | 84 | 0.02 | 4.9 ± 0.2g | 0.0 ± 0.0g | |
| polyUV+ΔT(CHDM-PA-mea/mac3) | Pristine | 124 | 83 | 0.08 | 2.3 ± 0.4f | 0.5 ± 0.3f |
| MR | 137 | 88 | 0.09 | 1.3 ± 0.3g | 0.0 ± 0.1g | |
In summary, dual cured materials are recyclable by hot pressing. However, the rate of methacrylation influences the vitrimeric behaviour of the final material. This is illustrated by the short relaxation times and good recyclability of polyUV+ΔT(CHDM-PA-mea/mac1) and polyUV+ΔT(CHDM-PA-mea/mac2). PolyUV+ΔT(CHDM-PA-mea/mac3) has more limited reshaping and recycling properties due to the lower amount of aliphatic –OH groups able to react through dynamic TERs and the higher crosslink density generated by permanent polymethacrylate parts.
3.6 3D printing of benzoxazine monomers
CHDM-PA-mea/mac monomers were 3D printed by MEX to achieve honeycomb-like structures as shown in Fig. 6d. For this aim, the fluid material is applied to a flat surface using an extrusion head that can move in a Cartesian plane to build the structure layer by layer, while being simultaneously exposed to UV irradiation. This triggers the photopolymerisation of the methacrylate groups, providing mechanical support to the printed material to withstand thermal exposure during the benzoxazine crosslinking step. Each precursor was printed using this method to form a hexagonal honeycomb. Due to the low viscosity of the precursor, 5 phr of fumed silica was added as a viscosity modifier to the printing formulations containing the resin and the radical initiator (DMPA, 0.5 phr). The impact of silica on resin viscosity was assessed by viscosity measurements (Fig. S38†). The printing parameters used to build honeycombs with these resins are summarised in Table S4.†According to the UV isothermal rheokinetic measurements, shorter gelation times are achieved with higher mac content. In practice, it is illustrated by the better definition and resolution of the honeycomb structures, both before and after thermal curing. For CHDM-PA-mea/mac1, the methacrylate content is too low to achieve a well-defined structure. Moreover, in this configuration only, the printing formulation tends to stick to the nozzle, leading to heterogeneities during the deposition process and defects in the printed part. A minimum amount of UV-active functionality is therefore required to ensure fast gelation and acceptable dimensional stability. CHDM-PA-mea/mac2 and CHDM-PA-mea/mac3 are suitable for this type of application. Printed parts were thermally cured for 1 h at 170 °C to complete the ROP of benzoxazines, validating the approach.
3.7 Properties of 3D printed parts
Among the formulations, only CHDM-PA-mea/mac2 and CHDM-PA-mea/mac3 enable 3D printing with good resolution (Fig. 6d). In addition, CHDM-PA-mea/mac2 offers the most significant vitrimeric properties (Fig. 6a–c). Therefore, it was selected for printing a demonstrator to undergo testing.μCT analyses were performed to determine the effective volume of a printed honeycomb before and after the completion of thermal curing (Fig. 7a). The results revealed a volumetric shrinkage of 6%, comparable to other low shrinkage resins used for 3D printing applications.53,65 It is important to note that the shrinkage observed does not result from the curing process—known to be minimal in benzoxazines—but rather from chain orientation and stress relaxation phenomena occurring during the extrusion and deposition phases of the 3D printing process. No delamination or defects between the layers were observed by optical microscopy, attesting to the good adhesion of the material layers during deposition and the dimensional stability of the printed part during the thermal treatment (Fig. 7b).
3.8 Reshaping, mechanical and chemical recycling of 3D printed parts
The benefit of having dynamic bonds within the network was revealed through reshaping, welding and both mechanical and chemical recycling of printed honeycombs. Fig. 7c illustrates that the printed and dual-cured honeycomb can be deformed without fracture at 170 °C over 5 min, demonstrating the ease of reshaping of such 3D printed structures. Fig. 7d shows the successful welding of two halves of a honeycomb into a single self-supported piece after maintaining both parts in contact for 30 min at 170 °C.Mechanical recycling was assessed by grinding 3D printed parts and then successfully reconsolidating them via hot pressing. 3D printed parts were also degraded under acidic conditions at 80 °C. After drying to remove the reaction media, a powder was recovered and reprocessed under conditions identical to those used for mechanical recycling, leading to fully self-supported materials. Recycled samples are all well reconsolidated, with internal porosities of 2.33% and 3.65% for the mechanically and chemically recycled specimens, respectively (Fig. 7e).
