 Open Access Article
Open Access ArticleInsights into hierarchical porous titanium(IV) phosphonates: synthesis, structure & applications
Archana K.
Pattnaik
 a,
Gobinda
Chandra Behera
a,
Gobinda
Chandra Behera
 b and
Kulamani
Parida
b and
Kulamani
Parida
 *a
*a
aCentre for Nanoscience and Nanotechnology, Siksha ‘O’ Anusandhan (Deemed to be University), Bhubaneswar 751030, Odisha, India. E-mail: kulamaniparida@soa.ac.in; arcpattnaik@gmail.com; akp18@iitbbs.ac.in
bDepartment of Chemistry, Maharaja Purna Chandra Autonomous College, Takhatpur, Baripada 757003, Odisha, India. E-mail: bcgobind@gmail.com
First published on 2nd May 2025
Abstract
Hierarchically porous titanium(IV) phosphonates (HPTPs) have recently attracted significant attention as a novel class of hybrid materials that achieve remarkable flexibility in structural porosity, chemical strength, and multifunctionality, offering broad applicability compared to traditional hybrid materials. Unlike carboxylate-based metal–organic frameworks (MOFs), they exhibit robust hydrolytic stability due to their multimodal coordinating ability and strong Ti–O–P bonding. This makes them suitable candidates for catalysis, pollutant adsorption, ion exchange, and energy storage. However, despite their promising capabilities, the unpredictable self-assembly of Ti(IV) metal nodes, poor structural crystallinity, and challenges in hierarchical pore size control hinder the tunability of these materials for targeted applications. Furthermore, the relationships between porosity, stability, and catalytic efficiency necessitate further research to improve overall performance. This review critically examines synthetic methodologies, structural features, and emerging applications of HPTPs, highlighting strategies to address these limitations. Despite advancements in synthetic procedures, the crystallization of HPTPs with precise pore topology and desirable electronic characteristics remains challenging. The combination of computational modeling and machine learning may enhance material design, stability, and porosity. Additionally, solventless strategies alongside green fabrication and nanomaterial integration, could further expand the potential applications of HPTPs in energy storage, photocatalysis, and wastewater treatment, paving the way for industrial-scale utilization.
Sustainability spotlightThe intricate synthesis, hierarchical structure, and multifaceted applications of titanium phosphonates underscore their pivotal role in addressing global challenges across various scientific and industrial domains. By providing a comprehensive review, this article sheds light on the tunable properties of porous titanium phosphonates, which have significant implications for advancing sustainable technologies in industry, innovation and infrastructure. Their applications in catalysis, energy storage, and environmental remediation offer promising avenues for reducing environmental impact, enhancing energy efficiency, and promoting green chemistry practices as well as sustainable, inclusive economic growth. By bridging fundamental insights with practical applications, this work inspires interdisciplinary collaboration and sets a foundation for future breakthroughs that benefit society and the environment with value-added partnerships for goals. |
1. Introduction
Hierarchical porous organic–inorganic hybrid materials have experienced significant advancements in the last two decades, driven by the development of tunable structures that incorporate metal nodes and suitable linkers. These materials are tailored depending on the dimensions and functionality of targeted complexes.1,2 Moreover, these materials achieve versatility through an impressive research platform that leverages their unique properties, including organic functionality and flexible inorganic scaffolds. Open framework structures targeting cavities within the complexes require the use of bulky ligands with aromatic rings or alkyl chain derivatives. The key concept of pore chemistry is to incorporate structural voids that induce distinctive features, which help in integrating the framework backbone of hybrid materials and facilitating mass transfer through the interface.3The use of polydentate ligands, tailoring metal–ligand geometry, and incorporating guest molecules/templating agents has proven to be a successful strategy for designing hybrid porous solids.4,5 Among these, metal complexes with organophosphorus acids6 and their derivatives as O-donors (Scheme 1) stand out as paradigms in these hybrid network structures due to their diverse coordination modes and potential applications in catalysis,7,8 sensing,9,10 sorption,11,12 metal extraction13 and optoelectronics.14 Unlike the widely explored carboxylate-based metal–organic frameworks (MOFs), phosphonate-based MOFs/clusters remain relatively underexplored. The rational design of phosphonate MOFs via an isoreticular approach has emerged as a key synthetic strategy to broaden the scope of organophosphate materials.15,16 While carboxylate- and multicarboxylate-based frameworks have been extensively investigated, with structures ranging from zero-dimensional clusters to three-dimensional porous frameworks,17 phosphonate-based frameworks offer inherent structural flexibility and enhanced stability. To further diversify hybrid materials and to unlock novel functionalities, other phosphorus- and sulphur-based linkers such as phosphonates, phosphinates, phosphates, and sulphonates have also been explored. These linkers can mimic the carboxylate's coordination ability, and thereby serve as pioneering building units for the assembly of advanced porous materials.18
Since the 18th century, traditional porous structures such as zeolites, mesoporous silica, and activated carbons have been well-known as porous materials. However, they often encounter limitations in applications due to their rigid structural framework and lack of atomic-level structural tunability.19,20 This rigidity hinders optimal interaction with reactants or gases, thereby limiting practical applications in specific fields such as catalysis or adsorption.21 Later, new molecular sieve materials were developed for the first time featuring interconnected octahedral and tetrahedral oxide polyhedra, and showing novel compositions with larger pores and new topologies.22,23 Subsequently, the introduction of hetero atoms into these frameworks emerged as one of the successful strategies for the formation of hybrid materials. Microporous titanium silicates (TS-1, ETS; Engelhard Corporation Titanosilicate) are used as active base catalysts in the dehydration of tertbutyl alcohol or the dehydrogenation of alcohols.24 Simultaneously, other titanium hybrids like crystalline mono25 and di-hydrated titanium phosphates26 were investigated with a detailed study of their catalytic, ion exchange and proton conduction behaviour. Upon disclosure, their crystal structures exhibited significant differences in structural arrangement, while the degree of hydration influenced both the structure and the extent of ion exchange properties in both compounds. With high stability towards hydrolysis, di-hydrated titanium phosphates show greater ion exchange capacity. Later in 1997, Bortun et al. further studied these compounds with newly identified crystalline fibrous titanium(IV) oxophosphates.27 This extended work resulted in MIL-n (MIL; Materials of Institut Lavoisier) series of compounds synthesized in the presence of fluorine and/or amines or surfactants. For example, Ti2(PO4)2F4·(N2C2H10) (MIL-62) and Ti2(PO4)2F4·(N2C3H12)·H2O (MIL-63) consist of TiO4F2 octahedra connected by PO4 tetrahedra with the interlayer space occupied by diammonium, bonded strongly with terminal fluorine atoms via hydrogen bonding.28 It was observed that the charged templates are not a good option for developing these materials as the removal of organic groups leads to structural collapse. So, to avoid this difficulty, Alberti and Clearfield et al. proposed synthesis methods without using templates, and, thus, they started using diphosphonic acid instead of phosphoric acids, resulting into nonporous compounds like MIL-22 and MIL-25n (n = 2,3)25,29(Fig. 1a–c).
 | ||
| Fig. 1 Crystalline structures based on diphosphonate linkers: (a) MIL-22,35 (b) MIL-252,36 (c) MIL-253 (ref. 36) and (d) MIL-91(Ti)34,37 color code: Ti, blue; O, red; C, grey; P, orange; N, dark blue. H-atoms of C-atoms were omitted for clarity. Adapted with permission from ref. 37. Copyright of The Royal Society of Chemistry, 2017. | ||
As porous materials are quintessential in most scientific and technical fields, with useful applications ranging from catalysis,30 separation,31 energy storage,32 and biomedical applications,33 developing zeolite-type porous structures using various organic linkers has gained attention over the past two decades. In 2006, the first porous titanium(IV) MOF was fabricated by Serre et al. using N,N′-piperazinebismethylenephosphonate (O3P-CH2-NC4H8N-CH2-PO3), designated as MIL-91 (TiivO(H2-L)·nH2O).34 Structural analysis revealed, as shown in Fig. 1d, a tancoite architecture featuring a framework of TiO6 octahedral units surrounded by HPO3C moieties, forming a channel-like structure (4 Å), with a reported surface area of approximately 500 m2 g−1. The study demonstrated that organic linkers enhance tunability, which in turn enables the tailoring of organic–inorganic hybrid materials or clusters composed of metal ions bridged by organic linkers—introducing modularity to porous materials. Additionally, these phosphonate-based compounds exhibited well-defined control over intriguing structural characteristics to a greater extent and became highly sought-after in the field of porous materials.
The growing number of zeolite-like porous structures featuring phosphonates has received significant attention over the past two decades. While these materials address stability issues, they still encounter the challenging problem of nonporous, layered, and unpredictable structures due to extensive metal–oxygen binding through all three accessible oxygen atoms.38 Thus, the challenge lies in synthesising phosphonate MOFs/clusters with imbued porosity. According to HSAB theory, phosphonate groups exhibit a strong affinity for hard metal ions such as Ti(IV), leading to thermally and chemically robust Ti(IV)phosphonates with hierarchical porous framework structures.39 Additionally, the association of tetravalent Ti(IV) with phosphonate results in Ti(IV)–O–P bonds, providing high chemical stability even under extreme conditions of pH ≤ 1.37 While this stability is beneficial for metal recovery and separation processes, it also limits control over reaction conditions due to inherent stability. On the other hand, phosphonates, [RPO3]2− and their related ligands derived from phosphonic acids are particularly significant due to the presence of the P–C bond, which enhances stability and prevents hydrolysis, along with the +5 oxidation state of phosphorus (Scheme 1). Furthermore, the available P–O bonds allow oxygen to coordinate with metal ions, increasing the specificity of the complex through its multidentate behaviour, thereby enabling variations in the coordination environment of titanium phosphonates (TPs).38
-R, as an alkyl or aryl group, contributes to the orientation of the complexes in space and their electronic behaviour largely affects the intermolecular interactions, such as widely studied hydrogen bonds, pi–pi interactions, etc. Apart from this, many discrete units reported to date, such as titanium oxo clusters (TOCs), with diverse nuclearity show the highly oxophillic nature of the titanium metal ion.40 Generally, titanium oxo core-based clusters are developed from a metal ion solution of Ti(OR)4 in the presence of carboxylate/phosphate/phosphonate ligands, with control over parameters like reaction time, pressure, presence of water, reaction conditions like an inert atmosphere, etc. Various types of clusters with different TixOy cores are presented in Scheme 2 which shows the rich structural chemistry of oxo compounds with μ2, μ3, and μ4 bridged oxo groups.41,42 Although its preferred coordination number is 6 it also exhibits 5- or rarely 4- and 7-coordinated structures; as a result, more than 500 polynuclear homometallic Ti(IV) compounds have been analysed by Single Crystal XRD structure analysis. In addition to this, in the coordination environment, apart from neutral ligands, anionic ligands play a dual role in these compounds, often stabilising the structure by balancing the residual charge of the oxo core TixOy as y < 2×, while simultaneously satisfying the coordination sphere of the metal ion.41
 | ||
| Scheme 2 Schematic core structures of various titanium oxo clusters.41 | ||
The high reactivity of Ti(IV) makes the formation of crystalline coordination polymers and MOFs particularly challenging. However, these materials hold significant promise for photocatalytic and optoelectronic applications. Among various tetravalent phosphonates reported, titanium(IV) is an impressive candidate due to its low cost, and redox, semiconducting,43,44 and photocatalytic properties.45 Titanium phosphonates have been widely reported as discrete units, clusters, 1D, 2D and 3D coordination polymers and MOFs. This precision arises from the ability to alter pore size, shape, and functional groups by selecting specific metal nodes and linkers as well as modifying the resulting frameworks post-synthetically to achieve the desired properties. This unique feature bestows MOFs/hybrid porous materials with several advantages, such as high surface area, tunable porosity, and specific interaction sites, enabling a wide range of potential applications. Hierarchical porous titanium phosphonates (HPTPs), characterised by multiple pore scales (micropores, mesopores, and macropores), overcome diffusion limitations typically observed in conventional microporous MOFs or single-sized porous materials by facilitating enhanced mass transport.46 Interconnected porous structures in hierarchical architectures enhance the practical utility of hybrid materials, making them ideal for applications that require rapid molecular diffusion, such as catalysis of large-molecule reactions, gas storage, air filtration, wastewater treatment, etc.1 These properties can be further modified, incorporating new functional groups (–OH, –NH2, and –COOH) into the organic linkers, aiding precise control over electronic structures at the molecular level. In some cases, mixed linkers offer additional advantages, by introducing multiple characteristics into the hybrid materials, such as tunable pore size, increased surface area, and enhanced electronic behaviour, complemented by more accessible active sites. Moreover, these materials influence the adsorption behaviour due to the enhanced interactions occurring with the guest molecules during the process. The introduction of hydrophilic/hydrophobic groups in linkers is particularly beneficial for solvent separation applications.5 An overview of the porous titanium phosphonates discussed in this review is provided in Table 1.
