Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Highly efficient mechanochemical depolymerisation of bio-based polyethylene furanoate and polybutylene furanoate†
Divya
Jain
 a,
Florian
Cramer
a,
Florian
Cramer
 bc,
Pauline
Shamraienko
bc,
Pauline
Shamraienko
 b,
Hans-Joachim
Drexler
b,
Hans-Joachim
Drexler
 a,
Brigitte
Voit
a,
Brigitte
Voit
 *bc and
Torsten
Beweries
*bc and
Torsten
Beweries
 *a
*a
aLeibniz Institute for Catalysis (LIKAT Rostock), Albert-Einstein-Str. 29a, 18059 Rostock, Germany. E-mail: torsten.beweries@catalysis.de
bLeibniz-Institut für Polymerforschung Dresden (IPF Dresden), Hohe Straße 6, 01069 Dresden, Germany. E-mail: voit@ipfdd.de
cOrganic Chemistry of Polymers, TUD Dresden University of Technology, Dresden 01062, Germany
First published on 24th June 2025
Abstract
The challenge of producing new environmentally friendly and fossil-free polyesters has strongly encouraged the development of bio-based alternatives such as polyethylene furanoate (PEF) and polybutylene furanoate (PBF) as alternatives to commodity plastics such as polyethylene terephthalate (PET) for everyday applications. In this contribution, we report the mechanochemical depolymerisation of these polymers using NaOH in the presence of NaCl as an additive along with the synthesis of high-molecular weight PEF and PBF. Efficient depolymerisation, producing 2,5-furandicarboxylic acid (FDCA) and the corresponding diols in quantitative yields after aqueous acidic workup, is possible within 30 minutes milling time. Using slightly modified reaction conditions, transesterification with MeOH produces the 2,5-furandicarboxylic acid dimethyl ester (FuMe2), which can potentially be reused for polymer synthesis. Notably, the furan ring remains stable under the mechanochemical conditions used. The applicability of these straight-forward, environmentally friendly protocols on a large scale is demonstrated through multigram scale reactions.
Sustainability spotlightThis work demonstrates an efficient mechanochemical protocol for the full and selective depolymerisation of bio-based polymers polyethylene furanoate and polybutylene furanoate, two polyesters with an excellent low CO2 footprint that are promising candidates for the replacement of commonly used fossil-based polyethylene terephthalate and polybutylene terephthalate. The reported reactions are carried out under solvent-free and mild conditions and proceed selectively and quantitatively within less than one hour, producing highly pure furan-based and diol monomers after workup that can be used for repolymerisation. Scalability of this approach is demonstrated through multigram scale reactions. Our work contributes to the UN sustainable development goals SDG 9 (Industry, Innovation and Infrastructure) and SDG 12 (Responsible Consumption and Production). |
Introduction
Bio-based polyesters are a class of polymers derived from renewable biological sources, such as plant oils, sugars, or agricultural by-products, rather than fossil fuels. These materials are gaining attention as eco-friendly alternatives to conventional petrochemical-based polyesters such as polyethylene terephthalate (PET) due to their lower environmental impact, reduced carbon footprint, and potential for biodegradability.1,2 Common bio-based polyesters include polylactic acid (PLA) and polyhydroxyalkanoates (PHA), which are used in a variety of applications, from packaging and textiles to medical devices. As promising, structurally similar substitutes of PET, furan-based polyesters such as polyethylene furanoate (PEF) and polybutylene furanoate (PBF) are emerging bio-based polymers that can be synthesised from plant-derived sugars via the production of 5-(hydroxymethyl)furfural and 2,5-furandicarboxylic acid (FDCA).3 PEF offers several advantages over traditional polyethylene terephthalate (PET), such as enhanced barrier properties against gases and liquids, making it an ideal candidate for food and beverage packaging. It also has an approximately 10–15 K higher glass transition and provides better ambient thermal stability and a 40 K lower melting point compared to PET, thus reducing the energy demand in existing post-processing steps in the melt.3b As a fully recyclable material, PEF is seen as a promising alternative to fossil-fuel-based plastics, thus supporting the development of a circular economy.4,5 In the past, several approaches have been discussed for the depolymerisation of polyesters using chemical recycling methods,6 including photocatalysis,7 enzymatic hydrolysis,8 and methanolysis (Fig. 1a).9Mechanochemistry offers a readily scalable, solvent-free, environmentally friendly, and energy-efficient alternative to traditional synthetic processes by reducing the use of hazardous chemicals and minimising waste. For this reason, this approach is often discussed as a promising contribution to a circular economy10 in general and specifically for polymer degradation.11 Recently, mechanochemical depolymerisation of PET was demonstrated by Štrukil via solid state saponification of the polyester with sodium hydroxide, followed by acidic workup (Fig. 1b).12a Further kinetic analysis by Sievers and co-workers showed that the mechanochemical depolymerisation follows a two-stage scenario in which first the conversion increases linearly with milling time, followed by a second stage where the reaction mixture transforms from a fine powder to a dense wax with a significant increase in reaction rate.12b Mathematical modelling of this conversion by Boukouvala and co-workers later allowed the fast estimation of monomer yields.12c These studies therefore represent an ideal starting point for the investigation of related bio-based polycondensates such as PEF and PBF. An interesting question in this context is whether similarly good results can be achieved under identical conditions, or whether the presence of the furan ring leads to undesirable reactivities under mechanochemical conditions.