The potential to close the recycling loop was demonstrated by reusing powder obtained from grinding printed honeycomb structures as a viscosity modifier in new printing tests. As shown in Fig. 7f, 15 phr of mechanically ground powder was added to fresh resin containing 0.5 phr of a radical initiator to adjust the viscosity of the formulation (Fig. S38†), enabling the printing of new honeycombs. Compression testing of the initially printed honeycombs (Fig. 7g) revealed a compressive modulus of 810 MPa and a strength at break of 80 MPa, comparable to other polymeric 3D-printed honeycombs.66–69 The sample printed with recycled powder achieved a compressive modulus of 690 MPa and an ultimate strength of 120 MPa, demonstrating the feasibility of producing high-performance parts using this recycling strategy. μCT imaging of the printed bead revealed no porosity, indicating strong interactions between recycled material grains and the matrix that likely contributed to the retention of mechanical properties. The lower compressive modulus and increased strength at break for the recycled powder-based part can be explained by the reduction in silica content, which made the initial honeycomb more rigid and brittle.70 For context, Nomex-resin honeycombs—commonly used in structural applications—exhibit compressive moduli around 1.5 GPa due to the reinforcement provided by the Nomex paper structure. In contrast, the material developed in this study achieves a compressive modulus of 810 MPa without such reinforcement. This performance, combined with its reprocessability and simplified fabrication, positions the material as a promising alternative for lightweight, recyclable structural applications.
4 Conclusions
This study is at the forefront of research on circular materials, integrating the benefits of additive manufacturing with bio-based and recyclable vitrimeric polybenzoxazines. These polymers are designed for recyclability by leveraging the introduction of dynamic bonds into their molecular structure. The result is a combination of attractive thermo-mechanical properties and vitrimeric behaviour that enables the material to be reshaped and recycled. Methacrylate functionalization expands the application of these materials to UV-assisted 3D printing, enabling UV curing and the creation of honeycomb structures as a printability demonstration. While excessive methacrylation reduces the amount of aliphatic –OH groups involved in transesterification, impacting vitrimeric properties, insufficient methacrylation hampers UV-assisted 3D printability. Our work identifies a good balance between these competing requirements, leading to the production of honeycomb structures that can be reshaped and recycled, with a Tα of 105 °C, 6% shrinkage, and a compressive modulus of 810 MPa. In addition, the reuse of printed material powder as a viscosity modifier for new printing tests has been successfully demonstrated, resulting in parts that retain their mechanical properties thanks to excellent filler–matrix interactions.The manufacturing process developed in this work is much simpler than that of widely used alternatives, for instance, for Nomex honeycombs, which require several complex steps including polymer processing, paper formation, resin impregnation, and curing—often at similar temperatures. In contrast, our monocomponent vitrimer can be shaped directly and reprocessed without additional formulation or treatment, offering a more energy-efficient and practical approach. Moreover, while the thermal post-treatment step requires a temperature of 170 °C, this condition must be considered in the context of the material entire life cycle. Unlike conventional thermosets, which typically require harsh, energy-intensive processes for end-of-life treatment with very low recycling efficiency, the vitrimer system presented here is reprocessable and reusable. This significantly reduces overall material waste and extends service life, aligning with sustainability goals.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Charles Jehl: conceptualization, investigation, methodology, validation, writing – original draft – review & editing. Antoine Adjaoud: conceptualization, investigation, writing – review & editing. Ambre Meyer: investigation, validation. Vincent Boulic: investigation, writing – review & editing. Channya Hesse: investigation, writing – review & editing. Laura Puchot: methodology, writing – review & editing. Joamin Gonzalez-Gutierrez: data curation, investigation, validation, writing – review & editing. Alexander S. Shaplov: conceptualization, methodology, writing – review & editing. Daniel F. Schmidt: conceptualization, funding acquisition, methodology, writing – review & editing. Pierre Verge: conceptualization, methodology, supervision, writing – original draft – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Luxembourg National Research Fund (FNR) through the Sustainable Polymer Composites - SusPoCo project (agreement number PRIDE21/16748260). The authors are also extremely thankful to Benoit Marcolini, Sebastien Gergen, Dr Reiner Dieden, and Dr Doriane Delfrari for their help in characterisation of the obtained polymer materials. The authors would also like to thank Tinatin Kouprava and Anaë Girault-Fodil for their helpful advice on the conceptual design of figures.Notes and references
- E. Morici and N. T. Dintcheva, Polymers, 2022, 14, 4153 CrossRef CAS PubMed.
