Two-dimensional Cu(I)-MOF with mesoporous architecture towards chemiresistive NO2 sensing†
Dilip
Pandey
a,
Trivedi
Samarth
a,
Vikash Kumar
Verma
b,
Chandrabhan
Patel
b,
L.
Ponvijayakanthan
c,
Neeraj K.
Jaiswal
*c,
Shaibal
Mukherjee
 *bde and
Abhinav
Raghuvanshi
*bde and
Abhinav
Raghuvanshi
 *a
*a
aDepartment of Chemistry, Indian Institute of Technology Indore, Madhya Pradesh 453552, India
bHybrid Nanodevice Research Group (HNRG), Department of Electrical Engineering, Indian Institute of Technology Indore, Madhya Pradesh 453552, India
c2-D Materials Research Laboratory, Discipline of Physics, PDPM Indian Institute of Information Technology Design and Manufacturing, Jabalpur, Madhya Pradesh 482005, India
dCentre for Advanced Electronics (CAE), Indian Institute of Technology Indore, Madhya Pradesh 453552, India
eSchool of Engineering, RMIT University, Melbourne, VIC 3001, Australia. E-mail: neeraj@iiitdmj.ac.in; shaibal@iiti.ac.in; r.abhinav@iiti.ac.in
First published on 13th March 2025
Abstract
Conducting metal–organic frameworks (c-MOFs) have emerged as a promising platform for chemiresistive gas sensors due to their intrinsic porosity and ability to facilitate charge transfer upon gas adsorption. In this study, we report a semiconducting copper(I)-MOF (Cu-MOF) formed by the self-assembly of CuI and N-phenyl-N-(pyridin-4-yl)pyridin-4-amine. The Cu-MOF consists of a 2D network comprising Cu4I4 secondary building units, which forms an intercalating 3D structure driven by multiple weak interactions. The semiconducting nature and mesoporous structure motivated the exploration of its chemiresistive gas sensing capabilities. The chemiresistive device fabricated with Cu-MOF displays high selectivity and efficient room temperature NO2 sensing with a lower limit of detection (3.5 ppb) and a swift response/recovery time (∼11/13 s), one of the fastest among the reported state-of-the-art MOF-based NO2 sensors. Experimental and theoretical analysis reveals that the adsorption of NO2 on Cu-MOF withdraws electrons from the Cu(I) center, leading to a change in electrical response. The rapid response/recovery without any external stimuli, repeatability and material robustness further enhance its potential applications.
Introduction
Metal–organic frameworks (MOFs) have gained significant interest due to their porosity along with chemical and structural diversity, leading to multifunctional properties. Although the majority of MOFs are electrical insulators, the past decade has witnessed the emergence of several electrically conductive MOFs (c-MOFs).1–3 c-MOFs are usually composed of π-conjugated ligands capable of effective charge delocalization and are highly attractive for various electronic applications, including electrocatalysis, sensing, energy storage, and quantum information.4–10 In particular, 2D c-MOFs have emerged as promising candidates for the development of chemiresistive sensors.11 Their porous structures and electrical properties improve gas adsorption and interactions while maintaining high electrical conductivity under ambient conditions.4,7,10,12–15 Moreover, the presence of diverse binding sites in these materials enables unique interactions with specific target molecules, significantly enhancing selectivity. Therefore, the incorporation of c-MOFs has significantly addressed the key limitations associated with the selectivity and room temperature operation of traditional sensing materials for some toxic gases.16–20 Recently, semiconducting Cu(I) frameworks have attracted considerable attention in the field of chemiresistive gas sensing due to their potential for detecting a wide range of volatile organic compounds and inorganic toxic gases such as NH3, MeOH, NO2, and others.21–24 The tendency of CuI salts to assemble into different high-nuclearity clusters, such as [Cu2I2], [Cu3I3], [Cu4I4], etc., and their structural diversity lead to attractive platforms for various applications.25–29 These clusters contribute to the formation of semiconducting, tunable and robust frameworks, enhancing their potential for sensing applications. Furthermore, cost-effectiveness, low toxicity, and convenient synthesis make these materials even more attractive.A significant discharge of industrial waste gases and automobile exhaust has caused considerable damage to the environment and human health. NO2 is one of the commonly emitted toxic gases, contributing to ground-level ozone formation, acid rain, eutrophication, reduced visibility and climate change.17,30 Low-level NO2 exposure to humans can cause respiratory and cardiovascular issues, including bronchitis, pulmonary edema, and olfactory paralysis, whereas prolonged exposure may lead to severe brain disorders such as Parkinson's disease, which can be life-threatening.31–34 Toxicity by NO2 arises due to its reaction with hemoglobin to form methemoglobin, which is unable to carry oxygen.35 Therefore, quick and real-time detection of NO2 is essential for public health and environmental monitoring. Several materials have shown promising results for room temperature NO2 sensing, but response time and reversibility are still a major challenge. The irreversibility is attributed to NO2's strong tendency to extract electrons from metal nodes and form stable complexes.36,37 Strategies such as photoactivation and high-temperature operation have been employed to address this issue, leading to additional power requirements in the device. The success of these approaches cannot be undermined, but for real-world applications, sensors that can operate at room temperature without the requirement of any external stimuli would be more appropriate.31,38 Recently, some copper(II) MOFs have been reported for NO2 gas sensing; however, most of these materials either exhibit relatively long response times or require external stimuli for efficient sensing performance.39,40
In this study, we report a 2D copper(I)-MOF (Cu-MOF), synthesized from N-phenyl-N-(pyridin-4-yl)pyridin-4-amine (PDPA) and CuI. The Cu-MOF consists of a Cu4I4 secondary building unit (SBU). The semiconducting behaviour and mesoporous structure intrigued us to explore its gas-sensing capabilities. The chemiresistive gas sensor fabricated from Cu-MOF demonstrated a high selectivity and response for NO2 gas with an exceptionally fast response/recovery time of approximately 11/13 seconds at room temperature. The sensing mechanism is thoroughly investigated using experimental and theoretical studies.
