Self-powered, online, highly sensitive lubricating oil acidity monitoring driven by a triboelectric sensor†
Bin
Ge‡
a,
Dong
Liang‡
a,
Yi
Lei
a,
Yajie
Zhang
*b,
Yi
Liu
a,
Wei
Wu
a,
Yijun
Shi
*c and
Jun
Zhao
 *a
*a
aSchool of Mechanical Engineering, Beijing Institute of Technology, Beijing 100081, PR China. E-mail: zhaojun@bit.edu.cn
bKey Laboratory for the Physics and Chemistry of Nanodevices, School of Electronics, Peking University, Beijing 100871, China. E-mail: yjzhang11@pku.edu.cn
cDivision of Machine Elements, Luleå University of Technology, Luleå SE-971 87, Sweden. E-mail: yijun.shi@ltu.se
First published on 14th May 2025
Abstract
Lubricating oil as the “lifeblood” of industry can improve the performance and longevity of machines by orders of magnitude. However, the acidity of oils occurring during operation always results in fatal failures of machine elements. It is a great challenge to monitor oil acidity online in a highly sensitive machine system. In this study, a self-powered, real-time, online triboelectric sensor was developed for detecting oil acidity based on oil-solid triboelectricity (OA-TENG). By engineering a tribo-surface molecular structure with fluorocarbon chains and amino groups, the contact angle of the optimized triboelectric surface changed to ∼155°, which was much higher than that of commercial polytetrafluoroethylene (PTFE, ∼40°). Furthermore, the real-time adsorption of aged oils on the optimized surface was achieved using quartz crystal microgravimetry with dissipation (QCM-D) for oil-solid contact. It was found that the amino group on the surface has the capability of capturing oxygen-containing contaminants generated during aging through hydrogen and covalent bonds, which is able to adsorb with another 0.3 μg cm−1 compared with that without amino groups. Thus, typical acidity contaminants in aged oils can be successfully monitored. The triboelectric output of the optimized OA-TENG was similar to that of the PTFE-based OA-TENG, but the monitoring sensitivity of the optimized OA-TENG (∼0.62 V per TAN) was much higher than that of the PTFE-based OA-TENG (∼0.2 V per TAN). The triboelectric sensor's sensitivity reported in this study was also much better than that of the previous method for monitoring oil acidity (∼0.25 V per TAN). This work not only provides new pathways for oil condition monitoring with high sensitivity but also greatly expands the applicability of TENGs.
1. Introduction
Energy loss is a critical problem in our energy-shortage world, the main causes of which are friction and wear. It was reported that about 3 trillion USD are spent annually worldwide on fuel used to overcome friction by passenger cars.1 Frictional losses among advanced manufacturing countries can be even higher, e.g., China, the USA, Germany, and Japan.2 Using lubricants is the most effective way to control friction and wear. Proper lubrication ensures that machines work under safe and healthy conditions, which is of great significance for energy conservation and emission reduction.3 At the same time, lubricating oil is an important source of information for monitoring machine working conditions, which is similar to the role of human blood in health condition monitoring. Under operation, the heat produced in the engine segment would influence oil performance. Lubricating oils usually operate in contact with air, and therefore, their chemical compounds react with oxygen in air during high-temperature and long-service processes. In our previous study,4 it has been found that oxoacids are common types of acids produced in high-temperature aged oils. Many oxygen-containing components (carboxylate, carbonyl, and hydroxy groups) are generated in lubricating oils after a long aging time. The low oxidation stability of lubricating oils may result in oil acidity, which not only damages the oil quality and lubrication performance but also corrode the machine. Oil acidity is thus usually the most important life-limiting factor.Early warning systems through the monitoring of machines become increasingly more important.4,5 Thus, oil acidity monitoring has played an irreplaceable role in ascertaining the health condition of machinery. Oil acidity is typically quantified by the total acid number (TAN), which is widely used as an indicator of oil acidity conditions. Oil acidity can be detected based on the analysis of the number of oil properties, which can be conducted either offline by taking oil samples from a system or online by incorporating appropriate sensors in machinery. Over the years, off-line analysis has become an essential means to monitor the oil aging process, providing guidance on oil changing for machine protection. Conventionally, oil acidity has been quantified using colorimetric6 and potentiometer titration methods7 that have been widely accepted by industry. These methods are time-consuming, costly and off-line. It is every industry's desire to be able to monitor oil acidity in real time to optimize oil changing and machinery maintenance. Infrared (IR) analysis became a popular and powerful practice in monitoring oil acidity, but its application is restricted to precisely controlled off-line conditions and static measurements.8 They use periodic manual sampling analysis, which has information lag, making it difficult to capture transient information of operation. It comprises a broadband IR light source, collimating mirrors, an ATR crystal, a filter-chopper, and a pyroelectric detector, and it is thus very complex and expensive and still in a prototype stage and has not been miniaturized and tested in real time.8,9 In addition, oil acidity can be reflected by a change in physical properties. Therefore, the oil acidity can be detected timely by monitoring the dielectric constant, viscosity, and conductivity, which have been employed to monitor the level of oxidation.10 However, most conventional detection sensors are quite large and unwieldy, and need installation or attachment to equipment systems, potentially causing interference with the monitoring system. Due to reliance on external power sources, energy consumption is a challenge for their miniaturization and weight reduction. It is highly desirable to develop a self-powered, high-sensitivity, and small detection system for real-time, online monitoring of oil acidity.