- K. L. Forsdyke and T. F. Starr, Thermoset Resins Market Report, iSmithers Rapra Publishing, 2002 Search PubMed.
- Md. G. Kibria, N. I. Masuk, R. Safayet, H. Q. Nguyen and M. Mourshed, Int. J. Environ. Res., 2023, 17, 20 CrossRef CAS PubMed.
- D. Montarnal, M. Capelot, F. Tournilhac and L. Leibler, Science, 2011, 334, 965 CrossRef CAS PubMed.
- W. Denissen, J. M. Winne and F. E. Du Prez, Chem. Sci., 2016, 7, 30–38 RSC.
- M. Guerre, C. Taplan, J. M. Winne and F. E. Du Prez, Chem. Sci., 2020, 11, 4855–4870 RSC.
- W. Zou, J. Dong, Y. Luo, Q. Zhao and T. Xie, Adv. Mater., 2017, 29, 1606100 CrossRef PubMed.
- M. Hayashi, Polymers, 2020, 12, 1322 CrossRef CAS PubMed.
- A. Adjaoud, L. Puchot and P. Verge, Polymer, 2023, 287, 126426 CrossRef CAS.
- A. Adjaoud, D. A. Boina, V. Boulic, C. Hesse, C. Jehl, C. Ziane, L. Puchot, A. S. Shaplov, D. F. Schmidt and P. Verge, in Sustainable Green Chemistry in Polymer Research. Volume 2. Sustainable Polymers and Applications, American Chemical Society, 2023, vol. 1451, pp. 49–84 Search PubMed.
- H. Ishida and D. J. Allen, J. Polym. Sci., Part B: Polym. Phys., 1996, 34, 1019 CrossRef CAS.
- H. Ishida and H. Y. Low, Macromolecules, 1997, 30, 1099 CrossRef CAS.
- L. Bonnaud, B. Chollet, L. Dumas, A. A. M. Peru, A. L. Flourat, F. Allais and P. Dubois, Macromol. Chem. Phys., 2019, 220, 1800312 CrossRef.
- D. Wang, B. Li, Y. Zhang and Z. Lu, J. Appl. Polym. Sci., 2013, 127, 516–522 CrossRef CAS.
- B. Lochab, I. K. Varma and J. Bijwe, J. Therm. Anal. Calorim., 2010, 102, 769 CrossRef CAS.
- K. Zhang, J. Liu, S. Ohashi, X. Liu, Z. Han and H. Ishida, J. Polym. Sci., Part A: Polym. Chem., 2015, 53, 1330 CrossRef CAS.
- H.-D. Kim and H. Ishida, J. Phys. Chem. A, 2002, 106, 3271–3280 CrossRef CAS.
- S. B. Shen and H. Ishida, Polym. Compos., 1996, 17, 710 CrossRef CAS.
- H. Xiang, H. Ling, J. Wang, L. Song and Y. Gu, Polym. Compos., 2005, 26, 563–571 CrossRef CAS.
- C. Barile, C. Casavola and F. De Cillis, Composites, Part B, 2019, 162, 122–128 CrossRef CAS.
- Y. Lyu and H. Ishida, Prog. Polym. Sci., 2019, 99, 101168 CrossRef CAS.
- L. Puchot, P. Verge, T. Fouquet, C. Vancaeyzeele, F. Vidal and Y. Habibi, Green Chem., 2016, 18, 3346 RSC.
