Open Access Article
Open Access ArticleA geothermal energy techno-economic analysis for downhole wellbore hydrogen production from biogas with subsurface carbon retention
S.
Gillick
 *a and
M.
Babaei
*a and
M.
Babaei
 *b
*b
aMetharc ApS, Copenhagen, Denmark
bDept. Chemical Engineering, University of Manchester, Manchester, UK. E-mail: stuart@metharc.com; masoud.babaei@manchester.ac.uk
First published on 16th June 2025
Abstract
Improving overall resource efficiency enhances energy security. Biogas is an important asset within waste management, transforming a range of organic waste into a higher-value product. By creating integrated partnerships, sector coupling highlights the synergies of Geothermal Energy, District Heating, Industry-CO2, Biowaste and Agriculture. This paper offers a perspective on a novel geothermal methodology for the wellbore reformation of biogas to generate hydrogen production with in situ carbon capture and storage (CCS) and proposes a new disruptive approach with a more immediate, direct and effective route to net zero. The methodology is referred to here as Carbon Injection and Gasification Geothermal (CIGG). The CIGG process combines several processes (i.e., hydrogen generation, carbon capture and biogas upgrading) with low-grade heat geothermal to eliminate process steps, saving process energy, costs, and materials, to create one, combined, sustainable solution. To capture these synergies, a wellbore methane reformation tool is proposed that exploits the natural geo-pressure from geothermal reservoirs and their associated formation fluid (hereafter power fluid). The hot injected CO2 waste stream eliminates the temperature depletion of the formation that is normally associated with geothermal power fluids. The immediate, in situ, downhole capture of CO2 will also enable improved geothermal power efficiencies from any CO2 partially recirculated within the power fluid. With geothermal wells having an expected life span of 15–25 years these synergies will enhance energy security for the long term. The CIGG process is proposed as a true win–win for both the energy economy and environmental stewardship, future-proofing biogas assets against emerging climate laws that restrict carbon production. It is climate-beneficial while creating a more holistic, sustainable CCS system that is a free byproduct of a net-energy production system, which simultaneously reduces carbon footprint to accelerate net zero goals. A techno-economic analysis was performed to estimate the cost of hydrogen generation, together with analysis supported by chemical reactions simulation covering energy and mass balance. These estimates show that with a biogas delivery of 4 MMSCFD (with 50% CO2 content), from 4 to 5 medium–high volume biomass Anaerobic Digestion plants (each generating 0.8–1.0 MMSCFD of biogas), it is possible to generate hydrogen at around 3 to 4 USD per kg from feeding 2 geothermal wells. Using a CIGG methodology, geothermal wells do not need to be drilled deep (e.g., 5000–7000 m) to reach hot reservoirs at >200 °C with normal geothermal temperature gradients. These high temperatures can now be realized using power fluids from shallower (e.g., 1500–2000 m), better quality, sedimentary reservoirs through heat recovery from the wellbore methane reformation tool. Importantly, geothermal power is now not limited by the geothermal depth of hot reservoirs. With a corresponding reduction in geothermal well costs by >50%, well depths will no longer dictate geothermal project economics. CIGG will create unrealized global scaling into geographical zones with high agricultural (or urban) biowaste and shallow sedimentary reservoirs of low geothermal gradient, enabling development of marginal projects, and expanding each sector in tandem.
1 Introduction
With the energy transition still in the starting blocks, increased focus on energy security, and gridlock in decarbonization technologies, more focus is required to develop and accelerate novel methodologies for renewable energy and sustainable exploitation of our biogas energy resources. This focus can be reduced to the following main areas: geothermal energy production, the decarbonization of methane for the generation of hydrogen at volume, and the capture and permanent storage of carbon (CCS).Existing technologies provide individual, part-solutions. The hydrogen industry buys its methane from the oil & gas industry and the biogas industry upgrades its biogas to biomethane (both venting or capturing their CO2), large industrial CCS projects inject CO2 waste from industry, and geothermal energy projects typically suffer from high well costs which dictate poor commerciality. To improve their climate credentials and commerciality, we can combine all these operations into one sustainable solution, simultaneously reducing climate damage and providing energy security. It is possible to future-proof biogas asset exploitation against climate laws that may restrict or stop carbon production, by creating a true green, circular economy. A combined approach would provide better overall energy efficiencies and economics than each of these individual energy-environment solutions alone, while proactively reducing climate damage.
This paper provides a perspective on this research gap with a proposed methodology that directly combines all the above points through the focus of geothermal energy and its exploitation of biogas† for the generation of wellbore hydrogen production with simultaneous subsurface carbon sequestration. The methodology is referred to here as Carbon Injection and Gasification Geothermal (CIGG). It is designed as a win–win solution for both the energy economy and environmental stewardship.
Geothermal energy meets the requirements of global climate restrictions which demand clean, carbon-free, energy sources. However, current geothermal projects are restricted geographically to high geothermal gradient regions and have high up-front capital costs for the wells (typically 2500–3000 m vertical depth) which are expensive to construct (especially for agricultural or urban biowaste communities). Although industry experience has demonstrated that wells can be maintained over a typical lifespan of 15-to-25 years, the commerciality of geothermal energy needs improving to encourage its global utilization, and so cheaper wells with additional revenue streams need to be developed.
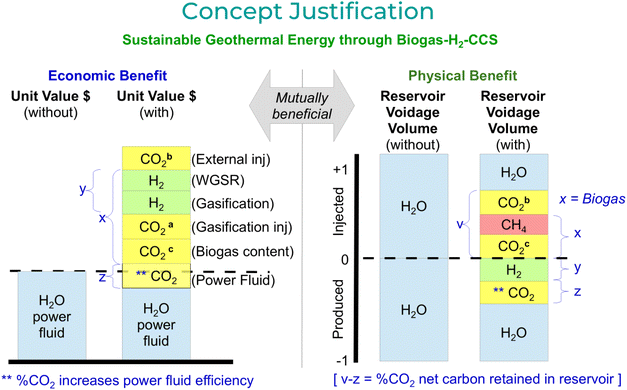
The proposed CIGG methodology offers to resolve this commercial shortfall by enhancing geothermal energy within a biogas context, increasing the economic longevity of existing infrastructures. It brings together and simplifies the above points by reducing the number of overlapping or duplicated process steps required to reach the products (i.e., enhanced geothermal energy, hydrogen and CCS). These energy savings through process step omissions also reduce the environmental impact of energy generation, benefiting society. A percentage of the captured CO2 can also be recycled within the power fluid‡ to enable improved geothermal power efficiency. Locating new geothermal wells close to biogas resources provides an opportunity to develop geothermal energy in tandem with a biogas-hydrogen economy, improving overall resource efficiency. A decentralization of power production creates a ‘behind-the-meter’ independence for local communities where hydrogen, power & water requirements are better serviced. The unique synergy between geothermal energy, district heating, industry-CO2 and biogas (from agriculture, landfill or urban biowaste) enhances all sectors in parallel, reducing greenhouse gas (GHG) transportation and allowing one sector to benefit from the climate issues generated by the other [Fig. 1].
 | ||
| Fig. 1 An illustrated flow diagram of enhanced geothermal energy within the context of a green circular agricultural economy. CCS = carbon capture and storage. | ||
Fig. 2 illustrates how a cluster of farms could become self-sustaining. By establishing separate ring networks to and from a central group of geothermal wells, several essential utilities could be shared. This could include, for example,
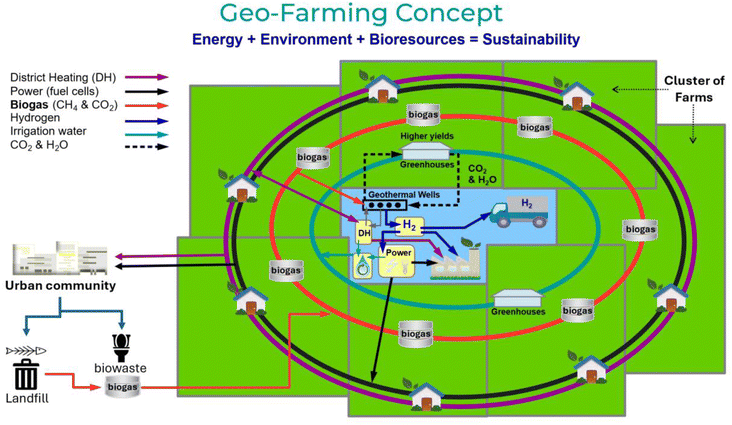 | ||
| Fig. 2 An illustrated example of a cluster of farms with centrally located geothermal wells. The biogas labels refer to anaerobic digesters (AD) which create biogas and digestate eco-fertilizers. | ||
• District heating (DH) from geothermal heat exchange
• Power generation (from enhanced power fluid temperatures and hydrogen fuel cell combustion)
• Biogas delivery to geothermal wells (for hydrogen generation and CCS)
• Irrigation water (biproduct from hydrogen fuel cell combustion)
• CO2 and H2O circulation to greenhouses (improving crop yields)
with the hydrogen generated also providing fossil-free fuels for transportation and factory processing.
