Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Man-made textile fibres from pectin†
Aleksandra M.
Kozlowski
 *a,
Kristina E.
Lindgren
*a,
Kristina E.
Lindgren
 a,
Gino
Mangiante
a,
Gino
Mangiante
 b and
Tobias
Köhnke
a
b and
Tobias
Köhnke
a
aRISE Research Institutes of Sweden, Materials and Production, Argongatan 30, 431 53 Mölndal, Sweden. E-mail: aleksandra.kozlowski@ri.se
bProduct & Process Development Center, Cargill Starches Sweeteners & Texturizers, Carentan, 50500, France
First published on 10th April 2025
Abstract
Low-methyl esterified pectin with a degree of methylation of 33% was shaped into continuous textile fibres via wet spinning with a calcium chloride coagulation bath. The resulting fibres exhibit mechanical properties approaching those of viscose and show promise as biobased, scalable, and green-produced alternatives to conventional man-made fibres.
Sustainability spotlightIn an era of resource scarcity, the textile industry remains heavily reliant on virgin materials, leading to significant environmental burdens. This work demonstrates a wet spinning process for pectin-based fibres, offering an alternative to conventional synthetic or resource-intensive fibres. With pectin extracted from agricultural by-products, and mild wetspinning conditions, the work addresses resource efficiency and opens for circular production, thus aligns with sustainable development goals 12 (responsible consumption and production) and 9 (industry, innovation, and infrastructure). The resulting fibres exhibit mechanical properties approaching those of conventional man-made fibres, presenting a solution toward more sustainable textile materials. Overall, the described study exemplifies how resource-efficient approach can reduce reliance on virgin feedstocks, paving the way for a greener textile industry. |
Introduction
European consumption of textiles has the fourth-highest impact on the environment and climate change, constitutes one of the top three pressures on water and land, and ranks within the top five for raw material use and greenhouse gas emissions.1 Moreover, approximately 65% of globally produced textile fibres are synthetic, characterised by limited recyclability, non-biodegradability, and prolonged lifespans, all of which contribute to persistent microplastic pollution and long-term environmental impacts.2 Biobased fibres (e.g. cotton, hemp, linen) and man-made cellulosic fibres (MMCFs, e.g., viscose, modal, lyocell) are viable alternatives to synthetics, generally associated with a reduced environmental footprint—particularly in terms of greenhouse gas emissions, reliance on fossil-based precursors, and end-of-life biodegradability.2 However, these textiles are almost always produced from virgin biomass (wood or agricultural streams), raising questions about the overall process sustainability and the responsible management of natural resources.3,4In this context, shifting to biomass already in circulation is a way to reduce pressure on virgin resources and foster a more circular textile economy. Ideally, textile production should operate in a closed loop, sourcing precursors from biomass today considered waste (e.g. biorefinery side streams) and engineering them into high-quality, durable fibres that are either recyclable or biodegradable. To meet these requirements, the spinning processes and production of man-made fibres could be effectively designed, and in alignment with the key principles of green chemistry.5 Natural biomacromolecules (like polysaccharides and proteins) are available in large quantities and are relatively easily tunable for spinning. Recently, cellulose-rich agricultural biomass (e.g. pineapple and banana leaves, wheat straw),6,7 keratinous waste from slaughterhouses (e.g. fur, feathers and low-quality wool),8,9 and marine-derived biopolymers such as alginate and chitin/chitosan (from, for example, seaweed and shrimp shells)10,11 have gained attention for textile production. Nevertheless, there is one biopolymer, not yet explored in textile applications, that is a leading example of resourceful utilisation of circulating biomass: pectin.
