Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nanostructured Fe2O3/CuxO heterojunction for enhanced solar redox flow battery performance†
Jiaming
Ma
 ,
Milad
Sabzehparvar
,
Milad
Sabzehparvar
 ,
Ziyan
Pan
,
Ziyan
Pan
 and
Giulia
Tagliabue
and
Giulia
Tagliabue
 *
*
Laboratory of Nanoscience for Energy Technologies (LNET), STI, École Polytechnique Fédérale de Lausanne, 1015 Lausanne, Switzerland. E-mail: giulia.tagliabue@epfl.ch
First published on 27th November 2024
Abstract
Solar redox flow batteries (SRFB) have received much attention as an alternative integrated technology for simultaneous conversion and storage of solar energy. Yet, the photocatalytic efficiency of semiconductor-based single photoelectrodes, such as hematite, remains low due to the trade-off between fast electron hole recombination and insufficient light utilization, as well as inferior reaction kinetics at the solid/liquid interface. Herein, we present an α-Fe2O3/CuxO p–n junction, coupled with a readily scalable nanostructure, that increases the electrochemically active sites and improves charge separation. Thanks to light-assisted scanning electrochemical microscopy (photo-SECM), we elucidate the morphology-dependent carrier transfer process involved in the photo-oxidation reaction at an α-Fe2O3 photoanode. The optimized nanostructure is then exploited in the α-Fe2O3/CuxO p–n junction, achieving an outstanding unbiased photocurrent density of 0.46 mA cm−2, solar-to-chemical (STC) efficiency over 0.35% and a stable photocharge–discharge cycling. The average solar-to-output energy efficiency (SOEE) for this unassisted α-Fe2O3-based SRFB system reaches 0.18%, comparable to previously reported DSSC-assisted hematite SRFBs. The use of earth-abundant materials and the compatibility with scalable nanostructuring and heterojunction preparation techniques offer promising opportunities for cost-effective device deployment in real-world applications.
Introduction
Solar energy conversion offers a promising solution to meet the steadily increasing energy demand sustainably. Through the combination of photoelectrochemical cells (PEC) and redox flow batteries (RFB), solar energy can be efficiently converted and stored as chemical fuels by oxidizing or reducing various redox couples.1–3 The success of this all-in-one solar redox flow battery (SRFB) mainly depends on the design of the cell structure4,5 and the development of high-performance photoelectrodes.6,7Theoretically, the maximum solar-to-chemical (STC) efficiency of a single photoelectrode SRFB system can reach 16–18% if the bandgap of the absorber material is within 1.4–2 eV and the thermodynamic cell voltage is around 0.9 V and 0.7 V.8 Yet, practical realizations have not surpassed 3.9% STC efficiencies9 with limited upscale fabrication. This is related to band-alignment constraints for SRFB photocharging up to high states of charge, which favor wide-bandgap materials with limited solar light absorption.10 Additionally, low efficiency of charge separation dramatically reduces the performance of real devices. STC conversion efficiencies up to 21.1% have been instead achieved by exploiting complex device structures (e.g. dual photoelectrodes11) or more expensive tandem photoelectrodes (e.g. multi-junction solar cells12,13). Thus, the development of photoelectrodes with low cost and scalable manufacturing necessitates further investigation.14–18
Hematite (α-Fe2O3) is a promising photoanode material due to its stability, non-toxicity, low cost, abundance on Earth, and attractive band gap19,20 (1.9–2.2 eV). However, its performance is hindered by the trade-off between light-absorption and charge separation/transport, due to the short hole diffusion length21 and poor charge carrier conductivity.6,22 Hematite thin films23,24 have been used to reduce the charge carrier diffusion distance to the electrode/electrolyte interface at the expense of complete light absorption (a thickness of 40–100 nm is needed to absorb 450–550 nm light25). An effective approach to overcome this trade-off is the use of nanoengineered structures, which can shorten the charge carrier transfer length,26–28 increase the electrochemically active surface area,29 achieve light trapping,30 and induce optical resonances within the active photocatalyst material itself.31 Additionally, properly engineered heterojunction photoelectrodes, e.g. based on a p–n junction,32 can further improve the spatial separation of photogenerated electron–hole pairs,33 enhancing the photocatalytic activity. In the context of water-splitting or photosynthetic devices, a significant amount of effort has been devoted to proposing and designing heterojunctions aimed at efficiently extracting photo-holes from α-Fe2O3 catalysts.19,34–37 Yet, this approach has not been thoroughly explored in semiconductor-based SRFBs, despite major advantages in enabling higher state-of-charge and higher voltages during photocharging and discharging, respectively. Overall, nanoengineering and heterojunction design have a large untapped potential for improving single photoelectrode SRFB PEC performance.
In this work, we present a scalable, nanostructured α-Fe2O3/CuxO p–n junction and demonstrate its largely improved unassisted photocharging of an integrated solar redox flow battery (Fig. 1a). First, α-Fe2O3/CuxO films with varying thicknesses were systematically investigated to elucidate the impact of the p–n junction on the photoelectrochemical performance (Fig. 1b). Concurrently, light-assisted scanning electrochemical microscopy (photo-SECM) was employed to reveal enhanced charge separation in α-Fe2O3 nanopillar arrays (Fig. 1c). Finally, guided by the results from SECM, the optimized α-Fe2O3 nanostructure was integrated with the p–n junction strategy to enhance charge carrier separation while improving electrochemically active sites, thus resulting in a high performance photoanode. Our SRFB, featuring the nanostructured α-Fe2O3/CuxO p–n junction, demonstrates record values of unassisted photocurrent (0.46 mA cm−2), along with STC efficiency ∼ 0.35% and SOEE ∼ 0.18%, comparable to solar cell-assisted hematite-based devices (Table S1†). Overall, this α-Fe2O3-based SRFB shows a stable photocharge–discharge cycling performance and presents opportunities to drive real-world deployment of more cost-effective devices.