Results and discussion
The ligand, PDPA, was synthesized in 70% yield by reacting 4,4′-dipyridylamine with bromobenzene (Scheme S1†) and characterized with various spectroscopic techniques (Fig. S1–S4†). The synthesized ligand was reacted with CuI in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio to obtain Cu-MOF as a white solid in 80% yield (Scheme 1). The synthesized MOF was initially analyzed using infrared spectroscopy, which showed slight shifts from the ligand spectrum, substantiating the coordination of the ligands with CuI (Figure S5†).
2 ratio to obtain Cu-MOF as a white solid in 80% yield (Scheme 1). The synthesized MOF was initially analyzed using infrared spectroscopy, which showed slight shifts from the ligand spectrum, substantiating the coordination of the ligands with CuI (Figure S5†).
Crystals suitable for single-crystal X-ray diffraction (SCXRD) were obtained by slow diffusion of acetonitrile solution of CuI into a dichloromethane solution of the ligand over a week. Cu-MOF crystallizes in the monoclinic system with the Cc space group. Other important crystallographic parameters are given in Table S1.† The asymmetric unit consists of four copper, four iodides, and two ligand units (Fig. S6a†). The expanded structure reveals a two-dimensional network with Cu4I4 distorted cubane tetrameric SBUs. Each copper has distorted tetrahedral geometry and is bridged with µ3-I and pyridyl nitrogens of the ligand. Both pyridyl rings of the ligand are coordinated to two different Cu4I4 clusters. These Cu4I4 SBUs are connected to adjacent Cu4I4via the bridging of ligands, leading to a 2D framework with 44-membered metallacycles forming rectangular voids (Fig. 1a and b). The distances between the centroid of adjacent clusters are 13.356 Å along vertical and 13.214 Å along horizontal axes (Fig. S6b†). In the Cu4I4 distorted cubane tetramer, Cu–I, Cu–N, and Cu–Cu distances are in the range of 2.624–2.748 Å, 2.023–2.047 Å and 2.624–2.698 Å, respectively (Fig. S7†). The angle of Cu–I–Cu is around 59°. These bond metrics are consistent with previous reports on Cu(I) iodide frameworks having Cu4I4 SBUs.29,41–43 C–H–π interactions (2.805–2.898 Å) involving ortho and meta hydrogen of the phenyl ring lead to another entangled plane which is chemically equivalent to the 2D framework (Fig. 1d), partially filling the voids generated by the rhombus-grid structure. In addition, some solvent molecules are also found trapped in the cavity. Thus entangled planes and solvent molecules in the voids generated by the 2D network led to close packing in the crystal structure. Both sheets are nearly perpendicular to each other with a sliding angle of 77.75° (Fig. 1d). Such crystal structures packing in CuI clusters are previously reported with some other ligands.43–46 Moreover, weak C–H⋯I (3.133 Å) interactions were also identified from para-hydrogen of the phenyl ring to iodine, leading to parallel stacking of sheets with interplanar distances of 12.776 Å (Fig. 1c). Thus, infinitely parallel 2D stacked sheets and 77.75° tilted 2D sheets form an intercalated close-packed 2D–3D mixed network.
 | ||
| Fig. 1 (a) 2D sheet of the rhombus grid network of Cu-MOF (b) Space filled model of 2D Cu-MOF. (c) C–H⋯I interactions leading to parallel stacking of sheets. (d) Interpenetration of two sheets. | ||
The experimental powder X-ray diffraction (PXRD) patterns confirmed the phase purity of the bulk sample, which correlates well with the simulated pattern obtained from SCXRD analysis (Fig. S8a†). Cu-MOF remains stable for several months under ambient conditions. The thermal stability of Cu-MOF was assessed using thermogravimetric analysis (TGA) (Fig. S8b†), which suggests thermal stability until 250 °C. The Brunauer–Emmett–Teller (BET) analysis showed a type IV isotherm with a surface area of 15.033 m2 g−1, indicating a mesoporous structure for Cu-MOF (Fig. S9a†). Additionally, the pore size of Cu-MOF was determined to be 5.77 nm using the Barrett–Joyner–Halenda (BJH) method (Fig. S9b†). The surface morphology of the synthesized MOF was assessed using field emission scanning electron microscopy (FE-SEM). FE-SEM images suggest the formation of irregular agglomerated petals (Fig. 2). These multiple petals pile up to form nanoflower structures of different sizes. The petals have curved ending sides while they seem to have grown over each other (Fig. 2a–c). Energy dispersive X-ray (EDX) analysis confirms the uniform presence of Cu, C, N, and I elements in an appropriate portion in MOF (Fig. S10†). Moreover, elemental mapping analysis verified the uniform distribution of elements over the surface, demonstrating a consistent composition (Fig. 2d–g). Transmission electron microscopy (TEM) images also suggest the formation of agglomerated nano-particles of sizes ranging from 100 to 800 nm, with shades of darker color in a single particle, which could show the extent of accumulation (Fig. 2h and i). The selected area electron diffraction (SAED) pattern indicates rings corresponding to different diffraction planes present in Cu-MOF, pointing to the crystalline nature of Cu-MOF (Fig. 2j).