Pectin is predominantly extracted from citrus peels or apple pomace which are abundant by-products of the food industry.12 The extraction process is well-established, yielding a high-purity biopolymer and contributing to waste valorisation.13 The food-grade pectin is further used as a texturiser and gelling agent.12 It has also found numerous applications in biomedicine, drug delivery systems, wound healing dressings, and tissue engineering scaffolds.14 Due to its gel-forming capability, pectin shows potential to be shaped into textile fibres via wet spinning. Specifically, low-methyl-esterified (LM) pectin, which undergoes simple, divalent-ion-driven gelation, could be suitable for an environmentally friendly, aqueous-based wet spinning process.15,16 Furthermore, biocompatible pectin could be functionalised with bioactive compounds and further used in medical fiber applications.17 However, research into these properties remains limited. Aside from a 1997 patent exploring LM pectin with the degree of methylation (DM) below 15% and amidated LM pectin with DM below 50% and degree of amidation (DA) below 40%, spun from neutral polymer dope into calcium-based coagulation bath (30% CaCl2),18 investigations have largely been confined to small-scale electrospinning and solution blow spinning trials using additives.19,20
Here we present the process utilising LM pectin with a 33% DM, dissolved in an acidic environment (pH 3) and continuously wet spun into textile fibres through stable coagulation in 10% (w/w) CaCl2. We communicate the results of research that, if taken further, could open up for efficient and tunable textile fibres with the potential for a wide range of applications, including (but not limited to) biomedical fibres or functional textiles. Furthermore, the presented solution relies on abundant by-products and offers mild processing conditions and minimal chemical inputs, reinforcing the sustainability potential of pectin-based textile fibres.
Experimental
Materials
Not-standardised LM pectin (DM: 33%, GPC recorded Mw: 108![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) was kindly provided by Cargill SAS (Baupte, France) and used as received. Hydrochloric acid (1.0 M), calcium chloride, and ethanol (96%) were purchased from Fisher Scientific. A commercially available textile conditioner (Neutral, Unilever) was used as a fibre finish.
000 g mol−1) was kindly provided by Cargill SAS (Baupte, France) and used as received. Hydrochloric acid (1.0 M), calcium chloride, and ethanol (96%) were purchased from Fisher Scientific. A commercially available textile conditioner (Neutral, Unilever) was used as a fibre finish.
Fibre spinning
Pectin was added to a portion of acidified water, with HCl added for pH 3.0, dispersed and kept for 60 min in a vacuum below 10 kPa and at 40 °C. Thereafter, the mixture was transferred to the closed reactor with the remaining solvent for the final polymer concentration of 6% (w/w), offering a practical balance between dissolution efficiency, viscosity, and spinnability. Then, pectin was heated to 60 °C and stirred at 200 rpm for 60 min using a propeller shaft. Dissolution was controlled with optical microscopy (Nikon ECLIPSE Ci-POL, Nikon Instruments Co., Ltd, Tokyo, Japan), while the spinnability of the prepared solution (i.e., spinning dope) was assessed with a stress-controlled rheometer (Nova, Rheologica Instruments AB, Sweden). A more detailed description of the analytical assessment of spinning dope is given in ESI.†A bench piston pump setup was used for fibre spinning. The filtered dope (20 μm filter mesh), heated in the pump shaft to 50 °C, was extruded via a 100-hole spinneret with a capillary diameter of 60 μm each and a length-to-diameter ratio (L/D) of 1. The extrusion speed was set up to 3 m min−1. The neutral aqueous solution of 10% (w/w) CaCl2 at room temperature (RT) was used as a coagulation bath. The fibres were collected on the take-up rollers (Ø 10 cm) for 12 turns, with a draw ratio of 2.1. The initially water-washed rollers with fibres were stored in (i) a deionised H2O, (ii) a mixture of EtOH/dH2O at a ratio 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w), and (iii) EtOH over 96 h (with triple medium exchange), to wash the excess of salt and assess the effect of these media on fibre properties. Afterwards, rollers with washed fibres were immersed in 0.17% (w/w) aqueous conditioner for approximately 5 minutes and finally dried with airflow at a maximum of 80 °C (Dyson Supersonic™, Dyson, UK).