To the best of our knowledge, the depolymerisation of PEF was so far only studied in solution, using bases13 or enzymes.14 In the latter context, von Langermann and co-workers have systematically studied a larger set of esterases and have characterised the product profile using ESI-TOF analysis. The development of a process for high-temperature alkaline hydrolysis of PEF was reported in a recent paper by the Lee group.15 In this contribution we present a first study of the mechanochemical depolymerisation of bio-based PEF and its analogue PBF (Fig. 1c). We demonstrate the facile and highly selective formation of ethylene glycol, FDCA and the dimethyl ester FuMe2, the latter two compounds being of excellent purity for repolymerisation.
Results and discussion
PEF and PBF were synthesised starting from FDCA. For this purpose, FDCA was transformed into the corresponding dimethyl ester (FuMe2) by a Fischer type esterification utilising sulfonic acid as a catalyst, and methanol as both the reagent and solvent. In a stirring autoclave, FuMe2 and an excess of ethylene glycol or 1,4-butanediol, respectively, were melted under a nitrogen atmosphere. Following a two-stage approach, FuMe2 was first glycolysed in a transesterification step setting free methanol and finally polymerised via polycondensation, where excess diol was removed under vacuum conditions (Scheme 1). | ||
| Scheme 1 Melt polycondensations of FuMe2 with ethylene glycol and 1,4-butanediol yielding PEF and PBF. | ||
Both steps were catalysed by titanium or antimony-based Lewis acid catalysts. The exact procedures including temperature and pressure regimens were derived from the literature16 and can be found in the ESI.† Nuclear magnetic resonance (NMR) spectroscopy was carried out, verifying the molecular structure of the resulting bio-based polyesters as expected.
The materials were analysed by size exclusion chromatography (SEC), thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) revealing molecular weights and different thermal properties, which are depicted in Table 1. Melting and glass transition temperatures are in accordance with the literature.17,18 The molecular weights and thermal properties are comparable to those of similar technical products, such as commercial PET used in drinking bottle production, which shows weight average molecular weights between 19 and 66 kg mol−1. At the same time, PET shows a glass transition around 80 °C and melting of the crystalline domains between 250 and 260 °C.19,20
| Properties | PEF | PBF |
|---|---|---|
a Mean value representing the mixture of two similar polymer batches.
b Obtained from SEC in pentafluorophenol/chloroform (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2).
c Defined as Mw/M(repeating unit).
d Obtained from TGA (maximum weight loss).
e Obtained from DSC. 2).
c Defined as Mw/M(repeating unit).
d Obtained from TGA (maximum weight loss).
e Obtained from DSC.
|
||
| Number average molecular weight Mn [g mol−1]a,b | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
| Weight average molecular weight Mw [g mol−1]a,b | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
| Degree of polymerisation DPwc | 202 | 328 |
| Dispersity Đ (Mw/Mn)a,b | 1.82 | 2.06 |
| Degradation temperature Td [°C]d | >250 | >250 |
| Glass transition temperature Tg [°C]e | 85 | 46 |
| Melting temperature Tm [°C]e | 218 | 171 |
Following the mechanochemical approach reported before for PET,12 in a typical depolymerisation experiment, 0.25 g of PEF, NaOH (0.24 g) and NaCl (0.175 g) were weighed into the reaction vessel, a 10 mL milling jar along with a 10 mm ball, followed by ball milling. NaCl was used in previous studies as an inert and safe additive that keeps the reaction mixture in powder form throughout the milling, facilitating the recovery of the crude reaction products, and thus ensuring high reproducibility.12,21,22 Work-up of the reaction mixture, containing disodium furanoate and ethylene glycol, is done by dissolving the crude reaction products in a minimum amount of water (10 mL), followed by acidification with 5 mL 1.0 M HCl solution in water that produces FDCA as a white precipitate, which can be separated by filtration followed by drying at 60 °C overnight (Scheme 2). Analysis of the metal content in the crude product shows that there is a slight increase in the Fe content, suggesting limited metal leaching from the mechanochemical apparatus (see ESI† for details). Excellent conversion of PEF into FDCA (>98%) was observed, producing highly pure FDCA under optimised conditions. The product was characterised by NMR and FTIR-ATR spectroscopic analysis. The conversion of PEF and the yield of FDCA were quantified by measuring the remaining amount of the PEF polymer before acidification and the amount of FDCA formed, respectively.