| Sl no. | HPTPs | Precursors | Morphology/type of structure | Crystallinity | Pore size/peak maxima (nm) | Surface area m2 g−1 | Year | Ref. | Application |
|---|---|---|---|---|---|---|---|---|---|
| a NB: the numbers mentioned in Sl no. 1, 2, and 3 are the cluster numbers for the compounds as shown in Scheme 3. | |||||||||
| 1 | Arylphosphonic acid(ArPO3H2) | Cluster | Crystalline | — | — | 2018 | 51 | Study of solubility and thermal stability | |
| Ti(OiPr)2X2 | |||||||||
| 1–5,7a | Ar=Ph(1), 1-Nap(2), 4-MeOPh(3), 4-FPh(4), 4-ClPh(5),4-BrPh(7) | Ti4P3 type | Crystalline | ||||||
| X = OiPr, Solv = THF(for 1 and 2) iPrOH(for 3–5, 7a) | |||||||||
| H2O | |||||||||
| 6,7b | 4-BrBn(6) | Ti7P6 type | Crystalline | ||||||
| 4-BrBn(7b) | |||||||||
| X = OiPr | |||||||||
| H2O | |||||||||
| 8 | Ar=Ph | Cage type | Crystalline | ||||||
| X = acac | |||||||||
| 2 | 1–5,7 | tert-Butylphosphonic acid(tBPA) | Cluster | Partial crystalline | 2–50, >50 | — | 2020 | 52 | Controlling the hydrolysis conditions of complex structured oxide materials synthesis |
| Titanium(IV) ethoxide | |||||||||
| Toluene-ethanol | |||||||||
| 3 | 6 | tert-Butylphosphonic acid(tBPA) | |||||||
| Titanium(IV)(diisopropoxide)bis(2,4-pentadionate) | |||||||||
| Acetone | |||||||||
| 4 | HM-TiPPh | 1-Hydroxyethylidene-1,1-diphosphonic acid (HEDP) | Nanosphere | Amorphous | 3–30 | 448 | 2018 | 53 | Photocatalysis; H2 evolution |
| TiCl4 | |||||||||
| 5 | PS-Ti-DEPP | Titanium tetraacetate (Ti(OAc)4) | Fillers with a platelet-like structure | Amorphous | — | — | 2023 | 54 | Impact of solvent on morphology |
| PS-Ti-DEBP | Polypropylene (PP) | ||||||||
| PP-Ti-DEOP | Polystyrene (PS) | ||||||||
| Diethyl octylphosphonate (DEOP) | |||||||||
| Diethyl benzylphosphonate (DEBP) | |||||||||
| Diethyl phenylphosphonate (DEPP) | |||||||||
| 6 | HTiP-7 | Benzene-1,3,5-triphosphonic acid (BTPA) | Nanosphere | Crystalline | 1.4, 3.0, 4.8 | 255 | 2013 | 55 | Optoelectronic |
| Titanium(IV) isopropoxide | |||||||||
| 7 | TOP | Tetrabutyl titanate, β-cyclodextrin (β-CD: (C6H10O5)7) | Reverse opal | Amorphous | 400 | 23 | 2008 | 56 | Photocatalysis; organic pollutant degradation |
| TOPβ | 1-Hydroxy ethylidene-1,1-diphosphonic acid (HEDP: H2PO3–C(OH)(CH3)-PO3H2) | 25 | Heavy metal adsorption | ||||||
| 8 | TPPhA-Ti | Tetrakis-1,3,5,7-(4-phosphonate phenyl) | Layered structures and film-like folded structures nanosphere | para crystalline | 1.3–3.8, 180–300 | 557 | 2006 | 57 | Structural study |
| Adamantane | |||||||||
| Ti{OCH(CH3)2}4 | |||||||||
| 9 | PMTP-2 | 1-Hydroxy ethylidene-1,1-diphosphonic acid (HEDP) | Monolith | Crystalline | 2.4 | 1034 | 2010 | 58 | Ion exchange |
| TiCl4 | |||||||||
| 10 | H8L-Ti-MOF | 1,1,2,2-Tetraphenylethylene (TPE)-phosphonate ester | Nanosphere | Crystalline | 1.2, 8.81 | 382 | 2021 | 9 | Sensing, energy storage device |
| Ti[OCH(CH3)2]4 | |||||||||
| TiCl4 | |||||||||
| 11 | PMTP-1 | Ethylene diamine tetra(methylene phosphonic acid) (EDTMPS) | 1D channel | Crystalline | 2.8 | 1066 | 2010 | 59 | Photocatalysis and metal ion adsorption |
| 12 | Ti–X (X = HE,BH,HP,PA,DT,PB) | Tetrabutyl titanate (Ti(OC4H9)4) | Macro channels | Amorphous | 100–2000 | 2017 | 60 | Ammonia adsorption | |
| Ti–HE | 1-Hydroxy ethylidene-1,1-diphosphonic acid (HEDP) | 102 | |||||||
| Ti–BH | bis(hexamethylene triamine penta(methylene phosphonic acid) (BHMTPMPA) | 115 | |||||||
| Ti–HP | 2-Hydroxyphosphonocarboxylic acid (HPAA) | 401 | |||||||
| Ti–PA | Polyamino polyether methylene phosphonate (PAPEMP) | 9 | |||||||
| Ti-DT | Diethylene triamine penta (methylenephosphonic Acid) (DTPMPA) | 85 | |||||||
| Ti–PB | 2-Phosphonobutane-1,2,4-tricarboxylic acid (PBTCA) tetrabutyl titanate | 3 | |||||||
| 13 | TPPH | 1-Hydroxyethylidene-1,1-diphosphonic acid (HEDP) tetrabutyltitanate (Ti(OC4H9)4) | Channels | Amorphous | 2–3, 800–1200 | 511 | 2010 | 61 | Metal ion adsorption/CO2 adsorption |
| P123 | |||||||||
| F127 | |||||||||
| 14 | CuO-dispersed titanium phosphonate | Ethylene diamine tetra(methylene phosphonic acid) | Hexagonal channels | Partially crystalline | ∼2.2 | 606 | 2010 | 62 | Catalytic application & adsorption |
| TiCl4 | |||||||||
| CuO | |||||||||
| 15 | TiPNW | Etidronic acid (EA) | Nanowire | Amorphous | ∼2 | 160 | 2020 | 63 | Photocatalysis |
| Titanium isopropoxide | |||||||||
| 16 | Ti-AST | Alendronate sodium trihydrate (AST) | 1D channel | Amorphous | 2.4 | 540 | 2014 | 64 | Acid–base bifunctional heterogeneous catalysis in CO2 conversion |
| TiCl4 | |||||||||
| 17 | Titanium phosphonate hybrids | Tetrabutylorthotitanate (Ti(OBu)4) | Partly crystalline | 2022 | 65 | Tuned sorption behaviour, solvent separation | |||
| TiO2–C3 | 1,3-Propyldiphosphonic acid (PrDPA) | Less layered | Mesoporous | 379 | |||||
| TiO2–C8 | 1,8-Octyldiphosphonic acid (ODPA) | Layered | 329 | ||||||
| TiO2–Ph | 1,4-Phenyldiphosphonic acid (PhDPA) | Layered | 334 | ||||||
The limited number of reviews on hierarchical porous materials of metal phosphonates or phosphate underscores the need for a comprehensive compilation of titanium phosphonates, which are yet to be reviewed, to provide insights into this emerging field of research. While our group has extensively studied MOF-based photocatalytic applications47–50 and has contributed numerous review articles on this topic, many remarkable porous hybrid materials remain unexplored and require a broader, more comprehensive analysis. This review aims to explore recent advancements in titanium phosphonate MOFs, clusters and hybrid complexes, focusing on their coordination environment and hierarchical porosity. Additionally, it emphasises synthetic strategies to overcome the unpredictable behaviour of the Ti(IV) metal ion in forming targeted structures, along with an analysis of structure–property relationships.
2. Rational fabrication of porous titanium(IV) phosphonates
Various synthetic strategies have been developed to produce porous titanium(IV) phosphonates (PTPs) based on different precursors as linkers. Template-assisted synthesis is the most versatile and convenient approach to generate hybrid porous structures with significant performance in photocatalytic hydrogen evolution. HPTPs are synthesized via a facile hydrothermal process using a polyelectrolyte–surfactant system, for example, the cationic surfactant cetyltrimethylammonium bromide (CTAB) and an anionic polymer like poly(acrylic acid) (PAA). These components facilitate electrostatic interactions, enabling the co-assembly of precursors into well-defined hierarchical mesoporous structures.63 Nanostructured HPTPs are difficult to synthesise using the conventional hydrothermal method. Instead, a stirring hydrothermal technique is employed, in which mechanical force plays a crucial role in structural orientation along the axial direction, facilitating mass transfer and leading to the formation of nanowires. Titanium phosphonate adsorbent materials are synthesized from tetrabutyl titanate and various organophosphonic acids including (1-hydroxy ethylidene-1,1-phosphonic acid (HEDP), bis(hexamethylene triamine penta(methylene phosphonic acid)) (BHMTPMPA), 2-hydroxyphosphonocarboxylic acid (HPAA), 2-phosphonobutane-1,2,4-tricarboxylic acid (PBTCA), polyamino polyether methylene phosphonate (PAPEMP), and diethylenetriamine penta(methylene phosphonic acid) (DTPMPA) via a hydrothermal route. After the complete hydrolysis of titanium alkoxide, the homogeneous mixture is transferred to an autoclave and heated at 80 °C for 24 h, yielding HPTPs.60Hybrid porous titania phosphonate sorption materials are also synthesized following the above method with post synthetic treatment using bridged diphosphonic acids (DPAs) and Ti(OBu)4 in a water–ethanol mixture at 30 °C. The successful incorporation of DPAs into the titania matrix is observed with quantitative yield of the hybrid materials based on the Ti/2P ratio using different types of filtration tools to separate them.65 A surfactant-assisted hydrothermal method is used, where Brij 56 acts as a structure-directing agent along with Alendronate Sodium Trihydrate (AST) and TiCl4 to prepare mesoporous Ti-AST. Controlled hydrolysis and condensation conditions are maintained at a P/Ti molar ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and pH ∼4.0, adjusted using ammonia and hydrochloric acid, followed by stirring for 2 h. Thereafter, the solution mixture is sealed in a Teflon-lined autoclave and aged at 120 °C for 48 hours under autogenous pressure.64
3 and pH ∼4.0, adjusted using ammonia and hydrochloric acid, followed by stirring for 2 h. Thereafter, the solution mixture is sealed in a Teflon-lined autoclave and aged at 120 °C for 48 hours under autogenous pressure.64
In situ synthesis of titanium phosphonate/polymer composites via a nonhydrolytic solgel route is another remarkable process that highlights the importance of solvent in the growth and morphology of nanocomposites. Unlike the traditional water-based hydrolytic sol–gel processes, this method employs organic solvents to control polycondensation reactions, leading to materials with higher hydrothermal stability and controlled porosity. A viscous polymer medium, polypropylene (PP) or polystyrene (PS), was used to incorporate layered metal phosphonate fillers, which were synthesized in a Haake® internal mixer, achieving 10 wt% titanium phosphonate in the final composite with titanium oxide precursor Ti(OAc)4. The molten polymer, fabricated by modulation of the aspect ratio confirms the presence of P–O–Ti bonds.54 Similarly by maintaining a constant molar ratio of Ti and BTPA (benzene-1,3,5-triphosphonic acid) without altering the reaction time (24 h), several HPTP complexes (HTiP-7A, HTiP-7B, HTiP-7C and HTiP-7D) were developed by varying the pH of the synthetic gel and hydrothermal temperature. The synthetic gel was prepared by the slow addition of phosphonic acid solution to the alcoholic titanium-isopropoxide solution, followed by overnight stirring before transferring it into an autoclave for thermal agitation at 453 K. The mixture was then centrifuged at 5000 rpm and suspended in dry ethanol to obtain the desired nanoparticles. This self-assembly process yields the product without the use of any surfactant or templating agent.55
Macroporous titanium phosphonate materials were also synthesised via the hydrothermal method, using the inverse opal method with a microwave-treated PS sphere as a hard template. This technique uses colloidal crystals (often silica spheres or polystyrene spheres) as a sacrificial template for the production of extremely ordered macroporous structures upon removal of the template. HEDP (1-hydroxyethylidene-1,1-diphosphonic acid) and tetrabutyl titanate were used as precursors to form a homogenous solution, which was statically agitated at 60 °C, followed by extraction of spheres with a THF/acetone(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of v/v) mixture solution for 48 h.56
1 of v/v) mixture solution for 48 h.56
The dendritic tetrakis-1,3,5,7-(4-phosphonatophenyl)adamantane precursor, along with titanium(IV) isopropoxide, is used to overcome the challenges of close pillar disposition and limited control over pore size in the organic–inorganic hybrid HPTPs. Instead of ditopic building blocks, tetratopic ligands with extended tetrahedral symmetry, are used via a non-hydrolytic condensation pathway producing insoluble materials with a Ti/P ratio of ∼1. HPTPs exhibited different morphologies depending on the solvent used in the condensation reactions, for example, from layered compounds to hollow nanospheres.57
Evaporation-Induced Self-Assembly (EISA) utilizes a soft template approach, where surfactants spontaneously diffuse towards the vapor phase and utilize solvent evaporation to create mesoporous structures. This differs from the mild solvent evaporation technique, in which the evaporation of solvent is slow and controlled in order to deposit precursors, which is known to yield denser, less porous materials compared to EISA. In the synthesis of HPTPs, self-assembly strategies such as autoclaving followed by a slow evaporation process are often employed. Additionally, EISA can be functionalised by ClSO3H treatment due to its strong catalytic behaviour and ion exchange capability. In this process, HEDP and TiCl4 are used in combination with the non-ionic surfactant Brij 56. After autoclaving, white gel blocks are produced, and upon surfactant removal during extraction, a yellowish hue with hard texture is developed. However, the shape of the monoliths remains well preserved, retaining periodic mesoporosity.58 In some instances, an unprecedented polydentate claw molecule, sodium salt of ethylenediamine tetra(methylene phosphonic acid) (EDTMPS), is also used as a coupling agent. These organic–inorganic hybrids proved to be more tunable compared to purely inorganic porous materials.59 In a similar process EDTMPS and TiCl4 (P/Ti molar ratio: 4/3) are placed in a reactor kept in a cryosel bath to slow down the hydrolysis process. The pH value is maintained at 4.0 throughout the reaction using ammonia and HCl solution, ultimately yielding HPTPs with well-defined structures.62
A mild solvent evaporation strategy was employed using tetrabutyl titanate and HEDP as an organophosphorus coupling molecule to yield HPTPs. In this process, triblock copolymers F127 and P123 acted as structure-directing agents. The reaction mixture was stirred for 24 h and then transferred into Petri dishes to allow ethanol evaporation under controlled relative humidity at 40 °C in the air for 2 days. The surfactant was subsequently removed by ethanol extraction over 96 h.61 Beyond MOFs, hybrid networks, for example, clusters (1–7) were developed by using titanium tetraisopropoxide (Ti(OiPr)4) and various aryl phosphonic acids, both in the presence and absence of solvent. Additionally, titanium bis(acetylacetonate) diisipropoxide [Ti(acac)2(OiPr)2] was used to develop cluster 8 under solvent free conditions, as depicted in Scheme 3.51 Arylphosphonic acids (ArPO3H2) were prepared via the alcoholysis of phosphonate esters, which were synthesized via the Arbuzov reaction.66,67
 | ||
| Scheme 3 Synthesis of clusters (1–8), illustrating the stepwise formation and structural variations in polyhedral metal clusters Ti4P3,Ti7P6-type and cluster 8. Adapted with permission from ref. 51. Copyright of The Elsevier, 2018.51 | ||
High porosity depends on both the denticity of the ligand and its geometric pattern, which determine how it coordinates with the metal ion in a MOF structure. For example, tri and tetratopic ligands with rigid structures yield porous frameworks. Similarly, a nanoporous Ti-phosphonate MOF was reported using a novel tetradentate phosphonate ligand and TiCl4 as precursors, synthesized via a hydrothermal method at 180 °C for 48 h.9
3. Structure and morphology
Owing to variable oxidation states, the unpredictable reactivity of the Ti(IV) metal centre is often associated with the intriguing coordination modes. However, the challenging synthesis process results in poor control over the structure and properties of hybrid materials, such as Ti-based oxo-clusters, coordination polymers, and MOFs. Despite these challenges, titanium complexes continue to fascinate the research community due to their promising photocatalysis and optoelectronic properties. While divalent and trivalent transition metal ions have been extensively studied, yielding thousands of framework structures, their stability, remains a significant limitation. These frameworks are often susceptible to hydrolysis or exhibit unprecedented porosity, with less ordered structures, limiting their practical applications. To address these challenges, tetravalent frameworks have been explored. However, their high reactivity often leads to precipitation or poorly crystallised materials. In this context, Zr(IV) is an exception, as UiO-66 has proven to be one of the most robust materials. It is not only tunable but also facilitates the successful synthesis of hybrid materials and MOFs through an isoreticular approach. Similarly, titanium as a tetravalent cation, has been utilised to produce MOFs, oxo clusters, and organic–inorganic hybrid matrices, although with lower crystallinity.Titanium phosphonates (TPs) exhibit diverse morphologies such as platelets, nanowires, nanospheres, channels, layers, monoliths, etc. (Fig. 6) each contributing uniquely to hierarchical porosity, which is crucial for applications in catalysis, adsorption, energy storage, and separation. Platelet-like TPs feature layered plate-like structures with tunable interlayer spacing, where microporosity arises from interlayer gaps, which contribute to a high surface area and are also responsible for the interaction of small molecules. Other types of porosity like mesoporosity from platelet aggregation, and macroporosity from stacking voids are observed, largely depending on the synthetic process followed. These properties make them well-suited for catalysis, adsorption, and composite materials. Nanowire TPs, characterized by their one-dimensional, high-aspect-ratio structures, exhibit microporosity due to surface defects, mesoporosity from interwire gaps, and macroporosity resulting from assembly of subunits. These materials excel in energy storage, catalysis, and adsorption applications. Nanosphere TPs, on the other hand, form spherical architectures that can be solid or hollow, with their size and porosity tunable through synthetic techniques. Their hierarchical porosity includes microporosity resulting from ligand selection, mesoporosity introduced by templating or etching, and macroporosity resulting from interparticle voids, making them effective in drug delivery, catalysis, and energy storage. The hierarchical porous network is built by the arrangement of nanospheres on the bulk scale and can be tailored under controlled conditions for various potential applications.