1 (w/w), and (iii) EtOH over 96 h (with triple medium exchange), to wash the excess of salt and assess the effect of these media on fibre properties. Afterwards, rollers with washed fibres were immersed in 0.17% (w/w) aqueous conditioner for approximately 5 minutes and finally dried with airflow at a maximum of 80 °C (Dyson Supersonic™, Dyson, UK).
Fibre properties
The fibre samples (out of rollers) were conditioned overnight and tested at a temperature of 20 ± 2 °C and a relative humidity of 65 ± 4%. Linear mass density and mechanical properties of the fibres were assessed with a Vibroskop-Vibrodyn set-up (Lenzing Instruments, Lenzing, Austria), where measured titer (tex) is further applied to assess parameters such as tenacity (cN per tex) and modulus. Twenty separate measurements were performed for each sample.Fibre morphology and alignment
The spun fibres were washed with different media and analysed using scanning electron microscopy (SEM) in a JEOL 7800F, with an accelerating voltage of 5 kV. Secondary electrons were used for imaging in the SEM. The working distance was 10 mm. Before imaging, 1.6 nm of Pt was deposited on the surface of the fibres to avoid charging during imaging. Sample cross sections were ion milled in a JEOL IB-19520CCP broad ion beam (BIB) at 4 kV and cooled to −60 °C. The fibres were glued to a glass substrate, and 4.4 nm of Pt was deposited before BIBing.The brightfield and polarised microscopy images were collected with a Nikon ECLIPSE Ci-POL (Nikon Instruments Co., Ltd, Tokyo, Japan) polarised light microscope (POM), equipped with Nikon TV-lens (C-0.38x) digital camera. Images were captured with Nikon NIS-Elements BR software, with exposure time set to 15 ms for bright-field and 1 s for POM. Individual filaments were fixed with double-sided tape to microscopic slides and oriented 45° relative to the direction of the microscope polarisers; once captured, the orientation of fibres in POM was changed to 90°. In each case, light adjustment for sharp images was conducted only in bright fields.
Results & discussion
To obtain a spinning-grade solution of LM pectin with a relatively high degree of methylation (33%), the pH of the aqueous medium was decreased to approximately 3.0. Under these acidic conditions, the carboxyl groups of pectin become protonated (pH of solvent < pectin pKa ∼3.5),21 thereby reducing electrostatic repulsion between polymer chains, improving molecular chain alignment and resulting in a solution rheology suitable for wet spinning.22,23 To enable solvent penetration into polymer agglomerates formed at the beginning of dissolution, pectin mixtures were stored in a vacuum. This resulted in uniform impregnation of polymer (with solvent) and particle-free, well-mixed solution at the pectin concentration of 6% (w/w), as presented in Fig. 1. However, regardless of good dissolution, the viscosity of the prepared pectin solution was too high to allow spinning at room temperature. The conformational order (ergo, the viscosity) of LM pectin under acidic conditions is temperature-dependent.22,24 Thus, rheological analyses were performed over a range of temperatures to determine the optimal conditions for pectin dope extrusion. As summarized in ESI and Fig. S1,† the 6% (w/w) solution of 33% DM pectin at pH 3 exhibited predominantly viscous behaviour (G′′ > G′) at both 50 and 60 °C. Since the complex viscosity at 60 °C was too low for wet spinning, the pectin dope was heated to 50 °C before extrusion. The rheological properties of the pectin dope at the spinning temperature are presented in Fig. 2. | ||
| Fig. 2 Rheological properties, i.e., dynamic moduli and complex viscosity as a function of the oscillatory frequency of 6% 33DE pectin solution at 50 °C. | ||
The extrusion of the pectin dope at 50 °C through a 100-hole spinneret (each hole ∼60 μm in diameter) into a 10% (w/w) aqueous CaCl2 bath at room temperature led to immediate fibre formation. This rapid transition is considered the result of two key factors:
(i) The ionotropic gelation of LM pectin in the presence of divalent cations, which is particularly pronounced when the pH shifts from ∼3 (below the pKa of pectin in dope) to neutral conditions (above the pKa in the coagulation bath). Upon this transition, carboxyl groups of pectin deprotonates (–COOH → –COO−) facilitate interaction with Ca2+ and characteristic ‘egg-box’ complexation between paired pectin molecules;15,25
(ii) The enhanced conformational ordering of the pectin molecules, caused by the sudden temperature drop (from 50 °C in the dope to RT in the coagulation bath).
