 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Unlocking the carbon dioxide photoreduction potential of graphene-derived catalysts: mechanisms, product selectivity, and challenges
Manisha
Sain†
 a,
Debanjali
Dey†
b,
Ramkrishna
Sen
b and
Shamik
Chowdhury
a,
Debanjali
Dey†
b,
Ramkrishna
Sen
b and
Shamik
Chowdhury
 *a
*a
aSchool of Environmental Science and Engineering, Indian Institute of Technology Kharagpur, West Bengal 721302, India. E-mail: shamikc@iitkgp.ac.in
bDepartment of Bioscience and Biotechnology, Indian Institute of Technology Kharagpur, West Bengal 721302, India
First published on 19th May 2025
Abstract
The escalating concentration of carbon dioxide (CO2) in the atmosphere necessitates innovative strategies to address global warming and simultaneously harness its potential as a valuable resource. To offset CO2 emissions, heterogeneous photocatalysis has emerged as an effective technology to photochemically reduce CO2 into value-added chemicals using specially designed photocatalysts. However, photocatalysts mediating CO2 reduction often encounter some intrinsic challenges like low specific surface area, inefficient charge separation, narrow visible light absorption, and inadequate stability. Graphene-based materials are widely regarded as a promising solution to address these limitations, offering an enormous specific surface area, excellent electron mobility, and robust chemical stability, which collectively enhance CO2 conversion efficiency and ensure durable photocatalyst performance. This review delves into the forefront of visible light assisted photocatalytic reduction of CO2, with a particular focus on graphene-based photocatalysts. The goal is to uncover sustainable solutions that utilize visible light to catalyze the reduction of CO2, offering an eco-friendly alternative to fossil fuels, while simultaneously acting as a carbon sink by capturing atmospheric CO2. This review discusses the constraints and challenges of graphene-based composites, encompassing their synthesis techniques and performance efficacy, and provides an outlook on the various product selectivities during CO2 photoreduction. A brief overview of the potential products obtained from CO2 photoreduction, with an insight into their plausible mechanism for the production of solar fuel and value-added chemicals, is provided. This timely review, therefore, aspires to expatiate on the recent advances in CO2 capture and sequestration using graphene-based heterogeneous photocatalysis.
Sustainability spotlightThis review underscores the transformative potential of graphene-based photocatalysts for visible light-driven CO2 reduction, highlighting their role in promoting sustainable solutions for reducing greenhouse gas emissions and producing value-added chemicals. Due to their advantages such as efficient charge separation, broad light absorption, and long-term stability, graphene-based materials offer a promising approach to addressing the current limitations of photocatalytic technologies. The discussed advancements are closely aligned with the UN Sustainable Development Goals (SDGs), particularly SDG 7 (Affordable and Clean Energy), SDG 13 (Climate Action), and SDG 12 (Responsible Consumption and Production). This approach not only drives innovation in renewable energy but also fosters a circular carbon economy, emphasizing environmentally sustainable strategies to combat climate change and support the development of green technologies. |
1. Introduction
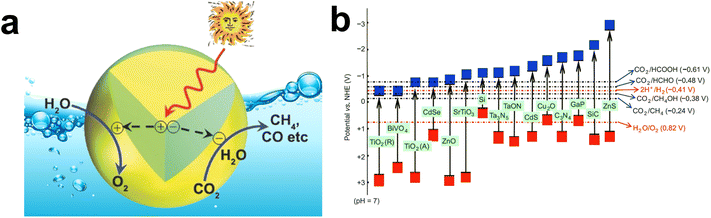
The recent surge in technological advancements due to rapid industrialization has significantly elevated global energy demands. Fossil fuels remain the primary energy source for the majority of sectors, resulting in a substantial increase in atmospheric carbon dioxide (CO2) concentrations. Because CO2 is a major greenhouse gas, urgent measures are imperative to curtail its release into the atmosphere.1–3 Strategies to stabilize atmospheric CO2 concentration generally fall into three categories: (a) reducing CO2 emissions at the source, (b) enhancing CO2 removal via capture and storage, and (c) CO2 utilization through converting it into valuable chemicals.4–6 The first strategy emphasizes phasing out fossil fuels and promoting renewable energy sources like solar, hydro, wind, and geothermal power, leading to a substantial reduction in CO2 emissions from the energy sector.7,8 The second category involves carbon capture and storage technologies, wherein CO2 emitted from power plants and industrial processes is captured and stored in underground geological formations, preventing its release into the atmosphere.9,10 The third category explores CO2 reduction into chemicals/fuels, a promising approach in the realm of sustainable and clean energy technologies, especially using solar light as an energy source.10–12 Such an artificial photosynthesis method harnesses solar energy to transform CO2 into value-added chemicals or fuels in an aqueous medium, providing a sustainable energy source while contributing to CO2 emission mitigation.13,14 However, CO2 photoreduction remains an evolving technology, with challenges such as higher product yield and scalability still to be addressed.15 Nevertheless, research and investment in this area contribute to the broader sustainable goals of addressing climate change, mitigating greenhouse gas emissions, and transitioning to a more sustainable and renewable energy-based economy.Photocatalytic CO2 reduction has garnered significant scientific attention under the name of artificial photosynthesis.16–19 Mimicking natural photosynthesis in an artificial system by virtue of photocatalysis implies the usage of earth-abundant semiconductor materials that absorb light in the visible spectrum. Various semiconductor materials, including zinc oxide (ZnO), iron oxide (Fe2O3), russellite (Bi2WO6), titanium dioxide (TiO2), graphitic carbon nitride (g-C3N4), and cadmium sulphide (CdS), are widely investigated to facilitate photocatalytic CO2 reduction.20–30 However, each of them has certain limitations that impel researchers to explore novel, versatile materials with exceptional physicochemical properties.
Graphene has garnered the curiosity of the scientific community due to its exceptional mechanical, optical, electrical, and thermal properties.31–34 The unique atomic arrangement of graphene in a hexagonal lattice grants it extraordinary properties as depicted in Fig. 1, making it one of the most promising materials of the 21st century. Graphene has high electron mobility and excellent conductivity that facilitates electron transfer through its π-conjugated two-dimensional (2D) structure, thereby improving charge carrier transfer to the photocatalyst surface. The large π-conjugated 2D structure of graphene supports CO2 activation and destabilization due to π—π conjugate interaction with CO2.35,36 Due to these attributes, graphene-based photocatalysts have emerged as promising candidates for the photocatalytic reduction of CO2. Graphene derivatives and their diverse properties have led to a surge in their usage in technological and scientific areas, as evidenced by the substantial increase in publications on graphene-based photocatalysis.37–43 To analyze the research trends in graphene-based composites for photocatalytic CO2 reduction, a Scopus database search was conducted using the keywords “photocatalytic reduction”, “graphene”, and “CO2”, and the resulting publication data from 2014 to 2024 are presented in Fig. 2a. The current research progress pertaining to graphene evinces that coupling graphene derivatives with suitable semiconductors raises the prospect of fabricating novel multifunctional composite materials for augmenting CO2 photocatalytic reduction activity. Additionally, there are ample opportunities for ameliorating the performance efficacy of graphene-based composites through proper optimization and tuning of surface chemistry.
In a nutshell, this review aims to summarize CO2 photocatalytic reduction using graphene-based composites and their probable product formation. To begin with, it briefly iterates the principles and activation mechanism of CO2 photocatalytic reduction, followed by a concise summary of the fine-tuned and robust graphene-based composites manifesting remarkable CO2 photoreduction activity. In particular, a clear acumen on the tailored product selectivity during the photocatalytic reduction of CO2 is provided. Finally, the challenges and future prospects for graphene-based photocatalysts in CO2 reduction are thoroughly discussed.
2. Basic principle and the activation mechanism of CO2 photocatalytic reduction
The linear structure of the CO2 molecule, characterized by its chemical inertness and thermodynamic stability, poses a challenge to photocatalytic reduction since it requires high energy input to break the σ and π bonds of O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. The standard Gibbs-free energy (ΔG0) required for the photocatalytic reduction of CO2 in an aqueous medium (i.e., water, H2O) into value-added compounds, including carbon monoxide (CO), methane (CH4), methanol (CH3OH), formaldehyde (HCHO), and formic acid (HCOOH) is significantly positive, as depicted in Fig. 2b. This suggests that substantial external energy input is required to convert CO2 into value-added compounds and fuels. This energy is essential for overcoming reaction barriers, breaking the C
O. The standard Gibbs-free energy (ΔG0) required for the photocatalytic reduction of CO2 in an aqueous medium (i.e., water, H2O) into value-added compounds, including carbon monoxide (CO), methane (CH4), methanol (CH3OH), formaldehyde (HCHO), and formic acid (HCOOH) is significantly positive, as depicted in Fig. 2b. This suggests that substantial external energy input is required to convert CO2 into value-added compounds and fuels. This energy is essential for overcoming reaction barriers, breaking the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, and facilitating the formation of C–C/C–H bonds, which ultimately result in the desired chemical products.44
O bond, and facilitating the formation of C–C/C–H bonds, which ultimately result in the desired chemical products.44
Furthermore, CO2 photocatalytic reduction relies on the absorption of photons by semiconductor photocatalysts, leading to the generation of electron–hole pairs. These photoinduced charges thereupon participate in redox reactions with CO2, driving the conversion of the greenhouse gas into compounds with lower environmental impact.45 Solar light can serve as the energy source that initiates the photocatalytic reduction of CO2 into chemicals/fuels through a variety of chemical transformations, utilizing appropriate photocatalysts. The photogenerated charge carriers drift to the active sites on the photocatalyst surface, where holes oxidize H2O into O2 thereby liberating H+ that further assists in electron-mediated reduction of CO2via a series of reactions (Fig. 3a). However, volumetric or surface recombination of electrons and holes during this process diminishes the photocatalytic reduction efficiency.46,47 Effective electron–hole separation, critical for CO2 photocatalytic reduction, can be achieved using nanostructured photocatalysts such as nanorods, nanobelts, nanotubes, and various types of junctions.48–51
 | ||
| Fig. 3 (a) Schematic of the photocatalytic reduction of CO2 in an aqueous medium over a graphene-based photocatalyst. Reproduced with permission from ref. 34, copyright 2014, Wiley. (b) Redox potential and band position of different semiconductors for CO2 reduction into value-added products in aqueous medium. Reproduced with permission from ref. 45, copyright 2014, Springer Nature. | ||
To escalate the CO2 reduction efficiency, the reactivity of CO2 can be increased through various activation mechanisms. Stable CO2 molecule activation can be achieved through any of the five modes: (a) bending of linear arrangement of the CO2 molecule (O–C–O) with attachment of the O atom, (b) at least one C–O bond elongation or both, (c) charge (electron) transfer to CO2 due to polarization of charges on C and O atoms, (d) hydride transfer, and (e) charge redistribution.52
Typically, the activation of the CO2 molecule over heterogeneous catalysts entails a charge transfer (mode c) from the catalyst to the molecule. This transfer elongates the C–O bond length and reduces the O–C–O bond angle (modes a and b).53,54 The activation of CO2 at the molecular level occurs due to a partial transfer of electrons into the lowest unoccupied molecular orbital (LUMO).55 The bending of CO2 results in a notable reduction of its energy of the LUMO and enhances the electron density of the carbon associated with it, thereby promoting the transfer of an electron to the molecule. Consequently, the bending results in the C–O bond weakening when compared to its linear configuration. This could result in the dissociation of CO2 on the catalyst surface into CO and O species. These characteristics improve the capacity for CO2 reduction through electron acceptance.
