Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Direct air capture (DAC): molten carbonate direct transformation of airborne CO2 to durable, useful carbon nanotubes and nano-onions†
Gad
Licht
*a,
Ethan
Peltier
b,
Simon
Gee
b and
Stuart
Licht
 *abc
*abc
aDirect Air Capture LLC, A4 188 Triple Diamond Blvd, North Venice, FL 34275, USA. E-mail: slicht@gwu.edu
bCarbon Corp, 1035 26 St NE, Calgary, AB T2A 6K8, Canada
cDept. of Chemistry, George Washington University, Washington DC 20052, USA
First published on 7th February 2025
Abstract
This study introduces the concept and first demonstration of an effective molten carbonate chemistry for Direct Air Capture (DAC). Molten carbonate electrolysis is a high-temperature decarbonization process within Carbon Capture, Utilization and Storage (CCUS) that transforms chemistry transforming flue gas CO2 into carbon nanotubes and carbon nano-onions. The key challenge for molten carbonate DAC is to split air's 0.04% CO2 without heating the remaining 99.6%. This is accomplished by integrating a diffusive, insulating membrane over an electrolyte with a high affinity for CO2.
Sustainability spotlightThe concept and first demonstration of an effective direct air capture molten carbonate chemistry is presented for removal of the greenhouse gas carbon dioxide to mitigate global warming which is an existential threat to the planet. Molten carbonate electrolysis is an CCUS high-temperature decarbonization chemistry transforming flue gas CO2 to graphenes. Its direct air capture challenge is to split air's 0.04% CO2 without heating the remaining 99.6% of the air, which is accomplished here by integration of a membrane over a high CO2 affinity electrolyte. |
Introduction
Direct air capture technologies
Atmospheric Greenhouse gas removal is a necessary component to limit climate change.1,2 Today's chemical Direct air capture decarbonization technologies2 typically rely on active CO2-concentration, often using sorbents such as amine binding, or lime reactions, as described in the ESI.†3,4 CO2-concentration is energy-intensive, produces rather than reduces CO2, and provides just the initial step in Carbon Capture and Storage (CCS) or Carbon Capture, Utilization, and Storage resulting in concentration, rather than storage. In contrast, this demonstration presents an efficient chemical DAC process that eliminates the need for active CO2 concentration.C2CNT: transition metal nucleated electrolytic CCUS
We've developed a large-scale transition metal nucleated molten carbonate chemistry to electrolytically split CO2 into carbon nanotubes (CNTs) and nano-onions (CNOs) and other Graphene NanoCarbon allotropes (GNCs) (ESI Section†). This C2CNT (CO2 to Carbon Nano Technology) process is highly effective for flue gas decarbonization. Graphite, as a multi-layered graphene, demonstrates the long-term sequestration potential of structures built from graphene. The challenge for C2CNT as a DAC Technology is to efficiently split air's 0.04% CO2 without heating the remaining 99.96% composition to the ∼750 °C of the C2CNT electrolysis. This study presents the concept and first demonstration of an effective DAC molten carbonate chemistry.CNTs possess the highest tensile strength ever recorded (93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 MPa) and feature exceptional thermal conductivity, high charge storage, flexibility, and catalytic properties.5,6 As described in the ESI,† they enhance structural materials such as cement and steel, and are used in a variety of applications including in medical and electrochemical fields, electronics, batteries, supercapacitors, sensing, plastics, textiles, hydrogen storage, and water treatment.
900 MPa) and feature exceptional thermal conductivity, high charge storage, flexibility, and catalytic properties.5,6 As described in the ESI,† they enhance structural materials such as cement and steel, and are used in a variety of applications including in medical and electrochemical fields, electronics, batteries, supercapacitors, sensing, plastics, textiles, hydrogen storage, and water treatment.