This novel process is distinctly different from in situ, reservoir hydrogen generation techniques associated with hydrocarbon reservoirs,1 in that geothermal reservoirs contain no commercial hydrocarbon. The CIGG methodology utilizes a tool placed in the geothermal wellbore completion to gasify the surface-injected biogas methane content. It is suggested that a completion tool provides a more controllable and less uncertain reformation process, in an accessible wellbore space, without the need for hydrocarbon reservoir management techniques to produce hydrogen.
The methane (CH4) content of biogas typically ranges from 45% to 75% by volume, with most of the remainder being carbon dioxide (CO2).2 Currently, biogas is upgraded to biomethane§, which is then blended into natural gas networks, producing CO2 when burned. As climate-driven government policies mandate reductions in the use of carbon-based, CO2-emitting fuels, this will lead to problems with the future generation, use and disposal of biogas. Using the proposed CIGG methodology, there is a significant opportunity for the geothermal energy sector to couple with the biogas sector to future-proof the generation of these methane reserves while improving both CCS sustainability and geothermal commerciality. Biogas will remain part of our energy mix, so it makes sense to adapt and repurpose this valuable energy resource and utilize it in the most climate-beneficial way possible.
In the next sections, we described our methodology (the wellbore tool), a basic analysis of the tool's efficiency, and discuss the benefits of the tool for CCS. Finally, we present the conclusions of the paper.
2 Methodology
2.1 Carbon injection and gasification geothermal (CIGG)
The CIGG methodology is an enhanced geothermal process that enables capture within the geothermal reservoir of both the biogas' own CO2 content and the CO2 generated from its CH4 reformation. As the process simultaneously captures all the carbon downhole, climate and carbon footprint gains are immediate. The total CO2 is injected into the reservoir, where it is partly sequestered within the formation, and (dependent on well spacings) partly produced within an adjacent geothermal well's power fluid. Depending on the well pattern, the temperature of the injection fluid, and the percentage of CO2 produced within the power fluid, this could increase the overall energy efficiency by 15 to 50%,3 and thereby the energy economics of the geothermal operations.This innovative geothermal process4,5 radically shifts the biogas (methane) reformation for the production of hydrogen from surface to downhole. Once injected at surface into the tubing, the biogas composition flows down to the tool, set deep within the wellbore. The methane content is subjected to a reformation process where it is converted into hydrogen and CO2. The flow diagram [Fig. 3] shows an outline of the process from surface facilities to wellbore and reservoir.
The process takes maximum advantage of the free energy provided by the elevated wellbore temperatures and pressures within the surrounding fluid-connected formation. This elevated environment reduces the process energy input requirements for the biogas reformation, as the deep wellbore becomes both pressure vessel (naturally compressing fluids) and thermal insulator (a ‘thermos flask’ effect), saving energy. As natural reservoir temperatures (e.g., 80–150 °C) will not be hot enough to initiate methane reformation reactions (e.g., 400–600 °C), additional, but lower, process heat input is required and provided by an ignition source fed by an electric cable (e.g., analogous to an electric submerged pump)6 [ref. 7, and references therein]. The hotter the reservoir, the lower the additional process heat input requirement from the electric cable, saving energy. Once at steady-state, the reactions can be maintained through Auto-Thermal Reforming (ATR) through the introduction of air content within the surface biogas injection stream, with the reformation process conducted with the aid of catalysts.
At reservoir depths of 2000–3000 meters most of the biogas reaction components (i.e., CH4, CO2 and H2) are naturally in their supercritical states. Water only requires an additional boost of temperature to reach supercritical conditions¶ as its supercritical pressure is already achieved at well depths > 2200 m TVD. This incremental boost in temperature is initially provided by the ignition source from the electric cable and subsequently maintained by the heat generated from the ATR (>400 °C). Due to this naturally provided wellbore supercritical environment, the methane reformation reactions are more rapid and use less overall process energy to generate the hydrogen when compared to a surface-based Steam-Methane Reforming (SMR) reaction chamber. The injected biogas composition fluids are mixed within the wellbore tool, and a supercritical water oxidation (SCWO) process provides methane reformation. Hydrothermal flames are produced in aqueous environments at supercritical conditions. Such flames are formed when fuel and oxidant streams are mixed in conditions that enable autoignition. The role of the high pressure is to reduce the temperature needed for autoignition and allow controlling hydrothermal flames at temperatures around 400–500 °C. Oxidizing at these temperatures permits a reduction in the concentration of combustible material (biogas) in the feed, so more methane is available for hydrogen generation. The operation under hydrothermal flames allows total oxidation within milliseconds of residence times with lower process energy,8 leading to a further reduction in process energy input from the surface to initiate or propagate the process.
The methane reformation reaction products then move into the separation stage. The pre-generated hydrogen is then filtered out of the methane reformation product stream via an electrochemical separation process, which electronically sieves the hydrogen into an isolated chamber, where it is then produced independently to the surface. There will be an upper limit to the rate of hydrogen transfer, which will depend on the tool size (available membrane surface area), electrochemical membrane material composition and its operating parameters. The electrochemical hydrogen separation (EHS) process provides hydrogen at high purity and maintains its ambient deep wellbore pressure. This approach does not promote an electrolysis process to generate hydrogen, as the hydrogen is previously generated in an indirect internal reforming (IIR) process within a previous tool stage. The EHS process consumes far less power in transferring the pre-generated hydrogen into an isolated chamber, than if the membrane itself were used to either generate the hydrogen through the electrolysis of a water phase or used in direct internal reforming (DIR) of biogas. Siqens9 suggests a reduction in the power requirement by around 90% per kilogram of hydrogen for an EHS process when compared to electrolysis. It is worth noting that 1 kg of hydrogen contains 33 kW h of energy. A typical range of values for each process are shown below.
• Electrolysis = 45–55 kW h per kg H2 (e.g., 136–166% of the H2 energy content)
• Surface based SMR (without CCS) = 12–17 kW h per kg H2 (e.g., 36–51% of the H2 energy content)
• EHS = 3–5 kW h per kg H2 (e.g., 9–15% of the H2 energy content)
The electricity for the tool's electrochemical process is provided via the surface cable and an internal heat energy recovery system (HERS) within the tool (analogous to a turbocharger or dynamo). A HERS further reduces process energy input from the surface and potentially provides an opportunity for the export of electricity once steady-state conditions are met.
The CO2 waste flow stream is diverted downhole and directly injected into a suitable formation of choice. This avoids the unnecessary CO2 journey to the surface, mitigating the need for surface re-compression equipment, costly specialist CO2 metallurgy in the wellbore and surface separation and processing equipment.
2.2 CIGG well design
An illustration of a standard geothermal well design and completion is shown [Fig. 4], where colder groundwater is injected into one well while hotter ground water (power fluid) is produced from another.In comparison, the CIGG well design incorporates biogas injection and hydrogen generation with in situ CCS within its process, giving immediate climate, energy, and cost advantages [Fig. 5].