At the molecular level, activation of stable CO2 molecules for photocatalytic reduction involves an electron transfer, which initiates multistep chemical reactions on the surface of the photocatalyst. Upon excitation, an electron is transferred from the highest occupied molecular orbital (HOMO) to the LUMO of CO2, resulting in the formation of the surface-bound CO2 radical anion (CO2˙−).55 The bent structure of the CO2 molecule, because of electron transfer from the photocatalyst to the π* antibonding molecular orbital of CO2, activates the CO2 molecule for photocatalytic reduction. However, experimental evidence through scanning tunnelling electron microscopy indicates that a single electron transfer to CO2 in the gaseous phase is thermodynamically unfavorable, as this creates a negative redox potential in the LUMO of CO2 (CO2 + e− → CO2˙−, E0 = −1.9 V vs. NHE, pH = 7).56 The type of product formation depends on the number of electrons participating in chemical reactions, leading to the production of CO (2e−), HCHO (4e−), CH3OH (6e−), and CH4 (8e−).57 Product selectivity in CO2 photocatalytic reduction is one of the significant concerns that may vary according to changes in reaction conditions, photocatalyst selection, and thermodynamic reduction potential. The redox potential (E0) of different products with different semiconductors for CO2 reduction is shown in Fig. 3b.58Table 1 outlines the potential chemical reactions and corresponding E0 values for CO2 photocatalytic reduction, highlighting the desired products formed under pH 7 conditions.58–62
| Product | Reaction | E° (V) |
|---|---|---|
| CO2 radical anion | CO2 + e− → CO2˙− | −1.90 |
| Formic acid | CO2 + 2H+ + 2e− → HCOOH | −0.61 |
| Carbon mono-oxide | CO2 + 2H+ + 2e− → CO + H2O | −0.53 |
| Formaldehyde | CO2 + 4H+ + 4e− → HCHO + H2O | −0.48 |
| Methanol | CO2 + 6H+ + 6e− → CH3OH + H2O | −0.38 |
| Ethane | 2CO2 + 14H+ + 14e− → C2H6 + 4H2O | −0.27 |
| Methane | CO2 + 8H+ + 8e− → CH4 + 2H2O | −0.24 |
| Oxygen | 2H2O + 4h+ → O2 + 4H+ | 0.81 |
| Hydrogen | 2H+ + 2e− → H2 | −0.41 |
| Hydroxyl radical | H2O + h+ → H+ + ˙OH | 2.32 |
Surface functional groups on graphene-derived materials, especially hydroxyl, epoxy, and carboxyl groups, play a central role in CO2 adsorption and activation during photocatalysis. These functionalities create localized polar sites that enhance CO2 chemisorption through hydrogen bonding and dipole–quadrupole interactions. Once adsorbed, the linear CO2 molecule undergoes structural distortion into a bent configuration, forming a CO2˙− radical intermediate, a critical step for initiating reduction reactions. These surface interactions not only reduce the energy barrier for activation but also influence the selectivity of photogenerated electrons toward specific reduction pathways, such as CO, CH4, or CH3OH production.
Furthermore, heteroatom doping (e.g., N, S, or B) into the graphene lattice introduces additional active sites with modified electronic structures, improving the overlap between the conduction band of the photocatalyst and the LUMO of CO2. For instance, nitrogen-doped graphene materials have been shown to facilitate better CO2 activation due to the lone-pair electrons on pyridinic and graphitic nitrogen, which act as coordination centers and enhance electron transfer to CO2.55,63 Experimental studies have demonstrated that the presence of carboxyl and hydroxyl groups correlates with increased photocatalytic efficiency, especially in CO and HCOOH production pathways.64 Such functionalization strategies are increasingly employed to enhance charge carrier separation, as supported by both theoretical and experimental investigations.65,66
3. Graphene-derived photocatalysts for CO2 reduction
In recent decades, several strategies have been developed to improve the semiconductor performance for CO2 photocatalytic reduction under visible light. Approaches to optimize the bandgap include doping, altering surface properties, and dye sensitization.67–69 Additionally, various junctions, including heterojunctions, homojunctions, and Schottky junctions, have been utilized to facilitate charge separation and transport for CO2 photocatalytic reduction.70 Graphene plays a pivotal role in this, as it efficiently absorbs a broad spectrum of light, including visible and ultraviolet (UV) wavelengths,71 improving solar light utilization and making the process more energy efficient. The surface of graphene provides numerous active sites that can support or anchor semiconducting catalytic species, typically non-metal or metal oxide nanoparticles, significantly enhancing the overall CO2 photocatalytic reduction efficiency. Furthermore, the high electrical conductivity and huge specific surface area of graphene enable rapid charge carrier separation and transfer to active sites, driving the CO2 reduction reactions on graphene-based photocatalysts.47,72,73 In recent advancements, researchers have combined graphene with other nanomaterials or co-catalysts to form hybrid structures that exhibit synergistic effects, resulting in improved photocatalytic activity and selectivity for CO2 reduction. Various types of graphene derivatives have been reported in the literature, such as graphene oxide (GO), graphene nanocrystals, and graphene-based composites that offer a promising route for converting CO2 emissions into valuable products, contributing to carbon capture and utilization. Therefore, it is anticipated that graphene-based composites will diversify opportunities and provide exceptional properties to photoactive materials, thereby advancing value-added chemical production by CO2 photocatalytic reduction using solar energy. Table 2 provides a summary of the various graphene-based composites employed for CO2 photocatalytic reduction.| Photocatalyst | Synthesis method | Light source (wavelength) | Product | Reference |
|---|---|---|---|---|
| Graphene-g-C3N4 | Impregnation-thermal reduction process | Daylight bulb, 15 W | Methane (5.87 μmol g−1) | 26 |
| Modified graphene oxide (GO) | Improved Hummer's method | Halogen lamp, 300 W | Methanol (0.172 μmol gcat−1 h−1 | 74 |
| GO-tungsten trioxide | Facile hydrothermal method (180 °C, 12 h) | Xenon lamp, 300 W | Methane (0.11 μmol h−1) | 75 |
| Platinum modified rGO with TiO2 nanotubes | Hydrothermal synthesis (120 °C, 24 h) | Xenon lamp, 300 W | Alcohol and carboxylic acid (1130 nmol h−1 cm−2 | 76 |
| GO decorated with copper nanoparticles | Rapid microwave process (one-pot) | 2 h of visible light irradiation | Acetaldehyde 3.88 μmol gcat−1 h−1 & methanol 2.94 μmol gcat−1 h−1 | 77 |
| Noble metal Ag, Au, Pd, & Pt modified rGO/TiO2 | Polyol process | Xenon arc lamp, 500 W | Methane (1.70 μmol gcat−1) in 6 h | 78 |
| Graphene derivative TiO2 | Liquid phase deposition method | Mercury vapour lamp | Methanol (47 μmol g−1 h−1), ethanol (144.7 μmol g−1 h−1) | 79 |
| rGO-copper oxide | Visible light (λ > 420 nm) | Methanol (max 1225 μmol gcat−1) | 80 | |
| Graphene supported TiO2 nanocrystal −001/101 | Solvothermal method | Xenon arc lamp, 300 W | Carbon monooxide (70.8 μmol g−1 h−1) | 81 |
| CuO/Cu2O nanowire with rGO graft | Thermal oxidation method | Xenon arc lamp, 500 W | Carbon monoxide (0.31 and 0.20 μmol cm−2) | 82 |
| GO-supported oxygen-TiO2 | Precipitation method followed by the impregnation method | Xenon arc lamp, 500 W | Methane (3.45 μmol gcat−1) | 83 |
| GO/modified cobalt | Xenon lamp, 300 W (λ > 420 nm) | Formic acid (96.49 μmol for 2 h) | 84 | |
| Blue titania/graphene/platinum | Xenon lamp, 300 W (λ > 420 nm) | Methane (259 μmol g−1 h−1), ethane (77 μmol g−1 h−1) | 85 | |
| α-Ferric oxide-zinc oxide/rGO | Electrochemical process | Xenon lamp, 300 W (λ > 420 nm) | Methanol (5.3 μmol g−1 in 3 h) | 86 |
| Graphene-chlorophyll copper | Visible light | Ethane (68.23 μmol m−2 h−1) | 87 | |
| In2O3/rGO | Mercury lamp, 250 W (λ, 400–700 nm) | Methane (953.72 μmol g−1) | 88 | |
| p-type nickel oxide/n-type ceric oxide/rGO | Hydrothermal process | Xenon lamp, 300 W | Formaldehyde (421.09 μmol g−1 h−1) | 89 |
| Poly(3-hexylthiophene-2,5-diyl) (P3HT)/GO hybrid | Mini-emulsion method | Halogen lamp 300 W | Methanol and acetaldehyde | 90 |
| g-C3N4/rGO | Hydrothermal method | Xenon lamp, 300 W (PLS-SXE300D), AM 1.5G filter | Methanol (114 μmol g−1 h−1), H2 (68 μmol g−1 h−1) | 41 |
| GO/copper oxide/copper organic frame | Hydrothermal method | Xenon lamp150 W, AM 1.5 filter (100 mW cm−2) | Alcohol (methanol, ethanol, propanol) (2217 nmol h−1 cm−2) | 91 |
| Gold/TiO2/N-graphene | Hydrothermal method | Xenon lamp, 300 W (λ > 420 nm) | Methane (742.39 μmol g−1 h−1) | 92 |
3.1. Graphene–inorganic composites
Graphene-based inorganic composites are formed by integrating graphene or its derivatives with inorganic materials, such as metal oxides or metal nanoparticles, in order to improve their photocatalytic performance. In particular, these composites have shown promising results in improving light absorption capacity, charge carrier separation, and catalytic activity. Additionally, the large specific surface area of graphene provides exceptional support for catalysts. The presence of graphene increases the adsorption of CO2 on the catalyst surface along with the enhancement of electron—hole separation in the composite photocatalyst.93 Although TiO2 is widely used for driving photocatalytic reactions, it faces challenges such as a wide bandgap energy of 3.2 eV (limiting excitation to the UV range) and rapid electron—hole recombination.94 Modification of TiO2 through graphene incorporation addresses these challenges by altering the bandgap, minimizing charge recombination, increasing specific surface area, and enhancing photocatalytic efficiency in the visible light range.95 In a notable study, a TiO2/nitrogen-doped reduced graphene oxide (rGO) composite (TiO2/NrGO) was synthesized via a one-step hydrothermal method for CO2 photocatalytic reduction.96 The incorporation of nitrogen (N2) dopants produced a synergistic effect, improving CO2 adsorption on the catalyst surface and facilitating the transfer of photogenerated electrons. Furthermore, this study focused on CO2 reduction at a gas–solid interface, where CO was found to be the primary product in the flow reactor system. This is because CO requires fewer electrons and protons and is kinetically favored for production. The absence of CH4 in this study may be due to the fact that the photoreduction of CO2 to CH4 demands more electrons and protons, making its formation more challenging than CO. Notably, in addition to quaternary-N moieties functioning as electron-transfer mediator, both pyridinic-N and pyrrolic-N motifs serve as active sites for CO2 reduction, enhancing the interfacial photocatalytic activity. As a result, the modified catalyst exhibited a significant total CO production yield of 356.5 μmol g−1, which is a 4.4 times increase compared to pure TiO2 (81.1 μmol g−1) and a 2.2 fold increase over TiO2/reduced graphene oxide (TiO2/rGO) (160.5 μmol g−1) as shown in Fig. 4a and b. Additionally, Fig. 4c and d show the CO production rate with respect to the O2/N2 volume ratio and the CO2 photocatalytic reduction rate over recycled TiO2/NrGO-300, respectively.96 It is noteworthy to mention that doping