Experimental section
Sample preparation
The summary of the sample preparation process is shown in Table S2.†
Material characterization
The morphology and crystal structure of photoanodes were characterized by a scanning electron microscope (Zeiss Gemini SEM 300) and X-ray diffractometer (XRD, Rigaku Synergy-I single crystal). Ultraviolet photoelectron spectroscopy (UPS) was performed using a PHI VersaProbe II scanning XPS microprobe (Physical Instruments AG, Germany) equipped used with He(I) and He(II) UV source.Optical measurements
The UV-vis test for α-Fe2O3/CuxO film and nanostructured α-Fe2O3/CuxO were performed under a solar simulator (Newport 66984-300XF-R1 Xe lamp) with an AM 1.5 G filter as the light source, using a monochromator (Newport, CS260B-2-MC-A) connected with an integrating sphere (Newport, 819D-IS-5.3). The absorption data was obtained following our previous work.41For the α-Fe2O3 nanopillars, an inverted microscope (Nikon Eclipse Ti2) was used in combination with a grating spectrometer (Princeton Instruments Spectra Pro HRS-500) equipped with a Peltier-cooled 2D CCD detector (Princeton Instruments PIXIS 256) to record reflection (R) and transmission (T) spectra. The absorption (A) was determined as A = 1 − R − T. The detailed process is in accordance with previous reports.42,43
Photoelectrochemical measurements and photocharge–discharge
Linear Sweep Voltammetry (LSV), photocurrent density–time (j–t) and Electrochemical Impedance Spectroscopy (EIS) were used to evaluate the photoelectrochemical performance of different photoanodes and were recorded on a Biologic SP-300 potentiostat. LSV were tested both in dark and under illumination with a scan rate of 10 mV s−1; J–t was recorded without bias and the photo response signal were obtained with 20–20 s light on/off. EIS analyses were carried out at a perturbation amplitude of 10 mV with the frequency ranging from 0.01 Hz to 10 kHz. EC-Lab software was used to fit the measured EIS results. All tests were carried out by using a two-electrode configuration of the SRFB device44 (one photoanode in anolyte and one 39 AA carbon felt in catholyte, as shown in Fig. S2†) under 1 sun illumination (AM 1.5 G filter, 100 mW cm−2). All samples were back-illuminated through the ITO glass, and the illuminated area is 0.785 cm2.The photocharge–discharge behavior of our solar redox flow batteries was demonstrated via three electrodes integrated SRFB and was recorded by two potentiostats. During photocharging process, potentiostat 1 (SP-300) was connected to the PEC part to monitor the photocurrent, while potentiostat 2 (CHI 760E), connecting the RFB part, measured the evolution of the cell potential. During the discharging process, the solar simulator and potentiostat 1 were turned off, while a discharging current of 0.4 mA was applied by potentiostat 2 to RFB part until the cell potential reached 0 V.
During all these tests, the electrolyte recirculation was guaranteed by using two peristaltic pumps (30 ml min−1), both the anolyte (0.2 M Na4Fe(CN)6/1 M NaOH) and catholyte (0.1 M 2,7-AQDS/1 M NaOH) volume of the running system were 4 ml.
Scanning electrochemical microscopy
Light-assisted scanning electrochemical microscopy (photo-SECM) was implemented to investigate the photocatalytic activity of micro-array structures (100 × 100 μm2) of α-Fe2O3 nanopillars, using a previously described home-built instrument.42 Briefly, a home-built SECM was coupled with an inverted optical microscope (Nikon Eclipse Ti2) and a white light source (Energetiq EQ-99X-FC LDLS) for back-illumination of the structures. A three-electrode configuration was employed with Pt and Ag/AgCl as counter and reference electrodes, and Pt ultra-microelectrode (Pt UME) tip as the working electrode. The structured samples were unbiased and grounded. Tip to substrate distance was controlled by monitoring the tip current during its fine approach towards the substrate and lifting up the tip by 3 μm higher than the distance corresponding to 30% change from the bulk state. All experiments were performed in a 4 mM K4Fe(CN)64− and 0.4 M KOH solution, using a 1.2 μm radius UME tip (RG value = 14.5) biased at a reductive 0 V vs. Ag/AgCl tip potential, and modulating a ∼90 μm diameter collimated light beam having 80 mW m−2 power density.Numerical simulation
Electromagnetic simulations were performed using the RF module of COMSOL Multiphysics v6.1 to obtain the absorption spectra of α-Fe2O3 nanopillars and α-Fe2O3 film. For α-Fe2O3 nanopillars, a 3D unit cell model with periodicity of 300 nm, consisting of one α-Fe2O3 nanopillar (50 nm in height) on top of 10 nm α-Fe2O3 film/100 nm ITO film/fused silica substrate surrounded with a top layer of air, was simulated by setting the diameter of nanopillars from 100 nm to 250 nm with 10 nm step size. For α-Fe2O3 film simulations, a similar 3D unit cell model without the α-Fe2O3 nanopillar was performed by varying the film thickness from 20 to 180 nm with 10 nm step size. In both cases, perfect magnetic conductor and perfect electric conductor boundary conditions were used at the side walls of the unit cell. A port boundary condition was used at the bottom of the unit cell. The back illumination was applied with a normal incident plane wave (300–850 nm) with electric field polarization perpendicular to the film plane as well as recording the reflected wave. At the top of the unit cell, a second port boundary condition without excitation was used to record the transmitted wave. The refractive indices for α-Fe2O3 and ITO were taken from literatures45,46 Then the absorbed power was calculated by volume integration of the electromagnetic power loss density over the α-Fe2O3 volume.Results and discussion