 | ||
| Scheme 2 Mechanochemical depolymerisation of PEF (1 equiv. is based on the repeating monomer unit). Further experimental details are depicted in Table 1 and in the ESI.† | ||
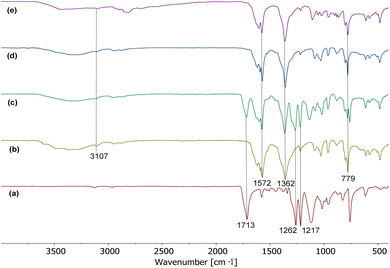
The characteristic absorption bands in the FTIR-ATR spectrum of FDCA disodium salt were obtained at 3107, 1572, 1362, and 779 cm−1 (Fig. 2b), in line with the spectra of the crude reaction mixture, indicating the complete conversion of the reaction mixture into the product under optimised conditions (Fig. 2d and Table 2, entry 2).
| Entry | Reaction conditionsa | PEF conversion into product [%] | Yield FDCA [%] |
|---|---|---|---|
| a Standard reaction conditions: 0.25 g PEF, 2 equiv. NaOH, 2 equiv. NaCl, milling frequency 30 Hz, 10 mL jar and 10 mm ball (both stainless steel if not mentioned otherwise). 25 μL solvent was used as a LAG. NaCl has been used as an inert surface for all the experiments (unless if mentioned, entry 24). Pure FDCA was obtained. b Unoptimised workup procedure for these conditions. | |||
| 1 | 10 min | 34 | <10 |
| 2 | 0.5 h | >98 | >98 |
| 3 | 1 h | >98 | >98 |
| 4 | 1.5 h | >98 | >98 |
| 5 | NaOH (1 equiv.) | 50 | 48 |
| 6 | NaOH (3 equiv.) | 95 | 90 |
| 7 | Without NaOH | 0 | 0 |
| 8 | KOH (2 equiv.) | >95 | 92 |
| 9 | Na2CO3 (2 equiv.) + H2O (74 μL) | <10 | <5 |
| 10 | Cs2CO3 (2 equiv.) + H2O (74 μL) | <5 | 0 |
| 11 | MeOH (25 μL), LAG | 96 | 94 |
| 12 | H2O (25 μL), LAG | >97 | 96 |
| 13 | DMSO (25 μL), LAG | 98 | 95 |
| 14 | Ethylene glycol (25 μL), LAG | >90 | 90 |
| 15 | 10 Hz | 0 | 0 |
| 16 | 20 Hz | 0 | 0 |
| 17 | 25 Hz | <40 | 20 |
| 18 | 26 Hz | 57 | 38 |
| 19 | 28 Hz | 68 | 46 |
| 20 | 15 mm SS ball, 25 Hzb | 96 | 81 |
| 21 | 10 mm ZrO2 ball | 91 | 86 |
| 22 | 10 mL ZrO2 jar | 90 | 85 |
| 23 | 10 mm ZrO2 ball, 10 mL ZrO2 jar | >90 | 84 |
| 24 | Without NaCl | >92 | 91 |
NMR analysis of the isolated product in DMSO-d6 solvent shows characteristic resonances at δ 7.27 and 13.60 ppm, which verify the formation of highly pure FDCA (Fig. 3, see ESI† for details). Of note, ethylene glycol can be recovered as a by-product of mechanochemical PEF hydrolysis from the aqueous phase used during workup (see ESI† for details). Although ethylene glycol undergoes rapid biodegradation in aerobic and anaerobic environments with approximately 100% removal within 24 h to 28 days,23 its full recovery from depolymerisation reactions could potentially reduce the environmental footprint of this bulk chemical.