of noble metal nanoparticles into rGO/TiO2 (GT) resulted in an enhanced photoactivity towards CO2 reduction to CH4.78 Thus, a set of noble metal (NM)-doped GT nanocomposites, including platinum (Pt), palladium (Pd), silver (Ag), and gold (Au), were successfully prepared using a simple polyol method. Among the NM–GT samples, the Pt–GT nanocomposite exhibited the highest photocatalytic activity, achieving a total CH4 yield of 1.70 μmol gcat−1 after 6 h of light irradiation. This is attributed to the strong dependence of the photonic efficiency of NM-GT on the electron affinity and work function of the metal, which favors its contact with TiO2. Pt has a higher work function (−5.65 eV) compared to Au (−5.1 eV), Ag (−4.7 eV), and Pd (−5.2 eV). Consequently, the photogenerated electrons can transfer more efficiently from TiO2 to Pt nanoparticles, while the reverse process is significantly hindered. Furthermore, the Pt nanoparticles significantly contributed to the CO2 reduction potential by enhancing charge separation and transfer while extending the absorption band into the visible light spectrum.78 In a subsequent study by Deerattrakul and coworkers, Cu–Zn/rGO was prepared with varying weight percentages of Cu–Zn, using an equimolar ratio of Cu and Zn on an rGO support through the incipient wetness impregnation method.97 The rGO-supported nanosheets significantly improved the catalytic performance and facilitated the dispersion of Cu–Zn bimetallic particles. The catalyst achieved a CH3OH production rate of 424 mg CH3OH gcat−1 h−1, indicating its potential for practical CO2 conversion to CH3OH.97 In another study, the integration of β-Ga2O3 nanorods with rGO nanosheets presented a highly efficient catalytic architecture. The innovative β-Ga2O3–rGO composite demonstrated a significant enhancement in CO production yield along with an impressive 98% CO selectivity. This exceptional performance highlights the substantial enhancements achieved through the innovative rGO integration approach.98 | ||
| Fig. 4 (a) CO production rate via photocatalytic reduction of CO2 with respect to irradiation time, and (b) total yield of CO under visible light irradiation using rGO, TiO2 and TiO2/NrGO-X. (c) Rate of CO production and the volumetric ratio of O2/N2 over TiO2/NrGO-300. (d) Recyclability test over TiO2/NrGO-300 for the CO2 photocatalytic reduction rate through four consecutive cycles. Reproduced with permission from ref. 80, copyright 2017, Elsevier Ltd. | ||
Researchers are actively investigating novel combinations of graphene with various inorganic materials, whereby tailoring the structural and electronic properties of composite materials may aim to improve CO2 adsorption, electron transfer, and selectivity for desired products, such as fuels and chemicals.
3.2. Graphene–2D material composites
The integration of graphene with other 2D materials takes advantage of the complementary properties of both graphene and the selected 2D material to improve the efficiency and selectivity of the CO2 photocatalytic reduction process. Various 2D materials, such as g-C3N4, molybdenum disulfide (MoS2), and tin disulfide (SnS2), have been explored in combination with graphene.36,41,99–103For instance, g-C3N4, a metal-free semiconductor, with a medium bandgap energy, is widely employed in the realm of photocatalysis. Both g-C3N4 and graphene have sp2 hybridized π bonds that aid in π—π interaction among them and also destabilize CO2 molecules because of delocalized π-conjugate binding with CO2.26,104 When g-C3N4 is combined with graphene, the composite can provide enhanced light absorption and charge transport properties, making it suitable for CO2 photocatalytic reduction. In a recent study, a composite of g-C3N4 with graphene was synthesized for CH3OH production via CO2 photocatalytic reduction.41 Herein, g-C3N4 was synthesized using the co-polymerization method by annealing guanidine carbonate (G) and ammonium thiocyanate (A) together at 5 wt% each, termed G5A5. Initially, hydrogen (H2) was the sole product obtained when G5A5 (as-synthesized g-C3N4 with the lowest bandgap) was used as the photocatalyst, while the G5A5/rGO composites produced both CH3OH and H2. Thus, it is evident that the addition of rGO to G5A5 facilitated the formation of CH3OH.41 Furthermore, the experimental results suggest that the conduction band of G5A5 likely lies below the CO2/CH3OH reduction potential, which thermodynamically prevents CO2 from being reduced to CH3OH. However, incorporating rGO with semiconductors (like g-C3N4) causes an upward shift in the G5A5 bands due to electron transfer from rGO. This band shifting allows the band edges to align with the CO2/CH3OH and H2O/O2 redox potentials, enabling the generation of CH3OH. Fig. 5a and b illustrate H2 and CH3OH yields on employing a series of composites at different concentrations under optimal conditions.41 The composite giving the maximum yield is identified as the combination of g-C3N4 and rGO at a 5 wt% concentration, denoted as G5A5/rGO5. Fig. 5c depicts H2 and CH3OH evolution using G5A5/rGO5 at a concentration of 3 mg mL−1 under 12 h of solar light. The results showed that over six effective cycles, nearly 114 μmol g−1 h−1 of CH3OH and 68 μmol g−1 h−1 of H2 were collected.41
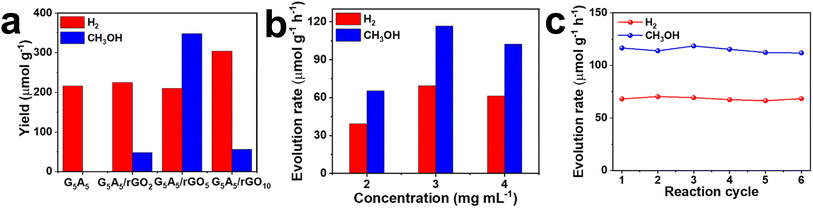 | ||
| Fig. 5 (a) Hydrogen and methanol yields using G5A5 and its rGO composites, (b) hydrogen and methanol yields at varying concentrations using G5A5 and its rGO composites, and (c) hydrogen and methanol evolution rates using G5A5/rGO5 (3 mg mL−1) under 12 h solar light. Reproduced with permission from ref. 74, copyright 2022, Elsevier Ltd. | ||
MoS2, a widely studied 2D semiconductor known for its exceptional catalytic properties, shows enhanced performance when combined with graphene. This composite effectively facilitates charge separation and has demonstrated good stability and reusability across multiple photocatalytic cycles.102,105–107 The combination has exhibited promising results in augmenting the production of value-added chemicals and fuels from CO2 photocatalytic reduction. To this end, a highly efficient, metal-free, and stable photocatalyst with a hierarchical porous structure was developed using a one-pot hydrothermal method as depicted in Fig. 6a.105 The composite, denoted as TGM, comprised TiO2 (T), graphene (G), and MoS2 (M) nanosheets, which contributed to the formation of its porous architecture. MoS2, known for its robustness and layer-dependent catalytic activity, acted as a co-catalyst, whereas graphene served as an electron channel component. This assembly provided a large specific surface area and expedited efficient mass transfer through its randomly distributed porous backbone. The electron transfer from TiO2 through graphene to the few-layered MoS2 effectively reduced charge recombination and boosted the CO2 reduction potential. As a result, the composite achieved a remarkable CO production rate of 92.33 μmol CO g−1 h−1.105 In another study, a ternary composite of MoS2, g-C3N4, and graphene was synthesized via an ultrasonication-mediated calcination process by Otgonbayar and co-workers.100 The investigation revealed a positive change in the electronic structure without altering the internal crystal and electronic structures of individual nanocomposites. The application of an aqueous solvent containing a basic salt and a donor scavenger enhanced the photocatalytic CO2 reduction through the type-II heterojunction. This facilitates the efficient supply of a large number of electrons and hydrated CO2 molecules necessary for the complex reduction reaction of CO2 to alcohol.100
 | ||
| Fig. 6 (a) Illustration of the synthesis approach of a TiO2–graphene–MoS2 composite. Reproduced with permission from ref. 89, copyright 2018, the American Chemical Society. (b) Schematic of the MoS2/SnS2/r-GO fabrication procedure. Reproduced with permission from ref. 85, copyright 2019, the American Chemical Society. | ||
Another interesting 2D semiconductor with a layered structure, i.e., SnS2, possesses a favorable conduction band position and an optimal bandgap, enhancing its ability to efficiently reduce CO2 and improve sunlight absorption. A 3D MoS2/SnS2/rGO nanocomposite, synthesized via a solvothermal method (Fig. 6b), demonstrated effective CO2 photoreduction under UV light.101 The distinctive structure of metal sulfides within the same family enhances defect formation and minimizes electron transport barriers, facilitating efficient electron transfer at the interface through electron tunneling and offering more active sites for CO2 reduction. The constructed S–C–S heterojunction exhibits a multilevel electron transport mechanism and synergistic interactions, increasing the potential for producing a higher yield of organic fuels. Thus, the unique design of the Z-type heterojunction (Fig. 7) provided lower diffusion resistance and faster ion diffusion channels, thereby forming CO and CH4 at an impressive rate of 68.63 μmol g−1 h−1 and 50.55 μmol g−1 h−1, respectively.101
 | ||
| Fig. 7 A plausible mechanism of the energy band structure and charge separation over MoS2/SnS2/r-GO during photocatalytic reduction of CO2. Reproduced with permission from ref. 85, copyright 2019, the American Chemical Society. | ||
Recently, a novel composite consisting of porous carbon-doped hexagonal boron nitride nanoribbons (c-BNNR) combined with photosensitizing graphene quantum dots (GQDs) was developed.108 The presence of a 0D/1D interaction between GQDs and c-BNNR facilitates electron transfer from GQDs to the c-BNNR surface. Notably, the introduction of GQDs effectively reduced electron–hole recombination, enhanced the generation of surface-active electrons, and selectively reduced CO2 to CO (123.81 μmol g−1). The improved stability and faster electron migration kinetics, enabled by the addition of GQDs, significantly enhanced the CO2 reduction efficiency of the nanocomposite.108 Interestingly, a graphene nanoflake (GNF)-decorated zeolitic imidazole framework (ZIF-67), denoted as GNF(X)/ZIF-67, has been synthesized to enhance the efficiency of visible light-driven photocatalytic CO2 reduction. The composite demonstrated an impressive performance, achieving a CH3OH production rate of 50.93 μmol g−1 and an ethanol (C2H5OH) production rate of 33.97 μmol g−1 after 8 h of visible light irradiation. These results significantly surpass the performance of pure ZIF-67, thereby serving as a testament to highlight the efficacy of GNF decoration in boosting photocatalytic activity.109
The selection of a 2D material to pair with graphene is guided by several factors, such as the specific target reaction, the required photocatalytic properties (e.g., bandgap alignment and charge transfer efficiency), and the feasibility of synthesis techniques. Additionally, considerations like the stability, scalability, and environmental compatibility of the materials play a critical role. Researchers are continuously exploring diverse combinations of graphene with 2D materials to enhance the efficiency, selectivity, and durability of these composites for CO2 photocatalytic reduction, aiming to maximize the production of value-added chemicals and sustainable fuels.
3.3. Graphene–polymer composites
Graphene–polymer composites have been extensively explored to harness solar energy for CO2 photocatalytic reduction to chemicals/fuels. Various polymers have been employed in conjunction with graphene, each offering unique properties to enhance the overall performance of the composite in this process. Commonly used polymers with graphene for CO2 photocatalytic reduction include polypyrrole, poly(3-hexylthiophene) (P3HT), poly(3,4-ethylenedioxythiophene) (PEDOT), poly(ethylene oxide) (PEO), poly(vinyl alcohol) (PVA), poly(4-vinylpyridine) (P4VP), polyaniline, and polyimide.For instance, a composite of rGO and MoS2 with varying concentrations of polypyrrole, exhibited enhanced photocatalytic performance for CO2 reduction in aqueous media under simulated sunlight.110 This composite demonstrated significant production rates of CH4 (1.5 μmol g−1 h−1), CO (3.95 μmol g−1 h−1) and H2 (4.19 μmol g−1 h−1). The polymerization of the composite effectively facilitated charge transfer, light absorption, CO2 adsorption, and minimized charge carrier recombination due to synergistic effects.110 Additionally, P3HT is highly favored as a polymeric donor material due to its excellent electrical conductivity and solvent solubility. When integrated with graphene, it improves carrier mobility, conductivity, and hole collection, whereas reducing the bandgap with increasing graphene content.111 Similar to P3HT, PEDOT is another conjugated polymer that can be incorporated with graphene to facilitate the production of value-added chemicals through CO2 photocatalytic reduction. PEDOT is known for its high electrical conductivity and stability, which are beneficial for enhancing the performance and durability of the composite.112 Other polymers, such as PVA, a biocompatible and water-soluble polymer, have been used with graphene in aqueous CO2 photocatalytic reduction studies. The hydrophilic nature and film-forming properties of PVA make it suitable for such applications.113,114 Additionally, polyaniline, recognized for its conductive and redox properties, has been shown to synergistically improve charge transfer, light absorption, and catalytic activity in graphene-polyaniline nanocomposites, contributing to more effective CO2 reduction. For example, Liu and colleagues synthesized a composite of ZnO, GO, and polyaniline for efficient conversion of CH4 into CH3OH and HCOOH. This transformation of gaseous fuel into liquid chemicals is advantageous due to the ease of storage and transportation.115 Furthermore, polyimide, a high-temperature-resistant polymer, demonstrated exceptional performance when combined with graphene in a composite featuring silver chromate and N-rGO, achieving a CO2 photocatalytic reduction rate of 352.1 μmol gcat−1 h−1. The hetero-linkage structure between silver chromate and polyimide created a Z-scheme heterojunction, enhancing light absorption and overall photocatalytic efficiency. Moreover, the presence of pyridinic-N, serving as a unique selective site, facilitated the generation of CO. This feature lowered the free energy barrier for the potential-limiting step, further enhancing the overall efficiency of the photocatalytic process.116
The aforementioned examples represent a subset of the polymers explored, and the selection depends on the specific application, environmental conditions, and desired properties of the graphene–polymer composite. Researchers must continue to explore new polymers and optimize existing ones to improve the performance and efficiency of CO2 photocatalytic reduction systems for sustainable chemical/fuel production.
3.4. Long-term performance and cycling stability of graphene-integrated composites
Long-term cycling tests reveal that, with a robust composite design, graphene-derived photocatalysts can sustain activity over extended operation. For instance, a TiO2/3D-graphene–MoS2 composite preserved over 80% of its initial CO2 to CH4 conversion rate after 15 consecutive 3 h runs (∼45 h total), and a WSe2–graphene nanocomposite exhibited negligible loss in methanol yield across six 48 h irradiation cycles (∼288 h cumulative).105,117 Deactivation was primarily attributed to photochemical deoxygenation of GO, defect-mediated photocorrosion of the hybrid interface, and accumulation of carbonaceous residues. Furthermore, a novel N-doped GO–wrapped TiO2 nanotube catalyst retained over 90% of its initial CH4 yield throughout a continuous 35 h run. Similarly, CoO/rGO hybrids retained consistent CO evolution across six 24 h cycling tests.118 These studies confirm that with appropriate composite design and mitigation strategies, graphene-derived materials can achieve day-long photoreduction stability. However, pilot-scale continuous-flow demonstrations remain to be developed. These findings underscore both the promise and the remaining challenges in achieving day-long operational durability under solar-driven conditions.4. Product selectivity during CO2 photocatalytic reduction and analysis
The photocatalytic reduction of CO2 yields major gaseous products, primarily CH4 and CO, with the presence of H2O leading to the production of H2 and O2 as H2O splitting byproducts.92,119–121 In an aqueous environment, a significant challenge lies in product selectivity, particularly as H2, a major competitor in H2O splitting, diminishes the selectivity and efficiency of chemical/fuel production during photocatalytic reduction, thereby efforts to suppress H2 evolution reaction are necessary. Coupling CO2 photocatalytic reduction with H2O splitting reactions in an aqueous medium helps identify the rate-determining step, contributing to enhanced selectivity and efficiency.58 A study demonstrated the effectiveness of binary co-catalysts designed to selectively reduce CO2 in the presence of H2O. The core–shell-structured Pt@Cu2O cocatalyst with TiO2 can effectively suppress the reduction of H2O to H2, while significantly promoting the selective reduction of CO2 to CO and CH4. The selectivity for CO2 reduction achieved an impressive 85%.122The product selectivity in CO2 photocatalytic reduction is intricately linked to its complex hydrogenation and deoxygenation processes. Karamian and Sharifnia have outlined a general pathway for CO2 photocatalytic reduction reaction, emphasizing the formation of various oxidizing species and the reduction of CO2 by different reductants such as H2O, H2, CH3OH, and CH4.123 The choice of reductant significantly affects the product formation, as can be seen in Fig. 8a. In most instances, CO is the primary product, with subsequent potential products including CH4 and other hydrocarbons such as acetic acid, HCOOH, CH3OH, C2H5OH, and acetaldehyde (CH3CHO).123 Achieving high product selectivity is essential for maximizing target yield in CO2 photocatalytic reduction, as low selectivity leads to multiple byproducts, complicating product separation processes. The potential products formed during CO2 photocatalytic reduction are outlined below, with the corresponding reactions responsible for their formation detailed in Table 1. While gaseous phase products are typically analyzed using a gas chromatograph equipped with flame ionization and thermal conductivity detectors, products in the liquid phase, such as alcohols, are detected through direct injection of liquid or heating gasification.124
 | ||
| Fig. 8 (a) General pathway for CO2 photocatalytic reduction in the presence of a mixture of reductants. Reproduced with permission from ref. 105, copyright 2016, Elsevier Ltd. (b) Gibbs free energy (ΔG) diagram for CO2 reduction into CH4 and CO on a Au–TiO2 decorated nitrogen-doped graphene photocatalyst, along with intermediate product adsorption configuration. Reproduced with permission from ref. 76, copyright 2022, Elsevier Ltd. (c) Possible pathways for the production of methanol during photocatalytic reduction of CO2 in aqueous media. Reproduced with permission from ref. 105, copyright 2016, Elsevier Ltd. | ||
4.1. Methane production
CH4 stands out as a prominent gaseous product in the realm of CO2 photocatalytic reduction, representing a vital solar fuel utilized in various applications such as electricity production through steam-generated machines and gas turbines. Kamal and colleagues used TiO2 decorated N-rGO with Au nanoparticles to achieve selective production of CH4 at an impressive rate of approximately 742.39 μmol g−1 h−1 under visible irradiation for 4 h in a gas-phase batch reactor.92 The composite demonstrated a remarkable 60-fold increase in electron consumption, significantly enhancing CH4 production, as verified through gas chromatography equipped with flame ionization and thermal conductivity detectors. Density functional theory analysis of the product distribution during CO2 photocatalytic reduction revealed a significant role played by positive spin density with nitrogen and carbon, contingent upon the utilization of pyridinic-N, pyrrolic-N, and negative spin basal plane of carbon. Mapping the spin density of N-rGO unveiled the formation of the carboxylic radical (˙COOH) as a reactive intermediate during the initial electron–proton transfer in the CO2 photocatalytic reduction process, leading to CH4 formation. The stabilization of ˙COOH depends on the specific reaction pathway during its subsequent reduction to solar fuels, as depicted in Fig. 8b for CH4 formation.92 In another study, an impressive CH4 production rate (953.72 μmol g−1) was achieved using a nanocomposite of indium oxide (In2O3) with rGO.88 This nanocomposite outperformed pure In2O3 in CH4 production, which can be attributed to prolonged charge carrier separation duration and enhanced charge transfer from In2O3 to rGO under visible light irradiation. The oxidation of H2O was found to generate H2 ions (H+), which, when combined with photogenerated electrons, facilitated the formation of CH4 and C2H5OH. A reduced recombination rate and a shift in energy bandgap contributed to the increased yield of CH4 as a primary product. Additionally, rGO significantly enhanced O vacancy defects and altered bandgaps, creating active sites for CO2 adsorption and thereby boosting CH4 production.88,125 These findings offer valuable insights into various strategies for achieving efficient photocatalytic reduction of CO2 into CH4.4.2. Alcohol production