- N. K. Sini, J. Bijwe and I. K. Varma, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 7 CrossRef CAS.
- P. Thirukumaran, A. Shakila Parveen and M. Sarojadevi, ACS Sustain. Chem. Eng., 2014, 2, 2790 CrossRef CAS.
- L. Dumas, L. Bonnaud, M. Olivier, M. Poorteman and P. Dubois, J. Mater. Chem. A, 2015, 3, 6012 RSC.
- M. Monisha, N. Amarnath, S. Mukherjee and B. Lochab, Macromol. Chem. Phys., 2019, 220, 1800470 CrossRef.
- M. Monisha, N. Yadav, S. B. Srivastava, S. P. Singh and B. Lochab, J. Mater. Chem. A, 2018, 6, 2555–2567 RSC.
- A. Adjaoud, B. Marcolini, R. Dieden, L. Puchot and P. Verge, J. Am. Chem. Soc., 2024, 146, 13367–13376 CrossRef CAS PubMed.
- A. Adjaoud, L. Puchot and P. Verge, ACS Sustain. Chem. Eng., 2022, 10, 594–602 CrossRef CAS.
- A. Adjaoud, A. Trejo-Machin, L. Puchot and P. Verge, Polym. Chem., 2021, 12, 3276 RSC.
- A. Adjaoud, L. Puchot, C. E. Federico, R. Das and P. Verge, Chem. Eng. J., 2023, 453, 139895 CrossRef CAS.
- A. Trejo-Machin, L. Puchot and P. Verge, Polym. Chem., 2020, 11, 7026 RSC.
- B. Akkus, B. Kiskan and Y. Yagci, Polym. Chem., 2019, 10, 5743–5750 RSC.
- S. Sriharshitha, K. Krishnadevi and D. Prasanna, RSC Adv., 2022, 12, 26934–26944 RSC.
- X. Wang, S. Zhang, Y. He, W. Guo and Z. Lu, Polymers, 2022, 14, 2234 CrossRef CAS PubMed.
- Z. Qiao, M. Wang, J. Jiang, H. Liu, M. Wang, W. Zhao and Z. Wang, ACS Sustain. Chem. Eng., 2022, 10, 9113–9122 CrossRef CAS.
- X.-L. Sha, L. Yuan, G. Liang and A. Gu, Polymer, 2020, 202, 122673 CrossRef CAS.
- S. Gao, Y. Liu, S. Feng and Z. Lu, J. Mater. Chem. A, 2019, 7, 17498 RSC.
- P. Wang, S. Zhang, W. Xu, T. Xiao and Q. Ran, ACS Sustain. Chem. Eng., 2021, 9, 7913–7921 CrossRef CAS.
- S. Ford and M. Despeisse, J. Clean. Prod., 2016, 137, 1573–1587 CrossRef.
- T. D. Ngo, A. Kashani, G. Imbalzano, K. T. Q. Nguyen and D. Hui, Composites, Part B, 2018, 143, 172–196 CrossRef CAS.
- A. H. Alami, A. Ghani Olabi, A. Alashkar, S. Alasad, H. Aljaghoub, H. Rezk and M. A. Abdelkareem, Ain Shams Eng. J., 2023, 14, 102516 CrossRef.
- M. H. Mobarak, Md. A. Islam, N. Hossain, Md. Z. Al Mahmud, Md. T. Rayhan, N. J. Nishi and M. A. Chowdhury, Appl. Surf. Sci. Adv., 2023, 18, 100462 CrossRef.
- J. Hoerber, J. Glasschroeder, M. Pfeffer, J. Schilp, M. Zaeh and J. Franke, Proced. CIRP, 2014, 17, 806–811 CrossRef.
- A. D. Nugraha, M. Syahril and M. A. Muflikhun, Heliyon, 2023, 9, e14706 CrossRef CAS PubMed.
- F. Craveiro, J. P. Duarte, H. Bartolo and P. J. Bartolo, Autom. Constr., 2019, 103, 251–267 CrossRef.
- A. Vyatskikh, A. Kudo, S. Delalande and J. R. Greer, Mater. Today Commun., 2018, 15, 288–293 CrossRef CAS.