Although a more thorough analysis of possible spinning conditions and their relation to fibre formation is necessary, the herein applied conditions surely yield a stable fibre structure.
The coagulated filaments were picked up at a draw ratio of 2.1 (48.4% stretch) and collected on rollers. The spinning process is shown in ESI in Fig. S2.† To remove the excess calcium chloride, the collected fibres were washed for a prolonged time (96 h); three types of washing baths, i.e., dH2O, EtOH/H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w), and EtOH, were tested. The application of ethanol was followed by the literature suggestion that alcohols improve final properties and separation of pectin fibres.18 However, such treatment has proven insufficient, as fibres produced herein fused without the possibility of separating filaments (ESI and Fig. S3†). Thus, to improve filaments separation, washed samples (all three baths) were further treated with commercially available surfactant (0.17% aqueous solution) and dried; after conditioning, the filaments separation and final feeling of fibres were considerably improved, as presented in Fig. 3d.
1, w/w), and EtOH, were tested. The application of ethanol was followed by the literature suggestion that alcohols improve final properties and separation of pectin fibres.18 However, such treatment has proven insufficient, as fibres produced herein fused without the possibility of separating filaments (ESI and Fig. S3†). Thus, to improve filaments separation, washed samples (all three baths) were further treated with commercially available surfactant (0.17% aqueous solution) and dried; after conditioning, the filaments separation and final feeling of fibres were considerably improved, as presented in Fig. 3d.
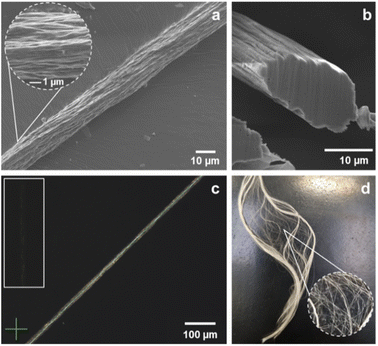
The properties of produced samples, in relation to the tested washing bath and choice of biobased man-made fibres, are summarised in Table 1. Fibres washed solely in water exhibit marginally higher elongation and tenacity than those washed in ethanol/water or ethanol. Importantly, these results suggest that pectin fibres can be safely washed with water without affecting the final performance of the material. This is specifically important from the economy and sustainability point of view, as relying on water-only washing further minimises the price and chemical footprint of potential scaled processes. Furthermore, the tenacity of pectin fibres washed with water (18.2 cN per tex) approaches the lower boundary of viscose's characteristic range (18–35 cN per tex), whilst the elongation (14.2%) is slightly below typical viscose levels (15–30%).26 Importantly, the mechanical performance of pectin fibres exceeds typical values reported for alginate (8–14 cN per tex)26,27 and compares favourably with chitosan fibres (6–25 cN per tex).26,28 Considering that alginate and chitosan fibres are already established in biomedical applications, such as wound dressings or tissue scaffolds,29 the superior mechanical properties of pectin fibres highlight their promising potential within similar biomedical contexts. While further optimisation is needed, appropriate post-spinning treatments could position pectin-based fibres competitively within the broader bio-based fibre market. The collected samples were analysed in SEM to assess if the type of washing bath affected the structure of produced pectin fibres. The non-smooth surface was observed for all the analysed samples. However, in comparison to fibres treated with alcohol (ESI, Fig. S4†), the morphology of water-washed samples is relatively more uniform, as presented in Fig. 3a.