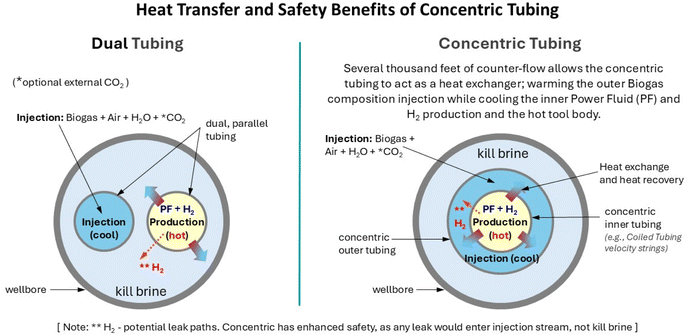
A dual, or concentric, tubing string would be required to provide the separate wellbore counter-flow of the produced hydrogen and the surface-injected biogas composition. The illustration [Fig. 6] shows an example of the heat exchange benefits of using concentric tubing within a 9–5/8′′ wellbore casing. It can be seen that there is an increase in cross-sectional areas (with an associated reduction in pressure drop), when concentric tubing versus parallel tubing strings are used. For example, parallel 3–1/2′′ tubings will each typically have a cross-sectional area of 7.4 inches2. In comparison, the cross-sectional area between typical 7′′ × 4–1/2′′ concentric tubings is 12.3 inches2 while a typical 4–1/2′′ tubing itself has a cross-sectional area 12.3 inches2. This concentric flow geometry provides a 66% increase in cross-sectional flow area for both the hot, inner, hydrogen production and cold, outer, biogas composition injection flow streams. Different tubing sizes depending on individual flow rate capacity requirements will change the benefits for either flow stream.
From a safety perspective, the use of concentric tubing reduces the risk of hydrogen leakage into the wellbore annulus kill brine (which has a greater potential if parallel tubing strings were used). Any hydrogen leak path from the inner concentric production tubing would then flow into the outer concentric injection tubing and enter the biogas (CH4, CO2, air and H2O) injection stream, impeding leakage into the kill brine. The concentric tubing will also act as a ∼2000 m long heat exchange system. Heat is transferred from the inner, hotter hydrogen string to preheat the outer, cooler biogas injection string, further lowering process input energy requirements. This injection stream will also help regulate the external temperature of the wellbore reformation tool through heat recovery, in addition to the tool's internal HERS.
The CO2 waste fluids from the hot wellbore tool are hotter than the reservoir when injected into the formation and so are slightly more viscous compared to typical CO2 streams recommended at 40 °C (and so less likely to override the reservoir resident fluid).10 Moreover, with a hotter injection fluid and the cooler reservoir resident fluid, there is no thermal expansion of the injected CO2 composition.11 This leads to a lower Joule–Thompson cooling effect due to gas expansion within the reservoir, and injection pressure does not increase.
For shallower, cooler reservoirs, or cooler CO2 injection temperatures, any pore space reductions due to potential CO2 hydrate formation in the near wellbore area will reduce permeability, lower injection rates, and increase injection pressures. Reservoir temperature reductions can also exist in the near wellbore area due to extended periods of colder water injection.12 Current industry solutions exist to manage this hydrate risk (e.g., hydrate chemical inhibitors, surface warming of flow stream with thermally insulated tubing); however, they are costly and energy intensive. The CIGG hotter injection stream of supercritical CO2 and water contrasts with normal, colder injection streams of similar or higher CO2 content fluids. For pressure depleted, or shallower, lower-pressured reservoirs below the hydrate stability temperature the risk of CO2 hydrate formation is reduced in the near wellbore area of the reservoir when injecting hotter fluids. The CIGG process more efficiently manages reservoir entry temperature risks associated with CO2 hydrate formation, with potential for reducing or eliminating the amount of chemical additives used for hydrate inhibition. As the volumetric ratio of CO2 increases, the time of decomposition and equilibration becomes longer and leads to the creation of dense and relatively stable hydrates.13 For shallower cooler reservoirs, the formation of CO2 hydrates (instead of CO2 gas) could lead to increased CO2 storage stability (i.e., leak reduction) for CCS reservoirs. Any leaks in wells, depending on the CO2 flow rates, could potentially convert to CO2 hydrates prior to reaching the surface.14 For the hot CIGG waste stream, CO2 hydrates formation could be chemically induced15 to form laterally deeper into the shallower, cooler reservoir (i.e., further away from the hotter wellbore area), where the hot injected flow streams cool down to ambient reservoir temperature. More research is required to quantify this chemically induced CO2 hydrate storage potential.
As an optimization (depending on existing well designs, their location and number, and the availability of additional reservoirs with good characteristics), a CIGG tool could be placed in all geothermal wells, converting all wells into dual injection and production wells. Using CIGG in all wells would increase total H2 production while also thermally boosting the geothermal power fluid by heat recovery from the hot CIGG tool (methane reformation > 400 °C). This would increase well utility, reduce the required well count and improve project cost efficiency [Fig. 7]. The surface plant water treatment would be required to follow environmental standards and regulations for mixing reservoir fluids from different aquifers.
Depending on reservoir wellbore spacing, the geothermal production of a free aqueous phase will occur only for an initial limited time (a few years) from an Enhanced Geothermal System (EGS) operation with CO2 injection, but water will persist in the CO2 production stream for decades.16 Similar to the Negative Saturation (NEGSAT) and the Two Stage Integrated Geothermal-CCS approaches, stage one is formation brine extraction to provide pressure relief (i.e., reservoir voidage) for the CO2 composition injection, and stage two begins when the CO2 composition reaches production wells. Co-produced brine and CO2 are then the working power fluid. Injecting moderate amounts of CO2 combined with production water into geothermal reservoirs has several advantages; enhances residual trapping, reduces mobility ratio, enhances spreading, and also takes advantage of single-phase dissolved CO2 injection which avoids confining the gaseous CO2 to the upper part of the reservoir hence decreasing the leak risk via the cap rock.16
The concept of Active CO2 Reservoir Management (ACRM) combines brine extraction and treatment and residual-brine re-injection with CO2 injection.17 This approach was named tandem-formation ACRM. If the reservoir has sufficient trapping characteristics, brine disposal options, reasonable formation temperature, and proximity to CO2 emitters, then ACRM can be applied to the separate formations with one formation being utilized for CO2 storage and a separate formation can be utilized for brine re-injection. Previous research estimated permanent CO2 storage after 30 years is between 10 and 85%, dependent on well spacing.17 The CIGG proposed methodology would take the tandem-formation ACRM, or CO2-plume Geothermal (CPG) systems proposed for high permeability and high porosity reservoirs, one step further to improve commercial, geographical scalability.
At water subcritical conditions, catalysts can improve the thermal efficiency of the SMR/ATR by reducing the activation energy and the operating temperature to around 400 °C. The use of precious metals such as radium (Rd) has been documented to reduce the temperature for the onset of hydrogen production further to 240 °C, with peak production at higher temperatures.18
It should be noted that if the temperature of produced hydrogen gas is too high, i.e., above 584.8 °C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 the hydrogen would spontaneously combine with dissolved oxygen in the water to create more water. This auto-ignition is a process whereby a substance spontaneously ignites without any external flame or spark. The proposed CIGG process must therefore have an upper-temperature limit, including a temperature safety margin, below this auto-ignition temperature. Hence a heat recovery system using the cooler, reservoir power fluid, externally cooling the tool and its exhaust, is paramount. The blending of the reservoir power fluid with the hydrogen production above the tool (ref. Fig. 7), will further serve to maintain the hydrogen flow temperature below this critical temperature.
19 the hydrogen would spontaneously combine with dissolved oxygen in the water to create more water. This auto-ignition is a process whereby a substance spontaneously ignites without any external flame or spark. The proposed CIGG process must therefore have an upper-temperature limit, including a temperature safety margin, below this auto-ignition temperature. Hence a heat recovery system using the cooler, reservoir power fluid, externally cooling the tool and its exhaust, is paramount. The blending of the reservoir power fluid with the hydrogen production above the tool (ref. Fig. 7), will further serve to maintain the hydrogen flow temperature below this critical temperature.
Any thermally induced formation fracturing, by injecting the hot CO2 waste fluids into the reservoir rock (from tool temperatures > 400 °C), could serve to improve the near wellbore connectivity (i.e., permeability), reducing skin|| (improving injectivity) and therefore potentially the number of wells required, or it can assist with increasing the well spacing. It may also reduce the need for expensive hydro-fracturing used in deep hot rocks.