Based on the conjunction of triboelectrification and electrostatic effects, a triboelectric nanogenerator (TENG) has been developed for energy harvesting and self-powered monitoring since 2012 after the first work reported by Wang and co-workers.11 TENG-based sensors have successfully been used as self-powered mechanical sensors for detecting water waves,12–17 liquid flow rate,18–20 and organic21–23 and ion concentration24–27 based on liquid–solid contact electrification. For the first time, we developed oil-based TENG sensors for oil condition monitoring.4 Electron transfer is the dominating mechanism for the triboelectrification process in cases of solid–solid and liquid–solid contact.28,29 Lubricating oils can generate a certain amount of charge by electron transfer at the oil-solid interface. The contaminants in a lubricating oil will change the electrification process performance of the oil and can be reflected in the TENG electric output. However, there are no TENG-based sensors that are able to detect the oil acidity contaminants in lubricants.
Thus, it is of great importance to achieve real-time, online monitoring of oil acidity contaminants, which can eliminate unnecessary machine shutdowns and avoid the possibility of catastrophic component failure during operation. In this study, a self-powered, online, and highly sensitive triboelectric sensor was developed for detecting oil acidity based on oil-solid triboelectricity (OA-TENG). Through optimizing the surface chemical structure, modification with different functional groups can be formed to regulate its triboelectric performance. The grafting of fluorine groups enhances its electron-withdrawing ability, while the introduction of amino groups enhances the hydrogen ion-capturing ability. Accordingly, the OA-TENG has very good sensitivity to oil acidity. Therefore, engineering the chemical structure is an efficient strategy to optimize the electric performance of TENGs and enlarge the functionalization of triboelectric sensors.
2. Results and discussion
2.1 Fabrication and structure of the developed OA-TENG
The fabrication diagram of the OA-TENG is demonstrated in Fig. 1. The nano-SiO2 and fluorocarbon chains have a wide range of applications in the development of superoleophobic surfaces.30 The SiO2 and fluorocarbon surfactant (FC) are used, respectively, for increasing the roughness and decreasing the surface energy of TENGs. SiO2 nanoparticles are used to increase the surface roughness, which enhances the oleophobic properties and charge transfer efficiency.31 Fluorocarbon chains further reduce the surface energy, creating a superoleophobic surface that minimizes oil adhesion.30,32 Amino groups are introduced to capture oxygen-containing functional groups in aged oils through hydrogen and covalent bonds,33,34 which is crucial for detecting oil acidity, as shown in Fig. 1. (3-Aminopropyl)triethoxysilane (APTES) having an amino group (–NH2) is then grafted onto the triboelectric surface, which has the capability of capturing protons from the acidified liquid, e.g., aged lubricating oils (Fig. 1f). It should be noted that the interaction between the APTES and fluorocarbon surfactant can be stably adsorbed onto the SiO2-based surface via the interaction of covalent bond and electrostatic force (Fig. 1e). The amino-functionalized surface has distinct advantages over other polar surface groups such as hydroxyl, carboxyl, and amine derivatives in the context of oil acidity detection. Amino groups (–NH2) are highly effective in capturing and interacting with oxygen-containing functional groups in aged oils, such as carboxyl, hydroxyl, and carbonyl groups, via hydrogen bonding and covalent bonding. This interaction enhances the adsorption of acidic contaminants on the sensor surface, thereby improving the sensitivity of oil acidity detection.35 Although other polar groups such as hydroxyl (–OH) and carboxyl (–COOH) also have the potential to interact with acidic contaminants, studies have indicated that amino groups generally exhibit stronger binding capabilities and higher reactivity.36 Accordingly, via optimizing the concentration of SiO2, FC, and APTES on the triboelectric surface, a series of OA-TENGs were obtained, as shown in Tables S1–S3,† named FCNH-n, in which n means the spraying times of APTES onto the surface. FC is particularly effective in reducing the surface energy, which is crucial for creating superoleophobic surfaces. This property minimizes oil adhesion and ensures efficient charge transfer in the TENG.30,32 FC is soluble in water, which simplifies the preparation process and ensures uniform coating on the triboelectric surface. In addition, FC exhibits excellent chemical stability.37 In this regard, FC was chosen as the electron-withdrawing material to optimize the performance of the OA-TENG.More details on the fabrication process are provided in the Experiment section. The triboelectric rig tested in this study is shown in Fig. 1f and S1.† The oil waves will be generated because of the reciprocating motion of the oil tank. Therefore, charge transfer occurs at the oil-solid interface when oil waves pass through the triboelectric surface. The electric signal generation from the developed OA-TENG is based on both triboelectrification and electrostatic induction. The triboelectric behaviors of the OA-TENG are greatly influenced by the interaction between the oil and the contacting surface, by which the oil acidity will be monitored effectively (Fig. 1g).