 | ||
| Fig. 3 1H NMR spectrum of FDCA, isolated after aqueous workup of the mechanochemical reaction (400 MHz, DMSO-d6, 297 K). | ||
For the mechanochemical depolymerisation reaction, the reaction time has been optimised from 10 min to 1.5 h, where the FDCA disodium salt was obtained in excellent yields (Table 2, entries 2–4). Most importantly, even after prolonged reaction times the furan ring in the FDCA disodium salt did not show further reactivity or decomposition. Upon changing the amount of NaOH with respect to optimised conditions from one equivalent to three equivalents (Table 2, entries 5–7), only approximately 50% of the PEF was converted into FDCA disodium salt using one equivalent, whereas two equivalents of NaOH are required for full conversion (Fig. 2c–e). Further optimisation was done using different bases. When KOH (2 equiv.) was used instead of NaOH, sticky reaction mixtures (Fig. 4c) were obtained rather than powder formation (Fig. 4a), thus resulting in a slightly decreased yield of 89% (Table 2, entry 8). The hydrolysis was also carried out using sodium carbonate and cesium carbonate with water. In these cases, only trace amounts of the product were obtained (Table 2, entries 9 and 10).
 | ||
| Fig. 4 Texture of the reaction mixture obtained after the reaction, (a) without LAG, MeOH or EG as a LAG additive, (b) using DMSO as a LAG additive, and (c) water as a LAG additive or KOH as a base without LAG additives, under optimised conditions (see Table 2 for details). | ||
Next, the role of liquid assisted grinding (LAG) in the hydrolysis reaction was studied (Table 2, entries 11–14). It was observed that the presence of 25 μL of a LAG additive does not significantly affect the reaction yield but has an impact on the texture of the reaction mixture (Fig. 4). Methanol and ethylene glycol, both have no impact on the texture of the reaction mixture. However, when water was used as an additive the crude reaction mixture became very hard and sticky, while the use of DMSO as an additive led to a texture that varied between powdery and sticky (Fig. 4). As a powdered form of the reaction mixture was found to be optimal for further analysis and processing, LAG additives were excluded from further studies.
Further optimisation was done by varying the milling frequency. Using a frequency of 25 Hz with a 10 mL stainless steel jar and 10 mm stainless steel ball, only 40% conversion of PEF into FDCA was obtained with a very low yield of 20% due to dilution, while no product formation occurred at 10 Hz and 20 Hz (Table 2, entries 15–17). Only 57% and 68% PEF was converted into product at frequencies of 26 Hz and 28 Hz respectively. It can be concluded that a frequency of 30 Hz is needed when using a 10 mm ball and a small 10 mL milling jar to produce the amount of energy inside the milling jar which allows for complete conversion of PEF into the FDCA disodium salt.24 In line with related studies, a depolymerisation reaction that was carried out using a 15 mm (13.1 g) stainless steel ball instead of a 10 mm (4 g) stainless steel ball at a lower frequency of 25 Hz showed complete conversion of the polymer into the product (Table 2, entry 20). This can be rationalised by an increase of the ball weight by a factor of more than three, which in combination with a reduction of the frequency by 5 Hz does not decrease the impact energy.24 A comparison of different ball and jar materials shows that very good yields can be obtained when using ZrO2 balls and milling jars, indicating that the stainless steel of milling jar and balls does not take part in the reaction (Table 2, entries 21–23). The hydrolysis reaction was performed in the absence of NaCl using the optimised conditions for 0.25 g of PEF, resulting in a slightly lower product yield of 91% (Table 2, entry 24). This highlights the role of NaCl in achieving full conversion by providing an optimal reaction surface and facilitating complete recovery of the product from the milling jar. Thus, NaCl shows significant potential as an additive for a large scale NaOH assisted mechanochemical hydrolysis reaction of PEF producing FDCA.
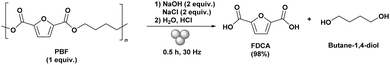
The described protocol was also applied for the mechanochemical hydrolysis of polybutylene furanoate (PBF) into FDCA using the optimised reaction conditions. For this, PBF, 2 equiv. of NaOH, and NaCl were ball milled for 0.5 h at a frequency of 30 Hz using a 10 mL stainless steel jar and 10 mm stainless steel ball (Scheme 3). Similarly, as for PEF, an excellent yield of 98% FDCA was obtained after acidic workup. The products were characterised by NMR and FTIR-ATR spectroscopic analysis (see ESI† for details).