High-temperature molten carbonate electrolytic CO2-splitting into C and O2 as a mitigation strategy was first introduced by our group in 2009–2010.7 By 2015, it was demonstrated that this process could convert CO2 into valuable, specialized graphene carbons, GNCs, through transition metal nucleated growth.8–10 The type of useful GNCs, such as CNTs and CNOs produced depends on the electrochemical conditions (ESI†), which offers a promising climate change mitigation approach. The process follows a 4-electron redox reaction of carbonate:
| CO32−(molten) → C(GNC) + O2(gas) + O2−(dissolved) | (1) |
The oxide reacts with CO2 to regenerate carbonate:
| CO2(gas) + O2−(dissolved) → CO32−(molten) | (2) |
These reactions combine to split CO2 into carbon products and O2:
| CO2(gas) → C(GNC) + O2(gas) | (3) |
The CCUS molten carbonate electrolysis process for CNT and CNO production has evolved into a sophisticated technology. By adjusting the CO2 electrolysis conditions, specific GNCs can be produced, including doped, thin, thick, long, magnetic, nano-bamboo, and helical CNTs, as well as nano-scaffold, graphene, nano-pearl, and nano-onion morphologies as described in the ESI.†9,10
We have explored alternative alkali carbonate mixtures and lower electrolysis temperatures, which can produce 3D-symmetry graphene scaffolds (ESI†). Electrolysis current densities ranging from 0.03–0.6 A cm−2 affect GNC growth; higher densities favour the formation of helical CNTs (ESI†). Electrolysis requires a voltage range from 0.8–2 V. Using renewable energy sources can further reduce the CO2 footprint (ESI†). High purity GNCs form directly on the cathode and are separated from the molten electrolyte through high-temperature filtration, as described in the ESI.†
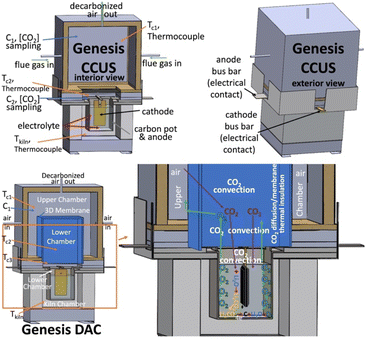
C2CNT process modules are called the Genesis Device®, and large-scale CCUS Genesis modules transforming the 5% CO2 flue gas from the Shepard Natural Gas Power Plant are operating in Calgary, Canada. Today, large-scale Genesis electrolysis uses cathodes over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm2. The oxidation resistance of the CNTs produced is high, with a 97.1% TGA purity. SEM and TEM images of the CNT product are detailed in the ESI.† The units tested in this study are bench-top scale, using similar Muntz brass cathodes, 304SS anodes, and 99.5% purity Li2CO3 electrolyte. These units are housed in a 12 × 12 × 15 cm carbon pot electrolyzer, which contains the molten electrolyte and serves as the electrolysis chamber, housed within a converted Caldera Paragon kiln. The CO2- concentration is measured with a CO2meter (http://co2meter.com/) sensor situated externally from the hot chamber connected via a 304SS tube which allows sufficient cooling to facilitate the room-temperature sensor performance.
000 cm2. The oxidation resistance of the CNTs produced is high, with a 97.1% TGA purity. SEM and TEM images of the CNT product are detailed in the ESI.† The units tested in this study are bench-top scale, using similar Muntz brass cathodes, 304SS anodes, and 99.5% purity Li2CO3 electrolyte. These units are housed in a 12 × 12 × 15 cm carbon pot electrolyzer, which contains the molten electrolyte and serves as the electrolysis chamber, housed within a converted Caldera Paragon kiln. The CO2- concentration is measured with a CO2meter (http://co2meter.com/) sensor situated externally from the hot chamber connected via a 304SS tube which allows sufficient cooling to facilitate the room-temperature sensor performance.