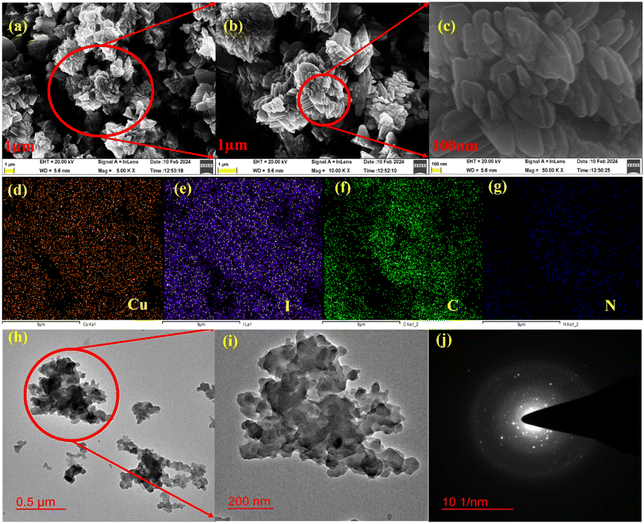 | ||
| Fig. 2 (a–c) FE-SEM images of Cu-MOF. (d–e) Elemental mapping of elements present in Cu-MOF. (h and i) TEM images of Cu-MOF. (j) SAED patterns from TEM (g and h). | ||
The optical band gap of Cu-MOF was measured from ultraviolet-visible diffuse reflectance spectroscopy (UV-Vis DRS) using the Tauc plot function (Fig. S11†). A band gap (Eg) of 2.04 eV was determined, suggesting the semiconducting nature of Cu-MOF. Furthermore, the electrical conductivity of the pressed pellet of Cu-MOF was measured using a Keithley 6517B electrometer across a voltage range of −10 V to +10 V. The electrical conductivity was found to be 3.3 × 10−7 S cm−1, confirming the semiconductor characteristics of Cu-MOF (Fig. S12†).
Gas sensing measurements
The 2D structure with accessible voids, favorable nanostructure morphology with the mesoporous nature of the material, and semiconducting behavior of Cu-MOF prompted us to explore its chemiresistive gas sensing behavior. Cu-MOF was mixed in ethanol, drop-cast onto interdigitated electrodes (IDEs, 1.5 × 1 cm), and then dried in the oven for 12 h. This sensing device was kept inside a sensing chamber to perform the sensing experiment at 27 °C. Changes in resistivity were assessed with different concentrations of gases. The response of the sensor was obtained using the following equation: | (1) |
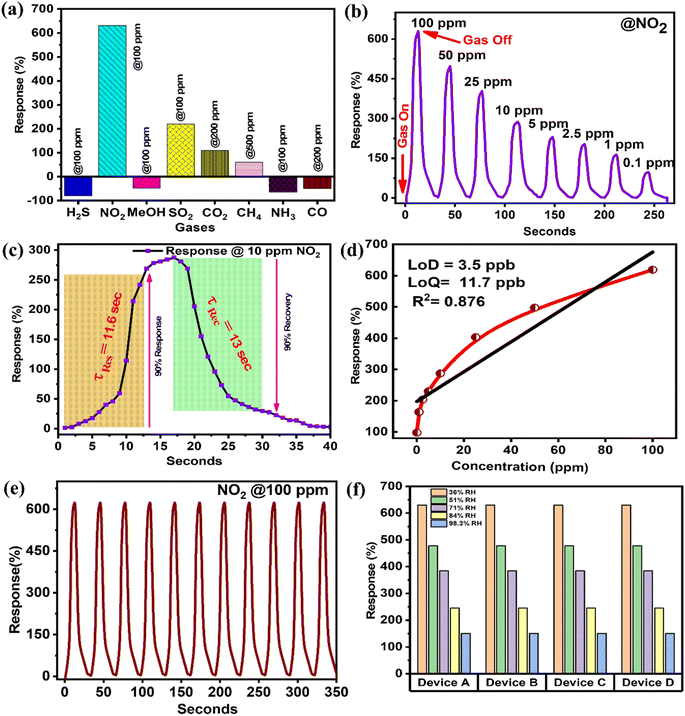
Initially, the responses of the Cu-MOF sensor toward different reducing and oxidizing gases were investigated (Fig. 3a). The Cu-MOF sensor shows response values of −80%, 630%, −48%, 220%, 110%, 60%, −64% and −49% to interfering gases such as H2S (100 ppm), NO2 (100 ppm), MeOH (100 ppm), SO2 (100 ppm), CO2 (200 ppm), CH4 (500 ppm), NH3 (100 ppm) and CO (200 ppm), respectively. The selectivity factors of the Cu-MOF sensor for 100 ppm of NO2 are calculated to be SNO2/SH2S = 7.8, SNO2/SMeOH = 13.1, SNO2/SSO2 = 2.9, SNO2/SCO2 = 5.7, SNO2/SCH4 = 10.5, SNO2/SNH3 = 9.49 and SNO2/SCO = 12.8 representing a significantly high selectivity toward NO2 compared to other oxidizing and reducing gases. Afterward, the dynamic sensing