The scalability of this reaction was demonstrated by performing larger-scale reactions of mechanochemical hydrolysis of PEF and PBF under similar reaction conditions, confirming its suitability for larger-scale applications. For this, 2.5 g of PEF was ball milled for 60 minutes at 30 Hz using a 25 mL stainless steel jar and two 15 mm stainless steel balls, yielding 1.95 g of FDCA, corresponding to a very good, isolated yield of 83%, with a high purity similar to that obtained in the small-scale reaction (see ESI† for details). In this reaction, complete conversion of PEF was observed but the diminished product yield might be due to dilution, which can be minimized in larger scale reactions using a minimal amount of water. A similar procedure was followed for larger scale mechanochemical hydrolysis of PBF (1.94 g), yielding 1.4 g of FDCA (90% yield). In these reactions, the byproduct recovery procedure could be optimised to enhance the overall efficiency of the reaction, ensuring maximum reutilisation (see ESI† for details). This approach contributes to the sustainability of the process and demonstrates its feasibility for large scale applications.
Further applicability of the mechanochemical depolymerisation protocol was explored for the transformation of PEF and PBF into the dimethyl ester FuMe2, i.e., the starting material for potential re-polymerisation (vide supra).16,25 In a related study of mechanochemical methanolysis of PET and polylactic acid, Borchardt, Kim and co-workers could demonstrate the transesterification of this polymer using an excess of MeOH in a planetary milling setup.9 For the optimisation of our methanolysis reaction, initially the reaction of 0.166 g PEF with MeOH (10 equiv.) was carried out in the presence of NaOH (2 equiv.) for 60 min at 30 Hz using a 10 mL SS jar with 10 and 15 mm SS balls, respectively, but FuMe2 could not be detected by 1H and 13C NMR analysis of the reaction mixture. Substitution of NaOH with NaOMe (2 equiv.) using the same reaction conditions, followed by quenching the reaction mixture with 5 mL 1 M HCl solution and extraction using water and chloroform solvent produced a mixture of monomethyl and dimethyl furan-2,5-dicarboxylate FuMe2 (see ESI† for details). Further optimisation of the reaction involved reducing the amount of NaOMe to 0.5 equivalents and using 10 equivalents of MeOH as a methylating reagent, resulting in quantitative conversion of the polymer (Scheme 4).
 | ||
| Scheme 4 Mechanochemical methanolysis of PEF and PBF (1 equiv. is based on the repeating monomer unit). | ||
After quenching the reaction mixture with 2 mL 1 M HCl solution and dissolving in ethanol:H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) upon heating up to 80 °C, colourless crystals of FuMe2, showing the expected 1H NMR signature, can be obtained in a very good yield of 74% by recrystallisation from saturated solution at 5 °C (Fig. 5). The molecular structure determined by single-crystal X-ray diffraction analysis of single crystals shows the expected diester motif with two different rotational conformers of the ester group at the furan ring (see ESI† for details). The mechanochemical methanolysis of PBF (0.194 g) required a further adjustment of the reaction conditions (20 equiv. MeOH, 2.5 h reaction time) and yielded FuMe2 after work-up in excellent purity, but a reduced yield of only 39%. Possible reasons for this significantly lower yield could be the presence of the longer alkyl group which might induce different transesterification reactivity and the less efficient separation of the by-product 1,4-butanediol formed (higher viscosity, melting point of 20 °C). The scalability of this highly useful transesterification/methanolysis reaction was again demonstrated by performing a large-scale reaction under similar reaction conditions. For this, 1.66 g of PEF (10 mmol), 108 mg NaOMe (0.2 equiv.), and 3 mL of MeOH were ball milled for 1 h at 30 Hz in a 25 mL stainless steel jar using a 15 mm stainless steel ball, yielding highly pure FuMe2 in an unoptimised yield of 65% (1.2 g FuMe2) after recrystallisation. This decrease of the product yield can be assigned to the solubility of the product during filtration. Further product can be collected from the filtrate after evaporating the solvent. We would like to emphasise that these results demonstrate the potential for the development of more general upcycling strategies for these bio-based polyesters.
3) upon heating up to 80 °C, colourless crystals of FuMe2, showing the expected 1H NMR signature, can be obtained in a very good yield of 74% by recrystallisation from saturated solution at 5 °C (Fig. 5). The molecular structure determined by single-crystal X-ray diffraction analysis of single crystals shows the expected diester motif with two different rotational conformers of the ester group at the furan ring (see ESI† for details). The mechanochemical methanolysis of PBF (0.194 g) required a further adjustment of the reaction conditions (20 equiv. MeOH, 2.5 h reaction time) and yielded FuMe2 after work-up in excellent purity, but a reduced yield of only 39%. Possible reasons for this significantly lower yield could be the presence of the longer alkyl group which might induce different transesterification reactivity and the less efficient separation of the by-product 1,4-butanediol formed (higher viscosity, melting point of 20 °C). The scalability of this highly useful transesterification/methanolysis reaction was again demonstrated by performing a large-scale reaction under similar reaction conditions. For this, 1.66 g of PEF (10 mmol), 108 mg NaOMe (0.2 equiv.), and 3 mL of MeOH were ball milled for 1 h at 30 Hz in a 25 mL stainless steel jar using a 15 mm stainless steel ball, yielding highly pure FuMe2 in an unoptimised yield of 65% (1.2 g FuMe2) after recrystallisation. This decrease of the product yield can be assigned to the solubility of the product during filtration. Further product can be collected from the filtrate after evaporating the solvent. We would like to emphasise that these results demonstrate the potential for the development of more general upcycling strategies for these bio-based polyesters.