α-Fe2O3/CuxO heterojunctions were fabricated via a facile and scalable method to enhance electron/hole separation in SRFB photoanodes. Specifically, after sputtering 15 nm Fe and between 15 nm and 45 nm Cu onto ITO, a NaOH treatment was used to convert Cu to Cu2O.38 Subsequently, annealing of the as-prepared composite leads to the formation of α-Fe2O3–Cu2O–CuO, concisely referred to α-Fe2O3/CuxO (see Experimental section). In the following, samples are named according to the thickness of the initial Fe and Cu film (e.g. 15/30F-CuxO for the heterostructure). We note that an approximately 2 fold expansion is expected for conversion from Fe to Fe2O3 (ref. 47) while a significant reduction in thickness, from 30 nm to 15 nm, is observed for the Cu to CuxO conversion duo to the partially dissolution of Cu into NaOH38 (Fig. S3, ESI†). As a control sample, we use a 15 nm Fe film that is annealed under the same conditions without NaOH treatment (sample 15F, see Experimental section).Fig. 1b shows the estimated band alignment for our α-Fe2O3/CuxO heterojunction, obtained by combining the measured bandgap from Tauc plot, work function from the Mott–Schottky technique and the valence band maxima from UPS (Fig. S4, ESI†). This is indeed crucial to assess the energy levels compatibility between the electrode/electrolyte. We confirmed that CuxO is a p-type semiconductor and that a p–n junction is formed at the α-Fe2O3/CuxO interface. While this is expected to promote charge separation, and hence the photoelectrochemical performance of the photoanode,33 it can reduce the possible theoretical discharge cell voltage (Fig. 1b), as the oxidation and reduction potentials of the chosen redox couples must lie within the p-type semiconductor valence band and the n-type semiconductor conduction band energies.33 We chose Fe(CN)64−/Fe(CN)63− as the anolyte (0.60 V vs. normal hydrogen electrode (NHE)) and AQDS/AQDS2− (−0.14 V vs. NHE) as the catholyte to evaluate the performance of the designed α-Fe2O3-based photoanodes.5
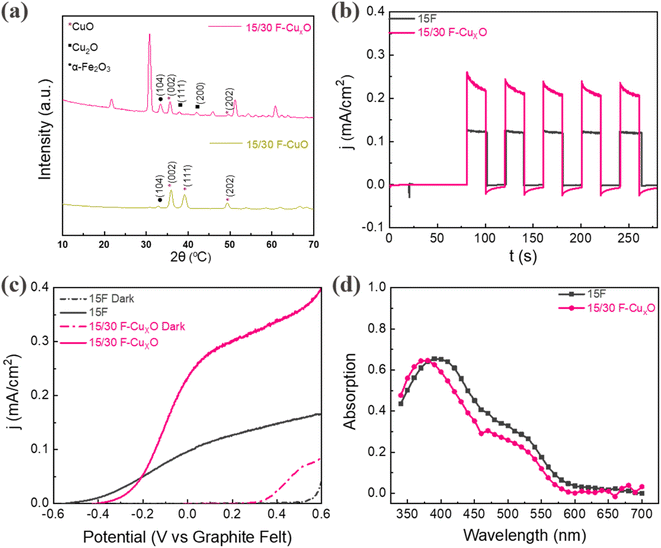
A two-electrode SRFB was used to perform photocurrent density–time (j–t) measurements and investigate the PEC performance of the photoanodes under 1 sun illumination. The NaOH treatment time and the thickness of sputtered Cu have been optimized, identifying 15/30F-CuxO as the best p–n junction film (Fig. S5, ESI†). XRD measurements (Fig. 2a, pink curve) confirm that the 15/30F-CuxO sample present the hematite phase of α-Fe2O3 and a mixture of Cu2O–CuO (CuxO). The sharp diffraction peaks at 2θ of 33.1° indicate the (104) plane of the rhombohedral structure of hematite.48 The peaks observed at 35.4° and 48.8° correspond to the (002) and (202) planes of CuO, while the peaks at 36.6° (111) and 42.2° (200) are characteristic peaks of Cu2O.49 The rest of the peaks can be attributed to ITO.50 Additionally, we observed that excluding the NaOH treatment of the Cu film (sample 15/30F-CuO, see Experimental section) results only in CuO diffraction peaks without any trace of Cu2O (Fig. 2a, green curve). From a morphological point of view, scanning electron micrographs (Fig. S6, ESI†) interestingly show that the 15/30F-CuxO heterojunction film exhibits a smoother surface and more uniform grains than the control hematite sample 15F. This can be attributed to the formation of CuxO during the annealing process of Cu2O on top of α-Fe2O3.
As shown in Fig. 2b, the unbiased photocurrent density for 15/30F-CuxO is 0.24 mA cm−2, two times higher than the control hematite sample (0.12 mA cm−2). Both 15/30F-CuxO and 15F show a stable pulse signal with instantaneous photoresponse, indicating their excellent photoactivity. The long-term operation (1 h) of 15/30F-CuxO also exhibits remarkable photocurrent stability, with a current retention of approximately 98% (Fig. S7, ESI†), indicating its robust structure as well as enduring photocatalytic stability. Additionally, linear sweep voltammetry (LSV) curves of these two photoanodes were measured in the same set up both under illumination and dark conditions with a sweeping rate of 10 mV s−1 (Fig. 2c). Indeed, the photocurrent onset of 15/30F-CuxO is observed around −0.42 V vs. carbon felt, slightly higher than that of 15F (−0.52 V), owing to the partial sacrifice of oxidation and reduction potentials by the p–n junction as discussed above. Given their consistent dark current onset (0.3 V), the photovoltage (defined here as the potential difference between dark and light current onset) of 15/30F-CuxO is smaller than that of 15F as expected. The unbiased photocurrent at 0 V in linear sweep voltammetry of these two photoanodes are in line with j–t tests, further demonstrating the significantly increased photocatalytic activity of the heterojunction.