| Washing bath | Titre (dtex) | Elongation (%) | Tenacity (cN per tex) |
|---|---|---|---|
| Man-made pectin fibres (this study) | |||
| H2O | 1.6 ± 0.2 | 14.2 ± 0.8 | 18.2 ± 0.7 |
| EtOH: H2O | 1.7 ± 0.2 | 12.5 ± 2.8 | 16.5 ± 1.8 |
| EtOH | 1.7 ± 0.1 | 13.8 ± 1.4 | 15.8 ± 1.2 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Reference biobased man-made fibres (literature values) | |||
| Viscose26 | 1.3–22 | 15–30 | 18–35 |
| Alginate26,27 | n.a | 4–8 | 8–14 |
| Chitosan26,28 | 1.4–1.7 | 6–10 | 6–25 |
The serrated cross-sections of water-washed pectin fibres slightly resembled those of viscose.26,30 In viscose, such morphology is well-documented and contributes to known mechanical and sorption characteristics of the fibre. For the newly developed pectin fibres, it is necessary to further investigate the spinning process to understand if its optimisation could result in more controlled structures and potentially more predictable properties.
The alignment of collected samples was assessed using POM. Although naturally occurring citrus pectin exhibits low crystallinity (ca. 6%),17 all spun fibres showed characteristic birefringence, as in the example in Fig. 3c, suggesting some degree of internal ordering. At this stage, no direct correlation between the washing medium and fibre orientation could be established, as all samples presented similar characteristics (ESI and Fig. S5†). To further clarify how spinning conditions influence molecular alignment and properties of pectin fibres, more in-depth structural analysis is needed.
Conclusions
This study demonstrates that low-methyl esterified pectin with a 33% degree of methylation can be effectively transformed into continuous textile fibres using a wet spinning process. Adjusting the solution's pH to approximately 3.0 and employing vacuum impregnation enabled the preparation of a uniform spinning dope with a polymer concentration of 6%. The resulting fibres, particularly those washed solely in water, exhibited tenacity values (18.2 cN per tex) approaching the lower limit of the viscose range. This suggests that fine-tuning process parameters could yield properties closer to established man-made cellulosic fibres. Moreover, the observed birefringence and serrated cross-sections indicated internal ordering, highlighting the potential for further improvement of fibre structure. Furthermore, a deeper understanding of the underlying dissolution and coagulation mechanisms is necessary to guide more precise control over fibre morphology and mechanical properties. Although additional optimisation is still required, these findings establish a promising foundation to further develop pectin-based textile fibres.Data availability
The authors confirm that the data supporting the findings of this study are available within the article and ESI.† Should any raw data files be needed in another format, they are available from the corresponding author upon reasonable request.Author contributions
All authors read, reviewed and approved the final manuscript. Specifically, A. M. K.: conceptualisation, methodology, analysis, investigation and visualisation, writing – original draft; K. E. L.: methodology and analysis. G. M. writing – review and editing. T. K.: writing – review and editing.Conflicts of interest
There are no conflicts to declare.References
- European Commission, Textiles factsheet European Commission, 2022.
- Textile Exchange, Materials Market Report, 2023 Search PubMed.
- O. Arodudu, B. Holmatov and A. Voinov, Clean Technol. Environ. Policy, 2020, 22, 1591–1611 CrossRef.
- E. A. R. Zuiderveen, K. J. J. Kuipers, C. Caldeira, S. V. Hanssen, M. K. van der Hulst, M. M. J. de Jonge, A. Vlysidis, R. van Zelm, S. Sala and M. A. J. Huijbregts, Nat. Commun., 2023, 14, 8521 CrossRef CAS PubMed.
- M. Hann, Textile Design, 2020, pp. 5–36 Search PubMed.
- K. Jayaprakash, A. Osama, R. Rajagopal, B. Goyette and O. P. Karthikeyan, Bioengineering, 2022, 9, 1–12 CrossRef PubMed.