 | ||
| Fig. 5 1H NMR spectrum of FuMe2 from mechanochemical depolymerisation of PEF (300 MHz, CDCl3, 297 K). | ||
Conclusions
In summary, we have described a mechanochemical protocol for the efficient depolymerisation of the bio-based polyesters polyethylene furanoate (PEF) and polybutylene furanoate (PBF). Our procedure comprises the use of readily available reagents NaOH, NaOMe and NaCl and a simple aqueous acidic workup procedure, allowing for the recovery of 2,5-furandicarboxylic acid (FDCA) and the corresponding diols, as well as the 2,5-furandicarboxylic acid dimethyl ester (FuMe2) in very good to quantitative yields. The latter reaction is of special interest as the final product can potentially be re-subjected to the production of furan-based polycondensates. The applicability of the reported procedure on a large scale could be shown through multigram scale reactions. Of note, the high-energy mechanochemical conditions do not induce reactivity at the furan heterocycle, making these procedures promising methods for the large-scale depolymerisation of furan-based polycondensates. Thus, we believe that our results will contribute to the implementation of these and related bio-based polyesters in a circular economy. Further studies will focus on depolymerisation and upcycling reactions of these and related polymers. The results of these studies will be reported in due course.Data availability
The data supporting this article have been included as part of the ESI.† Additional data are available from the authors.Author contributions
DJ: conceptualisation, data curation, formal analysis, investigation, project administration, visualisation, writing (original draft and editing). FC: coordination of polymer synthesis and characterisation, writing. PS: funding acquisition, supervision, writing (editing). HJD: supervision, writing (editing). BV: funding acquisition, supervision, writing. TB: conceptualisation, funding acquisition, supervision, writing (original draft and editing).Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank our technical and analytical staff, in particular Benjamin Andres and Cornelia Pribbenow, for assistance. We thank Andreas Korwitz for syntheses of the polymers and NMR analysis, Petra Treppe for SEC and Kerstin Arnhold for TGA and DSC measurements. Financial support by the Leibniz Association (project SUSTAIN, grant no. 419924354) is gratefully acknowledged.Notes and references
- C. Shi, E. C. Quinn, W. T. Diment and E. Y. X. Chen, Recyclable and (Bio)degradable Polyesters in a Circular Plastics Economy, Chem. Rev., 2024, 124, 4393–4478 CrossRef CAS PubMed.
- C. Vilela, A. F. Sousa, A. C. Fonseca, A. C. Serra, J. F. J. Coelho, C. S. R. Freire and A. J. D. Silvestre, The quest for sustainable polyesters – insights into the future, Polym. Chem., 2014, 5, 3119–3141 RSC.
- (a) G. Z. Papageorgiou, V. Tsanaktsis and D. N. Bikiaris, Synthesis of poly(ethylene furandicarboxylate) polyester using monomers derived from renewable resources: thermal behavior comparison with PET and PEN, Phys. Chem. Chem. Phys., 2014, 16, 7946–7958 RSC; (b) J.-G. Rosenboom, D. K. Hohl, P. Fleckenstein, G. Storti and M. Morbidelli, Bottle-grade polyethylene furanoate from ring-opening polymerisation of cyclic oligomers, Nat. Commun., 2018, 9, 2701 CrossRef PubMed.
- Selected overviews: (a) A. F. Sousa, C. Vilela, A. C. Fonseca, M. Matos, C. S. R. Freire, G.-J. M. Gruter, J. F. J. Coelho and A. J. D. Silvestre, Biobased polyesters and other polymers from 2,5-furandicarboxylic acid: a tribute to furan excellency, Polym. Chem., 2015, 6, 5961–5983 RSC; (b) Q. Zhang, M. Song, Y. Xu, W. Wang, Z. Wang and L. Zhang, Bio-based polyesters: Recent progress and future prospects, Prog. Polym. Sci., 2021, 120, 101430 CrossRef CAS; (c) K. R. Kunduru, R. Hogerat, K. Ghosal, M. Shaheen-Mualim and S. Farah, Renewable polyol-based biodegradable polyesters as greener plastics for industrial applications, Chem. Eng. J., 2023, 459, 141211 CrossRef CAS.