- A. Oleff, B. Küster, M. Stonis and L. Overmeyer, Prog. Addit. Manuf., 2021, 6, 705–730 CrossRef.
- M. a. S. R. Saadi, A. Maguire, N. T. Pottackal, M. S. H. Thakur, M. Md. Ikram, A. J. Hart, P. M. Ajayan and M. M. Rahman, Adv. Mater., 2022, 34, 2108855 CrossRef CAS PubMed.
- B. Krishna Kumar and T. J. Dickens, J. Appl. Polym. Sci., 2023, 140, e53304 CrossRef CAS.
- X. Wan, L. Luo, Y. Liu and J. Leng, Adv. Sci., 2020, 7, 2001000 CrossRef CAS PubMed.
- Y. Lu, K. W. J. Ng, H. Chen, X. Chen, S. K. J. Lim, W. Yan and X. Hu, Chem. Commun., 2021, 57, 3375–3378 RSC.
- Y. Yang, X. Xu, Z. Li, X. Yao, D. Kang, Y. Lu, Y. Wang, Y. Guo and X. Wang, Mater. Lett., 2023, 345, 134448 CrossRef CAS.
- J. J. Weigand, C. I. Miller, A. P. Janisse, O. D. McNair, K. Kim and J. S. Wiggins, Polymer, 2020, 189, 122193 CrossRef CAS.
- N. D. Arun, H. Yang, L. Yao and A. W. Feinberg, Adv. Mater. Technol., 2023, 8, 2201542 CrossRef CAS PubMed.
- L. Jin, T. Agag, Y. Yagci and H. Ishida, Macromolecules, 2011, 44, 767–772 CrossRef CAS.
- A. Trejo-Machin, P. Verge, L. Puchot and R. Quintana, Green Chem., 2017, 19, 5065–5073 RSC.
- C. Berti, E. Binassi, M. Colonna, M. Fiorini, G. Kannan, S. Karanam, M. Mazzacurati, I. Odeh and M. Vannini, ChemSusChem, 2016, 9, 3102–3112 CrossRef PubMed.
- X. Guo, J. Xin, X. Lu, B. Ren and S. Zhang, RSC Adv., 2014, 5, 485–492 RSC.
- B. Kiskan, B. Koz and Y. Yagci, J. Polym. Sci., Part A:Polym. Chem., 2009, 47, 6955–6961 CrossRef CAS.
- R. Kudoh, A. Sudo and T. Endo, Macromolecules, 2010, 43, 1185–1187 CrossRef CAS.
- B. Lochab, M. Monisha, N. Amarnath, P. Sharma, S. Mukherjee and H. Ishida, Polymers, 2021, 13, 1260 CrossRef CAS PubMed.
- F. I. Altuna, C. E. Hoppe and R. J. J. Williams, Eur. Polym. J., 2019, 113, 297 CrossRef CAS.
- J. Han, T. Liu, C. Hao, S. Zhang, B. Guo and J. Zhang, Macromolecules, 2018, 51, 6789–6799 CrossRef CAS.
- Y. Cui, J. Yang, D. Lei and J. Su, Ind. Eng. Chem. Res., 2020, 59, 11381–11388 CrossRef CAS.
- C. Wang, S. Ma, D. Li, J. Zhao, H. Zhou, D. Wang, D. Zhou, T. Gan, D. Wang, C. Liu, C. Qu and C. Chen, ACS Appl. Mater. Interfaces, 2021, 13, 15690–15700 CrossRef CAS PubMed.
- Z. C. Eckel, C. Zhou, J. H. Martin, A. J. Jacobsen, W. B. Carter and T. A. Schaedler, Science, 2016, 351, 58–62 CrossRef CAS PubMed.
- Z. Wen and M. Li, Materials, 2021, 14, 4410 CrossRef CAS PubMed.
- S. Antony, A. Cherouat and G. Montay, Appl. Compos. Mater., 2020, 27, 935–953 CrossRef CAS.
- P. Dittanet and R. A. Pearson, Polymer, 2012, 53, 1890–1905 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: See DOI: https://doi.org/10.1039/d5ta01478f |
| This journal is © The Royal Society of Chemistry 2025 |