Channel-based TPs form interconnected porous networks in 1D, 2D, or 3D frameworks, where microporosity originates from the intrinsic framework, mesoporosity from templating methods, and macroporosity from hard templating techniques. Their highly organized channels make them especially suitable for ion exchange, catalysis, adsorption and separation processes. Similarly, layered TPs consist of alternate inorganic Ti–O and organic–P layers forming lamellar structures. These TP layers are staked through non-covalent interactions like van der Waals forces or hydrogen bonding. Interlayer distances can be tailored using various phosphonic acid linkers and intercalating guest molecules. Furthermore, functionalisation in the organic linkers affects the layer structure, porosity and functionality. A self-supporting, robust, interconnected, continuous architecture forms TP monoliths which on integration with a well tuned pore hierarchy makes them highly effective for applications in separation and ion exchange processes. Unlike other forms of TPs, they produce a single framework which enhances structural integrity and prevents self-aggregation, an issue observed in other morphologies. The various morphological forms discussed in this review are elaborated categorically as follows.
Cluster titanium phosphonates (TPs) form a unique subclass of these materials, characterized by discrete Ti–O–P clusters that function as well-defined nanoscale building blocks. These clusters can be integrated into larger frameworks, contributing to hierarchical porosity by creating micropores within the clusters and meso to macropores through their arrangement pattern in the bulk material. However, the full extent of cluster-based porosity remains relatively unexplored, especially concerning the impact of aggregation and surface modifications influencing pore connectivity and accessibility. Gunji and co-workers reported eight single crystal clusters featuring three distinct core structures: Ti4P3, Ti7P6, and a distorted cubic framework, all exclusively composed of Ti–O–P bonds as shown in Scheme 3. Clusters 1 and 3 are crystallised in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group, cluster 7 adopted the trigonal P
space group, cluster 7 adopted the trigonal P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) space group, while the remaining clusters crystallised in a monoclinic system.51 In Ti4P3, four titanium metal centres exhibit octahedral geometry, each coordinated by five terminal and three bridging isopropoxy groups. Moreover, the presence of solvent adducts contributes to the stability of the coordination environment. The Ti7P6-type cluster consists of two [Ti3(μ3-O)(OiPr)3(μ-OiPr)3](Ti3O) units, six aryl groups (4-bromobenzyl for cluster 6 and 4-bromophenyl for cluster 7b) and a central titanium atom aligned along the C2-symmetry axis. Notably, cluster 7 is a cocrystal comprising two Ti4P3 clusters and one Ti7P6 type cluster. In contrast, cage-type cluster 8 is a distorted-cubic framework formed by four titanium atoms, four phenyl phosphonate groups and four isopropoxy groups, all chelated by acetylacetonato groups. Each metal centre is coordinated by one chelating acac group, one isopropoxy group and three bridging phenyl phosphonato groups connecting to other metal centres. This coordination environment highlights the influence of chelating groups and the steric hindrance introduced by para-substituents, which significantly affect the core structure of the clusters, as observed in both Ti4P3 and Ti7P6 type clusters. Similarly, Kessler and co-workers reported seven oxo alkoxide multinuclear clusters synthesized using solvothermal or reflux condensation methods, labelled 1 to 7, showing phosphonate ligand's bidentate and tridentate nature in coordinating metal ions. These clusters exhibit varying nuclearities, such as tetranuclear (2, 3, 4 and 6), pentanuclear (1 and 7), and hexanuclear (5) structures. The clusters are stabilized by various oxocores with suitable μ2, μ3 and μ4 bridges contributed by phosphonate and alkoxide (ethoxide/isopropoxide) groups.52 Bridging the titanium atom with μ2, μ3, and μ4 oxo groups plays a crucial role by terminating the coordination sites, making the core more compact and condensed, thus enhancing the stability of the complexes. Clusters 1, 3, 4 and 6 are crystallised in the monoclinic system while clusters 2, 5 and 7 adopt the triclinic crystal system. The pentanuclear oxo core cluster 1 consists of Ti3O units connected by μ3-O bridges, with phosphonate ligands coordinating to the metal centre in both tridentate and bidentate fashions. These examples demonstrate that incorporating multiple oxo bridges significantly influences the structural features of titanium–oxo complexes as they affect the nuclearity, geometry and stability of the cluster. Notably, upon hydrolysis, these clusters undergo a topotactic transformation, contracting from crystalline materials to densified structures featuring a crystalline core and an amorphous outer shell, as depicted in Fig. 2.
space group, while the remaining clusters crystallised in a monoclinic system.51 In Ti4P3, four titanium metal centres exhibit octahedral geometry, each coordinated by five terminal and three bridging isopropoxy groups. Moreover, the presence of solvent adducts contributes to the stability of the coordination environment. The Ti7P6-type cluster consists of two [Ti3(μ3-O)(OiPr)3(μ-OiPr)3](Ti3O) units, six aryl groups (4-bromobenzyl for cluster 6 and 4-bromophenyl for cluster 7b) and a central titanium atom aligned along the C2-symmetry axis. Notably, cluster 7 is a cocrystal comprising two Ti4P3 clusters and one Ti7P6 type cluster. In contrast, cage-type cluster 8 is a distorted-cubic framework formed by four titanium atoms, four phenyl phosphonate groups and four isopropoxy groups, all chelated by acetylacetonato groups. Each metal centre is coordinated by one chelating acac group, one isopropoxy group and three bridging phenyl phosphonato groups connecting to other metal centres. This coordination environment highlights the influence of chelating groups and the steric hindrance introduced by para-substituents, which significantly affect the core structure of the clusters, as observed in both Ti4P3 and Ti7P6 type clusters. Similarly, Kessler and co-workers reported seven oxo alkoxide multinuclear clusters synthesized using solvothermal or reflux condensation methods, labelled 1 to 7, showing phosphonate ligand's bidentate and tridentate nature in coordinating metal ions. These clusters exhibit varying nuclearities, such as tetranuclear (2, 3, 4 and 6), pentanuclear (1 and 7), and hexanuclear (5) structures. The clusters are stabilized by various oxocores with suitable μ2, μ3 and μ4 bridges contributed by phosphonate and alkoxide (ethoxide/isopropoxide) groups.52 Bridging the titanium atom with μ2, μ3, and μ4 oxo groups plays a crucial role by terminating the coordination sites, making the core more compact and condensed, thus enhancing the stability of the complexes. Clusters 1, 3, 4 and 6 are crystallised in the monoclinic system while clusters 2, 5 and 7 adopt the triclinic crystal system. The pentanuclear oxo core cluster 1 consists of Ti3O units connected by μ3-O bridges, with phosphonate ligands coordinating to the metal centre in both tridentate and bidentate fashions. These examples demonstrate that incorporating multiple oxo bridges significantly influences the structural features of titanium–oxo complexes as they affect the nuclearity, geometry and stability of the cluster. Notably, upon hydrolysis, these clusters undergo a topotactic transformation, contracting from crystalline materials to densified structures featuring a crystalline core and an amorphous outer shell, as depicted in Fig. 2.
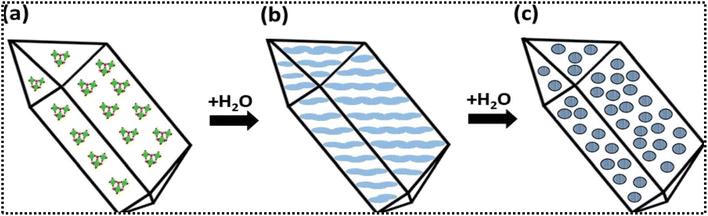 | ||
| Fig. 2 Transformation of an (oxo) alkoxide crystal (a) to a crystalline anatase core (b) results in the disordering of the amorphous phase (c) upon hydrolysis. Adapted with permission from ref. 52. Copyright of Royal Society of Chemistry, 2020. | ||
The development of platelet like titanium phosphonate-based nanocomposites via a non-hydrolytic sol–gel process within a viscous polymer medium is influenced by both the choice of phosphonate precursors and the polymer matrices employed. Specifically, diethyl phosphonates serve as the precursors, while polypropylene (PP) and polystyrene (PS) are utilised as the polymer matrices.54 The resulting nanocomposites exhibit distinct morphologies: in PP, fillers display amorphous platelet-like structures (less organised), whereas in PS, a layered (more organised) configuration is observed. The variation is attributed to the molecular mobility provided by the linker in DEBP/DEPP, which is associated with the PS matrix and facilitates π–π stacking interactions between aromatic groups. Such interactions promote better compatibility and integration between the filler and polymer matrix, leading to more organised layered structures. In contrast, the PP matrix lacks such interactions, resulting in less organised structures. Layered titanium phosphonates in the PS matrix result in an interlayer spacing of about 13.2 Å, leading to improved filler dispersion and reduced aggregate formation. The presence of P–O–Ti bonds within the MOF is effectively confirmed by FTIR and 31P NMR spectroscopy. When DEBP is used as a phosphonate precursor, the resulting PS-Ti-DEBP fillers are smaller (500 nm in length and 85 nm in width) and exhibit uniform dispersion. In contrast, when DEPP is employed, larger PS-Ti-DEPP fillers are formed. The introduction of a flexible methylene (CH2) group in the diethyl benzylphosphonate (DEBP) linker enhances molecular mobility within the polystyrene (PS) matrix. This increased flexibility facilitates more effective π–π stacking interactions among aromatic groups, leading to the formation of smaller, well-dispersed fillers. In contrast, the absence of the CH2 group in diethyl phenylphosphonate (DEPP) results in reduced molecular mobility, contributing to the formation of larger aggregates within the PS matrix. This structural design prioritizes thermal stability and solubility over porosity.
The extensively condensed HM-TiPPh MOF, characterised by hierarchical porosity, and amorphous nature, demonstrates high efficiency in photocatalytic hydrogen evolution. Mesomorphous liquid-crystalline phases, composed of building units, formed by Ti(IV) metal centres and 1-hydroxyethylidene-1,1-diphosphonic acid (HEDP), are coassembled through an ionic self-assembly mechanism. These formations are further stabilised by interactions with the surfactant cetyltrimethylammonium bromide (CTAB) and an oppositely charged polyelectrolyte, poly(acrylic acid) (PAA).53Fig. 3(a–d) show nanospheres with interstitial pores (left) alongside a schematic illustration of the assembly process (right), which follows the dual template method to achieve hierarchical porosity. The compositional homogeneity enhances light absorption, facilitates charge separation, and increases active site accessibility while also reducing material wastage and mitigating the recombination of photogenerated electrons and holes through the strategic distribution of electron traps.
 | ||
| Fig. 3 (a) SEM image of high yield spheres of HM-TiPPh. (b) Higher resolution of nanospheres showing a rough surface. (c) TEM image showing contrasting interstitial pores. (d) Secondary mesopores with larger size. The dual template method used in the formation of hierarchically porous titanium phosphonates (right). Adapted with permission from ref. 53. Copyright of Wiley, 2018. | ||
HTiP-7, another titanium phosphonate nanosphere, crystallises in the triclinic system, where titanium ions are interconnected with phosphonate ligands. The formation of P(O–Ti)3 units occurs as each phosphonate group from the BTPA ligand coordinates with three titanium atoms.55 As a result, a porous network emerges, integrating both micropores and mesopores with pore sizes centred around 1.4 nm, 3.0 nm, and 4.8 nm. Self-assembled nanoparticles with spherical morphology are facilitated by π–π stacking interactions between the aromatic rings of the BTPA ligands. The presence of mesopores, ranging from approximately 3.0 to 5.0 nm, subsequently helps in the adsorption of guest molecules. Macroporous titanium phosphonate materials (TOP), as reported by Yuan and co-workers, feature a three-dimensional ordered network synthesized using 1-hydroxyethylidene-1,1-diphosphonic acid (HEDP) as the phosphonate precursor.56 After the removal of the PS spheres (template) a void network is created, which is subsequently filled with titanium phosphonate gel, leading to a highly ordered macroporous structure with pore sizes around 400 nm. The titanium phosphonate framework adopts a reverse opal pattern, where the voids left by the original template spheres remain interconnected, forming large cages with internal spaces that enhance light distribution and facilitate mass transfer throughout the material (Fig. 4). Efficient filling of the voids with titanium phosphonate gel was achieved due to the thin pore walls (∼10–20 nm). In TOPβ (a β-cyclodextrin; β-CD derivative), the pore walls considerably thicken to around 200–400 nm, leading to a less ordered structure, with an increase in adsorption sites for metal ion uptake.
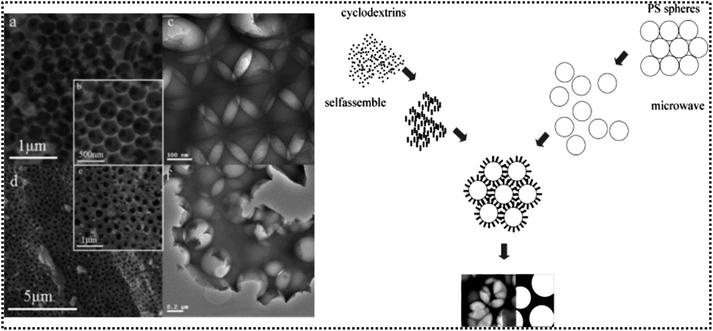 | ||
| Fig. 4 (a and b) SEM and (c) TEM images of TOP; (d and e) SEM and (f) TEM images of TOPβ (left). Scheme of formation of TOPβ (right). Adapted with permission from ref. 56. Copyright of American Chemical Society, 2008. | ||
HEDP enhances the mechanical strength and ductility compared to purely inorganic phosphonate precursors, which are more commonly used and exhibit distinct structures as reported in the literature.68–70 The well-ordered mesoporous framework, monolith PMTP-2, is an inorganic–organic hybrid with hexagonal symmetry, composed of a titanium phosphonate framework that incorporates HEDP. It has a pore size of approximately 2.4 nm, with a pore wall thickness of around 1.8 nm. This mesoporous structure, characterised by its unique composition, structural features, and potential for functionalization, presents promising applications in ion exchange, catalysis, and as an electrolyte in fuel cells.58 Yuan et al. developed another HPTP, a titanium organophosphonate hybrid framework, utilising structure-directing agents F127 and P123, along with a titanium precursor and HEDP, which acts as an organophosphorus coupling agent molecule.61 The triblock copolymers function as templating agents and aid in the self-assembly process to form a one dimensional macro channel structure. The macroporous framework (800–1200 nm in diameter) integrates a mesophase (2–3 nm in diameter), facilitating gas and liquid transport, while enabling metal ion binding through coordination. This structural hierarchy enhances adsorption efficiency, contributing to improved porosity and functionality. A single broad diffraction peak in the low-angle region confirms the aggregation of titanium phosphonate nanoparticles, which fill the mesovoids along the macroporous channel wall. This structural arrangement plays a crucial role in forming an interconnected network that improves adsorption by increasing the surface area and accessibility for on-site reactions in HPTPs.
