Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Depolymerization of lignin into cycloalkanes over a hydrotalcite-derived NiFe alloy catalyst†
Hairui
Jiao
b,
Yushuai
Sang
c,
Hong
Chen
 *a and
Yongdan
Li
*a and
Yongdan
Li
 *c
*c
aSchool of Environmental Science and Engineering, Tianjin University, Tianjin 300072, China. E-mail: chenhong_0405@tju.edu.cn
bCollaborative Innovation Center of Chemical Science and Engineering, Tianjin Key Laboratory of Applied Catalysis Science and Technology, State Key Laboratory of Chemical Engineering, School of Chemical Engineering, Tianjin University, Tianjin 300072, China
cDepartment of Chemical and Metallurgical Engineering, School of Chemical Engineering, Aalto University, Kemistintie 1, Espoo FI-00076, Finland. E-mail: yongdan.li@aalto.fi
First published on 12th December 2024
Abstract
Herein, an Ni9Fe1/NiAlOz catalyst is reported to be an efficient catalyst for conversion of enzymatic hydrolysis lignin (EHL) and 2-phenethyl phenyl ether (PPE), a lignin dimer model compound. Interspecies electron transfer within the alloy phase enhances the intrinsic hydrogenation activity of Ni, while Fe doping generates abundant interfacial oxygen vacancies (OVs). The combination of H* from high-intensity hydrogen spillover and abundant OVs promotes the depolymerization of EHL into cycloalkanes. Because of this combination, Ni9Fe1/NiAlOz achieves 99.4% of PPE conversion under mild reaction conditions (100 °C, 0.8 MPa H2, and 3 h) and achieves the yield of cycloalkanes (169.5 mg per g EHL at 300 °C) from EHL that exceeds the theoretical yield. The catalyst is also stable for 6 runs without much deactivation. This strategy provides new insights into the rational design of an efficient and stable transition metal catalyst using a simple preparation method for the valorization of EHL.
1 Introduction
Biomass is the most abundant renewable carbon resource of organic origin and the ideal feedstock for renewable fuel and value-added chemical production.1,2 Lignin, the primary component of lignocellulose, is the only renewable feedstock for aromatic fuels and chemicals.3 In recent years, there has been an increased interest in the depolymerization of lignin via C–O bond cleavage as a promising approach to derive valuable chemicals from natural and renewable resources.4,5Lignin is a natural polymer made of benzene and propane-like units linked together with C–C and C–O bonds. Most aromatic monomers in lignin are connected via phenolic ether bonds (50–85%, depending on the plant species), such as β-O-4, α-O-4, and 4-O-5 bonds, with β-O-4 bonds being the most abundant.6,7 The selective cleavage of the ether bond in lignin to yield monomers is particularly challenging. Given the complexity and variability of real lignin, model compounds have been intensively used in research to selectively cleave specific bonds in technical lignin.8,9 Among the model compounds utilized, 2-phenethyl phenyl ether (PPE) is deemed suitable. Various types of Ni-catalysts have been reported for C–O bond cleavage. Chen et al.10 used a defect-rich layered silicate-like nickel nanosheet catalyst for PPE conversion at 160 °C and 3 MPa H2 and obtained 98.3% cycloalkanes and trace amounts of aromatic hydrocarbon products. Several studies have revealed that Ni metal alloys efficiently catalyze the transformation of lignin model molecules and facilitate C–O bond cleavage via hydrogenolysis. Mauriello et al.11 achieved hydrogenolysis of PPE in isopropanol at 210 °C with a 3 wt% Pd/Ni catalyst and proposed that the synergy of palladium and nickel as alloys is crucial for enabling C–O bond scission and the subsequent formation of aromatic compounds. Chen et al.12 designed a NixLay/CNT (CNT, carbon nanotube) catalyst to achieve PPE C–O bond breaking at 240 °C, 4 h, and 2.0 MPa H2 and observed that aryl groups played an important role in the competitive hydrogenation of phenol. Han et al.13 obtained cyclohexanol and cyclohexane from phenol at 5 MPa H2 and 250 °C using an NiFe alloy catalyst, demonstrating the synergy of Ni sites and Ni–Fe alloy sites in H2 activation and C–O bond cleavage.
Layered double hydroxide (LDH), often called hydrotalcite-like compounds, and its derivatives are versatile materials with unique properties and wide variety of applications. The multiphase catalyst, a composite of metal nanoparticles and mixed metal oxides (MOs), obtained from LDH precursors has demonstrated excellent catalytic behavior.14 Moreover, LDH-derived catalysts have shown considerable promise in lignin depolymerization reactions, achieving complete lignin liquefaction and high yields of monomers.15,16 In addition, lignin-derived materials have been successfully utilized in advanced applications, such as strong bioadhesive hydrogels, conductive nanocapsules, and wearable bioelectrodes, showcasing the potential of lignin-based materials in functional and biomedical fields.17,18
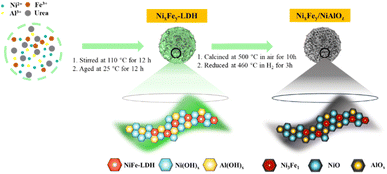
Herein, a co-precipitation technique is adopted for the preparation of highly active hydrotalcite-derived NiFe-based catalysts for the complete conversion of PPE and enzymatic hydrolysis lignin (EHL) to cycloalkanes. The incorporation of Fe species results in a catalyst with high oxygen affinity and an NiFe alloy with Niδ−–Feδ+, which exhibits excellent hydrodeoxygenation (HDO) and aromatic ring saturation performance.
2 Experimental
2.1 Catalyst preparation
All catalyst precursors were synthesized using a homogeneous co-precipitation technique with urea decomposition at 110 °C, as shown in Scheme 1. To prepare Ni9Fe1/NiAlOz, 21.1 g Ni(NO3)2·6H2O (99 wt%, Aladdin Co., Ltd), and 3.3 g Fe(NO3)3·9H2O (99 wt%, Aladdin Co., Ltd) were dissolved in 75 mL deionized water with oxygen and CO2 removed, followed by the addition of 9.2 g Al(NO3)3·9H2O (98 wt%, Shanghai Macklin Biochemical Co., Ltd). After full dissolution, the liquid was denoted as solution A. Urea (42.2 g, 99 wt%, Shanghai Macklin Biochemical Co., Ltd) was dissolved in 50 mL deionized water and added to solution A, resulting in solution B. Solution B was transferred to a round bottom flask and stirred at 120 °C for 12 h under reflux. Subsequently, the slurry was aged for 12 h at 25 °C, and the resulting powder obtained via filtration, with a Ni to Fe ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, was roasted for 12 h at 500 °C in a muffle oven to obtain the catalyst precursor, denoted as Ni9Fe1AlOz. Before the experiment, the Ni9Fe1AlOz catalyst was reduced at 460 °C for 3 h in 5 vol% H2/Ar to obtain Ni9Fe1/NiAlOz. The catalyst samples Ni/NiAlOz, Fe/AlOz, Ni9Fe0.5/NiAlOz and Ni9Fe1.5/NiAlOz were synthesized using a similar technique. In this context, NixFey/NiAlOz refers to all the catalyst samples.
2, was roasted for 12 h at 500 °C in a muffle oven to obtain the catalyst precursor, denoted as Ni9Fe1AlOz. Before the experiment, the Ni9Fe1AlOz catalyst was reduced at 460 °C for 3 h in 5 vol% H2/Ar to obtain Ni9Fe1/NiAlOz. The catalyst samples Ni/NiAlOz, Fe/AlOz, Ni9Fe0.5/NiAlOz and Ni9Fe1.5/NiAlOz were synthesized using a similar technique. In this context, NixFey/NiAlOz refers to all the catalyst samples.
2.2 Catalyst characterization
X-ray diffraction (XRD) patterns were recorded at 10–80° at an 8° min−1 scanning rate using an X-pert powder diffractometer (Smartlab, Rigaku Corp., Japan). X-ray absorption fine structure (XAFS) K-edge spectra for Ni and Fe were collected using a table XAFS-500-A instrument from Anhui Chuangpu Instrument Technology Co. The elemental contents of Ni, Fe, and Al were determined using an inductively coupled plasma-optical emission spectroscopy (ICP-OES) instrument (model: 730-ES; Varian; Palo Alto, USA). 0.1 g of the sample was digested and subjected to the ICP test. The H2-TPR was measured using an Altamira Instruments (AMI-300, USA) instrument and the catalyst was heated from 50 to 900 °C with a ramp of 10 °C min−1 under a flow of H2/Ar, 5 vol% (v/v) at 30 mL min−1, STP. The H2-TPD tests of the samples were recorded using a MicrotracBEL (BELCAT-B, Japan) machine. Prior to the H2-TPD test, pure H2 was used for pretreatment at 300 °C for 0.5 h and maintained under a stream of He for 0.5 h. The sample was cooled to 100 °C and maintained with flowing H2 (30 mL min−1) for 0.5 h. The system was purged with inert gas at 100 °C until the baseline was stabilized. Finally, the temperature was ramped up to 800 °C at a rate of 10 °C min−1. The WO3 color change experiment was used to illustrate the hydrogen spillover of the catalyst. Photographs of samples (0.1 g) made with WO3 (0.5 g) were mixed with the catalysts before treatment (0 s) and after treatment with 5% H2/Ar at 180 °C for 30 s and 180 s. The thermogravimetric (TG) curves were measured using an STA449 F3 analyzer (PerkinElmer Diamond, USA) in an air stream, 50 mL min−1, STP, and a temperature range of 50–800 °C at a heating rate of 5 °C min−1. In situ FT-IR measurements of the PPE hydrogenation reaction were performed with the mixture of catalyst and PPE dried at 40 °C in a vacuum for 12 h. The sample was heated up to 100 °C under a flow of H2 and kept for 3 h, and the spectra during the reaction were collected with a fixed time interval. The Raman spectra of the samples were obtained using a spectrometer, Horiba LabRAM HR Evolution, France, equipped with a DXR microscope at an excitation wavelength of 532 nm as the light source and a scan range of 1400–1700 cm−1.High-performance X-ray photoelectron spectroscopy (XPS) was used to reveal the valence states of the elements on the catalyst surface using a VG Scientific ESCALAB 250 spectrometer (Waltham, Massachusetts, USA). The morphology and structure of the samples were observed using a scanning electron microscope (SEM, S-4800, JEOL) and a transmission electron microscope (TEM, JEM-2100F, Hitachi). The sample microstructure and elemental distribution were also determined using the above two instruments. A Nicolet iS10 FTIR spectrometer (ThermoFisher Scientific Inc., UK) was used to measure the CO adsorption on the catalyst surface. The sample was heated to 200 °C and maintained for 1 h under a He atmosphere, 60 mL per min (STP), and then cooled to 30 °C to start recording the background. After that, a 30 min of CO adsorption was performed (1 vol% CO–He, 60 mL min−1, STP), with one reading every 3 min. Finally, the CO desorption was done with a 30 minute He gas purge and 15 minutes of data collection. Two-dimensional heteronuclear single quantum coherence-nuclear magnetic resonance (2D-HSQC NMR) was obtained using a Bruker 600 MHz Avance III spectrometer. A 40 mg sample was dissolved in 0.5 mL dimethyl sulfoxide (DMSO) and tested after being completely dissolved. The 1H resonance frequency was 600 MHz, and 13C was 150.91 MHz.
2.3 Lignin model compound and EHL conversion
500 mg dimers (PPE and 4,4′-dihydroxydiphenylmethane, 98.0%, Tianjin Heowns Biochem Technologies; diphenyl ether and biphenyl, 99.0%, Shanghai Aladdin Biochemical Technology Co., Ltd) or 500 mg EHL, 500 mg catalyst, and 70 mL n-dodecane (99.0%, Shanghai Dibai Biotechnology Co., Ltd) as solvent were mixed in an autoclave (Anhui Kemi Instrument Co., Ltd). There was no catalyst in the blank reaction, and the other conditions were retained. After sealing and purging, the reactor was charged with the prescribed H2 pressure and heated to a certain temperature, 60–300 °C, under 550 rpm stirring for a certain time, 0–10 h; thereafter, the reactor was cooled to room temperature. Subsequently, the reaction mixture was collected and mixed with the solution obtained by washing the autoclave with dodecane. An internal standard, 70 mg trimethylbenzene, was added. In the cycling experiments, the isolated catalyst was washed with ethanol, dried in a vacuum oven at 60 °C for 2 h, and used in the next reaction without the addition of a new catalyst. After recovery, the catalyst samples were marked as P–Ni9Fe1/NiAlOz after the sixth cycle reaction with PPE.The analysis of liquid products was carried out using the same equipment and method reported previously.17 The liquid products were identified and quantified using a gas chromatograph-mass (GC-MS) spectrometer and a gas chromatograph equipped with a flame ionization detector (GC-FID), respectively. The gaseous products were qualitatively analyzed using a Hiden Analytical HPR20 mass spectrometer.
PPE conversion and product yield were calculated using eqn (1) and (2) (monomer), and (3) (dimer). Moreover, the conversion and yield for EHL are calculated according to eqn (4) and (5).
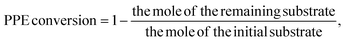 | (1) |
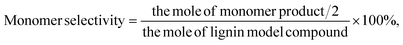 | (2) |
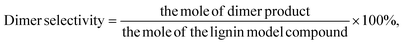 | (3) |
 | (4) |
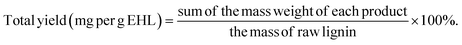 | (5) |
3 Results
3.1 Catalytic activity
| Catalysts | Conv. (%) | Selec. (%) | |||
|---|---|---|---|---|---|
| Cyclohexane | Cyclohexanol | Ethylbenzene | Ethylcyclohexane | ||
| Fe/AlOx | — | — | — | — | — |
| Ni/NiAlOx | 21.3 | 0.8 | 10.3 | 4.1 | 6.1 |
| Ni9Fe0.5/NiAlOx | 91.3 | 2.1 | 37.6 | 20.8 | 30.8 |
| Ni9Fe1/NiAlOx | 99.4 | 2.1 | 48.0 | — | 49.3 |
| Ni9Fe1.5/NiAlOx | 63.2 | 1.4 | 26.4 | 10.2 | 25.2 |
| Blank | — | — | — | — | — |
The results of the PPE hydrogenation reaction in different solvents and with or without hydrogen pressure are presented in Table S1.† In the absence of externally supplied hydrogen, the reaction with alcohol solvents and water did not proceed, indicating that the hydrogen supply is crucial for low-temperature hydrogenation. As displayed by the data in Table S1,† when hydrogen pressure was applied, the reaction occurred, but PPE conversion was significantly lower than that in the reaction in n-dodecane, decreasing to 33.4% in isopropanol, 19.8% in ethanol, 10.1% in methanol, and 8.5% in water.