C2CNT decarbonization is a unique process, unlike other CCS processes in that it directly (without pre-concentration) transforms CO2 to high-purity, high-yield GNCs at ∼750 °C. This decarbonization process, which eliminates the need for CO2 concentration, as the electrolyte is a carbon sink that draws in CO2 with a high affinity, applies to both C2CNT industrial CCUS (Carbon Capture, Utilization and Storage) and C2CNT DAC. However, the feed gas for industrial gas typically ranges from 5% CO2 (natural gas power plant stack emissions) to over 30% CO2 (cement plant emissions), and furthermore is generally hot coming off the industrial process. In contrast, DAC utilizes ambient temperature air containing a very low CO2 concentration. Unlike C2CNT CCUS, DAC may require more energy to heat the entire air feed to >700 °C electrolysis temperatures. Air contains 0.042% CO2, with the remaining >99.95% consisting of N2, O2, H2O and Ar. While the latter are highly insoluble in molten carbonates, CO2 dissolves reactively as shown in eqn (2). For effective DAC decarbonization, the C2CNT process energy should not be used to heat the 99.95% non-CO2 components of air.
The first DAC technology is presented that simultaneously (1) directly (without preconcentration) removes CO2 from the air, (2) transforms the CO2 into a valuable product (such as CNTs) provides a strong incentive to remove this greenhouse gas, and (3) unlike previous regular C2CNT processes, insulates the feed air from the hot electrolysis chamber while still allowing CO2 to diffuse into the electrolysis chamber.
CO2 diffusion through high temperature insulation
The known diffusion coefficient of CO2 in air varies as:11| DCO2(TK) = 2.7 × 10−5TK1.59/e(102.1/TK) | (4) |
We recently characterized the rate of CO2 diffusion at room temperature through a variety of high temperature porous insulations acting as membranes.11 These diffusion results are now used to develop a new high temperature direct air capture, in which these membranes are placed between the feed air and a high-temperature molten electrolyte that removes CO2via electrolysis in the carbon pot. Insulation materials tend to be porous and low-density. The majority of high-temperature alumina or CaO/MgO silicate insulations we recently studied have densities ranging from 0.06–0.14 g cm−3, with a measured porosity, of ε ranging from 0.89–0.96. Higher density-insulation (0.59 to 0.70 g cm−3) had a measured porosity of ε = 0.45–0.67.11 As expected, the manufacturer's thermal insulation values tend to vary linearly with thickness and, to a lesser extent, with density. For example, 1/4′′ insulation with densities of 0.10 and 0.13 g cm−3 have respective R-values of 0.3 and 0.4, while 4× thicker insulation has R-values of 1.1 to 1.5. Alumina and CaO/MgO silicate insulations had similar R-values.
In that room temperature study, we determined that CO2 readily diffuses through porous ceramic thermal insulators. The measured CO2 diffusion coefficient for the silicate membranes was found to correlate with the measured open-channel porosity as follows:11
| DM-porous-CO2 = DCO2·ε(M)3/2 | (5) |
These diffusion rates were further increased by approximately 50% with turbulent flow (either parallel or perpendicular flow) over the upper membrane surface.11 These findings, combined with eqn (4) and (5), are now used to configure a DAC C2CNT process that enables the chemical permeation of CO2 while insulating air's other components from the hot molten carbonate electrolysis.
Experimental section
Electrolyses were conducted using a 750 °C Li2CO3 (99.85%, Shanghai Seasongreen Chemical Co.) electrolyte, with or without a Li2O additive (99.5% Alfa Aesar), in a 12 × 12 × 15 cm tall 304SS pot and a Muntz brass cathode (Marmetal Industries). In these small-scale experiments, samples were collected from the cooled cathode, and acid-washed to remove electrolyte interspersed with the cooled product. In larger-scale experiments (ESI Section†) the molten electrolyte is directly extracted from the product using high temperature presses.The Genesis DAC configuration was tested according to the lower left-illustration of Fig. 1 using the 12 × 12 × 15 cm tall 304SS pot. Air is fed into the upper chamber at a low flow rate, proportional to the applied constant electrolysis eqn (8). Higher flow rate conditions (×1.5 and ×2 flow rate) conditions were also investigated.
Two aluminosilicate insulations were used as membranes in this configuration, a 1′′ (CeraBlanket 8254870040000) and a 1/4′′ (CeraBlanket 825680600200P2), with measured porosities of ε = 0.93 and ε = 0.91, respectively.11 Each was configured in the 3D open-bottom structure shown in the lower left of Fig. 1 with four 20 × 20 cm walls and a top, providing a total membrane surface area of 2000 cm2.