performance of the Cu-MOF sensor at different concentrations of NO2 was investigated at 27 °C (Fig. 3b). As the concentration of NO2 decreases from 100 ppm to 100 ppb, the response value of Cu-MOF sensors also decreases from 630 to 98%. Most importantly, the Cu-MOF sensor exhibited a quick response/recovery time of only 11.6/13 seconds and 9.1/10.8 seconds at 10 ppm and 100 ppm NO2 concentrations, respectively (Fig. 3c). These transient times are one of the quickest among the reported MOF-based NO2 sensors (Fig. 4a and Table S2†).
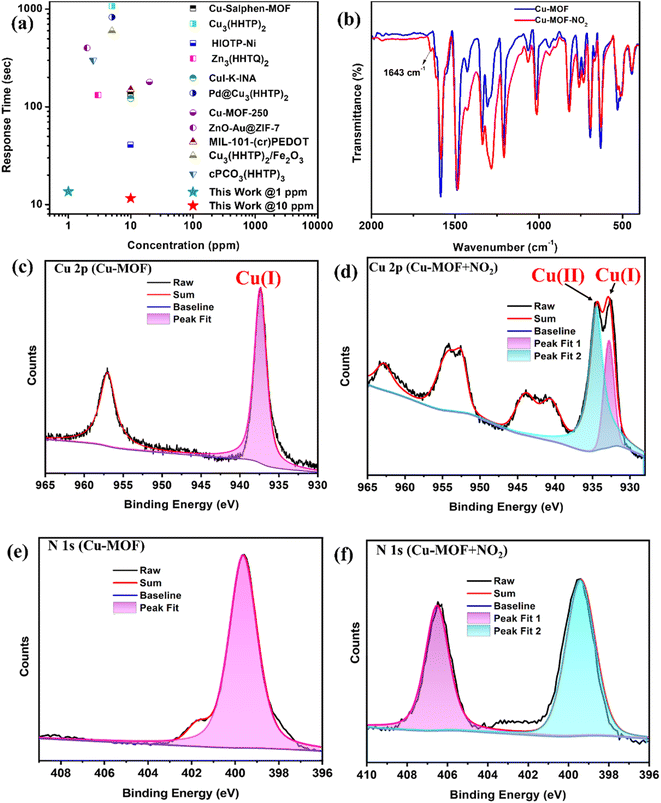 | ||
| Fig. 4 (a) Response time comparison of the Cu-MOF sensor with the reported MOFs and hybrid MOF-based NO2 sensors at room temperature (Tables S2 and S3†). (b) IR-spectra of the Cu-MOF sensor before and after NO2 exposure. High-resolution Cu2p XPS spectra (c) before NO2 exposure and (d) after NO2 exposure; high-resolution N1s XPS spectra (e) before NO2 exposure and (f) after NO2 exposure. | ||
Furthermore, the LOD and LOQ values of the Cu-MOF sensor were found to be 3.5 and 11.7 ppb, respectively, highlighting its excellent sensitivity (Fig. 3d). The repeatability of the sensor was also studied up to 11 cycles, and complete consistency was observed with no significant drop in performance (Fig. 3e). To evaluate the reproducibility and selectivity of the sensor towards NO2 under varying relative humidity (RH) conditions, we measured the response of four different devices A, B, C, and D, prepared similarly. The response was recorded at room temperature with RH levels of 36%, 51%, 71%, 84%, and 98.3% (Fig. 3f). All four devices showed minimal variation in response, with only a small deviation in performance. However, as RH increased from 36% to 98%, the response of the Cu-MOF sensor gradually decreased from 630% to 151%. Despite this, the sensor maintained a significant response, demonstrating its robustness. The decline in response can be attributed to the reduced availability of active sites for analyte interaction. Furthermore, long-term stability of the devices was assessed, which showed minor changes in response during 60 days of analysis (Fig. S13†).