- M. Shadhin, M. Rahman, R. Jayaraman, Y. Chen, D. Mann and W. Zhong, Ind. Crops Prod., 2023, 204, 117252 CrossRef CAS.
- M. Lebedytė and D. Sun, J. Text. Inst., 2022, 113, 1750–1766 CrossRef.
- D. Wang, X. H. Yang, R. C. Tang and F. Yao, Polymers, 2018, 10, 993 CrossRef PubMed.
- J. Huang, Y. Zhong, X. Zhang, H. Xu, C. Zhu and J. Cai, Macromol. Rapid Commun., 2021, 42, 1–9 Search PubMed.
- J. Ju, J. Yang, W. Zhang, Y. Wei, H. Yuan and Y. Tan, J. Mater. Sci. Technol., 2023, 140, 1–18 CrossRef CAS.
- A. Roman-Benn, C. A. Contador, M.-W. Li, H.-M. Lam, K. Ah-Hen, P. E. Ulloa and M. C. Ravanal, Food Chem. Adv., 2023, 2, 100192 CrossRef.
- D. Mohnen, Curr. Opin. Plant Biol., 2008, 11, 266–277 CrossRef CAS PubMed.
- R. K. Mishra, A. K. Banthia and A. B. A. Majeed, Asian J. Pharm. Clin. Res., 2012, 5, 1–7 CAS.
- L. Cao, W. Lu, A. Mata, K. Nishinari and Y. Fang, Carbohydr. Polym., 2020, 242, 116389 CrossRef CAS PubMed.
- H. Lotzkar, T. H. Schultz, H. S. Owens and W. D. Maclay, J. Phys. Chem., 1946, 50, 200–210 CrossRef CAS PubMed.
- R. M. Gohil, J. Appl. Polym. Sci., 2011, 120, 2324–2336 CrossRef CAS.
- T. C. Gerrish and G. A. Luzio, US Pat., 5688923, 1997 Search PubMed.
- L. J. R. Foster and B. J. Tighe, Polym. Int., 2009, 58, 1442–1451 CrossRef CAS.
- A. M. V. Martin, D. C. Flores, F. D. C. Siacor, E. B. Taboada and N. P. B. Tan, Nanotechnology, 2022, 33, 495602 CrossRef CAS PubMed.
- A. Ström, E. Schuster and S. M. Goh, Carbohydr. Polym., 2014, 113, 336–343 CrossRef PubMed.
- D. Lootens, D. Durand, T. Nicolai, P. Boulenguer and V. Langendorff, Food Hydrocolloids, 2003, 17, 237–244 CrossRef CAS.
- T. J. Lopes, G. R. Rosa, L. S. da Silva, C. W. Scheeren, F. S. Antelo and M. L. Martins, in Fundamentals of Natural Fibres and Textiles, Elsevier, 2021, pp. 473–513 Search PubMed.
- G. A. Morris, J. Castile, A. Smith, G. G. Adams and S. E. Harding, Polym. Degrad. Stab., 2010, 95, 2670–2673 CrossRef CAS.
- B. R. Thakur, R. K. Singh and A. K. Handa, Crit. Rev. Food Sci. Nutr., 1997, 37, 47–73 CrossRef CAS PubMed.
- D. Veit, Fibers, Springer International Publishing, Cham, 2022 Search PubMed.
- C. Var and S. Palamutcu, Polymers, 2024, 16, 1817 CrossRef CAS PubMed.
- T. Hahn, L. Bossog, T. Hager, W. Wunderlich, R. Breier, T. Stegmaier and S. Zibek, in Chitin and Chitosan, Wiley, 2019, pp. 395–428 Search PubMed.
- A. M. Smith, S. Moxon and G. A. Morris, in Wound Healing Biomaterials, Elsevier, 2016, vol. 2, pp. 261–287 Search PubMed.
- S. M. Burkinshaw, Physico–Chemical Asp. Text. Color, 2016, pp. 27–34 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5su00048c |
| This journal is © The Royal Society of Chemistry 2025 |