Open Access Article
Open Access ArticleLayered sodium vanadate (NaV8O20) nanobelts: a new high-performing pseudocapacitive material for sodium-ion storage applications†
Amol S.
Vedpathak
 ab,
Shrishreshtha A.
Sahu
c,
Tanuja N.
Shinde
b,
Shubham S.
Kalyane
b,
Sambhaji S.
Warule
d,
Ramchandra S.
Kalubarme
c,
Aditya Narayan
Singh
e,
Ravindra N.
Bulakhe
*f,
Ji Man
Kim
ab,
Shrishreshtha A.
Sahu
c,
Tanuja N.
Shinde
b,
Shubham S.
Kalyane
b,
Sambhaji S.
Warule
d,
Ramchandra S.
Kalubarme
c,
Aditya Narayan
Singh
e,
Ravindra N.
Bulakhe
*f,
Ji Man
Kim
 *f and
Shrikrishna D.
Sartale
*f and
Shrikrishna D.
Sartale
 *b
*b
aSymbiosis Centre for Nanoscience and Nanotechnology, Symbiosis International (Deemed University), Pune 412115, India
bThin Films and Nanomaterials Laboratory, Department of Physics, Savitribai Phule Pune University, Pune 411 007, India. E-mail: shrikrishna.sartale@unipune.ac.in
cCentre for Materials for Electronics Technology (C-MET), Panchavati, Off. Dr. Homi Bhabha Road, Pashan, Pune 411008, India
dDepartment of Physics, Nowrosjee Wadia College, Pune 411 001, India
eDepartment of Energy and Materials Engineering, Dongguk University-Seoul, Seoul, 04620, Republic of Korea
fDepartment of Chemistry, Sungkyunkwan University, Suwon, 440-746, Republic of Korea. E-mail: jimankim@skku.edu; bulakhe@skku.edu
First published on 18th March 2025
Abstract
Multifunctional layered nanostructures have attracted great attention for next-generation electrochemical supercapacitors and metal-ion batteries. Herein, we use a hydrothermal method to demonstrate the synthesis of 1D and layered sodium vanadate (NaV8O20) nanobelts architecture. These NaV8O20 nanobelts demonstrate outstanding electrochemical performance in supercapacitors (SCs) and sodium-ion batteries (SIBs). The possible formation mechanism of NaV8O20 nanobelts is briefly discussed. Benefiting from the 1D and layered nanostructure, pre-inserted cations, significantly enhanced electrochemical conductivity, and high electroactive surface area, the prepared NaV8O20 electrode material exhibited excellent charge storage capacity, favorable rate, and cyclic stability performance. The NaV8O20 nanobelts displayed outstanding electrochemical characteristics, including 676 F g−1 of specific capacitance, 45 W h kg−1 of energy density and 5224 W kg−1 of power density. Additionally, on testing in Na-ion batteries, the NaV8O20 nanobelts exhibit a discharge capacity of 110 mA h g−1 at 10 mA g−1 and retain ∼52% capacity after 100 cycles. Along with this, the galvanostatic intermittent titration technique (GITT) measurements reveal a high diffusion coefficient for Na+ ions, highlighting the efficient Na+ ions transportation within the NaV8O20 structure. To our knowledge, this is the first report on the use of NaV8O20 nanobelts for both SIBs and SCs, marking a significant contribution to the development of multifunctional materials for energy storage applications.
1. Introduction
The rapid advancement and proliferation of electrochemical energy storage (EES) technologies are reshaping modern life while laying the foundation for a sustainable energy future. The growing demand for portable/wearable electronics, electric vehicles, and stationary energy storage systems has sparked significant global interest in EES technologies, particularly supercapacitors (SCs) and metal-ion batteries (MIBs). These devices have emerged as critical components for addressing the energy challenges of modern technology due to their distinct capabilities in energy storage and power delivery.1,2 However, to address the growing requirements for energy storage systems, the performance of these EES devices must be significantly improved. The working electrode material is one of the most essential parameters for EES applications.3 Therefore, enhancing the charge storage capacity of electrode materials has become a primary focus in recent EES research. One-dimensional (1D) and layered nanostructures have garnered significant scientific and technical attention for EES systems, attributed to their high electroactive surface area, structural stability, short charge carrier transport pathways, and favorable intercalation/de-intercalation processes.4 Various 1D and layered nanostructures, including carbon nanostructures, conducting polymers, metal phosphides and metal chalcogenides, have been investigated as potential electrode materials (both cathode and anode) for SC and MIB applications.5–10 Among these, transition metal oxides (TMOs) stand out as highly favorable electrodes for EES due to their multifarious oxidation states, high theoretical specific capacitance, diverse 1D/2D nanostructured morphologies, high electroactive surface area, and cost-effective fabrication processes.11–13Transition metal vanadates, a class of bimetallic TMOs, have gathered great scientific attention as electrode materials for SCs and MIBs. Their appeal stems from their high surface area, low-dimensional and layered structures, excellent pseudocapacitive properties, enhanced electrical conductivity, and superior cyclic stability. These characteristics enable rapid and reversible surface redox reactions of electroactive species, making them highly suitable for advanced energy storage applications.14,15 Currently, different binary transition metal vanadates such as Co3V2O8, FeVO4, MnV2O8, Ni3V2O8, and Cu3V2O8 have proven their ability in SCs and MIBs applications owing to their wide working potential window, high specific capacitance, and energy storage capacity compared to their pristine metal oxide counterparts.16–20 For example, quasi-cuboidal CoV2O6 has been shown to exhibit a specific capacitance of 223 F g−1 at 1 A g−1, along with remarkable cyclic stability of 123% after 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, making it suitable for SC applications.21 Similarly, 3D flower-like Ni3V2O8@CNTs have proven effective in enhancing energy storage capacity due to their high specific capacitance of 1054 F g−1 at 1 A g−1 and retain high cyclic stability of 89% after 10
000 cycles, making it suitable for SC applications.21 Similarly, 3D flower-like Ni3V2O8@CNTs have proven effective in enhancing energy storage capacity due to their high specific capacitance of 1054 F g−1 at 1 A g−1 and retain high cyclic stability of 89% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.22 Pseudocapacitive layered Fe5V15O39(OH)9·9H2O nanosheets exhibit significant potential as a cathode material for lithium-ion batteries, achieving a high reversible capacity of 350 mA h g−1 at 100 mA g−1, indicating its capability for long-term cycling stability.23 Similarly, Zr–NH4V4O10 has been identified as a promising cathode material for sodium-ion batteries, achieving a high specific capacity of 342 mA h g−1 at 100 mA g−1, positioning it as an appealing option for large-scale energy storage systems.24 However, despite these advancements, several challenges persist, including the complex synthesis procedures, use of toxic and hazardous materials and high cost of precursor materials, which hinder the feasibility of these materials for widespread industrial applications.15 Due to its numerous significant benefits, the hydrothermal method is the most frequently utilized for synthesizing metal vanadates. This approach enables accurate regulation of the morphology and particle size of resultant nanostructures, which is essential for enhancing the electrochemical performance of energy storage devices. This process is environmentally friendly and scalable, capable of being conducted at relatively low temperatures and pressures, contributing to its energy efficiency and cost-effectiveness. Furthermore, the hydrothermal approach facilitates the creation of well-crystallised nanostructures that exhibit high purity, thereby improving their applicability in energy storage applications. Considering these aspects, low-cost and environment-friendly alkali metals (Li, Na, K) can be appropriate alternatives to toxic and expensive metals for developing metal vanadate materials. However, alkali metal-inserted vanadate materials have been less explored for electrochemical SC and MIB applications.