Dendritic tetraphosphonates, such as tetrakis-1,3,5,7-(4-phosphonate phenyl)adamantane (TPPhA), serve as tetrahedral organic building blocks that significantly impact structural organisation of the hybrid organic–inorganic framework.57 The phosphonate groups (P–O) from the TPPhA units coordinate to titanium atoms in a bidentate manner, as confirmed by FT-IR and NMR spectroscopy, which indicate bonding between phosphonate oxygen atoms and titanium, while excluding interactions between the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group and titanium. This coordination follows a characteristic P(O)O2Ti2O2(O)P pattern, preventing close packing and promoting the hierarchical porous structure with both microporosity (13.5 Å) and mesoporosity (38 Å). The material primarily exhibits two primary morphologies: (i) a plate-like layered structure, where the plate exhibits a lamellar arrangement, and (ii) folded films, appearing as thin film-like structures that occasionally curl or roll at the edges. Additionally, by varying synthetic routes, hollow nanospheres (180–300 nm in diameter) composed of a titanium oxide-phosphonate mixture have been synthesized, highlighting the influence of solvent selection on the assembly process. This porous material possesses both amorphous and partially ordered (paracrystalline) characteristics, as indicated by broad peaks in X-ray diffraction patterns, signifying a disordered framework with localised periodicity in certain regions.
O group and titanium. This coordination follows a characteristic P(O)O2Ti2O2(O)P pattern, preventing close packing and promoting the hierarchical porous structure with both microporosity (13.5 Å) and mesoporosity (38 Å). The material primarily exhibits two primary morphologies: (i) a plate-like layered structure, where the plate exhibits a lamellar arrangement, and (ii) folded films, appearing as thin film-like structures that occasionally curl or roll at the edges. Additionally, by varying synthetic routes, hollow nanospheres (180–300 nm in diameter) composed of a titanium oxide-phosphonate mixture have been synthesized, highlighting the influence of solvent selection on the assembly process. This porous material possesses both amorphous and partially ordered (paracrystalline) characteristics, as indicated by broad peaks in X-ray diffraction patterns, signifying a disordered framework with localised periodicity in certain regions.
A novel nanoporous Ti-phosphonate MOF (H8L-Ti-MOF) has been synthesized using another tetradentate phosphonate ligand, 1,1,2,2-tetraphenylethylene (TPE)-phosphonate ester (H8L). The tetratopic arms coordinate with titanium ions, leading to a monoclinic crystalline phase with high chemical and thermal stability and adopting a nanosphere morphology.9 This three-dimensional crystalline structure exhibits hierarchical porosity, comprising micropores with a pore size of approximately 1.2 nm and mesopores with a pore size of about 8.81 nm, facilitating the accommodation of guest molecules, making the MOF suitable for sensing applications. Furthermore, the extensive π-conjugation in the ligand structure improves ion transport, thereby enhancing the material's performance as a supercapacitor by boosting conductivity and charge storage capabilities, as illustrated in Fig. 5.
 | ||
| Fig. 5 pi–pi interaction between the targeted TNP and aromatic moieties of the H8L-Ti MOF as shown by the yellow circle. Adapted with permission from ref. 9. Copyright of American Chemical Society, 2021. | ||
The mesoporous titanium phosphonate material (PMTP-1) is synthesized using ethylenediamine tetra(methylene phosphonic acid) (EDTMP) ligands, which coordinate with titanium (Ti) centers to form a stable 1D channel framework.59 The phosphonate groups serve as coupling molecules, providing both structural integrity and substantial functionality to the framework. EDTMPS can be protonated or alkylated by adding HCL or NaOH, respectively. Additionally, the organic bridge containing the ethylene diamine moiety plays a crucial role in influencing the physicochemical properties of the material. The presence of a hexagonal (p6mm) mesophase is confirmed by X-ray diffraction (XRD), which shows a primary peak corresponding to the (100) reflection, indicating well-defined mesoporosity. The average pore size is approximately 2.8 nm, with a pore wall thickness of 1.8 nm, enabling effective transport and interaction of guest molecules.
PMTP-1 exhibits a robust nature, as it is thermally stable up to 450 °C, and upon calcination above 550 °C, it converts into an amorphous titanium phosphate network, while partially preserving its mesoporosity. The material exhibits a one-dimensional (1-D) channel structure and its morphological versatility allows for the fabrication of various forms, such as monoliths, cubes, and powders, making it suitable for diverse applications. EDTMP also serves as a phosphonate precursor in mesoporous inorganic–organic hybrids (MTPs), which have been integrated as a support for CuO nanoparticles.62 In addition to mesopores, irregular macrovoids (large voids or cavities) are dispersed throughout the microspheres, which are formed by the accumulation of titanium phosphonate nanoparticles. This structural arrangement forms hexagonal channels which enhance the surface area and promote diffusion, facilitating efficient mass transfer of reactants and products in catalytic reactions. The spherical morphology and uniform pore distribution with an average size of 2.2 nm, provide a large surface area-to-volume ratio, ensuring effective dispersion in catalytic systems. The titanium oxide framework, stabilised by P–O bonds, plays a crucial role in coordinating metal ions such as copper. Following calcination, the mesophase remains intact, allowing the incorporation of well-dispersed CuO nanoparticles (CuO-MTPs), further enhancing catalytic efficiency. Moreover, the material's excellent thermal stability is essential for high-temperature catalytic applications, as it ensures structural integrity under extreme conditions. The high surface area and stability of this HPTP make it an effective support for catalytic processes, particularly by promoting the dispersion and stabilisation of CuO nanoparticles.
Titanium phosphonate adsorbent materials featuring a hierarchical porous structure have been reported, exhibiting macropores ranging from 100 to 2000 nm. These macropores create a macrochannel framework that facilitates the movement of gas molecules, enhancing mass transport and improving the accessibility of adsorption sites.60 Various phophonate linkers as listed in Table 2 have been utilised to synthesize adsorbent materials, including Ti–HE, Ti–BH, Ti–HP, Ti–PA, Ti–DT and Ti–PB. The P–O–Ti bonding enhances the stability of the adsorbent material and its capacity to interact with ammonia through acid–base reactions. This hierarchical design, integrating large macropores for effective gas diffusion with mesopores for increased surface area, significantly enhances the material's ammonia adsorption efficiency. Transmission electron microscopy (TEM) images (A–D) confirm the presence of microchannels with walls composed of nanoparticles. The enhanced surface area is attributed to macropores interspersed with a wormhole-like mesophase (2 to 8 nm in size), which further optimizes the adsorption process. Notably, in Ti–HP, the macroporous network walls are composed of mesophase structures formed by aggregated nanoplates, which are approximately 25 nm in size.
Alshareef et al. reported the synthesis of one-dimensional titanium phosphonate nanowires (TiPNWs), where the unique morphology facilitates charge carrier transport, playing a pivotal role in photocatalysis.63 These nanowires, approximately 80 nm in diameter, exhibit an amorphous nature with smooth surfaces. The homogeneous incorporation of etidronic acid (EA) into the Ti–O cluster influences the structural integrity, contributing to the material's robustness and electronic properties. Phosphonate groups derived from the organophosphonic acid form strong bonds with the titanium-oxo clusters, imparting superior stability, compared to MOFs utilising carboxylate linkers. Most intriguing aspect reported in this study is the bandgap engineering, attributed to electron-donating hydroxyl groups (–OH), which enable narrowing the bandgap more effectively than –Br in TiPNW–Br.
Hybrid porous titania phosphonate materials incorporating various functional groups (octyl, propyl, and phenyl) are synthesized using titania scaffolds that serve as the inorganic backbone of the hybrid network.65 The titania framework is functionalized with diphosphonic acids modified with organic groups such as octyl (C8), propyl (C3) and phenyl (Ph), which are bonded strongly to TiO2via Ti–O–P linkages. These organic linkers are responsible for both the chemical stability and the surface functionalization of the material. For instance, a long alkyl chain introduces hydrophobicity and leads to the formation of layered structures with large pores whereas a shorter alkyl chain is responsible for flexible structures with controlled porosity. The layered morphology is further influenced by van der Waals interactions between extended organic chains, while the rigidity and potential π–π stacking interactions induced by aromatic groups impact molecular packing and porosity. The hybrid materials form nanosized particles with porous architectures, and it is observed that the porosity depends on both the type and concentration of the phosphonate precursor. 31P NMR analysis confirms multiple binding modes (mono-, bi-, and tridentate) for the phosphonate groups, significantly influencing the structural integrity and functional properties of the framework materials. These materials exhibit mesoporous architectures, with tunable pore sizes ranging from microporous to mesoporous depending on the functional groups and synthesis conditions employed. Furthermore, the study reveals that the crystallinity of the sample depends on higher ratios of Ti to phosphonate (Ti/2P). The incorporation of organic components increases the crystallinity, promoting the formation of a prominent anatase phase, with rutile and brookite phases also observed; this affects the porous nature, surface area, and catalytic efficiency. However, at lower Ti/2P ratios, the titania phase remains predominantly amorphous due to the extensive surface coverage by diphosphonic acids, which impedes crystalline phase formation. This effect is more pronounced with alkyl-based diphosphonic acid compared to phenyl phosphonic acid. The structural features underscore their morphological versatility (Fig. 6) and highlight their potential applications in separation technologies, catalysis, adsorption studies, etc.
4. Role of ligand selection in achieving well-defined structure and pore chemistry
The rational design of porous framework structures emphasizes the careful selection of metal nodes and organic precursors, as these components are fundamentally linked to the structural features and functionality of hybrid materials. The key challenge lies in identifying the optimal combination to achieve the desired intrinsic properties in functionalized target molecules, with precise control over position and orientation.71 Another critical aspect of ligand selection is its influence on the coordination geometry within the first coordination sphere, which directly controls activity regulation, while the second coordination sphere facilitates non covalent bonding and structure stability. Phosphonate precursors (Table 2) acting as potential O-donors successfully generate organic/inorganic building blocks with diverse topologies, including MOFs, clusters, coordination polymers, etc. The nature of the phosphonate functional groups further modulates the material's properties: alkyl groups/chains increase the flexibility within the hybrid framework, whereas aromatic ring-substituted phosphonic acids impart rigidity due to their delocalised pi electrons, reinforcing structural stability.Organophosphonic linkers (Table 2) play a crucial role in the band gap, due to the strong donating ability of –OH functionality. The incorporation of multifunctional groups within these linker such as di, tri or tetra phosphonic acids enhances the material's performance. Additionally, heterofunctional moieties are sometimes introduced to achieve hierarchical structural advantages. In ligands such as HPAA and PBTCA, the phosphonate and carboxylate groups may not coordinate simultaneously, leaving unsaturated coordination sites available as active centers for pollutant or heavy metal ion capture. Similarly, polydentate claw-type molecules, including EDTMPS, DTPMPA, BHMTPMPA, and PAPEMP, contain alkyl-bridged nitrogen donors, which enhance coordination and interaction, ultimately improving catalytic and adsorption efficiency in cost-effective multifunctional materials. The strong P–O–Ti bonding in various PTPs highlights the robust coordination ability of these linkers, often forming doubly or triply bridged binding modes observed in cage or cluster-type compounds. In HTiP-7, the BTPA ligand, featuring three –P(O)(OH)2 groups, is designed to form –P(O–Ti)3 units in the absence of Ti–O–Ti bonds, demonstrating the ligand's high affinity for titanium coordination. In polymer/titanium phosphonate (TP) nanocomposites, alkyl phosphonates such as DEOP act as fillers, while aryl phosphonates combined with a PS matrix generate layered fillers. In addition to this, in DEBP, the presence of –CH2 enhances molecular mobility, reducing aggregation and leading to a well-defined structure. TiO2–C8, utilizing alkyl-bridged diphosphonic acid (DPA), exhibits a flexible framework that enhances porosity and promotes the formation of layered structures—an effect not observed in propyl-bridged DPA. The reason may be the absence of van der Waals interaction in short propyl moieties associated with PrDPA, whereas PhDPA induces rigid, low-porosity structures.
Bulky polydentate ligands such as TPE and TPPhA, despite their inherent rigidity, contribute to intriguing porous architectures. Their organically pillared layered structures reduce close packing due to cross-linking effects, leading to the formation of macro-/mesoporous channel walls interspersed with micropores. This hierarchical arrangement, developed with diverse surface functionalities, further enhances the material's applicability in adsorption and catalysis.
5. Potential applications of PTPs
Due to their hierarchical porosity ranging from microporous to macroporous phases, porous titanium phosphonates (PTPs) have demonstrated remarkable potential for a variety of applications. Although receiving less attention compared to other hybrid materials, their applicability and performance relative to traditional hybrids possessing uniform porosity are astounding. This section reports the novel applications of hierarchical porous titanium phosphonates (HPTPs) in energy storage, catalysis, ion exchange, sensing, adsorption, and others relying on the structural hierarchy.5.1 PTP/HPTPs as catalysts
Hierarchical titanium phosphonates are of considerable interest as catalysts due to their unique porosity, which facilitates efficient mass transfer and diffusion of reactants and products. Further optimization of the porous architecture enhances the functional efficiency by selectively tailoring the catalytic environment, increasing the number of active sites, and improving the accessibility of the catalytic centres for practical applications. Photocatalytic hydrogen evolution using hybrid porous materials, such as clusters or Metal–Organic Frameworks (MOFs) as catalysts, is a burgeoning arena in sustainable energy research, offering viable alternatives to minimise resource consumption and promote green reactions.72,73 Photocatalysts absorb light with energy equal to or greater than their bandgap energy, causing electron excitation with electron–hole pair formation followed by charge migration and inhibition of recombination to retain catalytic activity. In processes, such as the hydrogen evolution reaction (HER) from water, under light irradiation, the goal is to produce hydrogen, a clean and renewable energy source, by harnessing solar energy. The enhanced catalytic efficiency enabled by the hierarchical porosity of the HPTPs plays a crucial role in this reaction, as their well-defined porosity facilitates optimal light absorption, charge separation, and mass transport, ultimately improving hydrogen production efficiency.Li et al. reported titanium phosphonate-based MOFs for enhancing the photocatalytic hydrogen evolution reaction (HER).53 Hierarchically mesoporous titanium phosphonates (HM-TiPPh) and mesoporous titanium phosphonate materials (M-TiPPh) were synthesized for comparative studies, highlighting the critical role of the anionic polymer poly(acrylic acid) (PAA) in forming hierarchical porosity. They found that the materials favoured mass transfer and exhibited strong optical absorption during the photocatalytic water splitting for hydrogen production. Under visible light irradiation (λ > 400 nm, Fig. 7a), a remarkable H2 production rate of 945 μmol h−1 g−1 is achieved on HM-TiPPh, surpassing that of TiO2 (249 μmol h−1 g−1). In contrast, M-TiPPh exhibits a lower mass-specific activity (698 μmol h−1 g−1) than HM-TiPPh, underscoring the significant role of large secondary nanopores in enhancing the performance. The HM-TiPPh materials exhibited remarkable visible-light-driven hydrogen evolution activity and were even found to be superior to most of the TiO2-based photocatalysts74 and other catalytic systems.75,76
 | ||
| Fig. 7 (a) Typical time course of hydrogen evolution catalyzed by the synthesized photocatalysts under visible light irradiation and (b) under simulated sunlight. Adapted with permission from ref. 53. Copyright of Wiley, 2018. | ||
On subsequent exposure to full-spectrum simulator illumination (Fig. 7b), HM-TiPPh exhibits a remarkable hydrogen production rate of 1873 μmol h−1 g−1, 1.58 and 3.21 times greater than that of MTiPPh (1185 μmol h−1 g−1) and TiO2 (584 μmol h−1 g−1), respectively, aligning with the pattern observed under visible light irradiation. HM-TiPPh exhibited excellent long-term stability, maintaining its activity after four consecutive cycles under prolonged light exposure for 16 h. These findings highlight material's superior hydrogen evolution performance under both visible light and simulated full spectrum illumination, as shown in Fig. 7.