Results and discussion
A new C2CNT chemistry for DAC rather than CCUS
Fig. 1 compares Genesis CCUS to the new Genesis DAC decarbonization configuration. CO2 transfer between the ambient gas feed and the C2CNT electrolysis electrolyte interface is dominated by convection. The placement of the flue gas, exit, CO2-sensor, and thermocouple placement are illustrated, as well as the external electrical connectors to the anode and cathode. A matrix of GNCs, with interspersed electrolyte grows at the cathode and is periodically lifted to harvest the GNC product.The Genesis DAC configuration in the bottom of Fig. 1 utilizes the recently characterized CO2 diffusion properties of open-channel porous thermal insulation, and reduces heat loss while sustaining CO2 transfer between an ambient air feed gas and the interior of the C2CNT electrolysis chamber, which acts as a carbon sink. CO2-transport in the upper air feed chamber and in both the lower electrolysis chamber are dominated by convection. As illustrated at the bottom portion of Fig. 1, Genesis DAC positions a high-surface area porous insulation as a membrane creating a quiescent zone between the air feed and the electrolysis chamber. In this quiescent zone CO2 mass transport by diffusion, rather than by convection, dominates. By maintaining a high 3D surface area of this interfacial diffusion zone, sufficient CO2 enters the electrolysis chamber to replenish the CO2 consumed.
Sustainable DAC rates and feed gas air flow
The membrane diffusion-limited sustainable CO2-splitting current, JMCO2, is determined from DM-porous-CO2, eqns (4) and (5), and the volume V(TK) of 1 mole of CO2 at temperature TK. JMCO2 has units of mA cm−2 when calculated from the CO2 transported per cm2 of the membrane, the membrane thickness lM (cm), the CO2Co = 0.042% concentration in the air, and Faraday's constant F = 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485/mole e−. For the n = 4e CO2-reduction is then:
485/mole e−. For the n = 4e CO2-reduction is then:| JMCO2(TK, mA cm−2 membrane) = 1000·4F·CoDM-CO2(TK)·1 cm2/(lM × Videal(TK)) |
V(TK) = 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 414 cm3 mole−1 × TK/273.15 K 414 cm3 mole−1 × TK/273.15 K | (6) |
These experiments are constant-current electrolyses at Ielectrolysis (A) conducted for a time telectrolysis (s). This charge passed is then converted to the max moles of CO2 at 20 °C, which can be split by the n = 4-electron electrolysis, molmax-CO2:
| molmax-CO2 = (Ielectrolysis (amps)/4F)·telectrolrysis (s) | (7) |
This ideal conversion of CO2 will produce an equal number of maximum moles of carbon and oxygen, i.e., molmax-CO2 = molmax-C = molmax-O2. From the respective molar masses, the maximum masses are calculated as mmax-CO2 = 44.01 × molmax-CO2; mmax-C = 12.01 × molmax-CO2; mmax-O2 = 32.00 × molmax-CO2. Oxygen (O2) from the electrolysis evolves as a gas, and exits the electrolysis chamber.
The required 20 °C airflow (LPM) per amp of electrolysis charge, fA, is given by:
| fA(LPM/A) = Co−1·1 mol CO2·Videal (298.15 K) (60 s min−1)/4F | (8) |
From eqn (8)fA is 9 LPM air per A of CO2 splitting electrolysis.