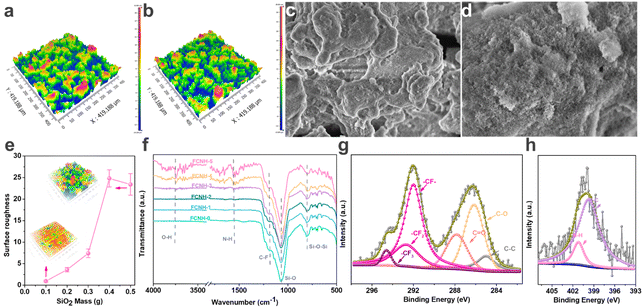
The surface morphologies and chemical structures of the developed OA-TENG are shown in Fig. 2. The OA-TENG (FCNH-0) has a surface roughness of ∼7.3 μm (Fig. 2a), and it undergoes few changes after grafting with APTES (FCNH-3–7.4 μm) (Fig. 2b). It was also found that with the increase in added SiO2, the surface roughness increases from 1 μm to 24 μm (Fig. 2d and S2†). For comparison, commercially received polytetrafluoroethylene (PTFE) was also used for fabricating an OA-TENG. PTFE is a widely used material in triboelectric applications due to its excellent electrical properties and robustness.30,38,39 Therefore, the PTFE-based TENG was used as a comparison sample. The surface of the PTFE-based OA-TENG is very smooth, the roughness of which is ∼0.31 μm, as shown in Fig. S3.† According to Fig. 2c and S4,† the OA-TENG (FCNH-3) displays a hierarchical cellular-like structure. The elements of F and Si uniformly appeared on the OA-TENG surface, indicating the successful deposition of SiO2 and FC. To verify the chemical structure of the OA-TENG (FCNH-n), FTIR spectra were recorded, and are shown in Fig. 2f. The OA-TENG show typical absorption peaks at 801 cm−1 and 1072 cm−1 attributed to the Si–O–Si bending vibration.40 The OA-TENG also has an additional absorption band at 1185 cm−1, which is due to the C–F stretching.41 The characteristic absorption intensity of the –NH2 group appeared (1544 cm−1) strongly with the increase in APTES, which further confirms the successful grafting of APTES onto the SiO2 substate.42 It should be noted that at a higher APTES (spraying mass > 90 mg), obvious O–H bands appeared at 3670 cm−1 and 3743 cm−1.43 It is because APTES can be hydrolyzed within the aqueous solution and generate hydroxyl groups, which then are created into ethanol when in contact with the surface of aqueous SiO2, but the hydroxyl groups are much higher on the OA-TENG (FCNH-5) that cannot be reacted fully. Thus, the OA-TENG (FCNH-3) with a proper modification of the –NH2 group plays a key role in capturing the oxygen-containing components in the lubricating oils. Further, the XPS survey spectra of the OA-TENG (FCNH-3) in Fig. 2g display that the primary elements are C, N, O, Si, and F (Fig. S5a†). It is obvious that the peaks at 284.8 eV, 285.4 eV, and 286.9 eV are observed in the C 1s spectrum, referring to C–C, C–O, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively. The peaks at 292 eV, 292.8 eV, and 295 eV are attributed to C–F related bonds (–CF–, –CF2−, and –CF3), which originated from the FC. It is noted that the intensity of the N element obviously increases with the spraying mass of APTES (Fig. S5b†), and the atomic concentration of N atoms increases to 2.65% after spraying 150 mg of APTES (Table S4†). According to the XPS data of N 1s shown in Fig. 2h, the peaks at 399.7 and 401.1 eV are, respectively, assigned to N–C and N–H in amino groups. The existence of C–N and N–H bonds demonstrate the presence of amino groups grafted onto the surface of the OA-TENG (FCNH-3).
O bonds, respectively. The peaks at 292 eV, 292.8 eV, and 295 eV are attributed to C–F related bonds (–CF–, –CF2−, and –CF3), which originated from the FC. It is noted that the intensity of the N element obviously increases with the spraying mass of APTES (Fig. S5b†), and the atomic concentration of N atoms increases to 2.65% after spraying 150 mg of APTES (Table S4†). According to the XPS data of N 1s shown in Fig. 2h, the peaks at 399.7 and 401.1 eV are, respectively, assigned to N–C and N–H in amino groups. The existence of C–N and N–H bonds demonstrate the presence of amino groups grafted onto the surface of the OA-TENG (FCNH-3).
2.2 Wetting and adsorption behavior of the OA-TENG
According to Fig. 3a and S6,† the oil contact angle of the OA-TENG first increases with the increase in SiO2 because of the positive contribution of SiO2 on the surface roughness (Fig. 2e). When the SiO2 mass is more than 0.2 g, the OA-TENG (FCNH) achieves great superoleophobicity. The contact angle of the OA-TENG (FCNH) increases and then decreases with the increase in FC mass. The optimized mass of FC is around 6 g regarding the value of contact angle (Fig. 3b and S7†). It can also be seen in Fig. 3c and S8† that the contact angle of the OA-TENG (FCNH-0) is almost similar to that of the OA-TENG (FCNH-3), which means that the spraying of APTES with low masses (≤90 mg) has little influence on wetting performance, i.e., superoleophobicity. It means that the contact angles of these FCNH-based surfaces are much higher than that of the PTFE surface (40°) (Fig. S9†). The contact angle of the surface also affects the interaction between the oil and the triboelectric surface.32 As the contact angle increases, the surface oleophobicity is enhanced, which can maintain a high charge transfer efficiency30 and exhibit a high output voltage, whereas the value of the contact angle of OA-TENG (FCNH) decreases significantly at a high spraying mass of APTES (>90 mg), hence the OA-TENG with a proper modification of APTES plays a key role in controlling superoleophobicity performance.Further, for analyzing the oil adsorption on the modified triboelectric surface, a quartz crystal microbalance with dissipation (QCM-D) was used in this study. It was found from Fig. 3d that there was a typical frequency-shift (ΔF) response for the adsorption of aged oils onto selective surfaces i.e., Au substrate, FCNH-0, and FCNH-3. The frequency rapidly decreased when the oils were injected into the Au substrate, meaning quick adsorption of the base oil (aging 0 h) on the substrate surface, as shown in Fig. 3d. The stable adsorption mass of the base oil on the substrate is about 4.6 μg cm−1. Oils aged for different periods (60 h, 120 h, 180 h) were then investigated in sequence. With the increase in aging time, there was a significant decrease in the frequency observed for the three tested surfaces. Compared with the Au substrate surface, the modified surfaces with FC (FCNH-0, and FCNH-3) have a slower frequency decrease and a lower adsorption mass (Fig. 3e) due to the high drag reduction efficiency in the case of the oleophobic surface. However, the surface modified with FCNH-3 displays an obvious decrease in frequency and dissipation in comparison to that of the FCNH-0-modified surface (inset of Fig. 3d), resulting in a high adsorption mass of aged oils onto the FCNH-3 modified surface (inset of Fig. 3e). The adsorption masses for the surface of FCNH-0 and FCNH-3 are 0.6 μg cm−1and 0.9 μg cm−1, respectively. It is probably because the amino group in APTES has the capability of capturing oxygen-containing components in aged oils (inset of Fig. 3e). Aged oil contaminants such as carboxyl, hydroxyl, and carbonyl groups can significantly affect charge retention by altering the surface properties of the triboelectric materials. These contaminants can adsorb onto the triboelectric surface, creating a layer that hinders electron transfer.35 Accordingly, it can be qualitatively determined that the introduced APTES (spraying 90 mg) has a great contribution on the adsorption of aging contaminants. The enhanced adsorption of FCNH-3 determines the electric output performance of TENGs. Accordingly, highly sensitive oil-acidity monitoring can be achieved enabled by the OA-TENG, which will be further discussed in the next part.