- Z. Tu, X. Zhang, J. Li, L. Li, F. Zhou, H. Ma and Z. Wei, Fully biobased poly(butylene furanoate) copolyesters from renewable 2,3-butanediol: Much more than just strengthening and toughening, Eur. Polym. J., 2024, 220, 113477 CrossRef CAS.
- (a) T. El Darai, A. Ter-Halle, M. Blanzat, G. Despras, V. Sartor, G. Bordeau, A. Lattes, S. Franceschi, S. Cassel, N. Chouini-Lalanne, E. Perez, C. Déjugnat and J.-C. Garrigues, Chemical recycling of polyester textile wastes: shifting towards sustainability, Green Chem., 2024, 26, 6857–6885 RSC; (b) M. Babaei, M. Jalilian and K. Shahbaz, Chemical recycling of Polyethylene terephthalate: A mini-review, J. Environ. Chem. Eng., 2024, 12, 112507 CrossRef CAS; (c) E. Gabirondo, B. Melendez-Rodriguez, C. Arnal, J. M. Lagaron, A. Martínez de Ilarduya, H. Sardon and S. Torres-Giner, Organocatalyzed closed-loop chemical recycling of thermo-compressed films of poly(ethylene furanoate), Polym. Chem., 2021, 12, 1571–1580 RSC.
- S. Jiang, M. Wang, Y. Huang, J. Wen and P. Hu, Selective Degradation of Polyethylene Terephthalate Plastic Waste Using Iron Salt Photocatalysts, ChemSusChem, 2024, 17, e202401920 Search PubMed.
- S. Kaabel, J. Arciszewski, T. H. Borchers, J. P. D. Therien, T. Friščić and K. Auclair, Solid-State Enzymatic Hydrolysis of Mixed PET/Cotton Textiles, ChemSusChem, 2023, 16, e202201613 CrossRef CAS PubMed.
- H. W. Lee, K. Yoo, L. Borchardt and J. G. Kim, Chemical recycling of polycarbonate and polyester without solvent and catalyst: mechanochemical methanolysis, Green Chem., 2024, 26, 2087–2093 RSC.
- J. Alić, M.-C. Schlegel, F. Emmerling and T. Stolar, Meeting the UN Sustainable Development Goals with Mechanochemistry, Angew. Chem., Int. Ed., 2024, 63, e202414745 CrossRef PubMed.
- (a) J. Zhou, T.-G. Hsu and J. Wang, Mechanochemical Degradation and Recycling of Synthetic Polymers, Angew. Chem., Int. Ed., 2023, 62, e202300768 CrossRef CAS PubMed; (b) S. Aydonat, A. H. Hergesell, C. L. Seitzinger, R. Lennarz, G. Chang, C. Sievers, J. Meisner, I. Vollmer and R. Göstl, Leveraging mechanochemistry for sustainable polymer degradation, Polym. J., 2024, 56, 249–268 CrossRef CAS.
- (a) V. Štrukil, Highly Efficient Solid-State Hydrolysis of Waste Polyethylene Terephthalate by Mechanochemical Milling and Vapor-Assisted Aging, ChemSusChem, 2021, 14, 330–338 CrossRef PubMed; (b) A. W. Tricker, A. A. Osibo, Y. Chang, J. X. Kang, A. Ganesan, E. Anglou, F. Boukouvala, S. Nair, C. W. Jones and C. Sievers, Stages and Kinetics of Mechanochemical Depolymerization of Poly(ethylene terephthalate) with Sodium Hydroxide, ACS Sustainable Chem. Eng., 2022, 10, 11338–11347 CrossRef CAS; (c) E. Anglou, Y. Chang, W. Bradley, C. Sievers and F. Boukouvala, Modeling Mechanochemical Depolymerization of PET in Ball-Mill Reactors Using DEM Simulations, ACS Sustainable Chem. Eng., 2024, 12, 9003–9017 CrossRef CAS PubMed.
- G. Dargó, D. Kis, A. Ráduly, V. Farkas and J. Kupai, Furandicarboxylic Acid (FDCA): Electrosynthesis and Its Facile Recovery From Polyethylene Furanoate (PEF) via Depolymerization, ChemSusChem, 2025, e202401190 CrossRef PubMed.