The Genesis DAC efficiency
Δmelec is the measured mass change of the system during the electrolysis. The maximum Δmelec, corresponding to a DAC efficiency of 100%, represents a full absorption of CO2, i.e. mmax-CO2 = 44.01 × molmax-CO2, its conversion to carbonate, and the maximum amount of carbon, i.e. mmax-C = 12.01 × molmax-CO2. The worst-case scenario occurs when DAC Efficiency = 0%, meaning there is no CO2 absorption during the electrolysis. In this case, all carbon formed reacts with the O2 produced and is then released as CO2; i.e. Δmelec = – mmax-CO2. The max DAC efficiency (100%) case occurs when all the electrolysis required CO2 is absorbed during that electrolysis at Ielectrolysis for telectrolrysis, and is converted by the electrolysis, and retained as pure carbon GNC product on the cathode, i.e. Δmelc = mmax-CO2, while O2 is evolved. This yields the equation for the molten carbonate direct air capture efficiency:| DAC eff (%) = 100%(Δmelec + mmax-CO2)/(mmax-C + mmax-CO2) | (9) |
Measurement of the genesis DAC efficiency
During electrolysis, the 1-inch thick membrane had an outer surface temperature of 40 °C and an inner surface temperature of 560 °C, providing substantial thermal insulation for the air feed gas, above the hot 750 °C electrolysis chamber. The 1/4-inch membrane offered less insulation, with an outer surface temperature of 160 °C and an inner surface temperature of 450 °C.The membrane temperature is estimated as the average temperature of its upper and lower surfaces, which is 300 °C for both the 1-inch and 1/4-inch thick membranes The electrochemical current density from eqn (6), JMCO2 (300 °C = 573 K), is 0.67 and 2.5 mA cm−2 for the respective 1-inch and 1/4-inch membranes. The Amembrane = 2000 cm2, which multiplied by the area current densities, should support respective electrolysis currents of 1.3 and 5.0 A, respectively.
Fig. 2 presents Genesis DAC Efficiencies from eqn (9) based on the measured mass change, Δmelec at the start and finish of 16 hours 750 °C electrolyses with brass cathodes, and with the carbon pot walls acting as anodes. In the Genesis DAC configuration using a 1-inch insulation as a membrane in the pure Li2CO3 electrolyte, a maximum DAC efficiency of 87% was achieved with an electrolysis current of 1 A. However, as shown in the black curve, the efficiency decreases rapidly at higher currents due to insufficient CO2 reaching the electrolysis chamber.
 | ||
| Fig. 2 DAC efficiency as a function of the electrolysis current in DAC Genesis as configured in the lower left of Fig. 1. Black data is measured with eqn (9) required airflow, and red and blue data with 2× higher flow. | ||
We have previously noted that in accordance with eqn (2), the addition of 1 m Li2O increases the rate of CO2 uptake.8 At an electrolysis current of 1 A, this addition increases DAC efficiency to 91%. In medium or high airflow (1.5× or 2× the eqn (8) flow), the efficiency rises further to 95% and 99% DAC (red curve). Even with high airflow, however, the DAC efficiency, as shown in the Fig. 2 red curve, decreases again at cell currents >1 A. The systematic increase in cell current (1, 2, 3, 5 and 9 amps) through the 2000 cm2 surface area membrane demonstrates that DAC efficiency in Fig. 2 decreases with increasing membrane diffusion limited current density. Lower current densities support higher levels of DAC efficiency.
A 1/4-inch membrane supports greater CO2 diffusion to the electrolysis chamber improving DAC efficiency. This is measured with turbulent (2×) air flow using a pure electrolysis Li2CO3 configuration. This configuration supports the highest electrolysis current of 2 A at 98% DAC efficiency, as shown in the Fig. 2 blue curve. Both this, and the 1-inch membrane, measured maximum DAC efficiencies, but these currents occur lower than the calculated eqn (6) supporting currents. The cathode bus bar (seen in Fig. 1) interferes with downward CO2 flow in the lower chamber and appears to create a bottleneck for CO2 mass transport to the electrolyte. Future experiments will explore configurations to mitigate this bottleneck, as well as surface-enhanced membranes, such as zig-zag, dimpled and multiple membrane layer designs, which increase the effective membrane area.