2.3 Global geothermal resources and sustainable biogas – accelerating the green transition
Global geothermal resources can provide a secure source of continuous green power for the long term that would help protect national economies against fuel price fluctuations or geopolitical supply disruptions. For example, if the US could capture just 2% of the thermal energy available between two and six miles beneath its surface, it could produce more than 2000 times the nation's total annual energy consumption in 2005.20,21Geothermal temperatures increase with depth and vary in different parts of the Earth, ranging from 10 to over 80 °C km−1, with an average increase of about 30 °C km−1.22 Higher geothermal temperature gradients mean that geothermal boreholes can be drilled to shallower depths to achieve the same reservoir temperature. In general, reservoir temperature resources above 150 °C are used for electric power generation, while reservoirs below 150 °C are usually used in direct-use projects for heating.22–24 In addition, some thermal waters also have a small natural methane content which could be utilized within this proposed CIGG methodology, providing an additional hydrogen volume. Although shallower reservoir rocks may not be hot enough, they are still attractive in that they will have better reservoir characteristics (i.e., permeabilities and porosities) enabling higher flow rates and so require less expensive hydraulic fracturing to achieve this. In the upper part of the crust, there are more than 8 orders of magnitude of permeability variation. However, by depths of 5 km, the variation is down to about 5 orders; and by 10 km, the range is closer to 2 orders of magnitude.20 This depth dependency of increasing geothermal temperature with deteriorating reservoir properties clearly has a direct correlation to increasing well construction and completion costs.
With a combined public/private investment of about 800 million to 1 billion USD over a 15-year period, EGS technology could be deployed commercially on a timescale that would produce more than 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MWe (or 100 GWe) of new capacity by 2050.20 However, because of geographical and geological constraints, high up-front capital costs and other challenges, geothermal resources are barely utilized at all. For example, in Oct 2023, geothermal energy accounted for only 0.4%
000 MWe (or 100 GWe) of new capacity by 2050.20 However, because of geographical and geological constraints, high up-front capital costs and other challenges, geothermal resources are barely utilized at all. For example, in Oct 2023, geothermal energy accounted for only 0.4%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 of US electricity generation.
25 of US electricity generation.
Biogas plays an important part in waste management, improving overall resource efficiency, and yielding energy security benefits. Its energy contribution can be developed at scale through integrated partnerships with geothermal energy and district heating working closely with biowaste (agricultural and urban) and industry-CO2 to highlight their synergies. Through the transformation of a range of organic wastes into higher-value products, biogas fits well into the concept of the circular economy.
Global biogas resources are expanding rapidly. Data from the International Energy Agency (IEA) lists the biogas production in Mtoe (million tonnes of oil equivalent) by region and by feedstock. In 2018 Europe's biogas resources were 18 Mtoe (with 14 Mtoe (77%) provided by a combination of crops and animal manure).26 The regions Asia Pacific, North America, Central and South America, and Europe are each forecast with the potential to produce more than 100 Mtoe biogas per year, with more than half coming from crop residues and animal manure. Asia Pacific is alone in surpassing an expectation of more than 200 Mtoe.27 Similarly, a report from the World Biogas Association28 states, that if the available manure from all the 1.5 billion cattle, 1 billion pigs, 22 billion chickens and 0.2 billion buffaloes living today were anaerobically digested, there is a potential to generate 250 to 370 bcm (billion cubic meters) of biomethane per year. By comparison, a total of 1.94 bcm of biomethane was produced from European biomethane plants in 2017.29
While food demands increase in line with a growing population, the land area available to farming does not, so an increase is needed in resource utilization and efficiency to improve the productivity of farmland. This means better use and recycling of water (for irrigation), and the controlled distribution of heat and CO2 for increased greenhouse crop yields.30 The CIGG methodology would provide hot water and CO2 to enable this increased food production, energy efficiency and water use in synergy. It would also eliminate the requirement for greenhouse CO2 generators running on propane or natural gas (removing another non-capturable source of CO2 from the carbon emissions network) [Fig. 8].
3 Analysis of the proposed downhole tool
A methane reformation tool is currently under development at TRL-4, and we currently plan to have a TRL-6 prototype tool available for lab testing by end of 2026.As many of the tool components are available from mature industries, it is felt that the tool overall technical risk is low. However, there still remain technical challenges with the integration of components and the choice of suitable tool materials to operate under the supercritical operations within the deep, hostile wellbore environment. This high-pressure, corrosive, bottom-hole environment is very common within oil & gas wells and affects wellbore tools by reducing their run-life (mean time to failure) and their operability. Current industry solutions exist to manage many flow assurance risks (e.g., high operating temperatures, high internal flow rates, chemical inhibitor injection via control lines or chemical injection lines, process staging/zoning etc.). Our tool design will follow similar flow assurance and is currently being modelled using a digital twin of components in computational fluid dynamics (CFD) to help anticipate scaling, solids deposition and corrosion risks.
There will be necessary compromises required in materials choice between optimal tool performance and manufacturability, due to the restrictions in manufacturing processes. Additive manufacturing (AM) 3D printing of multi-materials is planned for key components. This is a new approach to downhole tool manufacturing and comes with its own present set of limitations in component size and materials used. Continued industrial research in this area is required to remove these manufacturing limitations.
While the supercritical reformation of the methane that generates the hydrogen is rapid, the key to minimising the overall tool length is the electrochemical hydrogen separation stage. In contrast to the methane reformation stage, the hydrogen separation is much slower and requires sufficient residence time for the hydrogen to be removed from the reformation product stream. Optimizing the rate of hydrogen transfer across the electrochemical membrane requires materials that will survive both the manufacturing process and the tool operation within the hostile wellbore environment. Continued materials research is required in this area to improve on these limitations.
3.1 Thermodynamic and reactions analysis
The wellbore reformation of methane was previously analyzed31 for natural gas wells, where a 1D reactor MATLAB computer code was developed to verify downhole H2 production. It was found that resident time and hydrogen production through reforming and conversion, are not adversely impacted by high-pressure and high-temperature conditions. The simulations showed a fast reaction time of a few seconds for the dynamic case, where the reactions become steady state. The model was deemed sufficient as proof of concept for downhole/wellbore H2 production. Other research has shown that operation under SCWO allows total oxidation within milliseconds of residence times with lower process energy.8 However, further detailed analysis is required to accurately reflect operational complexities, and a more detailed 3D analysis of the process at supercritical temperature and pressure conditions is currently in progress.The CIGG technical analysis is similar to the natural gas well process. The differences arise from the biogas (methane) being injected from the surface and the operation of a geothermal aquifer in place of a natural gas reservoir. For the heat-loss to the surrounding formations, the assumption in this analysis is that we are operating this downhole device deep within the wellbore, close to (or at) reservoir depth. This will likely mean, for most geothermal reservoirs, that nearly all reformation chemical components are naturally at (or close to) their supercritical condition. The heat transfer from the reformation reactions is a mixture of exothermic and endothermic reactions. This mixture of heat source together with heat sink was considered in the initial verification study. The process proposed is an ATR system, where the overall reformation reaction energy itself provides the excess energy required to propagate the process. There will naturally be heat transfer to the wellbore fluids and surrounding reservoir through the casing and cement, however, the computer code simulations indicate that the wellbore will provide a degree of insulation, creating a ‘thermos flask’ effect. As the temperature of the reformation products leaving the tool exceeds that of the injected fluids entering it, this leads to a thermal build-up over time that further enhances energy efficiency. With the use of heat recovery mechanisms, the following assumptions were made in the previous work:31
(1) A reformer/reactor chamber with a volume of 1 m3.
(2) The operating temperature is 600 °C, with a pressure of 40 bar.
(3) Overall molar flow rate is 2 × 104 kmol h−1 for a mixture of 40% CH4 and 60% H2O amounting to ∼35 kg s−1 of CH4 (162 MMSCFD). This upper limit rate exceeds many natural gas production wells, but this high magnitude rate was chosen to demonstrate that the methane reformation resident time of the tool was still only a few seconds based on the code.
(4) The energy required for the reactions is assigned as 1 × 109 kJ h−1.
(5) The following reactions are taking place in the reformer.
| CH4 + H2O → CO + 3H2 Steam Methane Reforming (SMR): endothermic (ΔH = 206 kJ mol−1) |
| CO + H2O → CO2 + H2 water–gas shift (WGS) reaction: exothermic (ΔH = −41 kJ mol−1) |
| CH4 + 2H2O → CO2 + 4H2 Direct Steam Reforming (DSR) reaction: endothermic (ΔH = 165 kJ mol−1) |
The co-produced CO2 from WGS (via SMR) and DSR are injected via immediate downhole sequestration. The hydrogen production rate based on the above input is ∼4 kg s−1 (145 MMSCF/D). Therefore, we can convert n volume of CH4 to almost 0.9n volume of H2 using the tool at 600 °C and 40 bar. The simulations showed promising results on residence time, with no major drawback from the reformation volume of the tool, together with high pressure and overall thermal efficiency of the process. Optimal thermal efficiency can therefore be achieved through a mixture of heat recovery, electrical power and autothermal reforming and the effective use of catalysts.
To adapt the code for CIGG, we adjusted the feed from the high-rate natural gas reservoir to that of a biomass AD, and its biogas feedstock composition rates. We can assume, based on a standard volume (at surface), for each 1 MMSCF of biogas-methane, we can produce an almost equal volume of hydrogen. The general composition of biogas is 45–60% methane (CH4), 40–55% carbon dioxide (CO2) and a minuscule quantity of 0.001–2% nitrogen (N2), 0–1% ammonia (NH3), and 0.005–2% hydrogen sulphide (H2S).32 In terms of volume, biogas generated from individual commercial AD biomass waste plants and facilities average in the order of ∼0.5–1.0 MMSCFD levels, with some reaching ∼5 MMSCFD levels.
The magnitude of the enhancement of the geothermal energy generated will depend on each reservoir's flow characteristics, and the associated field's wellbore number, placement, and design specifics.
The variation of CO2 content within biogas was highlighted above. The corresponding methane content variation within a biogas composition will affect the methane reformation performance and result in different concentrations of hydrogen being generated. Detailed analysis of this variation requires additional specific case studies which were not conducted. The aim of this paper is to illustrate the overall potential climate and energy gains that lay within the synergy of this methodology only, and not the degree of variation in the outcomes. Overall, there are positive gains to be made in hydrogen production and CCS climate benefits, and this paper serves as a starting point, calling for further investigation.
3.2 High-level economic analysis
SMR is the most widespread technology for large-scale hydrogen production from natural gas, though ATR is also in use. Natural gas in SMR facilities is both fuel and feedstock (together with water). Typically, 30–40% of the methane is combusted to fuel the process.33 The agricultural and biowaste industries have a clear feedstock advantage provided by their own AD biogas resources. For context, typical biomethane-to-grid capacities for individual operational AD plants in the UK range from 400–1000 m3 per h biogas (approx. 340![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–850
000–850![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ft3 per day), of which there are currently several hundred in existence.34
000 ft3 per day), of which there are currently several hundred in existence.34
To run a comparative economic analysis to the hydrogen production from natural gas wells,31 where the target hydrogen production was previously 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kg per day, we assumed a smaller capacity of 2000 kg per day H2 from the reformation tool in line with the lower biogas rates. This 2000 kg per day H2 is around 0.85 MMSCFD of H2 which requires around 1.0 MMSCFD of CH4 (i.e., 2 MMSCFD of biogas at 50% CO2). We assumed using the tool in two wells, so 4 MMSCFD of biogas would be required in total to generate 4000 kg per day H2. This is equivalent to 4 or more medium–high volume AD biogas plants each delivering 0.8–1.0 MMSCFD biogas feedstock to the centrally located geothermal wells (as suggested in Fig. 2).
000 kg per day, we assumed a smaller capacity of 2000 kg per day H2 from the reformation tool in line with the lower biogas rates. This 2000 kg per day H2 is around 0.85 MMSCFD of H2 which requires around 1.0 MMSCFD of CH4 (i.e., 2 MMSCFD of biogas at 50% CO2). We assumed using the tool in two wells, so 4 MMSCFD of biogas would be required in total to generate 4000 kg per day H2. This is equivalent to 4 or more medium–high volume AD biogas plants each delivering 0.8–1.0 MMSCFD biogas feedstock to the centrally located geothermal wells (as suggested in Fig. 2).
In order to make a direct comparison with our natural gas application,35 the same economic analysis method was used. This analysis was fully based on the standards and protocol of the H2A Production Model Version 3.2018 from the National Renewable Energy Laboratory (NREL) of the U.S. Department of Energy.36 The NREL H2A template for “current-central-natural-gas-with-CO2-sequestration-v3-2018” or SMR + WGS + PSA (Pressure Swing Adsorption) generates estimated and comparative costs for hydrogen production. The operational input parameters for the H2A template analysis were kept identical**. Due to uncertainty in the capacity for CO2 sequestration for any specific reservoir, we did not include any commercial gains for CO2 volumes injected. Therefore, depending on the specific reservoir, this can be a significant economic upside to the values quoted in this paper (noted in the results below).
Retrofitting the wells with the wellbore reformation tool are assumed to coincide with scheduled well maintenance well re-completion workovers†† to optimize cost efficiencies. The capital cost and replacement cost for workovers were given estimates of 5 million USD for each wellbore reformation tool and its associated equipment, with a replacement cost of 5 million USD every 5 years.6 This is a conservative cost estimate for a geothermal well based on the cost of the Direct Fuel Cell from FuelCell Energy.37 The incremental operating cost increase, for well production with the tool installed, is included in the NREL model's utility consumption.
To establish a minimum capital cost impact scenario, the hydrogen generated is assumed to be either consumed on-site within fuel cells for electric power generation or blended into a natural gas pipeline (with the H2 content held below 20% H2 to CH4 v/v). Currently, it is estimated that the maximum hydrogen content that existing natural gas facilities can tolerate without upgrading is 15–20% H2 by volume.38 This avoids any requirement for major capital cost upgrades for surface facility equipment and pipelines to transport the H2. Given the difference in orders of magnitude in the volumes of natural gas produced versus biogas, the blending limitation for hydrogen from biogas should not cause immediate concern.
The economics of this geothermal methodology were not limited by the CIGG tool's capacity, as higher CH4 rates were analyzed for the natural gas methodology.35 This CIGG economics is limited by the assumed biogas delivery rates; however, these rates can be higher when more biogas resources become available, improving economics further.
Importantly the CIGG revenue streams are intended to supplement the existing geothermal economics and not replace them. For existing projects where the tool could be retrofitted into the wellbore, there would still be revenue streams from the original geothermal power and District Heating streams. Additional revenues would depend on countries of operation, and would come from H2 sales, CCS carbon credits sales (e.g., 45Q), and CO2 tax deductions and/or hydrogen subsidies (e.g., 45VH2-GREET). The further benefit of enhanced power fluid temperatures could be achieved in low-grade heat geothermal projects for district heating. The benefits of reduced (shallower) well costs would only come with new well projects. This improved commerciality is intended to accelerate the expansion of the geothermal sector in tandem with the biogas sector by enabling previously economically marginal projects to proceed in geographical areas of low geothermal gradient and high agricultural (or urban) activity.
The H2A results for the cost of hydrogen generation alone are shown in Fig. 9(a) and do not include the additional benefits mentioned above. The cost to generate hydrogen for the base case is estimated to be only 3.07 USD per kg. The sensitivity analysis in Fig. 9(b) shows this cost can increase to 3.47 USD per kg if the target rate of H2 production reduces to 1600 kg per day per well and rises to 3.16 USD per kg if the total capital investment is increased by 10%. Doubling the hydrogen rate to 8000 kg per day per well would require 4 MMSCFD per well of biogas delivery, however, this would reduce the hydrogen cost from 3.07 USD to under 2.20 USD per kg. Therefore, we can conclude that the main influential parameters in the total cost of H2 production are the capacity of production per day and the capital workover cost. Any improvement in these parameters can substantially decrease the cost of H2 production per kg.
 | ||
| Fig. 9 Results of biogas scenario (a) the economic analysis and hydrogen production cost per kg for the base case, and (b) the tornado chart for sensitivity analysis of production cost. | ||
Finally, for this section, sector coupling significantly enhances the commerciality of both geothermal and biogas when analyzed in synergy, with the whole being greater than the sum of the parts [Fig. 10].
Geothermal project economics always needs to be balanced between the depth drilled to obtain a desired geological temperature (for the associated power fluid) versus the affordable well cost. As an illustration only, using the cost information for completed wells,20 a typical US geothermal well cost to a depth of 2500 m (8200 ft) was estimated at approximately 3 million USD (yr 2004). For comparison, an equivalent well to 1500 m (∼5000 ft) was shown as approximately 2 million USD (yr 2004), which would save a significant percentage of the well cost (i.e., 30%). With the well costs estimated as roughly 30% of the total costs for a 110 MW geothermal project,24 any reduction in well construction costs significantly benefits project economics. Using the above example of a 30% reduction in well costs, this would imply a 10% overall project cost reduction. Minimising construction costs will be critical, especially by reducing subsurface expenses – namely for drilling – which today constitute an estimated 60–80% of the total, including for the power plant and all other infrastructure.39 The proposed CIGG process encourages well construction at shallower well depths.
4 Discussion: CCS and accelerating net zero
The majority of CCS processes do not generate energy but are separate energy-commodity consuming processes themselves. Currently, most CCS projects are analogous to landfill garbage disposal sites. To create a financially and environmentally self-sustaining, cost-efficient CCS system it needs to be part of a net-energy generation process, with the capture of CO2 done at point source. This would mitigate any subsequent requirement for individual high-cost, high-energy and commodity consuming, downstream CCS processes for this same carbon.It has been estimated from their simulation work that between 5 and 7% of CO2 injected could be permanently stored in the reservoir, giving an equivalent total amount of CO2 sequestrated of 2 × 107 tons over a 25-year period.16 Focusing more financial resources on incorporating CCS within a wellbore reformation process makes both economic and climate sense. At-source carbon capture within the context of the carbon life cycle, will use only a fraction of the comparable time, cost, and process energy consumption of downstream atmospheric CCS systems. Producing hydrogen close to the biogas source would also minimize greenhouse gases from CO2 and CH4 transportation leaks.
The post-burn, downstream, carbon capture narrative serves to rationalize huge budgets for atmospheric carbon capture with the consumption of high process energy to recapture far, far less than 100% of the carbon released globally. Processes that capture this downstream atmospheric CO2 often create synthetic hydrocarbon fuels, in an energy-intensive, self-perpetuating, CO2 cycle, which once burnt, re-release this same CO2 back into the atmosphere. Even if we assume that synfuel processes are ‘carbon neutral’ (in that they would subsequently release the same amount of carbon back into our atmosphere that was first taken out), they still consume additional cost, commodities, and process energy, in a self-perpetuating, CO2 cycle that does not actively reduce the carbon in our atmosphere.
Although CO2 capture is energy demanding in all cases, the energy usage for synthesizing CO2 into e-fuels is a lot higher than for transporting and storing it. The energy use estimated for CCU is > 7000 kW h per ton CO2 whereas for CCS is < 1000 kW h per ton CO2.40 All downstream CCS processes involve an additional external process for the capture of carbon. For comparison, the energy requirement for direct air capture (DAC) processes is estimated at between 2000 and 2400 kW h per ton CO2 (double that of CCS). In addition, a 1 million-ton CO2 per year DAC system is estimated to have a large requirement for land use of 0.4 km2, plus between 1.5 and 65.6 km2 (increasing with the greenness of its energy source),41 which makes a significant impact on land availability for other uses (e.g., farming).
In comparison, using the CIGG alternative, the CO2 is captured as a byproduct of the wellbore methane reformation process. The energy use for CCS is therefore included within the CIGG net-energy production process and essentially zero, and free, making it sustainable. The green energy currently being used for these other various downstream CCS processes could instead be put to better use decarbonizing the energy infrastructure. The hydrogen produced by CIGG is an alternative fuel which also eliminates the non-capturable carbon emissions from the energy network (e.g., domestic and transportation).
The following example illustrates the potential for emissions reduction. Assuming a single AD plant with an average capacity of 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ft3 per day biogas,34 with an average biogas content of 50% CO2
000 ft3 per day biogas,34 with an average biogas content of 50% CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, a conversion factor of 0.055 kg CO2 produced per ft3 of CH4
2, a conversion factor of 0.055 kg CO2 produced per ft3 of CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42, and assuming 1 kg of CO2 = 19.253 ft3 (SCF),16 then an equivalent of 0.01 Mt per year total CO2 (CH4 generated plus CO2 content) would be produced and available for capture from an average AD biogas plant. The IEA estimates that global CCUS facilities currently capture more than 45 Mt CO2 annually (equivalent to ∼0.12 million metric tons/day‡‡) from around 40 commercial capture facilities.43 This is equivalent to the production of 4500 average AD biogas plants. By the end of 2017, there were 17
42, and assuming 1 kg of CO2 = 19.253 ft3 (SCF),16 then an equivalent of 0.01 Mt per year total CO2 (CH4 generated plus CO2 content) would be produced and available for capture from an average AD biogas plant. The IEA estimates that global CCUS facilities currently capture more than 45 Mt CO2 annually (equivalent to ∼0.12 million metric tons/day‡‡) from around 40 commercial capture facilities.43 This is equivalent to the production of 4500 average AD biogas plants. By the end of 2017, there were 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 432 biogas plants already operating in Europe29 of various capacities.
432 biogas plants already operating in Europe29 of various capacities.
Capturing the carbon at source (time-zero), within CIGG is a quicker and cheaper path to reducing carbon emissions and footprint. It will have an immediate impact on climate as there is no requirement for the biogas to upgrade to biomethane use. No carbon production = no carbon to capture. This approach aligns with the principles of carbon neutrality and provides a more streamlined and efficient solution. True life-cycle climate savings would be gained as carbon is not produced back to the surface, eliminating the harm that these greenhouse gases (GHG) do during their time spent transitioning through the environment. Through direct biogas injection, the proposed CIGG methodology would also help to minimize the flaring of biomethane due to any calorific value mismanagement at AD plants (for natural gas blending) and lower the risk of not being able to obtain natural gas grid connections or other grid capacity issues; all of which exist today.
Although biogas is a renewable resource, we need to optimize its use and exploit it responsibly. An expanding biogas energy resource can still be provided from the same biomass or biowaste sources within the green transition if we adapt and repurpose our current carbon value chain business model and its infrastructure towards sustainability. Switching the larger part of our focus away from downstream carbon capture [Fig. 11] and towards at-source carbon capture will reduce emissions and accelerate Net Zero.
With perhaps the exception of the cement industry, reducing carbon emissions at source using a wellbore methane reformation tool installed in both natural gas and geothermal wells [Fig. 12] would greatly contribute to the environmental sustainability of our energy supplies.
5 Conclusions
Sector coupling of the geothermal and biogas industries takes advantage of their synergies to re-invent themselves in an alliance with industry-CO2 to add their weight to the hydrogen and carbon capture economies. An economic estimate of H2 production for a CIGG process showed a profitable cost that can lead to additional income from geothermal wells, improving their commerciality. With carbon not produced back to the surface, this enables a significant reduction in facilities, process energy and costs in O&M associated with downstream CCS. Economics is further boosted using CO2 offset income. The value of the geothermal wellbore decarbonization of biogas would be greater than the sum of its parts, as the positive environmental and climate consequences downstream of the wellbore are far-reaching within energy, agriculture and society.Due to the overlap and potential for a combination of process steps, decarbonizing biogas within geothermal wellbores can lead to significant overall process energy savings compared to traditional methods of methane decarbonization. These savings would be compounded by the free process energy provided by the wellbore and the elimination of all steps involved in CCS.
Most importantly, by using a CIGG methodology, geothermal wells do not need to be drilled deep to vertical depths of 5000–7000 m to reach hot reservoirs at >200 °C with normal geothermal temperature gradients. These high temperatures can now be realized using cooler power fluids from better quality, shallower, sedimentary reservoirs at vertical depths of 1500–2000 m through heat recovery from the wellbore methane reformation tool. As a consequence, geothermal power is now not limited by the geothermal depth of hot reservoirs. With a corresponding reduction in geothermal well costs by >50%, well depths will no longer dictate geothermal project economics. CIGG would create unrealized global scaling into geographical zones with high agricultural (or urban) biowaste and shallow sedimentary reservoirs of low geothermal gradient, enabling development of marginal projects, and expanding each sector in tandem.
A zero-carbon approach to energy production can be achieved with the technology available today. Sustainable geothermal biogas exploitation has the potential to feed the growing hydrogen economy. This mutually beneficial solution would shift focus away from stopping biogas production, to instead enabling continued, sustainable exploitation of these viable global energy reserves. This ensures energy security will be maintained, together with industrial knowledge, work experience, high levels of employment and government tax revenues, as the oil & gas companies could take the lead and expand into biogas-geothermal energy.
Nomenclature
| ACRM | Active CO2 Reservoir Management |
| AM | Additive Manufacturing (3D printing) |
| ATR | Autothermal Reformation |
| AD | Anaerobic Digesters |
| ADBA | Anaerobic Digestion and Bioresources Association |
| bcm | Billion Cubic Meters |
| CCS | Carbon Capture and Storage |
| CCU | Carbon Capture and Utilization |
| CCUS | Carbon Capture Utilization and Storage |
| CFD | Computational Fluid Dynamics |
| CIGG | Carbon Injection and Gasification Geothermal |
| CPG | CO2-plume Geothermal |
| DAC | Direct Air Capture (CO2 capture) |
| DIR | Direct Internal Reformation |
| DSR | Direct Steam Reformation |
| EHS | Electrochemical Hydrogen Separation |
| EGS | Enhanced Geothermal Systems |
| GHG | Greenhouse Gas |
| GWe | Giga Watt equivalent |
| HERS | Heat Energy Recovery System |
| IEA | International Energy Agency |
| IIR | Indirect Internal Reformation |
| ISO | International Organization for Standardization |
| MATLAB | Matrix Laboratory (a programming language and environment developed by MathWorks) |
| MMSCF | Million Standard Cubic Feet |
| MMSCFD | Million Standard Cubic Feet per Day |
| MWe | Mega Watt Equivalent |
| Mtoe | Million tonnes of Oil Equivalent |
| NEGSAT | Negative Saturation |
| NREL | National Renewable Energy Laboratory (U.S. Dept of Energy) |
| O&M | Operations and Maintenance |
| PF | Power Fluid (Geothermal) |
| SCWO | Supercritical Water Oxidation |
| SMR | Steam Methane Reformation |
| TVD | True Vertical Depth (not the measured depth, MD, along the hole) |
| USD | United States Dollar |
| WGSR | Water Gas Shift Reaction |
| ppg | lb/gal (a unit of density commonly used within the oil & gas industry) |
Data availability
Data for this article, including the H2A Production Model Version 3.2018 template Excel worksheet from the National Renewable Energy Laboratory (NREL) of the U.S. Department of Energy36 together with the 1D reactor MATLAB computer code developed for the chemical analysis are both available on the figshare repository at URL https://doi.org/10.6084/m9.figshare.29117465.v1.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors would like to thank Metharc for giving their permission to publish this article.References
- P. Song and Y. Li, Simulation of hydrogen generation via in situ combustion gasification of heavy oil, Int. J. Hydrogen Energy, 2023, 49, 925–936, DOI:10.1016/j.ijhydene.2023.09.248.
- IEA (International Energy Agency), An introduction to biogas and biomethane, Website accessed 25/03/2024, https://www.iea.org/reports/outlook-for-biogas-and-biomethane-prospects-for-organic-growth/an-introduction-to-biogas-and-biomethane.
- A. Waqas and A. Javed, CO2 as a Working Fluid in Geothermal Power Plants: Comparison of Recent Studies and Future Recommendations, PROCEEDINGS, Thirty-Ninth Workshop on Geothermal Reservoir Engineering, Stanford University, Stanford, California, February 24-26, 2014, https://pangea.stanford.edu/ERE/pdf/IGAstandard/SGW/2014/Ahmed2.pdf Search PubMed.
- S. Gillick and A. Metharc, System and Method for Providing a Hydrogen (H2) Composition, Denmark, Patent nr. DK 181125, 2023 Search PubMed.
- S. Gillick and A. Metharc, New Electrochemical Reactor Design. Patent Pending, International Publication Nr, WO 2023/227313 A2, World Intellectual Property Organization (WIPO/PCT), International Bureau, 2023 Search PubMed.
- M. Pflueger, Electric Submersible Pump Survival Analysis, Department of Statistics, Texas A&M, 2011, pages 11–12. Website accessed 10th Jan 2014, https://web.stat.tamu.edu/sheather/PDF/Pflueger_MasterProjectPaper.pdf.
- S. T. Wismann, Electrically heated steam methane reforming, PhD thesis, Danish Technical University (DTU), Department of Physics, Surface Science and Catalysts, 2019, website last accessed 17th March 2024, https://backend.orbit.dtu.dk/ws/portalfiles/portal/197988747/PhD_Thesis_Sebastian_Wismann.pdf Search PubMed.
- J. P. S. Queiroz, M. D. Bermejo, F. Mato and M. J. Cocero, Supercritical water oxidation with hydrothermal flame as internal heat source: Efficient and clean energy production from waste, J. Supercrit. Fluids, 2015, 96, 103–113 CrossRef CAS.
- L. Schwarzott, Siqens GmbH press article dated 4th October 2023, Website access 16/05/2024, https://siqens.de/en/news/siqens-ehs-most-economical-method-for-hydrogen-production/.
- K. Pruess, Enhanced Geothermal Systems (EGS): Comparing Water and CO2 as Heat Transmission Fluids, Lawrence Berkeley National Laboratory, 2007, (website access 14/03/2024), https://www.osti.gov/servlets/purl/922829 Search PubMed.
- L. Pan, C. Doughty and B. Freifeld, How to Sustain a CO2-Thermosiphon in a Partially Saturated Geothermal Reservoir: Lessons learned from field experiment and numerical modelling, Energy Geosciences Division, Lawrence Berkeley National Laboratory, 2017, (website access 14/03/2024), DOI:10.1016/j.geothermics.2017.10.004.
- M. Aghajanloo and L. Yan, et al., Impact of CO2 hydrates on injectivity during CO2 storage in depleted gas fields: A literature review, Gas Sci. Eng., 2024, 123, 205250, DOI:10.1016/j.jgsce.2024.205250 , https://www.sciencedirect.com/science/article/pii/S2949908924000463.
- M. Aminnaji, et al., CO2 gas hydrate for carbon capture and storage applications (Parts 1 & 2), Energy, 2024, 131579, DOI:10.1016/j.energy.2024.131580.
- IEA (international Energy Agency), IEA Greenhouse Gas R&D Programme, Gas Hydrates for Deep Ocean Storage of CO2, Report number PH4/26 (page iv), Feb 2004 Search PubMed.
- P. J. Herslund, Thermodynamic and Process Modelling of Gas Hydrate Systems in CO2 Capture Processes, PhD Thesis, Center for Energy Resources Engineering (CERE), Department of Chemical and Biochemical Engineering, Technical University of Denmark (DTU), 2013 Search PubMed.
- UIG (Universal Industrial Gases, inc), Unit Conversion Data for Carbon Dioxide, website accessed 14th Dec 2023, https://www.uigi.com/co2_conv.html.
- T. A. Buscheck, M. Chen, Y. Sun, Y. Hao, et al., Two-Stage, Integrated, Geothermal-CO2 Storage Reservoirs: An Approach for Sustainable Energy Production, CO2-Sequestration Security, and Reduced Environmental Risk, Lawrence Livermore National Laboratory, Livermore CA USA Department of Civil and Environmental Engineering, Princeton University, Princeton, NJ USA 2012, https://www.osti.gov/servlets/purl/1035606/ Search PubMed.
- A. Cherif, R. Nebbali, J. W. Sheffield, N. Doner and F. Sen, Numerical investigation of hydrogen production via autothermal reforming of steam and methane over Ni/Al2O3 and Pt/Al2O3 patterned catalytic layers, Int. J. Hydrogen Energy, 2021, 46, 37521–37532, DOI:10.1016/j.ijhydene.2021.04.032.
- ISO/TR 15916:2015(E) International Organisation for Standardization, Basic considerations for the safety of hydrogen systems, website accessed 04/11/2023, https://www.iso.org/standard/56546.html.
- INL (Idaho National Laboratory), The Future of Geothermal Energy, Impact of Enhanced Geothermal Systems (EGS) on the United States in the 21st Century, Idaho National Laboratory (INL/EXT-06-11746), pages 18, 20, 33 & 69, Fig 1.8, 2006, website accessed 02/11/2023, https://energy.mit.edu/wp-content/uploads/2006/11/MITEI-The-Future-of-Geothermal-Energy.pdf.
- MIT Technology Review, March 7, 2023, Website accessed 02/11/2023, https://www.technologyreview.com/2023/03/07/1069437/this-geothermal-startup-showed-its-wells-can-be-used-like-a-giant-underground-battery/.
- CONCERTO, Geothermal Communities (a project of the CONCERTO initiative, co-funded by the European Commission within the FP7), Website accessed 02/11/2023, https://geocom.geonardo.com/.
- G. S. Hamm, et al.DoE, GeoVision, Harnessing the Heat beneath Our Feet (Page 36), U.S. Department of Energy, 2019, https://www.osti.gov/biblio/1735644 Search PubMed.
- IRENE, Geothermal Power Technology Brief, pages 16 & 23, Sept, 2017, website accessed 02/11/2023, https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2017/Aug/IRENA_Geothermal_Power_2017.pdf.
- EIA (US Energy Information Administration), Independent Statistics and Analysis, Website accessed 02/11/2023, https://www.eia.gov/tools/faqs/faq.php?id=427%26t=3.
- IEA (International Energy Agency), Biogas Production by Region and by Feedstock Type, IEA, Paris, 2018, Website accessed 02/11/2023, https://www.iea.org/data-and-statistics/charts/biogas-production-by-region-and-by-feedstock-type-2018 Search PubMed.
- IEA (International Energy Agency), Outlook for biogas and biomethane: Prospects for organic growth, World Energy Outlook special report, IEA, Paris, 2020, website accessed 25/03/2024, https://www.iea.org/reports/outlook-for-biogas-and-biomethane-prospects-for-organic-growth Search PubMed.
- S. Jain, et al., Global Potential of Biogas, WBA (World Biogas Association), 2019, p. 17, Website accessed 13th Dec 2023, https://www.worldbiogasassociation.org/wp-content/uploads/2019/07/WBA-globalreport-56ppa4_digital.pdf Search PubMed.
- EBA (European Biogas Association), Annual Statistical Report of the European Biogas Association (Abridged Version), pages 2–11, Brussels, Belgium, 2018, Website accessed 13th Dec 2023, https://www.europeanbiogas.eu/wp-content/uploads/2019/05/EBA_Statistical-Report-<?pdb_no 2018?>2018<?pdb END?>_AbrigedPublic_web.pdf.
- M. Poudel and B. Dunn, Greenhouse Carbon Dioxide Supplementation, HLA-6723, Sept, 2023, Oklahoma State University, Website accessed 02/11/2023, https://extension.okstate.edu/fact-sheets/greenhouse-carbon-dioxide-supplementation.html.
- S. Gillick and M. Babaei, In Situ Hydrogen Production from Natural Gas Wells with Subsurface Carbon Retention, SPE J., 2024, 29(4), 2119–2129, DOI:10.2118/219449-PA.
- D. Bora Synthesis of a Low Cost, Natural Base Solution and its Comparison for CO2 Scrubbing with KOH from Biogas, Advances in Thermofluids and Renewable Energy: Select Proceedings of TFRE 2020, 2021, p. 365 Search PubMed.
- IEA (International Energy Agency), The Future of Hydrogen (pages 39), Report prepared by the IEA for the G20, Japan, 2019, Website accessed 04/11/2023, https://www.capenergies.fr/wp-content/uploads/2019/07/the_future_of_hydrogen.pdf.
- ADBA (Anaerobic Digestion and Bioresources Association), UK, Members only database, Website accessed 04/11/2023, https://adbioresources.org/.
- S. Gillick and M. Babaei, An Economic Analysis of In-Situ Hydrogen Production from Natural Gas Wells with Subsurface Carbon Retention, SPE J., 2024, 29(6), 3401–3411, DOI:10.2118/219485-PA.
- M. Penev, G. Saur, C. Hunter and J. Zuboy, H2A Production Model Version 3.2018 User Guide, National Renewable Energy Laboratory (NREL) of the U.S. Department of Energy, Website accessed 8th Dec 2023, https://www.nrel.gov/hydrogen/assets/pdfs/h2a-production-model-version-3-2018-user-guide-draft.pdf[nrel.gov] Search PubMed.
- F. C. Jahnke, A Novel Hybrid Reformer-Electrolyzer-Purifier (REP) for Distributed Production of Low-Cost, Low Greenhouse Gas Hydrogen, 2017 Search PubMed.
- M. W. Melaina, O. Antonia and M. Penev, Blending Hydrogen into Natural Gas Pipeline Networks: A Review of Key Issues, National Renewable Energy Laboratory (NREL) of the U.S. Department of Energy, 2013, Website accessed 8th Dec 2023, https://www.nrel.gov/docs/fy13osti/51995.pdf Search PubMed.
- IEA (International Energy Agency), The Future of Geothermal Energy, page 79, 2024, https://www.iea.org/reports/the-future-of-geothermal-energy.
- T. J. Sorensen and K. Capion, The potential for Carbon Capture and Storage in Denmark (2023), pages 13, 14. CONCITO, Denmark's Green Think Tank, Website accessed 21st Dec 2023, https://concito.dk/files/media/document/The-potential-for-Carbon-Capture-and-Storage-in-Denmark.pdf.
- K. Leblin, H. Leslie-Bole, Z. Byrum, et al., 6 Things to Know About Direct Air Capture, point 6, World Resources Institute, (May 2, 2022). Website Accessed 21st Dec 2023, https://www.wri.org/insights/direct-air-capture-resource-considerations-and-costs-carbon-removal Search PubMed.
- EPA (United States Environmental Protection Agency), Greenhouse Gases Equivalencies Calculator - Calculations and References, Website accessed 02/12/2023, https://www.epa.gov/energy/greenhouse-gases-equivalencies-calculator-calculations-and-references.
- IEA (International Energy Agency), Carbon Capture, Utilisation and Storage, website accessed 25/03/2024, https://www.iea.org/energy-system/carbon-capture-utilisation-and-storage.
- H. Zheng, T. Yu, C. Qu, W. Li and Y. Wang, Basic Characteristics and Application Progress of Supercritical Water, IOP Conf. Ser. Earth Environ. Sci., 2020, 555, 012036, DOI:10.1088/1755-1315/555/1/012036.
Footnotes |
| † Biogas – produced from a variety of sources; agricultural biodigesters (crop and animal waste), landfill gas recovery systems (industry and domestic organic garbage), and wastewater treatment plants (industry and domestic sewage). Biogas is produced when the organic matter (Biomass) is broken down by naturally occurring micro-organisms via anaerobic digestion (in an oxygen-free environment). |
| ‡ Power fluid. Warm fluids drawn from underground, geological reservoirs to the surface, which (depending on their temperature) either produce steam for the generation of electricity or provide heat to domestic systems. |
| § Biomethane: if biogas is subsequently upgraded, by concentrating its' methane content to that comparable to natural gas (through the removal of its CO2 content), the biogas becomes known as bio-methane (also commonly referred to as renewable natural gas). |
| ¶ Water becomes supercritical above 374.3 °C and 22.1 MPa (221 bars or 3205 psi).44 For context, this supercritical pressure is equivalent to an 8.6 ppg water column to a vertical tool depth of approximately 7200 ft (2200 m). Other reformation reactants, such as CO2, CH4 and H2, all reach supercritical conditions at far lower temperatures and pressures than water. |
| || Skin: a dimensionless factor that accounts for the difference between the actual and theoretical pressure dropin a well. It's a parameter used in Darcy’s Flow Equation to quantify the impact of near-wellbore conditions onfluid flow. It quantifies the altered permeability around a wellbore, often resulting from drilling, completion, orworkover procedures. This zone of reduced or enhanced permeability is often called the “skin effect”. |
| ** For the NREL H2A template Excel worksheet36 for this CIGG refer to Data Availability Statement (DAS). |
| †† Workover: the process of performing major maintenance or remedial treatments on a well. In many cases,workover implies the removal and replacement of the production tubing string with its associated wellbore equipment. A workover is a way to extend the life of a well or improve its performance after production declinesor there are wellbore equipment failures. |
| ‡‡ To put this into perspective, this capture is far less than 1% of global production of Natural Gas ∼11 billion m3 per day (∼388 billion ft3 per day, equivalent to ∼21 million metric tons per day CO2) or the additional global production of oil ∼100 million bbls per day (equivalent to ∼43 million metric tons per day CO2 when converting at 0.43 metric ton CO2 per bbl42). |
| This journal is © The Royal Society of Chemistry 2025 |