Macroporous titanium phosphonate (TOP) materials, with a pore size around 400 nm, exhibiting remarkable photocatalytic activity and heavy metal ion adsorption characteristics are reported.56 Three-dimensionally ordered macroporous titanium phosphonate materials developed by an inverse opal method using the HEDP precursor, TOP and β-cyclodextrin (β-CD) added derivative (TOPβ), containing hydroxyethylidene groups inside the frameworks are the focus of the comparative study. The inorganic–organic hybrid materials exhibit significant photocatalytic activity, as demonstrated by the photodecomposition of Rhodamine B (RhB).
Fig. 8 illustrates that while TOP exhibits slightly lower efficiency than the commercial P-25, TOPβ demonstrates superior catalytic activity, despite having a higher band gap of TOPβ (3.19 eV) compared to P-25 (3.10 eV). This discrepancy is likely attributed to the pore-wall structure of the synthesized photocatalysts. The macroporous titanium phosphonate material is comparable in size to the wavelength of light, facilitating enhanced photon energy supply to the inner surface. The 3D-ordered macropores with internal windows serve as light-transfer pathways, enabling deeper light penetration into the photocatalyst.75 Furthermore, doping phosphorus into the framework of the synthesized materials enhances photocatalytic efficiency.77–79 Due to the extended band gap energy of phosphonate based materials, mesoporous TiO2 exhibits higher photocatalytic activity than P-25.
 | ||
| Fig. 8 (a) Photocatalytic activities of the samples for RhB degradation under UV-light irradiation; (b) Plots of ln(C0/C) vs. the irradiation time, showing the fitting results using the pseudo-first-order reaction. Adapted with permission from ref. 56. Copyright of American Chemical Society, 2008. | ||
Similarly, EDTMPS, a polydentate claw molecule, forms a hexagonal structure PMTP-1 with well-ordered periodic mesopores with an average pore size of 2.8 nm, and a surface area of 1066 m2 g−1.59 The material exhibits enhanced photocatalytic activity, particularly in the degradation of organic compounds such as RhB, outperforming commercial photocatalysts like P-25. A degradation rate of 68.4% results from high absorption phenomena and a well-structured mesophase with a high surface area, providing more active sites for photoabsorption and mass transfer. CuO nanoparticles, dispersed on hybrid or inorganic supports, exhibit high oxidation activity, as shown in Fig. 9. CuO-MTPs demonstrate stability in low-temperature oxidation reactions compared to other phosphonate hybrids, whereas CuO-iTP shows a 4% decrease in the conversion rate during the first 2 h, followed by stability; this may be due to the reaction between the reactant and the catalyst.
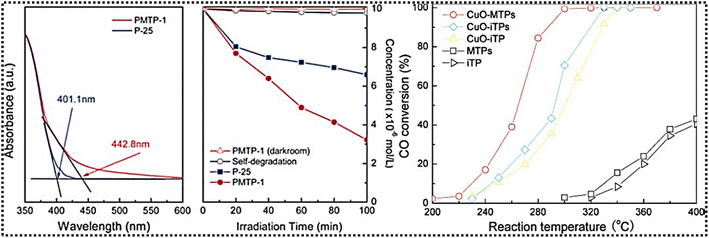 | ||
| Fig. 9 UV-vis diffuse reflectance spectra of PMTP-1 and commercial P-25 (left), and photocatalytic activities of PMTP-1 and P-25 for RhB degradation under analogous solar light irradiation (2nd left). Catalytic activity for CO oxidation (right). Adapted with permission from ref. 59. Copyright of Royal Society of Chemistry, 2010. | ||
The robust 1D nanowire morphology of titanium phosphonate photocatalysts facilitates charge carrier separation and transport while reducing the band gap. This structural advantage enables the development of advanced, rationally functionalized catalysts with superior structural compatibility and enhanced catalytic efficiency. As shown in Fig. 10c functionalisation of the phosphonate precursor by replacing –OH with a weak electron donor, such as –Br, results in reduced photocatalytic hydrogen production in TiPNW–Br compared to TiPNW, highlighting the crucial role of the –OH functionality.63 Moreover, the Ti–O–P bonding acts as a vital connection between phosphonic bridging groups and the titanium oxo core, contributing to the material's stability, outperforming TiO2. The study demonstrates the possibility of bandgap tuning in TiPNW materials, where structural modification followed by ligand functionalisation plays a crucial role in optimizing electronic properties (Fig. 10b). The presence of an electron-donating group (-OH) in the linker raises the Valence Band Maximum (VBM), effectively lowering the band gap relative to TiO2. In contrast, an electron electron-withdrawing group (–Br) lowers the VBM with an increase in the bandgap, which is confirmed by DFT calculations. The optimized VBM–CBM alignment in TiPNWs facilitates charge separation, reducing recombination losses. The lower bandgap enhances visible light absorption, ultimately increasing the photocatalytic conversion efficiency. TiPNWs exhibit superior photocatalytic activity compared to TiPNPs as evidenced by their higher hydrogen production rate of 1260 μmol h−1 g−1, significantly surpassing TiPNPs' 501 μmol h−1 g−1.
 | ||
| Fig. 10 (a) Typical time course of hydrogen evolution catalyzed by the synthesized photocatalysts in comparison with TiO2 under visible light irradiation. (b) Pictorial representation of the electronic band structure of TiO2, TiPNWs and TiPNW–Br. (c) Schematic view of the formation of TiPNW–Br. (d) Proposed mechanism for H2 production. Adapted with permission from ref. 63. Copyright of Wiley, 2020. | ||
Although both possess similar coordination environments and TiPNPs possess a higher surface area, the 1D nano wire morphology plays a pivotal role in the enhanced performance. A full spectrum simulator demonstrates a high hydrogen evolution rate which is 2346 μmol h−1 g−1 for TiPNWs and 955 μmol h−1 g−1 for TiPNPs. Hence this finding confirms that the surface area alone is not responsible for good catalytic activity as TiPNPs with a large surface area feature a lower hydrogen evolution rate in comparison to TiPNWs, underscoring the critical role of the nanostructure and morphology of the synthesized catalyst (Fig. 10a). Besides this the cocatalyst platinum (Pt) ensures efficient electron extraction at the conduction band of the Ti-oxo cluster by capturing and spatially separating the photoinduced electrons (Fig. 10d). This prevents rapid recombination with holes at organic ligands, thereby dramatically enhancing charge carrier dynamics and overall photocatalytic efficiency.
Furthermore, stability studies reveal that TiPNWs maintain their photocatalytic activity over five consecutive cycles of 3 h operation without degradation or deactivation. Additionally, the catalyst also retains its morphology after two months of storage, demonstrating robustness and long term stability of the material.
T. Ma and S. Z. Qiao explored the role of periodic mesoporous metal phosphonates (PMMPs), comprising titanium phosphonates, as highly effective acid–base bifunctional catalysts.64 These materials stand out due to their well-organized mesoporous structures, high surface areas, enhanced mass transfer and adjustable acid–base properties, making them valuable for heterogeneous catalysis. Mesoporous titanium phosphonates, Ti-AST, act as bifunctional catalysts, possessing both Lewis acid (P–OH) and Brønsted base (-NH2) sites within their framework, which activate aziridine and CO2, respectively, catalysing the cycloaddition reaction, as shown in Fig. 11. This dual functionality enables Ti-AST to facilitate acid–base cooperative reactions by forming reactive carbamate species. The cycloaddition process follows a highly regioselective path (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) due to the stability of the intermediate, resulting in selective oxazolidinone formation. Ti-AST exhibits outstanding catalytic performance, achieving a high conversion rate (>99%) and an impressive yield of 98%, surpassing most heterogeneous catalysts. Additionally, this reaction is both environment friendly and sustainable as it does not require halogens, organic solvents, or cocatalysts. Furthermore, Ti-AST is easily separable and reusable without any loss of activity, making it an efficient and practical catalyst for repeated use.
2) due to the stability of the intermediate, resulting in selective oxazolidinone formation. Ti-AST exhibits outstanding catalytic performance, achieving a high conversion rate (>99%) and an impressive yield of 98%, surpassing most heterogeneous catalysts. Additionally, this reaction is both environment friendly and sustainable as it does not require halogens, organic solvents, or cocatalysts. Furthermore, Ti-AST is easily separable and reusable without any loss of activity, making it an efficient and practical catalyst for repeated use.
 | ||
| Fig. 11 Proposed reaction mechanism initiated on the bifunctional Ti-AST catalyst for the cycloaddition reaction between CO2 and aziridine. Adapted with permission from ref. 64. Copyright of American Chemical Society, 2021. | ||
Overall, hierarchical porous titanium phosphonates (HPTPs) are very promising as highly effective catalysts, mainly due to their well-defined hierarchical porosity, high surface area, and structural tunability. These features synergistically enhance reactant diffusion, light harvesting, and catalytic site accessibility, leading to excellent catalytic efficiency and long-term stability. These advantages make HPTPs highly promising for practical applications in photocatalysis and other sustainable catalytic processes, surpassingthe limitations of conventional photocatalysts.
5.2. Environmental remediation; pollutant adsorption/separation
Adsorption phenomena in porous solids play a crucial role in environmental remediation, particularly in applications such as CO2 capture from industrial emissions, organic pollutant removal, heavy metal ion adsorption, etc. Phosphonate hybrid materials (PTPs) have emerged as effective adsorbents due to their unique structural attributes, combining organic and inorganic moieties to achieve high adsorption capacities, chemical stability, and selectivity for various pollutants, including heavy metal ions, etc. With increasing industrialization and urbanization, the release of heavy metal ions into the environment has become a major concern. When their concentrations exceed permissible limits set by the World Health Organization (WHO), they pose significant risks to ecological systems.80,81 To address this, functional groups such as thiols, thiourea, and amines have been employed as metal ion binding motifs for the efficient removal of toxic heavy metals like Hg(II), Cu(II), and Cd(II). However, the potential of porous metal-organophosphate materials for heavy metal adsorption remains underexplored.82,83 Porous structures are often considered as excellent adsorbents due to the availability of internal adsorption sites. In this process, the synthesis of crystalline nanoporous structures remains challenging as rapid aggregation often limits the development of permanent porosity, making it difficult to achieve stable and reusable materials.PMTP-1, a periodic mesoporous titanium phosphonate, proved to be an efficient adsorbent exhibiting Langmuir-type behaviour, with selective affinity towards Cu2+ ions over Pb2+ and Cd2+.54 Previously, mesoporous hydroxyethylidene-bridged metal phosphonate materials were investigated for their adsorption properties, but they exhibited relatively low surface areas (<511 m2 g−1).61 In contrast, PMTP-1 offers significantly improved performance featuring a high surface area of 1066 m2 g−1. A key factor contributing to this enhanced adsorption capability is the presence of ethylene diamine bridging groups from EDTMPS, which exhibit a strong affinity for Cu2+ ions. Additionally, the high distribution coefficient parameter (Kd) for Cu2+ further supports the preferential adsorption of Cu(II), making PMTP-1 a highly effective and selective material for heavy metal ion removal.84,85
The material remains reusable upto four cycles (96.4% uptake efficiency) even under strong acidic conditions (1 M HCl) sustaining multiple sorption cycles as depicted in Fig. 12 (right). The presence of external electrolytes, such as NaCl for Cu2+ and KNO3 for Pb2+ increases ionic strength, but results in only a minimal decrease in adsorption efficiency up to 1 mM strength;thereafter the adsorption efficiency shows static behaviour even at high electrolyte concentrations (Fig. 12, middle). Another mesoporous titanium phosphonate (MTP) integrated with the EDTMPS ligand possessing a surface area of 606 m2 g−1 and containing macrovoids within microspheres, has been reported to exhibit monolayer adsorption of Cu2+ due to the strong ligand coordination.62 The adsorption capacity was found to be 38.41 μmol g−1, comparable to those of previously reported periodic mesoporous organosilicas.86,87 Similarly, heavy metal ion adsorption by amorphous macroporous titanium organophosphonate materials (TOP and TOPβ adsorbents) in mixed solutions containing Cu(II), Cd(II), and Pb(II) ions at the same concentration (10 mg L−1), demonstrated a distinct preference for the uptake of Cu2+.51 The adsorption capacity for Cu2+(0.157 mmol g−1 for TOP and 0.166 mmol g−1 for TOPβ) was significantly higher than that for Cd2+ (0.064 mmol g−1 for TOP and 0.070 mmol g−1 for TOPβ) and Pb2+(0.045 mmol g−1 for TOP and 0.046 mmol g−1 for TOPβ). The percentage removal of Cu2+ was approximately 20%, whereas the removal of Cd2+ and Pb2+ was around 10%. Porous MOF materials have been widely studied for absorption and separation techniques, as organic/inorganic functional groups facilitate strong interaction with contaminants or pollutants. Yaghi et al. conducted extensive research on the porosity of various MOF materials.88–90 Later on, composite adsorbents with a combination of organic and inorganic phases demonstrated excellent adsorbent behaviour, particularly for ammonia gas adsorption (5.2 mol kg−1), as reported by Levan et al.91
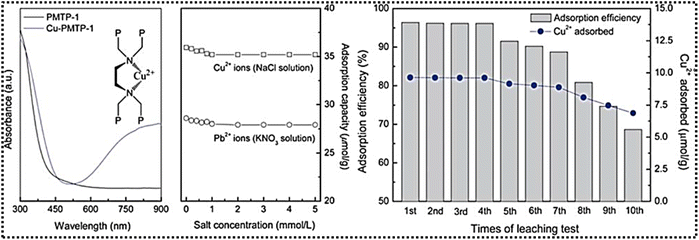 | ||
| Fig. 12 UV-vis diffuse-reflectance spectra of the PMTP-1 adsorbent before and after Cu2+ ion loading (Cu-PMTP-1) (left). Effect of ion strength on metal ion adsorption behaviour (middle). Reusability of the PMTP-1 adsorbent for Cu2+ ions (0.2 × 10−4 mol L−1) (right). Adapted with permission from ref. 59. Copyright of Royal Society of Chemistry, 2010. | ||
The hierarchical porosity of these materials enhances their adsorption efficiency, making them promising candidates for pollutant decontamination. Ammonia adsorbents based on phosphonate precursors (organophosphonic acids) and hydrolysed metal alkoxides have led to the development of hierarchical PTPs, which have been subjected to adsorption studies.60
Among the six HPTPs, Ti–PA and Ti–PB have monolith structures with relatively small surface areas, whereas Ti-HP possesses the highest surface area (401 m2 g−1) among all the Ti–X hybrids. Ti-HE, on the other hand, features the highest saturation adsorption capacity (75.2 mg g−1 (Fig. 13). The presence of hierarchical pores, surface adsorbed water, and hydroxyl groups enhances the saturation adsorption time and improves NH3 adsorption performance. Although both physisorption (via van der Waals forces) and chemisorption (via acid–base reactions between ammonia and acidic sites) contribute to the adsorption process, the overall efficiency primarily depends on chemisorption. This mechanism restricts desorption, preventing secondary pollution and making these materials highly effective for environmental remediation. Consequently, these innovative materials are categorised as adsorption-reaction-type materials.