The experimental data verifying the accuracy of the theoretical framework is shown in Fig. 3; specifically, the percentage of the applied cell current used for DAC conversion is compared to the theoretical membrane diffusion-limited current:
| DAC modelled achieved experimentally (%) = (DAC efficiency × cell current)/(Amembrane × JMCO2) | (10) |
 | ||
| Fig. 3 A comparison of the DAC modelled CO2 diffusion limited currents and the experimentally measured DAC currents. Black data is measured with eqn (9) required airflow, and red and blue data with 2× higher flow. | ||
As shown in Fig. 3, the product of the modelled membrane-limited rate of CO2 membrane diffusion limited current, (based on eqns (5) and (6)), generally underestimates the observed DAC capabilities by up to a factor of two, depending on the applied cell current. The observed DAC rate is higher for thinner membranes and tends to increase at higher currents. This two-fold underestimate in the model is a satisfactory preliminary approximation for CO2 membrane diffusion constants measured at room temperature and extrapolated for use with the 750 °C electrolysis of the Genesis DAC configuration. Future studies will explore whether the membrane porosity exponent in eqn (5) (ε(M)3/2) is itself a function of temperature, and the assumption of a linear temperature profile through the membrane's cross section. For example, the results suggest that the lower outer wall temperature of the membrane has a larger effect on the rate of CO2 diffusion.
CNTs and CNOs products from CO2 in the air
At 750 °C in Li2CO3, the CO2-splitting electrolysis consistently produces high-purity CNTs as exemplified by the SEM images of the washed product in Fig. 4 panels A & B.Under CO2-limiting conditions, where insufficient air CO2 is fed into the DAC system, an oxide buildup occurs as described by eqn (1) oxide. In this case, we observe that the product tends to be high-purity carbon nano-onions (CNOs) as shown in Fig. 5, panels A through I. When excess CO2 is reintroduced to the DAC Genesis, CNT production resumes.
As with CNTs,12 and as seen in the upper left corner of Fig. 5 (panel A), the CNO growth begins with a thin layer of graphene (observed as removed from the cathode) from which a matrix of CNO growth occurs. Under conditions of insufficient CO2, higher oxide concentration buildup in the electrolyte, this (i) reduces the solubility and prevalence of transition metal cations in the electrolyte, inhibiting CNT growth by transition metal nucleation, and (ii) induces sp3 defects in the sp2 graphene lattice. These sp3 sites favor the formation of spherical, rather than planar cylindrical, graphene growth.8,13 Thus, an oxide-rich electrolytic environment promotes CNO over CNT growth.13
Alternative graphene nanocarbon products from CO2 in the air
We have studied the morphology and purity of the GNC products using CO2 from the air since 2015,8 although previously without an effective means to separate heat losses between air and the molten electrolyte. In 2016, we demonstrated that using the 13C isotope resulted in the first pure 13C multiwalled carbon nanotube, and tracked the transformation of gas-phased CO2 into CNTs via electrolysis.14 In 2021, we applied high current density (0.6 A cm−2) and added oxides to the electrolyte, which induced sp3 defects and torsional effects transforming to CO2 to helical carbon nanotubes.15 By controlling electrolysis conditions – including variations in current density, anode, cathode and electrolyte composition, and temperature – we can produce high purity morphologies with a range of properties, including thin-walled, magnetic, doped, nano-bamboo, nano-pearl, and nano-tree CNTs as detailed in the ESI.†10,12–24Alternative electrolytes and an approximation of DAC costs
The CCUS Genesis Device® for decarbonization of flue gas is significantly more mature than the new DAC Genesis Device® for direct air capture. The CCUS electrodes have been scaled up from the original 5 cm2 area (ref. 8) to over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm2 in electrolysis modules, which comprise 1000 tons of CO2 decarbonization. These modules are directly fed with 5% CO2 flue emissions from the adjacent 860 MW Shepard Natural Gas Power Plant in Calgary Canada.25
000 cm2 in electrolysis modules, which comprise 1000 tons of CO2 decarbonization. These modules are directly fed with 5% CO2 flue emissions from the adjacent 860 MW Shepard Natural Gas Power Plant in Calgary Canada.25
In a recent advancement of the CCUS C2CNT decarbonization chemistry, the majority of the lithium carbonate electrolyte was replaced by strontium carbonate-based electrolytes. The thermodynamic and kinetic chemistry of the electrolytes has been shown to be equivalent for the electrolytic splitting of CO2 to GNCs. This is economically significant, as strontium salts are an order of magnitude less expensive and considerably more abundant than lithium salts. This shift also alleviates competition for limited lithium carbonate resources which are increasingly used for EVs and grid electric storage. An analysis of C2CNT decarbonization costs based on energy consumption, and the new strontium electrolytes, estimates $198 per tonne of CO2 ($791 per tonne GNC produced).25 In that study the equivalent affinities of SrCO3 and Li2CO3 for absorbing and releasing CO2 are demonstrated to be comparable, and are unlike all the other alkali and alkali earth carbonates. The temperature domain in which the CO2 transformation to GNCs can be effective is <800 °C. Although the solidus temperature of SrCO3 is 1494 °C, it is remarkably soluble in Li2CO3 at temperatures less than 800 °C, and the electrolysis energy is low. High purity CNTs are synthesized from CO2 respectively in SrCO3 based electrolytes containing 30% or less Li2CO3. These strontium-based electrolytes are also applicable to the DAC Genesis Device® for direct air capture. The components of DAC and CCUS C2CNT technologies are similar and the additional cost of the porous ceramic diffusion membranes is minor. As a first approximation, the cost per tonne of decarbonization will be similar, and will be analyzed in depth in a subsequent study as the technology is scaled. It is important to note that the C2CNT deployment involves not only the costs mentioned, but also revenue, as GNCs have high value. This provides an additional incentive for DAC C2CNT Genesis deployment.
Conclusions
In conclusion, we have achieved a high rate of CO2 direct air capture, without requiring active CO2 concentration. Molten carbonate electrolysis is a powerful method for high-temperature CCUS that converts flue gas CO2 into GNCs. The main challenge for its DAC counterpart has been extracting CO2 from air, which contains only 0.04% CO2, without needing to heat the remaining 99.6% of the air. This study introduces a novel concept and provides the initial demonstration of an effective molten carbonate chemistry for DAC. CO2 diffuses through high-temperature aluminosilicate insulation, where it is reactively consumed in a molten electrolyte carbon sink and electrolytically transformed into CNTs and CNOs, while the major components of air – N2, O2, and H2O – remain highly insoluble in the electrolyte. Low air feed gas temperatures and moderate electrolysis currents are maintained, ensuring high DAC efficiency.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- National Academies of Sciences, Engineering & Medicine, et al., Negative Emissions Technologies and Reliable Sequestration: A Research Agenda, National Academies Press, Washington, DC, 2019, DOI:10.17226/25259.
- H. Li, et al., Capturing carbon dioxide from air with charged-sorbents, nature, 2024, 630, 654–659 CrossRef CAS PubMed.
- G. Zhang, J. Liu, J. Qian, X. Zhang and Z. Liu, Review of research progress and stability studies of amine-based biphasic absorbents for CO2 capture, J. Ind. Eng. Chem., 2024, 134, 28–50 CrossRef CAS.
- Y. Tan, W. Liu, X. Zhang, W. Wei and S. Song, Conventional and optimized testing facilities of calcium looping process for CO2 capture: A systematic review, Fuel, 2024, 358, 130337 CrossRef CAS.
- C.-C. Chang, H.-K. Hsu, M. Aykol, W. Hung, C. Chen and S. Cronin, A New Lower Limit for the Ultimate Breaking Strain of Carbon Nanotubes, ACS Nano, 2010, 4, 5095–5100 CrossRef CAS PubMed.
- A. Islam, M. Hasan, M. Rahman, H. Mobarak, M. A. Mimona and N. Hossain, Advances and significances of carbon nanotube applications, Eur. Polym. J., 2024, 2024, 113443 CrossRef.
- S. Licht, B. Wang, S. Ghosh, H. Ayub, D. Jiang and J. Ganley, New solar carbon capture process: STEP carbon capture, J. Phys. Chem. Lett., 2010, 1, 2363–2368 CrossRef CAS.
- J. Ren, F.-F. Li, J. Lau, L. Gonzalez-Urbina and S. Licht, One-pot synthesis of carbon nanofibers from CO2, Nano Lett., 2015, 15, 6142–6148 CrossRef CAS PubMed.
- X. Wang, X. Liu, G. Licht, B. Wang and S. Licht, Exploration of alkali cation variation on the synthesis of carbon nanotubes by electrolysis of CO2 in molten carbonates, J. CO2 Util., 2019, 18, 303–312 CrossRef.