- (a) V. Kumar, A. Pellis, R. Wimmer, V. Popok, J. d. C. Christiansen and C. Varrone, Efficient Depolymerization of Poly(ethylene 2,5-furanoate) Using Polyester Hydrolases, ACS Sustainable Chem. Eng., 2024, 12, 9658–9668 CrossRef CAS PubMed; (b) T. Heinks, K. Hofmann, L. Zimmermann, I. Gamm, A. Lieb, L. Blach, R. Wei, U. T. Bornscheuer, J. Thiele, C. Hamel and J. von Langermann, Analysis of the product-spectrum during the biocatalytic hydrolysis of PEF (poly(ethylene furanoate)) with various esterases, RSC Sustainability, 2025, 3, 1346–1355 RSC; (c) A. Pellis, K. Haernvall, C. M. Pichler, G. Ghazaryan, R. Breinbauer and G. M. Guebitz, Enzymatic hydrolysis of poly(ethylene furanoate), J. Biotechnol., 2016, 235, 47–53 Search PubMed.
- Y.-H. Wang, H. L. Lee, H.-M. Lee, D. E. Pratama and T. Lee, Process Development and Optimization of Alkaline Hydrolysis for Depolymerization in Chemical Recycling of Poly(ethylene 2,5-furandicarboxylate) (PEF) Using Full Factorial Design, Ind. Eng. Chem. Res., 2025, 64, 5729–5742 CrossRef CAS.
- C. Mielke, D. Pospiech, J. Kuhnigk, A. Korwitz, H. Komber, R. Bernhardt, N. Krebs, R. Boldt, H. Ruckdäschel and B. Voit, Partially Bio-Based Polyester Bead Foams via Extrusion Foaming of Poly(butylene terephthalate)/Poly(butylene furanoate) Blends, Macromol. Mater. Eng., 2023, 308, 2300281 CrossRef CAS.
- G. Papamokos, T. Dimitriadis, D. N. Bikiaris, G. Z. Papageorgiou and G. Floudas, Chain Conformation, Molecular Dynamics, and Thermal Properties of Poly(n-methylene 2,5-furanoates) as a Function of Methylene Unit Sequence Length, Macromolecules, 2019, 52, 6533–6546 CrossRef CAS.
- Y. Wang, K. Su, C. Liu and Z. Li, Poly(butylene 2,5-furandicarboxylate) copolyester obtained using 1,6-hexanediamine with high glass transition temperature, RSC Adv., 2023, 13, 35816–35824 RSC.
- W. Romão, M. F. Franco, M. I. M. S. Bueno and M.-A. De Paoli, Distinguishing between virgin and post-consumption bottle-grade poly (ethylene terephthalate) using thermal properties, Polym. Test., 2010, 29, 879–885 CrossRef.
- H. Saechtling, Kunststoff-Taschenbuch, 26. Ausgabe, P. 452ff, Carl Hanser Verlag, 1995 Search PubMed.
- J.-L. Do, C. Mottillo, D. Tan, V. Štrukil and T. Friščić, Mechanochemical Ruthenium-Catalyzed Olefin Metathesis, J. Am. Chem. Soc., 2015, 137, 2476–2479 CrossRef CAS PubMed.
- J. Yang, X. Feng, G. Lu, Y. Li, C. Mao, Z. Wen and W. Yuan, NaCl as a solid solvent to assist the mechanochemical synthesis and post-synthesis of hierarchical porous MOFs with high I2 vapour uptake, Dalton Trans., 2018, 47, 5065–5071 RSC.
- C. A. Staples, J. B. Williams, G. R. Craig and K. M. Roberts, Fate, effects and potential environmental risks of ethylene glycol: a review, Chemosphere, 2001, 43, 377–383 CrossRef CAS PubMed.
- For a detailed study on the effects of ball and jar size and material, milling frequency, and mass of reagents on the efficiency of mechanochemical reactions, see: O. F. Jafer, S. Lee, J. Park, C. Cabanetos and D. Lungerich, Navigating Ball Mill Specifications for Theory-to-Practice Reproducibility in Mechanochemistry, Angew. Chem., Int. Ed., 2024, 63, e202409731 CrossRef PubMed.
- Z. Terzopoulou, E. Karakatsianopoulou, N. Kasmi, V. Tsanaktsis, N. Nikolaidis, M. Kostoglou, G. Z. Papageorgiou, D. A. Lambropoulou and D. N. Bikiaris, Effect of catalyst type on molecular weight increase and coloration of poly(ethylene furanoate) biobased polyester during melt polycondensation, Polym. Chem., 2017, 8, 6895–6908 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental, spectroscopic and characterisation details. CCDC 2448917 and 2448918. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5su00428d |
| This journal is © The Royal Society of Chemistry 2025 |