 | ||
| Fig. 13 NH3 adsorption efficiency of PTPs (left) and three cycles of NH3 adsorption measurements for Ti-HE. Adapted with permission from ref. 60. Copyright of Wuhan University of Technology and Springer Verlag Berlin Heidelberg, 2017. | ||
Another example of hierarchical macroporous morphology within a mesoporous framework is observed in TPPH-P123 and TPPH-F127, which exhibit high surface areas and exceptional sorption capacities for Cu2+ in the liquid phase and CO2 in the gas phase, highlighting their potential for environmental remediation.61 The hierarchical structure of these hybrid frameworks helps maintain the specific surface areas at the pore system level, ultimately enhancing sorption capacity. The high distribution coefficient (Kd) values (92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 405 ml g−1 for TPPH-P123 and 232
405 ml g−1 for TPPH-P123 and 232![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 273 ml g−1 for TPPH-F127) indicate a favorable environment for Cu2+ adsorption (Fig. 14a). Bridging phosphonates play a pivotal role with methylene –OH and P–O groups from HEDP providing coordination sites that enhance sorption ability. Additionally, macro channels combined with mesophases facilitate diffusion and mass transfer, further improving adsorption efficiency. Between the two hybrids, TPPH-P123 with its high surface area, proves to be a superior adsorbent. It also demonstrates excellent reusability, maintaining a 96.45% uptake efficiency even after four cycles (Fig. 14c). Interestingly pH studies reveal that the optimal absorption conditions occur in a neutral environment (pH = 7), with a sharp decrese in uptake capacity observed as the pH increases further (Fig. 14b).
273 ml g−1 for TPPH-F127) indicate a favorable environment for Cu2+ adsorption (Fig. 14a). Bridging phosphonates play a pivotal role with methylene –OH and P–O groups from HEDP providing coordination sites that enhance sorption ability. Additionally, macro channels combined with mesophases facilitate diffusion and mass transfer, further improving adsorption efficiency. Between the two hybrids, TPPH-P123 with its high surface area, proves to be a superior adsorbent. It also demonstrates excellent reusability, maintaining a 96.45% uptake efficiency even after four cycles (Fig. 14c). Interestingly pH studies reveal that the optimal absorption conditions occur in a neutral environment (pH = 7), with a sharp decrese in uptake capacity observed as the pH increases further (Fig. 14b).
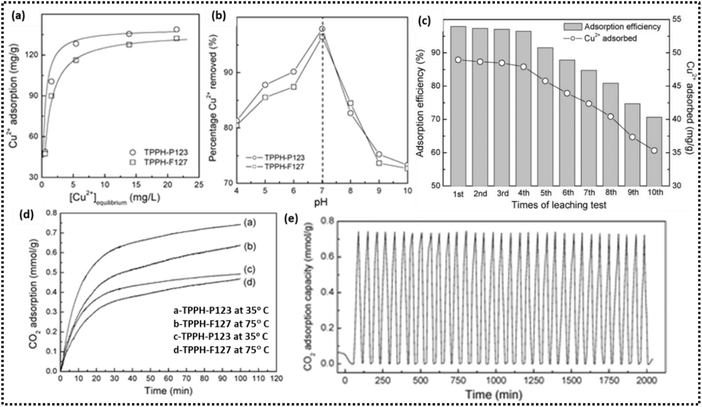 | ||
| Fig. 14 (a) Cu2+ adsorption isotherm for TPPH hybrid materials. (b) Effect of pH on Cu2+ removal by the TPPH material. (c) Reusability of the adsorbent TPPH-P123 for Cu2+ ions (10 mg L−1). (d) TGA records for CO2 adsorption. (e) Multiple cycles of CO2 adsorption measurements. Adapted with permission from ref. 61 Copyright of Royal Society of Chemistry, 2010. | ||
Similarly, various adsorbents like activated carbon, mesoporous silica and zeolite-type materials have been widely studied for gas phase adsorption and separation techniques in the environment remediation processes. However, PTPs are also gaining significant attention for greenhouse gas adsorption, particularly CO2, due to their advanced structure, functional properties, and superior adsorption performance. As depicted in Fig. 14d, thermogravimetric analysis (TGA) results for adsorption studies show that the initial adsorption rate is slow and thereafter it reaches equilibrium, likely due to the physisorption process. At 35 °C, TPPH-P123 exhibits a CO2 uptake of 0.75 mmol g−1 whereas TPPH-F127 shows 0.63 mmol g−1. Both values surpass the adsorption capacities of silica based adsorbents with high specific surface areas, highlighting the advantage of these hybrid materials. The presence of van der Waals forces between CO2 molecules and the hybrid framework further enhances the adsorption process. Additionally, the incorporation of the organic functional groups and the morphological properties of the materials, play a major role in enhancing adsorption efficiency. The micro-morphology of macrochannels reduces resistance and facilitates mass transfer, making these porous materials more effective for achieving adsorption/desorption equilibrium. Furthermore, hierarchical TPPH hybrids demonstrate excellent reusability, as their adsorption/desorption capacity remains undisturbed up to 33 consecutive cycles, proving their robustness and long-term efficiency as practical adsorbents.
The sorption behavior of alkyl-functionalized hybrid titania phosphonates, incorporating octyl (C8), propyl (C3), and phenyl groups, has also been investigated based on three different diphosphonic acids.65 Solid-phase extraction experiments were carried out using methanol/heptane for C3- and C8-functionalised systems, while a methyl/pyridine mixture was used for the phenyl functionalised system. Among these materials, TiO2–C8 demonstrated excellent separation performance due to its layered structure, which prevents strong interaction with heptane molecules, thereby facilitating separation. In contrast, TiO2-Ph-5 exhibited poor separation efficiency, retaining 100% of pyridine, which may be ascribed to the strong interaction between methanol and pyridine. Compared to commercially available silica C8-columns, alkyl functionalised titania hybrids show superior efficiency in purifying waste streams and contaminated water sources, making them promising materials for environmental remediation.
5.3. Miscellaneous applications
Chakraborty et al. reported a novel tetradentate phosphonate ligand-based porous metal−organic framework, H8L-Ti-MOF, which was for the selective sensing of 2,4,6-trinitrophenol (TNP) in the aqueous phase.9 The fluorescence intensity of the MOF was completely quenched upon coming in contact with TNP, while other nitroaromatic and nitroaliphatic compounds had only a minimal impact on its fluorescence profile. Although the quenching mechanism is attributed to one of the three possible pathways – Photoinduced Electron Transfer (PET), Resonance Energy Transfer (RET) and Intermolecular Charge Transfer (ICT) – the electron-rich polyaromatic system interacts with TNP via –C–H⋯π and π⋯π interactions (Fig. 5). The observed bathochromic shift in fluorescence titration spectra supports the ICT mechanism, indicating charge transfer upon interaction. Due to the close proximity of the analyte, RET can't be ruled out as a possible mechanism to describe the host–guest interaction. Moreover, a dynamic quenching mechanism is suggested for the interaction, as confirmed by fluorescence lifetime experiments. Reusability studies indicate that the material remains effective for up to four cycles, with a detection limit of 3.6 μM (0.82 ppm).
 | ||
| Fig. 15 Growth and decay current of BTP, HTiP-7, and HTiP-7RB (left). Schematic representation of electron flow in the dye-loaded HTiP-7 in the presence of light (right). Adapted with permission from ref. 55. Copyright of Royal Society of Chemistry, 2013. | ||
This interfacial electron transfer takes place when optically excited RB injects electrons into the conduction band at a rate significantly faster than the decay of its excited (Fig. 15 right). In contrast, this phenomenon is absent in the hybrid titanium phosphonate and bulk titanium phosphate due to their large band gaps, where charge transfer occurs via the valence band. Thus, the incorporation of a photosensitizer molecule enhances electron excitation and transfer within the micro/meso pores of PTPs, thereby significantly boosting visible light-driven photocurrent generation.
Hybrid networks can be precisely tailored by functionalizing organic moieties to achieve the desired shape and size. This customization enhances the stability of functional materials, enabling their effective use even under extreme environmental conditions. In addition, the hierarchical porosity enhances the material's suitability by increasing its surface area. A prime example is the mesoporous titanium phosphonate monolith, PMTP-2, synthesized using 1-hydroxy ethylidene-1,1-diphosphonic acid (HEDP) as an anchoring molecule through an autoclaving process. This self-assembled structure exhibits a remarkably high surface area of 1034 m2 g−1,58 making it highly effective for various applications. Furthermore, the synthesized PMTP-2 was functionalized by sulfation with ClSO3H, resulting in a significantly enhanced ion exchange capacity. This modification enables PMTP-2 to function not only as an efficient ion exchanger but also as a strong acid catalyst for certain low-temperature reactions. The facile sulfation process with ClSO3H leads to the formation of stable hydrosulfated esters, which is likely attributed to the specific alkyl hydroxyl structure of the coupling molecule HEDP. During the subsequent synthesis of the ordered mesophase, carbon-hydroxyl groups were uniformly distributed across the pore walls. These hydroxyl groups were then functionalized with SO3H groups through treatment with ClSO3H, enhancing the material's ion exchange and catalytic properties (Fig. 16).
 | ||
| Fig. 16 Synthesis, sulfation and ion exchange processes of the monolithic PMTP-2 material. Adapted with permission from ref. 58. Copyright of Royal Society of Chemistry, 2010. | ||
This functionalization significantly enhances the ion exchange capacity compared to the unfunctionalized PMTP-2 and previously reported titanium phosphate materials, making it more effective for catalytic and separation applications.56,96 For instance, sulfated PMTP-2s was employed for the esterification of oleic acid with methanol at ambient temperature and pressure, where PMTP-2s demonstrated superior activity compared to PMTP-2, achieving a conversion rate of 87.3%, significantly higher than that of unfunctionalized PMTP-2, which was only 4.9%. Furthermore, the conversion surpassed that of conventional sulfonic acid-functionalized mesoporous titania materials (81.7%).
Furthermore, the hydrosulfated groups in PMTP-2s act as proton carriers, enabling their potential use as an electrolyte for fuel cells.102 Similarly, H8L-Ti-MOF, used as an electrode in an asymmetric supercapacitor, delivers 20.03 F cm−2 @0.1 mA cm−2 current density with excellent recyclability.9 This energy storage capacity of the Ti-MOF was studied using an active material slurry coated on the cathode and a carbon black anode. While initial capacitance fading occurred up to 1000 cycles, only a 10% loss was observed over the next 900 cycles. The supercapacitive behaviour and 82.68% capacitance retention after 1000 cycles confirm the material's suitability for energy storage devices. As current density increases, galvanostatic charge/discharge (GCD) exhibits a slight deviation from ideal behaviour, indicating pseudocapacitance. The effect enhances the areal capacitance by enabling rapid, reversible redox processes combined with the usual electrostatic charge accumulation, increasing the energy storage ability.
| Materials | Thermal stability | Chemical stability | Mechanical stability | Hydrothermal stability | Key applications | Ref. |
|---|---|---|---|---|---|---|
| PTPs | Highly stable (>500 °C) | Excellent (Resistant to acids and bases) | High (rigid framework) | High (stable in hot water/steam) | Catalysis, ion exchange, adsorption, and drug delivery | 51 and 65 |
| Activated carbons | Moderate (∼800 °C under inert conditions) | Poor (oxidizes in strong acids/bases) | High (good mechanical strength) | Poor (degrades in humid environments) | Gas adsorption, water purification, and catalysis | 103 |
| Zeolites | High (>1000 °C) | Good (Resistant to acids but dissolves in strong bases) | High (robust crystalline structure) | Moderate (some structural degradation in hot water/steam) | Catalysis, ion exchange and molecular sieving | 105 and 106 |
| Metal–organic frameworks (MOFs) | Low to moderate (decomposes ∼200–400 °C) | Poor to moderate (sensitive to moisture, acids, and bases) | Low to moderate (soft structure, fragile) | Poor (degrades in water/humid environments) | Gas storage, separation, catalysis and drug delivery | 107 and 108 |
| Silica-based materials (e.g., MCM-41, SBA-15) | High (>800 °C) | Moderate (stable in weak acids but dissolves in strong bases) | High (porous but mechanically robust) | Moderate (some collapse in hot water) | Adsorption, catalysis and separation processes | 109 |
Overall, PTPs emerge as a superior alternative due to their exceptional stability under diverse environmental conditions, making them particularly advantageous for applications that require highly durable and chemically resilient materials. This comparative study highlights TPs as a highly preferred, well-suited option for demanding environments where chemical and hydrothermal stability are essential.
6. Summary, conclusion, and future perspectives
Hierarchical porous titanium phosphonates (HPTPs) are regarded as a promising class of hybrid materials due to their unique combination of hierarchical porosity and tunable chemical properties, making them ideal for a wide range of applications in photocatalysis, environmental remediation and energy storage. This review particularly emphasizes the advantages of phosphorus-based linkers in addressing the hydrolytic stability issues often faced by traditional carboxylate-based materials. This review discusses various synthesis strategies—such as sol–gel, hydrothermal, and template-assisted methods—used to produce crystalline, amorphous, and paracrystalline HPTPs. It also outlines their structural characteristics, such as pore size distribution, crystallinity and surface area, and how these factors influence their performance in specific applications.Furthermore, the review highlights recent advancements in the functionalization and modification of HPTPs to enhance their utility in industrial applications and environmental remediation processes such as pollutant adsorption and separation. Their high surface area, ordered mesostructures, and secondary pores enhance electrolyte and product transport, ensuring efficient light harvesting and exceptional photocatalytic performances. The pore architecture and the textural features can be finely tuned through various synthetic processes, ligand functionalisation and the incorporation of mixed ligands, such as N-donors and O-donors. Structure-directing agents significantly influence material porosity, enhancing pore size or inducing hierarchical structures for advanced photocatalytic systems. Multifunctional linkers with multiple binding modes further improve catalytic properties by directly affecting the coordination environment of the Ti(IV) centre.
High adsorption capacity and excellent reusability of HPTPs distinguish them as exceptional adsorbents for the environmental remediation process. Additionally, multiphase adsorption is observed, particularly in the liquid phase, where heavy metal ions and organic pollutants bind to functional groups within these hybrid materials. However, in gas phase adsorption, such as CO2 or other gaseous pollutants, pore size and physisorption play a crucial role, relying primarily on non-covalent interactions like van der Waal's forces between the adsorbate and hybrid adsorbent molecules. In some instances, a combined effect of physisorption and chemisorption is observed. Additionally, metal ion adsorption studies underscore the significant influence of pH on the adsorption efficiency of PTPs and HPTPs.