2.3 Electric output performance of the OA-TENG
Lubricating oils are the “lifeblood” of the machine industry. It is highly desirable to monitor their conditions using TENG-based sensors.4,30 Electron transfer is the dominating mechanism for the triboelectrification process in oil-solid cases. The triboelectric behavior of the fabricated OA-TENG was studied first. Via regulating the molecular structure of the solid surface with FC and APTES, the triboelectric performance of OA-TENGs was optimized finally. As shown in Fig. 4a, the FC mass used in the spraying process has a greatly positive influence on the triboelectric performance. Whereas, sparying with a higher mass (8 g) of FC, the fluorocarbon chains from FC can't effectively prick outward the surface due to the relative lack of SiO2 particles for electrostatic anchoring. Accordingly, the surface of the OA-TENG is not oleophobic (Fig. 3b and S7e†) and many residual oils adsorb on the triboelectric surface, hindering electron transfer. The output voltage and current are thus decreased at the higher value of FC mass (Fig. 4a and b). In comparison to the fluorocarbon in FC, the amino group in APTES has a greatly negative effect on the triboelectric output (Fig. 4c and d). For comparison, the triboelectric performance of the PTFE-based OA-TENG is shown in Fig. S10.† The output voltage of the PTFE-based sample is about 1 V, which is similar to that of the OA-TENG (FCNH-3), but the output current is much lower (15 nA). The applied force and reciprocation velocity are closely related in the context of triboelectric generation.30,32 The output voltage magnitude has a positive correlation with both the velocity of tank reciprocation and the volume of lubricating oils. The output voltage of the OA-TENG (FCNH-3) increases from 0.12 to 1.0 V by increasing the velocity from 2.4 to 7.2 cm s−1. The output voltage and current, respectively, increase up to 3.5 V and 40 nA at a high oil volume of 150 mL. To get a fair comparison, the following results are based on the parameter values of 7.2 cm s−1 and 100 mL (oil volume). The change in the dielectric constant of the oil is caused by acidic contaminants generated during the aging process.44,45 However, the magnitude of this change in the dielectric constant is relatively small. The decrease in voltage output is attributed to the shielding effect of the adsorption of acidic contaminants (Fig. S12†). With the increase in temperature, the output voltage is slightly decreased because the high temperature will cause thermionic emission,4,30 as shown in Fig. S15.† Nevertheless, the output performance is relatively stable when the temperature stays in the range from 20 °C to 40 °C. The OA-TENG output performance can still maintain a high voltage output at 100 °C. The high-temperature aging of oil leads to a decrease in its viscosity.46 A lower viscosity would increase the contact area47 and enhance charge transfer.30 However, the aged oil contains more contaminants adsorbed onto the triboelectric surface and creates a shielding layer,33,34 which reduces the effective charge transfer and leads to a decrease in the triboelectric output voltage (Fig. S14†). The adsorption effects of contaminants play a dominant role in modulating the triboelectric output of OA-TENGs.To further validate the charge transfer efficiency of our optimized triboelectric surfaces, we conducted Kelvin probe force microscopy (KPFM) measurements.48 The results presented in Fig. S13† provide critical insights into the surface potential changes due to different surface modifications. KPFM results (Fig. S13b and d†) show that the FC modification leads to a lower surface potential, which is correlated with a higher output voltage in the triboelectric nanogenerator (TENG). A lower surface potential, implying a more negative potential, indicates a stronger electron-withdrawing ability. The surface enhances the charge transfer efficiency from the oil to the TENG surface.49,50 In contrast, the FCNH-3 surface, which includes amino groups, exhibits a reduction in negative potential on the surface. This suggests that the amino groups may partially shield the electron-withdrawing effect of the fluorocarbon chains, leading to a reduced output voltage. This observation aligns with our findings that the FCNH-3 surface, despite having a higher contact angle and better superoleophobicity, shows a lower output voltage due to the adsorption and shielding effect of the amino group.51 However, because the surface potential is obviously negative (Fig. S13d†), the FCNH-3 surface still maintains a strong electron-withdrawing ability.