When considering solely the photoelectrode components (i.e. excluding considerations of battery resistance losses and redox couple reaction activities), the theoretically achievable photocurrent is decided by the light absorption and the charge carriers transfer from the bulk to the electrode/electrolyte interface of semiconductors.41 The optical response of the bare α-Fe2O3 film was measured and compared with that of the 15/30F-CuxO in Fig. 2d. The UV absorption (340–400 nm) increases from 15F to 15/30F-CuxO, while the visible light absorption (400–600 nm) of 15F is slightly higher than 15/30F-CuxO. Overall, the total light absorption of the 15F and 15/30F-CuxO samples in the range 340–700 nm is 0.27 and 0.25 respectively. As these values are comparable, the primary factor influencing photocurrent becomes the photogenerated electron–hole separation process, indicating that the 15/30F-CuxO p–n junction exhibits better charge transfer than 15F. As a comparison, we tested thicker hematite films (30F and 50F) with higher absorption as well as their p–n junction counterparts (Fig. S8–S10, ESI†). Yet, in all cases due to the limited charge mobility within hematite,21 the measured photocurrent was lower than for the 15/30F-CuxO film. Electrochemical Impedance Spectroscopy (EIS) analysis (Fig. S11, ESI†) further confirmed the photocurrent measurements. The internal charge transfer resistance (Rsc) of 15/30F-CuxO is 1530 Ω, significantly lower than that of 15F (5430 Ω), suggesting efficient charge carrier transport within the bulk facilitated by the p–n junction. Additionally, the charge transfer resistance at the interface (Rct) decreases from 15F (4060 Ω) to 15/30F-CuxO (1050 Ω), indicating faster photooxidation reaction dynamics at the 15/30F-CuxO/ferrocyanide interface compared to the 15F/ferrocyanide interface. Hence, these two factors contribute to the higher photocurrent density of the planar 15/30F-CuxO p–n junction.
To improve charge transfer in thicker hematite films that exhibit higher light absorption (Fig. S8, ESI†), we explored the impact of nanoengineering strategies, which increase the available surface area and reduce the charge transport distances. Indeed, nanoscale patterning of a high-index semiconducting catalyst can create resonant interactions between the nanometer-scale semiconductor and light (Mie modes), which are strongly dependent on the dimensions and periodicity of the structure.51–53 Specifically, we realized arrays of Fe nanopillars with well-defined diameter, D, and periodicity, P, which were subsequently oxidized to obtain α-Fe2O3 nanopillars. A thin (∼10 nm) α-Fe2O3 film was left to cover the ITO substrate (see Fig. S1b† and Experimental section). Electromagnetic simulations (COMSOL Multiphysics®) were used to quantify the impact of D on the absorption spectrum of α-Fe2O3 nanopillar arrays based on a 30 nm thick iron (P = 300 nm). We observe that, despite the reduction in overall absorbing material, nanostructuring allows the excitation of optical resonance modes that entail a high absorption level (Fig. S9b, ESI†). In order to explore the optimal balance between optical performance and electron transfer, α-Fe2O3 nanopillar arrays with diameters of 100, 150, and 200 nm (named as P100, P150, and P200 respectively) were patterned via e-beam lithography over an area of approximately 100 × 100 μm2. The SEM top-view images of nanopillar arrays with different diameters are reported in Fig. 3b and S12a–f (ESI),† showing the increase in the pillar diameter upon annealing, due to oxygen incorporation. Microscale absorption measurements43 of the different arrays (Fig. S13, ESI†) were consistent with simulations and showed a minimal decrease in absorption compared to the unpatterned film.
To investigate the effect of nanopatterning on the photocatalytic performance of the α-Fe2O3 photoanodes, we performed photo-SECM on both films and nanopillar array structures in a 4 mM Fe(CN)64− and 0.4 M NaOH electrolyte solution under white light illumination. The reductively biased Pt UME tip in our experiments locally detects the photo-generated oxidant species, i.e. Fe(CN)63− (Fig. 3a). For all the measurements, we controlled the tip to substrate distance by monitoring tip current versus distance and positioning the tip at a distance 3 μm higher than the 30% offset value54 (Fig. S14, ESI†). Fig. 3c shows the time trace of the tip current for the α-Fe2O3 films having different thicknesses when the light is modulated on and off at the same power density. Analyzing the IT,ON/IT,OFF values shows that the photocatalytic activity of the film structures decreases by increasing the film thickness from 15F to 30F, and then to 50F. This is in agreement with the lower charge transfer rates observed in the SRFB photoresponse as film thickness increases (Fig. S10a, ESI†). Comparing the IT,ON/IT,OFF values for the α-Fe2O3 nanopillar arrays (Fig. 3d) with the best performing film structure (15F) shows a significant enhancement (more than 5 folds) in photocatalytic activity, not achievable by only decreasing the film thickness. Most importantly, the results show that the P150 α-Fe2O3 nanopillar array has the highest photocatalytic activity among all the samples, realizing an optimum combination of surface area, light absorption, and charge transport dynamics.31 Based on these local PEC results, we chose 150 nm as the optimum Fe nanopillar diameter for realizing centimeter-scale, nanostructured α-Fe2O3/CuxO photoanodes for SRFBs.
The nanostructured α-Fe2O3/CuxO (P/CuxO) photoanode was fabricated employing a combination of nanoengineering and heterojunction design, as illustrated in Fig. S1c (ESI).† A Langmuir–Blodgett (LB) technique, rather than e-beam lithography, was exploited to fabricate nanopatterns over cm-scale samples.39 A nanostructured α-Fe2O3 (P) without copper coating nor NaOH treatment was also fabricated as a control sample. The addition of the CuxO layer results in distinct morphological changes. Specifically, the P/CuxO exhibits nanorod bundles-like structures on the surface which are absent on the P sample (Fig. S15, ESI†). Optically, the P sample exhibits a total light absorption (340–700 nm range) of 0.34, similar to the unpatterned 30F film (0.36) while the P/CuxO sample exhibits a small but broadband absorption increase that results in a larger total absorption of 0.39 (Fig. 4a).
