 Open Access Article
Open Access ArticleBiosurfactant/surfactant mixing properties at the air–water interface: comparing rhamnolipids and sophorolipids mixed with the anionic surfactant sodium dodecyl benzene sulfonate†
R. K.
Thomas
 a and
J.
Penfold
a and
J.
Penfold
 *ab
*ab
aPhysical and Theoretical Chemistry Laboratory, University of Oxford, Oxford, UK. E-mail: jeff.penfold@stfc.ac.uk
bISIS Facility, Rutherford Appleton Laboratory, STFC, Didcot, UK
First published on 7th April 2025
Abstract
There is an increasing interest in the use of biosurfactants in the development of more biocompatible and biosustainable surfactant-based products. To optimise performance and mitigate production costs, biosurfactants are commonly mixed with different synthetic surfactants. Understanding in detail their mixing properties at interfaces and in solution is key to the development of optimal formulations. Reported here is a detailed thermodynamic analysis, using the latest developments in the pseudo phase approximation, PPA, of the mixing behaviour at the air–water interface of two glycolipid biosurfactants, rhamnolipids, RL, containing the mono and di-rhamnose isomers R1 and R2, and the sophorolipids, SL, containing the lactonic and acidic isomers LS and AS, with the anionic surfactant sodium dodecyl benzene sulfonate, LAS. The analysis uses the previously reported adsorption data, from neutron reflectivity measurements, NR, for the associated binary and ternary mixtures. The different rhamnolipid and sophorolipid biosurfactant structures and their relative surface activities have a profound effect on their mixing properties at the air–water interface with the anionic surfactant LAS, due predominantly to the steric constraints of the different molecular structures. This results in different synergistic excess free energies of mixing and different optimal compositions.
1. Introduction
An extensive variety of different surfactant structures have been developed and synthesised for a wide range of diverse applications, and include a wide range of anionic, cationic, nonionic and zwitterionic structures.1 The nature of their preferential adsorption at different interfaces and their self-assembly in solution are key to their applications in home and personal care products, foods, pharmaceuticals, and a range of industrial processes. Hence a detailed characterisation of their solution structures and adsorption properties is an essential and important area of research.2,3In most cases the important properties and features are optimised and manipulated using surfactant mixtures.3–5 This has resulted in the treatment of the non-ideal mixing in surfactants becoming an extensive area of established research, and in which a number of different approaches have been developed.6–10 In particular it is the pseudo phase approximation, PPA, and the commonly used regular solution approximation, RST, which are the main basis of the thermodynamical treatment of the non-ideality.6,7 The application of the neutron scattering techniques of neutron reflectivity, NR, and small angle neutron scattering, SANS, in combination with deuterium/hydrogen, D/H, isotopic labelling, has transformed our ability to probe in detail the surfactant mixing in micelles and at surfaces; and this has led to further developments and applications of the PPA.11–13
Although there is an extensive range of synthetic surfactants available, there is an increasing interest in using surfactants derived from natural sources, biosurfactants, which are biocompatible and biosustainable. There is a wide range of such potential systems. Their origins and production have been extensively reviewed.14–20 Their applications in a variety of products has been discussed,21–28 and characterisation of their basic adsorption and self-assembly properties presented.29–41 Neutron and X-ray scattering methods have played an important role in the investigation of their interfacial and self-assembly properties,42–52 and adsorption data obtained by such methods are the focus of this paper.
As described above, to optimise performance and tailor specific properties, surfactants are extensively used as mixtures. For the same reasons, to mitigate production costs, and because of the occurrence of different isomers, biosurfactants will also be mostly used as mixtures with other biosurfactants and synthetic surfactants. This has led to an extensive literature relating to the different mixing properties and behaviour of biosurfactant related mixtures.47,48,53–65
However there are few detailed thermodynamic analyses of the mixing properties of multicomponent mixtures involving biosurfactants. The focus of this paper is a detailed thermodynamic analysis, based on surface tension and adsorption data derived from NR, and using the latest developments in the PPA,11–13 of the mixing properties of the binary and ternary mixtures of rhamnolipid/LAS and sophorolipid/LAS mixtures. The thermodynamics of mixing of the rhamnolipid/LAS mixtures was previously reported as part of a study of a quinary mixture containing rhamnolipids and three synthetic surfactants.58 This analysis is now extended here to the sophorolipid/LAS mixture, using adsorption data previously published.47 This has enabled a detailed comparison of the mixing properties of the two different sets of binary and ternary mixtures to be made. This comparison provides a detailed insight into the impact of the different relative surface activities and the different steric constraints due to the different molecular structures on the mixing behaviour of two of the key biosurfactants.
2. Thermodynamics of surfactant mixing
The non-ideal mixing of surfactant aggregates, micelles, and surfactant adsorption at interfaces has been extensively studied.2 The resulting synergistic phenomena are frequently described using the pseudo phase approximation, PPA,6,7 and this approach is briefly summarised here. This involves treating the micelles and the interfacial layers as separate pseudo phases, in which the Gibbs–Dunhem equation applies.11 It originates from Clint's treatment of ideal mixing,66 and was extended to the regular solution approach, RST, for micelles by Rubingh67 and applied to surfaces by Ingram.68 The non-ideality in the RST approach is described by a single non-ideality interaction parameter B, and is assumed to be symmetrical with composition. In this model the excess free energy of mixing for a binary mixture is then,| ΔGe = BRTx1x2 | (1) |
In the PPA the chemical potential of the components of the pseudo phase, micelles, interface and monomer, are assumed to be equal at equilibrium. So, for example, equating the chemical potential of the micelle and monomer gives rise to,
 | (2) |
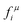 is the activity coefficient in the micelle and
is the activity coefficient in the micelle and  its critical micelle concentration, cmc.
its critical micelle concentration, cmc.
For a binary mixture, and assuming the micelle mole fraction equals unity, and at the cmc 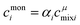 , where αi is the mole fraction of monomer in solution and
, where αi is the mole fraction of monomer in solution and  is the mixed cmc; and derived from the Butler equation,69 the following relationship is obtained,
is the mixed cmc; and derived from the Butler equation,69 the following relationship is obtained,
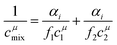 | (3) |
 | (4) |
In the regular solution approximation eqn (1) to (4) combine to produce,
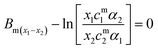 | (5) |
Eqn (5) can be solved iteratively to provide a value for the interaction parameter Bm, the micelle composition and monomer composition. In some cases the micelle composition can be determined by SANS,70 and this provides an important additional constraint to the solutions of eqn (5).
A version of eqn (4) can be applied to the surface, and cmi is replaced by  , where
, where  is the concentration at which the surface pressure π is obtained. From surface tension measurements at a fixed π but different compositions the surface interaction Bs, which can be different to Bm, and the surface composition can be obtained. The surface composition can be measured directly above and below the cmc by NR2 and this provides an important additional constraint in determining the surface interaction parameter.
is the concentration at which the surface pressure π is obtained. From surface tension measurements at a fixed π but different compositions the surface interaction Bs, which can be different to Bm, and the surface composition can be obtained. The surface composition can be measured directly above and below the cmc by NR2 and this provides an important additional constraint in determining the surface interaction parameter.
This approach results in a set of equations that can be solved iteratively to provide the micelle and surface interaction parameters, and the variation in the micelle, surface and monomer compositions with solution composition and concentration. This was initially extensively exploited on the basis of surface tension data.4–10 More recently the additional information and constraints of the micelle and surface compositions determined by SANS and NR make a more comprehensive and detailed evaluation of binary surfactant mixing possible.11–13 Using the approach of Redlich and Kister71 this has been extended to ternary and quinary mixtures.58,72
These studies, and in particular the NR data, have shown that the RST symmetrical approach to describe non-ideality is frequently inadequate and that the mixing is intrinsically asymmetrical. The asymmetry results from the competing impact of the electrostatic and steric interactions. This can be accounted for by an expansion of the excess free energy of mixing (eqn (1)), by including higher order terms in a way consistent with the Gibbs–Duhem relationship, to give for a binary mixture,
| ΔGe = x1x2B + x1x2(x1 − x2)C + x1x2(x1 − x2)2D | (6) |
In the RST approach only B is non-zero and the excess free energy of mixing is symmetrical with composition. Introducing a non-zero C parameter allows for the minimum in the excess free energy of mixing to be asymmetrical, not at a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 composition. For example, in a nonionic/ionic mixture at the surface with hexagonal symmetry, this minimum should occur at a 2
50 composition. For example, in a nonionic/ionic mixture at the surface with hexagonal symmetry, this minimum should occur at a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 composition ratio. A non-zero quartic term, D, provides some refinement to the shape of the excess free energy curve. In the analysis presented here the quartic term is not required.
1 composition ratio. A non-zero quartic term, D, provides some refinement to the shape of the excess free energy curve. In the analysis presented here the quartic term is not required.
This approach has been recently applied to the mixing properties of the rhamnolipids R1 and R2 and LAS mixtures.58 Here the same approach is applied to the sophorolipid AS and LS and LAS mixtures. Although the mixing properties of the sophorolipid/LAS mixtures, as determined by NR, have been previously reported,47,48 a detailed thermodynamic analysis has not been previously made. Here the contrasting behaviour of the two sets of binary and ternary mixtures are compared and discussed in the context of the different molecular structures and surface activities.
3. Analysis of mixing properties of RL/LAS and SL/LAS mixtures
3.1 RL/LAS
In this section the adsorption and self-assembly data presented and the discussion of that data are taken from ref. 42 and 43, and the detailed thermodynamic analysis of the mixing is from ref. 58.The predominant molecular structures of the rhamnolipids, R1 and R2, and LAS are shown in Fig. 1.
The nature of the adsorption of the R1 and R2 rhamnolipids, their mixtures, and their mixtures with LAS at the air–water interface were previously determined using NR.42,43 The mixtures were measured at a fixed solution concentration of 1 mM, above the mixed cmc, and in buffer (0.023 M borax and 0.008 M HCl) at pH 9. The key parameters which indicate their relative surface activities are summarised in Table 1.
| Surfactant | Area/molecule at saturation (±2 Å2) | cmc (±0.02 mM) | Surface tension at cmc (±0.05 mN m−1) |
|---|---|---|---|
| R1 | 60 | 0.36 | 31 |
| R2 | 88 | 0.18 | 37 |
| LAS | 48 | 0.34 | 28 |
The key features from the studies of Chen et al.42,43 are summarised in the following paragraphs.
The adsorption isotherms for R1 and R2, and the insensitivity in the adsorption with pH, are consistent with both surfactants being relatively weakly anionic, and can be considered as nonionic.42 R1 is more surface active than R2. In the R1/R2 mixtures R1 dominates the adsorption, predominantly because of the greater steric and packing constraints associated with the bulkier R2 headgroup and its greater relative hydrophobicity.42 In the mixing of LAS with R1 and R2, LAS adsorbs more strongly than R1 and R2, due to the greater surface activity of LAS.43 In the ternary mixtures the LAS and R1 adsorption dominate. The total adsorption is significantly enhanced due to the synergistic interactions,43 and goes through a pronounced maximum with composition.
As part of a more extensive study at the air–water interface at the air–water interface of a quinary surfactant mixture comprising of the R1 and R2 rhamnolipids, LAS, diethylene glycol monododecyl ether sodium sulfate, SLES, and octaethylene glycol monododecyl ether, C12E8, Liley et al.58 made a detailed thermodynamical analysis based on the PPA approach outlined in the previous section, using the adsorption and surface tension data presented in ref. 42, 43, and 58. The key thermodynamic parameters from that analysis are summarised in Table 2.
| Surfactant mixture | MIcelles | Surface | ||||||
|---|---|---|---|---|---|---|---|---|
| B m ±0.4 | C m ±0.4 | ΔGemin (kT) | x 1 | B s ±0.3 | C s ±0.3 | ΔGemin (kT) | x 1 | |
| LAS/R1 | −0.3 | 0.0 | −0.08 | 0.5 | −1.2 | 0.2 | −0.3 | 0.46 |
| LAS/R2 | 0.0 | 0.0 | — | — | −0.8 | −0.8 | −0.24 | 0.67 |
| R1/R2 | −0.5 | 0.0 | −0.13 | 0.5 | −1.7 | −1.4 | −0.48 | 0.65 |
The variations in the adsorbed mole fractions with composition, and the associated model fits (using the parameters in Table 2) for the binary mixtures are shown in Fig. 2.
 | ||
| Fig. 2 Variation in adsorbed mole fraction with bulk mole fraction for the binary mixtures (a) LAS-R1, (b) LAS-R2, and (c) R1-R2, using data from ref. 58. Solid line is calculated using the parameters in Table 2, and the dashed line is for ideal mixing. | ||
The variations in surface composition are well described by the thermodynamic model using the quadratic and cubic terms in eqn (6). In the adsorption data the variation from that expected for ideal mixing shows that the surface mixing is non-ideal. The values of the interaction parameters in Table 2 indicate that the non-ideal mixing is asymmetrical and synergistic (attractive). The R1/R2 interaction is the strongest and the LAS/R1 and LAS/R2 interactions are comparable. For ionic/nonionic mixtures the optimal composition (corresponding to the minimum in the excess free energy of mixing) to minimise interactions is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 nonionic/ionic, and although the rhamnolipids are only weakly ionic this might still be expected here. For the R1/R2 and LAS/R2 mixtures the optimal composition is close to 0.67 (mole fraction LAS), and reflects the greater steric hindrance associated with the R2 headgroup structure. For LAS/R1 the optimal composition is 0.45 (mole fraction LAS), due to the balance between the steric and electrostatic contributions to the interaction.
1 nonionic/ionic, and although the rhamnolipids are only weakly ionic this might still be expected here. For the R1/R2 and LAS/R2 mixtures the optimal composition is close to 0.67 (mole fraction LAS), and reflects the greater steric hindrance associated with the R2 headgroup structure. For LAS/R1 the optimal composition is 0.45 (mole fraction LAS), due to the balance between the steric and electrostatic contributions to the interaction.
The binary mixing parameters describe well the ternary mixing,58 without any further adjustment, and confirm the pair-wise nature of the interactions. This is shown by the variations in the ternary mixture adsorption and the comparison with the model calculations using the parameters in Table 2 in Fig. 3.