2.4 Monitoring the oil acidity using the OA-TENG
Lubricating oils are sensitive to temperature and will undergo oxidation during the operation of the actual equipment.52,53 The oxidation process leads to easy release of fatty acids and deterioration of the quality of the oil. This, in turn, will generate unexpected failures of critical dynamic components in machines.54,55 To address this issue, the fabricated OA-TENG in this study can effectively monitor the oil acidity. Fig. 5 shows the voltage outputs of the OA-TENG driven by oils aged at different times (0–180 h). For the case of the OA-TENG (FCNH-3), the output voltage decreases from 0.96 V to 0.5 V with the increase in aging time (up to 180 h) (Fig. 5a). For comparison, the voltage output of the PTFE-based OA-TENG has few changes (Fig. 5b and c), which is also less sensitive to oil acidity than the OA-TENG (FCNH-0) (Fig. S11a†). Similarly, with a high concentration of the aged oil (aged for 180 h) in pure base oil, the electric output decreases dramatically in the case of the OA-TENG (FCNH-3) (Fig. 5d). In particular, the output value of voltage decreases to 0.65 V when 40% aged oils are added in the pure base oil, which is much more sensitive to the oil acidity than the FCNH-0 sample (Fig. S11b†). Further, the output voltage of the PTFE-based OA-TENG is as high as 0.87 V in contact with the oil with 80% aged oils, which means that the PTFE-based sample cannot be utilized for monitoring the oil acidity (Fig. 5e). It is thus found the normalized voltage of the OA-TENGs (FCNH-3) is much higher than that of the PTFE-based sample at different volume concentrations of aged oils (Fig. 5f).As known, lubricating oil usage produces acidic by-products such as carboxylic acid via a number of aging processes.56 The TAN (total acid number) of lubricating oils increases significantly, which has been used as an important indicator of oil degradation.57 As shown in Fig. 5g–h, the TAN obviously increases with the increase in aging time and aged oil concentration in base oils, the value of which changes from about 0.3 to 1 mg KOH per g. It should be noted that through respective comparison of TAN, the output voltages of OA-TENGs are found to have a linear relationship with TAN (Fig. 5i and j). The normalized output voltage also has a similar relationship with TAN, as shown in Fig. 5. The FCNH-modified OA-TENG (FCNH-3) has a higher correlation than that of the PTFE-based OA-TENG according to the R2 value. Further, the slopes (or sensitivities) of the OA-TENG (FCNH-3) are 0.62 V per TAN and 0.82 V per TAN, respectively, for the cases of aged oils (Fig. 5i) and aged oil mixtures (Fig. 5j), whereas, the sensitivities of the OA-TENG (PTFE) are 0.2 V per TAN and 0.34 V per TAN, respectively. Thus, the OA-TENG (FCNH-3) has the highest sensitivity for the two types of oils, i.e., aged oils and aged oil mixtures, which is much better than that of the PTFE-based OA-TENG and sensors reported in previous studies (0.02 V per TAN,57 0.1–0.25 V per TAN10) and commercial ones (0.01–0.1 V per TAN10). It is difficult to monitor the oil acidity online using common sensors. Accordingly, dielectric constants are usually chosen to reflect the oil acidity.10 In this regard, a commercial sensor was introduced here for better comparison. As shown in Fig. S12,† the commercial sensor exhibited only a 2.7% change in dielectric constant with the increase in aged oil concentration. In contrast, the OA-TENG showed a 79.4% change in voltage output under the same conditions. This significant difference highlights the superior sensitivity of the OA-TENG in detecting oil acidity. Furthermore, the OA-TENG is used for real-time monitoring of oil acidity, which makes it a highly effective tool for applications. The peak voltage, current density, and power of this large OA-TENG with various load resistances are shown in Fig. S16.† The results show that the peak power of the OA-TENG reaches its maximum at a load resistance of 500 MΩ. This optimal resistance value ensures efficient energy transfer and maximizes the power output of the sensor. As the load resistance increases, the voltage is increased, whereas the current is reduced. At a load resistance of 500 MΩ, the maximum output power density (12.79 nW) was achieved.
The OA-TENG (FCNH-3) has a highly sensitive performance for monitoring the oil acidity. It is because the triboelectric surface modified by both fluorocarbon and amino groups not only can improve the electron transfer but also can effectively capture acidified components in the aged oils via electrostatic interaction, hydrogen bonds, and covalent bonds (Fig. 5k). For example, the fluorocarbon-based TENG's surface has a high electron-withdrawing capability, so that electrons are transferred from oil to the TENG's surface, making its surface to be negatively charged.30 Because of the electrostatic interaction, hydrogen ions released from aged oils easily adsorb onto the negatively charged TENG surface.58,59 It means the electric output decreases significantly when the TENG is in contact with aged oils compared with contacting pure base oils. Further, other oxygen-containing components such as hydroxyl, carboxyl, and aldehyde groups can anchor physically onto the electric surface due to the hydrogen bond with the surface's amino groups. The amino groups further chemically react with these components.60 Thus, the aged oil has a strong adhesive effect on the contact surface, which has been identified by measuring the adsorption mass by QCM-D (Fig. 3d and e). In particular, the OA-TENG (FCNH-3) has a higher adsorbed mass than that of the OA-TENG (FCNH-0), which confirms the key role of amino groups in capturing the oxygen-containing components that originated from the thermal oxidation of oils. The adsorbed components on the triboelectric surface have a shielding effect during the electrostatic induction process, resulting in the output signal decreasing quickly, by which the oil acidity can be monitored with a high sensitivity.