Photoelectrochemically, the nanostructured α-Fe2O3 (P) shows a photocurrent density of 0.2 mA cm−2 (Fig. 4b), nearly two folds higher than the best thin film sample, 15F, consistent with the SECM analysis. With the addition of the p–n junction, which improves both light harnessing and carrier transport, the P/CuxO sample exhibits an outstanding photocurrent density of 0.46 mA cm−2 as well as a good stability (Fig. S16, ESI†). Fig. 4b illustrates the variation of photocurrent density with applied voltage. At 0 V, the photocurrent density for P/CuxO and P are 0.49 mA cm−2 and 0.21 mA cm−2, respectively. The higher photocurrents compared to the photoresponse tests may arise from the transient current generated by the double-layer capacitance under voltage changes as well as the trapped photogenerated holes due to the existence of detrimental surface states at the electrode–electrolyte interface.55 Interestingly, the similar photovoltage (0.75 V) of both photoanodes suggest that the nanostructured p–n junction does not compromise its capability to drive these redox couples. EIS studies were also carried out under illumination to gain insight into the effect of the p–n junction on PEC redox oxidation reaction. The same electrical circuit was used as a model to fit the photooxidation process in the photoanodes (Fig. S17, ESI†). In contrast to P, the P/CuxO exhibits significantly lower values of Rsc (500 Ω) and Rct (757 Ω), indicating that the surface coverage of an additional CuxO layer can improve the charge separation inside bulk α-Fe2O3 as well as reduce the charge extraction barrier to create a facile carrier pathway at the electrode/electrolyte interface. Additionally, the space-charge capacitance (Csc) decreases from P/CuxO (1.4 × 10−3 F) to P (0.03 × 10−3 F), implying the broadening of the depletion layer of the P photoanode and thus much inferior carrier mobility.56–58
After demonstrating the photooxidation reaction activity and stability of the P/CuxO photoanodes, the photocharge/discharge test was finally performed using a fully integrated SRFB. The photocharge/discharge curves of the SRFB for the initial 20 cycles (approximately 22 h) are shown in Fig. 4c. The average photocurrent density is equal to 0.42 mA cm−2 (0.33 mA when considering the active area) under 1 sun illumination, with a discharging current applied of −0.4 mA. This integrated system exhibits a stable and high coulombic efficiency of around 90–98% and a stable average energy efficiency around 50% as shown in Fig. 4d. Overall, the P/CuxO-based SRFB achieves a stable solar-to-chemical efficiency of 0.35% and an average solar-to-output energy efficiency of 0.18% over 20 cycles, which is a significant progress for unassisted α-Fe2O3-based SRFB.
Conclusion
In summary, the synergistic design of nanostructuring and engineered heterojunction for photoelectrodes was realized to improve the SRFB performance. Firstly, we conducted a comprehensive investigation through band alignment engineering and various electrochemical techniques, elucidating the enhancement of carrier transport both within the bulk photoelectrode and at the photoelectrode/electrolyte interface facilitated by the CuxO–Fe2O3 p–n junction rather than CuO–Fe2O3. Secondly, leveraging the localized measurements of SECM, we are able to utilize microscale samples to seamlessly explore the nanostructure sizes effect on the photoelectrodes performance. Nanoengineering not only enables the manipulation of the optical properties of the photoelectrodes (including light trapping and Mie resonance), but also provides additional reactive sites, effectively mitigating losses attributable to charge recombination. Indeed, by combining these two strategies, the nanorod bundles-like P/CuxO exhibits the highest unbiased photocurrent density (0.46 mA cm−2) as well as good stability for unassisted α-Fe2O3-based SRFB. The average STC efficiency during photocharge process reaches 0.35% and the solar-to-output energy efficiency of the as-designed photoanode is 0.18%, a performance level previously achieved in hematite systems only with the assistance of external solar cells. Overall, the straightforward photoanode preparation process, the earth-abundant material choice along with the remarkable performance should be promising for the practical application of the solar rechargeable batteries. In addition, further advancements in the SRFB system can be anticipated, particularly in terms of microfluidic design to enhance mass transport and nanophotonic engineering to improve charge transfer and optical performance. These SRFB design concepts may open new avenues for reaching highly efficient solar redox flow batteries.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors acknowledge the support of the Swiss National Science Foundation (Eccellenza Grant #194181). J. M. acknowledges the support of the China Scholarship Council (201906210091). M. S. and G. T. thank the support of the EPFL Center for Imaging through the 2021 Interdisciplinary Project in Imaging. The authors also acknowledge the support of the following experimental facilities at EPFL: Interdisciplinary Centre for Electron Microscopy (CIME) and Center of MicroNano Technology (CMI). Finally, the authors would like to thank Dr Alan Bowman for his assistance with the discussion on absorption testing.References
- H.-C. Fu, W. Li, Y. Yang, C.-H. Lin, A. Veyssal, J.-H. He and S. Jin, An Efficient and Stable Solar Flow Battery Enabled by a Single-Junction GaAs Photoelectrode, Nat. Commun., 2021, 12(1), 156, DOI:10.1038/s41467-020-20287-w.
- K. Wedege, D. Bae, E. Dražević, A. Mendes, P. C. K. Vesborg and A. Bentien, Unbiased, Complete Solar Charging of a Neutral Flow Battery by a Single Si Photocathode, RSC Adv., 2018, 8(12), 6331–6340, 10.1039/C8RA00319J.
- H. Feng, D. Liu, Y. Zhang, X. Shi, O. C. Esan, Q. Li, R. Chen and L. An, Advances and Challenges in Photoelectrochemical Redox Batteries for Solar Energy Conversion and Storage, Adv. Energy Mater., 2022, 12(24), 2200469, DOI:10.1002/aenm.202200469.
- L. Cao, M. Skyllas-Kazacos and D.-W. Wang, Solar Redox Flow Batteries: Mechanism, Design, and Measurement, Adv. Sustainable Syst., 2018, 2(8–9), 1800031, DOI:10.1002/adsu.201800031.
- T. da Silva Lopes, P. Dias, R. Monteiro, A. Vilanova, D. Ivanou and A. Mendes, A 25 Cm2 Solar Redox Flow Cell: Facing the Engineering Challenges of Upscaling, Adv. Energy Mater., 2022, 12(5), 2102893, DOI:10.1002/aenm.202102893.