Future research should aim to develop novel and sustainable synthesis strategies to improve control over hierarchical structures, understanding the interaction mechanisms between HPTPs and different reactants. Sensing, ion-exchange, photoconductivity, and electrochemical potential in energy storage devices are among the highlighted applications, although they remain relatively unexplored for PTPs/HPTPs. The primary challenge lies in addressing issues while achieving permanent porosity in hybrid materials. However, several key challenges remain, such as precise structural control, scalable production, and ensuring long-term stability under extreme conditions. PTPs also face limitations, including lower surface areas compared to zeolites and MOFs, which may limit their adsorption capacity, and the need for further assessment of mechanical strength under high pressure. Despite these challenges, PTPs excel in applications that require stability, particularly in acid-catalyzed reactions, pollutant adsorption and sensing.
Subsequent efforts should focus on integrating computational modelling and machine learning to design materials that predict and enhance porosity and stability in functional hybrid materials. The formulation of green synthesis approaches utilizing bio-derived precursors and solventless strategies would further promote sustainability. Also, incorporating other nanomaterials into HPTPs heterostructures could increase the usefulness of these materials in energy storage, photocatalysis, and environmental remediation. By addressing these challenges, HPTPs can evolve into materials of choice for next-generation functional devices with large-scale industrial implementation. Their robustness and tunability make them highly remarkable for industrial, environmental, and biomedical applications. Finally, emerging trends, key applications, and future directions in HPTP research are outlined, addressing current challenges and expanding their potentialin advanced materials science.
Data availability
Data will be made available on request.Conflicts of interest
There are no conflicts to declare.References
- G. Cai, P. Yan, L. Zhang, H.-C. Zhou and H.-L. Jiang, Metal–organic framework-based hierarchically porous materials: synthesis and applications, Chem. Rev., 2021, 121, 12278–12326 CrossRef CAS PubMed.
- S. Kitagawa, Future porous materials, Acc. Chem. Res., 2017, 50, 514–516 CrossRef CAS PubMed.
- A. Ejsmont, J. Andreo, A. Lanza, A. Galarda, L. Macreadie, S. Wuttke, S. Canossa, E. Ploetz and J. Goscianska, Applications of reticular diversity in metal–organic frameworks: An ever-evolving state of the art, Coord. Chem. Rev., 2021, 430, 213655 CrossRef CAS.
- N. Hüsing, Porous Hybrid Materials, Wiley, Weinheim, 2006 Search PubMed.
- G. Férey, Hybrid porous solids: past, present, future, Chem. Soc. Rev., 2008, 37, 191–214 RSC.
- R. Murugavel, A. Choudhury, M. G. Walawalkar, R. Pothiraja and C. N. R. Rao, Metal complexes of organophosphate esters and open-framework metal phosphates: synthesis, structure, transformations, and applications, Chem. Rev., 2008, 108, 3549–3655 CrossRef CAS PubMed.
- P. Bhanja and A. Bhaumik, Organic–inorganic hybrid metal phosphonates as recyclable heterogeneous catalysts, ChemCatChem, 2016, 8, 1607–1616 CrossRef CAS.
- J. Panda, S. P. Tripathy, S. Dash, A. Ray, P. Behera, S. Subudhi and K. Parida, Inner transition metal-modulated metal organic frameworks (IT-MOFs) and their derived nanomaterials: a strategic approach towards stupendous photocatalysis, Nanoscale, 2023, 15, 7640–7675 RSC.
- D. Chakraborty, S. Bej, S. Sahoo, S. Chongdar, A. Ghosh, P. Banerjee and A. Bhaumik, Novel nanoporous Ti-phosphonate metal–organic framework for selective sensing of 2, 4, 6-trinitrophenol and a promising electrode in an energy storage device, ACS Sustainable Chem. Eng., 2021, 9, 14224–14237 CrossRef CAS.
- H.-B. Tai, S.-N. Lu, X. Zhang, Q.-W. Liu, Y.-N. Zhou, C.-Q. Jiao, Y.-Y. Zhu, C.-Y. Huang and Z.-G. Sun, Differently luminescent sensing abilities for Cu2+ ion of two metal phosphonates with or without the free Lewis basic pyridyl sites, J. Mol. Struct., 2021, 1234, 130175 CrossRef CAS.
- M. A. Nistor, S. G. Muntean, B. Maranescu and A. Visa, Phosphonate metal–organic frameworks used as dye removal materials from wastewaters, Appl. Organomet. Chem., 2020, 34, e5939 CrossRef CAS.
- S. S. Iremonger, J. Liang, R. Vaidhyanathan, I. Martens, G. K. Shimizu, T. Daff, D, M. Z. Aghaji, S. Yeganegi and T. K. Woo, Phosphonate monoesters as carboxylate-like linkers for metal organic frameworks, J. Am. Chem. Soc., 2011, 133, 20048–20051 CrossRef CAS PubMed.
- Y. Wei, K. A. Salih, M. F. Hamza, E. R. Castellón and E. Guibal, Novel phosphonate-functionalized composite sorbent for the recovery of lanthanum (III) and terbium (III) from synthetic solutions and ore leachate, Chem. Eng. J., 2021, 424, 130500 CrossRef CAS.
- Y. Zorlu, L. Wagner, P. Tholen, M. M. Ayhan, C. Bayraktar, G. Hanna, A. O. Yazaydin, Ö. Yavuzçetin and G. Yücesan, Electrically Conductive Photoluminescent Porphyrin Phosphonate Metal–Organic Frameworks, Adv. Opt. Mater., 2022, 10, 2200213 CrossRef CAS.
- P. Tholen, Y. Zorlu, J. Beckmann and G. Yücesan, Probing Isoreticular Expansions in phosphonate MOFs and their applications, Eur. J. Inorg. Chem., 2020, 2020, 1542–1554 CrossRef CAS.
- S. a. Ondrusova, M. Kloda, J. Rohlícek, M. Taddei, J. K. Zareba and J. Demel, Exploring the Isoreticular Continuum between Phosphonate-and Phosphinate-Based Metal–Organic Frameworks, Inorg. Chem., 2022, 61, 18990–18997 CrossRef CAS PubMed.
- R. Freund, O. Zaremba, G. Arnauts, R. Ameloot, G. Skorupskii, M. Dinca, A. Bavykina, J. Gascon, A. Ejsmont and J. Goscianska, The current status of MOF and COF applications, Angew. Chem., Int. Ed., 2021, 60, 23975–24001 CrossRef CAS PubMed.
- G. K. Shimizu, R. Vaidhyanathan and J. M. Taylor, Phosphonate and sulfonate metal organic frameworks, Chem. Soc. Rev., 2009, 38, 1430–1449 RSC.
- L.-H. Chen, M.-H. Sun, Z. Wang, W. Yang, Z. Xie and B.-L. Su, Hierarchically structured zeolites: from design to application, Chem. Rev., 2020, 120, 11194–11294 CrossRef CAS PubMed.
- W. Li, J. Liu and D. Zhao, Mesoporous materials for energy conversion and storage devices, Nat. Rev. Mater., 2016, 1, 1–17 Search PubMed.
- L.-B. Sun, X.-Q. Liu and H.-C. Zhou, Design and fabrication of mesoporous heterogeneous basic catalysts, Chem. Soc. Rev., 2015, 44, 5092–5147 RSC.
- D. W. Breck, Zeolite Molecular Sieves: Structure, Chemistry, and Use, 1974 Search PubMed.
- D. M. Chapman and A. L. Roe, Synthesis, characterization and crystal chemistry of microporous titanium-silicate materials, Zeolites, 1990, 10, 730–737 CrossRef CAS.
- M. Taramasso, G. Perego and B. Notari, Preparation of porous crystalline synthetic material comprised of silicon and titanium oxides, US Pat., US4410501A, 1983 Search PubMed.
- G. Alberti, P. Cardini-Galli, U. Costantino and E. Torracca, Crystalline insoluble salts of polybasic metals—I Ion-exchange properties of crystalline titanium phosphate, J. Inorg. Nucl. Chem., 1967, 29, 571–578 CrossRef CAS.
- S. Allulli, C. Ferragina, A. La Ginestra, M. Massucci and N. Tomassini, Preparation and ion-exchange properties of a new phase of the crystalline titanium phosphate, Ti (HPO4) 2· 2H2O, J. Inorg. Nucl. Chem., 1977, 39, 1043–1048 CrossRef CAS.
- A. I. Bortun, S. A. Khainakov, L. N. Bortun, D. M. Poojary, J. Rodriguez, J. R. Garcia and A. Clearfield, Synthesis and Characterization of Two Novel Fibrous Titanium Phosphates Ti2O (PO4) 2⊙ 2H2O, Chem. Mater., 1997, 9, 1805–1811 CrossRef CAS.
- C. Serre and G. Férey, Hydrothermal synthesis and ab initio structurfal approach of two new layered oxyfluorinated titanium (IV) phosphates: Ti 2 (PO 4) 2 F 4· N 2 C 2 H 10 (MIL-6) and Ti 2 (PO 4) 2 F 4· N 2 C 3 H 12· H 2 O, J. Mater. Chem., 1999, 9, 579–584 RSC.
- A. Clearfield and J. Stynes, The preparation of crystalline zirconium phosphate and some observations on its ion exchange behaviour, J. Inorg. Nucl. Chem., 1964, 26, 117–129 CrossRef CAS.
- C. M. Parlett, K. Wilson and A. F. Lee, Hierarchical porous materials: catalytic applications, Chem. Soc. Rev., 2013, 42, 3876–3893 RSC.
- R. L. Siegelman, E. J. Kim and J. R. Long, Porous materials for carbon dioxide separations, Nat. Mater., 2021, 20, 1060–1072 CrossRef CAS PubMed.
- S. Chuhadiya, D. Suthar, S. Patel and M. Dhaka, Metal organic frameworks as hybrid porous materials for energy storage and conversion devices: A review, Coord. Chem. Rev., 2021, 446, 214115 CrossRef CAS.
- H. Li, T.-Y. Ma, D.-M. Kong and Z.-Y. Yuan, Mesoporous phosphonate–TiO 2 nanoparticles for simultaneous bioresponsive sensing and controlled drug release, Analyst, 2013, 138, 1084–1090 RSC.
- C. Serre, J. A. Groves, P. Lightfoot, A. M. Slawin, P. A. Wright, N. Stock, T. Bein, M. Haouas, F. Taulelle and G. Férey, Synthesis, structure and properties of related microporous N, N ‘-piperazinebismethylenephosphonates of aluminum and titanium, Chem. Mater., 2006, 18, 1451–1457 CrossRef CAS.
- C. Serre and G. Férey, Hybrid Open Frameworks. 8. Hydrothermal Synthesis, Crystal Structure, and Thermal Behavior of the First Three-Dimensional Titanium (IV) Diphosphonate with an Open Structure: Ti3O2 (H2O) 2 (O3P−(CH2)− PO3) 2⊙(H2O) 2, or MIL-22, Inorg. Chem., 1999, 38, 5370–5373 CrossRef CAS.
- C. Serre and G. Férey, Hydrothermal Synthesis and Structure Determination from Powder Data of New Three-Dimensional Titanium (IV) Diphosphonates Ti (O3P−(CH2) n− PO3) or MIL-25 n (n= 2, 3), Inorg. Chem., 2001, 40, 5350–5353 CrossRef CAS PubMed.
- H. Assi, G. Mouchaham, N. Steunou, T. Devic and C. Serre, Titanium coordination compounds: from discrete metal complexes to metal–organic frameworks, Chem. Soc. Rev., 2017, 46, 3431–3452 RSC.
- J. Goura and V. Chandrasekhar, Molecular metal phosphonates, Chem. Rev., 2015, 115, 6854–6965 CrossRef CAS PubMed.
- K. J. Gagnon, H. P. Perry and A. Clearfield, Conventional and unconventional metal-organic frameworks based on phosphonate ligands: MOFs and UMOFs, Chem. Rev., 2012, 112, 1034–1054 CrossRef CAS PubMed.
- U. Schubert, Titanium-Oxo Clusters with Bi-and Tridentate Organic Ligands: Gradual Evolution of the Structures from Small to Big, Chem.–Eur. J., 2021, 27, 11239–11256 CrossRef CAS PubMed.
- U. Schubert and B. Stöger, Structural Chemistry of Titanium (IV) Oxo Clusters, Part 2: Clusters Without Carboxylate or Phosphonate Ligands, Chem.–Eur. J., 2024, 30, e202400744 CrossRef CAS PubMed.
- L. Rozes, N. Steunou, G. Fornasieri and C. Sanchez, Titanium-oxo clusters, versatile nanobuilding blocks for the design of advanced hybrid materials, Monatsh. Chem., 2006, 137, 501–528 CrossRef CAS.
- O. Auciello, W. Fan, B. Kabius, S. Saha, J. Carlisle, R. Chang, C. Lopez, E. Irene and R. Baragiola, Hybrid titanium–aluminum oxide layer as alternative high-k gate dielectric for the next generation of complementary metal–oxide–semiconductor devices, Appl. Phys. Lett., 2005, 86, 042904 CrossRef.
- S. Takagi, M. Eguchi, D. A. Tryk and H. Inoue, Porphyrin photochemistry in inorganic/organic hybrid materials: Clays, layered semiconductors, nanotubes, and mesoporous materials, J. Photochem. Photobiol., C, 2006, 7, 104–126 CrossRef CAS.
- L. Li, X. S. Wang, T. F. Liu and J. Ye, Titanium-based MOF materials: from crystal engineering to photocatalysis, Small Methods, 2020, 4, 2000486 CrossRef CAS.
- Y.-P. Zhu, T.-Y. Ma, Y.-L. Liu, T.-Z. Ren and Z.-Y. Yuan, Metal phosphonate hybrid materials: from densely layered to hierarchically nanoporous structures, Inorg. Chem. Front., 2014, 1, 360–383 RSC.
- S. Subudhi, S. P. Tripathy and K. Parida, Highlights of the characterization techniques on inorganic, organic (COF) and hybrid (MOF) photocatalytic semiconductors, Catal. Sci. Technol., 2021, 11, 392–415 RSC.
- J. Panda, J. Sahu and K. Parida, Zn 0.5 Cd 0.5 Se quantum dot-integrated MOF-derived C/N–CeO 2 photocatalyst for enhanced H 2 O 2 production and O 2 evolution reactions, Nanoscale, 2025, 17, 6580–6592 RSC.
- D. Behera, P. Priyadarshini and K. Parida, ZIF-8 metal–organic frameworks and their hybrid materials: emerging photocatalysts for energy and environmental applications, Dalton Trans., 2025, 54, 2681–2708 RSC.
- N. Priyadarshini, K. K. Das, S. Mansingh and K. Parida, Facile fabrication of functionalised Zr co-ordinated MOF: Antibiotic adsorption and insightful physiochemical characterization, Results Chem., 2022, 4, 100450 CrossRef CAS.
- R. Hayami, T. Sagawa, S. Tsukada, K. Yamamoto and T. Gunji, Synthesis, characterization and properties of titanium phosphonate clusters, Polyhedron, 2018, 147, 1–8 CrossRef CAS.
- F. G. Svensson, G. Daniel, C.-W. Tai, G. A. Seisenbaeva and V. G. Kessler, Titanium phosphonate oxo-alkoxide “clusters”: solution stability and facile hydrolytic transformation into nano titania, RSC Adv., 2020, 10, 6873–6883 RSC.