3. Conclusions
In this study, a self-powered, online, highly sensitive triboelectric sensor was developed for monitoring the lubricating oil acidity. Through the engineering of triboelectric surfaces with SiO2, fluorocarbon chains and amino groups, super-oleophobic OA-TENGs (FCNH) were achieved. By further spraying with APTES, the triboelectric surface chemically modified with amino groups was also able to capture the oxygen-containing functional groups, e.g., hydroxyl, carboxyl, and carbonyl, via hydrogen and covalent bonds. The FCNH-based surface-modified amino groups thus were able to adsorb with more oils (0.3 μg cm−1) in comparison to that without amino groups via QCM-D measurements for oil-solid contact. Therefore, the triboelectric outputs of the OA-TENGs (FCNH-n, PTFE) showed a decreasing trend with the increase in oil acidity because of the adsorption of aged oils onto the electric surface, resulting in a shielding effect, by which typical acidity contaminants in aged oils can be successfully monitored. In particular, it was found that the monitoring sensitivity of the OA-TENG (FCNH-3) (∼0.62 V per TAN) was much higher than that of the PTFE-based OA-TENG (∼0.2 V per TAN). The triboelectric sensor's sensitivity in this study was also much better than of the previous method (∼0.25 V per TAN) and commercial ones (0.01–0.1 V per TAN) for monitoring the oil acidity. This work provides new pathways for smart monitoring of lubricating oil conditions.4. Experimental section
4.1 Materials
Hydrophilic nano-SiO2, deionized (DI) water, (3-aminopropyl)triethoxysilane, and other chemical reagents were supplied by Aladdin, and paraffin oil was supplied by Macklin. Anionic fluorocarbon surfactant (noted as FC) was provided by DuPont. All the materials and chemicals were used as received without any treatment.4.2 Preparation of OA-TENGs
In this study, 3 M double-sided tape (4 × 5 cm2) served as the substrate for the OA-TENG. The polyimide (PI) single tape was placed directly on the 3 M double-sided tape and then an aluminum (Al) electrode tape, 4 cm in width and 100 μm in thickness, was affixed to the PI surface. Each side of the Al electrode was completely covered with a 100 μm-thick PI double tape to complete the OA-TENG structure.The preparation of the OA-TENG (FCNH-base) involved two steps. Initially, SiO2 nanoparticles were dissolved in DI water in a ratio specified in Table S3.† After stirring for 10 minutes, FC was added to the SiO2 solution, which was then stirred for an additional hour to create the waterborne solution. This solution was sprayed onto the final FC-SiO2 coating and dried in the air, with the number of repetitions varying to achieve the final OA-TENG, as indicated in Table S3.† The mass change of each spraying time is 30 mg. The prepared SiO2 suspension was sprayed onto the film surfaces and allowed to dry in the air. This spraying process was repeated five times to form the final FC-SiO2 coating. Subsequently, (3-aminopropyl)triethoxysilane was mixed with acetone in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and stirred for 30 minutes to form a mixed solution. This solution was sprayed onto the final FC-SiO2 coating and dried in air, with the number of repetitions varying to achieve the final OA-TENG, as indicated in Table S3.†
1 and stirred for 30 minutes to form a mixed solution. This solution was sprayed onto the final FC-SiO2 coating and dried in air, with the number of repetitions varying to achieve the final OA-TENG, as indicated in Table S3.†
4.3 Characterization and measurement
The chemical compositions of the OA-TENGs were analyzed using a Fourier transform infrared spectrometer (Vertex 70 V, NETZSCH, Germany) and an X-ray photoelectron spectrometer (PHI Quantera II, Ulvac-Phi Inc., Japan). The surface morphology and microstructure of the OA-TENGs were characterized using a three-dimensional white-light interferometry microscope (Nexview, ZYGO Lambda, USA) and a scanning electron microscope (Quanta 200 FEG, FEI, USA). The contact angles were measured using a video optical contact angle measuring instrument (OCA25, Dataphysics, Germany) with 4 μL droplets of paraffin oil. The acid numbers (TAN) of all types of oil were determined using a TAN tester (MiniVisc 3050, Spectro, USA). The changes in the adsorption of oil components on normative Au substrates coated with the FCNH coating surface were measured using a QCM-D instrument (Q-sense E1 system, Biolin Scientific, Sweden). KPFM was measured using a Dimension FastScan (Bruker, USA).Aged oil was obtained by heating at 150 °C for different durations. Different concentrations of aged oil were prepared by mixing with pure paraffin oil. In the QCM-D test, all oils were diluted with petroleum ether (40 wt%) to achieve low viscosity. The oil tank (stainless steel) was driven by a linear motor in the test system. The volume of oils used in the triboelectric test increases from 50 mL to 150 mL in an oil tank, and the average velocity range is 2.4–7.2 cm s−1 (0.6 Hz–1.8 Hz), as shown in Fig. 4e and f. The electric signals from all OA-TENGs were measured using an electrometer (Keithley 6514). The size of the tank is 13.5 cm (length) × 10.5 cm (weight) × 6 cm (height). The sliding distance of the tank is 4 cm.
Data availability
Data will be made available upon request.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors are grateful for the support received from the National Natural Science Foundation of China (grant no. 52275170 and U24A20114) and the Tribology Science Fund of the State Key Laboratory of Tribology in Advanced Equipment (grant no. SKLTKF24A05).References
- K. Holmberg and A. Erdemir, Friction, 2017, 5, 263–284 Search PubMed
.
- J. B. Luo and X. Zhou, Friction, 2020, 8, 643–665 Search PubMed
.
- J. Zhao, Y. Y. He, Y. F. Wang, W. Wang, L. Yan and J. B. Luo, Tribol. Int., 2016, 97, 14–20 CrossRef CAS
.