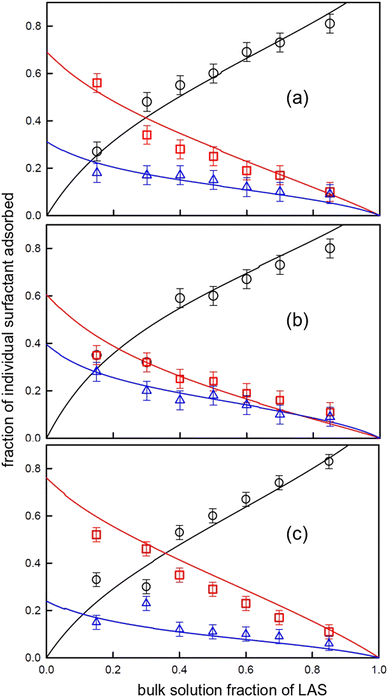 | ||
Fig. 3 Variation in adsorbed mole fraction with bulk mole fraction of LAS, for each of the ternary mixtures (a) LAS/2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 R1/R2, (b) LAS/1 1 R1/R2, (b) LAS/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 R1/R2, and (c) LAS/1 1 R1/R2, and (c) LAS/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 R1/R2, using data from ref. 58. The data points are (black) LAS, (red) R1, and (blue) R2, and the solid lines are calculated curves using the parameters in Table 2. 2 R1/R2, using data from ref. 58. The data points are (black) LAS, (red) R1, and (blue) R2, and the solid lines are calculated curves using the parameters in Table 2. | ||
In the ternary mixtures the strongest R1/R2 interaction dominates, and this results in a slight reduction in the LAS adsorption. This is a phenomena which is consistent with other recent studies58,72,73 on ternary mixtures, which showed that if one of the binary interactions is much stronger their composition at the surface is enhanced compared to the third component. However due to their greater surface activities R1 and LAS compete most effectively for the surface, and the R2 adsorption is slightly reduced. This gives to the optimal packing that results in the adsorption maximum described earlier.
In contrast, as illustrated in Table 2, from the cmc variation, the micelle mixing is ideal or relatively close to ideal, and hence the optimal composition is close to equimolar. This implies that the packing constraints in the micelles are more relaxed than at the surface. Chen et al.42,43 and Liley et al.59 studied the solution self-assembly of R1, R2, and LAS, and their binary and ternary mixtures using SANS. In solution, at relatively low surfactant concentrations, the rhamnolipids R1 and R2 are initially micellar, and R1 becomes lamellar at higher concentrations. LAS is micellar at low concentrations and lamellar at higher concentrations. In their binary mixtures LAS/R2 is primarily micellar and in the R1/R2 and LAS/R1 mixtures the lamellar phase dominates. In the ternary mixtures this results in a more complex evolution in structures from micellar to lamellar. At even higher concentrations Motta et al.52 have interpreted small angle X-ray scattering data in terms of a wider range of elongated and planar aggregates. However at the concentration close to that used for the adsorption measurements the solutions are micellar, and the changes in self-assembly have no significant impact upon the surface thermodynamical parameters in Table 2.
3.2 SL/LAS
In this section the adsorption and self-assembly data and the initial discussion of that data is taken from ref. 47 and 48. However the detailed thermodynamic analysis of that data and subsequent discussion of that data are newly presented here.The predominant molecular structures of the sophorolipids, AS and SL, are shown in Fig. 1.
The adsorption of the acidic and lactonic sophorolipids, their mixtures and their binary and ternary mixtures with LAS, at the air–water interface was previously investigated using NR by Chen et al.47 The measurements were made at a fixed concentration of 1 mM at the air–water interface. The mixed surfactant solutions were mostly at concentrations above the cmc, except for the solutions rich in LAS, with a solution mole fraction of LAS > 0.9. As discussed later, for the LAS and AS rich compositions the relative proximity to the mixed cmc will have some consequences. This is in contrast to the rhamnolipid/LAS mixtures which were all at concentrations much greater than the mixed cmc, and results from the latter being measured in buffer. In a complementary study Chen et al.48 also characterised the self-assembly of the LAS/sophorolipid mixtures at low surfactant concentrations using SANS. Their respective key surface activity parameters are summarised in Table 3.
| Surfactant | Area/molecule at saturation (±2 Å2) | cmc (±0.02 mM) | Surface tension at cmc (±0.5 mN m−1) |
|---|---|---|---|
| Acidic SL, AS | 86 | 0.67 | 39 |
| Lactonic SL, LS | 73 | 0.06 | 36 |
| LAS | 54 | 1.60 | 34 |
The LS sophorolipid was in the form of the diacetyl version, whereas the AS sophorolipid was in the diacetyl form for the NR measurements, but in the non-acetyl form for the ST measurements, Chen et al.47 showed that the acetylisation had a minimal impact upon the cmc and surface coverage.
The main observations from the study by Chen et al.47 are summarised as follows. The more hydrophobic LS has the lowest cmc and is more surface active than the AS sophorolipid. The AS sophorolipid is more ionic than the LS, and this in part contributes to the larger area/molecule at saturation for the AS sophorolipid. However the different structures would imply greater packing constraints for the LS, but this does not impact greatly upon its saturation adsorption.
In the binary mixtures, involving LS, LAS/LS and LS/AS, there is a pronounced partitioning at the surface in favour of the LS component. Whereas the LAS/AS mixture has a surface composition close to the solution composition over the concentration and composition range studied. In the ternary mixtures, LAS/AS/LS, the surface composition is dominated by the LS component. For the solutions richer in the AS component, the surface composition implies that the presence of the AS component eases the packing constraints.
Chen et al.47 measured the surface tension variations for the AS and LS sophorolipids, the LS/AS mixtures and the LAS/AS and LAS/LS mixtures, The variation in the cmc for the three mixtures are shown in Fig. 4, and the associated thermodynamic parameters, based on the analysis of that data alone, are summarised in Table 4.
 | ||
| Fig. 4 Variation in cmc with solution composition for (a) LAS/AS, (b) LAS/LS and (c) LS/AS mixtures. The solid lines are model calculations using the PPA approach outlined earlier and for (black line) the parameters in Table 4, (red dotted line) parameters in Table 6, and (dashed line) ideal mixing. | ||
| Surfactant mixture | B m (±0.4) | C m (±0.4) | ΔGemin (kT) | x 1 |
|---|---|---|---|---|
| LAS/AS | −1.3 | 0.3 | −0.33 | 0.45 |
| LAS/LS | −1.4 | −0.2 | −0.33 | 0.45 |
| LS/AS | 0.6 | 0.5 | 0.17 | 0.65 |
The large difference in the LAS and SL cmc values dominates the cmc variation with composition, and this results in a greater uncertainty in the evaluation of the thermodynamic parameters.
From the analysis of the cmc data only the micelle mixing parameters are broadly similar to those reported earlier (see Table 3) for the rhamnolipid/LAS mixtures, in that they are relatively weak. The LS/AS micelle mixing is slightly non-ideal and antagonistic (repulsive), with an optimal composition dominated by the LS component. The LAS/AS and the LAS/LS micelle mixing is synergistic (attractive). The optimal composition is close to equimolar, due to the balance between the steric constraints and the electrostatic contributions to the interaction. In the subsequent analysis of the surface data, which provides a more accurate description of the mixing properties, the cmc derived parameters require some adjustment, and this will be discussed in more detail in the subsequent discussion later in the paper.
The different sophorolipid structures result in a rather different pattern of self-assembly compared to the rhamnolipids, as illustrated by Chen et al.48 The AS sophorolipid is micellar at the relatively low surfactant concentrations studied, and this contrasts with the LAS self-assembly which evolves from micellar to lamellar. However the rather different LS molecular structure results in the formation of nano-vesicles which evolve into larger vesicles and a sponge phase at higher concentration. In the binary mixtures involving the AS sophorolipid, LAS/AS and AS/LS, the micellar structure dominates. In the LAS/LS binary mixtures and in the ternary mixtures more planar structures dominate, with an interplay between lamellar, vesicular and nano-vesicle formation, depending upon the solution composition and concentration.
Following the initial analysis of the micelle mixing a detailed thermodynamical analysis of the adsorption data derived from NR and surface tension data for the AS, LS and LAS binary and ternary mixtures, following the procedures outlined earlier and described in more detail elsewhere,58,72,74 was made. The key thermodynamic parameters from the analysis are summarised in Table 5.
| Surfactant mixture | B s(±0.3) | C s (±0.3) | ΔGemin | x 1 |
|---|---|---|---|---|
| LS/AS | −2.1 | 0.0 | −0.53 | 0.50 |
| LAS/AS | −1.9 | −0.9 | −0.50 | 0.55 |
| LAS/LS | −1.8 | 0.4 | −0.45 | 0.45 |
The corresponding variations in the surface composition with solution composition for the three binary mixtures are shown in Fig. 5.
 | ||
| Fig. 5 Variation in adsorbed mole fraction with bulk mole fraction for the binary mixtures, (a) LAS/AS, (b) LAS/LS, and (c) LS/AS. The solid lines are calculated using the parameters in Tables 5 and 6. The dashed lines are curves corresponding to ideal mixing. | ||
The variation in the surface mole fraction with solution mole fraction for the three binary mixtures at the air–water interface is well described by the PPA thermodynamic model described earlier, and in general requires the quadratic, and cubic terms in eqn (6). The surface mixing is non-ideal and asymmetrical with solution composition. The mixtures all have synergistic (attractive) interactions. The interactions between each pair of components is broadly similar, and the optimal composition is approximately equimolar. This implies that the reduction in the electrostatic interaction and the steric constraints are broadly similar for all three mixtures.
The evaluation of the micelle mixing, based on the cmc variation, is dominated by the relatively large difference between the cmc of LAS and the sophorolipids, see Table 3. This results in a relative insensitivity of the cmc data to the mixing parameters, and result in the best fits shown in Fig. 4 and summarised in Table 4. This was not the case for the equivalent variations for the LAS/rhamnolipid mixtures. Furthermore cmc measurements were, as stated earlier, made using the non-acetylated AS, whereas the NR measurements used the diacetylated versions. Although the cmc's and surface coverage were similar it may well have some impact upon the interactions. These factors result in the requirement to adjust the micelle mixing parameters to best fit the surface data, which then provide a much more accurate description of the mixing properties. This was done by refining the model fits to the cmc and adsorption data simultaneously. The result of this adjustment is summarised in Table 6 below, and the calculated variation in the cmc for the three binary mixtures is also shown in Fig. 4.
| Mixture | B m (±0.4) | C m (±0.4) | ΔGe | x 1 |
|---|---|---|---|---|
| LS/AS | −2.3 | 0.0 | −0.58 | 0.50 |
| LAS/AS | −0.9 | −1.0 | −0.26 | 0.45 |
| LAS/LS | −0.8 | 0.4 | −0.21 | 0.40 |
Fig. 4 shows the relative insensitivity of the cmc variation with composition to the different thermodynamic parameters summarised in Tables 4 and 6, and illustrates the importance in this case of refining the model parameters using the cmc and surface composition data.
Comparing Table 6 with Table 4 shows that there is a relatively small adjustment required for the LAS/AS and LAS/LS mixing, in which the interaction is relatively strong. For the LS/AS mixing the change is more significant, and now the micelle mixing is much more strongly non-ideal and synergistic.
The micelle mixing for the LAS/LS and LAS/AS mixtures is less non-ideal than for the surface. However the micelle and surface mixing for the LS/AS mixture is similar, and imply similar packing constraints in the micelles and at the surface.
The variations in the ternary, LAS/AS/LS, mixture adsorption are shown in Fig. 6.
 | ||
Fig. 6 Variation in adsorbed mole fraction with bulk mole fraction of LAS for (a) LAS/2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 LS/AS, (b) LAS/1 1 LS/AS, (b) LAS/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 LS/AS, (c) LAS 1 1 LS/AS, (c) LAS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 LS/AS, using data from ref. 46. The data points are (black) LAS, (red) LS, and (blue) AS, and the solid lines are model calculations, as described in the text using the parameters in Tables 4 and 5. 2 LS/AS, using data from ref. 46. The data points are (black) LAS, (red) LS, and (blue) AS, and the solid lines are model calculations, as described in the text using the parameters in Tables 4 and 5. | ||
The greater sensitivity of the surface mixing data requires that the thermodynamic parameters are refined for the cmc variations, and the binary and ternary surface mixture adsorption simultaneously. This was not the case for the LAS/rhamnolipid mixtures, where the micelle and surface mixing was analysed separately, and the ternary mixture behaviour could be predicted based only on the binary properties. However the model parameters, summarised in Tables 5 and 6, do describe well the variations in the binary and ternary surface mixing, as seen in Fig. 6. The deviations are greatest for the LAS and AS rich solutions, where the closer proximity to the cmc has an impact on the variation in the monomer and micelle composition and concentration which drive the adsorption.
Although the three binary interaction parameters are broadly similar, the adsorption in the ternary mixtures is largely dominated by the LS component, due to its greater surface activity. The LAS competes least favourably for the surface, apart from in the AS richer solutions. The packing constraints are such that unlike the rhamnolipid/LAS mixtures, the LAS/sophorolipid mixtures do not result in as enhancement in the total adsorption.
4. Discussion
The micelle mixing for both the rhamnolipid/LAS and sophorolipid/LAS mixtures is closer to ideal mixing than the equivalent surface mixing. For the rhamnolipid/LAS mixtures the micelle mixing is closer to ideal than the sophorolipid/LAS mixtures. The presence of buffer for the rhamnolipid/LAS mixtures will further reduce the already weakly ionic nature of the rhamnolipids and hence the electrostatic contribution to the interaction, and the slight departure from ideality further implies that the steric constraints are minimal. The sophorolipid/LAS mixtures had no additional electrolyte to screen the electrostatic interactions, but the weakly ionic sophorolipids will reduce the impact of the charge on the LAS molecules. Furthermore the LAS appears to ease the packing constraints. However the greatest impact on the packing constraints is seen in the LS/AS mixtures.In the LAS/rhamnolipid surface mixing the R1/R2 mixture has the strongest departure from ideal mixing at the surface, whereas that for the LAS/R1 and LAS/R2 mixtures are broadly comparable and weaker. This suggests that the LAS/R1 and LAS/R2 interactions are a balance between the electrostatic and steric contributions, whereas for the R1/R2 mixture the steric constraints dominate. For the sophorolipid/LAS mixtures the departure from ideal mixing is equally strong for all three of the binary mixtures. This implies that the electrostatic contribution is minimal and that the steric constraints dominate and reflect the greater packing constraints associated with both sophorolipid components. All the three interactions involving the SL's are as strong as the R1/R2 interaction. In the rhamnolipid/LAS mixtures the dominance of the R1/R2 interaction and the greater surface activities of R1 and LAS results in R1 and LAS dominating the adsorption trends in the ternary mixtures. In the sophorolipid/LAS mixtures all the binary surface mixing interactions are very similar, and the greater surface activity of the LS component dominates the ternary adsorption trends. Comparing the rhamnolipid/LAS and the sophorolipid/LAS ternary mixtures, the different relative strengths of interaction result in different patterns of adsorption, and this suggest that a different composition is required to optimise the adsorption for the required application.
The micelle mixing shows a different pattern of behaviour for both the rhamnolipid/LAS and the sophorolipid/LAS mixtures. For the rhamnolipid/LAS mixtures the departure from ideal mixing is much reduced compared to the surface mixing, and implies that the packing constraints are much less severe for the micelles. Indeed the evolution in the self-assembly structures is a balance between the preferred curvature of LAS and R1 tending towards planar structures and of R2 towards more globular micellar structures. As such there is an evolution from globular to lamellar structures depending upon surfactant concentration and composition.42,43,59 For the sophorolipid/LAS mixtures the micelle mixing is closer to ideal for the LAS/LS and LAS/AS mixtures than for the surface, and implies that the LAS reduces the packing constraints in the micelles compared to the surface. However for the LS/AS mixture the departure from ideality is similarly strong in the micelles and at the surface, and implies similarly strong packing constraints. This is also manifest in the solution self-assembly, where the LS sophorolipid favours a range of vesicle-like structures and the AS sophorolipid micellar structures; whereas in the LS/AS mixtures micellar structures dominate. This more complex evolution reflects the greater degree of packing constraints associated with the sophorolipid molecular structures.
At the interface for the rhamnolipid/LAS mixtures the broadly similar dialkyl chain structures for R1 and R2 and LAS imply relatively favourable packing. The disparity in the R2 headgroup size compared to R1 and LAS is responsible for the greater packing constraints associated with mixtures involving R2. This also implies the greatest impact on the R1/R2 mixing. In buffer and as a result of the weakly ionic nature of the rhamnolipids, the electrostatic contributions are minimal. For the sophorolipid/LAS mixtures, although the LS and AS molecular structures are different, they impose similar packing constraints on all three binary mixtures. Although the sophorolipid/LAS measurements were not in buffer, the weakly ionic nature of the sophorolipids at neutral pH means that electrostatic contribution may be slightly higher than for the rhamnolipid/LAS mixtures, but will not be significant. The different pattern of interactions for the sophorolipid/LAS and rhamnolipid/LAS mixtures reflect a different balance between these relative contributions. Without detailed measurements at different levels of added electrolyte and pH it is difficult to further separate those relative contributions.71
From a series of NR measurements in which both the contrast of the surfactants and solvent are varied it is possible to determine the structure of the surface layer in terms of of the distribution of the different components perpendicular to the surface.3 Hence the surfactant and solvent distributions normal to the surface and their overlaps obtained can provide some insights into the packing and the changes in packing on mixing. This was done by Chen et al.42,47 for the rhamnolipid/LAS and sophorolipid/LAS mixtures for a limited range of combinations.
Chen et al.42 determined the structure of R1, R2 and the R1/R2 mixtures. The significant feature is the width of the solvent distributions. The solvent distribution is narrower for R2 compared to R1, and is broadest for the R1/R2 mixture. Furthermore the position of the surfactant distribution relative to the solvent distribution is unaltered for R1, R2 and the R1/R2 mixture. As the width of the solvent distribution is predominantly associated with the headgroup, this implies that the larger R2 headgroup has a different conformation to R1 resulting in a less efficient lateral packing and hence the larger area/molecule. In the mixture the broader distribution shows how the optimal packing results in some disorder or a staggered conformation of the two headgroup, but does not significantly affect the alkyl chain distributions. These observations are consistent with the main contributions to the interactions on mixing being due to changes in the headgroup packing and hydration.
Similar structural measurements were made for the sophorolipids, and specifically for the LS and AS sophorolipids and for the LS/AS and LS/LAS mixtures by Chen et al.46 The structural measurements give significantly different surfactant distributions widths, and this implies different surface conformations for the AS and LS components, in which it is narrower for the LS than for the AS component. Furthermore the solvent distributions are narrower compared to the RL's and is the most narrow for the more hydrophobic LS component. This is consistent with the greater hydrophobicity for both components and especially for the LS component. The position of the surfactant distribution relative to the solvent distribution is similar for the AS and LS, and this implies that AS and LS occupy similar positions at the interface. The mixed structure was measured for LS/AS and LS/LAS mixtures. The LS component dominates the structure with a narrower solvent distribution, and the greater hydrophobicity of the LS reduces the hydration of the mixed layer. These distributions and the more rigid surfactant structure arising from the double bond and in the case of the LS component the lactone ring, imposes more rigid packing constraints when mixed with other components.
Studies on a number of biosurfactant/surfactant and biosurfactant mixtures have also been reported in the recent literature, in addition to those reported by the authors in studies directly related to and preceding this paper.42,43,47,48,58,59,72,75 These broader related studies involve mixtures closely but not directly related to the ternary mixtures reported here, and for completeness they are summarised briefly here. They do not in general provide a comparable thermodynamic analysis, and mostly use commercial surfactants of uncertain composition and purity;26,28,40,53,56,60–64 but nevertheless provide some supporting complementary insights. Esposito et al.62 reported non-ideal antagonistic interactions in rhamnolipid/SLES (sodium lauryl ether sulfate), based on surface tension and dynamic light scattering, DLS, evaluation of mixed micelle formation. Manko et al.,64 in contrast, reported synergistic adsorption and micellization in RL/SDS (sodium dodecyl sulfate) mixtures, from surface tension and viscosity measurements, but with no quantification of the magnitude of the synergy. Lui et al.28 and Rekiel et al.57 reported synergistic interactions in the adsorption and micellization of impure RL mixtures with the nonionic surfactants Triton X-100 and Triton X-165 respectively. The thermodynamic analysis, based on RST, showed an interaction parameter which varied with solution composition, consistent with some asymmetry in the interactions. Xu et al.60 and Zhou et al.63 used predominantly rheological measurements to identify synergistic interactions in RL/SLES/CAPB (cocamidopropyl betaine) and RL/SL/CAPB. However no quantification of the extent of the departure from ideal mixing was provided, and both the RL and SL biosurfactants were impure commercial mixtures. Song et al.56 studied R2/lactonic SL mixtures and reported synergistic interactions from ST and DLS/cryoTEM data. The extent of the interaction was shown to vary with composition, using the RST approach, again implying asymmetry in the interactions. They also report micelle growth, from micelles to larger aggregates resulting from the synergy, but with aggregation numbers at variance with other reported values in the literature. Ngygen et al.26 studied the interfacial tension variation in a commercial mixtures of RL's without further purification with an alkyl propoxyl ethoxy sulfate surfactant. They reported that the development of ultra low interfacial tension was enhanced on mixing, and on its relationship to environmental remediation. Ikizler et al.53 studied the adsorption and self-assembly of R1/R2 mixtures by ST, DLS, Turbidity and SEM. No quantification of the mixing was reported, and the structural evolution reported is at variance with the current literature. Imura et al.40 reported synergistic mixing in the Langmuir monolayers of the LS and AS sophorolipids, and again the extent of the synergism was not quantified. Onaizi et al.61 reported non-ideal mixing, based on ST data, in surfactin/LAS mixtures. They reported attractive interfacial and repulsive solution interactions.
Although not directly comparable to the studies reported in this paper, the studies summarised in the previous paragraph all report some degree of non-ideal mixing and imply some asymmetry in the mixing. They report variations that depend upon the surfactant structures, and differences in the mixing at the surface and in self-assembly in solution. However they do highlight the need for systematic studies with well defined components and a detailed thermodynamic analysis which goes beyond the symmetrical RST approach. This has been the approach for the results presented here for the rhamnolipid/surfactant and sophorolipid/surfactant mixtures and the comparison between the two systems, and this has provided an insight into the key factors affecting the mixing behaviour.
5. Conclusions
In their widespread use in a range of applications of surfactants, mixtures are invariably used to optimise particular features and properties.3–5,22–29 This is especially relevant for applications involving biosurfactants, where their mixture with different synthetic surfactants will be important.53–65 Characterising and understanding those mixing properties in self-assembly and at interfaces is hence of central importance.3–9,11–13 Here a detailed thermodynamical analysis has been presented, using the latest developments in the applications of the pseudo phase approximation,11–13 of the surface mixing behaviour of rhamnolipid/LAS and sophorolipid/LAS binary and ternary mixtures.The departure from ideal mixing in both mixtures is synergistic and asymmetrical with composition. The non-ideality in both mixtures results primarily from the relief on mixing of the packing constraints due to the different molecular structures of the different component surfactants. The asymmetry arises from the balance between the packing constraints and electrostatic interactions. That balance is different for the two different mixtures, and results in different optimal compositions corresponding to the minimum in the excess free energy of mixing.
In the rhamnolipid/LAS mixtures this is primarily due to the packing constraints associated with the larger R2 headgroup. In the sophorolipid/LAS mixtures the surface mixing is more strongly synergistic due to the greater steric constraints associated with the structure of the LS and AS sophorolipids. These differences result in different synergistic excess free energies of mixing and different optimal compositions.
This detailed approach provides important insights into how to optimise the mixing properties of complex multi-component mixtures involving biosurfactants and to their potential applications. The more optimal packing for the rhamnolipid/LAS mixtures results in a maximum in the adsorption with composition, and is a key factor in the potential applications in areas such as detergency and remediation. The impact of the greater packing constraints associated with the sophorolipid on the adsorption and self-assembly/LAS mixtures point to a greater opportunity to manipulate and optimise these properties with different mixed systems. The results also suggests applications where the solution structures are more important and maximising the adsorption less important, such as pharmaceuticals and cosmetics.
Author contributions
JP and RKT have both contributed to the analysis and interpretation of previously published data, and to the writing and editing of the manuscript.Data availability
The data used in this paper have been previously published in ref. 41, 42, 46 and 56, and in their associated ESI.†Conflicts of interest
There are no competing interests to declare.Acknowledgements
There are no associated funding declarations in addition to those already stated in ref. 42, 43, 47, 48, 72 and 73. The continued support from the Department of Chemistry at the University of Oxford and STFC's ISIS facility is acknowledged. The previous contributions of the co-authors in ref. 42, 43, 47, 72, and 74 in prior publication of the data used in this study are acknowledged.References
- K. L. Mittal, Solution Chemistry of Surfactants, Plenum Press, New York, 1979 Search PubMed.
- R. Nagarajan and E. Ruckenstein, Theory of surfactant self-assembly; a predictive molecular thermodynamic approach, J. Phys. Chem., 1991, 7, 2969 Search PubMed.
- J. R. Lu, R. K. Thomas and J. Penfold, Surfactant layers at the air-water interface: structure and composition, Adv. Colloid Interface Sci., 2000, 84, 143–304 CrossRef CAS.
- M. Abe and J. F. Scamehorn, Mixed Surfactant Systems, Surfactant Science Series, Marcel Dekker, New York, vol. 124, 2005 Search PubMed.
- B. P. Grady, Surfactant Mixtures: a short review, J. Surfact. Deter., 2023, 26, 250 Search PubMed.
- P. M. Holland and D. N. Rubnigh, Non-ideal mixed micelle model, J. Phys. Chem., 1984, 87, 1990 Search PubMed.
- P. M. Holland, Non-ideal mixed micelle solutions, Adv. Colloid Interface Sci., 1986, 26, 129 CrossRef.
- R. E. Kamrath and E. I. Franses, Mass action model of mixed micellisation, J. Phys. Chem., 1984, 88, 1048 CrossRef.
- R. Nagarajan, Micellisation, mixed micellization and solubilisation: the role of interfacial interactions, Adv. Colloid Interface Sci., 1986, 26, 264 CrossRef.
- J. Zawala, A. Wiertel-Pochopien and P. B. Kowalcruk, Critical synergistic concentration of binary surfactant mixtures, Minerals, 2020, 192, 1–9 Search PubMed.
- J. Penfold and R. K. Thomas, Recent development and application of the thermodynamics of surfactant mixing, Mol. Phys., 2019, 117, 3376–3386 CrossRef CAS.
- J. Penfold and R. K. Thomas, Neutron reflection and the thermodynamics of the air-water interface, Phys. Chem. Chem. Phys., 2022, 24, 8553 RSC.
- J. Penfold and R. K. Thomas, The adsorption and self-assembly of surfactant mixtures: how the detailed evaluation of the adsorption properties provides access to bulk behaviour, Adv. Colloid Interface Sci., 2022, 319, 102984 Search PubMed.
- J. D. Desai and I. M. Banat, Microbial production of surfactants and their commercial potential, Microbiol. Mol. Biol. Rev., 1997, 61, 47–64 CAS.
- I. M. Banat, R. S. Mukkar and S. S. Cameotra, Potential commercial applications of microbial surfactants, Appl. Microbiol. Biotechnol., 2000, 53, 495–509 CrossRef CAS.
- E. Z. Ron and E. Rosenberg, Natural role of biosurfactants, Environ. Microbiol., 2001, 31, 229–239 CrossRef.
- N. Nitschke, S. G. V. A. Costa and J. Contiero, Rhamnolipid surfactants, an update on general aspects, commercial production and properties, Biotechnol. Prog., 2003, 21, 1593–1600 CrossRef.
- K. Mutkesamy, S. Gopalkrishnan, T. Kochupappy, R. Sivachidambaram and P. Sivachidambaram, Biosurfactant properties, commercial production and applications, Curr. Opin. Colloid Interface Sci., 2008, 94, 736–747 Search PubMed.
- C. E. Drakonis and S. Amin, Biosurfactants, formulation properties and applications, Curr. Opin. Colloid Interface Sci., 2020, 48, 77–90 CrossRef.
- R. Jahan, A. M. Bodratti, M. Tsianou and P. L. Alexandridis, Biosurfactants, natural alternatives to synthetic surfactants; physicochemical properties and applications, Adv. Colloid Interface Sci., 2020, 275, 102061 CrossRef CAS PubMed.
- E. Guzman, F. Ortega and R. G. Rubio, Exploring the world of rhamnolipids: a critical review of their production, interfacial properties and potential applications, Curr. Opin. Colloid Interface Sci., 2024, 69, 101780 Search PubMed.
- A. Kashif, R. Rehman, A. Fuwad, M. K. Shahid, H. M. Dayarathne, A. Jamal, M. N. Aftab, B. Mainali and Y. Choi, Current advances in the classification, production, properties and applications of microbial biosurfactants, Adv. Colloid Interface Sci., 2022, 306, 102718 CrossRef CAS.
- S. Pal, N. Chatterjee, A. K. Das, D. J. McClemens and P. Dhar, Sophorolipids: a comprehensive review of properties and applications, Adv. Colloid Interface Sci., 2023, 313, 102856 CrossRef CAS.
- K. K. S. Randhawa and P. K. S. M. Raham, Rhamnolipid biosurfactant: past, present and future scenario of the global market, Front. Microbiol., 2014, 5, 107 Search PubMed.
- I. N. A. Bogaert, K. Saerens, C. De Muynck, D. Develter, W. Soetaert and E. J. Van Damme, Microbiological production and application of sophorolipids, Appl. Microbiol. Biotechnol., 2007, 76, 23–34 CrossRef.
- T. T. Nguyen, W. H. Youssef, M. J. McInerney and D. A. Sabatini, Rhamnolipid biosurfactant mixtures for environmental remediation, Water Res., 2006, 42, 1735–1747 CrossRef.
- W. H. Noordman, M. L. Brusseau and D. B. Janssa, Adsorption of multicomponent rhamnolipid surfactants to soil, Environ. Sci. Technol., 2000, 34, 832–838 CrossRef CAS.
- J. Liu, Y. Wang and H. Li, Synergistic solubilisation of phenantharene by mixed micelles composed of biosurfactants and a conventional nonionic surfactant, Molecules, 2020, 25, 4327 CrossRef CAS.
- G. Zhu, Effect and mechanism of different surfactants mixed with rhamnolipids on the solubilisation of pyrene, Env. Sci. Tech., 2021, 44, 93–99 Search PubMed.
- Y. Zhang, T. L. Placek, J. Jahan, P. Alexandridis and M. Tsianou, Rhamnolipid micellization and adsorption properties, Int. J. Mol. Sci., 2022, 23, 11090 CrossRef CAS PubMed.
- R. Esposito, I. Speciale, C. De Castro, G. D’Enrico and I. R. Krauss, Rhamnolipid self-assembly in aqueous media, a long journey towards the determination of structure-property relationship, J. Mol. Sci., 2023, 24, 5395 CrossRef CAS.
- A. Zdziennicka and B. Janczuk, Thermodynamic parameters of some biosurfactant and surfactant adsorption at the water-air interface, J. Mol. Liq., 2017, 243, 236–244 CrossRef CAS.
- G. Ozdemir, S. Peker and S. S. Helvaci, Effect of pH on surface and interface behaviour of R1 and R2, Colloids Surf., A, 2004, 234, 135143 CrossRef.
- S. S. Helvaci, S. Peker and G. Ozdemir, Effect of electrolyte on the surface behaviour of R1 and R2, Colloids Surf., B, 2004, 35, 225–233 CrossRef CAS PubMed.
- S. Peker, S. S. Helvaci and G. Ozdemir, Interface-subphase interaction of rhamnolipid with aqueous rhamnolipid solution, Langmuir, 2003, 19, 5838–5845 CrossRef CAS.
- M. Sanchez, F. J. Aranda, M. J. Espuny, A. Marques, J. A. Teruel, A. Manresa and A. Ortiz, Aggregation behaviour of dirhamnolipid biosurfactant secreted by Pseudomonas aeruginosa in aqueous media, J. Colloid Interface Sci., 2007, 307, 246–252 CrossRef CAS.
- L. Zhang, P. Somarundaran, S. K. Singh, A. P. Felse and R. Gross, Synthesis and interfacial properties of sophorolipid derivatives, Colloids Surf., A, 2004, 204, 75–87 CrossRef.
- P. Dhasaiyan, P. Le Giel, S. Roelants, E. Redant, I. N. A. Bogaert, S. Prevost, B. L. Prasad and N. Baccile, Micelles vs ribbons, how congegers drive the self-assembly of sophorolipid biosurfactants, Chem. Phys. Chem., 2017, 18, 643–652 CrossRef CAS PubMed.
- Y. Hirata, M. Ryu, K. Igarashi, A. Nagatsuka, T. Furuta, S. Kanaya and M. Sugara, Natural synergies of acidic and lactonic type mixed sophorolipids: interfacial activity and cytotoxicities, Biochem. Biotechnol., 2009, 58, 565–572 CAS.
- T. Imura, D. Kawamura, T. Taira, T. Morita, T. Fukuota, K. Abrurai, H. Sakai, M. Abe and D. Kitamoto, Monolayer behaviour of binary systems of lactonic and acidic forms of sophorolipids: thermodynamic analysis of Langmuir monolayers and AFM study of Langmuir-Blodgett films, J. Oleo Sci., 2014, 63, 67–73 CrossRef CAS.
- G. Li, X. Li, J. Jiang, Y. Zhang and Y. Li, Dynamic surface properties and dilational rheology of acidic and lactonic sophorolipids at the air-water interface, Colloids Surf., B, 2020, 195, 111248 CrossRef CAS.
- M. L. Chen, J. Penfold, R. K. Thomas, T. J. P. Smyth, A. Perfumo, R. Marchant, I. M. Banat, P. Stevenson, A. Parry, I. Tucker and I. Grillo, Solution self-assembly and adsorption at the air-water interface of the monorhamnose and dirhamnose rhamnolipids and their mixtures, Langmuir, 2020, 26, 18281–18292 CrossRef.
- M. L. Chen, J. Penfold, R. K. Thomas, T. J. P. Smyth, A. Perfumo, R. Marchant, I. M. Banat, P. Steveson, A. Parry, I. Tucker and I. Grillo, Mixing behaviour of the biosurfactant rhamnolipid with a conventional anionic surfactant, sodium dodecyl benzene sulfonate, Langmuir, 2010, 28, 17958–17968 CrossRef.
- B. Dahrazma, C. N. Milligan and M. P. Nieh, Effects of additives on the structure of rhamnolipids (biosurfactants): a SANS study, J. Colloid Interface Sci., 2008, 319, 590–597 CrossRef PubMed.
- N. Baccile, C. Seyrig, A. Poirier, S. A. deCastro, S. L. K. W. Roelants and A. Abel, Self-assembly, interfacial properties, interactions with macromolecules and molecular modelling and simulation of microbial based amphiphiles (biosurfactants): a tutorial review, Green Chem., 2021, 630, 404–415 Search PubMed.
- N. Baccile, A. Poirier, J. Perez, P. Pernot, D. Hermida-Merino, P. LeGriel, C. C. Bleston, C. Muller, L. M. Blank and T. Tise, Self-assembly of rhamnolipid biosurfactants; understanding structure-property relationship using SAXS, Langmuir, 2023, 39, 9273–9299 CrossRef PubMed.
- M. L. Chen, C. C. Dong, J. Penfold, R. K. Thomas, T. J. P. Smyth, A. Perfumo, R. Marchant, I. M. Banat, P. Stevenson, A. Parry, I. M. Tucker and R. A. Campbell, Adsorption of sophorolipid biosurfactant on their own and mixed with LAS at the air-water interface, Langmuir, 2011, 27, 8854–8866 CrossRef PubMed.
- M. L. Chen, J. Penfold, R. K. Thomas, C. C. Dong, T. J. P. Smyth, A. Perfumo, R. Marchant, I. M. Banat, P. Stevenson, A. Parry, I. Tucker and I. Grillo, Solution self-assembly of sophorolipid and its mixture with the anionic surfactant LAS, Langmuir, 2011, 27, 8867–8877 CrossRef PubMed.
- N. Baccile, J. S. Pedersen, G. Pehau-Amandet and I. Van Bogaert, Surface charge of acidic sophorolipid micelles: effect of base and time, Soft Matter, 2013, 9, 4911–4927 RSC.
- N. Baccile, A. S. Cuvier, S. Prevost, C. V. Stevens, E. Derbeke, J. Borton, W. Soetaert, I. N. A. Van Bogeart and S. Roelants, Self-assembly mechanisms in pH responsive glycolipids; micelles, fibers, vesicles, and bilayers, Langmuir, 2016, 32, 10888–10894 Search PubMed.
- N. Baccile, F. Barbonneau, J. Jestin and J. Pehan-Arnaudet, I Van Bogaert, Unusual pH induced self-assembly of sophorolipid biosurfactants, ACS Nano, 2012, 6, 4763–4776 CrossRef PubMed.
- A. M. Motta, P. Mariani, R. Hri and F. Spinozar, Self-assembly properties of mono and di-rhamnolipids characterised using small angle x-ray scattering, Colloids Surf., B, 2024, 241, 114038 CrossRef PubMed.
- B. Ikizier, G. Arslan, E. Kipcak, C. Dilek, D. Celenk, T. Aktuglu, S. S. Helvaci and S. Peker, Surface adsorption and spontaneous aggregation in rhamnolipid mixtures in aqueous media, Colloids Surf., A, 2017, 519, 125–136 CrossRef.
- T. T. Nguyen and P. A. Sabatini, Characterisation and emulsification properties of rhamnolipids and sophorolipids and their mixtures, Int. J. Mol. Sci., 2011, 12, 1232–1244 CrossRef PubMed.
- Q. Bao, L. Huang, J. Xu, L. Yi, Y. Zhang and B. Wu, Study of the thermal washing of oily sludge using rhamnolipid/sophorolipid binary mixed biosurfactant systems, Ecotoxicol. Environ. Saf., 2022, 240, 113696 CrossRef CAS PubMed.
- D. Song, Y. Li, S. Liang and J. Wang, Micelle behaviour of sophorolipid/rhamnolipid binary mixed biosurfactants, Colloids Surf., A, 2013, 436, 201–206 CrossRef CAS.
- E. Rekiel, A. Zdziennicka, K. Szymczyk and B. Janczuk, Thermodynamic analysis of the adsorption and micellization of mixtures of rhamnolipids and surfactin with Triton X-165, Molecules, 2022, 27, 3600 CrossRef CAS.
- J. R. Liley, R. K. Thomas, J. Penfold, I. M. Tucker, J. T. Petkov, P. Stevenson, I. M. Banat, R. Marchant, M. Rudden and J. R. P. Webster, Adsorption at the air-water interface in biosurfactant mixtures: quantitative analysis of 5-component mixture, Langmuir, 2017, 33, 13027–13039 CrossRef CAS PubMed.
- J. R. Liley, J. Penfold, R. K. Thomas, I. M. Tucker, J. T. Petkov, P. S. Stevenson, I. M. Banat, R. Marchant, M. Rudden, A. Terry and I. Grillo, Self-assembly in dilute mixtures of nonionic and anionic surfactants and rhamnolipid biosurfactants, J. Colloid Interface Sci., 2017, 487, 493–503 CrossRef CAS PubMed.
- L. Xu and S. Amin, Microrheological study of tertiary surfactant/biosurfactant mixtures, Int. J. Cosmet. Sci., 2019, 41, 364–370 CrossRef CAS.
- S. A. Onaizi, M. S. Nasser and F. A. Twaiq, Micellisation and interfacial behaviour of synthetic -biosurfactant mixtures, Colloids Surf., A, 2012, 415, 388–393 CrossRef CAS.
- R. Esposito, L. Ingenito, D. Cavasso, A. Siciliano, M. L. Alfieri, L. Chiappisi, G. Fragneto, M. F. Ottaiani, M. Guiuda, L. Paduano and G. D’Errico, Rhamnolipid – SLES aqueous mixtures, from molecular self-assembly to the functional and ecotoxicological properties, J. Mol. Liq., 2022, 367, 120547 CrossRef CAS.
- Y. Zhou, S. Harne and S. Amin, Optimisation of surface activity of biosurfactant/surfactant mixtures, J. Cosmet. Sci., 2019, 70, 127 Search PubMed.
- D. Manko, A. Zdziennicka and B. Janczuk, Adsorption and self-assembly of sodium dodecyl sulfate and rhamnolipid mixtures, J. Surfactants Deterg., 2017, 20, 411–423 CrossRef CAS.
- A. Zdziennicka and B. Janczuk, Thermodynamic parameters of some biosurfactant and surfactants adsorbed at the air-water interface, J. Mol. Liq., 2017, 243, 236–244 CrossRef CAS.
- J. H. Clint, Micellisation of mixed nonionic surface active agents, J. Chem. Soc. Faraday Trans 1, 1975, 71, 1327–1334 RSC.
- D. N. Rubnigh, Solution Chemistry of Surfactants, in Non-ideal mixing in micellar solutions, ed. K. L. Mittal, Plenum Press, NY, 1979, pp. 337–354 Search PubMed.
- B. Ingram, Surface tension of non-ideal surfactant mixtures, Colloid Polym. Sci., 1980, 258, 191–192 CrossRef CAS.
- J. A. V. Butler, The thermodynamics of the surfaces of solutions, Proc. R. Soc. London, Ser. A, 1932, A135, 348–375 Search PubMed.
- J. Penfold, E. Staples, L. Thompson, I. Tucker, J. Hines, R. K. Thomas and J. R. Lu, The solution and adsorption behaviour of the mixed surfactant system SDS/C12E6, Langmuir, 1995, 11, 2496 CrossRef CAS.
- G. Redlich and A. T. Kister, Algebraic representation of the thermodynamic properties and the classification of solutions, Ind. Eng. Chem., 1948, 40, 3345–3348 Search PubMed.
- J. R. Liley, R. K. Thomas, J. Penfold, I. M. Tucker, J. T. Petkov, P. Stevenson and J. R. P. Webster, Surface adsorption in ternary surfactant mixtures above the cmc: effects of asymmetry on the composition dependence of the excess free energy, J. Phys. Chem. B, 2017, 121, 2825–2838 CrossRef CAS.
- J. R. Liley, R. K. Thomas, J. Penfold, I. M. Tucker, J. T. Petkov, P. Stevenson and J. R. P. Webster, Impact of electrolyte on the adsorption at the air-water interface for ternary surfactant mixtures above the cmc, Langmuir, 2017, 33, 4301–4312 CrossRef CAS.
- P. X. Li, K. Ma, R. K. Thomas and J. Penfold, Analysis of asymmetric synergy in the adsorption of zwitterionic-ionic surfactant mixtures at the air-water interface below and above the cmc, J. Phys. Chem. B, 2016, 120, 3677–3691 CrossRef CAS.
- I. M. Tucker, A. Burley, R. E. Petkova, S. L. Hosking, M. Evans, C. E. Blythe, O. Beckingham, J. Penfold, R. K. Thomas, P. X. Li, J. R. P. Webster and R. Welbourn, Surfactant/biosurfactant mixing: adsorption of saponin/nonionic surfactant mixtures at the air-water interface, J. Colloid Interface Sci., 2020, 574, 385–392 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sm00147a |
| This journal is © The Royal Society of Chemistry 2025 |