- J. Li, S. K. Cushing, P. Zheng, F. Meng, D. Chu and N. Wu, Plasmon-Induced Photonic and Energy-Transfer Enhancement of Solar Water Splitting by a Hematite Nanorod Array, Nat. Commun., 2013, 4(1), 2651, DOI:10.1038/ncomms3651.
- S. Yun, Y. Qin, A. R. Uhl, N. Vlachopoulos, M. Yin, D. Li, X. Han and A. Hagfeldt, New-Generation Integrated Devices Based on Dye-Sensitized and Perovskite Solar Cells, Energy Environ. Sci., 2018, 11(3), 476–526, 10.1039/C7EE03165C.
- D. Bae, G. M. Faasse, G. Kanellos and W. A. Smith, Unravelling the Practical Solar Charging Performance Limits of Redox Flow Batteries Based on a Single Photon Device System, Sustainable Energy Fuels, 2019, 3(9), 2399–2408, 10.1039/C9SE00333A.
- J. R. McKone, F. J. DiSalvo and H. D. Abruña, Solar Energy Conversion, Storage, and Release Using an Integrated Solar-Driven Redox Flow Battery, J. Mater. Chem. A, 2017, 5(11), 5362–5372, 10.1039/C7TA00555E.
- D. Bae, G. Kanellos, G. M. Faasse, E. Dražević, A. Venugopal and W. A. Smith, Design Principles for Efficient Photoelectrodes in Solar Rechargeable Redox Flow Cell Applications, Commun. Mater., 2020, 1(1), 1–9, DOI:10.1038/s43246-020-0020-7.
- S. Liao, X. Zong, B. Seger, T. Pedersen, T. Yao, C. Ding, J. Shi, J. Chen and C. Li, Integrating a Dual-Silicon Photoelectrochemical Cell into a Redox Flow Battery for Unassisted Photocharging, Nat. Commun., 2016, 7(1), 11474, DOI:10.1038/ncomms11474.
- W. Li, J. Zheng, B. Hu, H.-C. Fu, M. Hu, A. Veyssal, Y. Zhao, J.-H. He, T. L. Liu, A. Ho-Baillie and S. Jin, High-Performance Solar Flow Battery Powered by a Perovskite/Silicon Tandem Solar Cell, Nat. Mater., 2020, 19(12), 1326–1331, DOI:10.1038/s41563-020-0720-x.
- W. Li, H.-C. Fu, Y. Zhao, J.-H. He and S. Jin, 14.1% Efficient Monolithically Integrated Solar Flow Battery, Chem, 2018, 4(11), 2644–2657, DOI:10.1016/j.chempr.2018.08.023.
- W. Li and S. Jin, Design Principles and Developments of Integrated Solar Flow Batteries, Acc. Chem. Res., 2020, 53(11), 2611–2621, DOI:10.1021/acs.accounts.0c00373.
- D. Liu, Z. Wei, C. Hsu, Y. Shen and F. Liu, Efficient Solar Energy Storage Using A TiO2/WO3 Tandem Photoelectrode in An All-Vanadium Photoelectrochemical Cell, Electrochim. Acta, 2014, 136, 435–441, DOI:10.1016/j.electacta.2014.05.129.
- G. Tian, R. Jervis, J. Briscoe, M. Titirici and A. J. Sobrido, Efficient Harvesting and Storage of Solar Energy of an All-Vanadium Solar Redox Flow Battery with a MoS2@TiO2 Photoelectrode, J. Mater. Chem. A, 2022, 10(19), 10484–10492, 10.1039/D2TA00739H.
- Z. Zhang, P. Lu, Z. Gu, Q. Ma, Z. Guo, H. Su and Q. Xu, Cu2O-Electrodeposited TiO2 Photoelectrode for Integrated Solar Redox Flow Battery, Processes, 2023, 11(9), 2631, DOI:10.3390/pr11092631.
- Y. Zhou, S. Zhang, Y. Ding, L. Zhang, C. Zhang, X. Zhang, Y. Zhao and G. Yu, Efficient Solar Energy Harvesting and Storage through a Robust Photocatalyst Driving Reversible Redox Reactions, Adv. Mater., 2018, 30(31), 1802294, DOI:10.1002/adma.201802294.
- Y. Lin, G. Yuan, S. Sheehan, S. Zhou and D. Wang, Hematite-Based Solar Water Splitting: Challenges and Opportunities, Energy Environ. Sci., 2011, 4(12), 4862–4869, 10.1039/C1EE01850G.
- A. Khataee, J. Azevedo, P. Dias, D. Ivanou, E. Dražević, A. Bentien and A. Mendes, Integrated Design of Hematite and Dye-Sensitized Solar Cell for Unbiased Solar Charging of an Organic-Inorganic Redox Flow Battery, Nano Energy, 2019, 62, 832–843, DOI:10.1016/j.nanoen.2019.06.001.
- K. R. Tolod, S. Hernández, E. A. Quadrelli and N. Russo, Chapter 4 – Visible Light-Driven Catalysts for Water Oxidation: Towards Solar Fuel Biorefineries, in Studies in Surface Science and Catalysis, ed. S. Albonetti, S. Perathoner and E. A. Quadrelli, Horizons in Sustainable Industrial Chemistry and Catalysis, Elsevier, 2019, vol. 178, pp. 65–84, DOI:10.1016/B978-0-444-64127-4.00004-5.
- R.-T. Gao, J. Zhang, T. Nakajima, J. He, X. Liu, X. Zhang, L. Wang and L. Wu, Single-Atomic-Site Platinum Steers Photogenerated Charge Carrier Lifetime of Hematite Nanoflakes for Photoelectrochemical Water Splitting, Nat. Commun., 2023, 14(1), 2640, DOI:10.1038/s41467-023-38343-6.