- J. Zhao, D. Wang, F. Zhang, Y. Liu, B. D. Chen, Z. L. Wang, J. S. Pan, R. O. L. Larsson and Y. J. Shi, Acs Nano, 2021, 15, 11869–11879 CrossRef CAS PubMed
.
- C. H. Wei, W. Wu, T. Li, F. Massi and J. Zhao, Tribol. Int., 2025, 201, 110281 CrossRef
.
- American Society for Testing Materials, Annual Book of ASTM Standards, ASTM D974-912, 2009 Search PubMed.
- American Society for Testing Materials, Annual Book of ASTM Standards, ASTM D664-609a, 2009 Search PubMed.
- K. Katarzyna, P. Andreas and K. Alexander, Proceedings of the IEEE Sensors 2003, Toronto, Canada, 2003 Search PubMed.
- A. Agoston, C. Ötsch, J. Zhuravleva and B. Jakoby, Proceedings of the IEEE Sensors 2004, Vienna, Austria, 2004 Search PubMed.
- M. Soleimani, M. Sophocleous, M. Glanc, J. Atkinson, L. Wang, R. J. K. Wood and R. I. Taylor, Tribol. Int., 2013, 65, 48–56 CrossRef CAS
.
- F. R. Fan, Z. Q. Tian and Z. L. Wang, Nano Energy, 2012, 1, 328–334 CrossRef CAS
.
- A. Ahmed, Z. Saadatnia, I. Hassan, Y. L. Zi, Y. Xi, X. He, J. Zu and Z. L. Wang, Adv. Energy Mater., 2017, 7, 1601705 CrossRef
.
- Y. B. Nan, X. T. Wang, H. Xu, H. Zhou, Y. A. Sun, M. X. Wang, W. L. Liu, C. Q. Ma and T. Yu, Infomat, 2024, e12621 Search PubMed
.
- Y. J. Zou, M. Z. Sun, S. Li, X. Y. Zhang, L. Feng, Y. Wang, T. L. Du, Y. L. Ji, P. T. Sun and M. Y. Xu, Nano Energy, 2024, 122, 9620 CrossRef
.
- A. Kulandaivel, S. Potu, R. K. Rajaboina and U. K. Khanapuram, ACS Appl. Mater. Interfaces, 2024, 16, 58029–58059 CrossRef CAS PubMed
.
- S. M. Hu, Z. J. Shi, R. Z. Zheng, W. L. Ye, X. Gao, W. W. Zhao and G. Yang, ACS Appl. Mater. Interfaces, 2020, 12, 40021–40030 CrossRef CAS PubMed
.
- A. Kulandaivel, S. Potu, A. Babu, N. Madathil, M. Velpula, R. K. Rajaboina and U. K. Khanapuram, Nano Energy, 2024, 120, 21 CrossRef
.
- D. L. Vu, Q. T. Nguyen, P. S. Chung and K. K. Ahn, Polymers, 2024, 16, 536 CrossRef CAS PubMed
.
- W. Kim, D. Choi, J. Y. Kwon and D. Choi, J. Mater. Chem. A, 2018, 6, 14069–14076 RSC
.
- K. Munirathinam, A. Shanmugasundaram, Y.-J. Jeong, J.-Y. Kim and D.-W. Lee, Chem. Eng. J., 2024, 159106 Search PubMed
.
- D. Z. Zhang, L. N. Zhou, Y. Wu, C. Q. Yang and H. Zhang, Small, 2024, 20 CAS
.
- J. Y. Zhang, S. Q. Lin, M. L. Zheng and Z. L. Wang, Acs Nano, 2021, 15, 14830–14837 CrossRef CAS PubMed
.
- R. K. Rajaboina, U. K. Khanapuram and A. Kulandaivel, Adv. Sensor Res., 2024, 3, 18 Search PubMed
.
- L. E. Helseth, Langmuir, 2023, 1826 Search PubMed
.
- S. H. Wang, L. Lin and Z. L. Wang, Nano Energy, 2015, 11, 436–462 CrossRef CAS
.
- G. Khandelwal and R. Dahiya, Adv. Mater., 2022, 34, 2200724 CrossRef CAS PubMed
.
- Z. K. Zhou, H. F. Qin, P. Cui, J. J. Wang, J. J. Zhang, Y. Ge, H. M. Liu, C. Feng, Y. Meng, Z. Y. Huang, K. Yang, G. Cheng and Z. L. Du, ACS Appl. Mater. Interfaces, 2024, 16, 4763–4771 CrossRef CAS PubMed
.
- D. Choi, Y. Lee, Z.-H. Lin, S. Cho, M. Kim, C. K. Ao, S. Soh, C. Sohn, C. K. Jeong and J. W. Lee, ACS nano, 2023, 17, 11087–11219 Search PubMed
.
-
S.-W. Kim, J.-K. Kim, S. Jung, J. W. Lee, C. Yang and J. M. Baik, in Handbook of Triboelectric Nanogenerators, Springer, 2023, pp. 455–504 Search PubMed
.
- J. Zhao, D. Wang, F. Zhang, J. S. Pan, P. Claesson, R. Larsson and Y. J. Shi, Nano-Micro Lett., 2022, 14, 2313794 Search PubMed
.
- W. J. Li, Y. Y. Xiang, W. Zhang, K. Loos and Y. T. Pei, Nano Energy, 2023, 113, 9 Search PubMed
.
- I. Y. Suh, J. Jeon, M. J. Park, H. Ryu, Y. J. Park and S. W. Kim, ACS Appl. Electron. Mater., 2024, 6, 4826–4842 Search PubMed
.
- C. Liu, R. G. Guan, W. Z. Shen and Z. J. Li, Adsorpt. Sci. Technol., 2008, 26, 303–309 CrossRef CAS
.
- X. Y. Li, X. G. Wang, Q. Y. Chen, H. S. Wu, W. J. Huang, W. X. Zhang, S. H. Ren, R. Z. Ouyang, Y. Q. Jiang and C. F. Yao, Sep. Purif. Technol., 2025, 358, 13 Search PubMed
.
- B. Song, Z. T. Zeng, G. M. Zeng, J. L. Gong, R. Xiao, M. Chen, X. Tang, S. J. Ye and M. C. Shen, Chemosphere, 2021, 262, 8 CrossRef PubMed
.
- X. Y. Shi, W. Lesniak, M. T. Islam, M. C. Muñiz, L. P. Balogh and J. R. Baker, Colloids Surf., A, 2006, 272, 139–150 CrossRef CAS
.
- C. U. I. Hongchao, L. I. Decai and W. Cui, Funct. Mater., 2011, 42, 1403–1406 Search PubMed
.
- L. Wang, J. Yu, P. Wang, L. Yang, X. J. Yu, J. L. Qiu, K. P. Tan, Y. B. Tang and Y. J. Guo, Chem. Eng. J., 2025, 503, 12 Search PubMed
.
- Z. H. Zhao, L. L. Zhou, S. X. Li, D. Liu, Y. H. Li, Y. K. Gao, Y. B. Liu, Y. J. Dai, J. Wang and Z. L. Wang, Nat. Commun., 2021, 12, 8 CrossRef PubMed
.
- Y. H. Liu, Q. Fu, J. L. Mo, Y. X. Lu, C. C. Cai, B. Luo and S. X. Nie, Nano Energy, 2021, 89, 106369 CrossRef CAS
.
- P. Liu, S. Q. Liu, X. Q. Yu and Y. F. Zhang, Chem. Eng. J., 2021, 406, 126807 CrossRef
.
- Y. X. Ma, C. H. Wei, Z. X. Wang, T. M. Lv, Y. X. Tan, J. L. He, X. Peng and K. Dong, J. Mater. Chem. A, 2024, 12, 17702–17713 RSC
.
- K. Chakarova and K. Hadjiivanov, J. Phys. Chem. C, 2011, 115, 4806–4817 CrossRef CAS
.
- S. R. Valantina, S. Uma, B. G. J. Prakash, D. R. P. Angeline, A. A. Maxwell and R. Aravindhan, J. Food Sci. Technol., 2019, 56, 571–579 CrossRef PubMed
.
-
L. Yang, C. Qi, Y. Lu, J. Hao, R. Liao, C. Gong and F. Meng, Proceedings of the Chinese Society of Electrical Engineering, 2013, vol. 33, pp. 162–169 Search PubMed
.
- R. Su, W. Cao, Z. L. Jin, Y. F. Wang, L. T. Ding, M. Maqsood and D. Wang, Lubricants, 2024, 12, 18 Search PubMed
.
- S. T. Liu, J. C. Lv and C. B. Liu, Lubricants, 2024, 12, 11 CrossRef
.
- K. Indira, M. E. Vizhi, B. Kathirvel and A. Pugazhendhi, Prog. Org. Coat., 2021, 150, 6 CrossRef
.
- H. H. Singh and N. Khare, Adv. Mater. Interfaces, 2021, 8, 2100032 CrossRef CAS
.
- V. Mohan, V. K. Mariappan, P. Pazhamalai, K. Krishnamoorthy and S. J. Kim, Carbon, 2023, 205, 328–335 CrossRef CAS
.
- X. J. Li, L. Q. Zhang, Y. G. Feng, X. L. Zhang, D. A. Wang and F. Zhou, Adv. Funct. Mater., 2019, 29, 10 Search PubMed
.
- X. J. Zhang, X. Huang, J. Li, Z. P. Tang and J. B. Wang, Asia-Pac. J. Chem. Eng., 2024, 19, e3114 CrossRef CAS
.
- X. T. Zhang, C. H. Li, Z. M. Zhou, B. Liu, Y. B. Zhang, M. Yang, T. Gao, M. Z. Liu, N. Q. Zhang, Z. Said, S. Sharma and H. M. Ali, Chin. J. Mech. Eng., 2023, 36, 76 CrossRef CAS
.
- Y. Du, T. H. Wu, L. X. Wang and R. J. Gong, Mech. Ind., 2017, 18, 402 CrossRef
.
- R. Sanga, M. Sivaramakrishna, V. S. Srinivasan and G. P. Rao, IEEE INDICON: 15th IEEE India Council International Conference, Amrita Vishwa Vidyapeetham, Coimbatore, India, 2018 Search PubMed.
- B. Jakoby, M. Scherer, M. Buskies and H. Eisenschmid, IEEE Sens. J., 2003, 3, 562–568 CrossRef CAS
.
- K. Yang, L. Wang and J. Atkinson, Sens. Bio-Sens. Res., 2019, 23(84), 100273 CrossRef
.
- S. Xiao, H. Y. Wu, N. Li, X. Y. Tan, H. C. Deng, X. X. Zhang, J. Tang and Y. Li, Adv. Sci., 2023, 10, 11310 Search PubMed
.
- S. Q. Lin, X. Y. Chen and Z. L. Wang, Chem. Rev., 2022, 122, 5209–5232 CrossRef CAS PubMed
.
- Q. Zhang, F. Q. Wang, R. Y. Wang, J. L. Liu, Y. P. X. Ma, X. R. Qin and X. X. Zhong, Adv. Sci., 2023, 10, 2207566 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ta00241a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |