Open Access Article
Open Access ArticleTunable mechanical properties of PDMS–TMPTMA microcapsules for controlled release in coatings†
Congwang
Ye
b,
Bianka
Pajo
a and
Carlos J.
Martinez
 *a
*a
aSchool of Materials Engineering, Purdue University, 701 West Stadium Ave, West Lafayette, IN 47907, USA. E-mail: martin19@purdue.edu
bMaterials Engineering Division, Lawrence Livermore National Lab, Livermore, CA 94550, USA
First published on 2nd January 2025
Abstract
Within coating formulations, microcapsules serve as vehicles for delivering compounds like catalysts and self-healing agents. Designing microcapsules with precise mechanical characteristics is crucial to ensure their contents' timely release and minimize residual shell fragments, thereby avoiding adverse impacts on the coating quality. With these constraints in mind, we explored the use of 1 cSt PDMS oil as a diluent (porogen) in trimethylolpropane trimethacrylate (TMPTMA)-based to fabricate microcapsules with customized mechanical properties and submicrometer debris size after shell breakup that can encapsulate a wide range of compounds. Microcapsules were fabricated from double emulsion templates featuring an aqueous core and shells with different PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA volume percent ratios. Their mechanical properties under compression and their capacity for encapsulation were characterized. PDMS
TMPTMA volume percent ratios. Their mechanical properties under compression and their capacity for encapsulation were characterized. PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA ratios exceeding 20
TMPTMA ratios exceeding 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 caused phase separation during crosslinking, leading to a porous shell structure of TMPTMA clusters. The strength of the microcapsules decreased as the PDMS:TMPTMA ratio increased, with ratios above 30
80 caused phase separation during crosslinking, leading to a porous shell structure of TMPTMA clusters. The strength of the microcapsules decreased as the PDMS:TMPTMA ratio increased, with ratios above 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 resulting in mechanically fragile microcapsules fracturing into fragments <10 μm in size. Microcapsules produced with a 0
70 resulting in mechanically fragile microcapsules fracturing into fragments <10 μm in size. Microcapsules produced with a 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 PDMS
100 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA ratio exhibited strong mechanical properties and were capable of encapsulating small volatile compounds like 1,4-diazabicyclo[2.2.2]octane (a catalyst), while those with ratios >30
TMPTMA ratio exhibited strong mechanical properties and were capable of encapsulating small volatile compounds like 1,4-diazabicyclo[2.2.2]octane (a catalyst), while those with ratios >30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 were only suitable to encapsulate larger molecules. The combination of PDMS:TMPTMA chemistry and the precise control provided by the double emulsion generation process in microcapillary devices makes PDMS:TMPTMA a versatile system suitable for various pressure-sensitive encapsulation applications.
70 were only suitable to encapsulate larger molecules. The combination of PDMS:TMPTMA chemistry and the precise control provided by the double emulsion generation process in microcapillary devices makes PDMS:TMPTMA a versatile system suitable for various pressure-sensitive encapsulation applications.
1 Introduction
Microcapsules are used in a wide range of applications such as drug delivery,1 organic phase change materials2 and in coating formulations to control the release of additives and more recently for self-healing coating applications.3–6 The efficacy of microcapsules in delivering its content depends on the ratio of the thickness of the shell to the capsule radius, as well as its material properties.7–9 Advanced applications such as the delivery of catalysts in coatings require microcapsules with custom mechanical properties so that they are easily broken before or during the coating application process.10–12 Furthermore, the fate of the residual shell material after breakage must be considered in the microcapsule design to ensure that it does not interfere with the aesthetics and properties of the coating. One approach is to fabricate microcapsules with sub-micrometer-thick shells and diameters significantly smaller than the coating thickness. However, in some cases, larger and thicker microcapsules are needed due to the chemistry of the encapsulated material or application requirements. Therefore, there is interest in finding shell chemistries that facilitate encapsulation and disintegrate under pressure.Microcapsules can be fabricated using liquids and solids as templates. For example, Pickering and surfactant-stabilized single emulsions fabricated via shear-driven emulsification have been used to fabricate polymeric or ceramic microcapsules via interfacial polymerization.13,14 Another approach is using particle templates coated with polyelectrolytes.15–17 Both techniques yield microcapsules with highly elastic shells and thicknesses ranging from hundreds of nanometers to tens of micrometers. Microcapsules with thicker shells and a wider range of materials can be obtained from double emulsion drops generated in microfluidic devices.18–20 Drops are generated by carefully tuning the dispersed and continuous phase flow rates to induce drop breakup in the dripping regime where their diameters are highly uniform.10,18 Once the drops are generated, the fluid shells are solidified through crosslinking or polymerization.21,22 Acrylates have gained popularity as shell materials in microfluidic double emulsion-based capsule generation due to their low viscosity, high reactivity, a wide range of chemistries, and ease of UV-crosslinking. Acrylates are usually used as the oil phase to form water/oil/water (w/o/w) double emulsion templates. For example, Chen et al.,12,21 successfully used silica-reinforced acrylates to encapsulate amines for self-healing applications. One modification to the acrylate composition that has not received much attention is the addition of diluents to form porous shells. Porous polymer particles and films can be obtained by adding a diluent such as acetone, toluene, or hexane. These porous particles belong to a class of microporous polymers formed due to the polymers’ phase separation during crosslinking.23 Macroporous polymers composed of styrene and divinyl benzene monomers have been extensively studied in the presence of solvating and non-solvating diluents and a wide range of crosslinker concentrations to form porous structures with tailored porous microstructures.24 More recently, Loiseau et al.,25 fabricated highly porous glycidyl methacrylate and ethylene glycol dimethacrylate microcapsules via double emulsion microfluidics. Mixtures of butanol and undecanol served as a diluent, forming porous shells composed of polymer aggregates similar to colloidosomes. The microcapsules’ shell porosity was tailored by the amount and solubility of the diluent. Higher porosity decreased the shell strength and increased its permeability. Despite their high porosity, the capsules were stronger than similar colloidosome capsules. These results show that carefully introducing porosity to an otherwise strong polymer can be a novel route to control the mechanical properties of shells. Moreover, using a porous, brittle polymer can reduce the size of the residual shell material, which can be beneficial for coating applications. One of the main considerations in selecting the materials for tuning the microcapsule shells’ porosity and mechanical properties is the polymer and diluent phase solubility parameters. Matching the solubility parameters between the materials is crucial to ensure phase separation between the two and, therefore, porosity and mechanical properties of the shell. According to Mohamed et al.,26 if the diluent has good miscibility with the monomer and its polymeric form, it will result in a low pore volume. The opposite is true when the diluent is a bad solvent for the polymer phase, which results in the formation of macropores and an increase in polymer porosity.
In this work, we explore the use of 1 cSt PDMS oil as a diluent (porogen) in trimethylolpropane trimethacrylate (TMPTMA) with the aim of fabricating microcapsules with tailored mechanical properties and submicrometer residual shell size that can encapsulate a wide range of chemical compounds. TMPTMA is a highly reactive UV-cross-linkable methacrylate that is widely used as a strength modifier in acrylate-based formulations.27–29 1 cSt PDMS oil was chosen as the diluent (porogen) because of its ease of emulsification, non-reactivity with polar compounds, low viscosity, Newtonian behavior, and volatility.30 Microcapsules with different PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA vol% ratios as the shell material were successfully fabricated from double emulsion drops generated in microcapillary devices. Soon after generation, the double emulsion drops were UV-crosslinked to solidify the shell, followed by harvesting from the solution and removal of the 1 cSt PDMS oil by evaporation. An in-house-built compression testing system was used to find the load to failure of hollow microcapsules with shells fabricated using different PDMS oil concentrations. The encapsulation capabilities of PDMS:TMPTMA microcapsules and the residual shell size after disintegration under pressure were also investigated.
TMPTMA vol% ratios as the shell material were successfully fabricated from double emulsion drops generated in microcapillary devices. Soon after generation, the double emulsion drops were UV-crosslinked to solidify the shell, followed by harvesting from the solution and removal of the 1 cSt PDMS oil by evaporation. An in-house-built compression testing system was used to find the load to failure of hollow microcapsules with shells fabricated using different PDMS oil concentrations. The encapsulation capabilities of PDMS:TMPTMA microcapsules and the residual shell size after disintegration under pressure were also investigated.
2 Experimental setup
2.1 Materials
Three fluids, inner, middle, and outer, are needed to fabricate the double emulsions that served as precursors for the PDMS:TMPTMA microcapsules. Drop generation was tested using an aqueous solution (DI-H2O) as the inner fluid while the middle fluid (shell) was composed of mixtures of octamethyltrisiloxane (1 cSt PDMS oil, Clearco Products, Bensalem, PA), trimethylolpropane trimethacrylate (TMPTMA, stabilized with hydroquinone monomethyl ether) (TCI, Tokyo, Japan) and photoinitator 2,2-dimethoxy-2-phenyl-acetophenone (Sigma, St. Louis, MO). The octamethyltrisiloxane will be referred to as PDMS through the document. Decamethylcyclopentasiloxane with trimethylated silicia (749 fluid, Dow Corning, Midland, MI) served as a surfactant and 2 wt% was added to all PDMS:TMPTMA mixtures. The outer fluid that served as the continuous phase was 40 wt% glycerol (99% (GC), Sigma, St. Louis, MO), 2 wt% poly(vinyl alcohol) (PVA) (Mw = 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–23
000–23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, 87–89% hydrolyzed, Sigma, St. Louis, MO) in DI-H2O. The encapsulation capabilities of the solid and porous TMPTMA microcapsules were demonstrated using 1,4-diazabicyclo[2.2.2]octane (DABCO) (Mw = 112.17 g mol−1), a catalyst used in coating formulations, resazurin dye (251.15 g mol−1) (Sigma, St. Louis, MO) and fluorescein isothiocyanate–dextran (FITC–dextran) (Mw = 4000 g mol−1) (Sigma, St. Louis, MO). Resazurin was chosen because of its intense purple color, which is easily seen by the naked eye. The viscosity of the PDMS:TMPTMA mixtures was measured using a rheometer (Gemini HR Nano, Malvern, Worcestershire, UK) using double-gap and concentric cylinder geometries.
000 g mol−1, 87–89% hydrolyzed, Sigma, St. Louis, MO) in DI-H2O. The encapsulation capabilities of the solid and porous TMPTMA microcapsules were demonstrated using 1,4-diazabicyclo[2.2.2]octane (DABCO) (Mw = 112.17 g mol−1), a catalyst used in coating formulations, resazurin dye (251.15 g mol−1) (Sigma, St. Louis, MO) and fluorescein isothiocyanate–dextran (FITC–dextran) (Mw = 4000 g mol−1) (Sigma, St. Louis, MO). Resazurin was chosen because of its intense purple color, which is easily seen by the naked eye. The viscosity of the PDMS:TMPTMA mixtures was measured using a rheometer (Gemini HR Nano, Malvern, Worcestershire, UK) using double-gap and concentric cylinder geometries.
2.2 Microcapillary devices
Microcapillary devices were fabricated following the work of Utada et al.,18 and Shirk et al.19 Briefly, two tapered round capillaries with 1 mm outer diameter were aligned and inserted in a square capillary of similar inner size, forming a coaxial structure suitable for double emulsion generation. A pipette puller (model P-97, Sutter Instruments, Novato, CA) was used to heat and pull the round glass capillaries (inner diameter: 0.580 mm, outer diameter: 1.000 mm, World Precision Instruments, Sarasota, FL) into two equal-sized tapered capillaries. A microforge station (Micro Forge MF830, Narishige, Japan) cleaved the tapered tips to the desired diameters. The surface of the input capillary was treated to be hydrophobic with a solution composed of 9.5 mL of 200-proof ethanol (KOPTEC, King of Prussia, PA) and 0.5 mL of chlorotrimethylsilane (Sigma, St. Louis, MO). The exit capillary was treated to be hydrophilic with a solution of 9.0 mL of 200 proof ethanol (KOPTEC, King of Prussia, PA), 0.5 mL of DI-H2O, 0.1 mL of acetic acid (Sigma, St. Louis, MO) and 0.2 mL of 2-[methoxy(polyethyleneoxy)propyl]trimethoxysilane (Sigma, St. Louis, MO). Three 20G Luer stubs (Intramedic Luer Stub Adapters, Beckton Dickinson, Sparks, MD) were used as input connectors for the three fluids. Each opening was closed with marine epoxy (Henkel, Rocky Hill, CT) and/or 5-minute epoxy (Permatex, Solon, OH), and the entire structure was fixed to a 6 x 2 inches glass slide.2.3 Double emulsion generation and characterization
Double emulsions were generated by first loading the respective solutions into glass syringes (Hamilton Gastight, Hamilton Co., Reno, NV) controlled by syringe pumps (PHD 2000, Harvard Apparatus, Holliston, MA) and connected to microcapillary devices using polyethylene tubing (Smiths Medical ASD, Inc., Keene, NH). The drop generation was controlled by independently adjusting the flow rates of the inner (Qinn), middle (Qmid), and outer (Qout) fluid and the process was visualized on an inverted microscope (Axio Observer, Zeiss America, Peabody, MA) equipped with a fast camera (Photron FASTCAM MINI UX100, Photron USA, Inc., San Diego, CA). The drops were irradiated with UV light (CE-6-BL, American Ultraviolet Co., Lebanon, IN) 10 seconds after generation to initiate shell crosslinking and reduce the diffusion of the inner fluid to the continuous phase due to an osmotic pressure gradient.19 Microcapsules were collected in a partially filled vial with DI-H2O, or in a funnel with filter paper to speed up the elimination and drying of the continuous phase. The collected microcapsules were rinsed with DI-H2O and removed from the funnel while adhering to the filter paper, followed by an hour of UV post-curing to ensure complete crosslinking of the shells. The dried capsules were then collected in vials for storage.An aliquot of the samples was collected in rectangular flat capillaries (Vitrocom, Mountain Lakes, NJ) with dimensions of 4 mm width, 0.4 mm height, and 55 mm length to optically visualize drops and microcapsules. The flat capillaries were glued to a glass slide, and the ends were covered with epoxy to reduce evaporation. Bright-field and fluorescence optical images were taken on an inverted microscope with a QICAM digital camera (QICAM 12-bit Cooled ColorFast 1394 Cooled, Qimaging, Surrey, BC, Canada). Optical images were analyzed using ImageJ (National Institutes of Health, Bethesda, MD) to measure the inner (dinn) and outer (dout) drop diameters. Dry microcapsules were placed on carbon tape fixed to an aluminum stub and sputter-coated with a 10-nm gold layer for SEM imaging (Phenom Pure, Phenom World, Eindhoven, The Netherlands).
2.4 Microcapsule compression testing
The load-to-failure and load–displacement curves for PDMS:TMPTMA microcapsules were measured using an “in-house” built compression apparatus inspired by the work of White et al.31 The apparatus, containing a load cell and a motorized stage, was mounted on an inverted microscope as shown in Fig. S1 (ESI†). Several studies1,2 on the mechanical properties of microcapsules with porous shells for controlled release of active components have reported applied loads on the 0–1000 mN range from compression tests performed on single capsules.25 This study also chose such loads to evaluate the strength and mechanical properties of the PDMS:TMPTMA microcapsules. The microcapsules were compressed by a cylindrical stainless steel tapered tip connected to the load cell, and the motorized stage controlled the motion of the tip. Data were collected and displayed using LabView. The final load–displacement curve was obtained after subtracting the machine compliance. Capsule compression and subsequent rupture were recorded using the fast camera described above.3 Results and discussion
3.1 Rheology of PDMS:TMPTMA solutions
PDMS oil readily mixed with TMPTMA at all concentrations without signs of phase separation. PDMS oil has a solubility parameter (δ1) of ∼15(MPa)1/2,32,33 while the solubility parameter for TMPTMA (δ2) is ∼18.3(MPa)1/2.34 The Δδ2 = (δ1 − δ2)2 for the PDMS:TMPTMA solutions is 8.19 MPa making PDMS oil a good to moderate solvent for the TMPTMA monomer. On the other hand, Δδ2 between PDMS oil and crosslinked TMPTMA (δ ∼ 23 MPa1/2) is ∼64 MPa, making PDMS oil a poor solvent for the TMPTMAs polymer and will result in an early phase separation between the two during UV crosslinking and as a result increasing the shell porosity. The behavior of the PDMS:TMPTMA solutions resembled that of a solvent/low molecular weight polymer mixture. To corroborate this, viscosity measurements were performed on solutions with different PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA vol% ratios, as shown in Fig. 1a. The apparent viscosity (ηapp) as a function of shear rate (
TMPTMA vol% ratios, as shown in Fig. 1a. The apparent viscosity (ηapp) as a function of shear rate (![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) ) is Newtonian for all mixtures ranging from 0.00087 Pa s for 100
) is Newtonian for all mixtures ranging from 0.00087 Pa s for 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 PDMS
0 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA ratio and 0.045 Pa s for a 0
TMPTMA ratio and 0.045 Pa s for a 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 PDMS
100 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA ratio. The Newtonian behavior of the mixtures suggests that the PDMS oil acts as a good solvent for the TMPTMA monomer. ηapp for each solution at 10 s−1 was plotted as a function of PDMS
TMPTMA ratio. The Newtonian behavior of the mixtures suggests that the PDMS oil acts as a good solvent for the TMPTMA monomer. ηapp for each solution at 10 s−1 was plotted as a function of PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA vol% ratio in Fig. 1b. The modified version of the Arrhenius rule of mixtures developed by Lederer35,36 to predict the viscosity of crude oil and solvent mixtures fit the data well as shown by the dashed line in Fig. 1b. The general form of the Lederer equation is ln
TMPTMA vol% ratio in Fig. 1b. The modified version of the Arrhenius rule of mixtures developed by Lederer35,36 to predict the viscosity of crude oil and solvent mixtures fit the data well as shown by the dashed line in Fig. 1b. The general form of the Lederer equation is ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η12 = x1
η12 = x1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η1 + x2
η1 + x2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η2, where η12 is the mixture viscosity, η1 and η2 are the viscosities of the two components and x1 and x2 are the modified volume fractions using
η2, where η12 is the mixture viscosity, η1 and η2 are the viscosities of the two components and x1 and x2 are the modified volume fractions using 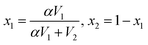 and
and 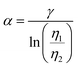 , where γ is 2.5, based on Einstein's diffusion equation for infinitely diluted suspensions and molecules. The low viscosity across the whole range of PDMS
, where γ is 2.5, based on Einstein's diffusion equation for infinitely diluted suspensions and molecules. The low viscosity across the whole range of PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA ratios ensures that the mixtures are suitable for generating drops in microfluidic devices because the pressure in the small channels will not be excessively high, which can lead to syringe pump stalling.
TMPTMA ratios ensures that the mixtures are suitable for generating drops in microfluidic devices because the pressure in the small channels will not be excessively high, which can lead to syringe pump stalling.
 | ||
Fig. 1 (a) Apparent viscosity (ηapp) as a function of shear rate (![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) ) for solutions with different PDMS:TMPTMA vol% ratios. (b) ηapp at ) for solutions with different PDMS:TMPTMA vol% ratios. (b) ηapp at ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) = 10−1 s as a function of PDMS = 10−1 s as a function of PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA vol% ratios. TMPTMA vol% ratios. | ||
3.2 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 100 PDMS
100 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) TMPTMA microcapsule fabrication
TMPTMA microcapsule fabrication
Microcapsule fabrication was started using a 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 PDMS
100 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA ratio. The purpose of these experiments was to understand the properties of the TMPTMA microcapsule without PDMS oil and serve as a baseline for other mixtures. Fabrication starts with double emulsion drop generation in microcapillary devices. An optical image of the generation of double emulsion drops composed of an aqueous core surrounded by a 0
TMPTMA ratio. The purpose of these experiments was to understand the properties of the TMPTMA microcapsule without PDMS oil and serve as a baseline for other mixtures. Fabrication starts with double emulsion drop generation in microcapillary devices. An optical image of the generation of double emulsion drops composed of an aqueous core surrounded by a 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 PDMS
100 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA shell near the entrance of the exit capillary is shown in Fig. 2a. The inner (DI-H2O) and middle (TMPTMA) fluids meet at the entrance of the exit capillary, where they are sheared by the outer aqueous fluid, breaking into double emulsion drops in a single step. Drop breakup occurs due to the interplay between viscous stress and interfacial tension between the fluids.37,38 Double emulsion drops were exposed to UV light as they traversed the exit tubing to partially crosslink the TMPTMA shell and prevent inner drop breakup or coalescence. The partially crosslinked microcapsules were collected in a glass vial and exposed to UV light for another 30 minutes to crosslink the shell fully. The crosslinked aqueous core/TMPTMA shell microcapsules are highly uniform with a coefficient of variation (CV) <3% for dinn and dout. One of the benefits of using a flow-focusing microcapillary device is that the inner and outer drop diameters can be adjusted by independently changing the three-fluid flow rates. A series of drop generation experiments were performed in which the Qout was fixed to 8000 μL h−1 and Qmid = 1500 μL h−1, while the Qinn was varied from 600 to 2400 μL h−1. A plot showing dinn and dout as a function of Qinn/(Qmid + Qout) and the shell thickness/outer radius ratio (tshell/rout) is shown in Fig. 2f. When the Qinn increased from 600 to 2400 μL h−1, the dinn value increased from 189 to 213 μm, while the dout value decreased from 280 to 255 μm. tshell decreased from 91 to 42 μm corresponding to a tshell/rout of 0.33 to 0.16. Optical images of a group of crosslinked TMPTMA microcapsules dispersed in DI-H2O and generated with different outer diameters and shell thicknesses are shown in Fig. 2b–e. Note that while the microcapsules’ shell thickness appears uniform in most of the optical images, they are not uniform along the z-axis (gravitational axis) due to differences in the density between the aqueous core (ρH2O= 1 g mL−1) and the TMPTMA (ρTMPTMA = 1.06 g mL−1) solution. The thinnest region of the shell is its weakest point, and it will influence the force at failure for microcapsules with similar shell thicknesses. Overall, the aqueous core/TMPTMA double emulsion drops are stable in solution, and due to the fast UV-crosslinking of the TMPTMA, >98% of the microcapsules survived the fabrication, crosslinking, and collection processes.
TMPTMA shell near the entrance of the exit capillary is shown in Fig. 2a. The inner (DI-H2O) and middle (TMPTMA) fluids meet at the entrance of the exit capillary, where they are sheared by the outer aqueous fluid, breaking into double emulsion drops in a single step. Drop breakup occurs due to the interplay between viscous stress and interfacial tension between the fluids.37,38 Double emulsion drops were exposed to UV light as they traversed the exit tubing to partially crosslink the TMPTMA shell and prevent inner drop breakup or coalescence. The partially crosslinked microcapsules were collected in a glass vial and exposed to UV light for another 30 minutes to crosslink the shell fully. The crosslinked aqueous core/TMPTMA shell microcapsules are highly uniform with a coefficient of variation (CV) <3% for dinn and dout. One of the benefits of using a flow-focusing microcapillary device is that the inner and outer drop diameters can be adjusted by independently changing the three-fluid flow rates. A series of drop generation experiments were performed in which the Qout was fixed to 8000 μL h−1 and Qmid = 1500 μL h−1, while the Qinn was varied from 600 to 2400 μL h−1. A plot showing dinn and dout as a function of Qinn/(Qmid + Qout) and the shell thickness/outer radius ratio (tshell/rout) is shown in Fig. 2f. When the Qinn increased from 600 to 2400 μL h−1, the dinn value increased from 189 to 213 μm, while the dout value decreased from 280 to 255 μm. tshell decreased from 91 to 42 μm corresponding to a tshell/rout of 0.33 to 0.16. Optical images of a group of crosslinked TMPTMA microcapsules dispersed in DI-H2O and generated with different outer diameters and shell thicknesses are shown in Fig. 2b–e. Note that while the microcapsules’ shell thickness appears uniform in most of the optical images, they are not uniform along the z-axis (gravitational axis) due to differences in the density between the aqueous core (ρH2O= 1 g mL−1) and the TMPTMA (ρTMPTMA = 1.06 g mL−1) solution. The thinnest region of the shell is its weakest point, and it will influence the force at failure for microcapsules with similar shell thicknesses. Overall, the aqueous core/TMPTMA double emulsion drops are stable in solution, and due to the fast UV-crosslinking of the TMPTMA, >98% of the microcapsules survived the fabrication, crosslinking, and collection processes.
3.3 Mechanical properties of 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 100 PDMS
100 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) TMPTMA microcapsules
TMPTMA microcapsules
The fully crosslinked 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 PDMS
100 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA microcapsules were strong with a dense microstructure, as shown by the partially broken capsules in Fig. 3(c and d). TMPTMA is known to be brittle and crack easily when cast on surfaces due to the residual stresses that develop during crosslinking.39 TMPTMA microcapsules do not show cracks when viewed under a SEM or an optical microscope, except for those cracked intentionally during testing or for imaging purposes. This is due to the unconstrained nature of the drops, which allows the shell to shrink freely during crosslinking resulting in defect-free microcapsules. As previously discussed, the microcapsule shell thickness is not uniform due to the difference in density between the aqueous core and the TMPTMA shell, as can be seen in Fig. 3c and d. Attempts were made to obtain more uniform shell thicknesses by moving the UV light closer to the entrance of the exit capillary, where drops are generated. This resulted in device clogging due to premature TMPTMA crosslinking.
TMPTMA microcapsules were strong with a dense microstructure, as shown by the partially broken capsules in Fig. 3(c and d). TMPTMA is known to be brittle and crack easily when cast on surfaces due to the residual stresses that develop during crosslinking.39 TMPTMA microcapsules do not show cracks when viewed under a SEM or an optical microscope, except for those cracked intentionally during testing or for imaging purposes. This is due to the unconstrained nature of the drops, which allows the shell to shrink freely during crosslinking resulting in defect-free microcapsules. As previously discussed, the microcapsule shell thickness is not uniform due to the difference in density between the aqueous core and the TMPTMA shell, as can be seen in Fig. 3c and d. Attempts were made to obtain more uniform shell thicknesses by moving the UV light closer to the entrance of the exit capillary, where drops are generated. This resulted in device clogging due to premature TMPTMA crosslinking.
The strength of the microcapsules depends on the shell tshell/rout ratio, microstructure, outer diameter, and composition. For example, microcapsules with thick shells will be stronger than those with thinner ones.21 The mechanical strength of 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 PDMS
100 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA microcapsules was assessed by evaluating the load displacement of microcapsules produced with varying tshell/rout ratios. Representative load vs. displacement curves of compression tests for microcapsules with a 0.169 tshell/rout ratio are shown in Fig. 3a. When the microcapsule cracks, there is a sudden decrease in the load due to their brittle nature. The load at failure increases with increasing tshell/rout as shown in Fig. 3b since a thicker shell can support the load over a large volume. The highest stress occurs at the compression contact poles, where the outer side of the shell is in compression while the inner side is in tension. The system's optics did not allow for a detailed image of the microcapsule surface before failure. However, high-speed video of the microcapsule-breaking process was captured, as shown in the sequence of images in Fig. S2 (ESI†), where the microcapsule appears as a black circle between two horizontal black regions corresponding to the compression tip and substrate. The microcapsule had a dinn = 179 μm and dout = 222 μm, corresponding to a tshell/rout = 0.096. The imposed load is dissipated once a crack starts propagating at high speed along the microcapsule in the longitudinal direction, shattering the shell into pieces seen as dark spots moving horizontally from the initial microcapsule location (Fig. S2, ESI†).
TMPTMA microcapsules was assessed by evaluating the load displacement of microcapsules produced with varying tshell/rout ratios. Representative load vs. displacement curves of compression tests for microcapsules with a 0.169 tshell/rout ratio are shown in Fig. 3a. When the microcapsule cracks, there is a sudden decrease in the load due to their brittle nature. The load at failure increases with increasing tshell/rout as shown in Fig. 3b since a thicker shell can support the load over a large volume. The highest stress occurs at the compression contact poles, where the outer side of the shell is in compression while the inner side is in tension. The system's optics did not allow for a detailed image of the microcapsule surface before failure. However, high-speed video of the microcapsule-breaking process was captured, as shown in the sequence of images in Fig. S2 (ESI†), where the microcapsule appears as a black circle between two horizontal black regions corresponding to the compression tip and substrate. The microcapsule had a dinn = 179 μm and dout = 222 μm, corresponding to a tshell/rout = 0.096. The imposed load is dissipated once a crack starts propagating at high speed along the microcapsule in the longitudinal direction, shattering the shell into pieces seen as dark spots moving horizontally from the initial microcapsule location (Fig. S2, ESI†).
As tshell/rout increases, so does the measurement standard deviation, as shown in Fig. 3b. This is due to the random orientation of the thinnest part of the shell with respect to the applied load-axis, and this effect becomes more pronounced as tshell/rout increases. The lowest load to failure is expected if the thinnest region of the shell is aligned with the applied load axis.21 Since the microcapsules are optically opaque, it is impossible to know the shell orientation; therefore higher variability is expected for microcapsules with thicker shells. As tshell/rout decreases, the microcapsules weaken, and the shell orientation effect diminishes.
Because of the microcapsule's brittle nature, their failure can be described using Weibull statistics. The probability of failure can be written as P(F) = 1 − exp[−(F0/F)m] where m is the Weibull modulus, and F0 is the characteristics failure load. The Weibull modulus m can be obtained from the linear fit slope of the double logarithmic Weibull plot, while F0 is the y-axis intercept. A summary of the Weibull statistics for the 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 PDMS
100 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA microcapsules is presented in Table 1. As the tshell/rout increases, F0 increases from 448 to 1121 mN while m decreases from 9.4 to 4.4, indicating a higher variability in microcapsule strength distribution. The microcapsule modulus (M) also increased from 10 to 36.7 mN μm−1 with increasing tshell/rout. The findings indicate that the mechanical properties of 0
TMPTMA microcapsules is presented in Table 1. As the tshell/rout increases, F0 increases from 448 to 1121 mN while m decreases from 9.4 to 4.4, indicating a higher variability in microcapsule strength distribution. The microcapsule modulus (M) also increased from 10 to 36.7 mN μm−1 with increasing tshell/rout. The findings indicate that the mechanical properties of 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 PDMS
100 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA microcapsules can be modified by altering the shell thickness. Significantly weaker microcapsules can be produced by reducing the TMPTMA shell thickness or outer diameter beyond the range explored in this study. Nevertheless, attempts to adjust the flow rates to make a thinner shell resulted in instability during the generation of double emulsion drops, and thus, this approach was not pursued further.
TMPTMA microcapsules can be modified by altering the shell thickness. Significantly weaker microcapsules can be produced by reducing the TMPTMA shell thickness or outer diameter beyond the range explored in this study. Nevertheless, attempts to adjust the flow rates to make a thinner shell resulted in instability during the generation of double emulsion drops, and thus, this approach was not pursued further.
PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA TMPTMA |
d inn (μm) | d out (μm) | t shell/rout | M (mN μm−1) | m | F 0 (mN) |
|---|---|---|---|---|---|---|
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
226 | 250 | 0.096 | 10 | 9.4 | 448.8 |
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
216 | 260 | 0.162 | 16.3 | 6.28 | 546.1 |
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
208 | 264 | 0.212 | 21.9 | 11.1 | 740.5 |
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
200 | 276 | 0.275 | 36.7 | 4.4 | 1121.5 |
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
150 | 181 | 0.171 | 4.98 | 6.40 | 145.9 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 90 |
154 | 187 | 0.176 | 4.94 | 8.19 | 139.5 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
158 | 191 | 0.173 | 4.79 | 14.3 | 119.97 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 70 |
182 | 220 | 0.173 | 4.53 | 2.09 | 92.7 |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60 |
185 | 229 | 0.192 | 2.55 | 4.16 | 12.78 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
199 | 248 | 0.198 | 1.58 | 4.70 | 4.85 |
3.4 PDMS:TMPTMA microcapsule fabrication
Microcapsules with an aqueous core and different PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA ratios were generated in microcapillary devices from double emulsion drops containing increasing concentrations of 1cSt PDMS oil mixed with TMPTMA. Optical images of a group of UV-crosslinked capsules with DI-H2O-cores and shells composed of (a) 10
TMPTMA ratios were generated in microcapillary devices from double emulsion drops containing increasing concentrations of 1cSt PDMS oil mixed with TMPTMA. Optical images of a group of UV-crosslinked capsules with DI-H2O-cores and shells composed of (a) 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, (b) 20
90, (b) 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80, (c) 30
80, (c) 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70, and (d) 40
70, and (d) 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 PDMS
60 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA vol% ratios are shown in Fig. 4. The microcapsules were generated with similar dinn, dout, and tshell by keeping a constant 1
TMPTMA vol% ratios are shown in Fig. 4. The microcapsules were generated with similar dinn, dout, and tshell by keeping a constant 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 rate Qmid/Qinn ratio and using the same microcapillary device. Double emulsions were stable, and the collected drops did not show signs of coalescence indicating that mixing PDMS oil with TMPTMA did not affect drop stability. Initially, all PDMS:TMPTMA solutions were optically clear, forming double emulsions with transparent cores and shells. As soon as the double emulsion drops were exposed to UV-light, the opacity of the shell increased with PDMS oil concentration, as shown in Fig. 4. The change in opacity is due to the difference in the refractive index between the PDMS oil and TMPTMA-crosslinked regions of the shell. A similar change in opacity has been reported in siloxane hydrogels containing a diluent phase.40 Mixtures with up to 80
1 rate Qmid/Qinn ratio and using the same microcapillary device. Double emulsions were stable, and the collected drops did not show signs of coalescence indicating that mixing PDMS oil with TMPTMA did not affect drop stability. Initially, all PDMS:TMPTMA solutions were optically clear, forming double emulsions with transparent cores and shells. As soon as the double emulsion drops were exposed to UV-light, the opacity of the shell increased with PDMS oil concentration, as shown in Fig. 4. The change in opacity is due to the difference in the refractive index between the PDMS oil and TMPTMA-crosslinked regions of the shell. A similar change in opacity has been reported in siloxane hydrogels containing a diluent phase.40 Mixtures with up to 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 PDMS
20 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA vol% ratios were tested (not shown). However, microcapsules made with PDMS oil concentrations above 50 vol% were mechanically weak and broke easily during harvesting from solution, therefore were not mechanically tested.
TMPTMA vol% ratios were tested (not shown). However, microcapsules made with PDMS oil concentrations above 50 vol% were mechanically weak and broke easily during harvesting from solution, therefore were not mechanically tested.
3.5 Mechanical properties of PDMS:TMPTMA microcapsules
Once generated, the PDMS:TMPTMA double emulsion drops were collected and fully UV-crosslinked for an additional 30 minutes. Microcapsules were harvested from the solution and dried for 24 hours under ambient conditions. During drying, the highly volatile 1 cSt PDMS oil evaporated from the shell leaving behind a porous microstructure composed of TMPTMA clusters as shown in Fig. 5c and d. Water also evaporated from the core. The porous structure is due to phase separation between the PDMS oil and the TMPTMA during UV-crosslinking. Similar phase separation has been observed in fabricating thin films composed of TMPTMA and different solvents.39,41,42 During UV-crosslinking of pure TMPTMA, nanoscale nucleation sites form, quickly growing and coalescing into a dense structure. When PDMS oil is added, the TMPTMA nucleation growth is suppressed, forming an interconnected network of TMPTMA particle aggregates. Porosity increases with PDMS oil concentration while the shell strength decreases. A close-up of a region of the shell showing the TMPTMA clusters is shown in Fig. 5d. The microstructure shown in Fig. 5d shows a higher porosity compared to the dense and microscale-pore-free microstructure shown in Fig. 3c and d for the 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 PDMS
100 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA microcapsules. Similar porosity has been reported for other polymer diluent mixtures.25,26
TMPTMA microcapsules. Similar porosity has been reported for other polymer diluent mixtures.25,26
A dense polymer layer with a thickness between 200 and 300 nm covered both the inner and outer surfaces of the shell for samples with PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA ratios above 30
TMPTMA ratios above 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 as shown in Fig. 5c and d. These dense, thin films have been previously reported and attributed to the presence of a poor solvent such as water.43 Work by Davidson et al.44 on polyvinyl chloride (PVC) particles showed that this type of skin layer is formed by adsorption and grafting of the agglomerated molecules at the interface.45 The TMPTMA monomers nucleate near the poor solvent interface that rapidly coalesce into a thin film, correlating to the roughness observed in the inner surface of the skin layer (see Fig. 5d). As shown later, these dense TMPTMA layers help to encapsulate molecules that would otherwise permeate through the porous TMPTMA shell.
70 as shown in Fig. 5c and d. These dense, thin films have been previously reported and attributed to the presence of a poor solvent such as water.43 Work by Davidson et al.44 on polyvinyl chloride (PVC) particles showed that this type of skin layer is formed by adsorption and grafting of the agglomerated molecules at the interface.45 The TMPTMA monomers nucleate near the poor solvent interface that rapidly coalesce into a thin film, correlating to the roughness observed in the inner surface of the skin layer (see Fig. 5d). As shown later, these dense TMPTMA layers help to encapsulate molecules that would otherwise permeate through the porous TMPTMA shell.
The load–displacement curves for the compression test of representative 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 PDMS
70 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA ratio microcapsules are shown in Fig. 5a. Note that the curves appear more linear than the 0
TMPTMA ratio microcapsules are shown in Fig. 5a. Note that the curves appear more linear than the 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 PDMS
100 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA microcapsules shown in Fig. 4a. The failure load vs. PDMS
TMPTMA microcapsules shown in Fig. 4a. The failure load vs. PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA vol% ratios are shown in Fig. 5b. As the PDMS
TMPTMA vol% ratios are shown in Fig. 5b. As the PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA ratio increases, the failure load decreases, ranging from 0.138 N for microcapsules with a 0
TMPTMA ratio increases, the failure load decreases, ranging from 0.138 N for microcapsules with a 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 PDMS
100 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA ratio to 0.004 N for those with a 50
TMPTMA ratio to 0.004 N for those with a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio. The reduction in strength stems from increased shell porosity and the aggregated nature of the TMPTMA clusters. The load to failure decreases slightly for microcapsules made with <30
50 ratio. The reduction in strength stems from increased shell porosity and the aggregated nature of the TMPTMA clusters. The load to failure decreases slightly for microcapsules made with <30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 PDMS
70 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA ratios, while a significant reduction is observed for higher ratios. This trend can also be observed in both F0 and M for the TMPTMA samples, as shown in Table 1. For example, M decreases moderately from 4.98 to 4.53 mN μm−1 for microcapsules with ratios less than 30
TMPTMA ratios, while a significant reduction is observed for higher ratios. This trend can also be observed in both F0 and M for the TMPTMA samples, as shown in Table 1. For example, M decreases moderately from 4.98 to 4.53 mN μm−1 for microcapsules with ratios less than 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70, but a notable drop to 1.58 is seen when a 50
70, but a notable drop to 1.58 is seen when a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio is used. Similarly, F0 declines from 145.9 mN at a 0
50 ratio is used. Similarly, F0 declines from 145.9 mN at a 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 ratio to 4.85 mN at a 50
100 ratio to 4.85 mN at a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio. Like the 0
50 ratio. Like the 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 PDMS
100 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA only microcapsules, there is high variability in the failure load measurements due to the inhomogeneous thickness of the microcapsules and porosity. Samples made with 40
TMPTMA only microcapsules, there is high variability in the failure load measurements due to the inhomogeneous thickness of the microcapsules and porosity. Samples made with 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 and 50
60 and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 PDMS
50 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA ratios show a narrower standard deviation in the load to failure since their shell microstructure is too weak to support the applied load, and the shell orientation becomes less relevant. Furthermore, the sensitivity of the load sensor is insufficient. The wide distribution of the shell strength results in low values of m ranging from 14.3 to 2.09 (see Table 1). In practical terms, using a PDMS
TMPTMA ratios show a narrower standard deviation in the load to failure since their shell microstructure is too weak to support the applied load, and the shell orientation becomes less relevant. Furthermore, the sensitivity of the load sensor is insufficient. The wide distribution of the shell strength results in low values of m ranging from 14.3 to 2.09 (see Table 1). In practical terms, using a PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA ratio greater than 30
TMPTMA ratio greater than 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 for the fabrication of dry microcapsules is not recommended, as these microcapsules may lack the necessary strength to survive processing and drying. Nevertheless, microcapsules with ratios exceeding 30
70 for the fabrication of dry microcapsules is not recommended, as these microcapsules may lack the necessary strength to survive processing and drying. Nevertheless, microcapsules with ratios exceeding 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 can be utilized if they are maintained in suspension under low shear conditions.
70 can be utilized if they are maintained in suspension under low shear conditions.
3.6 Microcapsules encapsulation efficiency
While this work focused on tailoring the mechanical properties of TMPTMA microcapsules, their encapsulation efficiency was also evaluated, but not exhaustively. Testing started with resazurin (Mw = 251 g mol−1), a dye with weak fluorescence used in biology that has an intense purple/blue color in aqueous solutions, so leakage from the microcapsules can be seen with the naked eye. Non-porous 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 PDMS
100 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA microcapsules were prepared with a 10−3 M resazurin aqueous solution in the core as shown in Fig. 6(a). In the initial encapsulation experiments, considerable leakage occurred from the double emulsion droplets prior to UV-crosslinking as they traveled through the collection tubing. It appeared that diffusion through the TMPTMA shell was the main cause of the resazurin leakage and crosslinking should be done as soon as the drops are generated. Hence, the UV light was moved to a distance of approximately 10 cm from the drop formation location within the microcapillary devices, ensuring it wasn’t so close as to cause early crosslinking and clogging of the microcapillary device. Following these adjustments, the encapsulation efficiency improved, and the sole cause of resazurin leakage was due to the occasional breakup of the inner drops before crosslinking. Furthermore, the resazurin aqueous solution was successfully encapsulated in UV-crosslinked 60
TMPTMA microcapsules were prepared with a 10−3 M resazurin aqueous solution in the core as shown in Fig. 6(a). In the initial encapsulation experiments, considerable leakage occurred from the double emulsion droplets prior to UV-crosslinking as they traveled through the collection tubing. It appeared that diffusion through the TMPTMA shell was the main cause of the resazurin leakage and crosslinking should be done as soon as the drops are generated. Hence, the UV light was moved to a distance of approximately 10 cm from the drop formation location within the microcapillary devices, ensuring it wasn’t so close as to cause early crosslinking and clogging of the microcapillary device. Following these adjustments, the encapsulation efficiency improved, and the sole cause of resazurin leakage was due to the occasional breakup of the inner drops before crosslinking. Furthermore, the resazurin aqueous solution was successfully encapsulated in UV-crosslinked 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 PDMS
40 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA microcapsules as shown in Fig. 6c where the shell has a white appearance while the resazurin core is purple. 60
TMPTMA microcapsules as shown in Fig. 6c where the shell has a white appearance while the resazurin core is purple. 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 PDMS
40 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA microcapsules required gentle cleaning and postprocessing to prevent breakup since they were fragile, but we estimate that >90% survived when left in solution after cleaning. On a different test, a 10−3 M FITC–dextran (Mw = 4000 g mol−1) aqueous solution was successfully encapsulated in 0
TMPTMA microcapsules required gentle cleaning and postprocessing to prevent breakup since they were fragile, but we estimate that >90% survived when left in solution after cleaning. On a different test, a 10−3 M FITC–dextran (Mw = 4000 g mol−1) aqueous solution was successfully encapsulated in 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 PDMS
100 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA microcapsules. The fluorescence intensity of the microcapsules was similar over a span of two months when stored in solution and covered to prevent FITC bleaching, as shown in Fig. 6d and e. Microcapsules with a PDMS
TMPTMA microcapsules. The fluorescence intensity of the microcapsules was similar over a span of two months when stored in solution and covered to prevent FITC bleaching, as shown in Fig. 6d and e. Microcapsules with a PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA ratio greater than 0
TMPTMA ratio greater than 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 and containing an aqueous FITC–dextran solution in the core were not produced due to the porous shell structure, which impeded fluorescence imaging. However, successful encapsulation is anticipated due to the higher molecular weight of FITC–dextran, which impedes diffusion through the shell regardless of its PDMS-oil content. Additionally, UV cross-linking could be performed after collection as the double emulsion drops were stable in the solution.
100 and containing an aqueous FITC–dextran solution in the core were not produced due to the porous shell structure, which impeded fluorescence imaging. However, successful encapsulation is anticipated due to the higher molecular weight of FITC–dextran, which impedes diffusion through the shell regardless of its PDMS-oil content. Additionally, UV cross-linking could be performed after collection as the double emulsion drops were stable in the solution.
The encapsulation of DABCO, a commonly used catalyst, was also investigated and presented challenges not encountered with resazurin and PEG-dextran. DABCO has a molecular weight of 112.17 g mol−1 and is volatile under ambient conditions.46 As noted earlier, water evaporates through the PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA shells at all vol% ratios. Due to the volatile nature of DABCO, the objective was to assess if the microcapsules could prevent its evaporation. The testing started by fabricating microcapsules with 0
TMPTMA shells at all vol% ratios. Due to the volatile nature of DABCO, the objective was to assess if the microcapsules could prevent its evaporation. The testing started by fabricating microcapsules with 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 PDMS
100 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA shells and 0.2 g mL−1 aqueous DABCO solution in the core. Like with resazurin, it was essential to position the UV light near the drop-generation area in the microcapillary devices to prevent DABCO diffusion out of the double emulsion drops. After making these changes, DABCO was successfully encapsulated in 0
TMPTMA shells and 0.2 g mL−1 aqueous DABCO solution in the core. Like with resazurin, it was essential to position the UV light near the drop-generation area in the microcapillary devices to prevent DABCO diffusion out of the double emulsion drops. After making these changes, DABCO was successfully encapsulated in 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 PDMS
100 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA microcapsules and, during drying, the water evaporated from the core and DABCO precipitated, as shown in Fig. 7a. Since the pH of the DABCO aqueous solutions changes with concentration, a calibration curve was measured as shown in Fig. S3 (ESI†) and this was used to estimate the remaining amount of DABCO in the microcapsules from pH measurements. A solution series was prepared with 0.257 g of crushed dried microcapsules mixed with 10 mL of DI-H2O. If DABCO did not leak or evaporate from the microcapsules, the concentration of the crushed microcapsule DABCO solution should be 0.004 g mL−1 at a pH = 10.37, according to the calibration graph shown in Fig. S3 (ESI†). A pH of 10.33 ± 0.2 was measured, which corresponds to a DABCO concentration of 0.0035 g mL−1, which means that 93% by weight of the initial DABCO concentration remained in the 0
TMPTMA microcapsules and, during drying, the water evaporated from the core and DABCO precipitated, as shown in Fig. 7a. Since the pH of the DABCO aqueous solutions changes with concentration, a calibration curve was measured as shown in Fig. S3 (ESI†) and this was used to estimate the remaining amount of DABCO in the microcapsules from pH measurements. A solution series was prepared with 0.257 g of crushed dried microcapsules mixed with 10 mL of DI-H2O. If DABCO did not leak or evaporate from the microcapsules, the concentration of the crushed microcapsule DABCO solution should be 0.004 g mL−1 at a pH = 10.37, according to the calibration graph shown in Fig. S3 (ESI†). A pH of 10.33 ± 0.2 was measured, which corresponds to a DABCO concentration of 0.0035 g mL−1, which means that 93% by weight of the initial DABCO concentration remained in the 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 PDMS
100 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA microcapsules. Furthermore, the microcapsules could encapsulate DABCO even at higher temperatures, as shown in Fig. 7c. In these experiments, the weight of 10 g of TMPTMA microcapsules was monitored at 22 °C and 50 °C for up to 100 hours and compared to non-encapsulated DABCO samples. The initial DABCO weight for the non-encapsulated samples was 1.32 g to match the DABCO weight inside the microcapsules. The weight% of non-encapsulated DABCO at 22 °C decreases with time due to evaporation, and the rate increases at 50 °C. The mass of the microcapsules remained constant over time, indicating their high efficiency in encapsulating DABCO.
TMPTMA microcapsules. Furthermore, the microcapsules could encapsulate DABCO even at higher temperatures, as shown in Fig. 7c. In these experiments, the weight of 10 g of TMPTMA microcapsules was monitored at 22 °C and 50 °C for up to 100 hours and compared to non-encapsulated DABCO samples. The initial DABCO weight for the non-encapsulated samples was 1.32 g to match the DABCO weight inside the microcapsules. The weight% of non-encapsulated DABCO at 22 °C decreases with time due to evaporation, and the rate increases at 50 °C. The mass of the microcapsules remained constant over time, indicating their high efficiency in encapsulating DABCO.
Comparable pH DABCO experiments were conducted using porous microcapsules composed of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 PDMS
60 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA, initially containing a 0.2 g mL−1 aqueous DABCO solution in their core. A series of solutions were prepared with 0.146 g of crushed dried microcapsules mixed with 10 mL of DI-H2O. The pH of the solutions was 9.87 ± 0.3, corresponding to a DABCO concentration of 0.0001 g mL−1 or 20% by weight of the initial DABCO concentration that remained in the microcapsules. It seems that the leakage of DABCO was mainly due to evaporation through the shell during drying since some crude pH measurements in the solution indicate that the initial encapsulation was successful; however, more research is needed to corroborate this. Since 40
TMPTMA, initially containing a 0.2 g mL−1 aqueous DABCO solution in their core. A series of solutions were prepared with 0.146 g of crushed dried microcapsules mixed with 10 mL of DI-H2O. The pH of the solutions was 9.87 ± 0.3, corresponding to a DABCO concentration of 0.0001 g mL−1 or 20% by weight of the initial DABCO concentration that remained in the microcapsules. It seems that the leakage of DABCO was mainly due to evaporation through the shell during drying since some crude pH measurements in the solution indicate that the initial encapsulation was successful; however, more research is needed to corroborate this. Since 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 PDMS
60 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA microcapsules are opaque, no visual conformation of encapsulation was possible. These results suggest that porous microcapsules made using ratios >30
TMPTMA microcapsules are opaque, no visual conformation of encapsulation was possible. These results suggest that porous microcapsules made using ratios >30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 PDMS
70 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTA are ineffective at encapsulating low-molecular-weight volatile molecules such as DABCO but can effectively encapsulate chemicals with higher molecular weights. A more comprehensive study is needed to understand the effects of shell porosity on DABCO or a similar volatile molecule from PDMS:TMPTMA microcapsules.
TMPTA are ineffective at encapsulating low-molecular-weight volatile molecules such as DABCO but can effectively encapsulate chemicals with higher molecular weights. A more comprehensive study is needed to understand the effects of shell porosity on DABCO or a similar volatile molecule from PDMS:TMPTMA microcapsules.
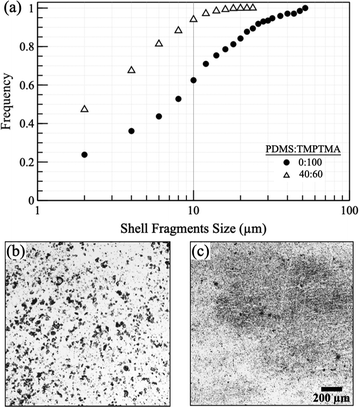
A potential advantage of porous shells is that they break into smaller fragments than nonporous ones. A series of experiments were conducted to quantify the difference in the size of the shell fragments between porous 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 and 0
60 and 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 PDMS
100 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTA microcapsules with a nominal shell thickness of ≈24 μm. Ten grams of each microcapsule were crushed in a mortar and pestle for 1 minute followed by optical imaging to digitally measure the size of the shell fragments according to their Feret diameter. Fig. 8 illustrates the cumulative size distribution of the crushed shell fragments, indicating that the 40
TMPTA microcapsules with a nominal shell thickness of ≈24 μm. Ten grams of each microcapsule were crushed in a mortar and pestle for 1 minute followed by optical imaging to digitally measure the size of the shell fragments according to their Feret diameter. Fig. 8 illustrates the cumulative size distribution of the crushed shell fragments, indicating that the 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 PDMS
60 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA sample exhibits a smaller fragment size distribution than the 0
TMPTMA sample exhibits a smaller fragment size distribution than the 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 PDMS
100 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA sample. For example, the arithmetic mean fragment sizes are 4.5 μm for the 40
TMPTMA sample. For example, the arithmetic mean fragment sizes are 4.5 μm for the 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 PDMS
60 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA and 11.4 μm for 0
TMPTMA and 11.4 μm for 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 PDMS
100 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA. Measurements coincide well with the visual size differences between the two samples as shown in Fig. 8b and c. These findings are anticipated because porous microcapsules are considerably less robust than their nonporous counterparts and can be broken with less force, as demonstrated in Fig. 5. The cumulative size data are limited by the resolving power of the 40× objective. Furthermore, reducing the fragment size of the 0
TMPTMA. Measurements coincide well with the visual size differences between the two samples as shown in Fig. 8b and c. These findings are anticipated because porous microcapsules are considerably less robust than their nonporous counterparts and can be broken with less force, as demonstrated in Fig. 5. The cumulative size data are limited by the resolving power of the 40× objective. Furthermore, reducing the fragment size of the 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 PMDS
100 PMDS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA sample could be achieved by increasing the crushing force, extending the crushing duration, or reducing the thickness of the shell. More detailed investigation is required to clarify the impact of these variables on the size of the shell fragment.
TMPTMA sample could be achieved by increasing the crushing force, extending the crushing duration, or reducing the thickness of the shell. More detailed investigation is required to clarify the impact of these variables on the size of the shell fragment.
4 Conclusions
Microcapsules with varying PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA ratios were successfully fabricated from double emulsion droplets produced in microcapillary devices. The strength of the microcapsules diminished as the PDMS
TMPTMA ratios were successfully fabricated from double emulsion droplets produced in microcapillary devices. The strength of the microcapsules diminished as the PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA vol % ratio increased, acting as a porogen during the UV-crosslinking of the shell. Microcapsules with a 0
TMPTMA vol % ratio increased, acting as a porogen during the UV-crosslinking of the shell. Microcapsules with a 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 PDMS
100 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA ratio exhibited no microscale porosity, were the most robust, and could encapsulate a low molecular weight volatile molecule such as DABCO. In contrast, microcapsules with PDMS
TMPTMA ratio exhibited no microscale porosity, were the most robust, and could encapsulate a low molecular weight volatile molecule such as DABCO. In contrast, microcapsules with PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA ratios exceeding 30
TMPTMA ratios exceeding 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 were mechanically weak and failed to encapsulate such molecules, but could encapsulate non-volatile molecules such as resazurin and FITC–dextran. Also, the shell fragment size was smaller for 40
70 were mechanically weak and failed to encapsulate such molecules, but could encapsulate non-volatile molecules such as resazurin and FITC–dextran. Also, the shell fragment size was smaller for 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 PDMS:TMPTMA microcapsules than 0
60 PDMS:TMPTMA microcapsules than 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 PDMS
100 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA ones.
TMPTMA ones.
The PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA system offers flexibility when designing and making microcapsules via double emulsion generation. Drop generation was stable for hours and grams of microcapsules were produced daily and production can be scaled up using a multinozzle microcapillary/microfluidic arrangment. It is possible to not only tailor the mechanical properties by mixing PDMS oil and TMPTMA, but also, the microcapillary setup permits the control of microcapsule diameter and shell thickness by adjusting the middle and inner fluid flow rates. If strong microcapsules are desired, then a mixture with a <30
TMPTMA system offers flexibility when designing and making microcapsules via double emulsion generation. Drop generation was stable for hours and grams of microcapsules were produced daily and production can be scaled up using a multinozzle microcapillary/microfluidic arrangment. It is possible to not only tailor the mechanical properties by mixing PDMS oil and TMPTMA, but also, the microcapillary setup permits the control of microcapsule diameter and shell thickness by adjusting the middle and inner fluid flow rates. If strong microcapsules are desired, then a mixture with a <30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 PDMS
70 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA ratio should be used. A >30
TMPTMA ratio should be used. A >30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 PDMS
70 PDMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMPTMA ratio works well when weaker microcapsules are needed in solution. Overall, the PDMS:TMPTMA chemistry combined with the exquisite control over the double emulsion generation process in microcapillary devices offers a robust system that could be used in a wide range of pressure-sensitive encapsulation applications.
TMPTMA ratio works well when weaker microcapsules are needed in solution. Overall, the PDMS:TMPTMA chemistry combined with the exquisite control over the double emulsion generation process in microcapillary devices offers a robust system that could be used in a wide range of pressure-sensitive encapsulation applications.
Author contributions
Congwang Ye – conceptualization, investigation, data curation, writing original draft, and writing review and editing. Bianka Pajo – data curation and writing review and editing. Carlos J. Martinez – funding acquisition, conceptualization, project administration, supervision, data curation, and writing review and editing.Data availability
Data for this article, including Excel datasets, optical images, and videos, are available at https://osf.io/vxqkh.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors wish to acknowledge the PPG corporation for their financial and technical support. Special thanks to David Walters and Dr. Scott Moravek for their invaluable technical guidance.References
- R. Kurapati, T. W. Groth and A. M. Raichur, ACS Appl. Bio Mater., 2019, 2, 5512–5527 CrossRef CAS PubMed.
- Y. Yoo, C. Martinez and J. P. Youngblood, ACS Appl. Mater. Interfaces, 2017, 9, 31763–31776 CrossRef CAS.
- E. Koh, N.-K. Kim, J. Shin and Y.-W. Kim, RSC Adv., 2014, 4, 16214–16223 RSC.
- T. Szabó, L. Molnár-Nagy, J. Bognár, L. Nyikos and J. Telegdi, Prog. Org. Coat., 2011, 72, 52–57 CrossRef.
- T. Nesterova, K. Dam-Johansen and S. Kiil, Prog. Org. Coat., 2011, 70, 342–352 CrossRef CAS.
- M. A. Trojer, L. Nordstierna, J. Bergek, H. Blanck, K. Holmberg and M. Nyden, Adv. Colloid Interface Sci., 2015, 222, 18–43 CrossRef.
- S. S. Bansode, S. K. Banarjee, D. D. Gaikwad, S. L. Jadhav and R. M. Thorat, Int. J. Pharm. Sci. Rev. Res., 2010, 1, 38–43 CAS.
- F. Cárdenas-Bailón, G. Osorio-Revilla and T. Gallardo-Velázquez, J. Microencapsulation, 2013, 30, 409–424 CrossRef PubMed.
- Y. Li, L. Ai, W. Yokoyama, C. F. Shoemaker, D. Wei, J. Ma and F. Zhong, J. Agric. Food Chem., 2013, 61, 3311–3319 CrossRef CAS.
- P. W. Chen, R. M. Erb and A. R. Studart, Langmuir, 2012, 28, 144–152 CrossRef CAS PubMed.
- M. W. Keller and N. R. Sottos, Exp. Mech., 2006, 46, 725–733 CrossRef CAS.
- P. W. Chen, G. Cadisch and A. R. Studart, Langmuir, 2014, 30, 2346–2350 CrossRef CAS PubMed.
- J. O. Zoppe, R. A. Venditti and O. J. Rojas, J. Colloid Interface Sci., 2012, 369, 202–209 CrossRef CAS PubMed.
- M. Kobašlija and D. T. McQuade, Macromolecules, 2006, 39, 6371–6375 CrossRef.
- A. P. Johnston, C. Cortez, A. S. Angelatos and F. Caruso, Curr. Opin. Colloid Interface Sci., 2006, 11, 203–209 CrossRef CAS.
- C. J. Martinez, B. Hockey, C. B. Montgomery and S. Semancik, Langmuir, 2005, 21, 7937–7944 CrossRef CAS.
- F. Caruso, R. A. Caruso and H. Mohwald, Science, 1998, 282, 1111–1114 CrossRef CAS.
- A. S. Utada, E. Lorenceau, D. R. Link, P. D. Kaplan, H. A. Stone and D. A. Weitz, Science, 2005, 308, 537–541 CrossRef CAS PubMed.
- K. Shirk, C. Steiner, J. W. Kim, M. Marquez and C. J. Martinez, Langmuir, 2013, 29, 11849–11857 CrossRef CAS PubMed.
- C. Ye, A. Chen, P. Colombo and C. Martinez, J. R. Soc., Interface, 2010, 7, S461–S473 CrossRef CAS.
- P. W. Chen, J. Brignoli and A. R. Studart, Polymer, 2014, 55, 6837–6843 CrossRef CAS.
- R. K. Shah, H. C. Shum, A. C. Rowat, D. Lee, J. J. Agresti, A. S. Utada and L.-Y. Chu, et al. , Mater. Today, 2008, 11, 18–27 CrossRef CAS.
- O. Okay, Prog. Polym. Sci., 2000, 25, 711–779 CrossRef CAS.
- W. L. Sederel and G. J. De Jong, J. Appl. Polym. Sci., 1973, 17, 2835–2846 CrossRef CAS.
- E. Loiseau, F. Niedermair, G. Albrecht, M. Frey, A. Hauser, P. A. Rühs and A. R. Studart, Langmuir, 2017, 33, 2402–2410 CrossRef PubMed.
- M. H. Mohamed and L. D. Wilson, Nanomaterials, 2012, 2, 163–186 CrossRef CAS.
- N. Z. Noriman, H. Ismail, C. T. Ratnam and A. A. Rashid, Polym.-Plast. Technol. Eng., 2010, 49, 228–236 CrossRef CAS.
- D. S. Kim and W. H. Seo, J. Appl. Polym. Sci., 2004, 92, 3921–3928 CrossRef CAS.
- S. K. Datta, A. K. Bhowmick, T. K. Chaki, A. B. Majali and R. S. Despande, Polymer, 1996, 37, 45–55 CrossRef CAS.
- R. Zivojnovic, Silicone oil in vitreoretinal surgery, Springer Science & Business Media, 2012, vol. 12 Search PubMed.
- J. D. Rule, N. R. Sottos and S. R. White, Polymer, 2007, 48, 3520–3529 CrossRef CAS.
- M. Roth, J. Polym. Sci., Part B: Polym. Phys., 1990, 28, 2715–2719 CrossRef CAS.
- H. Yang, Q. T. Nguyen, Y. Ding, Y. Long and Z. Ping, J. Membr. Sci., 2000, 164, 37–43 CrossRef CAS.
- P. D. Drumheller and J. A. Hubbell, J. Polym. Sci., Part A: Polym. Chem., 1994, 32, 2715–2725 CrossRef CAS.
- E. L. Lederer, Proc. World Pet. Cong. London, 1933, 2, 526–528 CAS.
- W. R. Shu, Soc. Pet. Eng. J., 1984, 24, 277–282 CrossRef CAS.
- B. Ambravaneswaran, H. J. Subramani, S. D. Phillips and O. A. Basaran, Phys. Rev. Lett., 2004, 93, 034501 CrossRef.
- C. Clanet and J. C. Lasheras, J. Fluid Mech., 1999, 383, 307–326 CrossRef.
- C. Viklund, E. Pontén, B. Glad, K. Irgum, P. Hörstedt and F. Svec, Chem. Mater., 1997, 9, 463–471 CrossRef CAS.
- P. C. Nicolson and J. Vogt, Biomaterials, 2001, 22, 3273–3283 CrossRef CAS.
- J. E. Rosenberg and P. Flodin, Macromolecules, 1986, 19, 1543–1546 CrossRef CAS.
- A. Schmid and P. Flodin, Die Makromol. Chem., 1992, 193, 1579–1589 CrossRef CAS.
- P. Reinholdsson, T. Hargitai, R. Isaksson and B. Törnell, Angew. Makromol. Chem., 1991, 192, 113–132 CrossRef CAS.
- J. A. Davidson and D. E. Witenhafer, J. Polym. Sci., Polym. Phys. Ed., 1980, 18, 51–69 CrossRef CAS.
- B. Törnell and J. Uustalu, J. Appl. Polym. Sci., 1988, 35, 63–74 CrossRef.
- A. Strachota, B. Strachotová and M. Šprková, Mater. Manuf. Processes, 2008, 23, 566–570 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sm01107d |
| This journal is © The Royal Society of Chemistry 2025 |