- Y. S. Piekner, D. A. Ellis, D. Grave, A. Tsyganok and A. Rothschild, Wasted Photons: Photogeneration Yield and Charge Carrier Collection Efficiency of Hematite Photoanodes for Photoelectrochemical Water Splitting, Energy Environ. Sci., 2021, 14(8), 4584–4598, 10.1039/D1EE01772A.
- F. Le Formal, M. Grätzel and K. Sivula, Controlling Photoactivity in Ultrathin Hematite Films for Solar Water-Splitting, Adv. Funct. Mater., 2010, 20(7), 1099–1107, DOI:10.1002/adfm.200902060.
- R. F. G. Gardner, F. Sweett and D. W. Tanner, The Electrical Properties of Alpha Ferric Oxide—II: Ferric Oxide of High Purity, J. Phys. Chem. Solids, 1963, 24(10), 1183–1196, DOI:10.1016/0022-3697(63)90235-X.
- R. Gao and D. Yan, Recent Development of Ni/Fe-Based Micro/Nanostructures toward Photo/Electrochemical Water Oxidation, Adv. Energy Mater., 2020, 10(11), 1900954, DOI:10.1002/aenm.201900954.
- R. Tang, S. Zhou, Z. Zhang, R. Zheng and J. Huang, Engineering Nanostructure–Interface of Photoanode Materials Toward Photoelectrochemical Water Oxidation, Adv. Mater., 2021, 33(17), 2005389, DOI:10.1002/adma.202005389.
- J.-W. Lee, K.-H. Cho, J.-S. Yoon, Y.-M. Kim and Y.-M. Sung, Photoelectrochemical Water Splitting Using One-Dimensional Nanostructures, J. Mater. Chem. A, 2021, 9(38), 21576–21606, 10.1039/D1TA04829E.
- L. Krause, K. Skibińska, H. Rox, R. Baumann, M. M. Marzec, X. Yang, G. Mutschke, P. Żabiński, A. F. Lasagni and K. Eckert, Hydrogen Bubble Size Distribution on Nanostructured Ni Surfaces: Electrochemically Active Surface Area Versus Wettability, ACS Appl. Mater. Interfaces, 2023, 15(14), 18290–18299, DOI:10.1021/acsami.2c22231.
- F. Kiani, F. Sterl, T. V. Tsoulos, K. Weber, H. Giessen and G. Tagliabue, Ultra-Broadband and Omnidirectional Perfect Absorber Based on Copper Nanowire/Carbon Nanotube Hierarchical Structure, ACS Photonics, 2020, 7(2), 366–374, DOI:10.1021/acsphotonics.9b01658.
- S. J. Kim, I. Thomann, J. Park, J.-H. Kang, A. P. Vasudev and M. L. Brongersma, Light Trapping for Solar Fuel Generation with Mie Resonances, Nano Lett., 2014, 14(3), 1446–1452, DOI:10.1021/nl404575e.
- F. Li, J. Li, J. Zhang, L. Gao, X. Long, Y. Hu, S. Li, J. Jin and J. Ma, NiO Nanoparticles Anchored on Phosphorus-Doped α-Fe2O3 Nanoarrays: An Efficient Hole Extraction p–n Heterojunction Photoanode for Water Oxidation, ChemSusChem, 2018, 11(13), 2156–2164, DOI:10.1002/cssc.201800571.
- J. Low, J. Yu, M. Jaroniec, S. Wageh and A. A. Al-Ghamdi, Heterojunction Photocatalysts, Adv. Mater., 2017, 29(20), 1601694, DOI:10.1002/adma.201601694.
- H. Zhang, P. Zhang, J. Zhao, Y. Liu, Y. Huang, H. Huang, C. Yang, Y. Zhao, K. Wu, X. Fu, S. Jin, Y. Hou, Z. Ding, R. Yuan, M. B. J. Roeffaers, S. Zhong and J. Long, The Hole-Tunneling Heterojunction of Hematite-Based Photoanodes Accelerates Photosynthetic Reaction, Angew. Chem., Int. Ed., 2021, 60(29), 16009–16018, DOI:10.1002/anie.202102983.
- N. Kodan, K. Agarwal and B. R. Mehta, All-Oxide α-Fe2O3/H:TiO2 Heterojunction Photoanode: A Platform for Stable and Enhanced Photoelectrochemical Performance through Favorable Band Edge Alignment, J. Phys. Chem. C, 2019, 123(6), 3326–3335, DOI:10.1021/acs.jpcc.8b10794.
- D. Barreca, G. Carraro, A. Gasparotto, C. Maccato, M. E. A. Warwick, K. Kaunisto, C. Sada, S. Turner, Y. Gönüllü, T.-P. Ruoko, L. Borgese, E. Bontempi, G. Van Tendeloo, H. Lemmetyinen and S. Mathur, Fe2O3–TiO2 Nano-Heterostructure Photoanodes for Highly Efficient Solar Water Oxidation, Adv. Mater. Interfaces, 2015, 2(17), 1500313, DOI:10.1002/admi.201500313.
- P. Zhang, L. Yu and X. W. D. Lou, Construction of Heterostructured Fe2O3-TiO2 Microdumbbells for Photoelectrochemical Water Oxidation, Angew. Chem., Int. Ed., 2018, 57(46), 15076–15080, DOI:10.1002/anie.201808104.
- J. Y. Zheng, T.-K. Van, A. U. Pawar, C. W. Kim and Y. S. Kang, One-Step Transformation of Cu to Cu 2 O in Alkaline Solution, RSC Adv., 2014, 4(36), 18616–18620, 10.1039/C4RA01174K.
- M. Thangamuthu, C. Santschi and O. J. F. Martin, Reliable Langmuir Blodgett Colloidal Masks for Large Area Nanostructure Realization, Thin Solid Films, 2020, 709, 138195, DOI:10.1016/j.tsf.2020.138195.
- M. Mireles, C. W. Soule, M. Dehghani and T. R. Gaborski, Use of Nanosphere Self-Assembly to Pattern Nanoporous Membranes for the Study of Extracellular Vesicles, Nanoscale Adv., 2020, 2(10), 4427–4436, 10.1039/D0NA00142B.
- J. Ma, K. Oh and G. Tagliabue, Understanding Wavelength-Dependent Synergies between Morphology and Photonic Design in TiO2-Based Solar Powered Redox Cells, J. Phys. Chem. C, 2023, 127(1), 11–21, DOI:10.1021/acs.jpcc.2c05893.
- F. Kiani, A. R. Bowman, M. Sabzehparvar, C. O. Karaman, R. Sundararaman and G. Tagliabue, Transport and Interfacial Injection of D-Band Hot Holes Control Plasmonic Chemistry, ACS Energy Lett., 2023, 8(10), 4242–4250, DOI:10.1021/acsenergylett.3c01505.
- A. R. Bowman, J. Ma, F. Kiani, G. G. Martínez and G. Tagliabue, Best Practices in Measuring Absorption at the Macro- and Microscale, APL Photonics, 2024, 9(6), 061101, DOI:10.1063/5.0210830.
- J. Ma, Z. Pan and G. Tagliabue, High-Performance Hematite Photoanodes for Unassisted Recharging of Solar Redox Flow Battery, Sol. RRL, 2024, 8(17), 2400477, DOI:10.1002/solr.202400477.
- Optical Constants, https://apps.dtic.mil/sti/citations/ADA158623, accessed, 04-18- 2024 Search PubMed.
- T. A. F. König, P. A. Ledin, J. Kerszulis, M. A. Mahmoud, M. A. El-Sayed, J. R. Reynolds and V. V. Tsukruk, Electrically Tunable Plasmonic Behavior of Nanocube–Polymer Nanomaterials Induced by a Redox-Active Electrochromic Polymer, ACS Nano, 2014, 8(6), 6182–6192, DOI:10.1021/nn501601e.
- M. Saleem, M. F. Al-Kuhaili, S. M. A. Durrani and I. A. Bakhtiari, Characterization of Nanocrystalline α-Fe2O3 Thin Films Grown by Reactive Evaporation and Oxidation of Iron, Phys. Scr., 2012, 85(5), 055802, DOI:10.1088/0031-8949/85/05/055802.
- T. C. Bhagya, A. Krishnan, A. Rajan S, A. Sha M, B. R. Sreelekshmy, P. Jineesh and S. M. A. Shibli, Exploration and Evaluation of Proton Source-Assisted Photocatalyst for Hydrogen Generation, Photochem. Photobiol. Sci., 2019, 18(7), 1716–1726, 10.1039/C9PP00119K.
- D. Ozaslan, O. Erken, M. Gunes and C. Gumus, The Effect of Annealing Temperature on the Physical Properties of Cu2O Thin Film Deposited by SILAR Method, Phys. B, 2020, 580, 411922, DOI:10.1016/j.physb.2019.411922.
- J. H. Kim, K. A. Jeon, G. H. Kim and S. Y. Lee, Electrical, Structural, and Optical Properties of ITO Thin Films Prepared at Room Temperature by Pulsed Laser Deposition, Appl. Surf. Sci., 2006, 252(13), 4834–4837, DOI:10.1016/j.apsusc.2005.07.134.
- O. L. Muskens, S. L. Diedenhofen, B. C. Kaas, R. E. Algra, E. P. A. M. Bakkers, J. Gómez Rivas and A. Lagendijk, Large Photonic Strength of Highly Tunable Resonant Nanowire Materials, Nano Lett., 2009, 9(3), 930–934, DOI:10.1021/nl802580r.
- L. Cao, J. S. White, J.-S. Park, J. A. Schuller, B. M. Clemens and M. L. Brongersma, Engineering Light Absorption in Semiconductor Nanowire Devices, Nat. Mater., 2009, 8(8), 643–647, DOI:10.1038/nmat2477.
- E. Cortés, F. J. Wendisch, L. Sortino, A. Mancini, S. Ezendam, S. Saris, L. d. S. Menezes, A. Tittl, H. Ren and S. A. Maier, Optical Metasurfaces for Energy Conversion, Chem. Rev., 2022, 122(19), 15082–15176, DOI:10.1021/acs.chemrev.2c00078.
- T. Sun, Y. Yu, B. J. Zacher and M. V. Mirkin, Scanning Electrochemical Microscopy of Individual Catalytic Nanoparticles, Angew. Chem., Int. Ed., 2014, 53(51), 14120–14123, DOI:10.1002/anie.201408408.
- T. R. Harris-Lee, F. Marken, C. L. Bentley, J. Zhang and A. L. Johnson, A Chemist's Guide to Photoelectrode Development for Water Splitting – the Importance of Molecular Precursor Design, EES Catal., 2023, 1(6), 832–873, 10.1039/D3EY00176H.
- X. Huang, Y. Li, X. Gao, Q. Xue, R. Zhang, Y. Gao, Z. Han and M. Shao, The Effect of the Photochemical Environment on Photoanodes for Photoelectrochemical Water Splitting, Dalton Trans., 2020, 49(35), 12338–12344, 10.1039/D0DT01566K.
- K. Zhang, S. Ravishankar, M. Ma, G. Veerappan, J. Bisquert, F. Fabregat-Santiago and J. H. Park, Overcoming Charge Collection Limitation at Solid/Liquid Interface by a Controllable Crystal Deficient Overlayer, Adv. Energy Mater., 2017, 7(3), 1600923, DOI:10.1002/aenm.201600923.
- Z. Xu, Z. Fan, Z. Shi, M. Li, J. Feng, L. Pei, C. Zhou, J. Zhou, L. Yang, W. Li, G. Xu, S. Yan and Z. Zou, Interface Manipulation to Improve Plasmon-Coupled Photoelectrochemical Water Splitting on α-Fe2O3 Photoanodes, ChemSusChem, 2018, 11(1), 237–244, DOI:10.1002/cssc.201701679.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta06302c |
| This journal is © The Royal Society of Chemistry 2025 |