To understand the sensing mechanism, we first recorded the ex situ PXRD and ATR-IR spectra of the Cu-MOF sensor after exposure to NO2 gas over the powder sample. After exposure to NO2, ATR-IR analysis revealed a new peak at 1643 cm−1, which corresponds to molecularly adsorbed NO2.47,48 Additionally, a slight shift in the rest of the spectra confirms the interaction between the analyte and the sensing material (Fig. 4b). The sharp PXRD patterns established the structural integrity of the Cu-MOF even after NO2 exposure (Fig. S14†). The presence of robust Cu4I4 SBUs does not allow any structural disintegration during gas interaction, which is probably also responsible for excellent reversibility during sensing experiments. XPS analysis was conducted before and after gas exposure for deeper insight into the changes in surface chemistry, oxidation states, and material–analyte interaction (Fig. S15†). Peaks at 952.08 eV and 932.08 eV indicate the presence of Cu(I) (Cu2p) and 618.88 eV and 630.28 eV correspond to iodine (I3d), while peaks at 400.28 eV and 284.28 eV correspond to nitrogen (N1s) and carbon (C1s), respectively (Fig. S16†). After exposing the material to NO2, two distinct peaks at 934 and 932 eV appear, suggesting the presence of both Cu2+ and Cu+ (Fig. 4c andd).13,38,49,50 Moreover, a new peak at 406.7 eV in the N1s spectrum can be designated to the presence of metal-coordinated NO2 molecules (Fig. 4e and f).13,19 Furthermore, UV-Vis DRS study suggests that exposing Cu-MOF to NO2 gas led to a decrease in the band gap of the material (Fig. S17†). These observations establish that NO2 accepts an electron from Cu(I) and oxidizes it to Cu(II) during the interaction, leading to a change in electrical response.
To elucidate the experimental findings, we conducted density functional theory (DFT) calculations to simulate the adsorption of NO2 on a 2D model of Cu-MOF. DFT calculations were performed for structural optimization and electronic property analysis. Structural optimizations were performed using the Linear Combination of Atomic Orbitals (LCAO) basis set as implemented in the QuantumATK software.51 All other calculations, such as adsorption energy, electronic properties, and charge density, were calculated using the plane wave basis Quantum Espresso code suite for enhanced accuracy.52,53 The exchange–correlation interactions were addressed using the revised Perdew–Burke–Ernzerhof (RPBE) functional, which was designed to describe the adsorption process more accurately within the Generalized Gradient Approximation (GGA) framework.54,55 A 2 × 2 × 1 k-point mesh was employed during the structural optimization process and all subsequent calculations utilized a denser 4 × 4 × 1 k-point mesh in sampling the Brillouin zone. Long-range interactions were accounted for using the Grimme-D3 dispersion correction method.56,57 Mulliken population analysis was calculated using the QuantumATK code suite which provided insights into the system's charge distribution.
The experimental findings from XPS suggested the adsorption of NO2 molecules onto the Cu site. Based on this observation, the optimization was done with initial configurations positioning the NO2 molecule at a distance of 2.00 Å above the cubane tetramer (Cu4I4) cluster. This starting point was chosen to reflect the likely interaction between the analyte and the substrate. The post-optimization analysis elucidated that the NO2 gas was effectively adsorbed onto the Cu atom of the Cu4I4. Furthermore, enhanced stability was observed when the N atom of the analyte interacts with the Cu atom compared to the O atom of the analyte. The distance between the adsorbed NO2 molecules and the Cu atom was found to be 2.74 Å. Therefore, the DFT structural optimization studies further support experimental observation that the NO2 analyte exhibits a preferential binding affinity towards the Cu sites of Cu4I4 within the Cu-MOF. The structures of bare and most stable NO2 adsorbed 2D Cu-MOF (NO2–Cu-MOF) are depicted in Fig. 5a and b.
The calculated adsorption energy of −0.286 eV for the most stable adsorbed configuration substantiates the energetic feasibility of the adsorption process. Additionally, theoretical calculations provided further predicts that NO2 forms a coordinate chemical bond with the Cu-MOF at a distance of 2.05 Å from Cu4I4 cluster, whereas NH3 and CO2, for example, interact only via physical adsorption, with larger distances of 3.26 Å and 3.92 Å, respectively. Moreover, the calculated adsorption energies underscore the differences in interaction strength. For NH3 and CO2, the adsorption energies are −0.214 eV and −0.116 eV, respectively, which are notably weaker than the −0.286 eV adsorption energy for NO2. This strong chemical interaction with NO2 results in a more significant impact on the MOF's conductivity compared to other physically adsorbed gases (Fig. S18†). To elucidate the charge transfer characteristics, we conducted a comprehensive Mulliken charge population analysis. Table S4† presents the calculated Mulliken charges of atoms within the Cu4I4 cubane tetramer and the adsorbate NO2 molecule. The analysis reveals that the Cu atom directly interacts with the NO2 gas with a subtle reduction in electron density, while NO2 acquires a marginally increased electron population compared to its gas phase molecular form. This observation implies a minor charge transfer from the Cu-MOF to the NO2 molecule upon adsorption. To further corroborate these findings, we plotted the charge density difference, comparing the electronic charge distribution before and after NO2 adsorption, as illustrated in Fig. 5. In the plot, regions of charge accumulation are depicted in golden yellow (Fig. 5c), while areas of charge depletion are represented in light green (Fig. 5d). The plot unambiguously demonstrates electron transfer from the Cu atom to the NO2 molecule, providing compelling evidence for the redistribution of charge occurring during the adsorption process.
We further investigated the electronic structure modifications induced by NO2 adsorption by calculating the density of states for both the bare Cu-MOF and NO2-Cu-MOF, as illustrated in Fig. S19.† Upon NO2 adsorption, a notable shift in the Fermi level (EF) towards the valence band states was observed. This downward shift of the EF is characteristic of p-type doping in semiconductor materials. Consequently, the adsorption of NO2 on the Cu-MOF appears to introduce hole carriers into the system. This observation along with experimental results suggests that the electrical response in the NO2-Cu-MOF is predominantly governed by hole conductivity. Overall, the theoretical study provides valuable insights into the preferential binding mechanism of NO2 on the Cu-MOF and the changes induced in its electronic properties, which corroborate the experimental results.
Conclusion
In this study, we have successfully demonstrated the potential of semiconducting Cu(I) MOF as a chemiresistive gas sensor for NO2 detection. The Cu-MOF, constructed from Cu4I4 SBUs and the PDPA ligand, forms a 2D network that was thoroughly characterized by IR, SCXRD, PXRD, FE-SEM, TEM, and XPS. The mesoporous nature and semiconducting behavior of Cu-MOF offer distinct advantages for chemiresistive gas sensing. The sensor exhibited excellent performance in detecting NO2 at room temperature, achieving a LOD of 3.5 ppb and exceptionally fast response and recovery times of less than 15 seconds. This performance surpasses that of many reported state-of-the-art NO2 sensors, making the Cu-MOF a strong candidate for practical sensing applications. The sensing mechanism was further investigated through IR, PXRD, XPS, and DFT analyses, indicating electron transfer from the Cu(I) center to NO2 during adsorption, resulting in a distinct and measurable change in electrical response. The straightforward synthesis and the outstanding performance of the Cu-MOF-based sensor in selective NO2 detection highlight its potential for industrial and environmental gas monitoring.Data availability
All the data will be made available on reviewers' request.Conflicts of interest
The authors declare no competing interests.Acknowledgements
Dilip Pandey acknowledges IIT Indore for providing a doctoral fellowship. Sophisticated Instrumentation Center (SIC) IIT Indore, the Department of Electrical Engineering, the Department of Chemistry, and the DST-FIST 500 MHz NMR facility of the Department of Chemistry, IIT Indore, are acknowledged for providing basic infrastructure and characterization facilities. Prof. Shaibal Mukherjee and Chandrabhan Patel thank the TIH-IOT CHANAKYA Group (PhD, PG, and UG) Fellowship Program 2021-2022 (TIH-IOT/12/2022/CHANAKYA/Group/Sanction Letter/004) and MHRD STARS (Project No. STARS/APR2019/NS/116/FS dated December 31, 2019) for providing financial support. Vikash Kumar Verma acknowledges CSIR-UGC for providing a doctoral fellowship. Prof. Shaibal Mukherjee acknowledges the TIH-IoT CHANAKYA Faculty Fellowship (No. TIH-IoT/2024-05/HRD/CHANAKYA/SL/CFF-003). Dr Neeraj K. Jaiswal acknowledges the National Supercomputing Mission (NSM) for providing computing resources of ‘PARAM Ganga’ at IIT Roorkee, which is implemented by C-DAC and supported by the Ministry of Electronics and Information Technology (MeitY) and the Department of Science and Technology (DST), Government of India.References
- L. S. Xie, G. Skorupskii and M. Dincă, Chem. Rev., 2020, 120, 8536–8580 CrossRef CAS PubMed.
- L. Sun, M. G. Campbell and M. Dincă, Angew. Chem., Int. Ed., 2016, 55, 3566–3579 CrossRef CAS PubMed.
- D. D. Medina, A. Mähringer and T. Bein, Isr. J. Chem., 2018, 58, 1089–1101 CrossRef CAS.
- M. Ko, L. Mendecki and K. A. Mirica, Chem. Commun., 2018, 54, 7873–7891 RSC.
- H. Banda, J.-H. Dou, T. Chen, N. J. Libretto, M. Chaudhary, G. M. Bernard, J. T. Miller, V. K. Michaelis and M. Dincă, J. Am. Chem. Soc., 2021, 143, 2285–2292 CrossRef CAS PubMed.
- M. Wang, R. Dong and X. Feng, Chem. Soc. Rev., 2021, 50, 2764–2793 RSC.
- Y. Lu, P. Samorì and X. Feng, Acc. Chem. Res., 2024, 57, 1985–1996 CrossRef CAS PubMed.
- W. Liu, G. Yuan, S. Jiang, Y. Shi and H. Pang, Chem. Eur J., 2024, 30, e202402747 CrossRef CAS.
- S. Mishra, M. K. Singh, D. Pandey, D. K. Rai and A. Raghuvanshi, J. Mater. Chem. A, 2024, 12, 4534–4543 RSC.
- C. Park, J. W. Baek, E. Shin and I.-D. Kim, ACS Nanosci. Au, 2023, 3, 353–374 CrossRef CAS PubMed.
- X. Wang, Y. Chen, Z. Zeng, M. Yan, X. Jia, P. Hu, J. Xu, Z. Xue and J. Xu, Mater. Sci. Eng. R Rep., 2024, 160, 100819 Search PubMed.
- X. Yan, J. Chen, X. Su, J. Zhang, C. Wang, H. Zhang, Y. Liu, L. Wang, G. Xu and L. Chen, Angew. Chem., Int. Ed., 2024, 63, e202408189 CAS.
- P. Chen, X. Su, C. Wang, G. Zhang, T. Zhang, G. Xu and L. Chen, Angew. Chem., Int. Ed., 2023, 62, e202306224 CrossRef CAS PubMed.
- Y. Jo, Y. K. Jo, J. Lee, H. W. Jang, I. Hwang and D. J. Yoo, Adv. Mater., 2023, 35, 2206842 CAS.
- W.-T. Koo, J.-S. Jang and I.-D. Kim, Chem, 2019, 5, 1938–1963 CAS.
- A. Sharma, S. B. Eadi, H. Noothalapati, M. Otyepka, H.-D. Lee and K. Jayaramulu, Chem. Soc. Rev., 2024, 53, 2530–2577 RSC.
- B. Mohan, Virender, R. K. Gupta, A. J. L. Pombeiro, A. A. Solovev and G. Singh, Adv. Funct. Mater., 2024, 34, 2405231 Search PubMed.
- M. G. Campbell, D. Sheberla, S. F. Liu, T. M. Swager and M. Dincă, Angew. Chem., Int. Ed., 2015, 54, 4349–4352 CAS.
- Z.-F. Wu, C. Wang, X. Liu, K. Tan, Z. Fu, S. J. Teat, Z.-W. Li, X. Hei, X.-Y. Huang, G. Xu and J. Li, J. Am. Chem. Soc., 2023, 145, 19293–19302 CAS.
- M. G. Campbell, S. F. Liu, T. M. Swager and M. Dincă, J. Am. Chem. Soc., 2015, 137, 13780–13783 CAS.
- Z. Meng, R. M. Stolz and K. A. Mirica, J. Am. Chem. Soc., 2019, 141, 11929–11937 CAS.
- D. Pandey, C. Patel, S. Mishra, S. Mukherjee and A. Raghuvanshi, ACS Appl. Nano Mater., 2024, 7, 15833–15840 CAS.
- S. Mishra, C. Patel, D. Pandey, S. Mukherjee and A. Raghuvanshi, Small, 2024, 20, 2311448 CAS.
- Z. Ma, Y. Zhang, Z. Xue, Y. Fan, L. Wang, H. Wang, A. Zhong and J. Xu, ACS Sens., 2024, 9, 1906–1915 CAS.
- D. Pandey, A. Mishra, L. S. Kharabe, S. K. Maurya and A. Raghuvanshi, Cryst. Growth Des., 2024, 24, 6051–6059 CAS.
- D. Pandey, M. K. Singh, S. Mishra, D. K. Rai and A. Raghuvanshi, J. Mater. Chem. A, 2024, 12, 27355–27363 CAS.
- E. Cariati, E. Lucenti, C. Botta, U. Giovanella, D. Marinotto and S. Righetto, Coord. Chem. Rev., 2016, 306, 566–614 CrossRef CAS.
- J. Troyano, F. Zamora and S. Delgado, Chem. Soc. Rev., 2021, 50, 4606–4628 RSC.
- A. Raghuvanshi, C. Strohmann, J. Tissot, S. Clément, A. Mehdi, S. Richeter, L. Viau and M. Knorr, Chem. Eur J., 2017, 23, 16479–16483 CrossRef CAS PubMed.
- H. E. Stokinger, J. Air Pollut. Control Assoc., 1958, 8, 129–137 CrossRef CAS.
- J. Zhang, X. Liu, G. Neri and N. Pinna, Adv. Mater., 2016, 28, 795–831 CrossRef CAS PubMed.
- T. L. Guidotti, Environ. Res., 1978, 15, 443–472 CrossRef CAS PubMed.
- C. Ren and S. Tong, Environ. Health, 2008, 7, 56 CrossRef PubMed.
- Q. Li, W. Zeng and Y. Li, Sens. Actuators, B, 2022, 359, 131579 CrossRef CAS.
- H. Kosaka, M. Uozumi and I. Tyuma, Free Radic. Biol. Med., 1989, 7, 653–658 CrossRef CAS PubMed.
- A. J. Timmons and M. D. Symes, Chem. Soc. Rev., 2015, 44, 6708–6722 RSC.
- A. M. Wright, C. Sun and M. Dincă, J. Am. Chem. Soc., 2021, 143, 681–686 CrossRef CAS PubMed.
- H. Roh, D. Kim, Y. Cho, Y. Jo, J. A. Del Alamo, H. J. Kulik, M. Dincă and A. Gumyusenge, Adv. Mater., 2024, 36, 2312382 CrossRef CAS PubMed.