- X. Liu, G. Licht, X. Wang and S. Licht, Controlled Growth of Unusual Nanocarbon Allotropes by Molten Electrolysis of CO2, Catalysts, 2022, 12, 137 CrossRef CAS.
- G. Licht, E. Peltier, S. Gee and S. Licht, Facile CO2 diffusion for decarbonization through thermal insulation membranes, DeCarbon, 2024, 5, 100063 CrossRef.
- M. Johnson, J. Ren, M. Lefler, G. Licht, J. V. X. Liu and S. Licht, Carbon nanotube wools made directly from CO2 by molten electrolysis: Value driven pathways to carbon dioxide greenhouse gas mitigation, Mater. Today Energy, 2017, 5, 230–236 CrossRef.
- X. Liu, J. Ren, G. Licht, X. Wang and S. Licht, Carbon nano-onions made directly from CO2 by molten electrolysis for greenhouse gas mitigation, Adv. Sustainable Syst., 2019, 3, 1900056 CrossRef CAS.
- J. Ren and S. Licht, Tracking airborne CO2 mitigation and low cost transformation into valuable carbon nanotubes, Sci. Rep., 2016, 6, 27760 CrossRef CAS PubMed.
- X. Liu, G. Licht and S. Licht, The green synthesis of exceptional braided, helical carbon nanotubes and nanospiral platelets made directly from CO2, Mater. Today Chem., 2021, 22, 100529 CrossRef CAS.
- X. Wang, X. Liu, G. Licht, B. Wang and S. Licht, Exploration of alkali cation variation on the synthesis of carbon nanotubes by electrolysis of CO2 in molten carbonates, J. CO2 Util., 2019, 18, 303–312 CrossRef.
- G. Licht, X. Wang, X. Liu and S. Licht, CO2 Utilization by Electrolytic Splitting to Carbon Nanotubes in Non-Lithiated, Cost-Effective, Molten Carbonate Electrolytes, Adv. Sustainable Syst., 2022, 10084 Search PubMed.
- H. Wu, Z. Li, D. Ji, Y. Liu, L. Li, D. Yuan, Z. Zhang, J. Ren, M. Lefler, B. Wang and S. Licht, One-pot synthesis of nanostructured carbon materials from carbon dioxide via electrolysis in molten carbonate salts, Carbon, 2016, 106, 208–217 CrossRef CAS.
- X. Wang, G. Licht, X. Liu and S. Licht, One pot facile transformation of CO2 to an unusual 3-D nano- fold morphology of carbon, Sci. Rep., 2020, 10, 21518 CrossRef CAS PubMed.
- X. Liu, G. Licht and S. Licht, Controlled Transition Metal Nucleated Growth of Carbon Nanotubes by Molten Electrolysis of CO2, Catalysts, 2022, 12, 137 CrossRef CAS.
- X. Wang, F. Sharif, X. Liu, G. Licht, M. Lefler and S. Licht, Magnetic carbon nanotubes: Carbide nucleated electrochemical growth of ferromagnetic CNTs, J. CO2 Util., 2020, 40, 101218 CrossRef CAS.
- M. Johnson, J. Ren, M. Lefler, G. Licht, J. Vicini and S. Licht, Data on SEM, TEM and Raman spectra of doped, and wool carbon nanotubes made directly from CO2 by molten electrolysis, Data Br., 2017, 14, 592–606 CrossRef CAS PubMed.
- J. Ren, M. Johnson, R. Singhal and S. Licht, Transformation of the greenhouse gas CO2 by molten electrolysis into a wide controlled selection of carbon nanotubes, J. CO2 Util., 2017, 18, 335–344 CrossRef CAS.
- X. Liu, X. Wang, G. Licht and S. Licht, Transformation of the greenhouse gas carbon dioxide to graphene, J. CO2 Util., 2020, 36, 288–294 CrossRef CAS.
- G. Licht, K. Hofstetter, X. Wang and S. Licht, A new electrolyte for molten carbonate decarbonization, Commun. Chem., 2024, 7, 211 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00679h |
| This journal is © The Royal Society of Chemistry 2025 |