CH3OH and C2H5OH have been identified as key alcohol products in the selective photocatalytic reduction of CO2. Interestingly, it was observed that conducting CO2 photocatalytic reduction in aqueous media often results in higher production rate of CH3OH compared to other products, as outlined in Table 1.123 The reaction can be triggered either by a conduction band electron of the photocatalyst reducing CO2 directly or by the dissociated form of CO2 in water, leading to the formation of carbonic acid, bicarbonate or carbonate ions, depending on the pH conditions. In aqueous media, CO2 predominantly exists as carbonic acid/CO2 at pH < 4, as carbonate ions at pH > 10, and as a mixture of all three forms at pH 7. Possible pathways for the production of CH3OH during the photocatalytic reduction of CO2 in aqueous media are illustrated in Fig. 8c.123 Given its direct usability as a fuel, CH3OH offers advantages, especially when applied in liquid systems. Studies, including those by Shih and coworkers highlight CH3OH and C2H5OH as ideal fuels in terms of storage and transportation, given their liquid state compared to other alternatives.126 Additionally, H2 evolution often competes during alcohol production, but its separation is feasible since H2 is obtained in the gas phase while alcohol remains in liquid form.126A group of researchers adopted a simple thermal copolymerization technique to synthesize g-C3N4 with a lowered bandgap, which was further combined with rGO for CH3OH production, achieving an impressive yield of 114 μmol g−1 h−1. The CH3OH yield notably improved as the rGO content increased from 0 to 5 wt%, resulting in a quantum yield of 0.63%. The composite denoted as G5A5/rGO5 showed 83% higher selectivity for CH3OH in 6 cycles, attributed to the increased charge carrier separation.41 The 2D/2D heterojunction formed between rGO and g-C3N4 increases charge transport, lowers recombination of charge carriers, and extends the electron lifetime for reduction reactions.41 In another study, a composite of GO and TiO2 was prepared using the liquid-phase deposition method for the production of CH3OH (47 μmol g−1 h−1, at pH 4) and C2H5OH (144.7 μmol g−1 h−1, at pH 11) under UV-visible irradiation. To mitigate the issue of H2 formation during photocatalytic reduction of CO2 with water, copper was used as a co-catalyst to trap more electrons in the conduction band.79 The effect of both copper(I)oxide and pH was considered during the photocatalytic reaction for alcohol production. The pH of the solution affects the solubility of CO2 in water, thus affecting carbonate ion production and protonation equilibrium. Furthermore, it was found that proton concentration is higher at lower pH, potentially reducing CO2 reduction potential with negative species protonation involving electron transfer to CO2.127 The carbonate ion accepts the electron from co-catalyst copper(I)oxide to form a CO2 radical (˙CO2), which then reacts with a H2 radical (˙H) to form a methoxyl radical. Under acidic conditions (pH 4), methoxyl radicals undergo protonation to form CH3OH, while under alkaline conditions (pH 11), C2H5OH is produced through a radical substrate reaction. Additionally, higher adsorption capacity of the photocatalyst and the movement of electrons between the two phases create synergistic interactions that augment the efficiency of the photocatalytic reduction reaction.79 Research has revealed that during CO2 photocatalytic reduction, H2 and carbon atoms can be attached, leading to the cleavage of C–O bonds and the transformation of ˙CO2 into CO over the catalyst surface. The presence of a co-catalyst bond plays a crucial role in CH3OH formation. If the bond is weak, the final product will be CO, and if the bond is relatively strong, the carbon radical attaches with four ˙H, leading to CH3OH formation.128 For the analysis of alcohol produced in the liquid phase, a gas chromatograph equipped with a flame ionization detector and helium as a carrier gas can be employed, while for qualitative analysis, nuclear magnetic resonance or gas chromatography-mass spectrometry techniques are suitable.90,120
4.3. Carboxylic acid production
Carboxylic acids, specifically HCOOH and acetic acid, are among the prominent products generated in CO2 photocatalytic reduction.76,129 A suitable photocatalyst, utilizing GO modified cobalt metallated aminoporphyrin (GO-Co-ATTP), was developed for the photocatalytic reduction of CO2 into HCOOH, achieving a yield of almost 96.49 μmol for 2 h under visible light irradiation.84 The GO-Co-ATPP material is a nanohybrid composed of GO covalently bonded with porphyrin, designed to facilitate charge-transfer processes. In this system, graphene serves as the electron donor, while porphyrin functions as the electron acceptor. As depicted in Fig. 9, the enzymatic conversion of CO2 to HCOOH by formate dehydrogenase is driven by the regenerated nicotinamide adenine dinucleotide (NADH). During the cyclic process, NAD+ released from the enzyme participates in the photoregeneration of NADH, which is subsequently reused for the reduction of CO2 to HCOOH.84 At times, the formation of HCOOH may initiate with hydrogenation, where a H atom combines with one O atom of ˙CO2 to form a carboxyl radical. In a highly polar aqueous environment, the carboxyl radical may react with ˙H to form HCOOH.128 Alternative pathways for carboxylic acid production may involve some anion radicals, aqueous electrons in solvated form, and other derivatives of CO2˙−.130 Another anticipated outcome of CO2 photocatalytic reduction is oxalic acid, resulting from one electron photocatalytic reduction of CO2˙− to the oxalate anion.119 After photocatalytic reduction of CO2, carboxylic acid obtained in the liquid phase can be analyzed by high-performance liquid chromatography.120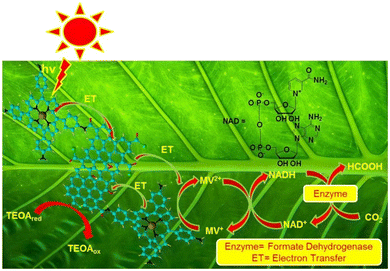 | ||
| Fig. 9 Plausible pathway for the conversion of CO2 into formic acid over graphene oxide modified cobalt metallated aminoporphyrin. Reproduced with permission from ref. 67, copyright 2018, Elsevier Ltd. | ||
4.4. Aldehyde production
Aldehydes, including CH3CHO and HCHO, are potential products of CO2 photocatalytic reduction. These compounds can be detected through gas chromatography, with quantification achieved using a flame ionization detector equipped column or Nash's colorimetry. High-performance liquid chromatography can be used to determine the concentration of aldehydes in the liquid phase after derivatization, as aldehydes are typically obtained at much lower concentrations.90,120 Shown and coworkers successfully obtained CH3CHO and CH3OH through the photocatalytic reduction of CO2 using a composite of copper nanoparticles (5–10 wt%) decorated over GO, synthesized via a one-pot microwave method. Under optimum conditions with 2 h of irradiation, the reported yields of CH3CHO and CH3OH were 3.88 μmol gcat−1 h−1 and 2.94 μmol gcat−1 h−1, respectively.77 In this scenario, the activation of CO2 occurs as electrons transfer from the d-orbitals of the metal to the π* orbital of the C–O bond, followed by multielectron reduction, resulting in the production of CH3CHO and CH3OH as products.77 In a recent study, a nanocomposite of p-type nickel oxide decorated over n-type ceric oxide/rGO produced nearly 4 times more HCHO than pure ceric oxide (CeO2).89 The study revealed that the p–n junction formed between nickel oxide and CeO2 modified the bandgap energy resulting in a red-shift in the nanocomposite. This heterojunction facilitated an increased generation of charge carriers, with the metallic properties of nickel enabling enhanced photon absorption. Additionally, oxygen vacancies in the nanocomposite, induced by CeO2 and evidenced by an increased Ce–O bond length, played a critical role in capturing CO2 molecules. CO2 is transformed into ˙CO2 after absorption, which lowers the activation energy and increases the reduction rate.89 Few studies in the literature have reported the conversion of alcohols into their corresponding aldehydes to meet specific demands in industries such as pharmaceutical, fragrance, and confectionery. For instance, Yang and Xu synthesized a composite from exfoliated GO and TiO2 that was employed under visible light to successfully produce benzaldehyde from benzyl alcohol.131The exploration of graphene-based composites in the production of value-added chemicals/fuels opens up new possibilities for efficient and sustainable catalytic processes, contributing to advancements in the synthesis of valuable chemical intermediates and fine chemicals. Continued research and optimization of these composite catalysts are essential to fully harness their potential and enable their practical implementation in the industrial sector.
5. Challenges and future prospects in CO2 photocatalytic reduction
CO2 photocatalytic reduction is a promising technology, but it faces several challenges, particularly related to the inert nature of CO2, a stable and unreactive molecule. Some of the key challenges include:(a) High activation energy: The conversion of CO2 into useful products necessitates overcoming a significant activation energy barrier. CO2 is a thermodynamically stable molecule making it challenging to initiate and drive the reaction using solar energy alone.132 Consequently, the photocatalytic reduction process often demands the use of catalysts to lower the activation energy.
(b) Low reaction rates: The kinetic inertness of CO2 renders its reduction to fuels via photocatalytic processes a slow and inefficient endeavor. Enhancing the reaction rates to improve the overall process efficiency remains a major challenge.
(c) Surface reaction kinetics: The kinetics of surface reactions, including adsorption and desorption of CO2 and reaction intermediates, can significantly impact the overall efficiency of the photocatalytic process. Understanding and optimizing these kinetics are essential.
(d) Selectivity and product separation: CO2 photocatalytic reduction can yield multiple products depending on the photocatalyst and reaction conditions. Efficient separation and extraction of the desired products from the reaction mixture can be challenging, especially when multiple products are formed. Thus, developing effective separation techniques is crucial for the practical implementation of this technology.133 Furthermore, there is currently no well-defined framework for tailoring photocatalysts to selectively drive the reduction process toward the formation of a single product. Additionally, in scenarios involving product distribution, computational studies exploring the reaction pathways and the factors influencing the generation of specific products are essential. Such studies could guide the design and modification of photocatalysts and elucidate the structural characteristics of active sites that promote the production of selective products.
(e) Understanding the reaction mechanism: Gaining a detailed understanding of the intricate reaction mechanisms underlying CO2 photocatalytic reduction is vital. Elucidating the various pathways and intermediates formed during the process is essential for targeted catalyst design and optimization.
(f) Photocatalyst development: The efficiency and selectivity of CO2 photocatalytic reduction are strongly influenced by the choice and design of photocatalysts. Developing efficient and stable photocatalysts that can not only perform under solar illumination but also withstand prolonged use is an ongoing challenge.