- W. Koo, S. Kim, J. Jang, D. Kim and I. Kim, Adv. Sci., 2019, 6, 1900250 CrossRef CAS PubMed.
- X. Su, Z. Zhong, X. Yan, T. Zhang, C. Wang, Y.-X. Wang, G. Xu and L. Chen, Angew. Chem., Int. Ed., 2023, 62, e202302645 CrossRef CAS PubMed.
- S.-Q. Bai, K. L. Ke, D. J. Young and T. S. A. Hor, J. Organomet. Chem., 2017, 849–850, 137–141 CrossRef CAS.
- S.-S. Zhao, L. Wang, Y. Liu, L. Chen and Z. Xie, Inorg. Chem., 2017, 56, 13975–13981 CrossRef CAS PubMed.
- P. K. Pal, A. Mukherjee, A. Ghosh and G. K. Patra, J. Mol. Struct., 2014, 1059, 332–337 CrossRef CAS.
- D. Sun, S. Yuan, H. Wang, H.-F. Lu, S.-Y. Feng and D.-F. Sun, Chem. Commun., 2013, 49, 6152 RSC.
- S. Khatua, S. Goswami, S. Biswas, K. Tomar, H. S. Jena and S. Konar, Chem. Mater., 2015, 27, 5349–5360 CrossRef CAS.
- H.-C. Fan, X. Xia, Y.-B. Zeng, Q.-F. Wang, D. Chen, S.-C. Qin, C.-Q. Wang, Q.-E. Cao and L.-Y. Zheng, Small Struct., 2022, 3, 2200030 CrossRef CAS.
- M. Schulz, A. Gehl, J. Schlenkrich, H. A. Schulze, S. Zimmermann and A. Schaate, Angew. Chem., Int. Ed., 2018, 57, 12961–12965 CrossRef CAS.
- K. Nakamoto, in Handbook of Vibrational Spectroscopy, ed. J. M. Chalmers and P. R. Griffiths, Wiley, 1st edn, 2001 Search PubMed.
- C. Park, W. Koo, S. Chong, H. Shin, Y. H. Kim, H. Cho, J. Jang, D. Kim, J. Lee, S. Park, J. Ko, J. Kim and I. Kim, Adv. Mater., 2021, 33, 2101216 CrossRef CAS.
- H. Lim, H. Kwon, H. Kang, J. E. Jang and H.-J. Kwon, Nat. Commun., 2023, 14, 3114 CrossRef CAS PubMed.
- S. Smidstrup, T. Markussen, P. Vancraeyveld, J. Wellendorff, J. Schneider, T. Gunst, B. Verstichel, D. Stradi, P. A. Khomyakov, U. G. Vej-Hansen, M.-E. Lee, S. T. Chill, F. Rasmussen, G. Penazzi, F. Corsetti, A. Ojanperä, K. Jensen, M. L. N. Palsgaard, U. Martinez, A. Blom, M. Brandbyge and K. Stokbro, J. Phys. Condens. Matter, 2020, 32, 015901 CrossRef CAS PubMed.
- P. Giannozzi, S. Baroni, N. Bonini, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli, G. L. Chiarotti, M. Cococcioni, I. Dabo, A. Dal Corso, S. De Gironcoli, S. Fabris, G. Fratesi, R. Gebauer, U. Gerstmann, C. Gougoussis, A. Kokalj, M. Lazzeri, L. Martin-Samos, N. Marzari, F. Mauri, R. Mazzarello, S. Paolini, A. Pasquarello, L. Paulatto, C. Sbraccia, S. Scandolo, G. Sclauzero, A. P. Seitsonen, A. Smogunov, P. Umari and R. M. Wentzcovitch, J. Phys. Condens. Matter, 2009, 21, 395502 CrossRef PubMed.
- P. Giannozzi, O. Andreussi, T. Brumme, O. Bunau, M. Buongiorno Nardelli, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli, M. Cococcioni, N. Colonna, I. Carnimeo, A. Dal Corso, S. De Gironcoli, P. Delugas, R. A. DiStasio, A. Ferretti, A. Floris, G. Fratesi, G. Fugallo, R. Gebauer, U. Gerstmann, F. Giustino, T. Gorni, J. Jia, M. Kawamura, H.-Y. Ko, A. Kokalj, E. Küçükbenli, M. Lazzeri, M. Marsili, N. Marzari, F. Mauri, N. L. Nguyen, H.-V. Nguyen, A. Otero-de-la-Roza, L. Paulatto, S. Poncé, D. Rocca, R. Sabatini, B. Santra, M. Schlipf, A. P. Seitsonen, A. Smogunov, I. Timrov, T. Thonhauser, P. Umari, N. Vast, X. Wu and S. Baroni, J. Phys. Condens. Matter, 2017, 29, 465901 CrossRef CAS PubMed.
- B. Hammer, L. B. Hansen and J. K. Nørskov, Phys. Rev. B, 1999, 59, 7413–7421 CrossRef.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- S. Grimme, S. Ehrlich and L. Goerigk, J. Comput. Chem., 2011, 32, 1456–1465 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2385865. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ta07702d |
| This journal is © The Royal Society of Chemistry 2025 |