- H. Li, Y. Sun, Z. Y. Yuan, Y. P. Zhu and T. Y. Ma, Titanium phosphonate based metal–organic frameworks with hierarchical porosity for enhanced photocatalytic hydrogen evolution, Angew. Chem., Int. Ed., 2018, 57, 3222–3227 CrossRef CAS PubMed.
- M. Besançon, Y. Wang, H. Mutin, J. G. Alauzun, J.-J. Robin, E. Espuche and V. Bounor-Legaré, In situ synthesis of titanium phosphonate by a non-hydrolytic sol-gel route within a viscous polymer medium, J. Sol-Gel Sci. Technol., 2023, 107, 227–243 CrossRef.
- M. Pramanik, A. K. Patra and A. Bhaumik, Self-assembled titanium phosphonate nanomaterial having a mesoscopic void space and its optoelectronic application, Dalton Trans., 2013, 42, 5140–5149 RSC.
- T.-Y. Ma, X.-J. Zhang, G.-S. Shao, J.-L. Cao and Z.-Y. Yuan, Ordered macroporous titanium phosphonate materials: synthesis, photocatalytic activity, and heavy metal ion adsorption, J. Phys. Chem. C, 2008, 112, 3090–3096 CrossRef CAS.
- M. V. Vasylyev, E. J. Wachtel, R. Popovitz-Biro and R. Neumann, Titanium phosphonate porous materials constructed from dendritic tetraphosphonates, Chem. - Eur. J., 2006, 12, 3507–3514 CrossRef CAS PubMed.
- T.-Y. Ma and Z.-Y. Yuan, Functionalized periodic mesoporous titanium phosphonate monoliths with large ion exchange capacity, Chem. Commun., 2010, 46, 2325–2327 RSC.
- T.-Y. Ma, X.-Z. Lin and Z.-Y. Yuan, Periodic mesoporous titanium phosphonate hybrid materials, J. Mater. Chem., 2010, 20, 7406–7415 RSC.
- G. Shao, L. Lu, X. Qian and Y. Zhang, Preparation and ammonia adsorption performance of titanium phosphonate adsorbent materials with hierarchically porous structure, J. Wuhan Univ. Technol., Mater. Sci. Ed., 2017, 32, 823–829 CrossRef CAS.
- T.-Y. Ma, X.-Z. Lin, X.-J. Zhang and Z.-Y. Yuan, High surface area titanium phosphonate materials with hierarchical porosity for multi-phase adsorption, New J. Chem., 2010, 34, 1209–1216 RSC.
- T.-Y. Ma and Z.-Y. Yuan, Periodic mesoporous titanium phosphonate spheres for high dispersion of CuO nanoparticles, Dalton Trans., 2010, 39, 9570–9578 RSC.
- Y. P. Zhu, J. Yin, E. Abou-Hamad, X. Liu, W. Chen, T. Yao, O. F. Mohammed and H. N. Alshareef, Highly stable phosphonate-based MOFs with engineered bandgaps for efficient photocatalytic hydrogen production, Adv. Mater., 2020, 32, 1906368 CrossRef CAS PubMed.
- T. Y. Ma and S. Z. Qiao, Acid–base bifunctional periodic mesoporous metal phosphonates for synergistically and heterogeneously catalyzing CO2 conversion, ACS Catal., 2014, 4, 3847–3855 CrossRef CAS.
- B. Pawlak, W. Marchal, B. M. Ramesha, B. Joos, L. Calvi, J. D'Haen, B. Ruttens, A. Hardy, V. Meynen and P. Adriaensens, Hybrid porous titania phosphonate networks with different bridging functionalities: Synthesis, characterization, and evaluation as efficient solvent separation materials, Microporous Mesoporous Mater., 2022, 341, 112080 CrossRef CAS.
- R. Rabinowitz, The Reactions of Phosphonic Acid Esters with Acid Chlorides. A Very Mild Hydrolytic Route, J. Org. Chem., 1963, 28, 2975–2978 CrossRef CAS.
- S. N. Tverdomed, J. Kolanowski, E. Lork and G.-V. Röschenthaler, An effective synthetic route to ortho-difluoromethyl arylphosphosphonates: studies on the reactivity of phosphorus- and fluorine-containing functions, Tetrahedron, 2011, 67, 3887–3903 CrossRef CAS.
- D. Wang, J. Yang, F. Xue, J. Wang and W. Hu, Experimental and computational study of zinc coordinated 1-hydroxyethylidene-1,1-diphosphonic acid self-assembled film on steel surface, Colloids Surf., A, 2021, 612, 126009 CrossRef CAS.
- J. Pan, Y.-J. Ma, S.-D. Han, J.-X. Hu, Y. Mu and G.-M. Wang, Construction of the Lanthanide Diphosphonates via a Template-Synthesis Strategy: Structures, Proton Conduction, and Magnetic Behavior, Cryst. Growth Des., 2019, 19, 3045–3051 CrossRef CAS.
- J. Rocha, F. A. Almeida Paz, F.-N. Shi, R. A. S. Ferreira, T. Trindade and L. D. Carlos, Photoluminescent Porous Modular Lanthanide–Vanadium–Organic Frameworks, Eur. J. Inorg. Chem., 2009, 2009, 4931–4945 CrossRef.
- F. Ishiwari, Y. Shoji and T. Fukushima, Supramolecular scaffolds enabling the controlled assembly of functional molecular units, Chem. Sci., 2018, 9, 2028–2041 RSC.
- S. Dash, S. P. Tripathy, S. Subudhi, P. Behera, B. P. Mishra, J. Panda and K. Parida, A Visible Light-Driven α-MnO2/UiO-66-NH2 S-Scheme Photocatalyst toward Ameliorated Oxy-TCH Degradation and H2 Evolution, Langmuir, 2024, 40, 4514–4530 CrossRef CAS PubMed.
- S. Dash, S. P. Tripathy, S. Subudhi, L. Acharya, A. Ray, P. Behera and K. Parida, Ag/Pd bimetallic nanoparticle-loaded Zr-MOF: an efficacious visible-light-responsive photocatalyst for H 2 O 2 and H 2 production, Energy Adv., 2024, 3, 1073–1086 RSC.
- L. Biswal, S. P. Tripathy, S. Dash, S. Das, S. Subudhi and K. Parida, Aggrandized photocatalytic H 2 O 2 and H 2 production by a TiO 2/Ti 3 C 2–TiC/mixed metal Ce–Zr MOF composite: an interfacial engineered solid-state-mediator-based Z-scheme heterostructure, Mater. Adv., 2024, 5, 4452–4466 RSC.
- L. Li, J. Yan, T. Wang, Z.-J. Zhao, J. Zhang, J. Gong and N. Guan, Sub-10 nm rutile titanium dioxide nanoparticles for efficient visible-light-driven photocatalytic hydrogen production, Nat. Commun., 2015, 6, 1–10 Search PubMed.
- J.-D. Xiao, Q. Shang, Y. Xiong, Q. Zhang, Y. Luo, S.-H. Yu and H.-L. Jiang, Boosting Photocatalytic Hydrogen Production of a Metal–Organic Framework Decorated with Platinum Nanoparticles: The Platinum Location Matters, Angew. Chem., Int. Ed., 2016, 55, 9389–9393 CrossRef CAS PubMed.
- Y. Liu, B. Zhang, L. Luo, X. Chen, Z. Wang, E. Wu, D. Su and W. Huang, TiO2/Cu2O core/ultrathin shell nanorods as efficient and stable photocatalysts for water reduction, Angew. Chem., 2015, 127, 15475–15480 CrossRef.
- F. Hoffmann, M. Cornelius, J. Morell and M. Fröba, Silica-based mesoporous organic–inorganic hybrid materials, Angew. Chem., Int. Ed., 2006, 45, 3216–3251 CrossRef CAS PubMed.
- Y. Maegawa, N. Mizoshita, T. Tani and S. Inagaki, Transparent and visible-light harvesting acridone-bridged mesostructured organosilica film, J. Mater. Chem., 2010, 20, 4399–4403 RSC.
- M. Jamshaid, A. A. Khan, K. Ahmed and M. Saleem, Heavy metal in drinking water its effect on human health and its treatment techniques-a review, Int. J. Biosci., 2018, 12, 223–240 CrossRef CAS.
- L. A. Malik, A. Bashir, A. Qureashi and A. H. Pandith, Detection and removal of heavy metal ions: a review, Environ. Chem. Lett., 2019, 17, 1495–1521 CrossRef CAS.
- A. Dutta, A. K. Patra and A. Bhaumik, Porous organic–inorganic hybrid nickel phosphonate: Adsorption and catalytic applications, Microporous Mesoporous Mater., 2012, 155, 208–214 CrossRef CAS.
- B. Maranescu, L. Lupa and A. Visa, Synthesis, characterization and rare earth elements adsorption properties of phosphonate metal organic frameworks, Appl. Surf. Sci., 2019, 481, 83–91 CrossRef CAS.
- S. Mishra, Adsorption–desorption of heavy metal ions, Curr. Sci., 2014, 601–612 CAS.
- S. S. Gupta and K. G. Bhattacharyya, Kinetics of adsorption of metal ions on inorganic materials: a review, Adv. Colloid Interface Sci., 2011, 162, 39–58 CrossRef PubMed.
- S. Dai, M. C. Burleigh, Y. Shin, C. C. Morrow, C. E. Barnes and Z. Xue, Imprint coating: a novel synthesis of selective functionalized ordered mesoporous sorbents, Angew. Chem., Int. Ed., 1999, 38, 1235–1239 CrossRef CAS PubMed.
- K. Z. Hossain and L. Mercier, Intraframework Metal Ion Adsorption in Ligand-Functionalized Mesoporous Silica, Adv. Mater., 2002, 14, 1053–1056 CrossRef CAS.
- H. Furukawa, N. Ko, Y. B. Go, N. Aratani, S. B. Choi, E. Choi, A. Ö. Yazaydin, R. Q. Snurr, M. O'Keeffe and J. Kim, Ultrahigh porosity in metal-organic frameworks, Science, 2010, 329, 424–428 CrossRef CAS PubMed.
- H. Li, M. Eddaoudi, M. O'Keeffe and O. M. Yaghi, Nature, 1999, 402, 276 CrossRef CAS.
- K. M. Choi, H. J. Jeon, J. K. Kang and O. M. Yaghi, Heterogeneity within order in crystals of a porous metal–organic framework, J. Am. Chem. Soc., 2011, 133, 11920–11923 CrossRef CAS PubMed.
- A. M. Furtado, J. Liu, Y. Wang and M. D. LeVan, Mesoporous silica–metal organic composite: synthesis, characterization, and ammonia adsorption, J. Mater. Chem., 2011, 21, 6698–6706 RSC.
- S. B. Adeloju, Progress and recent advances in phosphate sensors: A review, Talanta, 2013, 114, 191–203 CrossRef PubMed.
- Y. Tachibana, S. A. Haque, I. P. Mercer, J. R. Durrant and D. R. Klug, Electron injection and recombination in dye sensitized nanocrystalline titanium dioxide films: a comparison of ruthenium bipyridyl and porphyrin sensitizer dyes, J. Phys. Chem. B, 2000, 104, 1198–1205 CrossRef CAS.
- P. Kumar, A. Pournara, K.-H. Kim, V. Bansal, S. Rapti and M. J. Manos, Metal-organic frameworks: Challenges and opportunities for ion-exchange/sorption applications, Prog. Mater. Sci., 2017, 86, 25–74 CrossRef CAS.
- S. Sharma, A. V. Desai, B. Joarder and S. K. Ghosh, A Water-Stable Ionic MOF for the Selective Capture of Toxic Oxoanions of SeVI and AsV and Crystallographic Insight into the Ion-Exchange Mechanism, Angew. Chem., Int. Ed., 2020, 59, 7788–7792 CrossRef CAS PubMed.
- A. Bhaumik and S. Inagaki, Mesoporous titanium phosphate molecular sieves with ion-exchange capacity, J. Am. Chem. Soc., 2001, 123, 691–696 CrossRef CAS PubMed.
- X. W. Lv, C. C. Weng, Y. P. Zhu and Z. Y. Yuan, Nanoporous metal phosphonate hybrid materials as a novel platform for emerging applications: a critical review, Small, 2021, 17, 2005304 CrossRef CAS PubMed.
- N. Jabeen, A. Hussain and J. Ali, in Metal Phosphates and Phosphonates: Fundamental to Advanced Emerging Applications, Springer, 2023, pp. 245–266 Search PubMed.
- P. Priyadarshini and K. Parida, Two-dimensional metal-organic frameworks and their derived materials: Properties, synthesis and application in supercapacitors field, J. Energy Storage, 2024, 87, 111379 CrossRef.
- D. Chakraborty, T. Dam, A. Modak, K. K. Pant, B. K. Chandra, A. Majee, A. Ghosh and A. Bhaumik, A novel crystalline nanoporous iron phosphonate based metal–organic framework as an efficient anode material for lithium ion batteries, New J. Chem., 2021, 45, 15458–15468 RSC.
- P. Bhanja, T. Dam, S. Chatterjee, A. Bhaumik and A. Ghosh, Lithium embedded hierarchically porous aluminium phosphonate as anode material for lithium-polymer battery, Mater. Sci. Eng., B, 2021, 274, 115490 CrossRef CAS.
- S. Inagaki, S. Guan, T. Ohsuna and O. Terasaki, An ordered mesoporous organosilica hybrid material with a crystal-like wall structure, Nature, 2002, 416, 304–307 CrossRef CAS PubMed.
- C. Zhao, L. Ge, X. Li, M. Zuo, C. Xu, S. Chen, Q. Li, Y. Wang and C. Xu, Effects of the carbonization temperature and intermediate cooling mode on the properties of coal-based activated carbon, Energy, 2023, 273, 127177 CrossRef CAS.
- A. Galarneau, M. Nader, F. Guenneau, F. Di Renzo and A. Gedeon, Understanding the Stability in Water of Mesoporous SBA-15 and MCM-41, J. Phys. Chem. C, 2007, 111, 8268–8277 CrossRef CAS.
- R. Simancas, A. Chokkalingam, S. P. Elangovan, Z. Liu, T. Sano, K. Iyoki, T. Wakihara and T. Okubo, Recent progress in the improvement of hydrothermal stability of zeolites, Chem. Sci., 2021, 12, 7677–7695 RSC.
- G. Niu, Y. Huang, X. Chen, J. He, Y. Liu and A. He, Thermal and hydrothermal stability of siliceous Y zeolite and its application to high-temperature catalytic combustion, Appl. Catal., B, 1999, 21, 63–70 CrossRef CAS.
- L. P. L. Mosca, A. B. Gapan, R. A. Angeles and E. C. R. Lopez, Stability of metal–organic frameworks: recent advances and future trends, Eng. Proc., 2023, 56, 146 Search PubMed.
- A. J. Howarth, Y. Liu, P. Li, Z. Li, T. C. Wang, J. T. Hupp and O. K. Farha, Chemical, thermal and mechanical stabilities of metal–organic frameworks, Nat. Rev. Mater., 2016, 1, 1–15 Search PubMed.
- J. L. Blin, P. Riachy, C. Carteret and B. Lebeau, Thermal and hydrothermal stability of hierarchical porous silica materials, Eur. J. Inorg. Chem., 2019, 2019, 3194–3202 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |