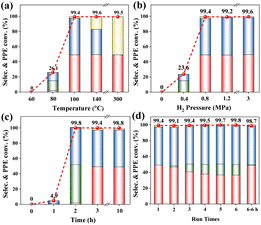
Fig. 1 illustrates the significant variation in product selectivity during the hydrogenation of PPE over time. Initially, at 0 min, no products are observed, indicating that the reaction has not yet begun. At 30 min, the conversion reaches 7.8%, with phenol (4%) and ethylbenzene (3.8%) as the primary products, while cyclohexanol and cyclohexanone are absent. At 60 min, conversion increases to 14.4%, with cyclohexanone (0.5%), phenol (6.9%), and ethylbenzene (7%) detected, marking the onset of phenyl group hydrogenation. By 90 min, conversion increases to 39.8%, yielding cyclohexanol (8.3%), phenol (10.6%), ethylbenzene (19.7%), and cyclohexanone (1.2%), suggesting an accelerated formation of cyclohexanol. At 120 min, conversion reaches 52.1%, with cyclohexanol (9%), phenol (15.3%), and ethylbenzene (27.8%) as major products, while cyclohexanone remains negligible. At 150 min, conversion climbs to 62.8%, accompanied by a shift toward saturated intermediates, with cyclohexanol (10.9%), phenol (17.5%), ethylbenzene (33.2%), and ethylcyclohexane (1.2%) detected. At 180 min, conversion further increases to 82.4%, with cyclohexanol (40.1%) dominating, phenol decreasing to 3.2%, and ethylbenzene at 35.1%, along with trace amounts of ethylcyclohexane. At 210 min, conversion reaches 96.8%, with cyclohexanol (51.4%) and ethylcyclohexane (20.9%) as the major products, while ethylbenzene decreases to 24.5%. Finally, between 240 and 300 min, conversion stabilizes at ∼99%, with cyclohexanol (50.3–50.5%) and ethylcyclohexane (48.4%) dominating, while phenol and ethylbenzene are no longer detected, indicating complete saturation of the aromatic substrate.
The H2 pressure effects are illustrated in Fig. 2b. Without hydrogen input, conversion does not occur. When the H2 pressure is 0.4 MPa, the PPE conversion is 23.6% and the product contains 8.4% cyclohexanol and 15.2% ethylbenzene. At 0.8 MPa H2, PPE conversion is 99.4% and the product has 48.0% cyclohexanol, 49.3% ethylcyclohexane, and 2.1% cyclohexane. Further increasing the H2 pressure to 1.2 and 3 MPa resulted in negligible changes in conversion and product selectivity, suggesting that an increase in H2 pressure does not lead to PPE hydrogenation and deep hydrogenation of the products.
The effect of the reaction time is plotted in Fig. 2c. During the first 1 h, only 4.9% PPE is converted, and the product contains cyclohexanol and ethylbenzene. At 2 h, the conversion increases drastically to 99.8%, with selectivity to 50.2% ethylbenzene, 48.0% cyclohexanol and 0.6% cyclohexane. At 3 h, the conversion is 99.4%, and the product has 49.3% ethyl cyclohexane, 48.0% cyclohexanol and 2.1% cyclohexane. When the reaction time is extended to 10 h, the conversion is measured as 99.8%, and the composition of the product is similar to that measured at 3 h. At this 100 °C temperature, the yield of cyclohexanol is stabilized, i.e. no deeper hydrogenation.
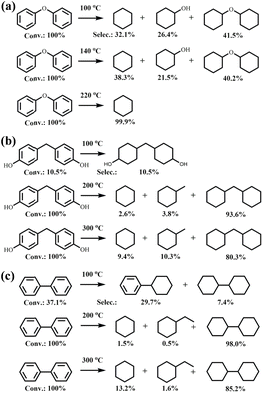
Moreover, the hydrogenation reactions of 4,4′-dihydroxydiphenylmethane and biphenyl at 100, 200, and 300 °C were carried out (Fig. 3b and c). With an increase in temperature, a gradual C–C bond cleavage occurs; however, the percentage of cleaved products is relatively low, indicating that the catalysts demonstrate high activity for the hydrogenation of aromatic rings. Further experiments were conducted with 4-phenylphenol, cyclohexyloxy-cyclohexane, phenylcyclohexane, and bicyclohexyl, and the results are shown in Fig. S1.† The conversion of 4-phenylphenol is only 13.4%, dominated by the hydrogenation product of the benzene ring; the hydroxyl group is not removed, and the C–C bond is not broken in this reaction. Partial bond-breaking products (7.8% cyclohexane) appear in the phenylcyclohexane reaction, while no C–O bond-breaking and C–C bond-breaking occur in the cyclohexyloxy-cyclohexane and bicyclohexyl reactions, respectively, which implies that when the benzene ring is fully hydrogenated, a further reaction does not occur, indicating low activity in cleaving Caliphatic–Caliphatic bonds despite its bond dissociation energy. Additionally, the weaker aliphatic peak in the in situ FT-IR spectra confirms the significant role of adsorption on the catalyst surface in its activation.19
3.2 Results of in situ characterization
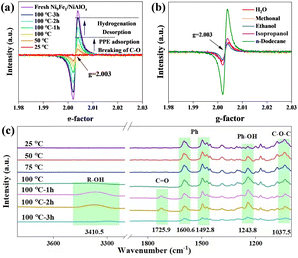
In situ EPR curves during the PPE hydrogenation reaction are shown in Fig. 5a. The catalyst before PPE adsorption shows a strong characteristic signal at g = 2.003, which has been assigned as the oxygen vacancies (OVs).20 The EPR curve gives a straight line for the mixture of PPE and Ni9Fe1/NiAlOz at 25 °C, without any signal in the range. In the temperature range of 25 to 100 °C, this signal characterizing the amount of OVs increases, indicating the surface reaction and desorption of the oxygen species. The measurement was prolonged at 100 °C, and an enhancement of the OVs signal with time online is observed, implying the intensive elimination of the oxygen species simultaneously with the formation/desorption of cyclohexanol and ethylcyclohexane. The maximum value of the OV signal is achieved at 3 h; however, this value is lower than that of the pristine catalyst. As shown in Fig. 5b, the post-reaction catalyst in oxygenated solvent has a lower OV content than that in dodecane solvent, and the post-reaction catalyst in dodecane solvent has the EPR signal closest to the EPR signal of the fresh catalyst.After adsorbing PPE on the surface of Ni9Fe1/NiAlOz, FT-IR spectra were collected at different temperatures and time intervals under H2 flow. In Fig. 5c, the peaks at 1037.5, 1492.8, and 1600.6 cm−1 are attributed to the symmetrical stretching vibration of the C–O–C ether bond, bending, and stretching vibration of the benzene ring, respectively.21,22 The peaks at 1243.8, 1725.9, and 3410.5 cm−1 correspond to the stretching vibration of –OH in the phenolic hydroxyl, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond in the aldehyde, and –OH in alcohol hydroxyl groups, respectively.22,23 With an increase in temperature from 25 to 100 °C, the intensity of the phenol peak gradually increases, whereas the peak of the C–O–C ether bond weakens. Subsequently, with the increase in time from 1 to 3 h at 100 °C, the peak corresponding to the alcohol hydroxyl group appears, and the peaks of the benzene ring, C–O–C ether bond, and phenolic hydroxyl group gradually weaken. The C–O–C ether bond of PPE is hydrogenated and broken, generating phenol and ethylbenzene. Meanwhile, the peaks due to the C
O bond in the aldehyde, and –OH in alcohol hydroxyl groups, respectively.22,23 With an increase in temperature from 25 to 100 °C, the intensity of the phenol peak gradually increases, whereas the peak of the C–O–C ether bond weakens. Subsequently, with the increase in time from 1 to 3 h at 100 °C, the peak corresponding to the alcohol hydroxyl group appears, and the peaks of the benzene ring, C–O–C ether bond, and phenolic hydroxyl group gradually weaken. The C–O–C ether bond of PPE is hydrogenated and broken, generating phenol and ethylbenzene. Meanwhile, the peaks due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond and alcohol hydroxyl group appear at 1 h, and the C
O bond and alcohol hydroxyl group appear at 1 h, and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond peak disappears at 3 h, whereas the peak due to the alcohol hydroxyl group remains. Phenol undergoes benzene ring hydrogenation, generating ketone intermediates and the formation of cyclohexanol. The gradual decrease in the peak intensity corresponding to the benzene ring demonstrates the hydrogenation of ethylbenzene. Additionally, the peaks corresponding to aliphatic hydrocarbons are not detected because of their weak adsorption.
O bond peak disappears at 3 h, whereas the peak due to the alcohol hydroxyl group remains. Phenol undergoes benzene ring hydrogenation, generating ketone intermediates and the formation of cyclohexanol. The gradual decrease in the peak intensity corresponding to the benzene ring demonstrates the hydrogenation of ethylbenzene. Additionally, the peaks corresponding to aliphatic hydrocarbons are not detected because of their weak adsorption.
3.3 Catalyst characterization
 | ||
| Fig. 6 XRD patterns of (a) NixFey-LDH, (b) NixFeyAlOz and (c) NixFey/NiAlOz. (d) Details of 40–50° in NixFey/NiAlOz. | ||
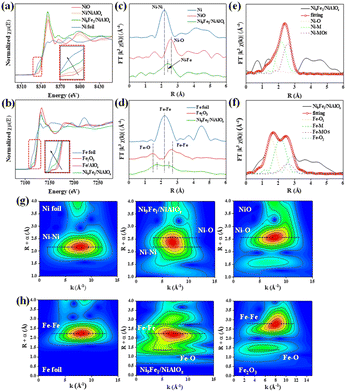
The XANES spectra at the Ni and Fe K-edges, depicted in Fig. 7a and b, reveal distinctive near-edge patterns situated between the reference curves of Ni foil, NiO, and Fe foil, Fe2O3. These unique features are different from those of Ni foil and Fe foil and coupled with the emergence of the absorption shoulder edges similar to oxides, suggesting a near-edge structure similar to those of oxides (Fig. S2†). In Fig. 7a, the white line intensities gradually decline in the order of Ni9Fe1/NiAlOz and Ni/NiAlOz, reflecting the electron-rich characteristics exhibited by the Ni species in Ni9Fe1/NiAlOz. Fig. 7b shows a lower white line intensity for the Fe K-edge in Ni9Fe1/NiAlOz compared to Fe/AlOz, which suggests that the Fe species in Ni9Fe1/NiAlOz exhibits a more electron-deficient character.25,26 Electron redistribution occurs between Ni and Fe and results in electron-rich Ni species in Ni9Fe1/NiAlOz.
Fig. 6c–f demonstrates the R-space data of the k2-weighted Ni and Fe K-edge Fourier transform EXAFS (FT-EXAFS) spectra of the corresponding reference samples, Ni9Fe1/NiAlOz and its fitting data. A single prominent coordination scattering peak at 2.4 Å, interposed between the Ni foil (2.1 Å) and NiO (2.6 Å) peak positions, is observed in Fig. 7c for Ni9Fe1/NiAlOz. In addition, the XANES threshold for Ni in the Ni9Fe1/NiAlOz shifted slightly to lower energies compared to the NiO, suggesting the formation of a Ni–M bond with a distance of 2.4 Å. Fe–Fe (2.5 Å) is present in Fe foil, while Fe–O (1.4 Å) and Fe–Fe (2.7 Å) in Fe2O3. In Fig. 7d, there are Fe–Fe and Fe–O bonds, but the Fe coordination environment changes and the corresponding bond lengths change to 1.7 Å for Fe–O and 2.4 Å for Fe–M, forming strong evidence for the existence of Ni–Fe bonds as the NiFe alloy phase. The absence of Ni–Ni and Fe–Fe peaks and the fact that the Ni–M and Fe–M bonds in Ni9Fe1/NiAlOz are displaced indicate the presence of a NiFe alloy phase and an M–O–M oxide structure. In Fig. 7d, the peak position of Fe–O in Fe2O3 is lower than that of Ni9Fe1/NiAlOz, and the Fe–Fe bond is not found. The changes in the Fe–O and Fe–Fe bonds are clear evidence of the formation of the NiFe alloy.
In Table S2,† the coordination numbers of Ni (Ni–M, Ni–O) in Ni9Fe1/NiAlOz are consistent with Ni and NiO, and this means that Ni species form the main part of the catalyst. The Fe–M coordination numbers of 6 and 5.8 in Table S3† indicate the absence of a full metal alloy, and a considerable number of Fe–O bonds appear, making it easier to generate multiple types of OVs. Fitting calculations to the R-space data of Ni9Fe1/NiAlOz catalyst are shown in Fig. 7e and f. In Fig. 6e, bonds located at 1.6 Å for Ni–O, 2.2 Å for Ni–M (distinguished from 2.1 Å for the Ni–Ni bond in the Ni foil), and 2.6 Å for Ni–MOs appear. In Fig. 7f, 1.5 Å of Fe–O1, 2.2 of Fe–M (distinguished from 2.17 Å of Fe–Fe bond in Fe foil), 2.6 Å of Fe–MO and 2.8 Å of Fe–O2 appear. The bond lengths of Ni–Fe in the fitted data for Ni and Fe–Ni in the fitted data for Fe are consistent with each other, as well as with Ni–MOs and Fe–MOs. The data indicate that Ni–Fe interacts closely with each other, forming the NiFe alloy and rich interface. The wavelet transform, with its pronounced differentiation in R spaces, serves as a complementary tool to FT-EXAFS for the microstructure determination of the catalyst metal phases. The K-edge WT-EXAFS for Ni and Fe are depicted in Fig. 7g and h, respectively. The coordination profiles of Ni and Fe change, and the regions of the corresponding bonds change. In this case, the Ni–M region in the catalyst is in the middle of Ni foil and NiO, and the change in the Fe coordination situation is more obvious, appearing in the Fe–O region and region change in Fe–M compared to Fe foil and Fe2O3. Moreover, quantitative FT-EXAFS fits were carried out to reveal structural parameters, as demonstrated in Tables S2 and S3.† The results indicate a high degree of fit, exhibiting an R factor of less than 0.009 and an internal potential correction (ΔE0) of less than ±10 eV. The catalysts show larger Ni–M bond lengths (2.6 Å/3.0 Å) than those of Ni foils (2.5 Å), with Fe showing a similar trend to that of Ni, and this directly proves the formation of the NiFe alloy phase.
In Fig. 8b, the H2-TPD profiles of the Ni/NiAlOz sample show two peaks at 193 and 390 °C, while the Ni9Fe1/NiAlOz catalyst exhibits two peaks at lower temperatures than those of the Ni/NiAlOz sample, at 181 and 374 °C. The presence of peaks at 181 and 374 °C in the TPD curve of the Ni9Fe1/NiAlOz sample may represent the desorption of chemisorbed hydrogen from the Ni–H species and from H* on the Ni surface, respectively.32 The Fe/AlOz sample demonstrates only one weak desorption peak at 514 °C. In Fig. 8b, Ni9Fe0.5/NiAlOz and Ni9Fe1.5/NiAlOz samples also give two peaks at 200 and 388 °C in their H2-TPD curves, but both the two peaks on the two curves appear later than those of Ni9Fe1/NiAlOz. Table 2 lists the overall H2 uptakes of the samples in H2-TPD. Ni/NiAlOz takes 0.41 mmol g−1 higher than that of Fe/AlOz sample, 0.34 mmol g−1. Higher H2 uptake is detected for the Ni9Fe0.5/NiAlOz, 0.44, and Ni9Fe1.5/NiAlOz, 0.42 mmol g−1, samples, suggesting that the optimized Fe amount favors H2 adsorption.33 Ni9Fe1/NiAlOz has the highest H2 absorption rate of 0.51 mmol g−1, which provides the possibility for low temperature and high efficiency HDO reaction.
| Catalysts | Ni/Fe ratioa | Ni/Fe ratiob | S BET (m2 g−1) | H2 uptake (mmolH2 g−1) |
|---|---|---|---|---|
| a The results are tested by ICP. b These results are obtained from SEM-EDS mapping. | ||||
| Ni/NiAlOz | — | — | 78.6 | 0.41 |
| Ni9Fe0.5/NiAlOz | 17.6 | — | 181.5 | 0.44 |
| Ni9Fe1/NiAlOz | 8.9 | 9.8 | 325.7 | 0.51 |
| Ni9Fe1.5/NiAlOz | 6.3 | — | 156.6 | 0.42 |
| Fe/AlOz | — | — | 28.9 | 0.34 |
The intensity of the hydrogen spillover was examined with the color change in the samples, as depicted in Fig. S3.† Initially, the mixture of catalyst samples and WO3 showed an identical bright yellow color. After 30 s of exposure to the hydrogen atmosphere, all the three samples changed their color. Fe/AlOz mixture still showed yellow, but the Ni/NiAlOz mixture had already turned grayish green, and the Ni9Fe1/NiAlOz mixture became dark blue. After 180 s, the Ni9Fe1/NiAlOz mixture was black, and the Ni/NiAlOz mixture turned into a bluish-gray, while Fe/AlOz had a sallow-like color. The rapidness of the color change indicates the strength of the hydrogen spillover phenomenon. Ni9Fe1/NiAlOz is the strongest, while Fe/AlOz is the weakest with Ni/NiAlOz in the middle.
 | ||
| Fig. 9 SEM images of (a) Ni9Fe1/NiAlOz, (b) Ni/NiAlOz and (c) Fe/AlOz. (d) HRTEM image and (e) TEM image and line scanning of Ni9Fe1/NiAlOz. (f) Elemental mappings of Ni9Fe1/NiAlOz. | ||
The Brunauer–Emmett–Teller (BET) specific surface area, catalyst composition and texture data are listed in Table 2. Ni9Fe1/NiAlOz sample has the largest specific surface area of 325.7 m2 g−1. The contents of Ni and Fe in Ni9Fe1/NiAlOz catalyst measured with the ICP technique are 47.83% and 10.89%, respectively, which are consistent with the prescription, indicating no loss of metals during the preparation procedure. Table 2 shows the SEM-EDS analysis of Ni9Fe1/NiAlOz catalyst surface composition, differing from the ICP analysis results of the bulk composition. The surface of the sample is Ni-enriched with a Ni/Fe ratio exceeding 9/1. Corroborating EDS mapping in Fig. 9f and ICP bulk analysis results, in Table 2, Fe is located in the interior of the particles.
For the Ni 2p3/2 XPS spectrum of the Ni9Fey/NiAlOz catalyst (Fig. 10a), the peaks at 852.6, 855.5, and 862.1 eV belong to Ni0, Ni2+, and a satellite peak, respectively.34 The Fe 2p3/2 XPS pattern (Fig. 10b) shows three peaks at 705.2, 711.3, and 714.3 eV, corresponding to Fe0, Fe2+, and Fe3+, respectively.35 The binding energy of Ni0 in Ni/NiAlOz is 0.3 eV higher than that in Ni9Fe1/NiAlOz, and the binding energy of Fe 2p3/2 in Ni9Fe1/NiAlOz catalyst exhibits a positive shift (0.4 eV) to that of the Fe/AlOz. The XPS results of Ni 2p and Fe 2p demonstrate that electrons are transferred from Fe to Ni.
 | ||
| Fig. 10 Deconvoluted XPS spectra of the (a) Ni 2p, (b) Fe 2p and (c) O 1s from the NixFey/NiAlOz samples. (d) EPR patterns; (e) CO-DRIFT curves of NixFey/NiAlOz. | ||
The high-resolution O 1s spectra of the NixFey/NiAlOz samples are illustrated in Fig. 10c. The peaks at 530.2 eV (O(I)), 531.8 eV (O(II)) and 532.8 eV (O(III)) are attributed to lattice oxygen, OVs, and chemisorbed oxygen, respectively.36,37 The ratios of OVs in different samples are presented in Fig. 10c. The OV ratios of Ni9Fey/NiAlOz samples are considerably higher than those of Ni/NiAlOz (0.33) and Fe/AlOz (0.31). The content of OVs increases and then decreases with the increase in doped Fe content, where it is 0.37 for Ni9Fe0.5/NiAlOz and 0.35 for Ni9Fe1.5/NiAlOz and takes the maximum with the Ni9Fe1/NiAlOz (0.41). The OV concentrations are validated using the EPR technique, and the results are plotted in Fig. 10d, exhibiting that the signal strengths of the OVs (g = 2.003) show the same sequence as shown by the XPS technique,20 which confirms that the incorporation of a suitable amount of Fe leads to more OVs.
For the NixFey/NiAlOz samples, FT-IR analysis was performed with CO adsorption (see Fig. 10e). The Fe/AlOz sample did not adsorb CO. Typically, CO adsorption on Ni sites can be categorized into two distinct types: bridge type, at frequencies below 1980 cm−1, and linear type, in the frequency range above 2050 cm−1.38 A linear adsorption peak, approximately 2058 cm−1, and a bridge peak, 1954 cm−1, of Ni/NiAlOz are observed in Fig. 10e. The linear, now at 2047 cm−1, and bridge, 1943 cm−1, adsorption peaks of Ni9Fey/NiAlOz are redshifted compared to those of the Ni/NiAlOz sample, also indicating electron transfer from Fe to Ni atom.
4 Discussion
A key finding of our study is the ability to fully depolymerize PPE, a common β-O-4 bonds that represent compounds, into alkanes under exceptionally mild conditions (100 °C, 0.8 MPa H2, and 3 h). Although previous efforts have achieved partial lignin depolymerization or the conversion of model linkages at higher temperatures (150–300 °C), none have shown complete decomposition to single compounds below 150 °C (Table S4†). Notably, our Fe–Ni catalyst surpasses the traditionally used monometallic systems. The synergistic hydrogen activation inferred from numerous characterized structures demonstrates that the catalytic configuration of the alloy-coupled oxygen vacancies greatly facilitates hydrogenation and deoxygenation reactions. The activity exhibited by our simple, scalable catalyst system compares favourably even with more advanced nanomaterials. In summary, this study demonstrates the most active lignin model compound depolymerization reported to date, highlighting the potential of multi-locus cooperativity between transition metals to advance biomass upgrading.4.1 Catalytic performance
Analysis of PPE deoxygenation product distributions at 100 °C, 140 °C, and 300 °C revealed that 100 °C selectively cleaves C–O bonds and saturates aromatic rings, whereas higher temperatures completely remove oxygen. Mono-Fe and mono-Ni catalysts exhibited substantially lower activity than the bifunctional Ni9Fe1/NiAlOz catalyst containing a Ni3Fe1 alloy phase, confirming that the alloy enables cooperative C–O activation via intimate Ni–Fe interfaces (Table 1).33 The predominant products at 80 °C (Fig. 2a) and 0.4 MPa H2 (Fig. 2b) cyclohexanol and ethylbenzene, respectively, suggest that the active sites selectively cleave the C–O bond to phenol while saturating the aromatic ring to cyclohexanol without hydrogenating the ethylbenzene ring. The combination of the reaction results with different hydrogen pressures and in situ FT-IR/EPR shows that temperature is crucial for the reaction, and both mobile and fixed hydrogen are efficiently involved in the reaction. Moreover, the reaction progress could be tuned by time to selectively optimize the yields of ethylbenzene, cyclohexanol, or cyclohexane–cyclohexanol mixtures (Fig. 2c). Near-complete conversion within 2 to 3 h (Fig. 2d) validated the high activity attributable to abundant active sites. Ethylbenzene hydrogenation at elevated temperatures benefited from sufficient energy and hydrogen supply. As the reaction intensity increases (higher temperature, higher H2 pressure and longer time), ethylcyclohexane/cyclohexane and cyclohexanol become the major products, indicating that the hydrogenation reaction occurs sequentially, rather than simultaneously, at different spatially active sites on the catalyst surface.The differential reactivity of dimers provides insight into the catalyst's distinct handling of C–O versus C–C bonds. At 100 °C, Fig. 3a shows that aromatic hydrogenation predominates for diphenyl ether over sluggish C–O cleavage, limited by ether oxygen ligation. In contrast, Fig. 3b demonstrates efficient dehydroxylation and ring hydrogenation for the 4,4′-dihydroxydiphenylmethane, attributed to phenolic hydroxyl group participation. Fig. 3c shows that although biphenyl did not undergo bond cleavage, aromatic hydrogenation alone enabled a conversion of 37.1%. Biphenyl has a higher reactivity at low temperatures compared to 4-phenylphenol (Fig. S1†), and this higher reactivity can be attributed to the lack of oxygen, which reduces competitive adsorption and thus promotes hydrogenation. Temperature effects observed for PPE transformations similarly impacted various dimers. Sufficient energy facilitated the complete cleavage of the C–O bond and fully hydrogenated products in diphenyl ether, alongside the cleavage of the C–C bond yielding alkylcycloalkanes. However, most C–C dimers converted to fully saturated bicyclic alkanes without bond cleavage.
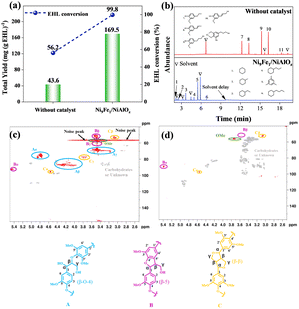
The EHL reaction produced a monomer of 169.5 mg per g EHL, with the remaining oligomer undetectable by GC-MS. However, the monomer yield exceeded the theoretical yield obtained from all C–O bond breaks in EHL, with excess coming from C–C bond break products. In the NMR results (Fig. 4c and d), the disappearance of the β-O-4 bond signal region and the attenuation of the –OMe signal region proved that the catalyst could achieve effective HDO, and the reduction of the C–C bond signal region provided evidence for monomer yields beyond the theoretical yields.8,15
4.2 Structure of the catalyst
The Ni9Fe1/NiAlOz catalyst exhibits superior hydrogenation and deoxygenation performance towards the conversion of PPE and EHL. This stems from the synergistic interplay between a high concentration of OVs and abundant Ni–Fe active sites. At the atomic scale, the mixed oxide phase features a unique two-dimensional layered (2DL) architecture in Ni9Fe1/NiAlOz, which is rich in interfacial OVs around the NiFe alloy clusters. This increases the contact probability of reactants by increasing the step, corner and edge densities. The enlarged surface area and defects give the catalyst a higher H2 adsorption capacity, which is a key to activating and supplying hydrogen. In addition, the core–shell structure, in which the Ni site is fully exposed and the Fe with weak hydrogenation activity is coated, is combined with the 2DL structure, which strengthens the H adsorption ability.10The specific adsorption of substrates and the activation of H are then related to the refined sites in the catalyst. According to the results of XAFS and XRD, Ni9Fe1/NiAlOz has Ni3Fe1 alloy phase and the appropriate distance of Ni–Fe bond, which is conducive to the stability of the catalyst. Nearby, enriched NiFe sites generated from Fe–doping induce the formation of dispersed ordered Ni3Fe1 alloy clusters, and these active phases readily dissociate H2 and spill over H, OH and oxygen species. In addition, the coordination of Ni and Fe contains Ni–O and Fe–O bonds, suggesting that a large amount of M–O is contained outside the coordinating atoms of Ni3Fe1, which directly leads to the formation of abundant interfacial OVs and promotes H transport and activation.33 The results of XPS and CO-DRIFTS also proved that electron transfer from Fe tunes the electronic states of Ni to further boost its hydrogenation functionality. The results of the H2-TPR, H2-TPD, and WO3 discoloration experiments particularly demonstrate this, with high-velocity hydrogen spillover occurring in the catalysts in conjunction with a high content of OVs, to the extent that it serves as high hydrotreating activity in the later hydrogenation reactions of EHL and PPE.32,41 Ni9Fe1/NiAlOz catalyst tends to saturate the carbon–oxygen bond more than the aromatic ring at first, thus simplifying the product distribution. Cooperative interactions between OVs, Ni–Fe alloy and adjusted surface chemistry collectively endowed the Ni9Fe1/NiAlOz with unrivalled activity for the challenging transformation of EHL and lignin derivatives.
4.3 Relationship of structural activity
Table 1 shows catalysts Ni9Fe1/NiAlOz and Fe/AlOz differing in activity for PPE conversion, with Ni9Fe1/NiAlOz (Ni3Fe1) significantly outperforming Fe/AlOz (FeAlOz). Catalysts Ni/NiAlOz and Ni9Fe1/NiAlOz contain different nickel species, and Ni9Fe1/NiAlOz (Ni3Fe1) catalyzes PPE at a much higher rate than Ni/NiAlOz catalyst (single Ni). Nickel sites facilitate C–O bond breaking. Ni3Fe1 contains both electron-rich Ni and electron-deficient Fe, and the ability to activate H is much higher than that of single Ni. Moreover, the Ni/NiAlOz catalyst has few OVs, limiting C–O bond adsorption and yielding irregular products.10Ni9Fe1/NiAlOz catalyst abundantly contains OVs around Ni3Fe1 that preferentially coordinate the C–O bond, directing its reaction through a streamlined pathway. The results in Fig. 5a and b illustrate the importance of OVs as important adsorption and hydrogenation sites. The former is shown by the in situ EPR results that the disappearance of OVs due to the complete adsorption of PPE at the beginning of the reaction, but the huge increase in the number of OVs at 2 h and 3 h is due to the desorption of cyclohexanol in the product. The latter is evident from the experimental data of PPE conversion in different solvents (Table S1†), which shows that the occupation of OVs greatly slows down the hydrogenation bond-breaking reaction. The core–shell structure of the Ni9Fe1/NiAlOz catalyst encapsulates the hydrogenation-inert Fe cores, exposing oxygen-vacancy-rich MO shell sites that promote surface H2 adsorption. As evidenced by temperature-programmed reduction and X-ray absorption fine structure spectroscopy, the robust interface between Ni3Fe1 cores and shells facilitates the stabilization of active catalytic sites with excellent reproducibility.42 The OV-rich shells are hypothesized to spillover hydrogen to Ni3Fe1 catalytic sites, generating highly reactive hydrogen species to enable regioselective C–O cleavage and aromatic ring hydrogenation.43,44 Notably, the synergistic cooperation between Ni3Fe1 and interfacial OVs in Ni9Fe1/NiAlOz catalyst is vital for efficient PPE conversion, outperforming single-component catalysts.
The specific reaction pathway for PPE in Scheme 2 is shown by summarizing the data in Subsection 3.1 (Fig. 1 and 2) and in situ FT-IR. The bond of C–O–C breakage followed this path: (1) C–O bond cleavage preferentially produced alkylphenols and arenes; (2) alkylphenols underwent ring saturation; (3) arenes underwent hydrogenative ring saturation; and (4) if the energy is sufficient, cyclohexanol dehydroxylates to form cyclohexane. (2-Cyclohexyloxy)benzene and (2-(cyclohexyloxy)ethyl)benzene were not detected throughout the reaction of PPE, indicating that the C–O bond in PPE is firmly adsorbed onto the interfacial oxygen vacancies composed of Fe atoms. This adsorption weakens the C–O bond energy, leading to the initial bond cleavage occurring before the hydrogenation of the aromatic ring. Even though the Ni sites have a good adsorption capacity for aromatic rings, the same is true for the preferential hydrogenation of phenol over ethylbenzene, and the involvement of the sites constituted by Fe is the fundamental reason the catalyst can produce specificity. Overall, judicious engineering of the bimetallic core–shell interface and attendant defects optimizes catalytic architectures for challenging biomass upgrading transformations. Further studies will provide deeper insights into reaction mechanisms and guide the design of specialized catalyst systems.
5 Conclusions
In this work, a hydrotalcite-derived NiFe catalyst was synthesized via co-precipitation and was partially reduced to produce a highly active and stable Ni9Fe1/NiAlOz catalyst. Using PPE as a lignin model, the Ni9Fe1/NiAlOz catalyst exhibited 99.4% PPE conversion with 56.8 and 40.5% yields of ethyl cyclohexane and cyclohexanol, respectively, under mild conditions of 100 °C and 0.8 MPa H2. This catalyst exhibited high activity with actual EHL with a total yield of monomers 169.5 mg per g EHL at 300 °C and 3 MPa H2. After recycling 6 times, 99.8% PPE conversion was still maintained.The results of catalyst characterization and controlled catalytic in situ experiments further revealed the structure of the catalyst and the synergistic effect of active sites. After the reduction of the catalyst, the metal structure in the LDH changes. Although the EXAFS results indicate that the present form of metal in the catalyst is dominated by oxides, partial reduction of Ni in the catalyst yielded Ni3Fe1 alloy. However, the remaining Ni species existed as NiAlOz, resulting in abundant OVs and a large surface area of about 325.7 m2 g−1. The presence of an alloy phase is necessary, leading to the electron-rich phenomenon of Ni and stabilizing it, which is an important basis for the existence of high activity of the catalyst. Moreover, the presence of rich OVs further promotes the hydrogenation reaction steps. In the Ni9Fe1/NiAlOz catalyst, the synergistic effects of reactive metal species and OVs supported its excellent EHL depolymerization and hydrogenation activities. In this work, a multi-active site synergistic high hydrogenation activity of a hydrotalcite-derived alloy catalyst strategy is established to decompose lignin into valuable monocyclic products with a high yield and provides new insights into the development of catalysts for abundant biomass conversion.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Author contributions
Hairui Jiao performed the catalyst preparation, characterization studies and reaction tests and wrote the draft. Yushuai Sang facilitates further in-depth analysis of catalyst characterization results Hong Chen and Yongdan Li conceived the idea and revised the paper.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work has received funding from the European Union's Horizon 2020 research and innovation program, (Building a low-carbon, climate resilient future: secure, clean and efficient energy) under Grant Agreement No. 101006744. The content presented in this document represents the views of the authors, and the European Commission has no liability in respect of the content. Dr Chen would like to express her gratitude to the National Natural Science Foundation of China (21690083 and 21808163).References
- Z. Cai, J. Long, Y. Li, L. Ye, B. Yin, L. J. France, J. Dong, L. Zheng, H. He, S. Liu, S. C. E. Tsang and X. Li, Chem, 2019, 5, 2365–2377 CAS
.
- C. Zhang and F. Wang, Acc. Chem. Res., 2020, 53, 470–484 CrossRef CAS PubMed
.
- X. Shen and R. Sun, Carbohydr. Polym., 2021, 261, 117884 CrossRef CAS PubMed
.
- J. Bai, Y. Zhang, X. Zhang, C. Wang and L. Ma, ACS Sustainable Chem. Eng., 2021, 9, 7112–7119 CrossRef CAS
.
- Y. Cao, G. Zhu, Y. Li, N. L. Breton, C. Gourlaouen, S. Choua, J. Boixel, H.-P. J. D. Rouville and J.-F. Soulé, J. Am. Chem. Soc., 2022, 144, 5902–5909 CrossRef CAS
.
- M. Oregui-Bengoechea, I. Agirre, A. Iriondo, A. Lopez-Urionabarrene-chea, J. M. Requies, I. Agirrezabal-Telleria, K. Bizkarra, V. L. Barrio and J. F. Cambra, Top. Curr. Chem., 2019, 377, 36 CrossRef
.
- G. Xu, J. Guo, Y. Qu, Y. Zhang, Y. Fu and Q. Guo, Green Chem., 2016, 18, 5510–5517 RSC
.
- L. Dong, L. Lin, X. Han, X. Si, X. Liu, Y. Guo, F. Lu, S. Rudić, S. F. Parker, S. Yang and Y. Wang, Chem, 2019, 5, 1521–1536 CAS
.
- D. Wu, Q. Wang, O. V. Safonova, D. V. Peron, W. Zhou, Z. Yan, M. Marinova, A. Y. Khodakov and V. V. Ordomsky, Angew. Chem., Int. Ed., 2021, 60, 12513–12523 CrossRef CAS PubMed
.
- B. Chen, C. He, M. Cao, X. Qiu, X. Ouyang and Y. Qian, Green Chem., 2022, 24, 846–857 RSC
.
- F. Mauriello, E. Paone, R. Pietropaolo, A. M. Balu and R. Luque, ACS Sustainable Chem. Eng., 2018, 6, 9269–9276 CrossRef CAS
.
- C. Chen, D. Wu, P. Liu, H. Xia, M. Zhou, X. Hou and J. Jiang, React. Chem. Eng., 2021, 6, 559–571 RSC
.
- Q. Han, M. U. Rehman, J. Wang, A. Rykov, O. Y. Gutierrez, Y. Zhao, S. Wang, X. Ma and J. A. Lercher, Appl. Catal., B, 2019, 253, 348–358 CrossRef CAS
.
- Z. Sun, G. Bottari, A. Afanasenko, M. C. A. Stuart, P. J. Deuss, B. Fridrich and K. Barta, Nat. Catal., 2018, 1, 82–92 CrossRef CAS
.
- D. Yang, J. Huang, Z. Hu, F. Miao, Z. Zhang, Y. Xie, S. Liu, Q. Wang and C. U. Pittamn Jr, Fuel, 2024, 357, 129982 CrossRef CAS
.
- F. Yan, R. Ma, X. Ma, K. Cui, K. Wu, M. Chen and Y. Li, Appl. Catal., B, 2017, 202, 305–313 CrossRef CAS
.
- O. Hu, M. Lu, M. Cai, J. Liu, X. Qiu, C. Guo, C. Zhang and Y. Qian, Adv. Mater., 2024, 36, 2407129 CrossRef CAS PubMed
.
- J. Shen, M. Cai, G. Li, C. Guo, X. Qiu and Y. Qian, Adv. Funct. Mater., 2024, 2413597 CrossRef
.
- H. Duan, J. Dong, X. Gu, Y.-K. Peng, W. Chen, T. Issariyakul, W. K. Myers, M.-J. Li, N. Yi, A. F. R. Kilpatrick, Y. Wang, X. Zheng, S. Ji, Q. Wang, J. Feng, D. Chen, Y. Li, J. C. Buffet, H. Liu, S. C. Edman Tsang and D. O'Hare, Nat. Commun., 2017, 8, 591 CrossRef PubMed
.
- H. Zhou, M. Wang and F. Wang, Joule, 2021, 5, 3031–3044 CrossRef CAS
.
- L. Huang, F. Tang, P. Liu, W. Xiong, S. Jia, F. Hao, Y. Lv and H. Luo, Fuel, 2022, 327, 125115 CrossRef CAS
.
- T. Qin, Q. Lu, H. Xiang, X. Luo and S. Yuan, Energy, 2023, 128374 CrossRef CAS
.
- G. S. Foo, A. K. Rogers, M. M. Yung and C. Sievers, ACS Catal., 2016, 6, 1292–1307 CrossRef CAS
.
- L. Nie, P. M. de Souza, F. B. Noronha, W. An, T. Sooknoi and D. E. Resasco, J. Mol. Catal. A: Chem., 2014, 388–389, 47–55 CrossRef CAS
.
- M. Chu, X. Wang, X. Wang, P. Xu, L. Zhang, S. Li, K. Feng, J. Zhong, L. Wang, Y. Li, L. He, M. Cao, Q. Zhang, L. Chi and J. Chen, J. Am. Chem. Soc., 2024, 146, 10655–10665 CrossRef CAS PubMed
.
- X. Wang, Y. Tong, W. Feng, P. Liu, X. Li, Y. Cui, T. Cai, L. Zhao, Q. Xue, Z. Yan, X. Yuan and W. Xing, Nat. Commun., 2023, 14, 3767 CrossRef PubMed
.
- R. Insyani, M.-K. Kim, J.-W. Choi, C.-J. Yoo, D. J. Suh, H. Lee, C. S. Kim, K. H. Kim, K. Kim and J.-M. Ha, Chem. Eng. J., 2022, 446, 136578 CrossRef CAS
.
- F. Liu, H. He and L. Xie, ChemCatChem, 2013, 5, 3760–3769 CrossRef CAS
.
- Y. Gao, Z. Zhao, H. Jia, X. Yang, X. Lei, X. Kong and F. Zhang, J. Mater. Sci., 2019, 54, 14515–14523 CrossRef CAS
.
- H. Fang, J. Zheng, X. Luo, J. Du, A. Roldan, S. Leoni and Y. Yuan, Appl. Catal., A, 2017, 529, 20–31 CrossRef CAS
.
- Y. Zhai, C. Li, G. Xu, Y. Ma, X. Liu and Z. Ying, Green Chem., 2017, 19, 1859–1903 RSC
.
- Z. Liu, Y. Yu, Y. Liu, A. Ying, X. Zhang and Y. Wang, ACS Catal., 2024, 14, 2115–2126 CrossRef CAS
.
- X. Yu, J. Chen and T. Ren, RSC Adv., 2014, 4, 46427–46436 RSC
.
- M. C. Biesinger, B. P. Payne, A. P. Grosvenor, L. W. M. Lau, A. R. Gerson and R. S. C. Smart, Appl. Surf. Sci., 2011, 257, 2717–2730 CrossRef CAS
.
- Z. Tian, X. Liang, R. Li, C. Wang, J. Liu, L. Lei, R. Shu and Y. Chen, Fuel, 2022, 308, 122034 CrossRef CAS
.
- J. Bao, X. D. Zhang, B. Fan, J. Zhang, M. Zhou, W. Yang, X. Hu, H. Wang, B. Pan and Y. Xie, Angew. Chem., Int. Ed., 2015, 54, 7399–7404 CrossRef CAS PubMed
.
- F. Cheng, T. Zhang, Y. Zhang, J. Du, X. Han and J. Chen, Angew. Chem., Int. Ed., 2013, 52, 2474–2477 CrossRef CAS PubMed
.
- W. Liu, Y. Yang, L. Chen, E. Xu, J. Xu, S. Hong, X. Zhang and M. Wei, Appl. Catal., B, 2021, 282, 119569 CrossRef CAS
.
- X. Zhang, J. Wu, T. Li, C. Zhang, L. Zhu and S. Wang, Chem. Eng. J., 2022, 429, 132181 CrossRef CAS
.
- Y. Ma, Y. Sang, K. Wu, Q. Liu, H. Chen and Y. Li, Catal. Today, 2023, 408, 194–203 CrossRef CAS
.
- Q. Hu, L. Yang, G. Fan and F. Li, J. Catal., 2016, 340, 184–195 CrossRef CAS
.
- H. Jiao, G. Xu, Y. Sang, H. Chen and Y. Li, Catal. Today, 2024, 430, 114542 CrossRef CAS
.
- J. Song, Z. Huang, L. Pan, J. Zou, X. Zhang and L. Wang, ACS Catal., 2015, 5, 6594–6599 CrossRef CAS
.
- J. Lu, J. Song, H. Niu, L. Pan, X. Zhang, L. Wang and J. Zou, Appl. Surf. Sci., 2016, 371, 61–66 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta07285e |
| This journal is © The Royal Society of Chemistry 2025 |