(g) Photocatalyst bandgap and stability: The ideal photocatalyst should have a bandgap that efficiently absorbs solar energy and promotes electron transfer to CO2. However, many photocatalysts suffer from degradation and reduced efficiency due to photocorrosion and stability issues.
(h) Mass transport limitations: Since CO2 is typically supplied as a gas, its availability at the catalytic sites is constrained by both its diffusion rate and solubility in the reaction medium.
(i) Photon absorption efficiency: To drive the photocatalytic reduction reaction, the photocatalyst must efficiently absorb solar photons. Enhancing light-harvesting capabilities and optimizing the photocatalyst design to utilize a broader visible light spectrum is an active area of research.
(j) Integrating with existing infrastructure: Integrating CO2 photocatalytic reduction technologies into existing industrial and energy infrastructure poses challenges. Thus, several factors, including compatibility, scalability, and ease of integration, need careful consideration.
(k) Real-world conditions: CO2 photocatalytic reduction must proceed effectively under varying real-world conditions, including changes in sunlight intensity, temperature, and humidity. Therefore, developing robust systems capable of withstanding environmental fluctuations is essential.
(l) Economic viability: Assessing and improving the economic viability of CO2 photocatalytic reduction processes is crucial for widespread adoption. Evaluating the costs associated with materials, catalysts, and energy input versus the benefits of fuel production is an enduring concern.
(m) Scale-up and cost: While promising at the lab scale, scaling up CO2 photocatalytic reduction processes for practical applications poses challenges. Additionally, evaluating the cost-effectiveness of these processes is crucial for commercial viability.
Addressing these challenges requires interdisciplinary research efforts in materials science, catalysis, photochemistry, and chemical engineering. Ongoing research and development are essential to optimize CO2 photocatalytic reduction technology, making it a viable and scalable option for sustainable CO2 reduction.
6. Challenges specific to graphene-derived photocatalysts and scalability of the CO2 photoreduction system
Although graphene-based materials have gained significant attention as photocatalysts for CO2 reduction, their long-term stability and performance are often hindered by several challenges. Specifically, GO-based photocatalysts suffer from photochemical and thermal deoxygenation of surface functional groups, including epoxy, hydroxyl, and carboxyl moieties, which disrupt the π-conjugated network and degrade conductivity under prolonged illumination.134,135 Variations in GO synthesis (e.g., classical versus modified Hummers' methods) result in materials with widely differing C/O ratios, defect densities, and lateral flake sizes, leading to pronounced batch-to-batch inconsistencies in activity.136,137 Furthermore, residual oxidants and carbonaceous byproducts from chemical reduction can foul active sites or leach into reaction streams, undermining both conversion efficiency and product selectivity.138 To overcome these issues, “green” reduction using L-ascorbic acid provides rGO with tunable oxygen content and minimal impurities.139 Furthermore, heteroatom doping, particularly N-doping in TiO2/rGO hybrids, reinforces the graphene lattice, anchors CO2 intermediates, and preserves more than 85% of initial activity over 35 h of continuous photoreduction.140,141 Moreover, careful control of nitrogen content and bonding configurations further stabilizes functional groups and supports long-term durability.140 These sustainable strategies collectively ensure reproducible and durable performance of graphene-derived photocatalysts under extended solar-driven operation.In parallel with addressing these material-specific challenges, scalability remains a critical hurdle in advancing CO2 photoreduction technologies toward practical application. Recent prototype demonstrations offer promising solutions at both the meso and pilot scales. A meso-scale continuous-flow photochemical reactor employing immobilized Pt/TiO2/rGO films achieved enhanced CO2 conversion rates by optimizing flow dynamics and light distribution over 12 h of operation.142 At a larger scale, a continuous-flow reactor system managing triple-phase interfaces via gas and liquid flow exhibited 10- to 24-fold increases in CO production rates compared to batch reactors, with a CO selectivity of 93.2% and long-term stability exceeding 780 min.143 These case studies underscore the importance of reactor design for uniform illumination, mass transfer, and catalyst immobilization in scaling up CO2 photoreduction technologies. Although most studies remain at the bench scale, recent prototype systems demonstrate practical feasibility. For instance, a mini-pilot photoreactor combining H2O splitting, H2 separation, and CO2 methanation operated outdoors under natural sunlight for three days, producing sufficient crude methane to power a Stirling engine.144 Reactor designs leveraging compound-parabolic collectors, panel reactors, and continuous-flow schemes, coupled with catalyst immobilization and optimized light management, are now being explored for pilot-scale deployment. These developments highlight a clear pathway from material innovation to real-world solar fuel production systems.
7. Conclusion
This review underscores the promising prospects of CO2 photocatalytic reduction for generating solar fuels and value-added chemicals, particularly through the use of graphene-based photocatalysts. Despite the progress in developing numerous photocatalysts over the years, the challenge of designing an efficient CO2 photoreduction system persists. The integration of graphene with appropriate semiconductors brings about a significant improvement in various physicochemical properties, including improved charge separation, enhanced electron transport, strong adsorption capabilities, and augmented photocatalytic performance. This synergistic effect ultimately enhances the overall performance of the composite materials.Nevertheless, graphene encounters inherent challenges that require fundamental and theoretical solutions. Approaches such as defect-induced modification and advanced doping methods can significantly enhance the properties of graphene-based composites. Since graphene acts as an electron acceptor and reduces recombination, detailed analyses such as photocurrent response and electron conductivity measurements are crucial for understanding and optimizing the charge carrier dynamics of graphene-based photocatalysts during CO2 photocatalytic reduction. Moreover, a comprehensive understanding of the mechanisms leading to the formation of various products during CO2 photocatalytic reduction is essential. This understanding can contribute to the further development of mechanisms that enhance product selectivity, ensuring that the carbon source in the products is derived from CO2 rather than graphene. The potential occurrence of the H2 evolution reaction, particularly during alcohol production, introduces an additional challenge that requires careful consideration. Furthermore, the stability of the composite against photocorrosion is a critical aspect that needs attention during CO2 photocatalytic reduction reactions. On the other hand, the accountability of graphene composites is substantial due to their diverse properties and unique structure. Therefore, overcoming challenges related to oxidation sites and defects in graphene to produce high-quality composites remains a formidable task. Additionally, the storage of solar fuels produced through CO2 photoreduction presents significant challenges that hinder the scalability and practicality of this technology. Considering the multiscale challenges, it seems particularly interesting to fortify the overall process efficiency and key material properties to achieve high conversion yields of CO2 to renewable fuels.
Data availability
Data will be made available upon request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Department of Science and Technology (DST), Government of India, through its Solar Energy Research and Development (SERD) initiative (File No. DST/TMD/CERI/RES/2020/41). Manisha Sain acknowledges the financial support provided by the Indian Institute of Technology Kharagpur, West Bengal, India, for her doctoral study. Debanjali Dey is thankful for the financial support provided by the Ministry of Education, Government of India, through its Prime Minister's Research Fellowship scheme for her doctoral study.References
- S. Chowdhury and R. Balasubramanian, J. CO2 Util., 2016, 13, 50–60 CrossRef CAS.
- S. Chowdhury and R. Balasubramanian, Sci. Rep., 2016, 6, 21537 CrossRef CAS PubMed.
- R. L. Singh and P. K. Singh, in Principles and Applications of Environmental Biotechnology for a Sustainable Future, Springer, Singapore, 2017, pp. 13–41 Search PubMed.
- K. Li, X. An, K. H. Park, M. Khraisheh and J. Tang, Catal. Today, 2014, 224, 3–12 CrossRef CAS.
- D. S. A. Simakov, Photocatalytic Reduction of CO2, in Renewable synthetic fuels and chemicals from carbon dioxide: Fundamentals, catalysis, design considerations and technological challenges, Springer, Cham, Switzerland, 2017, pp. 43–54 Search PubMed.
- S. Nagireddi, J. R. Agarwal and D. Vedapuri, ACS Eng. Au, 2024, 4, 22–48 CrossRef CAS.
- V. Khare, S. Nema and P. Baredar, Renew. Sustain. Energy Rev., 2016, 58, 23–33 CrossRef.
- K. K. Jaiswal, C. R. Chowdhury, D. Yadav, R. Verma, S. Dutta, K. S. Jaiswal, B. Sangmesh and K. S. K. Karuppasamy, Energy Nexus, 2022, 7, 100118 CrossRef CAS.
- D. Zhang and J. Song, Procedia IUTAM, 2014, 10, 319–327 CrossRef.
- W. M. Budzianowski, Int. J. Glob. Warm., 2017, 12, 272 CrossRef.
- J. Godin, W. Liu, S. Ren and C. C. Xu, J. Environ. Chem. Eng., 2021, 9, 105644 CrossRef CAS.
- P. Ganji, R. K. Chowdari and B. Likozar, Energy Fuels, 2023, 37, 7577–7602 CrossRef CAS PubMed.
- M. Bonchio, J. Bonin, O. Ishitani, T.-B. Lu, T. Morikawa, A. J. Morris, E. Reisner, D. Sarkar, F. M. Toma and M. Robert, Nat. Catal., 2023, 6, 657–665 CrossRef.
- Y. Huang, C.-F. Yan, C.-Q. Guo and S.-L. Huang, Int. J. Photoenergy, 2015, 2015, 1–11 Search PubMed.
- R. Xu, AIP Conf. Proc., 2024, 3144, 020004 CrossRef CAS.
- D. Gust, T. A. Moore and A. L. Moore, Acc. Chem. Res., 2009, 42, 1890–1898 CrossRef CAS PubMed.
- A. Kudo and Y. Miseki, Chem. Soc. Rev., 2009, 38, 253–278 RSC.
- D. Kim, K. K. Sakimoto, D. Hong and P. Yang, Angew. Chem., Int. Ed., 2015, 54, 3259–3266 CrossRef CAS PubMed.
- M. G. Kibria and Z. Mi, J. Mater. Chem. A, 2016, 4, 2801–2820 RSC.
- S. Patial, R. Kumar, P. Raizada, P. Singh, Q. Van Le, E. Lichtfouse, D. Le Tri Nguyen and V. H. Nguyen, Environ. Res., 2021, 197, 111134 CrossRef CAS PubMed.
- M. Lashgari, S. Soodi and P. Zeinalkhani, J. CO2 Util., 2017, 18, 89–97 CrossRef CAS.
- D. Zhang, Y. Wang, Y. Wang, Y. Zhang and X. M. Song, J. Alloys Compd., 2020, 815, 152377 CrossRef CAS.
- Y. Shen, Q. Han, J. Hu, W. Gao, L. Wang, L. Yang, C. Gao, Q. Shen, C. Wu, X. Wang, X. Zhou, Y. Zhou and Z. Zou, ACS Appl. Energy Mater., 2020, 3, 6561–6572 CrossRef CAS.
- H. Huang, K. Liu, K. Chen, Y. Zhang, Y. Zhang and S. Wang, J. Phys. Chem. C, 2014, 118, 14379–14387 CrossRef CAS.
- G. Zhang, J. Zhang, M. Zhang and X. Wang, J. Mater. Chem., 2012, 22, 8083–8091 RSC.
- W. J. Ong, L. L. Tan, S. P. Chai and S. T. Yong, Chem. Commun., 2015, 51, 858–861 RSC.
- N. Shehzad, M. Tahir, K. Johari, T. Murugesan and M. Hussain, J. Environ. Chem. Eng., 2018, 6, 6947–6957 CrossRef CAS.
- H. Y. Hafeez, S. K. Lakhera, N. Narayanan, S. Harish, Y. Hayakawa, B. K. Lee and B. Neppolian, ACS Omega, 2019, 4, 880–891 CrossRef CAS PubMed.
- A. Razzaq, A. Sinhamahapatra, T. H. Kang, C. A. Grimes, J. S. Yu and S. Il, Appl. Catal., B, 2017, 215, 28–35 CrossRef CAS.
- L. Cheng, Q. Xiang, Y. Liao and H. Zhang, Energy Environ. Sci., 2018, 11, 1362–1391 RSC.
- K. Chu, X. hu Wang, Y. biao Li, D. jian Huang, Z. rong Geng, X. long Zhao, H. Liu and H. Zhang, Mater. Des., 2018, 140, 85–94 CrossRef CAS.
- G. G. Naumis, S. Barraza-Lopez, M. Oliva-Leyva and H. Terrones, Rep. Prog. Phys., 2017, 80, 096501 CrossRef PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- D. G. Papageorgiou, I. A. Kinloch and R. J. Young, Prog. Mater. Sci., 2017, 90, 75–127 CrossRef CAS.
- J. Low, J. Yu and W. Ho, J. Phys. Chem. Lett., 2015, 6, 4244–4251 CrossRef CAS PubMed.
- R. Zhang, Z. Huang, C. Li, Y. Zuo and Y. Zhou, Appl. Surf. Sci., 2019, 475, 953–960 CrossRef CAS.
- M. Y. Akram, T. Ashraf, M. S. Jagirani, A. Nazir, M. Saqib and M. Imran, Catalysts, 2024, 14, 343 CrossRef CAS.
- Y. Liu, J. Shang and T. Zhu, J. Mater. Chem. C, 2024, 12, 9293–9304 RSC.
- Y. Liu, Y. Wang, J. Shang, J. Peng and T. Zhu, Appl. Catal. B Environ. Energy, 2024, 350, 123937 CrossRef CAS.
- D. Liu, Y. Hu, R. Chen, S. Guo, Y. Yang and X. Wang, Catal. Sci. Technol., 2025, 15, 427–434 RSC.
- R. C. Sahoo, H. Lu, D. Garg, Z. Yin and H. S. S. R. Matte, Carbon, 2022, 192, 101–108 CrossRef CAS.
- Y. Zhang, L. Zheng, J. Jia, K. Li, T. Zhang and H. Yu, Colloids Surf. A Physicochem. Eng. Asp., 2022, 639, 128321 CrossRef CAS.
- P. J. J. Sagayaraj, A. Augustin, M. Shanmugam, B. Honnappa, T. S. Natarajan, K. Wilson, A. F. Lee and K. Sekar, Energy Technol., 2023, 11, 2300563 CrossRef CAS.
- M. Q. Yang and Y. J. Xu, Nanoscale Horiz., 2016, 1, 185–200 RSC.
- V. P. Indrakanti, J. D. Kubicki and H. H. Schobert, Energy Environ. Sci., 2009, 2, 745 RSC.
- X. Chang, T. Wang and J. Gong, Energy Environ. Sci., 2016, 9, 2177–2196 RSC.
- W. Tu, Y. Zhou and Z. Zou, Adv. Mater., 2014, 26, 4607–4626 CrossRef CAS PubMed.
- H. Shi, G. Chen, C. Zhang and Z. Zou, ACS Catal., 2014, 4, 3637–3643 CrossRef CAS.
- M. Alhaddad and A. Shawky, Ceram. Int., 2021, 47, 9763–9770 CrossRef CAS.
- J. Jin and T. He, Appl. Surf. Sci., 2017, 394, 364–370 CrossRef CAS.
- D. A. Reddy, E. H. Kim, M. Gopannagari, R. Ma, P. Bhavani, D. P. Kumar and T. K. Kim, ACS Sustain. Chem. Eng., 2018, 6, 12835–12844 CrossRef CAS.
- U. J. Etim, C. Zhang and Z. Zhong, Nanomaterials, 2021, 11, 3265 CrossRef CAS PubMed.
- M. Nolan and M. Fronzi, Catal. Today, 2019, 326, 68–74 CrossRef CAS.
- K. M. Megha, A. Banerjee and T. K. Ghanty, Phys. Chem. Chem. Phys., 2020, 22, 16877–16886 RSC.
- J. M. Weber, Int. Rev. Phys. Chem., 2014, 33, 489–519 Search PubMed.
- J. Lee, D. C. Sorescu and X. Deng, J. Am. Chem. Soc., 2011, 133, 10066–10069 CrossRef CAS PubMed.
- C. Wang, X. Zhang and Y. Liu, Appl. Surf. Sci., 2015, 358, 28–45 CrossRef CAS.
- X. Li, J. Wen, J. Low, Y. Fang and J. Yu, Sci. China Mater., 2014, 57, 70–100 CrossRef.
- C. Hiragond, S. Ali, S. Sorcar and S. In, Catalysts, 2019, 9, 370 CrossRef CAS.
- S. Lu, F. Lou and Z. Yu, Catalysts, 2022, 12, 228 CrossRef CAS.
- X. Jiao, K. Zheng, Z. Hu, Y. Sun and Y. Xie, ACS Cent. Sci., 2020, 6, 653–660 CrossRef CAS PubMed.
- M. Isah, R. Lawal and S. A. Onaizi, Green Chem. Eng., 2025, 6, 305–334 CrossRef.
- R. S. Ruoff, Carbon, 2018, 132, 802 CrossRef CAS.
- S. Xing, Y. Liu, X. Liu, M. Li, J. Fu, P. Liu, P. Lv and Z. Wang, Appl. Catal., B, 2020, 269, 118718 CrossRef CAS.
- A. Atmanlı, B. Yüksel, E. İleri and A. Deniz Karaoglan, Energy Convers. Manag., 2015, 90, 383–394 CrossRef.
- W. Cao, W. Wang, H. Shi, J. Wang, M. Cao, Y. Liang and M. Zhu, Nano Res., 2018, 11, 1437–1446 CrossRef CAS.
- L. G. Devi and R. Kavitha, Appl. Catal., B, 2013, 140–141, 559–587 CrossRef CAS.
- V. Saxena and D. K. Aswal, Semicond. Sci. Technol., 2015, 30, 064005 CrossRef.
- S. G. Kumar and K. S. R. K. Rao, Appl. Surf. Sci., 2017, 391, 124–148 CrossRef CAS.
- W. Fan, Q. Zhang and Y. Wang, Phys. Chem. Chem. Phys., 2013, 15, 2632–2649 RSC.
- J. Li, L. Niu, Z. Zheng and F. Yan, Adv. Mater., 2014, 26, 5239–5273 CrossRef CAS PubMed.
- J. M. Barrera-Andrade, E. Albiter, M. A. Valenzuela and E. Rojas García, Graphene-Based Photocatalysts for CO2 Reduction, in Graphene-Based Photocatalysts, Adv. Struct. Mater., ed. M. R. Johan, M. N. Naseer, M. Ikram, A. A. Zaidi and Y. Abdul Wahab, Springer, Cham, Switzerland, 2024, vol. 217, pp. 709–729 Search PubMed.
- M. Khan, M. Khan, M. Khan, H. Javaid and S. Musaddiq, Harnessing the Power of Graphene: A Critical Analysis of Graphene-Based Photocatalysts for CO2 Reduction, in Graphene-Based Photocatalysts for Hydrogen Production and Environmental Remediation, Adv. Struct. Mater., ed. M. N. Naseer, M. Ikram, A. A. Zaidi, Y. Abdul Wahab and M. R. Johan, Springer, Cham, Switzerland, 2024, vol. 219, pp. 427–448 Search PubMed.
- H. Hsu, I. Shown, H. Wei, Y. Chang, H. Du, Y. Lin, C. Tseng, C. Wang, L. Chen, Y. Lin and K. Chen, Nanoscale, 2013, 5, 262–268 RSC.
- P.-Q. Wang, Y. Bai, P.-Y. Luo and J.-Y. Liu, Catal. Commun., 2013, 38, 82–85 CrossRef CAS.
- J. Cheng, M. Zhang, G. Wu, X. Wang, J. Zhou and K. Cen, Environ. Sci. Technol., 2014, 48, 7076–7084 CrossRef CAS PubMed.
- I. Shown, H. C. Hsu, Y. C. Chang, C. H. Lin, P. K. Roy, A. Ganguly, C. H. Wang, J. K. Chang, C. I. Wu, L. C. Chen and K. H. Chen, Nano Lett., 2014, 14, 6097–6103 CrossRef CAS PubMed.
- L. L. Tan, W. J. Ong, S. P. Chai and A. R. Mohamed, Appl. Catal., B, 2015, 166–167, 251–259 CrossRef CAS.
- L. M. Pastrana-Martínez, A. M. T. Silva, N. N. C. Fonseca, J. R. Vaz, J. L. Figueiredo and J. L. Faria, Top. Catal., 2016, 59, 1279–1291 CrossRef.
- R. Gusain, P. Kumar, O. P. Sharma, S. L. Jain and O. P. Khatri, Appl. Catal., B, 2016, 181, 352–362 CrossRef CAS.
- Z. Xiong, Y. Luo, Y. Zhao, J. Zhang, C. Zheng and J. C. S. Wu, Phys. Chem. Chem. Phys., 2016, 18, 13186–13195 RSC.
- Q. Zhang, L. Huang, S. Kang, C. Yin, Z. Ma, L. Cui and Y. Wang, RSC Adv., 2017, 7, 43642–43647 RSC.
- L. L. Tan, W. J. Ong, S. P. Chai and A. R. Mohamed, Chem. Eng. J., 2017, 308, 248–255 CrossRef CAS.
- S. Kumar, R. K. Yadav, K. Ram, A. Aguiar, J. Koh and A. J. F. N. Sobral, J. CO2 Util., 2018, 27, 107–114 CrossRef CAS.
- S. Sorcar, J. Thompson, Y. Hwang, Y. H. Park, T. Majima, C. A. Grimes, J. R. Durrant and S. Il, Energy Environ. Sci., 2018, 11, 3183–3193 RSC.
- X. Wang, Q. Li, C. Zhou, Z. Cao and R. Zhang, J. Colloid Interface Sci., 2019, 554, 335–343 CrossRef CAS PubMed.
- T. Wu, C. Zhu, D. Han, Z. Kang and L. Niu, Nanoscale, 2019, 11, 22980–22988 RSC.
- P. Devi and J. P. Singh, J. CO2 Util., 2021, 43, 101376 CrossRef CAS.
- H. R. Park, A. U. Pawar, U. Pal, T. Zhang and Y. S. Kang, Nano Energy, 2021, 79, 105483 CrossRef CAS.
- H. T. Lien, Y. C. Chang, C. Y. Huang, H. C. Hsu, S. T. Chang, D. P. Wong, C. H. Wang, C. H. Wang, K. H. Chen and L. C. Chen, J. Chem. Phys., 2021, 154, 164707 CrossRef CAS PubMed.
- N. Nandal, P. K. Prajapati, B. M. Abraham and S. L. Jain, Electrochim. Acta, 2022, 404, 139612 CrossRef CAS.
- K. M. Kamal, R. Narayan, N. Chandran, S. Popović, M. A. Nazrulla, J. Kovač, N. Vrtovec, M. Bele, N. Hodnik, M. M. Kržmanc and B. Likozar, Appl. Catal., B, 2022, 307, 121181 CrossRef CAS.
- A. Hasani, M. A. Teklagne, H. H. Do, S. H. Hong, Q. Van Le, S. H. Ahn and S. Y. Kim, Carbon Energy, 2020, 2, 158–175 CrossRef CAS.
- G. Žerjav, M. S. Arshad, P. Djinović, I. Junkar, J. Kovač, J. Zavašnik and A. Pintar, Nanoscale, 2017, 9, 4578–4592 RSC.
- P. Huo, X. Shi, W. Zhang, P. Kumar and B. Liu, J. Mater. Sci., 2021, 56, 6031–6051 CrossRef CAS.
- L. Y. Lin, Y. Nie, S. Kavadiya, T. Soundappan and P. Biswas, Chem. Eng. J., 2017, 316, 449–460 CrossRef CAS.
- V. Deerattrakul, P. Dittanet, M. Sawangphruk and P. Kongkachuichay, J. CO2 Util., 2016, 16, 104–113 CrossRef CAS.
- H. Jung, H. Choi, Y. Song, J. H. Kim and Y. Yoon, Nanoscale Adv., 2024, 6, 4611–4624 RSC.
- Y. Li, M. Zhou, B. Cheng and Y. Shao, J. Mater. Sci. Technol., 2020, 56, 1–17 CrossRef CAS.
- Z. Otgonbayar, Y. Liu and W. Oh, J. Environ. Chem. Eng., 2023, 11, 109884 CrossRef CAS.
- S. Yin, J. Li, L. Sun, X. Li, D. Shen, X. Song, P. Huo, H. Wang and Y. Yan, Inorg. Chem., 2019, 58, 15590–15601 CrossRef CAS PubMed.
- Y. Ding, Y. Zhou, W. Nie and P. Chen, Appl. Surf. Sci., 2015, 357, 1606–1612 CrossRef CAS.
- L. Zhu, Y. Wang, C. Qin and J. Cao, J. Phys. Chem. C, 2022, 126, 16702–16709 CrossRef CAS.
- J. Yu, J. Jin, B. Cheng and M. Jaroniec, J. Mater. Chem. A, 2014, 2, 3407 RSC.
- H. Jung, K. M. Cho, K. H. Kim, H.-W. Yoo, A. Al-Saggaf, I. Gereige and H. Jung, J. Mater. Chem. A, 2018, 6, 5718–5724 CAS.
- T. Van Khai, L. N. Long, M. T. Phong, P. T. Kien, L. Van Thang and T. D. Lam, J. Electron. Mater., 2020, 49, 969–979 CrossRef.
- C. Das, T. Shafi, S. Pan, Mu. Naushad, B. K. Dubey and S. Chowdhury, ACS Appl. Nano Mater., 2023, 6, 12991–13000 CrossRef.
- Z. Du, H. Cai, Z. Zhao, Z. Guo, J. Lin, Y. Huang, C. Tang, G. Chen and Y. Fang, Sep. Purif. Technol., 2023, 311, 123321 CrossRef CAS.
- R. Manna, G. Bhattacharya, S. Raj and A. N. Samanta, J. Environ. Chem. Eng., 2024, 12, 111722 CrossRef CAS.
- N. Kumar, S. Kumar, R. Gusain, N. Manyala, S. Eslava and S. S. Ray, ACS Appl. Energy Mater., 2020, 3, 9897–9909 CrossRef CAS.
- L. Velasco Davoise, R. Peña Capilla and A. M. Díez-Pascual, Polymers, 2022, 14, 1828 CrossRef CAS PubMed.
- X. Liu, X. Zhao, J. Yan, Y. Huang, T. Li and P. Liu, Carbon, 2021, 178, 273–284 CrossRef CAS.
- A. Sheelam, A. Muneeb, B. Talukdar, R. Ravindranath, S. Huang, C. Kuo and R. Sankar, J. Appl. Electrochem., 2020, 50, 979–991 CrossRef CAS.
- R. Castro-Muñoz, J. Buera-González, Ó. de la Iglesia, F. Galiano, V. Fíla, M. Malankowska, C. Rubio, A. Figoli, C. Téllez and J. Coronas, J. Membr. Sci., 2019, 582, 423–434 CrossRef.
- J. Liu, Y.-H. Zhang, Z.-M. Bai, Z.-A. Huang and Y.-K. Gao, Chin. Phys. B, 2019, 28, 048101 CrossRef CAS.
- L. Zhou, H. Kamyab, A. Surendar, A. Maseleno, A. Z. Ibatova, S. Chelliapan, N. Karachi and Z. Parsaee, J. Photochem. Photobiol. A, 2019, 368, 30–40 CrossRef CAS.
- A. Ali and W.-C. Oh, Sci. Rep., 2017, 7, 1867 CrossRef PubMed.
- R. Wang, L. Du, Y. Liu, Y. Gu, X. Li and Y. Li, 2D Mater., 2024, 11, 015014 CrossRef CAS.
- M. R. Hoffmann, J. A. Moss and M. M. Baum, Dalton Trans., 2011, 40, 5151–5158 RSC.
- J. Hong, W. Zhang, J. Ren and R. Xu, Anal. Methods, 2013, 5, 1086–1097 RSC.
- D. Liu, Y. Hu, R. Chen, S. Guo, Y. Yang and X. Wang, Catal. Sci. Technol., 2025, 15, 427–434 RSC.
- Q. Zhai, S. Xie, W. Fan, Q. Zhang, Y. Wang, W. Deng and Y. Wang, Angew. Chem., 2013, 125, 5888–5891 CrossRef.
- E. Karamian and S. Sharifnia, J. CO2 Util., 2016, 16, 194–203 CrossRef CAS.
- J. Fu, K. Jiang, X. Qiu, J. Yu and M. Liu, Mater. Today, 2020, 32, 222–243 CrossRef CAS.
- Y. Ji and Y. Luo, J. Am. Chem. Soc., 2016, 138, 15896–15902 CrossRef CAS PubMed.
- C. F. Shih, T. Zhang, J. Li and C. Bai, Joule, 2018, 2, 1925–1949 CrossRef CAS.
- A. Dhakshinamoorthy, S. Navalon, A. Corma and H. Garcia, Energy Environ. Sci., 2012, 5, 9217–9233 RSC.
- M. A. Gondal, A. Lais, M. A. Dastageer, D. Yang, K. Shen and X. Chang, Int. J. Energy Res., 2017, 41, 2162–2172 CrossRef CAS.
- S. Ali, R. Iqbal, A. Khan, S. U. Rehman, M. Haneef and L. Yin, ACS Appl. Nano Mater., 2021, 4, 6893–6902 CrossRef CAS.
- D. Vadivel, F. Ferraro, D. Merli and D. Dondi, Springer Nature, Photochem. Photobiol. Sci., 2022, 21, 863–878 CrossRef CAS PubMed.
- M. Q. Yang and Y. J. Xu, Phys. Chem. Chem. Phys., 2013, 15, 19102–19118 RSC.
- D. C. Grills and E. Fujita, J. Phys. Chem. Lett., 2010, 1, 2709–2718 CrossRef CAS.
- X. Li, J. Yu, J. Low, Y. Fang, J. Xiao and X. Chen, J. Mater. Chem. A, 2015, 3, 2485–2534 RSC.
- M. Minella, M. Demontis, M. Sarro, F. Sordello, P. Calza and C. Minero, J. Mater. Sci., 2015, 50, 2399–2409 CrossRef CAS.
- K. Spilarewicz-Stanek, A. Jakimińska, A. Kisielewska, M. Dudek and I. Piwoński, Mater. Sci. Semicond. Process., 2021, 123, 105525 CrossRef CAS.
- K.-Q. Lu, Y.-H. Li, Z.-R. Tang and Y.-J. Xu, ACS Mater. Au, 2021, 1, 37–54 CrossRef CAS PubMed.
- A. Badoni, S. Thakur, N. Vijayan, H. C. Swart, M. Bechelany, Z. Chen, S. Sun, Q. Cai, Y. Chen and J. Prakash, Catal. Sci. Technol., 2025, 15, 1702–1770 RSC.
- B. Anegbe, I. H. Ifijen, M. Maliki, I. E. Uwidia and A. I. Aigbodion, Environ. Sci. Eur., 2024, 36, 15 CrossRef CAS.
- M. Palomba, G. Carotenuto and A. Longo, Materials, 2022, 15, 6456 CrossRef CAS PubMed.
- L.-Y. Lin, Y. Nie, S. Kavadiya, T. Soundappan and P. Biswas, Chem. Eng. J., 2017, 316, 449–460 CrossRef CAS.
- C. B. Hiragond, J. Lee, H. Kim, J.-W. Jung, C.-H. Cho and S.-I. In, Chem. Eng. J., 2021, 416, 127978 CrossRef CAS.
- S. Nabil, E. A. Shalaby, M. F. Elkady, Y. Matsushita and A. H. El-Shazly, Catal. Lett., 2022, 152, 3243–3258 CrossRef CAS.
- H. Jung, C. Kim, H.-W. Yoo, J. You, J. S. Kim, A. Jamal, I. Gereige, J. W. Ager and H.-T. Jung, Energy Environ. Sci., 2023, 16, 2869–2878 RSC.
- T. Hisatomi, Q. Wang, F. Zhang, S. Ardo, E. Reisner, H. Nishiyama, A. Kudo, T. Yamada and K. Domen, Front. Sci., 2024, 2, 1411644 CrossRef.
Footnote |
| † Equal contribution. |
| This journal is © The Royal Society of Chemistry 2025 |