000 cycles.22 Pseudocapacitive layered Fe5V15O39(OH)9·9H2O nanosheets exhibit significant potential as a cathode material for lithium-ion batteries, achieving a high reversible capacity of 350 mA h g−1 at 100 mA g−1, indicating its capability for long-term cycling stability.23 Similarly, Zr–NH4V4O10 has been identified as a promising cathode material for sodium-ion batteries, achieving a high specific capacity of 342 mA h g−1 at 100 mA g−1, positioning it as an appealing option for large-scale energy storage systems.24 However, despite these advancements, several challenges persist, including the complex synthesis procedures, use of toxic and hazardous materials and high cost of precursor materials, which hinder the feasibility of these materials for widespread industrial applications.15 Due to its numerous significant benefits, the hydrothermal method is the most frequently utilized for synthesizing metal vanadates. This approach enables accurate regulation of the morphology and particle size of resultant nanostructures, which is essential for enhancing the electrochemical performance of energy storage devices. This process is environmentally friendly and scalable, capable of being conducted at relatively low temperatures and pressures, contributing to its energy efficiency and cost-effectiveness. Furthermore, the hydrothermal approach facilitates the creation of well-crystallised nanostructures that exhibit high purity, thereby improving their applicability in energy storage applications. Considering these aspects, low-cost and environment-friendly alkali metals (Li, Na, K) can be appropriate alternatives to toxic and expensive metals for developing metal vanadate materials. However, alkali metal-inserted vanadate materials have been less explored for electrochemical SC and MIB applications.
In this study, we address these above-mentioned challenges by developing a simple, cost-effective hydrothermal route to prepare the 1D and layered structural sodium vanadate (NaV8O20) nanobelts without any post-annealing treatment. Sodium vanadate is particularly gaining attention due to its pre-inserted sodium ions, which enhances electrochemical performance and its suitability as a low-cost charge storage material for SCs and sodium-ion battery (SIB) applications. To the best of our knowledge, no prior publication has been reported that explored the electrochemical SCs and sodium-ion battery (SIB) applications of the 1D and layered NaV8O20 structures. Owing to the unique 1D layered structure and nanobelts morphology features, the NaV8O20 electrode displayed outstanding electrochemical features with high specific capacitance (Csp) and cyclic stability. The NaV8O20 material was investigated using various electrochemical and physicochemical characterization techniques to evaluate its structural, morphological and electrochemical characteristics. We have also successfully demonstrated the practical applicability of NaV8O20 nanobelts in electrochemical applications, particularly in solid-state asymmetric SCs, highlighting their potential for advanced EES systems.
2. Experimental section
2.1 Synthesis of NaV8O20 nanobelts
The details of chemicals and reagents are provided in ESI, S1(a).† The chemicals and reagents used for all the experiments were of analytical grades. A hydrothermal method was used to synthesize NaV8O20 material (Fig. 1a). First, 0.75 g of V2O5 powder was dispersed in 40 ml of double distilled water and ultrasonicated for 90 min. Then, with continuous stirring, 1 M Na2SO4 (30 ml) aqueous solution was gradually added to the V2O5 suspension. After 30 min, the mixture was transferred into a 100 ml Teflon-lined stainless-steel autoclave and heated to 180 °C for 48 h. After hydrothermal treatment, the obtained greenish-colored product was washed several times with double distilled water. Finally, the obtained product was dried at 150 °C in a vacuum oven. | ||
| Fig. 1 Schematic representation of (a) hydrothermal synthesis of NaV8O20 nanobelts, (b) preparation of working electrode, (c) fabrication of ASC device and (d) fabrication of Na-ion battery. | ||
2.2 Preparation of working electrode, AC//NaV8O20 asymmetric SC and Na-ion battery
The schematic representation of the working electrode fabrication via the slurry casting method is illustrated in Fig. 1b. Initially, a homogeneous slurry was prepared by mixing NaV8O20 nanobelts (active material), polyvinylidene difluoride (PVDF) as a binder, and activated carbon (AC) as a conductive additive in a weight ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, using N-methyl-pyrrolidone (NMP) as the solvent. This slurry was then uniformly coated onto a Toray carbon paper substrate. The coated electrode was subsequently dried under vacuum at 150 °C for 24 hours. The final electrode had an active surface area of 1 × 1 cm2, with a mass loading of ∼1.2 mg.
1, using N-methyl-pyrrolidone (NMP) as the solvent. This slurry was then uniformly coated onto a Toray carbon paper substrate. The coated electrode was subsequently dried under vacuum at 150 °C for 24 hours. The final electrode had an active surface area of 1 × 1 cm2, with a mass loading of ∼1.2 mg.
Fig. 1c and d depicts the schematic for the device manufacture processes for SC and SIB coin cells of the NaV8O20 nanobelts material. As shown in Fig. 1c, the AC//NaV8O20 asymmetric SC (ASC) device was fabricated using NaV8O20 nanobelts as cathode (∼1.2 mg of mass loading) and AC as an anode (∼2 mg of mass loading) materials prepared onto a circular 16 mm carbon paper current collector. These electrodes were separated using a cellulose membrane. The PVA–NaClO4 gel electrolyte was prepared by mixing 2 g NaClO4 and 2 g PVA in 25 ml DDW and then heated at 80 °C for 3 h. Finally, the AC//NaV8O20 asymmetric SC device was fabricated with a CR2032 coin cell using the PVA–NaClO4 gel electrolyte.
The sodium-ion storage performance of NaV8O20 nanobelts as the active cathode material was investigated. The electrode was fabricated by combining NaV8O20, PVDF, and AC with a weight ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and thoroughly mixed in NMP solvent. The prepared homogeneous slurry was uniformly coated onto a current collector (aluminium foil) using the doctor blade method and subsequently dried under a vacuum oven at 100 °C for 12 hours. The dried film was then cut into 16 mm diameter discs with a mass loading of approximately 0.745 mg cm−2. Sodium metal was utilized as the counter and reference electrode, while a 19 mm diameter glass-fiber Whatman separator was employed. The 2032-type coin cells were assembled in an argon-filled glove box, where oxygen and moisture levels were controlled at 0.1 ppm. A 1 M solution of NaClO4 in ethylene carbonate (EC) and diethyl carbonate (DEC) (1
1 and thoroughly mixed in NMP solvent. The prepared homogeneous slurry was uniformly coated onto a current collector (aluminium foil) using the doctor blade method and subsequently dried under a vacuum oven at 100 °C for 12 hours. The dried film was then cut into 16 mm diameter discs with a mass loading of approximately 0.745 mg cm−2. Sodium metal was utilized as the counter and reference electrode, while a 19 mm diameter glass-fiber Whatman separator was employed. The 2032-type coin cells were assembled in an argon-filled glove box, where oxygen and moisture levels were controlled at 0.1 ppm. A 1 M solution of NaClO4 in ethylene carbonate (EC) and diethyl carbonate (DEC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v), with the addition of 5% fluoroethylene carbonate (FEC), was used as the electrolyte. A schematic representation of the Na+ ion battery assembly process is provided in Fig. 1d.
1 v/v), with the addition of 5% fluoroethylene carbonate (FEC), was used as the electrolyte. A schematic representation of the Na+ ion battery assembly process is provided in Fig. 1d.
2.3 Electrochemical measurements
All the electrochemical measurements of electrodes and fabricated devices, such as cyclic voltammetry (CV), galvanostatic charging–discharging (GCD), electrochemical impedance spectroscopy (EIS), rate capability, and cycling stability tests were conducted on the IVIUM-Vertex 1A electrochemical workstation (Ivium Technologies, Netherlands).2.4 Physicochemical characterization of 1D and layered nanostructured NaV8O20 nanobelts
The physicochemical characteristics of the synthesized material were investigated using various techniques, including X-ray diffraction (XRD), Brunauer–Emmett–Teller (BET) surface area analysis, field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), and X-ray photoelectron spectroscopy (XPS). Further details regarding these methods are provided in the ESI, S1(b).†3. Results and discussion
3.1 XRD studies
XRD characterization was carried out to identify the crystal structure and phase purity of the NaV8O20 material. Fig. 2a shows the comparative XRD pattern of bulk V2O5 and NaV8O20 powders. The diffraction pattern of bulk V2O5 powder displays sharp and intense diffraction peaks, which match well to α-V2O5 phase (JCPDS no. 77-2418) with an orthorhombic crystal structure. By comparing both the diffraction patterns, it is clear that, after hydrothermal treatment, the V2O5 phase is converted into the newly formed NaV8O20 phase. In the XRD pattern of NaV8O20 powder, all the observed peaks are consistent with the characteristic peaks of the NaV8O20 phase (JCPDS no. 45-1363) with a monoclinic crystal structure. Furthermore, no additional diffraction peaks related to V2O5 or any other sodium vanadate phases were observed, implying the high phase purity of the NaV8O20 phase. However, the NaV8O20 phase displays relatively low-intensity and broad diffraction peaks compared with bulk V2O5, corresponding to the small crystallite size and low crystallinity of the NaV8O20 nanobelts material. The intense diffraction from the (001) plane illustrates the preferential orientation of the NaV8O20 phase in the c direction, which possesses the layered crystal structure arrangement.25 The interlayer spacing (d-value) estimated from the (001) diffraction peak was ∼10 Å. This large interlayer spacing is favorable for accommodating Na+ ions during electrochemical processes. Rietveld refinement of the NaV8O20 phase was conducted to identify the position of the sodium ions in the NaV8O20 phase. Fig. 2b depicts the Rietveld refinement analysis of the NaV8O20 nanobelts, performed using the MAUD software. The XRD pattern of the NaV8O20 sample was fitted using the standard crystallography information file (CIF) consisting of (Na,Ca)(V,Fe)8O20·nH2O and JCPDS no. 45-1363.26 By replacing the Ca and Fe atomic sites with corresponding amounts of Na and V, the refined analysis resulted in good agreement factors (Rwp = 3.03, Rexp = 0.62 and goodness of fit, GoF = 4.89). The parameters that are refined are (1) microstructure, (2) basic phase parameters, (3) background and scale parameters, and (4) crystal structure (lattice parameter, occupancy, fractional coordinate, and isotropic displacement factor, Biso). The refined lattice parameters are: a = 11.74(2) Å, b = 3.6600(6) Å, and c = 11.117(5) Å with monoclinic phase, C2/m space group and β angle value = 102.4(1)°. The refinement data are mentioned in the ESI, Table S1.† | ||
| Fig. 2 (a) XRD patterns of V2O5 and NaV8O20 powder samples and (b) XRD refinement analysis of NaV8O20 powder sample. | ||
3.2 FE-SEM, BET and TEM studies
The morphological properties of NaV8O20 material were elucidated by FESEM techniques (Fig. 3a and b). The low-resolution FESEM image (Fig. 3a) shows a network of uniformly distributed nanobelts adjoining each other to establish the self-assembled architecture of NaV8O20 nanobelts. This 1D and densely attached nanobelt morphology will be favorable for electron transportation and will help to enhancing the diffusion of Na+ ions by serving maximum electroactive sites during the electrochemical interactions.27,28Fig. 3b represents the high-resolution FESEM image of the NaV8O20 nanobelts. The width of NaV8O20 nanobelts was measured using ImageJ software and the Gaussian function was used to calculate the average width of nanobelts. The calculated average width (Fig. 3c) of the NaV8O20 nanobelt was ∼49 nm. Additionally, to identify the surface area and pore size of the NaV8O20 nanobelts sample, N2 adsorption/desorption isotherm analysis was used. Fig. 3d shows the mesopores nature of NaV8O20 nanobelts with a specific surface area of 12.41 m2 g−1 and pore size of ∼43.3 nm (inset of Fig. 3d). Furthermore, crystallinity, morphological and elemental analysis of the NaV8O20 nanobelts sample was investigated through TEM analysis. The low and high-magnification TEM images (Fig. 3e and f) display the nanobelts-like morphology of the NaV8O20 material. The corresponding HRTEM images (Fig. 3g–i) depict the well-observable lattice fringes. The measured d-spacings of 0.22, 0.45, and 0.36 nm correspond to the (204), (201), and (003) lattice planes of the NaV8O20 nanobelts. The selected area electron diffraction (SAED) pattern (Fig. 3k) further confirms the crystallinity of the NaV8O20 nanobelts, revealing their polycrystalline nature. Furthermore, the elemental mapping images (Fig. 3l–o) show a uniform spreading of Na, V, and O elements all over the NaV8O20 nanobelts surface.
The X-ray photoelectron spectroscopy analysis of the NaV8O20 nanobelts sample is provided in the ESI, S2.†
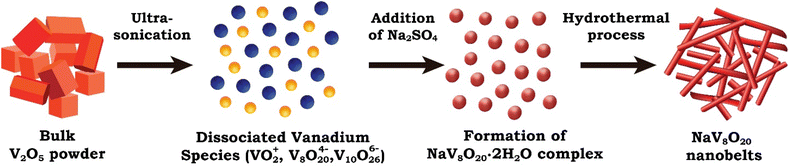
3.3 Formation mechanism of NaV8O20 nanobelts
The possible formation mechanism of the NaV8O20 nanobelts (Fig. 4) can be proposed based on their 1D nanobelt morphology and layered crystal structure, as confirmed by structural and morphological characterizations.A hydrothermal synthesis route was used to prepare 1D and layered NaV8O20 nanobelts. In the typical synthesis procedure, bulk V2O5 powder (vanadium precursor) was pre-treated with ultrasonication in double distilled water to break bulk vanadium particles into smaller particles. This ultrasonication process promotes the dissociation and formation of free vanadium ionic species such as decavanadate ([V10O28]6−), polyoxovanadate (V8O204−), vanadyl ions (VO2+), hydrated vanadium species etc., because of partial reduction and condensation of vanadium.29,30
Here, the presence of water plays a vital role in the hydrolysis of V2O5. When Na2SO4 is added to the ionic solution of vanadium, the Na+ ions interact with V8O204− ions, forming hydrated sodium vanadate complexes. Here, the free sulfate ions (SO42−) do not participate directly in the formation of the final product and water molecules (crystal water) help to stabilize these complexes by maintaining charge balance, leading to the formation of the NaV8O20·2H2O (complex), where water integrates sodium ions into the polyvanadate structure.
During the hydrothermal treatment, the system is subjected to high temperature and pressure, promoting dehydration and crystallization. The removal of crystal water during this step drives the organization of sodium vanadate species into well-defined and layered nanobelt structures. The dehydration process facilitates the reorganization of the vanadate layers, promoting anisotropic and preferentially growth NaV8O20 nanobelts along the c direction, confirmed by XRD with an intense peak at 8.8° with Miller indices (001).
Afterward, the FESEM image reveals that the NaV8O20 nanobelts adopt a self-assembled morphology. This may be due to the removal of crystal water during crystallization, facilitating the formation of smooth, well-defined nanobelts. The self-assembly process is further driven by the depreciation of structural energy during crystallization, with sodium ions playing a role in charge stabilization.31
4. Electrochemical investigation
4.1 Electrochemical performance of NaV8O20 nanobelts electrode
The electrochemical charge storage features of the NaV8O20 electrode material for supercapacitor studies were evaluated in 1 M Na2SO4 electrolyte within a 0 to 0.6 V voltage window. The CV curves of the NaV8O20 electrode were recorded for various scan rates ranging from 2.5 to 50 mV s−1, as shown in Fig. 5a. In Fig. 5a, the CV curves of the NaV8O20 electrode exhibit a typical extrinsic pseudocapacitive nature consisting of a nearly rectangular shape with broad oxidation and reduction peaks. This extrinsic pseudocapacitive nature mainly arises from surface faradaic charge transfer reactions due to the intercalation or diffusion of charge-compensating electrolyte ions.32 However, this nature is well retained despite decreasing the scan rate up to 2.5 mV s−1, implying outstanding rate performance of the NaV8O20 electrode in Na2SO4 aqueous solutions. From the CV curves, the Csp values for different scan rates of the NaV8O20 electrode were calculated using eqn (E1) (ESI, S1(b)†) and are given in the ESI, Table S2.†The CV curves of the NaV8O20 electrode reveal a hybrid charge storage behaviour, combining pseudocapacitive and electric double-layer (EDLC) characteristics. The Trasatti method is employed to understand the charge storage mechanism of the NaV8O20 nanobelts. This method helps distinguish between the contributions of surface-capacitive and diffusion-controlled processes. According to Trasatti, the overall capacitance arises from a blend of these two mechanisms: surface capacitive reactions and diffusion-driven charge storage.33,34Fig. 5b depicts the Trasatti plot, plotted using Csp against the square root of the scan rate. The y-axis intercept of the linear fit represents the surface-controlled contribution. Based on this intercept, the proportion of surface capacitive and diffusion-controlled charge storage components was calculated across various scan rates. These results are displayed in Fig. 5c as a histogram. This histogram shows that the surface capacitive (EDLC) contribution has the dominant charge storage mechanism over the diffusion-controlled contribution. By increasing the scan rate from 2.5 to 50 mV s−1, the surface capacitive contributions improved from 56 to 85% with no noticeable CV curve distortion detected, revealing a better rate performance of the NaV8O20 electrode. Furthermore, at lower scan rates (2.5 to 20 mV s−1), broad redox peaks and quasi-rectangular CV curves were observed (ESI, Fig. S3(d)†). This is due to surface faradaic reactions, clarifying the extrinsic pseudocapacitive nature. During the cathodic CV scans (ESI, Fig. S3(d)†), two reduction peaks at ∼0.2 and ∼0.35 V vs. SCE arose because of the reduction of vanadium ionic species from V5+ → V4+ and V4+ → V3+. Whereas, in the anodic CV scans, two oxidation peaks at ∼0.1 and ∼0.24 V vs. SCE were detected due to the oxidation of vanadium ionic species from V3+ → V4+ and V4+ → V5+. Interestingly, by increasing the scan rates, the cathodic peaks gradually shifted towards the higher potentials, indicating that rapid and scan rate-dependent redox reactions occurred near the NaV8O20 electrode surface.35Fig. 5d shows the GCD curves of the NaV8O20 electrode, performed 0.5 to 32 A g−1 of current densities. All the GCD curves show an ideal triangular and symmetric nature with a slight hump at ∼0.4 V vs. SCE, which indicates fast faradaic reactions and high reversibility of Na+ ions on the NaV8O20 electrode surface. Additionally, the GCD curves show extended charging–discharging characteristics with a negligible iR drop, signifying the outstanding rate capability. The Csp values were calculated using eqn (E2) (see ESI†) and summarized in the ESI, Table S3.† The NaV8O20 electrode displays an optimal Csp of 676 F g−1 at a current density of 0.5 A g−1; afterwards, it decreases with an increase in current density (as shown in Fig. 5e). At a 32 A g−1 current density, the NaV8O20 electrode still provides the admirable Csp of 142 F g−1. We believe these excellent Csp values mainly arise from the pre-insertion of Na+ into the V8O20 structure, which offers a high electroactive surface area, enhances the overall electronic conductivity and simplifies the Na+ ions insertion/de-insertion process. The electrode material's low electric and electrochemical resistances are typically preferred for the supercapacitor's longer cyclic lifespan and versatile applicability. The electrochemical resistance characteristics of the NaV8O20 electrode material were evaluated through EIS measurements. The Nyquist plot for the NaV8O20 electrode is presented in Fig. 5f. In the high-frequency region of the Nyquist plot, two key components are observed: the equivalent series resistance (Rs) and the charge transfer resistance (Rct). Rs represents the overall internal (ohmic) resistance, which encompasses the active electrode material's intrinsic resistance, the electrolyte's ionic resistance, and the contact resistance at the electrode/electrolyte interface.36–38 Meanwhile, the diameter of the semicircle represents the charge transfer resistance, which is associated with faradaic reactions. The simulated circuit provided that the NaV8O20 electrode possesses Rs = 4.9 Ω and Rct = 0.55 Ω. These low resistance values are attributed to the high electronic conductivity, large electroactive surface area, enhanced electron transport rate, and reduced charge transfer resistance of the NaV8O20 electrode, which collectively enable rapid faradaic reactions at the electrode/electrolyte interface. In the Nyquist plot, the Warburg impedance (W) is associated with the vertical line in the low-frequency region, corresponding to the intercalation or diffusion of electrolyte ions inside the active electrode material. The slope of the Warburg impedance approaches a 90° angle, signifying the capacitive behaviour of the NaV8O20 electrode.
Long cyclic life is one of the significant parameters for electrochemical supercapacitor applications. The electrochemical cycling stability over the NaV8O20 electrode (Fig. 5g) was performed at a scan rate of 50 mV s−1 over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. In the initial ∼1000 cycles, the Csp gradually increases and then tends to stabilize for further CV cycling. This is because of the activation of the NaV8O20 electrode through the insertion/de-insertion of Na+ ions and complete infiltration of Na+ electrolyte ions inside the active electrode material surface.21 The NaV8O20 electrode retains 90% of its initial Csp after 10
000 cycles. In the initial ∼1000 cycles, the Csp gradually increases and then tends to stabilize for further CV cycling. This is because of the activation of the NaV8O20 electrode through the insertion/de-insertion of Na+ ions and complete infiltration of Na+ electrolyte ions inside the active electrode material surface.21 The NaV8O20 electrode retains 90% of its initial Csp after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 CV cycles. This outstanding cycling stability is mainly ascribed to the layered and 1D nanobelts structured assembly of the NaV8O20 electrode, which offers significant redox active sites and efficiently accommodates the possible volume deviations through Na+ ions insertion/de-insertion process, which ultimately enhances the charge storage capacity of the NaV8O20 electrode material.
000 CV cycles. This outstanding cycling stability is mainly ascribed to the layered and 1D nanobelts structured assembly of the NaV8O20 electrode, which offers significant redox active sites and efficiently accommodates the possible volume deviations through Na+ ions insertion/de-insertion process, which ultimately enhances the charge storage capacity of the NaV8O20 electrode material.
4.2 Electrochemical performance of the AC//NaV8O20 asymmetric supercapacitor (ASC) device
To evaluate the practical applications of the NaV8O20 nanobelts for supercapacitors, the AC//NaV8O20 ASC coin cell device (Fig. 1d) was fabricated. Before the fabrication of the SC device, the NaV8O20 (cathode) and AC (anode) were electrochemically evaluated in a three-electrode system to assess the appropriate working voltage window.Fig. 6a shows the CV profile for the AC and NaV8O20 electrodes carried out in a three-electrode system at a scan rate of 50 mV s−1 in 1 M Na2SO4 electrolyte. The CV curves of NaV8O20 and AC electrodes are nearly rectangular, measured between the voltage windows of 0 to 0.6 V vs. SCE and −1 to 0 V vs. SCE, respectively. These results imply that integrating the cathode and anode can provide a 1.6 V working voltage. After the fabrication of the ASC device, first, CV measurements with different voltages from 1 to 1.6 V (Fig. 6b) were performed, which indicates the actual working voltage can be extended up to 1.6 V without showing any alteration in CV curves due to aqueous electrolyte redox processes. Fig. 6c displays the CV curves of the AC//NaV8O20 ASC device, measured at 10 to 100 mV s−1 scan rates. These CV curves retain the quasi-rectangular shape observed for NaV8O20 nanobelts electrodes in a three-electrode setup, confirming the pseudocapacitive behaviour of NaV8O20 nanobelt material. Fig. 6d displays the GCD curves of the AC//NaV8O20 ASC device measured at 0.5 to 16 A g−1 of current densities, demonstrating good symmetric and triangular GCD curves because of the high reversible charge/discharge processes. The Csp of the AC//NaV8O20 ASC device (ESI, Table S3†) was calculated using eqn (E2) (ESI†). Fig. 6e shows the change in Csp with current densities. The highest Csp of 126 F g−1 was achieved at 0.5 A g−1 of current density. Energy (Ed) and power densities (Pd) of the AC//NaV8O20 ASC device were calculated using the following equations:39
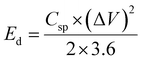 | (1) |
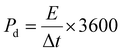 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles and presented in Fig. 6h. The AC//NaV8O20 ASC device displayed 89% of its initial capacitance value with ∼98% coulombic efficiency. This good cyclic stability can be accredited to (1) the proper combination of faradaic and pseudocapacitive electroactive material, (2) the appropriate interlayer spacing of the electrode materials, and (3) the large surface area of both electrode materials.
000 cycles and presented in Fig. 6h. The AC//NaV8O20 ASC device displayed 89% of its initial capacitance value with ∼98% coulombic efficiency. This good cyclic stability can be accredited to (1) the proper combination of faradaic and pseudocapacitive electroactive material, (2) the appropriate interlayer spacing of the electrode materials, and (3) the large surface area of both electrode materials.
| Vanadate material | Method | Morphology | Three-electrode system | Supercapacitor device | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Electrolyte | C sp (F g−1 @ A g−1) | Electrode stability (% @ cycles) | Voltage window (V) | E d (W h kg−1) | P d (W kg−1) | Device stability (% @ cycles) | ||||
| LiVO3 | Chemical route | Nanorods | 1 M Li2SO4 | 426 @ 0.5 | 80 @ 2000 | 1.6 | 25 | 3600 | 80 @ 5000 | 40 |
| Li3VO4/carbon | Solid-state | Nanofibers | 0.1 mol per L (NH4)2SO4 | — | — | 3.8 | 110 | 3870 | 86 @ 2400 | 41 |
| β-Na0.33V2O5 | Hydrothermal | Nanobelts | 1 M LiCO4/PC | 320 @ 5 mV s−1 | — | 1 | 47 | 5000 | 66 @ 4000 | 42 |
| β-Na0.33V2O5 | Spin coating | Nanowires | 1 M LiCO4/PC | 498 @ 0.4 | 90 @ 1500 | — | — | — | — | 43 |
| NaV3O8 | Electrochemical conversion | Nanosheets | 1 M Na2SO4 | 640.10 @ 0.5 | 87.7 @ 2000 | 1.6 | 62.6 | 7200 | 81 @ 5000 | 44 |
| Na2V6O16 | Chemical route | Nanobelts | 1 M Na2SO4 | 455 @ 0.5 | 90 @ 5000 | 1.6 | 42 | 4300 | 80 @ 5000 | 27 |
| Na6V10O28 | Chemical route | Microrods | 1 M LiClO4 + PC | 354 @ 0.1 | — | 2.8 | 73 | 6238 | 70 @ 5000 | 45 |
| KV3O8 | Chemical route | Nanobelts | 0.5 M K2SO4 | 677 @ 0.5 | 89 @ 5000 | 1.8 | 51 | 4127 | 90 @ 5000 | 46 |
| Cu3V2O8 | SILAR | Nanopebbles | 1.5 M NaClO4 | 443 @ 5 mV s−1 | 85 @ 2000 | 1.15 | 8.4 | 345 | 76 @ 4000 | 47 |
| S–Co3V2O8 | Hydrothermal | Nanosheets | 6 M KOH | 410 mA h g−1 at 2A g−1 | 94 @ 4000 | 1.5 | 36 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
98 @ 4000 | 48 |
| Mn2V2O7 | Hydrothermal | Nanoparticles | 3 M KOH | 327 C g−1 at 1 A g−1 | 93 @ 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.5 | 44.3 | 807.4 | 158 @ 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
49 |
| Ni3−xCoxV2O8 | Hydrothermal | Oval-shaped | 3 M KOH | 391 @ 0.5 | 98 @ 8000 | 1.3 | 25.5 | 7500 | 85 @ 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
50 |
| Ni3V2O8/Ni | Hydrothermal | Nanosheets | 3 M KOH | 1300 @ 1 | 80 @ 7000 | 1.2 | 33.2 | 2400 | 73 @ 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
51 |
| NiCo2V2O8 | Microwave-assisted | Microspheres | 2 M KOH | 113.2 mA h g−1 at 0.5 A g−1 | 82 @ 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.6 | 34.8 | 4000 | 80 @ 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
52 |
| NaV 8 O 20 | Hydrothermal | Nanobelts | 1 M Na 2 SO 4 | 676 @ 0.5 |
90 @ 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 000 000
|
1.6 | 45 | 5224 |
89 @ 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 000 000
|
This work |
A detailed comparative analysis of the NaV8O20 nanobelts developed in this study with the various metal vanadate electrodes for SC devices previously reported in the literature is presented in Table 1.
The comparative results suggest that, compared with reported metal vanadate electrode materials, the NaV8O20 nanobelts consistently demonstrate better electrochemical performance. The NaV8O20 nanobelts exhibit significantly higher capacitance with an operating window of 1.6 V, surpassing many vanadium-based alternatives. They offer excellent cyclic stability, retaining their electrochemical performance over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, making them ideal for high-performance applications. Here, the key contributor to this exceptional electrochemical performance is the use of the Na2SO4 electrolyte, which provides numerous advantages, such as high ionic conductivity, high ionic mobility, and less corrosive compared to KOH. It also ensures compatibility with the Na+ pre-insertion in the electrode material, allowing for efficient ion transfer and enhanced electrochemical reactions. Moreover, the excellent electrochemical performance of the NaV8O20 nanobelts can be primarily ascribed to the pre-insertion of Na+ ions during synthesis. This pre-insertion enhances the redox active sites, provides an efficient diffusion path for Na+ ions, and ensures optimal interlayer spacing, facilitating effective Na+ ion intercalation. Additionally, the high electrical conductivity of the NaV8O20 nanobelts further improves their overall electrochemical behaviour, leading to enhanced charge storage and cyclic stability. These features collectively position NaV8O20 nanobelts as an auspicious material for next-generation energy storage technologies.
000 cycles, making them ideal for high-performance applications. Here, the key contributor to this exceptional electrochemical performance is the use of the Na2SO4 electrolyte, which provides numerous advantages, such as high ionic conductivity, high ionic mobility, and less corrosive compared to KOH. It also ensures compatibility with the Na+ pre-insertion in the electrode material, allowing for efficient ion transfer and enhanced electrochemical reactions. Moreover, the excellent electrochemical performance of the NaV8O20 nanobelts can be primarily ascribed to the pre-insertion of Na+ ions during synthesis. This pre-insertion enhances the redox active sites, provides an efficient diffusion path for Na+ ions, and ensures optimal interlayer spacing, facilitating effective Na+ ion intercalation. Additionally, the high electrical conductivity of the NaV8O20 nanobelts further improves their overall electrochemical behaviour, leading to enhanced charge storage and cyclic stability. These features collectively position NaV8O20 nanobelts as an auspicious material for next-generation energy storage technologies.
4.3 Practical application of the AC//NaV8O20 ASC coin cell device
Fig. 7 displays the AC//NaV8O20 ASC coin cell device for its practical application by glowing the 28-red LED-contained panel. Each red LED bulb requires 1.2 V to shine; therefore, two AC//NaV8O20 coin cell devices were connected in series for lighting. Then, fabricated ASC coin cell devices were first charged up to 1.6 V and discharged through an LED panel. These two devices can easily illuminate LED panels up to ∼120 s (ESI Media File†), demonstrating the successful practical applicability of NaV8O20 nanobelts material for SC application.4.4 Electrochemical performance of NaV8O20 nanobelts for Na+ ion battery
To identify the electrochemical features of the NaV8O20 nanobelts for the Na-ion battery, half-cell devices were assembled using Na metal foil as the reference/counter electrode. Fig. 8 shows the electrochemical measurements of a fabricated Na-ion battery performed in the voltage range of 1.5–4.0 V vs. Na/Na+ to investigate the electrochemical redox reaction kinetics of the NaV8O20 nanobelts. Fig. 8a shows the initial three CV curves recorded at a scan rate of 0.1 mV s−1vs. Na/Na+. These CV curves show a significant degree of overlap, particularly during the initial three cycles, indicating excellent reversibility of Na+ ion insertion/extraction between the electrodes. The CV profiles exhibit two distinct intercalation peaks at 1.997 V and 3.631 V, along with two corresponding de-intercalation peaks at 3.528 V and 1.75 V. These peaks are associated with the reversible intercalation and de-intercalation of Na+ ions within the NaV8O20 structure, contributing to the material's high-capacity retention. Along with this, some minor oxidation peaks (3.075 and 3.204 V) and reduction (2.783 and 2.991 V) peaks were observed. These peaks may arise due to the multistep process of Na+ ions insertion and de-insertion.53,54The CV measurements for 0.1 to 1 mV s−1 scan rates were also performed (ESI, Fig. S3(e)†) to examine the rate performance and reaction kinetics. Fig. S3(e)† indicates that the peak positions slightly shift towards higher potentials, specifying that the Na+ ions' insertion and de-insertion kinetics are closely related at different scan rates. To evaluate the Na+ ions diffusion characteristics for the NaV8O20 electrode material, the diffusion coefficient (D) is determined by the Randles–Sevcik equation:55
 | (3) |
To calculate D from the CV measurements, the above formula can be modified as follows:
 | (4) |
 is the slope of the plot of peak current vs. the square root of the scan rate (Fig. 8b). Using this formula, the calculated Na+ ions diffusion coefficient value of the NaV8O20 cathode material was found to be 4.10 × 10−7 cm2 s−1, reflecting faster Na+ ion diffusion inside NaV8O20 cathode material. This excellent diffusion coefficient value results from (1) 1D nanobelts morphology and high surface area of NaV8O20 nanobelts material, which provides a long pathway for easier ion migration, and (2) high electrical conductivity, which is helpful for an efficient charge transfer process. Fig. 8c displays the GCD plot of the NaV8O20 material at a 10 mA g−1 of current densities for the initial three cycles. Here, the plateaus are well corroborated with the CV curves and possess a maximum reversible discharge capacity of 110 mA h g−1. These three GCD curves slightly overlapped, which showed excellent ionic diffusion features and structural stability, complementing the electronic conductivity of the NaV8O20 cathode material. The galvanostatic intermittent titration technique (GITT) was performed to analyze the diffusion kinetics of Na+ ions. During the measurement, closed-circuit voltage (CCV) was obtained by applying a current pulse of 100 mA g−1 for 300 s, followed by a relaxation period of 2 h to measure the quasi-open circuit voltage (QOCV). The diffusion coefficient of sodium ions (DNa+) of NaV8O20 was calculated from the obtained GITT data according to the following formula:
is the slope of the plot of peak current vs. the square root of the scan rate (Fig. 8b). Using this formula, the calculated Na+ ions diffusion coefficient value of the NaV8O20 cathode material was found to be 4.10 × 10−7 cm2 s−1, reflecting faster Na+ ion diffusion inside NaV8O20 cathode material. This excellent diffusion coefficient value results from (1) 1D nanobelts morphology and high surface area of NaV8O20 nanobelts material, which provides a long pathway for easier ion migration, and (2) high electrical conductivity, which is helpful for an efficient charge transfer process. Fig. 8c displays the GCD plot of the NaV8O20 material at a 10 mA g−1 of current densities for the initial three cycles. Here, the plateaus are well corroborated with the CV curves and possess a maximum reversible discharge capacity of 110 mA h g−1. These three GCD curves slightly overlapped, which showed excellent ionic diffusion features and structural stability, complementing the electronic conductivity of the NaV8O20 cathode material. The galvanostatic intermittent titration technique (GITT) was performed to analyze the diffusion kinetics of Na+ ions. During the measurement, closed-circuit voltage (CCV) was obtained by applying a current pulse of 100 mA g−1 for 300 s, followed by a relaxation period of 2 h to measure the quasi-open circuit voltage (QOCV). The diffusion coefficient of sodium ions (DNa+) of NaV8O20 was calculated from the obtained GITT data according to the following formula: | (5) |
| Electrode material | Current density | GITT-pulse duration/relaxation time | Diffusion coefficient (DNa+) | Ref. |
|---|---|---|---|---|
| NaVPO4F | 14.3 mA g−1 | 600 s/2 h | 10−11 to 10−10 cm2 s−1 | 57 |
| Na3MnTi(PO4)3 | 11.7 mA g−1 | 10 min/30 min | 10−10 to 10−15 m2 s−1 | 58 |
| Fe-HCF (iron-based hexacyanoferrate) | — | 600 s/2 h | ∼10−11 cm2 s−1 | 59 |
| NaFePO4 | 3.85 mA g−1 | 1 h/15 h | ∼10−17 cm2 s−1 | 60 |
| Na0.66Ca0.05[Ni0.25Li0.11Mn0.64]O1.95F0.05 | 13 mA g−1 | 30 min/5 h | 10−11 to 10−12 cm2 s−1 | 61 |
| Na8Fe5(SO4)9@rGO | — | — | 10−11 to 10−12 cm2 s−1 | 62 |
| NaV 8 O 20 | 100 mA g− 1 | 300 s/2 h | ∼10− 9 cm 2 s− 1 | This work |
Fig. 8e displays the GCD behavior of second cycles for different rates from 10 mA g−1 to 1 A g−1. The NaV8O20 cathode provides a maximum discharge capacity of 105, 87, 70, 58, 48, 36, and 28 mA h g−1 for the current densities of 10, 20, 50, 100, 200, 500, and 1000 mA g−1. Additionally, the rate capability of the NaV8O20 cathode is shown in Fig. 8f; three cycles with each rate were tested, and the nature shows the electrochemical stability at a particular rate, but the capacity decreases with an increase in the rate. As the current density reverts to 10 mA g−1, the NaV8O20 cathode recovers ∼83.2% (84 mA h g−1) of its initial capacity. In addition, the cyclic stability measurement was performed over the fabricated Na+ ion battery. Fig. 8g displays the cyclic stability of the Na+ ion battery performed for 100 cycles at a current density of 100 mA g−1. The Na+ ion battery device displays ∼100% coulombic efficiency while retaining ∼52.4% of the initial capacity. The observed capacity fading can be attributed to the forming of a passivation layer on the electrode surface and a reduction in the number of electroactive sites. Additionally, EIS was employed to analyze the reaction kinetics of the NaV8O20 cathode, both before and after cycling, as shown in Fig. 8h. By comparing both the EIS spectra, it can be found that the Rct (quasi-semicircle) for the diffusion of sodium ions around ∼650 Ω, which after cycling gradually decreased to ∼562 Ω and shows an increase in ion and electron transport mobility as cycling is undertaken. This may be due to the decrease in the charge transfer characteristic in terms of lack of the electrolyte ions at the surface of the electrodes, activation, or reduction in active surface area during a prolonged cycling test.
5. Conclusions
In summary, we have successfully synthesized one-dimensional (1D) and layered sodium vanadate (NaV8O20) nanobelts using a simple, cost-effective hydrothermal method and demonstrated their dual application as high-performance electrode materials for SC and SIB devices. This study represents a significant advancement as it is the first to explore the electrochemical potential of NaV8O20 nanobelts in both SCs and SIBs. Compared to previously reported metal vanadate-based electrode materials, the NaV8O20 nanobelts exhibit remarkable electrochemical performance owing to their unique 1D and layered structure. This architecture significantly improves the electrochemical properties, leading to excellent cycling stability and superior rate performance for both SCs and SIBs. For SCs, the NaV8O20 nanobelts exhibit an impressive Csp of 676 F g−1 at 0.5 A g−1 and remarkable cyclic stability, retaining ∼90% of the capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. Moreover, the ASC coin cell device (AC//NaV8O20) delivers an optimal Ed of 45 W h kg−1 and Pd of 5224.48 W kg−1, with 89% cyclic stability after 10
000 cycles. Moreover, the ASC coin cell device (AC//NaV8O20) delivers an optimal Ed of 45 W h kg−1 and Pd of 5224.48 W kg−1, with 89% cyclic stability after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, demonstrating its suitability for high-performance energy storage systems. In addition to SCs, the NaV8O20 nanobelts were employed as a cathode material for SIBs, showing a high initial charge/discharge capacity of 110 mA h g−1 and a retention of 52.4% capacity after 100 cycles at 100 mA g−1. The GITT measurements reveal a high diffusion coefficient for Na+ ions, indicating that the NaV8O20 nanobelts enable efficient ion transport during cycling, which is essential for high-rate performance and long-term stability. These results highlight the potential of NaV8O20 nanobelts for dual electrochemical applications, with a simple synthesis process which is both practical and sustainable.
000 cycles, demonstrating its suitability for high-performance energy storage systems. In addition to SCs, the NaV8O20 nanobelts were employed as a cathode material for SIBs, showing a high initial charge/discharge capacity of 110 mA h g−1 and a retention of 52.4% capacity after 100 cycles at 100 mA g−1. The GITT measurements reveal a high diffusion coefficient for Na+ ions, indicating that the NaV8O20 nanobelts enable efficient ion transport during cycling, which is essential for high-rate performance and long-term stability. These results highlight the potential of NaV8O20 nanobelts for dual electrochemical applications, with a simple synthesis process which is both practical and sustainable.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors express their gratitude to the Alexander von Humboldt Foundation for donating the potentiostat. ASV is thankful to Symbiosis International (Deemed University), Pune for the financial support provided through the Major Research Project (MJRP) grant (2024/4900). SSW acknowledges support from the SERB-TARE project (TAR/2020/000257) funded by the Department of Science and Technology (DST), India. SAS extends appreciation to the DST, Government of India, for the PhD fellowship provided under the DST-INSPIRE scheme. Also, this research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. RS-2019-NR040081).References
- R. Rajagopalan and L. Zhang, Advanced Materials for Sodium Ion Storage, CRC Press, 2019 Search PubMed.
- M. Wu, X. Hu, W. Zheng, L. Chen and Q. Zhang, Chem. Eng. J., 2023, 466, 143077 CrossRef CAS.
- S. Kumar, G. Saeed, L. Zhu, K. N. Hui, N. H. Kim and J. H. Lee, Chem. Eng. J., 2021, 403, 126352 CrossRef CAS.
- H. Sun, Y. Zhang, J. Zhang, X. Sun and H. Peng, Nat. Rev. Mater., 2017, 2, 17023 CrossRef CAS.
- L. Yu, X. He, B. Peng, F. Wang, N. Ahmad, Y. Shen, X. Ma, Z. Tao, J. Liang and Z. Jiang, Adv. Funct. Mater., 2024, 34, 2406771 CrossRef CAS.
- Q. Li, D. Yang, H. Chen, X. Lv, Y. Jiang, Y. Feng, X. Rui and Y. Yu, SusMat, 2021, 1, 359–392 CrossRef CAS.
- G. Wan, Y. Chen, B. Peng, L. Yu, X. Ma, N. Ahmad and G. Zhang, Battery Energy, 2023, 2, 20230022 CrossRef CAS.
- Y. F. Zhu, Y. Xiao, S. X. Dou, Y. M. Kang and S. L. Chou, Escience, 2021, 1, 13–27 CrossRef.
- A. Navarro-Suárez, J. Carretero-González, N. Casado, D. Mecerreyes, T. Rojo and E. Castillo-Martinez, Sustainable Energy Fuels, 2018, 2, 836–842 RSC.
- P. Mutadak, A. Vedpathak, S. Warule, N. Chaudhari, S. Sartale, M. More and D. J. Late, Nanoscale Horiz., 2024, 9, 2259–2272 RSC.
- B. B. Sahoo, V. S. Pandey, A. S. Dogonchi, P. K. Mohapatra, D. N. Thatoi, N. Nayak and M. K. Nayak, J. Energy Storage, 2023, 65, 107335 CrossRef.
- I. Melkiyur, Y. Rathinam, P. S. Kumar, A. Sankaiya, S. Pitchaiya, R. Ganesan and D. Velauthapillai, Renewable Sustainable Energy Rev., 2023, 173, 113106 CrossRef CAS.
- A. D. Narang, S. P. Gupta, P. P. Sanap, S. S. Kahandal, R. S. Tupke, H. Kim, Q. Wang, A. S. Vedpathak, S. D. Sartale and V. K. Gade, J. Mater. Sci.: Mater. Electron., 2024, 35, 1335 CrossRef CAS.
- D. Xia, H. Gao, M. Li, F. Gong and M. Li, Energy Storage Mater., 2021, 35, 169–191 CrossRef.
- G. Yao, N. Zhang, Y. Zhang and T. Zhou, J. Nanopart. Res., 2021, 23, 57 CrossRef CAS.
- G. Yang, H. Cui, G. Yang and C. Wang, ACS Nano, 2014, 8, 4474–4487 CrossRef CAS PubMed.
- A. Mishra, G. Bera, P. Mal, G. Padmaja, P. Sen, P. Das, B. Chakraborty and G. Turpu, Appl. Surf. Sci., 2019, 488, 221–227 CrossRef CAS.
- Y. Piffard, F. Leroux, D. Guyomard, J.-L. Mansot and M. Tournoux, J. Power Sources, 1997, 68, 698–703 CrossRef CAS.
- C. Lv, J. Sun, G. Chen, C. Yan and D. Chen, Nano Energy, 2017, 33, 138–145 CrossRef CAS.
- M. Li, Y. Gao, N. Chen, X. Meng, C. Wang, Y. Zhang, D. Zhang, Y. Wei, F. Du and G. Chen, Chem.–Eur. J., 2016, 22, 11405–11412 CrossRef CAS PubMed.
- Y. Wang, H. Chai, H. Dong, J. Xu, D. Jia and W. Zhou, ACS Appl. Mater. Interfaces, 2016, 8, 27291–27297 CrossRef CAS.
- Y. Zhang, X. Xu, Y. Gao, D. Gao, Z. Wei and H. Wu, J. Power Sources, 2020, 455, 227985 CrossRef CAS.
- Q. Wei, Q. Wang, Q. Li, Q. An, Y. Zhao, Z. Peng, Y. Jiang, S. Tan, M. Yan and L. Mai, Nano Energy, 2018, 47, 294–300 CrossRef CAS.
- A. Sarkar, S. Sarkar and S. Mitra, J. Mater. Chem. A, 2017, 5, 24929–24941 RSC.
- W. Avansi, C. Ribeiro, E. R. Leite and V. R. Mastelaro, Mater. Chem. Phys., 2011, 127, 56–61 CrossRef CAS.
- M. Du, C. Liu, F. Zhang, W. Dong, X. Zhang, Y. Sang, J. J. Wang, Y. G. Guo, H. Liu and S. Wang, Advanced Science, 2020, 7, 2000083 CrossRef CAS PubMed.
- A. Vedpathak, T. Shinde, M. A. Desai, B. R. Thombare, R. Humane, S. A. Raut, R. Kalubarme, S. D. Sartale and S. Bhagwat, ACS Appl. Energy Mater., 2023, 6, 4693–4703 CrossRef CAS.
- J. Mao, C. He, J. Qi, A. Zhang, Y. Sui, Y. He, Q. Meng and F. Wei, J. Electron. Mater., 2018, 47, 512–520 CrossRef CAS.
- M. Aureliano and D. C. Crans, J. Inorg. Biochem., 2009, 103, 536–546 CrossRef CAS PubMed.
- P. Chithaiah, G. Chandrappa and J. Livage, Inorg. Chem., 2012, 51, 2241–2246 CrossRef CAS PubMed.
- C.-J. Mao, H.-C. Pan, X.-C. Wu, J.-J. Zhu and H.-Y. Chen, J. Phys. Chem. B, 2006, 110, 14709–14713 CrossRef CAS.
- Y. Gogotsi and R. M. Penner, ACS Nano, 2018, 12, 2081–2083 CrossRef CAS PubMed.
- S. Ardizzone, G. Fregonara and S. Trasatti, Electrochim. Acta, 1990, 35, 263–267 CrossRef CAS.
- C. V. Khedkar, A. S. Vedpathak, A. V. Dhotre, K. D. Daware, Y. D. Kolekar, S. D. Sartale, S. W. Gosavi and S. I. Patil, Chem. Phys. Impact, 2023, 6, 100153 CrossRef.
- T. Liu, H. Chai, D. Jia, Y. Su, T. Wang and W. Zhou, Electrochim. Acta, 2015, 180, 998–1006 CrossRef CAS.
- D. Sun, J. Lang, X. Yan, L. Hu and Q. Xue, J. Solid State Chem., 2011, 184, 1333–1338 CrossRef CAS.
- A. S. Vedpathak, M. A. Desai, S. Bhagwat and S. D. Sartale, Energy Fuels, 2022, 36, 4596–4608 CrossRef CAS.
- M. A. Desai, A. S. Vedpathak, A. R. Bhapkar, G. D. Saratale and S. D. Sartale, J. Environ. Manage., 2021, 299, 113564 CrossRef CAS PubMed.
- C. Jagtap, V. Kadam, B. Kamble, P. Lokhande, A. Pakdel, D. Kumar, R. Udayabhaskar, A. Vedpathak, N. Chaure and H. Pathan, J. Energy Storage, 2024, 83, 110666 CrossRef.
- T. N. Shinde, A. Vedpathak, B. J. Nagare, D. M. Sapkal, M. Desai, P. P. Atre and S. D. Sartale, Energy Technol., 2024, 12, 2301056 CrossRef CAS.
- F. Wang, Z. Liu, X. Yuan, J. Mo, C. Li, L. Fu, Y. Zhu, X. Wu and Y. Wu, J. Mater. Chem. A, 2017, 5, 14922–14929 RSC.
- E. Khoo, J. Wang, J. Ma and P. S. Lee, J. Mater. Chem., 2010, 20, 8368–8374 RSC.
- N. T. H. Trang, N. Lingappan, I. Shakir and D. J. Kang, J. Power Sources, 2014, 251, 237–242 CrossRef.
- A. Vedpathak, M. Desai, B. Thombare, R. S. Kalubarme, G. Guan, S. Bhagwat and S. D. Sartale, J. Electroanal. Chem., 2024, 958, 118150 CrossRef CAS.
- H. Y. Chen, G. Wee, R. Al-Oweini, J. Friedl, K. S. Tan, Y. Wang, C. L. Wong, U. Kortz, U. Stimming and M. Srinivasan, ChemPhysChem, 2014, 15, 2162–2169 CrossRef CAS PubMed.
- A. S. Vedpathak, S. S. Kalyane, T. N. Shinde, Q. Wang, R. N. Bulakhe, J. M. Kim and S. D. Sartale, J. Power Sources, 2024, 621, 235315 CrossRef CAS.
- S. R. Sankapal, T. B. Deshmukh, A. G. Bagde, K. Patil, B. R. Sankapal and C. D. Lokhande, J. Phys. Chem. Solids, 2024, 195, 112286 CrossRef CAS.
- G. P. Sharma, P. K. Gupta, S. K. Sharma, R. G. S. Pala and S. Sivakumar, ACS Appl. Energy Mater., 2021, 4, 4758–4771 CrossRef CAS.
- K. Prasad, T. Sreekanth, K. Yoo and J. Kim, Inorg. Chem. Commun., 2024, 159, 111848 CrossRef CAS.
- D. Merum, N. Parvin, S. V. P. Vattikuti, R. R. Nallapureddy, R. Pitcheri, M. Shkir, M. A. Manthrammel, A. N. Banerjee and S. W. Joo, J. Energy Storage, 2023, 61, 106674 CrossRef.
- D. Merum, R. R. Nallapureddy, M. R. Pallavolu, T. K. Mandal, R. R. Gutturu, N. Parvin, A. N. Banerjee and S. W. Joo, ACS Appl. Energy Mater., 2022, 5, 5561–5578 CrossRef CAS.
- H. Sun, Y. Li, H. Chai, Y. Cao and W. Zhou, J. Alloys Compd., 2019, 805, 388–395 CrossRef CAS.
- J. Cheng, G. Gu, Q. Guan, J. M. Razal, Z. Wang, X. Li and B. Wang, J. Mater. Chem. A, 2016, 4, 2729–2737 RSC.
- J. K. Kim, B. Senthilkumar, S. H. Sahgong, J. H. Kim, M. Chi and Y. Kim, ACS Appl. Mater. Interfaces, 2015, 7, 7025–7032 CrossRef CAS PubMed.
- A. J. Bard, L. R. Faulkner and H. S. White, Electrochemical Methods: Fundamentals and Applications, John Wiley & Sons, 2022 Search PubMed.
- Y. Lu, C. Z. Zhao, R. Zhang, H. Yuan, L. P. Hou, Z. H. Fu, X. Chen, J.-Q. Huang and Q. Zhang, Sci. Adv., 2021, 7, eabi5520 CrossRef CAS PubMed.
- S. D. Shraer, N. D. Luchinin, I. A. Trussov, D. A. Aksyonov, A. V. Morozov, S. V. Ryazantsev, A. R. Iarchuk, P. A. Morozova, V. A. Nikitina and K. J. Stevenson, Nat. Commun., 2022, 13, 4097 CrossRef CAS PubMed.
- S. Lu, Y. Cai, Y. Li, X. Du, J. Wang, Y. Liu, K. Cao and Y. Fan, ACS Appl. Mater. Interfaces, 2024, 16, 38092–38100 CrossRef CAS PubMed.
- J. Zhong, L. Xia, S. Chen, Z. Zhang, Y. Pei, H. Chen, H. Sun, J. Zhu, B. Lu and Y. Zhang, Proc. Natl. Acad. Sci. U. S. A., 2024, 121, e2319193121 CrossRef CAS PubMed.
- Y. Zhu, Y. Xu, Y. Liu, C. Luo and C. Wang, Nanoscale, 2013, 5, 780–787 RSC.
- Z. Y. Li, X. Ma, K. Sun, F. Ning, L. Sun, G. Tian, J. Gao, H. Wang and D. Chen, J. Mater. Chem. A, 2024, 12, 26113–26124 RSC.
- C. Liu, K. Chen, H. Xiong, A. Zhao, H. Zhang, Q. Li, X. Ai, H. Yang, Y. Fang and Y. Cao, eScience, 2024, 4, 100186 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: S1: (a) chemicals and reagents, (b) formulae for Csp calculation of working electrode and ASC device, and (c) characterization techniques, S2: XPS studies of NaV8O20 nanobelts sample, S3: electrochemical measurements of carbon paper, bulk V2O5 and NaV8O20 electrode and Na ion battery, Table S1: crystallographic data of the NaV8O20 powder obtained from Rietveld refinement, Table S2: Csp values of NaV8O20 electrode from CV curves, Table S3: Csp values of NaV8O20 electrode from GCD curves, Table S4: Csp, energy and power density values of AC//NaV8O20 ASC coin cell device. See DOI: https://doi.org/10.1039/d4ta08624d |
| This journal is © The Royal Society of Chemistry 2025 |